Abstract
Background
Multiple infection outbreaks have been linked to contaminated duodenoscopes worldwide. However, the contamination rate of patient-ready duodenoscopes varies highly amongst published studies testing this subject. We aimed to estimate the contamination rate of reprocessed patient-ready duodenoscopes for endoscopic retrograde cholangio-pancreatography (ERCP) based on currently available data.
Methods
We searched the PubMed and Embase databases from January 1, 2010 until March 10, 2020, for citations investigating contamination rates of reprocessed patient-ready duodenoscopes. Studies not assessing other types of endoscopes than duodenoscopes were excluded from the analysis. Study eligibility and data extraction was evaluated by three reviewers independently. A random-effects model (REM) based on the proportion distribution was used to calculate the pooled total contamination rate of reprocessed patient-ready duodenoscopes. Subgroup analyses were carried out to assess contamination rates when using different reprocessing methods by comparing single high-level disinfection (HLD) with double HLD and ethylene oxide (EtO) gas sterilization. Additionally, we investigated the contamination rate between studies conducted following an outbreak compared to non-outbreak-initiated studies.
Findings
We identified 15 studies that fulfilled the inclusion, which included 925 contaminated duodenoscopes from 13,112 samples. The calculated total weighted contamination rate was 15.25% ± 0.018 (95% confidence interval [Cl]: 11.74% - 18.75%). The contamination rate after only using HLD was 16.14% ± 0.019 (95% Cl: 12.43% - 19.85%) and after using either dHLD or EtO the contamination rate decreased to 9.20% ± 0.025 (95% Cl: 4.30% - 14.10%). Studies conducted following an outbreak (n=4) showed a 5.72% ± 0.034 (95% Cl: 0.00% - 12.43%) contamination rate, and non-outbreak-initiated studies (n=11) revealed a contamination rate of 21.50% ± 0.031 (95% Cl: 15.35% - 27.64%).
Interpretation
This is the first meta-analysis to estimate the contamination rate of patient-ready duodenoscopes used for ERCP. Based on the available literature, our analysis demonstrates that there is a 15.25% contamination rate of reprocessed patient-ready duodenoscopes. Additionally, the analysis indicates that dHLD and EtO reprocessing methods are superior to single HLD but still not efficient in regards to cleaning the duodenoscopes properly. Furthermore, studies conducted following an outbreak did not entail a higher contamination rate compared to non-outbreak-initiated studies.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Keywords: Duodenoscopes, Cross infection, Equipment contamination, Drug Resistance, Multiple, Bacterial*, Infection Control
Abbreviations: AGA, American Gastroenterological Association; AORN, The Joint Commission and The Association of Perioperative Registered Nurses; CRE, carbapenem-resistant Enterobacteriaceae; CDC, Center for Disease Control and Prevention; Cl, confidence interval; CFU, colony-forming units; dHLD, double high-level disinfection; ERCI, Environmental Risk Communications, Inc.; ERCP, endoscopic retrograde cholangio-pancreatography; EtO, ethylene oxide; FDA, Food & Drug Administration; HLD, high-level disinfection; MDR, multi-drug-resistant; MeSH, medical subject headings; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; REM, random-effects model
Research in context.
Evidence before this study
Worldwide, multiple outbreaks have been reported due to contaminated patient-ready duodenoscopes. In the past years contaminated endoscopes have gained more and more attention, and The Food and Drug Administration (FDA) has recently they stated that healthcare facilities should consider transition to duodenoscopes with newer and innovative designs in order to minimize the risk of infection. Despite multiple outbreaks, various updated reprocessing guidelines, and safety communications, evidence is still missing within this topic. We aimed to estimate the contamination rate of reprocessed patient-ready duodenoscopes based on currently available data.
Added value of this study
Currently, no data exists on one generalizable contamination rate of duodenoscopes summarizing all published evidence. This study demonstrates a higher-than-expected contamination rate of patient-ready duodenoscopes. Current evidence is limited, but with the increased focus on cross-contamination an updated reference point is highly needed. Various studies and guidelines are currently citating a study from the 1990s stating that the risk of infection due to a contaminated duodenoscope is 1 out of 1.8 million. However, new evidence suggests that the risk is much higher which is also being support by the interim results from the FDA postmarket surveillance study showing a 9% contamination rate associated with reprocessed duodenoscopes.
Implications of all the available evidence
Our study suggests that patient-ready duodenoscopes are associated with a 15.25% contamination rate. Additionally, our study indicates that none of the current reprocessing methods are efficient in regards to cleaning duodenoscopes properly. The findings highlight the need for more studies to be conducted in the future to address issues related to contaminated reusable duodenoscopes that may potentially lead to cross-infections and patient harm following endoscopy.
Alt-text: Unlabelled box
1. Introduction
More than 600,000 endoscopic retrograde cholangio-pancreatography (ERCP) procedures are performed annually in the United States [1]. An ERCP procedure is an important and less invasive treatment alternative to open surgery in the bile duct and pancreatic duct [2,3]. At present, these highly beneficial ERCP procedures are primarily performed using reusable duodenoscopes; however, due to their complex design, duodenoscopes are difficult to clean properly [4], [5], [6]. Insufficient cleaning of duodenoscopes results in microbiological debris in patient-ready duodenoscopes, thus leading to patient-to-patient cross-contamination, the transfer of multi-drug-resistant (MDR) organisms, and subsequent infections [6,7]. Hence, contaminated duodenoscopes have resulted in multiple outbreaks and deaths involving MDR organisms across the world [8,9].
Awareness of the risk of post-endoscopic infections caused by contaminated duodenoscopes has increased significantly over the recent years. On the 19 February 2015, the Food & Drug Administration (FDA) published the first duodenoscope-related Safety Communication; this stated that the design of duodenoscopes may impede effective cleaning, and therefore lead to the potential transmission of microorganisms in the patient-ready high-level-disinfected (HLD) duodenoscopes used for ERCP. From 2015 to 2019, 17 out of 85 (20%) of the FDA's Medical Device Safety Communications have been related to endoscopes, predominantly targeting contaminated reusable duodenoscopes [10].
In October 2015, the FDA ordered manufacturers of duodenoscopes sold in the United States (Fujifilm Medical Systems USA, Inc; Olympus Medical Systems Corporation; Pentax of America) to conduct post-market surveillance studies to evaluate contamination rates and to clarify how duodenoscopes are reprocessed in real-world settings. The interim results from these post-market surveillance studies were presented in March 2019 and indicated higher than expected levels of contamination [11]. Prior to the post-market surveillance studies, the FDA expected a contamination rate lower than 0.4% [12]. In the post-market surveillance study, 5.4% of samples that were collected appropriately tested positive for organisms of high-concern (e.g., E. coli and P. aeruginosa). In addition, 3.6% of the collected samples tested positive for organisms that were of low to moderate concern (>100 colony-forming units (CFUs)); this constituted an overall contamination rate of 9% [12]. Controversies still remain with regards to the impact of contaminated duodenoscopes, and whether such equipment can cause post-endoscopic device-related infections that could impair patient safety [13]. Additionally, while previous studies have summarized research on duodenoscope contamination, no studies have provided an estimate of the actual contamination rate associated with patient-ready duodenoscopes. Therefore, the aim of this systematic review was to estimate the contamination rate of patient-ready duodenoscopes based on currently available literature.
2. Methods
2.1. Study selection
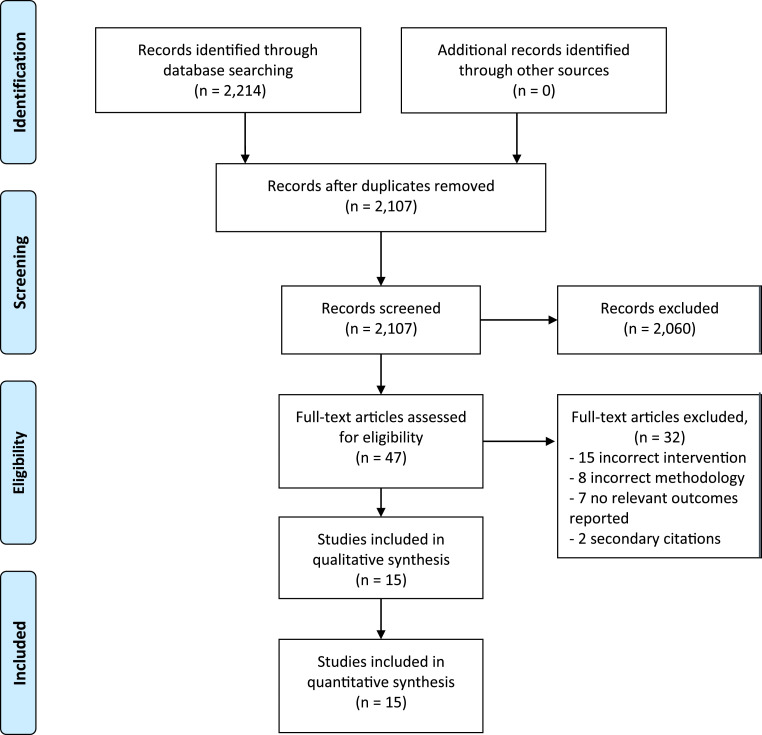
We carried out a comprehensive and systematic literature search to identify full-text human studies, published in English, investigating contamination rates associated with duodenoscopes. The complete literature search is presented in Figure 1. Methods of analysis, and inclusion criteria, were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guideline [14].
Fig. 1.
Flowchart describing the study process and the selection of publications.
Studies were identified through a systematic literature search from January 1, 2010 until March 10, 2020 in the PubMed and Embase electronic databases. To identify and include relevant studies, we conducted the search using the following medical subject headings (MeSH) and keywords: (“duodenoscop*” [All Fields] OR “gastrointestinal endoscop*” [All Fields]) AND ((“infection*” [MeSH Terms] OR “cross infection*” [MeSH Terms] OR “outbreak*” [All Fields] OR “device contamination*” [All Fields] OR “hospital infection*” [All Fields] OR “disinfection*” [MeSH Terms] OR “bacteria*” [MeSH Terms]) AND English [lang]. Truncation was deployed after some keywords to include different variations of the term.
2.2. Inclusion and exclusion criteria
The search was conducted to identify all relevant randomized controlled trials, systematic reviews, meta-analyses, case reports, surveillance studies, and prospective or retrospective cohort studies assessing the contamination rate associated with reprocessed duodenoscopes. The search was limited to studies published after 2010 since a time horizon of 10 years was considered reasonable due to various updated endoscope reprocessing guidelines [15], [16], [17], [18]. For inclusion, studies needed to state the total number of duodenoscopes sampled (N) and the number of contaminated duodenoscopes (positive cultures). There was also the requirement that all samples were acquired from a duodenoscope and not from a patient. Studies performed in animals or in vitro models were excluded. Other exclusion criteria were abstracts, editorials, and letters that did not report original results. Studies published in grey literature were excluded as well. Lastly, studies with less than 10 samples were excluded to avoid bias in the random effects model that may have arisen due to small sample sizes [19].
The titles and abstracts of the identified studies were independently reviewed by two authors (SL, HT). Studies that did not fulfill the abovementioned criteria were excluded, and the full-texts of the remaining publications were carefully independently evaluated by three authors (SL, HT, RR). Disagreements were resolved by consensus assessment.
2.3. Data extraction
All included studies were assessed for eligibility by three independent reviewers (SL, HT, RR). None of the authors was blinded to any information within the studies. For each included study, we extracted the following data: author, year of publication, study design, country, hospital, number of annual ERCPs, type of microorganism, number of contaminated duodenoscopes (e.g., the number of positive samples), total number of sampled duodenoscopes, reprocessing method, colony forming unit (CFU) level, and sampling setting (e.g., outbreak vs. non-outbreak).
2.4. Outcomes
The primary outcome of the pooled analysis was the total weighted contamination rate based on the number of contaminated duodenoscopes (the number of positive samples) relative to the number of duodenoscopes sampled. A subgroup analysis was carried out to assess the contamination rate amongst duodenoscopes reprocessed using HLD and duodenoscopes reprocessed with either double HLD (dHLD) or ethylene oxide (EtO) gas sterilization to see if there were any significant differences between the cleaning methods. Another subgroup analysis was carried out to assess whether studies conducted following an outbreak entailed a higher contamination rate compared to non-outbreak initiated studies.
Patient-specific data were not assessed since the analysis only focus on the duodenoscopes. There were no missing data for any of the data points used to calculate the weighted contamination rate.
2.5. Data analysis and statistical methods
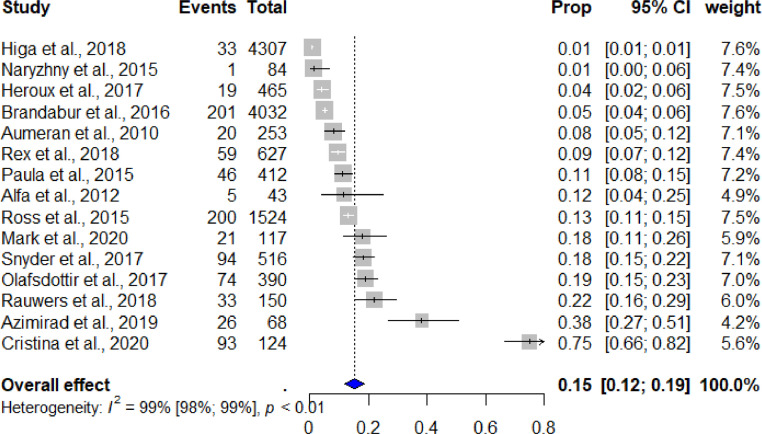
The meta-analysis analyzed data from studies where a duodenoscope contamination rate was assessed. The primary objective of the analysis was to assess the total contamination rate of reprocessed patient-ready duodenoscopes. Two subgroup analyses were carried out to 1) assess the contamination rate of patient-ready duodenoscopes reprocessed using HLD and assess the contamination rate of patient-ready duodenoscopes reprocessed using either dHLD or EtO gas sterilization to compare the different reprocessing methods, and 2) assess the contamination rate for outbreak-initiated studies and non-outbreak-initiated studies. For all statistical analyses we used the meta package (metafor) in RStudio V.3.6.2. Data were pooled using a random effects model based on proportions. We applied the random effects model because we anticipated heterogeneity, particularly arising from variations in both sample size and population. We estimated the level of heterogeneity between the included studies using the inconsistency index (I2) test, which indicates the proportion (%) of variation between the studies that is linked to heterogeneity rather than being a coincidence [20,21]. Heterogeneity values below 25% were defined as being indicative of low levels of heterogeneity [21]. Publication bias is known to impact the validity and generalizability of conclusions based on meta-analyses [22]. We assessed publication bias using funnel plots and evaluated the asymmetry of the funnel using Egger's regression test. A forest plot of all outcomes was created using the random effects model, as shown in Fig. 2.
Fig. 2.
Pooled estimates of contamination rates. Cl: confidence interval.
2.6. Role of the funding source
This study did not receive any funding.
3. Results
3.1. Characteristics of included studies
We identified a total of 2214 studies, of which 2107 of these studies were screened based on title and abstract review. After applying our inclusion and exclusion criteria, the search was narrowed to 47 studies, which were reviewed in full detail. The PRISMA flowchart of the literature search, and the selection process, is demonstrated in Fig. 1. After assessing the full-text articles for eligibility, 15 studies were included in the final analysis.
All of the 15 studies included in the final analysis were published between January 1, 2010 and March 10, 2020. The included studies yielded a sample size of 13,112 duodenoscopes that had been sampled for culture testing. The total number of contaminated duodenoscopes (the positive samples) was 925.
The baseline characteristics of the included studies are provided in Table 1. The majority of the included studies were conducted in the United States (n=8, 53.3%), while the remaining 46.7% of the studies were conducted in the Netherlands (n=1, 6.7%), France (n=1, 6.7%), Canada (n=1, 6.7%), Italy (n=1, 6.7%), Iran (n=1, 6.7%), and in Austria (n=1, 6.7%). Twelve out of 15 (80.0%) studies report using HLD as the reprocessing method, one study report using EtO (6.7%), and two studies have tested a combination of both HLD, dHLD, and EtO (13.3%). Six out of 15 (40%) studies report the CFU threshold used to determine the contamination rate. Four studies (26.7%) are using the recommended CFU threshold of >10 stated in the Center for Disease Control and Prevention (CDC) protocol, while one study (6.7%) uses a threshold of >20 CFU, and a study (6.7%) reports a CFU threshold of >100 CFU. Four out of 15 studies (26.7%) were conducted following an outbreak, while the remaining 11 studies were conducted independent of any outbreak detected.
Table 1.
Study characteristics of included studies.
| First author, year | Study design | Country | Hospital | Annual ERCPs | Contaminated duodenoscopes, n* | Cultures, n | Type of microorganism | Reprocessing method | CFU threshold | Sampling setting |
|---|---|---|---|---|---|---|---|---|---|---|
| Snyder, 2017 [23] | Parallel group randomized study | USA | Beth Israel Deaconess Medical Center |
1,500 | 94 | 516 | N/A | HLD, dHLD, HLD/EtO | >0 CFU | Non-outbreak |
| Rauwers, 2018 [24] | Descriptive study | Netherlands | 67 Dutch ERCP centers1 | N/A | 33 | 150 | Yeasts, Moraxella spp., Klebsiella pneumoniae, Streptococcus salivarius, Enterobacter cloacae, Moraxella osloensis, Escherichia coli, Streptococcus mitis, Klebsiella oxytoca, Neisseria flavescens, Enterococcus faecium, Rothia spp., Enterococcus faecalis, Streptococcus mutans, Pseudomonas aeruginosa, Streptococcus oralis, Staphylococcus aureus, Streptococcus spp. Bacillus spp., Stenotrophomonas maltophilia, Micrococcus luteus, Acinetobacter spp., Staphylococcus epidermidis, Agrobacterium radiobacter, Kocuria spp., Paracoccus yeeii, Staphylococcus hominis, Achromobacter xylosoxidans, Staphylococcus warneri, Alternaria spp., Kocuria rhizophila, Pseudomonas monteilii, Micrococcus spp., Pseudomonas putida, Staphylococcus auricularis, Sphingomonas paucimobilis, Staphylococcus spp. (CNS), Rhizobium spp. or Sphingobium spp. |
HLD | ≥ 20 CFU | Non-outbreak |
| Rex, 2018 [25] | Parallel group randomized study | USA | N/A | 3,000 | 59 | 627 | Enterococcus spp. Candida spp., Zygomycete, Micrococcus spp., Staphylococcus (CNS), Bacillus spp. Corynebacterium spp. | dHLD | N/A | Non-outbreak |
| Heroux, 2017 [26] | Parallel group randomized study | USA | Beth Israel Deaconess Medical Center |
1,500 | 19 | 465 | N/A | HLD, dHLD, EtO | ≥10 CFU | Non-outbreak |
| Olafsdottir, 2017 [27] | Parallel group randomized study | USA | Beth Israel Deaconess Medical Center |
1,500 | 74 | 390 | N/A | HLD | >0 CFU | Non-outbreak |
| Paula, 2015 [28] | Descriptive study | Austria | Vienna University Hospital | 700 | 46 | 412 | Unspecified skin bacteria and aerobe spore-forming bacilli |
HLD | >100 CFU | Non-outbreak |
| Ross, 2015 [29] | Descriptive study | USA | Virginia Mason Medical Center | 1,500 | 200 | 1524 | Acinetobacter, Enterococcus, Escherichia coli, Enterobacter, Pseudomonas aeruginosa, Staphylococcus aureus (methicillin sensitive), Staphylococcus aureus (methicillin resistant) | HLD | N/A | Outbreak |
| Naryzhny, 2015 [30] | Descriptive study | USA | N/A | N/A | 1 | 84 | Carbapenem-resistant Enterobacteriaceae | EtO | N/A | Outbreak |
| Mark, 2020 [31] | Descriptive study | USA | Children's Hospital Colorado | N/A | 21 | 117 | Pseudomonas aeruginosa, fungal organisms, Staphylococcus aureus, Coagulase negative staphylococcus, Viridans streptococcus | HLD | >10 CFU | Non-outbreak |
| Alfa, 2012 [32] | Descriptive study | Canada | St Boniface General Hospital |
N/A | 5 | 43 | gram-positive Bacilli, gram-positive Cocci | HLD | N/A | Non-outbreak |
| Higa, 2018 [33] | Parallel group randomized study | USA | N/A | N/A | 33 | 4307 | Staphylococcus aureus, Streptococcus viridans, Enterococcus, and other pathogenic enteric gramnegative organisms. | HLD | N/A | Outbreak |
| Azimirad, 2019 [34] | Descriptive study | Iran | N/A | N/A | 26 | 68 | Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus spp., Staphylococcus epidermidis, Escherichia coli, Enterobacter spp., Clostridium perfringenes |
HLD | N/A | Non-outbreak |
| Brandabur, 2016 [35] | Parallel group randomized study | USA | 21 unspecified facilities | N/A | 201 | 4032 | Coagulase-negative staphylococci, Bacillus spp, coryneform gram-positive bacilli, gram-negative glucose-nonfermenters, enteric gram-negative bacilli, Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Enterococcus spp, and Stenotrophomonas maltophilia | HLD | N/A | Non-outbreak |
| Cristina, 2020 [36] | Descriptive study | Italy | N/A | 350 | 93 | 124 | Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Klebsiella oxytoca, Stenotrophomonas maltophilia, Escherichia coli, Citrobacter freundii, Enterobacter spp | HLD | >10 CFU | Non-outbreak |
| Aumeran, 2010 [37] | Descriptive study | France | N/A | N/A | 20 | 253 | Klebsiella pneumoniae ESBL CTX-M-15, Escherichia coli ESBL, Other multiresistant E. coli, Nonmultiresistant Enterobacteriaceae, Multiresistant Pseudomonas aeruginosa, Nonmultiresistant P. aeruginosa, Coagulase -negative staphylococci, Enterococci, Anaerobes, yeasts | HLD | N/A | Outbreak |
Number of positive sampled cultures; colony forming units (CFU); Species (spp.); coagulase - negative staphylococci (CNS).
Academic Medical Center (AMC), Amsterdam; Albert Schweitzer Hospital, Dordrecht; Alrijne Hospital, Leiden/Leiderdorp; Amphia Hospital, Breda; Antoni van Leeuwenhoek, Amsterdam; Antonius Zorggroep, Sneek; Beatrix Hospital, Gorinchem; Bernhoven, Uden; Bravis Hospital, Roosendaal; Canisius Wilhelmina Hospital, Nijmegen; Catharina Hospital Eindhoven, Eindhoven; Deventer Hospital, Deventer; Diakonessenhuis, Utrecht; Elkerliek Hospital, Helmond; Erasmus University Medical Center (Erasmus MC), Rotterdam; FlevoHospital, Almere; Groene Hart Hospital, Gouda; Hospital Amstelland, Amstelveen; Hospital De Tjongerschans, Heerenveen; Hospital Gelderse Vallei, Ede; Hospital St. Jansdal, Harderwijk; IJsselland Hospital, Capelle aan Den IJssel; Ikazia, Rotterdam; Isala Diaconessenhuis, Meppel; Jeroen Bosch Hospital, ’s Hertogenbosch; Laurentius Hospital, Roermond; Leiden University Medical Center (LUMC), Leiden; Maasstad Hospital, Rotterdam; Maastricht University Medical Center (MUMC), Maastricht; Martini Hospital, Groningen; Maxima Medical Center, Veldhoven; Meander Medical Center, Amersfoort; Medical Center Alkmaar, Alkmaar; Medical Center Haaglanden, Den Haag; Medical Center Leeuwarden, Leeuwarden; Medical Center Slotervaart, Amsterdam; Medical Center Zuiderzee, Lelystad; Medisch Spectrum Twente, Enschede; Nij Smellinghe Hospital, Drachten; Ommelander Hospital Group, Delfzijl; Onze Lieve Vrouwe Gasthuis—Location East/West, Amsterdam; Radboud University Medical Center (Radboudumc), Nijmegen; Reinier de Graaf Hospital, Delft; Rijnstate Hospital, Arnhem; Rode Kruis Hospital, Beverwijk; Slingeland Hospital, Doetinchem; St. Anna Hospital, Eindhoven; St. Antonius Hospital, Nieuwegein; St. Elisabeth Hospital, Tilburg; St. Franciscus Gasthuis, Rotterdam; Tergooi Hospital, Hilversum; Treant Zorggroep, Location Scheper, Emmen; TweeSteden Hospital, Tilburg/ Waalwijk; University Medical Center Groningen (UMCG), Groningen; University Medical Center Utrecht (UMCU), Utrecht; VieCuri Medical Center, Venlo; VU Medical Center, Amsterdam; Westfriesgasthuis, Hoorn; Wilhelmina Hospital Assen, Assen; Zaans Medical Center, Zaandam; Ziekenhuisgroep Twente, Almelo; ZorgSaam Hospital, Terneuzen; Zuwe Hofpoort Hospital, Woerden; Zuyderland Medical Center, Heerlen.
3.2. Analysis of primary outcomes
Meta-analysis of the included studies demonstrated an overall pooled contamination rate of 15.25% ± 0.018 (95% confidence interval [Cl]: 11.74% - 18.75%; I2 = 98.6%). Heterogeneity between the included studies (n=15) was considered to be high. Funnel plot analysis, and Egger's regression test, for the included contamination studies indicated significant publication bias (Egger's test of publication bias: p<0.001) (Fig. 2). Funnel plots are presented in Supplementary Figure 1.
3.3. Subgroup analyses
Meta-analysis of the included studies only assessing the contamination rate after the duodenoscopes have been reprocessed using HLD demonstrated a pooled contamination rate of 16.14% ± 0.019 (95% Cl: 12.43% - 19.85%; I2 = 98.8%). Meta-analysis of the included studies only assessing the contamination rate after the duodenoscopes have been reprocessed using either dHLD or EtO sterilization demonstrated a pooled contamination rate of 9.20% ± 0.025 (95% Cl: 4.30% - 14.10%; I2 = 92.3%). Heterogeneity between the included studies for both populations (HLD vs. dHLD/EtO) was considered to be high. Meta-analysis of outbreak-initiated studies (n=4) demonstrated a pooled contamination rate of 5.72% ± 0.034 (95% Cl: 0.00% - 12.43%). Non-outbreak-initiated studies (n= 11) showed a contamination rate of 21.50% ± 0.031 (95% Cl: 15.35% - 27.64%). Heterogeneity in both populations was considered high.
4. Discussion
This study is the first to estimate the contamination rate of patient-ready duodenoscopes used for ERCP based on currently available literature. To estimate the contamination rate, we performed a systematic literature review and meta-analysis. The results of our study identified a 15.25% contamination rate of reprocessed patient-ready duodenoscopes.
Multiple outbreaks in the past decades have, to some extent, created an awareness of duodenoscope-related infections caused by contaminated duodenoscopes. However, a number of guidelines continuously underestimate the actual endoscope-related risk of infection; consequently, the present study aimed to quantify the contamination rate associated with patient-ready duodenoscopes [38], [39], [40]. An endoscope-related infection risk can be difficult to identify in some cases, due to the baseline risk of infection associated with ERCP. Approximately 7% of complications are directly related to ERCP procedures; furthermore, 1% out of these complications is categorized as severe and often requires intervention, blood transfusion, or hospitalization for more than 10 days [41]. Additionally, the harm of a duodenoscope-related infection following ERCP might be difficult to quantify as the procedure itself is often lifesaving. A previous review by Rubin et al. identified 32 outbreaks between 2000 and 2017, involving almost 400 patients. These authors stated that the acquisition of precise data related to duodenoscope-related infections is difficult, since many of the existing studies failed to perform screening cultures on exposed asymptomatic patients, or simply failed to include these data. Lastly, Rubin et al. highlighted that the list of studies they compiled may have underestimated the morbidity and mortality associated with infections caused by contaminated duodenoscopes; this was because their analysis only captured outbreaks that had been reported [8]. These results indicate the difficulties in attributing an infection risk to the amount of contaminated duodenoscopes.
Because it is difficult to identify the endoscope-related infection risk caused by contaminated duodenoscopes, the severe consequences of infections transmitted through a contaminated duodenoscope are often underestimated. A study published by Humphries et al., reported that six out of nine patients with duodenoscope-related carbapenem-resistant Enterobacteriaceae (CRE) infections had died one year after an outbreak had been identified, although only two deaths were directly related to a CRE infection caused by a contaminated duodenoscope [42]. The overall mortality associated with ERCP is estimated to be approximately 0.3% [41,43]. However, mortality rates can be difficult to link directly to contaminated duodenoscopes; thus, these data are rarely published, and the actual mortality rate related to contaminated duodenoscopes may be underestimated [8]. Our findings suggest a higher than expected contamination rate which may also indicate a higher-than expected endoscope-related infection risk, and potentially mortality rate, associated with ERCP [44,45]. Multiple US guidelines, including the Centers for Disease Control and Prevention (CDC) state that “Even though endoscopes represent a valuable diagnostic and therapeutic tool in modern medicine and the incidence of infection associated with their use reportedly is very low (about 1 in 1.8 million procedures), more healthcare-associated outbreaks have been linked to contaminated endoscopes than to any other medical device” [38]. The reported incidence of 1 infection in 1.8 million procedures was originally published in 1993 by Kimmery et al., and may not necessarily reflect the current status of duodenoscope-related outbreaks [46]. In addition, since 2010, reusable flexible endoscopes have featured on every annual list of the ‘Top 10 Technology Hazards’ published by the Environmental Risk Communications, Inc. (ERCI) Institute, due to issues related to cross-contamination, inadequate reprocessing, and infection risk [47]. In general, quality problems in healthcare tend to be overlooked, and that problems are in fact much larger than we normally assume. This also seems to be the case for contaminated duodenoscopes, and other endoscopes such as bronchoscopes, gastroscopes, and colonoscopes [48], [49], [50], [51], [52], [53].
Despite many gastroenterology societies being aware of the current obstacles associated with reprocessing duodenoscopes, the lack of evidence for this association over the past 25 years suggests that the issue has been neglected. In October 2019, the official newspaper of the American Gastroenterological Association (AGA) Institute commented on the risk of acquiring a MDR infection with a duodenoscope by saying that “the chance of getting an identified “superbug infection” with a duodenoscope is very low, currently estimated at 1 per 20,000 ERCPs performed in more than 650,000 ERCP procedures each year in the U.S” [54]. The infection risk announced by the AGA was the highest infection risk reported this far but was still considered “very low.” However, once presented, MDR infection is expensive to treat and is also associated with a reduction in the quality of life [55]. Therefore, it is debatable as to whether 1 MDR infection per 20,000 ERCPs is considered ‘low’ relative to the harm caused by the specific infected patient. In addition, there is no scientific evidence to support this risk of infection, thus highlighting the urgent need for further studies and the acquisition of robust evidence. The AGA statement was announced shortly after the FDA published a Safety Communication on the 29th August 2019, which recommended that hospitals and endoscopy facilities needed to replace fixed endcap duodenoscopes with those with newer design features that facilitate or eliminate the need for reprocessing [56].
The rate of contamination observed in our study is consistent with the rates of contamination for other endoscopes, including bronchoscopes, gastroscopes, and colonoscopes [57], [58], [59], [60]. However, our contamination rate is higher than the interim results from the post-market surveillance study undertaken by the FDA. The interim results published by the FDA revealed a 9% contamination rate; 5.4% of the identified organisms were classified as ‘high concern’ due to a high association with disease caused by bacteria such as E. coli and P. aeruginosa. In November 2019, the FDA released the results of their ‘Human Factors Studies’, which were designed to investigate human errors associated with manual reprocessing. These studies involved the three major manufacturers of duodenoscopes (Olympus, Pentax, and Fujifilm). The results of this study indicated an overall poor adherence to reprocessing guidelines. This was illustrated by the fact that 87% of the participants failed to complete the elevator brushing task that was described in the user manual provided by Olympus. Furthermore, the user manual of the Fujifilm duodenoscope described 33 critical manual cleaning tasks; only two of these tasks were carried out by all of the participants [61]. Furthermore, the ‘Human Factors Studies’ revealed that all cleaning personnel participating in the study failed to flush the surfaces and inspect the movable parts, and that most participants expressed difficulty adhering to the reprocessing manual [61]. These new results indicated that the issues associated with the appropriate reprocessing of duodenoscopes is caused by multiple factors, including their complex design, but also by human factors which can be very difficult to overcome. The fact that educated cleaning personnel are unable to reprocess the duodenoscopes in an appropriate manner further increases the risk of contamination, and thereby, the risk of cross-infection. Due to a high staff turnover, cleaning personnel tend to lack appropriate training, and never become highly skilled in reprocessing the endoscopes in a robust manner. According to The Joint Commission and The Association of Perioperative Registered Nurses (AORN), this practice leads to high rates of non-compliance with reprocessing guidelines [62,63]. Since 2009, the annual proportion (%) of facilities not complying with these standards have increased; in 2015, up to 60% of facilities were non-compliant due to breaches related to HLD and sterilization [62]. Although one contaminated duodenoscope does not necessarily lead to an infection, it does create a higher risk of infection; such infections are related to hospital admission, additional healthcare costs, and expenses for each ERCP patient [64].
Duodenoscopes have a complex design, which makes adequate reprocessing a challenge. Various mechanical parts inside the duodenoscope render the device heat-labile. These systems therefore require robust cleaning routines that do not require heat sterilization [65], [66], [67]. The current standard for reprocessing duodenoscopes is HLD; this standard recommends over 100 cleaning steps, including precleaning, leak-testing, manual cleaning, and drying [68], [69], [70]. Precleaning is an important step as this avoids the formation of biofilm. Manual cleaning is generally effective but difficult to control in practice. Furthermore, biofilm is not always successfully removed because of the resistance of biofilms to antibiotics, disinfectants, and biocides [71]. Therefore, despite strict adherence to the recommended reprocessing guidelines, outbreaks of MDR infections still occur. These outbreaks have led to the FDA recommending stricter reprocessing measures as an addition to the current standards provided by the manufacturers [8,72]. In 2015, The FDA recommended that healthcare facilities repeat HLD, using either EtO gas sterilization or liquid chemical sterilization. It is also important to check whether the duodenoscopes have been cleaned properly by performing microbiological cultures [72]. Reusable endoscopes have been linked to far more outbreaks than any other reusable medical devices in healthcare, thus suggesting that problems with reprocessing endoscopes, and especially duodenoscopes, remain a huge challenge, even after the FDA have recommended stricter and more thorough reprocessing measures [73]. In our analysis, 12 out of 15 (80.0%) studies report that HLD was the reprocessing method used prior to sampling and culturing the duodenoscopes. However, when only looking at the pooled contamination rate for these studies, the total weighted contamination rate increased to 16.14% from 15.25%. When only looking at studies reporting either dHLD or EtO as the reprocessing method, the contamination rate decreased to 9.20%. This supports the FDA recommendations on either using dHLD or EtO, since these reprocessing methods appear to be more effective compared to single HLD. However, even after dHLD and EtO sterilizaton duodenoscopes still remain contaminated which is a concern. Duodenoscopes become highly contaminated because they enter the gastrointestinal tract, and therefore come into contact with a very high microbial load. This, combined with the fact that duodenoscopes have a very complex design, render these systems very difficult to clean properly [73]. Therefore, reprocessing strategies are unlikely to solve the problem alone. Redesigning the duodenoscopes might represent the next step towards eliminating the cross-infections associated with contaminated reprocessed duodenoscopes [8]. Findings form our subgroup analyses comparing contamination rates between outbreak-initiated studies and non-outbreak-initiated studies showed a lower contamination rate amongst outbreak-initiated studies, indicating that duodenoscopes are contaminated even in non-outbreak settings.
While the data presented in this study are informative for decision-making and clinical guidelines, there are several limitations that should be considered. The main limitation of this review is the low quality of published evidence. There are several reasons for this shortfall. First, the included studies were associated with methodological limitations that were related to their non-randomized design, mainly because some studies were initiated because of the suspicion of an outbreak due to contaminated duodenoscopes. Secondly, the existing literature is inconsistent in regards to methodology, reprocessing methods, and especially CFU limits making the results more difficult to compare. Thirdly, there is the possibility of publication bias with regards to unpublished studies or studies publishing negative results. Publication bias represents a substantial limitation in this study, since we have to assume that several issues with contaminated duodenoscopes is unreported, especially in Europe where only a very limited number of studies have been reported. Funnel plots and Egger's regression test indicated that significant publication bias existed amongst the included studies. Lastly, limitations exist in regards to the two subgroup analyses carried out in this study. The subgroup analysis investigating the effect of different reprocessing methods include studies not directly investigating this subject which increase the risk of confounding factors affecting our findings. Additionally, both subgroup analyses were based on rather small sample sizes questioning the validity of the findings. Despite these limitations, the findings of the present study are novel and may help to create awareness about the issues concerning cross-contamination associated with reprocessed patient-ready duodenoscopes.
This study is the first to quantify the contamination rate of patient-ready duodenoscopes used for ERCP based on currently available data. Our results showed a 15.25% contamination rate of reprocessed patient-ready duodenoscopes. Additionally, the analysis indicates that dHLD and EtO reprocessing methods are superior to single HLD but still not efficient in regards to cleaning the duodenoscopes properly. Lastly, a subgroup analysis indicates that studies conducted in outbreak settings do not entail a higher contamination compared to studies initiated in non-outbreak settings. We recommend that more studies will be conducted in the future to address issues related to contaminated reusable duodenoscopes that may potentially lead to cross-infections and patient harm following ERCP.
Declaration of Competing Interest
SL, RVR, and LKO are employed by Ambu A/S, Ballerup, Denmark. SS has previously been employed by Ambu Inc, Maryland, United States. AM has previously been employed by Ambu A/S, Denmark.
Acknowledgments
Acknowledgements
The authors did not receive any specific funding for this research from any funding agency in the public, commercial or not-for-profit sectors.
Contributors
Data collection, data analysis and manuscript writing was performed by SL. Statistical analyses were performed by SL and LKO. Revision of manuscript for important intellectual content was performed by all authors.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100451.
Appendix. Supplementary materials
References
- 1.iData Research Inc.US Market Report Suite for Gastrointestinal Devices. 2016;1–535.
- 2.Adler D.G., Lieb J.G., II, Cohen J., Pike I.M., Park M.S.W.G., Rizk M.K. Quality indicators for ERCP. Gastrointest Endosc. 2015;81:54–66. doi: 10.1016/j.gie.2014.07.056www.giejournal.org. cited 2019 Oct 30Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 3.Othman M.O., Guerrero R., Alhanafi S., Davis B., Hernandez J., Houle J., Mallawaarachchi I., Dwivedi A.K., Zuckerman M.J. A prospective study of the risk of bacteremia in directed cholangioscopic examination of the common bile duct. Gastrointest Endosc. 2016;83(1):151–157. doi: 10.1016/j.gie.2015.05.018. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L604952604 Available from: [DOI] [PubMed] [Google Scholar]
- 4.Visrodia K.H., Ofstead C.L., Yellin H.L., Wetzler H.P., Tosh P.K., Baron T.H. The use of rapid indicators for the detection of organic residues on clinically used gastrointestinal endoscopes with and without visually apparent debris. Infect Control Hosp Epidemiol. 2014;35(8):987–994. doi: 10.1086/677148. [DOI] [PubMed] [Google Scholar]
- 5.Marques Ribeiro M., Cristina de Oliveira A., Maria Cunha Pinheiro Ribeiro S., Watanabe E., Aparecida de Resende Stoianoff M., Antonio Guimaraes Ferreira J. Effectiveness of flexible gastrointestinal endoscope reprocessing. Infect Control Hosp Epidemiol. 2013;34(3):309–312. doi: 10.1086/669518. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L368327072 cited 2019 Jan 24Available from: [DOI] [PubMed] [Google Scholar]
- 6.Kovaleva J., J. K., Kovaleva J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract Res Clin Gastroenterol. 2016;30(5):689–704. doi: 10.1016/j.bpg.2016.09.008. http://www.embase.com/search/results?subaction=viewrecord&from =export&id=L613199150 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Brandabur J.J., Leggett J.E., Wang L., Bartles R.L., Baxter L., Diaz G.A. Surveillance of guideline practices for duodenoscope and linear echoendoscope reprocessing in a large healthcare system. Gastrointest Endosc. 2016;84(3):392–399. doi: 10.1016/j.gie.2016.03.1480. e3. [DOI] [PubMed] [Google Scholar]
- 8.Rubin Zachary A., Kim Stephen, Thaker Adarsh M., Muthusamy R.V. Safely reprocessing duodenoscopes: current evidence and future directions. Lancet Gastroenterol Hepatol. 2018;3(1):499–508. doi: 10.1016/S2468-1253(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 9.US Senate. Preventable tragedies: superbugs and how ineffective monitoring of medical device safety fails patients. 2016.
- 10.Medical Device Safety | FDA. [cited2019]. Available from:https://www.fda.gov/medical-devices/medical-device-safety
- 11.U.S. Food and Drug Administration. Infections Associated with Reprocessed Duodenoscopes . [cited2019]. Available from:https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices/infections-associated-reprocessed-duodenoscopes
- 12.The FDA continues to remind facilities of the importance of following duodenoscope reprocessing instructions: FDA safety communication | FDA . [cited2019]. Available from:https://www.fda.gov/medical-devices/safety-communications/fda-continues-remind-facilities-importance-following-duodenoscope-reprocessing-instructions-fda
- 13.Rubin Z.A, Murthy R.K. Outbreaks associated with duodenoscopes: New challenges and controversies. Curr Opin Infect Dis. 2016;29(4):407–414. doi: 10.1097/QCO.0000000000000290. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L610891702 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264. doi: 10.7326/0003-4819-151-4-200908180-00135. http://annals.org/article.aspx?doi=10.7326/0003-4819-151-4-200908180-00135 cited 2019 Oct 9Available from: [DOI] [PubMed] [Google Scholar]
- 15.SGNA guideline for use of high level disinfectants & sterilants for reprocessing flexible gastrointestinal endoscopes . 2013 [cited 2019 Nov 14]. Available from:www.SGNA.org
- 16.Calderwood A.H., Eisen G.M. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87(5):1167–1179. doi: 10.1016/j.gie.2017.12.009. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2000570891 Available from: [DOI] [PubMed] [Google Scholar]
- 17.Beilenhoff U., Biering H., Blum R., Brljak J., Cimbro M., Dumonceau J.-M. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Up. Endoscopy. 2018;50(12):1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 18.Guideline for use of high-level disinfectants and sterilants for reprocessing flexible gastrointestinal endoscopes. Gastroenterol Nurs. 2015;38(1):70–80. doi: 10.1097/SGA.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 19.Chen H.Y., Shen D.T., Ji D.Z., Han P.C., Zhang W.M., Ma J.F. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68(3):512–521. doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Aert R.C.M., Wicherts J.M., Van Assen M.A.L.M. Publication bias examined in meta-analyses from psychology and medicine: a meta-meta-analysis. PLoS One. 2019 Apr 1;14(4) doi: 10.1371/journal.pone.0215052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder G.M., Wright S.B., Smithey A., Mizrahi M., Sheppard M., Hirsch E.B., Chuttani R., Heroux R., Yassa D.S., Olafsdottir L.B., Davis R.B., Sawhney M.S. Randomized Comparison of 3 High-Level Disinfection and Sterilization Procedures for Duodenoscopes. Gastroenterology. 2017;153(4):1018–1025. doi: 10.1053/j.gastro.2017.06.052. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L618941107 Available from: [DOI] [PubMed] [Google Scholar]
- 24.Rauwers A.W., Voor In ’t Holt A.F., Buijs J.G., de Groot W., Hansen B.E., Bruno M.J. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut. 2018;67(9):1637–1645. doi: 10.1136/gutjnl-2017-315082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex D.K., Sieber M., Lehman G.A., Webb D., Schmitt B., Kressel A.B. A double-reprocessing high-level disinfection protocol does not eliminate positive cultures from the elevators of duodenoscopes. Endoscopy. 2018;50(6):588–596. doi: 10.1055/s-0043-122378. http://www.embase.com/search/results?subaction=viewrecord&from=export &id=L619860368 Available from: [DOI] [PubMed] [Google Scholar]
- 26.Heroux R., Sheppard M., Wright S.B., Sawhney M., Hirsch E.B., Kalaidjian R. Duodenoscope hang time does not correlate with risk of bacterial contamination. Am J Infect Control. 2017;45(4):360–364. doi: 10.1016/j.ajic.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Olafsdottir L.B., Wright S.B., Smithey A., Heroux R., Hirsch E.B., Chen A. Adenosine Triphosphate Quantification Correlates Poorly with Microbial Contamination of Duodenoscopes. Infect Control Hosp Epidemiol. 2017;38(6):678–684. doi: 10.1017/ice.2017.58. [DOI] [PubMed] [Google Scholar]
- 28.Paula H., Presterl E., Tribl B., Diab-Elschahawi M. Microbiologic surveillance of duodenoscope reprocessing at the vienna university hospital from november 2004 through march 2015. Infect Control Hosp Epidemiol. 2015;36(10):1233–1235. doi: 10.1017/ice.2015.146. http://www.embase.com/search/results?subaction=viewrecord&from=export &id=L608516746 Available from: [DOI] [PubMed] [Google Scholar]
- 29.Ross A.S., Baliga C., Verma P., Duchin J., Gluck M. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc. 2015;82(3):477–483. doi: 10.1016/j.gie.2015.04.036. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L604853307 Available from: [DOI] [PubMed] [Google Scholar]
- 30.Naryzhny I., Silas D., Chi K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest Endosc. 2016;84(2):259–262. doi: 10.1016/j.gie.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 31.Mark J., Underberg K., Kramer R. Results of duodenoscope culture and quarantine after manufacturer-recommended cleaning process. Gastrointest Endosc. 2020 Jan doi: 10.1016/j.gie.2019.12.050. https://linkinghub.elsevier.com/retrieve/pii/S0016510720300328 cited 2020 Jan 21Available from: [DOI] [PubMed] [Google Scholar]
- 32.Alfa M.J., Sepehri S., Olson N., Wald A. Establishing a clinically relevant bioburden benchmark: a quality indicator for adequate reprocessing and storage of flexible gastrointestinal endoscopes. Am J Infect Control. 2012;40(3):233–236. doi: 10.1016/j.ajic.2011.02.023. Available from: [DOI] [PubMed] [Google Scholar]
- 33.Higa J.T., Choe J., Tombs D., Gluck M., Ross A.S. Optimizing duodenoscope reprocessing: rigorous assessment of a culture and quarantine protocol. Gastrointest Endosc. 2018;88(2):223–229. doi: 10.1016/j.gie.2018.02.015. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2000624042 Available from: [DOI] [PubMed] [Google Scholar]
- 34.Azimirad M., Alebouyeh M., Sadeghi A., Khodamoradi E., Aghdaei H.A., Mohammad Alizadeh A.H., Zali M.R. Bioburden and transmission of pathogenic bacteria through elevator channel during endoscopic retrograde cholangiopancreatography: application of multiple-locus variable-number tandem-repeat analysis for characterization of clonal strains. Expert Rev Med Devices. 2019;16(5):413–420. doi: 10.1080/17434440.2019.1604215. http://www.embase.com/search/results?subaction=viewrecord&from=export &id=L627368240 Available from: [DOI] [PubMed] [Google Scholar]
- 35.Brandabur J.J., Leggett J.E., Wang L., Bartles R.L., Baxter L., Diaz G.A. Surveillance of guideline practices for duodenoscope and linear echoendoscope reprocessing in a large healthcare system. Gastrointest Endosc. 2016;84(3):392–399. doi: 10.1016/j.gie.2016.03.1480. e3Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 36.Cristina M.L., Sartini M., Schinca E., Ottria G., Dupont C., Bova P. Is Post-Reprocessing Microbiological Surveillance of Duodenoscopes Effective in Reducing the Potential Risk in Transmitting Pathogens? Int J Environ Res Public Health. 2019;17(1):140. doi: 10.3390/ijerph17010140. https://www.mdpi.com/1660-4601/17/1/140 cited 2020 Feb 4Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aumeran C., Poincloux L., Souweine B., Robin F., Laurichesse H., Baud O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42(11):895–899. doi: 10.1055/s-0030-1255647. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Disinfection of Healthcare Equipment Guideline for Disinfection and Sterilization in Healthcare Facilities . 2019 [cited 2019 Nov 20]. Available from:https://www.cdc.gov/infectioncontrol/guidelines/disinfection/healthcare-equipment.html
- 39.The Society of Gastroenterology Nurses and Associates. Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endos . 2012 [cited 2019 Nov 21]. Available from:www.SGNA.org
- 40.ANSI/AAMI. ANSI/AAMI:2015 Flexible and semi-rigid endoscope processing in health care facilities . 2015 [cited 2019 Nov 21]. Available from:www.aami.org.
- 41.Attia M.M.M.Endoscopic Retrograde Cholangiopancreatography (ERCP) . 2019[cited 2019 Nov 20]. Available from:https://www.ncbi.nlm.nih.gov/books/NBK493160/
- 42.Humphries R.M., Yang S., Kim S., Muthusamy V.R., Russell D., Trout A.M. Duodenoscope-related outbreak of a carbapenem-resistant klebsiella pneumoniae identified using advanced molecular diagnostics. Clin Infect Dis. 2017;65(7):1159–1166. doi: 10.1093/cid/cix527. http://www.embase.com/search/results?subaction=viewrecord &from=export&id=L618680646 Available from: [DOI] [PubMed] [Google Scholar]
- 43.Anderson M., Fisher L., Jain R., Evans J., Appalaneni V., Ben-Menachem T. Complications of ERCP. YMGE. 2012;75:467–473. doi: 10.1016/j.gie.2011.07.010. www.giejournal.org cited 2019 Nov 20Available from: [DOI] [PubMed] [Google Scholar]
- 44.Babich T., Naucler P., Valik J.K., Giske C.G., Benito N., Cardona R. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteremia – retrospective multicenter study. Int J Antimicrob Agents. 2019 doi: 10.1016/j.ijantimicag.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Babich T., Naucler P., Valik J.K., Giske C.G., Benito N., Cardona R. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteremia – retrospective multicenter study. Int J Antimicrob Agents. 2019 doi: 10.1016/j.ijantimicag.2019.11.004. http://www.ncbi.nlm.nih.gov/pubmed/31770625 cited 2019 Dec 18]; Available from: [DOI] [PubMed] [Google Scholar]
- 46.Kimmery M.B., Burnett D.A., Carr-Locke D.L., DiMarino A.J., Jensen D.M., Katon R. Transmission of infection by gastrointestinal endoscopy. Gastrointest Endosc. 1993;39(6):885–888. [Google Scholar]
- 47.ECRI Institute. ERCI Institute: Top 10 Health Technology Hazards . [cited 2019 Dec 12]. Available from:https://www.ecri.org/
- 48.Ofstead C.L., Wetzler H.P., Heymann O.L., Johnson E.A., Eiland J.E., Shaw M.J. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control. 2017;45(2):e26–e33. doi: 10.1016/j.ajic.2016.10.017. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L614256471 Available from: [DOI] [PubMed] [Google Scholar]
- 49.Ofstead C.L., Wetzler H.P., Johnson E.A., Heymann O.L., Maust T.J., Shaw M.J. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016;44(11):1237–1240. doi: 10.1016/j.ajic.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Wang P., Xu T., Ngamruengphong S., Makary M.A., Kalloo A., Hutfless S. Rates of infection after colonoscopy and osophagogastroduodenoscopy in ambulatory surgery centres in the USA. Gut. 2018;67(9):1626–1636. doi: 10.1136/gutjnl-2017-315308. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L622462377 Available from: [DOI] [PubMed] [Google Scholar]
- 51.Ofstead C.L., Quick M.R., Wetzler H.P., Eiland J.E., Heymann O.L., Sonetti D.A. Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes. Chest. 2018;154(5):1024–1034. doi: 10.1016/j.chest.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 52.Institute of Medicine . 2001. Crossing the quality chasm: a new health system for the crossing the quality chasm: a new health system for the 21st century; p. 360. [PubMed] [Google Scholar]
- 53.Institute of Medicine To err is human: Building a safer health system (Report Brief) Inst Med. 1999;(November):1–8. https://iom.nationalacademies.org/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx [cited 2019 Dec 18]Available from: [Google Scholar]
- 54.American Gastroenterological Association. FDA transition to disposable component duodenoscopes — talking points for your patients | GI and Hepatology News . 2019. [cited 2019 Nov 21]. Available from:https://www.mdedge.com/gihepnews/article/210654/society-news/fda-transition-disposable-component-duodenoscopes-talking
- 55.Rennert-May E., Conly J., Leal J., Smith S., Manns B. Vol. 7. BioMed Central Ltd.; 2018. Economic evaluations and their use in infection prevention and control: a narrative review. (Antimicrobial Resistance and Infection Control). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Food and Drug Administration. The FDA is Recommending Transition to Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication | FDA . 2019. [cited 2019 Nov 21]. Available from:https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication
- 57.Ofstead C.L., Wetzler H.P., Heymann O.L., Johnson E.A., Eiland J.E., Shaw M.J. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control. 2017;45(2):e26–e33. doi: 10.1016/j.ajic.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Ofstead C.L., Quick M.R., Wetzler H.P., Eiland J.E., Heymann O.L., Sonetti D.A. Effectiveness of Reprocessing for Flexible Bronchoscopes and Endobronchial Ultrasound Bronchoscopes. Chest. 2018;154(5):1024–1034. doi: 10.1016/j.chest.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 59.Ofstead C.L., Wetzler H.P., Doyle E.M., Rocco C.K., Visrodia K.H., Baron T.H. Persistent contamination on colonoscopes and gastroscopes detected by biologic cultures and rapid indicators despite reprocessing performed in accordance with guidelines. Am J Infect Control. 2015;43(8):794–801. doi: 10.1016/j.ajic.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Mouritsen J.M., Ehlers L., Kovaleva J., Ahmad I. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia. 2019 doi: 10.1111/anae.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The Food and Drug Administration. Reducing the Risk of Infection from Reprocessed Duodenoscopes. 2019. [DOI] [PubMed]
- 62.The Joint Commission. High-Level Disinfection (HLD) and Sterilization BoosterPak.
- 63.The Association of periOperative Registered Nurses (AORN). Improving Reprocessing Compliance: 5 Steps to Take - Association of periOperative Registered Nurses.
- 64.Haque M., Sartelli M., McKimm J., Bakar M.A. Vol. 11. Dove Medical Press Ltd.; 2018. Health care-associated infections – An overview; pp. 2321–2333. (Infection and Drug Resistance). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S., Muthusamy V.R. Current Practice of Duodenoscope Reprocessing. Curr Gastroenterol Rep. 2016;18(10):54. doi: 10.1007/s11894-016-0528-7. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L611979233 Available from: [DOI] [PubMed] [Google Scholar]
- 66.Food and Drug Administration. FDA Executive Summary Reducing the Risk of Infection from Reprocessed Duodenoscopes. 2019.
- 67.Ha J., Son B.K. Vol. 48. Korean Society of Gastrointestinal Endoscopy; 2015. Current issues in duodenoscope-associated infections: Now is the time to take action; pp. 361–363. (Clinical Endoscopy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.CDC, HHSEssential elements of a reprocessing program for flexible endoscopes-recommendations of the healthcare infection control practices advisory committee.
- 69.Petersen B.T., Cohen J., Hambrick R.D., 3rd, Buttar N., Greenwald D.A., Buscaglia J.M. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85(2):282–294. doi: 10.1016/j.gie.2016.10.002. e1. [DOI] [PubMed] [Google Scholar]
- 70.Ofstead C.L., Quick M.R., Eiland J.E., Adams S.J.A glimpse at the true cost of reprocessing andoscopes: Results of a pilot project . [cited 2018 Dec 14]. Available from:www.iahcsmm.org
- 71.Kovaleva J., Peters F.T.M., Van Der Mei H.C., Degener J.E.Transmission of Infection by Flexible Gastrointestinal Endoscopy and Bronchoscopy. 2013; [DOI] [PMC free article] [PubMed]
- 72.Food and Drug Administration. Safety Communications >Supplemental Measures to Enhance Duodenoscope Reprocessing: FDA Safety Communication . 2015. [cited 2019 Nov 21]. Available from:http://wayback.archive-it.org/7993/20170722150658/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm
- 73.Rutala W.A., Kanamori H., Sickbert-Bennett E.E., Weber D.J. What's new in reprocessing endoscopes: Are we going to ensure “the needs of the patient come first” by shifting from disinfection to sterilization? Am J Infect Control. 2019;47:A62–A66. doi: 10.1016/j.ajic.2019.01.017. http://www.embase.com/search/results?subaction=viewrecord&from=export &id=L2001702984 Available from: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.