Summary
Clostridioides difficile infection of the colon leads to severe inflammation and damage to the gastrointestinal epithelium due to the production of potent toxins. This inflammatory tissue damage causes the liberation of high concentrations of host heme at infection sites. Here, we identify the C. difficile heme sensing membrane protein system (HsmRA) and show this operon induces a protective response that repurposes heme to counteract antimicrobial oxidative stress responses. HsmR senses vertebrate heme, leading to increased expression of the hsmRA operon and subsequent deployment of HsmA to capture heme and reduce redox damage caused by inflammatory mediators of protection and antibiotic therapy. Strains with inactivated hsmR or hsmA have increased sensitivity to redox active compounds and reduced colonization persistence in a murine model of relapse C. difficile infection. These results define a mechanism exploited by C. difficile to repurpose toxic heme within the inflamed gut as a shield against antimicrobial compounds.
Keywords: Clostridioides difficile, heme utilization, antibiotic sensitivity, relapse infection, nutritional immunity, host-pathogen interactions
Graphical Abstract
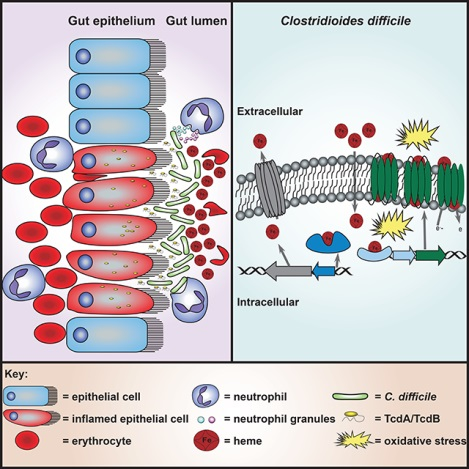
eTOC
Clostridioides difficile encounters high concentrations of heme at the host-pathogen interface. Knippel et al. discover that the HsmRA system sequesters heme and utilizes the heme bound by the membrane protein HsmA to protect against oxidative stress. The HsmRA system decreases sensitivity to vancomycin in the presence of heme during infection.
Introduction
Clostridioides difficile (formerly Clostridium difficile) is a Gram-positive, spore forming obligate anaerobe that is an urgent threat to public health as it is the leading cause of nosocomial diarrhea in the United States (Lessa et al., 2015). Following perturbation of the gut microbiome, commonly due to antibiotic therapy, C. difficile infects the colon, causing severe damage to the gastrointestinal epithelium as a result of the production of the toxins TcdA and TcdB (Carter et al., 2015). The resulting inflammation presents a hostile environment to microbial colonizers (Péchiné and Collignon, 2016). Furthermore, the toxins stimulate the production of proinflammatory cytokines and chemokines by resident immune cells and intoxicated epithelial cells, initiating the recruitment of circulating innate and adaptive immune cells (Abt et al., 2016). Recruited neutrophils, a key characteristic of the clinical pathophysiology of C. difficile infection (CDI) (Kelly and Kyne, 2011), release highly concentrated reactive oxygen species (ROS), reactive nitrogen species (RNS), and antimicrobial peptides (Ng et al., 2019) at the site of infection to restrict the proliferation of C. difficile (Péchiné and Collignon, 2016). Despite this, C. difficile thrives in the inflamed colon, causing disease that can progress to pseudomembranous colitis or, in serious cases, death (Rupnik et al., 2009). The initial treatment for CDI consists of an antibiotic regimen including vancomycin, metronidazole, or fidaxomicin (Guery et al., 2019). For reasons that remain unknown, 1 in 5 patients will suffer from recurrence requiring further antibiotic treatment (Guery et al., 2019).
The mechanisms C. difficile utilizes to survive in the inflamed colon are largely unknown. In a previous study, we detected a high concentration of hemoglobin from lysed erythrocytes in the gastrointestinal lumen during CDI (Knippel et al., 2018). Heme, the iron-containing protoporphyrin cofactor of hemoglobin, functions as a redox active molecule for a number of enzymes, including those involved in respiration and protection against oxidative stress. However, its reactivity causes heme to be toxic, including to C. difficile. To cope with heme toxicity, C. difficile encodes the HatRT system that senses excess heme and detoxifies the molecule through efflux (Knippel et al., 2018). HatRT is required for full pathogenicity in a murine model of CDI, underscoring the importance of this process during infection. However, strains inactivated for hatRT are not defective in colonization or persistence, suggesting C. difficile contains additional mechanisms to survive excess heme encountered during inflammation.
In this work, we discovered a C. difficile system that senses heme, and utilizes this molecule to provide resistance to oxidative stress. A heme-inducible operon was identified that contains a MarR family transcriptional regulator and a putative membrane protein. We have named these gene products HsmRA for heme sensing membrane protein (R = regulator, A = membrane protein). The total transcriptional response of C. difficile to a brief exposure of heme is restricted to the hsmRA and hatRT operons, and this effect is mediated exclusively by HsmR and HatR. HsmA reduces heme toxicity through sequestration, and heme-bound HsmA provides increased resistance to compounds that generate oxidative stress, including vancomycin and metronidazole. Lack of HsmA results in reduced colonization persistence in a murine model of relapse infection. Taken together, these results describe a mechanism by which C. difficile senses and utilizes heme liberated within the inflamed gastrointestinal tract to provide a protective defense against immune effectors and antibiotic therapy, enabling this organism to thrive at the host-pathogen interface during infection. The conservation of HsmA orthologues across diverse bacterial species suggests that this may be a broadly relevant microbial strategy to survival environmental stress.
Results
Transcription of the hsmRA operon occurs in response to heme.
Analysis of RNA-sequencing of transcripts from C. difficile R20291 exposed to a sublethal concentration of heme revealed two uncharacterized genes as being highly responsive to heme exposure (Knippel et al., 2018). More specifically, two genes separated by two nucleotides encoding for a MarR family transcriptional regulator (CDR20291_0782) and a putative membrane protein (CDR20291_0781) with predicted structural homology based on a PHYRE2 model to a human duodenal cytochrome b (65% confidence, 89% identity, PBD: 5ZLG) exhibited the most significant changes in transcript abundance in this data set (Figure 1A) (Ganasen et al., 2018; Kelley et al., 2015). To confirm CDR20291_0782 and CDR20291_0781 are heme responsive, quantitative reverse transcription PCR (qRT-PCR) was performed utilizing RNA harvested at the early exponential phase of growth (OD600 = 0.3) from cultures grown in equimolar concentrations of sodium hydroxide (NaOH, vehicle), protoporphyrin IX (PPIX, porphyrin ring without iron), iron (II) sulfate, or heme. Transcription of each gene was minimally altered in the samples treated with NaOH or iron (II) sulfate, in contrast to a 1-log increase in transcript abundance following protoporphyrin IX exposure, and 2 – 2.5-log increase in transcript abundance following heme exposure (Figure 1B). To examine the responsiveness of CDR20291_0781, CDR20291_0782, and the hatRT operon to heme, qRT-PCR was performed on RNA harvested from cultures grown to an early exponential phase of growth (OD600 = 0.3) and exposed to a range of low concentrations of heme (0.25 – 1 μM) for 5 min. There was no significant difference in the transcription of these four genes at 0.25 and 0.5 μM heme (Figure 1C). At 0.75 and 1 μM heme, transcription of CDR20291_0782 and CDR20291_0781 was 1 – 2-log higher than an untreated control. This transcriptional response was more intense than that of the previous characterized heme efflux system hatRT (Knippel et al., 2018), as this operon displayed minimal transcriptional change at 0.75 μM and a 1-log transcriptional increase at 1 μM heme compared to an untreated control (Figure 1C). Based on these data, as well as data described below, we have named CDR20291_0782 heme sensing membrane protein regulator (hsmR) and CDR20291_0781 heme sensing membrane protein (hsmA).
Figure 1. hsmRA transcriptionally responds to and detoxifies heme.

(A) Schematic of the hsmRA operon. (B) hsmR and hsmA transcription determined by qRT-PCR. cDNA was reverse transcribed from RNA harvested from C. difficile R20291 grown in the presence of sodium hydroxide (NaOH, 500 μM), protoporphyrin IX (PPIX, 50 μM), iron sulfate (50 μM) or heme (50 μM). (C) hsmR, hsmA, hatR, and hatT transcription determined by qRT-PCR of cDNA reverse transcribed from RNA harvested from C. difficile grown in the presence of a low concentration range of heme (0.25 – 1 μM). Transcription is graphed as the fold change relative to an untreated control. The data are a representative of three independent experiments with standard deviation shown. Statistical significance was determined using the multiple comparison two-way ANOVA test with the Sidak correction for multiple comparisons * denotes p < 0.05. (D) Growth of WT, hsmR::CT, and hsmA::CT strains in the presence or absence of heme (25 μM). The data are a representative from three independent experiments each in biological triplicate with standard error of the mean. See also Figure S1 and Tables S1 and S2.
HsmR and HsmA reduce heme toxicity.
To investigate the contribution of the hsmRA operon to heme detoxification, two strains of C. difficile were generated that are inactivated for hsmR (hsmR::CT) or hsmA (hsmA::CT) using the ClosTron system (Francis et al., 2013). The lack of either hsmR or hsmA renders the bacteria sensitive to heme toxicity, as growth over time in the presence of 25 μM heme is delayed in the mutant strains compared to wild-type (WT, Figure 1D). The growth of hsmR::CT and hsmA::CT returned to WT levels by expressing the relevant gene in trans under the control of the native promoter of the operon (Figure S1A). Notably, the growth delay of the hsmR::CT and hsmA::CT strains in 25 μM heme coincided with a compensatory induction of hatR expression during both early- and mid-exponential phase growth (Figure S1B). Taken together, these data suggest that HsmR and HsmA coordinate to function as an acutely sensitive mechanism of heme sensing and detoxification in C. difficile.
HsmR and HsmA coordinate to reduce heme toxicity through sequestration.
Orthologs of hsmR were identified using the SEED database (Overbeek et al., 2005) in other clostridial species that are pathogenic to vertebrates and therefore may experience heme stress during infection (Figure 2A). A scan of the HsmR sequence against the Pfam profile hidden Markov model (HMM) library reveals that HsmR is a member of the MarR family of winged helix-turn-helix DNA-binding proteins (Perera and Grove, 2010). A multiple sequence alignment of selected MarR family proteins with known structure and at least 20% sequence identity to HsmR shows a relatively well-conserved DNA-binding helix α4, consistent with the other members of this family (Figure S2A). A structural model of homodimeric HsmR (Figure S2B) derived from this alignment (Finn et al., 2011) reveals that HsmR, like other MarR family members, is a pyramid-shaped homodimer with each protomer consisting of six α-helices with a pair of β-strands that form a “β-wing” between the α4 and α5 helices (Capdevila et al., 2018; Guerra et al., 2011). Helices α1 and α6 form the dimerization interface, while helices α2, α3, and α4 form a DNA binding domain on each protomer, with α4 poised to read the base pairs of successive major grooves in the palindromic DNA operator.
Figure 2. HsmR binds and senses heme and HsmA reduces heme toxicity through sequestration.
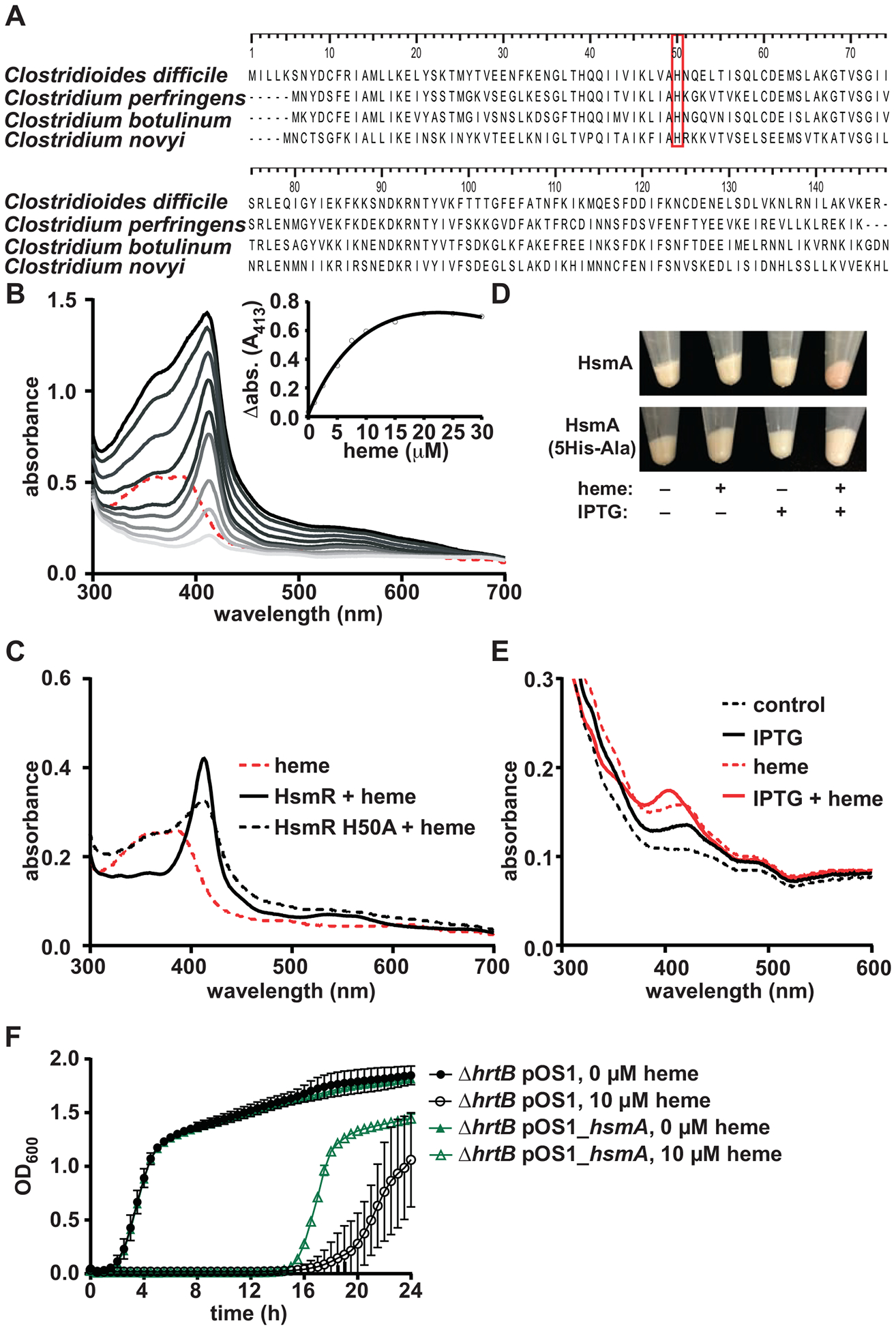
(A) Alignment of HsmR homologues in other pathogenic clostridial species. Red box denotes conserved histidine residue. (B) Absorption spectra of heme binding to recombinant HsmR. Increasing concentrations of heme (1 to 30 μM) were added to 10 μM protein. The spectrum corresponding to 10 μM heme is shown as a dashed red line. HsmR with increasing concentrations of heme are shown as gray lines. The inset displays change in absorbance at 413 nm for HsmR bound to heme minus the corresponding heme alone peak. (C) Absorption spectra of 10 μM heme binding to HsmR or HsmR H50A. (D) E. coli pET15b_hsmA or pET15b_hsmA_5His-Ala cell pellets in the presence or absence of heme (10 μM) and IPTG (1 mM). (E) Absorption spectra of solubilized membrane fractions of the cell pellets from D. (F) Growth of S. aureus ΔhrtB pOS1 and pOS1_hsmA strains in the presence or absence of heme (10 μM). The data are a representative from three independent experiments with standard error of the mean. See also Figures S2, S3, and S4 and Tables S1 and S2.
Considering the responsiveness of hsmRA to both heme and PPIX, and the putative assignment of HsmR as a transcriptional regulator, we examined the ability of HsmR to bind heme. Recombinant HsmR (10 μM) was incubated with heme (1–30 μM), resulting in the appearance of a Soret peak at 413 nm (Figure 2B), indicative of HsmR-heme complex formation (Stryer, 1961). Differential absorption spectroscopy at 413 nm over a range of heme concentrations was used to determine that HsmR binds heme at a 1:1 ratio using a single site binding model (kd = 6.6 ± 1.1 μM; Figure 2B insert). The conserved histidine residue at position 50 was identified as a potential axial ligand to bind heme (Figure 2A red box). Purified recombinant HsmR containing the substitution of His50 to Ala (H50A) exhibited a reduced ability to bind heme (Figures 2C and S3A). Alanine substitutions of other conserved residues with the potential to bind heme (K33A, K46A, and Y86A) did not significantly alter heme binding (Figure S4B–D). It is interesting to note that His50 is positioned at the C-terminus of the α2 helix in our structural model of HmsR, near the middle of the α5 helix (Figure S2B). This would place the coordinated heme in a cleft between the dimer interface and the DNA-binding domain that is a common effector binding site in the MarR family (Perera and Grove, 2010).
Based on the homology between HsmA and heme-containing cytochromes, we investigated the ability of HsmA to bind heme. Attempts to purify HsmA were unsuccessful; therefore, we developed a whole cell assay to measure HsmA heme binding. HsmA expression was induced by IPTG in an expression strain of E. coli (E. coli pET15b_hsmA), and this strain was treated with excess heme. The presence of excess heme in HsmA-expressing strains resulted in a red cell pellet, indicative of bound heme, and this color change is not observed in E. coli lacking the HsmA expression vector upon heme exposure, or in the un-induced E. coli pET15b_hsmA strain (Figure 2D). Moreover, this color change was not observed in cells expressing a mutant hsmA in which all five putative heme-binding His residues were changed to Ala (Figure 2D). Absorption spectroscopy of the solubilized membranes from these cells resulted in a unique peak at ~405 nm that was only present in the E. coli pET15b_hsmA strain treated with both heme and IPTG (Figure 2E). These data suggest that HsmR and HsmA bind heme.
The ability of HsmA to bind heme could reduce toxic free heme concentrations through sequestration, as has been observed in other heme detoxification systems (Choby and Skaar, 2016). To test this, a Staphylococcus aureus strain lacking the hrtB (ΔhrtB) heme efflux pump (Torres et al., 2007) was transformed with a plasmid containing a constitutively expressed hsmA (ΔhrtB pOS1_hsmA). S. aureus ΔhrtB was utilized for these experiments due to this strain’s high sensitivity to heme (Wakeman et al., 2012). The S. aureus ΔhrtB pOS1_hsmA displayed increased resistance to heme toxicity when compared to the S. aureus ΔhrtB strain harboring empty vector (Figure 2F). Together these data suggest that HsmR and HsmA coordinate to reduce heme toxicity through direct binding to HsmA.
HsmR is a transcriptional activator of the hsmRA operon.
Members of the MarR family of transcriptional regulators can function as repressors or activators of genes (Grove, 2013). We investigated the regulation of the hsmRA operon by HsmR utilizing qRT-PCR of WT and hsmR::CT grown to early exponential phase (OD600 = 0.3) and exposed to 50 μM heme for 30 min. Heme-treated WT exhibit a 1–1.5-log activation of hsmRA, and this is entirely dependent on a functional HsmR, as hsmRA transcription was unaffected by heme in hsmR::CT when compared to an untreated WT control (Figure 3A). Moreover, we find that HsmR directly binds to a region encompassing the 5’ UTR of hsmR and extending twelve basepairs into the coding region of HsmR, as determined by electrophoretic mobility shift assay (EMSA). However, DNA binding by HsmR was not observed for the 5’ UTR and coding region of an unrelated gene, CDR20291_0783, suggesting HsmR DNA binding is specific for the hsmRA promoter (Figure 3B). These data suggest that HsmR acts as an activator of the hsmRA operon.
Figure 3. HsmR is an activator of the hsmRA operon.

(A) hsmR and hsmA transcription determined by qRT-PCR. cDNA was reverse transcribed from RNA harvested WT or hsmR::CT grown to early exponential phase (0.3 abs) and exposed to heme (50 μM) for 30 min. Data are represented as fold change relative to untreated WT. (B) Electrophoretic mobility shift assay (EMSA) demonstrates direct and specific DNA binding by HsmR to the 5’ UTR of hsmR, but not the 5’ UTR of the unrelated gene CDR20291_0783 (see Figure 1A). (C) RNA-sequencing analysis comparing RNA from heme treated (25 μM for 30 min) WT to an untreated WT control. (D) RNA-sequencing analysis comparing RNA from heme treated (25 μM for 30 min) hsmR::CT to an untreated hsmR::CT control. Dashed lines represent genes of fold change > 2. Samples with p-value > 1 × 10−5 are represented as 5 on the graph. Solid black line denotes p < 0.05. Statistical significance was determined using the multiple comparison two-way ANOVA test with the Sidak correction for multiple comparisons comparing the means of each group to one another. * denotes p-value < 0.05, n.s. denotes not significant. See also Figure S1 and Tables S1, S3, S4 and S5.
To identify additional genes regulated by HsmR, we performed an RNA-sequencing experiment comparing the total relative mRNA transcript abundance of WT and hsmR::CT grown to early exponential phase (OD600 = 0.3) and exposed to 50 μM heme for 30 min. In WT C. difficile, hsmR, hsmA, hatR, and hatT were the only four genes that displayed a significant induction above log2 of 2 following exposure to heme (Figure 3C, Table S3). The comparison between heme-exposed hsmR::CT and the untreated hsmR::CT control replicated the observation that HsmR acts as an activator of the hsmRA operon. Upregulation of hsmR and hsmA transcripts were not observed in the hsmR::CT strain in response to heme (Figure 3D, Table S3), whereas hatR and hatT retained significant transcriptional induction in this strain. Additional comparisons between untreated and heme treated WT and hsmR::CT samples further revealed the absence of significant heme induced transcriptional changes outside of the hatRT and hsmRA operons (Figure S1C–D, Tables S4 and S5). Taken together, these data demonstrate that the heme responsive regulon of HsmR is solely comprised of hsmRA. Moreover, these studies show that the transcriptional response of C. difficile to heme is largely limited to the hsmRA and hatRT operons.
HsmA employs exogenous heme to confer resistance to oxidative stress and antibiotics.
HsmA contains homology to the cytochrome b561 family (Bérczi and Zimányi, 2014), but C. difficile does not respire. Therefore, we investigated other functions of cytochromes to determine the functional role of this protein. One function of cytochromes is to detoxify redox molecules by shuttling electrons through bound heme cofactors (Asard et al., 2013). We investigated whether HsmA may function to diminish damage caused by redox active molecules (Wang et al., 2018). To test this, WT, hsmR::CT, and hsmA::CT cultures were treated with the ROS-generating molecule paraquat in the presence or absence of heme (Wu et al., 2012). No toxicity was observed when C. difficile was treated with paraquat alone (2 mM; Figure S4A), whereas a delay in growth occurred in heme and paraquat treated hsmR::CT and hsmA::CT, but not WT (Figure S4A).
Considering C. difficile is an obligate anaerobe, we sought to investigate the contribution of HsmA to survival in the presence of atmospheric oxygen. WT and hsmA::CT were grown for 6 hours prior to treatment with heme (25 μM) for 30 min. Samples were exposed to atmospheric oxygen, and the heme treated WT samples displayed a 2.5-fold decrease in cellular ROS compared to the untreated WT strain (Figure 4A). By contrast, heme-treated hsmA::CT displayed a 2.5 fold increase in cellular ROS compared to the untreated hsmA::CT strain (Figure 4A). There were no statistically significant differences in cellular ROS between the untreated WT and hsmA::CT strains (Figure 4A).
Figure 4. HsmA reduces oxidative stress.

(A) C. difficile (CD) WT and hsmA::CT strains were grown for 6 hours followed by treatment with or without heme (25 μM) for 30 min. Samples were exposed to atmospheric oxygen and oxidative stress generation was determined by measuring fluorescence of dihydrorhodamine 123 (DHR123; ex. 507 nm, em. 529). (B) Growth of S. aureus (SA) ΔΔsod pOS1 and pOS1_hsmA strains in the presence or absence of paraquat (2 mM) and dihydrorhodamine 123. (C) Oxidative stress generation was quantified by measuring fluorescence of DHR123 from SA ΔΔsod pOS1 (empty vector), SA ΔΔsod pOS1_hsmA, or SA ΔΔsod pOS1_hsmA_5His-Ala (point mutant defective for heme binding) in the presence or absence of paraquat. The data are a representative from three independent experiments with standard error of the mean. (D) S. aureus ΔΔsod strains carrying the empty pOS1 vector or pOS1_hsmA were co-incubated with neutrophils derived from murine bone marrow (MOI of 1) for 12 hours. S. aureus CFUs were enumerated and compared to untreated controls. Statistical significance was determined using the multiple comparison two-way ANOVA test with the Sidak correction for multiple comparisons comparing the means of each group to one another. * denotes p-value < 0.05. See also Figure S4 and Table S2.
To decouple the ability of HsmA to decrease oxidative stress from other oxidative stress reducing proteins in C. difficile, a S. aureus strain lacking both genes encoding for superoxide dismutase enzymes (ΔΔsod) (Karavolos et al., 2003) was transformed with a plasmid containing a constitutively expressed hsmA (ΔΔsod pOS1_hsmA) or with an empty vector control plasmid (ΔΔsod pOS1). S. aureus ΔΔsod is acutely sensitive to superoxide stress and this organism synthesizes heme, which is bound by HsmA, thereby removing the requirement for heme supplementation. The ΔΔsod pOS1_hsmA strain displayed a recovery in aerobic growth over time in 2 mM paraquat when compared to the ΔΔsod pOS1 strain (Figure 4B). Notably, paraquat toxicity was not observed under anaerobic conditions (Figure S4B). Measuring the cellular ROS generated over 6 h displayed a decrease in ΔΔsod pOS1_hsmA strain compared to the ΔΔsod pOS1 strain (Figure 4C). However, protection conferred by HsmA against ROS was lost in the ΔΔsod pOS1_hsmA_5His-Ala strain (Figure 4C), which is defective for heme binding (Figure 2D). To determine if the protective effect is specific to superoxide, a S. aureus strain lacking catalase (ΔkatA) (Painter et al., 2015) that cannot detoxify hydrogen peroxide was transformed with a plasmid containing a constitutively expressed hsmA (ΔkatA pOS1_hsmA) or empty vector control (ΔkatA pOS1). The expression of HsmA did not confer resistance to hydrogen peroxide (Figure S4C). As neutrophils are a potent producer of superoxide during infection, we sought to test whether HsmA promotes survival of S. aureus ΔΔsod after phagocytosis by neutrophils. Indeed, ΔΔsod pOS1_hsmA exhibited complete protection against neutrophil killing, whereas ΔΔsod pOS1 exhibited only ~25% survival (Figure 4D). Taken together, these data suggest that in addition to detoxifying heme, HsmA provides protection against atmospheric, chemical, and immunogenic superoxide stress.
HsmR and HsmA promote resistance to vancomycin during CDI.
The ability of HsmA to reduce oxidative stress could protect against antibiotic toxicity, as it has previously been suggested that bactericidal antibiotics generate considerable oxidative stress resulting from hyper-induced metabolism (Van Acker and Coenye, 2017). To investigate the protective effects of the hsmRA operon against antibiotics, we determined the sensitivities of WT, hsmR::CT, and hsmA::CT to vancomycin as measured by growth over time. All three strains were equally sensitive to vancomycin treatment alone (Figures 5A and 5B). When heme was added in combination with vancomycin, the WT strain displayed a significant growth recovery, while the hsmR::CT strain exhibited a significant delay in growth and the hsmA::CT strain did not grow (Figures 5A and 5B). The difference in sensitivity suggests there may be basal transcription of hsmA present in the hsmR::CT mutant. This growth recovery is specific to heme as PPIX did not rescue growth in the WT strain (Figure S4D). Heme treatment additionally rescued growth of the WT strain but not hsmR::CT and hsmA::CT in the presence of metronidazole (Figure S4E–F). These results demonstrate that HsmA reduces toxicity against redox damage produced directly (Edwards, 1993) or indirectly (Van Acker and Coenye, 2017) by clinically relevant glycopeptide and nitroimidazole antibiotics.
Figure 5. The hsmRA operon decreases sensitivity to vancomycin in the presence of heme during infection.
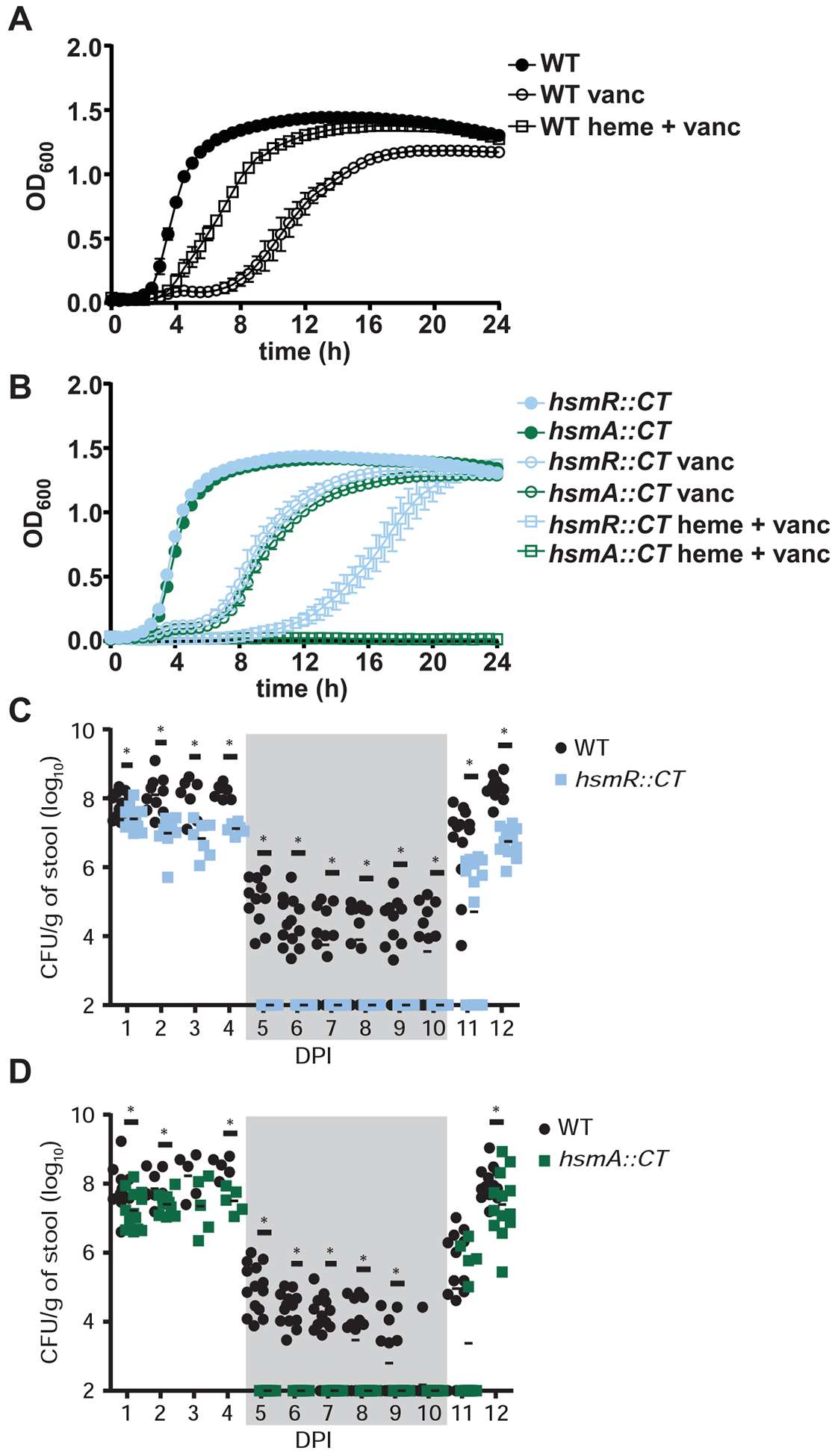
(A, B) Growth of WT, hsmR::CT and hsmA::CT in the presence or absence of vancomycin (100 μg/mL) and heme (10 μM). The data are a representative from three independent experiments with standard error of the mean. (C, D) CFU analysis of mice coinfected with WT and hsmR::CT or WT and hsmA::CT strains for 12 days post infection (DPI) with standard error of the mean (n=14/group). Vancomycin treatment (0.4 mg/mL) was administered on days 5–10 (denoted by gray shading) and removed on day 11. The data are presented with standard error of the mean. Statistical significance was determined using the multiple comparison two-way ANOVA test with the Bonferroni correction for multiple comparisons comparing the means of each group to one another. * denotes p-value < 0.05. See also Figure S4 and S5.
C. difficile infection leads to a significant accumulation of heme in the lumen of the gut (Knippel et al., 2018). To investigate the involvement of the hsmRA operon in persistence during infection, mice were infected with a 1:1 ratio of WT and hsmR::CT or WT and hsmA::CT spores. Disease was monitored for 4 days, followed by a 5-day treatment of vancomycin (0.2 mg/mL) to clear the infection, after which mice were monitored for relapse. During the acute phase of infection, hsmR::CT and hsmA::CT strains displayed reduced colonization relative to WT, as exhibited by and the ~106 −107 colony-forming units (CFU) of the hsmR::CT and hsmA::CT strains recovered per gram of stool, compared to the ~108 CFU of the WT strain recovered per gram of stool (Figures 5C and 5D). During vancomycin treatment, hsmR::CT and hsmA::CT colonization levels were below the limit of detection for the length of the antibiotic treatment in contrast to the transient reduction (~106 to ~102) in WT CFUs per gram of stool (Figures 5C and 5D). Upon removal of vancomycin, hsmR::CT and hsmA::CT exhibited a 2–3-log defect in recovery compared to the WT in the first day of relapse. On the final day of infection, the mutant strains displayed a 0.5 – 2-log reduction of CFUs per gram of cecal contents compared to WT (Figures 5C and 5D). These results demonstrate the hsmRA operon provides protection against vancomycin treatment. In support of our data showing that HsmR and HsmA function to limit heme toxicity, we detected heme in murine stool samples during the course of CDI (Figure S5A), and a re-analysis of a publicly available RNA-sequencing dataset from Fletcher et al. reveals active transcription of the hsmRA operon during CDI (Figure S5B). Despite their role in heme detoxification, hsmR and hsmA are dispensable for full virulence during CDI induced by monoinfections with WT, hsmR::CT, or hsmA::CT (Figure S5C). Taken together, these data establish HsmR as a sensor of host heme that induces the production of HsmA which binds heme and prevents toxicity associated with heme accumulation, while also providing protection against front-line antibiotics and oxidative stress. Combined, these activities contribute to the ability of C. difficile to persist during infection despite the oxidative burst of phagocytes or antimicrobial treatment.
To predict the generalizability of HsmA-mediated protection against oxidative stress across the Bacterial kingdom, we investigated the conservation of HsmA across species. A phylogenetic tree was created using HsmA from C. difficile as a seed. This analysis revealed that HsmA is widespread in Clostridia and candidate HsmA orthologues can be found in multiple genera including Bacteroides, Bacillus, Lactobacillus, Enterococcus, and Geobacter (Figure S6A, Table S6), suggesting that HsmA may represent a conserved strategy for dealing with environmental oxidants across numerous organisms.
Discussion
C. difficile thrives in the colon during infection despite creating a hostile inflammatory environment through toxin-mediated damage of the gastrointestinal epithelium (Carter et al., 2015; Chumbler et al., 2012; Kuehne et al., 2014). Robust inflammation leads to high levels of heme at the host-pathogen interface during CDI (Figure 6A) (Knippel et al., 2018). Herein, we identified an acutely heme responsive transcription factor HsmR which activates expression of the membrane protein HsmA that incorporates the reactivity of heme to defend against redox stress while simultaneously detoxifying excess heme through sequestration.
Figure 6. C. difficile utilization of host heme for protection against oxidative stress.

(A) Graphical representation of the C. difficile host pathogen interface during infection. Toxin mediated inflammation induces translocation and lysis of erythrocyte in the gastrointestinal lumen resulting in high concentrations of heme. (B) Host heme is sensed by HsmR and incorporated into HsmA, providing protection against oxidative stress produced by host immune cells and environment. Concurrently, HatR binds heme derepressing the hatRT operon and leading to subsequent efflux of heme by HatT.
In our previous study we identified the HatRT system in C. difficile that senses and detoxifies excess intracellular heme through efflux (Knippel et al., 2018). The results herein further refine our current model of heme homeostasis in C. difficile. HsmR senses low concentrations of heme and activates expression of the hsmRA operon which leads to the integration of heme into HsmA. Heme-bound HsmA within the membrane shields the bacterium against redox active molecules. Concurrently, HatR binds heme, derepressing the hatRT operon, leading to subsequent efflux of free heme through HatT, and resulting in a relief from heme toxicity. Together these systems function to maintain a tolerable concentration of intracellular heme for C. difficile to protect itself against the stressors encountered within the host during CDI (Figure 6B).
The inflamed gut contains a multitude of environmental (Rivera-Chávez et al., 2017), microbiota (Kang et al., 2019), and host mediated stressors (Péchiné and Collignon, 2016). In addition to heme toxicity at the host pathogen interface, C. difficile as an obligate anaerobe encounters oxidative stress in various forms ranging from oxygenation of the colonic epithelium due to inflammation (Hill et al., 2017) and ROS produced by host immune cells (Figure 6A) (Péchiné and Collignon, 2016). The HsmA-dependent decrease of oxidative stress may be the result of a serendipitous evolutionary event, as heme toxicity and oxidative stress coincide temporally. The mechanism by which HsmA protects against oxidative stress remains unknown. HsmA may function to enzymatically convert a radical species into a harmless form or it may shuttle electrons through the bound heme to act as an electron sink, consistent with its homology to cytochrome b561. The ability of heme-bound HsmA to protect against different classes of antibiotics may be due to the elicitation of oxidative stress by bactericidal antibiotics (Lopatkin et al., 2019; Van Acker and Coenye, 2017). While C. difficile does not aerobically respire, the altered metabolism induced by antibiotics, as observed in other bacterial species (Pericone et al., 2003), might generate oxidants that eventually lead to death. This accumulation of oxidants is countered by heme-HsmA complexes, which reduce their concentrations below lethal limits. In total, coating of the membrane with heme-HsmA complexes provides a shield against redox damage produced in the inflamed gastrointestinal tract during infection.
A bioinformatic analysis of the C. difficile genome reveals an incomplete heme biosynthesis pathway, as there are no identified ferrochelatase or δ-amino-levulinic acid synthesis genes despite the presence of genes required for siroheme (Dailey et al., 2017) and cobalamin synthesis (Moore and Warren, 2012). These data indicate that C. difficile acquires heme exogenously during infection, presumably from the host due to toxin-mediated damage of the gastrointestinal epithelial layer. Additionally, as C. difficile cannot use heme as a sole iron source (Cernat and Scott, 2012), the limited transcriptional response of C. difficile to heme exposure and lack of identified heme cofactor proteins suggests HsmA is the primary protein to utilize intracellular heme in this organism. However, the mechanism by which heme enters C. difficile remains unknown. The phenomenon of host-heme utilization by bacteria that cannot synthesize endogenous heme has been observed (Yamamoto et al., 2005). In Enterococcus faecalis, exogenous heme induces the production of a heme containing catalase (Frankenberg et al., 2002). Numerous lactic acid bacteria, such as Lactococcus lactis, acquire exogenous heme to establish aerobic respiratory chains (Lechardeur et al., 2011). In this study, we demonstrate C. difficile uses heme from the host as a cofactor for HsmA, which provides resistance against antimicrobial stressors.
Heme sequestration to reduce heme toxicity is a conserved strategy in multiple pathogenic organisms (Choby and Skaar, 2016). Most sequestration proteins have been described in Gram-negative pathogens and consist of intracellular heme binding proteins such as the HemS family in Yersinia enterocolitica (Stojiljkovic and Hantke, 1994), Shigella dysenteriae (Wyckoff et al., 2005), Pseudomonas aeruginosa (Marvig et al., 2014), and E. coli (Suits et al., 2005). These proteins often have additional functions dependent upon the organism such as storage, trafficking, or degradation but all contribute to heme detoxification (Choby and Skaar, 2016). In our current model, HsmA utilizes intracellular heme obtained from the host in a manner similar to populating a cytochrome or other heme-binding membrane proteins with heme rather than an extracellular heme import protein. HsmA is widespread in the Clostridial species, but it is not limited to this genus nor to anaerobes as orthologs are present in Bacteroidetes and Bacilli as well as the Proteobacteria (Figure S6A). In several Bacillus cereus species, a physical clustering exists between genes encoding an ortholog of HsmA and the heme efflux pump HrtAB (Torres et al., 2007) suggesting evolutionary pressure to genetically cluster heme detoxification systems in certain species (Figure S6B). The heme detoxification proteins encoded by hsmRA represent a unique mechanism of sequestration that appears to be widespread among bacteria that interact with vertebrate blood or environmental heme.
Together, these results demonstrate that C. difficile HsmRA capitalizes on the toxin-induced inflammation of the gastrointestinal tract to utilize heme from the host to protect against antibiotic therapy and immune cell mediated oxidative stress produced at the host-pathogen interface (Figure 4D and Figures 6AB). In our previous study, a strain lacking hatT::CT displayed reduced pathogenicity in a toxin-independent manner. However, there was no reduction in disease in strains lacking hsmR or hsmA (Figure S5C) suggesting these strains may adapt to heme toxicity during infection by inducing hatRT expression (Figure S1B). The primary treatment for CDI in patients is a vancomycin regimen wherein 20% of patients have recurrent infection resulting in additional antibiotic treatment or eventually a fecal microbiota transplant (Cammarota et al., 2014). In a murine model of relapsing CDI, the hsmR::CT and hsmA::CT mutant strains were significantly more sensitive to vancomycin treatment (Figure 5CD). These data suggest the development of a drug targeting HsmR or HsmA as a therapy for CDI could be used in combination with these antibiotics. Further studies will elucidate the biochemical mechanisms of HsmA-induced protection against oxidative stress in C. difficile and other organisms.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Eric Skaar (eric.skaar@vumc.org).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
RNA-sequencing data
Raw RNA-sequence data are deposited on the NCBI Sequence Read Archive (accession code: PRJNA576216). For analyses of the published RNA-sequencing dataset from (Fletcher et al., 2018), raw data from their publicly available RNA-sequencing experiment characterizing the C. difficile transcriptome during colonization of a murine model of infection were retrieved from NCBI Sequence Reads Archive (submission number SRP134023).
HsmR multiple sequence alignment
HsmR multiple sequence alignment was performed using Clustal Omega (http://www.clustal.org/omega [accessed August 2019]) using Clostridioides difficile R20291 (NCBI accession no. CBE02826.1), Clostridium perfringens ATCC 13124 (NCBI accession no. ABG82197.1), Clostridium botulinum A str. Hall (NCBI accession no. YP_001388019.1) and Clostridium novyi (NCBI accession no. WP_011722139.1).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains
Bacterial strains used in this study are listed in the Key Resource Table. C. difficile strains were grown at 37 °C in an anaerobic chamber (85% nitrogen, 10% hydrogen, 5% carbon dioxide, Coy Lab Products) in brain-heart-infusion broth (BD Life Sciences) supplemented with 0.5% yeast extract (BD Life Sciences) and 0.1% cysteine (Sigma-Aldrich) (BHIS) or in C. difficile minimal media (CDMM) as described previously (Cartman and Minton, 2010). Escherichia coli strains were grown in lysogeny broth (LB) or agar (LBA), supplemented with 50 μg/mL kanamycin or 50 μg/mL carbenicillin when necessary. Bacillus subtilis strains were grown on LBA or in BHI broth supplemented with 5 μg/mL tetracycline and/or 2.5 μg/mL chloramphenicol. Staphylococcus aureus strains were grown on tryptic soy agar (TSA) or in broth (TSB) supplemented with 10 μg/mL chloramphenicol when needed. All antibiotics were purchased from Sigma-Aldrich.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Clostridioides difficile R20291 | Stabler et al., 2009 | |
| Clostridioides difficile hsmR::CT | This study | |
| Clostridioides difficile hsmA::CT | This study | |
| Bacillus subtilis JH BS2 | Francis et al., 2013 | |
| Escherichia coli DH5α | Hanahan, 1983 | |
| Escherichia coli MG1655 | Blattner et al., 1997 | |
| Escherichia coli BL21(DE3) | Jeong et al., 2009 | |
| Staphylococcus aureus RN4220 | Kreiswirth et al., 1983 | |
| Staphylococcus aureus Newman | Duthie and Lorenz, 1952 | |
| Staphylococcus aureus ΔhrtB | Attia et al., 2010 | |
| Staphylococcus aureus ΔΔsod | Kehl-Fie et al., 2011 | |
| Staphylococcus aureus ΔkatA | Choby et al., 2018 | |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Hemin from porcine | Sigma-Aldirch | Cat#51280–5G |
| Protoporphyrin IX | Sigma-Aldrich | Cat#P8293–1G |
| Paraquat dichloride hydrate | Sigma-Aldrich | Cat#36541–100MG |
| Vancomycin | Fisher Scientific | Cat#BP2958 |
| Metronidazole | Fisher Scientific | Cat#AC210340050 |
| Iron (II) sulfate | Sigma-Aldrich | Cat#215422–250 |
| RQ1 DNase | Thermo Fisher Scientific | Cat#M6106 |
| Ribolock RNase inhibitor | Promega | Cat#EO0381 |
| M-MLV reverse transcriptase | Thermo Fisher Scientific | Cat#M1705 |
| iQ SYBER green supermix | Biorad | Cat#1708880 |
| HisPur cobalt Resin | Thermo Fisher Scientific | Cat#25230 |
| HsmR | This paper | |
| HsmR H50A | This paper | |
| Cefoperazone | Sigma-Aldrich | Cat#C4292 |
| SYBR-safe | Invitrogen | Cat#S33102 |
| Dihydrorhodamine 123 | Invitrogen | Cat#DS2806 |
| Critical Commercial Assays | ||
| Thrombin Cleancleave kit | Sigma-Aldrich | Cat#RECOMT-1KT |
| RNeasy Mini Kit | Qiagen | Cat#74104 |
| Ribo-Zero rRNA removal kit | Illumina | Cat#2003715 |
| TruSeq RNA library prep kit v2 | Illumina | Cat#RS-122–2001 |
| Deposited Data | ||
| Raw RNA-sequence data | This Study | PRJNA576216 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | The Jackson Laboratory | Cat #664 |
| Oligonucleotides | ||
| Primers for cloning and qRT-PCR: Table S1 | ||
| Recombinant DNA | ||
| Plasmids for complementation and expression: Table S2 | ||
| Software and Algorithms | ||
| TargeTronics algorithm | Targetrons | |
| PATRIC database | PATRIC | Version 3.5.43 |
| iTOL | iTOL | Version 4 |
| PubSEED | The SEED | Version 2.0 |
| GeneGraphics | Katlabs | |
| Prism | Graph Pad | Version 8.2 |
| CLC Genomics Workbench | Qiagen | Version 20.0.1 |
| HMMER | EMBL-EBI | Version 2.41.1 |
| MODELLER | University of California San Francisco | Version 9.24 |
| Canvas X16 | Canvas | Version 16 |
Primary Cell Culture
Polymorphonuclear leukocyte (PMN) were isolated from 10–12 week old male murine bone marrow single cell-suspensions. Using density centrifugation and Histopaque® 1119 and Histopaque® 1077, the granulocyte layer was isolated from between the two density layers. After isolation, the neutrophils were rested on ice for 1 hr in D10 media (DMEM + 10% (vol/vol) FBS) before being transferred into ultra-low cluster round bottom 96-well plates (Costar) and incubated in an anaerobic chamber (0% O2, 1.6% H2, 37 °C) for 1 hour prior to experimentation. Cells isolated through this technique have been found to be 90–95% neutrophils (CD11b+Ly6G+) via flow cytometry.
Animal Models
All animal experiments under protocol M1700053 were reviewed and approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Procedures were performed according to the institutional policies, Animal Welfare Act, NIH guidelines, and American Veterinary Medical Association guidelines on euthanasia. Adult (6–8 week old) age-matched male C57Bl/6 (Jackson Laboratories) mice were housed in groups of five and maintained at Vanderbilt University Medical Center Animal Facilities.
METHOD DETAILS
hsmR::CT and hsmA::CT strain generation
Gene inactivations were achieved using the ClosTron system, as described previously (Francis et al., 2013). Briefly, gBlocks containing specific modifications for insertion into the genome were generated using the TargeTronics algorithm (http://www.targetrons.com) and synthesized by Integrated DNA Technologies. The gBlocks were cloned into pCR-Blunt vector using the Zero Blunt PCR cloning kit (ThermoFisher Scientific) followed by restriction digest with BsrgI and HindIII (NEB) and ligation (NEB T4 ligase) into pJS107. Plasmids were transformed into the recA+ E. coli MG1655 through a standard heat shock protocol followed by transformation into B. subtilis JH2 using an established method (Francis et al., 2013). B. subtilis strains containing the pJS107_hsmR or pJS107_hsmA plasmids were mated with C. difficile R20291 overnight at 37 °C by plating and mixing together 100 μL of each strain onto a BHIS plate in the anaerobic chamber. Plates were scraped and transferred into 2 mL of BHIS prior to plating 200 μL onto BHIS plates containing 20 μg/mL thiamphenicol and 50 μg/mL kanamycin (BHISthiamp20kan50). Colonies from these plates were patched onto new BHISthiamp20kan50 and BHIS plates containing 5 μg/mL tetracycline (BHIStet5). Patched colonies that were tetracycline sensitive were patched again onto new BHISthiamp20kan50 and BHIStet5 plates. Colonies that remained tetracycline sensitive were streaked onto BHIS plates containing 20 μg/mL lincomycin (BHISlinc20). Inactivation of the hsmR or hsmA gene was confirmed by performing PCR to identify a 1.5 kbp shift in size using gDNA extracted as previously described on colonies that were lincomycin resistant (Francis et al., 2013).
Complementation plasmids
Complementation plasmids (Table S2) were created by GenScript using the pJS116 plasmid as a backbone for the synthesized intergenic (271 bp) and full coding region of hsmR, and intergenic region of hsmR fused to the full coding region of hsmA. C. difficile strains were transformed as described above with the removal of the lincomycin selection and were maintained on BHISthiamp20 to ensure plasmid retention.
Protein expression plasmids
Protein expression plasmids for HsmR and HsmA (Table S2) were generated by amplifying hsmR flanked by BamHI and NdeI or hsmA flanked by XhoI and NdeI (Table S1) and cloning into the multiple cloning site of pET15b after restriction digest. Point mutant generation in pET15b_hsmR was performed with NEB Q5 Site Directed Mutagenesis kit according to the manufacturer’s instructions, using the primers listed in Table S1. Point mutations in pET15b_hsmA_5His-Ala was generated by amplifying and inserting hsmA_5His-Ala flanked by NdeI and BamHI from a custom construct (IDT) into pET15b by Gibson assembly (NEB), using oligos listed in Table S1.
Heme, paraquat, and antibiotic toxicity C. difficile growth assays
Freshly streaked C. difficile colonies were used to inoculate 5 mL of BHIS or BHISthiamp20 and grown for 16 h at 37 °C. Cultures were subcultured 1:50 into fresh BHIS or BHISthiamp20 and grown for 6 h at 37 °C prior to 1:50 inoculation into BHIS, CDMM or CDMMthiamp20 containing heme, PPIX, paraquat, vancomycin, or metronidazole at the indicated concentrations. All growth assays were performed in a 96-well plate in 200 μL of media at 37 °C. Optical density at 600 nm (OD600) served as a measurement of growth and was measured every 30 min for the indicated total time in an EpochII microplate reader (BioTek).
qRT-PCR
C. difficile were grown anaerobically in triplicate in CDMM at 37 °C to an OD600 of 0.3 abs. Hemin (Sigma) was solubilized in 0.1 M NaOH and added to 50 μM or the indicated concentration. After 5 or 30 min, a 1:1 solution of acetone:ethanol was added to an equal volume of the culture. For activation analysis, C. difficile were grown in CDMM containing the indicated concentrations of NaOH, protoporphyrin-IX, iron (II) sulfate, or heme to an OD600 of 0.3 abs prior to addition of acetone:ethanol. Samples were stored at −80 °C until used for RNA extraction. Samples were thawed on ice, pelleted, and resuspended in 750 μL of LETS buffer (1 M LiCl, 0.5 M EDTA, 1 M Tris pH7.4). Cells were transferred to tubes containing lysing matrix B beads (MP Biomedicals) and lysed by a FastPrep-24 (MP Biomedicals) bead beater for 45 s at 6 m/s. Lysed samples were heated for 5 min at 55 °C and pelleted by centrifugation for 10 min. The supernatant was transferred to a fresh tube and 1 mL TRIzol (Thermo Scientific) was added. Chloroform (200 μL) was added to each sample and vortexed prior to separation of the aqueous and organic layers by centrifugation for 15 min. The aqueous (upper) layer was transferred to a fresh tube and the RNA was precipitated through the addition of 1 mL isopropyl alcohol. Samples were incubated for 10 min and RNA was pelleted by centrifugation for 10 min. Supernatant was removed and the RNA pellet was washed with 200 μL of 70% ethanol. Samples were air dried for 1 min, then resuspended in 100 μL RNase free water. DNA contamination was removed through the addition of 8 μL RQ1 DNase, 12 μL 10x RQ1 buffer, and 2 μL RNase inhibitor (Promega) to the purified RNA. Samples were DNase treated for 2 h and purified using the RNeasy miniprep RNA cleanup kit (Qiagen). RNA concentration was determined using the Synergy 2 with Gen 5 software (BioTek) and 2 μg was reverse transcribed by M-MLV reverse transcriptase (Fisher Scientific) in the presence of RNase inhibitor (Promega) and random hexamers (Promega). Reactions lacking the reverse transcriptase were used to control for DNA contamination. Newly created cDNA was diluted 1:100 and was used in qRT-PCR using iQ SYBR green supermix (BIO-RAD) utilizing the primer pairs in Table S1. Amplification was achieved using a 3-step melt cure program on a CFX96 qPCR cycler (BIORAD). Transcript abundance was calculated using the ΔΔCT method normalized by the rpoB gene.
HsmA taxonomic distribution and physical clustering
HsmA (CDR20291_0781) is part of the Interprofamily IPR023813 that contains 750 proteins with only ~ 100 from Clostridioides or Clostridium derivatives. To visualize the genetic spread of the family the corresponding protein sequence was used as input to search all representative and reference genomes using the internal BlastP search tool in the Patric database version 3.5.43 (Wattam et al., 2017). One hundred eighty-one proteins with alignment scores > 55 were extracted and were confirmed for membership to the IPR023813 family. Forty-two of these were found in organisms with complete genome sequences. This group of 42 bacteria was merged to the group of 120 reference genomes present in the Patric database and these 157 genomes (5 were found in the two groups) were used to build a species phylogeny using the internal Patric CodonTree tool. The output file in newick format was used as input in Itol v4 (Letunic and Bork, 2019) with added HsmA homolog presence/absence data. Physical clustering analysis was performed using the gene neighborhood tool of PubSEED (Overbeek et al., 2014) and can be visualized in the CD0851 SubSystem (http://pubseed.theseed.org//SubsysEditor.cgi?page=ShowSpreadsheet&subsystem=CD0851). The cluster figure was created using GeneGraphics (Harrison et al., 2018).
Protein expression and purification
E. coli BL21 (DE3) pREL containing the pET15b_hsmR plasmids were grown overnight in 5 mL of LBcarb50 at 37 °C. Cells were subcultured into Terrific broth (ThermoFisher Scientific) containing 50 μg/mL carbenicillin and grown to the mid-logarithmic phase of growth (0.5 abs measured at 600 nm) at 37 °C prior the addition of 1 mM isopropyl-1-thiol-D-galactopyranoside (IPTG). Growth was continued at 16 °C for 16 h. Cells were harvested by centrifugation (6000 × g for 10 min) and resuspended in 1 X PBS. Cells were lysed by passage through an EmulsiFlex homogenizer (Avestin) three times at 20,000 lb/in2. The insoluble debris was removed by centrifugation at 40,000 × g for 1 h and the supernatant was filtered using a 0.22-μM-pore sizer filter. Filtered lysate was added to HisPur cobalt resin (ThermoFisher Scientific) and allowed to bind at 4 °C for 30 min prior to transfer to a gravity column. The column was washed with four column volumes of wash buffer (100 mM HEPES, 500 mM NaCl, pH 7.8) three times followed by 2 column volumes of elution buffer (100 mM HEPES, 500 mM NaCl, 200 mM imidazole, pH 7.8) twice. The hexahistidine tag was cleaved using the Thrombin Cleancleave kit (SigmaAldrich) by following the manufacturer’s instructions. After cleavage, buffer was exchanged utilizing overnight dialysis at 4 °C in 4 L of wash buffer (100 mM HEPES, 500 mM NaCl, pH 7.8).
Solubilization of membrane fractions
E. coli BL21 (DE3) pREL containing the pET15b_hsmA plasmids were grown overnight in 5 mL of LBcarb50 at 37 °C. Cells were subcultured into 25 mLs of fresh LBcarb50 and grown to the midlogarithmic phase of growth (0.5 abs measured at 600 nm) at 37 °C prior to the addition of 1 mM IPTG and/or 10 μM heme. Growth was continued at 16 °C for 16 h. Cells were harvested by centrifugation (6000 × g for 10 min), photographed using a dual 12-megapixel camera (Apple) and resuspended in 1 X PBS. Membranes were solubilized for 1 h at 4 °C by adding octyl-β-glucoside to a final concentration of 1.5% with gentle rocking. Insoluble fraction was pelleted by centrifugation (20,000 × g for 3 min) and soluble membrane fraction was removed.
Absorption spectroscopy
Heme binding by HsmR was determined by measuring the absorption spectrum of increasing amounts of hemin (0 – 30 μM) after addition to a cuvette containing 10 μM recombinant HsmR in 1 mL of Tris-buffered saline (TBS) and a reference standard containing 1 mL TBS on a Varian Cary 50BIO. Samples were mixed and allowed to incubate at room temperature in the dark for 5 min prior to collecting the spectrum between 300 – 800 nm with 10 nm increments. Binding ratio of heme to HsmR was determined by plotting the change in absorbance at 413 nm between the reference standard and the HsmR sample. A curve fit and ratio was obtained by performing the one-site binding model non-linear regression function on Graph Pad Prism 8.2 using the following equation:
Heme binding by HsmA was performed on 1 mL of solubilized membrane fractions isolated as described above collecting the spectrum between 300 – 800 nm with 10 nm increments.
Heme and oxidative stress toxicity S. aureus growth assays
Freshly streaked S. aureus colonies were used to inoculate 5 mL of TSB or TSBcm10 in 15 mL round-bottom polypropylene tubes with aeration lids and grown for 16 h at 37 °C at a 45° angle in an Innova 44 incubator shaking at 180 rpm. Cultures were subcultured 1:50 into fresh TSB or TSBcm10 and grown for 6 h at 37 °C prior to 1:50 inoculation into TSB or TSBcm10 containing heme, paraquat, or hydrogen peroxide at the indicated concentrations. All growth assays were performed in a 96-well plate in 200 μL of media shaking linearly at 567 cpm (3 mm) at 37 °C. Optical density at 600 nm (OD600) served as measurement of growth and was measured every 30 min for the indicated total time in an EpochII microplate reader (BioTek).
For anaerobic assays, cultures were grown for 16 h at 37 ˚C in an anaerobic chamber (85% nitrogen, 10% hydrogen, 5% carbon dioxide, Coy Lab Products). Cultures were subcultured 1:50 into fresh TSB or TSBcm10 and grown for 6 h at 37 °C prior to 1:50 inoculation into TSB with or without 2 mM paraquat. All growth assays were performed in a 96-well plate in 200 μL of media shaking linearly at 567 cpm (3 mm) at 37 °C. Optical density at 600 nm (OD600) served as measurement of growth and was measured every 30 min for the indicated total time in an EpochII microplate reader (BioTek).
RNA-sequencing analysis
RNA was isolated and purified as described above. RNA sequencing was performed by the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core using the Illumina HiSeq 3000 platform (Illumina). The integrity and concentration of total RNA were determined using an Agilent 2100 Bioanalyzer system in combination with an RNA 6000 Nano kit (Agilent). rRNA was depleted using the Ribo-Zero rRNA removal kit (for bacteria) (Illumina) and paired-end cDNA libraries were prepared with a TruSeq RNA library prep kit v2 (Illumina). Data analysis for sequencing experiments was performed on the CLC Genomics workbench (version 20.0.1; Qiagen) using the reference C. difficile R20291 genome. Prior to analysis, rRNA reads were removed in order to account for variations in rRNA depletion procedure among samples. Standard settings were used for adapter and quality trimming, as well as transcriptome sequencing (RNA-seq) analysis. Expression values were calculated as RPKM (reads per kilobase per million mapped reads) (Benjamini and Hochberg, 1995), and a lower cutoff of 5 RPKM was introduced for subsequent analysis.
Analyses of the published RNA-sequencing dataset from (Fletcher et al., 2018), was performed using CLC Genomics Workbench Version 20.0.1 (Qiagen). Standard settings were employed if not stated otherwise. After quality and adapter trimming, reads with ≥95% sequence identity were aligned to the C. difficile VPI 10463 genome (NZ_CM000604). Reads from samples collected 24h and 36h post-infection were combined to increase coverage. Expression values were generated as Transcripts Per Million (TPM) using the CLC Genomics Workbench RNASeq Analysis tool (95% similarity fraction).
Relapse mouse model of CDI
Mice were subjected to a previously described model of CDI (Seekatz et al., 2015). Briefly, mice were treated with 0.5 mg/mL cefoperazone in their drinking water for 5 days. Mice were given a 2 day recovery period prior to administration of 105 spores of WT, hsmR::CT, or hsmA::CT C. difficile strains in PBS via oral gavage. Prior to infection, mice were confirmed to be C. difficile negative. After infection, mice were monitored for signs of disease, including diarrhea and weight loss. At day 4 post infection, mice were treated with 0.2 mg/mL vancomycin in their drinking water for 5 days. Mice were monitored for relapse after removal of vancomycin. On the final day of infection and necropsy, cecal contents were harvested. Mice that displayed severe disease or weight loss greater than 20% were humanely euthanized.
Bacterial burden determination
C. difficile CFUs were quantified daily from fecal or cecal samples. Samples were diluted and homogenized in PBS and serial plated onto taurocholate cycloserine cefoxitin fructose agar (TCCFA) for enumeration as CFU per gram of feces.
Heme quantification
Weighed fecal pellets were suspended in 200 μL water and allowed to incubated 1 h at 25 °C to disperse the fecal pellets. 1 mL ethyl acetate + 1 % trifluoroacetic acid was added to each sample to extract the heme. Samples were turned end-over-end at 25 °C for 30 min. Phase separation was accelerated by centrifugation at 16,000 xg for 5 min. 900 μL of the organic layer was transferred to clean glass vials and dried under a stream of nitrogen gas. Extracted heme was dissolved in 200 μL methanol. 15 μL of each sample and standard were analyzed on an Agilent 1260 Infinity II system. Analytes were separated by gradient HPLC on a Supelco Ascentis Express C18 column (50 × 2.1 mm, 5 μm) with a Phenomenex SecurityGuard C18 cartridge (3.2 × 8 mm) at a flow rate of 0.4 mL/min using 0.1 % trifluoroacetic acid in water and 0.1 % trifluoroacetic acid in acetonitrile as the A and B mobile phases, respectively. The gradient was held at 25 % B for 0.5 min, then ramped to 100 % B over the next 9.5 min. The column was washed at 100 % B for 6 min, then equilibrated to 25 % B for 4 min. Heme was detected using absorbance at 384 nm, and the retention time of 5.8 min was confirmed with a heme standard. Quantification was performed by comparing analyte AUC values to those of an external calibration line of hemin at 0, 1, 3, 10, and 30 μM.
MarR-family structural modeling
An hmmscan search on the HMMER web server (Finn et al., 2011) of the HsmR sequence against the Pfam profile hidden Markov model (HMM) library reveals that HsmR belongs to the helix-turn-helix clan of DNA binding proteins, and more specifically, the multiple antibiotic resistance (MarR) family of winged helix-turn-helix DNA-binding proteins (Perera and Grove, 2010a). No structure has been solved for any close homolog of HsmR, but the Protein Data Bank contains numerous structures of MarR family proteins with at least 20% sequence identity to HsmR, several of which are well-characterized. Lacking an ideal template with high sequence identity, we opted to use a collection of structures with lower sequence identity. A structural model of homodimeric HsmR was derived from this multiple sequence alignment, constructed using MODELLER (Eswar et al., 2008).
Electrophoretic Mobility Shift Assay
0.5 μg of each DNA probe (Table S1) was individually mixed with 0, 0.1, 0.5, or 1 μM recombinant HsmR in binding buffer (20 mM Tris HCl pH 7.5, 2.5 mM MgCl2, 0.45 mM EDTA, 0.05% IGEPAL, and 10% glycerol) for 30 minutes at 37 ˚C before being loaded onto a Bio-Rad Mini-PROTEAN TGX PAGE gel (4–20% gradient) in tris-glycine buffer (25 mM Tris, 192 mM glycine). Gel was run at 80 V for 30 minutes, followed by 130 V for 1 hour. Gel was rinsed briefly with water before staining with SYBR-safe (Invitrogen) for 5 minutes in tris-glycine buffer at room temperature in the dark. Gel was imaged using a Bio-Rad ChemiDoc MP Imaging System.
Cytotoxicity assay
Neutrophils isolated through the above methodology were diluted to a final concentration of 1×104 cells/well. S. aureus cultures were both diluted from overnight cultures, grown to mid exponential phase, and then back diluted to 104 CFU/ul. The diluted bacterial cultures were then opsonized in non-heat treated fetal bovine serum (FBS) (Atlanta biologicals) for 15 minutes. Neutrophils were then co-cultured at a MOI of 1 (10,000 CFU per 10,000 neutrophils) in D10 (0% O2, 1.6% H2, 37 °C). After 30 min, 2, 6, 12, and 24 hr, samples were serially diluted plated onto tryptic soy agar (TSA) plates (S. aureus). The bacterial colonies were enumerated, and percent growth was quantified by dividing the CFU of the S. aureus-neutrophil co-culture by the S. aureus alone culture.
Reactive oxygen species measurements
Freshly streaked C. difficile colonies were used to inoculate 5 mL of BHIS grown for 16 h at 37 °C. Cultures were subcultured 1:50 into fresh BHIS and grown for 6 h at 37 °C prior to the addition of heme and dihydrorhodamine 123 (Invitrogen) for 30 min at the indicated concentrations. Two hundred μL of culture was transferred to a 96-well plate and sealed with a Breathe-Easy gas permeable membrane (Diversified Biotech). Sealed plates were removed from the chamber and OD600 and fluorescence (excitation = 507 nm; emission = 529 nm) was measured at 10 min intervals on a Cytation 5 (BioTek) shaking in a double orbital at 567 cpm (3 mm) at 37 °C in atmospheric oxygen.
S. aureus ΔΔsod pOS1, pOS1_hsmA, and pOS1_hsmA_5His-Ala were diluted 1:50 into 200 μL TSB containing paraquat and dihydrorhodamine 123 (Invitrogen) at the indicated concentrations. OD600 and fluorescence (excitation = 507 nm; emission = 529 nm) were measured at 15 min intervals on a Cytation 5 (BioTek) shaking linearly at 567 cpm (3 mm) at 37 °C. The data displayed are background corrected for the wells with all components except cells and normalized to OD600.
QUANTIFICATION AND STATISICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism 8 and Microsoft Excel. Statistical significance was assessed using the multiple comparison two-way ANOVA test with the Sidak correction for multiple comparisons, the multiple comparison two-way ANOVA test with the Bonferroni correction for multiple comparisons, or the multiple comparison one-way ANOVA test with the Tukey correction for multiple comparisons. Significance was defined as p < 0.05, and the data were only excluded on the basis of technical errors associated with the experiment. Exact statistical tests used, significance values, group sizes, and dispersion and precision of measurements are defined in the figure legends.
Supplementary Material
Table S6. Bacterial Species containing HsmA orthologs related to STAR Methods and Figure S6.
Highlights.
In the presence of high heme concentrations C. difficile expresses the HsmRA system.
HsmA sequesters heme from the host and protects against oxidative stress.
The hsmRA operon decreases sensitivity to vancomycin during infection.
Acknowledgements
We thank the members of the Skaar lab for their critical review of this manuscript. This work was supported by National Institute of Allergy and Infectious Diseases grant R01AI073843 (to E.P.S.), the National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK058404 (to E.P.S.), and the National Institute of General Medical Sciences grant R35GM118157 (to D.P.G.), and grant R01GM129793 (to V.C.L.). R.J.K. was supported by the National Institute of General Medical Sciences training grant T32GM065086 and by the National Institute of Allergy and Infectious Diseases training grant T32AI007281. A.G.W. is supported by a Helen Hay Whitney Foundation Research Fellowship and the National Institute for Biomedical Imaging and Bioengineering training grant T32EB001628. W.N.B. is supported by American Heart Association Postdoctoral Fellowship 18POST34030426.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Authors declare no competing interests.
References
- Abt MC, McKenney PT, and Pamer EG (2016). Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asard H, Barbaro R, Trost P, and Bérczi A (2013). Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid Redox Signal 19, 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia AS, Benson MA, Stauff DL, Torres VJ, and Skaar EP (2010). Membrane damage elicits an immunomodulatory program in Staphylococcus aureus. PLoS Pathog 6, e1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, and Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57, 289–300. [Google Scholar]
- Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, et al. (1997). The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462. [DOI] [PubMed] [Google Scholar]
- Bérczi A, and Zimányi L (2014). The trans-membrane cytochrome b561 proteins: structural information and biological function. Curr Protein Pept Sci 15, 745–760. [DOI] [PubMed] [Google Scholar]
- Cammarota G, Ianiro G, and Gasbarrini A (2014). Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 48, 693702. [DOI] [PubMed] [Google Scholar]
- Capdevila DA, Huerta F, Edmonds KA, Le MT, Wu H, and Giedroc DP (2018). Tuning site-specific dynamics to drive allosteric activation in a pneumococcal zinc uptake regulator. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L, Howarth P, Clare S, Cunningham B, Sambol SP, et al. (2015). Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections. MBio 6, e00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartman ST, and Minton NP (2010). A mariner-based transposon system for in vivo random mutagenesis of Clostridium difficile. Appl Environ Microbiol 76, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernat RC, and Scott KP (2012). Evaluation of novel assays to assess the influence of different iron sources on the growth of Clostridium difficile. Anaerobe 18, 298–304. [DOI] [PubMed] [Google Scholar]
- Choby JE, Grunenwald CM, Celis AI, Gerdes SY, DuBois JL, and Skaar EP (2018). HemX Modulates Glutamyl-tRNA Reductase Abundance To Regulate Heme Biosynthesis. MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choby JE, and Skaar EP (2016). Heme Synthesis and Acquisition in Bacterial Pathogens. J Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Haslam DB, Haslam D, Goldenring JR, and Lacy DB (2012). Clostridium difficile Toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog 8, e1003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, and Warren MJ (2017). Prokaryotic Heme Biosynthesis: Multiple Pathways to a Common Essential Product. Microbiol Mol Biol Rev 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie ES, and Lorenz LL (1952). Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6, 95–107. [DOI] [PubMed] [Google Scholar]
- Edwards DI (1993). Nitroimidazole drugs--action and resistance mechanisms. II. Mechanisms of resistance. J Antimicrob Chemother 31, 201–210. [DOI] [PubMed] [Google Scholar]
- Eswar N, Eramian D, Webb B, Shen MY, and Sali A (2008). Protein structure modeling with MODELLER. Methods Mol Biol 426, 145–159. [DOI] [PubMed] [Google Scholar]
- Finn RD, Clements J, and Eddy SR (2011). HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39, W29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JR, Erwin S, Lanzas C, and Theriot CM (2018). Shifts in the Gut Metabolome and Clostridium difficile Transcriptome throughout Colonization and Infection in a Mouse Model. mSphere 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MB, Allen CA, Shrestha R, and Sorg JA (2013). Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog 9, e1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg L, Brugna M, and Hederstedt L (2002). Enterococcus faecalis heme-dependent catalase. J Bacteriol 184, 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganasen M, Togashi H, Takeda H, Asakura H, Tosha T, Yamashita K, Hirata K, Nariai Y, Urano T, Yuan X, et al. (2018). Structural basis for promotion of duodenal iron absorption by enteric ferric reductase with ascorbate. Commun Biol 1, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove A (2013). MarR family transcription factors. Curr Biol 23, R142–143. [DOI] [PubMed] [Google Scholar]
- Guerra AJ, Dann CE, and Giedroc DP (2011). Crystal structure of the zinc-dependent MarR family transcriptional regulator AdcR in the Zn(II)-bound state. J Am Chem Soc 133, 19614–19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery B, Galperine T, and Barbut F (2019). Clostiridioides difficle: diagnosis and treatments. BMJ 366, l4609. [DOI] [PubMed] [Google Scholar]
- Hanahan D (1983). Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Harrison KJ, Crécy-Lagard V, and Zallot R (2018). Gene Graphics: a genomic neighborhood data visualization web application. Bioinformatics 34, 1406–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Huang S, Nagy MS, Yadagiri VK, Fields C, Mukherjee D, Bons B, Dedhia PH, Chin AM, Tsai YH, et al. (2017). Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Barbe V, Lee CH, Vallenet D, Yu DS, Choi SH, Couloux A, Lee SW, Yoon SH, Cattolico L, et al. (2009). Genome sequences of Escherichia coli B strains REL606 and BL21(DE3). J Mol Biol 394, 644–652. [DOI] [PubMed] [Google Scholar]
- Kang JD, Myers CJ, Harris SC, Kakiyama G, Lee IK, Yun BS, Matsuzaki K, Furukawa M, Min HK, Bajaj JS, et al. (2019). Bile Acid 7α-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit Clostridium difficile: Role of Secondary Bile Acids. Cell Chem Biol 26, 27–34.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolos MH, Horsburgh MJ, Ingham E, and Foster SJ (2003). Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 149, 2749–2758. [DOI] [PubMed] [Google Scholar]
- Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, and Skaar EP (2011). Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, and Sternberg MJ (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CP, and Kyne L (2011). The host immune response to Clostridium difficile. J Med Microbiol 60, 1070–1079. [DOI] [PubMed] [Google Scholar]
- Knippel RJ, Zackular JP, Moore JL, Celis AI, Weiss A, Washington MK, DuBois JL, Caprioli RM, and Skaar EP (2018). Heme sensing and detoxification by HatRT contributes to pathogenesis during Clostridium difficile infection. PLoS Pathog 14, e1007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, and Novick RP (1983). The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305, 709–712. [DOI] [PubMed] [Google Scholar]
- Kuehne SA, Collery MM, Kelly ML, Cartman ST, Cockayne A, and Minton NP (2014). Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J Infect Dis 209, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechardeur D, Cesselin B, Fernandez A, Lamberet G, Garrigues C, Pedersen M, Gaudu P, and Gruss A (2011). Using heme as an energy boost for lactic acid bacteria. Curr Opin Biotechnol 22, 143–149. [DOI] [PubMed] [Google Scholar]
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al. (2015). Burden of Clostridium difficile infection in the United States. N Engl J Med 372, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, and Bork P (2019). Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47, W256–W259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatkin AJ, Stokes JM, Zheng EJ, Yang JH, Takahashi MK, You L, and Collins JJ (2019). Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvig RL, Damkiær S, Khademi SM, Markussen TM, Molin S, and Jelsbak L (2014). Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. MBio 5, e00966–00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, and Warren MJ (2012). The anaerobic biosynthesis of vitamin B12. Biochem Soc Trans 40, 581–586. [DOI] [PubMed] [Google Scholar]
- Ng LG, Ostuni R, and Hidalgo A (2019). Heterogeneity of neutrophils. Nat Rev Immunol 19, 255–265. [DOI] [PubMed] [Google Scholar]
- Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de CrécyLagard V, Diaz N, Disz T, Edwards R, et al. (2005). The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33, 56915702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, et al. (2014). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42, D206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter KL, Strange E, Parkhill J, Bamford KB, Armstrong-James D, and Edwards AM (2015). Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant smallcolony variants via the SOS response. Infect Immun 83, 1830–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IC, and Grove A (2010). Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. Journal of Molecular Cell Biology 2, 243–254. [DOI] [PubMed] [Google Scholar]
- Pericone CD, Park S, Imlay JA, and Weiser JN (2003). Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol 185, 6815–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péchiné S, and Collignon A (2016). Immune responses induced by Clostridium difficile. Anaerobe 41, 68–78. [DOI] [PubMed] [Google Scholar]
- Rivera-Chávez F, Lopez CA, and Bäumler AJ (2017). Oxygen as a driver of gut dysbiosis. Free Radic Biol Med 105, 93–101. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Wilcox MH, and Gerding DN (2009). Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7, 526–536. [DOI] [PubMed] [Google Scholar]
- Schneewind O, Model P, and Fischetti VA (1992). Sorting of protein A to the staphylococcal cell wall. Cell 70, 267–281. [DOI] [PubMed] [Google Scholar]
- Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, and Young VB (2015). Fecal Microbiota Transplantation Eliminates Clostridium difficile in a Murine Model of Relapsing Disease. Infect Immun 83, 3838–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, et al. (2009). Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol 10, R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic I, and Hantke K (1994). Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol 13, 719–732. [DOI] [PubMed] [Google Scholar]
- Stryer L (1961). A conformation-dependent Cotton effect in the Soret band of hemin:poly-Llysine. Biochim Biophys Acta 54, 395–397. [DOI] [PubMed] [Google Scholar]
- Suits MD, Pal GP, Nakatsu K, Matte A, Cygler M, and Jia Z (2005). Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc Natl Acad Sci U S A 102, 16955–16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, and Skaar EP (2007). A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 1, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, and Coenye T (2017). The Role of Reactive Oxygen Species in Antibiotic-Mediated Killing of Bacteria. Trends Microbiol 25, 456–466. [DOI] [PubMed] [Google Scholar]
- Wakeman CA, Hammer ND, Stauff DL, Attia AS, Anzaldi LL, Dikalov SI, Calcutt MW, and Skaar EP (2012). Menaquinone biosynthesis potentiates haem toxicity in Staphylococcus aureus. Mol Microbiol 86, 1376–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Branicky R, Noë A, and Hekimi S (2018). Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217, 1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, et al. (2017). Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45, D535–D542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Vulić M, Keren I, and Lewis K (2012). Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56, 4922–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff EE, Lopreato GF, Tipton KA, and Payne SM (2005). Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity. J Bacteriol 187, 5658–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Poyart C, Trieu-Cuot P, Lamberet G, Gruss A, and Gaudu P (2005). Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol Microbiol 56, 525–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S6. Bacterial Species containing HsmA orthologs related to STAR Methods and Figure S6.
Data Availability Statement
RNA-sequencing data
Raw RNA-sequence data are deposited on the NCBI Sequence Read Archive (accession code: PRJNA576216). For analyses of the published RNA-sequencing dataset from (Fletcher et al., 2018), raw data from their publicly available RNA-sequencing experiment characterizing the C. difficile transcriptome during colonization of a murine model of infection were retrieved from NCBI Sequence Reads Archive (submission number SRP134023).
HsmR multiple sequence alignment
HsmR multiple sequence alignment was performed using Clustal Omega (http://www.clustal.org/omega [accessed August 2019]) using Clostridioides difficile R20291 (NCBI accession no. CBE02826.1), Clostridium perfringens ATCC 13124 (NCBI accession no. ABG82197.1), Clostridium botulinum A str. Hall (NCBI accession no. YP_001388019.1) and Clostridium novyi (NCBI accession no. WP_011722139.1).


