Abstract
Background
A high prevalence of primary bile acid diarrhoea (BAD) has been reported for Rome III defined irritable bowel syndrome (IBS)-diarrhoea and functional diarrhoea. We determined whether this still applies under the contemporaneous Rome IV criteria, given that the latter characterises IBS-diarrhoea as having more frequent abdominal pain compared with previous iterations, whilst no longer recognising abdominal discomfort.
Methods
Patients referred for a 75SeHCAT test completed a baseline questionnaire comprising, i) demographic data, ii) risk factors for BAD (inflammatory bowel disease, bowel resection, cholecystectomy, microscopic colitis, celiac disease, abdominal-pelvic radiotherapy), iii) the Rome III and IV bowel disorder questionnaire, and iv) mood and somatisation scores. A diagnosis of BAD constituted a 75SeHCAT of ≤15%, with moderate to severe disease being defined as ≤10% and ≤5%, respectively.
Findings
Of 300 patients with complete dataset, 184 had no risk factors for BAD and fulfilled criteria for either IBS-diarrhoea or functional diarrhoea. The prevalence of primary BAD was 38% (n = 70/184), with almost half having moderate (n = 16) to severe (n = 17) disease. Using the Rome III criteria, the prevalence of primary BAD was 36% in IBS-diarrhoea (n = 63/173) and 64% (n = 7/11) in functional diarrhoea; p = 0.11. Using the Rome IV criteria, the prevalence of primary BAD was 38% (n = 53/139) in IBS-diarrhoea and 38% (n = 17/45) in functional diarrhoea; p = 0.97. Patients with primary BAD experienced more frequent loose stools (p = 0.01) and had a higher body mass index (p<0.0001) compared to those without BAD, but otherwise no significant differences were seen in age, gender, mood, somatisation, or abdominal pain. The presence of primary BAD in patients classified as overweight or obese was approximately 40% and 60%, respectively.
Interpretation
Over a third of patients with Rome IV IBS-diarrhoea or functional diarrhoea have primary BAD, similar to Rome III. We therefore recommend that, in secondary care settings, generic testing for primary BAD should be considered in patients presenting with chronic diarrhoea of presumed functional origin regardless of concomitant abdominal pain. Centres that lack tests for primary BAD, and who empirically treat instead, may consider targeting patients who are overweight or obese.
Keywords: Bile acid diarrhoea, ROME III, Rome IV, Irritable bowel syndrome, Functional diarrhoea, SeHCAT, Obesity
Research in the context.
Evidence before this study
Almost a third of patients presenting with symptoms compatible with functional diarrhoea or IBS-diarrhoea have primary bile acid diarrhoea (BAD). However, this data has been based on historic Rome I-III criteria. The Rome IV criteria, published in 2016, have undergone marked modifications with IBS now stringently defined as a chronic bowel disorder associated with frequent abdominal pain. Whether primary BAD can present as a painful bowel disorder, and therefore mimic the symptoms of Rome IV IBS-diarrhoea is not known. A few small studies demonstrate that colonic bile acids correlate positively with visceral hypersensitivity and the intensity of abdominal pain, whilst others report visceral hyposensitivity and no association with abdominal pain. Hence, evaluating the prevalence of primary BAD in patients with Rome IV functional diarrhoea and IBS-diarrhoea will help provide clinicians with clarity in whom to test. It will also aid in understanding the association between primary BAD and abdominal pain.
Added value of this study
Over a third of subjects with Rome IV defined IBS-diarrhoea or functional diarrhoea have primary BAD, as defined by a 75Selenium HomoCholic Acid Taurine (75SeHCAT) test of ≤15%. This is similar to the Rome III criteria. Moreover, almost half of those with primary BAD have moderate to severe disease. There was no correlation between abdominal pain frequency and primary BAD. Primary BAD was associated with increased body mass index, with 40% of overweight and 60% of individuals with obesity having an abnormal 75SeHCAT test, compared with around 20% who were of normal weight.
Implications of all the available evidence
Primary BAD commonly mimics the symptoms of Rome IV IBS-diarrhoea and functional diarrhoea, and therefore should be tested for in patients with chronic diarrhoea irrespective of abdominal pain. These findings support and update recent guidelines from the United Kingdom, Canada, and the United States which are promoting an increased awareness of primary BAD. Centres who currently do not have the facilities to test for BAD, but empirically treat with bile acid sequestrants instead, may consider targeting individuals who are overweight or obese. Randomised controlled trials of therapies for primary BAD need to performed, including whether obesity management could be an option
Alt-text: Unlabelled box
1. Introduction
Bile acids are synthesised in the liver, stored in the gallbladder, and released into the small intestine, where they aid digestion of lipids. Normally, 95% of bile acids are reabsorbed in the terminal ileum and recycled back to the liver via the entero-hepatic circulation. If there is a failure of reabsorption, excess bile acids spill over into the colon where they stimulate electrolyte and water secretion, resulting in chronic watery diarrhoea [1,2]. The concept of bile acid diarrhoea (BAD) being responsible for symptom generation is supported by colonic bile acid exposure correlating with colonic transit time and bowel habit, with subsequent treatment with bile acid sequestrants leading to clinical improvement [3]. The conditions causing BAD can be classified according to the aetiology. Secondary BAD relates to the malabsorption of bile acids from the terminal ileum (previously termed Type 1 BAD) due to either resection or localised disease (e.g. Crohn's disease, right hemicolectomy). It also encompasses miscellaneous intestinal disorders such as previous cholecystectomy, coeliac disease, microscopic colitis, or fibrosis following abdominal-pelvic radiotherapy (previously termed Type 3 BAD). Primary BAD - otherwise known as Type 2 BAD or idiopathic BAD - is where there is no anatomical abnormality or other risk factors apparent [1,2]. In the latter case, evidence suggests that absorption of bile acids within the terminal ileum is normal or increased [4], but impaired negative feedback of bile acids on fibroblast growth factor (FGF)−19 leads to dysregulation of the enterohepatic circulation and excessive bile acid synthesis [5].
A systematic review and meta-analysis has shown that between a quarter to a third of cases with chronic diarrhoea of presumed functional origin - i.e. diagnosed as irritable bowel syndrome with diarrhoea (IBS-diarrhoea) or functional diarrhoea - actually have primary BAD [6]. As such, recent guidelines from the United Kingdom, Canada, and United States recommend testing (where possible) for primary BAD in patients with suspected IBS-diarrhoea or functional diarrhoea [[7], [8], [9]]. However, this guidance is based upon data derived using the historic Rome I-III criteria for IBS-diarrhoea and functional diarrhoea which, following the recent publication of the Rome IV criteria in 2016, have undergone substantial modifications [10]. Whilst the previous Rome III criteria were relatively lax and defined the symptoms of IBS as abdominal pain or discomfort at least 2–3 days a month associated with altered bowel habit, the Rome IV criteria for IBS are far more stringent in that discomfort is no longer a recognised term whilst abdominal pain frequency has increased to at least one day per week [10]. Consequently, a proportion of patients previously fulfilling criteria for IBS-diarrhoea under the Rome III criteria will no longer satisfy criteria for IBS-diarrhoea under the Rome IV criteria, and instead be allocated a diagnosis of functional diarrhoea [11].
Following this update in criteria, it may be envisaged that the prevalence of primary BAD will still remain high for patients with Rome IV functional diarrhoea. However, whether primary BAD can present as a painful disorder and thus mimic Rome IV IBS-diarrhoea is less clear. By means of its nomenclature, some physicians may merely view primary BAD as a relatively painless diarrhoeal disorder, a concept that would be supported by data showing primary BAD to be associated with rectal hyposensitivity and without any association for abdominal pain [3,12]. In contrast, a few small studies have demonstrated colonic bile acids to correlate positively with visceral hypersensitivity and abdominal pain intensity [13,14]. In view of this discrepant data, large studies to confirm a possible association between primary BAD and abdominal pain are needed, with the Rome IV criteria for IBS-diarrhoea and functional diarrhoea providing an opportunity to study this from a clinically meaningful perspective.
Hence, our primary aim was to determine whether the high prevalence of primary BAD reported for Rome III defined IBS-diarrhoea and functional diarrhoea is still applicable using the contemporaneous Rome IV criteria. Secondary outcomes were to identify any clinical characteristics that may be predictive of primary BAD.
2. Methods
2.1. Study design and participants
This observational study was undertaken at Sheffield Teaching Hospitals, United Kingdom, during the course of 2019. The hospital provides secondary care services to a local population of 500,000 people. All adults, aged 18 years and over, referred at the clinical discretion of their GI physician to the Nuclear Medicine department to test for the possibility of BAD were eligible to participate. Individuals were invited to complete a baseline questionnaire collecting demographic and symptom-based data, followed by undergoing a 75Selenium HomoCholic Acid Taurine (75SeHCAT) retention test to assess for BAD.
Baseline questionnaire - The following items were collected on the day of the 75SeHCAT retention test:
-
a)
Demographic data – participants entered their age, sex, ethnicity, alcohol and tobacco use, and weight in kg and height in metres which was subsequently used to calculated body mass index (BMI). This was further classified in accordance with the World Health Organization criteria as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9) and obese (≥30) [15].
-
b)
Past medical history – patients were asked whether they had any of the following illnesses or interventions; inflammatory bowel disease, celiac disease, microscopic colitis, cholecystectomy, bowel resection, or abdominal-pelvic radiotherapy. For verification, all clinical records were reviewed.
-
c)
Mood and somatisation data – individuals with bowel symptoms, in particular those of functional origin, can exhibit psychological distress and somatic symptoms [16]. To collect information on psychological distress we used the hospital anxiety and depression scale (HADS), which is a 14-item instrument containing 7 questions for anxiety and 7 questions for depression [17]. Each question is scored from 0 to 3, providing a minimum score of 0 (no symptoms) and a maximum score of 21 (maximal severity of symptoms) on each subscale. A subscale score of ≥11 is used to indicate a clinically significant level of anxiety or depression.
The Patient Health Questionnaire (PHQ)−12 evaluates the severity of extra-intestinal somatic symptoms [18]. The twelve non-GI symptoms assessed are back pain, limb pain, headaches, chest pain, dizziness, fainting spells, palpitations, breathlessness, menstrual cramps, dyspareunia, insomnia and lethargy. Each item is scored from 0 to 2, with the total score ranging from 0 to 24, and high levels of somatisation categorised as a PHQ-12 score ≥13.
-
d)
The Rome III/IV bowel disorder questionnaire [19] – Participants were asked to score the frequency of mushy or watery loose stools over the last 3 months, with answers ranging from 0% – and increasing by 10% increments – to a maximum score of 100%. Mushy and loose stools denote type 6 and type 7 on the Bristol Stool form scale [10], respectively, with the Rome bowel disorder questionnaire collecting this information as a single item [19].
Subjects were also asked to complete two separate questions to ascertain the presence of abdominal discomfort and abdominal pain, respectively. Specifically, patients were asked a) “In the last 3 months, how often did you have discomfort anywhere in your abdomen?” and b) “In the last 3 months how often did you have pain anywhere in your abdomen?” The answers available for these questions were: never, fewer than 1 day a month, 1 day a month, 2–3 days a month, 1 day a week, 2–3 days a week, most days, everyday, multiple times per day or all the time.
Based on these answers – and in the absence of organic disease - it was possible to identify which subjects fulfilled Rome III/IV criteria for IBS-diarrhoea and functional diarrhoea. To satisfy Rome III criteria for IBS-diarrhoea, subjects recorded having abdominal pain or discomfort at least 2–3 days a month, with the others deemed to have Rome III functional diarrhoea. In contrast, to satisfy Rome IV criteria for IBS-diarrhoea, patients would record having abdominal pain at least 1 day per week, with the remaining classed as Rome IV functional diarrhoea.
The 75SeHCAT retention test - This is a simple and highly sensitive method of testing for BAD, where retention of radio-labelled bile acids of less than 15% after 7 days is abnormal. The degree of BAD can be further classified as mild if retention ≤15%, moderate if ≤10%, and severe if ≤5%. The results of the 75SeHCAT retention tests were reported by the Nuclear Medicine department who were blinded to the questionnaire data.
2.2. Statistical analysis
The primary analysis determined the prevalence of primary BAD in patients with symptoms compatible with functional diarrhoea and IBS-diarrhoea, according to the Rome III and Rome IV criteria. We also compared difference in characteristics in those with primary BAD versus those without, whilst also extending to compare characteristics across mild, moderate, and severe BAD. Finally, we determined the association between the frequency of abdominal pain and the prevalence of primary BAD.
Statistical analysis was carried out using SPSS version 25.0 software, with significance set at a p-value of <0.05. Categorical variables were summarised by descriptive statistics, including total numbers and percentages, with comparisons between groups performed using the chi-square test or Fisher exact test. Continuous variables were summarised by mean and standard deviation, with difference between two independent groups performed using the unpaired student T-test, and between multiple groups using 1-way analysis of variance. Correlations were assessed using Spearman's test.
2.3. Ethics
The study commenced following ethical approval by Sheffield Teaching Hospital (protocol number: STH20572) and the Health Research Authority (IRAS project ID: 253210). The study was done in accordance with the STROBE statement.
2.4. Role of funder
This study was carried out independently and did not receive funding.
3. Results
3.1. Study participants
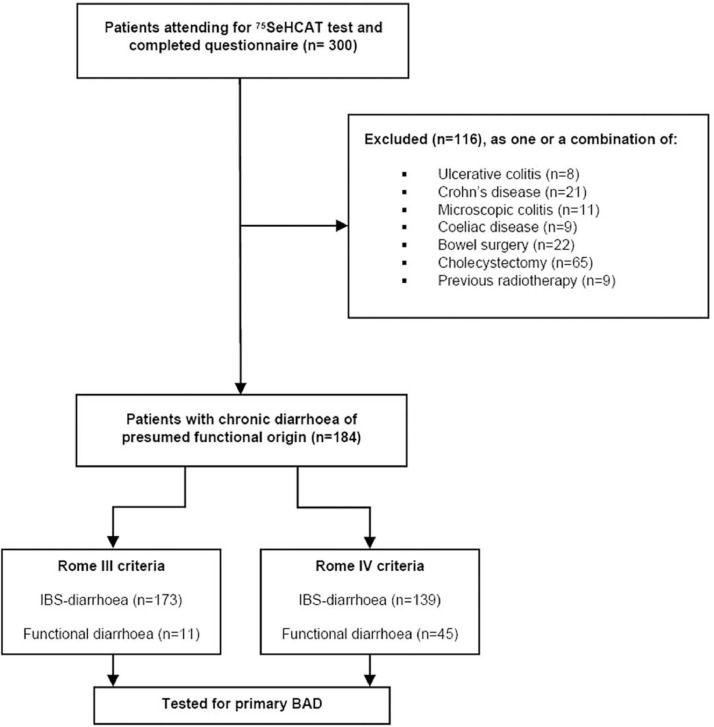
Complete baseline demographic and symptom data was obtained from 300 of 310 patients who attended for a 75SeHCAT retention test. We subsequently excluded 116 patients from further analysis as they disclosed risk factors for secondary BAD, as shown in the study flow chart (Fig. 1).
Fig. 1.
Study flow chart.
The remaining 184 patients (female 65%, mean-age 47yrs, white race 94%) were of interest, to assess for the possibility of primary BAD, as they had symptoms consistent with chronic diarrhoea which was presumed to be of functional origin, i.e. either functional diarrhoea or IBS-diarrhoea.
3.2. Prevalence of primary BAD in patients with chronic diarrhoea of presumed functional origin
Following a 75SeHCAT test the prevalence of primary BAD in patients with chronic diarrhoea of presumed functional origin was 38% (n = 70/184); of these, 53% (n = 37) had mild disease, 23% (n = 16) had moderate disease, and 24% (n = 17) had severe disease.
Subjects with primary BAD were significantly more likely to have a higher BMI and report increased stool frequency than those without BAD, but otherwise no significant differences were seen in basic demographics, mood scores, somatic severity, or abdominal discomfort or pain frequency (Table 1). There was also no difference seen in clinical characteristics according to the severity of primary BAD (Table 2).
Table 1.
Characteristics of individuals with primary BAD compared to those without.
| Baseline characteristics | No BAD (n = 114) | Primary BAD (n = 70) | P value |
|---|---|---|---|
| Demographics | |||
| Female | 78 (68%) | 41 (59%) | 0.2 |
| Mean age (SD) | 47 (18) | 48 (15) | 0.7 |
| White race | 107 (94%) | 66 (94%) | 0.9 |
| Smoker | 18 (16%) | 15 (21%) | 0.4 |
| Alcohol intake | 74 (65%) | 50 (71%) | 0.4 |
| Mean BMI (SD) | 26 (5.9) | 30 (6) | <0.0001 |
| Symptoms | |||
| Abnormal HADS-anxiety score | 42 (37%) | 29 (42%) | 0.5 |
| Abnormal HADS-depression score | 19 (17%) | 14 (20%) | 0.6 |
| High PHQ-12 somatisation score | 18 (16%) | 12 (17%) | 0.8 |
| Abdominal discomfort | |||
| ≤ One day per month | 4 (3.5%) | 8 (11.4%) | |
| Two-three days per month | 4 (3.5%) | 1 (1.4%) | |
| One day per week | 3 (2.6%) | 3 (4.3%) | 0.3 |
| Two-three days per week | 29 (25.4%) | 11 (15.7%) | |
| Most days | 21 (27.2%) | 17 (24.3%) | |
| Everyday | 19 (16.7%) | 13 (18.6%) | |
| Multiple times per day | 24 (21.1%) | 17 (24.3%) | |
| Abdominal pain | |||
| ≤ One day per month | 14 (12.3%) | 10 (14.3%) | |
| Two-three days per month | 14 (12.3%) | 7 (10%) | |
| One day per week | 9 (7.9%) | 4 (5.7%) | 0.3 |
| Two-three days per week | 27 (23.7%) | 9 (12.9%) | |
| Most days | 25 (21.9%) | 21 (30%) | |
| Everyday | 16 (14%) | 8 (11.4%) | |
| Multiple times per day | 9 (7.9%) | 11 (15.7%) | |
| % of stools reported as mushy or watery loose (SD) | 66% (25) | 76% (21) | 0.01 |
Table 2.
Characteristics of individuals with primary BAD stratified according to severity.
| Baseline characteristics | Mild BAD (n = 37) | Moderate BAD (n = 16) | Severe BAD (n = 17) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Female | 20 (54%) | 9 (56%) | 12 (71%) | 0.6 |
| Mean age (SD) | 48 (16) | 52 (13.5) | 44 (13) | 0.3 |
| White race | 36 (97%) | 16 (100%) | 14 (82%) | 0.1 |
| Smoker | 8 (22%) | 3 (19%) | 4 (24%) | 1.0 |
| Alcohol intake | 26 (70%) | 13 (81%) | 11 (65%) | 0.6 |
| Mean BMI (SD) | 30 (6.7) | 28 (5.3) | 31 (4.7) | 0.5 |
| Symptoms | ||||
| Abnormal HADS-anxiety score | 17 (47%) | 5 (31%) | 7 (42%) | 0.6 |
| Abnormal HADS-depression score | 6 (17%) | 5 (31%) | 3 (18%) | 0.45 |
| High PHQ-12 somatisation score | 8 (22%) | 2 (12.5%) | 2 (12%) | 0.7 |
| Abdominal discomfort | ||||
| ≤ One day per month | 6 (16.2%) | 1 (6.3%) | 1 (5.9%) | |
| Two-three days per month | 0 (0%) | 1 (6.3%) | 0 (0%) | |
| One day per week | 3 (8.1%) | 0 (0%) | 0 (0%) | |
| Two-three days per week | 7 (18.9%) | 1 (6.3%) | 3 (17.6%) | 0.9 |
| Most days | 7 (18.9%) | 5 (31.3%) | 5 (29.4%) | |
| Everyday | 5 (13.5%) | 4 (25%) | 4 (23.5%) | |
| Multiple times per day | 9 (24.3%) | 4 (25%) | 4 (23.5%) | |
| Abdominal pain | ||||
| ≤ One day per month | 6 (16.2%) | 2 (12.6%) | 2 (11.8%) | |
| Two-three days per month | 5 (13.5%) | 2 (12.5%) | 0 (0%) | |
| One day per week | 2 (5.4%) | 1 (6.3%) | 1 (5.9%) | |
| Two-three days per week | 6 (16.2%) | 0 (0%) | 3 (17.6%) | 0.75 |
| Most days | 11 (29.7%) | 4 (25%) | 6 (35.3%) | |
| Everyday | 3 (8.1%) | 3 (18.8%) | 2 (11.8%) | |
| Multiple times per day | 4 (10.8%) | 4 (25%) | 3 (17.6%) | |
| % of stools reported as mushy or watery loose (SD) | 72% (24) | 81% (21) | 80% (16) | 0.2 |
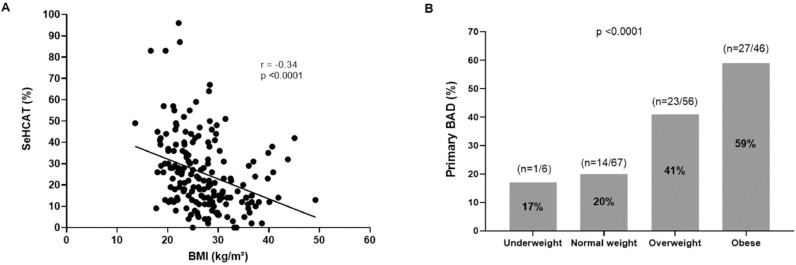
We further scrutinised the relationship between frequency of mushy or loose watery stool and 75SeHCAT value, noting a negative correlation (r=−0.18, p = 0.001). With regards to BMI, we noted a) that it was negatively associated with 75SeHCAT values (r=−0.37, p <0.0001) and b) that prevalence of Primary BAD increased from ~20% in those who were underweight to almost 60% in those classed as obese (p<0.0001); Fig. 2.
Fig. 2.
Evaluating the association between BMI and primary BAD in patients with chronic diarrhoea of presumed functional origin. A) The correlation between BMI and 75SeHCAT result. B) The prevalence of primary BAD according to weight category.
3.3. Prevalence of primary BAD according to Rome III and Rome IV criteria
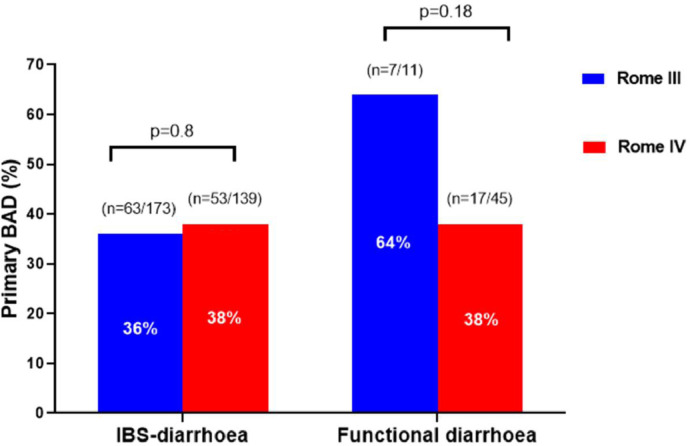
The number of patients fulfilling symptom criteria for Rome III chronic diarrhoea disorders was 173 cases for IBS-diarrhoea and 11 cases for functional diarrhoea. Following a 75SeHCAT retention test, the prevalence of primary BAD in this Rome III defined sample was 36% (n = 63/173) in IBS-diarrhoea and 64% (n = 7/11) in functional diarrhoea; p = 0.11. The presence of BAD in Rome III IBS-diarrhoea was mild in 18.5%, moderate in 9%, and severe in 9%. The presence of BAD in Rome III functional diarrhoea was mild in 45%, moderate in 9%, and severe in 9%.
When the Rome IV criterion was applied to the same cohort, the number of patients with suspected IBS-diarrhoea was 139, a significant reduction of 34 cases (20%) from the 173 IBS-diarrhoea cases under Rome III classification; p<0.0001. Instead, the remaining 34 cases fulfilled criteria from Rome IV functional diarrhoea, leading to the number of cases to rise from 11 (under Rome III) to 45. The prevalence of primary BAD in this Rome IV defined sample was 38% (n = 53/139) in IBS-diarrhoea and 38% (n = 17/45) in functional diarrhoea; p = 0.97. The presence of BAD in Rome IV IBS-diarrhoea was mild in 19%, moderate in 9%, and severe in 11%. The presence of BAD in Rome III functional diarrhoea was mild in 24%, moderate in 9%, and severe in 4%.
Fig. 3 summarises the prevalence of primary BAD according to Rome III and Rome IV criteria. This shows no difference in the prevalence of primary BAD from Rome III to Rome IV criteria for IBS-diarrhoea, which was 36% and 38% (p = 0.8), respectively. There was a non-significant reduction of primary BAD from Rome III to Rome IV functional diarrhoea, which was 60% and 38% respectively (p = 0.18), although the former was limited by a relatively small sample size.
Fig. 3.
Prevalence of primary BAD in patients fulfilling symptom criteria for IBS-diarrhoea and functional-diarrhoea, based on Rome III and Rome IV criteria.
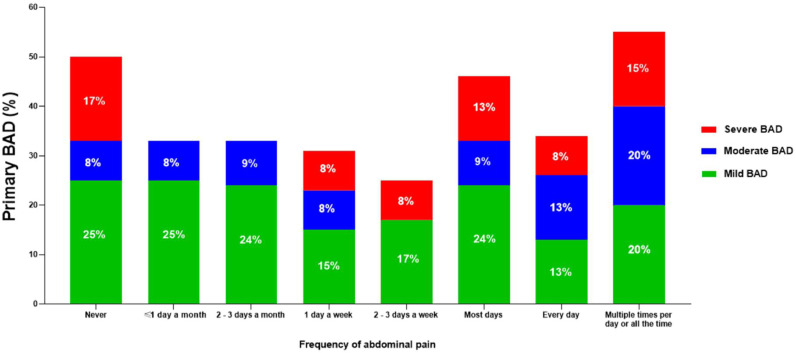
3.4. Prevalence of primary BAD according to the frequency of abdominal pain
We found there to be no association between the frequency of abdominal pain and the prevalence of primary BAD, with values ranging between 25% and 50% (p = 0.36, Fig. 4). Moreover, the differing severities of BAD were present regardless of abdominal pain frequency.
Fig. 4.
Prevalence of primary BAD in patients with chronic diarrhoea of presumed functional origin, according to the frequency of abdominal pain.
4. Discussion
This study builds on the existing literature, whereby a systematic review found that in excess of a quarter of patients with symptoms compatible with IBS-diarrhoea have primary BAD [6]. However, these findings were based on historic Rome I-III criteria and extrapolating to Rome IV required clarification given that the latter criteria for IBS has undergone substantial modifications and is characterised by frequent abdominal pain. The association between primary BAD and abdominal pain has previously been conflicting [ 3,[12], [13], [14]], thereby potentially leaving physicians in uncertainty as to the role of primary BAD in those with Rome IV IBS-diarrhoea. By undertaking a large observational study we have shown that over a third of patients with symptoms compatible with either functional diarrhoea or IBS-diarrhoea have primary BAD, a finding seen across both the Rome III and Rome IV criteria. Moreover, almost half of cases with primary BAD were categorised as being moderate to severe. In summary, these findings confirm that primary BAD is common, and generic testing for its possibility should be considered in patients presenting to secondary care settings with chronic diarrhoea of presumed functional origin regardless of whether or not they have abdominal pain.
Strengths of the study include its large sample, prospective recruitment, patients being referred for SeHCAT test by different physicians from across the hospital trust, careful exclusion of organic disease, and being the first to evaluate the prevalence of primary BAD using the Rome III and Rome IV criteria in tandem. However, a potential weakness that may be attributed to our study is its generalisability or selection bias given that patients were recruited within the Nuclear Medicine department having been referred specifically for the evaluation of primary BAD, and the results may not be applicable to most patients with chronic diarrhoea seen in the out-patient clinic setting. We acknowledge that this is a reasonable argument in primary care where there is no data on the prevalence of primary BAD. However, we believe this argument can be refuted within secondary care settings on the basis of the previously mentioned systematic review [6] and in particular from a large prospective dual-centre out-patient secondary-care study conducted within the UK, which found that a third of all consecutive patients with Rome III IBS-diarrhoea referred for a 75SeHCAT had primary BAD [20]. Similarly, a study from the United States reported that 38% of patients with IBS-diarrhoea had increased levels of serum bile acid precursors compared with healthy controls [21]. More recently, a large retrospective study from the United States found that of 936 patients with chronic unexplained diarrhoea, over 50% had increased faecal bile acid excretion whilst, in comparison, other diagnostic tests performed for organic diseases (e.g. endoscopies and cross-sectional radiological imaging) had a yield of less than 10% [22]. Finally, most Rome III-positive IBS patients seeking healthcare fulfil Rome IV IBS criteria [23], suggesting that the high prevalence rates of primary BAD reported for Rome III can be transferred over to Rome IV; in our study, 80% of patients with Rome III IBS retained the diagnosis under Rome IV whilst the other 20% were reassigned as functional diarrhoea.
Our study strongly supports recent international guidelines from the United Kingdom, Canada and the United States that are promoting increased awareness of primary BAD [[7], [8], [9]]. The potential scale of this disorder may be estimated from a large survey across these 3 countries which has shown that approximately 5% (i.e. 1-in-20) of adults within the general population fulfil symptom criteria for either Rome IV functional diarrhoea or IBS-diarrhoea [11]. Unfortunately, the high gastrointestinal illness burden and associated poor quality of life reported by such individuals is despite many having previously sought healthcare for their symptoms [11]. A recently published worldwide study, performed across 30 countries and six continents, has yielded similar results [24]. However, it could be argued that over of third of these individuals may have primary BAD instead, which would imply that an estimated 1.5% of the general population are suffering from the condition. Hence, testing for primary BAD is important given that it is common, frequently overlooked, and detrimentally impacts on physical and mental well-being, with open-label treatment leading to improved patient-related outcomes and a reduction in the number of subsequent diagnostic investigations [3,22,[25], [26], [27]]. Unfortunately, testing modalities for primary BAD are not readily available across the globe. Currently, the 75SeHCAT test is available in the United Kingdom, certain European countries, and Canada, but not in the United States where some centres test for BAD via alternate means, for example, serum bile acid precursors (i.e. 7a‑hydroxy‑4-cholesten-3-one) or measurement of faecal bile acids following a 48-hour stool collection [28]. There have been recent advances in developing a simple, cheap, and readily available biomarker to screen for BAD, with promising data for serum FGF19 or detection of volatile organic compounds (presumed to be due to dysbiosis of colonic bile acids), however all require further validation [[28], [29], [30]].
In the meantime, some clinicians without resources to test for BAD may consider a therapeutic trial of a bile acid sequestrant [31]. This approach may be limited by the palatability of bile acid sequestrant and clinical uncertainty regarding diagnosis [32]. In such circumstances, selecting the patient with the highest pre-test probability of primary BAD may be a thoughtful option, with our data suggesting those who are overweight or obese as potentially reasonable candidates in view of the prevalence of primary BAD in this cohort reaching almost 60%. Previous studies have also shown a link between increasing BMI and primary BAD although – unlike ours – have not performed direct comparisons of BAD prevalence rates in patients with chronic diarrhoea stratified according to the World Health Organisation weight classification system [20,21,33]. The link between increasing BMI and primary BAD is intriguing, with a recent systematic review and meta-analysis confirming altered bile acid metabolism in obesity [34]. This is evidenced by individuals with obesity having lower serum FGF19, elevated serum bile acid precursors, increased bile acid synthesis and luminal excretion, with shorter colonic transit times [3,21,[33], [34], [35]]. The pathophysiological basis for this association is unclear, although some plausible explanations have been provided. There is data showing FGF19 to increase metabolic rates in mouse models [36]; therefore, deficient FGF19 levels could theoretically lead to a lower metabolic rate and subsequently cause obesity. However, conversely, a rise in FGF19 levels has been noted following bariatric surgery [37]. Further mechanistic insights into this association are needed and - from a clinical perspective - it would of interest to establish whether targeting obesity can be considered a therapeutic option in primary BAD. Current treatment options for BAD are limited to bile acid sequestrants or a low fat diet, albeit hampered by a paucity of randomised controlled trials. This is clearly an exciting area for future research.
In conclusion, over a third of subjects with Rome IV defined IBS-diarrhoea or functional diarrhoea referred for a 75SeHCAT retention test have primary BAD, similar to the Rome III criteria. Testing for primary BAD should be considered in patients with chronic diarrhoea of presumed functional origin irrespective of associated abdominal pain.
Funding
None.
Data sharing statement
Available on reasonable request.
Declaration of Competing Interest
Nothing to declare.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100465.
Appendix. Supplementary materials
References
- 1.Walters J.R. Defining primary bile acid diarrhea: making the diagnosis and recognizing the disorder. Expert Rev Gastroenterol Hepatol. 2010;4(5):561–567. doi: 10.1586/egh.10.54. [DOI] [PubMed] [Google Scholar]
- 2.Mottacki N., Simrén M., Bajor A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2016;43(8):884–898. doi: 10.1111/apt.13570. [DOI] [PubMed] [Google Scholar]
- 3.Bajor A., Törnblom H., Rudling M., Ung K.A., Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64(1):84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 4.Bajor A., Kilander A., Fae A. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur J Gastroenterol Hepatol. 2006;18(4):397–403. doi: 10.1097/00042737-200604000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Walters J.R., Tasleem A.M., Omer O.S., Brydon W.G., Dew T., le Roux C.W. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7(11):1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Slattery S.A., Niaz O., Aziz Q., Ford A.C., Farmer A.D. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42(1):3–11. doi: 10.1111/apt.13227. [DOI] [PubMed] [Google Scholar]
- 7.Arasaradnam R.P., Brown S., Forbes A. Guidelines for the investigation of chronic diarrhoea in adults: british Society of Gastroenterology, 3rd edition. Gut. 2018;67(8):1380–1399. doi: 10.1136/gutjnl-2017-315909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadowski D.C., Camilleri M., Chey W.D. Canadian association of gastroenterology clinical practice guideline on the management of bile acid diarrhea. Clin Gastroenterol Hepatol. 2020;18(1):24–41. doi: 10.1016/j.cgh.2019.08.062. e21. [DOI] [PubMed] [Google Scholar]
- 9.Smalley W., Falck-Ytter C., Carrasco-Labra A., Wani S., Lytvyn L., Falck-Ytter Y. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D) Gastroenterology. 2019;157(3):851–854. doi: 10.1053/j.gastro.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Lacy B.E., Mearin F., Chang L. Bowel Disorders. Gastroenterology. 2016;150(6):1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Palsson O.S., Whitehead W., Törnblom H., Sperber A.D., Simren M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158(5):1262–1273.e1263. doi: 10.1053/j.gastro.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Duboc H., Rainteau D., Rajca S. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):513–520. doi: 10.1111/j.1365-2982.2012.01893.x. e246-517. [DOI] [PubMed] [Google Scholar]
- 13.Coremans G., Tack J., Vantrappen G., Janssens J., Annese V. Is the irritable bowel really irritable. Ital J Gastroenterol. 1991;23(8 Suppl 1):39–40. [PubMed] [Google Scholar]
- 14.Dior M., Delagrèverie H., Duboc H. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28(9):1330–1340. doi: 10.1111/nmo.12829. [DOI] [PubMed] [Google Scholar]
- 15.Physical status: the use and interpretation of anthropometry Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 16.Aziz I., Palsson O.S., Törnblom H., Sperber A.D., Whitehead W.E., Simrén M. The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: a cross-sectional general population study in three countries. Am J Gastroenterol. 2018;113(1):86–96. doi: 10.1038/ajg.2017.421. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiller R.C., Humes D.J., Campbell E. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32(6):811–820. doi: 10.1111/j.1365-2036.2010.04402.x. [DOI] [PubMed] [Google Scholar]
- 19.Palsson O.S., Whitehead W.E., van Tilburg M.A. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016;150(6):1481–1491. doi: 10.1053/j.gastro.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Aziz I., Mumtaz S., Bholah H., Chowdhury F.U., Sanders D.S., Ford A.C. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on Rome III criteria. Clin Gastroenterol Hepatol. 2015;13(9):1650–1655. doi: 10.1016/j.cgh.2015.03.002. e1652. [DOI] [PubMed] [Google Scholar]
- 21.Wong B.S., Camilleri M., Carlson P. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–1015.e1003. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijayvargiya P., Gonzalez Izundegui D., Calderon G., Tawfic S., Batbold S., Camilleri M. Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol. 2020;115(7):1094–1102. doi: 10.14309/ajg.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz I., Törnblom H., Palsson O.S., Whitehead W.E., Simrén M. How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol. 2018;113(7):1017–1025. doi: 10.1038/s41395-018-0074-z. [DOI] [PubMed] [Google Scholar]
- 24.Sperber A.D., Bangdiwala S.I., Drossman D.A. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.014. in press. [DOI] [PubMed] [Google Scholar]
- 25.Turner J.M., Pattni S.S., Appleby R.N., Walters J.R. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol. 2017;8(4):279–283. doi: 10.1136/flgastro-2017-100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bannaga A., Kelman L., O'Connor M., Pitchford C., Walters J.R., Arasaradnam R.P. How bad is bile acid diarrhoea: an online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol. 2017;4(1) doi: 10.1136/bmjgast-2016-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wedlake L., A'Hern R., Russell D., Thomas K., Walters J.R., Andreyev H.J. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 28.Vijayvargiya P., Camilleri M. Current Practice in the diagnosis of bile acid diarrhea. Gastroenterology. 2019;156(5):1233–1238. doi: 10.1053/j.gastro.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 29.Walters J.R. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2014;11(7):426–434. doi: 10.1038/nrgastro.2014.32. [DOI] [PubMed] [Google Scholar]
- 30.Vijayvargiya P., Camilleri M., Shin A., Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11(10):1232–1239. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendy P., Florin T. Letter: therapeutic trial is more informative than SeHCAT to diagnose bile acid malabsorption. Aliment Pharmacol Ther. 2015;42(6):780. doi: 10.1111/apt.13320. [DOI] [PubMed] [Google Scholar]
- 32.Schiller L.R. Good news about BAD. Clin Gastroenterol Hepatol. 2020;18(1):45–47. doi: 10.1016/j.cgh.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Sadik R., Abrahamsson H., Ung K.A., Stotzer P.O. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99(4):711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 34.So S.S.Y., Yeung C.H.C., Schooling C.M., El-Nezami H. Targeting bile acid metabolism in obesity reduction: a systematic review and meta-analysis. Obes Rev. 2020;21(7):e13017. doi: 10.1111/obr.13017. [DOI] [PubMed] [Google Scholar]
- 35.Pattni S.S., Brydon W.G., Dew T. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38(8):967–976. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 36.Fu L., John L.M., Adams S.H. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 37.Pournaras D.J., Glicksman C., Vincent R.P. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.