Abstract
Polymorphisms in drug transporters, like the adenosine triposphate‒binding cassette (ABC) and solute carrier (SLC) superfamilies, may contribute to the observed diversity in drug response in African patients. This review aims to provide a comprehensive summary and analysis of the frequencies and distributions in African populations of ABC and SLC variants that affect drug pharmacokinetics (PK) and pharmacodynamics (PD). Of polymorphisms evaluated in African populations, SLCO1B1 rs4149056 and SLC22A6 rs1158626 were found at markedly higher frequencies than in non‐African populations. SLCO1B1 rs4149056 was associated with reduction in rifampin exposure, which has implications for dosing this important anti‐tuberculosis therapy. SLC22A6 rs1158626 was associated with increased affinity for antiretroviral drugs. Genetic diversity in SLC and ABC transporters in African populations has implications for conventional therapies, notably in tuberculosis and HIV. More PK and PD data in African populations are needed to assess potential for a different response to drugs compared with other global populations.
African populations have disproportionately high incidences of adverse drug reactions, 1 and one of the potential contributing factors may be a higher genetic diversity in Africa than in other continental populations. 2 For example, members of the cytochrome P450 (CYP) superfamily of enzymes, which play a substantial role in oxidative metabolism of therapeutic drugs, have been shown to have greater genetic variability in African populations than in Asian and Caucasian populations, and certain CYP alleles may be unique to Africa. 2
Polymorphisms in drug transporters, such as those in the adenosine triphosphate (ATP)‐binding cassette (ABC) and solute carrier (SLC) superfamilies, may also contribute to the diversity in drug response observed in African patients, owing to the potential of the polymorphisms to affect the absorption and disposition of select drug molecules or classes differently. In particular, agents that are used to treat infectious diseases (bacterial or viral), which have higher prevalence in sub‐Saharan Africa, tend to exist in a distinct chemical class (low permeability, moderate to high solubility). 3 As such, the disposition pathways of these molecules tend to be heavily influenced by drug transporter activity. Notably, diversity in ABC and SLC transporters has been implicated in the variability in response of different global populations to drugs for the treatment of HIV infection and tuberculosis (TB), 4 diseases that constitute a particularly high burden in Africa. 5
This review aims to provide a comprehensive summary and analysis of the frequencies and distributions in African populations of ABC and SLC variants that affect drug pharmacokinetics (PK) and pharmacodynamics (PD), using three approaches: (i) identifying ABC and SLC polymorphisms that have been shown to have a different impact on PK and PD in African populations compared with the wild‐type or compared with non‐African populations; (ii) collating allele frequency distributions for these polymorphisms from African studies; and (iii) reporting allele frequencies in African populations for other ABC and SLC genes known to be clinically relevant from studies in non‐African populations. We also aimed to collate allele frequency distributions in African populations for all reported ABC and SLC polymorphisms, to assess progress in quantifying genetic diversity in Africa and to establish what new data are needed to inform treatment choices and to guide new drug development.
LITERATURE SEARCH STRATEGY, ARTICLE SELECTION CRITERIA, AND LITERATURE SEARCH OUTPUTS
Embase and Ovid MEDLINE were searched for publications (published from January 1995 to February 2019) reporting ABC and SLC allele frequencies in African populations. Key search terms relating to transporters included “organic anion transporter,” “organic anion transporting polypeptide,” “organic cation transporter,” “multidrug and toxin extrusion protein,” “ABC transporter,” “ABC transporter subfamily B,” “breast cancer resistance protein,” multidrug resistance protein 1,” and “carrier protein.” Key search terms relating to genetic variation included “genetics,” “genetic variability,” “genetic variation,” “DNA polymorphism,” and “genetic polymorphism.” The search term “Africa” was included to ensure the correct populations were identified. Full search terms are reported in Table S1 . After selection of relevant publications from this initial search, reference lists from articles identified were reviewed for publications of interest. Additional targeted searches were performed in PubMed to provide data on the impact of specific alleles on drug PK. Reviews and editorials were excluded. Figure 1 illustrates the flow of publications through the search and selection process.
Figure 1.
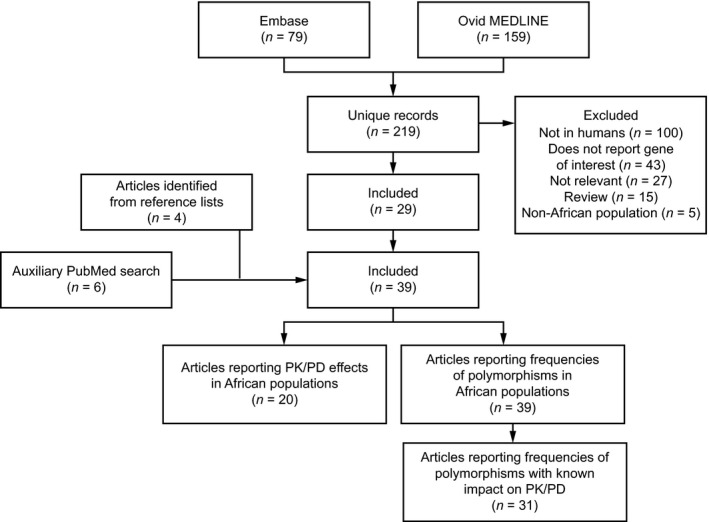
Flow of studies through the search and selection process. PD, pharmacodynamics; PK, pharmacokinetics.
Embase and Ovid MEDLINE searches returned 79 and 159 publications, respectively, with a total of 219 unique articles. Of these, 29 articles were included in the analysis (Figure 1 ). Ten additional articles were identified from the review of reference lists and additional targeted searches.
SLC ALLELE FREQUENCIES
Human SLC transporters constitute a family of 395 membrane‐bound proteins that are primarily involved in the uptake of small molecules into cells. SLC transporters interact with a broad range of substrates, and vary in specificity. 4 Table 1 comprehensively summarizes SLC transporter tissue distribution, substrate drugs, and examples of allelic variants that are relevant to drug translocation. Table S2 shows the frequencies of all SLC alleles in African populations from the studies included in this review. Some polymorphisms showed notably wide variation in frequency across different populations. For example, SLCO1B1 rs4149056 was present at a frequency range of 0.0–0.99 (across 23 populations), SLCO1B1 c.597C>T at 0.0–0.66 (across nine populations) and SLCO1B1 c.571C>T at 0.0–0.42 (across nine populations).
Table 1.
SLC polymorphisms and their effects on drug transport 4
| Transporter subfamily | Gene | Protein | Expression site | Substrate drug(s) | Known polymorphisms | Known effect |
|---|---|---|---|---|---|---|
| SLCO | SLCO1B1 |
SLCO1B1, OATP1B1 |
Intestine, liver, kidney, brain | Statins, rifampin | N130D, V174A (rs4149056; T521C) | Decreased transporter activity; higher drug plasma concentration |
| SLCO1C1 |
SLCO1C1, OATP1C1 |
Brain, testis | — |
PDE3A, −SLCO1C1 locus (rs3794271) |
Reduced efficacy | |
| SLCO1A2 |
SLCO1A2, OATP1A2 |
Ubiquitous | — | I3T | Increased transporter activity | |
| SLCO2B1 |
SLCO2B1, OATP2B1 |
Ubiquitous | — | R312Q | Reduced transporter activity | |
| SLC22 | SLC22A1 | SLC22A1, OCT1 | Liver | Metformin, procainamide | C88R, G465R | Complete loss of transporter activity |
| A270S | Reduced transporter activity; higher drug plasma concentration | |||||
| SLC22A2 |
SLC22A2, OCT2 |
Kidney | Metformin, procainamide | T400I | Reduced transporter activity | |
| SLC22A3 | SLC22A3, OCT3 | Kidney | Metformin, procainamide | L503F | Increased transporter activity | |
| SLC22A4 |
SLC22A4, OCTN1 |
Kidney, liver, lung, intestine | — |
D165G, R282X |
Loss of transporter activity | |
| SLC22A5 |
SLC22A5, OCTN2, CDSP |
Wide distribution, including apical membranes, muscle, heart, lungs, eye | — | F17L | Reduced transporter activity | |
| SLC22A12 | SLC22A12, URAT1, OAT4L | Kidney | — | G65W | Reduced transporter activity; lower drug plasma concentration | |
| SLC22A6 |
SLC22A6, OAT1 |
Kidney | Diuretics, NSAIDs, β‐lactam antibiotics | R454Q | Complete loss of transporter activity | |
| SLC22A8 |
SLC22A8, OAT3 |
Kidney, brain, eye, testis | — | T723A | Altered transporter activity; increased substrate uptake | |
| SLC22A11 |
SLC22A11, OAT4 |
Kidney, brain, placenta | — | L29P | Reduced expression; reduced substrate uptake | |
| SLC47 | SLC47A1 |
SLC47A1, MATE1 |
Kidney | Metformin |
G64D, V480M |
Complete loss of transporter activity |
| SLC47A2 |
SLC47A1, MATE2‐K |
Kidney | Metformin |
K64N, G211V |
Reduced expression; reduced transporter activity |
MATE, multidrug and toxin extrusion protein; NSAID, nonsteroidal anti‐inflammatory drug; OAT, organic anion transporter; OATP, organic anion‐transporting polypeptide; OCT, organic cation transporter; OCTN, organic cation transporter novel; SLC, solute carrier; SLCO, solute carrier organic anion transporter.
SLCO1B1
SLCO1B1 encodes organic anion‐transporting polypeptide 1B1 (OATP1B1), which mediates sodium‐independent transport of organic anions across the cell membrane. This gene was the most frequently described among the African studies included in our analysis. Polymorphisms in this gene have been found to affect the PK and PD of statins, moxifloxacin, rifampin, rifabutin, and lopinavir (Table 1 ). 6
Eight studies investigated the impact of SLCO1B1 allelic variants on drug PK and PD in African populations, and reported 11 loci (Table 2 ). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Of these loci, five were found to have an impact in the African populations studied compared with the wild‐type, and one has been implicated in altered drug response in other studies in non‐African populations (rs4149056). Observations about these variants from studies with more than 50 participants are reported in detail below. Frequencies of these variants in African populations were reported in 15 studies 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 and are summarized in Table 3 .
Table 2.
Studies reporting impact of SLC polymorphisms on drug PK, PD, and outcomes in African populations
| Reference | N | Population | Substrate drug(s) | Polymorphism | Effect of polymorphism |
|---|---|---|---|---|---|
| SLC22A6 | |||||
| 7 | 92 | African (n = 24), Asian (n = 24), Caucasian (n = 24), anonymous (n = 20) | Adefovir, cidofovir, tenofovir | rs11568626 (R50H; 728G>A) | Decrease in K m for all drugs vs. wild‐type |
| R525I (8347A>T) | No change in K m for all drugs vs. wild‐type | ||||
| SLCO1B1 | |||||
| 8 | 57 | South African, black African ancestry (n = 23), mixed race (n = 34) | Rifampin | rs4149032 | Reduced bioavailability of rifampin; simulations suggested that increasing the daily rifampin dose by 150 mg in patients with the polymorphism would result in plasma concentrations similar to those of individuals with the wild‐type allele, and reduce the proportion of patients with maximum plasma concentrations (Cmax) below 8 mg/L from 63% to 31% |
| 9 | 113 | Ghanaian | Rifampin | rs2306283 (388A>G) *1b |
Potential association with rifampin PK parameters; two patients with the *1b homozygous variant (AA genotype) had significantly lower rifampin Cmax and AUC0–8 and higher CL/F and V/F than those with the wild‐type variant (GG genotype) in pairwise analysis |
| rs4149032 | No effect | ||||
| rs11045819 (463C>A)*4 | No effect | ||||
| rs4149056 (521T>C)*5 | No effect | ||||
| 10 | 57 | South African | Rifampin | rs4149032 | Low median rifampin C2.5, 3.7 μg/mL (range 2.8–5.0 μg/mL) in the heterozygous and 3.4 μg/mL (range 2.7–4.7 μg/mL) in the homozygous variant carriers; of the eight patients in whom TB recurred, seven had the polymorphism |
| 11 | 35 | South African | Rifabutin |
rs11045819 (463C>A)*4 |
Associated with a 30% increase in bioavailability of rifabutin |
| rs4149032 | No effect | ||||
| rs4149056 (521T>C)*5 | No effect | ||||
| rs2306283 (388A>G) *1b | No effect | ||||
| 12 | 86 | Bantu (black South African, n = 34; Malawians, n = 52) | Lopinavir | rs2306283 (388A>G) *1b | No effect |
| rs4149044A>T | No effect | ||||
| rs4149045G>A | No effect | ||||
| rs4149057T>C | No effect | ||||
| 13 | 30 | Zimbabwean | Rosuvastatin | rs34671512 (1929A>C) | 75% reduction in rosuvastatin exposure compared with wild‐type (P < 0.001) |
| 21 | 88 | Patients with TB: black African (n = 37), US and Spain (n = 5 black; n = 29 white; n = 1 Asian); healthy individuals from North America (n = 4 black; n = 11 white; n = 8 Hispanic) | Rifampin | rs11045819 (463C>A) | Lower rifampin exposure was associated with the polymorphism |
| 14 | 49 | Ugandan (n = 24), South African (n = 2), American (n = 23) | Moxifloxacin | rs4149015 (‐1187G>A) | AG genotype associated with significantly higher AUC0–24 and Cmax than GG genotype |
| rs59502379 (1463G>C) | No effect | ||||
| rs2306283 (388A>G) *1b | No effect | ||||
| rs11045819 (463C>A) | No effect | ||||
| rs4149056 (521T>C)*5 | No effect | ||||
| rs4149117 (334T>G) | No effect | ||||
A separate color is used to identify each polymorphism.
AUC0–8, area under the time‒concentration curve from 0 to 8 hours postdose; AUC0–24, area under the time‒concentration curve from 0 to 24 hours postdose; C2.5, concentration at 2.5 hours; Cmax, maximum plasma concentration; CL/F, apparent oral clearance; K m, affinity; PD, pharmacodynamics; PK, pharmacokinetics; SLC, solute carrier; TB, tuberculosis; V/F, apparent predicted volume of distribution.
Table 3.
SLC gene polymorphisms of relevance for variability in drug PK and PD: frequencies in African populations
| Reference | N | Population | SLCO1B1 | SLC22A6 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
rs4149032 38664 C>T |
*1b rs2306283 388A>G |
rs11045819 463C>A | rs34671512 1929A>C | rs4149015 11187G>A |
rs11568626 R50H, 728G>A |
R525I, 8347A>T | |||
| 15 | 632 | Ethiopian | 0.481 | 0.603 | 0.028 | — | — | — | — |
| 361 | Tanzanian | 0.519 | 0.868 | 0.047 | |||||
| 7 | 48 | African | — | — | — | — | — | 0.17 | 0.02 |
| 8 | South African: | — | — | — | — | — | — | — | |
| 23 | Xhosa | 0.93 | — | — | — | — | — | — | |
| 34 | Cape Admixed | 0.59 | — | — | — | — | — | — | |
| 57 | Total | 0.7 | — | — | — | — | — | — | |
| 16 | 44 |
Black African/African American |
— | 0.886 | — | — | — | — | — |
| 9 | 113 | Ghanaian | 0.27 | 0.17 | 0.08 | — | — | — | — |
| 10 | 57 | South African | 0.76 | — | — | — | — | — | — |
| 11 | 35 | South African | 0.642 | 0.886 | 0.071 | — | — | — | — |
| 17 | South African: | ||||||||
| 162 | Zulu | 0.642 | 0.991 | 0.006 | — | — | — | — | |
| 130 | Cape Admixed | 0.640 | 1.0 | 0.215 | — | — | — | — | |
| Unknown | Luhya (Kenya) | 0.16 | 0.03 | — | — | — | — | ||
| Unknown | Yoruba (Nigeria) | 0.19 | 0.05 | — | — | — | — | ||
| 12 | 86 | South African and Malawian | — | 0.86 | — | — | — | — | — |
| Luhya (Kenya) | — | 0.83 | — | — | — | — | — | ||
| 97 | Yoruba (Nigeria) | — | 0.82 | — | — | — | — | — | |
| 88 | — | — | — | — | — | — | — | ||
| 18 | 115 | Ugandan | — | 0.78 | 0.022 | 0.073 | |||
| 19 | 285 | Ethiopian | — | 0.387 | — | — | — | — | — |
| 209 | Tanzanian | — | 0.135 | — | — | — | — | — | |
| 20 | 23 | Biaka Pygmy | — | 0.85 | 0.02 | 0.22 | — | — | — |
| 13 | Mbuti Pygmy | — | 0.46 | 0.15 | 0.15 | — | — | — | |
| 22 | Mandinka | — | 0.75 | 0.05 | 0.02 | — | — | — | |
| 22 | Yoruba | — | 0.91 | 0.02 | 0.05 | — | — | — | |
| 11 | Bantu (NE) | — | 0.82 | 0.0 | 0.09 | — | — | — | |
| 6 | San | — | 0.83 | 0.08 | 0.08 | — | — | — | |
| 8 | Bantu (NE, SW) | — | 0.88 | 0.06 | 0.06 | — | — | — | |
| 29 | North Africa: Mozabite | — | 0.64 | 0.035 | 0.16 | — | — | — | |
| 13 | 30 | Zimbabwean | — | — | — | 0.06 | — | — | — |
| 21 | 37 | Black African | — | 0.878 | 0.095 | — | 0.054 | — | — |
| 14 | 26 | Black African | — | 0.904 | 0.096 | — | 0.019 | — | — |
NE, north‐east; PD, pharmacodynamics; PK, pharmacokinetics; SLC, solute carrier; SW, south‐west.
SLCO1B1 rs4149032 38664C>T
Chigutsa et al. 8 collected samples from 57 South African patients with TB who were undergoing treatment with rifampin, pyrazinamide, isoniazid, or ethambutol. Patients who were heterozygous and homozygous for the rs4149032 polymorphism had reductions in bioavailability of rifampin of 18% and 28%, respectively. The median area under the time–concentration curve from 0 to 24 hours postdose (AUC0–24) was significantly lower among individuals heterozygous or homozygous for the polymorphism than those without it (43 mg/h per liter vs. 56 mg/h per liter; P < 0.05). PK simulations suggested that increasing the daily rifampin dose by 150 mg in patients with this polymorphism would result in plasma concentrations similar to those of individuals with the wild‐type allele.
Gengiah et al. 10 also reported that the rs4149032 polymorphism affected rifampin PK among a population of 57 South African patients with both TB and HIV infection. A low median rifampin concentration at 2.5 hours postdose (C2.5) of 3.7 μg/ml (interquartile range 2.8–5.0 μg/ml) was recorded in heterozygous variant carriers and 3.4 μg/ml (interquartile range 2.7–4.7 μg/ml) in homozygous variant carriers (a maximum plasma concentration (Cmax) target range of 8–24 μg/ml is recommended for optimal bactericidal activity and postantibiotic effect). Of the eight patients in whom TB recurred, seven were carriers of the polymorphism. However, in two further studies (one in Ghana and one in South Africa), one of which was larger than those reported above (n = 113 individuals), rs4149032 was not found to have a significant impact on PK parameters of rifampin 9 or rifabutin. 11
It is notable that the frequencies reported for rs4149032 are higher and show greater variability in African populations ( Table 3 ) than in European populations. 15 The lowest frequency was 0.27 and was reported among 113 Ghanaian children. 9 Among four studies of South African populations, the majority of individuals had the allele, and the frequency was particularly high (0.59–0.93). 8 , 10 , 11 , 17 In the largest South African study (n = 292) the frequency of the allele was 0.64. 17 In contrast, the reported frequency in a European population (n = 57) was only 0.198. 15
SLCO1B1 rs11045819 463C>A
The reported frequency of the rs11045819 allele was similar in African populations (Table 3 ), 9 , 11 , 14 , 15 , 17 , 18 , 20 , 21 and non‐African populations. 12 , 15 , 17 , 18 , 20 , 21 The 463C>A allele leads to an amino acid change in the substrate recognition domain of the OATP1B1 transporter, resulting in altered transporter kinetics for several substrates, including statins. 22 , 23 Among 72 patients with TB (37 African; 35 Caucasian) and 16 healthy Caucasian individuals, the 463C>A polymorphism significantly affected rifampin PK parameters in a univariate analysis. 21 Patients with the polymorphism had 42% lower rifampin exposure (P = 0.0002), 34% lower peak rifampin levels (P = 0.03), and 63% greater apparent oral clearance of rifampin (P = 0.0009) than those without this polymorphism. In multivariate analyses, the rifampin AUC0–24 was significantly affected by the 463C>A polymorphism. In contrast, a study of 113 Ghanaian children reported no impact of rs11045819 on rifampin PK. 9
SLCO1B1 rs2306283 388A>G
Dompreh et al. 9 found an association between presence of the rs2306283 388A>G polymorphism and rifampin Cmax (P = 0.052), area under the time–concentration curve from 0 to 8 hours postdose (P = 0.085), apparent oral clearance time per hour (P = 0.093), and apparent predicted volume of distribution (P = 0.067) in a study of 113 Ghanaian children. In three further studies, (two in South African populations and one in a South African population and a Ugandan population), this polymorphism had no discernible impact on drug PK. 11 , 12 , 14 It is notable that greater variability in the frequency of rs2306283 is reported among African populations (from 0.135 among Tanzanians 19 to 1.0 in a South African population 17 ) (Table 3 ) than non‐African populations (from 0.2 to 0.938). 12 , 15 , 16 , 17 , 18 , 20 , 21
SLC22A6
SLC22A6 encodes organic anion transporter 1 (OAT1), which is involved in the transport of diuretics, non‐steroidal anti‐inflammatory drugs, and β‐lactam antibiotics (Table 1 ). 4 In a study of 92 individuals of African, Asian, or Caucasian origin, two nonsynonymous SLC22A6 polymorphisms (728G>A (rs11568626) and 8347A>T) were found only in individuals of African descent. 7 728G>A appeared to confer a lower affinity for the nucleoside phosphate analogs cidofovir, adefovir, and tenofovir than the wild‐type; however, the potential effect on drug PK and PD was not investigated. Among the African participants, the allele frequencies were 0.17 for 728G>A and 0.02 for 8347A>T (Table 3 ) but, considering the unique presence in African subjects, further assessment of their functional activity would be of interest.
Polymorphisms in other clinically relevant SLC transporters
Two further SLC transporters play clinically significant roles in drug transport: OCT1 encoded by SLC22A1 and OCT2 encoded by SLC22A2, primarily located in the liver and kidney, respectively (Table 1 ). OCT1 is involved in the transport of the antiretroviral efavirenz (used to treat HIV infection/acquired immunodeficiency syndrome) 24 and OCT2 mediates the cellular uptake of metformin (a treatment for type 2 diabetes) and procainamide (a treatment for cardiac arrhythmia). 4 It is notable that SLC22A1 reduced‐function variants may influence efficacy and toxicity potential without a direct effect on drug plasma concentration. 25
SLC22A1
Only two studies documented the PK and PD effects of single nucleotide polymorphisms (SNPs) in SLC22A1. In 69 Tunisian patients with chronic myeloid leukemia, SLC22A1 was significantly downregulated in those who were resistant to imatinib mesylate (IM) compared with those who responded to IM. 26 However, the studied SLC22A1 allelic variants (rs628031 and rs36056065, showing complete linkage disequilibrium and both present at a frequency of 0.57) did not correlate with IM response.
Nineteen protein‐altering SLC22A1 SNPs and one intronic SNP with documented impact on response to metformin were evaluated within a healthy Cape Admixed population (n = 100) and the allele frequencies were compared with those in other ethnic groups (Luhya, Yoruba, Native American/Hispanic, Caucasian, and Han Chinese). 27 Variants rs34447885 and rs36103319 were only present in African participants (both at a frequency of 0.01 in the Cape Admixed population), whereas rs34130495 and rs12208357 were not observed in African populations but were present in other populations (Table S2 ). Therefore, drugs that are substrates for OCT1 may have different response profiles in the Cape Admixed and African populations than in Caucasian and Asian populations.
SLC22A2
Jacobs et al. 28 found that 16 variants of SLC22A2 (rs59695691, rs572296424, rs150063153, rs112210325, rs8177511, rs112710522, rs112425400, rs115889347, rs11967308, rs114897022, rs139045661, rs8177516, rs8177517, rs17588242, rs3103352, and rs617217) were predominantly or exclusively observed in African populations or populations with an African origin. In a South African study (n = 440), Pearce et al. 29 identified three nonsynonymous SNPs, rs316019 (A270S), rs8177516 (R400C), and rs8177517 (K432Q), which had previously been found to affect drug transport in a Korean population (rs316019), and Caucasian and African American populations (rs8177516 and rs8177517). These were present at frequencies of 0.06, 0.03, and 0.007, respectively. Wilson et al. 6 also evaluated SNP frequencies in a South African population, noting three missense polymorphisms that were more common among this population than non‐African populations (Table S2 ).
ABC ALLELE FREQUENCIES
Encoded by 49 genes in humans, ABC transporters include proteins of high clinical relevance in severe inherited disorders such as cystic fibrosis, as well as translocation of numerous drugs and metabolites. 30 Table 4 summarizes ABC transporter tissue distribution, substrate drugs, and examples of relevant allelic variants. Table S3 shows the frequencies of all reported ABC alleles in African populations from the studies included in this review. Overall, ABC polymorphisms appeared to have lower variability in frequency distribution than SLC alleles. ABC polymorphisms showing the greatest variability in frequency across different populations were ABCB1*6 (0.048–0.4; across 35 populations), ABCB1 2677G>T (0.0–0.19; across 12 populations), and ABCC2 rs2273697 (0.11–0.29; across eight populations).
Table 4.
ABC polymorphisms with known effects on drug transport 4
| Transporter subfamily | Gene | Protein | Expression site | Substrate drug(s) | Known polymorphisms | Known effect |
|---|---|---|---|---|---|---|
| ABCB | ABCB1 | ABCB1, MDR1, P‐gp, CD243 | GI tract, liver, brain, kidney, testis, other tissues | Rifampin, digoxin, statins |
C1236T, C3435T, G2677T/A |
Reduced protein expression; higher drug plasma concentration |
| ABCC | ABCC1 | ABCC1, MRP1 | Lung, skin, intestine, kidney, placenta | Numerous chemotherapeutic drugs | R433S | Reduced transporter activity; higher drug plasma concentration |
| ABCC2 | ABCC2 | Apical membranes | Irinotecan, methotrexate | R768W, S789F, Q1382R |
No mRNA expression |
|
| ABCC3 | ABCC3, CMOAT2, MOAT‐D, MRP3 | Intestine | ― | −211C>T | Reduced protein expression; reduced ATP‐dependent bile acid sulfates/glucuronides export | |
| ABCC4 | ABCC4 | Blood‒brain barrier, liver, kidney, platelets | Nucleoside analogs | G187W | Reduced protein expression and activity; higher intracellular drug accumulation | |
| ABCC10 |
ABCC10, MRP7 |
Liver | Nevirapine | rs2125739 | Reduced substrate plasma concentrations | |
| ABCG | ABCG2 |
ABCG2, CDw338, BCRP |
GI tract, biliary tract, brain, placenta, testis | Mitoxantrone and camptothecin analogs |
Q141K |
Reduced protein expression, impaired transporter activity; higher drug plasma level |
| Q126stop, S441N, F489L | Loss of transporter activity | |||||
| −15994C>T | Increased protein expression; increased drug clearance |
ABC, adenosine triphosphate‒binding cassette; ATP, adenosine triphosphate; BCRP, breast cancer resistance protein; CMOAT, canalicular multispecific organic anion transporter; CD, cluster of differentiation; GI, gastrointestinal; mRNA, messenger RNA; MOAT‐D, multispecific organic anion transporter; MRP, multidrug resistance‒associated protein; P‐gp, permeability glycoprotein 1.
ABCB1
ABCB1 (also known as MDR1, P‐gp and CD243) is an ATP‐dependent transmembrane efflux pump, which has been shown to affect response to several drugs, including rifampin (Table 4 ). 4 Eleven studies investigated the impact of ABCB1 allelic variants on drug PK and PD, with an impact reported for three loci: ABCB1*7, ABCB1*8, and rs3842 (Table 5 ). 8 , 16 , 19 , 31 , 32 , 33 , 34 Eighteen studies reported the frequencies of ABCB1 allelic variants in African populations (Table 6 ). 8 , 19 , 21 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
Table 5.
Studies reporting effect of ABCB1 polymorphisms on drug PK, PD, and outcomes in African populations
| Reference | N | Study population | Substrate drug(s) | Polymorphism | Effect of polymorphism |
|---|---|---|---|---|---|
| 36 | 26 | Malawian | Nevirapine | ABCB1*6 3435C>T | No effect |
| ABCB1*7 2677G>T/A | No effect (2677G>T) | ||||
| 16 | 84 | Black African or African American (n = 44); others (n = 40): white, Mestizo; Native American/ Hawaiian/Pacific Islander | Atazanavir | ABCB1*7 2677G>T/A | Lower atazanavir C24 in participants with 2 copies of C‐G‐C haplotype vs one copy |
| 8 | 57 | South African | Rifampin | ABCB1*6 3435C>T | No effect |
| ABCB1*7 2677G>T/A | 19% increase in oral clearance; 19% increase in mean transit time (2677G>T) | ||||
| ABCB1*8 1236C>T | No effect | ||||
| 39 | 161 | South African; nevirapine‐associated hepatotoxicity (n = 53); healthy individuals (n = 108) | Nevirapine | ABCB1*6 3435C>T | Decreased risk of hepatotoxicity (risk ratio 0.30; P = 0.016) |
| 41 | 94 | South African; HIV‐positive | Efavirenz, lamivudine, stavudine | ABCB1*6 3435C>T | No effect on immune recovery or decline in viral load |
| 31 | 121 | Ugandan | Efavirenz | rs3842 4036A>G | 26% higher relative bioavailability in individuals homozygous for rs3842 |
| ABCB1*6 3435C>T | No effect | ||||
| 33 | 197 | Ugandan; HIV‐positive | Efavirenz | ABCB1*6 3435C>T | No effect |
| rs3842 4036A>G | 4036A>G genotype associated with higher plasma concentration at day 3 (P = 0.06) and week 8 (P = 0.04) | ||||
| 19 | 494 | Ethiopian (n = 285), Tanzanian (n = 209) | Efavirenz | rs3842 4036A>G | G allele associated with higher plasma concentrations; G allele frequency significantly higher in Tanzanians than Ethiopians; Tanzanians had significantly higher efavirenz plasma concentration at week 4 (P < 0.0002) and week 16 (P = 0.006) than Ethiopians |
| 34 | 182 | South African (112 Xhosa, 70 mixed ancestry); HIV‐positive | Efavirenz, lamivudine, stavudine | ABCB1*7 2677G>T/A | Effect on immune recovery (P < 0.01) (2677G>A); significant increase in CD4‐cell count in (2677G>A) heterozygous patients |
| rs3213619 T‐129C | Effect on immune recovery (P = 0.03); patients homozygous for the C allele had greatest increase in CD4‐cell count | ||||
| 41, 42 | 23 | Ugandan | ABCB1*6 3435C>T | No effect | |
| ABCB1*7 2677G>T/A | No effect | ||||
| 32 | 282 | South African; HIV‐positive | Efavirenz | ABCB1*6 3435C>T | No significant difference in efavirenz concentration between C/C and C/T genotypes |
| ABCB1*7 2677G>T/A | No significant difference in efavirenz concentration between G/G and G/T or G/A genotypes | ||||
|
rs3842 4036A>G |
Significant association between low plasma efavirenz concentrations and 4036A/G or 4036G/G genotypes (P = 0.0236) | ||||
| ABCB1*8 1236C>T | 1236C/T and 126T/T genotypes associated with high efavirenz concentrations (P = 0.0282) |
A separate color is used to identify each polymorphism.
ABCB1, ATP‐binding cassette transporter B1; C24, concentration at 24 hours postdose; CD4, cluster of differentiation 4; PD, pharmacodynamics; PK, pharmacokinetics.
Table 6.
ABC gene polymorphisms with effects on drug PK, PD, and outcomes: frequencies in African populations
| Reference | N | Population | ABCB1 | ABCC1 | ABCG2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| *6 (rs1045642) 3435C>T | *7 (rs2032582) |
*8 (rs1128503) 1236C>T |
rs3842 4036A>G |
rs3213619 −129T>C |
rs11642957 |
rs2231142 421C>A Q141K |
||||
| 2677G>T | 2677G>A | |||||||||
| 35 | 111 | Beninese | 0.14 | 0.009 | 0.0 | — | — | — | — | — |
| 36 | 26 | Malawian | 0.21 | 0.0 | — | — | — | — | — | — |
| 8 | 57 | South African | 0.26 | 0.19 | — | 0.26 | 0.21 | — | — | — |
| 37 | 993 | South African | 0.12 | — | — | 0.09 | — | 0.16 | — | — |
| 38 | 103 | Zanzibari | 0.16 | 0.029 (T/A) | — | — | — | — | — | |
| 39 | 53 | South African; nevirapine‐associated hepatotoxicity | 0.106 | 0.094 | — | — | — | — | — | — |
| 108 | Healthy individuals | 0.194 | 0.107 | — | — | — | — | — | — | |
| 40 | 109 | Xhosa | 0.11 | — | — | — | — | — | — | — |
| 67 | Cape Admixed | 0.2 | — | — | — | — | — | — | — | |
| 60 | Yoruba | 0.12 | — | — | — | — | — | — | — | |
| 89 | Luhya | — | — | — | — | — | — | — | — | |
| 143 | Maasai | 0.16 | — | — | — | — | — | — | — | |
| 41 | 94 | South African | 0.11 | — | — | — | — | — | — | — |
| 31 | 121 | Ugandan | 0.048 | 0.037 | — | 0.119 | 0.168 | — | — | — |
| 33 | 197 | Ugandan | 0.114‒0.13 | — | — | — | 0.184—0.196 | — | — | — |
| 19 | 285 | Ethiopian | 0.22 | — | — | — | 0.145 | — | — | — |
| 209 | Tanzanian | 0.155 | — | — | — | 0.22 | — | — | — | |
| 34 | 112 | Xhosa | 0.0982 | 0.023 | 0.0089 | 0.1295 | — | 0.1875 | — | — |
| 70 | South African mixed ancestry | 0.2071 | 0.164 | 0.0143 | 0.2429 | — | 0.2429 | — | — | |
| 42 | 23 | Ugandan | 0.087 | 0.0217 | — | — | — | — | — | — |
| 45 | 719 | Ethiopian | — | — | — | — | — | — | 0.18 | — |
| 43 | 172 | Ghanaian | 0.10 | — | — | — | — | — | — | — |
| 32 | 282 | South African: | ||||||||
| 127 | Sotho/Tswana | 0.110 | 0.012 | 0.004 | 0.106 | 0.165 | — | — | — | |
| 107 | Xhosa | 0.210 | 0.098 | 0.005 | 0.178 | 0.224 | — | — | — | |
| 139 | Zulu | 0.140 | 0.022 | 0.000 | 0.119 | 0.209 | — | — | — | |
| 21 | 37 | Black African | 0.07 | — | — | — | — | — | — | — |
| 44 | 934 | Ghanaian (total) | 0.12 | — | — | — | — | — | — | 0.01 |
| 90 | Akwapim | 0.15 | — | — | — | — | — | — | 0.01 | |
| 103 | Ashanti | 0.09 | — | — | — | — | — | — | 0.00 | |
| 183 | Ewe | 0.13 | — | — | — | — | — | — | 0.01 | |
| 160 | Fante | 0.13 | — | — | — | — | — | — | 0.00 | |
| 183 | Ga | 0.09 | — | — | — | — | — | — | 0.00 | |
| Unknown | Yoruba (Nigeria; HapMap) a | 0.11 | — | — | — | — | — | — | — | |
| Unknown | Ghanaian | 0.17 | — | — | — | — | — | — | — | |
| Unknown | Egyptian b | 0.4 | — | — | — | — | — | — | — | |
| Unknown | Kenyan b | 0.17 | — | — | — | — | — | — | — | |
| Unknown | Sudanese b | 0.27 | — | — | — | — | — | — | — | |
ABC, adenosine triphosphate‒binding cassette; PD, pharmacodynamics, PK, pharmacokinetics.
Data are from individuals in the HapMap database.
Data cited in the reference as being from the Pharmacogenetics for Every Nation Initiative (numbers of individuals unknown).
ABCB1*8 (rs1128503) 1236C>T
The frequency of the ABCB1*8 1236C>T allele varied from 0.09 to 0.26 in African populations (five studies) (Table 6 ). 8 , 31 , 32 , 34 , 37 No frequency data were reported for non‐African populations in these studies. Swart et al. 32 reported a significant difference in plasma efavirenz levels in South African patients with HIV infection (n = 282) with C/T or T/T genotypes compared with patients with the wild‐type (C/C) genotype. In a multivariate analysis, efavirenz concentration was 24.2% higher (95% confidence interval, 7.81–40.6%; P = 0.004) among patients with 1236C>T genotypes vs. the wild‐type. In a smaller South African study (n = 57), Chigutsa et al. 8 found no significant impact of 1236C>T on rifampin clearance, distribution, or bioavailability.
ABCB1*7 (rs2032582) 2677G>T/A
The impact of two alternative polymorphisms at this locus has been assessed by six studies (Table 5 ). 8 , 16 , 32 , 34 , 36 , 41 , 42 In African populations, the frequency of the ABCB1*7 2677G>T allele varied from 0.0 to 0.19 (eight studies), although the 2677G>A allele was rarer, ranging from 0.0 to 0.014 (three studies) (Table 6 ). One study only reported the frequency of 2677G>T/A (0.029). 38 No comparative frequency data from non‐African populations were reported in these studies.
A South African study (n = 57) found that ABCB1*7 resulted in a 19% higher increase in rifampin oral clearance and 19% higher increase in the mean transit time than the wild‐type. 8 Addition of the allele in the model used in this study had a significant impact on the goodness‐of‐fit criterion used (p < 0.05), but it is unclear whether this change would be clinically relevant. In addition, Parathyras et al. 34 found that ABCB1*7 had an impact on immune recovery (P < 0.01) among patients with HIV infection receiving efavirenz in South Africa (112 Xhosa and 70 South African mixed ancestry). In contrast, Swart et al. 32 reported no difference in plasma efavirenz levels in South African patients with HIV infection (n = 282) with G/T or G/A genotypes compared with those with the wild‐type (G/G) genotype. Two small studies (both fewer than 30 individuals) also found no correlation between ABCB1*7 and nevirapine exposure. 36 , 41 , 42
ABCB1 rs3842 4036A>G
The frequency of the 4036A>G allele varied from 0.145 to 0.224 (five studies) (Table 6 ), 8 , 19 , 31 , 32 , 33 similar to the frequency among Asians and Europeans (~ 0.20). 31 Several studies suggest an association between ABCB1 4036A>G (rs3842) and efavirenz PK. In a large study of individuals with HIV infection from Ethiopia (n = 285) and Tanzania (n = 209), Ngaimisi et al. 19 found that the ABCB1 4036A>G (rs3842) genotype was significantly associated with higher plasma efavirenz concentrations than the wild‐type (P = 0.002), but did not report the specific plasma efavirenz concentrations detected in the study participants. Modeling showed that 4036A>G contributed 0.6% and 8.3% of the interindividual variability in plasma efavirenz concentration in Ethiopian and Tanzanian patients, respectively. A study of 121 healthy Ugandan individuals found that the relative bioavailability of efavirenz was 26% higher in individuals homozygous for ABCB1 4036A>G than in carriers of the wild‐type allele.s 31 Simulations of a single 600 mg efavirenz dose indicated that typical individuals homozygous for the mutant alleles of ABCB1 4036, CYP2B6*6, and CYP3B6*11 had an area under the curve (AUC) equivalent to 943 μM/h‐1, approximately double that of individuals homozygous for the wild‐type allele (475 μM/h‐1). In a study of 197 Ugandan patients with HIV infection, the 4036A>G genotype was associated with higher efavirenz plasma concentrations at day 3 (P = 0.06) and week 8 (P = 0.04) compared with the wild‐type; however, the plasma efavirenz concentrations detected in study participants were not reported. 33 In contrast, Swart et al. 32 reported lower plasma efavirenz levels in South African patients with HIV infection (n = 282) with A/G or G/G genotypes than those with the wild‐type genotype (P = 0.0236). Additional studies in African populations are needed to clarify these contrasting observations, and the potential impact of CYP2B6*6, which is expressed at different frequencies across Africa, in efavirenz metabolism should be considered.
Polymorphisms in other clinically relevant ABC transporters
ABCC1 and ABCG2 are known to affect drug PK and PD from studies in non‐African populations. 4 Among the studies included, there were two polymorphisms in these genes for which allele frequencies in African populations were reported (Table 6 ).
In an Ethiopian population (n = 719), the frequency of ABCC1 rs11642957 was 0.18, 45 and in a large Ghanaian study (n = 934) the frequency of ABCG2 rs2231142 (Q141K) was 0.01. 44 Nevirapine has been shown to be transported by ABCC10 (MRP7), which is expressed in the liver, and genetic variants influence its plasma concentrations. 46 In a German study of 163 HIV‐infected patients receiving nevirapine, marked differences in the haplotype structure of ABCC10 were observed between white and black patients. In white patients, an exonic SNP of ABCC10 (rs2125739) was significantly associated with nevirapine plasma concentration (P = 0.02); however, no such association was found in black patients. 46 We did not find any studies reporting the frequency of this polymorphism, which emphasizes the requirement for more studies of SNPs in ABCC10 in African populations and their potential impact on drug PK and PD. Matsson et al. 47 compared the frequencies of four other ABCC10 polymorphisms in Nigerian (Yoruba) (n = 60), US Caucasian (n = 60) and combined Japanese/Chinese (n = 90) populations. The frequencies of rs2185631 were 0.13, 0.49, and 0.44; of rs12195350 were 0.42, 0.17, and 0.26; of rs2487663 were 0.49, 0.20, and 0.31; and rs9394952 were 0.0, 0.46, and 0.34, respectively. 47 The notable differences in the frequencies of all four polymorphisms between African populations and Caucasian or Asian populations highlights the need for more studies assessing their potential impact on drug PK and PD in African individuals.
FINDINGS IN CONTEXT
Overall genetic diversity is greater in African populations than in other continental populations, as demonstrated by whole genome analysis 48 as well as in studies of specific loci, such as our previous analysis of CYP genes. 2 Sub‐Saharan African populations and countries along the banks of the Nile River experience a high disease burden from infectious and parasitic agents. 49 , 50 , 51 , 52 , 53 Compared with other drugs, many drugs used for the treatment of infectious diseases possess distinct physicochemical properties such as higher aqueous solubility and appreciably lower permeability across biologic membranes. 3 , 54 As a consequence, such drugs tend to undergo minimal CYP‐mediated metabolism after administration to humans, and are eliminated unchanged via renal or biliary secretory pathways with the active participation of drug transporters that reside on the membranes of cells that serve a barrier function. 55 , 56 Therefore, genetic differences that affect the activity of the transport process may be of greater significance in therapeutic outcomes in African populations where there is a high need for the use of the anti‐infective drugs that are substrates for drug transporters.
This review highlights that there is much less published data on drug transporters in African populations than for CYP genes, with a smaller number of studies identified from only 13 African countries; the majority of these studies had fewer than 100 participants. Comparatively, our previous analysis of CYP genes identified 80 studies reporting CYP polymorphism frequencies in 37 different African populations. In addition, the studies of drug transporter genes often reported contradictory results, making it challenging to interpret the clinical relevance of the data and highlighting the need for more research in African populations.
Of the drug transporter alleles for which the impact on drug PK and PD was evaluated, SLCO1B1 rs4149032 and SLC22A6 rs1158626 were particularly relevant because their frequency was different in African populations compared with non‐African populations. SLCO1B1 rs4149032 (38664C>T) is an intronic SNP in linkage disequilibrium with the functional variant allele rs11045819, and was the most common allele in African populations (up to 0.93 among six African studies in our analysis, compared with 0.29 in Caucasians and 0.56 in Asians). 8 It was associated with reduced bioavailability and plasma concentration of rifampin in two studies in some African populations. 8 , 10 The reduction in rifampin exposure associated with this SLCO1B1 polymorphism has implications for this cornerstone anti‐TB therapy in Africa. Chigutsa et al. 8 considered 38664C>T an important determinant of the low rifampin plasma concentrations in South African patients and recommended an increase in the current standard daily dose. In applied simulations, they demonstrated that increasing the daily dose by 150 mg would produce concentrations similar to those observed in individuals with the wild‐type variant, and would reduce the proportion of patients with rifampin plasma concentrations below 8 μg/ml by 50%. 8 It is worth noting that the observed decrease in rifampin exposure may not be directly related to the effect of the mutation on OATP1B1 activity, but may instead be the result of linkage association with a mutation in P‐glycoprotein or a mutation in the regulatory components of OATP1B1 itself, resulting in increased transporter activity. This may explain why rs4149032 was reported to have no effect on rifampin exposure in a South Indian population. 57
Another notable polymorphism is SLC22A6 rs1158626 R50H 728G>A, associated with higher affinity for the nucleoside phosphate analogs cidofovir, adefovir, and tenofovir than the wild‐type. 7 Bleasby et al. 7 included African, Asian, and Caucasian individuals in their study, and this polymorphism was only found in African participants, at a frequency of 0.17. Similarly, in a study of five ethnic groups (n = 450), the minor allele was not detected in any European American, Korean, Han Chinese, or Japanese individuals, and at a frequency of 0.06 among African Americans. 58
Drug disposition depends upon extensive interplay among multiple transporters as well as metabolic enzymes. A greater understanding of this interplay is starting to be unlocked for some drugs, including statins. 59 For example, pravastatin is known to be transported by ABCB1, ABCC2, ABCG2, ABCB11, SLC22A8, SLCO2B1, and SLCO1B3, with combinations of polymorphisms having additive impacts on transport. 60 ABCB1 also plays a role in preventing the neurotoxicity of avermectin pesticides used to treat parasitic infections, by limiting transport at the blood–brain barrier and placental barrier. 61 However, the specific impact of ABCB1 polymorphisms is unclear, with no consistent data showing that known polymorphisms or haplotypes have an adverse effect on ABCB1 function at the blood–brain barrier or placental barrier. 61 In terms of efficacy, a study of Ghanaian participants, (42 ivermectin‐treated patients and 204 randomly selected healthy individuals), found significantly higher ABCB1*6 3435C>T allele frequency among suboptimal responders to ivermectin (0.21) than responders (0.12) or the healthy participants (0.11). 62
Our review suggests that ABC and SLC polymorphisms may show high diversity in African populations. For example, several of the studies compared allele frequencies across different African populations. Ngaimisi et al. 19 found significant differences in the frequency distributions of all common SLCO1B1 and ABCB1 genotypes and variant alleles between Ethiopian and Tanzanian patients with HIV infection. Similarly, the frequencies of the ABCB1 4036G, 2677T, and 2677A alleles in a South African population were significantly different to the frequencies reported in Yoruba individuals (P < 0.0001). 32 Pasanen et al. 20 also found higher genetic diversity across African populations than others; they compared the frequency of SLCO1B1 genes across 52 populations from Africa, the Middle East, Asia, Europe, Oceania, and the Americas. The highest genetic diversity was found in Africa, with all studied SLCO1B1 polymorphisms appearing in the African populations. We note that, in the present review, comparative data from non‐African populations were primarily sourced from the included African studies, which restricts our ability to draw firm conclusions about the genetic diversity in drug transporter genes in Africa compared with other global populations. A thorough analysis of genetic diversity in ABC and SLC in non‐African populations would allow for a more detailed comparison, although the primary aim of this study was not to systematically review global genetic diversity in these transporters, but to provide a comprehensive summary and analysis of the frequencies and distributions of allelic variants in African populations.
RECOMMENDATIONS FOR FUTURE RESEARCH, AND ASSOCIATED CHALLENGES
Allelic variants of metabolic enzymes and transporter proteins involved in drug metabolism and transport may result in substantial variability in drug PK and PD. This may lead to individual differences in drug safety and efficacy, manifesting at a population level as interethnic disparities in drug response. Therefore, genotype and ethnicity information have the potential to be used to guide appropriate therapeutic drug doses. 63 Furthermore, understanding individual and ethnic differences in drug PK and PD is increasingly an expectation for modern global drug development, as highlighted by guidance from the European Medicines Agency and the US Food and Drug Administration on pharmacogenomic studies in early clinical stages. The present analysis supports the inclusion of African populations in drug PK and PD studies, given that individuals in these populations may respond differently from other global populations. The high genetic diversity in ABC and SLC genes found across different African populations highlights the need to collect data from more regions in Africa. This is particularly important for polymorphisms for which data on PK and PD impact in African populations are lacking, e.g., ABCC1 rs11642957, ABCG2 alleles such as rs2231142 (Q141K) and several SNPs found in ABCC10, SLC22A1, and SLC22A2. There are other clinically relevant SLC transporters for which we could not identify any data from African populations, either in terms of allele frequencies or data on the PK and PD impact of polymorphisms: SLCO1B3, SLCO2B1, and SLC22A8.
Assessing the impact of genotype on drug response in African populations presents challenges. Most pharmacogenetic studies are in Asian and Caucasian populations. Data from African American populations are sometimes extrapolated to African populations, but these data are not representative of the genetic diversity across all African populations. 64 This review has therefore focused on studies in African populations. However, in some cases, these data are limited by small sample sizes, and there are a number of polymorphisms with known impacts on drug PK and PD for which there are no available data for African populations. There is a lack of knowledge about additional polymorphisms in redundant transporters and drug metabolizing enzymes that may either mask or amplify effects.
CONCLUSIONS
As has previously been observed for CYP drug metabolism enzymes, 2 the level of genetic diversity in SLC and ABC drug transporters in African populations appears to be high compared with other global populations. This has important implications for conventional therapies, particularly for treatment of tuberculosis and HIV infection. African populations should be included in drug PK and PD studies because individuals in these populations may respond differently from other global populations.
Funding
Funding for this review was provided by Novartis Pharma AG.
Conflict of interest
I.R. is an employee of Novartis NKK, Tokyo, Japan. L.K. is an employee of PharmaGenesis London, London, UK. I.H. is an employee of Novartis, East Hanover, NJ, USA. All authors declared no additional competing interests for this work.
Author Contributions
I.R., L.K., and I.H. wrote the manuscript; I.R. and L.K. designed the research; I.R. and L.K. performed the research; and I.R., L.K., and I.H. analyzed the data.
Supporting information
Table S1. Embase and Ovid MEDLINE search terms and results.
Table S2. SLC allele frequencies in African populations.
Table S3. ABC allele frequencies in African populations.
References
- 1. Mekonnen, A.B. , Alhawassi, T.M. , McLachlan, A.J. & Brien, J.E. Adverse drug events and medication errors in African hospitals: a systematic review. Drugs Real World Outcomes 5, 1–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajman, I. , Knapp, L. , Morgan, T. & Masimirembwa, C. African genetic diversity: implications for cytochrome P450‐mediated drug metabolism and drug development. EBioMedicine 17, 67–74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camp, D. , Garavelas, A. & Campitelli, M. Analysis of physicochemical properties for drugs of natural origin. J. Nat. Prod. 78, 1370–1382 (2015). [DOI] [PubMed] [Google Scholar]
- 4. Ahmed, S. , Zhou, Z. , Zhou, J. & Chen, S.Q. Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genomics Proteomics Bioinform. 14, 298–313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Global tuberculosis report. <www.who.int/tb/publications/global_report/en/> (2018). Accessed July 31, 2019.
- 6. Wilson, N.C. , Choudhury, A. , Carstens, N. & Mavri‐Damelin, D. Organic cation transporter 2 (OCT2/SLC22A2) gene variation in the South African Bantu‐speaking population and functional promoter variants. OMICS 21, 169–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleasby, K. , Hall, L.A. , Perry, J.L. , Mohrenweiser, H.W. & Pritchard, J.B. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J. Pharmacol. Exp. Ther. 314, 923–931 (2005). [DOI] [PubMed] [Google Scholar]
- 8. Chigutsa, E. et al The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob. Agents Chemother. 55, 4122–4127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dompreh, A. et al Effect of genetic variation of NAT2 on isoniazid and SLCO1B1 and CES2 on rifampin pharmacokinetics in Ghanaian children with tuberculosis. Antimicrob. Agents Chemother. 62, e02099–02017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gengiah, T.N. , Botha, J.H. , Soowamber, D. , Naidoo, K. & Abdool Karim, S.S. Low rifampicin concentrations in tuberculosis patients with HIV infection. J. Infect. Dev. Countries 8, 987–993 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Hennig, S. et al Effect of SLCO1B1 polymorphisms on rifabutin pharmacokinetics in African HIV‐Infected patients with tuberculosis. Antimicrob. Agents Chemother. 60, 617–620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mpeta, B. et al Differences in genetic variants in lopinavir disposition among HIV‐infected Bantu Africans. Pharmacogenomics 17, 679–690 (2016). [DOI] [PubMed] [Google Scholar]
- 13. Soko, N.D. , Masimirembwa, C. & Dandara, C. A cost effective RFLP method to genotype Solute carrier organic anion 1B1 (SLCO1B1) c.1929A>C (p.Leu643Phe, rs34671512); a variant with potential effect on rosuvastatin pharmacokinetics. BMC Res. Notes 11, 384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiner, M. et al Elevated plasma moxifloxacin concentrations and SLCO1B1 g.‐11187G>A polymorphism in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 62, e01802–01817 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aklillu, E. et al SLCO1B1 gene variations among Tanzanians, Ethiopians, and Europeans: relevance for African and worldwide precision medicine. OMICS 20, 538–545 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Castillo‐Mancilla, J.R. et al Pharmacogenetics of unboosted atazanavir in HIV‐infected individuals in resource‐limited settings: a sub‐study of the AIDS Clinical Trials Group (ACTG) PEARLS study (NWCS 342). J. Antimicrob. Chemother. 71, 1609–1618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoosain, N. , Pearce, B. , Jacobs, C. & Benjeddou, M. Mapping SLCO1B1 genetic variation for global precision medicine in understudied regions in Africa: a focus on Zulu and Cape Admixed populations. OMICS 20, 546–554 (2016). [DOI] [PubMed] [Google Scholar]
- 18. Mwinyi, J. , Kopke, K. , Schaefer, M. , Roots, I. & Gerloff, T. Comparison of SLCO1B1 sequence variability among German, Turkish, and African populations. Eur. J. Clin. Pharmacol. 64, 257–266 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Ngaimisi, E. et al Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel‐group prospective cohort study in two sub‐Saharan Africa populations. PLoS One 8, e67946 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasanen, M.K. , Neuvonen, P.J. & Niemi, M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics 9, 19–33 (2008). [DOI] [PubMed] [Google Scholar]
- 21. Weiner, M. et al Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 54, 4192–4200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lubomirov, R. et al ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet. Genomics 20, 217–230 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Hermann, M. et al Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several‐fold in patients with atorvastatin‐induced myopathy. Clin. Pharmacol. Ther. 79, 532–539 (2006). [DOI] [PubMed] [Google Scholar]
- 24. Moss, D.M. , Liptrott, N.J. , Siccardi, M. & Owen, A. Interactions of antiretroviral drugs with the SLC22A1 (OCT1) drug transporter. Front. Pharmacol. 6, 78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yee, S.W. et al Influence of transporter polymorphisms on drug disposition and response: a perspective from the International Transporter Consortium. Clin. Pharmacol. Ther. 104, 803–817 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben Hassine, I. et al hOCT1 gene expression predict for optimal response to Imatinib in Tunisian patients with chronic myeloid leukemia. Cancer Chemother. Pharmacol. 79, 737–745 (2017). [DOI] [PubMed] [Google Scholar]
- 27. Du Plessis, M. , Pearce, B. , Jacobs, C. , Hoosain, N. & Benjeddou, M. Genetic polymorphisms of the organic cation transporter 1 gene (SLC22A1) within the Cape Admixed population of South Africa. Mol. Biol. Rep. 42, 665–672 (2015). [DOI] [PubMed] [Google Scholar]
- 28. Jacobs, C. , Pearce, B. , Du Plessis, M. , Hoosain, N. & Benjeddou, M. Single nucleotide polymorphisms of the SLC22A2 gene within the Xhosa population of South Africa. Drug Metab. Pharmacokinet. 30, 457–460 (2015). [DOI] [PubMed] [Google Scholar]
- 29. Pearce, B. , Jacobs, C. , Hoosain, N. & Benjeddou, M. SLC22A2‐mapping genomic variations within South African indigenous and admixed populations. Drug Metab. Pers. Ther. 31, 213–220 (2016). [DOI] [PubMed] [Google Scholar]
- 30. Vasiliou, V. , Vasiliou, K. & Nebert, D.W. Human ATP‐binding cassette (ABC) transporter family. Hum. Genomics 3, 281–290 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mukonzo, J.K. et al A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single‐dose efavirenz population pharmacokinetics in Ugandans. Br. J. Clin. Pharmacol. 68, 690–699 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swart, M. , Ren, Y. , Smith, P. & Dandara, C. ABCB1 4036A>G and 1236C>T polymorphisms affect plasma efavirenz levels in South African HIV/AIDS patients. Front. Genet. 3, 236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mukonzo, J.K. et al Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV‐positive patients with or without tuberculosis: a prospective cohort study. BMC Infect. Dis. 13, 261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parathyras, J. , Gebhardt, S. , Hillermann‐Rebello, R. , Grobbelaar, N. , Venter, M. & Warnich, L. A pharmacogenetic study of CD4 recovery in response to HIV antiretroviral therapy in two South African population groups. J. Hum. Genet. 54, 261–265 (2009). [DOI] [PubMed] [Google Scholar]
- 35. Allabi, A.C. , Horsmans, Y. , Issaoui, B. & Gala, J.L. Single nucleotide polymorphisms of ABCB1 (MDR1) gene and distinct haplotype profile in a West Black African population. Eur. J. Clin. Pharmacol. 61, 97–102 (2005). [DOI] [PubMed] [Google Scholar]
- 36. Brown, K.C. et al Exploration of CYP450 and drug transporter genotypes and correlations with nevirapine exposure in Malawians. Pharmacogenomics 13, 113–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dandara, C. , Lombard, Z. , Du Plooy, I. , McLellan, T. , Norris, S.A. & Ramsay, M. Genetic variants in CYP (‐1A2, ‐2C9, ‐2C19, ‐3A4 and ‐3A5), VKORC1 and ABCB1 genes in a black South African population: a window into diversity. Pharmacogenomics 12, 1663–1670 (2011). [DOI] [PubMed] [Google Scholar]
- 38. Ferreira, P.E. et al Polymorphism of antimalaria drug metabolizing, nuclear receptor, and drug transport genes among malaria patients in Zanzibar, East Africa. Ther. Drug Monit. 30, 10–15 (2008). [DOI] [PubMed] [Google Scholar]
- 39. Haas, D.W. et al Pharmacogenetics of nevirapine‐associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin. Infect. Dis. 43, 783–786 (2006). [DOI] [PubMed] [Google Scholar]
- 40. Ikediobi, O. , Aouizerat, B. , Xiao, Y. , Gandhi, M. , Gebhardt, S. & Warnich, L. Analysis of pharmacogenetic traits in two distinct South African populations. Hum. Genomics 5, 265–282 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masebe, T.M. , Bessong, P.O. , Nwobegahay, J. , Ndip, R.N. & Meyer, D. Prevalence of MDR1 C3435T and CYP2B6 G516T polymorphisms among HIV‐1 infected South African patients. Dis. Markers 32, 43–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Penzak, S.R. et al Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV‐infected patients in Uganda. HIV Med. 8, 86–91 (2007). [DOI] [PubMed] [Google Scholar]
- 43. Schaeffeler, E. et al Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet 358, 383–384 (2001). [DOI] [PubMed] [Google Scholar]
- 44. Yen‐Revollo, J.L. et al Influence of ethnicity on pharmacogenetic variation in the Ghanaian population. Pharmacogenomics J 9, 373–379 (2009). [DOI] [PubMed] [Google Scholar]
- 45. Petros, Z. et al Genome‐wide association and replication study of anti‐tuberculosis drugs‐induced liver toxicity. BMC Genomics 17, 755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liptrott, N.J. et al Association of ABCC10 polymorphisms with nevirapine plasma concentrations in the German Competence Network for HIV/AIDS. Pharmacogenet. Genomics 22, 10–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsson, P. et al Discovery of regulatory elements in human ATP‐binding cassette transporters through expression quantitative trait mapping. Pharmacogenomics J 12, 214–226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Genomes Project Consortium . A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liao, C.W. et al Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of Sao Tome and Principe, West Africa. Afr. Health Sci. 16, 690–697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee, Y.H. , Lee, J.S. , Jeoung, H.G. , Kwon, I.S. , Mohamed, A. & Hong, S.T. Epidemiological survey on schistosomiasis and intestinal helminthiasis among village residents of the rural river basin area in white Nile state, Sudan. Korean J. Parasitol. 57, 135–144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ismail, H.A. et al Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit. Vectors 7, 478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. M'Bondoukwe, N.P. et al Prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co‐infections in different settlements of Gabon, Central Africa. Infect. Dis. Poverty 7, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roberts, D.J. Hematology: Basic Principles and Practice 7th edn (Elsevier, New York, 2018). [Google Scholar]
- 54. O'Shea, R. & Moser, H.E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 51, 2871–2878 (2008). [DOI] [PubMed] [Google Scholar]
- 55. Hosey, C.M. & Benet, L.Z. Predicting the extent of metabolism using in vitro permeability rate measurements and in silico permeability rate predictions. Mol. Pharm. 12, 1456–1466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li, J. , Larregieu, C.A. & Benet, L.Z. Classification of natural products as sources of drugs according to the biopharmaceutics drug disposition classification system (BDDCS). Chin. J. Nat. Med. 14, 888–897 (2016). [DOI] [PubMed] [Google Scholar]
- 57. Ramesh, K. et al SLCO1B1 gene polymorphisms do not influence plasma rifampicin concentrations in a South Indian population. Int. J. Tuberc. Lung Dis. 20, 1231–1235 (2016). [DOI] [PubMed] [Google Scholar]
- 58. Cheong, H.S. et al Screening of genetic variations of SLC15A2, SLC22A1, SLC22A2 and SLC22A6 genes. J. Hum. Genet. 56, 666–670 (2011). [DOI] [PubMed] [Google Scholar]
- 59. Rodrigues, A.C. Efflux and uptake transporters as determinants of statin response. Expert Opin. Drug Metab. Toxicol. 6, 621–632 (2010). [DOI] [PubMed] [Google Scholar]
- 60. Kitzmiller, J.P. , Mikulik, E.B. , Dauki, A.M. , Murkherjee, C. & Luzum, J.A. Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers. Med. 9, 97–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Macdonald, N. & Gledhill, A. Potential impact of ABCB1 (p‐glycoprotein) polymorphisms on avermectin toxicity in humans. Arch. Toxicol. 81, 553–563 (2007). [DOI] [PubMed] [Google Scholar]
- 62. Kudzi, W. , Dodoo, A.N. & Mills, J.J. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC Med. Genet. 11, 111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shah, R.R. & Gaedigk, A. Precision medicine: does ethnicity information complement genotype‐based prescribing decisions? Ther. Adv. Drug Saf. 9, 45–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tishkoff, S.A. et al The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Embase and Ovid MEDLINE search terms and results.
Table S2. SLC allele frequencies in African populations.
Table S3. ABC allele frequencies in African populations.


