Abstract
Background
The integration of residual cancer burden (RCB) and post‐treatment Ki67 as residual proliferative cancer burden (RPCB) has been proposed as a stronger predictor of long‐term outcome in unselected patients with breast cancer (BC) undergoing neoadjuvant chemotherapy (NACT), as compared with RCB. However, no specific analysis in hormone‐receptor‐positive (HR+) human epidermal growth receptor 2‐negative (HER2−) BC is available so far.
Materials and Methods
A cohort of 130 patients with HR+/HER2− BC who underwent NACT between 2000 and 2014 was included. Archival surgical specimens were evaluated for RCB. RPCB was calculated by combining RCB and Ki67 as previously described. Patients were categorized in four RCB and RPCB categories (pathological complete response and tertiles). Disease‐free survival (DFS) and overall survival (OS) estimates were determined by Kaplan‐Meier analysis and compared using the log‐rank test. Overall change of χ2 and c‐indexes were used to compare the performance of the prognostic models.
Results
RPCB was calculated for 85 patients. After a median follow up of 8.5 years, RCB was associated with OS (p = .048) but not with DFS (p = .152); RPCB was instead significantly associated with both DFS and OS (p = .034 and p < .001, respectively). In terms of OS, RPCB provided a significant amount of prognostic information beyond RCB (∆χ2 5.73, p < .001). In addition, c‐index for OS prediction was significantly higher for RPCB as compared with RCB (0.79 vs. 0.61, p = .03).
Conclusion
This is the first study evaluating RPCB in patients with HR+/HER2− BC treated with NACT. In this independent cohort, RPCB was a strong predictor of DFS and OS. The better performance of RPCB versus RCB was in part due to the ability of RPCB to discriminate a subgroup of patients with a particularly worse prognosis after NACT, who may be candidates for clinical trials evaluating novel adjuvant strategies.
Implications for Practice
The present work validated residual proliferative cancer burden (RPCB) as a strong predictor of long‐term outcome in patients with hormone receptor‐positive human epidermal growth receptor 2‐negative (HR+/HER2−) breast cancer (BC) treated with neoadjuvant chemotherapy. In addition, results from the present study suggest RPCB as a promising tool to identify patients with HR+/HER2− BC who might potentially benefit from the inclusion in clinical trials evaluating novel or escalated postneoadjuvant treatment strategies because it allowed to discriminate a subgroup of patients with particularly poor prognosis despite having received subsequent endocrine therapy in the adjuvant setting.
Keywords: Breast cancer, Residual cancer burden, Residual proliferative cancer burden, Ki67, Neoadjuvant chemotherapy
Short abstract
This article reports on the prognostic value of the Residual Proliferative Cancer Burden index in a cohort of patients with HR‐positive HER2‐negative breast cancer undergoing neoadjuvant chemotherapy.
Introduction
The achievement of a pathologic complete response (pCR) represents an established surrogate endpoint for long‐term outcome in patients with breast cancer (BC) undergoing neoadjuvant chemotherapy (NACT) [1]. On this basis, the Food and Drug Administration endorsed the use of pCR in neoadjuvant clinical studies for accelerated drug approval [2]. However, the prognostic significance of a simple dichotomization in pCR versus non‐pCR is suboptimal because a proportion of patients achieving pCR still relapse and a fraction of patients with residual disease after NACT may have an excellent prognosis. In addition, rates and prognostic value of pCR vary considerably across BC subtypes. In particular, hormone receptor‐positive (HR+) human epidermal growth receptor 2‐negative (HER2−) patients are associated with lower pCR rates than triple‐negative (TN) and HER2+ subtypes [3]. However, the presence of residual disease after NACT does not necessarily translate to poor outcome in this BC subtype [1, 4].
Residual cancer burden (RCB) index, which takes into account bidimensional measurements of residual tumor bed, invasive tumor cellularity, and nodal disease burden, has been validated as a stronger predictor of long‐term outcome in patients with BC undergoing NACT than pCR [5, 6]. However, patients’ stratification may be further improved by integrating RCB with postneoadjuvant Ki67—which in turn represents a recognized independent prognostic marker [7, 8, 9, 10, 11, 12]—as residual proliferative cancer burden (RPCB) index. Indeed, it has recently been suggested that RPCB may be capable of providing more prognostic information in unselected patients with BC undergoing NACT, as compared with RCB alone [5].
Nonetheless, although an exploratory analysis according to estrogen receptor (ER)‐positive versus ER‐negative subpopulation has been conducted in the discovery series, no specific analysis in the subgroup of patients with HR+/HER2− phenotype has been conducted so far.
We investigated the prognostic value of RPCB in an independent cohort of patients with HR+/HER2− BC treated with NACT at our institution.
Materials and Methods
Patient Cohort
A total of 130 consecutive patients with stage II–III HR+/HER2− BC who underwent NACT at our institution between 2000 and 2014 were included. HR was considered positive in case of positive immunohistochemistry (IHC) staining in ≥10% of tumor cells; HER2 was considered negative if score 0, 1+, or 2+ at IHC and/or in situ hybridization nonamplified.
Patients were identified from a prospectively maintained database in which clinicopathologic characteristics, treatment, time of recurrence, and follow‐up data were recorded.
Main exclusion criteria were stage IV disease, HR‐negative BC, HER2‐positive BC (3+ at IHC and/or amplified at fluorescence in situ hybridization), or unavailability or inadequacy of post‐NACT surgical specimens.
The study protocol was approved by the local ethics committee.
Pathologic Evaluation of Residual Disease
Formalin‐fixed paraffin‐embedded post‐NACT surgical specimens were retrieved from our Anatomy and Histology Department Archive.
RCB was evaluated on H&E‐stained as proposed by Symmans et al. [6] (Fig. 1), and calculated by using the RCB online source (http://www.mdanderson.org/breastcancer_RCB). In detail, primary tumor bed area, overall cancer cellularity, percentage of in situ disease, number of positive nodes, and diameter of the largest nodal metastasis were assessed in order to compute RCB score. For survival analysis, RCB was categorized in four classes according to pCR and RCB tertile values, for the ease of comparison with RPCB, consistently with previous studies [5, 6]:
pCR = absence of invasive cancer cells in breast and lymph nodes
I tertile
II tertile
III tertile
Figure 1.
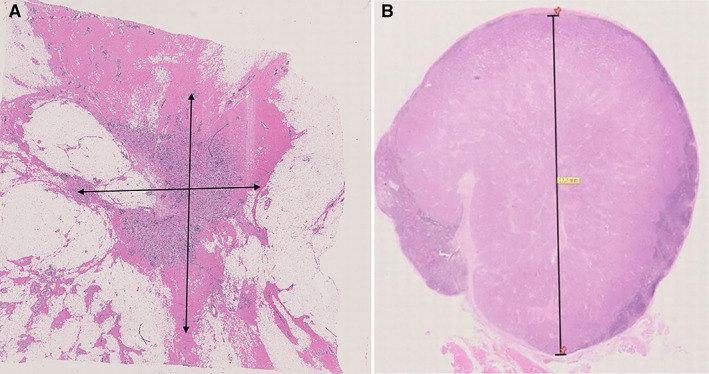
Parameters for residual cancer burden evaluation (A): Bidimensional diameter of residual tumor bed in the breast. (B): Diameter of the largest lymph node metastasis.
Cutoffs between I–II and II–III tertiles were 2.78 and 3.81, respectively.
Evaluation of post‐treatment Ki67 was performed by a pathologist, blinded for clinical data. Post‐treatment Ki67 was evaluated in MIB1‐clone stained slides and scored as the percentage of carcinoma cells with positive nuclear staining, consistently with Ki67 evaluation in RPCB pivotal study [5].
RPCB was calculated by combining RCB and Ki67 as previously described by Sheri et al. [5]. In particular, it was calculated as follows:
RPCB = b1(RCB) + b2(ln[Ki67 + 0.1]), where b1 and b2 represent Cox‐derived coefficients from Sheri et al. [5, 6] multivariate analysis for RCB and Ki67, respectively (b1 = 0.48, hazard ratio = 1.62 [95% confidence interval (CI) 1.53–2.57); b2 = 0.45, hazard ratio = 1.57 [95% CI 1.29–1.92]).
For survival analyses, patients were categorized into four categories for both RCB and RPCB. The four categories included pCR and RCB or RPCB tertiles. The categorization into four groups according to pCR and RPCB tertiles was consistent with the pivotal study [5]. However, we did not use the original cut‐points but instead adopted tertiles based on the distribution of RPCB in our cohorts. The rationale for this choice was based on two main considerations: (a) the original cut‐points were derived from a cohort of patients unselected for tumor phenotype; and (b) by applying the original cut‐points, only two patients would have been categorized as III tertile, limiting the power of prognostic analyses.
Therefore, the categories of RPCB were as follows:
pCR = absence of invasive cancer cells in breast and lymph nodes
I tertile
II tertile
III tertile
Cutoffs between I–II and II–III tertiles were 2.49 and 3.06, respectively.
RCB was considered as four categories according to pCR and tertiles and not as the standard classes defined by Symmans et al. in order to maintain consistency with the methodology used to categorize RPCB.
Statistical Analysis
Statistical analysis was carried out using IBM SPSS Statistics (version 22.0) software (IBM Corp, Armonk, NY) and R project [7].
Descriptive statistics were performed for patient demographics and clinical characteristics. For continuous variables, median, range values, and quartiles were computed. The Mann‐Whitney nonparametric test was used to study the distribution of continuous variables across groups defined by clinicopathologic characteristics.
Disease‐free survival (DFS) was defined as the time from surgery to first relapse (locoregional or distant) or death from any cause. Overall survival (OS) was defined as the time from surgery to death from any cause. Alive patients were censored at the date of last follow‐up. The Kaplan‐Meier method was used to estimate survival curves, and the log‐rank test was used to compare between groups. Univariate Cox regression modeling for proportional hazards was used to calculate hazard ratio and 95% CI.
Overall change of χ2 were computed to compare the performance of the prognostic models. We also evaluated c‐indexes of the two separate models and calculated the difference by compare C function in R [7]. The comparison between the prognostic models as continuous variables was conducted on the cohort of patients with both RCB and RPCB available and included only patients with residual disease (consistently with the methodology used by the original paper by Sheri et al. [5]).
All reported p values are two‐sided, and significance level was set at p < .05.
Results
Patient Characteristics
Overall, 130 patients with HR+/HER2− BC were included (clinicopathologic characteristics of the overall cohort are reported in supplemental online Table I).
RCB and RPCB were calculated for 105 and 85 patients, respectively (Fig. 2). No significant differences in baseline clinicopathologic characteristics were observed between RCB and RPCB cohorts.
Figure 2.
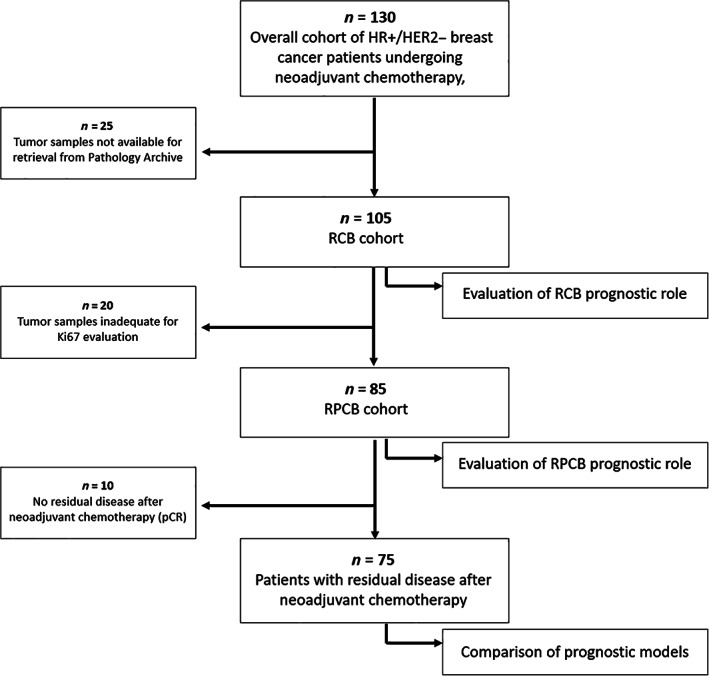
Flow diagram of the study. Abbreviations: HER2, human epidermal growth receptor 2; HR, hormone receptor; pCR, pathologic complete response; RCB, residual cancer burden; RPCB, residual proliferative cancer burden.
Residual Cancer Burden Evaluation and Prognostic Value
RCB was calculated for 105 patients. The baseline clinicopathologic features of the RCB cohort are reported in Table 1.
Table 1.
Clinicopathologic characteristics in the residual cancer burden cohort
| Characteristics | Total or n (%) |
|---|---|
| Patients, n | 105 |
| Age at BC diagnosis, yr | |
| Median (Q1–Q3) | 50.1 (44.0–59.4) |
| Mean | 51.7 |
| Histology | |
| Ductal | 74 (70.4) |
| Lobular | 20 (19.0) |
| Other | 6 (5.7) |
| NA | 5 (4.9) |
| Grade | |
| G1 | 1 (1.0) |
| G2 | 35 (33.3) |
| G3 | 41 (39.0) |
| X | 2 (1.9) |
| NA | 26 (24.8) |
| AJCC stage at diagnosis | |
| II | 43 (40.9) |
| III | 57 (54.3) |
| NA | 5 (4.8) |
| PgR, % | |
| Median (Q1–Q2) | 65.0 (5.0–90.0) |
| Mean | 50.9 |
| <20% | 28 (26.7) |
| ≥20% | 74 (70.5) |
| NA | 3 (2.8) |
| Ki67, % | |
| Median (Q1–Q3) | 28.0 (18.0–40.0) |
| Mean | 28.0 |
| <20% | 29 (27.6) |
| ≥20% | 63 (60.0) |
| NA | 13 (12.4) |
| NACT | |
| Anthracycline | 5 (4.8) |
| Anthracycline + taxane | 99 (94.2) |
| NA | 1 (1.0) |
| pCR | 10 (9.5) |
| AJCC post‐NACT pathologic stage | |
| 0 | 10 (9.5) |
| I | 16 (15.2) |
| II | 34 (32.4) |
| III | 45 (42.8) |
| Grade post‐NACT | |
| G1 | 2 (2.1) |
| G2 | 12 (12.6) |
| G3 | 9 (9.5) |
| X | 69 (72.6) |
| NA | 3 (3.2) |
| ER post‐NACT a | |
| ≤10% | 1 (1.0) |
| >10% | 83 (87.4) |
| NA | 11 (11.6) |
| Ki67 post‐NACT, median (Q1–Q3), % | 12.5 (5.0–25.0) |
| Adjuvant systemic therapy a | |
| Chemotherapy | 39 (30) |
| Endocrine therapy | 125 (96.2) |
| Relapse | |
| Total | 35 (33.3) |
| Local | 4 (3.8) |
| Distant | 33 (31.4) |
| Local + distant | 2 (1.9) |
| Death | 23 (21.9) |
Percentage has been computed in relation to the total number of patients with residual disease (n = 95).
Abbreviations: AJCC, American Joint Committee on Cancer; BC, breast cancer; ER, estrogen receptor; NA, not available; NACT, neoadjuvant chemotherapy; pCR, pathologic complete response; PgR, progesterone receptor; Q, quartile.
Median age at diagnosis was 50.1 years. The majority of patients had ductal histology (70.4%), grade 3 (39.0%), and stage III BC (54.3%). Median and mean baseline Ki67 were 28% (Q1–Q3 18%–40%) and 28.0%, respectively. Almost 20% of patients had luminal‐B BC, based on the surrogate definition of Ki67 ≥20% and/or progesterone receptor <20% [8, 9]. More than 94% of patients received anthracycline plus taxane‐based NACT. Ten patients achieved pCR after NACT (pCR rate = 11.7%). Median post‐treatment Ki67 was 12.5% (Q1–Q3 5.0%–25.0%). Almost all patients received adjuvant endocrine therapy (96.2%). Additional chemotherapy in the adjuvant setting has been administered in a third of the cases.
In this cohort, after a median follow‐up time of 8.5 years (95% CI 8.0–9.0), 3.8% and 31.4% of patients experienced local and distant relapse, respectively; 21.9% of patients died.
RCB did not significantly predict DFS in our cohort. In particular, 5‐year DFS according to RCB categories was 100% for pCR, 75.7% for I tertile, 67.5% for II tertile, and 65.1% for III tertile (p = .152). Kaplan‐Meier curves for DFS are shown in Figure 3A.
Figure 3.
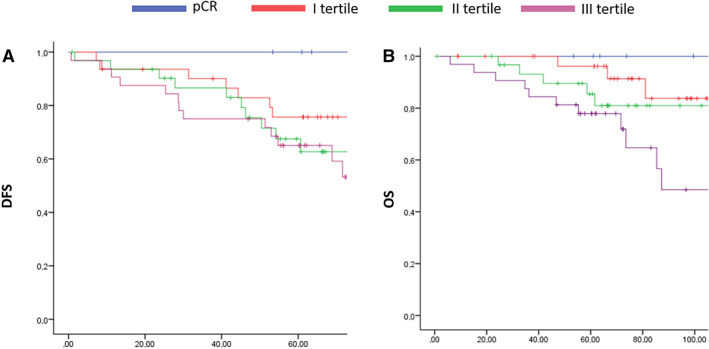
Kaplan Meier curves according to residual cancer burden evaluation. (A) DFS, ( B) OS. Abbreviations: DFS, disease‐free survival; OS, overall survival; pCR, pathologic complete response.
RCB was instead associated with OS. In detail, 5‐year OS was 100%, 96.2%, 85.4%, and 77.9% for pCR, I tertile, II tertile, and III tertile, respectively (p = .048). Kaplan‐Meier curves for OS are shown in Figure 3B.
Residual Proliferative Cancer Burden Evaluation and Prognostic Value
RPCB was calculated for 85 patients. The main baseline clinicopathological characteristics of the RPCB cohort are reported in Table 2.
Table 2.
Clinicopathologic characteristics in the residual proliferative cancer burden cohort
| Characteristics | Total or n (%) |
|---|---|
| Patients, n | 85 |
| Age at BC diagnosis, yr | |
| Median (Q1–Q2) | 49.4 (44.5–59.4) |
| Mean | 52.0 |
| Histology | |
| Ductal | 64 (75.3) |
| Lobular | 14 (16.5) |
| Other | 4 (4.7) |
| NA | 3 (3.5) |
| Grade | |
| G1 | 1 (1.2) |
| G2 | 31 (36.5) |
| G3 | 36 (42.3) |
| X | 1 (1.2) |
| NA | 16 (18.8) |
| AJCC stage at diagnosis | |
| II | 35 (41.2) |
| III | 45 (52.9) |
| NA | 5 (5.9) |
| PgR pre‐NACT, % | |
| Median (Q1–Q2) | 65.5 (9–90) |
| Mean | 52.2 |
| <20% | 22 (25.9) |
| ≥20% | 60 (70.6) |
| NA | 3 (3.5) |
| Ki67 pre‐NACT, % | |
| Median (Q1–Q2) | 30 (15.7–40) |
| Mean | 28.37 |
| <20% | 24 (28.2) |
| ≥20% | 52 (61.2) |
| NA | 9 (10.6) |
| NACT | |
| Anthracycline | 4 (4.7) |
| Anthracycline + taxane | 80 (94.1) |
| NA | 1 (1.2) |
| pCR | 10 (11.7) |
| AJCC post‐NACT pathologic stage | |
| 0 | 10 (10.6) |
| I | 14 (17.6) |
| II | 24 (28.2) |
| III | 37 (43.5) |
| Grade post‐NACT a | |
| G1 | 1 (1.3) |
| G2 | 11 (14.7) |
| G3 | 8 (10.7) |
| X | 54 (72.0) |
| NA | 1 (1.3) |
| ER post‐NACT a | |
| ≤10 | 1 (1.3) |
| >10 | 73 (97.4) |
| NA | 1(1.3) |
| Ki67 post‐NACT, median (Q1–Q3), % | 13 (5.0–28.5) |
| Adjuvant systemic therapy a | |
| Chemotherapy | 17 (20.0) |
| Endocrine therapy | 82 (96.5) |
| Recurrence | |
| Total | 31 (36.4) |
| Local | 2 (6.5) |
| Distant | 27 (87) |
| Local + distant | 2 (6.5) |
| Death | 20 (23.5) |
Percentage has been computed in relation to the total number of patients with residual disease (n = 95)
Abbreviations: AJCC, American Joint Committee on Cancer; BC, breast cancer; ER, estrogen receptor; NA, not available; NACT, neoadjuvant chemotherapy; pCR, pathologic complete response; PgR, progesterone receptor; Q, quartile.
RPCB was significantly associated with both DFS and OS in our HR+/HER2− BC cohort. In particular, 5‐year DFS was 100%, 70.3%, 78.6%, and 46.7% for pCR, I tertile, II tertile, and III tertile, respectively (p = .034), whereas 5‐year OS was 100%, 100%, 90.7%, and 59.4% for pCR, I tertile, II tertile, and III tertile, respectively (p < .001).
Kaplan‐Meier curves for DFS and OS according to RPCB categories are shown in Figure 4.
Figure 4.

Kaplan Meier curves according to residual proliferative cancer burden. (A) DFS, (B) OS. Abbreviations: DFS, disease‐free survival; OS, overall survival; pCR, pathologic complete response.
Cox analysis for DFS and OS according to RPCB is reported in Table 3.
Table 3.
Cox analysis for DFS and OS according to residual proliferative cancer burden
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| RPCB categories | HR | 95% CI | p value | HR | 95% CI | p value |
| pCR | Ref | .034 | Ref | <.001 | ||
| I tertile | 3.27 | 0.70–15.21 | 2.10 | 0.23–18.97 | ||
| II tertile | 2.64 | 0.53–13.10 | 2.44 | 0.25–24.19 | ||
| III tertile | 6.22 | 1.37–28.34 | 12.69 | 1.61–100.21 | ||
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; pCR, pathologic complete response.
Comparison of Prognostic Models
In terms of DFS, both RCB and RPCB provided limited prognostic information: χ2 0.62 for RCB and χ2 0.90 for RPCB. Nevertheless, the ∆χ2 of 0.28 was statistically significant (p < .001).
In terms of OS, RPCB provided a significant amount of prognostic information beyond RCB (χ2 9.3 for RPCB vs. 3.57 for RCB, ∆χ2 5.73, p < .001).
The c‐index for DFS prediction was numerically higher for RPCB than RCB (0.58 vs. 0.55, p = .475); c‐index for OS prediction was significantly higher for RPCB as compared with RCB (0.76 vs. 0.64, p = .031).
Discussion
The present study retrospectively evaluated the prognostic role of RPCB in a cohort of patients with HR+/HER2− BC who underwent NACT.
pCR and RCB are well‐recognized surrogate predictors for long‐term outcome after NACT; however, a more subtle definition of residual disease, accounting for not only residual tumor burden but also post‐treatment proliferative index Ki67 in the RPCB score, has been suggested as being capable of better predicting long‐term outcome in unselected patients with BC [5]. Indeed, several authors have consistently reported that post‐NACT Ki67 is capable of independently predicting long‐term survival in patients failing to achieve pCR, thus possibly representing a surrogate marker of NACT efficacy [10, 11, 12, 13, 14, 15].
Results from the present study provide an independent validation of RPCB as a strong predictor of long‐term survival in patients with HR+/HER2− BC treated with NACT. In particular, consistently with Sheri et al. [5], a significant association between RPCB and both DFS and OS has been observed.
Indeed, the comparison of prognostic model performance revealed that RPCB was a stronger predictor for both DFS and OS than RCB. One should consider that whereas in TN and HER2+ subtypes pCR and RCB allow a fairly reliable stratification of patients at different risk of relapse and/or death [1, 3, 4], in the HR+/HER2− subgroup, the survival advantage resulting from the administration of endocrine therapy in the postneoadjuvant setting may consistently dilute the prognostic impact of residual disease extent after NACT. In this context, an integrated evaluation of residual disease through RPCB assessment also encompassing tumor biology may provide more clinically useful information than RCB and pCR, thus ultimately optimizing patient prognostic stratification.
Interestingly, in our cohort of patients with BC, the prognostic impact of RPCB evaluation was mainly driven by the third tertile, thus allowing to discriminate a subgroup of patients with particularly poor long‐term DFS and OS rates despite having received adjuvant endocrine therapy (5‐year DFS 46.7% and 8‐year OS 25%), who may actually benefit from the inclusion in clinical trials evaluating novel and/or escalated postneoadjuvant treatment strategies (the comparison between first and second tertile should be interpreted with caution given the relatively small sample size)
In this respect, the presence of residual disease after NACT has been adopted as the main inclusion criteria for several clinical trials—some of which are still ongoing ([16, 17, 18, 19, 20] NCT01864746, NCT03155997)—testing diverse postneoadjuvant strategies, including, among others, CDK 4/6 inhibitors and various chemotherapy agents/regimens. In this context, postneoadjuvant capecitabine has been reported to improve both DFS and OS in patients with HER2− BC failing to achieve pCR after NACT. However, among patients with HR+ disease, no significant survival benefit has been observed [16]. Similarly, several trials testing other postneoadjuvant strategies in patients with residual disease after NACT failed to report any survival advantage in the HR+/HER2− subgroup [18, 19, 20]. These observations highlight that although in HER2+ and TN BC the simple dichotomization in pCR versus residual disease may still be considered appropriate to select high‐risk patients suitable for enrollment in postneoadjuvant clinical trials, as recently demonstrated in the KATHERINE and CREATE‐X trials, respectively [16, 17], in HR+ disease, the identification of a more reliable tool capable of properly detecting high‐risk patients who may benefit from additional postneoadjuvant treatments is mandatory. In this context, RPCP or other composite scores incorporating parameters that reflect both tumor burden and tumor biology, such as the preoperative endocrine prognostic index score [21], may represent good candidates. Interestingly, some effort in this direction is already ongoing [22].
The present study has several strengths: the inclusion of a selected population of patients with HR+/HER2− BC, where pCR and RCB proved to be suboptimal surrogates for long‐term outcome as compared with more aggressive BC subtypes [1, 3, 4], provided the opportunity to further dissect the prognostic impact of residual disease evaluation after NACT. Notably, in our HR+/HER2− BC population, a not negligible proportion of patients were luminal‐B, which is relatively less endocrine‐sensitive and more chemo‐sensitive—as well as being associated with poorer prognosis—as compared with luminal‐A phenotype [23], thus representing a challenging clinical scenario. In this context, the evaluation of RPCB could be an additional and clinically useful tool for optimizing the prognostic stratification of patients with HR+ BC. Moreover, a prolonged follow‐up time (8.5 years) allowed exploring the long‐term prognostic impact of RPCP, which is mandatory when analyzing the outcome of patients with HR+/HER2− BC who may still be at risk of relapse several years after diagnosis [24, 25]. The major limitations of the present study are represented by its retrospective and mono‐institutional nature and heterogeneity in neoadjuvant and postneoadjuvant treatments. Nonetheless, the vast majority of patients received anthracycline plus taxane‐based NACT and subsequent adjuvant endocrine therapy.
Conclusion
This represents the first study specifically evaluating RPCB in patients with HR+/HER2− BC treated with NACT. In this independent cohort of patients with BC, after a median follow‐up of 8.5 years, RPCB proved to better predict long‐term outcome in terms of both DFS and OS than RCB, probably owing to its ability to discriminate a subgroup of patients with a particularly unfavorable outcome after NACT despite the subsequent administration of endocrine therapy and who may be candidates for clinical trials in the adjuvant setting.
Of course, the evaluation of RPCB in the context of larger retrospective studies and, more importantly, well‐designed prospective clinical studies including patients with HR+ HER2+ BC treated with NACT is needed not only to further validate the prognostic significance of RPCB but also to explore its reproducibility and feasibility in sight of a broad application.
Author Contributions
Conception/design: Federica Miglietta, Maria Vittoria Dieci, Valentina Guarneri
Provision of study material or patients: Federica Miglietta, Maria Vittoria Dieci, Vassilena Tsvetkova, Gaia Griguolo, Grazia Vernaci, Alice Menichetti, Giovanni Faggioni, Tommaso Giarratano, Eleonora Mioranza, Elisa Genovesi, Enrico Cumerlato, Michele Bottosso, Tania Saibene, Silvia Michieletto, Marcello Lo Mele, Pierfranco Conte, Valentina Guarneri
Collection and/or assembly of data: Federica Miglietta, Maria Vittoria Dieci, Vassilena Tsvetkova, Gaia Griguolo, Grazia Vernaci, Alice Menichetti, Giovanni Faggioni, Tommaso Giarratano, Eleonora Mioranza, Elisa Genovesi, Enrico Cumerlato, Michele Bottosso, Tania Saibene, Silvia Michieletto, Marcello Lo Mele, Pierfranco Conte, Valentina Guarneri
Data analysis and interpretation: Federica Miglietta, Maria Vittoria Dieci, Pierfranco Conte, Valentina Guarneri
Manuscript writing: Federica Miglietta, Maria Vittoria Dieci, Vassilena Tsvetkova
Final approval of manuscript: Federica Miglietta, Maria Vittoria Dieci, Vassilena Tsvetkova, Gaia Griguolo, Grazia Vernaci, Alice Menichetti, Giovanni Faggioni, Tommaso Giarratano, Eleonora Mioranza, Elisa Genovesi, Enrico Cumerlato, Michele Bottosso, Tania Saibene, Silvia Michieletto, Marcello Lo Mele, Pierfranco Conte, Valentina Guarneri
Disclosures
Maria Vittoria Dieci: Eli Lilly and Company, Genomic Health (C/A), Eli Lilly and Company, Celgene (SAB); Pierfranco Conte: Roche, Novartis, Eli Lilly and Company, AstraZeneca, Tesaro‐GlaxoSmithKline (C/A), Roche, Novartis, Merk (RF), Eli Lilly and Company, Novartis (SAB); Valentina Guarneri: Roche (RF), Eli Lilly and Company, Novartis (SAB, C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 2. Food and Drug Administration, HHS . Pathologic complete response in neoadjuvant treatment of high‐risk early‐stage breast cancer: Use as an endpoint to support accelerated approval; guidance for industry availability. DFed Regist 2014;79:60476–60477. [Google Scholar]
- 3. Esserman LJ, Berry DA, DeMichele A et al. Pathologic complete response predicts recurrence‐free survival more effectively by cancer subset: Results from the I‐SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012;30:3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–1804. [DOI] [PubMed] [Google Scholar]
- 5. Sheri A, Smith IE, Johnston SR et al. Residual proliferative cancer burden to predict long‐term outcome following neoadjuvant chemotherapy. Ann Oncol 2015;26:75–80. [DOI] [PubMed] [Google Scholar]
- 6. Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 7.The R foundation. R: The R project for statistical computing. Available at https://www.r-project.org. Accessed October 2018.
- 8. Lee J, Im YH, Lee SH et al. Evaluation of ER and Ki‐67 proliferation index as prognostic factors for survival following neoadjuvant chemotherapy with doxorubicin/docetaxel for locally advanced breast cancer. Cancer Chemother Pharmacol 2008;61:569–577. [DOI] [PubMed] [Google Scholar]
- 9. Prat A, Cheang MC, Martin M et al. Prognostic significance of progesterone receptor‐positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Minckwitz G, Schmitt WD, Loibl S et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res 2013;19:4521–4531. [DOI] [PubMed] [Google Scholar]
- 11. Tanei T, Shimomura A, Shimazu K et al. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol 2011;37:155–161. [DOI] [PubMed] [Google Scholar]
- 12. Miglietta L, Vanella P, Canobbio L et al. Prognostic value of estrogen receptor and Ki‐67 index after neoadjuvant chemotherapy in locally advanced breast cancer expressing high levels of proliferation at diagnosis. Oncology 2010;79:255–261. [DOI] [PubMed] [Google Scholar]
- 13. Guarneri V, Piacentini F, Ficarra G et al. A prognostic model based on nodal status and Ki‐67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol 2009;20:1193–1198. [DOI] [PubMed] [Google Scholar]
- 14. Jones RL, Salter J, A'Hern R et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009;116:53–68. [DOI] [PubMed] [Google Scholar]
- 15. Curigliano G, Burstein HJ, Winer EP et al. De‐escalating and escalating treatments for early‐stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2018;29:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Huang CS, Mano MS et al. Trastuzumab Emtansine for Residual Invasive HER2‐Positive Breast Cancer. N Engl J Med 2019;380:617–628. [DOI] [PubMed]
- 17. Masuda N, Lee SJ, Ohtani S et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147–2159. [DOI] [PubMed] [Google Scholar]
- 18. von Minckwitz G, Rezai M, Tesch H et al. Zoledronate for patients with invasive residual disease after anthracyclines‐taxane‐based chemotherapy for early breast cancer ‐ The Phase III NeoAdjuvant Trial Add‐oN (NaTaN) study (GBG 36/ABCSG 29). Eur J Cancer 2016;64:12–21. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez‐Angulo AM, Lei X, Alvarez RH et al. Phase II randomized study of ixabepilone versus observation in patients with significant residual disease after neoadjuvant systemic therapy for HER2‐negative breast cancer. Clin Breast Cancer 2015;15:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas E, Holmes FA, Smith TL et al. The use of alternate, non‐cross‐resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin‐based regimen in women with operable breast cancer: Long‐term results from a prospective randomized trial. J Clin Oncol 2004;22:2294–2302. [DOI] [PubMed] [Google Scholar]
- 21. Ellis MJ, Tao Y, Luo J et al. Outcome prediction for estrogen receptor‐positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suman VJ, Ellis MJ, Ma CX. The ALTERNATE trial: Assessing a biomarker driven strategy for the treatment of post‐menopausal women with ER+/Her2‐ invasive breast cancer. Chin Clin Oncol 2015;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voduc KD, Cheang MC, Tyldesley S et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 2010;28:1684–1691. [DOI] [PubMed] [Google Scholar]
- 25. Ignatov A, Eggemann H, Burger E et al. Patterns of breast cancer relapse in accordance to biological subtype. J Cancer Res Clin Oncol 2018;144:1347–1355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table


