Abstract
The aim of this study was to examine the risk of falls associated with the use of non-gamma amino butyric acid (GABA) sleep medications, suvorexant and ramelteon. This case-control and case-crossover study was performed at the Kudanzaka Hospital, Chiyoda Ward, Tokyo. A total of 325 patients who had falls and 1295 controls matched by sex and age were included. The inclusion criteria for the case group were hospitalized patients who had their first fall and that for the control were patients who were hospitalized and did not have a fall, between January 2016 and November 2018. The internal sleep medications administered were classified as suvorexant, ramelteon, non-benzodiazepines, benzodiazepines, or kampo. In the case-control study, age, sex, clinical department, the fall down risk score, and hospitalized duration were adjusted in the logistic regression model. In the case-control study, multivariable logistic regression showed that the use of suvorexant (odds ratio [OR]: 2.61, 95% confidence interval [CI]: 1.29–5.28), nonbenzodiazepines (OR: 2.49, 95% CI: 1.73–3.59), and benzodiazepines (OR: 1.65, 95% CI: 1.16–2.34) was significantly associated with an increased OR of falls. However, the use of ramelteon (OR: 1.40, 95% CI: 0.60–3.16) and kampo (OR: 1.55, 95% CI: 0.75–3.19) was not significantly associated with an increased OR of falls. In the case-crossover study, the use of suvorexant (OR: 1.78, 95% CI: 1.05–3.00) and nonbenzodiazepines (OR: 1.63, 95% CI: 1.17–2.27) was significantly associated with an increased OR of falls. Similar patterns were observed in several sensitivity analyses. It was suggested that suvorexant increases the OR of falls. This result is robust in various analyses. This study showed that the risk of falls also exists for non-GABA sleep medication, suvorexant, and thus it is necessary to carefully prescribe hypnotic drugs under appropriate assessment.
Introduction
Falls are a major cause of fractures and accidents and are also associated with reduced activities of daily living (ADL) in elderly people [1–3]. It has been reported that over 70% of hip fractures are caused by falls [1] and some fractures occur in approximately 5–10% of all falls. Hip fractures occur in 1–2% cases of falls [2]. Furthermore, even if patients do not get injured, repeated falling is associated with reduced ADL [3]. When viewed from the perspective of hospital management, 70% of hospital accidents are caused by falls, and therefore prevention of falls is an important issue [4].
Oral intake of sleep medications is a major risk factor for falls. For example, a systematic review found that the use of four or more internal medicines was associated with about 1.7–2.7 times increased risk of falls in the elderly [5]. Particularly, sedatives or hypnotics are the most strongly linked to increased risk of falls among oral medications, and it is suggested that there is an increased risk of falls in internal medicine patients with one or more sedatives or hypnotics [5]. In the past, non-benzodiazepines were not considered to be associated with an increased risk of falls and fractures [6]. In recent years, however, several studies have shown that the risk of falls and fractures associated with non-benzodiazepines intake has increased similarly to that of benzodiazepines and it is apparent that the risk associated with non-benzodiazepines intake is higher compared to benzodiazepines [7, 8]. In a previous study, the prevalence of insomnia was 8–18% in the general population, and the prevalence of insomnia symptoms generally increased with age [9]. Many people with insomnia use sleep medications [10, 11]. There appears to be a trade-off here. Though the elderly are at a high risk of falling due to lack of focus of attention, muscle weakness, and sensory deficits [12], at the same time the prevalence of insomnia also increases [9, 11].
Since the mechanism of the orexin receptor antagonist is different from that of existing sleep medications, suvorexant has been studied as an option for insomnia [13–15]. In randomized control trials (RCTs), suvorexant has not been found to be associated with an increased risk of falls and fractures [16–19]. However, there are no studies that have investigated falls as a main outcome, and in previous research, fall cases were very few in the intervention group and the non-intervention group [15–19]. In addition, research on side effects of suvorexant, including falls and fractures are few [20], so the fact that only results of pre-approved clinical trials are available makes it difficult to estimate the cost-effectiveness of suvorexant [21]. Ramelteon, a melatonin receptor agonist, is increasingly being used in the United States and Japan and has become one of the options for treating insomnia [20]. Nonetheless, there are few reports investigating the risk of falls associated with the use of ramelteon [22]. A study that evaluated the standing balance after oral administration of ramelteon found no adverse effect on balance [23]. However, to our knowledge, there are no real clinical reports that have reviewed falls associated with ramelteon [24, 25]. Suvorexant and ramelteon are non-gamma amino butyric acid (GABA) receptor agonist sleep medications, so they are considered to have little muscle relaxant action and little risk for falls [24–26]. However, the half-life of suvorexant is longer than previous medications like zolpidem [14, 27], and some studies have showed that suvorexant and ramelteon have carry-over effects [14, 23]. Regardless, the risk of falling associated with suvorexant and ramelteon is unclear. Thus, the aim of this study was to examine the risk of falls associated with the use of non-GABA receptor agonist sleep medications. We hypothesized that the use of suvorexant and ramelteon would be associated with increased risk of fall.
Methods
Target population, study design, and strategy for matching
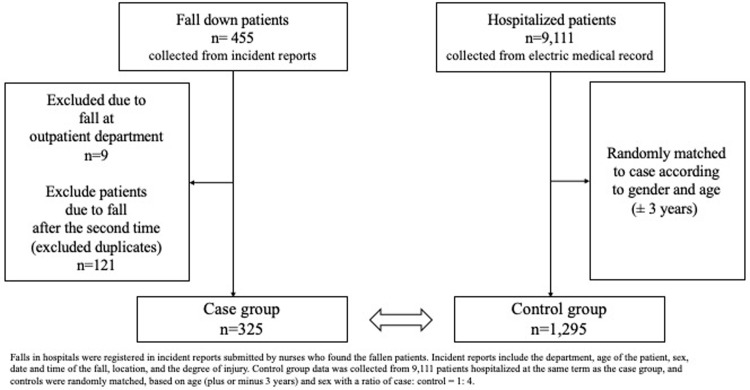
Retrospective case-control and case-crossover studies were conducted at the Kudanzaka Hospital (231 beds) in Chiyoda-ward, Tokyo. The inclusion criteria for the case group were hospitalized patients who had their first fall between January 2016 and November 2018. Second or subsequent falls after hospitalization or outpatient falls were excluded. The clinical departments of Kudanzaka hospital were orthopedic surgery, internal medicine, general surgery, rehabilitation department, psychosomatic medicine, gynecology, urology, and dermatology. Inclusion criteria for the control group were patients who were hospitalized between January 2016 and November 2018 and had not fallen. Control group data were collected from 9,111 patients, and controls were randomly matched, based on age (plus or minus 3 years) and sex with a ratio of case:control = 1:4 (Fig 1). Source population in this study is the group who did not fall but was hospitalized in Kudanzaka hospital; so, we sampled the control from the group randomly [28]. In previous studies, matching was performed using gender and age [29, 30], and to reduce overmatching and bias, this study matched only with gender and age [31, 32]. We confirmed the robustness of the result in the case-crossover analysis to eliminate the risk that the matching is overmatching or limited and to reduce the bias of control selection [33].
Fig 1. Design of case-control study.
Data collection and definition in the case-control and case-crossover study
Falls
The fall of a patient was defined as the incident in which the body of a patient suddenly or unintentionally hits the ground or another location. Falls in hospitals were registered in incident reports submitted by nurses who found the fallen patients. Incident reports included the department, age of the patient, sex, date and time of the fall, and location. Incident reports were checked by the group leader, and two researchers confirmed the incident via checking the electronic medical records.
Sleep medications
The internal sleep medications administered were classified as suvorexant, ramelteon, non-benzodiazepines, benzodiazepines, or the oriental medicine (called kampo, Yokukansan, or Yi-gan san). Kampo is a traditional and popular herbal drug in Japan and yokukansan is a kind of kampo [34]. Although, there is little medical evidence of its efficacy for insomnia, yokukansan is widely used in Japan [35, 36]. We analyzed these medications as exposure for falls. Adjustment for each medication was performed for patients taking multiple sleep medications, and the risk of fall was analyzed. The medications of the patients were extracted from the electronic medical record.
Other variables
The fall down risk score (it consists of 8 items namely age, medical history, vision or hearing impairment, motor dysfunction, activity, cognitive ability, medication, and excretion and was evaluated at 39 points as full score with 16 points or more considered as high risk of falls) [37, 38] (S1 Table). The fall down risk score was evaluated by nurses at hospitalized day and in every week. Hospitalized duration was defined as the date from hospitalization and discharge. The department of hospitalization, hospitalization duration, the fall down risk score, age, and sex were extracted from the electronic medical record.
Variable measurement date in the case-control and case-crossover study
In the case and control groups, the department was collected at hospitalized day, and hospitalization duration was recorded at discharge date. In the case group, we collected data of internal medicine measured on falling date, and the nearest fall down risk score before falls was collected. In the control group, since there were reports that falls tend to occur in approximately 1 week after hospitalization [39, 40], oral medications used within 1 week after hospitalization were investigated and the score of 1 week after hospitalization was collected. In the case-crossover study, internal medications of hospitalization and that of the middle date between hospitalization and falling were measured as an exposure of controls, and internal medications at falling was measured as an exposure of cases (S1 Fig). We summarized the variable information in the case-control and case-crossover study in the S2 Table.
Ethical review
This study was approved by the Kudanzaka Hospital Ethics Committee. This research is part of a hospital-based quality improvement project, and data were dealt anonymously. The need for written informed consent from patients was waived because this study was conducted as part of a hospital-based quality improvement project and posed no risk to patients or their privacy.
Statistical analysis
Sample size was calculated statistically, we needed approximately 1,000 patients at a significant level = 0.05, power = 0.9. For sensitivity analysis and case-crossover study, we collected data for more than 1,000 patients. Age, hospitalized duration, and the fall down risk score were analyzed as quantitative variables. Other data were analyzed as categorical variables. We conducted a complete-case analysis, so those with missing data were not included in the analysis. In the case-control study, age, sex, clinical department, the fall down risk score, and hospitalized duration were adjusted. In the case-crossover study, age, sex, clinical department, and the fall down risk score were adjusted. The association between sleep medications usage and falls was assessed by conditional logistic regression analysis using odds ratio (OR) and 95% confidence interval (CI).
In the case-control study, there were four analyses for sensitivity analysis. Firstly, since previous studies have shown a high risk of falls in orthopedic patients and those with musculoskeletal problems [5, 41, 42], an analysis excluding orthopedic surgery was conducted. Secondly, to eliminate the influence of multiple oral medications or long hospitalized duration, we excluded patients using multiple sleep medications or had long hospitalization. Thirdly, evaluating the onset of action of benzodiazepines, analysis of benzodiazepines by separating them into long-acting type and short-acting type was carried out. Lastly, considering dose response, the analysis of the fall OR of non-benzodiazepines and benzodiazepines was conducted in the case-control study. We could not conduct the dose response analysis of suvorexant and ramelteon because the adoption of suvorexant in Kudanzaka Hospital was only 15 mg tablets and that of ramelteon was only 8 mg. All patients who were prescribed suvorexant and ramelteon were prescribed those of 15 mg and 8 mg. For multivariate analysis, the models considered the multicollinearity with the variance inflation factor of which the cutoff was 2.5. Statistical analysis was performed with R (http://www.r-project.org/) version 3.4.2. We conducted a two-sided test, and the analysis was at α level 0.05.
Results
Case-control and case-crossover study
There were 455 cases of falls at the Kudanzaka Hospital during the period of study (Fig 1). We excluded nine cases that occurred at outpatient service and 121 cases that occurred after the second time of falls, and 325 patients were assigned as cases of study. Of the 325 patients, 181 (55.7%) were taking sleep medications. Of the 1,295 patients who matched on age and sex, 416 (32.1%) were taking sleep medications and were selected as controls. Two patients, a 100-year-old male and a 102-year-old male, could not be matched to four controls (we chose two and one patients for controls corresponding to these cases).
Details of internal medicine and the number of patients who took sleep medications are shown in Table 1. Non-benzodiazepine medications included zolpidem, eszopiclone, and zopiclone. Benzodiazepine medications included triazolam, etizolam, brotizolam, rilmazafone, alprazolam, lorazepam, quazepam, flutoprazepam, clotiazepam, diazepam, flunitrazepam, nitrazepam, clonazepam, and lormetazepam. Across all sleep medications, the percentage of patients taking a medication was higher in the case group compared to the control group (p<0.001). Table 2 shows the measured background factors of the patients. Significant difference (p<0.001) was observed between the case group and the control group in terms of medical departments (except for general surgery and other departments), hospitalized duration, and the fall risk score. Therefore, the department of medical treatment, hospitalization duration, and the fall risk score were included in the multivariate analysis in the case-control study. Table 3 shows the result of conditional logistic regression analysis for falls. All variables were significant in univariate regression results. When the Bonferroni correction was performed in consideration of the multiple tests, almost the same result as the multivariate regression analysis was obtained. In the multivariate regression analysis, the use of suvorexant (OR: 2.61, 95% CI: 1.29–5.28), non-benzodiazepines (OR: 2.49, 95% CI: 1.73–3.59), and benzodiazepines (OR: 1.65, 95% CI: 1.16–2.34) was significantly associated with an increased OR of falls. However, the use of ramelteon (OR: 1.40, 95% CI: 0.60–3.16) and kampo (OR: 1.55, 95% CI: 0.75–3.19) was not significantly associated with an increased OR of falls. Table 4 shows the results of the case-crossover analysis, which showed that in the multivariate regression analysis, the use of suvorexant (OR: 1.78, 95% CI: 1.05–3.00) and non-benzodiazepines (OR: 1.63, 95% CI: 1.17–2.27) was significantly associated with an increased OR of falls. The use of ramelteon (OR: 1.19, 95% CI: 0.55–2.46), benzodiazepines (OR: 1.09, 95% CI: 0.79–1.50), and kampo (OR: 1.46, 95% CI: 0.75–2.68) was not significantly associated with an increased OR of falls.
Table 1. Medication prescribed to cases and controls.
| Medication | Cases n (%) | controls n (%) | P value |
|---|---|---|---|
| Suvorexant | 30 (9.2) | 20 (1.5) | <0.001 |
| Ramelteon | 13 (4.0) | 22 (1.7) | 0.017 |
| Non-benzodiazepines | 84 (25.8) | 151 (11.6) | <0.001 |
| Benzodiazepines | 96 (29.5) | 255 (19.7) | <0.001 |
| Kampo (yokukansan) | 21 (6.5) | 26 (2.0) | <0.001 |
| No sleep medication prescribed | 144 (44.3) | 879 (67.9) | <0.001 |
| Total patients | 325 | 1295 |
Non-benzodiazepines include: Zolpidem, Eszopiclone, Zopiclone.
Benzodiazepines include: Triazolam, Etizolam, Brotizolam, Rilmazafone, Alprazolam, Lorazepam, Quazepam, Flutoprazepam, Clotiazepam, Diazepam, Flunitrazepam, Nitrazepam, Clonazepam, Lormetazepam.
Variables are analyzed by Fisher's exact probability test.
Table 2. Patient background variables.
| Characteristics | Case group | Control group | P value |
|---|---|---|---|
| Age, mean (±SD) | 76.7 (±12.0) | 76.1 (±11.7) | 0.391 |
| Sex n (%) (male) | 155 (47.7) | 615 (47.5%) | 0.951 |
| Department | |||
| Orthopaedic surgery n (%) | 199 (61.2) | 649 (50.1) | <0.001 |
| Internal medicine n (%) | 59 (18.2) | 414 (32.0) | <0.001 |
| General surgery n (%) | 21 (6.5) | 123 (9.5) | 0.101 |
| Rehabilitation n (%) | 40 (12.3) | 62 (4.8) | <0.001 |
| Others(Gynecology, Urology, Dermatology, Psychology) n (%) | 6 (1.8) | 47 (3.6) | 0.118 |
| Hospitalized duration mean (SD) (day) | 70.1 (63.5) | 21.0 (27.9) | <0.001 |
| Fall down risk score mean (SD) (point) | 15.7 (4.18) | 11.3 (5.6) | <0.001 |
Continuous variables are analysed by student's t-test.
Percentage variables are analyzed by Fisher's exact probability test.
SD: standard deviation.
Table 3. Logistic regression model for sleep medications and covariances.
| Univariate analysis for fall OR (95%CI, P value) | Multivariate analysis for fall OR (95%CI, P value) | |
|---|---|---|
| Sleep medications use | ||
| Suvorexant | 6.48 (3.66–11.7, <0.001) | 2.61 (1.29–5.29, 0.008) |
| Ramelteon | 2.41 (1.17–4.78, 0.013) | 1.40 (0.60–3.16, 0.429) |
| Non-benzodiazepines | 2.64 (1.95–3.56, <0.001) | 2.49 (1.73–3.59, <0.001) |
| Benzodiazepines | 1.71 (1.30–2.25, <0.001) | 1.65 (1.16–2.34, 0.005) |
| Kampo (yokukansan) | 3.37 (1.85–6.06, <0.001) | 1.55 (0.75–3.19, 0.233) |
| Department | ||
| Orthopaedic surgery (Reference) | ||
| Internal medicine | 0.46 (0.34–0.63, <0.001) | 1.19 (0.81–1.77, 0.367) |
| General surgery | 0.56 (0.33–0.89, 0.019) | 1.75 (0.93–3.17, 0.074) |
| Rehabilitation | 2.10 (1.36–3.21, <0.001) | 1.20 (0.69–2.06, 0.510) |
| Others(Gynecology, Urology, Dermatology, Psychology) | 0.42 (0.16–0.92, 0.047) | 1.42 (0.48–3.52, 0.481) |
| Hospitalized duration (per 1day) | 1.031 (1.027–1.035, <0.001) | 1.024 (1.020–1.029, <0.001) |
| Fall down risk score (per 1point) | 1.18 (1.15–1.21, <0.001) | 1.15 (1.11–1.19, <0.001) |
Multiple logistic regression adjusted age, sex, all sleep medications, department, hospitalized duration, fall down risk score.
OR: odds ratio, CI: confidence interval.
Table 4. Case-crossover analysis.
| Univariate analysis for fall OR (95%CI, P value) | Multivariate analysis for fall OR (95%CI, P value) | |
|---|---|---|
| Suvorexant | 1.84 (1.10–3.07, 0.019) | 1.78 (1.05–3.00, 0.031) |
| Ramelteon | 1.14 (0.54–2.33, 0.707) | 1.19 (0.55–2.46, 0.640) |
| Non-benzodiazepines | 1.60 (1.16–2.20, 0.004) | 1.63 (1.17–2.27, 0.004) |
| Benzodiazepines | 1.13 (0.84–1.51, 0.419) | 1.09 (0.79–1.50, 0.585) |
| Kampo (yokukansan) | 1.55 (0.83–2.85, 0.159) | 1.46 (0.75–2.68, 0.277) |
Multiple logistic regression adjusted age, sex, hospitalized department, the fall down risk score and all sleep medications.
OR: odds ratio, CI: confidence interval.
Sensitivity analysis
The results of the sensitivity analysis are shown in S3–S5 Tables. In the analysis excluding orthopedic surgery data, consistency of the results was maintained, and an increased OR of falls was observed for suvorexant (OR: 5.43, 95% CI: 1.59–20.1) and non-benzodiazepines (OR: 1.81, 95% CI: 0.92–3.45) (S3 Table). In order to eliminate the influence of multiple oral medications, patients using multiple sleep medications were excluded and there was also an increased OR of falls for suvorexant (OR: 2.48, 95% CI: 0.87–6.85), non-benzodiazepines (OR: 2.32, 95% CI: 1.44–3.70), and benzodiazepines (OR: 1.62, 95% CI: 1.05–2.47) intake (S4 Table). The analysis was performed excluding those whose length of hospital stay was above the median (S5 Table). An increase in the OR of falls was observed for suvorexant (OR: 9.09, 95% CI: 1.05–65.6) and non-benzodiazepines (OR: 2.34, 95% CI: 0.91–5.51). Ramelteon and Kampo could not be analyzed because the data were too sparse. However, the consistency of the results was maintained. The analysis in which benzodiazepines were divided into short-acting type and long-acting type is shown in S6 Table. Based on the adjusted analysis, there was an increased OR for falls for long-acting benzodiazepines (OR: 2.17, 95% CI: 1.30–3.57). Considering dose of medications, the consistency of the results was maintained (S7 Table). The multicollinearity test was performed, and the results are presented in S8 Table.
Discussion
Summary of results and differences in results from previous studies: Risk of falls with non-GABA medications
In the case-control study, the OR of falls was significantly higher in patients who used suvorexant, non-benzodiazepines, and benzodiazepines. The use of ramelteon and kampo was not associated with the significantly increased OR of falls. In the case-crossover study, the significance of benzodiazepines disappeared, but similar results were observed elsewhere. Benzodiazepines and non-benzodiazepines have a muscle relaxant action, for which the α1–3 subunits of the GABA receptor [43, 44] are responsible, and even in previous studies, an increased risk of falls has been shown [6–8].
Suvorexant is a sleep drug released in Japan and the United States in 2014 and has a new mechanism, blocking the orexin receptor that controls arousal [13, 15, 45]. Since it does not act on GABA but acts selectively on the orexin system, it is considered not to have a muscle relaxant action or carry-over effect [26]. In some studies, its association with the risk of adverse events such as falls and fractures was negative [16–19]. However, in this study, an increase in the OR of falls was observed. In previous RCTs [17–19], it was difficult to statistically analyze for falls because the number of patients with falls was extremely small. The study that recorded most falls compared 12 (2.3%) and 8 (3.1%) falls [17], and it is necessary to collect falling patients and to design the case-control study for rare events such as falls. There is a possibility that risk determination is affected by the fact that the average age of the samples in this study is older (case group, mean age: 76.7 years), and the proportion of patients with orthopedic surgery is large. However, because the results did not change even in the analysis excluding orthopedic surgery, influence by the medical department is not anticipated. Analysis focused on elderly people has not been conducted in previous studies, and the risk of falls may be selectively high in elderly people. Clinical trials are performed for outpatients who have insomnia, but this study was performed in hospital settings and patients with comorbidities other than insomnia (no patients hospitalized for insomnia) were the subjects. For hospitalized patients, the risk of falls may be higher than that in outpatients. As far as we know, no research for suvorexant has been conducted on hard outcomes such as hip fracture, and further studies are needed. From the perspective of pharmacology, the reason falls are increased with suvorexant may be due to the half-life of the drug in the blood, which is about 9–13 hours [14]. Additionally, to maintain attention, the cholinergic system centered on the forebrain base plays an important role, and the orexin neural system interacts with it [46, 47]. Inhibition of the orexin receptor may reduce attention, and thus the orexin receptor antagonist, suvorexant, may increase the risk of falls by impairing attention [46]. In the elderly, orexin receptor and cholinergic neuron actions decrease, which may further increase the risk of falls [47]. S9 Table shows when patients fell in the case group. Patients who were prescribed suvorexant did not fall more at night than at daytime. There might be some residual sedation and carry over in the pharmacology of suvorexant [14].
Ramelteon is a melatonin receptor agonist, and it is increasingly being used by medical doctors [20, 22]. This case-control study did not show an increased OR for falls with the use of ramelteon. A similar result was obtained with the case-crossover analysis. Ramelteon might be less sedating, and previous studies have found less sedative adverse effects [23]; similar results were obtained in this study. Because melatonin stimulates daytime arousal and nighttime sleep [48, 49], it might not have raised the risk of falls. The result of this study, which was not significant, however, does not indicate the absence of risk [50]; therefore, attention must be paid to adverse effects such as falls. It is necessary to accumulate further research.
In insomnia guidelines, it is generally recommended to first consider cognitive-behavioral-therapy and psychological behavioral interventions and then consider hypnotic formulation [51, 52]. It is suggested that the risk of falls also exists for the non-GABA sleep medications as per the results in this study, and thus it is necessary to carefully prescribe hypnotic drugs under appropriate assessment.
Evaluation of bias by sensitivity analysis and limitation of this research
In hospital studies, it is common that people with low severity are selected as controls, and the OR may be overestimated. Even in the case-crossover study, the same result as the case-control study indicates that bias by control selection is adjusted and the result is robust [33]. In the multivariate analysis, even if the fall risk score or hospitalized duration was added, the result did not change, and among those who are considered to have the same possibility of falls, the influence of sleep medications remained. Furthermore, conditional logistic regression analysis excluding patients taking two or more kinds of sleep medications was similar, and it was considered that the effect of multiple sleep medications could also be eliminated.
There are several limitations in this research. First, we did not consider if patients were taking other medications, which have an increased risk of falls. This could be the bias of the result for this study. Second, it was impossible to evaluate the state of sleep and comorbidities in individuals. It cannot be denied that people with insomnia or chronic disease (like chronic obstructive pulmonary disease and obstructive sleep apnea) often take sleep medications and fall. However, most of the bias due to each patient's condition is controlled in a case-crossover study because the status of patients who have some chronic diseases is the same in the case-crossover comparison and the hospitalized duration. In future, a more planned prospective study is needed. We plan to conduct a validation study using instrumental variables to control potential confounders in this study. Third, this study might suffer from Berkson's bias due to recruitment from the hospitalized patients. Finally, the measurement and documentation of falls may not be entirely accurate and may be a limiting factor in this study.
In conclusion, sleep medications are associated with an increased OR of falls. This result is robust in various analyses, case-crossover studies, and sensitivity analyses. In this study, it was found that suvorexant was associated with an increased OR of falls. Although ramelteon was not associated with an increased OR of falls, further evaluation of its effect on the risk of falls and fractures in the future are needed. This study showed that the risk of falls also exists for non-GABA sleep medications, and thus it is necessary to carefully prescribe hypnotic drugs under appropriate assessment. The results of this study reaffirmed the importance of guidelines to optimize prescriptions. The preconception that there will be no falls because there is no muscle relaxation is dangerous. Physicians need to be cautious when prescribing sleep medications, even if they are not GABA receptor agonists.
Supporting information
(PPTX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors are grateful to Prof. T Takebayashi and Pro. T Okamura for helpful discussions. We also thank M. Abe and Ms. Miura for sharing their dataset with us.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hagino H, Sakamoto K, Harada A, et al. Committee on Osteoporosis of The Japanese Orthopaedic Association. Nationwide one-decade survey of hip fractures in Japan. J Orthop Sci. 15(6); 737–745, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Nevitt MC. Falls in the elderly: Risk factors and prevention. Masdeu JC, et al eds. Gait disorders of aging. Falls and therapeutic strategies. Lippincott–Raven, Philadelphia, 1997, pp13-36.
- 3.Sekaran NK, Choi H, Hayward RA, Langa KM. Fall-associated difficulty with activities of daily living in functionally independent individuals aged 65 to 69 in the United States: a cohort study. J Am Geriatr Soc. 2013. January;61(1):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton JC, Standen PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994. Mar-Apr;48(2):63–6. [PubMed] [Google Scholar]
- 5.Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". JAMA. 2010. January 20;303(3):258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989. December 15;262(23):3303–7. [PubMed] [Google Scholar]
- 7.Treves N, Perlman A, Kolenberg Geron L, Asaly A, Matok I. Z-drugs and risk for falls and fractures in older adults-a systematic review and meta-analysis. Age Ageing. 2018. March 1;47(2):201–208. [DOI] [PubMed] [Google Scholar]
- 8.Brand Jaden and Leong Christine. Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research. Drugs R D. 2017. Dec; 17(4): 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiology of insomnia: what we know and what we still need to learn. Ohayon MM. Sleep Med Rev. 2002. April;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 10.Chong Y, Fryar CD, Gu Q. Prescription Sleep Aid Use Among Adults: United States, 2005–2010. Hyattsville, Maryland: National Center for Health Statistics; 2013. NCHS Data Brief No. 127. [PubMed] [Google Scholar]
- 11.Doi Y, Minowa M, Okawa M, Uchiyama M.. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol, 10 (2000), pp. 79–86 [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine (US) Division of Health Promotion and Disease Prevention, Berg RL, Cassells JS, eds. The Second Fifty Years: Promoting Health and Preventing Disability. Washington (DC): National Academies Press (US); 1992. [PubMed]
- 13.Lee-Iannotti JK, Parish JM. Suvorexant: a promising, novel treatment for insomnia. Neuropsychiatr Dis Treat. 2016. February 25;12:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013. February 1;36(2):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kripke DF. Is suvorexant a better choice than alternative hypnotics? F1000Res. 2015. August 3;4:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas RH, Katz R, Illoh K, et al.: Application Number 204569Orig1s000: Medical Review(s).2013.
- 17.Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014. May;13(5):461–71. [DOI] [PubMed] [Google Scholar]
- 18.Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in Patients With Insomnia: Results From Two 3-Month Randomized Controlled Clinical Trials. Biol Psychiatry. 2016. January 15;79(2):136–48. [DOI] [PubMed] [Google Scholar]
- 19.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012. December 4;79(23):2265–74. [DOI] [PubMed] [Google Scholar]
- 20.Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, et al. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clin Ther. 2016. November;38(11):2340–2372. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura S, Nakao M. Cost-effectiveness analysis of suvorexant for the treatment of Japanese elderly patients with chronic insomnia in a virtual cohort. J Med Econ. 2018. July;21(7):698–703. [DOI] [PubMed] [Google Scholar]
- 22.Gooneratne NS, Gehrman P, Gurubhagavatula I, Al-Shehabi E, Marie E, Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo-controlled pilot study. J Clin Sleep Med. 2010. December 15;6(6):572–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Mets MA, de Vries JM, de Senerpont Domis LM, Volkerts ER, Olivier B, Verster JC. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011. October 1;34(10):1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchiyama M, Hamamura M, Kuwano T, Nagata H, Hashimoto T, Ogawa A, et al. Long-term safety and efficacy of ramelteon in Japanese patients with chronic insomnia. Sleep Med. 2011. February;12(2):127–33. [DOI] [PubMed] [Google Scholar]
- 25.Mets MA, van Deventer KR, Olivier B, Verster JC. Critical appraisal of ramelteon in the treatment of insomnia. Nat Sci Sleep. 2010. November 10;2:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA. CENTER FOR DRUG EVALUATION AND RESEARCH. APPLICATION NUMBER:204569Orig1s000. June 29 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204569Orig1s000MedR.pdf (accessed 2 July 2019).
- 27.Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf. 2003;26(4):261–82. [DOI] [PubMed] [Google Scholar]
- 28.Kenneth J. Rothman, Sander Greenland, Timothy L. Lash. Modern Epidemiology. 3rd Edition. 2008 Lippincott Williams & Wilkins. Chapter 8 p111-127.
- 29.Pierfitte C, Macouillard G, Thicoïpe M, Chaslerie A, Pehourcq F, Aïssou M, et al. Benzodiazepines and hip fractures in elderly people: case-control study. BMJ. 2001. March 24;322(7288):704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivertsen B, Salo P, Pentti J, Kivimäki M, Vahtera J. Use of sleep medications and risk of cancer: a matched case-control study. Sleep Med. 2015. December;16(12):1552–5. [DOI] [PubMed] [Google Scholar]
- 31.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009. January 6;5(1):Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007. November;18(6):805–35. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S. A unified approach to the analysis of case-distribution (case-only) studies. Stat Med. 1999. January 15;18(1):1–15. [DOI] [PubMed] [Google Scholar]
- 34.Ikarashi Y, Iizuka S, Imamura S, Yamaguchi T, Sekiguchi K, Kanno H, et al. Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biol Pharm Bull. 2009. October;32(10):1701–9. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi K, Ikarashi Y. Multiple Psychopharmacological Effects of the Traditional Japanese Kampo Medicine Yokukansan, and the Brain Regions it Affects. Front Pharmacol. 2017. March 21;8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analysis for the behavioral sciences. Second Edition. 1988. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers. [Google Scholar]
- 37.Japanese Nursing Association 2002. White paper nursing. Japanese Nursing Association Publishing Company, 2002. p.170-196. In Japanese.
- 38.Akiko H, Keioko N. Prediction accuracy of fall risk check tool for hospitalized patients. Journal of the Japan Society for Healthcare administration. No.53-1 Page31–39. 2016.01. In Japanese. [Google Scholar]
- 39.Mahoney JE, Palta M, Johnson J, Jalaluddin M, Gray S, Park S. Temporal association between hospitalization and rate of falls after discharge. Arch Intern Med. 2000. October 9;160(18):2788–95. [DOI] [PubMed] [Google Scholar]
- 40.de Paiva MC, de Paiva SA, Berti HW, Campana AO. Characterization of patient falls according to the notification in adverse event reports. Rev Esc Enferm USP. 2010. March;44(1):134–8. [DOI] [PubMed] [Google Scholar]
- 41.Mandl LA, Lyman S, Quinlan P, Bailey T, Katz J, Magid SK. Falls among patients who had elective orthopaedic surgery: a decade of experience from a musculoskeletal specialty hospital. J Orthop Sports Phys Ther. 2013. February;43(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013. Summer;13(2):214–23. [PMC free article] [PubMed] [Google Scholar]
- 43.Vinkers Christiaan H., Olivier Berend. Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABAA Receptor Modulators? Adv Pharmacol Sci. 2012; 2012: 416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang YH, Lai JN, Lee CH, Wang JD, Chen PC. Increased risk of hospitalization related to motor vehicle accidents among people taking zolpidem: a case-crossover study. J Epidemiol. 2011;21(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010. February 16;1314:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol. 2013. October;23(5):752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabata H, Kuriyama A, Yamao F, Kitaguchi H, Shindo K. Suvorexant-Induced Dream Enactment Behavior in Parkinson Disease: A Case Report. J Clin Sleep Med. 2017. May 15;13(5):759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cajochen C, Münch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005. March;90(3):1311–6. [DOI] [PubMed] [Google Scholar]
- 49.Zisapel Nava. The Role of Melatonin in Sleep Regulation. Neuroendocrine Correlates of Sleep/Wakefulness pp 295–309. [Google Scholar]
- 50.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016. April;31(4):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Academy of Sleep Medicine. Five Things Physicians and Patients Should Question. Choosing Wisely. Released December 2, 2014; #1 sources updated June 1, 2017.
- 52.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001. June;158(6):892–8. [DOI] [PubMed] [Google Scholar]