Abstract
Echium plantagineum L. (Boraginaceae) is an invasive species in Australia and contains medicinal shikonins in its roots. In this study, the hairy root lines of E. plantagineum were established using Agrobacterium rhizogenes strain ATCC15834 and confirmed by the amplification of the rolB gene. Results showed significant difference in shikonin production between the hairy root lines in the 1/2B5 and M9 media. The biomass of the lines in the 1/2B5 medium was fivefold of that in the M9 medium. However, the components of detected shikonins were similar in these two liquid media. By contrast, different accumulation profiles appeared in the hairy root lines. HPLC analysis revealed the presence of nine possible related compounds, including shikonins, and acetylshikonin was the most abundant shikonin derivative. The content of acetylshikonin in the 1/2B5 medium (36.25 mg/L on average) was twofold of that in the M9 medium. Our results showed that the hairy root cultures of E. plantagineum can be used in enhancing the production of potential pharmaceutical compounds, such as acetylshikonin.
Keywords: Culture medium, Echium plantagineum L., Hairy root, In vitro biosynthesis, Shikonins
Introduction
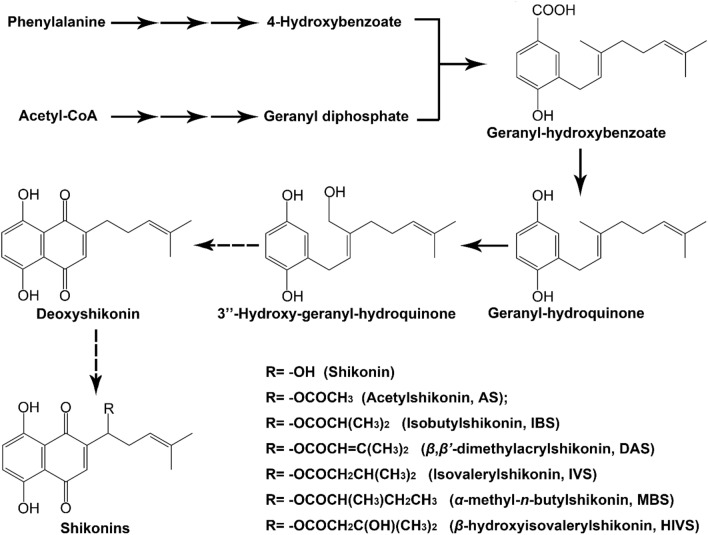
Plants can produce a range of secondary metabolites at different developmental stages or in specific tissues. These natural products contribute to the interaction of plants with the environment and serve as pharmaceuticals, cosmetics, and dyes (Verpoorte and Alfermann 2000). Naphthoquinones constitute a class of secondary metabolites with a wide distribution among higher plant families. Boraginaceae has a broad distribution, with more than 2000 species in 146 genera, including shrubs, trees, and herbs (Babula et al. 2009). Shikonins are the most important naphthoquinones detected in this family. These red pigments are abundant in the roots of some genera, such as Lithospermum, Arnebia, Echium, and Onosma (Malik et al. 2016). Shikonin biosynthesis starts from geranyl-hydroxybenzoate, which is coupled by geranyl diphosphate from the mevalonate pathway and 4-hydroxybenzoate from the phenylpropanoid pathway, and undergoes a series of oxidation and reduction reactions to yield shikonin and its derivatives (Fig. 1) (Malik et al. 2016). Apart from dyes and food additives, shikonins have a broad spectrum of biological and pharmacological properties, such as antimicrobial, antitumor, antiviral, and anti-inflammatory effects (Papageorgiou et al. 2008; Dresler et al. 2017; Guo et al. 2019).
Fig. 1.
A brief biosynthetic pathway of shikonins. Dotted arrows: uncharacterized reactions
Echium plantagineum L., commonly known as purple viper’s-bugloss or Paterson's curse, is an annual flowering plant of Boraginaceae (Katelaris et al. 1982). It is native to western and southern Europe, northern Africa, and southwestern Asia, and was introduced to Australia in the 1800s as an ornamental plant (Piggin 1982; Grigulis et al. 2001). The flowers, leaves, and stems of E. plantagineum contain pyrrolizidine alkaloids (PAs), which are poisonous to the livers and kidneys and even cause death in herbivores (Weston et al. 2011). Oil extracted from the seeds has a high concentration of valuable fatty acids, such as alpha-linolenic acid, which are good for human health (Cequier-Sánchez et al. 2011; Zárate et al. 2016). Apart from PAs and fatty acids, E. plantagineum root periderm contains naphthoquinones. The first study on shikonins from E. plantagineum dates back to 1974, when the pigment extracted from the roots was identified as shikonin with the use of chromatograms (Shcherbanovskii and Luks 1974). Not until 2011, when Weston et al. (2011) have started their research on E. plantagineum in Australia, did shikonins were found only in the roots of this plant. According to their findings, red naphthoquinones can be extracted from seedlings and young roots, while mature and aged roots contain lower amounts of shikonins due to possible oxidation or polymerization (Weston et al. 2011).
In the past, shikonins were mainly extracted from the aged roots of Lithospermum erythrorhizon and Arnebia euchroma, and the whole process would take more than 3 years (Yazaki 2017). However, biotechnological methods made the production of shikonins in a less time span under in vitro conditions possible (Malik et al. 2016). One such method is using Agrobacterium rhizogenes in inducing the production of hairy roots with stable inheritance and rapid growth in a hormone-free medium (Giri and Narasu 2000). The metabolic profiles of hairy roots are similar to those of mother plants (Kim et al. 2002; Srivastava and Srivastava 2007). The in vitro production of secondary metabolites by hairy roots is influenced by many culture conditions, including temperature, pH, light, elicitors, and nutrient elements (Guillon et al. 2006). The effects of medium ingredients on the production of shikonins by callus cells proposed a new medium, the M9 medium (Mizukami et al. 1977; Fujita et al. 1981a, 1981b). Subsequently, a two-stage culture system composed of a growth medium (Linsmaier and Skoog or Murashige and Skoog, LS or MS) and a production medium (M9) was established (Fujita et al. 1981b). This system is widely used in in vitro shikonin biosynthesis in the tissue cultures of L. erythrorhizon (Zhao et al. 2015; Tatsumi et al. 2016), Lithospermum canescens (Pietrosiuk et al. 2006), A. euchroma (Singh et al. 2010), and Onosma paniculatum (Qi et al. 2008). Notably, an important medium component is a nitrogen source with an ammonium-to-nitrate ratio that is beneficial to the growth of callus cells and negatively correlated with the biosynthesis of shikonins (Fujita et al. 1981b). Thus, the M9 medium with no ammonium is unsuitable for the growth of hairy roots as a separate application. By contrast, the B5 medium has a lower ammonium content than MS and LS, especially 1/2B5 medium with half ammonium concentration, and is thus more conducive to shikonin biosynthesis (Sathyanarayana and Verghese 2007; Renouard et al. 2018; Khazaei et al. 2019).
In this study, we examined A. rhizogenes-mediated hairy root induction in E. plantagineum and compared hairy roots cultured in 1/2B5 medium and M9 medium in terms of shikonin production. The obtained results indicated that the 1/2B5 medium was more efficient for the growth of hairy roots and accumulation of shikonins.
Materials and methods
Plant materials and culture conditions
Mature seeds of E. plantagineum L. were surface sterilized with 75% ethanol for 30 s and 0.1% HgCl2 for 5 min successively. The treated seeds were cultured on a solid and hormone-free 1/2MS basal medium at pH 5.8 supplemented with 3% sucrose and 0.8% agar and grown in a growth incubator maintained at 25 °C with a 16 h light /8 h dark cycle.
Hairy root induction of E. plantagineum
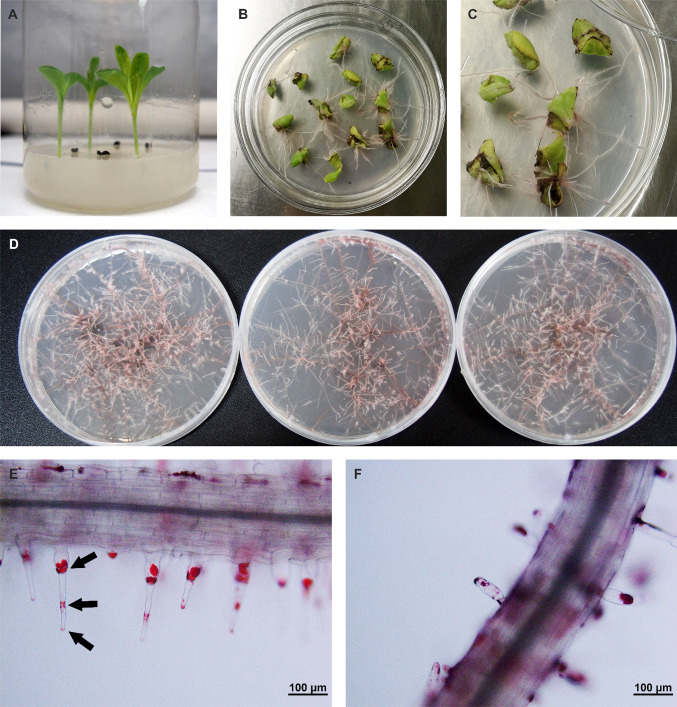
For hairy root induction, A. rhizogenes ATCC15834 was used as the infection strain. Young leaves and stems from sterile E. plantagineum seedlings were used to induce hairy roots (Fig. 2a). First, the cutting explants were precultured on an MS solid medium supplemented with 0.2 mg/L NAA and 2.0 mg/L 6-BA and incubated for 48 h at 23 °C in the dark. A. rhizogenes ATCC15834 was cultured in a liquid YEB medium at 28 °C in a rotary shaker at 120 rpm. Acetosyringone (final concentration of 100 μM) was added for the evaluation of the transformation efficiency when the OD600 of the cells reached 0.4, and cultivation was stopped after OD600 doubled. Then, the cultured bacterial stain was centrifuged and resuspended using an MS liquid medium. The explants were submerged in A. rhizogenes suspension and co-cultured for 15 min. Excess bacteria were removed by placing the explants on sterile filter paper. The infected explants were incubated on the same medium as the precultivation in darkness at 23 °C for 4 days. Then the explants were transferred into a liquid MS medium with 500 mg/L cefotaxime for 20 min with occasional shaking. The plain liquid MS medium was used in washing off residual antibiotic. The explants were cultured on a solid MS medium at 23 °C for 5 days with cefotaxime, whose concentration was reduced from 500 mg/L to 250, 100, 50, and 0 mg/L gradually. After 4–5 weeks, germ-free hairy roots were isolated and cultured on a hormone-free 1/2B5 solid medium at 25 °C in the dark. The hairy roots were subcultured every 30 days. The microscopic observation of transformed hairy roots was performed using OLYMPUS BX51.
Fig. 2.
Hairy root culture of E. plantagineum. a Aseptic seedlings of wild E. plantagineum. b, c Close-up image of induced hairy roots. d Hairy root lines of WT2, WT3, and WT11 in order. e, f Microscopy of transformed hairy roots; Arrows point to red naphthoquinones; Scale bar = 100 μm
Identification of transformed hairy roots
The genomic DNA of E. plantagineum was isolated from the hairy roots and plant samples using TIANGEN Hi-DNA secure plant Kit (TIANGEN, China). The integration of T-DNA from Ri plasmid was confirmed by the amplification of the rolB gene (780 bp), and A. rhizogenes ATCC15834 was used as the positive control. The primer pairs used were as follows:
rolB-F: ATGGATCCCAAATTGCTATTCCCCCACGA;
rolB-R: TAGGCTTCTTTCATTCGGTTTACTGCAGC.
Cultivation of transgenic hairy roots
Wild-type (WT) hairy roots were harvested from the solid medium and transferred to a 40 mL 1/2B5 liquid medium at 25 °C on a shaker at 100 rpm in the dark. After fast proliferation, the hairy roots were transferred into sterile water to wash off the medium, and the central older roots were cut off. The rest fresh hairy roots were placed on sterile filter paper and divided into parts with almost the same volume. Approximately 0.2 g of the drained hairy roots was transferred to a 40 mL 1/2B5 or M9 liquid medium at 25 °C with constant shaking at 100 rpm in the dark. The hairy roots in the two liquid nutrient media were harvested after 7 days of culture and compared in terms of shikonin biosynthesis.
Extraction and HPLC analysis of shikonins
The shikonins in different hairy root lines harvested at day 7 of growth were extracted using the modified method and subjected to HPLC analysis (Wu et al. 2017; Eruygur 2018). The frozen samples were powdered, and 0.1 g of each sample was mixed with 2 mL of ethanol. The mixture was violently blended and incubated at 40 °C for 48 h with occasional shaking. Subsequently, the extraction was centrifuged at 12,000 g for 10 min. The supernatant was then filtered with a 0.45 μm fiber column for HPLC analysis. The spent liquid medium (15 mL) was freeze dried and dissolved in 500 μL of ethanol. The solution was centrifuged, and the supernatant was used for further analysis.
HPLC was performed with an Agilent 1200 Series system (Agilent Technologies, USA). Samples were loaded on an Ultimate XB-C18 column (4.6 × 100 mm, 5 μm, Welch Materials, Inc, China). The mobile phase was composed of solvent A (H2O with 0.1% trifluoroacetic acid) and solvent B (acetonitrile), and the A-to-B ratio was 30:70. The injection volume was 10 μL, and the flow rate was 0.8 mL/min. Peaks were identified by comparing standard retention times. The standard samples of shikonins were purchased from Nanjing PuYi Biological Technology Co. Ltd (Nanjing, China).
Results
Establishment of E. plantagineum hairy roots
Putative hairy roots emerged at wounding sites within two weeks after inoculation (Fig. 2b, c). The excised hairy roots were cultured on an MS solid medium with cefotaxime for the elimination of residual A. rhizogenes. Aseptic hairy roots with extensive branching grew rapidly on the antibiotic-free and hormone-free 1/2B5 solid medium (Fig. 2d). Red granules accumulated in the root hairs of the induced hairy roots (Fig. 2e). The older hairy roots appeared to have deep red epidermal surfaces and shorter root hairs than the new ones (Fig. 2f). The stable transformation of hairy roots were confirmed by amplifying the rolB gene. In the PCR results, rolB gene fragments were observed in transgenic hairy roots, demonstrating a successful transformation (Fig. 3).
Fig. 3.
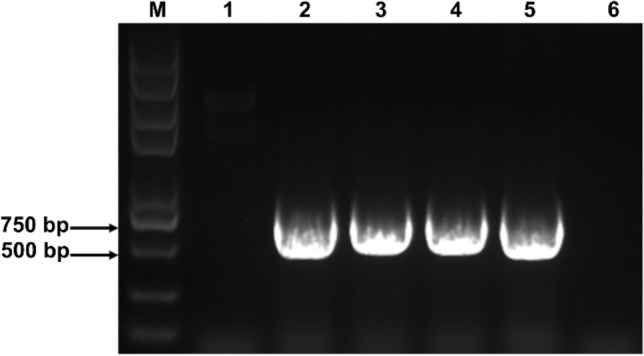
PCR results of rolB gene in hairy root lines. Lane M, marker (8000 bp); lane 1, negative control 1 (leaves of the plant); lane 2–4 transformed hairy root lines; lane 5, positive control (bacteria of ATCC15834); lane 6, negative control 2 (roots of the plant)
Shikonin production of hairy roots grown in 1/2B5 and M9 nutrient liquid media
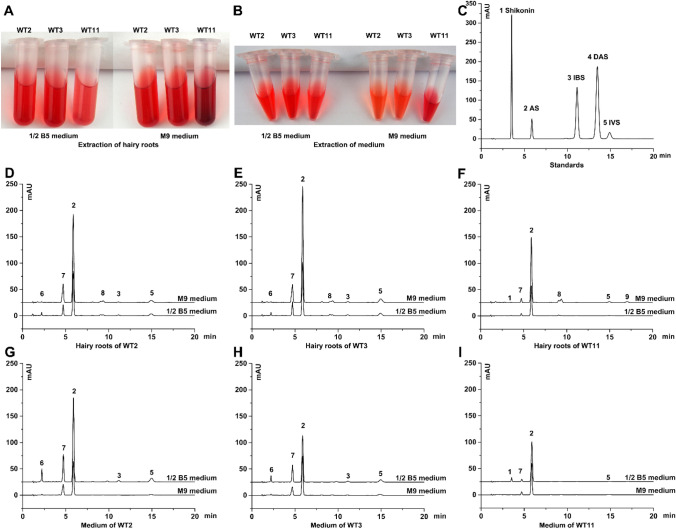
Approximately 0.2 g of the randomly selected hairy root (WT2, WT3, or WT11) was cultured in 40 mL liquid media for 7 days (Fig. 4). Obvious red pigment accumulation was found in all the hairy roots (Fig. 5a). The intercellular and extracellular production of shikonins was determined by HPLC analysis, and five analytical standards, namely, shikonin (Rt = 3.5 min); acetylshikonin (AS, Rt = 5.8 min); isobutylshikonin (IBS, Rt = 11.1 min); β, β’-dimethylacrylshikonin (DAS, Rt = 13.5 min); and isovalerylshikonin (IVS, Rt = 14.9 min; Fig. 4c), were used. HPLC analysis of the pigment extraction from the hairy roots and culture medium revealed nine compounds (Fig. 4). Apart from the identified standard shikonins, compound 7 was deduced to be β-hydroxyisovalerylshikonin (HIVS) (Fujita et al. 1983; Wu et al. 2017). The peaks between AS and IBS were named as compounds 8, which may include propionylshikonin and deoxyshikonin (Albreht et al. 2009; Ito et al. 2011). In addition, the shikonin profiles of E. plantagineum roots (Skoneczny et al. 2017; Wu et al. 2017) and hairy roots were compared, and the obtained results showed HIVS and AS to be consistently present in all samples. The intact plant roots were devoid of two unknown compounds (compounds 6 and 9), while no DAS peaks were observed in the hairy roots. The components of shikonins differed among hairy root lines rather than the culture media. The hairy roots of WT2 and WT3 had similar shikonin accumulation patterns in the 1/2B5 and M9 media (Fig. 4d, e, g, h). By contrast, shikonin and compound 9 were only detectable in line WT11, whereas IBS and compound 6 were not detected (Fig. 4f, i).
Fig. 4.
HPLC analysis for the shikonin production of the hairy roots in 1/2B5 and M9 liquid nutrient media. a, b Physical appearance of ethanol-extracted samples from hairy roots and spent liquid medium. c Chromatogram of standard shikonins (1 shikonin; 2 AS, acetylshikonin; 3 IBS, isobutylshikonin; 4 DAS, β, β’-dimethylacrylshikonin; 5 IVS, isovalerylshikonin). d–f Chromatogram of different shikonins from transformed hairy root extracts. g–i Chromatogram of different shikonins from liquid medium extracts. 7 HIVS, β-hydroxyisovalerylshikonin (inferential); 8 propionylshikonin and deoxyshikonin (inferential); 6 and 9 unknown compounds
Fig. 5.
Influence of different liquid nutrient media on the growth and acetylshikonin (AS) accumulation in the hairy roots. a Morphological observation of harvested hairy roots after 7 days of growth in two different liquid nutrient media. b Biomass of hairy roots in different liquid nutrient media harvested at day 7. c The impact of two growth media on the production of acetylshikonin (AS) by E. plantagineum hairy roots. d Acetylshikonin (AS) contents of hairy root strains grown in liquid 1/2B5 and M9 nutrient media at day 7. Results are the mean values ± SE with three replicates. Heat map was generated using Heml 1.0 Software. Significant difference indicates as *p < 0.05, **p < 0.01, and ***p < 0.001, respectively, when compared between different liquid nutrient media of each hairy root line
Despite variations among samples, AS was the most abundant shikonin derivative. The intercellular yield of AS in the M9 medium was approximately 2.2-fold of that in the 1/2B5 medium for all the three lines. The yields of the extractions from the 1/2B5 medium were 2.0-fold of those from the spent M9 medium, except for line WT11 which seemed to release more AS in the medium (Fig. 5c). Different hairy root lines displayed variations in AS yields with a higher accumulation in WT3. The total production of intercellular and extracellular AS in the 1/2B5 media was twofold (36.25 mg/L on average) of that in the M9 media (18.06 mg/L on average; Fig. 5d).
Effects of culture media on the growth of the transformed hairy roots
The growth of hairy roots was evaluated in hormone-free conditions in 1/2B5 and M9 media. The fresh weight of hairy roots harvested from the 1/2B5 medium was nearly fivefold of that from the M9 medium (Fig. 5b). Furthermore, growth rate varied among the investigated hairy root lines in the 1/2B5 medium, that is, WT11 had twofold increase in biomass compared with the other two lines after 7-day cultivation. However, no increase in biomass was observed in the hairy roots cultured in M9 media.
Discussion
A. rhizogenes-transformed hairy roots have been widely used in the in vitro biosynthesis of desired secondary metabolites. Shikonins are the most common naphthoquinones among the family Boraginaceae and have antimicrobial, anti-inflammatory, and antitumor properties (Zare et al. 2011). Hairy roots systems have been successfully established in L. canescens and L. erythrorhizon for the production of shikonins (Sykłowska-Baranek et al. 2012; Fang et al. 2016). E. plantagineum belongs to the genus Echium, close to these Lithospermum plants (Chacón et al. 2016). However, studies that used hairy root systems for the biosynthesis of polyunsaturated fatty acid and PAs in Echium acanthocarpum and Echium rauwolfii are few (Abd El-Mawla 2010; Cequier-Sánchez et al. 2011; Zárate et al. 2013), and little is known about shikonin production in the hairy roots of Echium plants. In the current investigation, we aimed to establish the hairy roots of E. plantagineum to study the biosynthesis of shikonins. The ATCC15834 strain, which is mostly used in the hairy root induction of Lithospermeae, was used to infect the explants of E. plantagineum. The induction conditions, such as growth media and bacterial concentrations, have a significant effect on transformed efficiency (Giri and Narasu 2000). The explants used for hairy root induction are essential (Sykłowska-Baranek et al. 2012; Renouard et al. 2018). Notably, hairy roots were hardly induced with the stems of E. plantagineum, but a good transformation rate was observed using leaves. E. plantagineum is an invasive species in Australia, with vigorous growth in field and laboratory environments. The characteristics, such as branching roots, rapid growth, and loss of geotropism, are useful in identifying putative transformed hairy roots. The formation of hairy roots is a consequence of the integration of T-DNA from A. rhizogenes into the genome of plant cells. Given that spontaneous adventitious root emission was observed in some wounded leaf fragments that were not treated with A. rhizogenes, the T-DNA of Ri plasmid containing the rolB gene was amplified for the confirmation of transformation.
The induced hairy roots showed some red droplets in the root hairs and reddish-violet periderm (Fig. 2e, f). The cellular location of shikonins in the hairy roots of L. erythrorhizon involves root hairs and root border cells (Brigham et al. 1999). These red droplets remained at a certain distance from the epidermis of the root hairs, indicating the release of shikonins into the rhizosphere (Tsukada and Tabata 1984). Tatsumi et al. (2016) reported that the secretion of shikonins required actin and the pathway of the ADP-ribosylation factor/guanine nucleotide exchange factor system in some way. The release of shikonins from root hairs may be involved in defense against herbivores and plays an important role in antimicrobial activity (Yazaki 2004). Bioactive shikonins extracted from the living roots and rhizosphere of E. plantagineum implicate important roles in plant defense and invasive success (Zhu et al. 2016; Skoneczny et al. 2017). Skoneczny et al. (2019) proposed that naphthoquinones produced by Paterson's curse enhances intraspecific competition and resistance to drought or high temperatures. However, despite the pharmacological effects of shikonins and their ability to respond to stress, the exact mechanism of transportation and secretion of shikonins is still unknown.
The nitrogen sources in basal media have a considerable influence on shikonin production. Ammonium inhibits the production of shikonins, whereas nitrate increases the yield (Fujita et al. 1981b). Basal MS or LS media with high concentrations of ammonium enhance the growth of tissue cultures but suppress shikonin accumulation. The B5 medium is conducive to the growth of tissues, with a lower ammonium content than MS and LS media (Sathyanarayana and Verghese 2007; Renouard et al. 2018; Khazaei et al. 2019). It has been used for the study of shikonins in Echium italicum callus cells with liquid paraffin (Zare et al. 2010). The M9 medium is modified according to the effects of nutrient factors for the improvement of naphthoquinones’ productivity regardless of the cell growth of L. erythrorhizon (Fujita et al. 1981b).
Hence, we compared shikonin biosynthesis in the transformed hairy root cultures in a 1/2B5 medium (low ammonium content) with that in an M9 medium (most usage in shikonin accumulation). The hairy roots of E. plantagineum were rapidly grew after being transferred to the 1/2B5 medium. The harvested biomass of line WT11 was twofold of that of WT2 or WT3 (Fig. 5b). The callus cells of L. erythrorhizon can be subcultured for some cycles (Fujita et al. 1981b), and the hairy root cultures of L. canescens were maintained in an M9 medium for 3 weeks (Sykłowska-Baranek et al. 2012). However, the growth of the E. plantagineum hairy roots in the M9 medium barely increased in our investigation (Fig. 5b), indicating that the M9 medium is ineffective in scaling-up cultures for the production of shikonins with E. plantagineum hairy roots.
Shikonins were detected by HPLC in all hairy roots and their corresponding media. The peaks of pigment extracted from 1/2B5 medium resembled those from the M9 medium but differed among the hairy root lines (Fig. 4d–i). The root lines of WT2 and WT3 produced compound 6, which was not detectable in line WT11 and the roots of the intact plant. Interestingly, compound 6 had a shorter retention time than shikonin, indicating a new naphthoquinone with a small molecular weight. Compound 9 was only detected in the WT11 hairy roots. Compounds 6 and 9 need to be identified precisely. AS was the dominant component in the pigment extraction of hairy roots or the media. The WT3 hairy roots had the highest amount of accumulated AS (42.69 mg/L), followed by WT11 and WT2 lines in the 1/2B5 medium (Fig. 5d). The accumulation of AS in the M9 medium was approximately 18.06 mg/L for all the three lines. In summary, the different yields of AS between the two liquid nutrient media indicated that the M9 medium favored shikonin accumulation but with serious growth inhibition. The 1/2B5 medium was beneficial for the biomass increase of hairy roots with considerable AS production and thus more suitable for industrial application.
Our investigation demonstrated an alternative method for in vitro shikonin production. Some limitations on the industrial exploration of the transformed hairy roots need to be investigated: First, the extraction of the pigments secreted into the media could be improved by the use of a biphasic culture or by the addition of macroreticular adsorbents. Second, culture conditions, elicitors, and hormones can be examined for maximization of shikonin production. Third, a careful selection of hairy root lines should be performed for the accumulation of some special shikonins from root cultures.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (U1903201, 31970321, 31771413, 31670298), the Open Project Program from MOE Key Laboratory of Molecular Epigenetics of China, and the Program for Changjiang Scholars and Innovative Research Team in University from the Ministry of Education of China (IRT_14R27).
Author contributions
YHY, JLQ, and GHL conceived and designed the experiments. JYF, HZ, and JXB performed the experiments. JYF, HZ, ZLW, and RJF analyzed the data. AF, MKY, BL, TMY, and YJP contributed to resources. JYF and HZ wrote the draft of manuscript. YHY, JLQ, and GHL contributed to review and edit the manuscript. All the authors carefully checked and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Jiang-Yan Fu and Hua Zhao contributed equally to this work.
Contributor Information
Gui-Hua Lu, Email: ghlu@hytc.edu.cn.
Jin-Liang Qi, Email: qijl@nju.edu.cn.
Yong-Hua Yang, Email: yangyh@nju.edu.cn.
References
- Abd El-Mawla A. Effect of certain elicitors on production of pyrrolizidine alkaloids in hairy root cultures of Echium rauwolfii. Pharmazie. 2010;65(3):224–226. [PubMed] [Google Scholar]
- Albreht A, Vovk I, Simonovska B, Srbinoska M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J Chromatogr A. 2009;1216(15):3156–3162. doi: 10.1016/j.chroma.2009.01.098. [DOI] [PubMed] [Google Scholar]
- Babula P, Adam V, Havel L, Kizek R. Noteworthy secondary metabolites naphthoquinones-their occurrence, pharmacological properties and analysis. Curr Pharm Anal. 2009;5(1):47–68. [Google Scholar]
- Brigham LA, Michaels PJ, Flores HE. Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiol. 1999;119(2):417–428. doi: 10.1104/pp.119.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier-Sánchez E, Rodríguez C, Dorta-Guerra R, Ravelo ÁG, Zárate R. Echium acanthocarpum hairy root cultures, a suitable system for polyunsaturated fatty acid studies and production. BMC Biotechnol. 2011;11(1):42. doi: 10.1186/1472-6750-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón J, Luebert F, Hilger HH, Ovchinnikova S, Selvi F, Cecchi L, Guilliams CM, Hasenstab-Lehman K, Sutorý K, Simpson MG. The borage family (Boraginaceae s. str.): a revised infrafamilial classification based on new phylogenetic evidence, with emphasis on the placement of some enigmatic genera. Taxon. 2016;65(3):523–546. [Google Scholar]
- Dresler S, Szymczak G, Wójcik M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm Biol. 2017;55(1):691–695. doi: 10.1080/13880209.2016.1265986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruygur N. A simple isocratic high-perfomance liquid chromatography method for the simultaneous determination of shikonin derivatives in some Echium species growing wild in Turkey. Turk J Pharm Sci. 2018;15(1):38–43. doi: 10.4274/tjps.40316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Zou A, Zhao H, Wu F, Zhu Y, Zhao H, Liao Y, Tang R-J, Pang Y, Yang R. Transgenic studies reveal the positive role of LeEIL-1 in regulating shikonin biosynthesis in Lithospermum erythrorhizon hairy roots. BMC Plant Biol. 2016;16(1):121. doi: 10.1186/s12870-016-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Hara Y, Ogino T, Suga C. Production of shikonin derivatives by cell suspension cultures of Lithospermum etythrorhizon. I. Effects of nitrogen sources on the production of shikonin derivatives. Plant Cell Rep. 1981;1:59–60. doi: 10.1007/BF00269272. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hara Y, Suga C, Morimoto T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. II. A new medium for the production of shikonin derivatives. Plant Cell Rep. 1981;1(2):61–63. doi: 10.1007/BF00269273. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Maeda Y, Suga C, Morimoto T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. III. Comparison of shikonin derivatives of cultured cells and Ko-shikon. Plant Cell Rep. 1983;2(4):192–193. doi: 10.1007/BF00270101. [DOI] [PubMed] [Google Scholar]
- Giri A, Narasu ML. Transgenic hairy roots recent trends and applications. Biotechnol Adv. 2000;18(1):1–22. doi: 10.1016/s0734-9750(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Grigulis K, Sheppard A, Ash J, Groves R. The comparative demography of the pasture weed Echium plantagineum between its native and invaded ranges. J App Ecol. 2001;38(2):281–290. [Google Scholar]
- Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol. 2006;9(3):341–346. doi: 10.1016/j.pbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C. Pharmacological properties and derivatives of shikonin: a review in recent years. Pharmacol Res. 2019;2019:104463. doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- Ito Y, Onobori K, Yamazaki T, Kawamura Y. Tigloylshikonin, a new minor shikonin derivative, from the roots and the commercial root extract of Lithospermum erythrorhizon. Chem Pharm Bull. 2011;59(1):117–119. doi: 10.1248/cpb.59.117. [DOI] [PubMed] [Google Scholar]
- Katelaris C, Baldo B, Howden M, Matthews P, Walls R. Investigation of the involvement of Echium plantagineum (Paterson’s curse) in seasonal allergy: IgE antibodies to Echium and other weed pollens. Allergy. 1982;37(1):21–28. doi: 10.1111/j.1398-9995.1982.tb04113.x. [DOI] [PubMed] [Google Scholar]
- Khazaei A, Bahramnejad B, Mozafari A-A, Dastan D, Mohammadi S. Hairy root induction and Farnesiferol B production of endemic medicinal plant Ferula pseudalliacea. 3 Biotech. 2019;9(11):407. doi: 10.1007/s13205-019-1935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wyslouzil BE, Weathers PJ. Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol-Plant. 2002;38(1):1–10. [Google Scholar]
- Malik S, Bhushan S, Sharma M, Ahuja PS. Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol. 2016;36(2):327–340. doi: 10.3109/07388551.2014.961003. [DOI] [PubMed] [Google Scholar]
- Mizukami H, Konoshima M, Tabata M. Effect of nutritional factors on shikonin derivative formation in Lithospermum callus cultures. Phytochemistry. 1977;16(8):1183–1186. [Google Scholar]
- Papageorgiou V, Assimopoulou A, Ballis A. Alkannins and shikonins: a new class of wound healing agents. Curr Med Chem. 2008;15(30):3248–3267. doi: 10.2174/092986708786848532. [DOI] [PubMed] [Google Scholar]
- Pietrosiuk A, Sykłowska-Baranek K, Wiedenfeld H, Wolinowska R, Furmanowa M, Jaroszyk E. The shikonin derivatives and pyrrolizidine alkaloids in hairy root cultures of Lithospermum canescens (Michx.) Lehm. Plant Cell Rep. 2006;25(10):1052–1058. doi: 10.1007/s00299-006-0161-2. [DOI] [PubMed] [Google Scholar]
- Piggin C. The biology of Australian weeds. 8. Echium plantagineum L. J Austral Inst Agric Sci. 1982;48:3–16. [Google Scholar]
- Qi J-L, Zhang W-J, Liu S-H, Wang H, Sun D-Y, Xu G-H, Shi M-W, Liu Z, Zhang M-S, Zhang H-M. Expression analysis of light-regulated genes isolated from a full-length-enriched cDNA library of Onosma paniculatum cell cultures. J Plant Physiol. 2008;165(14):1474–1482. doi: 10.1016/j.jplph.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Renouard S, Corbin C, Drouet S, Medvedec B, Doussot J, Colas C, Maunit B, Bhambra A, Gontier E, Jullian N. Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int J Mol Sci. 2018;19(4):990. doi: 10.3390/ijms19040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana B, Verghese DB. Plant tissue culture: practices and new experimental protocols. New Delhi: I. K. International Publishing House; 2007. [Google Scholar]
- Shcherbanovskii L, Luks YA. Shikonin from Echium lycopsis. Chem Nat Compd. 1974;10(4):517–517. [Google Scholar]
- Singh RS, Gara RK, Bhardwaj PK, Kaachra A, Malik S, Kumar R, Sharma M, Ahuja PS, Kumar S. Expression of 3-hydroxy-3-methylglutaryl-CoA reductase, p-hydroxybenzoate-m-geranyltransferase and genes of phenylpropanoid pathway exhibits positive correlation with shikonins content in arnebia [Arnebia euchroma (Royle) Johnston] BMC Mol Biol. 2010;11(1):88. doi: 10.1186/1471-2199-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoneczny D, Weston P, Zhu X, Gurr G, Callaway R, Barrow R, Weston L. Metabolic profiling and identification of shikonins in root periderm of two invasive Echium spp. weeds in Australia. Molecules. 2017;22(2):330. doi: 10.3390/molecules22020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoneczny D, Zhu X, Weston PA, Gurr GM, Callaway RM, Weston LA. Production of pyrrolizidine alkaloids and shikonins in Echium plantagineum L. in response to various plant stressors. Pest Manag Sci. 2019;75(9):2530–2541. doi: 10.1002/ps.5540. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK. Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol. 2007;27(1):29–43. doi: 10.1080/07388550601173918. [DOI] [PubMed] [Google Scholar]
- Sykłowska-Baranek K, Pietrosiuk A, Gawron A, Kawiak A, Łojkowska E, Jeziorek M, Chinou I. Enhanced production of antitumour naphthoquinones in transgenic hairy root lines of Lithospermum canescens. Plant Cell Tissue Organ Cult. 2012;108(2):213–219. [Google Scholar]
- Tatsumi K, Yano M, Kaminade K, Sugiyama A, Sato M, Toyooka K, Aoyama T, Sato F, Yazaki K. Characterization of shikonin derivative secretion in Lithospermum erythrorhizon hairy roots as a model of lipid-soluble metabolite secretion from plants. Front Plant Sci. 2016;7:1066. doi: 10.3389/fpls.2016.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Tabata M. Intracellular localization and secretion of naphthoquinone pigments in cell cultures of Lithospermum erythrorhizon. Planta Med. 1984;50(04):338–341. doi: 10.1055/s-2007-969725. [DOI] [PubMed] [Google Scholar]
- Verpoorte R, Alfermann AW. Metabolic engineering of plant secondary metabolism. Dordrecht: Springer; 2000. [Google Scholar]
- Weston LA, Weston PA, McCully M. Production of bioactive napthoquinones by roots of Paterson’s curse (Echium plantagineum)—Implications for invasion success. Pak J Weed Sci Res. 2011;18:677–686. [Google Scholar]
- Wu F-Y, Tang C-Y, Guo Y-M, Bian Z-W, Fu J-Y, Lu G-H, Qi J-L, Pang Y-J, Yang Y-H. Transcriptome analysis explores genes related to shikonin biosynthesis in Lithospermeae plants and provides insights into Boraginales’ evolutionary history. Sci Rep. 2017;7(1):4477. doi: 10.1038/s41598-017-04750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K (2004) Natural products and metabolites. In: Christou P and Klee H (ed) Handbook of plant biotechnology. Doi: 10.1002/0470869143.kc039
- Yazaki K. Lithospermum erythrorhizon cell cultures: present and future aspects. Plant Biotechnol. 2017;34(3):131–142. doi: 10.5511/plantbiotechnology.17.0823a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate R, Cequier-Sánchez E, Rodríguez C, Dorta-Guerra R, El Jaber-Vazdekis N, Ravelo ÁG. Improvement of polyunsaturated fatty acid production in Echium acanthocarpum transformed hairy root cultures by application of different abiotic stress conditions. ISRN Biotechnol. 2013;2013:169510. doi: 10.5402/2013/169510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate R, Cequier-Sánchez E, El Jaber-Vazdekis N, Rodríguez C, Dorta-Guerra R, Ravelo ÁG. Healthy omega-3 enhancement in Echium acanthocarpum transformed hairy roots by overexpression of a 6-desaturase gene from Primula vialli. SCIREA J Bio. 2016;1:1–40. [Google Scholar]
- Zare K, Nazemiyeh H, Movafeghi A, Khosrowshahli M, Motallebi-Azar A, Dadpour M, Omidi Y. Bioprocess engineering of Echium italicum L.: induction of shikonin and alkannin derivatives by two-liquid-phase suspension cultures. Plant Cell Tissue Organ Cult. 2010;100(2):157–164. [Google Scholar]
- Zare K, Khosrowshahli M, Nazemiyeh H, Movafeghi A, Azar AM, Omidi Y. Callus culture of Echium italicum L. towards production of a shikonin derivative. Nat Prod Res. 2011;25(16):1480–1487. doi: 10.1080/14786410902804857. [DOI] [PubMed] [Google Scholar]
- Zhao H, Chang Q, Zhang D, Fang R, Wu F, Wang X, Lu G, Qi J, Yang Y. Overexpression of LeMYB1 enhances shikonin formation by up-regulating key shikonin biosynthesis-related genes in Lithospermum erythrorhizon. Biol Plantarum. 2015;59(3):429–435. [Google Scholar]
- Zhu X, Skoneczny D, Weidenhamer JD, Mwendwa JM, Weston PA, Gurr GM, Callaway RM, Weston LA. Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson's curse (Echium plantagineum), a noxious invader. J Exp Bot. 2016;67(12):3777–3788. doi: 10.1093/jxb/erw182. [DOI] [PMC free article] [PubMed] [Google Scholar]