Abstract
Background:
Individuals are exposed to air pollution and ionizing radiation from natural sources through inhalation of particles. This study investigates the association between cardiac arrhythmias and short-term exposures to fine particulate matter (PM2.5) and particle radioactivity.
Methods:
Ventricular arrhythmic events were identified among 176 patients with dual-chamber implanted cardioverter-defibrillators (ICDs) in Boston, Massachusetts between September 2006 and June 2010. Patients were assigned exposures based on residential addresses. Daily PM2.5 level was estimated at 1-km×1-km grid cells from a previously validated prediction model. Particle gross β activity was used as a surrogate for particle radioactivity and was measured from several monitoring sites by the U.S. Environmental Protection Agency’s monitoring network. The association of the onset of ventricular arrhythmias (VA) with 0–21 day moving averages of PM2.5 and particle radioactivity (two single-pollutant models and a two-pollutant model) prior to the event were examined using time-stratified case-crossover analyses, adjusted for dew point and air temperatures.
Results:
A total of 1,050 VA were recorded among 91 patients, including 123 sustained VA among 25 of these patients. In the single-pollutant model of PM2.5, each interquartile range (IQR) increase in daily PM2.5 levels for a 21-day moving average was associated with 39% higher odds of a VA event (95% CI: 12% to 72%). In the single-pollutant model of particle radioactivity, each IQR increase in particle radioactivity for a 2-day moving average was associated with 13% higher odds of a VA event (95% CI: 1% to 26%). In the two-pollutant model, for the same averaging window of 21-days, each IQR increase in daily PM2.5 was associated with an 48% higher odds of a VA event (95% CI: 15 to 90%), and each IQR increase of particle radioactivity with a 10% lower odds of a VA event (95% CI: −29% to 14%). We found that with higher levels of particle radioactivity, the effect of PM2.5 on ventricular arrhythmias is reduced.
Conclusions:
In this high-risk population, intermediate (21-day) PM2.5 exposure was associated with higher odds of a ventricular arrhythmia event onset among patients with known cardiac disease and indication for ICD implantation independently of particle radioactivity.
Keywords: Ventricular arrhythmia, Implanted cardioverter-defibrillators, Fine particulate matter, Particle radioactivity
Introduction
Both short and long-term exposure to particulate air pollution have been associated with cardiovascular morbidity1–3 and mortality4,5. Studies have shown that air pollution can influence the autonomic nervous system and in turn affect heart rate variability leading to arrhythmias6–9. While some studies examine acute air pollution effects on ventricular arrhythmias, few have explored both short and intermediate effects.
More recent research has tried to identify the relevant toxic components of particulate matter, which lead to cardiovascular events. Here we perform a novel analysis, assessing particle radioactivity as a contributing component in the association of air pollution with ventricular arrhythmias.
Individuals receive exposure to ionizing radiation from a variety of natural sources: decay products of radon (222Rn) and thoron (220Rn), cosmic radiation and natural radioactivity found in soil and food10,11. Exposure to this natural radiation can occur externally from cosmic or terrestrial radiation or internally through inhalation or ingestion. The National Council on Radiation Protection and Measurements found that individuals in the U.S. received 73% of their average annual dose of natural radiation through the inhalation of radon and thoron and their progeny12,13. Radon and thoron formed by the decay of radium and thorium diffuse through the ground, enter the atmosphere, and decay to solid progeny. These can attach to existing aerosol particles to form radioactive aerosols. Studies have shown that the majority of radioactive progeny attach to fine particles (particulate matter ≤2.5 μm aerodynamic diameter; PM2.5)14–16. Since PM2.5 can penetrate deep into the lung and enter circulation17,18, these radioactive aerosols may deposit ionizing radiation into the bronchial passages and alveoli and induce adverse health effects.
Many studies have highlighted the increased risk of cardiovascular diseases and mortality related to both short- and long-term exposure to high levels of ionizing radiation from the atomic bomb, nuclear spills, and occupational hazards at nuclear power plants or uranium mining19–24. Radiation therapy for the treatment of benign or cancerous tumors has also been associated with the incidence and progression of cardiovascular disease25–27. In particular, radiation therapy for breast cancer and Hodgkin’s lymphoma have been implicated in the development of cardiovascular disease, even though the targeted organs did not include the heart28–30. Recently, a few studies have looked at the potential cardiovascular effects of low-level radiation associated with air pollution particles31,32. None of these studies, however, has looked at arrhythmias specifically.
We examined the associations of short- and medium-term PM2.5 and particle radioactivity with the odds of ventricular arrhythmias both independently and together in a two-pollutant model. We used the detected ventricular arrhythmic onset events from dual-chamber implanted cardioverter-defibrillators (ICDs) from a longitudinal study of cardiac patients in Massachusetts. This is the first study to assess the effects of radioactive properties of particle matter on cardiovascular health through increases in ventricular arrhythmias and the first to report the effects of fine particulate matter in the ICD cohort.
Methods
Patient Population
Our patient population has been previously described33. In brief, patients were recruited from the Tufts Medical Center’s Cardiac Arrhythmia Center in Boston, Massachusetts between September 2006 and March 2010. The study included patients with prior implantation of a dual (atrial and ventricular) chamber ICD and who were older than 18 years of age. Patient exclusion criteria included chronic atrial fibrillation (AF), diagnosis of a terminal disease, or the inability to provide informed consent.
During their first study visit, after obtaining informed consent, patients participated in an interview-administered questionnaire collecting individual characteristics and sociodemographic factors. To obtain a complete medical history, information from each of the patient’s medical records was recorded on a form based on the National Cardiovascular Disease Data ICD Registry form33. Authors will not make their data available to other researchers due to the sensitive nature of the data collected for this study. The Institutional Review Boards at Tufts Medical Center and at the Harvard T.H. Chan School of Public Health approved the study protocol.
Ventricular arrhythmias
Information was collected from the implanted ICD devices beginning at a patient’s enrollment until June 30, 2010. The ICD provided an arrhythmia logbook and electrograms by direct download during a follow-up visit at the clinic or wirelessly via trans-telephonic transmission33. These records recorded information of any detected atrial or ventricular arrhythmic event and classified each episode as sustained or non-sustained. The treating physician programmed each device to detect and respond to certain heart rate thresholds according to the patient’s needs33.
Once clinicians downloaded the information, an electrophysiologist blinded to the particle radioactivity and air pollution data reassessed any suspected arrhythmia. Each confirmed ventricular arrhythmia was classified as a ventricular tachycardia (VT) or ventricular fibrillation (VF) that was treated by the ICD (sustained), non-sustained VT or VF (not treated by the ICD), sinus tachycardia, atrial fibrillation (AF), atrial arrhythmia other than AF, or not an arrhythmia. Sinus tachycardia events, noise, or oversensing recordings were disregarded following previous study protocols that also utilized the same cohort33. Further information about the classification of arrhythmias for this cohort can be found in Link et al. (2013).
The primary endpoint was all detected ventricular arrhythmias (sustained and non-sustained ventricular arrhythmias) and our secondary endpoint was sustained ventricular arrhythmias that required intervention by the ICD. The study excluded events that arose during the first 6 weeks after implantation of the ICD or events when the individual was admitted to a health care facility. Multiple events could occur on the same calendar day, but were only included if they were separated by a period of at least 60 minutes. An event day was characterized as a calendar day when one or more ventricular arrhythmias occurred.
Individuals were assigned exposures by linking their residential addresses to the closest PM2.5 or meteorological grid cell or the nearest particle radiation monitoring station.
PM2.5 and meteorological variables
We retrieved daily PM2.5 predictions at 1-km × 1-km grid cells in the continental U.S. using a well-validated model incorporating land use, meteorology, chemical transport models, and satellite remote sensing. Three models were trained using a neural network model, a random forest, and gradient boosting, and then ensemble averaged using a geographically weighted regression34. To obtain daily PM2.5 predictions, we linked each patient’s residential zip code to the nearest center of a 1-km × 1-km grid cell for their exposure estimate.
Dew point and air temperatures were obtained from the National Center for Environmental Prediction (NCEP) and the National Center for Atmospheric Research (NCAR) Reanalysis project at 32 km × 32 km grid cells in the continental US35. Values for these variables were assigned to each patient by linking their residential zip code to the closest 32 km × 32 km grid cell.
Particle radioactivity
The study utilized particle gross β activity as a proxy for total particle radioactivity. Hernández et al. (2005) found a significant linear correlation of R=0.72 between gross β and gross α activity36. The strong correlation between β and α radiation suggests that gross β activity can represent all long-lived radon progeny (including α emitter 210Po, not just 210Pb). Previous studies using methods similar to RadNet have shown that levels of gross β activity are a good qualitative indicator of radiation activity for particles collected on air sampling, and specifically radiation due to 210Pb, a long-lived radon progeny32,37.
The Environmental Protection Agency (EPA)’s RadNet monitoring network, which includes approximately 140 radiation air monitors around the United States, provided the information on gross β activitity38. RadNet started collecting data on radioactivity in 1973 when several different radiation systems were consolidated into one network. Current RadNet stationary sampling stations use a high-volume air sampler to collect total suspended particles (TSP) on 10-cm-diameter synthetic fiber filters38,39. Integrated samples are collected by monitor operators over 5 to 7 days and are then sent to the National Analytical Radiation Environmental Laboratory (NAREL) for analysis. Measurement occurs several days after sample collection, which allows time for short-lived radon progenies (including 214Pb and 214Bi) to decay39. Outlier values, identified as values greater than 1.5 times the IQR from the median after log-transforming beta concentrations to ensure normality, were excluded from the dataset. All days within each sampling period were assigned to the β gross activity measured for that sample. This created a pseudo-daily time series. On days where one sample was completed and another sample began, the daily value was calculated as the mean of the two measured concentrations.
This study improved upon earlier studies of beta radiation32,37 by assigning particle radioactivity exposure based on each participant’s closest RadNet monitoring station, rather than using a regional particle gross β activity exposure. Each participant was matched to their closest RadNet site and was assigned the corresponding daily measurement. Data was obtained from the following three RadNet stations that encompassed the possible range of residential locations for the participants: Boston, MA (83%); Worcester, MA (14%) and Providence, RI (3%). Missing values at each monitor were imputed using random forest models based on nearby monitors and meteorological variables. Prediction results were cross-validated using ten-fold cross validation and showed good predictive ability (CV- R2 between 0.77 and 0.85).
Statistical analysis
We examined whether same-day and moving average of PM2.5 and particle radioactivity (single pollutant models) and then PM2.5 and particle radioactivity together (two-pollutant model) were associated with ventricular arrhythmias using a time-stratified case-crossover analysis adjusted for temperature and dew point. Case-crossover designs have been used to study various ambient air pollutant effects on acute cardiovascular events33,40–42. Case days occur on a calendar day when a patient experiences one or more VA event. Control days where chosen to match the cases’ by day of the week within the matching calendar month. In this study design, time invariant variables that do not vary daily such as race, sex, age, smoking status, diabetic status and other chronic conditions are eliminated as potential confounders. Matching by day of the week within the same calendar month helped control for potential confounding that varied within week and seasonality by month. The bi-directional selection of control days before and after the case day helped eliminate potential bias induced by long-term time trends43.
We estimated odds ratios assessing the association of ventricular arrhythmias with the exposure of interest using conditional logistic regression, controlling for the matched sets. A matched-set in this study is defined as a single case-day with all its matched controls. It was possible for a single individual to have multiple matched sets. Based on previous studies with the same population, all models were adjusted for dew point and air temperature. The effect estimates are reported as odds ratios of an event for an interquartile range (IQR) increase in PM2.5 or particle radioactivity33.
In sensitivity analyses, we included an indicator variable for multiple events on the same day to evaluate whether patients with multiple events on the same day are more susceptible to ventricular arrhythmias. The first event in that 24-hour period was given a value of 0, while following events within that one calendar day were assigned a value of 1. In the two-pollutant model (PM2.5 and particle radioactivity) to investigate the combined exposure of PM2.5 and particle radioactivity, we utilized multiplicative interactions terms.
Data management and all statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, 2013) and R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Population
Descriptions of the participants included in this analysis have previously been published33. Briefly, 1,143 patients were screened and 843 were excluded due to either implantation of a single chamber ICD and/or chronic AF. From the 300 eligible patients, 200 enrolled and 176 subjects were followed for at least 90 days. The mean follow-up time in the final cohort was 1.9 years. The study population that experienced any type of VA was mostly male (77%) and Caucasian (91%) with a median age of 65 years and the median body mass index of 27.7 kg/m2 (Table 1). Only 4% reported different residential zip codes during their one year follow up. Patients reported spending a median of 6 hours per weekend (range: 0–48 hours) and 6 hours in the last 48 hours away (range: 0–40 hours) from their homes.
Table 1:
Patient Population (91 subjects that experienced an event and were followed for at least 90 days) in the ICD cohort during the study period from September 1, 2006 until June 30, 2010
| Subjects with any type of VA | Subjects with sustained VA | |||
|---|---|---|---|---|
| N (Total) |
N (with characteristics) |
N (Total) |
N (with characteristics) |
|
| Age (years) | 91 | 65.0 (33–89) | 25 | 62.0 (37–86) |
| Gender (male) | 91 | 70 (77%) | 25 | 21 (84%) |
| Other | 1 (1%) | |||
| BMI (kg/m2) | 91 | 27.7 (15.6–56.7) | 25 | 29.6 (21.7–55.6) |
| Other | 9 (10%) | 2 (8%) | ||
| Left ventricular ejection fraction (%) | 90 | 25.0 (10–70) | 25 | 25 (10–55) |
| IV | ||||
| Hypertension | 88 | 52 (59%) | 25 | 18 (72%) |
| Platelet Aggregation Inhibitors | 88 | 66 (75%) | 25 | 19 (76%) |
| Lived with Smoker | 91 | 57 (63%) | 25 | 17 (68%) |
Values are median (range) or n (%). Values may not always add up to 91 or 25 because of missing data. VA= ventricular arrhythmias; BMI= body mass index; CHF= congestive heart failure
Their median left ventricular ejection fraction was 25%, while 60% had a history of congestive heart failure. The subset of the study population that experienced a sustained VA event (>30 seconds) had similar demographic characteristics to the general ICD population that experienced any type of ventricular arrhythmic event. Seven individuals experienced more than one event per day for all VAs accounting for 18% of all events while two individuals experienced more than one event per day for sustained VAs accounting for 17% of sustained VAs.
Arrhythmias
During the study period, twenty-five patients had 123 sustained ventricular arrhythmia events categorized as ventricular tachycardia (VT) (n=112) and ventricular fibrillation (VF) (n=11). Ninety-one patients had 1050 sustained or non-sustained ventricular arrhythmias events categorized as non-sustained ventricular tachycardia (NSVTs) (n=913) and non-sustained ventricular fibrillations (NSVFs) (n=14).
Air quality and weather covariates
Table 2 presents the daily mean air pollution concentrations and weather covariates, as well as the estimated daily particle radioactivity levels during the study period. During this time, the median PM2.5 was 8.42 μg/m3 and the median particle radioactivity was 0.19 mBq/m3. None of the air quality or weather covariates were highly correlated (Pearson correlation <0.5). PM2.5 was positively correlated with particle radioactivity, dew point and air temperature. On the other hand, particle radioactivity was negatively correlated with dew point and air temperature.
Table 2:
Summary statistics and Pearson’s correlation coefficient of daily mean air pollutant concentrations, particle radioactivity levels and meteorological variables in Boston, USA, during the study period from September 1, 2006 until June 30, 2010.
| Summary statistics | Pearson’s correlation coefficient | |||||||
|---|---|---|---|---|---|---|---|---|
| # Days | Min | 25th percentile | 50th percentile | 75th percentile | Max | PM2.5 | PR | |
| PM2.5 (μg/m3) | 1128 | 2.23 | 6.27 | 8.42 | 12.52 | 55.21 | 1 | 0.35 |
| PR (mBq/m3) | 1128 | 0.06 | 0.14 | 0.19 | 0.23 | 0.56 | 0.35 | 1 |
| Temperature (°C) | 1128 | −12.74 | 1.94 | 11.35 | 18.62 | 28.05 | 0.20 | −0.11 |
| Dew point temperature (°C) | 1128 | −20.08 | −1.37 | 6.22 | 14.31 | 23.57 | 0.24 | −0.14 |
Single-pollutant models
Exposure to higher levels to PM2.5 was associated with higher odds of any ventricular arrhythmic event with 4, 5, or 21-day moving average prior to the event, in models adjusted for dew point and temperature (Figure 1A). For the 4 and 5-day moving averages, the increased odds were very similar. The strongest increased odds was for the 21-day moving average, odds 39% higher (95% CI: 12 to 72%) for each IQR (3.37 μg/m3) increase in PM2.5. In a sensitivity analysis, we included a multiple event indicator to test whether patients with multiple events on a given day are more susceptible to PM2.5. We did not see evidence of an interaction with the 4- or 5-day moving averages. However, for the 21-day average, the interaction reported a nominally larger association for subsequent events (75% higher odds; 95% CI: −2% to 214%).
Figure 1:
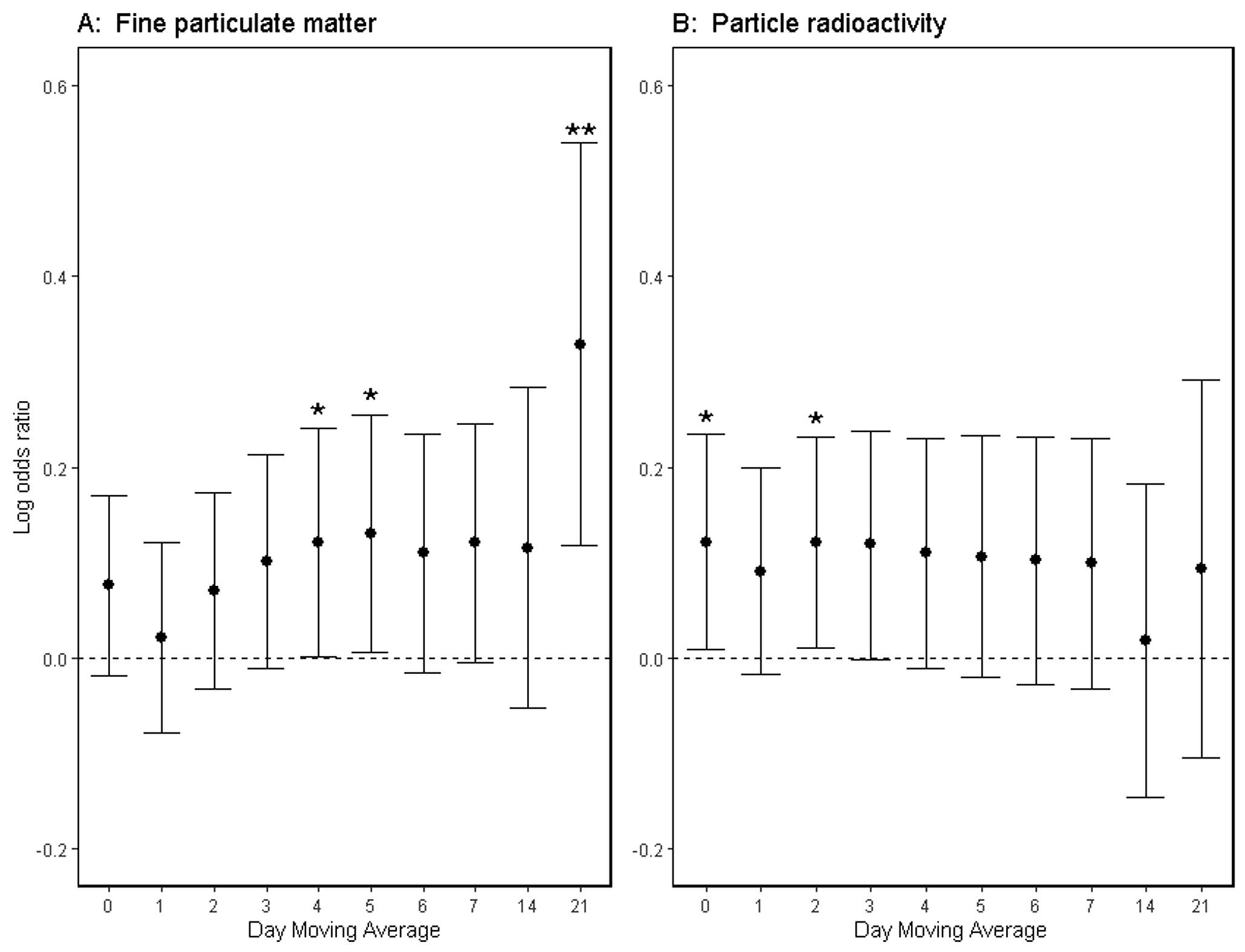
Log odds ratios of ICD Detected Ventricular Arrhythmias Associated with Each Interquartile Range Increase in Mean (A) Fine Particulate Matter (PM2.5) or (B) Particle radioactivity 0–21 days prior to the Arrhythmic Event, Adjusted for Temperature and Dew Point in the ICD cohort from September 1, 2006 to June 30, 2010. * p<0.05; ** p<0.01
Higher exposure to particle radioactivity was associated with higher odds of any ventricular arrhythmic event with same-day exposure and 2-day moving average prior to the event, in models adjusted for dew point and temperature (Figure 1B). For the 0, 2 and 3-day moving average, the increased odds were similar, except the 3-day moving average did not meet the threshold for statistical significance. Specifically, for the 2-day moving average there was a 13% higher odds of ventricular arrhythmic events (95% CI: 1% to 26%) for each IQR (0.08 mBq/m3) increase in particle radioactivity. We did not see evidence that patients with multiple events on a given day are more susceptible to particle radioactivity.
PM2.5 and particle radioactivity (Two-pollutant models)
In models including both PM2.5 and particle radioactivity, only the 21-day moving average of PM2.5 remained statistically significant for any of the time windows (Table 3). For each 3.37 μg/m3 increase in daily mean PM2.5 levels for a 21-day moving average, the odds of a ventricular arrhythmic event was 48% higher (95% CI: 15 to 90%) when adjusted for particle radioactivity, dew point and air temperature. In sensitivity analyses, we included an interaction term between the multiple event indicator and the 21-day PM2.5 moving average. The interaction reported a stronger association for subsequent events (76% higher odds; 95% CI: −2% to 215).
Table 3:
Odds ratios of ICD Detected Ventricular Arrhythmias Associated with Each Interquartile Range Increase in Mean Exposure levels (PM2.5 and PR) 0–21 days prior to the Arrhythmic Event, Model includes both PM2.5 and PR, and is adjusted for Temperature and Dew Point in the ICD cohort from September 1, 2006 to June 30, 2010 . * p<0.01
| Moving average (day) |
PM2.5 (95% CI) |
Particle radioactivity (95% CI) |
|---|---|---|
| 0 | 1.04 (0.94–1.16) | 1.11 (0.98–1.25) |
| 1 | 0.99 (0.88–1.10) | 1.10 (0.98–1.24) |
| 2 | 1.03 (0.91–1.15) | 1.12 (0.99–1.26) |
| 3 | 1.07 (0.94–1.21) | 1.09 (0.95–1.25) |
| 4 | 1.10 (0.96–1.25) | 1.07 (0.93–1.23) |
| 5 | 1.11 (0.96–1.28) | 1.06 (0.92–1.22) |
| 6 | 1.09 (0.94–1.25) | 1.07 (0.92–1.23) |
| 7 | 1.10 (0.96–1.27) | 1.06 (0.91–1.22) |
| 14 | 1.15 (0.95–1.40) | 0.95 (0.79–1.15) |
| 21 | 1.48* (1.15–1.90) | 0.90 (0.71–1.14) |
To assess whether particle radioactivity modified the effects of PM2.5, we added an interaction term between 21-day PM2.5 and 21-day particle radioactivity. We found a significant interaction between 21-day PM2.5 and 21-day particle radioactivity (estimate −0.28; 95% CI: −0.45 to −0.11).
We did not find any significant association between PM2.5 or particle radioactivity with sustained ventricular arrhythmias that required intervention by the ICD implant, our secondary endpoint (Table 4).
Table 4:
Odds ratios of ICD Detected Sustained Ventricular Arrhythmias Associated with Each Interquartile Range Increase in Mean Exposure levels (PM2.5 and PR) 0–21 days prior to the Arrhythmic Event, Adjusted for Temperature and Dew Point in the ICD cohort from September 1, 2006 to June 30, 2010.
| Moving average (day) |
PM2.5 (95% CI) |
Particle radioactivity (95% CI) |
|---|---|---|
| 0 | 1.14 (0.76–1.69) | 0.95 (0.65–1.39) |
| 1 | 1.20 (0.82–1.76) | 0.84 (0.59–1.19) |
| 2 | 1.19 (0.80–1.77) | 0.89 (0.60–1.32) |
| 3 | 1.21 (0.78–1.88) | 0.82 (0.53–1.27) |
| 4 | 1.22 (0.78–1.91) | 0.78 (0.49–1.25) |
| 5 | 1.17 (0.72–1.87) | 0.83 (0.51–1.37) |
| 6 | 1.05 (0.64–1.71) | 0.98 (0.61–1.59) |
| 7 | 1.07 (0.65–1.74) | 0.98 (0.61–1.57) |
| 14 | 1.43 (0.78–2.62) | 1.00 (0.54–1.83) |
| 21 | 1.17 (0.52–2.62) | 1.33 (0.53–3.29) |
Discussion
This is the first study that we know of to explore the association of PM2.5 and particle radioactivity with ventricular arrhythmias and finds a direct correlation between them. We found that in the single PM2.5 pollutant models that were individually adjusted for dew point and air temperature, higher exposure was associated with a higher odds of ventricular arrhythmias during 4, 5 and 21 days prior. On the other hand, the single pollutant particle radioactivity models found an association between higher exposure and higher odds of ventricular arrhythmias on the day of exposure and 2 days prior.
In the two-pollutant models including both PM2.5 and particle radioactivity, only the 21-day moving average exposure of PM2.5 was independently associated with a higher odds of a ventricular arrhythmic event (48% higher odds; 95% CI: 15 to 90%). However, the associations in the two-pollutant models for the 4 and 5-day moving averages of PM2.5 remain very similar in magnitude to the estimates in the single pollutant models of PM2.5, but with slightly wider confidence intervals. This suggests that PM2.5 has an effect on the risk of VA, which is independent of particle radioactivity for both an acute and intermediate effect (4, 5 and 21-day exposure). By conducting a sensitivity analysis with a multiple event indicator, we found that having multiple events on the same calendar day PM2.5 could potentially have a nominally larger effect on subsequent events.
Although particle radioactivity did not cross the significance threshold in the two-pollutant models, the effect estimates for the 0, 2 and 3-day moving averages were very similar to the one-pollutant models, but with slightly wider confidence intervals. Indicating that PM2.5 could have an intermediate effect (21-day) while particle radioactivity could have a more acute impact (<3 days) on ventricular arrhythmias.
While many studies have found that air pollution factors into mortality and morbidity rates across the globe44–48, instead of focusing exclusively on PM2.5 as a single exposure, our study included particle radioactivity. The significant interaction between 21-day PM2.5 and 21-day particle radioactivity suggests that the risk of ventricular arrhythmias due to PM2.5 increases at a steeper rate at lower concentrations of particle radioactivity after adjusting for dew point and temperature. Thus, there is a weaker effect of PM2.5 in the presence of higher levels of particle radiation. This provides evidence that particle radioactivity modifies the association between PM2.5 and the risk of ventricular arrhythmias. So far, no study has investigated how the combined exposure to both particulate matter and particle radiation affects this high-risk population and how they interact, although this could have important implications for cardiac health and preventative strategies.
The World Health Organization (WHO) estimates that in 2016 ambient air pollution caused 4.2 million premature deaths across the globe49. From these premature deaths, cardiovascular disease accounts for the majority of deaths from air pollution49,50. Since this is the first study to examine how particle radioactivity affects the risk of ventricular arrhythmias, we are unable to directly compare our particle radioactivity results with other studies. Nevertheless, epidemiological studies have found evidence of a positive association between circulatory disease mortality and low doses of ionizing radiation51. A recent longitudinal study in the Normative Aging cohort employed the same exposure metric of gross β activity as a surrogate of particle radioactivity and found a positive association with an increase in both diastolic and systolic blood pressure32. While this study did not find an independent effect of particle radioactivity on VA, these scientific studies support the growing literature looking at the association between cardiovascular diseases, fine particle mass and particle radioactivity20,32.
Biological Mechanism
While inhaled radon gas has been associated with higher lung cancer risk, few studies have explored the association between low background levels of ionizing radiation and cardiovascular disease52,53. Radioactive aerosols emit α and β particles and transmit γ and X-rays. The deposition of radioactive materials inside the human body can cause biophysical harm depending on the dose, deposition site and the different types of radiation emitted throughout the decay process54–56.
Many studies have reported on possible biological mechanisms associated with the effects of radiation on cardiovascular morbidity and mortality21,57,58. Radiation therapy utilizes high doses of ionizing radiation which can induce cardiovascular toxicity through radiation induced fibrosis, microvascular injury and neovascularization, and atherosclerosis59,60. At lower doses, pro-inflammatory markers are upregulated after exposure to radiation21,61. Specifically, a recent study found moderate associations of regional mean particle β radioactivity with several oxidative stress and inflammatory biomarkers after adjusting for PM2.5 concentrations in The Framingham Heart Study37. The literature supports the theory that low background levels of ionizing radiation contribute to cardiovascular disease through a heightening of the immune response and systemic inflammation.
Strengths and Limitations
Our study had several limitations. We did not find an association between sustained ventricular arrhythmias and PM2.5, but this could be due to insufficient power. There is potential for non-differential measurement error in our exposure assessment, which has previously been described32,37. Since measurements of particle gross β activity were measured on samples collected over a period of several days and then used to create a pseudo-daily time series, we may not have enough temporal resolution to estimate short-term exposures at windows of less than five days. This study improves upon earlier studies of particle gross β activity32,37 by assigning particle radioactivity exposure based on each participant’s closest RadNet site, rather than using a regional beta value. This reduces exposure misclassification and improves the spatial and temporal variability of our particle radioactivity exposures. It is unlikely that any measurement error in either particle radioactivity or PM2.5 is associated with the participant’s VA events since the exposure was measured independently from the ventricular arrhythmic events.
This study also improves on previous air pollution measurements and weather covariate information utilized for this ICD cohort population. Instead of using a single monitoring site like previous studies,33,62 this study assigned exposures based on patients’ residential address using spatio-temporal models for PM2.5, particle radioactivity, dew point temperature and air temperature, which reduced the amount of potential measurement error. The assigned exposures do not take into account a patient’s mobility outside of their residential zip code. This potential misclassification is nondifferential because patients with lower exposure are not likely to have more misclassification error than patients with higher exposure. This suggests that adjusting for the nondifferential measurement error would result in a larger effect estimate with smaller confidence intervals.
The implantable ICD devices allow for accurate diagnosis and timing of events (all VAs and sustained VAs). Precise time measurements were recorded for every event, which were independently verified by an electrophysiologist increasing the accuracy of the outcome measurement. The study assessed the temporal association of ventricular arrhythmias captured by implantable defibrillators with fine particulate matter and particle radioactivity. By including patients with dual-chambered ICD, we reduce the potential for outcome misclassification by distinguishing between ventricular and atrial arrhythmias.
Whether the association of radiation and PM2.5 with arrhythmias is a direct arrhythmogenic response to these agents or whether the arrhythmias are secondary to radiation- or particle-induced myocardial ischemia or heart failure is not addressed by this study. Many of the patients had a history of coronary artery disease and congestive heart failure. These patients characterize an at-risk population because their previous history of cardiovascular disease could make them more susceptible to air pollution. By utilizing a case-crossover method, the self-matching design eliminates confounding by time invariant or relatively constant characteristics such as a patient’s chronic or average risk factors43. Nevertheless, the generalizability of the results is limited by the characteristics of a high-risk patient population for subclinical and clinical cardiac events. It is uncertain whether the associations would be the same among younger, non-white, or less at-risk patients.
Conclusions
In this high-risk population, intermediate (21-day) PM2.5 exposure was associated with higher odds of a ventricular arrhythmia event onset among patients with known cardiac disease and indication for ICD implantation independently of particle radioactivity. For shorter term associations (less than 7 days), we may not be able to distinguish the effect of PM2.5 from particle radioactivity, but in models only accounting for PM2.5, associations between fine particulate matter and ventricular arrhythmias were significant for 4 and 5 day moving averages.
We found that exposure to fine particulate matter independent of low levels of background radiation contributes to the risk of ventricular arrhythmias. Furthermore, particle radioactivity reduces the effect of fine particulate matter in the presence of higher levels of particle radiation on ventricular arrhythmias.
Clinical Perspective.
1). What is new?
Study found that radioactive properties of particle matter and total fine particle mass were associated with cardiovascular health (ventricular arrhythmias) in patients with implanted cardioverter-defibrillators
Study population consisted of patients at high risk for ventricular arrhythmias
To address combined associations, study includes a dual pollutant model.
2). What are the clinical implications?
Particle air pollution and its radioactive components contribute significantly to the risk of acute clinically relevant electrophysiologic cardiac outcomes in high risk patients.
Cardiovascular patients and those at high risk for cardiovascular events should be informed about the risks associated with air pollution and the onset of arrhythmias.
Funding Sources
This publication was made possible by the United States Environmental Protection Agency (USEPA) grant RD-835872 and the National Institute of Environmental Health Sciences (NIEHS) P01ES009825. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA or NIEHS. Further, USEPA and NIEHS does not endorse the purchase of any commercial products or services mentioned in the publication. This publication was also funded by the National Institutes of Health grant T32ES007069 and ES000002.
Non-standard Abbreviations and Acronyms
- ICDs
Implanted cardioverter-defibrillators
- VA
ventricular arrhythmias
- PM2.5
fine particulate matter
- IQR
interquartile range
- AF
atrial fibrillation
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- NSVT
non-sustained ventricular tachycardia
- NSVF
non-sustained ventricular fibrillations
- TSP
total suspended particles
- BMI
body mass index
- CHF
congestive heart failure
Footnotes
Disclosures
The authors have no conflict of interests.
References
- 1.Schwartz J Air pollution and hospital admissions for heart disease in eight US counties. Epidemiology. 1999;10:17–22. [PubMed] [Google Scholar]
- 2.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdi MD, Wang Y, Di Q, Zanobetti A, Schwartz J. Long-term exposure to PM2. 5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int . 2019;130:104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz J, Marcus A. Mortality and air pollution in London: a time series analysis. Am J Epidemiol. 1990;131:185–194. doi: 10.1093/oxfordjournals.aje.a115473 [DOI] [PubMed] [Google Scholar]
- 5.Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Zareba W, Schneider A, Rückerl R, Ibald-Mulli A, Cyrys J, Wichmann H-E, Peters A. Runs of ventricular and supraventricular tachycardia triggered by air pollution in patients with coronary heart disease. J Occup Environ Med. 2006;48:1149–1158. [DOI] [PubMed] [Google Scholar]
- 7.Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. [DOI] [PubMed] [Google Scholar]
- 8.Mann JK, Tager IB, Lurmann F, Segal M, Quesenberry CP Jr, Lugg MM, Shan J, Van Den Eeden SK. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ Health Perspect. 2002;110:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Verrier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–674. doi: 10.1289/ehp.7767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amrane M, Oufni L, Misdaq MA. Attached and unattached fractions of short-lived radon decay products in outdoor environments: effect on the human respiratory system. Radiat Prot Dosimetry. 2014;162:400–409. doi: 10.1093/rpd/nct338 [DOI] [PubMed] [Google Scholar]
- 11.Nations United. Scientific Committee on the Effects of Atomic Radiation Sources and Effects of Ionizing Radiation: Sources. Vol 1 United nations publications; 2000. [Google Scholar]
- 12.Properties Porstendörfer J. and behaviour of radon and thoron and their decay products in the air. J Aerosol Sci. 1994;25:219–263. doi: 10.1016/0021-8502(94)90077-9 [DOI] [Google Scholar]
- 13.NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States; 2006. [DOI] [PubMed] [Google Scholar]
- 14.Mohery M, Abdallah AM, Al-Amoudi ZM, Baz SS. Activity size distribution of some natural radionuclides. Radiat Prot Dosimetry. 2013;158:435–441. [DOI] [PubMed] [Google Scholar]
- 15.Moriizumi J, Yamada S, Xu Y, Matsuki S, Hirao S, Yamazawa H. Indoor/outdoor radon decay products associated aerosol particle-size distributions and their relation to total number concentrations. Radiat Prot Dosimetry. 2014;160:196–201. doi: 10.1093/rpd/ncu080 [DOI] [PubMed] [Google Scholar]
- 16.Papastefanou C Radon decay product aerosols in ambient air. Aerosol Air Qual Res. 2009;9:385–393. [Google Scholar]
- 17.Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SM, Kim HR, Park YJ, Lee SY, Chung KH. Organic extracts of urban air pollution particulate matter (PM2.5)-induced genotoxicity and oxidative stress in human lung bronchial epithelial cells (BEAS-2B cells). Mutat Res Toxicol Environ Mutagen. 2011;723:142–151. doi: 10.1016/j.mrgentox.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. [DOI] [PubMed] [Google Scholar]
- 20.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys. 2013;52:435–449. doi: 10.1007/s00411-013-0484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the Mortality of Atomic Bomb Survivors, Report 14, 1950–2003: An Overview of Cancer and Noncancer Diseases. Radiat Res. 2011;177:229–243. doi: 10.1667/RR2629.1 [DOI] [PubMed] [Google Scholar]
- 23.Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae J-M, Engels H, Gilbert E, Gulis G, Habib RR, Howe G, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–1135. doi: 10.1093/ije/dym138 [DOI] [PubMed] [Google Scholar]
- 24.Kreuzer M, Auvinen A, Cardis E, Hall J, Jourdain J-R, Laurier D, Little MP, Peters A, Raj K, Russell NS, et al. Low-dose ionising radiation and cardiovascular diseases – Strategies for molecular epidemiological studies in Europe. Mutat Res Mutat Res. 2015;764:90–100. doi: 10.1016/j.mrrev.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. [DOI] [PubMed] [Google Scholar]
- 26.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JE, Moulder JE, Hopewell JW. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal. 2011;15:1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZS, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029 [DOI] [PubMed] [Google Scholar]
- 29.Hooning MJ, Botma A, Aleman BMP, Baaijens MHA, Bartelink H, Klijn JGM, Taylor CW, van Leeuwen FE. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- 30.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1 [DOI] [PubMed] [Google Scholar]
- 31.Blomberg AJ, Coull BA, Jhun I, Vieira CLZ, Zanobetti A, Garshick E, Schwartz J, Koutrakis P. Effect modification of ambient particle mortality by radon: A time series analysis in 108 U.S. cities. J Air Waste Manage Assoc. 2019;69:266–276. doi: 10.1080/10962247.2018.1523071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyhan MM, Coull BA, Blomberg AJ, Vieira CLZ, Garshick E, Aba A, Vokonas P, Gold DR, Schwartz J, Koutrakis P. Associations between ambient particle radioactivity and blood pressure: the NAS (Normative Aging Study). J Am Heart Assoc. 2018;7:e008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F. Acute Exposure to Air Pollution Triggers Atrial Fibrillation. J Am Coll Cardiol. 2013;62:816 LP–825. doi: 10.1016/j.jacc.2013.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A. An ensemble-based model of PM2. 5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int . 2019;130:104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalnay E, Kanamitsu M, Kistler R, Collins W, Deaven D, Gandin L, Iredell M, Sana S, White G, Woollen J. The NCEP/NCAR 40-Year Reanalysis Project Ш. Bull Am Meteorol Soc. 1996;77:437–471. [Google Scholar]
- 36.Hernández F, Hernández-Armas J, Catalán A, Fernández-Aldecoa JC, Karlsson L. Gross alpha, gross beta activities and gamma emitting radionuclides composition of airborne particulate samples in an oceanic island. Atmos Environ. 2005;39:4057–4066. [Google Scholar]
- 37.Li W, Nyhan MM, Wilker EH, Vieira CLZ, Lin H, Schwartz JD, Gold DR, Coull BA, Aba AM, Benjamin EJ. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham Heart Study. Environ Int . 2018;121:1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Environmental Protection Agency. Learn About RadNet. Accessed September 18, 2018 https://www.epa.gov/radnet/learn-about-radnet
- 39.Environmental Protection Agency. RadNet Sampling and Analyses Schedules. Accessed September 18, 2018 https://www.epa.gov/radnet/radnet-sampling-and-analyses-schedules
- 40.Rich DQ, Dockery DW, Speizer FE, Luttmann-Gibson H, Schwartz J, Link M, Mittleman MA, Catalano PJ. Association of Short-term Ambient Air Pollution Concentrations and Ventricular Arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143 [DOI] [PubMed] [Google Scholar]
- 41.Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–123. doi: 10.1289/ehp.8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert CM, Mittleman MA, Chae CU, Lee I-M, Hennekens CH, Manson JE. Triggering of Sudden Death from Cardiac Causes by Vigorous Exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902 [DOI] [PubMed] [Google Scholar]
- 43.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43:1645–1655. doi: 10.1093/ije/dyu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An Association between Air Pollution and Mortality in Six U.S. Cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401 [DOI] [PubMed] [Google Scholar]
- 45.Pope III CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine Particulate Air Pollution and Mortality in 20 U.S. Cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401 [DOI] [PubMed] [Google Scholar]
- 47.Seaton A, Godden D, MacNee W, Donaldson K. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/S0140-6736(95)90173-6 [DOI] [PubMed] [Google Scholar]
- 48.Künzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, Herry M, Horak F, Puybonnieux-Texier V, Quénel P, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356:795–801. doi: 10.1016/S0140-6736(00)02653-2 [DOI] [PubMed] [Google Scholar]
- 49.Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, Dingenen R van, Estep K, Amini H, Apte JS, et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ Sci Technol. 2016;50:79–88. doi: 10.1021/acs.est.5b03709 [DOI] [PubMed] [Google Scholar]
- 50.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Little MP, Azizova TV., Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta, de Vathaire, Hall, et al. Systematic Review and Meta-analysis of Circulatory Disease from Exposure to Low-Level Ionizing Radiation and Estimates of Potential Population Mortality Risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:223. doi: 10.1136/bmj.38308.477650.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, William Field R, Klotz JB, Létourneau EG, Lynch CF, Lyon JL, et al. A Combined Analysis of North American Case-Control Studies of Residential Radon and Lung Cancer. J Toxicol Environ Heal Part A. 2006;69:533–597. doi: 10.1080/15287390500260945 [DOI] [PubMed] [Google Scholar]
- 54.The 2007 Recommendations of the International Commission on Radiological Protection ICRP publication 103; Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 55.United Nations. Scientific Committee on the Effects of Atomic Radiation Effects of Ionizing Radiation: UNSCEAR 2006 Report to the General Assembly, with Scientific Annexes. Vol 2 United nations publications; 2008. [Google Scholar]
- 56.IARC Working Group on the Evaluation of Carcinogenic Risk to Humans Ionizing Radiation, Part 2: Some Internally Deposited Radionuclides. Monograph Volume 78 International Agency for Research on Cancer; 2001. [PMC free article] [PubMed] [Google Scholar]
- 57.McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu N-N, Li Y-X, Wu R-Y, Zhang X-M, Wang W-H, Jin J, Song Y-W, Fang H, Ren H, Wang S-L, et al. Dosimetric and Clinical Outcomes of Involved-Field Intensity-Modulated Radiotherapy After Chemotherapy for Early-Stage Hodgkin’s Lymphoma With Mediastinal Involvement. Int J Radiat Oncol Biol Phys. 2012;84:210–216. doi: 10.1016/j.ijrobp.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 59.Schultz-Hector S, Trott K-R. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071 [DOI] [PubMed] [Google Scholar]
- 60.Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation-Induced Cardiovascular Disease. Curr Atheroscler Rep. 2017;19:22. doi: 10.1007/s11883-017-0658-x [DOI] [PubMed] [Google Scholar]
- 61.Mitchel REJ, Hasu M, Bugden M, Wyatt H, Little MP, Gola A, Hildebrandt G, Priest ND, Whitman SC. Low-dose radiation exposure and atherosclerosis in ApoE−/− mice. Radiat Res. 2011;175:665–676. doi: 10.1667/RR2176.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen JL, Laden F, Link MS, Schwartz J, Luttmann-Gibson H, Dockery DW. Weather and triggering of ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Expo Sci Environ Epidemiol. 2015;25:175–181. doi: 10.1038/jes.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]


