Abstract
Aim: To evaluate the long-term prognostic significance of right ventricular (RV) deformation and RV-arterial coupling in a cohort of patients with heart failure (HF) due to severe aortic stenosis (AS) candidate for trans-catheter aortic valve implantation (TAVI). Methods: The study is a retrospective analysis of 56 patients undergoing echocardiography before TAVI execution. RV function was defined by tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), peak systolic myocardial velocity by tissue Doppler imaging (RVSm) and RV longitudinal strain (RVLS). RV-arterial coupling were defined as TAPSE and RVLS normalized for systolic pulmonary artery pressure (sPAP) to obtain afterload-independent parameters: TAPSE/sPAP and RVLS/sPAP, respectively. All-cause mortality was the primary endpoint of survival analysis; composite of death and hospitalization for HF was the secondary endpoint. Results: All patients underwent TAVI from femoral access. Mean age was 81.6±6.3 years and left ventricular ejection fraction was preserved in most patients (51±15%). At 10 years, using Cox regression analysis adjusted for the parameters related to prognosis at univariate analysis, we found that only pre-procedural RVLS was independently associated with all-cause mortality (aHR 1.53, 95% CI 1.10-2.12, P=0.011). RVLS (aHR 7.542, 95% CI 1.325-42.921, P=0.023), sPAP (aHR 1.421, 95% CI 1.045-1.932, P=0.025), TAPSE/sPAP (aHR 4.977, 95% CI 5.425-21.99, P=0.044) and RVLS/sPAP (aHR 2.333, 95% CI 3.9677-12.999, P=0.046) were independently associated with the secondary endpoint. Conclusions: Among patients with HF due to severe AS undergoing TAVI, deformation imaging (i.e., RVLS) and RV-arterial coupling (i.e., TAPSE/sPAP and RVLS/sPAP) provide better risk stratification at long-term follow up of 10 years than other RV echocardiographic parameters.
Keywords: Right ventricular function, right ventricle longitudinal strain, trans-catheter valve implantation, heart failure, right ventricular-arterial coupling
Introduction
Trans-catheter aortic valve implantation (TAVI) was demonstrated to reduce mortality and morbidity in patients with severe aortic stenosis (AS), considered at prohibitive, high and intermediate surgical risk [1,2]. Moreover, it was recently shown that TAVI is also a valid alternative to surgery in patients at low surgical risk [3,4]. However, according to current guidelines, patients candidate for TAVI still have high rates of short- and long-term mortality, because of their comorbidities and procedure-related complications [5-9]. To now, there is no TAVI risk predicting model comparable to the Logistic EuroScore used in cardiac surgery, even if several clinical and procedural characteristics have been shown to affect the outcome after TAVI. Baseline cardiovascular characteristics (e.g., low left ventricular ejection fraction (LVEF), moderate-to-severe mitral regurgitation (MR)) and procedural complications (e.g., vascular complications, residual aortic regurgitation (AR), need for cardiac stimulation) are now recognized to play a central role in acute and early mortality. Regarding long-term mortality, non-cardiac comorbidities (such as anemia, liver disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease) seem to be more important [2,10]. Pre-procedural full echocardiographic assessment of patients with AS candidate for TAVI or surgery should be performed to identify anatomical and functional detailed features of the aortic valve, ascending aorta and LV [11-13]. Importantly, lack of knowledge does still exist about the role of right ventricular (RV) dysfunction. Given its particular position and shape, cardiovascular magnetic resonance (CMR) is the gold standard to evaluate RV volumes, mass and function, but it has limited availability [14-16]. For this reason, echocardiography is still the most used imaging technique in this field [17]. Tissue Doppler imaging (TDI) and speckle tracking echocardiography (STE) estimate ventricular contractility which is less influenced by passive motion of the myocardium and loading conditions [18]. In particular, load-independent indices of RV function are able to determine RV-arterial coupling and can be obtained normalizing RV longitudinal systolic parameters to RV afterload (i.e., systolic pulmonary artery pressure (sPAP)) [19]. The idea that such parameters could be more efficacious prognostic markers in HF than other conventional echocardiographic parameters is now well recognized [20-22]. We aimed to assess the role of pre-procedural RV STE and RV-arterial coupling in predicting long-term outcome in patients with HF undergoing TAVI.
Materials and methods
Study population
The present study is a retrospective evaluation of a cohort of 56 patients with severe degenerative AS in tricuspid valves (valve area ≤ 1.0 cm2 or peak velocity > 4 m/s) who were referred for TAVI between September 2009 and September 2012 at the Cardiology Department of the University Hospital ASST Spedali Civili of Brescia, Italy. Inclusion criteria were: diagnosis of severe degenerative AS and recent onset of symptoms and sign of HF not due to other concomitant conditions than AS; age > 18 years; adequate image quality for post-hoc analysis of STE measurements; planned TAVI with trans-femoral access during hospitalization. Exclusion criteria were: concomitant diagnosis of coronary artery disease requiring revascularization during hospitalization for TAVI; idiopathic cardiomyopathy; history of pulmonary embolism; recent acute coronary syndrome or revascularization (in the previous 3 months); other life-threatening comorbidities with adverse prognosis at time of hospitalization for symptomatic AS; any disease causing precapillary pulmonary hypertension (PH). The study was carried out according to the principles of the Declaration of Helsinki and approved by local ethics committee; all patients provided written informed consent. TAVI was performed under general anesthesia. Patients underwent CoreValve implantation due to high/prohibitive risk for surgical operation.
Data collecting and echocardiographic parameters
For each patient we collected pre-operative clinical and demographic characteristics (at time of hospitalization for TAVI): age, sex, body mass index (BMI), biochemical data, functional class (New York Hear Association, NYHA), clinical history and risk factors (i.e., hypertension, diabetes, coronary heart disease, COPD), preoperative cardiac surgery risk score assessed as Logistic EuroScore. We used all pre-operative echocardiographic images of study participants for post-hoc analysis of parameters. The echocardiographic evaluation was performed in a blinded way by two specialists and included mono- and two-dimensional evaluation, continuous Doppler, pulsed Doppler, TDI. All LV and RV parameters were evaluated and analyzed according to the more recent international standards defined by the American Society of Echocardiography and the European Association of Cardiovascular Imaging [17,23]. LVEF was calculated using the Simpson’s biplane method. The mean pressure gradient across the aortic valve was estimated using the simplified Bernoulli equation. Using Doppler echocardiography, peak aortic velocity, peak left ventricular outflow tract (LVOT) velocity, aortic and LVOT velocity time integral (VTI) and mean pressure gradient were determined [24]. The effective orifice area (EOA) was calculated from the continuity equation. MR, AR, and tricuspid valve regurgitation (TR) were evaluated using spectral and color-Doppler images and graded as trivial, mild, moderate, and severe, as recommended [25]. LV and RV diastolic function was obtained as the ratio of the early and late diastolic trans-mitral and trans-tricuspidal flow velocities (LV E/A ratio and RV E/A ratio), by the deceleration time of the early diastolic trans-mitral and trans-tricuspidal flow velocity (LV E deceleration time, RV E deceleration time) [17,26]. The sPAP was calculated by measurement of the TR velocity and estimation of the right atrial pressure by dimension and collapsibility of the inferior vena cava [17]. RV systolic quantitative parameters were consistent with current guidelines and acquired as follows: fractional area change (FAC), peak systolic myocardial velocity by TDI (RVSm), tricuspid annular plane systolic excursion by M-Mode (TAPSE), RV longitudinal strain (RVLS) [17]. FAC was computed as: (RV diastolic area-RV systolic area)/RV diastolic area × 100% [17]. Echocardiographic images for deformation analyses had been stored at a frame rate between 50 and 70 frames/sec, for at least three cardiac cycles. Loops were processed with an ad hoc software (EchoPAC BT12; GE Medical Systems), allowing offline STE analyses. For RVLS loops had been acquired using 4 chamber view, RV endocardial border was traced at the end-systolic frame and RV was partitioned into 6 standard segments at 3 levels (i.e., the basal, middle, and apical levels), correspondingly generating 6 time-strain curves. We calculated the global RVLS values by averaging the values computed at the segmental level, as recommended in the consensus document of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [27]. TAPSE/sPAP ratio and RVLS/sPAP ratio were derived as indexes of RV-arterial coupling to obtain afterload-independent parameters [19-22].
Follow-up and endpoint of the study
The follow-up was conducted either at the hospital during a routine clinical evaluation or by telephone contact with the patients, their relatives or family doctors, and was 100% complete. Long-term mortality included death for any cause. Readmission at follow-up included any episode of re-hospitalization for HF. The primary endpoint of the present study was long-term (10-year) mortality from any cause. Secondary endpoint was long-term freedom from cardiac events, defined as combined of death of any cause and HF readmission (combined endpoint).
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD). Frequencies are reported as number (%). We randomly selected 10 patients and evaluated intra-observer and inter-observer variabilities using Pearson’s correlation coefficient (R), Bland-Altman with limit of agreement (LOA) statistics and intraclass correlation coefficient. Univariate analysis was performed to identify the correlates of RV dysfunction described as TAPSE < 17 mm or RVLS < median value (given the absence of consensus about a cut-off for normality in the general population): variables tested included those known to cause or contribute to RV dysfunction, including age, history of coronary artery disease, arterial pressure, LVEF, sPAP. Odds ratios (OR) and their 95% confidence intervals (CI) were computed by means of logistic models. Cumulative survival was calculated based on Kaplan-Meier estimates; the endpoint of survival analysis was all-cause death and combined endpoint of all-cause death and hospitalization for HF. Cox regression model was used for defining multivariate analysis with variables and survival (time-free event). All statistical analyses were performed using SPSS V.21.0, IBM, Chicago, Illinois, USA), with a two-sided significance level of p 0.05.
Results
Patients population and echocardiographic measurements
56 patients were included in this retrospective analysis. Patients underwent TAVI during hospitalization and 50% of them were treated with 26 mm valve and other 50% with 29 mm valve, according to pre-operative evaluation. TAVI was performed by femoral access in all patients, so they were homogeneous under this point of view. Clinical and demographic characteristics of patients are listed in (Table 1). Mean age was 81.6±6.3 years and patients were at high risk for surgical replacement according to Logistic EuroScore (mean value more than 20%). Most patients had hypertension (70%) and coronary artery disease (50%). At time of evaluation most patients were hemodynamically stable (mean systolic pressure: 128 mmHg, mean diastolic pressure: 68 mmHg) and most had functional class NYHA III (41 patients, 73%).
Table 1.
Clinical features of patients population
| Variable | Value (n=56) |
|---|---|
| Age (years) | 81.6±6.3 |
| Male gender | 24 (42.9%) |
| Body mass index (kg/m2) | 26.6±4.6 |
| Logistic EuroScore (%) | 24.1±17.9 |
| Creatinine clearance (mL/min) | 47±22 |
| Hemoglobin (g/dL) | 11.8±2.2 |
| NYHA Class III-IV | 42 (75.0%) |
| Systolic arterial pressure (mmHg) | 127.7±20.2 |
| Diastolic arterial pressure (mmHg) | 67.5±10.9 |
| Hypertension | 39 (69.6%) |
| Diabetes mellitus | 16 (28.6%) |
| Coronary artery disease | 28 (50.0%) |
| Chronic obstructive pulmonary disease | 12 (21.4%) |
| Previous cardiac surgery | 15 (26.8%) |
NYHA, New York Heart Association.
Echocardiographic parameters are listed in Table 2. Patients had LV hypertrophy and the mean of values of LVEF was 51±15%, so no one of patients enrolled had severe systolic dysfunction (LVEF < 35%). Mean trans-aortic gradient was 51 mmHg; AR was mild in 32% of patients, moderate in 53% and severe only in 5% of patients. Concomitant MR was mild in 38% of patients, moderate in 62% and no one had severe MR. About TR, there was more heterogeneity: 50% of patients had mild TR, 27% moderate and 23% severe. Most patients had PH pre-TAVI (sPAP was 39±16 mmHg). Continuous Doppler on tricuspid valve showed reduced early diastolic flow (E/A < 1), with mean deceleration time 216±47 ms. Tricuspid annulus diameter, FAC, TAPSE, RVSm had normal values. Before TAVI we observed 42.9% (n=24) of prevalence of RV dysfunction detected by TAPSE < 17 mm.
Table 2.
Echocardiographic characteristics of patients population
| Variable | Value (n=56) |
|---|---|
| Aortic annulus diameter (mm) | 22.8±2.0 |
| Sinus of Valsalva diameter (mm) | 32.9±3.6 |
| Tubular tract diameter (mm) | 36.0±3.4 |
| LA antero-posterior diameter (mm) | 45±8 |
| LA area (cm2) | 26±10 |
| EDD (mm) | 55±11 |
| IVS (mm) | 14±2 |
| PWT (mm) | 15±3 |
| LV mass (g) | 348.3±110.8 |
| LV mass index (g/m2) | 198.8±54.5 |
| EDV (mL) | 114±50 |
| ESV (mL) | 54±31 |
| LVEF (%) | 51±15 |
| Peak transaortic gradient (mmHg) | 86±24 |
| Mean transaortic Gradient (mmHg) | 51±17 |
| Aortic regurgitation | |
| - trivial | 21 (37.5%) |
| - mild | 18 (32.1%) |
| - moderate | 14 (25.0%) |
| - severe | 3 (5.4%) |
| Mitral regurgitation | |
| - trivial | 0 (0.0%) |
| - mild | 21 (37.5%) |
| - moderate | 35 (62.5%) |
| - severe | 0 (0.0%) |
| Tricuspidal regurgitation | |
| - trivial | 0 (0.0%) |
| - mild | 28 (50.0%) |
| - moderate | 15 (26.8%) |
| - severe | 13 (23.2%) |
| sPAP (mmHg) | 39.1±15.7 |
| RV E (m/s) | 0.34±0.14 |
| RV A (m/s) | 0.5±0.2 |
| RV E/A | 0.9±0.9 |
| RV DT (ms) | 216±47 |
| Tricuspid anulus diameter (mm) | 34.9±6.6 |
| IVC diameter (mm) | 18.4±4.7 |
| FAC (%) | 35.8±13.4 |
| RVLS (%) | -17.6±4.8 |
| TAPSE (mm) | 18.6±4.8 |
| RVSm (m/s) | 0.1±0.03 |
| TAPSE/sPAP (mm/mmHg) | 0.50±0.23 |
| RVLS/sPAP (%/mmHg) | -0.48±0.23 |
A, late diastolic flow velocity; DT, deceleration time; E, early diastolic flow velocity; EDD, end-diastolic diameter; EDV, end-diastolic volume; ESV, end-systolic volume; FAC, fractional area change; IVC, inferior vena cava; IVS, interventricular septum thickness; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; PWT, posterior wall thickness; RV, right ventricular; RVLS, right ventricular longitudinal strain; RVSm, peak systolic myocardial velocity by TDI; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
Intra-observer and inter-observer variabilities
Intra-observer and inter-observer variabilities for our measures, obtained from 10 patients of study population, are summarized in Table 3 and Supplementary Figure 1. The intra-class correlation coefficients for RVLS was 0.891 (95% CI 0.721-0.960, P < 0.001) for intra-observer variability and 0.827 (95% CI 0.519-0.939, P=0.001) for inter-observer variability. With Bland-Altman plots we found the following bias: for intra-observer variability -0.75% (range from -5.13% to 3.63% for 95% LOA) and for inter-observer variability -0.31% (from -7.13% to 6.50% for 95% LOA).
Table 3.
Intraobserver and interobserver variabilities using Bland-Altman statistics, intraclass correlation coefficient (ICC) and Pearson’s correlation coefficient (R)
| Intra | Measure 1 | Measure 2 | Bland-Altman (95% CI) | ICC (95% CI) | R |
|
| |||||
| RVLS (%) | -17.3±4.6 | -16.6±5.1 | -0.75% (-5.13%-3.63%) | 0.891 (70.721-0.960), P < 0.001 | 0.9, P=0.04 |
|
| |||||
| Inter | Measure 1 | Measure 3 | Bland-Altman (95% CI) | ICC (95% CI) | R |
|
| |||||
| RVLS (%) | -17.3±4.6 | -17.1±4.1 | -0.31% (-7.13%-6.50%) | 0.827 (0.519-0.939), P=0.001 | 0.7, P=0.03 |
Two specialists reviewed the images of 10 randomly selected patients; both specialists performed RVLS measure; the first performed 2 measurements (Measure 1 and 2) for evaluating the intraobserver variability, the second specialist performed only one measure (Measure 3), that was compared with Measure 1 for the interobserver variability.
Correlates of RV dysfunction
We tested clinical and echocardiographic variables in multivariate analysis for evaluating factors associated with RV dysfunction at baseline, expressed as TAPSE < 17 mm and RVLS < median value of our cohort (> -17%) (Table 4). For TAPSE, we found significant relations with: gender (OR 0.235, 95% CI 0.076-0.727, P=0.012), coronary artery disease (OR 2.8, 95% CI 0.617-5.251, P=0.049), previous cardiac surgery (OR: 5.923, 95% CI 1.582-22.172, P=0.008), and sPAP (OR 2.600, 95% CI: 1.2-6.333, P=0.042). A weak correlation was found with LV mass (OR 1.007, 95% CI 1.000-1.014, P=0.050), not maintained after indexation for body surface area. No correlation was observed between TAPSE and sPAP in our cohort, nor between TAPSE and grade of TR or tricuspid annulus diameter. Predictors of reduced RVLS were found to be: hypertension (OR 0.290, 95% CI 0.085-0.985, P=0.047), coronary artery disease (OR 2.800, 95% CI 0.617-5.251, P=0.049), previous cardiac surgery (OR 3.882, 95% CI 1.056-14.276, P=0.041), sPAP (OR 3.622, 95% CI 0.988-12.333, P=0.007).
Table 4.
Independent predictors of reduced TAPSE < 17 mm and RVLS > -17%
| Clinical and demographic characteristics | TAPSE < 17 mm | RVLS > -17% | ||||
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
|
| ||||||
| Age | 1.04 | 0.947-1.134 | 0.44 | 0.94 | 0.85-1.03 | 0.18 |
| Sex (male vs female) | 0.24 | 0.076-0.727 | 0.01 | 1.00 | 0.14-1.22 | 0.11 |
| Body mass index | 1.01 | 0.899-1.137 | 0.85 | 1.00 | 0.89-1.12 | 0.99 |
| NYHA class | 1.81 | 0.595-5.517 | 0.30 | 0.57 | 0.19-1.66 | 0.30 |
| Hypertension | 0.39 | 0.122-1.259 | 0.12 | 0.29 | 0.085-0.985 | 0.05 |
| Diabetes mellitus | 1.50 | 0.467-4.816 | 0.50 | 0.70 | 0.22-2.26 | 0.55 |
| Coronary artery disease (yes vs no) | 2.80 | 0.617-5.251 | 0.05 | 2.80 | 0.62-5.25 | 0.05 |
| Logistic EuroScore | 1.02 | 0.985-1.049 | 0.30 | 1.02 | 0.99-1.05 | 0.21 |
| COPD | 2.22 | 0.60-8.104 | 0.23 | 2.40 | 0.63-9.16 | 0.20 |
| Previous cardiac surgery | 5.92 | 1.58-22.17 | 0.01 | 3.88 | 1.06-14.28 | 0.04 |
| Creatinine clearance | 0.98 | 0.95-1.00 | 0.17 | 1.00 | 0.98-1.03 | 0.72 |
| Hemoglobin | 0.90 | 0.69-1.15 | 0.40 | 1.18 | 0.88-1.57 | 0.26 |
| Systolic arterial pressure | 1.00 | 0.97-1.029 | 0.89 | 1.02 | 0.99-1.05 | 0.24 |
| Diastolic arterial pressure | 0.98 | 0.93-1.031 | 0.45 | 1.06 | 1.01-1.12 | 0.02 |
|
| ||||||
| Echocardiographic measurements | OR | 95% CI | P | OR | 95% CI | P |
|
| ||||||
| Aortic annulus diameter | 1.32 | 1-1.75 | 0.05 | 0.96 | 0.74-1.25 | 0.77 |
| Sinus of Valsalva diameter | 1.02 | 0.872-1.18 | 0.84 | 0.94 | 0.81-1.10 | 0.44 |
| Tubular tract diameter | 1.14 | 0.964-1.352 | 0.13 | 0.99 | 0.85-1.16 | 0.91 |
| LA antero-posterior diameter | 1.06 | 0.96-1.16 | 0.24 | 1.04 | 0.95-1.14 | 0.40 |
| LA area | 0.95 | 0.839-1.073 | 0.40 | 1.19 | 0.95-1.50 | 0.13 |
| EDD | 1.03 | 0.96-1.091 | 0.36 | 1.02 | 0.96-1.08 | 0.45 |
| IVS | 1.01 | 0.76-1.337 | 0.96 | 1.01 | 0.77-1.33 | 0.95 |
| PWT | 1.02 | 1.053-1.914 | 0.22 | 1.14 | 0.90-1.44 | 0.27 |
| LV mass | 1.01 | 0.99-1.014 | 0.05 | 1.00 | 0.99-1.01 | 0.29 |
| EDV | 1.01 | 0.99-1.02 | 0.29 | 1.01 | 0.99-1.02 | 0.44 |
| ESV | 1.03 | 0.998-1.062 | 0.06 | 1.02 | 0.99-1.04 | 0.16 |
| LVEF | 0.99 | 0.957-1.029 | 0.67 | 0.99 | 0.95-1.03 | 0.58 |
| Peak trans-aortic gradient | 1.00 | 0.972-1.019 | 0.69 | 1.01 | 0.98-1.03 | 0.41 |
| Medium trans-aortic gradient | 1.00 | 0.965-1.035 | 0.98 | 1.02 | 0.99-1.06 | 0.19 |
| Aortic regurgitation | 1.19 | 0.724-1.941 | 0.50 | 0.97 | 0.59-1.58 | 0.90 |
| Mitral Regurgitation | 0.67 | 0.322-1.390 | 0.28 | 1.21 | 0.59-2.46 | 0.59 |
| sPAP | 2.60 | 1.200-6.333 | 0.04 | 3.62 | 0.988-12.333 | 0.01 |
| Tricuspid regurgitation | 1.63 | 0.84-3.180 | 0.15 | 1.31 | 0.68-2.52 | 0.41 |
| Tricuspid annulus diameter | 1.08 | 0.99-1.182 | 0.08 | 0.93 | 0.85-1.01 | 0.08 |
EDD, end-diastolic diameter; EDV, end-diastolic volume; ESV, end-systolic volume; IVS, interventricular septum thickness; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; PWT, posterior wall thickness; sPAP, systolic pulmonary artery pressure.
Long-term outcomes
The mean follow-up length in the overall population was 8.5±0.5 years. During follow-up, 46 patients died (82% of our cohort). At univariate analysis predictors of survival were (Table 5): low grade of TR (HR 0.585, 95% CI 0.392-0.875, P=0.009), tricuspid annulus diameter (HR 0.929, 95% CI 0.882-0.978, P=0.005); TAPSE (HR 0.943, 95% CI 0.890-0.999, P=0.047), TAPSE/sPAP (HR 0.195, 95% CI 0.050-0.765, P=0.019), RVLS (HR 1.140, 95% CI 1.072-1.213, P < 0.001), RVLS/sPAP (HR 11.432, 95% CI 2.837-46.070, P=0.001). The Kaplan-Meier curve of 10-year survival according to RVLS value in our cohort is presented in Figure 1 (chi-square 7.8, log-rank; P=0.05). We performed a Cox regression multivariable analysis, adjusted for the parameters related to prognosis at univariate analysis, and we found that only RVLS was independently associate with all-cause mortality (adjusted HR 1.53, 95% CI 1.10-2.12, P=0.011).
Table 5.
Cox proportional hazard model for all-cause mortality; variable adjusted included each one of variables related to the endpoint at univariate analysis
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Tricuspid regurgitation | 0.585 | 0.392-0.875 | 0.009 | 0.471 | 0.17-1.30 | 0.147 |
| Tricuspidal annulus diameter | 0.929 | 0.882-0.978 | 0.005 | 1.023 | 0.87-1.20 | 0.781 |
| FAC | 0.986 | 0.964-1.008 | 0.209 | 0.6 | 0.89-1.2 | 0.89 |
| RVLS | 1.140 | 1.072-1.213 | < 0.001 | 1.53 | 1.10-2.12 | 0.011 |
| TAPSE | 0.943 | 0.890-0.999 | 0.047 | 1.154 | 0.92-1.43 | 0.201 |
| sPAP | 1.017 | 0.998-1.037 | 0.086 | - | - | - |
| TAPSE/sPAP | 0.195 | 0.050-0.765 | 0.019 | 0.019 | 0.00023-1.64 | 0.082 |
| RVsm | 0.001 | 0.000-10.817 | 0.137 | 0.6 | 0.13-1.06 | 0.12 |
| RVLS/sPAP | 11.432 | 2.837-46.070 | 0.001 | 0.5 | 0.6-0.99 | 0.14 |
FAC, fractional area change; RVLS, right ventricular longitudinal strain; RVSm, peak systolic myocardial velocity by TDI; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
Figure 1.
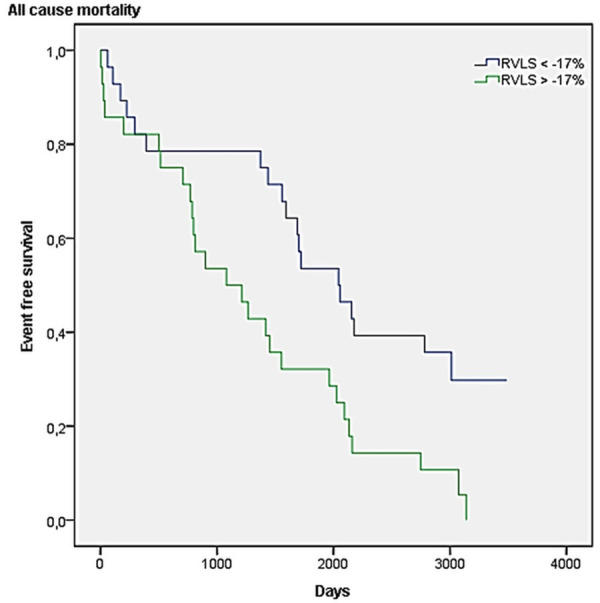
Kaplan-Meier 10-year survival curves according to RVLS value in our cohort (chi-square 7.8, log-rank P=0.05).
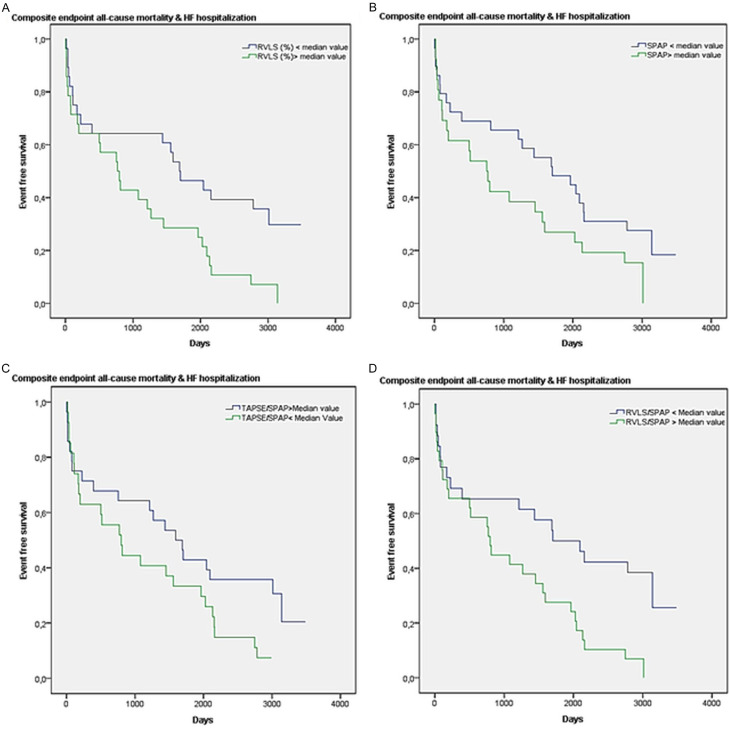
The combined endpoint was reached by 82% of patients. At univariate analysis the predictors were (Table 6): grade of TR (HR 0.634, 95% CI 0.427-0.942, P=0.024), tricuspid annulus diameter (HR 0.929, 95% CI 0.882-0.978, P=0.037); TAPSE/sPAP (HR 0.193, 95% CI 0.048-0.775, P=0.020), RVLS (HR 1.23, 95% CI 1.058-1.192, P < 0.001), sPAP (HR 1.020, 95% CI 1.001-1.040, P=0.042), RVLS/sPAP (HR 12.3, 95% CI 2.9-52.031, P=0.001). Even for this endpoint, we performed a multivariable Cox regression analysis, adjusted for the parameters related to prognosis at univariate analysis, and we found correlations with RVLS (adjusted HR 7.542, 95% CI 1.325-42.921, P=0.023), sPAP (adjusted HR 1.421, 95% CI 1.045-1.932, P=0.025), TAPSE/sPAP (adjusted HR 4.977, 95% CI 5.425-21.99, P=0.044), RVLS/sPAP (adjusted HR 2.333, 95% CI 3.9677-12.999, P=0.046). Figure 2 shows the Kaplan Meier curves of survival free from the composite endpoint according to these four parameters.
Table 6.
Cox proportional hazard model for all-cause mortality + heart failure hospitalization; variable adjusted included each one of variables related to the endpoint at univariate analysis
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Tricuspid regurgitation | 0.634 | 0.427-0.942 | 0.024 | 5.412 | 0.54-54.24 | 0.151 |
| Tricuspidal annulus diameter | 0.929 | 0.882-0.978 | 0.037 | 1.514 | 0.985-2.326 | 0.059 |
| FAC | 0.986 | 1.058-1.192 | 0.340 | - | - | - |
| RVLS | 1.23 | 1.058-1.192 | < 0.001 | 7.542 | 1.325-42.921 | 0.023 |
| TAPSE | 0.953 | 0.899-1.009 | 0.100 | - | - | - |
| sPAP | 1.020 | 1.001-1.040 | 0.042 | 1.421 | 1.045-1.932 | 0.025 |
| TAPSE/sPAP | 0.193 | 0.048-0.775 | 0.020 | 4.977 | 5.425-21.99 | 0.044 |
| RVsm | 0.001 | 0.000-10.817 | 0.400 | - | - | - |
| RVLS/sPAP | 12.3 | 2.9-52.031 | 0.001 | 2.333 | 3.9677-12.999 | 0.046 |
FAC, fractional area change; RVLS, right ventricular longitudinal strain; RVSm, peak systolic myocardial velocity by TDI; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
Figure 2.
Kaplan-Meier 10-year curves of survival free of composite endpoint according to: (A) RVLS; (B) sPAP; (C) TAPSE/sPAP; (D) RVLS/sPAP.
Discussion
The main finding of this study is that pre-operative careful and comprehensive evaluation of RV function (including deformation) and RV-arterial coupling can predict long-term outcomes of patients with HF and AS who are candidate for TAVI. Death for any cause was independently predicted by RVLS, while the composite endpoint of death and HF hospitalization was independently associated to each one of RVLS, sPAP, TAPSE/sPAP and RVLS/sPAP. Our patients were homogeneous regarding their demographic characteristics, aortic valve grade of degeneration, LVEF, type of intervention (all patients had femoral access), so these parameters were not related to long-term incidence of events. Indeed, all patients in our cohort had LV hypertrophy and a mean LVEF of 51±15%, so no one of patients enrolled had severe systolic dysfunction (LVEF < 35%). Recently, Sultan et al. found that TAPSE/sPAP quartiles have a linear relationship with 2-year all-cause mortality after TAVI in a larger population with similar characteristics to ours [28]. Our study is the first with a so long follow-up (8.5±0.5 years), and the results are even more important because of the recent moving to treatment with TAVI from higher to lower-risk groups, which have a longer life-expectancy [3,4]. These results highlight the importance of RV function assessment by different methods for better stratification of prognosis of patients admitted for HF due to severe AS and candidate for TAVI.
As well known, TAPSE and RVSm reflect the function of the basal segment of the RV free wall, which is assumed to represent RV global function; for the same reason, given the base-apex motion gradient of right fibers, they are mostly load-dependent. This could be an important limitation for these parameters in predicting long-term prognosis when regional dysfunction is supposed and, moreover, when a reduction in RV afterload is forecast, such as after TAVI [29]. In other previous studies we demonstrated the superiority of RVSm compared with TAPSE and FAC in predicting prognosis of patients with chronic HF [30,31]. Indeed, in 31 patients with HF with no or mild RV dysfunction we correlated all echocardiographic parameters with the CMR-calculated RVEF, stroke volume, end-diastolic volume, and end-systolic volume and we found that, given the importance of longitudinal deformation of RV fibers in whole RV function, there was a strongest correlation between RVLS and not only RVEF, but also RV volumes [32]. In addition, RVLS is an independent predictor of first HF hospitalization and death for any cause in patients with asymptomatic left-sided structural heart disease at 5-year follow-up [33]. PH itself is an accepted predictor of poor outcome both after cardiac surgery and TAVI [34,35]. Gerges et al. analyzed RV function in patients with HF and PH, and demonstrated that TAPSE/sPAP, which is validated against invasive hemodynamics [19], was a predictor of combined pre-capillary and post-capillary PH on univariate and multivariate analyses [36,37]. O’Sullivan et al. enrolled 606 consecutive patients undergoing TAVI and pre-procedural right heart catheterization and demonstrated that, when compared with no PH, a higher 1-year mortality rate was observed in both pre-capillary and combined pre-capillary and post-capillary, but not isolated post-capillary PH patients [38]. Indeed, in the setting of HF, a growing evidence suggests the role of RV-arterial coupling in predicting early and long-term outcomes of patients, regardless of TAPSE, sPAP and RVEF [39,40]. In patients with HF and both reduced and preserved LVEF, TAPSE/sPAP and RVLS/sPAP have shown to strongly predict long-term mortality and re-hospitalization [20-22]; more importantly, patients with preserved LVEF have less frequently RV dysfunction, but similar TAPSE/sPAP when compared with patients with reduced LVEF. Our findings are in line with recent evidences about the role of RV function and RV-arterial coupling in patients with HF and preserved LVEF [21]. Myocardial deformation imaging is more sensitive and specific than other methods in detecting RV global dysfunction, taking into account not only the basal-lateral segment; but, more important, this method is load independent, so it could maintain its value even after TAVI. Our findings highlight the importance of serial evaluation of RV function corrected for afterload, for early detection of pulmonary vascular remodeling leading to irreversible PH that could affect long-term prognosis of patients with AS. RVLS and RV-arterial coupling assessed as TAPSE/sPAP and RVLS/sPAP could detect the grade of global (rather than segmental) involvement of right chambers in chronic adaptation to AS and the grade of non-reversible cardio-pulmonary remodeling despite correction of severe AS by TAVI. These parameters, irrespective to other echocardiographic and clinical features, could reveal an important hemodynamic involvement so they should be periodically assessed during the echocardiographic follow-up of patients with severe AS without symptoms or evident PH. These results on composite endpoint including hospitalization for HF reaffirm, as expected, the role of both loading condition and RV contractility parameters on symptoms of congestion even after TAVI.
In the setting of cardiac surgery, the prognostic role of the preoperative RV dysfunction in predicting both early ad long-term outcomes have been widely underlined by several authors [41,42]. The more, when compared with aortic replacement, TAVI results in better preservation of RV volumes and function [43], and also RV deformation [44]. In addition, RVLS was demonstrated to be more impaired after surgical replacement than TAVI in a small population of patients [45]. These evidences support the importance of carefully evaluating RV function before strategy decision in patients with severe AS. Patients with pre-existing RV dysfunction could have worse outcomes after aortic valve replacement because of the well-known impact of surgery on this chamber. Comparing traditional RV systolic parameters (TAPSE, FAC) with RVLS, Ternacle et al. found that 34% of patients with normal FAC but abnormal RVLS were at higher risk for post-operative mortality after surgical valve replacement, demonstrating that conventional parameters are less effective compared with STE [46]. Indeed, in another study of patients candidate for TAVI, RV function assessed using TAPSE and RVSm did not correlate with prognosis at a 2-year follow-up [47]. Conversely, CMR-derived RVEF has been demonstrated to have a significant prognostic value in severe AS, with both normal and low gradients [48]. In series of patients with low flow-low gradient AS and reduced LVEF, at multivariable Cox analysis stratified for the type of treatment (aortic valve replacement vs conservative) and adjusted for age, AS severity, previous myocardial infarction and LV longitudinal strain, RVLS > -13% was independently associated with all-cause mortality [49]. All these evidences support the idea that using more sensitive tools, a full assessment of RV function could be central in long-term risk stratification of patients candidate for TAVI (especially when trans-femoral approach is the final choice), given the less impact of the procedure on RV volumes and function themselves.
The present study has some limitations. It is a retrospective study of a small size cohort. Second, complete data about cardiovascular drugs before the procedure of TAVI are not available, so we cannot assess if therapies could affect echocardiographic measurements. Third, biomarkers of myocardial function or neurohormonal activation (such as natriuretic peptide) were not available, too.
In conclusion, in patients with HF due to severe AS candidate for TAVI the evaluation of RV function and RV-arterial coupling could add important prognostic information, similarly to what happens in all patients with HF without AS. This concept is important because of even more patients with not high surgical risk undergo TAVI, and have a long life expectancy. Our study is the first one with a so long follow-up. Deformation imaging allows a sensitive evaluation of RV function and RV-arterial coupling, and earlier identification of pulmonary remodeling leading to irreversible PH. The identification of RV involvement in patients with AS, irrespective to other echocardiographic and clinical features, could reveal most important hemodynamic alterations. Future larger studies are necessary to develop risk models for TAVI outcomes including RV function parameters.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Sinning JM, Hammerstingl C, Vasa-Nicotera M, Adenauer V, Lema Cachiguango SJ, Scheer AC, Hausen S, Sedaghat A, Ghanem A, Müller C, Grube E, Nickenig G, Werner N. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:1134–1141. doi: 10.1016/j.jacc.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 9.Giannini C, De Carlo M, Tamburino C, Ettori F, Latib AM, Bedogni F, Bruschi G, Presbitero P, Poli A, Fabbiocchi F, Violini R, Trani C, Giudice P, Barbanti M, Adamo M, Colombo P, Benincasa S, Agnifili M, Petronio AS. Transcathether aortic valve implantation with the new repositionable self-expandable evolut R versus corevalve system: a case-matched comparison. Int J Cardiol. 2017;243:126–131. doi: 10.1016/j.ijcard.2017.05.095. [DOI] [PubMed] [Google Scholar]
- 10.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a national heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S, Tuzcu EM, Krishnaswamy A, Schoenhagen P, Stewart WJ, Svensson LG, Kapadia SR. Transcatheter aortic valve replacement: current perspectives and future implications. Heart. 2015;101:169–177. doi: 10.1136/heartjnl-2014-306254. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Delgado V, Bapat V, Baumgartner H, Collet JP, Erbel R, Hamm C, Kappetein AP, Leipsic J, Leon MB, MacCarthy P, Piazza N, Pibarot P, Roberts WC, Rodés-Cabau J, Serruys PW, Thomas M, Vahanian A, Webb J, Zamorano JL, Windecker S. Open issues in transcatheter aortic valve implantation. Part 1: patient selection and treatment strategy for transcatheter aortic valve implantation. Eur Heart J. 2014;35:2627–2638. doi: 10.1093/eurheartj/ehu256. [DOI] [PubMed] [Google Scholar]
- 13.Vivas D, Perez de Isla L, Zamorano J. Using echocardiography to guide interventional procedures. Curr Cardiovasc Imaging Rep. 2008;1:9–15. [Google Scholar]
- 14.Simsek E, Nalbantgil S, Ceylan N, Zoghi M, Kemal HS, Engin C, Yagdi T, Ozbaran M. Assessment of right ventricular systolic function in heart transplant patients: correlation between echocardiography and cardiac magnetic resonance imaging. Investigation of the accuracy and reliability of echocardiography. Echocardiography. 2017;34:1432–1438. doi: 10.1111/echo.13650. [DOI] [PubMed] [Google Scholar]
- 15.Dore Reyes M, De La Torre C, Bret Zurita M, Triana Junco P, Jiménez Gómez J, Romo Muñoz M, Vilanova Sánchez A, Parrón Pajares M, Pérez Vigara A, Encinas Hernández JL, Martínez Martínez L, Hernández Oliveros F, López-Santamaría M. Beneficios de la resonancia magnética para el estudio del pectus excavatum en niños: experiencia inicial [benefits of magnetic resonance for the study of pectus excavatum in children: initial experience] . Cir Pediatr. 2017;30:71–76. [PubMed] [Google Scholar]
- 16.Hamilton-Craig CR, Stedman K, Maxwell R, Anderson B, Stanton T, Chan J, Yamada A, Scalia GM, Burstow DJ. Accuracy of quantitative echocardiographic measures of right ventricular function as compared to cardiovascular magnetic resonance. Int J Cardiol Heart Vasc. 2016;12:38–44. doi: 10.1016/j.ijcha.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of echocardiography endorsed by the european association of echocardiography, a registered branch of the European society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-788. [DOI] [PubMed] [Google Scholar]
- 18.Visentin S, Palermo C, Camerin M, Daliento L, Muraru D, Cosmi E, Badano LP. Echocardiographic techniques of deformation imaging in the evaluation of maternal cardiovascular system in patients with complicated pregnancies. Biomed Res Int. 2017;2017:4139635. doi: 10.1155/2017/4139635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL All investigators. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–879. doi: 10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 21.Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi: 10.1002/ejhf.873. [DOI] [PubMed] [Google Scholar]
- 22.Santas E, Palau P, Guazzi M, de la Espriella R, Miñana G, Sanchis J, Bayes-Genís A, Lupón J, Chorro FJ, Núñez J. Usefulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am J Cardiol. 2019;124:567–572. doi: 10.1016/j.amjcard.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the european association of cardiovascular imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Sultan I, Cardounel A, Abdelkarim I, Kilic A, Althouse AD, Sharbaugh MS, Gupta A, Xu J, Fukui M, Simon MA, Schindler JT, Lee JS, Gleason TG, Cavalcante JL. Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart. 2019;105:117–121. doi: 10.1136/heartjnl-2018-313385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longobardo L, Suma V, Jain R, Carerj S, Zito C, Zwicke DL, Khandheria BK. Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr. 2017;30:937–946. e6. doi: 10.1016/j.echo.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Vizzardi E, D’Aloia A, Caretta G, Bordonali T, Bonadei I, Rovetta R, Quinzani F, Bugatti S, Curnis A, Metra M. Long-term prognostic value of longitudinal strain of right ventricle in patients with moderate heart failure. Hellenic J Cardiol. 2014;55:150–155. [PubMed] [Google Scholar]
- 31.Vizzardi E, D’Aloia A, Bordonali T, Bugatti S, Piovanelli B, Bonadei I, Quinzani F, Rovetta R, Vaccari A, Curnis A, Dei Cas L. Long-term prognostic value of the right ventricular myocardial performance index compared to other indexes of right ventricular function in patients with moderate chronic heart failure. Echocardiography. 2012;29:773–778. doi: 10.1111/j.1540-8175.2012.01703.x. [DOI] [PubMed] [Google Scholar]
- 32.Vizzardi E, Bonadei I, Sciatti E, Pezzali N, Farina D, D’Aloia A, Metra M. Quantitative analysis of right ventricular (RV) function with echocardiography in chronic heart failure with no or mild RV dysfunction: comparison with cardiac magnetic resonance imaging. J Ultrasound Med. 2015;34:247–255. doi: 10.7863/ultra.34.2.247. [DOI] [PubMed] [Google Scholar]
- 33.Gavazzoni M, Badano LP, Vizzardi E, Raddino R, Genovese D, Taramasso M, Sciatti E, Palermo C, Metra M, Muraru D. Prognostic value of right ventricular free wall longitudinal strain in a large cohort of outpatients with left-side heart disease. Eur Heart J Cardiovasc Imaging. 2019;21:1013–1021. doi: 10.1093/ehjci/jez246. [DOI] [PubMed] [Google Scholar]
- 34.Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, Rezvanieh S, Veglia F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol. 2007;115:86–89. doi: 10.1016/j.ijcard.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Kukulski T, Hübbert L, Arnold M, Wranne B, Hatle L, Sutherland GR. Normal regional right ventricular function and its change with age: a Doppler myocardial imaging study. J Am Soc Echocardiogr. 2000;13:194–204. doi: 10.1067/mje.2000.103106. [DOI] [PubMed] [Google Scholar]
- 36.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 37.Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–337. doi: 10.1016/j.healun.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan CJ, Wenaweser P, Ceylan O, Rat-Wirtzler J, Stortecky S, Heg D, Spitzer E, Zanchin T, Praz F, Tüller D, Huber C, Pilgrim T, Nietlispach F, Khattab AA, Carrel T, Meier B, Windecker S, Buellesfeld L. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: insights from the new proposed pulmonary hypertension classification. Circ Cardiovasc Interv. 2015;8:e002358. doi: 10.1161/CIRCINTERVENTIONS.114.002358. [DOI] [PubMed] [Google Scholar]
- 39.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 40.Ghio S, Temporelli PL, Klersy C, Simioniuc A, Girardi B, Scelsi L, Rossi A, Cicoira M, Tarro Genta F, Dini FL. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail. 2013;15:408–414. doi: 10.1093/eurjhf/hfs208. [DOI] [PubMed] [Google Scholar]
- 41.Maslow AD, Regan MM, Panzica P, Heindel S, Mashikian J, Comunale ME. Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg. 2002;95:1507–1518. doi: 10.1097/00000539-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, Lambert J, Tardif JC. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr. 2007;20:1065–1072. doi: 10.1016/j.echo.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Musa TA, Uddin A, Fairbairn TA, Dobson LE, Steadman CD, Kidambi A, Ripley DP, Swoboda PP, McDiarmid AK, Erhayiem B, Garg P, Blackman DJ, Plein S, McCann GP, Greenwood JP. Right ventricular function following surgical aortic valve replacement and transcatheter aortic valve implantation: a cardiovascular MR study. Int J Cardiol. 2016;223:639–644. doi: 10.1016/j.ijcard.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 44.Keyl C, Schneider J, Beyersdorf F, Ruile P, Siepe M, Pioch K, Schneider R, Jander N. Right ventricular function after aortic valve replacement: a pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur J Cardiothorac Surg. 2016;49:966–971. doi: 10.1093/ejcts/ezv227. [DOI] [PubMed] [Google Scholar]
- 45.Kempny A, Diller GP, Kaleschke G, Orwat S, Funke A, Schmidt R, Kerckhoff G, Ghezelbash F, Rukosujew A, Reinecke H, Scheld HH, Baumgartner H. Impact of transcatheter aortic valve implantation or surgical aortic valve replacement on right ventricular function. Heart. 2012;98:1299–1304. doi: 10.1136/heartjnl-2011-301203. [DOI] [PubMed] [Google Scholar]
- 46.Ternacle J, Berry M, Cognet T, Kloeckner M, Damy T, Monin JL, Couetil JP, Dubois-Rande JL, Gueret P, Lim P. Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J Am Soc Echocardiogr. 2013;26:721–726. doi: 10.1016/j.echo.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Poliacikova P, Cockburn J, Pareek N, James R, Lee L, Trivedi U, de Belder A, Hildick-Smith D. Prognostic impact of pre-existing right ventricular dysfunction on the outcome of transcatheter aortic valve implantation. J Invasive Cardiol. 2013;25:142–145. [PubMed] [Google Scholar]
- 48.Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, Flecher E, Mabo P, Donal E. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2015;16:531–538. doi: 10.1093/ehjci/jeu290. [DOI] [PubMed] [Google Scholar]
- 49.Cavalcante JL, Rijal S, Althouse AD, Delgado-Montero A, Katz WE, Schindler JT, Crock F, Harinstein ME, Navid F, Gleason TG, Lee JS. Right ventricular function and prognosis in patients with low-flow, low-gradient severe aortic stenosis. J Am Soc Echocardiogr. 2016;29:325–333. doi: 10.1016/j.echo.2015.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.