Abstract
The hippocampus and its extended network contribute to encoding and recall of episodic experiences. Drawing from recent anatomical, physiological, and behavioral studies, we propose that hippocampal engrams function as indices to mediate memory recall. We broaden this idea to discuss potential relationships between engrams and hippocampal place cells, as well as the molecular, cellular, physiological and circuit determinants of engrams that permit flexible routing of information to intra- and extra-hippocampal circuits for re-instatement, a feature critical to memory indexing. Incorporating indexing into frameworks of memory function opens new avenues of study and even therapies for hippocampal dysfunction.
Keywords: index, hippocampus, engram, place cell, learning and memory, reinstatement
Introduction
A few keywords typed into the Google search bar will, more often than not, immediately lead to the exact piece of information we are seeking. While the details of how this magic happens are proprietary, the general idea is transparent; Google has managed to index vast swaths of the internet and uses our search terms to quickly point to the most appropriate information (https://www.google.com/search/howsearchworks). The system is surprisingly flexible, using history, context or location to hone results, completing or anticipating partial bits of information, and finding and separating similar items by detecting small differences. These properties, which underlie both its efficiency and popularity, echo the abilities of the memory systems operating in our own brains, particularly the episodic memory circuits dependent on the hippocampus (Squire et al., 2004; Tulving, 2002). The hippocampus is crucial for the encoding of memory, it is adept at integrating and interpreting contextual cues to drive recall, and it is efficient at both discrimination and association (Maren et al., 2013). Thus, much like how Google works as an index of information, one parsimonious explanation for hippocampal function is that it functions as an index of memories (Guo et al., 2018; Miller and Sahay, 2019; Tanaka, 2020; Tanaka and McHugh, 2018; Tanaka et al., 2018; Tonegawa et al., 2018). This is not a new idea (McClelland et al., 1995; Teyler and DiScenna, 1985, 1986; Teyler and Rudy, 2007), however recent work has begun to lend direct experimental evidence to this theory and edifying putative, underlying circuit mechanisms. Here we will explore and examine these findings in depth and discuss possible indexing mechanisms, as well as how these ideas could shape a better understanding of memory processes in both the healthy and diseased brain.
Space and Memory
The discovery of place cells, neurons in the hippocampus that have receptive fields tuned to discrete locations within a context (O’Keefe and Dostrovsky, 1971), revolutionized the experimental approach to studying hippocampal function (Moser et al., 2017). One of the earliest and most influential theories to emerge linking place cell activity and episodic memory was the Cognitive Map Theory (O’Keefe and Nadel, 1978), positing that the primary role of the hippocampus is to provide a spatial framework that permits the location and association of the items and events that constitute a given experience. While the authors suggested that this cognitive map may not be limited to physical space and could be applied to map episodic experience more broadly, place cells and their properties have proved a useful substrate to examine and test these ideas. For example, as different ensembles of neurons are recruited to represent different spatial experiences, the hippocampus could continuously provide a new underlying scaffolding across space (and time) that would allow memories to remain both related and distinct. Subsequent retrieval could then be triggered by a reinstatement of the original spatial map triggered by the cues that define a given context (Wikenheiser and Redish, 2015). Over time, other theoretical models have built on and expanded these ideas, and, as noted in other sections below, have proposed anatomical substrates for hippocampal functions that include novelty detection (Lisman and Otmakhova, 2001; Vinogradova, 2001), rapid encoding, pattern completion and separation (Kesner and Rolls, 2015; Knierim and Neunuebel, 2016; Nadel and Moscovitch, 1997), as well as their relation to spatial coding. Moreover, frameworks that encompass the place cell data, but are not tied to a specific spatial function of the hippocampus, have also been described. These posit that place cells may reflect a broader functionality related to general-purpose sequence generation (Buzsáki and Tingley, 2018) or relational memory (Cohen and Eichenbaum, 1993; Eichenbaum et al., 1994), allowing the extension of both hippocampal memory and physiology (Aronov et al., 2017; MacDonald et al., 2011; Pastalkova et al., 2008) beyond the domain of physical space.
Hand in hand with the growing characterization of place cell physiology, there developed a deeper understanding of the anatomical, behavioral and computational properties of the hippocampal circuit. This resulted in specific mnemonic functions being linked to the anatomic and physiological properties of discrete subregions of the structure (Fanselow and Dong, 2010; Nadel et al., 2013; Strange et al., 2014). In this framework, the classic model of sequential processing along the trisynaptic loop has the large number and sparse activity of granule cells in the dentate gyrus (DG) providing orthogonalization of similar cortical inputs leading to pattern separation (Hainmueller and Bartos, 2020; Leal and Yassa, 2018; McHugh et al., 2007); the DG providing input to the recurrent CA3 network to facilitate autoassociation and pattern completion (Cayco-Gajic and Silver, 2019; Kesner and Rolls, 2015; Knierim and Neunuebel, 2016; McHugh et al., 2007; Nakazawa et al., 2002); and finally, CA1 broadcasting the results back to the cortex (Soltesz and Losonczy, 2018; Valero and de la Prida, 2018). This framework has served as the backbone of relating place cell activity to memory processing, insofar as place cells may coordinate the pattern separation, completion, and reinstatement properties noted above.
Building on this framework, advances in genetic approaches have led to activity-dependent memory tagging systems in rodent models, which allow for the examination and artificial reactivation or inhibition of distinct memory traces (Josselyn and Tonegawa, 2020). These traces fulfill the properties of the memory engram, a moniker for the physical basis of memory first proposed by zoologist and biologist Richard Semon (Schacter et al., 1978; Semon, 1921), in that they can be viewed as the physical instantiation of an experience registered in enduring changes in synaptic connectivity and physiology of an ensemble of neurons. Tagging systems employed include the tetracycline-regulated transcriptional activation system, in which time-locked expression of actuators such as opsins or chemogenetic receptors are induced via activity-dependent promoters [e.g., c-Fos-or Arc-expressing; (Liu et al., 2012)] or immediate early gene (IEG)-binding elements (Sun et al., 2020), as well as the Cre-ERT transcription system (TRAP mice), which utilizes a tamoxifen-sensitive modified estrogen receptor to drive experience-driven expression of Cre-dependent constructs in activated cells (Guenthner et al., 2013). Such methods have allowed for the artificial triggering of memory-related behavior even in contexts where no such behavior would be expected. While these methods are not without their caveats (discussed further in sections below), engram-labeling studies have shown that optogenetic or chemogenetic stimulation or inhibition of excitatory neurons in the dentate gyrus (DG) [e.g., (Guo et al., 2018; Lacagnina et al., 2019; Liu et al., 2012)], CA3 [e.g., (Denny et al., 2014)], or CA1 [e.g., (Ghandour et al., 2019; Ryan et al., 2015; Tanaka et al., 2014)] reinstates or impedes (respectively) behavioral recall of that experience. While many engram studies have focused on measures of conditioned fear, these and other studies report hippocampal engram-driven behavior for a variety of context-specific behaviors, including place [e.g., (Ramirez et al., 2013)] or social avoidance [e.g., (Zhang et al., 2019)], as well as appetitive conditioning and place preference [e.g., (Redondo et al., 2014)].
These findings raise several key questions. First, how can a small number of experience-tagged hippocampal engram cells, much fewer in fraction than that of active place cells [e.g., (Tanaka et al., 2018)], encode a complex behavioral experience? Indeed, activation of a very small percentage (2–3%) of DG granule cells labeled during learning can reproduce context-appropriate behaviors [see (Liu et al., 2012) for examples]. Further, activation in the DG could harness the pattern completion abilities of the downstream CA3 network to amplify their activity via attractor dynamics (Colgin et al., 2010; Knierim and Neunuebel, 2016) and lead to a robust brain-wide reinstatement of a memory-related ensemble. However, reactivation of a subset of CA1 neurons, which lack recurrent connectivity, can also trigger behavioral reinstatement, and presumably memory recall [e.g., (Ryan et al., 2015)]. It is plausible that downstream activation of the entorhinal cortex and/or re-entrant excitation of the DG and CA3 adds these features thereby functioning like a recurrent network, although experimental evidence supporting this interpretation is lacking.
Additionally, 50 years of hippocampal physiology in rodents has revealed that place cell activity is exquisitely structured not only across space, but also across time (Howard and Eichenbaum, 2015). During exploration, the dominant theta oscillation in the hippocampal local field potential (LFP) organizes ensembles of place cells with spatially adjacent receptive fields into sequences, expressed on the time scale of a single theta cycle of ~125 ms (Burgess and O’Keefe, 2011). These sequences can be re-expressed during sharp-wave ripples (SWR) that occur during pauses in movement on an even shorter timescale, compressed into fast events lasting only 10’s of milliseconds (Foster, 2017). This precise temporal arrangement of activity has made the gap between place cell and engram-based memory studies difficult to bridge, as the latter have demonstrated that simultaneous optogenetic activation of ensembles of neurons in temporal and spatial patterns that are not observed under natural physiological conditions are sufficient to evoke behaviors mimicking memory recall. One can interpret this gap in the temporal dynamics between optically induced behavioral reinstatement and place cell activity as reflecting the dispensability of these temporal pattern for behaviors driven by contextual recognition, or perhaps this disconnect could simply be due to technical limitations in the place cell recording, as the retrieval of a hippocampal-dependent memory can occur very rapidly and in the absence of the exploration needed to drive extensive place cell activity, precluding a robust sampling of activity. For instance, when rodents receive a footshock immediately after placement in a previously exposed chamber, context exploration may be minimal, yet animals successfully retrieve the contextual memory and associate it with shock, resulting in context-dependent behavior during subsequent memory tests (Wiltgen et al., 2001). One possibility is that reinstatement of even a single place field is sufficient for memory reinstatement. However, long-term monitoring of the stability of place cell representations across repeated visits on the timescale of weeks, now possible due to advances in in vivo imaging approaches in mice, suggests that there exists a hitherto unappreciated high degree of instability in the spatial representation of a familiar environment [(Ziv et al., 2013); but, also see (Gonzalez et al., 2019)]. If only a fraction (~15%) of place cells show stability across several weeks, it becomes more difficult to draw a direct connection between memory recall and a completely stable spatial representation. It is possible that a small fraction of stable place cells can serve as a partial cue to reinstate the full representation of memory through a process of pattern completion in downstream regions, however this view is challenged by a recent study examining the physiological nature of engram cells in the hippocampus, which is discussed below (Tanaka et al., 2018). Thus, while place cell studies have provided insight into the anatomical organization and potential memory mechanisms of the hippocampus, we, like many others, struggle to reconcile these potential spatial coding properties with the core role of the system as a memory storage device (Tanaka, 2020). Further, it is important, both through hypothesis and experiments, to attempt to identify the rules of transformation that allow simultaneous activation of hippocampal engrams to generate appropriate patterns of downstream activation and behavior (Lisman et al., 2017). Perhaps then, we should reconsider what the activity of hippocampal neurons during memory formation and recall truly represent and how place cells that have driven much of the thinking in the field for the last fifty years can inform us about the hippocampus as a memory system (Figure 1).
Figure 1. Comparing and contrasting hippocampal place cells and engram-tagged (immediate early gene-expressing) neurons.

Place cell activity has precise temporal structure both during exploration and rest, whereas engram-tagged neurons are simultaneously and experimentally reactivated. Place cell density appears moderate, and this density of active place cells is relatively stable in any given context. Conversely, engram-tagged cells in the hippocampus are sparse, with familiar contexts exhibiting low levels of tagged expression. Engram cells exhibit considerably less stability in their context-dependent reinstatement over time as compared to place cells, although both are highly unstable with time. Remote timepoints for reactivation of experience-dependent engram cells remain unknown. While there are overlapping behavioral correlates of place and engram cell activity, place cell research has led the field in its correlation to behavior.
Instantiating the Hippocampal Index
The Hippocampal Memory Indexing Theory posits that the hippocampus does not “contain” the episodic memory itself, rather it generates a code or “index” that binds neuronal activity patterns underlying an experiential event, which is stored across distributed neocortical (and potentially subcortical) modules (Teyler and DiScenna, 1985, 1986; Teyler and Rudy, 2007). In other words, the hippocampus encodes a linked representation of brain activity at the time of an experience or episode, which can subserve subsequent recall via activation of that hippocampal representation. What is presumed to make these patterns of activity unique from other experience-induced patterns in the brain, such as ensemble activity in the sensory cortex activated by a stimulus, is their conjunctive and associational nature and the ability of the hippocampal ensemble to reinstate the original spatial and temporal patterns of cortical/subcortical activity of an experience (McClelland et al., 1995; Teyler and Rudy, 2007). Important to note is that the Indexing Theory is not mutually exclusive to the Cognitive Map Theory. Instead, it simply remains agnostic to what, if anything, the hippocampal neurons involved in memory indexing must represent in terms of behaviorally relevant information; spatial coding would be acceptable if these neurons had properties consistent with that of an index, as summarized and presented in Figure 2. In the following and subsequent sections, we elaborate on these features and discuss how the brain’s circuit architecture support a view of hippocampal function through the lens of indexing.
Figure 2.
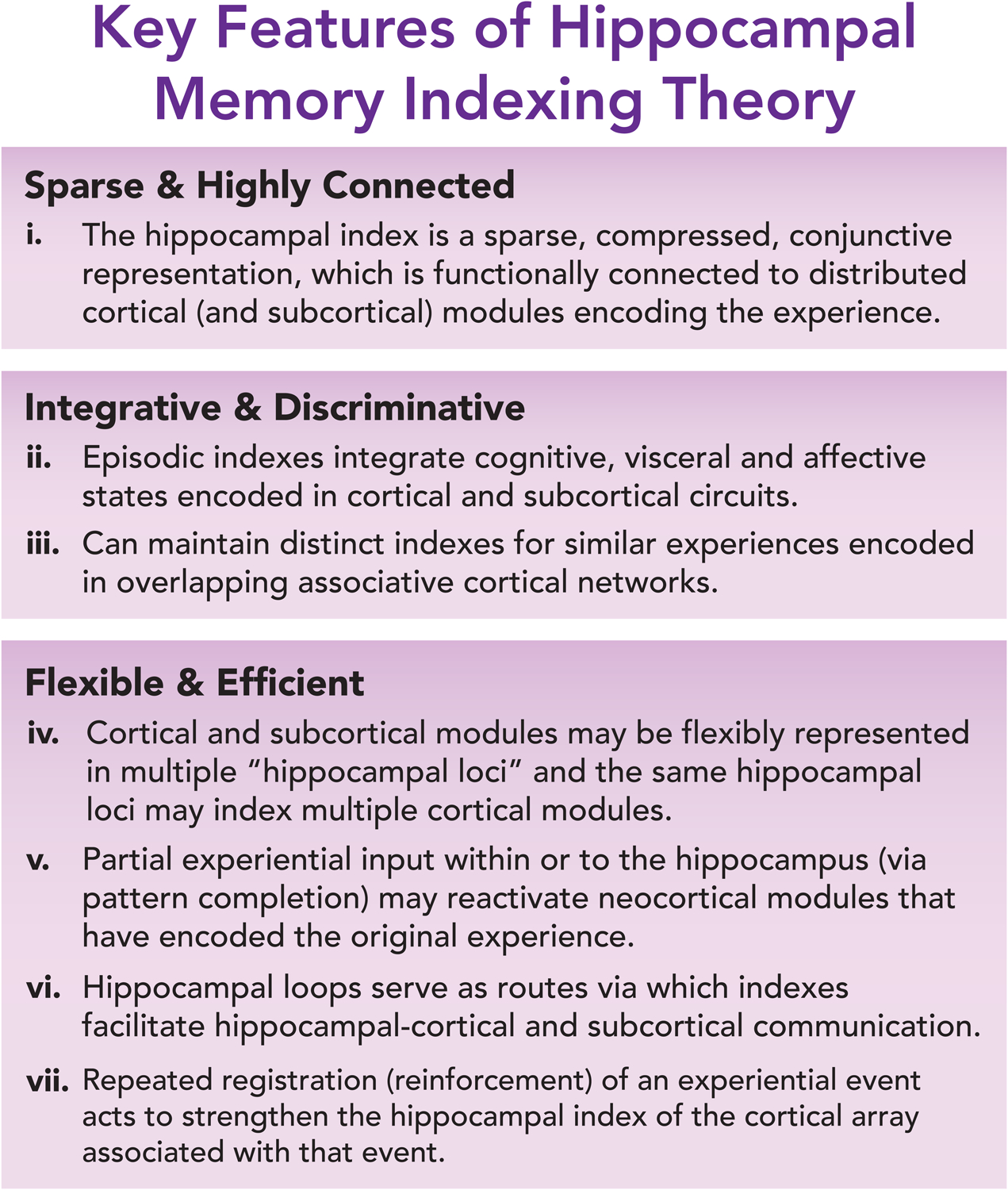
Hippocampal Memory Indexing Theory.
Engrams as Indices
Numerous studies have now shown that photoactivation of a sparse hippocampal engram drives IEG activity in select downstream brain regions thought to be involved in the original learning [e.g., (Ramirez et al., 2013, 2015; Roy et al., 2017)]. Such observations, together with the reinstatement of behavior following optogenetic engram stimulation, support the idea that the hippocampus is capable of indexing and triggering memory recall by reinstating learning-dependent activity in memory-related extrahippocampal brain systems. However, increased IEG induction in extrahippocampal structures following artificial activation of engram-bearing hippocampal cells does not necessarily indicate that these are the precise extrahippocampal neurons involved in the original learning. Moreover, specific controls, such as untagged, context-exposed and nonreinforced animals, are often essential to address issues of memory vs. performance; indeed, animals may be able to use alternative learning or generalization strategies to achieve task-dependent behavior [e.g., (Wiltgen et al., 2010)], even in the absence of the hippocampus [see (Maren et al., 2013) for discussion]. So, what is the evidence for learning-specific and hippocampus-dependent reinstatement of neural activity? To this end, one study has shown that photoinhibition of learning-tagged CA1 pyramidal cells resulted in the reduction of fear behavior in a shock-associated context and that this coincided with reduced reinstatement of c-Fos expression specifically in other c-Fos-tagged and presumably, engram-bearing, cortical and subcortical cells of the brain (Tanaka et al., 2014). In a separate study (Guo et al., 2018), it was found that contextual fear learning increased mossy fiber synaptic contacts of tagged engram-bearing dentate granule cells (DGCs) with stratum lucidum parvalbumin-positive inhibitory neurons (PV+ SLINs) to a significantly greater extent than a random population of DGCs. This engram-dependent recruitment of PV+ SLINs returned to pre-learning levels with time-dependent memory generalization. By genetically enhancing the coupling of engram-bearing DGCs with PV+ SLINs, the authors increased feed-forward inhibition in DG-CA3 circuitry and stabilized the hippocampal engram. Critically, this was shown to confer optogenetic behavioral reinstatement and context-specific reactivation of a distributed fear memory trace in hippocampal-cortical-subcortical networks at remote time points. Collectively, these findings mirror natural recall, insofar as contextual fear memory retrieval in the original learning context is associated with the specific reactivation of learning-dependent tagging in the hippocampus and some extrahippocampal targets. However, formal demonstration for how hippocampus may in fact coordinate extrahippocampal reinstatement in an experience-dependent manner is absent.
In light of understanding hippocampal engram functions through the lens of indexing, it is important to emphasize that although behavioral reinstatement does not equate to neural reinstatement, the behavioral outcomes of optogenetic manipulations of hippocampal engrams appear experience-dependent. For example, as noted above, stimulation of contextual fear conditioning-tagged cells in the DG results in increased freezing in a safe (no shock) context [e.g., (Liu et al., 2012)]. However, if such stimulation of the DG occurs for neurons that were tagged following the extinction of fear in a shock-associated context, then this manipulation results in reduced freezing in a shock-associated context and decreased spontaneous recovery of contextual fear (Lacagnina et al., 2019). Likewise, inhibition of context fear-tagged DG cells attenuates freezing in a shock-associated context (Tanaka et al., 2014), but inhibition of extinction-tagged DG cells can increase defensive responding in a previously extinguished context (Lacagnina et al., 2019). These experience-specific findings are complemented by other studies where the behavioral response (beyond defensive behavior) of DG engram reactivation reflects the valence of the reinforcing stimuli associated the context or engram [e.g., (Ramirez et al., 2015; Redondo et al., 2014)]. Also, consider that simply reactivating engram cells that were tagged during homecage exploration or exposure to a context in which shock never occurred does not appear to induce abnormal locomotion or overt defensive behaviors [e.g., (Ghandour et al., 2019)].
While indexing may explain the capacity for photoactivation of tagged hippocampal engram cells to trigger memory-specific behavior in different contexts, reinstatement of behavior, is often notably less than what would be expected through natural recall (i.e., returning the animal to the original training context). In the framework of indexing, we propose there are number of reasons why this may be the case beyond the fact that natural recall is presumably most effective in reactivating the index. Importantly, the abovementioned tagging systems, while experimentally time-locked, are still thought to open a window of tagging that may be on the order of at least several hours. Thus, when artificially reactivating these cells, it is possible that the experimenter may also be triggering activation of other non-specific indices and/or experiences such as activity in the homecage and pre-or post-training handling. These patterns are not specific to the primary learning episode in question and thus may compete for behavioral expression. Contextual stimuli present in the test context may also trigger interference as well, acting as external inhibitors. Thereby, a number of controls (e.g., nonreinforced, homecage, etc.) for better isolating and assessing the degree of experienced-dependent behavioral reinstatement should be performed. Additionally, hippocampal indices are proposed not only to encode the relevant brain systems activated during an experience but may also represent the sequential patterns of such activation (Buzsáki and Tingley, 2018). Current methods of optogenetic reactivation of hippocampal engram cells lack such sequence-based reactivation (Carrillo-Reid et al., 2019), beyond what is inherently structured in the linkage of hippocampal engram-bearing circuits. Further technological advancements, which may better constrain the window of tagging to a particular experience or may be able to reactivate cells in sequence-dependent manners, are crucial to improve the readouts and interpretation of this reinstatement.
Is the hippocampus alone in its potential capacity for indexing? Association cortices such as sensory association cortex and entorhinal cortex (EC) may also exhibit indexing properties due to their convergence of sensory input, thereby contributing to a hierarchical indexing scheme (McClelland et al., 1995). Thus, the hippocampus may serve to some extent as an index of indices in the EC and other input structures as information is routed in and then back out again. Assuming such hippocampal signals can be decompressed to reinstate activity in cortical and subcortical nuclei (as noted above), an exact one-to-one representation in the hippocampus of cortical modules (for example) seems unlikely and may not be necessary. In fact, convergence of neural activity into the hippocampus might be essential for its abilities to form conjunctive contextual representations (Rudy and O’Reilly, 1999). Other critical targets of the hippocampus, such as the retrosplenial cortex (RSC) (Cowansage et al., 2014; Mao et al., 2018) or lateral septum (LS) (Bender et al., 2015; Besnard et al., 2019; Tingley and Buzsáki, 2018), may also maintain such convergence of processing, and may thereby be part of a hierarchical indexing scheme, assuming these structures are capable of reinstating patterns of experience-specific assembly patterns. Indeed, one study found that reactivation of cells of the RSC that were tagged at conditioning is sufficient to induce behavioral expression of fear, even in the absence of a fully functional hippocampus (Cowansage et al., 2014). Importantly, this study showed that optogenetic activation of the RSC engram, like natural recall, recruited overlapping downstream circuits in the amygdala and EC, demonstrative of reinstatement of experiential activity. Thus, indexing may not necessarily be unique to the hippocampus, however, the hippocampus may be uniquely positioned to index episodic events, given the significantly greater extent to which it integrates complex and hierarchical sensory information from across the brain (see sections below), as well as due to its discriminative coding and circuit architecture. Unpublished findings indicating that reactivation of engram-tagged neural structures, outside the hippocampus, does not equally reinstate behavior may support the particular importance of hippocampal indexing (Roy et al., 2019).
Memory Indexing in Humans?
Electrophysiological studies in humans have suggested that the human hippocampus also possesses properties consistent with indexing. For example, in epileptic patients implanted with depth electrodes into the medial temporal lobe, free recall of an audiovisual experience was shown to follow the selective reactivation of hippocampal and EC cells that were active during the prior experience (Gelbard-Sagiv et al., 2008). Likewise, successful retrieval in an object association task coincided with reinstatement of spiking activity in hippocampal and EC cells, with hippocampal activity preceding EC firing, and with decoding analyses of EC activity predicting the identity of the recalled object (Staresina et al., 2019). Other intracranial recordings have shown that behavioral recall was linked to coordinated hippocampal-lateral temporal cortical representational reinstatement of item-context associations (Pacheco Estefan et al., 2019). In this study, hippocampal reinstatement preceded that seen in the neocortex and hippocampal-cortical gamma phase synchrony during hippocampal reinstatement predicted neocortical reinstatement. Moreover, these findings are mirrored in additional studies that have found memory-related reinstatement in the human hippocampus is underscored by a sparse and distributed set of active cells (Wixted et al., 2014, 2018).
Human functional magnetic resonance imaging (fMRI) studies support a similar interpretation. For example, one fMRI study (Harand et al., 2012) reported that hippocampal BOLD (blood-oxygen-level-dependent) activity during an episodic learning experience matched its activity at recall (i.e., recognition of previously shown visual cues), particularly when subjects reported the remembering of episodic details of the learning event. Interestingly, this episodic reinstatement of hippocampal activity occurred for remembered cues at three days and even three months following learning. Moreover, for successful retrieval of experiential memory (in tasks such as object recall and recognition), regions including the RSC, parahippocampal cortex (PHC), perirhinal cortex (PRC), and prefrontal cortex (PFC) have all been shown to exhibit recall-dependent reinstatement along with or in close temporal proximity to hippocampal reinstatement, suggestive of hippocampal-dependent routing [e.g., (Arnold et al., 2018; Jonker et al., 2018; Schultz et al., 2019)]. Again, while reinstatement and temporal patterns of activation alone do not demonstrate indexing, these findings are consistent with data from rodents and leaves open the possibility that future experiments may directly test this idea in humans.
An Integrated Circuit Model of the Hippocampal Index
In the decades since the introduction of the Hippocampal Memory Indexing Theory, considerable advances have been made in our understanding of the complexity and diversity of hippocampal circuits. If experience-tagged hippocampal engrams serve as episodic indices, how might the circuit architecture of the brain be employed for encoding and recall? To these ends, the conjunctive, sparse and compressed code generated in the DG via pattern separation would support indexing by minimizing memory interference (Feature i of Figure 2) (Cayco-Gajic and Silver, 2019; Hainmueller and Bartos, 2020; Knierim and Neunuebel, 2016; McHugh et al., 2007), while DG outputs, together with direct EC inputs (alongside the diverse afferents described below), onto CA3 cells would bias attractor dynamics in the recurrent network to store an experience as a new memory, or catalyze the retrieval or updating of a previously encoded memory by pattern completion (Features ii, iii, and iv of Figure 2). Accordingly, the experience is registered in a sparse hippocampal code or engram composed of principal cells (and inhibitory neurons) across the different subregions (DG, CA3, CA2 and CA1), with their coordinated activity permitting intra- and extrahippocampal reinstatement of the original experience through dynamic routing (Features v, vi, and vii of Figure 2; Figure 3–4). We elaborate on this idea with recent examples in the next sections.
Figure 3. Hippocampal (DG-CA3-CA2-CA1) circuit architecture and anatomical loops permits flexible integration and routing of experiential information.
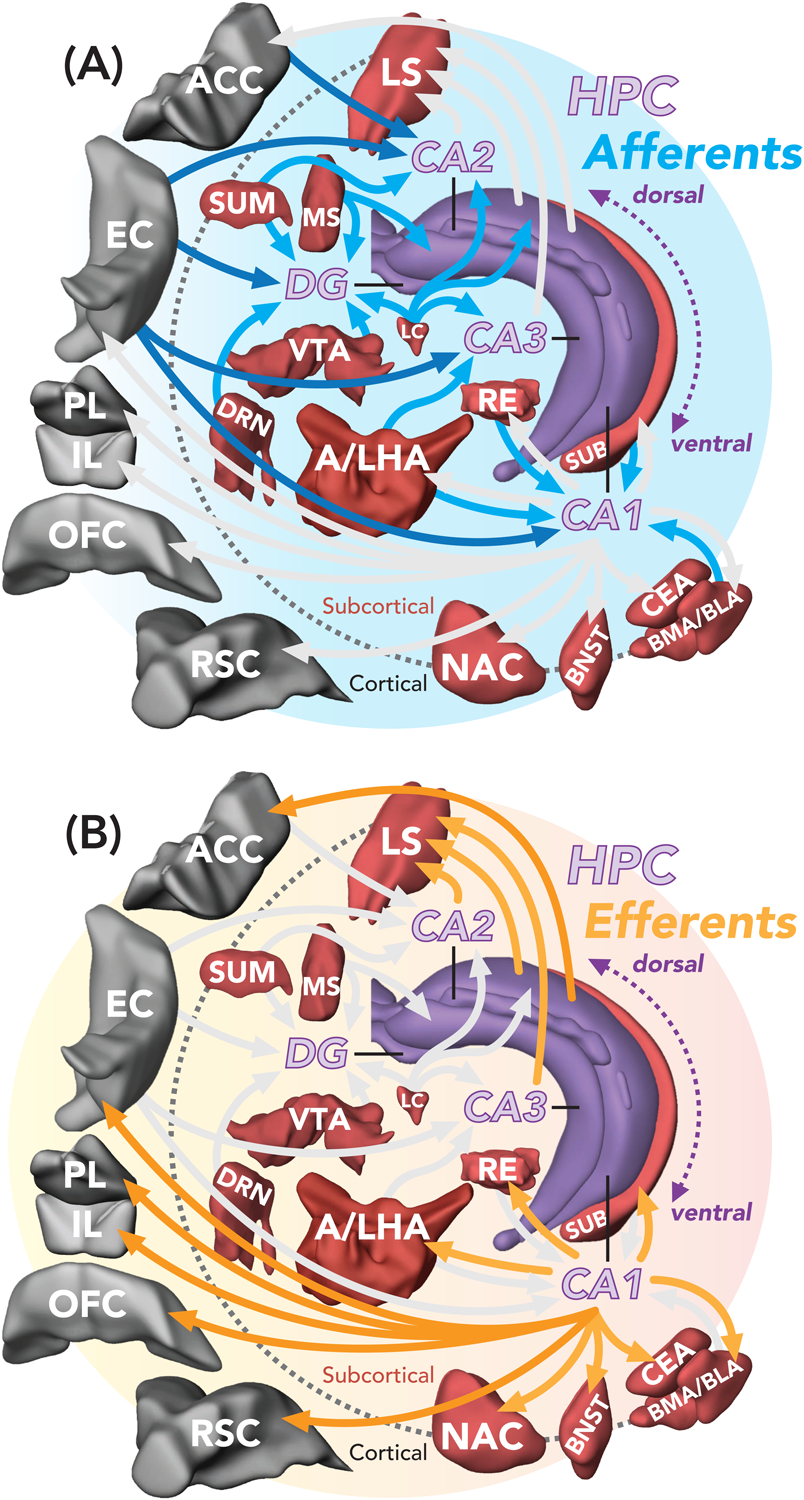
(A) Examples of hippocampal inputs (blue arrows). (B) Examples of hippocampal outputs (orange arrows). Note that the projections shown are not exhaustive. Brain regions: anterior cingulate cortex (ACC); bed nucleus of the stria terminalis (BNST); basolateral/basomedial amygdala (BLA/BMA); central amygdala (CEA); dentate gyrus (DG); dorsal raphe nucleus (DRN); entorhinal cortex (EC); cornu ammonis regions (CA1–3); infralimbic cortex (IL); locus coeruleus (LC); anterior/lateral hypothalamic area (A/LHA); lateral septum (LS); medial septum (MS); nucleus accumbens (NAC); orbital frontal cortex (OFC); prelimbic cortex (PL); nucleus reuniens (RE); retrosplenial cortex (RSC); subiculum (SUB); supramammillary nucleus (SUM); ventral tegmental area (VTA). Brain region images were generated using Brain Explorer 2.0 [Allen Mouse Brain Atlas; (Lein et al., 2007)].
Figure 4. Hippocampal engrams may index experience to reinstate experiential memory.
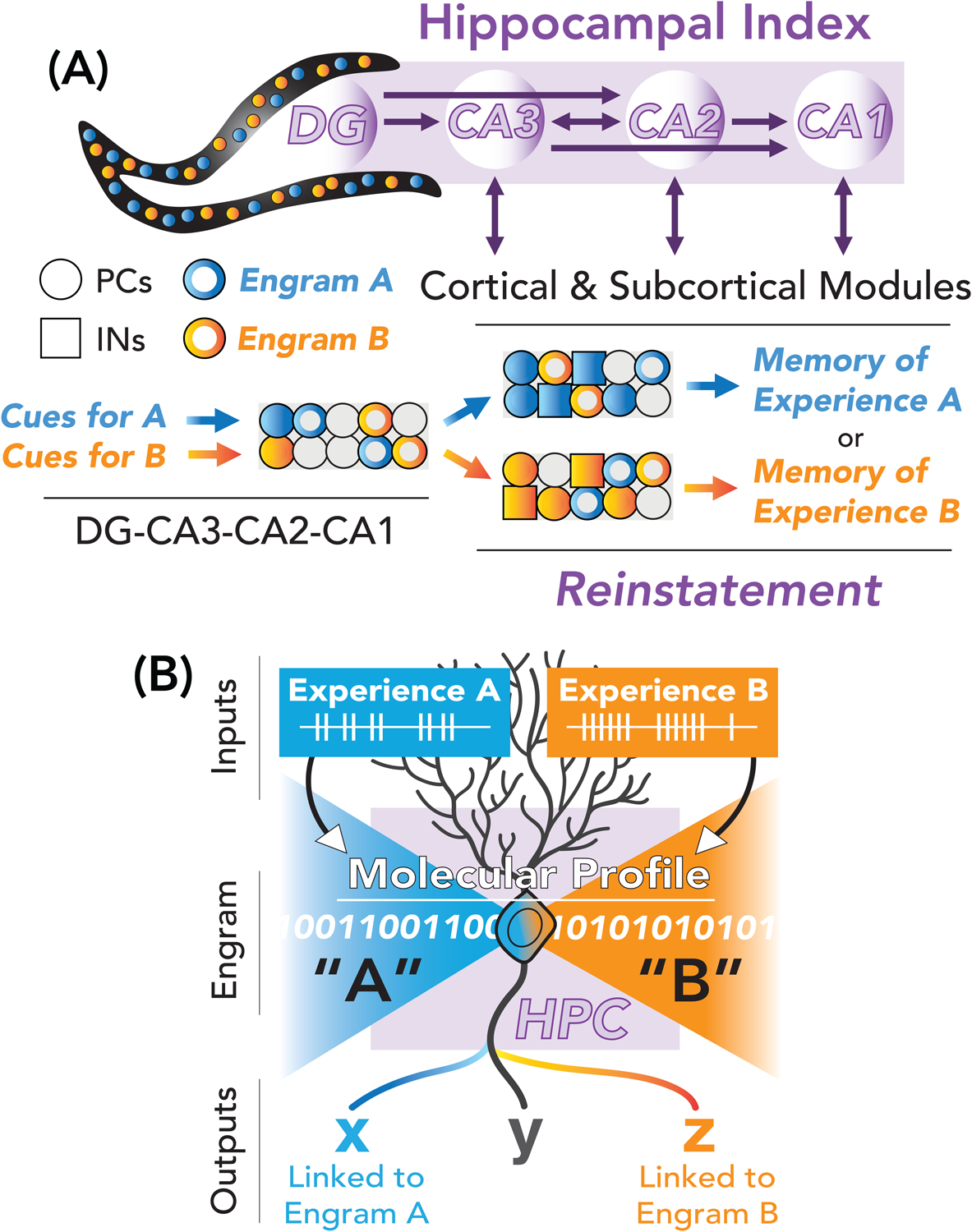
(A) Distinct experiences are thought to be encoded within DG-CA3-CA2-CA1 connections, with engram-bearing cells being functionally linked to other neurons for the same episode. Recall can then be driven by partial input (cues) that reinstate activity (filled-in circles/squares) within hippocampal circuits to drive extrahippocampal reinstatement via its diverse outputs and connectivity to other engram-bearing cells. PCs: principal cells; INs: inhibitory neurons. (B) Hippocampal cells may register more than one experience in distinct patterns of connectivity prescribed by activity-dependent gene expression (shown here as combinations of 1 and 0’s).
Dynamic Routing: Hippocampal Afferents
In this framework, the DG-CA3-CA2-CA1 circuit can be perceived as a template of nodes, with each node receiving diverse intra- and extra-hippocampal inputs allowing for the integrative, dynamic and flexible incorporation of cognitive and visceral information into memory representations (Figure 3A). These properties would enable the hippocampus to participate in many “types” of memories—spatial, goal-oriented, social, future-planning—all which may comprise diverse episodic experiences. For example, direct long-range GABAergic projections from the lateral hypothalamus to CA3 have been recently identified (Zhou et al., 2019a); these neurons synapse onto CA3 interneurons and appear to have critical functions in tasks of object recognition and discrimination, revealing a direct pathway by which CA3 may integrate endocrine signals in learning and memory processes. CA3 neurons also incorporate locus coeruleus (LC) input, and one study found that LC projections to dorsal CA3 (dCA3; but not to CA1 or DG) are required for encoding (but not retrieval; which may be mediated by CA3-CA1, see below) of a contextual representation (as assessed by distance traveled in a previously explored context or via single-trial contextual fear conditioning) (Wagatsuma et al., 2018). Additionally, parallel circuits projecting from neurons in the supramammillary nucleus (SUM) to the DG and CA2 have been found to carry contextual and social novelty signals respectively, allowing hypothalamic sculpting of hippocampal memory in a task specific manner (Chen et al., 2020); see also (Li et al., 2020; Hashimotodani et al., 2018). These recent discoveries broaden our understanding of the diversity of mammalian hippocampal afferents and point to multiple sources via which the hippocampus may integrate signals for memory formation or recall. Activity of these distinct sets of inputs (alongside other important inputs, including from the amygdala and anterior cingulate cortex), recruited based on ongoing experience, may govern which hippocampal routes are deployed for encoding and/or recall.
Dynamic Routing: Hippocampal Efferents
Further arguing against a simple sequential processing loop, it is clear that each node within the DG-CA3-CA2-CA1 circuit projects to distinct outputs (Figure 3B). In the framework of Index Theory, these outputs may be flexibly deployed by engram cells to reinstate an experience (whether that experience is appetitive, aversive, etc.). An example of this potential selective routing can be found in ventral CA1 (vCA1) neurons (Ciocchi et al., 2015). In this study, vCA1 cells were tracked based on their projections to the PFC, nucleus accumbens (NAc), and amygdala during behavior, ask- and pathway-dependent. These findings critically suggest that signals out of CA1 are not uniformly transmitted to its targets; rather, it supports the idea of that efferent hippocampal signals are routed based on task and mnemonic demands. With particular relevance to indexing, dorsal CA1 (dCA1) tetrode recordings paired with circuit-specific optogenetics have shown that expression of conditioned place preference (CPP) depends on the reinstatement of dCA1 representations that were active during training (Trouche et al., 2019). Furthermore, CPP expression is lost if dCA1 terminals in NAc are photoinhibited, despite dCA1 pyramidal cells maintaining their context-dependent cell assemblies during testing [also, see (Zhou et al., 2019b)].
This routing ability is not restricted to CA1; CA3 output neurons may route information via projections to CA1, CA2, or the DLS. Indeed, brain-wide analyses of co-activated circuits accompanying contextual fear discrimination identified a CA3-DLS module (Besnard et al., 2019). This pathway appears to recruit somatostatin (SST)+ DLS cells to gate conditioned freezing, as in vivo calcium imaging found SST+ DLS cell activity reliably discriminated shock-associated vs. safe contexts. In support of these findings, optogenetic terminal specific silencing of dCA3 terminals in dCA1 and DLS have suggested distinct roles for dCA3-CA1 and dCA3-DLS projections to contextual fear learning (or consolidation) and discrimination, respectively (Besnard et al., 2020).
For CA2, its efferent network positions it strongly for memories involving social recognition, discrimination and aggression (Middleton and McHugh, 2019). Indeed, CA2 (and CA3) efferents do not uniformly regulate discrimination (Raam et al., 2017). Optogenetic experiments have demonstrated that anterior/dorsal CA2 (dCA2) neurons targeting dCA1 are essential for novel object recognition but not for discrimination between novel and familiar conspecifics. The opposite was true for dCA2/dCA3 projections to posterior CA1. Photoinhibition of dCA2/dCA3 projections to the DLS were instead shown to somewhat enhance social discrimination, but with no effect on object discrimination or recognition. In social behaviors, axons from dCA2 neurons targeting ventral hippocampus were shown to be critically involved in maintaining memory of a familiar animal (Meira et al., 2018; Raam et al., 2017), while pharmacogenetic inhibition of CA2 terminals in the DLS attenuates social aggression (Leroy et al., 2018), a pathway that, when active, appeared to invoke DLS-innervation of the ventromedial hypothalamus to drive attack behavior.
In total, multiple non-overlapping engrams within these diverse hippocampal routes may compete through updating or ongoing learning to modify behavioral output. For example, 2P imaging of DG and CA3 engrams in vivo revealed that the updating of a reward location promoted activity remapping in CA1 and CA3 but not in the DG (Hainmueller and Bartos, 2018). Recent work has also demonstrated that contextual fear extinction recruits a distinct DG engram from that encoding the original context-shock association, which reduces levels of freezing in the training context, but can be overcome by the original engram to induce relapse (Lacagnina et al., 2019). Likewise, prolonged optogenetic or chemogenetic reactivation of a DG fear memory engram in the training context without the unconditioned stimulus promoted extinction of the conditioned response (Khalaf et al., 2018). Of course, the hippocampus is not unique in this type of broad connectedness, other hubs in the brain, including the claustrum (Jackson et al., 2020) and the thalamus (Halassa and Sherman, 2019) may surpass it in terms of total connectivity. However, these data suggest that the hippocampus is capable of integrating and routing complex information from a variety of source structures, supporting its role in the binding of cognition and emotion to subserve memory (Figure 4A).
Dynamic Routing: Inhibitory Microcircuits
How might these diverse communication channels running through the hippocampus be managed? Hippocampal inhibitory neurons (INs) are well-positioned to function as arbiters of information flow in the hippocampus as they target different cellular compartments of principal neurons, are reciprocally connected with other interneurons and project locally within- and across different subregions and lamellae and out of the hippocampus to association cortices and subcortical circuits (long-range INs) (Caroni, 2015). Moreover, hippocampal INs modulate neuronal excitability, summation of excitatory inputs and neuronal firing in addition to generation of network oscillations (theta and gamma oscillations) and as such, are thought to play critical roles in local circuit computations underlying exploration and encoding, action selection, memory consolidation, retrieval and reinstatement (Cardin, 2018; Makino et al., 2019; Roux and Buzsáki, 2015; Sosa et al., 2018). Indeed, recent studies have uncovered a diverse population of INs in CA1 and CA3 that exert perisomatic and dendritic inhibition on dentate granule cells, and which are modulated by sharp-wave ripples.
Local INs may regulate information flow within a hippocampal subregion by biasing recruitment of principal cells, thereby creating parallel channels as evidenced in a study that identified biased PV+ BCs connectivity with deep and superficial CA1 neurons of the ventral hippocampus (Lee et al., 2014). The authors found that PV+ BCs preferentially innervated deep CA1 pyramidal neurons but received greater excitatory inputs from superficial CA1 pyramidal neurons. At the level of output, PV+ BCs exert greater inhibition onto BLA-projecting deep CA1 neurons than those that projected to the PFC and, in turn, received greater excitatory input from PFC than BLA. These data suggest that PV+ BCs do not uniformly inhibit CA1, but instead, it is likely that PV+ BC-principal cell microcircuits bias information flow to distinct ventral CA1 outputs including PFC, BLA, NAc, DLS and LH (serving dynamic and flexible routing). Local INs may also differentially regulate theta phase-locking and burst firing of CA1 neurons through somatic or dendritic inhibition, respectively (Royer et al., 2012). Because burst firing of pyramidal cells is thought to increase synaptic communication by increasing excitation of downstream targets, local INs may modulate CA1 outputs through this mechanism (Graves et al., 2012; Lisman, 1997; Takahashi and Magee, 2009). Importantly, rhythmic optogenetic activation of PV+ INs in CA1 to mimic that seen during learning enhanced theta, delta and ripple oscillations, stabilized functional connectivity between CA1 neurons and reliably promoted ensemble reactivation (Ognjanovski et al., 2014). The exact role these oscillations play in the ability of an index to reactivate downstream targets remains largely untested, however evidence suggests the coherence or coordination of activity they provide may facilitate both the encoding and recall of memories across various structures by ensuring temporal coordination of activity (Buzsáki, 2015; Corcoran et al., 2016; Igarashi et al., 2014; Joo and Frank, 2018; Lin et al., 2017; Makino et al., 2019; Wirt and Hyman, 2019).
Pioneering in vivo recordings and imaging studies in rats identified extensively connected inhibitory neurons with extra-hippocampal (septum, subiculum, para and pre-subiculum, RSC)-projections that coordinate network oscillations (Bonifazi et al., 2009; Jinno, 2009). Long-range inhibitory projection neurons of the LEC suppress CA1 CCK+ interneurons that relay feed-forward inhibition from CA3 to CA1 ex vivo (Basu et al., 2016). This disinhibition of CA1 interneurons induced enhanced dendritic spiking within a specific temporal window, a mechanism by which sensory information and mnemonic information arriving from excitatory LEC inputs and CA3, respectively may be integrated. More recently, a class of long-range inhibitory nNOS-expressing cells in CA1 (LINC neurons) has been identified that project both locally and extra-hippocampally (Christenson Wick et al., 2019). These neurons inhibit superficial and deep principal cells and other INs in CA1 and project to diverse extra-hippocampal targets including tenia tecta, subiculum, hypothalamus, olfactory bulb and EC. Optogenetic activation of LINC neurons entrained hippocampal oscillations and hippocampal-frontal cortex (tenia tecta) coherence. Thus, converging evidence has begun to illuminate how cell physiology, activity-dependent gene expression, and microcircuit connectivity support hippocampal engram cell-dependent indexing (i.e., encoding of experiences and routing of information to mediate reinstatement). We discuss these features of engram cell identity next.
Index Cell Identity
Given the long-standing focus on rodent hippocampal place cells, an obvious question is what aspect of contextual memory is encoded within the hippocampal engram? Behavioral studies using variations of contextual fear conditioning suggest that the hippocampus generates a conjunctive representation of multimodal sensory information formed through physical exploration of a context [(Fanselow, 2000); also, see (Krasne et al., 2015)]. For example, one study preexposed rats to either the conditioning context or independent features of that context, and found context fear after an immediate shock is facilitated only when these multimodal cues are presented together, suggesting that the hippocampus represents the conjunction of the cues defining the context (Rudy and O’Reilly, 1999). Indeed, temporary pharmacological inactivation of the hippocampus during the context preexposure prevents the contextual fear conditioning of immediate shock (Matus-Amat et al., 2004). Past studies of IEG expression in the hippocampus support this this view [e.g., (Huff et al., 2006; Zhu et al., 1997)]; the strongest IEG response is achieved when a novel combination of multimodal cues is presented to the animal. Conversely, hippocampal IEG expression is weak or non-existent when a highly habituated stimulus is given. Note that immediate shock upon context entry in the absence of pre-exposure (and extensive post-exposure) does not appear to elevate levels of IEGs in the hippocampus relative to a habituated homecage [also, see: (Erwin et al., 2020)]. These data suggest that IEG-expressing engram neurons may not necessarily or exclusively store spatial information, as rodents will have active place cells even in the most familiar of contexts, but rather hippocampal circuits detect novelty in the combination of sensory cues and encode it as a contextual representation supporting the episodic experience. Indeed, optogenetic stimulation of CA1 neurons tagged in a novel context the day prior to immediate shock delivery in the same context, but not a different context, resulted in retrieval of the contextual fear memory [(Ghandour et al., 2019); also, see (Ramirez et al., 2013)]. Thus, a memory engram, defined by active principal cells during contextual learning, may preferentially encode conjunctive contextual information, as opposed to specific locations that could be biased by a specific cue or subset of cues.
Physiology
Key insights into the precise identity of engram-bearing cells came from in vivo recording of CA1 neurons in a mouse in which c-Fos-positive neurons labelled during a novel context exposure were tagged with channelrhodopsin (ChR2) and subsequently optically identified (Tanaka et al., 2018). As expected, roughly 50% of all CA1 pyramidal cells could be classified as place cells, however only a quarter of these place cells also expressed c-Fos (optogenetically identified); in short, engram cells were place cells, but only a quarter of place cells were engram cells. During memory encoding, these engram-bearing (c-Fos) cells are distinguished by higher mean firing rates [as also seen in a calcium imaging study (Ghandour et al., 2019)], repetitive bursts of action potentials at the theta frequency and higher entrainment by the local fast gamma oscillation compared to the non-c-Fos-expressing place cells; again, highlighting the role inhibitory circuits and oscillations may play in the formation of the index. Interestingly, when mice were returned to the context the next day, c-Fos-positive engram neurons, while remaining place cells, meaning they still demonstrated a reliable in-session spatially receptive field, showed a much higher degree of spatial instability compared to the encoding session (remapping) than the c-Fos negative place cell population. These data can be seen as paradoxical; how is it that the neurons shown to be capable of reinstating context appropriate behavior show relatively lower spatial specificity than the remaining active cells? Importantly, when only firing rate, and not location, was considered, it was clear that the engram cells faithfully encoded contextual identity, but not specific location. A return to the encoding context resulted in c-Fos-positive neurons reinstating their average firing rates, which were highly correlated between the first and second visits, while the firing rates in a distinct context were strongly altered. It worth noting that this strong correlation of activity emerges as soon as animal is placed in the environment, suggesting their activity could support rapid retrieval of contextual memory, consistent with the Indexing Theory. Further, these findings of a unique physiology suggest the importance of the temporal relationship between input from CA3 and the EC in triggering CA1 pyramidal cell plasticity and activity in vivo (Bittner et al., 2017; Ketz et al., 2013).
Complementary results were also found in a physiological study in which c-Fos-positive CA1 neurons were inhibited during recall (Trouche et al., 2016). In this study, engram neurons, defined by c-Fos expression associated with the acquisition of a cocaine-rewarded CPP, were labeled with an inhibitory opsin. Inactivation of this ensemble during a subsequent recall session reduced conditioned place preference behavior and interestingly, led to a global remapping of the c-Fos-negative active place cell population. Together, these data suggest that the role of the c-Fos-positive place cells is to serve as a context-specific memory index and their activity is crucial for the stable reinstatement of a more detailed spatial map, consisting of the remainder of the place cell population, that would permit animals precise navigation.
While it may seem odd at first that the neurons indispensable for inducing memory recall in CA1 show spatial instability, recent computational modeling lends support to this view (Benna and Fusi, 2019). Based on an assumption that the hippocampus encodes correlations of incoming sensory information, similar to the interpretation from contextual conditioning and IEG studies, the model predicted instability in the spatial representation of the hippocampus. Their ultrametric tree-like network generated sparse and compressed representations of inputs to efficiently store uncorrelated patterns in a hippocampal-like network. When spatial navigation in a 2D open field is simulated, activities of hippocampal cells in the model exhibit strong modulation by the animal’s location within the environment (i.e., place cells). However, similar to the experimental observations above, these place fields significantly remapped between epochs in the same environment, suggesting instability of firing location as a reflection of correlation coding rather than spatial coding. Taken together, these studies support a view that activity of the hippocampal engram reflects more than just space and suggest at least a subset of neurons are dedicated to capturing the conjunctive correlations that define the larger context of the experience.
Activity-Dependent Regulation of Gene Expression and Connectivity
Hippocampal engrams are generated and maintained by strengthening or modification of synapses among activated cells within and across subregions. One study found that c-Fos-tagged CA3 cells preferentially responded to stimulation of engram-tagged DGCs (Ryan et al., 2015), results indicative of experience-driven connectivity of DG-CA3. Likewise, context fear-dependent increases in the number and size of spines in engram-bearing cells of CA1 coincides with direct input from engram-tagged cells from CA3 (Choi et al., 2018).
Additionally, DG engram cells were shown to exhibit greater connectivity with stratum lucidum PV inhibitory neurons than non-engram DG cells (Guo et al., 2018). These observations have motivated investigations into how developmental programs and activity-dependent gene expression prescribes engram formation. First, principal neurons may have differing propensities towards recruitment into engrams based on developmentally programmed intrinsic firing properties, and connectivity (Cembrowski and Spruston, 2019; Soltesz and Losonczy, 2018). Second, activity-dependent transcription factors and combinations thereof, enable neurons to read patterns of neural activity and transcribe molecular specifiers of connectivity to facilitate strengthening or modification of synapses (Tyssowski et al., 2018). For example, cAMP (cyclic adenosine monophosphate) response element binding protein (CREB)-overexpression studies have revealed that enhancing basal activity and excitability of principal cells in the hippocampus (or subsets of amygdalar neurons, etc.) prior to learning can bias the allocation and tagging process to these cells, without altering the overall size of the engram per se (Josselyn and Frankland, 2018; Josselyn and Tonegawa, 2020). A recent report using RNA sequencing of engram-tagged DG cells (following contextual fear conditioning) identified a unique learning-dependent genetic profile for engram-bearing cells, with CREB-dependent transcription networks being differentially regulated and required for consolidation (Rao-Ruiz et al., 2019). Interestingly, many of these genes were previously shown to regulate somatic inhibition [e.g., neuronal PAS domain protein 4 (NPAS4), proenkephalin (Penk), and brain-derived neurotrophic factor (BDNF)] (Bloodgood et al., 2013). Consistent with these findings, within the DG, contextual fear learning regulates CREB-dependent levels of neuropeptide Y (NPY) in SST+ hilar perforant path-associated (HIPP) interneurons, which may regulate SST+ HIPP-mediated feedback and feedforward inhibition in the DG to govern the size of the engram (Raza et al., 2017; Stefanelli et al., 2016). Not surprisingly, different immediate early gene transcription factors (including NPAS4) have been functionally implicated in linking principal cells with distinct inhibitory neuron networks to support engram formation (Sun et al., 2020). Thus, experiential input may drive unique IEG expression to govern functional allocation of engram-bearing cells to work in concert for memory expression (Figure 4B).
Hippocampal Index Stability and Memory Fidelity
Memory consolidation is thought to involve transformation and reorganization of hippocampal-linked cognate cortical representations and a gradual decay of the hippocampal engram over time (DeNardo et al., 2019; Guskjolen et al., 2018; Kitamura et al., 2017; Roy et al., 2017; Tayler et al., 2013; Winocur et al., 2007). Consolidated memories have been shown to generalize or lack detail, including the extent to which they may elicit visceral or physiological reactions, leading to the suggestion that the role the hippocampus plays in memory is to contribute episodic detail (Yonelinas et al., 2019). If this contribution relies on the initial memory trace or not remains a contested topic. For example, it has been argued that hippocampal memory traces remain, even for older memories (Moscovitch and Nadel, 2019), but others have argued there is a shift in the role the hippocampus, from one of recall to reconstruction in the absence of the original trace, with the activity serving to index consolidated neocortical traces (Barry and Maguire, 2019a, 2019b). These observations raise the following questions: is the hippocampal index always necessary for memory retrieval or whether cortical indexes acquire this function over time? Is the cortical index equivalent to the hippocampal index?
Several lines of evidence support the notion that the hippocampal index may be necessary for maintenance and retrieval of only highly precise memories. First, although hippocampal damage at remote timepoints may still permit retrieval of detailed contextual representations, the extent of memory retrieval is often much less robust (Wang et al., 2009), suggesting that extra-hippocampal indices may not fully compensate for the loss of the hippocampal index. Indeed, while a similar degree of hippocampal activation is seen for recent and remote memories, the reactivation patterns are different [(Tayler et al., 2013); also, see (Guskjolen et al., 2018)].
Second, artificially stabilizing the engram within DG-CA3 decreases remote memory generalization [and maintains behavioral reinstatement of remote DG engram stimulation; (Guo et al., 2018)], providing a direct link between maintenance of the hippocampal index and remote memory precision.
However, maintenance of separate hippocampal indices for all episodic memories is thought to require significantly greater capacity than is available to avoid memory interference (McClelland et al., 1995; Miller and Sahay, 2019; Skaggs and McNaughton, 1992). In CA1, the various methods employed to genetically label engram neurons typically capture about 20% of the pyramidal cells in the region (Tanaka et al., 2018) and in vivo imaging suggested the identity of these allocated neurons shifts over the timescale of hours (Cai et al., 2016); thus, it appears that natural decay of hippocampal indices ensures the time-dependent re-organization of memory traces to support different degrees of generalization and generation of schema to facilitate new learning. It may be that some hippocampal indexes, perhaps for salient life events, are maintained for longer periods of time thereby permitting recall of remote memories with high fidelity.
The integrity and composition of the cortical indices depends on how competition for representation of episodic memories and abstraction of statistical commonalities across ensembles dictates the balance between preservation of details versus generation of schema to facilitate memory generalization. This may involve time-dependent changes in the exact number of cells and patterns of efferent connectivity of cortical ensembles and linkage of distinct engrams of separate experiences via some degree of overlapping and synchronous activation (during recall or reconsolidation) of engram-bearing cells (Abdou et al., 2018; DeNardo et al., 2019; Kitamura et al., 2017; Ohkawa et al., 2015; Oishi et al., 2019; Pignatelli et al., 2019; Ramirez et al., 2013; Redondo et al., 2014).
While no one model can explain all the current data, from the perspective of engrams and indexing we favor the hypothesis of a time-dependent shift in the indexing function from the hippocampus to cortical traces concurrent with a silencing or loss of the original hippocampal index (Tonegawa et al., 2018). Ultimately, time-dependent shifts in hippocampus-dependent episodic detail may be useful in the development of experiential schemas and broader knowledge.
Hippocampal Dysfunction
“Indexopathies”
Memory deficits and hippocampal dysfunction accompany traumatic brain injury, epilepsy, age-related cognitive decline, Alzheimer’s Disease (AD) and numerous other psychiatric disorders, including posttraumatic stress disorder (PTSD) and schizophrenia (Besnard and Sahay, 2016; Haberman et al., 2017; Small et al., 2011). Can these disorders of experiential memory be classified as “indexopathies”, insofar as they are marked by an inability to accurately or precisely encode or effectively implement hippocampus-dependent routing of information (i.e., indexing)? For example, recent work documented declining hippocampal and cortical reinstatement in aging individuals (Trelle et al., 2020). Disease- or aging-induced excitation-inhibitory imbalance in hippocampal circuits, which may impede indexing, may underlie much of these memory dysfunctions. Indeed, excitation-inhibition imbalance (hypo- or hyperactivity) at the level of CA1 [e.g., (Oh et al., 2013)] and CA3 [e.g., (Simkin et al., 2015; Wilson et al., 2005)] and loss of feedforward inhibition in DG-CA3 [e.g., (Guo et al., 2018)] are associated with memory imprecision in preclinical models of aging and memory disorders. Human imaging studies have further reported similar activity changes (e.g., hyperactivity) of hippocampal structures (Haberman et al., 2017), such as in presymptomatic familial AD (FAD) individuals (Quiroz et al., 2010) or in patients with amnestic mild cognitive impairment (Bakker et al., 2012).
Such cellular, circuit and network level alterations may disrupt the balance between pattern separation and pattern completion, thereby promoting aberrant index-dependent reinstatement and recall. What previously may have been subthreshold to trigger CA3-dependent memory recall prior to disease, may be sufficient after disease onset, thereby promoting excessive reinstatement and memory expression in contexts that may not be optimal. For example, aberrant or excessive retrieval of past experiences has been suggested to underlie psychosis in schizophrenia (Tamminga et al., 2010). Additionally, degradation of flexible routing to extrahippocampal targets, due to connectivity losses in disease or injury, may also impede the abilities of the hippocampus to effectively integrate cortical and subcortical information for proper encoding and retrieval. Perhaps related to such circuit loss, AD is considered, in part, a disease of memory retrieval failure (Leal and Yassa, 2018; Roy et al., 2016).
Promoting Indexing: New Directions in Therapy
Recent advances in technology and medicine have led to a number of new therapeutic avenues for memory disorders—strategies that may be effective, in part, because they promote or reestablish hippocampal indexing functions. For example, growing interest has centered on deep-brain and closed-loop feedback neuroprosthetics for symptom management in individuals where other lines of treatment have failed (Grosenick et al., 2015; Takeuchi and Berényi, 2020). Perhaps by restoring context-dependent routing (and thereby indexing), these real-time electrophysiological (or opto- or chemogenetic, potentially) methods could dynamically normalize aberrant activity or restore cell excitability in perturbed brain circuits (e.g., in hippocampal-amygdalar or hippocampal-prefrontal loops). Additionally, recent developments in targeted gene-editing approaches (Knott and Doudna, 2018) may permit molecular re-allocation or re-specification of connectivity aimed at promoting memory precision and accuracy. Indeed, quieting disease-related hyperexcited CA3 pyramidal cells may involve targeting feedforward inhibitory mechanisms of DG-CA3 (Guo et al., 2018; Viana da Silva et al., 2019), CA2–CA3 connections (Boehringer et al., 2017), or inhibiting aberrant LH-CA3 activity (Zhou et al., 2019a), for example. Other pharmaco- or gene therapies that promote neurogenesis in aging or disease states may also exist as beneficial therapeutic avenues for memory impairments (Miller and Sahay, 2019).
Novel treatments for memory impairments may not be limited to invasive techniques and may involve supplementing existing procedures to best tap into the indexing properties of the hippocampus. For example, although the use of mnemonic devices for memory treatments is not new, recent developments in technology, such as augmented reality or 3D interactive environments, may provide novel avenues for improving memory recall within and beyond the clinic. Indeed, the growing ubiquity of personal handheld devices may make mobile reminders or mnemonic cues (to facilitate reinstatement of memory) for treatments or symptom management for memory impairments more accessible or specialized. When combined with psychological treatments in the clinic, these and the abovementioned possibilities may yield new successes in treatment-resistant memory disorders. Moving forward, indexing may be a useful framework for improving clinical therapies for memory dysfunction.
Conclusions: Moving Memory Forward
Perhaps the brain’s most powerful search engine, the hippocampus sits at the center of the acquisition and recall of episodic memory. While the mechanisms of how this is achieved has been the focus of decades of research across many species and disciplines, it is often challenging to relate disparate lines of inquiry. Here we have highlighted human and animal work based on recent genetic, physiological, anatomical and computational approaches that together support an expanded view of the Hippocampal Memory Indexing Theory. In particular, we argue that (1) the functional roles of putative hippocampal engram cells include indexing, which may facilitate detailed recall of episodic experiences, (2) this episodic recall is facilitated by the reinstatement of engram cell activity and in their experience-sculpted connectivity, (3) indexing may not be unique to the hippocampus, but the hippocampus may be uniquely positioned to index experiential memory, (4) disease of the hippocampus may impede truly episodic memory by disrupting its capacity for precise context-specific reinstatement. Nonetheless, there remain a number of outstanding questions for the field and for future work (Figure 5). Future work that integrates these levels of analyses will be required to understand how the dynamics of information flow in the hippocampal circuit contributes to the encoding and recall processes it supports.
Figure 5.

Outstanding challenges for hippocampal indexing and memory research.
Acknowledgements
T.D.G. acknowledges support from Harvard Brain Science Initiative. K.Z.T acknowledges support from MEXT Grant-in-Aid for Young Scientists (19K16305), Grant-in-Aid for JSPS fellows (19J00974), and a Nakajima Foundation research grant. A.S. acknowledges support from US National Institutes of Health Biobehavioral Research Awards for Innovative New Scientists (BRAINS) 1-R01MH104175, NIH-NIA 1R01AG048908-01A1, NIH 1R01MH111729-01, the James and Audrey Foster MGH Research Scholar Award, Ellison Medical Foundation New Scholar in Aging, Whitehall Foundation, the Inscopix Decode Award, the NARSAD Independent Investigator Award, Ellison Family Philanthropic support, Blue Guitar Fund, Harvard Neurodiscovery Center/MADRC Center Pilot Grant Award, a Alzheimer’s Association research grant, the Harvard Stem Cell Institute (HSCI) Development grant and a HSCI seed grant. T.J.M acknowledges support from MEXT Grant-in-Aid for Scientific Research (19H05646), MEXT Grant-in-Aid for Scientific Research on Innovative Areas (17H05591, 17H05986, 19H05233) and the RIKEN Center for Brain Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu S-I, and Inokuchi K (2018). Synapse-specific representation of the identity of overlapping memory engrams. Science 360, 1227–1231. [DOI] [PubMed] [Google Scholar]
- Arnold AEGF, Ekstrom AD, and Iaria G (2018). Dynamic neural network reconfiguration during the generation and reinstatement of mnemonic representations. Front. Hum. Neurosci 12, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Nevers R, and Tank DW (2017). Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, and Gallagher M (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DN, and Maguire EA (2019a). Consolidating the case for transient hippocampal memory traces. Trends Cogn. Sci (Regul. Ed.) 23, 635–636. [DOI] [PubMed] [Google Scholar]
- Barry DN, and Maguire EA (2019b). Remote memory and the hippocampus: A constructive critique. Trends Cogn. Sci (Regul. Ed.) 23, 128–142. [DOI] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, and Siegelbaum SA (2016). Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351, aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, and Ponomarenko A (2015). Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat. Commun 6, 8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benna MK, and Fusi S (2019). Are place cells just memory cells? Memory compression leads to spatial tuning and history dependence. BioRxiv. [DOI] [PMC free article] [PubMed]
- Besnard A, and Sahay A (2016). Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 41, 24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A, Gao Y, TaeWoo Kim M, Twarkowski H, Reed AK, Langberg T, Feng W, Xu X, Saur D, Zweifel LS, et al. (2019). Dorsolateral septum somatostatin interneurons gate mobility to calibrate context-specific behavioral fear responses. Nat. Neurosci 22, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A, Miller SM, and Sahay A (2020). Distinct dorsal and ventral hippocampal CA3 outputs govern contextual fear discrimination. Cell Rep. 30, 2360–2373.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Milstein AD, Grienberger C, Romani S, and Magee JC (2017). Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, and Greenberg ME (2013). The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer R, Polygalov D, Huang AJY, Middleton SJ, Robert V, Wintzer ME, Piskorowski RA, Chevaleyre V, and McHugh TJ (2017). Chronic loss of CA2 transmission leads to hippocampal hyperexcitability. Neuron 94, 642–655.e9. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, and Cossart R (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424. [DOI] [PubMed] [Google Scholar]
- Burgess N, and O’Keefe J (2011). Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol 21, 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, and Tingley D (2018). Space and time: the hippocampus as a sequence generator. Trends Cogn. Sci (Regul. Ed.) 22, 853–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, et al. (2016). A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA (2018). Inhibitory interneurons regulate temporal precision and correlations in cortical circuits. Trends Neurosci. 41, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P (2015). Inhibitory microcircuit modules in hippocampal learning. Curr. Opin. Neurobiol 35, 66–73. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Han S, Yang W, Akrouh A, and Yuste R (2019). Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 447–457.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayco-Gajic NA, and Silver RA (2019). Re-evaluating Circuit Mechanisms Underlying Pattern Separation. Neuron 101, 584–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, and Spruston N (2019). Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat. Rev. Neurosci 20, 193–204. [DOI] [PubMed] [Google Scholar]
- Chen S, He L, Huang AJY, Boehringer R, Robert V, Wintzer ME, Polygalov D, Weitemier AZ, Tao Y, Gu M, Middleton SJ, Namiki K, Hama H, Therreau L, Chevaleyre V, Hioki H, Miyawaki A, Piskorowski RA, McHugh TJ (2020) A hypothalamic novelty signal modulates hippocampal memory. Nature, in press. [DOI] [PubMed]
- Choi J-H, Sim S-E, Kim J-I, Choi DI, Oh J, Ye S, Lee J, Kim T, Ko H-G, Lim C-S, et al. (2018). Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435. [DOI] [PubMed] [Google Scholar]
- Christenson Wick Z, Tetzlaff MR, and Krook-Magnuson E (2019). Novel long-range inhibitory nNOS-expressing hippocampal cells. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, and Klausberger T (2015). Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, and Eichenbaum H (1993). Memory, Amnesia, and The Hippocampal System (Cambridge, MA: MIT Press; ). [Google Scholar]
- Colgin LL, Leutgeb S, Jezek K, Leutgeb JK, Moser EI, McNaughton BL, and Moser M-B (2010). Attractor-map versus autoassociation based attractor dynamics in the hippocampal network. J. Neurophysiol 104, 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Frick BJ, Radulovic J, and Kay LM (2016). Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiol. Learn. Mem 127, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, and Mayford M (2014). Direct reactivation of a coherent neocortical memory of context. Neuron 84, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, Guenthner CJ, Tessier-Lavigne M, and Luo L (2019). Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci 22, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, and Hen R (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, and Cohen NJ (1994). Two functional components of the hippocampal memory system. Behav. Brain Sci 17, 449–472. [Google Scholar]
- Erwin SR, Sun W, Copeland M, Lindo S, Spruston N, and Cembrowski MS (2020). A Sparse, Spatially Biased Subtype of Mature Granule Cell Dominates Recruitment in Hippocampal-Associated Behaviors. Cell Rep. 31, 107551. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res 110, 73–81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, and Dong H-W (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ (2017). Replay comes of age. Annu. Rev. Neurosci 40, 581–602. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, and Fried I (2008). Internally generated reactivation of single neurons in human hippocampus during free recall. Science 322, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandour K, Ohkawa N, Fung CCA, Asai H, Saitoh Y, Takekawa T, Okubo-Suzuki R, Soya S, Nishizono H, Matsuo M, et al. (2019). Orchestrated ensemble activities constitute a hippocampal memory engram. Nat. Commun 10, 2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez WG, Zhang H, Harutyunyan A, and Lois C (2019). Persistence of neuronal representations through time and damage in the hippocampus. Science 365, 821–825. [DOI] [PubMed] [Google Scholar]
- Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, and Spruston N (2012). Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 76, 776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Marshel JH, and Deisseroth K (2015). Closed-loop and activity-guided optogenetic control. Neuron 86, 106–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner CJ, Miyamichi K, Yang HH, Heller HC, and Luo L (2013). Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Soden ME, Herber C, Kim MT, Besnard A, Lin P, Ma X, Cepko CL, Zweifel LS, and Sahay A (2018). Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med 24, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskjolen A, Kenney JW, de la Parra J, Yeung B-RA, Josselyn SA, and Frankland PW (2018). Recovery of “lost” infant memories in mice. Curr. Biol 28, 2283–2290.e3. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Branch A, and Gallagher M (2017). Targeting Neural Hyperactivity as a Treatment to Stem Progression of Late-Onset Alzheimer’s Disease. Neurotherapeutics 14, 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller T, and Bartos M (2018). Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller T, and Bartos M (2020). Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci 21, 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, and Sherman SM (2019). Thalamocortical circuit motifs: A general framework. Neuron 103, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harand C, Bertran F, La Joie R, Landeau B, Mézenge F, Desgranges B, Peigneux P, Eustache F, and Rauchs G (2012). The hippocampus remains activated over the long term for the retrieval of truly episodic memories. PLoS One 7, e43495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Karube F, Yanagawa Y, Fujiyama F, and Kano M (2018). Supramammillary Nucleus Afferents to the Dentate Gyrus Co-release Glutamate and GABA and Potentiate Granule Cell Output. Cell Rep. 25, 2704–2715.e4. [DOI] [PubMed] [Google Scholar]
- Howard MW, and Eichenbaum H (2015). Time and space in the hippocampus. Brain Res. 1621, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, and Rudy JW (2006). Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J. Neurosci 26, 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser M-B, and Moser EI (2014). Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 510, 143–147. [DOI] [PubMed] [Google Scholar]
- Jackson J, Smith JB, and Lee AK (2020). The Anatomy and Physiology of Claustrum-Cortex Interactions. Annu. Rev. Neurosci [DOI] [PubMed]
- Jinno S (2009). Structural organization of long-range GABAergic projection system of the hippocampus. Front. Neuroanat 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker TR, Dimsdale-Zucker H, Ritchey M, Clarke A, and Ranganath C (2018). Neural reactivation in parietal cortex enhances memory for episodically linked information. Proc. Natl. Acad. Sci. USA 115, 11084–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HR, and Frank LM (2018). The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci 19, 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, and Frankland PW (2018). Memory allocation: mechanisms and function. Annu. Rev. Neurosci 41, 389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, and Tonegawa S (2020). Memory engrams: Recalling the past and imagining the future. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, and Rolls ET (2015). A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci. Biobehav. Rev 48, 92–147. [DOI] [PubMed] [Google Scholar]
- Ketz N, Morkonda SG, and O’Reilly RC (2013). Theta coordinated error-driven learning in the hippocampus. PLoS Comput. Biol 9, e1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf O, Resch S, Dixsaut L, Gorden V, Glauser L, and Gräff J (2018). Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, and Tonegawa S (2017). Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, and Neunuebel JP (2016). Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem 129, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GJ, and Doudna JA (2018). CRISPR-Cas guides the future of genetic engineering. Science 361, 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne FB, Cushman JD, and Fanselow MS (2015). A Bayesian context fear learning algorithm/automaton. Front. Behav. Neurosci 9, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA, and Drew MR (2019). Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci 22, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, and Yassa MA (2018). Integrating new findings and examining clinical applications of pattern separation. Nat. Neurosci 21, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, and Soltesz I (2014). Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, Buss EW, Kandel ER, and Siegelbaum SA (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bao H, Luo Y, Yoan C, Sullivan HA, Quintanilla L, Wickersham I, Lazarus M, Shin Y-YI, and Song J (2020). Supramammillary nucleus synchronizes with dentate gyrus to regulate spatial memory retrieval through glutamate release. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-J, Rugg MD, Das S, Stein J, Rizzuto DS, Kahana MJ, and Lega BC (2017). Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans. Hippocampus 27, 1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE (1997). Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 20, 38–43. [DOI] [PubMed] [Google Scholar]
- Lisman JE, and Otmakhova NA (2001). Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus 11, 551–568. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, and Redish AD (2017). Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci 20, 1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, and Tonegawa S (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, and Eichenbaum H (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Polygalov D, Bolaños F, Benucci A, and McHugh TJ (2019). Physiological Signature of Memory Age in the Prefrontal-Hippocampal Circuit. Cell Rep. 29, 3835–3846.e5. [DOI] [PubMed] [Google Scholar]
- Mao D, Neumann AR, Sun J, Bonin V, Mohajerani MH, and McNaughton BL (2018). Hippocampus-dependent emergence of spatial sequence coding in retrosplenial cortex. Proc. Natl. Acad. Sci. USA 115, 8015–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, and Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, and Rudy JW (2004). The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J. Neurosci 24, 2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, and O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev 102, 419–457. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, and Tonegawa S (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99. [DOI] [PubMed] [Google Scholar]
- Meira T, Leroy F, Buss EW, Oliva A, Park J, and Siegelbaum SA (2018). A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun 9, 4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton SJ, and McHugh TJ (2019). CA2: A highly connected intrahippocampal relay. Annu. Rev. Neurosci [DOI] [PubMed]
- Miller SM, and Sahay A (2019). Functions of adult-born neurons in hippocampal memory interference and indexing. Nat. Neurosci 22, 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, and Nadel L (2019). Sculpting Remote Memory: Enduring Hippocampal Traces and vmPFC Reconstructive Processes. Trends Cogn. Sci (Regul. Ed.) 23, 634–635. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser M-B, and McNaughton BL (2017). Spatial representation in the hippocampal formation: a history. Nat. Neurosci 20, 1448–1464. [DOI] [PubMed] [Google Scholar]
- Nadel L, and Moscovitch M (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol 7, 217–227. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hoscheidt S, and Ryan LR (2013). Spatial cognition and the hippocampus: the anterior-posterior axis. J. Cogn. Neurosci 25, 22–28. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, et al. (2002). Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanovski N, Maruyama D, Lashner N, Zochowski M, and Aton SJ (2014). CA1 hippocampal network activity changes during sleep-dependent memory consolidation. Front. Syst. Neurosci 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Waters J, and Disterhoft JF (2013). Altered calcium metabolism in aging CA1 hippocampal pyramidal neurons. J. Neurosci 33, 7905–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa N, Saitoh Y, Suzuki A, Tsujimura S, Murayama E, Kosugi S, Nishizono H, Matsuo M, Takahashi Y, Nagase M, et al. (2015). Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11, 261–269. [DOI] [PubMed] [Google Scholar]
- Oishi N, Nomoto M, Ohkawa N, Saitoh Y, Sano Y, Tsujimura S, Nishizono H, Matsuo M, Muramatsu S-I, and Inokuchi K (2019). Artificial association of memory events by optogenetic stimulation of hippocampal CA3 cell ensembles. Mol. Brain 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, and Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, and Nadel L (1978). The Hippocampus as a Cognitive Map (Oxford University Press; ). [Google Scholar]
- Pacheco Estefan D, Sánchez-Fibla M, Duff A, Principe A, Rocamora R, Zhang H, Axmacher N, and Verschure PFMJ (2019). Coordinated representational reinstatement in the human hippocampus and lateral temporal cortex during episodic memory retrieval. Nat. Commun 10, 2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, and Buzsáki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, and Tonegawa S (2019). Engram cell excitability state determines the efficacy of memory retrieval. Neuron 101, 274–284.e5. [DOI] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillón G, Lopera F, and Stern CE (2010). Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol 68, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, and Sahay A (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun 8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, Ryan TJ, and Tonegawa S (2013). Creating a false memory in the hippocampus. Science 341, 387–391. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, and Tonegawa S (2015). Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Couey JJ, Marcelo IM, Bouwkamp CG, Slump DE, Matos MR, van der Loo RJ, Martins GJ, van den Hout M, van IJcken WF, et al. (2019). Engram-specific transcriptome profiling of contextual memory consolidation. Nat. Commun 10, 2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza SA, Albrecht A, Çalışkan G, Müller B, Demiray YE, Ludewig S, Meis S, Faber N, Hartig R, Schraven B, et al. (2017). HIPP neurons in the dentate gyrus mediate the cholinergic modulation of background context memory salience. Nat. Commun 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, and Tonegawa S (2014). Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, and Buzsáki G (2015). Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, and Tonegawa S (2016). Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Muralidhar S, Smith LM, and Tonegawa S (2017). Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. USA 114, E9972–E9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Park Y-G, Ogawa SK, Cho JH, Choi H, Kamensky L, Martin J, Chung K, and Tonegawa S (2019). Brain-wide mapping of contextual fear memory engram ensembles supports the dispersed engram complex hypothesis. BioRxiv.
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, and Buzsáki G (2012). Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci 15, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, and O’Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav. Neurosci 113, 867–880. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, and Tonegawa S (2015). Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Eich JE, and Tulving E (1978). Richard Semon’s theory of memory. J. Verbal Learning Verbal Behav 17, 721–743. [Google Scholar]
- Schultz H, Tibon R, LaRocque KF, Gagnon SA, Wagner AD, and Staresina BP (2019). Content Tuning in the Medial Temporal Lobe Cortex: Voxels that Perceive, Retrieve. Eneuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon RW (1921). The mneme (New York: The Macmillan Company; ). [Google Scholar]
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, Oh MM, Nicholson DA, and Disterhoft JF (2015). Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression. J. Neurosci 35, 13206–13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, and McNaughton BL (1992). Computational approaches to hippocampal function. Curr. Opin. Neurobiol 2, 209–211. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, and Barnes CA (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci 12, 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, and Losonczy A (2018). CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci 21, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M, Gillespie AK, and Frank LM (2018). Neural activity patterns underlying spatial coding in the hippocampus. Current Topics in Behavioral Neurosciences 37, 43–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, and Clark RE (2004). The medial temporal lobe. Annu. Rev. Neurosci 27, 279–306. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Reber TP, Niediek J, Boström J, Elger CE, and Mormann F (2019). Recollection in the human hippocampal-entorhinal cell circuitry. Nat. Commun 10, 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli T, Bertollini C, Lüscher C, Muller D, and Mendez P (2016). Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89, 1074–1085. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, and Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Sun X, Bernstein MJ, Meng M, Rao S, Sørensen AT, Yao L, Zhang X, Anikeeva PO, and Lin Y (2020). Functionally Distinct Neuronal Ensembles within the Memory Engram. Cell 181, 410–423.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, and Magee JC (2009). Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, and Berényi A (2020). Oscillotherapeutics - Time-targeted interventions in epilepsy and beyond. Neurosci. Res 152, 87–107. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, and Wagner AD (2010). The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178–1193. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ (2020). Heterogeneous representations in the hippocampus. Neurosci. Res [DOI] [PubMed]
- Tanaka KZ, and McHugh TJ (2018). The hippocampal engram as a memory index. J. Exp. Neurosci 12, 1179069518815942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, and Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ, He H, Tomar A, Niisato K, Huang AJY, and McHugh TJ (2018). The hippocampal engram maps experience but not place. Science 361, 392–397. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, and Wiltgen BJ (2013). Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol 23, 99–106. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, and DiScenna P (1985). The role of hippocampus in memory: a hypothesis. Neurosci. Biobehav. Rev 9, 377–389. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, and DiScenna P (1986). The hippocampal memory indexing theory. Behav. Neurosci 100, 147–154. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, and Rudy JW (2007). The hippocampal indexing theory and episodic memory: updating the index. Hippocampus 17, 1158–1169. [DOI] [PubMed] [Google Scholar]
- Tingley D, and Buzsáki G (2018). Transformation of a Spatial Map across the Hippocampal-Lateral Septal Circuit. Neuron 98, 1229–1242.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Morrissey MD, and Kitamura T (2018). The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci 19, 485–498. [DOI] [PubMed] [Google Scholar]
- Trelle AN, Carr VA, Guerin SA, Thieu MK, Jayakumar M, Guo W, Nadiadwala A, Corso NK, Hunt MP, Litovsky CP, et al. (2020). Hippocampal and cortical mechanisms at retrieval explain variability in episodic remembering in older adults. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, Black SL, Reijmers LG, and Dupret D (2016). Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat. Neurosci 19, 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-Dos-Santos V, Reeve HM, Perestenko PV, Garas FN, Magill PJ, et al. (2019). A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell 176, 1393–1406.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (2002). Episodic memory: from mind to brain. Annu. Rev. Psychol 53, 1–25. [DOI] [PubMed] [Google Scholar]
- Tyssowski KM, DeStefino NR, Cho J-H, Dunn CJ, Poston RG, Carty CE, Jones RD, Chang SM, Romeo P, Wurzelmann MK, et al. (2018). Different neuronal activity patterns induce different gene expression programs. Neuron 98, 530–546.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M, and de la Prida LM (2018). The hippocampus in depth: a sublayer-specific perspective of entorhinal-hippocampal function. Curr. Opin. Neurobiol 52, 107–114. [DOI] [PubMed] [Google Scholar]
- Viana da Silva S, Zhang P, Haberl MG, Labrousse V, Grosjean N, Blanchet C, Frick A, and Mulle C (2019). Hippocampal mossy fibers synapses in CA3 pyramidal cells are altered at an early stage in a mouse model of alzheimer’s disease. J. Neurosci 39, 4193–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS (2001). Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11, 578–598. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A, Okuyama T, Sun C, Smith LM, Abe K, and Tonegawa S (2018). Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. USA 115, E310–E316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, Teixeira CM, Wheeler AL, and Frankland PW (2009). The precision of remote context memories does not require the hippocampus. Nat. Neurosci 12, 253–255. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, and Redish AD (2015). Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr. Opin. Neurobiol 32, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, and Tanila H (2005). Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci 25, 6877–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, and Fanselow MS (2001). Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav. Neurosci 115, 26–32. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, and Silva AJ (2010). The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol 20, 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, and Sekeres M (2007). Memory consolidation or transformation: context manipulation and hippocampal representations of memory. Nat. Neurosci 10, 555–557. [DOI] [PubMed] [Google Scholar]
- Wirt RA, and Hyman JM (2019). ACC Theta Improves Hippocampal Contextual Processing during Remote Recall. Cell Rep. 27, 2313–2327.e4. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR, Jang Y, Papesh MH, Goldinger SD, Kuhn JR, Smith KA, Treiman DM, and Steinmetz PN (2014). Sparse and distributed coding of episodic memory in neurons of the human hippocampus. Proc. Natl. Acad. Sci. USA 111, 9621–9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Goldinger SD, Squire LR, Kuhn JR, Papesh MH, Smith KA, Treiman DM, and Steinmetz PN (2018). Coding of episodic memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 115, 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Ranganath C, Ekstrom AD, and Wiltgen BJ (2019). A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat. Rev. Neurosci 20, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TR, Larosa A, Di Raddo M-E, Wong V, Wong AS, and Wong TP (2019). Negative memory engrams in the hippocampus enhance the susceptibility to chronic social defeat stress. J. Neurosci 39, 7576–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, He Y, Rehman AU, Kong Y, Hong S, Ding G, Yalamanchili HK, Wan Y-W, Paul B, Wang C, et al. (2019a). Loss of function of NCOR1 and NCOR2 impairs memory through a novel GABAergic hypothalamus-CA3 projection. Nat. Neurosci 22, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, Tian Z, Huang B, Yan E, Liu X, et al. (2019b). A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat. Neurosci 22, 1986–1999. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, and Brown MW (1997). Differential activation of the rat hippocampus and perirhinal cortex by novel visual stimuli and a novel environment. Neurosci. Lett 229, 141–143. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, and Schnitzer MJ (2013). Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci 16, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]


