Abstract
The pharmacokinetics (PKs) of sodium oxybate (SXB) was evaluated in a subset of participants from a study of SXB treatment in children (aged 7–11 years; n = 11) and adolescents (aged 12–17 years; n = 18) with narcolepsy with cataplexy. PK evaluation was conducted over 2 nights during the period when participants received a stable nightly SXB dose. The SXB dose on night 1 was half of night 2 and was administered in two equally divided doses: dose 1 was administered > 2 hours after the evening meal, and dose 2 was administered ≥ 4 hours after dose 1. Noncompartmental PK analysis demonstrated higher plasma concentrations post‐dose 2 vs. post‐dose 1, higher than dose‐proportional increases in area under the concentration‐time curve from 0 to 4 hours (AUC0–4h) after dose 1, indicating nonlinear clearance, and better correlation between exposure and mg/kg than exposure and gram dose. To confirm the noncompartmental findings, identify factors affecting SXB PK, and compare with prior results in adults, a population PK (PopPK) model was established combining PK data from the current study with prior data from adults (132 healthy volunteers and 13 with narcolepsy). A two‐compartment PopPK model with first‐order absorption and nonlinear clearance from the central compartment described the data well. PopPK identified weight as the main intrinsic factor and food as the main extrinsic factor affecting SXB PK, and predicts similar PK profiles on a mg/kg basis across ages. These results, along with previously reported efficacy and safety outcomes, support weight‐based SXB dose initiation in pediatric patients.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The pharmacokinetics (PKs) of sodium oxybate (SXB) has been studied in adults but not in pediatric patients.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The study addressed (i) whether the PKs of SXB in pediatric participants is similar to what is known in adults and (ii) whether the PK data support weight‐based dosing.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Noncompartmental analysis demonstrated nonlinear clearance of SXB, approximately linear increases in maximum plasma concentration (Cmax) and area under the concentration‐time curve (AUC) with mg/kg but not gram dose, and lower peak plasma concentrations with the first dose > 2 hours after eating vs. with the second dose. Population PK analysis demonstrated that SXB had common PK attributes in adults and pediatric individuals; weight was the main intrinsic and food the main extrinsic factor.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOL‐OGY OR TRANSLATIONAL SCIENCE?
☑ The close association between SXB plasma concentrations and mg/kg dosage supports the weight category‐based dosing regimen implemented and approved for SXB.
Narcolepsy is an incurable life‐long neurologic disorder estimated to affect 0.02–0.18% of the population worldwide. 1 Narcolepsy commonly begins in childhood, with most adults reporting first symptoms between 5 and 15 years of age. 2 The core symptoms of narcolepsy are excessive daytime sleepiness (EDS), cataplexy, sleep paralysis, sleep‐related hallucinations, and disrupted nighttime sleep. 3 Patients may have type 1 or type 2 narcolepsy; the main difference is that type 1 narcolepsy is characterized by cataplexy, which usually manifests as short‐term loss of muscle tone in adults, whereas type 2 narcolepsy is not associated with cataplexy. 1 In pediatric patients, cataplexy may manifest as complex movement disorders. 4 The symptoms of narcolepsy confer a substantial burden of illness in adults and children, severely affecting quality of life. 5 , 6
The treatment of narcolepsy is based on symptoms and includes anticataplectics, traditional stimulants, and wake‐promoting agents. 4 , 7 , 8 Sodium oxybate (SXB), the sodium salt of gamma‐hydroxybutyrate, is a central nervous system depressant whose oral solution dosage form is recognized in the United States and Europe as a standard of care for the treatment of adults with narcolepsy. 3 , 9 Multiple randomized trials demonstrated the efficacy and safety of SXB in the treatment of EDS and cataplexy in this population. 10 , 11 , 12 , 13 The recommended dosing schedule for adults with narcolepsy is to initiate SXB at 4.5 g nightly, divided in two doses 2.5–4 hours apart, and increase the dose by 1.5 g per night at weekly intervals to the effective dose range of 6–9 g/night. 14
Until recently, no drugs were approved for the treatment of EDS and cataplexy in children and adolescents with narcolepsy. In a placebo‐controlled, double‐blind, randomized, multicenter study of children and adolescents with narcolepsy with cataplexy, the efficacy, safety, and tolerability of SXB were found to be comparable with those previously demonstrated in adults. 15 This study led to the approval of SXB for the treatment of EDS and cataplexy in children and adolescents (aged ≥ 7 years) with narcolepsy. 14 Similar to adult dosing, SXB is administered in children and adolescents in two divided nightly doses at least 2.5 hours apart. In pediatric patients, the recommended starting dose (2–4.5 g per night), titration regimen (1–1.5 g per night), and maximum nightly dose (6–9 g/night) are based on the patient’s weight category (20 to < 30 kg, 30 to < 45 kg, and ≥ 45 kg). 14 As in adults, individual titration is recommended based on efficacy and tolerability to optimal dose. 14
The pharmacokinetics (PKs) of SXB was characterized in healthy adults and adults with hepatic impairment, alcohol dependency, and narcolepsy. 14 , 16 , 17 , 18 , 19 , 20 , 21 SXB was well absorbed (absolute oral bioavailability, 88%) and was mainly eliminated from the body via metabolism, with only 5% excreted unchanged in the urine. SXB metabolism begins primarily via conversion to succinic acid in two enzymatic steps: the primary pathway involves the cytosolic NADP+‐linked enzyme gamma‐hydroxybutyrate dehydrogenase, which catalyzes the conversion of gamma‐hydroxybutyrate to succinic semialdehyde, which is then metabolized by succinic semialdehyde dehydrogenase to succinic acid. Succinate then enters the tricarboxylic acid cycle and is eventually breathed out as carbon dioxide. 14 This is part of the reason for a dosage reduction in patients with hepatic impairment, 14 in whom metabolism may be reduced, and (because metabolism is saturable) explains why exposure increases more than proportionally with increasing doses. A 2‐fold greater SXB dose resulted in a 3.7‐fold increase in exposure, as measured by area under the concentration‐time curve (AUC), indicating nonlinear PK. 14 A combination of rapid clearance and a relatively small volume of distribution results in a short elimination half‐life of < 1 hour, which may partially explain the short duration of action of SXB, 21 necessitating twice‐nightly dosing. Dosing SXB immediately after a high‐fat meal reduced maximal concentration (Cmax) by 59% and AUC by 37%. 14
The PKs of SXB in children and adolescents with narcolepsy has not been previously characterized. The objectives of this PK study were (i) to characterize the PK of SXB in a subset of participants from the study of SXB in children and adolescents with narcolepsy with cataplexy 15 ; (ii) to develop a structural population PK (PopPK) model to confirm the characteristics of SXB in pediatric patients compared with adults; and (iii) to identify intrinsic and extrinsic covariates in the model that contribute to PK variability. The PK characterization and PopPK analysis would ultimately help to support the proper dose regimen in pediatric patients with narcolepsy.
METHODS
Overall study design
In the double‐blind, placebo‐controlled, randomized withdrawal study conducted at 30 sites in 5 countries, the efficacy and safety of SXB oral solution were evaluated in pediatric participants (aged 7–16 years at screening) with narcolepsy with cataplexy. 15 Following screening, patients who were naive to SXB underwent titration to a stable dose over 3 to 10 weeks (Figure 1 ). For SXB‐naive participants, a weight category‐based dosing regimen was used for initiation, titration rate, and maximal nightly dose (Table S1 ). SXB doses were administered by or under the supervision of qualified study site personnel. Participants took SXB diluted with 60 mL of water and/or flavorant, followed by 180 mL of water. The actual timing of each administered dose was recorded. During titration, the dose of SXB was adjusted for each SXB‐naive participant according to the investigator’s clinical judgment. The optimal dose for each SXB‐naive patient was the one that improved the frequency of cataplexy attacks and achieved stability in cataplexy frequency and severity without need for further adjustments. Once the optimal dose was defined during the titration period, that regimen was continued during the stable‐dose period. Participants who were already taking SXB at study entry did not undergo dose titration, but instead continued their usual dose and proceeded to the stable‐dose period directly after screening. Participants subsequently entered a 2‐week double‐blind period and then an open‐label period for up to 52 weeks on study.
Figure 1.
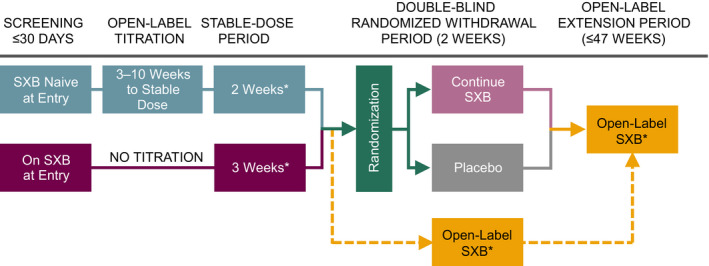
PK study design in context of overall study. A pre‐planned interim analysis of 35 participants showed that efficacy was achieved (P = 0.0002) based on the primary end point (change in weekly cataplexy attacks). Therefore, the double‐blind randomized withdrawal period was terminated early, and participants entered the open‐label period from the stable‐dose period (dashed lines). SXB, sodium oxybate; PK, pharmacokinetics. *Indicates times when PK data could be collected. Adapted from Plazzi et al. 15
The study was registered at ClinicalTrials.gov (NCT02221869) and was conducted in accordance with the Declaration of Helsinki. All participants provided assent and their parent(s) or guardian(s) gave written informed consent in accordance with local institutional review board/independent ethics committee requirements before the performance of any study‐related procedures.
PK study design
A subset of 29 participants from the entire study population (N = 106) underwent PK evaluation while they were consistently taking a stable dose of SXB during the open‐label periods of the study (Figure 1 ). These participants spent 2 nights in the clinic. On PK night 1, they received approximately half their nightly stable SXB dose. On PK night 2, they returned to the clinic and received their full stable dose. Each night, SXB was administered as two equally divided doses, given while in bed at bedtime (dose 1) and 4 hours later (dose 2). Participants were required to eat a light dinner > 2 hours before dose 1. Following the light dinner, no food was allowed until the following morning. Water was allowed at night but could not be taken 1 hour before or after dosing (60 mL of water to dilute the dosing solution, immediately followed by 180 mL of water, was part of the dosing). Participants were required to remain in bed for 8 hours after dose 1. Blood samples to measure plasma SXB concentrations were collected at 0 (predose 1), 0.75, 1.5, 2.5, 4 (predose 2), 4.75, and 8 hours after the first dose on each night. PK samples were taken within ± 5 minutes of the specified time points. The actual time of blood collection was recorded for all samples. SXB concentrations in the plasma samples were determined using a validated liquid chromatography/tandem mass spectrometry method according to US Food and Drug Administration (FDA) guidance. Briefly, this method has a linear range of 1.0 to 160 µg/mL of SXB and a limit of quantitation of 1.0 µg/mL.
Noncompartmental PK analysis in pediatric participants
PK parameters
Descriptive summary statistics were presented for the entire PK population and by evenly split age groups (children 7–11 years and adolescents 12–17 years). For each PK night, SXB concentrations were summarized by sampling time point. Concentrations below the limit of quantification were imputed as 0. The PK parameters for plasma SXB concentrations included AUC from 0 to 4 hours (AUC0–4h), Cmax, and time to Cmax (Tmax) over the first 4‐hour dosing interval. Additionally, SXB concentrations at 4.75 and 8 hours were measured to estimate peak (pseudo Cmax) and residual exposure, respectively, after dose 2, which was administered at 4 hours.
Dose proportionality assessment
Analyses for dose proportionality were performed for AUC0–4h and Cmax. For each parameter, the natural log‐transformed value on PK night 2 minus the natural log‐transformed value on PK night 1 was the response variable. The estimated mean difference and 90% confidence interval (CI) were back‐transformed to ratio scale by exponentiation to interpret the results. A value of 2 within the 90% CI indicated proportionality.
PopPK modeling in pediatric participants and adults
In the pediatric study, PK sampling was performed before dose 1 (0 hours) and at 0.75, 1.5, 2.5, 4 (before dose 2), 4.75, and 8 hours after dose 1; therefore, data for dose 1 were well‐suited for noncompartmental analysis. In contrast, as only three of those samples were collected at dose 2, data for dose 2 could not be well characterized by noncompartmental analysis. To address this limitation, a separate PopPK analysis was performed to comprehensively evaluate the full data set and to compare pediatric and adult PK. The PopPK analysis model was based on data pooled from the current pediatric study 15 and four adult PK studies (three in healthy participants and one in participants with narcolepsy; Table S2 ). 14 , 21 SXB oral solution was administered as a single 4.5‐g dose or multiple doses ranging from 1 to 4.5 g twice nightly. 14 , 21 Unlike the pediatric PK study, healthy adults and adults with narcolepsy received a fixed SXB dose and did not undergo initial individual titration to the PK dose.
Compartmental models were fit to SXB concentration‐time data with a nonlinear fixed‐effects method using NONMEM version 7.3 (ICON, Dublin, Ireland). 22 , 23 , 24 The following intrinsic factors were explored as covariates: age and age category, body surface area, 25 body weight, lean body mass, 26 body mass index, sex, race, and disease status (i.e., with narcolepsy or healthy; Table S3 ). The following extrinsic factors were explored as covariates: prandial status, diurnal factor (i.e., time of dosing), and bioanalytical method (Table S3 ). For the pediatric PK data, the first nightly dose was considered to be in the fed state, whereas the second nightly dose was considered to be in the fasted state.
In the PopPK model, a forward addition and backward elimination process (using goodness‐of‐fit plots and change in the objective function value (OFV)) was used to evaluate the effect of covariates on maximum velocity/rate of metabolism (Vmax), first‐order absorption rate constant (Ka), central volume of distribution (VC), concentration of substrate at half Vmax (Km), and fraction absorbed. Continuous (body surface area, body weight, body mass index, and age) and categorical (age category, prandial status, and diurnal factor) covariates were tested. Covariate additions were based on a likelihood ratio test if the decrease in OFV was ≥ 3.84 (equivalent to P < 0.05 for χ2 distribution with 1 degree of freedom) and elimination if the increase in OFV was ≥ 10.828 (equivalent to P < 0.001 for χ2 distribution with 1 degree of freedom). The final model was evaluated using goodness‐of‐fit plots, bootstrap analysis, and visual predictive checks.
Simulations
Simulations were performed in NONMEM version 7.3. The final validated covariate model was used to perform simulations to assess the impact of statistically significant covariates on SXB PK and to evaluate the clinical relevance. Simulations were performed for a range of age groups (child, adolescent, and adult), for a range of dosing regimens, including fixed dose for an age range (1–4.5 g twice nightly) and weight‐based dosing (16.6–113 mg/kg), under both fed and fasted states. The covariates from the available human studies in all age groups were used for the simulation by resampling, with replacement to create the respective populations (child (7–11 years), adolescent (12–17 years), and adult (≥ 18 years)).
RESULTS
PK characterization in the pediatric study (noncompartmental analysis)
Demographic and baseline characteristics
The demographics and baseline characteristics of the 29 participants in the pediatric PK population were similar to those of the efficacy and safety populations in the same study, as previously reported. 15 At study entry, 37.9% of participants were children and 62.1% were adolescents (Table 1).
Table 1.
Demographic and baseline characteristics of the PK population
| Variable | Children (n = 11) | Adolescents (n = 18) | All (n = 29) |
|---|---|---|---|
| Age a (years) | 9.0 (8–11) | 13.0 (12–16) | 12.0 (8–16) |
| Male, n (%) | 9 (81.8) | 13 (72.2) | 22 (75.9) |
| Nightly dosage a (mg/kg) | 150.0 (93–226) | 108.4 (70–184) | 112.4 (70–226) |
| Weight a (kg) | 43.0 (30–78) | 65.1 (39–129) | 60.0 (30–129) |
PK, pharmacokinetics.
Median (range).
Stable dose
The median stable dose was 7 g/night and ranged from 4 to 9 g/night, or 69.8 to 225.8 mg/kg/night.
Plasma concentrations at stable dose
SXB plasma concentrations were generally greater after dose 2 compared with dose 1 for the equivalent postdose time points (Figure 2 ). The mean (SD) SXB concentration 0.75 hours after dose 1 and dose 2 was 74.7 (42.0) and 128 (49.7) µg/mL, respectively. The mean (SD) SXB concentration 4 hours after dose 1 and dose 2 was 29.5 (24.7) and 47.4 (44.9) µg/mL, respectively.
Figure 2.
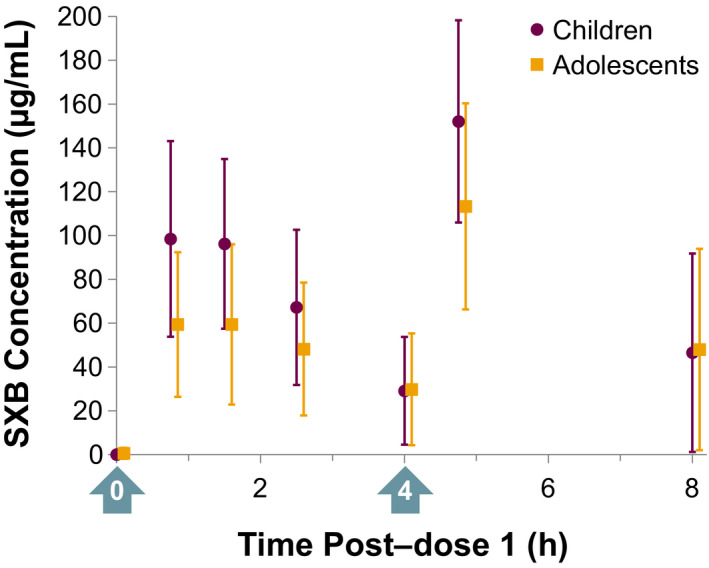
Mean (±SD; n = 29) plasma SXB concentration‐time data by age group for participants following administration of SXB at 4–9 g/night as two equally divided doses. Arrows indicate the two doses administered. SXB, sodium oxybate.
PK parameters based on noncompartmental analysis
PK parameters are presented in Table 2 for children, adolescents, and overall. Median Tmax, Cmax, and AUC0–4h were, respectively, 0.8 hours, 109.0 µg/mL, and 267.9 µg∙h/mL in children, and 1.2 hours, 72.9 µg/mL, and 160.0 µg∙h/mL in adolescents. C4.75h was greater than C8h in both age groups. No formal statistical analysis was performed to compare the PK parameters between the two age groups due to heterogeneous doses. Rather, the effects of age and body weight on PK were assessed in the PopPK analysis.
Table 2.
SXB PK parameters following the stable dosage of 4–9 g/night as 2 equally divided doses
| Parameter a | Children (n = 11) | Adolescents (n = 18) | All (n = 29) |
|---|---|---|---|
| Tmax, h | 0.8 (1–2) | 1.2 (1–3) | 0.8 (1–3) |
| Cmax, µg/mL | 109.0 (43–173) | 72.9 (21–136) | 78.8 (21–173) |
| AUC0–4h, µg∙h/mL | 267.9 (112–470) | 160.0 (52–406) | 189.1 (52–470) |
| C4.75h, µg/mL | 167.0 (77–220) | 109.5 (24–189) | 118.0 (24–220) |
| C8h, µg/mL | 35.3 (2–121) | 26.6 (2–130) | 32.6 (2–130) |
AUC0–4h, area under the concentration‐time curve at 0–4 hours; C4.75h, concentration at 4.75 hours after dose 1 or 0.75 hours post‐dose 2; C8h, concentration at 8 hours after dose 1 or 4 hours post‐dose 2; Cmax, maximal concentration; PK, pharmacokinetics; SXB, sodium oxybate; Tmax, time to Cmax.
Median (range).
Exposure–dose relationship
A linear correlation was observed between SXB exposure (Cmax and AUC0–4h) and dose normalized to body weight (mg/kg), but there was no correlation between exposure and fixed‐gram dose (Figure 3 ). The 90% CIs of the mean ratio of within‐subject exposure for dose 2 (full dose) vs. dose 1 (half dose) encompassed 2.00 for Cmax (children: ratio, 2.33 (90% CI, 1.95–2.79); adolescents: ratio, 1.77 (90% CI, 1.40–2.25); and overall: ratio, 1.97 (90% CI, 1.67–2.31)), indicating dose proportionality, but exceeded 2.00 for AUC0–4h in children and overall (children: ratio, 3.02 (90% CI, 2.52–3.61); adolescents: ratio, 2.28 (90% CI, 1.84–2.82); and overall: ratio, 2.53 (90% CI, 2.18–2.94)), indicating that exposure was more than dose proportional (i.e., supra‐dose proportional).
Figure 3.
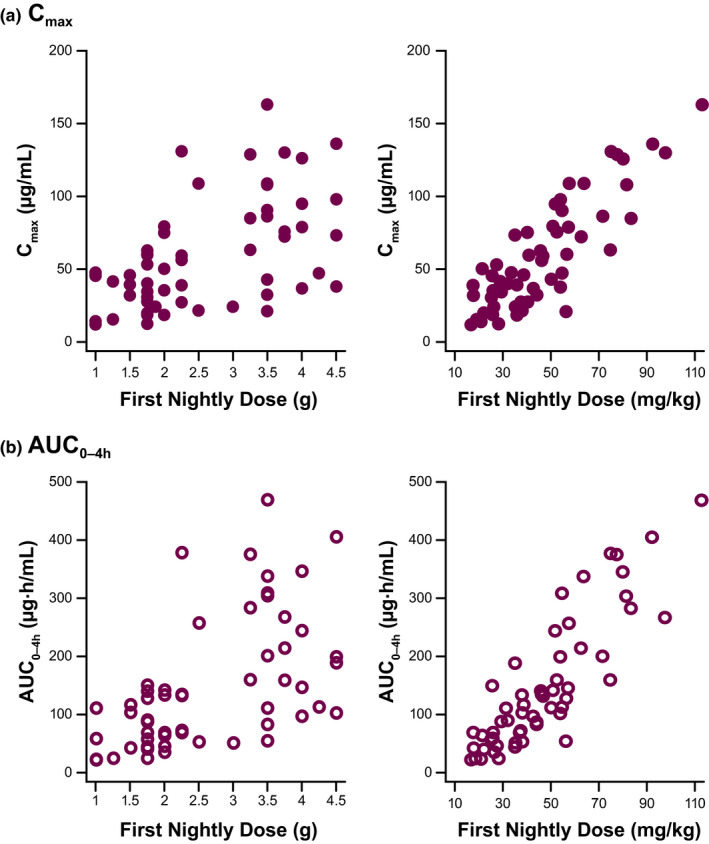
Sodium oxybate demonstrated linear correlation of (a) Cmax and (b) AUC0–4h with mg/kg dosage but not fixed‐gram dose. AUC0–4h, area under the concentration‐time curve at 0–4 hours; Cmax, maximal concentration.
PK characterization in pediatric and adult studies (PopPK modeling)
Analysis population
The PK modeling population comprised 174 participants: 29 (16.7%) from the pediatric study (aged 7–16 years at screening) 15 and 145 (83.3%) from studies of adult participants with narcolepsy (n = 13 (7.5%)) and healthy participants (n = 132 (75.9%)) 14 , 21 (Table S2 ). There were 3,775 total PK observations: 333 (8.8%) from pediatric participants and 3,442 (91.2%) from adults (with narcolepsy, 326 (8.6%); healthy, 3,116 (82.5%)). One pediatric participant was later excluded from the data set because of unusual SXB concentrations, whose cause was unclear, that precluded convergence of the model.
Development of compartmental model
The plasma concentration–time data of SXB were fit to a two‐compartment model with first‐order absorption and Michaelis–Menten clearance using NONMEM (Figure S1). The data supported the addition of interindividual variability terms on Vmax, Km, Ka, and VC. Interoccasion variability was included on Vmax and Km to account for any within‐participant differences in PKs between visits. This interoccasion variability was an empiric finding that improved the fit of the model. The final model contained a food effect that significantly reduced Ka, allometric scalars (based on body weight) on VC and Vmax, pediatric and child age‐category effects on VC (relative to adults, children had a 12% reduction, and adolescents had a 22% increase), and a diurnal effect on Vmax (Table S4 ). Eta shrinkage values were 5.86%, 14.97%, 55.51%, 52.06%, 71.63%, 71.67%, 17.63%, and 25.34%. Epsilon shrinkage was 4.42%. The NONMEM model performance was considered good based on evaluation of visual predictive checks and goodness‐of‐fit plots (Figure S2 ). SXB plasma concentrations calculated by the model fit well with observed values in individual patients (Figure S3 ).
Simulated PK
Model‐based simulations demonstrated that SXB concentrations and resulting PK parameters increased in a supra‐dose‐proportional manner that was more pronounced with AUC than Cmax (Figure S4 ). Based on the results of PK simulations, when SXB was dosed on a mg/kg basis, mean Cmax (after dose 2 of a twice‐nightly, 4‐hours‐apart regimen) and AUC from time 0 to 24 hours postdose in children and adolescents were predicted to be 80% to 101% of the respective mean values compared with adults, which fall within the standard bioequivalence range of 80% to 125%.
Based on NONMEM simulations for pediatric participants and adults, Cmax values were predicted to be ~ 30% lower after administration of SXB under fed vs. fasted conditions (Figure 4 ). The simulated AUC values were likewise decreased, but only by ~ 15%, and within the standard bioequivalence range of 80% to 125% (Figure 4 ). Additionally, based on simulations, pediatric participants and adults receiving the same gram dose had different plasma concentration–time profiles, whereas pediatric participants and adults receiving the same mg/kg dosages had comparable plasma concentration–time profiles, including the simulated C8h values (Figure 5 ).
Figure 4.
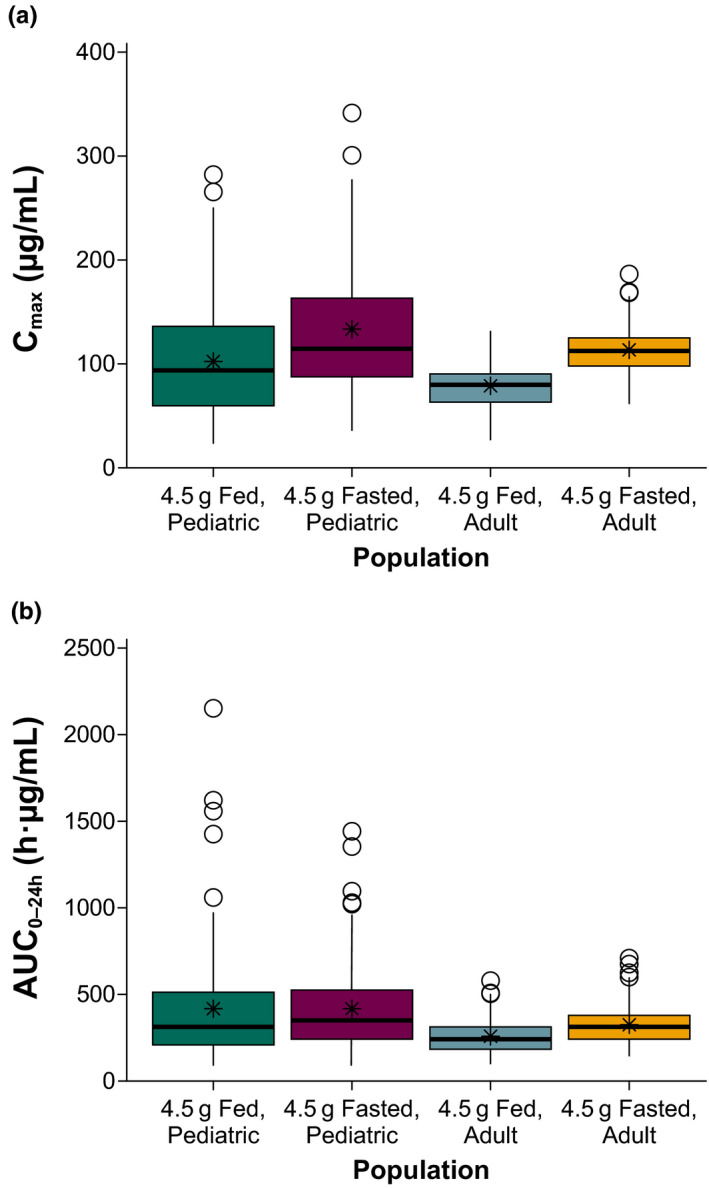
Predicted food effects on sodium oxybate (a: Cmax and b: AUC0–24h) in pediatric participants and adults. In each panel, the elements represent values as follows: heavy horizontal line, median; bottom and top of colored box, 25% and 75% quartiles, respectively (i.e., interquartile range (IQR)); whiskers, lowest and highest values within 1.5 × IQR of lower and upper quartiles; circles, observations beyond whiskers; and asterisk, mean. AUC0–24h, area under the plasma concentration–time curve from time 0 to 24 hours postdose; Cmax, maximum observed plasma concentration.
Figure 5.
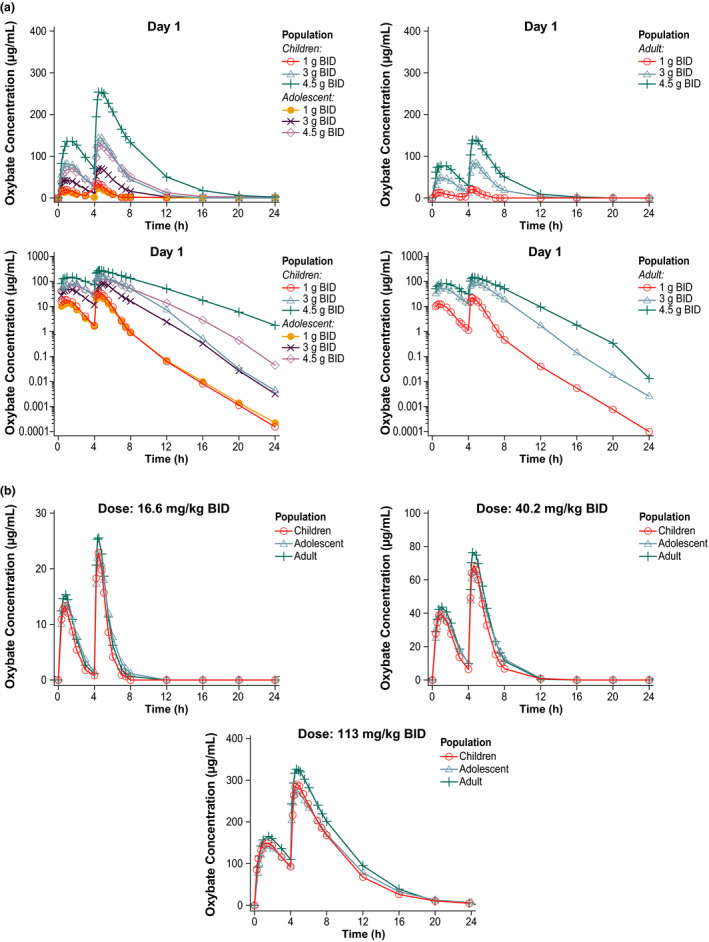
Simulated PK profiles of SXB based on (a) gram doses or (b) mg/kg dosages. PK, pharmacokinetics; SXB, sodium oxybate.
DISCUSSION
This is, to the best of our knowledge, the first report on the evaluation of the PKs of SXB in pediatric participants with narcolepsy. Noncompartmental analyses were performed on PK data from a subset of participants from a study on the efficacy and safety of SXB in children and adolescents. 15 PopPK analyses were conducted on combined PK data from this pediatric study 15 and previous adult studies 14 , 21 to confirm the noncompartmental results and examine the major intrinsic and extrinsic factors that affect SXB PK.
In general, the major PK attributes of SXB (e.g., nonlinear clearance and supra‐dose proportionality) in pediatric participants were the same as reported in prior PK studies in healthy adults or adults with narcolepsy. 14 , 21 , 27 There was linear correlation in the pediatric population between exposure as measured by Cmax and AUC0–4h and mg/kg dosage but no correlation between exposure and flat gram dose of SXB. These observations suggest that body weight was an important determinant of SXB exposure in the pediatric PK population.
The PopPK analyses, with a larger total sample, confirmed that body weight significantly affects SXB PK (VC and Vmax) and accounts for most of the observed differences in PKs between pediatric participants and adults. When the dose was normalized by weight, PopPK simulations indicated that plasma concentration–time profiles, including trough (C8h) values, would be similar in pediatric and adult participants at the same mg/kg dosages. Although age categories, but not continuous age, were statistically significant covariates on VC, there were no clinically meaningful differences in simulated PK profiles among age categories when SXB was administered at similar mg/kg dosages. The mean Cmax (after dose 2 of a twice‐nightly, 4‐hours‐apart regimen) and AUC from time 0 to 24 hours postdose in children and adolescents were predicted to fall within the standard bioequivalence range of 80% to 125% based on simulation. Moreover, it is expected that the PKs of SXB would be similar in pediatric patients without cataplexy, as disease state did not affect SXB PKs.
Because similar PKs on a mg/kg basis is expected for pediatric participants compared with adults, these data support treatment initiation based on body weight category as implemented in the pediatric study (Table S1). The recommended initiation dosing based on 3 weight categories (20 to < 30, 30 to < 45, and ≥ 45 kg) resulted in the same maximal initiation dosage of 100 mg/kg and, thus, a similar PK profile regardless of body weight. In the current label, no specific dosing recommendation is provided for patients aged 7 years and older with body weight < 20 kg. However, similar PK on a mg/kg basis is likely, as comparable PK profiles were observed between a 2‐day‐old boy (< 20 kg) and a 15‐year‐old boy when both were administered 30 mg/kg of SXB intravenously. 28 Thus, this dose initiation strategy (i.e., maximal 100 mg/kg) would be reasonable for children with weight below 20 kg.
In pediatric analyses, SXB plasma concentrations were higher after the second nightly dose compared with the first for the same time points after the first vs. the second dose (e.g., 0.75 hour vs. 4.75 hours after first dose, assessed at 0.75 hours after the each of the two doses). Such observations were consistent with PK data in adults when the first dose was given at bedtime and the second dose 4 hours later. 27 It is well known that fed conditions (dosed within 30 minutes after a high‐fat breakfast) significantly reduce Cmax (59%) and AUC (37%) compared with fasting conditions for SXB. 14 , 17 PopPK modeling indicated that this was largely due to a reduction of ~ 70% in Ka for the first nightly dose, which was under relatively fed conditions because it was closer to the evening meal than was the second nightly dose. The study records support the theory of food effects as a partial reason for lower concentrations after dose 1 than at the corresponding time points after dose 2. The average elapsed time between eating and dosing in the pediatric study was about 3 hours (based on mean time of the first dose of 9:51 pm and assumed mealtime of 7:00 pm), supporting a more fed state for dose 1 than dose 2, and, thus, lower exposure after dose 1 than dose 2. In addition to the food effect, the greater exposure to SXB after dose 2 compared with dose 1 could have resulted from “carryover” from dose 1 to dose 2 due to a 4‐hour interval between the 2 doses. The choice to treat the first nightly dose in pediatric participants as “fed” and the second nightly dose as “fasted” could have introduced a confounding factor for the assessment of prandial and diurnal effects in PopPK modeling. However, overall, a similar degree of food effect on modeled exposure (≈ 30% Cmax reduction with little effect on AUC) was predicted from PopPK analysis for both pediatric participants and adults. Part of the reason for taking SXB at least 2 hours after a meal is to minimize Cmax variability arising from varying food effects for both pediatric and adult patients with narcolepsy. In adult healthy volunteers, taking SXB within 30 minutes after a high‐fat, high‐calorie breakfast resulted in reduction of Cmax by 59% and AUC by 37% compared with fasting conditions. 14 A possible reason for reduced exposure is lower gastric motility and emptying rate under fed vs. fasted conditions. 29
Consistent with adult data, both noncompartmental PK results and PopPK modeling demonstrated a more than dose‐proportional increase of AUC (i.e., supra‐dose proportionality), suggesting nonlinear and saturable clearance. Supra‐dose proportionality requires caution during the dose titration process, which was implemented in the pediatric study and recommended in the label through an individually tailored incremental upward titration that decreased the frequency and severity of cataplexy attacks to a stable level while maintaining tolerability.
Trough SXB concentrations (C8h) around the time of awakening in the pediatric PK study seemed to be more variable within the same dosage range (median, ≈ 100–150 mg/kg) compared with those typically seen in adult PK studies, in which a fixed dose of SXB (highest single dose, 4.5 g) was administered. However, no obvious association between higher C8h SXB plasma concentrations and incidences of adverse events was observed in this study. 15 Moreover, as described previously, PopPK simulation indicated that a similar PK profile, including C8h, was expected in adults and pediatric participants following the same mg/kg dosage. Nevertheless, as individual responses vary, caution should be exercised by watching for alertness in the morning when daily activities start.
In conclusion, similar PK characteristics (supra‐dose proportionality, reduced Cmax under fed compared with fasting conditions) for SXB were observed in children, adolescents, and adults. Furthermore, weight was the main intrinsic factor affecting SXB PK; when normalizing the dose by weight (i.e., on mg/kg basis), similar plasma concentration–time profiles were expected across all ages and weights. Collectively, these findings support a weight category‐based dose regimen in children and adolescents with narcolepsy, with the same initiation regimen of SXB ≤ 100 mg/kg across children, adolescents, and adults, individually tailored upward titration, and administration of SXB at least 2 hours after a meal.
Funding
This study was supported by Jazz Pharmaceuticals.
Conflict of Interest
C.C., R.P., and K.Z. are employees of Jazz Pharmaceuticals who, in the course of their employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. C.L.R. has been a consultant for Jazz Pharmaceuticals. She acknowledges that this publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, 4UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. C.R. has served as an advisory board member and unpaid consultant for Jazz Pharmaceuticals. L.H.B. has nothing to disclose. S.B. and M.S. have been compensated consultants for Jazz Pharmaceuticals. G.P. has received consultancy fees from UCB, Jazz Pharmaceuticals, and Bioprojet, and is an editorial board member of Sleep Medicine and Sleep Medicine Reviews.
Author Contributions
All authors wrote the manuscript. C.C., C.L.R., C.R., L.H.B., R.P., K.Z., and G.P. designed the research. C.L.R., C.R., L.H.B., and G.P. performed the research. C.C., K.Z., and G.P. analyzed the data. C.C., S.B., and M.S. designed the population PK analysis; S.B. and M.S. performed and interpreted the population PK analysis; R.P. performed statistical analysis; C.C. and G.P. interpreted results.
Supporting information
Figure S1. Population PK model schema.
Figure S2. Population PK (A) visual predictive checks and (B) goodness of fit plots.
Figure S3. Representative sample of observed plasma oxybate concentrations vs. values predicted by the population PK model in individual study participants.
Figure S4. Simulated SXB exposure in pediatric and adult participants by mg/kg dosage.
Table S1. Weight category‐based SXB dosing regimen for initiation of treatment, titration rate, and maximum dose per study protocol.
Table S2. Studies included in population PK analysis.
Table S3. Population PK modeling: intrinsic and extrinsic factors evaluated.
Table S4. PK parameter estimates from the population PK model22‐24.
Acknowledgments
The authors thank all of the study investigators, study staff, nursing team, and patients for their participation in this research. Under the direction of the authors, Kirsty Nahm, MD, employee of The Curry Rockefeller Group, LLC (CRG), and Michael J. Theisen, PhD, employee of Peloton Advantage, LLC, an OPEN Health company, provided medical writing assistance for this publication. Editorial assistance in formatting, proofreading, copyediting, and fact‐checking was also provided by CRG and Peloton Advantage. Jazz Pharmaceuticals provided funding to CRG and Peloton Advantage for writing and editorial support.
References
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd edn. (American Academy of Sleep Medicine, Darien, IL, 2014). [Google Scholar]
- 2. Dauvilliers, Y. et al Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 57, 2029–2033 (2001). [DOI] [PubMed] [Google Scholar]
- 3. Morgenthaler, T.I. et al Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 30, 1705–1711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postiglione, E. , Antelmi, E. , Pizza, F. , Lecendreux, M. , Dauvilliers, Y. & Plazzi, G. The clinical spectrum of childhood narcolepsy. Sleep Med. Rev. 38, 70–85 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Black, J. et al Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med. 33, 13–18 (2017). [DOI] [PubMed] [Google Scholar]
- 6. Plazzi, G. , Clawges, H.M. & Owens, J.A. Clinical characteristics and burden of illness in pediatric patients with narcolepsy. Pediatr. Neurol. 85, 21–32 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Mignot, E.J. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 9, 739–752 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aran, A. , Einen, M. , Lin, L. , Plazzi, G. , Nishino, S. & Mignot, E. Clinical and therapeutic aspects of childhood narcolepsy‐cataplexy: a retrospective study of 51 children. Sleep 33, 1457–1464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Billiard, M. et al EFNS guidelines on management of narcolepsy. Eur. J. Neurol. 13, 1035–1048 (2006). [DOI] [PubMed] [Google Scholar]
- 10. U.S. Xyrem Multicenter Study Group . A randomized, double blind, placebo‐controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 25, 42–49 (2002). [PubMed] [Google Scholar]
- 11. Harvey, P.D. et al Improvement in cognitive function following a switch to ziprasidone from conventional antipsychotics, olanzapine, or risperidone in outpatients with schizophrenia. Schizophr. Res. 66, 101–113 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Xyrem International Study Group . A double‐blind, placebo‐controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J. Clin. Sleep Med. 1, 391–397 (2005). [PubMed] [Google Scholar]
- 13. Black, J. & Houghton, W.C. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep 29, 939–946 (2006). [DOI] [PubMed] [Google Scholar]
- 14. Xyrem® (sodium oxybate) oral solution Prescribing Information (Jazz Pharmaceuticals, Palo Alto, CA, 2018). [Google Scholar]
- 15. Plazzi, G. et al Treatment of paediatric narcolepsy with sodium oxybate: a double‐blind, placebo‐controlled, randomised‐withdrawal multicentre study and open‐label investigation. Lancet Child. Adolesc. Health 2, 483–494 (2018). [DOI] [PubMed] [Google Scholar]
- 16. Palatini, P. et al Dose‐dependent absorption and elimination of gamma‐hydroxybutyric acid in healthy volunteers. Eur. J. Clin. Pharmacol. 45, 353–356 (1993). [DOI] [PubMed] [Google Scholar]
- 17. Borgen, L.A. , Okerholm, R. , Morrison, D. & Lai, A. The influence of gender and food on the pharmacokinetics of sodium oxybate oral solution in healthy subjects. J. Clin. Pharmacol. 43, 59–65 (2003). [DOI] [PubMed] [Google Scholar]
- 18. Ferrara, S.D. et al Pharmacokinetics of gamma‐hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br. J. Clin. Pharmacol. 34, 231–235 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrara, S.D. et al Effect of moderate or severe liver dysfunction on the pharmacokinetics of gamma‐hydroxybutyric acid. Eur. J. Clin. Pharmacol. 50, 305–310 (1996). [DOI] [PubMed] [Google Scholar]
- 20. Scharf, M.B. , Lai, A.A. , Branigan, B. , Stover, R. & Berkowitz, D.B. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep 21, 507–514 (1998). [DOI] [PubMed] [Google Scholar]
- 21. Borgen, L.A. , Okerholm, R.A. , Lai, A. & Scharf, M.B. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J. Clin. Pharmacol. 44, 253–257 (2004). [DOI] [PubMed] [Google Scholar]
- 22. Beal, S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28, 481–504 (2001). [DOI] [PubMed] [Google Scholar]
- 23. Jonsson, E.N. & Karlsson, M.O. Xpose—an S‐PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58, 51–64 (1999). [DOI] [PubMed] [Google Scholar]
- 24. Lindbom, L. , Pihlgren, P. & Jonsson, E.N. PsN‐Toolkit—a collection of computer intensive statistical methods for non‐linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79, 241–257 (2005). [DOI] [PubMed] [Google Scholar]
- 25. Gehan, E.A. & George, S.L. Estimation of human body surface area from height and weight. Cancer Chemother. Rep. 54, 225–235 (1970). [PubMed] [Google Scholar]
- 26. DHSS/MRC Group on Obesity Research , Waterlow, J.C. & James, W.P.T. Research on Obesity: A Report of the DHSS/MRC Group (Her Majesty's Stationery Office, London, 1976). [Google Scholar]
- 27. Thorpy, M.J. Sodium oxybate for the treatment of narcolepsy. Expert Opin. Pharmacother. 6, 329–335 (2005). [DOI] [PubMed] [Google Scholar]
- 28. van den Bogert, A.G. , Vree, T.B. , van der Kleijn, E. & Damsma, J. Placenta transfer of 4‐hydroxybutyric acid in man In: Frey R. ed. Neue Untersuchungen mit Gamma‐Hydroxibuttersäure (Springer, Berlin, Heidelberg, 1978, 55–64). [Google Scholar]
- 29. Marathe, P.H. , Wen, Y. , Norton, J. , Greene, D.S. , Barbhaiya, R.H. & Wilding, I.R. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br. J. Clin. Pharmacol. 50, 325–332 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Population PK model schema.
Figure S2. Population PK (A) visual predictive checks and (B) goodness of fit plots.
Figure S3. Representative sample of observed plasma oxybate concentrations vs. values predicted by the population PK model in individual study participants.
Figure S4. Simulated SXB exposure in pediatric and adult participants by mg/kg dosage.
Table S1. Weight category‐based SXB dosing regimen for initiation of treatment, titration rate, and maximum dose per study protocol.
Table S2. Studies included in population PK analysis.
Table S3. Population PK modeling: intrinsic and extrinsic factors evaluated.
Table S4. PK parameter estimates from the population PK model22‐24.


