Abstract
LL‐37 is a cationic antimicrobial peptide and the sole human member of cathelicidins. Besides its bactericidal properties, LL‐37 is known to have direct immunomodulatory effects, among which enhancement of antiviral responses via endosomal toll‐like receptors (TLRs). Omiganan pentahydrochloride is a synthetic cationic peptide in clinical development. Previously, omiganan was primarily known for its direct bactericidal and antifungal properties. We investigated whether omiganan enhances endosomal TLR responses, similar to LL‐37. Human peripheral blood mononuclear cells were treated with endosomal TLR3, −7, −8, and −9 ligands in the presence of omiganan. Omiganan enhanced TLR‐mediated interferon‐α release. Subsequent experiments with TLR9 ligands showed that plasmacytoid dendritic cells were main contributors to omiganan‐enhanced IFN production. Based on this type I interferon‐enhancing effect, omiganan may qualify as potential treatment modality for virus‐driven diseases. The molecular mechanism by which omiganan enhances endosomal TLR responses remains to be elucidated.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Cathelicidin LL‐37, a cationic peptide of 37 amino acids long, enhances interferon responses to viral triggers. Presumably, LL‐37 binds to DNA and RNA, altering the endosomal trafficking of toll‐like receptor (TLR) ligands.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We studied whether omiganan pentahydrochloride, a synthetic cationic peptide in clinical development, is also capable of enhancing endosomal TLR‐mediated interferon responses.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Omiganan enhances interferon responses induced by different endosomal TLR ligands. Plasmacytoid dendritic cells are key contributors in the omiganan‐enhanced interferon production.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These results show that cationic peptides can be used for modulation of type I responses, and, as such, may qualify as potential treatment modality for virus‐driven diseases.
Cathelicidins are a group of cationic antimicrobial peptides occurring in human and many other species. 1 About 30 different cathelicidins are currently known in mammalian species. Cathelicidins are part of the innate immune system and exert antimicrobial activity by permeating and disintegrating the membranes of pathogens. 2 In man, LL‐37 is the sole identified member of the cathelicidin family. 1 LL‐37 is a cationic peptide of 37 amino acids long, expressed primarily in circulating neutrophils and in the gastrointestinal tract, lungs, and the epithelial cells of the skin. 3 , 4 , 5
Besides the direct antimicrobial effect, 2 LL‐37 has other mechanisms of action. LL‐37 stimulates wound closure, 6 neutralizes lipopolysaccharide, 7 and there is growing evidence for direct immunomodulatory effects of LL‐37.
LL‐37 affects the response of human neutrophils to viruses, 8 and the expression of pro‐inflammatory and anti‐inflammatory cytokines in macrophages during mycobacterial infection. 9 Another well‐described effect of LL‐37 is the enhancement of interferon responses to viral triggers. 10 The peptide enhances interferon production induced by toll‐like receptor (TLR) 3 ligand polyI:C in keratinocytes. 11 This effect is not exclusive for TLR3‐driven responses, and is also observed for other endosomal TLRs. 10 , 12 The binding of LL‐37 to DNA and RNA is supposed to be the mechanism behind this effect, altering the endosomal trafficking of TLR ligands. 13 LL‐37 is known to bind to polyI:C, thereby changing the structure of the ligand and enhancing its recognition by TLR3. 14
Based on the enhancement of TLR‐driven interferon responses by LL‐37, pharmacological modulation of viral diseases by synthetic cationic peptides is an interesting concept. Several cationic peptides are in (pre)clinical development, 15 such as omiganan pentahydrochloride. 16 Omiganan is a 12 amino acids long, antimicrobial peptide with bactericidal 17 and antifungal 18 properties. This paper describes a series of experiments conducted on primary human immune cells, wherein we investigated the potential interferon‐enhancing effects of omiganan. Further, we investigated the cell types contributing to omiganan‐enhanced interferon production.
MATERIALS AND METHODS
Materials
Omiganan pentahydrochloride (sequence: ILRWPWWPWRRK) was supplied by Cutanea Life Sciences. Scrambled omiganan (sequence: WLIPRWRPKWWR) was obtained from JPT (Berlin, Germany). LL‐37 was obtained from Anaspec (Eurogentec, Belgium). TLR ligands imiquimod, PolyI:C (HMW), imiquimod, ssRNA40/lyovec, CpG class A (ODN2216), CpG class B (ODN2006), and CpG class C (ODN2395) were all obtained from Invivogen, Toulouse, France.
Peripheral blood mononuclear cell isolation
Blood was collected from healthy volunteers via venipuncture into cell preparation tubes containing sodium heparin (Becton Dickinson, Franklin Lakes, NJ) after written informed consent in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The cell preparation tubes were centrifuged at 1800 xg for 30 minutes at room temperature. The peripheral blood mononuclear cell (PBMC) layer was removed and washed twice with phosphate‐buffered saline. PBMCs were counted using the MACSQuant 10 flow cytometer, using propidium iodide (Miltenyi Biotec, Bergisch‐Gladbach, Germany) for viability staining.
PBMC cultures
PBMCs were cultured at 0.5 × 10^6 cells/well in round‐bottom 96‐well plates in RPMI1640 (Gibco; Thermo Fisher Scientific, Waltham, MA), supplemented with 10% heat inactivated fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific), RPMI1640 supplemented with 5% autologous plasma or X‐Vivo 15 Serum‐free Hematopoietic Cell Medium (Lonza, Amboise, France). PBMCs were incubated with omiganan (25 µg/mL), scrambled omiganan (25 µg/mL), or LL‐37 (62.5 µg/mL) 30 minutes prior to TLR stimulation. Supernatants were collected after 24 hours and stored at −80°C before assaying. Cultures including imiquimod were performed in either RPMI1640 + 5% autologous serum or serum free medium, because the omiganan enhancing effect on IFNα is not observed when FBS is present in the culture medium.
Dendritic cell isolation
Plasmacytoid and myeloid dendritic cells (pDCs and mDCs) were isolated from PBMCs using negative selection kits from Stemcell (Vancouver, British Columbia, Canada), and the RoboSep (Stemcell) according to the user manual. Purity of isolated cells was assessed by flow cytometry using CD3‐PE‐Vio770, CD20‐PE‐Vio770, CD56‐PE‐Vio770, CD14‐VioBlue, HLA‐DR‐FITC, CD11c‐PE, and CD123‐APC antibodies (all Miltenyi Biotec), and measured on a MACSQuant 10 analyzer (Miltenyi Biotec). See Figure S1 for gating strategy.
Interferon measurements
Culture supernatants were assayed for IFNα by enzyme‐linked immunosorbent assay (PBL Assay Science, Piscataway, NJ).
Statistical analysis
A one‐way analysis of variance was performed on log transformed data using GraphPad Prism, version 6.05 (GraphPad Software, San Diego, CA). The effect of omiganan, scrambled omiganan, or LL37 was compared with TLR ligand alone (Figure 1 ). Results in Figure 2a were analyzed with a mixed effect model with treatment, CpG and treatment by CpG as fixed effects, subject, subject by treatment, and subject by CpG as random effects using SAS software (Cary, NC). Results are log transformed before analysis to correct for log‐normal distribution of the data. P values < 0.05 were considered significant.
Figure 1.
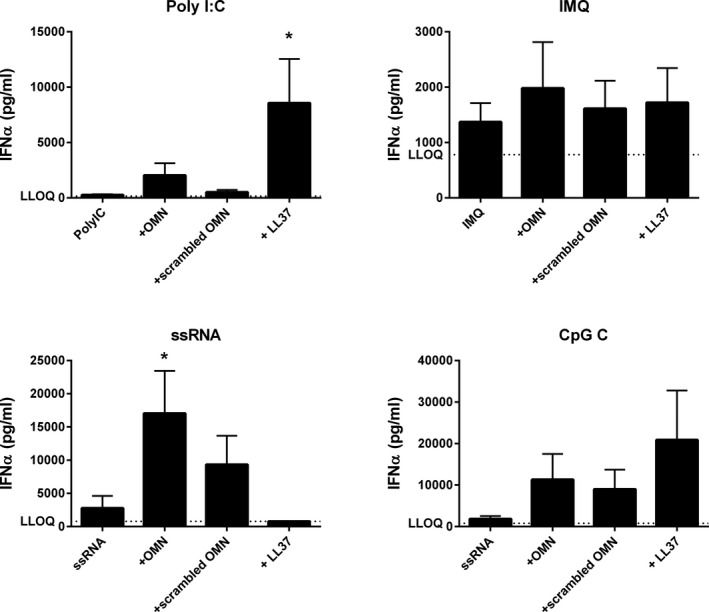
Omiganan, LL‐37 and scrambled omiganan enhance endosomal toll‐like receptor (TLR)‐driven interferon‐α (IFNα) release, but not for all TLRs. Peripheral blood mononuclear cells from four subjects were treated with either 25 µg/mL omiganan, 25 µg/mL scrambled omiganan, or 63.2 µg/mL LL‐37 (molecular equivalent to omiganan) for 30 minutes prior to simulation with 50 µg/mL polyI:C, 1 µg/mL imiquimod, 2.5 µg/mL ssRNA40/lyovec, or 2.5 µM CpG class C for 24 hours. IFNα was quantified in cell supernatant (mean + SEM). *P < 0.05. IMQ, imiquimod; LLOQ, lower limit of quantification; OMN, omiganan.
Figure 2.
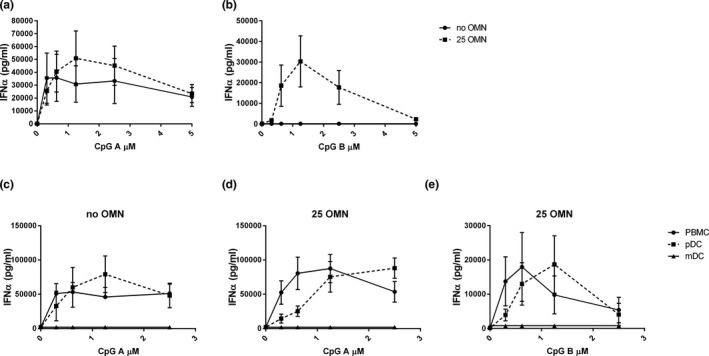
Omiganan enhances different toll‐like receptor 9 (TLR9)‐driven responses. In 3 independent experiments, peripheral blood mononuclear cells (PBMCs) from 6 subjects were pretreated with or without 25 µg/mL omiganan for 30 minutes prior to addition of CpG‐A (a) or CpG‐B (b). In two independent experiments, plasmacytoid and myeloid dendritic cells (pDCs and mDCs) were isolated from PBMC fractions of four subjects. PBMCs, pDCs, and mDCs were incubated with 0, 6.25, or 25 µg/mL omiganan for 30 minutes prior to addition with CpG‐A (c) or CpG‐B (d). Interferon‐α was quantified in cell supernatant (mean + SEM). OMN, omiganan, PBMC, peripheral blood mononuclear cell.
RESULTS
Omiganan enhances endosomal TLR‐driven IFNα production
The potential effect of omiganan on endosomal TLR‐driven responses was investigated by incubating PBMCs in RPMI1640 + 5% autologous plasma or RPMI1640 + 10% FBS with 25 µg/mL omiganan, 25µg/mL scrambled omiganan, or 62.5µg LL37 (molar equivalent to omiganan) prior to stimulation with polyI:C (TLR3), imiquimod (TLR7), ssRNA (TLR8), or CpG class C (TLR9) for 24 hours (Figure 1 ). IFNα was measured in the culture supernatants. IFNα release induced by the TLR ligands alone was modest (maximally 500–1,500 pg/mL), but reached levels of 1,800–18,000 pg/mL in the presence of omiganan. Scrambled omiganan enhanced IFNα release as well, although not as strongly as omiganan. LL‐37 enhanced polyI:C‐mediated and CpG‐C‐mediated responses (and more strongly than omiganan did), but not ssRNA‐mediated and imiquimod‐mediated responses. Due to the small sample size of this experiment, statistically significant contrasts were only observed for enhancement of ssRNA‐driven IFNα release by omiganan, and for enhancement of polyI‐C‐driven IFNα release by LL‐37. Additional experiments evaluating the omiganan concentration‐effect relationship substantiated these findings for polyI:C‐driven, ssRNA‐driven, and CpG‐C‐driven responses (Figure S2 four additional donors). Given the fact that these were separate experiments, it was decided to not pool the data.
Omiganan enhances TLR9‐driven responses
Subsequently, the effect of omiganan on responses driven by TLR9 ligands CpG‐A and CpG‐B was investigated (Figure 2a , b). Omiganan slightly enhanced CpG‐A IFNα production, although the contrast did not reach statistical significance (P = 0.09; omiganan pre‐incubation at 25 µg/mL; Figure 2a ). CpG‐B alone did not drive any IFNα production, but after pre‐incubation with omiganan CpG‐B stimulation resulted in a strong, bell‐shaped IFNα response (Figure 2b ). A next experiment investigated which cells in the PBMC fraction contribute to IFNα production after TLR9 stimulation. PBMCs, pDCs, and mDCs derived from the same donor were pre‐incubated with omiganan prior to stimulation with a dose range of CpG‐A or CpG‐B (Figure 2c – e ). Purities of 57–81% were observed for mDCs, 73–93% for pDCs, and the viability was always > 95%. Results of the preceding experiments could be confirmed, with a modest enhancement of CpG‐A‐driven IFNα responses by omiganan in PBMC cultures (Figure 2 : panel d vs. c), and a strong IFNα response upon omiganan/CpG‐B incubation (Figure 2e ). The mDCs did not produce IFNα in culture. The pDCs did release IFNα after CpG‐A stimulation, in the presence and absence of omiganan (Figure 2c , d). Combined incubation with omiganan and CpG‐B resulted in a strong IFNα release by isolated pDCs (Figure 2e ), indicating pDCs to be a key source of IFNα in CpG/omiganan‐stimulated PBMC cultures.
DISCUSSION AND CONCLUSIONS
In this series of experiments, pre‐incubation of omiganan enhanced TLR3‐driven, TLR7‐driven, TLR8‐driven, and TLR9‐driven IFNα release, following stimulation with the respective TLR ligands. This effect of omiganan was observed despite the small sample size (4–6 donors) and relatively large inter‐donor variability, which is inherent to culture experiments with primary human immune cells. Moreover, a part of the response variability may be attributed to the fact that PBMCs from both male as female donors were used, and sex differences in endosomal TLR‐driven IFNα production has been reported. 19 , 20 Scrambled omiganan also enhanced IFNα release, although not as strongly as omiganan.
LL‐37 also enhanced TLR‐driven responses, although the response pattern differed between omiganan and LL‐37. Whereas omiganan enhanced interferon responses to all selected TLR ligands (although only to a minor extend for imiquimod), LL‐37 only enhanced polyI:C‐driven and CpG‐C‐driven responses. For these experiments, equimolar levels of both cationic peptides were used. The enhancing effect of LL37 on CpG‐induced IFNα expression has been reported before. Morizane et al. 10 showed that coculture of CpG‐C with LL37 strongly drives mRNA IFNA2 expression. LL37 also enhanced PolyI:C‐induced (TLR3) and CpG‐C‐induced (TLR9) IFNB1 mRNA expression. 10 , 13
The inflammatory response to TLR9 stimulation largely depends on the selected TLR9 ligand, and is different for CpG classes A, B, and C. 21 CpG‐A (stretch of nucleotides with a palindrome motif) is known to induce interferon responses, whereas CpG‐B (single stretch of nucleotides) acts via late endosomal signaling resulting in a pro‐inflammatory cytokine response via nuclear factor κB. 21 CpG‐C (a combination of CpG‐A and CpG‐B) activates both interferon and nuclear factor κB‐driven responses. 22 Therefore, the effect of omiganan was investigated on responses driven by differently acting TLR9 ligands. Interestingly, pre‐incubation of PBMCs with omiganan preceding CpG‐B stimulation resulted in a strong IFNα release, suggesting activation of TLR9 in the early endosomes as described for CpG‐A. 23 Omiganan also enhanced the interferon response driven by CpG‐A, although this effect was much smaller. One potentially important observation is the bell‐shape of the omiganan‐enhanced CpG‐B response. This response shape implies that there may be an optimal molar ratio between omiganan and TLR ligand, and potentially an interaction between the two. A comparable bell‐shaped response has been observed for omiganan‐enhanced imiquimod responses, but not for other TLR ligands. A potential interaction between CpG and cationic peptides has been reported before, based on observations, such as colocalization in early endosomes, 24 and trafficking of LL‐37 with self‐RNA/DNA to endosomal TLRs. 25
To investigate which PBMC cell subset contributes to omiganan‐enhanced interferon production, we isolated PBMCs, pDCs, and mDCs and incubated the cells with CpG‐A or CpG‐B with or without omiganan. The omiganan‐dependent enhancement in CpG‐driven interferon release as observed in PBMCs was explained by a very strong interferon production by pDCs. No omiganan‐dependent interferon production by mDCs was observed. It should be noted that the number of pDCs and mDCs in the PBMC fraction is unknown and absolute counts may differ between PBMC fractions and isolated DCs. Interindividual variability in pDC number may explain the substantial interindividual variability in IFNα responses in PBMC cultures, as observed in preceding experiments. The purity of the isolated mDCs and pDCs was relatively low (57–81% and 73–93%, respectively). This is a limitation of the selected methodology, because these levels of purity are in line with the kit specifications set by the manufacturer. Importantly, the purity did not affect the conclusions of the experiment, because no interferon production was observed for the isolated mDCs.
The observed interferon‐enhancing effects of omiganan warrant further evaluation of the peptide as potential therapy for viral infections. The use of interferons for the treatment of such conditions is a growing area of interest. For example, hepatitis B and C virus infections are successfully treated with IFN‐α, and potential other applications of interferons are evaluated in the field of HIV, hepatitis, and influenza infections. 26
In summary, our experiments show that omiganan enhances IFNα responses to endosomal TLR ligands. The pDCs were identified as a major source of interferon production driven by omiganan and CpG. Based on these findings, omiganan may qualify as potential treatment modality for virus‐driven diseases. The molecular mechanism by which omiganan enhances endosomal TLR responses remains to be elucidated.
Funding
The research described in this paper was funded by Cutanea Life Sciences.
Conflicts of Interest
H.W.G., S.M.G., M.M., and G.F. are co‐inventors on a patent that includes the results with omiganan reported in this work. All other authors declared no competing interests for this work.
Author Contributions
All authors wrote the manuscript. H.W.G., S.M.G.J., R.R., K.E.M., G.F., and M.M. designed the research. H.W.G., S.M.G.J., T.D.W., and M.S. performed the research. H.W.G., S.M.G.J, and M.M. analyzed the data.
Supporting information
Fig S1
Fig S2
References
- 1. Kosciuczuk, E.M. et al. Cathelicidins: family of antimicrobial peptides. A review. Mol. Biol. Rep. 39, 10957–10970 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seil, M. et al. Spotlight on human LL‐37, an immunomodulatory peptide with promising cell‐penetrating properties. Pharmaceuticals 3, 3435–3460 (2010). [Google Scholar]
- 3. Bals, R. et al. The peptide antibiotic LL‐37/hCAP‐18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95, 9541–9546 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL‐37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997). [DOI] [PubMed] [Google Scholar]
- 5. Wan, M. et al. Leukotriene B4 triggers release of the cathelicidin LL‐37 from human neutrophils: novel lipid‐peptide interactions in innate immune responses. FASEB J. 21, 2897–2905 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Shaykhiev, R. et al. Human endogenous antibiotic LL‐37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L842–L848 (2005). [DOI] [PubMed] [Google Scholar]
- 7. Cirioni, O. et al. LL‐37 protects rats against lethal sepsis caused by gram‐negative bacteria. Antimicrob. Agents Chemother. 50, 1672–1679 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alalwani, S.M. et al. The antimicrobial peptide LL‐37 modulates the inflammatory and host defense response of human neutrophils. Eur. J. Immunol. 40, 1118–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torres‐Juarez, F. et al. LL‐37 immunomodulatory activity during Mycobacterium tuberculosis infection in macrophages. Infect. Immun. 83, 4495–4503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morizane, S. et al. Cathelicidin antimicrobial peptide LL‐37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Invest. Dermatol. 132, 135–143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh, D. et al. The human antimicrobial peptide LL‐37, but not the mouse ortholog, mCRAMP, can stimulate signaling by poly(I:C) through a FPRL1‐dependent pathway. J. Biol. Chem. 288, 8258–8268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilliet, M. & Lande, R. Antimicrobial peptides and self‐DNA in autoimmune skin inflammation. Curr. Opin. Immunol. 20, 401–407 (2008). [DOI] [PubMed] [Google Scholar]
- 13. Takiguchi, T. et al. Cathelicidin antimicrobial peptide LL‐37 augments interferon‐beta expression and antiviral activity induced by double‐stranded RNA in keratinocytes. Br. J. Dermatol. 171, 492–498 (2014). [DOI] [PubMed] [Google Scholar]
- 14. Lai, Y. et al. LL37 and cationic peptides enhance TLR3 signaling by viral double‐stranded RNAs. PLoS One 6, e26632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lei, J. et al. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 11, 3919–3931 (2019). [PMC free article] [PubMed] [Google Scholar]
- 16. Naafs, M. The antimicrobial peptides: ready for clinical trials? Biomed. J. Sci. Tech. Res. 7, 6038–6042 (2018). [Google Scholar]
- 17. Sader, H.S. et al. Omiganan pentahydrochloride (MBI 226), a topical 12‐amino‐acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob. Agents Chemother. 48, 3112–3118 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fritsche, T.R. et al. Antimicrobial activity of omiganan pentahydrochloride against contemporary fungal pathogens responsible for catheter‐associated infections. Antimicrob. Agents Chemother. 52, 1187–1189 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torcia, M.G. et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One 7, e39853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berghofer, B. et al. TLR7 ligands induce higher IFN‐alpha production in females. J. Immunol. 177, 2088–2096 (2006). [DOI] [PubMed] [Google Scholar]
- 21. Lee, B.L. & Barton, G.M. Trafficking of endosomal toll‐like receptors. Trends Cell Biol. 24, 360–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vollmer, J. et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 34, 251–262 (2004). [DOI] [PubMed] [Google Scholar]
- 23. Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004). [DOI] [PubMed] [Google Scholar]
- 24. Song, Y.C. & Liu, S.J. A TLR9 agonist enhances the anti‐tumor immunity of peptide and lipopeptide vaccines via different mechanisms. Sci. Rep. 5, 12578 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chamilos, G. et al. Cytosolic sensing of extracellular self‐DNA transported into monocytes by the antimicrobial peptide LL37. Blood 120, 3699–3707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li, S.F. et al. Type I Interferons: distinct biological activities and current applications for viral infection. Cell Physiol. Biochem. 51, 2377–2396 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


