Abstract
Background
Neurodegenerative diseases, such as Alzheimer’s disease, cause a great deal of suffering for both patients and carers. Bacopa monnieri (L.) wettst. Is known for its memory-enhancing properties, and is of great interest in treating neurodegenerative disease.
Aims
This study aimed to evaluate B.monnieri against glutamate toxicity, and identify whether B.monnieri reduces mitochondrial and ER stress, as well as to measure B.monnieri’s effect on the life span and aging of Caenorhabditis elegans. We hypothesized that B.monnieri would prevent cellular oxidative stress, prevent mitochondrial/ER stress, and increase the life span while reducing signs of aging in C.elegans.
Experimental procedures
Glutamate toxicity was measured using viable cell staining assays and the MTT assay. ROS and mitochondrial stress were assessed by H2DCFDA and Rodamine123 staining, with fluorescence/confocal microscopy. C.elegans’ median and maximum life span were measured, in response to B.monnieri treatment, along with lipofuscin imaging to measure the health of the C.elegans population.
Results
B.monnieri hexane extract (but not ethanol extract) prevented the toxicity of 5 mM glutamate in HT-22 cells. We found that the mechanism involves the reduction of ROS production and the prevention of mitochondrial and ER stress. Furthermore, we showed that B.monnieri could increase the median and maximal lifespan of wild type C.elegans, maintain a younger appearing phenotype in the aged C.elegans.
Conclusions
In conclusion, B.monnieri prevents mitochondrial, and oxidative stress in the cultured cells. Furthermore, it can prolong the healthy lifespan of C.elegans, indicating that B.monnieri the potential for therapeutic and preventative use in neurodegenerative disease.
Keywords: Bhumi, Herbal medicine, Alzheimer’s disease, Neurodegeratative disease, Cytoprotective, Neuroprotective
Graphical abstract
Highlights
-
•
First B.monnieri study to investigate the HT-22 cell glutamate toxicity model.
-
•
B.monnieri protects HT-22 cells from oxidative stress caused by glutamate toxicity.
-
•
B.monnieri prevents ER stress, changing the expression s of ER Stress proteins CHOP and ERP57.
-
•
B.monnieri prevents mitochondrial stress, preventing mitochondrial leakage.
-
•
B.monnieri increases the median and maximal life span, and reduces aging in wild type C.elegans.
1. Introduction
Life expectancy is increasing throughout the developed world, resulting in more significant numbers of aged people, of whom many may require more health care, as the prevalence of disease increases with age.1 Neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and other forms of dementia are significant causes of suffering in aged populations around the world. There are currently 44 million who have Alzheimer’s disease worldwide; furthermore, with an aging population and no current effective cure, that number is expected to rise to 76 million by 2030, and 135 by 2050. The cost of treating and caring for Alzheimer’s patients is 605 billion dollars; equivalent to 1% of the world’s GDP.2
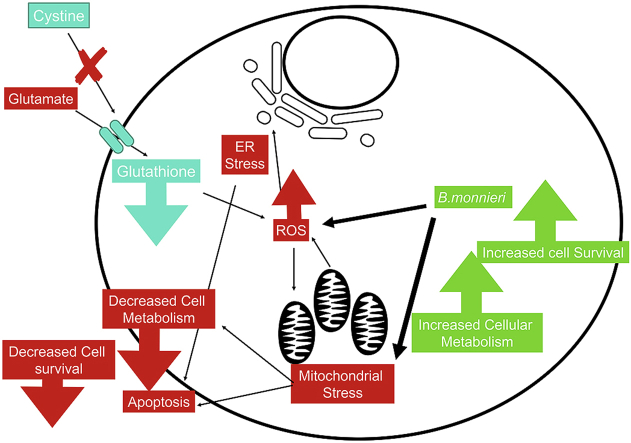
Glutamate toxicity is thought to be a factor in the pathology of neurodegeneration in aging patients, with the toxicity mediated through multiple pathways, one being a reactive oxygen species (ROS) mechanism. High concentrations of glutamate can block cystine uptake into the cells via the cysteine/glutamate anti-porter, which results in depletion of intracellular cysteine and glutathione (a cellular anti-oxidant), and subsequently, increasing ROS production.3 Increased ROS can have many detrimental effects in neuronal cells, resulting in endoplasmic reticulum stress, as proteins are miss-folded4 and mitochondrial stress and leakage, which can cause apoptosis and neurodegeneration.5
B.monnieri is a medicinal herb found throughout Asia and is known for its memory-enhancing effects.6 A list of commonly reported compounds found in B.monnieiri is shown in Supplementary Fig. 1, with the some of the main compounds reported being the mixture known as Bacoside A (consisting of Bacoside A3, Bacopaside II, Bacopaside X and Bacopasaponin C 5)7,8.
Animal studies have found that the herb can reverse the amnesic effects of scopolamine9,10 in mice and improve memory in the form of pain avoidance in rats.11 Furthermore, B.monnieri supplementation improves symptoms of paraquat treatment of mice in a Parkinson’s disease model12 and improves the Alzheimer’s like characteristics in mouse models, such as colchicine treatment,13 olfactory bulbectomy,14 and APP(SWE) expressing mice.14
A number of studies and clinical trials have investigated the memory-enhancing properties of B.monnieri, showing it to be some effect in the enhancement of the memories of healthy older individuals (average age 62 years). One such study showed that a 300 mg/day dose could improve, with statistical significance compared to the placebo, various measures (but not all) of working memory, over a 12 week period. Furthermore, it was observed that the effect continued after the administration of the B.monnieri extract was ceased.15 Other studies, investigating younger patients, have proved less convincing. In one study in patients with an average age of 59, showed no significant difference in working memory, although the study did show a significant improvement in recall of unrelated word pairs, and the authors suggest that this may be the result of a reduction in the amount of information lost from memory.16 One further study on much younger healthy individuals (19–22 years old) showed very little difference when compared to the placebo, with only two of the ten neuropsychological tests giving a significant changed when compared to the placebo.17 A meta-analysis of 9 randomized controlled trials concluded that B.monnieri has the potential to improve cognitive function, particularly in the speed of attention; however, the authors based their conclusions on nine small scale studies and suggest that a large scale study is required.18 It is interesting that B.monnieri appears to have greater effects on neuropsychological tests in older patients. There have been studies that have investigated B.monnieri’s effects in older patients with memory/cognitive disorders. One such study investigated the effects of a standardized B.monnieri extract (300 mg 2 times per day of Bacognize®) in newly diagnosed Alzheimer’s patients (average age 65 years). The study showed significant improvements in the orientation, attention, and language components of the mini-mental state examination scale (MMSES) but not the registration, recall, and design. The also patients reported, anecdotally, an improvement of quality of life.19 One study in older patients (predominantly 65 and over) with memory complaints (although not formally diagnosed with Alzheimer’s disease) showed that treatment with 125 mg day B.monnieri extract resulted in improvements in some aspects of memory (mental control, logical memory, paired associate learning, and to a lesser statistically significant extent digit forward), but not digit backward and visual reproduction, over a 12 week period20. Another study showed that a standardized extract (Bacomind® 450 mg/day) in patients between 50 and 75 years old was able to improve results in the digit span backward test and list learning delayed recall test; however, there was no improvement in various other neuropsychological measures.21 Although the clinical data is far from conclusive, with each of the above studies showing effects in only some of the neuropsychological measures, there is the possibility that B.monnieri can have a positive outcome in the treatment of Alzheimer’s. For an up to date review of the neuropharmacological and cognitive effects of B.monnieri, see Nimisha et al., (2019).7
There have been very few in vitro studies regarding B.monnieri and its effects on cultured neuronal cells, in particular, there is no study related to glutamate toxicity in HT-22 cells, a common in vitro model for measuring glutamate-induced ROS production in neuronal cells.22 Furthermore, to our knowledge, there has been no study on the effect of B.monnieri on the life span and aging of wild type Caenorhabditis elegans.
This study aimed to evaluate the protective effect of B.monnieri against glutamate toxicity in HT-22 cells, and identify whether mitochondrial and oxidative stress was reduced by B.monnieri treatment. We also aimed to measure the effect of B.monnieri on the life span and aging of the model organism Caenorhabditis elegans. We hypothesized that B.monnieri would prevent oxidative stress, protect the mitochondrial membrane integrity, in HT22 cells and that B.monnieri can increase the life span, and reduce signs of aging in wild type (wt) C.elegans.
2. Methods
2.1. Cell culture
HT22 cells (a gift from Professor David Schubert, Salk Institute, San Diego, CA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibthai, Bangkok, Thailand) supplemented with 10% fetal bovine serum (FBS) (Gibthai, Bangkok, Thailand), and maintained at 37 °C in a humidified, atmosphere containing 5% CO2. Cells were passed before reaching 80% confluence, and the culture media was changed every two days. Before the experiments, the cells were washed in Phosphate buffered saline (PBS) and detached from the culture flask using trypsin-EDTA (0.25%) (Gibthai, Bangkok, Thailand). The cells were then pelleted at 500 g, before being resuspended in fresh DMEM, and plated at a density of 15,000 cells per cm2 (unless otherwise stated).
2.2. Herb identification and extraction
B.monnieri was identified by, expert botanist, Nirun Vipunngeun, College of Pharmacy, Rangsit University, Thailand, voucher number 016299 (BUC). B.monnieri leaves were shade dried and powdered using a blender. The powdered leaves were then extracted with hexane, dichloromethane (DCM) or 95% Ethanol by soxhlet extraction. The resulting extract was concentrated, and solvents were removed. The dried extracts were resuspended in DMSO at a concentration of 20 mg/ml and filtered through a 0.2-μm filter and stored at −20 °C before use.
2.3. HPLC analysis
B.monnieri extract was analyzed by reversed-phase HPLC to confirm the identity of the herb. The content of Bacoside A (Bacoside A3, Bacopaside II, Bacopaside X and Bacopasaponin C 5) from the B.monnieri extract was compared to the Bacoside A reference standard (Merk Germany). The HPLC system consisted of a C18 column (Inertsil ODS-3: 4.6 × 250 mm, 5 mL I.D.) and diode array at 205 nm. The mobile phase HPLC conditions namely mobile phase composition in line A contained 0.05% Phosphoric acid mixed with water and Acetonitrile in line B. The flow rate was 20 μL/min, and the temperature 35 °C.
2.4. Trypan blue exclusion assay
HT22 cells were plated in 6 well tissue culture plates and allowed to adhere overnight. The following day the cells were treated with B.monnieri extracts (10 μg/ml) with or without glutamate (5 mM) and incubated for 18 h. The culture media from each treatment was removed and kept aside while the cells were detached from the culture plates with trypsin-EDTA. The cells were then recombined with the original culture media and mixed. Samples were then taken and diluted in trypan blue (0.02%) for 3 min, before being loaded onto a Neubauer hemocytometer for counting. Each treatment was carried out in triplicate; data shown are from at least three independent experiments, and represented as mean plus and minus the SEM.
2.5. MTT assay
The MTT cell viability assay was carried out as previously described23. Briefly, HT22 cells were plated in 96 well culture plates and allowed to adhere overnight. The cells were then treated with 5 mM glutamate with or without B.monnieri extracts, before being incubated overnight at 37 °C in a humidified 5% CO2 atmosphere. The following day, the media was replaced, with media containing 0.5 mg/ml MTT ((4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The cells were then incubated at 37 °C in a humidified 5% CO2 atmosphere for 4 h. The media was then carefully removed, and the formazan crystals dissolved in DMSO. Absorbance was read at 550 nm using an EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA, USA). Results are expressed as a percentage relative to untreated control.
2.6. Propidium iodide staining
HT-22 cells were plated in culture dishes containing glass coverslips and allowed to adhere overnight. The cells were then treated with 5 mM glutamate with or without B.monnieri extracts (10 μg/ml), and incubated for 18 h. Cells were then stained with propidium iodide (PI) 1 μg/mL and counterstained with Hoechst 33342 (2 μg/ml).
2.7. Mitochondrial membrane potential imaging
HT-22 cells were plated in culture dishes containing glass coverslips and allowed to adhere overnight. The cells were then treated with 5 mM glutamate with or without B.monnieri extracts (10 μg/ml) and incubated for 18 h. Cells were then stained with Rhodamine 123, (50 μM) and counterstained with Hoechst 33342 (2 μg/ml).
2.8. Mitochondrial function assay
HT-22 cells were seeded in 5 mM cell culture dishes and allowed to adhere overnight. The cells were then treated with 5 mM glutamate with or without B.monnieri extracts (10 μg/ml) or in the case of the control 0.5% DMSO and incubated for 18 h. Subsequently, the cells were detached from the dish using trypsin and washed with PBS. The cells were counted and then stained with rhodamine 123, (50 μM) for 30 min before being rewashed with PBS. The cells were then plated into 96 well plates at 20,000 cells per well. The fluorescence of the cells was measured using the EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) with excitation at 488 nm and emission at 525 nm.
2.9. Intracellular reactive oxygen species
HT-22 cells were seeded in 5 mM dishes and allowed to adhere overnight. The cells were then treated with 5 mM glutamate with or without B.monnieri extracts (10 μg/ml) or in the case of the control 0.5% DMSO and incubated for 18 h. The cells were then washed with PBS and stained with carboxy-H2DCFDA (Invitrogen) and incubated at 37 °C/5% CO2 for 30 min. The cells were then washed three times with PBS and imaged using Axio Observer A1 fluorescence microscope (Carl Zeiss, Jena, Germany). The images were then analyzed for mean cellular fluorescence using image J and the data represented as AU.
2.10. Western blot analysis
Protein was extracted from treated cells by cell scraping followed by incubation in a lysis buffer containing 20 mM Tris•HCL (pH 7.5) 1% Triton X, 150 mM sodium chloride, 10% glycerol 1 mM sodium orthovanadate, 50 mM sodium fluoride, 100 mM phenylmethylsulfonyl fluoride and commercial protease inhibitor cocktail (Roche Molecular Biochemicals) for 30 min on ice. The lysates were then stored at −80 °C until use. Total protein concentrations were standardized using the Bradford protein assay, and mixed with laemmli loading buffer and incubated at 95 °C for 5 min before loading into a 12% SDS-PAGE. The Protein was then transferred to PVDF membranes and blocked in 5% Blocking buffer (BioRad, Hercules, California, USA) in TBST ((25 mM Tris-HCl, pH 7.5, 125 mM NaCl, and 0.05% Tween 20). The Membranes were then probed with CHOP, ERP57 and b-actin (Cell Signaling, Danvers, Massachusetts, USA) at 4 °C overnight. Membranes were washed with TBST for 10 min, three times and incubated with horseradish peroxidase-conjugated anti-rabbit antibodies for 1 h at room temperature. Subsequently, the bands were exposed to X-ray film with the chemiluminescence detection system (ECL™ Select western blotting detection reagent: GE Healthcare, Piscataway, NJ, USA) and quantified using Image J software, then normalized to b-actin.
2.11. Caenorhabditis elegans propagation
Wild type C. elegans (Bristol N2) were obtained from the Caenorhabditis Genetics Center, (University of Minnesota, USA) and grown in nematode growth medium (NGM) agar plates seeded with E. coli OP50 as the food source at 15 °C.24
2.12. Caenorhabditis elegans longevity (lifespan/survival) assay
The lifespan of wild type C. elegans was measured as previously described.23 The nematodes were treated with different doses of B. monnieri (hexane extract) ranging from 1 to 100 μg/ml to analyze the effect of the extract in extending lifespan. In brief, a known number of young adult stage nematodes (∼10) were transferred to a 24 well plate with 300 μL of M9 buffer along with the laboratory food source E. coli OP50. Followed by different concentrations of B. monnieri (hexane extract), ranging between 1 and 100 μg/ml. The final volume was made up to 500 μL using M9 buffer and then maintained at 15 °C. The total number of worms was counted once in every 24 h, and it was considered dead when they do not respond to external stimuli such as a gentle tap or touch with the platinum loop. A parallel vehicle control of DMSO was also used, which was equivalent to the highest concentration of the solvent used. All the experiments were carried out in triplicate.
2.13. Caenorhabditis elegans lipofuscin imaging
C. elegans intestinal autofluorescence (sometimes referred to as Lipofuscin Imaging) was carried out using C. elegans (wt) treated with the hexane extract of B.monnieri for five days before imaging using ZEISS LSM 700 confocal microscope using the DAPI filter and 10x magnification at the objective lens. In each independent experiment, at least ten worms were randomly selected and photographed for analysis. The images were analyzed with Image J and the relative fluorescence represented as means ± SEM with arbitrary units (AU) from three independent experiments.
2.14. Statistical analysis
Statistical analysis was carried out using Graph Pad Prizm® for mac version 6.0 h. All results come from 3 or more independent experiments and are represented as means ± SEM. Unless otherwise stated data were analyzed using ANOVA, followed by Dunnet’s post hoc test for significance, P values lower than 0.05 were considered significant.
3. Results
3.1. HPLC analysis of B.monnieri
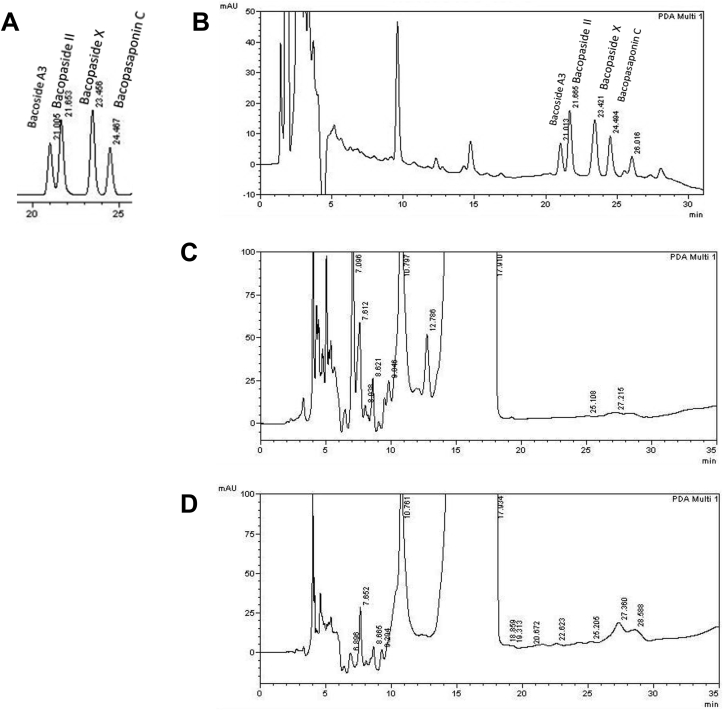
To standardize the extract of B.monnieri, making this study more comparable with others of the same herb, the bacoside A content was measured using HPLC. The HPLC fingerprint of bacoside A standards (a mixture of bacoside A3, bacopaside II, bacopaside X and bacopasaponin C standards) is shown in Fig. 1a. The HPLC fingerprint of B.monnieri is shown in Fig. 1b–d. It can be seen from Fig. 1, that all four constituents of bacoside A are present in the ethanol extract of B.monnieri. However, in the dichloromethane and hexane extracts these compounds appear to be missing or at very low concentrations (Fig. 1 c-d). The Peak Area of Bacoside A3, Bacopaside II, Bacopaside X, and Bacopasaponin C are shown in supplementary 2a. The concentration of Bacoside A3, Bacopaside II, Bacopaside X and Bacopasaponin C found in Bacopa monnieri (mg/g) are reported in Supplementary Fig. 2b. Bacopaside II was found to be the most abundant compound from those measured (0.38 ± 0.00001 mg/g).
Fig. 1.
HPLC analysis of B.monnieri. A HPLC fingerprint of Bacoside A3, Bacopaside II, Bacopaside X and Bacopasaponin C standards. B HPLC fingerprint from ethanol extract of Bacopa monnieri extract of B. monniera. C HPLC fingerprint from dichloromethane extract of Bacopa monnieri extract of B. monniera. D HPLC fingerprint from hexane extract of Bacopa monnieri extract of B. monniera.
3.2. The effect of B.monnieri extracts, on glutamate toxicity in HT-22 cells
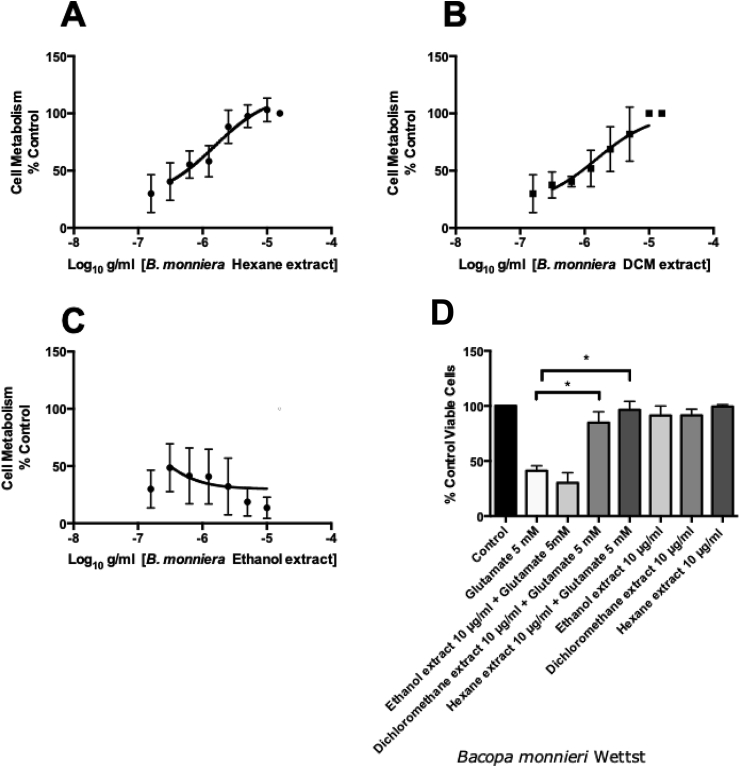
B.monnieri extracts (hexane, ethanol, and DCM) were assessed for their ability to prevent the effects of glutamate toxicity in the HT-22 cell line using the MTT assay (Fig. 2 a-c). Fig. 2a shows the dose-dependent protective effect of the hexane extract of B.monnieri against the toxicity caused by 5 mM glutamate compared to control cells (treated with PBS); the EC50 value for the hexane extract was 1.2 ± 1.3 μg/ml (n = 4). Fig. 2b shows the dose-dependent protective effect of the DCM extract of B.monnieri; with an EC50 value of 2.1 ± 1.37 μg/ml (n = 3). The ethanol extract of B.monnieri (Fig. 2c) did not affect (within the concentrations tested) the toxicity of 5 mM glutamate in HT-22 cells (n = 3).
Fig. 2.
HTT 22 cells exposed to 5 mM glutamate (Control Cells not treated with glutamate were used to normalize the data and set to 100%) and varying concentrations of A MTT assay B. monnieri (hexane extract), EC50 1.2 ± 1.3 μg/ml B MTT assay, B. monnieri (dichloromethane extract) EC50 2.1 ± 1.37 μg/ml & C MTT assay B. monnieri (ethanol extract) EC50 > 10 μg/ml. D Trypan blue counting of HT-22 cells exposed to glutamate and or 10 μg/ml B. monnieri extracts * significant difference between 5 mM glutamate and Bacopa monnieri 10 μg/ml (Hexane and DCM extracts) measured using ANOVA followed by Dunnett’s multiple comparisons post hoc test (n = 3).
In order to confirm the cell survival data collected from the MTT assay, viable cells were counted using the trypan blue exclusion assay (Fig. 2d). The highest effective concentration of B.monnieri from the MTT assay was chosen for this assay (10 μg/ml). Both the hexane and DCM extracts of B.monnieri returned the number of viable cells up to that of the control (statistically significant P= < 0.0001 (n = 4) using ANOVA followed by Dunnett’s multiple comparison post hoc test). As with the MTT assay, the ethanol extract had no cytoprotective effect in HT-22 cells treated with glutamate. Fig. 2d also shows that none of the extracts (at 10 μg/ml) affected viable HT-22 cell numbers when added alone.
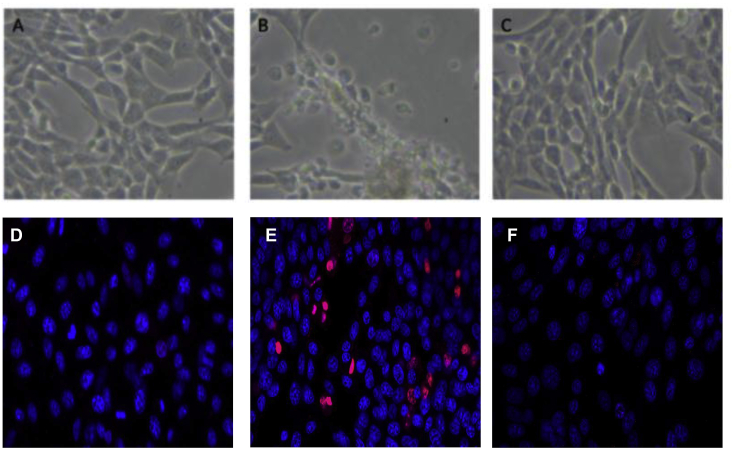
The morphology of the HT-22 cells (control, glutamate 5 mM, and glutamate 5 mM plus B.monnieri hexane extract respectively) are shown in Fig. 3(a–c). Glutamate has an apparent effect on cell morphology causing cell shrinkage, and death, Fig. 3(d–f) shows the confocal micrograph images with dead cells stained with propidium iodide (red) and live cells stained with Hoechst 33342 (blue). There is a clear difference between the cell treated with 5 mM glutamate and control or Glutamate plus 10 μg/ml B.monnieri (hexane extract). The B.monnieri hexane extract protected the HT-22 cells from the toxicity of 5 mM glutamate.
Fig. 3.
Reprasentative micrograph images of HT22 cells treated with Glutamate and/or Bacopa monnieri Hexane extract. A Phase contrast of control cells. B Phase contrast of 5 mM Glutamate treated cells. C Phase contrast 5 mM Glutamate +10 μg/ml Bacopa monnieri Hexane extract treated cells. D Confocal image of control HT22 cells stained with PI (Red) and Hoechst 33342 (blue). E Confocal image of 5 mM glutamate treated HT22 cells stained with PI (Red) and Hoechst 33342 (blue). F Confocal image of HT22 cells treated with 5 mM glutamate +10 μg/ml Bacopa monnieri stained with PI (Red) and Hoechst 33342 (blue).
3.3. B.monnieri extract’s effect on glutamate-induced mitochondrial stress
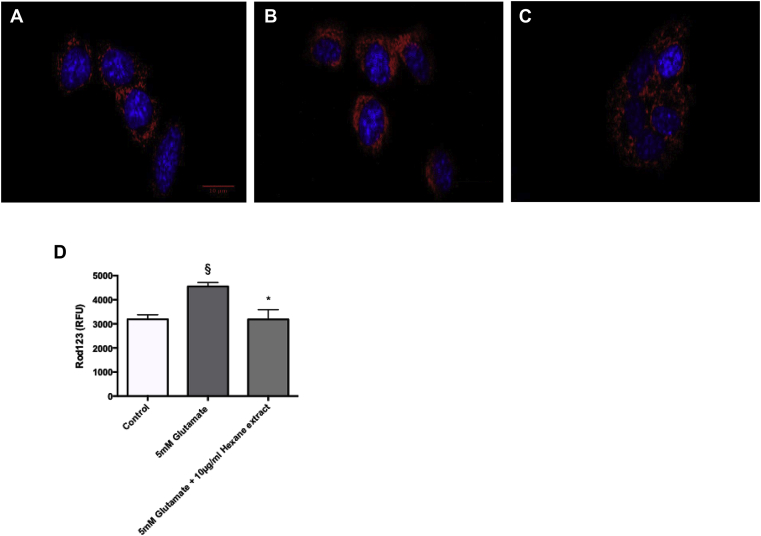
In order to assess the effect of 5 mM glutamate in the mitochondria of HT-22 cells, rhodamine123 staining was used. Fig. 4a shows a representative confocal micrograph of control cells stained with rhodamine 123, in which, clear and distinct staining of the mitochondria can be seen, indicating that the mitochondria are intact. Fig. 4b shows a representative confocal micrograph of HT-22 cells treated with 5 mM glutamate, and stained with rhodamine123; here, the staining is more diffuse, suggesting that the mitochondria are stressed and leaking. Fig. 4c shows a representative confocal micrograph of cells treated with 5 mM glutamate and 10 μg/ml B.monnieri hexane extract followed by rhodamine 123 staining. In contrast to glutamate alone, the B.monnieri hexane extract appears to protect the mitochondria from 5 mM glutamate-induced stress as the staining shows distinct mitochondria, and less diffuse.
Fig. 4.
Representative confocal images of HT22 cells stained with Rhodamine 123 (red) and Hoechst 33342 (blue). A Control HT22 cells. B HT22 cells treated with 5 mM glutamate C HT22 cells treated with 5 mM glutamate and 10 μg/ml Bacopa monnieri.D Rhodamine 123 fluorescence (total relative fluorescence units (RFU)) in HT22 cells measured in 96 well plates. § statistically significant difference between control cells and cells treated with 5 mM glutamate using ANOVA followed by Dunnett’s post hoc test P < 0.05 (n = 3). * Statistically significant difference between cells treated with 5 mM glutamate and cells treated with 5 mM glutamate and 10 μg/ml Bacopa monnieri Hexane extract using ANOVA followed by Dunnett’s post hoc test P < 0.05.
To quantify the effect of 5 mM glutamate and B.monnieri hexane extract on rhodamine123 staining of HT-22 cells were treated in the same way as the cells on the coverslips for imaging. The total fluorescence was then quantified using a plate reader (Fig. 4d). Cells treated with 5 mM glutamate showed a statistically significant higher fluorescence compared to control cells (ANOVA followed by Dunnett’s post hoc test P < 0.05 (n = 4)) indicating a significant increase in mitochondrial stress, and a loss of mitochondrial membrane integrity, in glutamate treated cells; whereas cells treated with 10 μg/ml B.monnieri Hexane extract and 5 mM glutamate, showed fluorescence more in line with control cells, and with statistically significant reduction in fluorescence compared to 5 mM glutamate alone (ANOVA followed Dunnett’s post hoc test P < 0.05 (n = 4)), which would suggest that B.monnieri is reducing mitochondrial stress, and increasing the mitochondrial membrane integrity.
3.4. B.monnieri’s effect on glutamate-induced ROS production
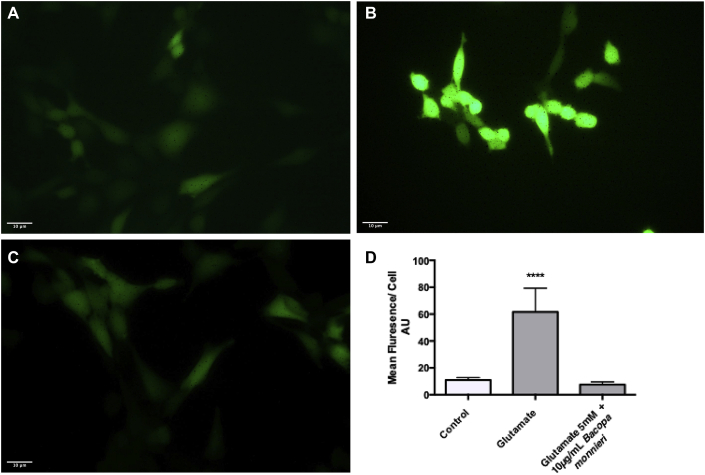
In order to evaluate the ROS production in response to glutamate (5 mM), HT-22 cells were stained with carboxy-H2DCFDA (Fig. 7a–c). Fig. 5a–c shows the apparent increase in H2DCFDA fluorescence when the HT-22 cells are treated with 5 mM glutamate, compared to the control, and 5 mM glutamate plus 10 μg/ml B.monnieri (hexane extract). This difference was quantified using Image J to measure the mean cellular fluorescence from multiple images from each of the groups Fig. 5d. The mean cellular fluorescences were 10.93 ± 1.85, 61.66 ± 17.60 and 7.55 ± 1.20 for the control, 5 mM glutamate treated, and 5 mM glutamate plus 10 μg/ml Hexane extract of B.monnieri respectively (n = 3). One-way ANOVA analysis showed that there was a significant difference between the mean cellular fluorescence of the control, 5 mM glutamate, and 5 mM glutamate plus 10 μg/ml Hexane extract of B.monnieri. Using Dunnet’s post hoc test for the comparison between glutamate 5 mM and glutamate plus 10 μg/ml hexane extract of B.monnieri P < 0.0001 (n = 3), while there was no significant difference between the control and glutamate plus 10 μg/ml hexane extract of B.monnieri.
Fig. 7.
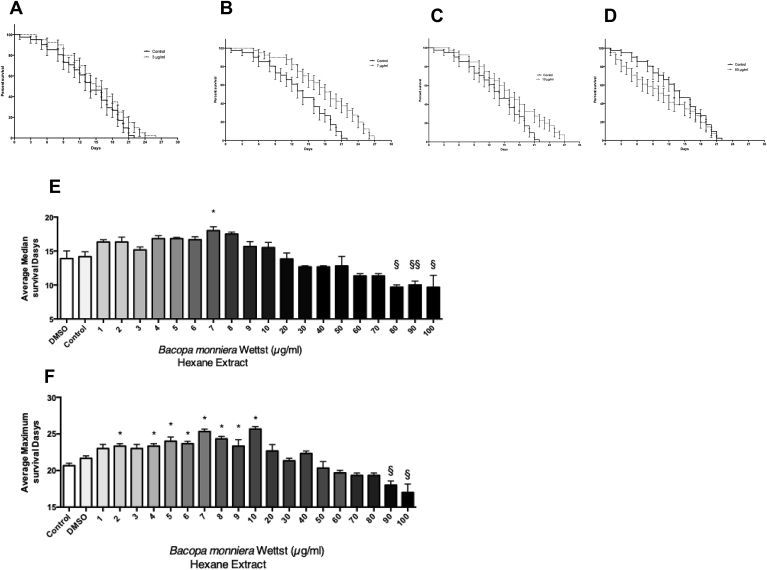
Selected survival curves for Caenorhabditis elegans (wild type) and median/maximal lifespansn when treated with varying concentrations of Bacopa monnieri Hexane extract. A Control vs 3 μg/ml Bacopa monnieri Hexane extract. No Significant difference between the curves using Log-rank (Mantel-Cox) test (p = 0.09). B Control vs 7 μg/ml Bacopa monnieri Hexane extract, Curves significantly different using Log-rank (Mantel-Cox) test (p < 0.0001) & Gehan-Breslow-Wilcoxon test (p = 0.0011). C Control vs 10 μg/ml Bacopa monnieri Hexane extract, Curves significantly different using Log-rank (Mantel-Cox) test (p < 0.0085). D Control vs 80 μg/ml Bacopa monnieri Hexane extract, Curves not significantly different using Log-rank (Mantel-Cox) test (p = 0.26).E Median survival of Caenorhabditis elegans (wild type) treated with varying concentrations of Bacopa monnieri hexane extract (mean ± SEM n = 3). * Median survival time in days significantly higher than control using ANOVA with Dunnett’s post hoc test p < 0.05. § Median survival time in days significantly lower than control using ANOVA with Dunnett’s post hoc test p < 0.05. F Maximum survival of Caenorhabditis elegans (wild type) treated with varying concentrations of Bacopa monnieri hexane extract (mean ± SEM n = 3). * Maximum survival time in days significantly higher than control using ANOVA with Dunnett’s post hoc test p < 0.05. § Maximum survival time in days significantly lower than control using ANOVA with Dunnett’s post hoc test p < 0.05.
Fig. 5.
The production of ROS in response to 5 mM glutamate. A control HT-22 cells stained with H2DCFDA. B HT-22 cells treated with 5 mM glutamate, stained with H2DCFDA. C Ht-22 cells treated with glutamate (5 mM) and Bacopa monnieri (10 μg/ml) stained with H2DCFDA. D quantification of H2DCFDA mean cellular fluorescence using image J. ****Statistically Significant difference between cells treated with 5 mM glutamate and cells treated with 10 μg/ml B.monnieri + glutamate using ANOVA followed by Dunnett’s post hoc test P < 0.05.
3.5. B.monnieri’s effect on glutamate-induced ER stress
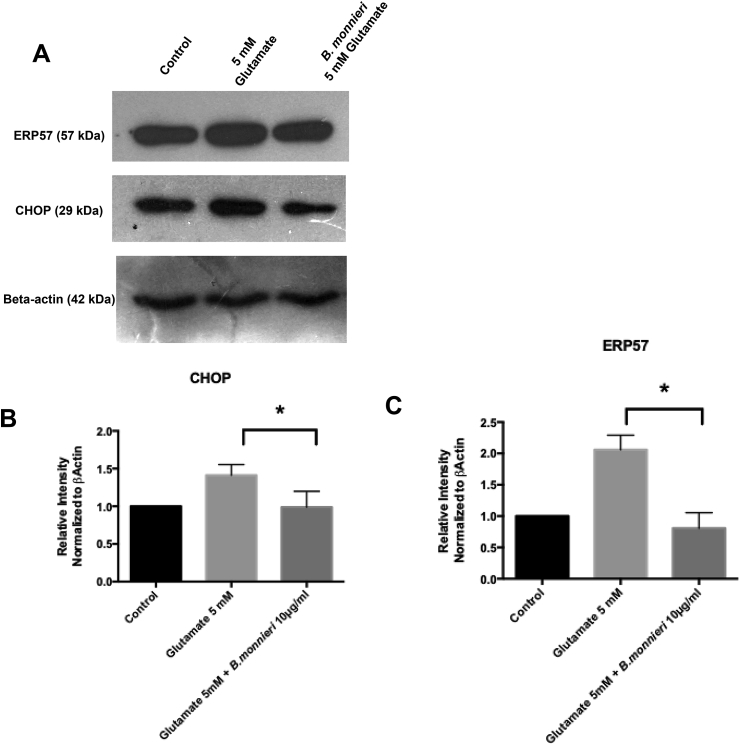
The effects of glutamate on the ER-localized proteins ERP57 and CHOP were analyzed using western blotting analysis (Fig. 6A). Density analysis shows that glutamate caused a doubling in the expression of ERP57, and an approximate 50% increase in CHOP expression, whereas, co-treatment with B.monnieri reduces the expression of these proteins back to control levels (Fig. 6B-C).
Fig. 6.
Western Blot analysis of HT22 cells treated with Glutamate and/or B.monnieri.A Blots for ERP 57, CHOP and beta-actin, using protein from HT22 cells treated with PBS(control), Glutamate (5 mM) and Glutamate + B.monnieri.hexane extract (10 μg/ml) B Density analysis for CHOP from 3 independent experiments, normalized to control and Beta actin. * Significant difference in density anaylysed using ANOVA followed by Dunnet’s post hoc test for significance C Density analysis for ERP 57 from 3 independent experiments, normalized to control and Beta actin. * Significant difference in density anaylysed using ANOVA followed by Dunnet’s post hoc test for significance.
3.6. B.monnieri’s effect on Caenorhabditis elegans life span
We analyzed the effect of B.monnieri (between 1 and 100 μg/ml) on the life span of C. elegans survival curves for selected doses are shown in Fig. 7. At lower doses (3 μg/ml shown in Fig. 7a) B.monnieri Hexane extract showed no significant difference between the control and B.monnieri treated using Log-rank (Mantel-Cox) test (p = 0.09, n = 3). However at doses between 7 and 10 μg/ml (Fig. 7b–c respectively) there was a significant increase in survival compared to control using the Log-rank (Mantel-Cox) test (p < 0.0001, n = 3) & Gehan-Breslow-Wilcoxon test (p = 0.0011, n = 3) for 7 μg/ml and for 10 μg/ml Log-rank (Mantel-Cox) test (p = 0.0085 n = 3). At higher doses, there was an apparent reduction in C. elegans survival, although analysis showed it not to be statistically significant, Log-rank (Mantel-Cox) test (P = 0.26 n = 3).
3.7. Caenorhabditis elegans intestinal autofluorescence
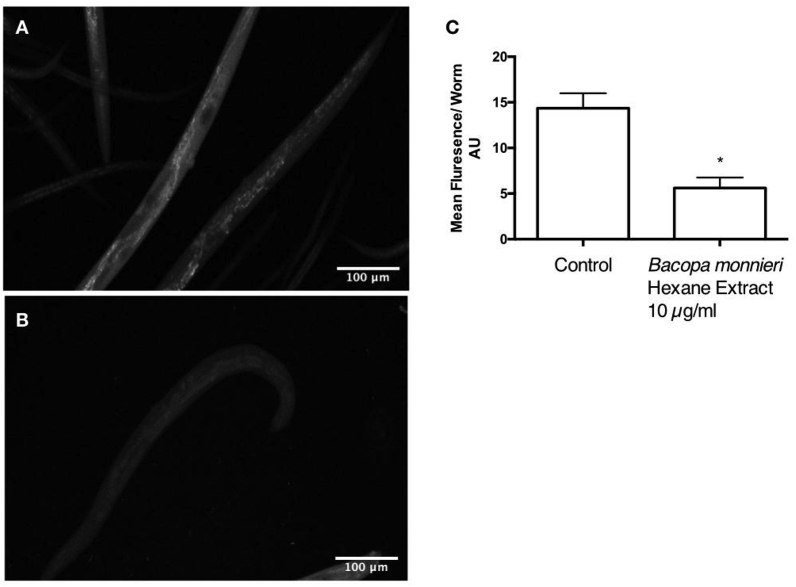
To assess whether the increase in lifespan of the population of C.elegans was accompanied by an increase in “healthspan” (i.e., reduction in the signs of aging caused by oxidative stress25), the intestinal fluorescence was measured. C.elegans intestinal autofluorescence is an indicator of health in terms of aging and oxidative stress in many species, including C.elegans. Lipofuscin consists of oxidized lipid peroxide and protein residues that increase within cells in a manner dependent on age26. Lipofuscin Imaging (Fig. 8a–c), showed that there was a significant reduction in Lipofuscin fluorescence in the worms treated with 10 μg/ml B.monnieri hexane extract (Fig. 8b) when compared to the control worms (Fig. 8a). The difference was quantified using Image J (Fig. 8c). After three independent experiments, the average fluorescence per worm for the control was 15.4 ± 1.7 (n = 3) fluorescence units, and for the B.monnieri treated worms 5.6 ± 1.1 (n = 3). This difference was statistically significant (unpaired t-test P = 0.0006 n = 3).
Fig. 8.
Caenorhabditis elegans intestinal autofluorescence. A Representative micrograph for control worms (untreated) B Representative micrograph for Worms treated with 10 μg/ml Bacopa monnieri hexane extract C Quantification of worm autofluorescence. Mean fluorescence/worm ± SEM (AU) was 14.4 ± 1.7 for the control and 5.6 ± 1.1 for treatment with 10 μg/mll Bacopa monnieri. * significant difference to control using an unpaired T-test P = 0.0006.
4. Discussion
In this study, we have shown that the Hexane and DCM extracts of B.monnieri protect against glutamate toxicity in HT-22 cells, whereas the ethanol extract does not. This is the first study to investigate the effects of glutamate-induced toxicity with different solvents used to extract the contents of the herb. Previous work by Limpeanchob et al., (2008)27 investigated the ethanol extract concerning glutamate-toxicity in primary cortical cells, found that the ethanol extract did not prevent glutamate toxicity, but did protect against the effects of amyloid β. It should also be noted that Limpeanchob et al., (2008) used at least 10 times higher concentrations of B.monnieri and that the glutamate-induced cell death in cortical neurons is likely to be caused by excitotoxicity, which is different mechanism to that in HT-22 cells; which is a block on the production of glutathione, resulting in ROS build up. Limpeanchob et al. (2008) did show that the ethanol extract of B.monnieri at 100 μg/ml could reduce the ROS content of the control cells (i.e., not stressed). We did not see any indication of a protective antioxidant effect with the dose we used with the Ethanol extract. However, we did see significant protection against glutamate-toxicity with the DCM and hexane extracts and furthermore, at much lower concentrations than in the previous study. This difference between the ethanol, DCM and hexane extracts is likely due to compounds other than the bacoside A constituents, due to the lack of bacoside A compounds in the dichloromethane and hexane extracts. While the % w/w of bacoside A compounds in the ethanol exract were relatively lower than other studies the ratio of compounds was comparable to previous studies.27,28 It is interesting that the hexane extract provided the greatest protection to the cells, since the non-polar copounds more likely to be extracted by hexane, will have a greater ability to be absorbed by the gut, and cross the blood brain barrier, making them more usefull in the treatment of neurodegernative diseases. Less polar compounds shown in suplamentary Fig. 1 such as monnieraside III and Plantainoside B are possible candidates for the protection seen in this study. Curbitacini E & I, are less likely to be protective compounds since they apear to induce cell death in various cancers29,30 One other possible compound found in B.monnieri8 and hexane extracts of other herbs is apigenin,31 and has potential neuroprotective effects with realtion to glutamate release.32 To identify such active compounds future studies may use mass spectrometry methods to identify the different compounds found in the extracts, particularly the difference between the hexane and Ethanol extracts.
The mitochondria, are a source of free radicals and ROS, as well as being susceptible to ROS damage.33 Neuronal cells in the brain have a particularly high metabolism, using 20% and 25% of the total consumed oxygen and glucose,34 this makes neurons and their mitochondria particularly susceptible to ROS damage. Mitochondrial damage in neurons is considered to be a risk factor for neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.33,35 In this study we have shown that mitochondrial damage/leakage and the build-up of ROS in cultured neurons can be prevented by treatment with the hexane extract of B.monnieri. Intact and functioning mitochondria are essential for proper neuronal function; it is well documented that a reduction in glucose metabolism in the brain is common in Alzheimer’s patients.36, 37, 38 B.monnieri’s ability to protect the mitochondria from ROS insult may explain why studies have shown positive behavioral changes in Alzheimer’s experimental models but no change in the size of plaques, even when amyloid β load is reduced.13 The protection of mitochondria in the surviving neurons, and the ability to maintain glucose metabolism at higher levels may explain B.monnieri’s effectiveness in improving memory in Alzheimer’s patients and healthy patients alike.
In this study we demonstrated that B.monnieri extract could extend the median and maximum lifespan of the C. elegans; however, such an effect would only be useful if the extended lifespan coincided with healthy individuals, and therefore extend the quality of life for the individual. C. elegans intestinal autofluorescence is an indicator of the individual’s apparent age and thus linked to the “health-span” of the population.39,40 From our results it would appear that the B.monnieri extract can extend the lifespan and reduce the effects of aging in C. elegans, resulting in younger appearing worms when compared to the control.
5. Summary and conclusions
In summary, the hexane extract (but not the ethanol extract) of B.monnieri protects cultured neuronal cells against the ROS production induced by glutamate treatment and prevents mitochondrial stress. This suggests that while the bacoside A compounds may be a defining feature of B.monnieri, they are not necessarily the only bioactive compounds found in the herb. Furthermore, the extract can extend the lifespan of the model organism, C.elegans, while maintaining a younger like phenotype in the aged worms. B.monnieri has previously been shown to have positive outcomes in the improvement of memory in both normal and aged/Alzheimer’s subjects. This study provides more evidence that B.monnieri, can potentially be of use as a therapeutic agent in aged patients. Furthermore, the fact that the ethanol extract and the hexane extract had differing efficacies could lead to the identification of new lead compounds for pharmaceutical study.
Declaration of competing interest
The Authors declare that they have no known conflict of interest with the publication of this manuscript in The Journal of Traditional and complimentary medicine.
Acknowledgments
This research was funded by the Royal Thai Government research fund. Dr. J.M.B and Dr. M.I.P were supported by the Rachadapisek Sompote Fund for Postdoctoral Fellowship, Chulalongkorn University, Thailand.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.10.001.
Contributor Information
James Michael Brimson, Email: james.b@chula.ac.th.
Mani Iyer Prasanth, Email: prasanth.I@chula.ac.th.
Waluga Plaingam, Email: waluga.p@rsu.ac.th.
Tewin Tencomnao, Email: tewin.t@chula.ac.th.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Christensen K., Doblhammer G., Rau R., Vaupel J.W. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M.J., Prina M., Guerchet M. Alzheimer’s Disease International; London: 2013. World Alzheimer Report 2013: Journey of Caring: An Analysis of Long-Term Care for Dementia. [Google Scholar]
- 3.Suh H.-W., Kang S., Kwon K.-S. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem. 2007;298(1-2):187–194. doi: 10.1007/s11010-006-9365-6. [DOI] [PubMed] [Google Scholar]
- 4.Kudo T., Kanemoto S., Hara H. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15(2):364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E., Davies K.J.A., Adenas E.N.C. Mitochonrial free radical generation,oxidative stress,and aging. Free Radic Biol Med. 2000;29(3/4):222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 6.Kean J., Downey L., Stough C. Systematic overview of bacopa monnieri (L.) wettst. Dominant poly-herbal formulas in children and adolescents. Medicine. 2017;4(4):86. doi: 10.3390/medicines4040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukumaran N.P., Amalraj A., Gopi S. Neuropharmacological and cognitive effects of Bacopa monnieri (L.) Wettst – a review on its mechanistic aspects. Complement Ther Med. 2019;44(February):68–82. doi: 10.1016/j.ctim.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Deepak M., Sangli G.K., Arun P.C., Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal An Int J Plant Chem Biochem Tech. 2005;16(1):24–29. doi: 10.1002/pca.805. [DOI] [PubMed] [Google Scholar]
- 9.Saraf M.K., Prabhakar S., Khanduja K.L., Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evidence-Based Complement Altern Med. 2010;2011:1–10. doi: 10.1093/ecam/neq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraf M.K., Anand A., Prabhakar S. Scopolamine induced amnesia is reversed by bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010;35(2):279–287. doi: 10.1007/s11064-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 11.Singh H.K., Dhawan B.N. Effect of Bacopa monniera Linn. (Brāhmi) extract on avoidance responses in rat. J Ethnopharmacol. 1982;5(2):205–214. doi: 10.1016/0378-8741(82)90044-7. [DOI] [PubMed] [Google Scholar]
- 12.Krishna G., Hosamani R. Bacopa monnieri supplements offset paraquat-induced behavioral phenotype and brain oxidative pathways in mice. Cent Nerv Syst Agents Med Chem. 2019;19(1):57–66. doi: 10.2174/1871524919666190115125900. [DOI] [PubMed] [Google Scholar]
- 13.Saini N., Singh D., Sandhir R. Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem Res. 2012;37(9):1928–1937. doi: 10.1007/s11064-012-0811-4. [DOI] [PubMed] [Google Scholar]
- 14.Le X.T., Pham H.T.N., Do P.T. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38(10):2201–2215. doi: 10.1007/s11064-013-1129-6. [DOI] [PubMed] [Google Scholar]
- 15.Peth-Nui T., Wattanathorn J., Muchimapura S. Effects of 12-week bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evidence-Based Complement Altern Med. 2012;2012:1–10. doi: 10.1155/2012/606424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roodenrys S., Booth D. Chronic effects of Brahmi ( bacopa monnieri ) on human memory. Neuropsychopharmacology. 2002;27(2):279–281. doi: 10.1016/S0893-133X(01)00419-5. Ph D. [DOI] [PubMed] [Google Scholar]
- 17.Thawani V., Venkat Ramana G., Abichandani L.G., Naidu M.U.R., Kumar N., Gharpure K.J. Efficacy of standardized extract of bacopa monnieri (Bacognize®) on cognitive functions of medical students: a six-week, randomized placebo-controlled trial. Evidence-Based Complement Altern Med. 2016;2016:1–8. doi: 10.1155/2016/4103423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman Scholfield C., Dilokthornsakul P., Limpeanchob N., Thanarangsarit P., Kongkeaw C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2013;151(1):528–535. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Goswami S., Saoji A., Kumar N., Thawani V., Tiwari M., Thawani M. Effect of Bacopa monnieri on Cognitive functions in Alzheimer’s disease patients. Ijcrimph. 2011;3(4):285–293. [Google Scholar]
- 20.Raghav S., Singh H., Dalal P., Srivastava J., Asthana O. Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment. Indian J Psychiatr. 2006;48(4):238. doi: 10.4103/0019-5545.31555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amit A., Wasim P., Allan J.J. Efficacy and tolerability of BacoMind®on memory improvement in elderly participants - a double blind placebo controlled study. J Pharmacol Toxicol. 2009;3(6):425–434. doi: 10.3923/jpt.2008.425.434. [DOI] [Google Scholar]
- 22.Schubert D., Kimura H., Maher P. Growth factors and vitamin E modify neuronal glutamate toxicity. Proc Natl Acad Sci. 1992;89(17):8264–8267. doi: 10.1073/pnas.89.17.8264. http://www.pnas.org/content/89/17/8264.short Accessed September 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasansuklab A., Meemon K., Sobhon P., Tencomnao T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement Altern Med. 2017;17(1):1–14. doi: 10.1186/s12906-017-2050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasanth M.I., Santoshram G.S., Bhaskar J.P., Balamurugan K. Ultraviolet-A triggers photoaging in model nematode Caenorhabditis elegans in a DAF-16 dependent pathway. Age (Omaha). 2016;38(1):1–13. doi: 10.1007/s11357-016-9889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Ma X., Li J., Cui X. Peptides from sesame cake extend healthspan of Caenorhabditis elegans via upregulation of skn-1 and inhibition of intracellular ROS levels. Exp Gerontol. 2016;82:139–149. doi: 10.1016/j.exger.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Jyotsna A., Mishra B.N., Asthana Pandey R., JGSI Free Radic Res. 2015;50(8):861–874. doi: 10.1080/10715762.2016.1187268. 2016. [DOI] [PubMed] [Google Scholar]
- 27.Limpeanchob N., Jaipan S., Rattanakaruna S., Phrompittayarat W., Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120(1):112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Ganzera M., Gampenrieder J., Pawar R.S., Khan I.A., Stuppner H. Separation of the major triterpenoid saponins in Bacopa monnieri by high-performance liquid chromatography. Anal Chim Acta. 2004;516(1-2):149–154. [Google Scholar]
- 29.Sörensen P.M., Iacob R.E., Fritzsche M. The natural product cucurbitacin E inhibits depolymerization of actin filaments. ACS Chem Biol. 2012;7(9):1502–1508. doi: 10.1021/cb300254s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan K.L.K., Duncan M.D., Alley M.C., Sausville E.A. Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochem Pharmacol. 1996;52(10):1553–1560. doi: 10.1016/s0006-2952(96)00557-6. [DOI] [PubMed] [Google Scholar]
- 31.Mallhi T.H., Qadir M.I., Khan Y.H. Determination of phytoconstituents of n-hexane extract of leaves of Morus nigra and evaluation of their effects on biochemical and histopathological parameters in paracetamol intoxicated mice liver. Brazilian J Pharm Sci. 2018;54(3) [Google Scholar]
- 32.Brimson J.M., Onlamoon N., Tencomnao T., Thitilertdecha P. Clerodendrum petasites S. Moore: the therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomed Pharmacother. 2019;118(July):109319. doi: 10.1016/j.biopha.2019.109319. [DOI] [PubMed] [Google Scholar]
- 33.Hirai K., Aliev G., Nunomura A. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on Astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Wang W., Li L. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Z., Rogers B., Shokouhi S. Longitudinal progression of cognitive decline correlates with changes in the spatial pattern of brain 18F-FDG PET. J Nucl Med. 2013;54(9):1564–1569. doi: 10.2967/jnumed.112.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamane T., Ikari Y., Nishio T. Visual-statistical interpretation of 18F-FDG-PET images for characteristic alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation. Am J Neuroradiol. 2014;35(2):244–249. doi: 10.3174/ajnr.A3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiman E.M., Foster N.L., Jagust W.J. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2009;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pincus Z., Mazer T.C., Slack F.J. Autofluorescence as a measure of senescence in C. elegans: look to red, not blue or green. Aging (Albany NY) 2016;8(5):889–898. doi: 10.18632/aging.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forge T.A., MacGuidwin A.E. Nematode autofluorescence and its sse as an indicator of viability. J Nematol. 1989;21(3):399–403. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.