Abstract
Despite the decline in death rate from breast cancer and recent advances in targeted therapies and combinations for the treatment of metastatic disease, metastatic breast cancer remains the second leading cause of cancer-associated death in U.S. women. The invasion-metastasis cascade involves a number of steps and multitudes of proteins and signaling molecules. The pathways include invasion, intravasation, circulation, extravasation, infiltration into a distant site to form a metastatic niche, and micrometastasis formation in a new environment. Each of these processes is regulated by changes in gene expression. Noncoding RNAs including microRNAs (miRNAs) are involved in breast cancer tumorigenesis, progression, and metastasis by post-transcriptional regulation of target gene expression. miRNAs can stimulate oncogenesis (oncomiRs), inhibit tumor growth (tumor suppressors or miRsupps), and regulate gene targets in metastasis (metastamiRs). The goal of this review is to summarize some of the key miRNAs that regulate genes and pathways involved in metastatic breast cancer with an emphasis on estrogen receptor α (ERα+) breast cancer. We reviewed the identity, regulation, human breast tumor expression, and reported prognostic significance of miRNAs that have been documented to directly target key genes in pathways, including epithelial-to-mesenchymal transition (EMT) contributing to the metastatic cascade. We critically evaluated the evidence for metastamiRs and their targets and miRNA regulation of metastasis suppressor genes in breast cancer progression and metastasis. It is clear that our understanding of miRNA regulation of targets in metastasis is incomplete.
Keywords: miRNAs, Breast cancer metastasis, EMT, TGFβ
1. Introduction
Depending on the breast cancer subtype, primary breast tumors are usually successfully treated by surgery, radiation, and targeted therapies. Where local disease has spread to adjacent lymph nodes or the tumor gene signature derived from multigene assays, e.g., MammaPrint(R) and Oncotype DX(R), show concordance with a high risk of recurrence, specific regimes of chemotherapy are applied [1]. Breast tumors are categorized into three major subtypes based on the expression of specific protein markers: estrogen receptor α (ERα), progesterone receptor (PR), ERBB2 (also referred to as HER2) versus triple negative breast cancer (TNBC) which lacks ERα, PR, and ERBB2. The five year survival rate is 94–99% for ERα/PR+ and ERBB2+ breast cancers whereas it is 85% for TNBC [2]. Patients with ERα+ tumors are successfully treated with endocrine therapies that block estrogen synthesis from adrenal androgens using aromatase inhibitors (AI), e.g., letrozole, in postmenopausal patients [3]. For premenopausal patients with ERα+ breast cancer, selective ER modulators (SERMs), e.g., tamoxifen (TAM), that compete with estrogens for ERα, are used in combination with ovarian function suppression therapy, i.e., GNRH/LHRH agonists, e.g., goserelin [4–6]. Patients whose breast tumors are ERBB2+ receive monoclonal antibody therapy: trastuzumab that targets the extracellular domain of ERBB2 or pertuzumab that targets the ERBB2 dimerization domain, or neratinib that is an oral tyrosine kinase inhibitor of ERB2 family members [2]. TNBC patients, proportionally ~ 15% of all breast cancers, receive chemotherapy, but have a high risk of relapse with distant metastasis in the first 3–5 years after diagnosis [2].
Unfortunately, ~ 30% of breast cancer patients succumb to metastatic disease, sometimes decades after initial treatment, highlighting the need to understand and develop anti-metastatic therapies [7]. Mechanisms involved in acquired endocrine-resistant breast cancer have been reviewed and include amplification of growth factor signaling pathways, altered expression of coactivators and corepressors, altered intracellular location and splice variants of ERα, increased G-protein coupled ER (GPER1, also called GPER and GPR30), and dysregulation of non-coding RNAs [8–11]. For patients with ERα+ tumors who receive AI therapy, ~ 25–40% of metastatic tumors from these patients have mutations in ESR1 (ERα) within the ligand binding domain (LBD) [12]. These LBD ‘hot spot’ mutations activate the mutant ERα in the absence of estrogen binding and reduce the efficacy of selective ER downregulators (SERDs) including fulvestrant, AZD9496, RU-58688, and GDC-0810 to block activation and degrade ERα [13]. ESR1 Y537S and D538G mutations are associated with shorter survival [14]. Recently, inactivating mutations in ARID1A, a subunit of the SWI/SNF chromatin remodeling complex, were identified in metastatic tumors from patients who had received endocrine therapies and these mutations resulted in reduced progression-free survival on SERDs [15]. Knockout of ARID1A in MCF-7 human luminal A (ERα +) breast cancer cells altered chromatin accessibility for transcription factor (TF) binding including reduced ER, FOXA1, and GATA2 sites and increased TEAD4 sites while altering the transcriptome with increased expression of basal-like/stemness genes [15]. FOXA1 is higher in ER+/PR+ vs. ER−/PR− or HER2+ breast tumors and high FOXA1 confers a benefit in OS in breast cancer patients [16].
Current clinically beneficial therapy for patients with metastatic ERα+ breast cancer includes addition of the CDK4/6 inhibitors palbociclib, ribociclib, or abemaciclib in combination with letrozole [17–20]. The combination of PI3K inhibitor alpelisib and fulvestrant is approved for postmenopausal women (and men) with ERα+/HER2− advanced breast cancer [21], including the ~ 40% of patients whose tumors have PIK3CA mutations [22,23]. The mTORC1 (mTOR) inhibitor everolimus is approved in combination with the AI exemestane for ERα+/HER2− postmenopausal women after treatment failure on AIs: letrozole or anastrozole [24]. Clinical trials support the efficacy of everolimus plus fulvestrant in prolonging progression-free survival (PFS) in postmenopausal women with ERα+ primary breast tumors after recurrence on AIs [25]
A variety of non-coding RNAs (ncRNAs) have reported roles in breast cancer progression and metastasis including circular RNAs (circRNAs), PIWI-interacting RNAs (piRNA), microRNAs (miRNAs), and long noncoding RNAs (lncRNAs) [26,27]. Although together these ncRNAs constitute only ~ 1% of total cellular ncRNA, they regulate transcription, post-transcriptional stability, splicing, covalent modification, and mRNA translation [28]. In addition, ncRNA may be packaged into extracellular vesicles (EV) including exosomes [29], thus providing intercellular communication by transfer of miRNA and lncRNA, as well as other molecules, to recipient adjacent and distant cells [30]. Here we will focus on the role of selected miRNAs in regulating gene targets in pathways that contribute to breast cancer metastasis. We have not included the role of metabolic alterations in metastasis as reviewed in [31,32,33{Blücher, 2017 #31679,34].
2. Overview of miRNA in breast cancer
The current miRBase (release 22.1) reports 2,654 mature miRNAs http://www.mirbase.org/ [35]. However, there is debate about how many of these are false positives and a recent study validated ~ 2,300 human miRNAs [36]. miRNAs are short (~ 22 nt), evolutionarily-conserved, single stranded RNAs that base-pair with ~ 7 bp miRNA recognition elements (MREs) in the 3’UTR of their target mRNAs within the RNA induced silencing complex (RISC) [37]. One estimate is that > 60% of human protein-coding genes have been under selective pressure to maintain miRNA binding sites for regulation [38]. One miRNA can directly or indirectly target hundreds of mRNA thus forming a complex miRNA-mRNA network to regulate cellular processes, including breast cancer metastasis.
The biogenesis of miRNAs has been reviewed [27,39]. In brief, most miRNAs are transcribed as primary (pri)-miRNAs by RNA polymerase II. Pri-miRNAs may be co-transcribed within introns of host genes or as independent genes from intergenic regions [40]. Fig. 1 summarizes selected aspects of miRNA biogenesis and their regulation in breast cancer and metastases. Pri-miRNAs are processed to precursor (pre-miRNAs) within the nucleus by the DROSHA-DGCR8 microprocessor complex, which includes additional proteins [41,42]. DROSHA is a class 2 ribonuclease III that cleaves the hairpin-loop pri-miRNA to generate the 60–70 nt pre-miRNA that is exported from the nucleus by the Exportin (XPO5) and Ran-GTP (RAN) or Exportin1 (XPO1) [43]. SNPs in DROSHA are associated with elevated risk of breast cancer [44]. DROSHA has oncogenic or tumor suppressor activity, depending on tumor type (reviewed in [45]). Heterogeneous Nuclear Ribonucleoprotein A2/B1 (HNRNPA2/B1) is a reader of the N(6)-methyladenosine (m6A) post-transcriptional modification of pri-miRNAs and promotes DROSHA processing to pre-miRNAs [46,47]. The DICER (DICER1), a Ribonuclease III,-TRBP cytoplasmic complex unwinds the double stranded pre-miRNA, allowing the one miRNA strand (called the ‘guide strand’) to be incorporated into the RNA-induced silencing (RISC) complex, composed of Argonaute 2 (AGO2), DICER, TRBP (TRBP2), PACT (PRKRA), and TNRC6 (TNRC6A) [48]. The other miRNA strand can also be incorporated into RISC or be degraded [49]. Selection of the miRNA strand is mediated by the relative thermodynamic stabilities within RISC [50]. There are data supporting the localization of the DROSHA microprocessor, DICER- TRBP, and RISC complexes with the adherens junction complex protein PLEKHA7 at the apical zonula adherens (ZA) of epithelial cells to regulate the expression of miRNAs, e.g., miR-30, let-7, and miR-24 families, that target SNAI1, MYC, and SOX2 to support epithelial homeostasis [51]. Whether this is true in breast ductal epithelial cells and the role for dysregulation of these interactions in breast cancer is unknown.
Fig. 1: A brief summary of miRNA biogenesis and aspects of regulation in breast cancer and metastasis.
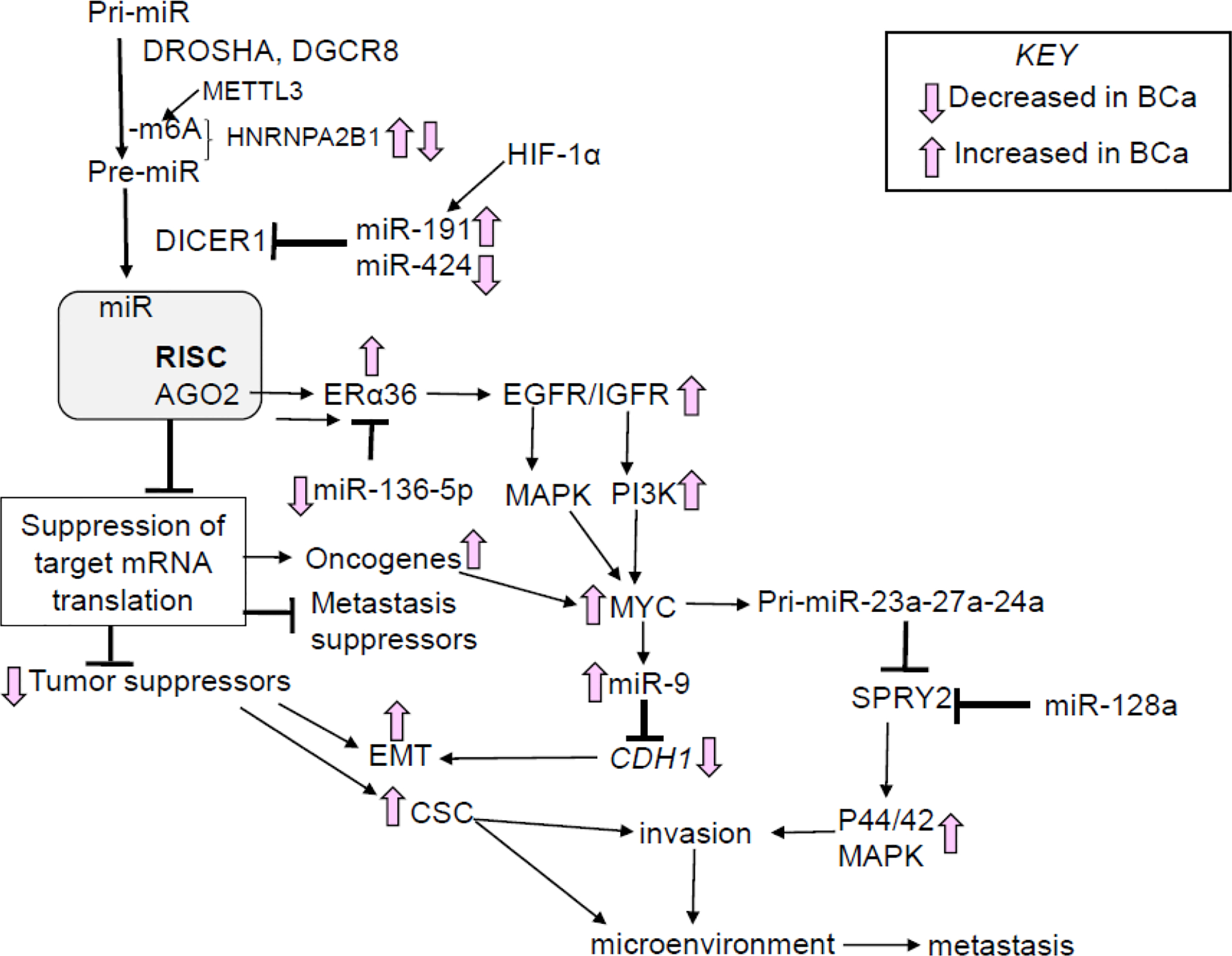
The pathway and proteins involved are described in the test. The upregulation of ERα36 in breast cancer is reviewed in [72]; the regulation of SPRY2 is reviewed in [336]; and MYC upregulation of miR-9 in [752].
Among the post-transcriptional modifications of nascent transcripts, N(6)-methyladenosine (m6A) is the most common modification and for mRNAs m6A plays a role in alternative splicing, transcript stability, and translation [52]. The methyl group is added to adenosine (forming m6A) by the RNA methyltransferase complex (WTAP, METTL3, METTL14, VIRMA, and RBM15, plus additional proteins), removed by the dioxygenases ALKBH5 and FTO, with the m6A ‘mark’ recognized by ‘readers’, including YTHDC1, HNRNPC,YTHDF1, YTHDF2, and HNRNPA2/B1 [53]. METTL3 methylation of m6A on pri-miRNAs [47] and RNA-dependent interaction of HNRNPA2/B1 with DGCR8, a component of the DROSHA complex, stimulate processing of selected pri-miRNA to pre-miRNAs [46]. METTL3 transcript expression was reported to be downregulated in breast tumors [54]. HNRNPA2B1 transcript and protein expression was higher in breast cancer cells and tumors compared with non-transformed cell lines and normal breast tissue [55,56]. Using data in Kaplan-Meier plotter (kmplot) for breast tumors [57], we reported that higher HNRNPA2B1 is significantly associated with lower OS in breast cancer patients [58].. HNRNPA2B1 also interacts with the lncRNA HOTAIR, a nuclear scaffold for transcriptional repression by interacting with the PRC2/EZHW and LSD1 complexes [59], in MCF-7 cells [60]. We reported higher expression of HNRNPA2B1 in TAM-resistant LCC9 and LY2 cells compared to parental MCF-7 cells and identified the miRome regulated by overexpression of HNRNPA2B1 in MCF-7 cells [58]. A recent study reported that HNRNPA2B1 expression was lower in breast tumors with LN metastasis than DCIS and high expression correlated with longer OS [61]. The authors reported that HNRNPA2B1 knockout in MDA-MB-231 TNBC cells reduced tumor growth, but stimulated metastasis when injected subcutaneously in nude mice [61]. It is clear that further studies of HNRNPA2B1’s role in breast metastasis are needed using additional clinical samples and in other breast cell and animal models.
DICER acts as a tumor suppressor in breast cancer [45]. Amplification of the miR-191/425 locus on chromosome 3p21.31 in breast tumors is associated with lower disease-free survival (DFS), and both miR-191 and miR-424 target the 3’UTR of DICER1 in breast cancer cells [62]. miR-191 expression was increased in breast tumors relative to normal breast tissue [63] and its expression is stimulated by HIF-1α [64]. In contrast, the miR-424(322)/503 locus on Chr X was deleted from the active allele in ~14% of breast tumors in METABRIC and TCGA data sets with loss of miR-424(322)/503 associated with poor prognosis, lower OS, and more aggressive tumor subtypes, i.e., basal and luminal B [65]. One study reported higher DICER was associated with TAM-resistance, which is associated with metastatic disease [66].
High AGO2 correlated with luminal B breast cancer in one study [67]. However, another study found higher AGO2 staining in HER2+ and basal breast tumors and the authors reported that reduced AGO2 is associated with poor patient survival [68]. Overexpression of AGO2 in MCF-7 luminal A human breast cancer cells increased levels of an ERα splice variant called ERα36 (36 kDa) and stimulated E2-induced xenograft tumor growth in vivo [67]. ERα36 lacks both N-terminal activation function (AF)-1 and C-terminal AF-2, contains the wildtype DBD, and has a unique 27aa C-terminal domain insertion that regulates coactivator interaction [69]. ERα36 is a plasma membrane-associated protein that is increased in breast tumors and breast cancer cells [70]. High ERα36 in primary breast tumors correlates with increased metastatic disease and reduced DFS and lung and brain metastasis have higher ERα36 IHC staining compared to the primary tumor, suggesting a role for ERα36 in metastasis [71]. ERα36 is activated by estradiol (E2) and 4-hydroxytamoxifen (4-OHT) and plays a role in endocrine resistance and cancer stem cells (CSC) [72]. ERα36 is uniquely targeted by miR-136–5p which is methylated and repressed in breast cancer cells and tumor samples [73]. Although ERα36 is increased in TAM-resistant patient-derived xenografts (PDX) [74], the expression and role of ERα36 in metastases from primary breast tumors is not yet known [70].
3. The metastatic trail in breast cancer
Metastasis is a sequential process that results from the evolutionary acquisition of features that allow tumor cells from a primary site to dissociate, disseminate, migrate, survive, extravagate, infiltrate into a distant site to form a metastatic niche, survive in a new environment, and proliferate. An overview of the pathway including selected gene and miRNA alterations is shown in Fig. 2. Processes involved in these events include activation of oncogenes, altered signaling events, upregulation of matrix metalloproteinases (MMPs), and inactivation of metastasis suppressor genes [75]. The miRNA regulation of MMPs has been reviewed [76]. Changes in the composition of the extracellular matrix (ECM) remodel epithelial-stromal-adipocyte-immune cell interactions and include mechanical stiffening in malignant transformation [77]. Increased expression of the transcription factor FRA-1 (FOSL) increases the transcription of genes in the mesenchymal transition, invasion, and metastasis, e.g., urokinase-type plasminogen activator (uPA, PLAU,)-urokinase receptor (uPAR, PLAUR), MMP1, MMP9 [78]. uPA is a serine protease that binds its receptor uPAR in breast cancer epithelial cell membranes (not stromal fibroblasts or myofibroblasts) to activate certain MMPs to degrade components of the ECM including fibrin, laminin, and fibronectin (reviewed in [79]). PAI-1 (SERPINE1) inhibits uPA by direct binding. Hypoxia activates HIF-1α that stimulates cell proliferation, EMT, and angiogenesis in BCa [80]. Tumors cells form clusters to metastasize [81]. The tumor microenvironment, composed of tumor cells, stromal cells, cancer-associated fibroblast (CAFs), adipocytes, endothelial cells, and infiltrating innate immune cells, i.e., M2-like macrophages, that inhibit or facilitate tumor cell intravasation, is critical in the process of metatastasis [82]. Tumor-associated macrophages (TAMs) in breast tumors secrete chemokines, cytokines, and growth factors that stimulate tumor cell migration, invasion, and metastasis, e.g., CSCL12, CCL2,IL-4, IL-6, IL-8, IL-13, NOS2 (to produce nitric oxide), TGFβ, VEGFA, and EGF [83,84]. TAMS also interact with adipocytes to stimulate CYP19A1 (aromatase) expression in adipose stromal cells (fibroblasts) to increase local estrogen (E2 and estrone (E1)) production from adrenal androgens [85]. The appearance of metastatic disease can occur many years, even decades after initial breast cancer diagnosis in a process termed “metastatic latency” [86]. Metastatic latency is correlated with ERα expression in the primary breast tumor and ~ 60% of ERα+ breast cancer patients develop bone metastasis, followed by lung, liver and brain [86].
Fig. 2: An overview of the metastatic process in breast cancer.
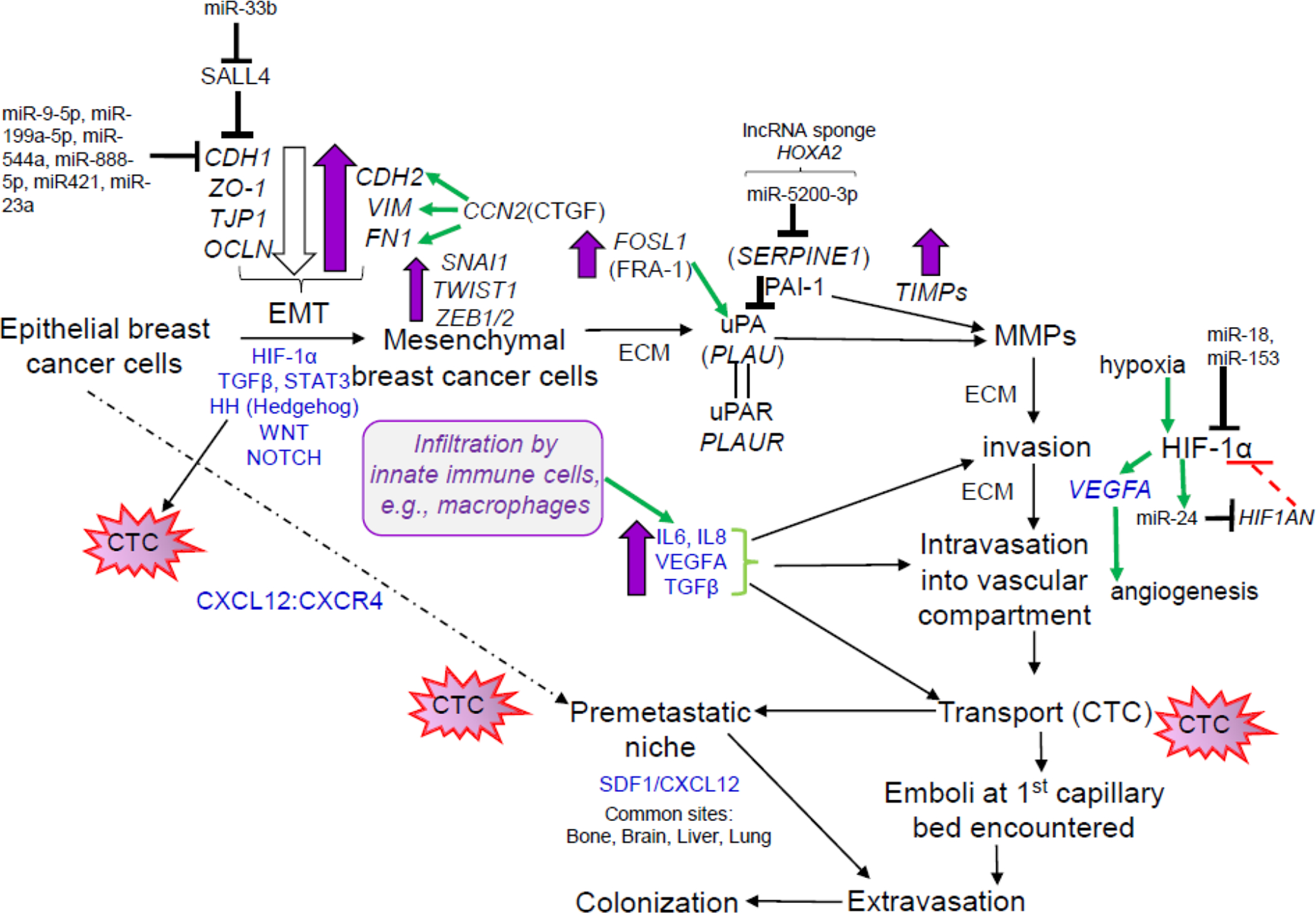
The overall metastatic pathway was recently reviewed in [87]. Purple-fill arrows indicate upregulation, open arrows indicate downregulation, and green arrows indicate stimulation. Growth factor, cytokine, signaling pathways are in blue.
Metastasis is an inefficient process that results from the acquisition of genetic and epigenetic changes that enable tumor cells to survive, migrate, and then survive in a new environment to establish macroscopic foci [87]. Although we usually think of metastatic cells in circulation, either directly or in the lymphatics system, some cancer cells, e.g., pancreatic duct adenocarcinoma, gastric carcinoma, head and neck cancer, prostate cancer, and colorectal cancer, migrate along nerve axons with the support of growth factors and chemokines secreted from cells in the perineural niche [88,89]. Note that higher nerve fiber density was reported in invasive ductal carcinoma (IDC) compared to ductal carcinoma in situ (DCIS) or invasive lobular carcinoma (ILC), suggesting an association with metastatic potential [90].
Only ~ <0.02% of disseminated tumor cells (DTCs) are estimated to successfully seed metastasis [91]. Notably, after colonization, DTCs either proliferate or can enter dormancy in protected niches such as bone marrow for an extended period until ‘awakened’ by intrinsic or extrinsic signals [86]. Interestingly, NR2F1 (COUP-TFI) drives a pro-dormancy gene program in ER+ breast cancer cells after hypoxia that allow cells to evade chemotherapy [92]. Follow-up studies reported that breast cancer patients with low nuclear NR2F1 in DTCs in bone marrow had systemic relapse within 12 month after bone marrow aspiration whereas those with high NR2F1 in DTCs had higher distant disease-free survival, implicating NR2F1’s role in DTC dormancy [93]. NR2F1 is translationally repressed by direct targeting by miR-21 to its 3’-UTR in mouse embryonic stem cell lines [94], but whether the known increase in miR-21 in breast cancer patients is involved in suppressing NR2F1 to release cells from dormancy is unknown.
Evidence supporting the model that metastatic spread occurs early in tumor progression and likely precedes detection of the primary tumor [95,91]. At a molecular level, early tumor cell dissemination results from tumor heterogeneity. Although tumor heterogeneity has long been recognized [96], next-generation sequencing (NGS) with single cell RNA sequencing (scRNA-seq) has revealed that within the non-treated primary tumor resides considerable cellular heterogeneity including small populations of cells already resistant to targeted therapies [97] as well as CSC, also called tumor-initiating cells [98]. Mutations in MYC, TP53, PIK3CA, HER2, and FGFR1 occur early in the development of TNBC [99]. Studies using multiple sublines of MDA-MB-468 TNBC cells identified IL11 and VEGFD (FIGF) as drivers of metastasis in vivo (from the mammary fat pad injection site to lung and bone marrow) by activating ‘effector’ neutrophils, thought to promote the metastatic niche, although the studies were performed in immunocompromised mice [100]. Chemotherapy can increase the metastatic potential of cancer by selecting those cells with innate resistance as well as driving the development of new mutations that endow chemotherapeutic resistance [101].
Genetic and epigenetic regulatory mechanisms contribute the phenotypic diversity of cells within a tumor, including cells that have undergone epithelial-mesenchymal transition (EMT) and transitioned to the CSC state [102]. CSC are defined by markers, e.g., CD44+/CD24low, EPCAM+, ALDH1+ [103]. Primary tumors also contain what are termed “metastasis-initiating cells (MICs)” that are defined as cancer cells capable of seeding clinically significant metastatic colonies in secondary organs [91]. MICs are highly plastic and retain tumor-initiating cell capabilities, including flexibility between EMT and mesenchymal-to-epithelial transition (MET), resistance to anoikis and apoptosis, evasion of immune surveillance, and metabolic adaptability [91].
A key step in metastasis is colonization of circulating tumor cells (CTCs) in a secondary site or distant organ. CTCs are rare: usually < 1 CTC/10 ml blood in nonmetastatic cancer and 1–5 CTC/7.5 ml blood in breast cancer patients with metastatic breast cancer and have short survival time of 1–3 h in blood [104]. CTC detection in blood has been repeatedly demonstrated to define a subgroup of breast cancer patients at higher risk of relapse [104]. This step is considered the best ‘open’ therapeutic window in metastasis [7]. For cells to extravasate from the bloodstream and colonize a distant site, primary tumors secrete factors that induce the formation of a “pre-metastatic niche” to support the growth of the CTCs in this distant site. The pre-metastatic niche is a supportive microenvironment, colloquially called the “soil” into which the CTC are the “seeds” which grow into a metastatic tumor [105].
Molecules secreted from the primary tumor regulate pre-metastatic niche formation, thus promoting metastasis. Among the molecules known to be secreted from tumors that stimulate pre-metastatic niche formation are EVs, including exosomes, as well as growth stimulatory factors. The C-X-C motif chemokine ligand 12 (CXCL12):C-X-C motif chemokine receptor 4 (CXCR4) signaling axis promotes breast cancer cell homing and colonization from the primary tumor to the bone [106,86]. Interestingly, in breast tumors, high miR-4784 is a bad prognostic indicator whereas higher expression of its target CXCL12 is a good prognostic indicator [107]. A recent gene expression profiling study of miRNA and mRNA expression in the spontaneous MMTV-NeuT mouse model of mammary carcinogenesis identified pre-invasive changes, i.e., upregulation of circulating miR-29 and miR-23 family prior to invasion of the bone marrow [108]. They determined that the increase in miR-29 family members was coming from circulating immune cells whereas miR-23 family members originated in the mammary epithelium. They found that miR-23a-3p was upregulated in the blood of breast cancer patients [108]. They reported that the increase in miR-29a-3p and miR-29c-3p downregulated SPARC (Osteonectin) and protein in the bone marrow which is associated with metastasis. This mouse study demonstrates that circulating miRNAs may impact genes that regulate bone marrow metastasis Interestingly, TGFβ increases miR-23a expression in MCF-7 and MDA-MB-231 cells and miR-23 also directly targets CDH1 [109]. Knockdown of miR-23a reduced MCF-7 and MDA-MB-231 cell migration and invasion in vitro and lung tumor metastases in mice injected with MDA-MB-231-anti-miR-23a inhibitor [109]. These data suggest a role for high miR-23a in breast tumors in invasion and migration in vivo.
4. MetastamiRs and their targets in breast cancer
Other investigators have previously [110,111] and more recently reviewed the role of specific miRNAs in pathways in breast cancer invasion and metastasis [112,80,113,114]. miRNAs dysregulated either by upregulation or downregulation and associated with metastasis are called “metastamiRs” and include reported upregulation of 13 miRNAs, e.g., miR-9–5p, miR-10b-5p, miR-21–5p, miR-29 family members, and miR-520–3p, and downregulation of 23 anti-metastatic miRNAs, e.g., miR-141–3p, miR-200 family members, miR-31–5p, and miR-15b-5p [115,110] (Table 1). We reviewed the literature in PubMed with respect to previously reported metastamiR expression in human breast tumors and their metastases, gene targets, and in vitro human breast cancer cell line studies to update our understanding of metastamiRs in breast cancer. Some of the papers identifying miRNAs as metastamiRs have been retracted; thus, necessitating reconsideration of the role of certain miRNAs. Specifically, two papers from Weinberg’s lab reporting that levels of anti-metastatic miR-31–5p primary breast tumors were inversely associated with the propensity for metastatic relapse were retracted [116,117]. There is one report of reduced miR-31–5p in invasive ductal carcinomas (IDC) vs. ductal carcinoma in situ (DCIS) [118]; however, another report came to the opposite conclusion [119]. The anti-invasive phenotype of miR-31–5p is mediated by its suppression of the expression of integrins, including ITGA2, ITGA5, ITGAV, ITGB1, ITGB3, and ITGB5 (242) (Table 1). Further study is needed to clarify the role of miR-31–5p in metastatic spread in breast cancer.
Table 1.
| miRNA | Bona fide validated Gene targets Those miRNA gene targets not cited by reference number were identified as validated targets in miRTarBase [387]. | Role-Pathways Phrases in red are from [136]. ? = more recent data do not support the original identification. |
Expression in human breast tumors and roles |
|---|---|---|---|
| miR-9–5p | CDH1 [124], LIFR [125] | Pro-metastatic oncomiR
|
|
| miR-10b-5p | TBX5, PTEN, HOXD10 [389], NF1, KLF4 [114], TBX5 [390] | Pro-metastatic EMT Adhesion, migration, invasion
|
|
| miR-21–5p | PTEN, PDCD4 [392,393]; NFIB [394]; TPM1 [395], RASA1 and RASA2 [396]; BTG2, FBXO11, MARCKS, RECK, and TPM1 [397]; TIMP3 [398]; LZTFL1 [399] | Pro-metastatic apoptosis |
|
| miR-143–3p | MAPK1 [405]; DNMT3A [406]; CIAPIN1 [407]; BCL2 [408]; KRAS, HRAS, CD44, AKT1 | Pro-metastatic
|
|
| miR-182–5p |
PFN1 [409] PLD1 [410] SNAI1 [138] FOXO3a [137] |
Pro-metastatic EMT
|
|
| miR-373 | ESR1 [144]; CD44 [139] TXNIP and RABEP1 [411]; RELA and TGFBR2 [141] | Pro-metastatic | |
| miR-520c-3p | RELA and TGFBR2 [141] IL8 [140]; CD44, MROP, SIRT1, GPC3, MICA, XPNPEP1 | Pro-metastatic |
|
| miR-29a/b/c | DICER1, TTP, PTEN, ARP1B1, KLF4, MYP, ANGPTL4, LOX, MMP, PDFGC, VEGFA, ADAM12, SERPINH1 (reviewed in [11,154,26]). ATP5G1 and ATPIF1 [335] | Pro-metastatic?
|
|
| miR-27a/b (share 20/21 nt) |
ZBTB10 [414] FOXO1 [252] SPRY2 [336] FBXW7 [415] miR-27b: CYP1B1 [416]; ST14, MMP13 [417] |
Pro-metastatic Pro-Angiogenesis |
|
| miR-19a-3p miR-19b-3p (miR-17/20 cluster on chromosome 13q31 encodes six miRNAs [418]). |
FOSL (Fra-1)[419]; IMPDH1 & NPEPL1 [420]; PTEN [421] BRCA2 [422] miR-19b: VEZF1 [155] |
Pro-metastatic? Pro-Angiogenesis? |
|
| miR-221–3p | ESR1/ERα [159], CDKN1B, FOXO3, KIT, PTEN and TIMP3, BRAP, ARIH2, FOS, ICAM1 | Pro-metastatic Angiogenesis |
|
| miR-222–3p | ESR1/ERα [159], TIMP3 [425], STAT5A [426], MMP1 [427], FOXO3, FOX, PTEN, KIT, SOCS1 and CDKN1B [428]; LBR in CAFs [429] | Pro-metastatic Angiogenesis |
|
| Let-7f | CYP19A1 [162] | Pro-metastatic Angiogenesis |
|
| miR-141–3p (a member of the miR-200 family [435]) |
ZEB1,ZEB2 [163,164]; TGFB2 [436], PTEN [437], EIF4E [438], CTNNB1 [439]; ANP32E [440], CDC25B [435], CTBP2, MAPK14, PPARA, BRD3, UBAP1, ZFPM2 | Anti-metastatic?
|
|
| miR-193b |
PLAU (UPA) [167] YWHAZ, SHMT2, AKR1C2 [446] |
Anti-metastatic |
|
| miR-200a/b/c | ZEB1 ZEB2 [317,376–379]; SLUG, BCL2, E2F2, RND3, VEGFA, KDR, FLT1, ETS1, SUZ12, BMI1, NOTCH, LIN28B, FOXM1 [447] |
Anti-metastatic
|
|
| miR-205–5p |
ZEB1, SIP1 [164]; ERBB2 [449]; SHP2, PTEN, E2F1,E2F5 PRKCE (PKC epsilon), VEGFA, ERBB3 [450]; NOTCH2 [451]; ITGA5 [452] |
Anti-metastatic |
|
| miR-429 (miR-200 family member [435]) |
LRP1 [455], PLCG1 [456] ZEB1 & ZEB2 [435] |
Anti-metastatic
|
|
| miR-146a/b | RHOA [458] |
Anti-metastatic
|
|
| miR-206 | NOTCH3 [459] |
Anti-metastatic
|
|
| miR-335 | SOX4, TNC, MERTK, PTPRN2 [460]; ESR1, IGF1R SP1 [461] |
Anti-metastatic
|
|
| miR-31–5p | ITGA2, ITGA5, ITGAV, ITGB1, ITGB3, ITGB5 [464]; FZD3, ITGA5, M-RIP, MMP16, RDX, RHOA, SATB2 [388]; DICER1 [465]; SATB2 [466] |
Anti-metastatic
|
|
| miR-15b-5p | MTSS1 [180]; CCNE1, RECK, BCL2, CCND1, VEGFA, EI4A1, AXIN2, IFNG, PURA | Anti-metastatic? [178] | |
| miR-16 miR-15a/miR-16 cluster |
CCDN1, CCNE1 [472], BCL2 [473]; EEF1AKNMT (METTL13) [474] | Anti-metastatic |
|
| miR-20a-5p (part of the miR-17–92 cluster) | TGFBR2 [478]; E2F1 [479]; CCND1 [418], CXCL8 (IL-8) [480], ZBTB4 [481]; BECN1, ATG16L1, SQSTM1 [482]; RUNX3 [483]; HMGA2 [484]; |
Anti-metastatic?
|
|
| miR-20b-3p and miR-20b-5p (from pri-mir-106a-363 on Chromosome X) | For 3p: ESR1 [487]; EPAS1 [488]; NCOA3 (SRC-3, AIB-1) [489]; BRCA1, PTEN [490]; For 5p: ARID4A, MYLIP [491]; HIF1A, VEGFA [492]; PPARG, BAMBI, CRIM1 [493] EPHB4, EFNB2 [494]; PTEN [495]; SOS1, ERK2 [496]. |
Anti-metastatic?
|
Abbreviations: BCa = breast cancer; ceRNA = competing endogenous RNA (‘miRNA sponge’); CSC = cancer stem cells; DCIS = ductal carcinoma in situ; DFS = disease-free survival; EMT = epithelial-to-mesenchymal transition; LN = lymph node; OS = overall survival; RFS = relapse-free survival; TNBC = triple negative breast cancer;
The pro-metastatic miRNAs show increased expressing in breast tumors with stage and correlate with reduced DFS, relapse free survival (RFS), and/or overall survival (OS). For example, miR-9–5p is overexpressed in high stage tumors, with high histological grade, and was reported to be higher in HER2+ and TNBC compared to other breast cancer (BCa) subtypes [120]. However, this increase in miR-9–5p was not found in an analysis of TCGA data [121]. Other reports found that patients whose breast tumor have high miR-9 show lower OS and DFS [122,123]. E-Cadherin (CDH1) [124] and leukemia inhibitor factor receptor (LIFR), a metastasis suppressor [125], are direct targets of miR-9–5p in breast cancer, contributing to EMT and metastasis.
miR-21–5p is a well-established oncomiR [126] that downregulates a number of tumor suppressors notably PTEN, PDCD4, and TPM1 (Table 1). The expression of miR-21 is increased in breast tumors and plasma from BCa patients [127]. High miR-21 in breast tumors correlates with lymph node status and tumor stage (150). miR-21–5p is also secreted by CAFs in BCa [128]. miR-21 is a potential plasma/serum biomarker for BCa with a sensitivity of 0.79 [129]. TGFβ and BMP signaling increase miR-21 transcription by activating SMAD2/3 [130]. Transient knockdown of miR-21 in TAM-resistant MCF-7 cells enhanced autophagy in response to TAM or fulvestrant and inhibited the PI3K-AKT-mTOR pathway [131]. miR-21 has been confirmed to maintain the malignant phenotype in various cancers and thus is of significant interest in the development of therapeutic ablation by DNAzymes, antimiRs, antagomiRs, miRNA sponges, or miRNases [132,133].
In contrast, miR-143–3p was reported to be pro-metastatic in BCa [75] although its expression is downregulated in BCa tissues [127,134]. The decrease in miR-143–3p expression induces RAS signaling to promote aggressive PTEN-deficient basal-like BCa [135]. With the decrease in miR-143–3p expression of its pro-metastatic targets, e.g., KRAS, HRAS, and CD44, would be increased (Table 1).
miR-182–5p was reported to be pro-metastatic [136]; however, overexpression of miR-182–5p inhibited invasively aggressive properties of MDA-MB-231 cells in vitro (167). Likewise, miR-182–5p overexpression in MCF-10A immortalized breast epithelial cells increased E-cadherin and decreased vimentin (VIM) in vitro (168). In agreement with its pro-metastatic role, hsa_circ_0025202 expression is decreased in correlation with histological grade and lymph node status and because circ_0025202 is a miRNA sponge for miR-182–5p, its decrease would increase miR-182–5p levels [137]. In a mouse model of metastatic breast cancer, injection of mouse mammary tumor 4T1 cells in the mammary fat pad resulted in lung metastases that had high miR-182 expression [138].
Overexpression of pro-metastatic miR-520c-3p induces MCF-7 and MDA MB-435 cell migration and invasion in vitro and promotes tumor metastasis in a tail vein injection mouse model in vivo [139]. In contrast, another paper found that miR-520c-3p overexpression inhibits invasion and migration of MCF-7 and T47D human luminal A breast cancer cells [140]. Overexpression of miR-520c-3p inhibits TGF-β signaling, i.e., phosphorylation of SMAD2 and SMAD3, and decreases target genes ANGPTL3, PTHLH, and SERPINE1 (PAI-1) in MDA-MB-231 TNBC cells in vitro [141]. lncRNA HOXA-AS2 acts as a miRNA sponge for miR-520c and its increased expression is detected in patients with distant metastasis [142]. The increase in HOXA-A2A would be expected to reduce miR-520c levels and increase miR-520c targets such as CD44 and XPNPEP1 (X-Prolyl Aminopeptidase APP1) (Table 1). XPNPEP1 is upregulated in BCa, is a predictor or poor prognosis and metastases, and overexpression of XPNPEP1 in MDA-MB-231, MT474, MCF-7 breast cancer cells increases cell migration [143].
Although miR-373 targets ESR1 (ERα) [144], it is a pro-metastatic as indicated in experiments demonstrating that its overexpression induces MCF-7 (luminal A) and MDA MB-435 (TNBC) cell migration and invasion in vitro and that it promotes tumor metastasis in a tail vein injection mouse model in vivo [139]. miR-373 directly targets integrin α2 (ITGA2), a collagen receptor on epithelial cells that leads to cell migration in BCa cells and ITGA2 protein is reduced in breast tumors [145]. Transfection of MCF-7 cells with miR-373 induced EMT by inhibiting TXNIP resulting in HIF-1α activation and increased TWIST1 [146]. Expression of miR-373 is higher in breast tumors with lymph node positive disease [139] and serum concentrations are higher in patients with metastatic disease at diagnosis [147].
There are four members of the miR-29a/b/c family located on 2 human chromosomes. Chromosome 1q32.2 is the location of miR-29b-2 and miR-29c [148,149]. miR-29b-1 and miR-29a are encoded on chromosome 7q32.3 [150–152]. All miR-29 family members have the same seed sequence [149]. miR-29 family members were reported to have higher expression in Luminal A and B breast tumors compared with basal and HER2+ tumors {Chou, 2013 #22972}. GATA3 overexpression in MDA-MB-231 TNBC cells increased the levels of luminal gene expression, e.g., CDH1, KRT8, and miR-29a/b-1 (from a common promoter), while reducing mesenchymal markers, e.g., ZEB1, ZEB2, FN1, SNAI1, SNAI2, and VIM, with miR-29b downregulating ANGPTL4, LOX, MMP2, MMP9, PDGF and VEGFA [153]. miR-29 family members have both tumor suppressor and oncogenic activities in breast cancer (reviewed in [154]). We reported that transient overexpression of miR-29a and miR-29b-1in MCF-7 cells and TAM-resistant LCC9 and LY2 cell lines derived from MCF-7 cells inhibited cell proliferation, migration, and colony formation of LY2 tamoxifen-resistant, luminal A breast cancer cells derived from MCF-7 cells [154].
miR-19a/b were named as metastamiRs by stimulating angiogenesis [136]. However, overexpression of miR-19b inhibited the proliferation of HUVECs, MCF-7, and MDA-MB-231 cells in vitro and inhibited MDA-MB-231 xenograft growth and caused growth arrest in vivo [155]. In breast tumors, two reports show opposite conclusions with one reporting lower miR-19b in invasive tumors [156]and one reporting higher expression in tumors associated with distant metastasis, TNM stage and reduced OS [157]. A target of miR-19b is Vascular Endothelial Zinc Finger 1 (VEZF1) [155], a transcription factor required for vascular system development in mice [158], thus, miR-19b was suggested to inhibit angiogenesis [155]. Thus, further study of the expression and role of miR-19a/b in breast cancer metastases is needed.
Both miR-221 and miR-222 are overexpressed in breast tumors, downregulate ESR1 (ERα), and are associated with endocrine-resistance [159,160] (Table 1). They are considered pro-metastatic and pro-angiogenic. Both miR-221 and miR-222 are involved in regulating genes involved in adherens junctions, PI3K and MAPK signaling, TGFβ signaling, apoptosis, and cell cycle [160] (Table 1). Thus, they contribute to cell proliferation and invasion.
Let-7f was also identified as metastamiR by stimulating angiogenesis [136]; however, members of the Let-7 family are documented tumor suppressors [161] and results of BCa tumor expression show both increased and reduced expression (Table 1). A bona fide target of Let-7f is CYP19A1 [162], the aromatase gene which is a target of AI therapy in postmenopausal BCa patients whose primary tumor is ER+, a result suggesting an inhibitory role for let-7 in this pathway. Indeed Let-7f is upregulated by letrozole treatment in clinical samples [162].
Among the metastamiRs reported to have anti-metastatic activity [136], miR-141–3p would seem to be a good candidate since it targets ZEB1 and ZEB2 [163,164]. ZEB1 and ZEB2 are established EMT-inducers downstream of TGFβ, WNT-β-catenin, and RAS-MAPK signaling pathways [165]. However, knockdown of miR-141 inhibited metastatic colonization to brain of SUM149 cells whereas overexpression of miR-141 in non-expressing MDA-MB-231 TNBC enhanced brain metastatic colonization after tail vein injection of mice [166]. Further, reports of tumor and blood expression levels of miR-141 in BCa patients show both upregulation and downregulation (Table 1). These data suggest the need for further examination of miR-141 as an anti-metastatic metastamiR.
The anti-metastatic activity of miR-193b is supported by a study reporting that mice injected with MDA-MB-231 overexpressing miR-193b cells developed smaller mammary tumors and had a 50% decrease in lung metastasis [167].
Concordant in vivo and in vitro data support the anti-metastatic roles of miR-200 family members, miR-205–5p, miR-429, miR-146a/b, miR-206, and miR-335 (Table 1). miR-146 is upregulated by BRMS1 [115], a known metastasis suppressor gene that suppresses NFκB-regulated gene transcription [168], interacts with the SIN3-HDAC-ARID4 corepressor complex [169], and with the LSD1-CoREST corepressor complex resulting in demethylation of H3K4me1/2 and histone acetylation on EMT target gene promoters for epigenetic repression, e.g., VIM [170]. Overexpression of miR-146a/b in MDA-MB-231 cells increased invasion, migration, and NFκB activity [171]. However, the roles of miR-15b-5p, miR-16, miR-20a-5p, miR-20b-3p and miR-20b-5p as anti-metastatic metastamiRs [136] is uncertain (Table 1). miR-335 is anti-metastatic and suppresses CSC biogenesis [172] and it was reported to be downregulated in BCa cell lines overexpressing BRMS1 [173]. However, miR-335 was increased in exosomes from plasma samples from TNBC patients [174]. miR-20a-5p is part of the miR-17–92 cluster. As reviewed in Table 1, miR-20a has been reported to be downregulated in breast tumors by some investigators and upregulated in ER− vs. ER+ breast tumors and expression correlates with lymph node involvement [175]. miR-20a is upregulated in sera from BCa patients vs normal controls [176]. BCa cell-derived exosomal miR-20a-5p promotes TNBC MDA-MB-231 cell migration and invasion the proliferation and differentiation of osteoclasts by targeting SRCIN1 [177]. These findings suggest that miR-20a is not anti-metastatic, but further studies are warranted.
Although miR-15b-5p was reported to be anti-metastatic [178], this conclusion needs reconsideration for BCa. In fact, miR-15 is among a 9 serum miRNA signature (miR-15a, miR-18a, miR-107, miR-133a, miR-139–5p, miR-143, miR-145, miR-425, and miR-365) that discriminated early stage ER+ BCa patients from healthy controls [179]. miR-15b-5p targets MTSS1 (Metastasis suppressor 1) [180] and MTSS1 expression is inversely associated with OS in breast tumors [181]. Another report found higher miR-15b-5p in aggressive breast tumors than in adjacent normal tissue and inversely correlated with MTSS1 [180]. Further, miR15b-5p was reported to be upregulated in brain metastasis compared with primary BCa tumors [182]. EGF-induced miR-15b suppresses the translation of MTSS1, and the loss of MTSS1 promotes migration of mammary MCF-10A epithelial cells [180].
5. Metastasis Suppressor regulation by miRNAs
Tumor formation and metastasis formation are distinct, as shown by studies identifying metastasis suppressor genes that block metastasis but not primary tumor formation [75]. Early studies showed that transfection of MDA-MB-435 TNBC cells with an expression vector for the metastasis suppressor KISS1 reduced the number of lung metastases observed, but not the growth of the mammary primary tumor in the mammary fat pad injection site, separating primary tumor growth vs. metastasis [183]. Further studies identifying and characterizing metastasis suppressors have been reviewed [184] [185] [75,87]. Here we searched the literature for eight identified metastasis suppressors and their potential role in breast cancer and regulation by miRNAs (Table 2). The genes and description of their regulation by miRNAs in breast cancer does not include all the information in Table 2, but focuses on those genes/proteins with clear roles in breast cancer metastasis.
Table 2: Metastasis suppressors identified by Welch and colleagues [75], their regulation by miRNAs, and roles in breast cancer.
Metastasis suppressor genes do not prevent primary tumor cell growth, but inhibit metastasis. Those miRNA gene targets not cited by reference number were identified as validated targets in miRTarBase [387].
| Metastasis suppressor | miRNA regulation | Role | Breast cancer |
|---|---|---|---|
| BRMS1 | None reported for BCa; miR-3200–5p in osteosarcoma [499]; miR-346 in hepatocellular carcinoma (HCC) [500] |
|
|
| CADM1 | None reported for BCa; miR-10b in HCC [503]; miR-214 in colorectal cancer cells [187] |
|
|
| KISS1 [75] | None experimentally reported for BCa; however, miR-137and miR-345 were identified as putative targets in silico [506] |
|
|
| NR1H4 = FXR | None reported for BCa; miR-421 in biliary tract cancer [194]; miR-192–3p and miR-192–5p in HCC cells [195] |
|
|
| GAS1 | miR-34a-5p in papillary thyroid carcinoma cells [509] |
|
• |
| CD82 KAI1 | miR-203 in H1299 lung adenocarcinoma cells [510]; miR-97 in gastric cancer [511]. |
|
|
| LIFR | miR-9 [125]; miR-629–3 [202] |
|
|
| KDM1A | miR-137 [513], miR-329–3p [514], miR-708–5p [515] |
|
|
| MTBP | None listed in miRTarBase |
|
|
| MAP2K4 | miR-27a-3p, miR-92a-3p |
|
|
| NDRG1 | miR-769–3p [216]; miR-182 in prostate cancer cells [521]; miR-449a in endometrial cancer cells [522] |
|
|
| NME1 (Nm23, NM23-H1) | None in miRTarBase miR-645 in osteosarcoma cells [523] |
|
|
| GPR68 (OGR1) | None in miRTarBase; miR-18a-3p in Leydig cells [531] |
|
|
| RRM1 (Rho-GDI beta) | miR-101–3p in pancreatic cancer [533] |
|
|
| AKAP12 (AKAP 250 or Gravin) | miR-186–5p [538]; miR-103 in HCC [539] |
|
|
| CST6 | None in miRTarBase |
|
|
| TIMP1 and TIMP2 |
TIMP1: miR-519a-3p, miR-181a-5p, miR-1293, miR-17–5p, miR-337–3p TIMP2: miR-519d-3p, miR-519c-3p, miR-200c-3p, miR-106–5p, miR-17–5p, miR-429 |
|
|
| TIMP3 | miR-181a-5p, miR-181b [548], miR-21–5p [549], miR-222–3p [550] |
|
|
| TIMP4 | miR-200b-3p in prostate ca cells [552] |
|
As described above metastasis suppressor BRMS1 interacts with corepressor complexes SIN3-HDAC-ARID4 [169], and LSD1-CoREST [170]. Although BRMS1 has been reported to be targeted by miRNAs in osteosarcoma and hepatocellular carcinoma (Table 2), its regulation by miRNAs in BCa is uncertain. BRMS1 represses expression of miR-10b by inhibiting HOXD10 and inhibits RAC1, a GTPase that is required for cell migration of ER+, TNBC, and HER2+ BCa cells [186], by suppressing TIAM1, which is a target of mTORC2-AKT [110].
Cell adhesion molecule 1 (CADM1, also called TSCL1 and NECL-2) mediates cell-cell adhesion and inhibits metastatic colonization [75]. CADM1 was reported to be a direct target of miR-214 in colorectal cancer cells [187]. Although CADM1 regulation by miRNAs in BCa cells is unknown, miR-214 is a breast tumor suppressor [188].
The KISS1 transcript encodes a 145 aa peptide that is processed to a number of shorter kisspeptins (kisspeptins 10, 13, 14, and 54) that bind the KISS1 receptor (KISS1, GPCR54) resulting in inhibition of NFκB activation and downregulation of MMP9 and IL8 [189]. Kisspeptins regulate neuroendocrine signaling controlling puberty and reproduction in humans [190]. SIRT1 deacetylates CREB which activates KISS1 transcription in CRC cells [191]. KISS1 expression is reduced in BCa metastasis in brain relative to the breast primary tumor and expression is reduced by CXCL12 from astrocytes [189].
Activation of the bile acid receptor (FXR, gene NR1H4) inhibits cell migration, stress fibers, and contraction of CAFs [192]. FXR is expressed in breast tumors and its expressed correlates with ERα/PR, i.e., the luminal A phenotype [193]. A recent report found that activation of FXR by the FXR agonist GW4064 inhibited the tumor-promoting activities of CAFs, in co-culture experiments with MCF-7 and T47D cells, suggesting that targeting FXR might be a useful molecular target to reduce cell migration and invasion as early steps in metastatic spread [192]. While no miRNAs targeting FXR in BCa have been reported, miR-421 acts as an oncomiR to downregulate FXR in biliary tract cancer [194]. FXR is downregulated by miR-192–3p and miR-192–5p in HCC cells [195]. Experiments examining miRNA regulation in breast cancer are needed.
Lentiviral overexpression of metastasis suppressor Growth arrest specific 1 (GAS1) inhibits MDA-MB-231 xenograft tumor growth in mice [196]. GAS1 inhibits GFRα3-ARTN and MAPK signaling pathways [196]. GAS1 is a positive regulator of CSC maintenance [197]. lncRNA-Hh, was found to directly target GAS1, an enhancer of hedgehog signaling to increase the SOX2 and OCT4 expression, thus played a critical role for TWIST-driven EMT cells and TWIST-positive breast cancer cells to gain the CSCs-like characteristics [197].
CD82 KAI1 is a plasma membrane glycoprotein that is a member of the transmembrane 4 superfamily with no intrinsic activity [198]. It is a metastasis suppressor that is associated with metastatic progression in a variety of cancers [184]. It is considered a tumor suppressor by its interaction with multiple protein partners, e.g., DARC (ACKR1, Atypical Chemokine Receptor 1 (Duffy Blood Group)) on endothelial cells interacts with KAI1 on cancer cells inhibiting cell proliferation and inducing senescence [199]. KAI1 suppresses cell motility and metastasis of MDA-MB-231 and MDA-MB-435 BCa cells in mouse models [198]
Leukemia inhibitory factor receptor (LIFR), a type I cytokine receptor family member, is in a complex with Gp130 (gene IL6ST, Interleukin 6 Signal Transducer) and activates JAK/STAT, MAPK, AKT, and mTOR pathways in BCa [200]. LIFR functions in the Hippo-Yap pathway to suppress metastasis [201]. It is downregulated by miR-9 [125] and miR-629–3p [202]. Novel LIFR inhibitor EC359 blocks binding of ligands, thus attenuating LIFR oncogenic signaling, reduced proliferation, invasion, CSC in TNBC cell lines and PDX assays [200].
Metastasis suppressor Lysine-specific demethylase 1A (LSD1, aka KDM1A) is a histone demethylase for H3K4me2/1 or H3K9me2/1, p53, DMNT, E2F1, HIF-1α, and STAT3 [203]. LSD1 has been reported to exhibit both oncogenic and metastasis suppressor actions in breast cancer. Inhibition of LSD1 strongly inhibits proliferation of breast cancer cells [204]. Thus, increased miR-127, miR-239–3p, and miR-708–5p would be expected to reduce LSD1 in BCa cells and be inversely correlated with LSD1 expression in breast tumors, although no one has yet investigated this. A recent report demonstrated that LSD1 interacts with GATA3, a key luminal-specific transcription factor in BCa, and coregulates 443 genes with 519 overlapping binding sites (determined by ChIP-seq) in MCF-7 cells [205]. LSD1 knockdown in T47D, MCF-7, and MCF-10A cells inhibited proliferation, reduced transcript expression of CDH1, and increased MCF-7 cell invasion in vitro. LSD1 also downregulated the transcription of TRIM37, a histone H2A ubiquitin ligase associated with PRC2 for gene repression. Conditional knockdown of Lsd1 in the MMTV-PyMT luminal breast cancer mouse model reduced survival with a significant increase in metastatic lung tumors, implicating Lsd1 as an in vivo metastasis suppressor in this model [205]. CARM1, an arginine methyltransferase, dimethylates LSD1 (R838) increasing LSD1 stability by promoting deubiquitylation of LSD1 in MDA-MB-231 TNBC cells by Ubiquitin Specific Protease 7 (USP7), thus blocking proteasome-mediated degradation, and resulting in reduced CDH1 transcription and increased EMT [206]. The authors reported that CARM1 expression is elevated in malignant breast tumors and positively correlated with LSD1R838me2 and LSD1 protein levels, suggesting a tumor promoting role for CARM1 and LSD1 in breast cancer metastasis [206].
MDM2 Binding Protein (MTBP, aka MDM2BP) suppresses cell migration and metastasis in human HCC [207]; however, it may not be metastasis suppressor in breast cancer. Overexpression was associated with reduced OS in BCa [208] and it was reported to be amplified in 19% of breast tumors with highest amplification in TNBC [209]. No miRNAs are listed as regulating MTBP in miRTarBase.
MAP2K4 plays a role in MAPK-PI3K crosstalk and is associated with drug resistance in several cancers [210]. It is regulated by miR-27a-3p and miR-92a-3p (Table 2), but there are no reports of miRNA regulation in breast cancer. The MAP3K1-MAP2K4-JNK cascade activates JUN for FOS heterodimerization to form AP1 to regulate gene transcription [211]. Loss-of-function mutations in MAPK2K4 sensitize to MAP2K4-mutant BCa cell lines to inhibition by MEK inhibitors selumetinib and dacomitinib in vitro and in vivo [212]. MAP2K4 interacts with Vimentin in BCa cells to promote cell migration and invasion [213]. Thus, MAP2K4 does not appear to have a role as a metastasis suppressor in BCa.
Transcription of MYCN (N-Myc) downstream regulated gene (NDRG1) is increased in response to cellular hypoxia by direct transcriptional regulation by HIF1α and XBP1 and is associated with ERα− BCa [214]. NDRG1 is a metastatic suppressor in prostate and colon cancers (reviewed in [215]). NDRG1 is a direct target of miR-769–3p in MCF-7 breast cancer cells [216]. However, others reported NDRG1 not to be a metastasis suppressor [214]. Elevated expression is correlated with an aggressive metabolic gene signature and in vitro studies show that NDRG1 alters lipid trafficking and metabolism in breast cancer [217,214]. Inhibiting lipid and fatty acid (FA) metabolism is a way to block breast cancer metastasis [218,219]. Breast cancer cells with cancer stem cell (CSC) properties show increased FA uptake and oxidation [220]. NDRG1 is targeted by miR-769–3p in MCF-7 cells and stimulates apoptosis [216]. The expression of NDRG1 is downregulated by the lncRNA NDRG1-OT1_v4 [221].
Tumor suppressor Nm23-H1, NME/NM23 Nucleoside Diphosphate Kinase 1 (NME1) has serine/threonine protein kinase activity, geranyl and farnesyl pyrophosphate kinase activity, histidine protein kinase activity, 3’−5’ exonuclease activity, and granzymeA-activated DNase activity [222]. It was first identified “metastasis suppressor gene” involved in the colonization stage of metastasis [223]. Early studies suggested that NME1 suppresses breast cancer metastasis, at least in part, through down-regulation of Lysophosphatidic Acid Receptor 1 (LPAR1, formerly called EDG2) expression [224].
Tissue inhibitors of metalloproteinases (TIMPs) consist of four endogenous proteins: TIMP1, TIMP2, TIMP3, and TIMP4 that are metastasis suppressors (56). TIMPs are regulators of ECM composition and structure. Each is targeted by specific miRNAs (Table 2). TIMPs regulate the enzymatic activity of metzincin proteinases (matrix metalloproteinases (MMPs)) and A disintegrin and a metalloproteases (ADAMs) that are dysregulated in BCa. TIMP1 transcript levels are increased in breast tumors whereas TIMP2, TIMP3, and TIMP4 transcript expression is reduced [225]. TIMP2 and TIMP3 share gene targets in the matrisome, an ensemble of genes that make up the ECM proteome [225].
6. EMT regulation by miRNAs in breast cancer
A number of steps are involved in the initial invasion of tumor cells from the primary tumor into the surrounding ECM including EMT, anoikis, changes in tumor-tumor and tumor ECM adhesion molecules, proteases, and activation of CSC cell pathways [7]. EMT is a reversible process initially characterized in embryonic morphogenesis that allows mesenchymal cells to migrate to new sites within the embryo and then undergo MET to switch back to an epithelial state [226]. In addition to development, EMT is also important in wound healing and inflammation where cell migration and invasion are needed [227]. In breast cancer, EMT is induced by signaling pathways including receptor tyrosine kinases (RTK, e.g., IL-1-R (IL1R1)), TGFβ, TNFα, activation of toll-like receptors (TLR), WNT, and NOTCH with significant correlations between the expression of these genes and metastatic breast cancer risk [228]. In addition, EMT is related to resistance to endocrine and other therapies including conventional chemotherapy in breast cancer [229].
During EMT reprogramming in breast cancer cells there is a disruption of the polarization of epithelial cells and loss of tight cell-to-cell junctions. During EMT, cells acquire the ability to migrate due to repression of epithelial markers including the adherens junction proteins (E-cadherin, CDH1), occludins, claudins, α6β4 integrin, and an increase in expression of neural cadherin (N-cadherin (CDH2), vimentin (VIM), an intermediate filament protein, and fibronectin (FN1), a glycoprotein that functions in migration [230]. Thus, markers of EMT include a decrease in CDH1, zona occludins protein 1 (ZO-1, TJP1), and occludin (OCLN) from adherens junctions, and increased VIM and CDH2). EMT is upregulated by TFs: ZEB family members (ZEB1 and ZEB2) [231], SNAIL (SNAI1), SLUG (SNAI2), and TWIST (TWIST1) (Table 3). These TFs inhibit transcription of genes associated with the epithelial state [230]. A meta-analysis of 14 studies concluded that overexpression of EMT-TFs TWIST (TWIST1), SNAIL (SNAI1), SLUG (SNAI2) was a prognostic factor in advanced MBC, with SLUG (SNAI2) “the most impactful” and demonstrating a higher hazard ratio for Asian MBC patients [228]. A recent study reported that PRKD1 is a direct target of TWIST1 that is required for cells to disseminate from organoid models of breast tumors by stimulating invasion, loss of adhesion, and cell migration [232]. The authors reported high PRKD1 in basal breast tumors was associated with reduced Distant Metastasis Free Survival (DMFS) [232].
Table 3. Regulation of EMT-related genes by miRNAs in breast cancer and metastasis.
Genes involved in EMT are from those listed in dbEMT 2.0 [236] or are from the indicated references. We have included only some of the 265 “dual role” (oncogenic and tumor suppressive) and oncogenic EMR-related genes. Those miRNA gene targets not cited by reference number were identified as validated targets in miRTarBase [387].
| EMT-related gene | miRNA regulation in BCa | Role and regulation | Breast cancer (BCa) |
|---|---|---|---|
| EPHA2 | miR-200a [241] |
|
|
| ETS2 | miR-320 [556] miR-320b [557] |
|
|
| EZH2 | miR-92b [559]; miR-26a [560]; miR-25, miR-214, miR-30d, miR-101 [187] | ||
| FOXO1 | miR-27a, miR-96, and miR-182 [252]; miR-9, miR-153, miR-183, and miR-186 [564]; miR-5188 [253] |
|
|
| RHOA | miR-155 [388]; miR-146a [458]; miR-490–3p [566]; miR-101 [567]; miR-150 [119]; miR-381 [568] |
|
|
| SKP2 | None for BCa, but miRNA-330–5p in pancreatic cancer [571]; miR-30d-5p in lung cancer [263]; miR-508–5p in gastric cancer [572] |
|
|
| CCN3 | miR-30c [575] | ||
| KLF4 | miR-10b-5p [114] [269];, miR-29a/b/c [580]; miR-7 [581]; |
|
|
| KLF5 | miR-590–5p [272] |
|
|
| GREM1 | miR-27a in rat bone marrow mesenchymal stem cells [585] |
|
|
| STAT3 | miR-519d [276]; miR-124 in TNBC [588] |
|
|
| SALL4 | miR-33b [589] |
|
|
| ILK | miR-625 in gastric cancer [590]; miR-145 in bladder cancer [591] |
|
|
| GLI1 | miR-361–3p in retinoblastoma [595] |
|
|
| SIRT1 | miR-22 [599]; miR-200a [600]; miR-34a [601]; miR-211–5p[602] |
|
|
| CCN2 (previously CTGF, connective tissue growth factor) | miR-124–3p, miR-18a-5p, miR-145–5p |
|
|
| PRKCE | miR-1–3p, miR-31–5p, miR-107, miR-146–5p |
|
|
| TP63 | miR-196a2* [304]; miR-203–3p [611] |
|
|
| SUZ12 | miR-200abc [447]; miR-200b [613]; miR-103/7 and miR15b/16 families [172] | • | |
| CDKN1B | miR-203 [309]; miR-455–5p [616] |
|
|
| WT1 | miR-193a in BCa [313]; |
|
|
| HDAC1 | miR-34a in BCa [623] |
|
|
| RUNX1 | miR-378 in BCa cells [625]; miR-139–5p in glioma cells [626] |
|
|
| SREBP1 | miR-18a-5p in BCa [629] |
|
|
| NME1 |
|
||
| CDKN1A | miR-106b in human mammary epithelial cells (HMECs) [636] |
|
|
| PPARG | miR-27b in neuroblastoma cells [640]; miR-130a-3p in cholangiocarcinoma [641] |
|
|
| CDH1 | miR-9–5p [124], miR-199a-5p [318], miR-544a [319], miR-888–5p [320], and miR-421 [337] in BCa; miR-92a-3p [647] | ||
| VIM | miR-124 [325] and miR-17–3p [326] in HCC cells; miR-378g [327]; miR-138 in renal carcinoma [651] | ||
| SPARC | miR-29a-3p and miR-29c-3p [108]. |
|
|
| E2F1 | miR-20a [479]; miR-149 [656]; miR-17–5p [657]; miR-124 [658]; miR-205 [659,660]; miR-149–3p [656] |
|
|
| YAP1 | miR-10a-5p in thyroid cancer [664]; miR-15a and miR-16–1 in gastric cancer [665] |
|
|
| RASSF1 | (miR-181b-5p in small cell lung cancer (SCC) was not verified by RASSF1–3’UTR luciferase assay [669]) |
|
|
| TGFB1 | miR-675 [331]; miR-181a-5p [673]; miR-133b [364]; miR-142–3p in granulosa cells [674] |
|
|
| TP73 | miR-193a-5p in prostate cancer [676] |
|
|
| ECT2 | miR-223–3p in BCa cells [680] |
|
|
| TCF3 | miR-506–3p in neural stem cells [683] |
|
|
| SPRY2 | miR-27a/b [336]; miR-128a [685]; miR-21 in gliomas [686] miR-23a and miR-24–2 [336] |
|
|
| NOTCH1 | miR-34a-5p [343]; miR-30a [344]; miR-139–5p [345]; miR-3178 [346] miR-10b [689] and miR-200b [690] in nasopharyngeal carcinoma cells |
|
|
| CAV1 | miR-203–3p [611]; miR-124-3p in bladder cancer cells [692] |
|
|
| WNT5A | miR-374a [696] |
|
|
| VEGFA | miR-20a [703]; miR-20b[703]; miR-205 [704]; miR-185 [705]; miR-126 [706]; miR-140–5p [707] |
|
|
| YWHAG | miR-181b-3p [713] | YWHAG is 14-3-3γ protein that mediates cell signaling and promotes BCa cell motility [714] | |
| TWIST1 | miR-580 [716]; miR-300, miR-539, and miR-543 [717]; miR-720 [718]; miR-33b [282]; miR-151–3p [719]; miR-34a [343]; miR-20a [720]; miR-490–3p which is sponged by lncRNA TP730 AS1 [721] |
|
|
| SNAI1 | miR-203 [726]; miR-129–5p [727]; miR-182 [138] |
|
|
| SNAI2 | miR-30a [730]; miR-497 [731]; miR-124 [732]; miR-452 [709] |
|
|
| MUC1 | miR-125b [735]; miR-145 [360]; miR-145 is sponged by LINC-ROR that is upregulated in TNBC cells and tumors [736]; miR-1226 [737]; miR-1226–3p is sponged by lncRNA MIR210HG [738]. |
|
|
| ZEB1 | miR-141–3p [163,164]; miR-200a [241]; miR-200 family including miR-141 and miR-429 [455,376] [740]; miR-205 [164]; miR-652–3p [741]; miR-101 [567]; miR-455–3p [380] | ||
| ZEB2 | 141–3p [163,164]; miR-200a [241]; miR-200 family-including miR-141 and miR-429 [163] [455,740] [376]; miR-155–5p [747]; miR-101 [567]; miR-124 [748]; miR-30a-5p and miR-30a-3p [749] |
Although EMT is a characteristic of metastatic cells, EMT is not essential for metastasis in every tumor type [87]. EMT is characterized by changes in cell morphology from epithelial, squamous, columnar, cuboidal shapes to fibroblastic-spindle-like elongated cells due to loss of epithelial cell-cell junctions, loss of cell apical-basal polarity, and gain of motility [102]. Activation of EMT also activates MMPs that degrade the extracellular matrix and promote cell invasion.
The role for ncRNA in regulating EMT has been reviewed [233–235]. The EMT Gene database dbEMT 2.0 [236] lists 371 genes that have either oncogenic or tumor suppressor functions in the EMT process. Fig. 3 summarizes aspects of TGFβ and NOTCH signaling, the EMT transition, and roles for miRNA regulation of selected gene/protein targets in this pathway in breast cancer. Cell-to-cell signaling via the NOTCH signaling cascade has been reviewed [237]. Components of NOTCH signaling and downstream targets are upregulated in breast cancer and stimulate EMT (reviewed in [238]). Table 3 lists selected genes from the EMT gene database, their direct miRNA regulation in breast and selected other cancers (if no established role in breast cancer was found in the literature), their roles and regulation in breast cancer, and expression and prognostic value in human breast tumors. This list is not comprehensive since there are a number of other reviews on this topic, as indicated above.
Figure 3: TGFβ stimulation of EMT, angiogenesis, and stemness in breast cancer progression toward metastasis.
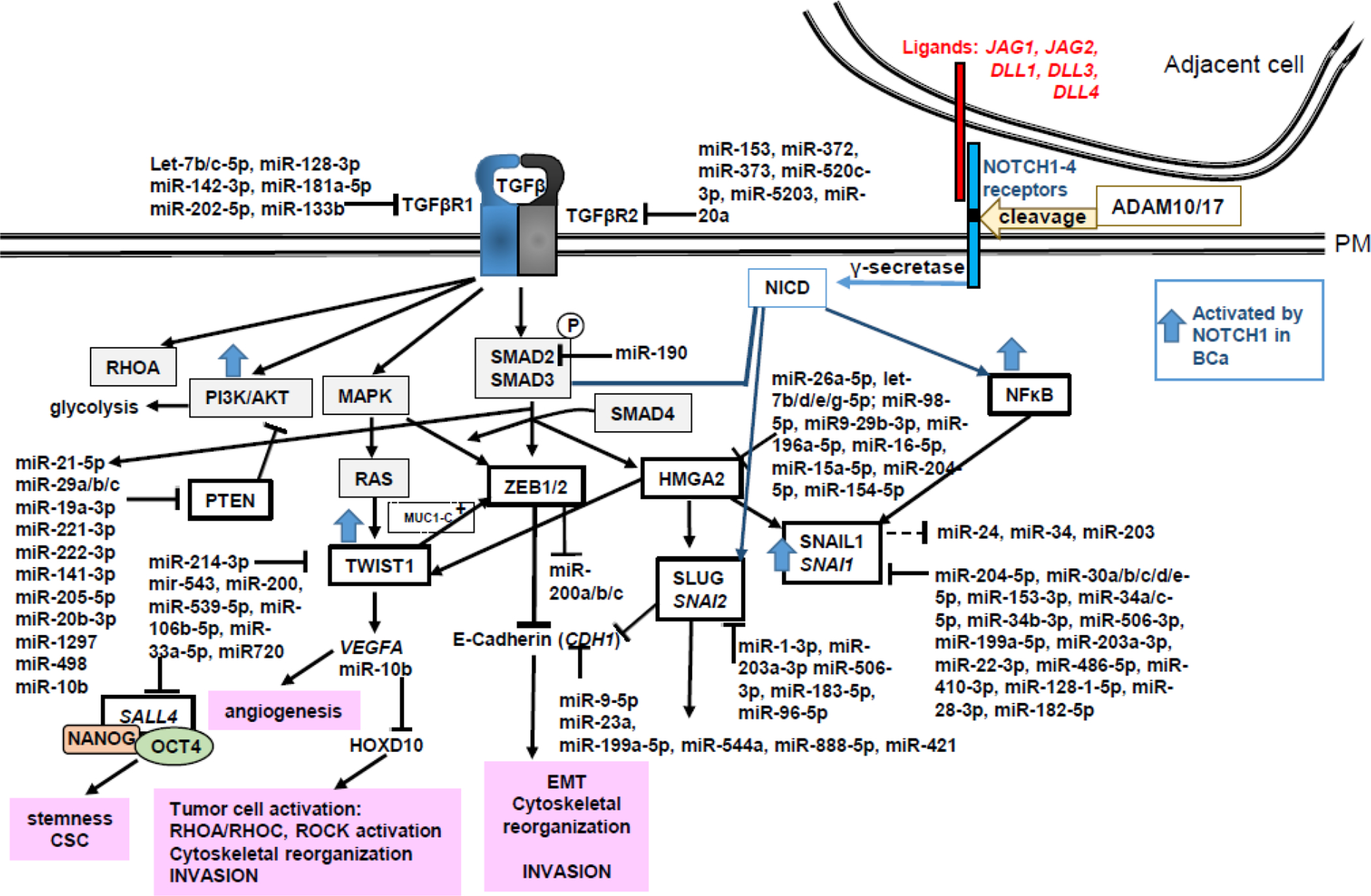
TGFβ binds TGFβ type I and II receptors and phosphorylates SMAD2 and SMAD3. SMADs 2/3 form a complex with SMAD4, translocate into the nucleus to bind HMGA2, a chromatin remodeling factor, and activate the transcription of SNAI1, SNAI2, ZEB1/2 and TWIST1. These transcription factors suppress CDH1 and increase VEGFA, in addition to regulating other genes in EMT (not shown). Canonical NOTCH signaling is initiated by interaction of ligands from one cell, e.g., Jagged (JAG1, JAG2), Delta-like (DLL1,2,3,4) and cognate NOTCH (NOTCH1,2,3,4,5) receptors on adjacent cells. The interaction activates cleavage of NOTCH receptor(s) by ADAM10 or ADAM17. γ-secretase cleavage releases NOTCH intracellular domain (NICD) that goes to the nucleus and interacts with RBPJ and MAML1 (Mastermind Like Transcriptional Coactivator 1) as a coactivator to stimulate gene transcription, including by interaction with NFκB. NICD also interacts with SMAD3 to regulate gene transcription. The indicated miRNAs and targets in this pathway are summarized in Tables 1–3.
EPH Receptor A2 (EPHA2) is an oncogenic cell-surface RTK located at sites of cell-cell contact that is regulated by AKT phosphorylation in the absence of ligand, resulting in enhanced pro-oncogenic signaling [239]. EFNA1 is a ligand for EPHA2 that inhibits cell proliferation and mammary tumor growth in HER2/Neu animal models and is downregulated in breast tumors leading to ligand-independent activation and tumor progression by activing glutaminolysis and lipid metabolism [240]. miR-200a targets EPHA1 [241].
ETS2 is an oncogenic transcription factor that is downstream of MAPK and PI3K/AKT pathways and regulates genes involved in apoptosis, cell cycle, and tumor progression [242]. ETS2 was reported to be a direct effector of CSF-1 signaling in TAMs that decreased primary and tail-vein injection lung “metastases” of BCa cell line growth in mice [243]. Activation of the CSF1-ETS2 pathway in TAMs represses TIMP3 and increases miR-21 and miR-29a that stimulates metastatic tumor growth and angiogenesis in mouse models of BCa [244]. Direct interaction between coactivator SRC-1 (NCOA1) and ETS2 increased expression of MYC and MMP2 in aromatase inhibitor (AI)-resistant MCF-7 cells [245]. Although miR-320 and miR-320b target ETS2 (Table 3), there were no reports of lower expression of these miRs in endocrine-resistant breast cancer [10,11]. An interesting observation is that higher nuclear phospho-ETS2 was detected in patients with AI-resistant lung metastasis (n =3) compared to matched primary breast tumor [245]. One might speculate that miR-320 might be a therapeutic in AI-resistant metastatic breast cancer and design appropriate experiments to test this hypothesis.
EZH2 is the catalytically active methyltransferase component of the polycomb repressive complex (PRC2) that represses gene transcription via methylation of H3K27me3 [246]. EZH2 was reported to interact with 276 chromatin-interacting lncRNAs including MEG3, which downregulated genes in the TGFβ pathway in BT-549 BCa cells [247]. EZH2 expression is increased in breast cancer (Table 3). EZH2 activates RAF1-β-catenin signaling to promote CSCs in BCa [248]. EZH2 activates NOTCH1 transcription in BCa cells and increases CSC [249]. EZH2 is induced by hypoxia in TNBC cells and high HIF1A, EXH2, and FOXM1 expression correlates with OS in BCa patients [250].
FOXO1 is a member of the Forkhead transcription factor gene family containing a 110 aa conserved DNA binding motif [251]. FOX family members regulate embryogenesis, organogenesis, metabolism, and are involved in cancer [251]. FOXO1 is a tumor suppressor whose expression is lower in breast tumors than normal breast [252]. FOXO1 suppressed β-catenin expression and nuclear localization, thus downregulating WNT and JUN signaling, cancer stemness, and metastasis in xenograft models of MCF-7 and MDA-MB-231 [253]. FOXO1 is downregulated by a number of miRNAs (Table 3) including miR-5188 which is elevated in BCa and predicts reduced DFS [253]. Overexpression of miR-5188 enhances tumorigenesis, CSC, metastasis, and chemoresistance in vivo in MCF-7 and MDA-MB-231 xenografts [253]. Interestingly, FOXO1’s 3’UTR acts as a competitive endogenous RNA (ceRNA) for miR-9 binding to the CDH1 3’UTR, thus increasing E-cadherin and inhibiting EMT in BCa cells [254].
RHOA is a GTP binding protein that activates cytoskeletal reorganization and stimulates BCa cell invasion [255]. miR-155 is an oncomiR that facilitates EMT through repressing RHOA expression (125). RHOA is also downregulated by other miRNAs in BCa, including miR-150 and miR-155 (Table 2). Expression of miR-150 and miR-155 was higher in invasive breast cancer (IBC) and lympho-vascular invasion (LVI) compared to DCIS in microdissected tumors and corresponded with reduced RHOA expression [119]. RHOA is targeted by miR-146a. miR-155 (Table 3) which are in a network of miRNAs, including miR-125b, miR-21, and miR-27a, that contribute to antiestrogen resistance [256]and reviewed in [10].
SKP2 (S-phase kinase-associated protein) is a component of the Skp1-Cul1-Roc1 (SCF) ubiquitin ligase complex [257]. SKP2 is an oncogene that specifically recognizes phosphorylated cell cycle regulator proteins, e.g., PDCD4 [258] and p27Kip1 (CDKN1B) [259], and mediates their ubiquitination and degradation, resulting in increased cell cycle progression and proliferation. SKP2 ubiquitylates AKT, resulting in its activation - independent of PI3K, providing a mechanism for resistance to PI3K inhibitors [260]; however, how SKP2 is increased in PI3K resistance is unknown [261]. High SKP2 was associated with increased risk of distant recurrence in breast cancer patients treated with radiation therapy [262] (Table 3), but no miRNAs have been reported to target SKP2 in breast cancer. Interestingly, miR-30d-5p was reported to target SKP2 in lung cancer [263] (Table 3) and miR-30d (as well as miR-30a/b/c/e) were identified as suppressors of breast cancer bone metastasis whose expression was downregulated in human osteotropic BCa cell lines [264].
Kruppel-like factor 4 (KLF4) is a zinc-finger transcription factor that has both oncogenic and tumor suppressor functions and inhibits ERα-DNA binding by direct interaction with the DBD [265]. KLF3 is upregulated by MYB in luminal breast cancer [266]. KLF4 is highly expressed in CSC-enriched populations of MCF-7 and MDA-MB-231 breast cancer cells [267]. KLF4 is downregulated by CpG island methylation in anti-estrogen resistant LCC9 BCa cells [268]. KLF4 is downregulated by miR-29a/b/c and miR-10b-5 (Tables 1 and 3). miR-10b was the top miRNA identified in exosomes isolated from MDA-MB-231 TNBC cell culture medium [269]. Treatment of miR-10b non-expressing normal human mammary epithelial cells (HMLE) with exosomes from MDA-MB-231 cells increased HMLE miR-10b expression ~ 6-fold and reduced KLF4 and HOXD protein expression (both mIR-10b targets) and increased cell invasion [269].
KLF5 appears to be oncogenic in breast tumors (Table 3). Metformin was reported to target KLF5 for degradation in TNBC cells, thus decreasing CSC [270]. KLF5 increased lncRNA RP1 −5O6.5 that repressed p27Kip1 (CDKN1B) translation in TNBC cells [271]. KLF5 is targeted by miR-590–5p [272] in breast cancer cells.
STAT3 is a transcription factor activated by phosphorylation downstream of TGFβ, IL-6, WNT, NOTCH, and Hedgehog (HH) signaling pathways [273]. More recent studies showed the leptin stimulated STAT3 activation, suggesting a pathway by which obesity stimulates breast cancer [274]. Cell stress activates p38 MAPK that phosphorylates EGFR leading to its internalization in endosomes which leads to STAT3 phosphorylation and triggers a TWIST1-dependent EMT transcription program [275]. STAT3 is targeted by miR-519d, which is reduced in breast tumors [276]. Phospho-STAT3 increased the transcription of MCL1 and BIRC4 [277]. STAT3 is constitutively active in TNBC and ChIP-seq identified 22 common transcripts in 5 TNBC cell lines that included upregulation of ANKRD2, BEAN1, CPA3, and GDF15 and downregulation of STAT3, NNMT, CPT1C, and C4A expression [278]. Activation of STAT3 in TNBC cells stimulates cell processes including actin cytoskeleton, adherens junction, extracellular vesicular exosome, basement membrane, and stress fibers [278].
SALL4 is a zinc finger-containing transcription factor that increases transcription of CCND1, CCND2, TWIST1, BMI1, and represses CDH1 to promote EMT (477). SALL4 is directly activated by TCF/LEF in the canonical WNT signaling pathway [279]. SALL4 interacts with OCT4 and NANOG [280] and enhances stemness and CSC in TNBC cell lines [281]. This is modeled in Fig. 3. miR-33b directly suppresses SALL4 translation and miR-33b expression is downregulated in breast tumors and is inversely correlated with lymph node metastatic status [282], providing a mechanism for upregulation of SALL4 in breast cancer progression and metastasis. SALL4 is directly activated by TCF/LEF in the canonical WNT signaling pathway [279].
GLI1 is a member of the Kruppel family of zinc-finger transcription factors that is activated by HH signaling [283]. GLI1 directly interacts with STAT3 and stimulates CSC [284]. Results examining GLI1 in breast tumors appear to be divergent in terms of association with luminal status (Table 3). Overall, increased GLI1 is reported in breast tumors. miRNA regulation of GLI1 in breast tumors has not been reported.
SIRT1 (Sirtuin 1) is an NAD+-dependent deacetylase for histones H1, H2, and H4 as well as p53, E2F1 [285], NFκB, selected NRs, e.g., AR, ERα, and LXRα (reviewed in[286]) and FOXO family members [287]. SIRT1 is involved in a range of cellular processes including aging, circadian rhythm, metabolism, and cancer [288]. SIRT1 transcription is induced by E2F1 and repressed by P53 [289]. SIRT1 is downregulated in aggressive breast tumors [290] and in TNBC compared to normal breast tissue [291]. SIRT1 is directly regulated by miR-22, miR-200a, miR-34a, and miR-211–5p (Table 3) and is a putative target of metastamiR miR-520c-3p (Table 1). Low miR-34a and high levels of SIRT1 have been reported in breast CSC (495). SIRT1 seems to have both pro- and anti-metastatic activity in breast cancer depending on the model system used. For example, SIRT1 suppresses EMT in breast cancer by suppressing TGF-β-driven EMT in SV40,h-rasV12-h,TERT immortalized normal human mammary epithelial cells (HMLER cells [292]) [293]. On the other hand, SIRT1 upregulates MMPs in BCa cells and interacts with SMAD proteins downstream of TGFβ signaling [288]. Knockdown of SIRT1, inhibited lysosomal activity and promoted exosome release from MDA-MB-231 TNBCs with the released exosomes promoting proliferation and an invasive phenotype of immortalized MCF-10A breast epithelial cells [291]
CCN2/CTGF is a secreted growth factor that is increased ~ 2-fold by TGFβ signaling in fibroblasts where it simulates angiogenesis, but it inhibits the migration and invasion of ovarian and CRC cancer cells [294]. CTCF is an oncogene in gastric cancer [295], glioma [296] and melanoma, but acts as a tumor suppressor lung adenocarcinoma [297]. Elevated CCN2 (CTGF) was reported in stroma-rich, TNBC tumors with poor clinical prognosis [298,299]. In breast tumors, CCN2 expression was correlated with EMT markers, i.e., increased CDH2, FN1, VIM, GSC, SNAI1, and TWIST1, and with downregulation of CDH1, KRT18, and KRT8 [299]. CTGF acts both as a paracrine factor to stimulate collagen fiber deposition and an autocrine factor by activing TNF receptor (TNFR1, TNFRSF1A) signaling and activating NFκB to increase the transcription of genes that stimulate EMT. Inhibiting miR-221 in MDA-MB-231 cells decreased the expression of CTGF by suppressing the expression of the ubiquitin-editing enzyme, A20, that ubiquitinylates CTGF for proteosomal degradation [300].
TP63 is a member of the p53 TF family with cell-specific oncogenic and tumor suppressor activities [301]. There are 6 protein isoforms of TP63 due to differential promoter use and alternative splicing [302]. The isoform TAp63 provides pro-apoptotic and senescence-inducing properties, whereas ΔNp63 isoforms stimulate cell survival [303]. Increased expression of ΔNp63 was associated with metaplastic and medullary TNBC tumors, and with a basal phenotype, whereas TAp63 was associated with AR, BRCA1/2 wild-type status, PTEN positivity, and with improved OS [303]. miR-196a2* targets TP63 and is upregulated by E2-stimulated ERα-ERK transcriptional activation in BCa cells [304].
Cyclin dependent kinase inhibitor 1 B (CDKN1B) encodes the protein referred to as p27 (p27Kip1). p27Kip1 is a tumor suppressor that inhibits cell cycle progression is regulated by the PTEN/AKT pathway [305]. Low levels of p27Kip1 in breast tumors is associated with lower OS [306]. Mutating the CDKN1B gene in MCF-7 cells results in increased proliferation and re-expressing p27Kip1 inhibits proliferation [307]. miR-222 targets PTEN resulting in increased phospho-AKT and decreased p27Kip1 [308]. Overexpressing miR-203 in MCF-7 cells suppresses p27Kip1 expression, resulting in increased cell growth, migration and invasion [309].
Wilms’ tumor 1 (WT1) is a transcription factor that regulates transcription of numerous human genes involved in WNT signaling, e.g., DKK2, JUN, and DACT1; cell growth, e.g., AREG, CX3CL1, EREG, VDR, IGF1, CCNE, PDGF1, SLC6A2, and MAPK signaling, e.g., DUSP16 and MAPKAPK2; epigenetic regulation, e.g. DNMT3A and SRPK1; and Apoptosis, e.g., BCL2A1, BCL2, and JUN [310]. WT1 inhibits transcription of STIM1 (Stromal Interaction Molecule 1) that triggers store-operated calcium channels to increase Ca+ entry which stimulates BCa cell progression and EMT [311]. WT1 interacts directly with ERα to suppress IGF-I receptor (IGR1R) transcription in BCa cells [312]. WT1 is a target of miR-193a in BCa [313] and miR-193a-5p was decreased BCa [314]. Accordingly, WT1 expression is increased in breast tumors (Table 3).
As described above, the loss of CDH1 is a marker of EMT. Reduced CDH1 expression in breast tumors is a poor prognostic marker (Table 3). Patients whose primary breast tumors showed loss of E-cadherin had increased CTCs with a mesenchymal phenotype and LN metastasis with increased expression of TWIST1, SNAI1 (SNAIL), and SNAI2 (SLUG) accompanied by decreased Ki67 labeling index [315]. CDH1 transcription is stimulated by EP300, FOXA1/2, and RUNX1 [316] and repressed by ZEB1/2 [317]. CDH1 is targeted by miR-9–5p [124], miR-199a-5p [318], miR-544a [319], miR-888–5p [320], and miR-421 [321].
Another marker of EMT is increased Vimentin (VIM) [322,323] and VIM promotes cell migration [324]. In breast cancer, VIM is targeted by miR-124 [325], miR-17–3p [326], and miR-378g [327] (Table 3).
Transforming Growth Factor Beta 1 (TGFβ1) is a secreted protein that binds TGFβ receptor type II (TβRII) receptor which then heterodimerizes with TGβRI to activate SMAD family transcription factors to regulate development and homeostasis [328]. TGFβ signaling in EMT is modeled in Fig. 3. TGFB1 transcript was upregulated and associated with low recurrence score in breast tumors, e.g., higher in non-metaplastic TNBC [329]. TGFB1 is an inhibitor of proliferation of primary HMECs and many breast cancer cell lines - thus acting as a tumor suppressor [330]. TGFB1 is a target of miR-675 [331] and represses miR-29b/c expression [332–334]. The repression of miR-29b/c may play a role in breast cancer progression since miR-29 has tumor suppressor targets (Table 1), although miR-29 has oncomiR targets as well (reviewed in [335,154]).
Sprouty (SPRY2) is a negative regulator in the EGF and FGF signaling that is a tumor suppressor downregulated in breast tumors and associated with poor RFS and OS (Table 3). In TNBC, increased EGF induced the expression of c-MYC, which increased the expression of mature miR-23a, miR-24–2, and miR-27a that decreased the expression of SPRY2; and promoted cell migration and invasion through activation of p44/42 MAPK [336]. SPRY2 is targeted by miR-27a/b, miR-23a, miR-24–2 [337]; and miR-128a (632) (Table 3).
NOTCH signaling promotes the self-renewal of CSC in various cancers and participates in tumor-stroma and tumor-endothelium interactions in CSC niches in primary and metastatic tumors [338,339]. Low NOTCH1 in breast tumors correlates lower OS [340] and high levels of EZH2 were associated with activated NOTCH1 protein and increased tumor initiating cells (TICs) in TNBC invasive carcinomas [249]. EZH2 increases NOTCH1 transcription and CSC in TNBC [249]. NOTCH1 is upregulated by EGF and ERBB3 in ERBB2/HER2+ BCa cells [341]. IL6 and hypoxia increase NOTCH1 transcription in MCF-7 cells in a C/EBPδ (CEBPD)-dependent manner [342]. NOTCH1 is targeted by miR-34a-5p [343]; miR-30a [344]; miR-139–5p [345]; miR-3178 [346] in breast cancer. The miR-106b-25 cluster represses the E3-ubiquitin ligase NEDD4L, thus increasing NOTCH1 in ERα+ and TNBC cells [347].
Mucin1 (MUC1) is a high MW, PM-bound, heavily O-glycosylated protein that has a single transmembrane domain and a 72 aa C-terminal domain (CD) that is cleaved forming an intracellular C-terminal domain (MUC1-C) that is regulated by tyrosine phosphorylation [348]. The N-terminal domain (NTD) of MUC1 protrudes from the apical surface of glandular epithelial cells in breast and other cell types [349]. Studies (at least 55 papers in PubMed) from the Kufe lab have identified roles for MUC1 and MUC1-C in EMT and CSC and as a target for breast cancer treatment [350,351]. The cellular distribution of the MUC1-C subunit (C-terminal) is dysregulated in cancer with a loss of cell polarity and a distribution of MUC1-C over the entire surface of the breast cancer cell where it interacts with EGFR and HER2 [352]. MUC1-C interacts with NFκB p65, acting as a coactivator to increase ZEB1 transcription [353,354]. MUC1-C interacts with other TFs including C/EBPβ [355] and ERα [356] as a coactivator. MUC1-C also interacts with EZH2 to decrease gene-specific H3K27me3 and repress transcription of CDH1 and BRCA1 in BT-549 and MDA-MB-468 TNBC cells [357]. MUC1-C interacts with STAT3 to increase TWIST1 transcription and with TWIST1 protein to increase transcription of EMT and CLC stemness genes, e.g., ZEB1, SNAI1, SOX2, BMI1, ALDH1, and CD44 [358]. MUC1-C interacts with MYC to drive stemness and with components of the NuRD complex on the ESR1 promoter to suppress transcription in TNBC cells [359]. miRNAs that regulate MUC1 are listed in Table 3. miR-145 is a bona fide regulator of MUC1 with reduced expression in breast cancer cells corresponding to increased MUC1 [360].
7. TGFβ signaling in breast cancer and metastasis
TGFβ has tumor suppressor roles in normal breast epithelial cells by activating cyclin-dependent kinase (CDK) inhibitors, e.g., CDKN2B (P15 CDK inhibitor) and CDKN1A (P21, in normal and pre-malignant cells, TGF-β inhibits proliferation [361]. However, after epigenetic and genetic changes occur that convert normal ductal epithelial cells to primary breast cancer cells, TGFβ takes on an oncogenic role driving EMT, motility, invasion, and metastasis (reviewed in [362]). Higher levels of TGFβ in serum of patients with advanced breast cancer is a prognostic indicator of cancer progression associated with lower OS [363].
Fig. 3 summarizes selected aspects of TGFβ signaling in breast cancer and EMT for metastasis. The 33 members of the TGFβ family of cytokines in humans includes TGFβ1 (TGFB1) that binds TGFβ type I and II receptors (TGFBR1, TGFBR2) which are ser/thr kinases that act as heterodimers to initiate an intracellular signaling pathway of SMAD intracellular effectors. TGFB1R1 transcript expression is higher in breast tumors compared to normal breast and is inversely correlated with miR-133b expression [364]. 3’UTR-luciferase reporter assays demonstrated that miR-133b directly targets TFGB1R1 in vitro [364]. miR-133b is downregulated in MCF-7, MDA-MB-231, MDA-MB-453, and MDA-MB-469 cells relative to MCF-10A cells and miR-133b was lower in breast tumors compared to normal breast tissue [364]. Knockdown of TGFB1R1 in MCF-7 and MDA-MB-231 cells inhibited phosphorylation of SMAD3, increased CDH1, and suppressed cell invasion and migration in vitro [364]. TGFB1R2 expression is also higher in breast tumors versus normal breast tissues and its expression is inversely correlated with miR-153 that is downregulated in breast tumors [365]. TFGBR2 3’UTR-luciferase reporter assays validated TFGB1R2 as a direct target of miR-153 [365]. Transient overexpression of miR-153 in MDA-MB-231 cells inhibited cell migration and invasion, increased protein levels of CDH1 (E-cadherin) and VIM (vimentin) [365]. TGFBR2 and RELA are directly targeted by miR-372, miR-373, miR-520c, and miR-520e, all of which show reduced expression in MDA-MB-231 TNBC cells and ERα− breast tumors [366].
TGFBR1 phosphorylates regulatory SMADs, SMAD2 and SMAD3, that form heterodimeric complexes with each other and SMAD4, translocate to the nucleus, bind DNA, recruit coactivators, and stimulate transcription of genes, e.g., HMGA2, DNMT1, SNAI1, SNAI2) ZEB1, ZEB2, TWIST1, and a number of miRs, including miR-155 [367,368] (Fig. 3). SMAD2 is a bona fide target of miR-190 that is decreased in breast tumors [369]. Cell-based studies demonstrated that transiently overexpressed ZEB1, TWIST1, and SNAI1/2 bind the miR-190 promoter to suppress its transcription in MCF-10A cells and overexpression of miR-190 decreased SMAD2 protein levels and inhibited TGFβ-induced MCF-10A migration in vitro [369].
HMGA2 binds AT-rich DNA sequences and is a positive effector to increase transcription of SNAI1, SNAI2, and TWIST1 [370]. TGFβ also activates non-SMAD pathways including the PI3K/AKT, MAPK, JNK, and P38 MAPK pathways [368]. Although the three isoforms of AKT (AKT1, AKT2, and AKT3) have ~ 80% similarity, AKT1 is the most mutated form and AKT2 is amplified in breast tumors and promotes migration and invasion by regulating β-integrins, EMT-related proteins and F-actin [371]. Integrins are a family of transmembrane cell adhesion receptors composed of 18 α and 8 β subunits that mediate cellular cytoskeletal interaction with the ECM [372].
SNAIL (SNAI1) and SLUG (SNAI2) are related C2H2 zinc finger transcriptional repressors that share structural homology, including an N-terminal SNAG (Snai.Gfi1) domain for nuclear localization and repression, and both bind E-box DNA sequences [373]. They have non-overlapping cellular functions in mammary gland development and in breast cancer progression in regulation of EMT and stemness (CSC) [373]. Increased expression of SNAI1/SNAI2 can induce resistance to endocrine therapies in breast cancer cells [374] with findings confirmed for increased SNAI2 in a separate cell-based study and in primary breast tumors and metastases [375].
ZEB1 and ZEB2 are Zinc Finger E-box-binding homeobox transcription factors overexpressed in breast tumors and in the serum of BCa patients compared to healthy controls (Table 2). The expression of ZEB1 and ZEB2 is induced by signaling pathways activated in breast tumor progression, e.g., NFκB, TGFβ, WNT-β-catenin, and RAS-MAPK g [165] and induce EMT by suppression of targets including CDH1 and miR-200 family members [230] [317,376–379]. ZEB1 and ZEB2 are downregulated by common and specific miRNAs (Table 3). GATA3 inhibits TGFβ-induced EMT in breast cancer cells by increasing the transcription of miR-455–3p which directly targets ZEB1, SMAD2, and HDAC2 in MCF-7 and MCF-10A cells [380]. ZEB1 reciprocally repressed miR-455 transcription in MCF-7 cells [380]. ZEB1 expression is increased and miR-200abc decreased in TAM-resistant LCC9 and LY2 breast cancer cells [381]. There is an inverse correlation between miR-200 family members and ZEB1 expression in TNBC cells, i.e., MDA-MB-231 and BT549 [163,382,383,164]. Studies in TNBC cells show that treatment with DNA methyltransferase inhibitors (DNMTi, e.g., 5-Azacytidine and Decitabine) and HDAC inhibitors (HDACi, e.g., Vorinostat and Entinostat) can reprogram the cells to a less aggressive phenotype, e.g., MET, by suppressing ZEB1, EZH2, and mutant p53 while increasing CDH1 expression [384]. Entinostat is in Phase I/II clinical trials for TNBC treatment [385].
8. Conclusions
Despite advances in treatment for breast cancer, the survival rate for patients with distant metastasis is only 27% [386]. Thus, a better understanding of dysregulated signaling pathways underlying metastasis is clearly needed. Proteins in the miRNA biogenesis pathway are targets of miRNAs that are dysregulated in breast cancer and contribute to metastatic pathways (Fig. 1). In advanced stage breast cancer, pro-metastatic miRNAs are reported to have increased expression, resulting in decreased expression of their tumor-suppressing targets. I n contrast, miRs that have anti-metastatic activity by targeting oncogenes have decreased expression in more advanced stage breast tumors. Recent research focuses on miRNA participation in crosstalk between signaling pathways that contribute to tumor progression and metastasis when dysregulated, e.g., TGFβ/SMAD/EMT, WNT-β-catenin pathway and CSC pathways. These studies are beginning to elucidate the mechanisms and effects of aberrant miRNA expression, but indicate a need for further investigation.
We reviewed the identity, regulation, human breast tumor expression, and reported prognostic significance of miRNAs that have been documented to directly target key genes in pathways contributing to the metastatic cascade. We critically evaluated the evidence for metastamiRs and their targets in breast cancer. Not all of the roles identified for specific metastamiRs was supported by recent reports as summarized in the text and in Table 1. We evaluated the expression and miRNA regulation of metastasis suppressor genes in breast cancer (Table 2) and identified the need for further studies on specific miRNAs, e.g., miR-31–5p and miR-19a/b in metastatic spread. We reviewed the miRNA regulation of EMT-related genes and their expression and prognostic value in breast cancer (Table 3). Additional miRNAs target proteins involved in TGFβ signaling, a central inducer of EMT, a hallmark of metastasis, were reviewed (Fig. 3, Table 3).
Although the literature on the topic of breast cancer metastasis and miRNAs is vast, our understanding of the complexities of these interactions remains to be resolved and computational modeling of these interactions will provide insight into potential ‘high yield’ targets for further study. Identification and validation of miRNAs that regulate the protein, and RNA levels, of genes that act as metastamiRs, metastasis suppressors, or in pathways including TGFβ signaling, and EMT is incomplete. Finally, the studies reviewed here suggest that miRNAs that are dysregulated in breast tumors or patient sera should be explored as novel diagnostic and potential targets for therapy to reduce metastatic disease and thus suffering and mortality in breast cancer patients..
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare that they have no competing interests.
REFERENCES
- 1.Vieira AF, & Schmitt F (2018). An Update on Breast Cancer Multigene Prognostic Tests—Emergent Clinical Biomarkers. [Review]. Front Med (Lausanne), 5(248), doi: 10.3389/fmed.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waks AG, & Winer EP (2019). Breast Cancer Treatment: A Review. JAMA, 321(3), 288–300, doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.van Hellemond IEG, Geurts SME, & Tjan-Heijnen VCG (2018). Current Status of Extended Adjuvant Endocrine Therapy in Early Stage Breast Cancer. Current Treatment Options in Oncology, 19(5), 26, doi: 10.1016/j.ctrv.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nourmoussavi M, Pansegrau G, Popesku J, Hammond GL, Kwon JS, & Carey MS (2017). Ovarian ablation for premenopausal breast cancer: A review of treatment considerations and the impact of premature menopause. Cancer Treatment Reviews, 55, 26–35, doi: 10.1016/j.ctrv.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 5.Mathew A, & Davidson NE (2015). Adjuvant endocrine therapy for premenopausal women with hormone-responsive breast cancer. The Breast, 24, S120–S125, doi: 10.1016/j.breast.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. (2016). Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. Journal of Clinical Oncology, 34(14), 1689–1701, doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 7.Steeg PS (2016). Targeting metastasis. [Review Article]. Nature Reviews Cancer, 16, 201, doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke R, Tyson JJ, & Dixon JM (2015). Endocrine resistance in breast cancer - An overview and update. Molecular and Cellular Endocrinology, 418, Part 3, 220–234, doi: 10.1016/j.mce.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignatov A, Ignatov T, Roessner A, Costa S, & Kalinski T (2010). Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Research and Treatment, 123(1), 87–96, doi: 10.1007/s10549-009-0624-6. [DOI] [PubMed] [Google Scholar]
- 10.Muluhngwi P, & Klinge CM (2015). Roles for miRNAs in endocrine resistance in breast cancer. Endocrine-Related Cancer, 22(5), R279–R300, doi: 10.1530/erc-15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muluhngwi P, & Klinge CM (2017). Identification of miRNAs as biomarkers for acquired endocrine resistance in breast cancer. Molecular and Cellular Endocrinology, 456, 76–86, doi: 10.1016/j.mce.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Jeselsohn R, De Angelis C, Brown M, & Schiff R (2017). The Evolving Role of the Estrogen Receptor Mutations in Endocrine Therapy-Resistant Breast Cancer. Current Oncology Reports, 19(5), 35, doi: 10.1007/s11912-017-0591-8. [DOI] [PubMed] [Google Scholar]
- 13.Toy W, Weir H, Razavi P, Lawson M, Goeppert AU, Mazzola AM, et al. (2017). Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov, 7(3), 277–287, doi: 10.1158/2159-8290.cd-15-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandarlapaty S, Chen D, He W, & et al. (2016). Prevalence of ESR1 mutations in cell-free dna and outcomes in metastatic breast cancer: A secondary analysis of the bolero-2 clinical trial. JAMA Oncology, 2, 1310–1315, doi: 10.1001/jamaoncol.2016.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE, et al. (2020). ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nature Genetics, doi: 10.1038/s41588-019-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BenAyed-Guerfali D, Dabbèche-Bouricha E, Ayadi W, Trifa F, Charfi S, Khabir A, et al. (2019). Association of FOXA1 and EMT markers (Twist1 and E-cadherin) in breast cancer. Molecular Biology Reports, 46(3), 3247–3255, doi: 10.1007/s11033-019-04784-w. [DOI] [PubMed] [Google Scholar]
- 17.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. (2016). Palbociclib and Letrozole in Advanced Breast Cancer. New England Journal of Medicine, 375(20), 1925–1936, doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 18.Ayyagari R, Tang D, Patterson-Lomba O, Zhou Z, Xie J, Chandiwana D, et al. (2018). Progression-free Survival With First-line Endocrine-based Therapies Among Postmenopausal Women With HR+/HER2− Metastatic Breast Cancer: A Network Meta-analysis. Clinical Therapeutics, 40(4), 628–639.e623, doi: 10.1016/j.clinthera.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Kornblum N, Zhao F, Manola J, Klein P, Ramaswamy B, Brufsky A, et al. (2018). Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. Journal of Clinical Oncology, JCO.2017.2076.9331, doi: 10.1200/JCO.2017.76.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo HS, Turner NC, Finn RS, Joy AA, Verma S, Harbeck N, et al. (2018). Palbociclib plus endocrine therapy in older women with HR+/HER2− advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. European Journal of Cancer, 101, 123–133, doi: 10.1016/j.ejca.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T, et al. (2019). Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol, 20(10), 1360–1369, doi: 10.1016/s1470-2045(19)30420-6. [DOI] [PubMed] [Google Scholar]
- 22.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. (2019). Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. New England Journal of Medicine, 380(20), 1929–1940, doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 23.Markham A (2019). Alpelisib: First Global Approval. Drugs, 79(11), 1249–1253, doi: 10.1007/s40265-019-01161-6. [DOI] [PubMed] [Google Scholar]
- 24.O’Shaughnessy J, Thaddeus Beck J, & Royce M (2018). Everolimus-based combination therapies for HR+, HER2− metastatic breast cancer. Cancer Treatment Reviews, 69, 204–214, doi: 10.1016/j.ctrv.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Schmid P, Zaiss M, Harper-Wynne C, Ferreira M, Dubey S, Chan S, et al. (2019). Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women With Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol, doi: 10.1001/jamaoncol.2019.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinge CM (2018). Non-Coding RNAs in Breast Cancer: Intracellular and Intercellular Communication. Noncoding RNA, 4(4), 40, doi: 10.3390/ncrna4040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klinge CM (2018). Non-coding RNAs: long non-coding RNAs and microRNAs in endocrine-related cancers. Endocrine-Related Cancer, 25(4), R259–R282, doi: 10.1530/ERC-17-0548. [DOI] [PubMed] [Google Scholar]
- 28.Cech TR, & Steitz JA (2014). The Noncoding RNA Revolution—Trashing Old Rules to Forge New Ones. Cell, 157(1), 77–94, doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Meldolesi J (2018). Exosomes and Ectosomes in Intercellular Communication. Current Biology, 28(8), R435–R444, doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, et al. (2018). Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer, 17(1), 82, doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madak-Erdogan Z, Band S, Zhao YC, Smith BP, Kulkoyluoglu-Cotul E, Zuo Q, et al. (2019). Free Fatty Acids Rewire Cancer Metabolism in Obesity-Associated Breast Cancer via Estrogen Receptor and mTOR Signaling. Cancer Research, 79(10), 2494, doi: 10.1158/0008-5472.CAN-18-2849. [DOI] [PubMed] [Google Scholar]
- 32.Walsh HR, Cruickshank BM, Brown JM, & Marcato P (2019). The Flick of a Switch: Conferring Survival Advantage to Breast Cancer Stem Cells Through Metabolic Plasticity. Front Oncol, 9, 753, doi: 10.3389/fonc.2019.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.San-Millán I, Julian CG, Matarazzo C, Martinez J, & Brooks GA (2020). Is Lactate an Oncometabolite? Evidence Supporting a Role for Lactate in the Regulation of Transcriptional Activity of Cancer-Related Genes in MCF7 Breast Cancer Cells. [Original Research]. Front Oncol, 9(1536), doi: 10.3389/fonc.2019.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blücher C, & Stadler SC (2017). Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. [Mini Review]. Frontiers in Endocrinology, 8(293), doi: 10.3389/fendo.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozomara A, & Griffiths-Jones S (2014). miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res, 42(Database issue), D68–73, doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, et al. (2019). An estimate of the total number of true human miRNAs. Nucleic Acids Research, 47(7), 3353–3364, doi: 10.1093/nar/gkz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, & Lin H (2014). Posttranscriptional Regulation of Gene Expression by Piwi Proteins and piRNAs. Molecular Cell, 56(1), 18–27, doi: 10.1016/j.molcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman RC, Farh KK, Burge CB, & Bartel DP (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Research, 19(1), 92–105, doi: 10.1101/gr.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drusco A, & Croce CM (2017). Chapter One - MicroRNAs and Cancer: A Long Story for Short RNAs In Croce CM, & Fisher PB (Eds.), Advances in Cancer Research (Vol. 135, pp. 1–24). Cambridge, MA, USA: Academic Press. [DOI] [PubMed] [Google Scholar]
- 40.Saini HK, Griffiths-Jones S, & Enright AJ (2007). Genomic analysis of human microRNA transcripts. Proceedings of the National Academy of Sciences, 104(45), 17719–17724, doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daugaard I, & Hansen TB (2017). Biogenesis and Function of Ago-Associated RNAs. Trends in Genetics, 33(3), 208–219, doi: 10.1016/j.tig.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Klinge CM (2015). miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Molecular and Cellular Endocrinology, 418, 273–297, doi: 10.1016/j.mce.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng P, Fields C, Aadland K, Wei T, Kolaczkowski O, Gu T, et al. (2018). Dicer cleaves 5′-extended microRNA precursors originating from RNA polymerase II transcription start sites. Nucleic Acids Research, 46(11), 5737–5752, doi: 10.1093/nar/gky306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S, Greco D, Michailidou K, Milne RL, Muranen TA, Heikkinen T, et al. (2014). MicroRNA Related Polymorphisms and Breast Cancer Risk. PLoS ONE, 9(11), e109973, doi: 10.1371/journal.pone.0109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hata A, & Kashima R (2016). Dysregulation of microRNA biogenesis machinery in cancer. Critical Reviews in Biochemistry and Molecular Biology, 51(3), 121–134, doi: 10.3109/10409238.2015.1117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alarcón Claudio R., Goodarzi H, Lee H, Liu X, Tavazoie S, & Tavazoie Sohail F. (2015). HNRNPA2B1 Is a Mediator of m6A-Dependent Nuclear RNA Processing Events. Cell, 162(6), 1299–1308, doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alarcón CR, Lee H, Goodarzi H, Halberg N, & Tavazoie SF (2015). N6-methyladenosine marks primary microRNAs for processing. [Letter]. Nature, 519(7544), 482–485, doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hock J, & Meister G (2008). The Argonaute protein family. Genome Biol, 9(2), 210, doi: 10.1186/gb-2008-9-2-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitchiaya S, Heinicke LA, Park JI, Cameron EL, & Walter NG (2017). Resolving Subcellular miRNA Trafficking and Turnover at Single-Molecule Resolution. Cell Reports, 19(3), 630–642, doi: 10.1016/j.celrep.2017.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi H, & Tomari Y (2016). RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1859(1), 71–81, doi: 10.1016/j.bbagrm.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Kourtidis A, & Anastasiadis PZ (2018). Close encounters of the RNAi kind: the silencing life of the adherens junctions. Current Opinion in Cell Biology, 54, 30–36, doi: 10.1016/j.ceb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer KD, & Jaffrey SR (2014). The dynamic epitranscriptome: N6-methyladenosine and gene expression control. [Review]. Nat Rev Mol Cell Biol, 15(5), 313–326, doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Licht K, & Jantsch MF (2016). Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. Journal of Cell Biology, 213(1), 15–22, doi: 10.1083/jcb.201511041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Wu D, Ning J, Liu W, & Zhang D (2019). Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer, 19(1), 326, doi: 10.1186/s12885-019-5538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J, Allred DC, Avis I, Martinez A, Vos MD, Smith L, et al. (2001). Differential expression of the early lung cancer detection marker, heterogeneous nuclear ribonucleoprotein-A2/B1 (hnRNP-A2/B1) in normal breast and neoplastic breast cancer. Breast Cancer Research and Treatment, 66(3), 217–224, doi: 10.1023/a:1010631915831. [DOI] [PubMed] [Google Scholar]
- 56.Hu Y, Sun Z, Deng J, Hu B, Yan W, Wei H, et al. (2017). Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biology, 39(3), 1010428317694318, doi: 10.1177/1010428317694318. [DOI] [PubMed] [Google Scholar]
- 57.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. (2010). An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Research and Treatment, 123(3), 725–731, doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 58.Klinge CM, Piell KM, Tooley CS, & Rouchka EC (2019). HNRNPA2/B1 is upregulated in endocrine-resistant LCC9 breast cancer cells and alters the miRNA transcriptome when overexpressed in MCF-7 cells. Sci Rep, 9(1), 9430, doi: 10.1038/s41598-019-45636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pawłowska E, Szczepanska J, & Blasiak J (2017). The Long Noncoding RNA HOTAIR in Breast Cancer: Does Autophagy Play a Role? International Journal of Molecular Sciences, 18(11), doi: 10.3390/ijms18112317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meredith EK, Balas MM, Sindy K, Haislop K, & Johnson AM (2016). An RNA matchmaker protein regulates the activity of the long noncoding RNA HOTAIR. RNA, 22(7), 995–1010, doi: 10.1261/rna.055830.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Li H, Liu F, Gao L-B, Han R, Chen C, et al. (2020). Heterogeneous nuclear ribonucleoprotein A2/B1 is a negative regulator of human breast cancer metastasis by maintaining the balance of multiple genes and pathways. EBioMedicine, 51, 102583, doi: 10.1016/j.ebiom.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Wu M, Chong Q-Y, Zhang W, Qian P, Yan H, et al. (2018). Amplification of hsa-miR-191/425 locus promotes breast cancer proliferation and metastasis by targeting DICER1. Carcinogenesis, 39(2), 1506–1516, doi: 10.1093/carcin/bgy102. [DOI] [PubMed] [Google Scholar]
- 63.Nagpal N, Ahmad HM, Molparia B, & Kulshreshtha R (2013). MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis, 34(8), 1889–1899, doi: 10.1093/carcin/bgt107. [DOI] [PubMed] [Google Scholar]
- 64.Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, & Kulshreshtha R (2015). HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFβ-signaling in hypoxic microenvironment. Sci. Rep, 13(5), 9650, doi: 10.1038/srep09650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Barrueco R, Nekritz EA, Bertucci F, Yu J, Sanchez-Garcia F, Zeleke TZ, et al. (2017). miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy. Genes & Development, 31(6), 553–566, doi: 10.1101/gad.292318.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selever J, Gu G, Lewis MT, Beyer A, Herynk MH, Covington KR, et al. (2011). Dicer-Mediated Upregulation of BCRP Confers Tamoxifen Resistance in Human Breast Cancer Cells. Clinical Cancer Research, 17(20), 6510–6521, doi: 10.1158/1078-0432.ccr-11-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conger AK, Martin EC, Yan TJ, Rhodes LV, Hoang VT, La J, et al. (2016). Argonaute 2 Expression Correlates with a Luminal B Breast Cancer Subtype and Induces Estrogen Receptor Alpha Isoform Variation. Noncoding RNA, 2(3), 8, doi: 10.3390/ncrna2030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casey MC, Prakash A, Holian E, McGuire A, Kalinina O, Shalaby A, et al. (2019). Quantifying Argonaute 2 (Ago2) expression to stratify breast cancer. BMC Cancer, 19(1), 712, doi: 10.1186/s12885-019-5884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, & Deuel TF (2005). Identification, cloning, and expression of human estrogen receptor-[alpha]36, a novel variant of human estrogen receptor-[alpha]66. Biochemical and Biophysical Research Communications, 336(4), 1023–1027, doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 70.Omarjee S, Jacquemetton J, Poulard C, Rochel N, Dejaegere A, Chebaro Y, et al. (2017). The molecular mechanisms underlying the ER[alpha]-36-mediated signaling in breast cancer. [Original Article]. Oncogene, 36(18), 2503–2514, doi: 10.1038/onc.2016.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q, Jiang J, Ying G, Xie X-Q, Zhang X, Xu W, et al. (2018). Tamoxifen enhances stemness and promotes metastasis of ERα36+ breast cancer by upregulating ALDH1A1 in cancer cells. Cell Research, 28, 336, doi: 10.1038/cr.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z-Y, & Yin L (2015). Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Molecular and Cellular Endocrinology, 418, 193–206, doi: 10.1016/j.mce.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Thiebaut C, Chesnel A, Merlin JL, Chesnel M, Leroux A, Harle A, et al. (2019). Dual Epigenetic Regulation of ERalpha36 Expression in Breast Cancer Cells. Int J Mol Sci, 20(11), doi: 10.3390/ijms20112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulard C, Jacquemetton J, Tredan O, Cohen PA, Vendrell J, Ghayad SE, et al. (2019). Oestrogen Non-Genomic Signalling is Activated in Tamoxifen-Resistant Breast Cancer. Int J Mol Sci, 20(11), doi: 10.3390/ijms20112773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bohl C, Harihar S, Denning W, Sharma R, & Welch D (2014). Metastasis suppressors in breast cancers: mechanistic insights and clinical potential. Journal of Molecular Medicine, 92(1), 13–30, doi: 10.1007/s00109-013-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abba M, Patil N, & Allgayer H (2014). MicroRNAs in the Regulation of MMPs and Metastasis. Cancers (Basel), 6(2), 625–645, doi: 10.3390/cancers6020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, et al. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology, 7(10), 1120–1134, doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Annis MG, Ouellet V, Rennhack JP, L’Esperance S, Rancourt C, Mes-Masson A-M, et al. (2018). Integrin-uPAR signaling leads to FRA-1 phosphorylation and enhanced breast cancer invasion. Breast Cancer Research, 20(1), 9, doi: 10.1186/s13058-018-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han B, Nakamura M, Mori I, Nakamura Y, & Kakudo K (2005). Urokinase-type plasminogen activator system and breast cancer (Review). Oncology Reports, 14(1), 105–112. [PubMed] [Google Scholar]
- 80.Cosentino G, Plantamura I, Cataldo A, & Iorio MV (2019). MicroRNA and Oxidative Stress Interplay in the Context of Breast Cancer Pathogenesis. Int J Mol Sci, 20(20), doi: 10.3390/ijms20205143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheung KJ, & Ewald AJ (2016). A collective route to metastasis: Seeding by tumor cell clusters. Science, 352(6282), 167–169, doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Franklin RA, & Li MO (2016). Ontogeny of Tumor-associated Macrophages and Its Implication in Cancer Regulation. Trends Cancer, 2(1), 20–34, doi: 10.1016/j.trecan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang M, McKay D, Pollard JW, & Lewis CE (2018). Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Research, 78(19), 5492, doi: 10.1158/0008-5472.CAN-18-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams CB, Yeh ES, & Soloff AC (2016). Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer, 2, doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermano E, Goldberg R, Rubinstein AM, Sonnenblick A, Maly B, Nahmias D, et al. (2019). Heparanase Accelerates Obesity-Associated Breast Cancer Progression. Cancer Research, 79(20), 5342, doi: 10.1158/0008-5472.CAN-18-4058. [DOI] [PubMed] [Google Scholar]
- 86.Clements ME, & Johnson RW (2019). Breast Cancer Dormancy in Bone. Current Osteoporosis Reports, 17(5), 353–361, doi: 10.1007/s11914-019-00532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welch DR, & Hurst DR (2019). Defining the Hallmarks of Metastasis. Cancer Research, doi: 10.1158/0008-5472.CAN-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amit M, Na’ara S, & Gil Z (2016). Mechanisms of cancer dissemination along nerves. Nature Reviews Cancer, 16, 399, doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesh H, & Monje M (2017). Neuronal Activity in Ontogeny and Oncology. Trends Cancer, 3(2), 89–112, doi: 10.1016/j.trecan.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pundavela J, Roselli S, Faulkner S, Attia J, Scott RJ, Thorne RF, et al. (2015). Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Molecular Oncology, 9(8), 1626–1635, doi: 10.1016/j.molonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Celià-Terrassa T, & Kang Y (2016). Distinctive properties of metastasis-initiating cells. Genes & Development, 30(8), 892–908, doi: 10.1101/gad.277681.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fluegen G, Avivar-Valderas A, Wang Y, Padgen MR, Williams JK, Nobre Ana R., et al. (2017). Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. [Article]. Nature Cell Biology, 19, 120, doi: 10.1038/ncb3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borgen E, Rypdal MC, Sosa MS, Renolen A, Schlichting E, Lønning PE, et al. (2018). NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Research, 20(1), 120, doi: 10.1186/s13058-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terrigno M, Bertacchi M, Pandolfini L, Baumgart M, Calvello M, Cellerino A, et al. (2018). The microRNA miR-21 Is a Mediator of FGF8 Action on Cortical COUP-TFI Translation. Stem Cell Reports, 11(3), 756–769, doi: 10.1016/j.stemcr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, et al. (2011). Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. International Journal of Cancer, 129(10), 2522–2526, doi: 10.1002/ijc.25895. [DOI] [PubMed] [Google Scholar]
- 96.Marjanovic ND, Weinberg RA, & Chaffer CL (2013). Cell Plasticity and Heterogeneity in Cancer. Clinical Chemistry, 59(1), 168, doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grosselin K, Durand A, Marsolier J, Poitou A, Marangoni E, Nemati F, et al. (2019). High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nature Genetics, 51(6), 1060–1066, doi: 10.1038/s41588-019-0424-9. [DOI] [PubMed] [Google Scholar]
- 98.Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, et al. (2017). Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. [Article]. Nature Genetics, 49, 708, doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 99.Yates LR (2017). Intratumoral heterogeneity and subclonal diversification of early breast cancer. Breast, 34 Suppl 1, S36–s42, doi: 10.1016/j.breast.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 100.Janiszewska M, Tabassum DP, Castaño Z, Cristea S, Yamamoto KN, Kingston NL, et al. (2019). Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nature Cell Biology, 21(7), 879–888, doi: 10.1038/s41556-019-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karagiannis GS, Condeelis JS, & Oktay MH (2018). Chemotherapy-induced metastasis: mechanisms and translational opportunities. Clinical & Experimental Metastasis, 35(4), 269–284, doi: 10.1007/s10585-017-9870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shibue T, & Weinberg RA (2017). EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature Reviews Clinical Oncology, 14, 611, doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alferez DG, Simoes BM, Howell SJ, & Clarke RB (2018). The Role of Steroid Hormones in Breast and Effects on Cancer Stem Cells. Curr Stem Cell Rep, 4(1), 81–94, doi: 10.1007/s40778-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thery L, Meddis A, Cabel L, Proudhon C, Latouche A, Pierga JY, et al. (2019). Circulating Tumor Cells in Early Breast Cancer. JNCI Cancer Spectr, 3(2), pkz026, doi: 10.1093/jncics/pkz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, & Cao X (2016). Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell, 30(5), 668–681, doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 106.Clements ME, & Johnson RW (2019). PREX1 drives spontaneous bone dissemination of ER+ breast cancer cells. Oncogene, doi: 10.1038/s41388-019-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao Y, Liu R, Gao C, Zhang T, Qi L, Liu G, et al. (2019). Identification of prognostic biomarkers for breast cancer based on miRNA and mRNA co-expression network. Journal of Cellular Biochemistry, 120(9), 15378–15388, doi: 10.1002/jcb.28805. [DOI] [PubMed] [Google Scholar]
- 108.Chiodoni C, Cancila V, Renzi TA, Perrone M, Tomirotti AM, Sangaletti S, et al. (2019). Transcriptional profiles and stromal changes reveal bone marrow adaptation to early breast cancer in association with deregulated circulating microRNAs. Cancer Research, canres.1425.2019, doi: 10.1158/0008-5472.CAN-19-1425. [DOI] [PubMed] [Google Scholar]
- 109.Ma F, Li W, Liu C, Li W, Yu H, Lei B, et al. (2017). MiR-23a promotes TGF-beta1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/beta-catenin signaling. Oncotarget, doi: 10.18632/oncotarget.18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L, & Wang J (2012). MicroRNA-mediated breast cancer metastasis: from primary site to distant organs. Oncogene, 31(20), 2499–2511. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Li Y, & Lai M (2010). The microRNA network and tumor metastasis. Oncogene, 29(7), 937–948. [DOI] [PubMed] [Google Scholar]
- 112.Weidle UH, Dickopf S, Hintermair C, Kollmorgen G, Birzele F, & Brinkmann U (2018). The Role of micro RNAs in Breast Cancer Metastasis: Preclinical Validation and Potential Therapeutic Targets. Cancer Genomics Proteomics, 15(1), 17–39, doi: 10.21873/cgp.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fridrichova I, & Zmetakova I (2019). MicroRNAs Contribute to Breast Cancer Invasiveness. Cells, 8(11), doi: 10.3390/cells8111361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim J, Yao F, Xiao Z, Sun Y, & Ma L (2018). MicroRNAs and metastasis: small RNAs play big roles. Cancer and Metastasis Reviews, 37(1), 5–15, doi: 10.1007/s10555-017-9712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hurst DR, Edmonds MD, Scott GK, Benz CC, Vaidya KS, & Welch DR (2009). Breast Cancer Metastasis Suppressor 1 Up-regulates miR-146, Which Suppresses Breast Cancer Metastasis. Cancer Research, 69(4), 1279–1283, doi: 10.1158/0008-5472.can-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC, et al. (2009). RETRACTED: A Pleiotropically Acting MicroRNA, miR-31, Inhibits Breast Cancer Metastasis. Cell, 137(6), 1032–1046, doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Retraction: Concurrent Suppression of Integrin alpha5, radixin, and RhoA phenocopies the effects of miR-31 on metastasis (2015). Cancer Research, 75(13), 2760, doi: 10.1158/0008-5472.Can-15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farazi TA, Horlings HM, ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, et al. (2011). MicroRNA Sequence and Expression Analysis in Breast Tumors by Deep Sequencing. Cancer Research, 71(13), 4443–4453, doi: 10.1158/0008-5472.can-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soon PS, Provan PJ, Kim E, Pathmanathan N, Graham D, Clarke CL, et al. (2018). Profiling differential microRNA expression between in situ, infiltrative and lympho-vascular space invasive breast cancer: a pilot study. Clinical & Experimental Metastasis, 35(1), 3–13, doi: 10.1007/s10585-017-9868-4. [DOI] [PubMed] [Google Scholar]
- 120.Gwak JM, Kim HJ, Kim EJ, Chung YR, Yun S, Seo AN, et al. (2014). MicroRNA-9 is associated with epithelial-mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Research and Treatment, 147(1), 39–49, doi: 10.1007/s10549-014-3069-5. [DOI] [PubMed] [Google Scholar]
- 121.Sporn JC, Katsuta E, Yan L, & Takabe K (2019). Expression of MicroRNA-9 is Associated With Overall Survival in Breast Cancer Patients. Journal of Surgical Research, 233, 426–435, doi: 10.1016/j.jss.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 122.Barbano R, Pasculli B, Rendina M, Fontana A, Fusilli C, Copetti M, et al. (2017). Stepwise analysis of MIR9 loci identifies miR-9–5p to be involved in Oestrogen regulated pathways in breast cancer patients. Sci Rep, 7(1), 45283, doi: 10.1038/srep45283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng CW, Yu JC, Hsieh YH, Liao WL, Shieh JC, Yao CC, et al. (2018). Increased Cellular Levels of MicroRNA-9 and MicroRNA-221 Correlate with Cancer Stemness and Predict Poor Outcome in Human Breast Cancer. Cellular Physiology and Biochemistry, 48(5), 2205–2218. [DOI] [PubMed] [Google Scholar]
- 124.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. (2010). miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. [10.1038/ncb2024]. Nat Cell Biol, 12, 247–256 doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, et al. (2012). LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nature Medicine, 18(10), 1511–1517, doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medina PP, Nolde M, & Slack FJ (2010). OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. [10.1038/nature09284]. Nature, advance online publication, doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 127.Adhami M, Haghdoost AA, Sadeghi B, & Malekpour Afshar R (2018). Candidate miRNAs in human breast cancer biomarkers: a systematic review. Breast Cancer, 25, 198–205, doi: 10.1007/s12282-017-0814-8. [DOI] [PubMed] [Google Scholar]
- 128.Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, et al. (2017). Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li S, Yang X, Yang J, Zhen J, & Zhang D (2016). Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Clin Exp Med, 16(1), 29–35, doi: 10.1007/s10238-014-0332-3. [DOI] [PubMed] [Google Scholar]
- 130.Dituri F, Cossu C, Mancarella S, & Giannelli G (2019). The Interactivity between TGFbeta and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells, 8(10), doi: 10.3390/cells8101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu X, Li R, Shi W, Jiang T, Wang Y, Li C, et al. (2016). Silencing of MicroRNA-21 confers the sensitivity to tamoxifen and fulvestrant by enhancing autophagic cell death through inhibition of the PI3K-AKT-mTOR pathway in breast cancer cells. Biomedicine and Pharmacotherapy, 77, 37–44, doi: 10.1016/j.biopha.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Larcher LM, Wang T, & Veedu RN (2019). Development of Novel antimiRzymes for Targeted Inhibition of miR-21 Expression in Solid Cancer Cells. Molecules, 24(13), doi: 10.3390/molecules24132489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patutina OA, Miroshnichenko SK, Mironova NL, Sen’kova AV, Bichenkova EV, Clarke DJ, et al. (2019). Catalytic Knockdown of miR-21 by Artificial Ribonuclease: Biological Performance in Tumor Model. [Original Research]. Frontiers in Pharmacology, 10(879), doi: 10.3389/fphar.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toda H, Seki N, Kurozumi S, Shinden Y, Yamada Y, Nohata N, et al. (2019). RNA-sequence-based microRNA expression signature in breast cancer: tumor-suppressive miR-101–5p regulates molecular pathogenesis. Mol Oncol, doi: 10.1002/1878-0261.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S, Liu JC, Ju Y, Pellecchia G, Voisin V, Wang DY, et al. (2017). microRNA-143/145 loss induces Ras signaling to promote aggressive Pten-deficient basal-like breast cancer. JCI Insight, 2(15), 93313, doi: 10.1172/jci.insight.93313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hurst DR, Edmonds MD, & Welch DR (2009). Metastamir: The Field of Metastasis-Regulatory microRNA Is Spreading. Cancer Research, 69(19), 7495–7498, doi: 10.1158/0008-5472.can-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sang Y, Chen B, Song X, Li Y, Liang Y, Han D, et al. (2019). CircRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182–5p/FOXO3a axis in breast cancer. Molecular Therapy - Nucleic Acids, 27(9), 1638–1652, doi: 10.1016/j.omtn.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhan Y, Li X, Liang X, Li L, Cao B, Wang B, et al. (2016). MicroRNA-182 drives colonization and macroscopic metastasis via targeting its suppressor SNAI1 in breast cancer. Oncotarget, 8(3), 4629–4641, doi: 10.18632/oncotarget.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, et al. (2008). The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol, 10(2), 202–210. [DOI] [PubMed] [Google Scholar]
- 140.Tang CP, Zhou HJ, Qin J, Luo Y, & Zhang T (2017). MicroRNA-520c-3p negatively regulates EMT by targeting IL-8 to suppress the invasion and migration of breast cancer. Oncology Reports, 38(5), 3144–3152, doi: 10.3892/or.2017.5968. [DOI] [PubMed] [Google Scholar]
- 141.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, et al. (2012). MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-[kappa]B and TGF-[beta] signaling pathways. Oncogene, 31(37), 4150–4163, doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 142.Fang Y, Wang J, Wu F, Song Y, Zhao S, & Zhang Q (2017). Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget, 8(28), 46090–46103, doi: 10.18632/oncotarget.17552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu X, Chen S, & Lu C (2019). Amyloid precursor protein promotes the migration and invasion of breast cancer cells by regulating the MAPK signaling pathway. International Journal of Molecular Medicine, doi: 10.3892/ijmm.2019.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eichelser C, Stuckrath I, Muller V, Milde-Langosch K, Wikman H, Pantel K, et al. (2014). Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget, 5(20), 9650–9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ding W, Fan X-L, Xu X, Huang J-Z, Xu S-H, Geng Q, et al. (2015). Epigenetic Silencing of ITGA2 by MiR-373 Promotes Cell Migration in Breast Cancer. PLoS ONE, 10(8), e0135128, doi: 10.1371/journal.pone.0135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen D, Dang BL, Huang JZ, Chen M, Wu D, Xu ML, et al. (2015). MiR-373 drives the epithelial-to-mesenchymal transition and metastasis via the miR-373-TXNIP-HIF1alpha-TWIST signaling axis in breast cancer. Oncotarget, 6(32), 32701–32712, doi: 10.18632/oncotarget.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K, & Schwarzenbach H (2013). Deregulated Serum Concentrations of Circulating Cell-Free MicroRNAs miR-17, miR-34a, miR-155, and miR-373 in Human Breast Cancer Development and Progression. Clinical Chemistry, 59(10), 1489–1496, doi: 10.1373/clinchem.2013.205161. [DOI] [PubMed] [Google Scholar]
- 148.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. (2008). Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet, 40(1), 43–50, 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eyholzer M, Schmid S, Wilkens L, Mueller BU, & Pabst T (2010). The tumour-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br J Cancer, 103(2), 275–284, 10.1038/sj.bjc.6605751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mott JL, Kurita S, Cazanave S, Bronk SF, Werneburg NW, & Fernandez-Zapico ME (2010). Transcriptional Suppression of mir-29b-1/mir-29a Promoter by c-Myc, Hedgehog, and NF-kappaB. J Cell Biochem, 110(5), 1155–1164, doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kriegel AJ, Liu Y, Fang Y, Ding X, & Liang M (2012). The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics, 44(4), 237–244, doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mishmar D, Rahat A, Scherer SW, Nyakatura G, Hinzmann B, Kohwi Y, et al. (1998). Molecular characterization of a common fragile site (FRA7H) on human chromosome 7 by the cloning of a simian virus 40 integration site. Proc Natl Acad Sci U S A, 95(14), 8141–8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chou J, Lin JH, Brenot A, Kim J. w., Provot S, & Werb Z (2013). GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. [10.1038/ncb2672]. Nat Cell Biol, 15(2), 201–213, doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Muluhngwi P, Krishna A, Vittitow SL, Napier JT, Richardson KM, Ellis M, et al. (2017). Tamoxifen differentially regulates miR-29b-1 and miR-29a expression depending on endocrine-sensitivity in breast cancer cells. Cancer Letters, 388, 230–238, doi: 10.1016/j.canlet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yin R, Guo L, Gu J, Li C, & Zhang W (2018). Over expressing miR-19b-1 suppress breast cancer growth by inhibiting tumor microenvironment induced angiogenesis. The International Journal of Biochemistry & Cell Biology, 97, 43–51, doi: 10.1016/j.biocel.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Thorne JL, Battaglia S, Baxter DE, Hayes JL, Hutchinson SA, Jana S, et al. (2018). MiR-19b non-canonical binding is directed by HuR and confers chemosensitivity through regulation of P-glycoprotein in breast cancer. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1861(11), 996–1006, doi: 10.1016/j.bbagrm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 157.Li C, Zhang J, Ma Z, Zhang F, & Yu W (2018). miR-19b serves as a prognostic biomarker of breast cancer and promotes tumor progression through PI3K/AKT signaling pathway. Onco Targets Ther, 11, 4087–4095, doi: 10.2147/ott.S171043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kuhnert F, Campagnolo L, Xiong J-W, Lemons D, Fitch MJ, Zou Z, et al. (2005). Dosage-dependent requirement for mouse Vezf1 in vascular system development. Developmental Biology, 283(1), 140–156, doi: 10.1016/j.ydbio.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao J-J, Lin J, Yang H, Kong W, He L, Ma X, et al. (2008). MicroRNA-221/222 negatively regulates ERalpha and associates with tamoxifen resistance in breast cancer. Journal of Biological Chemistry, 283(45), 31079–31086, doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, et al. (2011). MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene, 30(9), 1082–1097, doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Balzeau J, Menezes MR, Cao S, & Hagan JP (2017). The LIN28/let-7 Pathway in Cancer. [Review]. Frontiers in Genetics, 8(31), doi: 10.3389/fgene.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shibahara Y, Miki Y, Onodera Y, Hata S, Chan MS, Yiu CC, et al. (2012). Aromatase inhibitor treatment of breast cancer cells increases the expression of let-7f, a microRNA targeting CYP19A1. Journal of Pathology, 227(3), 357–366, doi: 10.1002/path.4019. [DOI] [PubMed] [Google Scholar]
- 163.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. (2008). A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep, 9(6), 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. (2008). The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol, 10(5), 593–601. [DOI] [PubMed] [Google Scholar]
- 165.Lamouille S, Xu J, & Derynck R (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nature Reviews Molecular Cell Biology, 15(3), 178–196, doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Deblois G, Smith HW, Tam IS, Gravel S-P, Caron M, Savage P, et al. (2016). ERR[alpha] mediates metabolic adaptations driving lapatinib resistance in breast cancer. [Article]. Nat Commun, 7, doi: 10.1038/ncomms12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li XF, Yan PJ, & Shao ZM (2009). Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene, 28(44), 3937–3948, doi: 10.1038/onc.2009.245. [DOI] [PubMed] [Google Scholar]
- 168.Cicek M, Fukuyama R, Welch DR, Sizemore N, & Casey G (2005). Breast Cancer Metastasis Suppressor 1 Inhibits Gene Expression by Targeting Nuclear Factor-{kappa}B Activity. Cancer Research, 65(9), 3586–3595, doi: 10.1158/0008-5472.CAN-04-3139. [DOI] [PubMed] [Google Scholar]
- 169.Hurst DR, Xie Y, Vaidya KS, Mehta A, Moore BP, Accavitti-Loper MA, et al. (2008). Alterations of BRMS1-ARID4A Interaction Modify Gene Expression but Still Suppress Metastasis in Human Breast Cancer Cells. Journal of Biological Chemistry, 283(12), 7438–7444, doi: 10.1074/jbc.M709446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiu R, Shi H, Wang S, Leng S, Liu R, Zheng Y, et al. (2018). BRMS1 coordinates with LSD1 and suppresses breast cancer cell metastasis. Am J Cancer Res, 8(10), 2030–2045. [PMC free article] [PubMed] [Google Scholar]
- 171.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, & Benz CC (2008). Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene, 27(42), 5643–5647, doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Polytarchou C, Iliopoulos D, & Struhl K (2012). An integrated transcriptional regulatory circuit that reinforces the breast cancer stem cell state. Proceedings of the National Academy of Sciences, 109(36), 14470–14475, doi: 10.1073/pnas.1212811109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hashemi ZS, Forouzandeh Moghadam M, Khalili S, Ghavami M, Salimi F, & Sadroddiny E (2019). Additive effect of metastamiR-193b and breast cancer metastasis suppressor 1 as an anti-metastatic strategy. Breast Cancer, 26(2), 215–228, doi: 10.1007/s12282-018-0915-z. [DOI] [PubMed] [Google Scholar]
- 174.Stevic I, Müller V, Weber K, Fasching PA, Karn T, Marmé F, et al. (2018). Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Medicine, 16(1), 179, doi: 10.1186/s12916-018-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luengo-Gil G, Gonzalez-Billalabeitia E, Perez-Henarejos SA, Navarro Manzano E, Chaves-Benito A, Garcia-Martinez E, et al. (2018). Angiogenic role of miR-20a in breast cancer. PLoS ONE, 13(4), e0194638, doi: 10.1371/journal.pone.0194638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan M, Liaw CS, Ji SM, Tan HH, Wong CY, Thike AA, et al. (2013). Identification of Circulating MicroRNA Signatures for Breast Cancer Detection. Clinical Cancer Research, 19(16), 4477–4487, doi: 10.1158/1078-0432.ccr-12-3401. [DOI] [PubMed] [Google Scholar]
- 177.Guo L, Zhu Y, Li L, Zhou S, Yin G, Yu G, et al. (2019). Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med, 0(0), doi: 10.1002/cam4.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao C, Wang G, Zhu Y, Li X, Yan F, Zhang C, et al. (2016). Aberrant regulation of miR-15b in human malignant tumors and its effects on the hallmarks of cancer. Tumor Biology, 37(1), 177–183, doi: 10.1007/s13277-015-4269-2. [DOI] [PubMed] [Google Scholar]
- 179.Bottani M, Banfi G, & Lombardi G (2019). Circulating miRNAs as Diagnostic and Prognostic Biomarkers in Common Solid Tumors: Focus on Lung, Breast, Prostate Cancers, and Osteosarcoma. J Clin Med, 8(10), doi: 10.3390/jcm8101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kedmi M, Ben-Chetrit N, Körner C, Mancini M, Ben-Moshe NB, Lauriola M, et al. (2015). EGF induces microRNAs that target suppressors of cell migration: miR-15b targets MTSS1 in breast cancer. Sci Signal, 8(368), ra29–ra29, doi: 10.1126/scisignal.2005866. [DOI] [PubMed] [Google Scholar]
- 181.Parr C, & Jiang WG (2009). Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. European Journal of Cancer, 45(9), 1673–1683, doi: 10.1016/j.ejca.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 182.Li Z, Peng Z, Gu S, Zheng J, Feng D, Qin Q, et al. (2017). Global Analysis of miRNA-mRNA Interaction Network in Breast Cancer with Brain Metastasis. Anticancer Research, 37(8), 4455–4468, doi: 10.21873/anticanres.11841. [DOI] [PubMed] [Google Scholar]
- 183.Lee JH, & Welch DR (1997). Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Research, 57(12), 2384–2387. [PubMed] [Google Scholar]
- 184.Yoshida BA, Sokoloff MM, Welch DR, & Rinker-Schaeffer CW (2000). Metastasis-suppressor genes: a review and perspective on an emerging field. Journal of the National Cancer Institute, 92(21), 1717–1730. [DOI] [PubMed] [Google Scholar]
- 185.Hurst DR, & Welch DR (2011). Chapter three - Metastasis Suppressor Genes: At the Interface Between the Environment and Tumor Cell Growth In Jeon KW (Ed.), International Review of Cell and Molecular Biology (Vol. 286, pp. 107–180): Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morrison Joly M, Williams MM, Hicks DJ, Jones B, Sanchez V, Young CD, et al. (2017). Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Research, 19(1), 74, doi: 10.1186/s13058-017-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Katoh M (2014). Cardio-miRNAs and onco-miRNAs: circulating miRNA-based diagnostics for non-cancerous and cancerous diseases. Front Cell Dev Biol, 2, 61, doi: 10.3389/fcell.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Derfoul A, Juan AH, Difilippantonio MJ, Palanisamy N, Ried T, & Sartorelli V (2011). Decreased microRNA-214 levels in breast cancer cells coincides with increased cell proliferation, invasion and accumulation of the Polycomb Ezh2 methyltransferase. Carcinogenesis, 32(11), 1607–1614, doi: 10.1093/carcin/bgr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kaverina N, Borovjagin AV, Kadagidze Z, Baryshnikov A, Baryshnikova M, Malin D, et al. (2017). Astrocytes promote progression of breast cancer metastases to the brain via a KISS1-mediated autophagy. Autophagy, 13(11), 1905–1923, doi: 10.1080/15548627.2017.1360466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Franssen D, & Tena-Sempere M (2018). The kisspeptin receptor: A key G-protein-coupled receptor in the control of the reproductive axis. Best Practice & Research Clinical Endocrinology & Metabolism, 32(2), 107–123, doi: 10.1016/j.beem.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 191.Shen ZL, Wang B, Jiang KW, Ye CX, Cheng C, Yan YC, et al. (2016). Downregulation of miR-199b is associated with distant metastasis in colorectal cancer via activation of SIRT1 and inhibition of CREB/KISS1 signaling. Oncotarget, 7(23), 35092–35105, doi: 10.18632/oncotarget.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Barone I, Vircillo V, Giordano C, Gelsomino L, Győrffy B, Tarallo R, et al. (2018). Activation of Farnesoid X Receptor impairs the tumor-promoting function of breast cancer-associated fibroblasts. Cancer Letters, 437, 89–99, doi: 10.1016/j.canlet.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 193.Journe F, Durbecq V, Chaboteaux C, Rouas G, Laurent G, Nonclercq D, et al. (2009). Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast Cancer Research and Treatment, 115(3), 523–535, doi: 10.1007/s10549-008-0094-2. [DOI] [PubMed] [Google Scholar]
- 194.Zhong X. y., Yu J. h., Zhang W. g., Wang Z. d., Dong Q, Tai S, et al. (2012). MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene, 493(1), 44–51, doi: 10.1016/j.gene.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 195.Krattinger R, Bostrom A, Schioth HB, Thasler WE, Mwinyi J, & Kullak-Ublick GA (2016). microRNA-192 suppresses the expression of the farnesoid X receptor. Am J Physiol Gastrointest Liver Physiol, 310(11), G1044–1051, doi: 10.1152/ajpgi.00297.2015. [DOI] [PubMed] [Google Scholar]
- 196.Jiménez A, López-Ornelas A, Estudillo E, González-Mariscal L, González RO, & Segovia J (2014). A soluble form of GAS1 inhibits tumor growth and angiogenesis in a triple negative breast cancer model. Experimental Cell Research, 327(2), 307–317, doi: 10.1016/j.yexcr.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 197.Zhou M, Hou Y, Yang G, Zhang H, Tu G, Du YE, et al. (2016). LncRNA-Hh Strengthen Cancer Stem Cells Generation in Twist-Positive Breast Cancer via Activation of Hedgehog Signaling Pathway. Stem Cells, 34(1), 55–66, doi: 10.1002/stem.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miller J, Dreyer TF, Bacher AS, Sinner EK, Heinrich C, Benge A, et al. (2018). Differential tumor biological role of the tumor suppressor KAI1 and its splice variant in human breast cancer cells. Oncotarget, 9(5), 6369–6390, doi: 10.18632/oncotarget.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, et al. (2006). Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nature Medicine, 12(8), 933–938, doi: 10.1038/nm1444. [DOI] [PubMed] [Google Scholar]
- 200.Viswanadhapalli S, Luo Y, Sareddy GR, Santhamma B, Zhou M, Li M, et al. (2019). EC359: A First-in-Class Small-Molecule Inhibitor for Targeting Oncogenic LIFR Signaling in Triple-Negative Breast Cancer. Molecular Cancer Therapeutics, 18(8), 1341, doi: 10.1158/1535-7163.MCT-18-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hergovich A (2012). YAP-Hippo signalling downstream of leukemia inhibitory factor receptor: implications for breast cancer. Breast Cancer Res, 14(6), 326, doi: 10.1186/bcr3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang J, Song C, Tang H, Zhang C, Tang J, Li X, et al. (2017). miR-629–3p may serve as a novel biomarker and potential therapeutic target for lung metastases of triple-negative breast cancer. Breast Cancer Research, 19(1), 72, doi: 10.1186/s13058-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang GJ, Lei PM, Wong SY, Ma DL, & Leung CH (2018). Pharmacological Inhibition of LSD1 for Cancer Treatment. Molecules, 23(12), doi: 10.3390/molecules23123194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pollock JA, Larrea MD, Jasper JS, McDonnell DP, & McCafferty DG (2012). Lysine-Specific Histone Demethylase 1 Inhibitors Control Breast Cancer Proliferation in ERα-Dependent and -Independent Manners. ACS Chemical Biology, 7(7), 1221–1231, doi: 10.1021/cb300108c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hu X, Xiang D, Xie Y, Tao L, Zhang Y, Jin Y, et al. (2019). LSD1 suppresses invasion, migration and metastasis of luminal breast cancer cells via activation of GATA3 and repression of TRIM37 expression. Oncogene, 38(44), 7017–7034, doi: 10.1038/s41388-019-0923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu J, Feng J, Li L, Lin L, Ji J, Lin C, et al. (2019). Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer. EMBO Rep, e48597, doi: 10.15252/embr.201948597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bi Q, Ranjan A, Fan R, Agarwal N, Welch DR, Weinman SA, et al. (2015). MTBP inhibits migration and metastasis of hepatocellular carcinoma. Clinical & Experimental Metastasis, 32(4), 301–311, doi: 10.1007/s10585-015-9706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grieb BC, Gramling MW, Arrate MP, Chen X, Beauparlant SL, Haines DS, et al. (2014). Oncogenic Protein MTBP Interacts with MYC to Promote Tumorigenesis. Cancer Research, 74(13), 3591, doi: 10.1158/0008-5472.CAN-13-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Grieb BC, Chen X, & Eischen CM (2014). MTBP Is Overexpressed in Triple-Negative Breast Cancer and Contributes to Its Growth and Survival. Molecular Cancer Research, 12(9), 1216, doi: 10.1158/1541-7786.MCR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mundt F, Rajput S, Li S, Ruggles KV, Mooradian AD, Mertins P, et al. (2018). Mass Spectrometry-Based Proteomics Reveals Potential Roles of NEK9 and MAP2K4 in Resistance to PI3K Inhibition in Triple-Negative Breast Cancers. Cancer Research, 78(10), 2732, doi: 10.1158/0008-5472.CAN-17-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sehgal V, & Ram PT (2013). Network Motifs in JNK Signaling. Genes Cancer, 4(9–10), 409–413, doi: 10.1177/1947601913507577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xue Z, Vis DJ, Bruna A, Sustic T, van Wageningen S, Batra AS, et al. (2018). MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Research, 28(7), 719–729, doi: 10.1038/s41422-018-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu S, Huang J, Zhang Y, Liu Y, Zuo S, & Li R (2019). MAP2K4 interacts with Vimentin to activate the PI3K/AKT pathway and promotes breast cancer pathogenesis. Aging (Albany NY), 11(22), 10697–10710, doi: 10.18632/aging.102485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sevinsky CJ, Khan F, Kokabee L, Darehshouri A, Maddipati KR, & Conklin DS (2018). NDRG1 regulates neutral lipid metabolism in breast cancer cells. Breast Cancer Research, 20(1), 55, doi: 10.1186/s13058-018-0980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wangpu X, Lu J, Xi R, Yue F, Sahni S, Park KC, et al. (2016). Targeting the Metastasis Suppressor, N-Myc Downstream Regulated Gene-1, with Novel Di-2-Pyridylketone Thiosemicarbazones: Suppression of Tumor Cell Migration and Cell-Collagen Adhesion by Inhibiting Focal Adhesion Kinase/Paxillin Signaling. Molecular Pharmacology, 89(5), 521, doi: 10.1124/mol.115.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Luo E-C, Chang Y-C, Sher Y-P, Huang W-Y, Chuang L-L, Chiu Y-C, et al. (2014). MicroRNA-769–3p Down-regulates NDRG1 and Enhances Apoptosis in MCF-7 Cells During Reoxygenation. Sci Rep, 4(1), 5908, doi: 10.1038/srep05908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pietiäinen V, Vassilev B, Blom T, Wang W, Nelson J, Bittman R, et al. (2013). NDRG1 functions in LDL receptor trafficking by regulating endosomal recycling and degradation. Journal of Cell Science, 126(17), 3961–3971, doi: 10.1242/jcs.128132. [DOI] [PubMed] [Google Scholar]
- 218.Kinlaw WB, Baures PW, Lupien LE, Davis WL, & Kuemmerle NB (2016). Fatty Acids and Breast Cancer: Make Them on Site or Have Them Delivered. Journal of Cellular Physiology, 231(10), 2128–2141, doi: 10.1002/jcp.25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. (2017). Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer, 16(1), 76, doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang T, Fahrmann JF, Lee H, Li Y-J, Tripathi SC, Yue C, et al. (2018). JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metabolism, 27(1), 136–150.e135, doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lin HC, Yeh CC, Chao LY, Tsai MH, Chen HH, Chuang EY, et al. (2018). The hypoxia-responsive lncRNA NDRG-OT1 promotes NDRG1 degradation via ubiquitin-mediated proteolysis in breast cancer cells. Oncotarget, 9(12), 10470–10482, doi: 10.18632/oncotarget.23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Valastyan S (2012). Endogenous anticancer mechanisms: metastasis. Front Biosci (Elite Ed), 4, 1888–1897, doi: 10.2741/510. [DOI] [PubMed] [Google Scholar]
- 223.Smith SC, & Theodorescu D (2009). Learning therapeutic lessons from metastasis suppressor proteins. Nature Reviews Cancer, 9(4), 253–264, doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, Maga TK, et al. (2007). Nm23-H1 Suppresses Tumor Cell Motility by Down-regulating the Lysophosphatidic Acid Receptor EDG2. Cancer Research, 67(15), 7238–7246, doi: 10.1158/0008-5472.can-07-0962. [DOI] [PubMed] [Google Scholar]
- 225.Peeney D, Fan Y, Nguyen T, Meerzaman D, & Stetler-Stevenson WG (2019). Matrisome-Associated Gene Expression Patterns Correlating with TIMP2 in Cancer. Sci Rep, 9(1), 20142, doi: 10.1038/s41598-019-56632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Baum B, Settleman J, & Quinlan MP (2008). Transitions between epithelial and mesenchymal states in development and disease. Seminars in Cell & Developmental Biology, 19(3), 294–308, doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 227.Derynck R, & Weinberg RA (2019). EMT and Cancer: More Than Meets the Eye. Developmental Cell, 49(3), 313–316, doi: 10.1016/j.devcel.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Imani S, Hosseinifard H, Cheng J, Wei C, & Fu J (2016). Prognostic Value of EMT-inducing Transcription Factors (EMT-TFs) in Metastatic Breast Cancer: A Systematic Review and Meta-analysis. Sci Rep, 6(1), 28587, doi: 10.1038/srep28587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Creighton CJ, Gibbons DL, & Kurie JM (2013). The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res, 5, 187–195, doi: 10.2147/cmar.S35171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dongre A, & Weinberg RA (2019). New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nature Reviews Molecular Cell Biology, 20(2), 69–84, doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 231.Vandewalle C, Van Roy F, & Berx G (2009). The role of the ZEB family of transcription factors in development and disease. [journal article]. Cellular and Molecular Life Sciences, 66(5), 773–787, doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Georgess D, Padmanaban V, Sirka OK, Coutinho K, Choi A, Frid G, et al. (2020). Twist1-Induced Epithelial Dissemination Requires Prkd1 Signaling. Cancer Research, 80(2), 204–218, doi: 10.1158/0008-5472.Can-18-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Iorio MV, & Croce CM (2012). microRNA involvement in human cancer. Carcinogenesis, 33(6), 1126–1133, doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stacy AJ, Craig MP, Sakaram S, & Kadakia M (2017). DeltaNp63alpha and microRNAs: leveraging the epithelial-mesenchymal transition. Oncotarget, 8(2), 2114–2129, doi: 10.18632/oncotarget.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Exposito-Villen A, A EA, & Franco D (2018). Functional Role of Non-Coding RNAs during Epithelial-To-Mesenchymal Transition. Noncoding RNA, 4(2), doi: 10.3390/ncrna4020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhao M, Liu Y, Zheng C, & Qu H (2019). dbEMT 2.0: An updated database for epithelial-mesenchymal transition genes with experimentally verified information and precalculated regulation information for cancer metastasis. Journal of Genetics and Genomics, Epub ahead of print, doi: 10.1016/j.jgg.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 237.LaFoya B, Munroe JA, Mia MM, Detweiler MA, Crow JJ, Wood T, et al. (2016). Notch: A multi-functional integrating system of microenvironmental signals. Developmental Biology, 418(2), 227–241, doi: 10.1016/j.ydbio.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kar R, Jha NK, Jha SK, Sharma A, Dholpuria S, Asthana N, et al. (2019). A “NOTCH” Deeper into the Epithelial-To-Mesenchymal Transition (EMT) Program in Breast Cancer. Genes (Basel), 10(12), doi: 10.3390/genes10120961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Miao H, & Wang B (2012). EphA receptor signaling—Complexity and emerging themes. Seminars in Cell & Developmental Biology, 23(1), 16–25, doi: 10.1016/j.semcdb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Youngblood VM, Kim LC, Edwards DN, Hwang Y, Santapuram PR, Stirdivant SM, et al. (2016). The Ephrin-A1/EPHA2 Signaling Axis Regulates Glutamine Metabolism in HER2-Positive Breast Cancer. Cancer Research, 76(7), 1825–1836, doi: 10.1158/0008-5472.can-15-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Aydoğdu E, Katchy A, Tsouko E, Lin C-Y, Haldosén L-A, Helguero L, et al. (2012). MicroRNA-regulated gene networks during mammary cell differentiation are associated with breast cancer. Carcinogenesis, 33(8), 1502–1511, doi: 10.1093/carcin/bgs161. [DOI] [PubMed] [Google Scholar]
- 242.Buggy Y, Maguire TM, McDermott E, Hill ADK, O’Higgins N, & Duffy MJ (2006). Ets2 transcription factor in normal and neoplastic human breast tissue. European Journal of Cancer, 42(4), 485–491, doi: 10.1016/j.ejca.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 243.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, et al. (2010). An Ets2-Driven Transcriptional Program in Tumor-Associated Macrophages Promotes Tumor Metastasis. Cancer Research, 70(4), 1323–1333, doi: 10.1158/0008-5472.Can-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mathsyaraja H, Thies K, Taffany DA, Deighan C, Liu T, Yu L, et al. (2015). CSF1-ETS2-induced microRNA in myeloid cells promote metastatic tumor growth. Oncogene, 34(28), 3651–3661, doi: 10.1038/onc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McBryan J, Theissen SM, Byrne C, Hughes E, Cocchiglia S, Sande S, et al. (2012). Metastatic Progression with Resistance to Aromatase Inhibitors Is Driven by the Steroid Receptor Coactivator SRC-1. Cancer Research, 72(2), 548–559, doi: 10.1158/0008-5472.can-11-2073. [DOI] [PubMed] [Google Scholar]
- 246.Lawrence CL, & Baldwin AS (2016). Non-Canonical EZH2 Transcriptionally Activates RelB in Triple Negative Breast Cancer. PLoS ONE, 11(10), e0165005, doi: 10.1371/journal.pone.0165005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mondal T, Subhash S, Vaid R, Enroth S, Uday S, Reinius B, et al. (2015). MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun, 6, 7743, doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chang C-J, Yang J-Y, Xia W, Chen C-T, Xie X, Chao C-H, et al. (2011). EZH2 Promotes Expansion of Breast Tumor Initiating Cells through Activation of RAF1-β-Catenin Signaling. Cancer Cell, 19(1), 86–100, doi: 10.1016/j.ccr.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gonzalez ME, Moore HM, Li X, Toy KA, Huang W, Sabel MS, et al. (2014). EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proceedings of the National Academy of Sciences, 111(8), 3098–3103, doi: 10.1073/pnas.1308953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mahara S, Lee PL, Feng M, Tergaonkar V, Chng WJ, & Yu Q (2016). HIFI-α activation underlies a functional switch in the paradoxical role of Ezh2/PRC2 in breast cancer. Proceedings of the National Academy of Sciences, 113(26), E3735–E3744, doi: 10.1073/pnas.1602079113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cirillo LA, & Zaret KS (2007). Specific Interactions of the Wing Domains of FOXA1 Transcription Factor with DNA. Journal of Molecular Biology, 366(3), 720–724, doi: 10.1016/j.jmb.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guttilla IK, & White BA (2009). Coordinate Regulation of FOXO1 by miR-27a, miR-96, and miR-182 in Breast Cancer Cells. Journal of Biological Chemistry, 284(35), 23204–23216, doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zou Y, Lin X, Bu J, Lin Z, Chen Y, Qiu Y, et al. (2019). Timeless-Stimulated miR-5188-FOXO1/β-Catenin-c-Jun Feedback Loop Promotes Stemness via Ubiquitination of β-Catenin in Breast Cancer. Molecular Therapy, doi: 10.1016/j.ymthe.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yang J, Li T, Gao C, Lv X, Liu K, Song H, et al. (2014). FOXO1 3′UTR functions as a ceRNA in repressing the metastases of breast cancer cells via regulating miRNA activity. FEBS Letters, 588(17), 3218–3224, doi: 10.1016/j.febslet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 255.Bourguignon LYW, Wong G, Earle C, Krueger K, & Spevak CC (2010). Hyaluronan-CD44 Interaction Promotes c-Src-mediated Twist Signaling, MicroRNA-10b Expression, and RhoA/RhoC Up-regulation, Leading to Rho-kinase-associated Cytoskeleton Activation and Breast Tumor Cell Invasion. Journal of Biological Chemistry, 285(47), 36721–36735, doi: 10.1074/jbc.M110.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Xin F, Li M, Balch C, Thomson M, Fan M, Liu Y, et al. (2009). Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics, 25(4), 430–434, doi: 10.1093/bioinformatics/btn646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Johansson P, Jeffery J, Al-Ejeh F, Schulz RB, Callen DF, Kumar R, et al. (2014). SCF-FBXO31 E3 Ligase Targets DNA Replication Factor Cdt1 for Proteolysis in the G2 Phase of Cell Cycle to Prevent Re-replication. Journal of Biological Chemistry, 289(26), 18514–18525, doi: 10.1074/jbc.M114.559930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li C, Du L, Ren Y, Liu X, Jiao Q, Cui D, et al. (2019). SKP2 promotes breast cancer tumorigenesis and radiation tolerance through PDCD4 ubiquitination. Journal of Experimental and Clinical Cancer Research, 38(1), 76, doi: 10.1186/s13046-019-1069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kossatz U, Dietrich N, Zender L, Buer J, Manns MP, & Malek NP (2004). Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes & Development, 18(21), 2602–2607, doi: 10.1101/gad.321004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Brandão M, Caparica R, Eiger D, & de Azambuja E (2019). Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Annals of Oncology, 30, x27–x42, doi: 10.1093/annonc/mdz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Clement E, Inuzuka H, Nihira NT, Wei W, & Toker A (2018). Skp2-dependent reactivation of AKT drives resistance to PI3K inhibitors. Sci Signal, 11(521), eaao3810, doi: 10.1126/scisignal.aao3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gee HE, Buffa FM, Harris AL, Toohey JM, Carroll SL, Cooper CL, et al. (2015). MicroRNA-Related DNA Repair/Cell-Cycle Genes Independently Associated With Relapse After Radiation Therapy for Early Breast Cancer. International Journal of Radiation Oncology*Biology*Physics, 93(5), 1104–1114, doi: 10.1016/j.ijrobp.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 263.Gao L, He RQ, Wu HY, Zhang TT, Liang HW, Ye ZH, et al. (2018). Expression Signature and Role of miR-30d-5p in Non-Small Cell Lung Cancer: a Comprehensive Study Based on in Silico Analysis of Public Databases and in Vitro Experiments. Cellular Physiology and Biochemistry, 50(5), 1964–1987, doi: 10.1159/000494875. [DOI] [PubMed] [Google Scholar]
- 264.Croset M, Pantano F, Kan CWS, Bonnelye E, Descotes F, Alix-Panabières C, et al. (2018). MicroRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. [10.1158/0008–5472.CAN-17–3058]. Cancer Research, 78(18), 5259–5273. [DOI] [PubMed] [Google Scholar]
- 265.Akaogi K, Nakajima Y, Ito I, Kawasaki S, Oie S. h., Murayama A, et al. (2009). KLF4 suppresses estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of ER[alpha]. Oncogene, 28(32), 2894–2902. [DOI] [PubMed] [Google Scholar]
- 266.Quintana A, Liu F, O’Rourke J, & Ness S (2011). Identification and Regulation of c-Myb Target Genes in MCF-7 Cells. BMC Cancer, 11(1), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. (2011). Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene, 30(18), 2161–2172, doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hilakivi-Clarke L, Wärri A, Bouker KB, Zhang X, Cook KL, Jin L, et al. (2017). Effects of In Utero Exposure to Ethinyl Estradiol on Tamoxifen Resistance and Breast Cancer Recurrence in a Preclinical Model. JNCI: Journal of the National Cancer Institute, 109(1), 1–11, doi: 10.1093/jnci/djw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Singh R, Pochampally R, Watabe K, Lu Z, & Mo YY (2014). Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer, 13, 256, doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shi P, Liu W, Tala Wang, H., Li F, Zhang H, et al. (2017). Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov, 3(1), 17010, doi: 10.1038/celldisc.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jia X, Shi L, Wang X, Luo L, Ling L, Yin J, et al. (2019). KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death & Disease, 10(5), 373, doi: 10.1038/s41419-019-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cai W, Xu Y, Yin J, Zuo W, & Su Z (2018). miR-590–5p suppresses osteosarcoma cell proliferation and invasion via targeting KLF5. Mol Med Rep, 18(2), 2328–2334, doi: 10.3892/mmr.2018.9173. [DOI] [PubMed] [Google Scholar]
- 273.Vafaizadeh V, Graab U, Darvishi T, Machado R, & Groner B (2012). Transforming growth factor beta signaling regulates the invasiveness of normal mammary epithelial cells and the metastasis formation of tumor cells. Horm Mol Biol Clin Investig, 10(1), 227–239, doi: 10.1515/hmbci-2012-0016. [DOI] [PubMed] [Google Scholar]
- 274.Chang C-C, Wu M-J, Yang J-Y, Camarillo IG, & Chang C-J (2015). Leptin-STAT3-G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression. Cancer Research, 75(11), 2375, doi: 10.1158/0008-5472.CAN-14-3076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 275.Balanis N, & Carlin CR (2017). Stress-induced EGF receptor signaling through STAT3 and tumor progression in triple-negative breast cancer. Molecular and Cellular Endocrinology, 451, 24–30, doi: 10.1016/j.mce.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Deng X, Zhao Y, & Wang B (2015). miR-519d-mediated downregulation of STAT3 suppresses breast cancer progression. Oncology Reports, 34(4), 2188–2194, doi: 10.3892/or.2015.4160. [DOI] [PubMed] [Google Scholar]
- 277.Hughes K, & Watson CJ (2018). The Multifaceted Role of STAT3 in Mammary Gland Involution and Breast Cancer. Int J Mol Sci, 19(6), doi: 10.3390/ijms19061695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.McDaniel JM, Varley KE, Gertz J, Savic DS, Roberts BS, Bailey SK, et al. (2017). Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget, 8(5), 8226–8238, doi: 10.18632/oncotarget.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Böhm J, Sustmann C, Wilhelm C, & Kohlhase J (2006). SALL4 is directly activated by TCF/LEF in the canonical Wnt signaling pathway. Biochemical and Biophysical Research Communications, 348(3), 898–907, doi: 10.1016/j.bbrc.2006.07.124. [DOI] [PubMed] [Google Scholar]
- 280.Dirican E, & Akkiprik M (2016). Functional and clinical significance of SALL4 in breast cancer. Tumor Biology, 37(9), 11701–11709, doi: 10.1007/s13277-016-5150-7. [DOI] [PubMed] [Google Scholar]
- 281.Matsumoto Y, Itou J, Sato F, & Toi M (2018). SALL4 - KHDRBS3 network enhances stemness by modulating CD44 splicing in basal-like breast cancer. Cancer Med, 7(2), 454–462, doi: 10.1002/cam4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang C, et al. (2015). MicroRNA-33b Inhibits Breast Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci. Rep, 5, doi: 10.1038/srep09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bhateja P, Cherian M, Majumder S, & Ramaswamy B (2019). The Hedgehog Signaling Pathway: A Viable Target in Breast Cancer? Cancers (Basel), 11(8), doi: 10.3390/cancers11081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sirkisoon SR, Carpenter RL, Rimkus T, Anderson A, Harrison A, Lange AM, et al. (2018). Interaction between STAT3 and GLI1/tGLI1 oncogenic transcription factors promotes the aggressiveness of triple-negative breast cancers and HER2-enriched breast cancer. Oncogene, 37(19), 2502–2514, doi: 10.1038/s41388-018-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Olmos Y, Brosens JJ, & Lam EWF (2011). Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resistance Updates, 14(1), 35–44, doi: 10.1016/j.drup.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 286.Bayele HK (2019). A conserved mechanism of sirtuin signalling through steroid hormone receptors. Bioscience Reports, 39(12), doi: 10.1042/bsr20193535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Huang H, & Tindall DJ (2007). Dynamic FoxO transcription factors. Journal of Cell Science, 120(15), 2479–2487, doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 288.Liarte S, Alonso-Romero JL, & Nicolás FJ (2018). SIRT1 and Estrogen Signaling Cooperation for Breast Cancer Onset and Progression. [Mini Review]. Frontiers in Endocrinology, 9(552), doi: 10.3389/fendo.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brooks CL, & Gu W (2009). How does SIRT1 affect metabolism, senescence and cancer? Nature Reviews Cancer, 9(2), 123–128, doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Shi L, Tang X, Qian M, Liu Z, Meng F, Fu L, et al. (2018). A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene, 37(49), 6299–6315, doi: 10.1038/s41388-018-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Latifkar A, Ling L, Hingorani A, Johansen E, Clement A, Zhang X, et al. (2019). Loss of Sirtuin 1 Alters the Secretome of Breast Cancer Cells by Impairing Lysosomal Integrity. Developmental Cell, doi: 10.1016/j.devcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, et al. (2001). Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes and Development, 15(1), 50–65, doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Simic P, Williams Eric O., Bell Eric L., Gong Jing J., Bonkowski M, & Guarente L (2013). SIRT1 Suppresses the Epithelial-to-Mesenchymal Transition in Cancer Metastasis and Organ Fibrosis. Cell Reports, 3(4), 1175–1186, doi: 10.1016/j.celrep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Capparelli C, Whitaker-Menezes D, Guido C, Balliet R, Pestell TG, Howell A, et al. (2012). CTGF drives autophagy, glycolysis and senescence in cancer-associated fibroblasts via HIF1 activation, metabolically promoting tumor growth. Cell Cycle, 11(12), 2272–2284, doi: 10.4161/cc.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Jiang CG, Lv L, Liu FR, Wang ZN, Liu FN, Li YS, et al. (2011). Downregulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritoneal dissemination. Mol Cancer, 10, 122, doi: 10.1186/1476-4598-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Edwards LA, Woolard K, Son MJ, Li A, Lee J, Ene C, et al. (2011). Effect of brain- and tumor-derived connective tissue growth factor on glioma invasion. Journal of the National Cancer Institute, 103(15), 1162–1178, doi: 10.1093/jnci/djr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chang CC, Shih JY, Jeng YM, Su JL, Lin BZ, Chen ST, et al. (2004). Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. Journal of the National Cancer Institute, 96(5), 364–375, doi: 10.1093/jnci/djh059. [DOI] [PubMed] [Google Scholar]
- 298.de Kruijf EM, van Nes JG, van de Velde CJ, Putter H, Smit VT, Liefers GJ, et al. (2011). Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Research and Treatment, 125(3), 687–696, doi: 10.1007/s10549-010-0855-6. [DOI] [PubMed] [Google Scholar]
- 299.Zhu X, Zhong J, Zhao Z, Sheng J, Wang J, Liu J, et al. (2015). Epithelial derived CTGF promotes breast tumor progression via inducing EMT and collagen I fibers deposition. Oncotarget, 6(28), 25320–25338, doi: 10.18632/oncotarget.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Santolla MF, Lappano R, Cirillo F, Rigiracciolo DC, Sebastiani A, Abonante S, et al. (2018). miR-221 stimulates breast cancer cells and cancer-associated fibroblasts (CAFs) through selective interference with the A20/c-Rel/CTGF signaling. Journal of Experimental and Clinical Cancer Research, 37(1), 94, doi: 10.1186/s13046-018-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Chen Y, Peng Y, Fan S, Li Y, Xiao ZX, & Li C (2018). A double dealing tale of p63: an oncogene or a tumor suppressor. Cellular and Molecular Life Sciences, 75(6), 965–973, doi: 10.1007/s00018-017-2666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gatti V, Bongiorno-Borbone L, Fierro C, Annicchiarico-Petruzzelli M, Melino G, & Peschiaroli A (2019). p63 at the Crossroads between Stemness and Metastasis in Breast Cancer. Int J Mol Sci, 20(11), doi: 10.3390/ijms20112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Coates PJ, Nenutil R, Holcakova J, Nekulova M, Podhorec J, Svoboda M, et al. (2018). p63 isoforms in triple-negative breast cancer: ΔNp63 associates with the basal phenotype whereas TAp63 associates with androgen receptor, lack of BRCA mutation, PTEN and improved survival. Virchows Archiv, 472(3), 351–359, doi: 10.1007/s00428-018-2324-2. [DOI] [PubMed] [Google Scholar]
- 304.Kim K, Madak-Erdogan Z, Ventrella R, & Katzenellenbogen BS (2013). A MicroRNA196a2* and TP63 circuit regulated by estrogen receptor-alpha and ERK2 that controls breast cancer proliferation and invasiveness properties. Horm Cancer, 4(2), 78–91, doi: 10.1007/s12672-012-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, et al. (1994). p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes & Development, 8(1), 9–22, doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 306.Kim GJ, Kim D-H, Min K-W, Kim YH, & Oh YH (2018). Loss of p27kip1 expression is associated with poor prognosis in patients with taxane-treated breast cancer. Pathology - Research and Practice, 214(4), 565–571, doi: 10.1016/j.prp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 307.Berton S, Cusan M, Segatto I, Citron F, D’Andrea S, Benevol S, et al. (2017). Loss of p27kip1 increases genomic instability and induces radio-resistance in luminal breast cancer cells. Sci Rep, 7(1), 595, doi: 10.1038/s41598-017-00734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wang DD, Yang SJ, Chen X, Shen HY, Luo LJ, Zhang XH, et al. (2016). miR-222 induces Adriamycin resistance in breast cancer through PTEN/Akt/p27(kip1) pathway. Tumour Biology, 37(11), 15315–15324, doi: 10.1007/s13277-016-5341-2. [DOI] [PubMed] [Google Scholar]
- 309.Zhao S, Han J, Zheng L, Yang Z, Zhao L, & Lv Y (2015). MicroRNA-203 Regulates Growth and Metastasis of Breast Cancer. Cellular Physiology and Biochemistry, 37(1), 35–42, doi: 10.1159/000430331. [DOI] [PubMed] [Google Scholar]
- 310.Toska E, & Roberts Stefan G. E. (2014). Mechanisms of transcriptional regulation by WT1 (Wilms’ tumour 1). Biochemical Journal, 461(1), 15–32, doi: 10.1042/bj20131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cheng H, Wang S, & Feng R (2016). STIM1 plays an important role in TGF-beta-induced suppression of breast cancer cell proliferation. Oncotarget, 7(13), 16866–16878, doi: 10.18632/oncotarget.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Reizner N, Maor S, Sarfstein R, Abramovitch S, Welshons WV, Curran EM, et al. (2005). The WT1 Wilms’ tumor suppressor gene product interacts with estrogen receptor-{alpha} and regulates IGF-I receptor gene transcription in breast cancer cells. Journal of Molecular Endocrinology, 35(1), 135–144. [DOI] [PubMed] [Google Scholar]
- 313.Xie F, Hosany S, Zhong S, Jiang Y, Zhang F, Lin L, et al. (2017). MicroRNA-193a inhibits breast cancer proliferation and metastasis by downregulating WT1. PLoS ONE, 12(10), e0185565, doi: 10.1371/journal.pone.0185565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tsai K-W, Leung C-M, Lo Y-H, Chen T-W, Chan W-C, Yu S-Y, et al. (2016). Arm Selection Preference of MicroRNA-193a Varies in Breast Cancer. [Article]. Sci Rep, 6, 28176, doi: 10.1038/srep28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Majewska H, Szade J, et al. (2014). Epithelial-mesenchymal transition markers in lymph node metastases and primary breast tumors - relation to dissemination and proliferation. Am J Transl Res, 6(6), 793–808. [PMC free article] [PubMed] [Google Scholar]
- 316.Liu Y-N, Lee W-W, Wang C-Y, Chao T-H, Chen Y, & Chen JH (2005). Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene, 24(56), 8277–8290, doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 317.Hurteau GJ, Carlson JA, Spivack SD, & Brock GJ (2007). Overexpression of the MicroRNA hsa-miR-200c Leads to Reduced Expression of Transcription Factor 8 and Increased Expression of E-Cadherin. Cancer Research, 67(17), 7972–7976, doi: 10.1158/0008-5472.can-07-1058. [DOI] [PubMed] [Google Scholar]
- 318.Chen J, Shin VY, Siu MT, Ho JCW, Cheuk I, & Kwong A (2016). miR-199a-5p confers tumor-suppressive role in triple-negative breast cancer. BMC Cancer, 16(1), 887, doi: 10.1186/s12885-016-2916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lu P, Gu Y, Li L, Wang F, & Qiu X (2016). miR-544a Promotes Breast Cancer Cell Migration and Invasion Reducing Cadherin 1 Expression. Oncology Research, 23(4), 165–170, doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Huang S, Cai M, Zheng Y, Zhou L, Wang Q, & Chen L (2014). miR-888 in MCF-7 Side Population Sphere Cells Directly Targets E-cadherin. Journal of Genetics and Genomics, 41(1), 35–42, doi: 10.1016/j.jgg.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 321.Zhang W, Shi S, Jiang J, Li X, Lu H, & Ren F (2017). LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer. Biomedicine and Pharmacotherapy, 91, 312–319, doi: 10.1016/j.biopha.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 322.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, & Waltham M (2007). Vimentin and epithelial-mesenchymal transition in human breast cancer--observations in vitro and in vivo. Cells Tissues Organs, 185(1–3), 191–203, doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 323.Satelli A, & Li S (2011). Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and Molecular Life Sciences, 68(18), 3033–3046, doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Snider NT, & Omary MB (2014). Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nature Reviews Molecular Cell Biology, 15(3), 163–177, doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Furuta M, Kozaki K.-i., Tanaka S, Arii S, Imoto I, & Inazawa J (2010). miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis, 31(5), 766–776, doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- 326.Shan SW, Fang L, Shatseva T, Rutnam ZJ, Yang X, Du W, et al. (2013). Mature miR-17–5p and passenger miR-17–3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. Journal of Cell Science, 126(6), 1517–1530, doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 327.Deng Z, Du WW, Fang L, Shan SW, Qian J, Lin J, et al. (2013). The Intermediate Filament Vimentin Mediates MicroRNA miR-378 Function in Cellular Self-renewal by Regulating the Expression of the Sox2 Transcription Factor. Journal of Biological Chemistry, 288(1), 319–331, doi: 10.1074/jbc.M112.418830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dijke P. t., & Hill CS (2004). New insights into TGF-β-Smad signalling. Trends in Biochemical Sciences, 29(5), 265–273, doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 329.Evans MF, Vacek PM, Sprague BL, Stein GS, Stein JL, & Weaver DL (2020). Microarray and RNA in situ hybridization assay for recurrence risk markers of breast carcinoma and ductal carcinoma in situ: Evidence supporting the use of diverse pathways panels. Journal of Cellular Biochemistry, 121(2), 1736–1746, doi: 10.1002/jcb.29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Buck MB, & Knabbe C (2006). TGF-beta signaling in breast cancer. [10.1196/annals.1386.024]. Annals of the New York Academy of Sciences, 1089, 119–126, doi: 10.1196/annals.1386.024. [DOI] [PubMed] [Google Scholar]
- 331.Vennin C, Spruyt N, Dahmani F, Julien S, Bertucci F, Finetti P, et al. (2015). H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget, 6(30), 29209–29223, doi: 10.18632/oncotarget.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, et al. (2011). TGF-β Regulates miR-206 and miR-29 to Control Myogenic Differentiation through Regulation of HDAC4. Journal of Biological Chemistry, 286(16), 13805–13814, doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, et al. (2012). Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. Journal of the American Society of Nephrology, 23(2), 252–265, doi: 10.1681/asn.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wang Y, Liu J, Chen J, Feng T, & Guo Q (2015). MiR-29 mediates TGFbeta 1-induced extracellular matrix synthesis through activation of Wnt/beta -catenin pathway in human pulmonary fibroblasts. Technology and Health Care, 23 Suppl 1, S119–125, doi: 10.3233/thc-150943. [DOI] [PubMed] [Google Scholar]
- 335.Muluhngwi P, Alizadeh-Rad N, Vittitow SL, Kalbfleisch TS, & Klinge CM (2017). The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells. Sci Rep, 7(1), 5205, doi: 10.1038/s41598-017-05727-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S, et al. (2013). c-MYC-regulated miR-23a/24–2/27a Cluster Promotes Mammary Carcinoma Cell Invasion and Hepatic Metastasis by Targeting Sprouty2. Journal of Biological Chemistry, 288(25), 18121–18133, doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zhang W, Shi S, Jiang J, Li X, Lu H, & Ren F (2017). LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer. Biomedicine and Pharmacotherapy, 91(Supplement C), 312–319, doi: 10.1016/j.biopha.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 338.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, et al. (2010). Targeting Notch to Target Cancer Stem Cells. Clinical Cancer Research, 16(12), 3141–3152, doi: 10.1158/1078-0432.Ccr-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. (2015). Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature Reviews Clinical Oncology, 12(8), 445–464, doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. (2005). High-level Coexpression of JAG1 and NOTCH1 Is Observed in Human Breast Cancer and Is Associated with Poor Overall Survival. Cancer Research, 65(18), 8530–8537. [DOI] [PubMed] [Google Scholar]
- 341.Zanetti A, Affatato R, Centritto F, Fratelli M, Kurosaki M, Barzago MM, et al. (2015). All-trans-retinoic Acid Modulates the Plasticity and Inhibits the Motility of Breast Cancer Cells: ROLE OF NOTCH1 AND TRANSFORMING GROWTH FACTOR (TGFβ). Journal of Biological Chemistry, 290(29), 17690–17709, doi: 10.1074/jbc.M115.638510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Balamurugan K, Mendoza-Villanueva D, Sharan S, Summers GH, Dobrolecki LE, Lewis MT, et al. (2018). C/EBPδ links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene, 38(20), :3765–3780, doi: 10.1038/s41388-018-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Imani S, Wei C, Cheng J, Khan MA, Fu S, Yang L, et al. (2017). MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget, 8(13), 21362–21379, doi: 10.18632/oncotarget.15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang HD, Jiang LH, Sun DW, Li J, & Tang JH (2017). miR-30a inhibits the biological function of breast cancer cells by targeting Notch1. International Journal of Molecular Medicine, 40(4), 1235–1242, doi: 10.3892/ijmm.2017.3084. [DOI] [PubMed] [Google Scholar]
- 345.Li H. c., Chen Y. f., Feng W, Cai H, Mei Y, Jiang Y. m., et al. (2017). Loss of the Opa interacting protein 5 inhibits breast cancer proliferation through miR-139–5p/NOTCH1 pathway. Gene, 603, 1–8, doi: 10.1016/j.gene.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 346.Kong P, Chen L, Yu M, Tao J, Liu J, Wang Y, et al. (2018). miR-3178 inhibits cell proliferation and metastasis by targeting Notch1 in triple-negative breast cancer. Cell Death & Disease, 9(11), 1059, doi: 10.1038/s41419-018-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Guarnieri AL, Towers CG, Drasin DJ, Oliphant MUJ, Andrysik Z, Hotz TJ, et al. (2018). The miR-106b-25 cluster mediates breast tumor initiation through activation of NOTCH1 via direct repression of NEDD4L. Oncogene, 37(28), 3879–3893, doi: 10.1038/s41388-018-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Carson DD (2008). The cytoplasmic tail of MUC1: a very busy place. Sci Signal, 1(27), pe35, doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 349.Gendler SJ (2001). MUC1, the renaissance molecule. Journal of Mammary Gland Biology and Neoplasia, 6(3), 339–353. [DOI] [PubMed] [Google Scholar]
- 350.Kufe DW (2013). MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene, 32(9), 1073–1081, doi: 10.1023/a:1011379725811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Panchamoorthy G, Jin C, Raina D, Bharti A, Yamamoto M, Adeebge D, et al. (2018). Targeting the human MUC1-C oncoprotein with an antibody-drug conjugate. JCI Insight, 3(12), doi: 10.1172/jci.insight.99880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kufe DW (2009). Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer, 9(12), 874–885, doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, et al. (2009). MUC1-C Oncoprotein Functions as a Direct Activator of the Nuclear Factor-{kappa}B p65 Transcription Factor. Cancer Research, 69(17), 7013–7021, doi: 10.1158/0008-5472.can-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rajabi H, Alam M, Takahashi H, Kharbanda A, Guha M, Ahmad R, et al. (2014). MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. [Original Article]. Oncogene, 33(13), 1680–1689, doi: 10.1038/onc.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Alam M, Ahmad R, Rajabi H, Kharbanda A, & Kufe D (2013). MUC1-C Oncoprotein Activates ERK→C/EBPβ Signaling and Induction of Aldehyde Dehydrogenase 1A1 in Breast Cancer Cells. Journal of Biological Chemistry, 288, 30892–30903, doi: 10.1074/jbc.M113.477158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wei X, Xu H, & Kufe D (2006). MUC1 Oncoprotein Stabilizes and Activates Estrogen Receptor [alpha]. Molecular Cell, 21(2), 295–305, doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 357.Rajabi H, Hiraki M, Tagde A, Alam M, Bouillez A, Christensen CL, et al. (2017). MUC1-C activates EZH2 expression and function in human cancer cells. Sci Rep, 7(1), 7481, doi: 10.1038/s41598-017-07850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hata T, Rajabi H, Yamamoto M, Jin C, Ahmad R, Zhang Y, et al. (2019). Targeting MUC1-C Inhibits TWIST1 Signaling in Triple-Negative Breast Cancer. Molecular Cancer Therapeutics, 18(10), 1744, doi: 10.1158/1535-7163.MCT-19-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hata T, Rajabi H, Takahashi H, Yasumizu Y, Li W, Jin C, et al. (2019). MUC1-C Activates the NuRD Complex to Drive Dedifferentiation of Triple-Negative Breast Cancer Cells. Cancer Research, 79(22), 5711, doi: 10.1158/0008-5472.CAN-19-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sachdeva M, & Mo Y-Y (2010). MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Research, 70(1), 378–387, doi: 10.1158/0008-5472.can-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Derynck R, & Zhang YE (2003). Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature, 425(6958), 577–584, doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 362.Gulei D, Mehterov N, Ling H, Stanta G, Braicu C, & Berindan-Neagoe I (2017). The “good-cop bad-cop” TGF-beta role in breast cancer modulated by non-coding RNAs. Biochimica et Biophysica Acta (BBA) - General Subjects, 1861(7), 1661–1675, doi: 10.1016/j.bbagen.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 363.Dumont N, & Arteaga CL (2003). Targeting the TGFβ signaling network in human neoplasia. Cancer Cell, 3(6), 531–536, doi: 10.1016/S1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 364.Wang S, Huang M, Wang Z, Wang W, Zhang Z, Qu S, et al. (2019). MicroRNA133b targets TGFbeta receptor I to inhibit TGFbetainduced epithelialtomesenchymal transition and metastasis by suppressing the TGFbeta/SMAD pathway in breast cancer. International Journal of Oncology, 55(5), 1097–1109, doi: 10.3892/ijo.2019.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wang J, Liang S, & Duan X (2019). Molecular mechanism of miR-153 inhibiting migration, invasion and epithelial-mesenchymal transition of breast cancer by regulating transforming growth factor beta (TGF-β) signaling pathway. Journal of Cellular Biochemistry, 120(6), 9539–9546, doi: 10.1002/jcb.28230. [DOI] [PubMed] [Google Scholar]
- 366.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, et al. (2012). MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene, 31(37), 4150–4163, doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 367.Heldin C-H, Vanlandewijck M, & Moustakas A (2012). Regulation of EMT by TGFβ in cancer. FEBS Letters, 586(14), 1959–1970, doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 368.Hao Y, Baker D, & ten Dijke P (2019). TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. International Journal of Molecular Sciences, 20(11), doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Yu Y, Luo W, Yang Z-J, Chi J-R, Li Y-R, Ding Y, et al. (2018). miR-190 suppresses breast cancer metastasis by regulation of TGF-β-induced epithelial-mesenchymal transition. [journal article]. Molecular Cancer, 17(1), 70, doi: 10.1186/s12943-018-0818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin C-H, & Moustakas A (2006). Transforming growth factor-β employs HMGA2 to elicit epithelial-mesenchymal transition. The Journal of Cell Biology, 174(2), 175–183, doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Hinz N, & Jücker M (2019). Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Communication and Signaling, 17(1), 154, doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zeltz C, Primac I, Erusappan P, Alam J, Noel A, & Gullberg D (2019). Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Seminars in Cancer Biology, in press, corrected proof, doi: 10.1016/j.semcancer.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 373.Phillips S, & Kuperwasser C (2014). SLUG: Critical regulator of epithelial cell identity in breast development and cancer. Cell Adh Migr, 8(6), 578–587, doi: 10.4161/19336918.2014.972740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Jiang Y, Zhao X, Xiao Q, Liu Q, Ding K, Yu F, et al. (2014). Snail and Slug mediate tamoxifen resistance in breast cancer cells through activation of EGFR-ERK independent of epithelial-mesenchymal transition. J Mol Cell Biol, 6(4), 352–354, doi: 10.1093/jmcb/mju019. [DOI] [PubMed] [Google Scholar]
- 375.Alves CL, Elias D, Lyng MB, Bak M, & Ditzel HJ (2018). SNAI2 upregulation is associated with an aggressive phenotype in fulvestrant-resistant breast cancer cells and is an indicator of poor response to endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Research, 20(1), 60, doi: 10.1186/s13058-018-0988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. (2008). A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Research, 68(19), 7846–7854, doi: 10.1158/0008-5472.can-08-1942. [DOI] [PubMed] [Google Scholar]
- 377.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, & Richer JK (2009). MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Molecular Cancer Therapeutics, 8(5), 1055–1066, doi: 10.1158/1535-7163.mct-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sossey-Alaoui K, Bialkowska K, & Plow EF (2009). The miR200 Family of MicroRNAs Regulates WAVE3-dependent Cancer Cell Invasion. Journal of Biological Chemistry, 284(48), 33019–33029, doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Cochrane D, Cittelly D, Howe E, Spoelstra N, McKinsey E, LaPara K, et al. (2010). MicroRNAs Link Estrogen Receptor Alpha Status and Dicer Levels in Breast Cancer. Hormones and Cancer, 1(6), 306–319, doi: 10.1007/s12672-010-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zeng Y, Gao T, Huang W, Yang Y, Qiu R, Hou Y, et al. (2019). MicroRNA-455–3p mediates GATA3 tumor suppression in mammary epithelial cells by inhibiting TGF-β signaling. Journal of Biological Chemistry, 294(43), 15808–15825, doi: 10.1074/jbc.RA119.010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Manavalan TT, Teng Y, Appana SN, Datta S, Kalbfleisch TS, Li Y, et al. (2011). Differential expression of microRNA expression in tamoxifen-sensitive MCF-7 versus tamoxifen-resistant LY2 human breast cancer cells. Cancer Letters, 313(1), 26–43, doi: 10.1016/j.canlet.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Hurteau GJ, Carlson JA, Spivack SD, & Brock GJ (2007). Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res, 67(17), 7972–7976, doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 383.Park SM, Gaur AB, Lengyel E, & Peter ME (2008). The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Genes Dev, 22(7), 894–907, doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Su Y, Hopfinger NR, Nguyen TD, Pogash TJ, Santucci-Pereira J, & Russo J (2018). Epigenetic reprogramming of epithelial mesenchymal transition in triple negative breast cancer cells with DNA methyltransferase and histone deacetylase inhibitors. Journal of Experimental and Clinical Cancer Research, 37(1), 314, doi: 10.1186/s13046-018-0988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tomaselli D, Lucidi A, Rotili D, & Mai A (2020). Epigenetic polypharmacology: A new frontier for epi-drug discovery. Medicinal Research Reviews, 40(1), 190–244, doi: 10.1002/med.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34, doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 387.Hsu S-D, Tseng Y-T, Shrestha S, Lin Y-L, Khaleel A, Chou C-H, et al. (2014). miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Research, 42(D1), D78–D85, doi: 10.1093/nar/gkt1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Iyevleva A, Kuligina E, Mitiushkina N, Togo A, Miki Y, & Imyanitov E (2011). High level of miR-21, miR-10b, and miR-31 expression in bilateral vs. unilateral breast carcinomas. Breast Cancer Research and Treatment, 131(3), 1–11, doi: 10.1007/s10549-011-1845-z. [DOI] [PubMed] [Google Scholar]
- 389.Negrini M, & Calin GA (2008). Breast cancer metastasis: a microRNA story. Breast Cancer Res, 10(2), 203, doi: 10.1186/bcr1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kim J, Siverly AN, Chen D, Wang M, Yuan Y, Wang Y, et al. (2016). Ablation of miR-10b Suppresses Oncogene-Induced Mammary Tumorigenesis and Metastasis and Reactivates Tumor-Suppressive Pathways. [10.1158/0008–5472.CAN-16–1571]. Cancer Research, 76(21), 6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhang J, Yang J, Zhang X, Xu J, Sun Y, & Zhang P (2018). MicroRNA-10b expression in breast cancer and its clinical association. PLoS ONE, 13(2), e0192509, doi: 10.1371/journal.pone.0192509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, et al. (2008). MicroRNA-21 Promotes Cell Transformation by Targeting the Programmed Cell Death 4 Gene. Oncogene, 27(31), 4373–4379. [DOI] [PubMed] [Google Scholar]
- 393.Wickramasinghe N, Manavalan T, Dougherty S, Riggs K, Li Y, & Klinge C (2009). Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res, 37(8), 2584–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Hannafon B, Sebastiani P, de las Morenas A, Lu J, & Rosenberg C (2011). Expression of microRNAs and their gene targets are dysregulated in pre-invasive breast cancer. Breast Cancer Research, 13(2), R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zhu S, Si M-L, Wu H, & Mo Y-Y (2007). MicroRNA-21 Targets the Tumor Suppressor Gene Tropomyosin 1 (TPM1). Journal of Biological Chemistry, 282(19), 14328–14336, doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 396.Queiros AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, et al. (2013). Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. International Journal of Cardiology, 169(5), 331–338, doi: 10.1016/j.ijcard.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 397.Kanwal R, Plaga AR, Liu X, Shukla GC, & Gupta S (2017). MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Letters, 407(Supplement C), 9–20, doi: 10.1016/j.canlet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 398.Petrovic N (2016). miR-21 Might be Involved in Breast Cancer Promotion and Invasion Rather than in Initial Events of Breast Cancer Development. Mol Diagn Ther, 20(2), 97–110, doi: 10.1007/s40291-016-0186-3. [DOI] [PubMed] [Google Scholar]
- 399.Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C, et al. (2019). microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer, 19(1), 738, doi: 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Liu L-Z, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. (2011). MiR-21 Induced Angiogenesis through AKT and ERK Activation and HIF-1α Expression. PLoS ONE, 6(4), e19139, doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, & Struhl K (2010). STAT3 Activation of miR-21 and miR-181b-1 via PTEN and CYLD Are Part of the Epigenetic Switch Linking Inflammation to Cancer. Molecular Cell, 39(4), 493–506, doi:DOI: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. (2005). MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Research, 65(16), 7065–7070. [DOI] [PubMed] [Google Scholar]
- 403.Markou A, Yousef GM, Stathopoulos E, Georgoulias V, & Lianidou E (2014). Prognostic Significance of Metastasis-Related MicroRNAs in Early Breast Cancer Patients with a Long Follow-up. Clinical Chemistry, 60(1), 197–205, doi: 10.1373/clinchem.2013.210542. [DOI] [PubMed] [Google Scholar]
- 404.De Mattos-Arruda L, Bottai G, Nuciforo PG, Di Tommaso L, Giovannetti E, Peg V, et al. (2015). MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget, 6(35), 37269–37280, doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Chang L, Zhang D, Shi H, Bian Y, & Guo R (2017). MiR-143 inhibits endometrial cancer cell proliferation and metastasis by targeting MAPK1. Oncotarget, 8(48), 84384–84395, doi: 10.18632/oncotarget.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhang Q, Feng Y, Liu P, & Yang J (2017). MiR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer. Tumour Biology, 39(7), 1010428317711312, doi: 10.1177/1010428317711312. [DOI] [PubMed] [Google Scholar]
- 407.Wang JH, Wang XW, Qu D, Sun JW, Guo FX, & Lu D (2017). Upregulation of microRNA-143 reverses drug resistance in human breast cancer cells via inhibition of cytokine-induced apoptosis inhibitor 1. Oncol Lett, 13(6), 4695–4700, doi: 10.3892/ol.2017.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ma Z, Luo Y, & Qiu M (2017). miR-143 Induces the Apoptosis of Prostate Cancer LNCap Cells by Suppressing Bcl-2 Expression. Med Sci Monit, 23, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Yang F, Zhang W, Shen Y, & Guan X (2015). Identification of dysregulated microRNAs in triple-negative breast cancer (review). International Journal of Oncology, 46(3), 927–932, doi: 10.3892/ijo.2015.2821. [DOI] [PubMed] [Google Scholar]
- 410.Fite K, & Gomez-Cambronero J (2016). Down-regulation of MicroRNAs (MiRs) 203, 887, 3619 and 182 Prevents Vimentin-triggered, Phospholipase D (PLD)-mediated Cancer Cell Invasion. Journal of Biological Chemistry, 291(2), 719–730, doi: 10.1074/jbc.M115.686006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Yan GR, Xu SH, Tan ZL, Liu L, & He QY (2011). Global identification of miR-373-regulated genes in breast cancer by quantitative proteomics. Proteomics, 11(5), 912–920, doi: 10.1002/pmic.201000539. [DOI] [PubMed] [Google Scholar]
- 412.Jiang H, Zhang G, Wu JH, & Jiang CP (2014). Diverse roles of miR-29 in cancer (review). Oncology Reports, 31(4), 1509–1516, doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 413.Milevskiy MJG, Sandhu GK, Wronski A, Korbie D, Brewster BL, Shewan A, et al. (2018). MiR-29b-1–5p is altered in BRCA1 mutant tumours and is a biomarker in basal-like breast cancer. Oncotarget, 9(71), 33577–33588, doi: 10.18632/oncotarget.26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Scott GK, Mattie MD, Berger CE, Benz SC, & Benz CC (2006). Rapid Alteration of MicroRNA Levels by Histone Deacetylase Inhibition. Cancer Research, 66(3), 1277–1281. [DOI] [PubMed] [Google Scholar]
- 415.Jiang G, Shi W, Fang H, & Zhang X (2018). miR-27a promotes human breast cancer cell migration by inducing EMT in a FBXW7dependent manner. Mol Med Rep, 18(6), 5417–5426, doi: 10.3892/mmr.2018.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, & Yokoi T (2006). MicroRNA Regulates the Expression of Human Cytochrome P450 1B1. Cancer Research, 66(18), 9090–9098, doi: 10.1158/0008-5472.can-06-1403. [DOI] [PubMed] [Google Scholar]
- 417.Vimalraj S, Miranda PJ, Ramyakrishna B, & Selvamurugan N (2013). Regulation of breast cancer and bone metastasis by microRNAs. Disease Markers, 35(5), 369–387, doi: 10.1155/2013/451248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, et al. (2008). A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. Journal of Cell Biology, 182(3), 509–517, doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang TH, et al. (2013). MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene, 33, 3014, doi: 10.1038/onc.2013.258. [DOI] [PubMed] [Google Scholar]
- 420.Ouchida M, Kanzaki H, Ito S, Hanafusa H, Jitsumori Y, Tamaru S, et al. (2012). Novel direct targets of miR-19a identified in breast cancer cells by a quantitative proteomic approach. PLoS ONE, 7(8), e44095, doi: 10.1371/journal.pone.0044095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wu J, Jiang Y, Cao W, Li X, Xie C, Geng S, et al. (2018). miR-19 targeting of PTEN mediates butyl benzyl phthalate-induced proliferation in both ER(+) and ER(−) breast cancer cells. Toxicology Letters, 295, 124–133, doi: 10.1016/j.toxlet.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 422.Mogilyansky E, Clark P, Quann K, Zhou H, Londin E, Jing Y, et al. (2016). Post-transcriptional Regulation of BRCA2 through Interactions with miR-19a and miR-19b. [Original Research]. Frontiers in Genetics, 7(143), doi: 10.3389/fgene.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Sochor M, Basova P, Pesta M, Dusilkova N, Bartos J, Burda P, et al. (2014). Oncogenic microRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer, 14, 448, doi: 10.1186/1471-2407-14-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, et al. (2008). MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27(Kip1). Journal of Biological Chemistry, 283(44), 29897–29903, doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Lu Y, Roy S, Nuovo G, Ramaswamy B, Miller T, Shapiro C, et al. (2011). Anti-microRNA-222 (Anti-miR-222) and −181B Suppress Growth of Tamoxifen-resistant Xenografts in Mouse by Targeting TIMP3 Protein and Modulating Mitogenic Signal. Journal of Biological Chemistry, 286(49), 42292–42302, doi: 10.1074/jbc.M111.270926. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 426.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, & Brizzi MF (2010). microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arteriosclerosis, Thrombosis, and Vascular Biology, 30(8), 1562–1568, doi: 10.1161/atvbaha.110.206201. [DOI] [PubMed] [Google Scholar]
- 427.Zhang Y, Lin X, Zhang L, Hong W, & Zeng K (2018). MicroRNA-222 regulates the viability of fibroblasts in hypertrophic scars via matrix metalloproteinase 1. Exp Ther Med, 15(2), 1803–1808, doi: 10.3892/etm.2017.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Li Y, Liang C, Ma H, Zhao Q, Lu Y, Xiang Z, et al. (2014). miR-221/222 promotes S-phase entry and cellular migration in control of basal-like breast cancer. Molecules, 19(6), 7122–7137, doi: 10.3390/molecules19067122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Chatterjee A, Jana S, Chatterjee S, Wastall LM, Mandal G, Nargis N, et al. (2019). MicroRNA-222 reprogrammed cancer-associated fibroblasts enhance growth and metastasis of breast cancer. British Journal of Cancer, 121(8), 679–689., doi: 10.1038/s41416-019-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Wu Q, Lu Z, Li H, Lu J, Guo L, & Ge Q (2011). Next-generation sequencing of microRNAs for breast cancer detection. J Biomed Biotechnol, 2011, 597145, doi: 10.1155/2011/597145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Kim C, Go EJ, & Kim A (2018). Recurrence prediction using microRNA expression in hormone receptor positive breast cancer during tamoxifen treatment. Biomarkers, 23(8), 84–811, doi: 10.1080/1354750x.2018.1499131. [DOI] [PubMed] [Google Scholar]
- 432.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, et al. (2006). Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer, 5(24), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. (2007). MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol, 8(10), R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Oztemur Y, Bekmez T, Aydos A, Yulug IG, Bozkurt B, & Dedeoglu BG (2015). A ranking-based meta-analysis reveals let-7 family as a meta-signature for grade classification in breast cancer. PLoS ONE, 10(5), e0126837, doi: 10.1371/journal.pone.0126837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Humphries B, & Yang C (2015). The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget, 6(9), 6472–6498, doi: 10.18632/oncotarget.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon Y-J, Volinia S, et al. (2011). p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. The Journal of Experimental Medicine, 208(5), 875–883, doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Menschikowski M, Hagelgans A, Nacke B, Jandeck C, Sukocheva O, & Siegert G (2015). Epigenetic control of phospholipase A2 receptor expression in mammary cancer cells. BMC Cancer, 15, 971, doi: 10.1186/s12885-015-1937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yao YS, Qiu WS, Yao RY, Zhang Q, Zhuang LK, Zhou F, et al. (2015). miR-141 confers docetaxel chemoresistance of breast cancer cells via regulation of EIF4E expression. Oncology Reports, 33(5), 2504–2512, doi: 10.3892/or.2015.3866. [DOI] [PubMed] [Google Scholar]
- 439.Abedi N, Mohammadi-Yeganeh S, Koochaki A, Karami F, & Paryan M (2015). miR-141 as potential suppressor of beta-catenin in breast cancer. Tumour Biology, 36(12), 9895–9901, doi: 10.1007/s13277-015-3738-y. [DOI] [PubMed] [Google Scholar]
- 440.Li P, Xu T, Zhou X, Liao L, Pang G, Luo W, et al. (2017). Downregulation of miRNA-141 in breast cancer cells is associated with cell migration and invasion: involvement of ANP32E targeting. Cancer Med, 6(3), 662–672, doi: 10.1002/cam4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Chen L, Li Y, Fu Y, Peng J, Mo M-H, Stamatakos M, et al. (2013). Role of Deregulated microRNAs in Breast Cancer Progression Using FFPE Tissue. PLoS ONE, 8(1), e54213, doi: 10.1371/journal.pone.0054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Antolin S, Calvo L, Blanco-Calvo M, Santiago MP, Lorenzo-Patino MJ, Haz-Conde M, et al. (2015). Circulating miR-200c and miR-141 and outcomes in patients with breast cancer. BMC Cancer, 15, 297, doi: 10.1186/s12885-015-1238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Debeb BG, Lacerda L, Anfossi S, Diagaradjane P, Chu K, Bambhroliya A, et al. (2016). miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer. JNCI: Journal of the National Cancer Institute, 108(8), doi: 10.1093/jnci/djw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zhang G, Zhang W, Li B, Stringer-Reasor E, Chu C, Sun L, et al. (2017). MicroRNA-200c and microRNA-141 are regulated by a FOXP3-KAT2B axis and associated with tumor metastasis in breast cancer. Breast Cancer Research, 19(1), 73, doi: 10.1186/s13058-017-0858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Finlay-Schultz J, Cittelly DM, Hendricks P, Patel P, Kabos P, Jacobsen BM, et al. (2015). Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. [Original Article]. Oncogene, 34(28), 3676–3687, doi: 10.1038/onc.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Leivonen S-K, Rokka A, Östling P, Kohonen P, Corthals GL, Kallioniemi O, et al. (2011). Identification of miR-193b targets in breast cancer cells and systems biological analysis of their functional impact. Molecular & Cellular Proteomics, 10(7), M110 005322, doi: 10.1074/mcp.M110.005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Chen Y, & Zhang L (2017). Members of the microRNA-200 family are promising therapeutic targets in cancer. Exp Ther Med, 14(1), 10–17, doi: 10.3892/etm.2017.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Yahya SMM, & Elsayed GH (2015). A summary for molecular regulations of miRNAs in breast cancer. Clinical Biochemistry, 48(6), 388–396, doi: 10.1016/j.clinbiochem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 449.De Cola A, Volpe S, Budani MC, Ferracin M, Lattanzio R, Turdo A, et al. (2015). miR-205–5p-mediated downregulation of ErbB/HER receptors in breast cancer stem cells results in targeted therapy resistance. Cell Death Dis, 6, e1823, doi: 10.1038/cddis.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Greene SB, Herschkowitz JI, & Rosen JM (2010). The ups and downs of miR-205: identifying the roles of miR-205 in mammary gland development and breast cancer. RNA Biol, 7(3), 300–304, doi:11837 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Chao C-H, Chang C-C, Wu M-J, Ko H-W, Wang D, Hung M-C, et al. (2014). MicroRNA-205 signaling regulates mammary stem cell fate and tumorigenesis. The Journal of Clinical Investigation, 124(7), 0–0, doi: 10.1172/JCI73351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Xiao Y, Li Y, Tao H, Humphries B, Li A, Jiang Y, et al. (2018). Integrin α5 down-regulation by miR-205 suppresses triple negative breast cancer stemness and metastasis by inhibiting the Src/Vav2/Rac1 pathway. Cancer Letters, 433(1), 199–209, doi: 10.1016/j.canlet.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 453.Hasegawa T, Adachi R, Iwakata H, Takeno T, Sato K, & Sakamaki T (2017). ErbB2 signaling epigenetically suppresses microRNA-205 transcription via Ras/Raf/MEK/ERK pathway in breast cancer. FEBS Open Bio, 7(8), 1154–1165, doi: 10.1002/2211-5463.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Zhang H, Li B, Zhao H, & Chang J (2015). The expression and clinical significance of serum miR-205 for breast cancer and its role in detection of human cancers. Int J Clin Exp Med, 8(2), 3034–3043. [PMC free article] [PubMed] [Google Scholar]
- 455.Korpal M, Lee ES, Hu G, & Kang Y (2008). The miR-200 Family Inhibits Epithelial-Mesenchymal Transition and Cancer Cell Migration by Direct Targeting of E-cadherin Transcriptional Repressors ZEB1 and ZEB2. Journal of Biological Chemistry, 283(22), 14910–14914, doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Uhlmann S, Zhang JD, Schwager A, Mannsperger H, Riazalhosseini Y, Burmester S, et al. (2010). miR-200bc/429 cluster targets PLC[gamma]1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene, 29(30), 4297–4306, doi: 10.1038/onc.2010.201. [DOI] [PubMed] [Google Scholar]
- 457.Castilla MÁ, Díaz-Martín J, Sarrió D, Romero-Pérez L, López-García MÁ, Vieites B, et al. (2012). MicroRNA-200 Family Modulation in Distinct Breast Cancer Phenotypes. PLoS ONE, 7(10), e47709, doi: 10.1371/journal.pone.0047709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Liu Q, Wang W, Yang X, Zhao D, Li F, & Wang H (2016). MicroRNA-146a inhibits cell migration and invasion by targeting RhoA in breast cancer. Oncology Reports, 36(1), 189–196, doi: 10.3892/or.2016.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Song G, Zhang Y, & Wang L (2009). MicroRNA-206 Targets notch3, Activates Apoptosis, and Inhibits Tumor Cell Migration and Focus Formation. Journal of Biological Chemistry, 284(46), 31921–31927, doi: 10.1074/jbc.M109.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. (2008). Endogenous human microRNAs that suppress breast cancer metastasis. Nature, 451(7175), 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Heyn H, Engelmann M, Schreek S, Ahrens P, Lehmann U, Kreipe H, et al. (2011). MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. International Journal of Cancer, 129(12), 2797–2806, doi: 10.1002/ijc.25962. [DOI] [PubMed] [Google Scholar]
- 462.Kasinski AL, & Slack FJ (2011). MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. [10.1038/nrc3166]. Nat Rev Cancer, 11(12), 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Martin EC, Conger AK, Yan TJ, Hoang VT, Miller DFB, Buechlein A, et al. (2017). MicroRNA-335–5p and −3p Synergize to Inhibit Estrogen Receptor Alpha Expression and Promote Tamoxifen Resistance. FEBS Letters, 591(2), 382–392, doi: 10.1002/1873-3468.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Augoff K, Das M, Bialkowska K, McCue B, Plow EF, & Sossey-Alaoui K (2011). miR-31 Is a Broad Regulator of β1-Integrin Expression and Function in Cancer Cells. Molecular Cancer Research, 9(11), 1500-, doi: 10.1158/1541-7786.mcr-11-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Chan YT, Lin YC, Lin RJ, Kuo HH, Thang WC, Chiu KP, et al. (2013). Concordant and discordant regulation of target genes by miR-31 and its isoforms. PLoS ONE, 8(3), e58169, doi: 10.1371/journal.pone.0058169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Luo L. j., Yang F, Ding J.-j., Yan D.-l., Wang D.-d., Yang S.-j., et al. (2016). MiR-31 inhibits migration and invasion by targeting SATB2 in triple negative breast cancer. Gene, 594(1), 47–58, doi: 10.1016/j.gene.2016.08.057. [DOI] [PubMed] [Google Scholar]
- 467.Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, et al. (2014). MicroRNA Profiling Implies New Markers of Chemoresistance of Triple-Negative Breast Cancer. PLoS ONE, 9(5), e96228, doi: 10.1371/journal.pone.0096228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Riaz M, van Jaarsveld MT, Hollestelle A, Prager-van der Smissen WJ, Heine AA, Boersma AW, et al. (2013). miRNA expression profiling of 51 human breast cancer cell lines reveals subtype and driver mutation-specific miRNAs. Breast Cancer Res, 15(2), R33, doi: 10.1186/bcr3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Viré E, Curtis C, Davalos V, Git A, Robson S, Villanueva A, et al. (2014). The Breast Cancer Oncogene EMSY Represses Transcription of Antimetastatic microRNA miR-31. Molecular Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Mulrane L, Gallagher WM, & O’Connor DP (2014). A novel mechanism of regulation of the anti-metastatic miR-31 by EMSY in breast cancer. Breast Cancer Res, 16(6), 467, doi: 10.1186/s13058-014-0467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kratassiouk G, Pritchard LL, Cuvellier S, Vislovukh A, Meng Q, Groisman R, et al. (2016). The WEE1 regulators CPEB1 and miR-15b switch from inhibitor to activators at G2/M. Cell Cycle, 15(5), 667–677, doi: 10.1080/15384101.2016.1147631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Rivas MA, Venturutti L, Huang Y-W, Schillaci R, Huang TH-M, & Elizalde PV (2012). Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Research, 14(3), R77, doi: 10.1186/bcr3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. (2005). miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences, 102(39), 13944–13949, doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Liang H, Fu Z, Jiang X, Wang N, Wang F, Wang X, et al. (2015). miR-16 promotes the apoptosis of human cancer cells by targeting FEAT. BMC Cancer, 15(1), 448, doi: 10.1186/s12885-015-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, & Kerin MJ (2010). Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Annals of Surgery, 251(3), 499–505, doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 476.Rinnerthaler G, Hackl H, Gampenrieder SP, Hamacher F, Hufnagl C, Hauser-Kronberger C, et al. (2016). miR-16–5p Is a Stably-Expressed Housekeeping MicroRNA in Breast Cancer Tissues from Primary Tumors and from Metastatic Sites. Int J Mol Sci, 17(2), doi: 10.3390/ijms17020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Stuckrath I, Rack B, Janni W, Jager B, Pantel K, & Schwarzenbach H (2015). Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget, 6(15), 13387–13401, doi: 10.18632/oncotarget.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, et al. (2006). A microRNA expression signature of human solid tumors defines cancer gene targets. Proceedings of the National Academy of Sciences, 103(7), 2257–2261, doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, et al. (2007). An E2F/miR-20a Autoregulatory Feedback Loop. Journal of Biological Chemistry, 282(4), 2135–2143, doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 480.Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, et al. (2010). microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proceedings of the National Academy of Sciences, 107(18), 8231–8236, doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kim K, Chadalapaka G, Lee SO, Yamada D, Sastre-Garau X, Defossez PA, et al. (2012). Identification of oncogenic microRNA-17–92/ZBTB4/specificity protein axis in breast cancer. Oncogene, 31(8), 1034–1044, doi: 10.1016/j.toxlet.2018.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Liu L, He J, Wei X, Wan G, Lao Y, Xu W, et al. (2017). MicroRNA-20a-mediated loss of autophagy contributes to breast tumorigenesis by promoting genomic damage and instability. [Original Article]. Oncogene, 36(42), 5874–5884, doi: 10.1038/onc.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Bai X, Han G, Liu Y, Jiang H, & He Q (2018). MiRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3. Biomedicine and Pharmacotherapy, 103, 1482–1489, doi: 10.1016/j.biopha.2018.04.165. [DOI] [PubMed] [Google Scholar]
- 484.Zhao W, Geng D, Li S, Chen Z, & Sun M (2018). LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med, 7(3), 842–855, doi: 10.1002/cam4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 485.Moi L, Braaten T, Al-Shibli K, Lund E, & Busund L-TR (2019). Differential expression of the miR-17–92 cluster and miR-17 family in breast cancer according to tumor type; results from the Norwegian Women and Cancer (NOWAC) study. Journal of Translational Medicine, 17(1), 334, doi: 10.1186/s12967-019-2086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Braicu C, Raduly L, Morar-Bolba G, Cojocneanu R, Jurj A, Pop L-A, et al. (2018). Aberrant miRNAs expressed in HER-2 negative breast cancers patient. Journal of Experimental and Clinical Cancer Research, 37(1), 257, doi: 10.1186/s13046-018-0920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, et al. (2009). The estrogen receptor-alpha induced microRNA signature regulates itself and its transcriptional response. Proceedings of the National Academy of Sciences, USA, 106(37), 15732–15737, doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Giraud-Triboult K, Rochon-Beaucourt C, Nissan X, Champon B, Aubert S, & Piétu G (2011). Combined mRNA and microRNA profiling reveals that miR-148a and miR-20b control human mesenchymal stem cell phenotype via EPAS1. Physiological Genomics, 43(2), 77–86, doi: 10.1152/physiolgenomics.00077.2010. [DOI] [PubMed] [Google Scholar]
- 489.Ao X, Nie P, Wu B, Xu W, Zhang T, Wang S, et al. (2016). Decreased expression of microRNA-17 and microRNA-20b promotes breast cancer resistance to taxol therapy by upregulation of NCOA3. [Original Article]. Cell Death Dis, 7, e2463, doi: 10.1038/cddis.2016.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Li D, Ilnytskyy Y, Kovalchuk A, Khachigian LM, Bronson RT, Wang B, et al. (2013). Crucial role for early growth response-1 in the transcriptional regulation of miR-20b in breast cancer. Oncotarget, 4(9), 1373–1387, doi: 10.18632/oncotarget.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Landais S, Landry S, Legault P, & Rassart E (2007). Oncogenic potential of the miR-106–363 cluster and its implication in human T-cell leukemia. Cancer Research, 67(12), 5699–5707, doi: 10.1158/0008-5472.can-06-4478. [DOI] [PubMed] [Google Scholar]
- 492.Xue TM, Tao LD, Zhang M, Zhang J, Liu X, Chen GF, et al. (2015). Clinicopathological Significance of MicroRNA-20b Expression in Hepatocellular Carcinoma and Regulation of HIF-1alpha and VEGF Effect on Cell Biological Behaviour. Disease Markers, 2015, 325176, doi: 10.1155/2015/325176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.He J, Zhang JF, Yi C, Lv Q, Xie WD, Li JN, et al. (2010). miRNA-mediated functional changes through co-regulating function related genes. PLoS ONE, 5(10), e13558, doi: 10.1371/journal.pone.0013558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Wang W, Feng L, Zhang H, Hachy S, Satohisa S, Laurent LC, et al. (2012). Preeclampsia Up-Regulates Angiogenesis-Associated MicroRNA (i.e., miR-17, −20a, and −20b) That Target Ephrin-B2 and EPHB4 in Human Placenta. The Journal of Clinical Endocrinology & Metabolism, 97(6), E1051–E1059, doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wang B, Yang J, & Xiao B (2016). MicroRNA-20b (miR-20b) Promotes the Proliferation, Migration, Invasion, and Tumorigenicity in Esophageal Cancer Cells via the Regulation of Phosphatase and Tensin Homologue Expression. PLoS ONE, 11(10), e0164105, doi: 10.1371/journal.pone.0164105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 496.Hong S, Yu S, Li J, Yin Y, Liu Y, Zhang Q, et al. (2016). MiR-20b Displays Tumor-Suppressor Functions in Papillary Thyroid Carcinoma by Regulating the MAPK/ERK Signaling Pathway. Thyroid, 26(12), 1733–1743, doi: 10.1089/thy.2015.0578. [DOI] [PubMed] [Google Scholar]
- 497.Pérez-Rivas LG, Jerez JM, Carmona R, de Luque V, Vicioso L, Claros MG, et al. (2014). A microRNA Signature Associated with Early Recurrence in Breast Cancer. PLoS ONE, 9(3), e91884, doi: 10.1371/journal.pone.0091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Li M, Zhou Y, Xia T, Zhou X, Huang Z, Zhang H, et al. (2018). Circulating microRNAs from the miR-106a-363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Research and Treatment, 170(2), 257–270, doi: 10.1007/s10549-018-4757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Li G, Li L, Sun Q, Wu J, Ge W, Lu G, et al. (2018). MicroRNA-3200–5p Promotes Osteosarcoma Cell Invasion via Suppression of BRMS1. Molecules and Cells, 41(6), 523–531, doi: 10.14348/molcells.2018.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Guo Z, Li J, Sun J, Sun L, Zhou Y, & Yu Z (2018). miR-346 Promotes HCC Progression by Suppressing Breast Cancer Metastasis Suppressor 1 Expression. Oncology Research, 26(7), 1073–1081, doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Meehan WJ, Samant RS, Hopper JE, Carrozza MJ, Shevde LA, Workman JL, et al. (2004). Breast Cancer Metastasis Suppressor 1 (BRMS1) Forms Complexes with Retinoblastoma-binding Protein 1 (RBP1) and the mSin3 Histone Deacetylase Complex and Represses Transcription. Journal of Biological Chemistry, 279(2), 1562–1569. [DOI] [PubMed] [Google Scholar]
- 502.Hicks DG, Yoder BJ, Short S, Tarr S, Prescott N, Crowe JP, et al. (2006). Loss of Breast Cancer Metastasis Suppressor 1 Protein Expression Predicts Reduced Disease-Free Survival in Subsets of Breast Cancer Patients. Clinical Cancer Research, 12(22), 6702–6708, doi: 10.1158/1078-0432.ccr-06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Li Q. j., Zhou L, Yang F, Wang G.-x., Zheng H, Wang D. s., et al. (2012). MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumor Biology, 33(5), 1455–1465, doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 504.Minami A, Shimono Y, Mizutani K, Nobutani K, Momose K, Azuma T, et al. (2013). Reduction of the ST6 β-Galactosamide α−2,6-Sialyltransferase 1 (ST6GAL1)-catalyzed Sialylation of Nectin-like Molecule 2/Cell Adhesion Molecule 1 and Enhancement of ErbB2/ErbB3 Signaling by MicroRNA-199a. Journal of Biological Chemistry, 288(17), 11845–11853, doi: 10.1074/jbc.M112.405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Takahashi Y, Iwai M, Kawai T, Arakawa A, Ito T, Sakurai-Yageta M, et al. (2012). Aberrant expression of tumor suppressors CADM1 and 4.1B in invasive lesions of primary breast cancer. Breast Cancer, 19(3), 242–252, doi: 10.1007/s12282-011-0272-7. [DOI] [PubMed] [Google Scholar]
- 506.Ulasov IV, Borovjagin AV, Timashev P, Cristofanili M, & Welch DR (2019). KISS1 in breast cancer progression and autophagy. Cancer and Metastasis Reviews, doi: 10.1007/s10555-019-09814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Martin TA, Watkins G, & Jiang WG (2005). KiSS-1 expression in human breast cancer. Clinical and Experimental Metastasis, 22(6), 503–511, doi: 10.1007/s10585-005-4180-0. [DOI] [PubMed] [Google Scholar]
- 508.Garcia M, Thirouard L, Sedès L, Monrose M, Holota H, Caira F, et al. (2018). Nuclear Receptor Metabolism of Bile Acids and Xenobiotics: A Coordinated Detoxification System with Impact on Health and Diseases. International Journal of Molecular Sciences, 19(11), 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Ma Y, Qin H, & Cui Y (2013). MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochemical and Biophysical Research Communications, 441(4), 958–963, doi: 10.1016/j.bbrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 510.Mine M, Yamaguchi K, Sugiura T, Chigita S, Yoshihama N, Yoshihama R, et al. (2015). miR-203 Inhibits Frizzled-2 Expression via CD82/KAI1 Expression in Human Lung Carcinoma Cells. PLoS ONE, 10(7), e0131350, doi: 10.1371/journal.pone.0131350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Xu L, Hou Y, Tu G, Chen Y, Du Y. e., Zhang H, et al. (2017). Nuclear Drosha enhances cell invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in gastric cancer. Cell Death & Disease, 8, e2642, doi: 10.1038/cddis.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Yang X, Wei L, Tang C, Slack R, Montgomery E, & Lippman M (2000). KAI1 protein is downregulated during the progression of human breast cancer. Clinical Cancer Research, 6(9), 3424–3429. [PubMed] [Google Scholar]
- 513.Nilsson EM, Laursen KB, Whitchurch J, McWilliam A, Odum N, Persson JL, et al. (2015). MiR137 is an androgen regulated repressor of an extended network of transcriptional coregulators. Oncotarget, 6(34), 35710–35725, doi: 10.18632/oncotarget.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Yang H, Li Q, Zhao W, Yuan D, Zhao H, & Zhou Y (2014). miR-329 suppresses the growth and motility of neuroblastoma by targeting KDM1A. FEBS Letters, 588(1), 192–197, doi: 10.1016/j.febslet.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 515.Ma L, Ma S, Zhao G, Yang L, Zhang P, Yi Q, et al. (2016). miR-708/LSD1 axis regulates the proliferation and invasion of breast cancer cells. Cancer Med, 5(4), 684–692, doi: 10.1002/cam4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Derr RS, van Hoesel AQ, Benard A, Goossens-Beumer IJ, Sajet A, Dekker-Ensink NG, et al. (2014). High nuclear expression levels of histone-modifying enzymes LSD1, HDAC2 and SIRT1 in tumor cells correlate with decreased survival and increased relapse in breast cancer patients. BMC Cancer, 14(1), 604, doi: 10.1186/1471-2407-14-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, et al. (2015). A Molecular Portrait Of High-Grade Ductal Carcinoma In Situ (DCIS). Cancer Research, doi: 10.1158/0008-5472.can-15-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Lefebvre C, Bachelot T, Filleron T, Pedrero M, Campone M, Soria JC, et al. (2016). Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med, 13(12), e1002201, doi: 10.1371/journal.pmed.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.De Mattos-Arruda L, Ng CKY, Piscuoglio S, Gonzalez-Cao M, Lim RS, De Filippo MR, et al. (2018). Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases. Oncotarget, 9(29), 20617–20630, doi: 10.18632/oncotarget.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Lopez-Knowles E, Pearson A, Schuster G, Gellert P, Ribas R, Yeo B, et al. (2019). Molecular characterisation of aromatase inhibitor-resistant advanced breast cancer: the phenotypic effect of ESR1 mutations. British Journal of Cancer, 120(2), 247–255, doi: 10.1038/s41416-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Liu R, Li J, Teng Z, Zhang Z, & Xu Y (2013). Overexpressed MicroRNA-182 Promotes Proliferation and Invasion in Prostate Cancer PC-3 Cells by Down-Regulating N-myc Downstream Regulated Gene 1 (NDRG1). PLoS ONE, 8(7), e68982, doi: 10.1371/journal.pone.0068982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Wu A-Y, Hu Y, Cang W, Li D, Wang W-J, Tian Q, et al. (2019). Suppressive effect of microRNA-449a on the NDRG1/PTEN/AKT axis regulates endometrial cancer growth and metastasis. Experimental Cell Research, 382(2), 111468, doi: 10.1016/j.yexcr.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 523.Jiao G-J, Zhang S-J, Li Y, Wu W-L, & Liu H-C (2018). MicroRNA-645 promotes metastasis of osteosarcoma via targeting tumor suppressor NM23 nucleoside diphosphate kinase 2. Clinical and Experimental Pharmacology and Physiology, 45(12), 1317–1324, doi: 10.1111/1440-1681.13006. [DOI] [PubMed] [Google Scholar]
- 524.Hartsough MT, Clare SE, Mair M, Elkahloun AG, Sgroi D, Osborne CK, et al. (2001). Elevation of Breast Carcinoma Nm23-H1 Metastasis Suppressor Gene Expression and Reduced Motility by DNA Methylation Inhibition. Cancer Research, 61(5), 2320–1878. [PubMed] [Google Scholar]
- 525.Radpour R, Kohler C, Haghighi MM, Fan AXC, Holzgreve W, & Zhong XY (2009). Methylation profiles of 22 candidate genes in breast cancer using high-throughput MALDI-TOF mass array. Oncogene, 28(33), 2969–2978. [DOI] [PubMed] [Google Scholar]
- 526.Xue R, Peng Y, Han B, Li X, Chen Y, & Pei H (2019). Metastasis suppressor NME1 promotes non-homologous end joining of DNA double-strand breaks. DNA Repair, 77, 27–35, doi: 10.1016/j.dnarep.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 527.Curtis CD, Likhite VS, McLeod IX, Yates JR, & Nardulli AM (2007). Interaction of the Tumor Metastasis Suppressor Nonmetastatic Protein 23 Homologue H1 and Estrogen Receptor {alpha} Alters Estrogen-Responsive Gene Expression. Cancer Research, 67(21), 10600–10607, doi: 10.1158/0008-5472.can-07-0055. [DOI] [PubMed] [Google Scholar]
- 528.Romani P, Ignesti M, Gargiulo G, Hsu T, & Cavaliere V (2018). Extracellular NME proteins: a player or a bystander? Laboratory Investigation, 98(2), 248–257, doi: 10.1038/labinvest.2017.102. [DOI] [PubMed] [Google Scholar]
- 529.Smagghe BJ, Stewart AK, Carter MG, Shelton LM, Bernier KJ, Hartman EJ, et al. (2013). MUC1* Ligand, NM23-H1, Is a Novel Growth Factor That Maintains Human Stem Cells in a More Naïve State. PLoS ONE, 8(3), e58601, doi: 10.1371/journal.pone.0058601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Charpin C, Garcia S, Bonnier P, Martini F, Andrac L, Horschowski N, et al. (1998). Prognostic significance of Nm23/NDPK expression in breast carcinoma, assessed on 10-year follow-up by automated and quantitative immunocytochemical assays. Journal of Pathology, 184(4), 401–407, doi:. [DOI] [PubMed] [Google Scholar]
- 531.Ribeiro MA, Estill MS, Fernandez GJ, Moraes LN, Krawetz SA, & Scarano WR (2018). Integrative transcriptome and microRNome analysis identifies dysregulated pathways in human Sertoli cells exposed to TCDD. Toxicology, 409, 112–118, doi: 10.1016/j.tox.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 532.Insel PA, Sriram K, Wiley SZ, Wilderman A, Katakia T, McCann T, et al. (2018). GPCRomics: GPCR Expression in Cancer Cells and Tumors Identifies New, Potential Biomarkers and Therapeutic Targets. [Original Research]. Frontiers in Pharmacology, 9(431), doi: 10.3389/fphar.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Fan P, Liu L, Yin Y, Zhao Z, Zhang Y, Amponsah PS, et al. (2016). MicroRNA-101–3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Letters, 373(1), 130–137, doi: 10.1016/j.canlet.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 534.Zhang Y-W, Jones TL, Martin SE, Caplen NJ, & Pommier Y (2009). Implication of Checkpoint Kinase-dependent Up-regulation of Ribonucleotide Reductase R2 in DNA Damage Response. Journal of Biological Chemistry, 284(27), 18085–18095, doi: 10.1074/jbc.M109.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Xu YC, Zhang FC, Li JJ, Dai JQ, Liu Q, Tang L, et al. (2015). RRM1, TUBB3, TOP2A, CYP19A1, CYP2D6: Difference between mRNA and protein expression in predicting prognosis of breast cancer patients. Oncology Reports, 34(4), 1883–1894, doi: 10.3892/or.2015.4183. [DOI] [PubMed] [Google Scholar]
- 536.Jørgensen CLT, Ejlertsen B, Bjerre KD, Balslev E, Nielsen DL, & Nielsen KV (2013). Gene aberrations of RRM1 and RRM2B and outcome of advanced breast cancer after treatment with docetaxel with or without gemcitabine. BMC Cancer, 13(1), 541, doi: 10.1186/1471-2407-13-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Dorman SN, Baranova K, Knoll JHM, Urquhart Brad L., Mariani G, Carcangiu ML, et al. (2016). Genomic signatures for paclitaxel and gemcitabine resistance in breast cancer derived by machine learning. Molecular Oncology, 10(1), 85–100, doi: 10.1016/j.molonc.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Jones DZ, Schmidt ML, Suman S, Hobbing KR, Barve SS, Gobejishvili L, et al. (2018). Micro-RNA-186–5p inhibition attenuates proliferation, anchorage independent growth and invasion in metastatic prostate cancer cells. BMC Cancer, 18(1), 421, doi: 10.1186/s12885-018-4258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Xia W, Ni J, Zhuang J, Qian L, Wang P, & Wang J (2016). MiR-103 regulates hepatocellular carcinoma growth by targeting AKAP12. The International Journal of Biochemistry & Cell Biology, 71, 1–11, doi: 10.1016/j.biocel.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 540.Gelman IH (2012). Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/Gravin/AKAP12. [journal article]. Cancer and Metastasis Reviews, 31(3), 493–500, doi: 10.1007/s10555-012-9360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Soh RYZ, Lim JP, Samy RP, Chua PJ, & Bay BH (2018). A-kinase anchor protein 12 (AKAP12) inhibits cell migration in breast cancer. Experimental and Molecular Pathology, 105(3), 364–370, doi: 10.1016/j.yexmp.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 542.Kioulafa M, Balkouranidou I, Sotiropoulou G, Kaklamanis L, Mavroudis D, Georgoulias V, et al. (2009). Methylation of cystatin M promoter is associated with unfavorable prognosis in operable breast cancer. International Journal of Cancer, 125(12), 2887–2892, doi: 10.1002/ijc.24686. [DOI] [PubMed] [Google Scholar]
- 543.Roll JD, Rivenbark AG, Sandhu R, Parker JS, Jones WD, Carey LA, et al. (2013). Dysregulation of the epigenome in triple-negative breast cancers: Basal-like and claudin-low breast cancers express aberrant DNA hypermethylation. Experimental and Molecular Pathology, 95(3), 276–287, doi: 10.1016/j.yexmp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 544.Chimonidou M, Strati A, Malamos N, Kouneli S, Georgoulias V, & Lianidou E (2017). Direct comparison study of DNA methylation markers in EpCAM-positive circulating tumour cells, corresponding circulating tumour DNA, and paired primary tumours in breast cancer. Oncotarget, 8(42), 72054–72068, doi: 10.18632/oncotarget.18679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Li Q, Zheng Z-C, Ni C-J, Jin W-X, Jin Y-X, Chen Y, et al. (2018). Correlation of Cystatin E/M with Clinicopathological Features and Prognosis in Triple-Negative Breast Cancer. Annals of Clinical and Laboratory Science, 48(1), 40–44. [PubMed] [Google Scholar]
- 546.Clarke MR, Imhoff FM, & Baird SK (2015). Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and −2. Molecular Carcinogenesis, 54(10), 1214–1219, doi: 10.1002/mc.22178. [DOI] [PubMed] [Google Scholar]
- 547.Daniele A, Abbate I, Oakley C, Casamassima P, Savino E, Casamassima A, et al. (2016). Clinical and prognostic role of matrix metalloproteinase-2, −9 and their inhibitors in breast cancer and liver diseases: A review. The International Journal of Biochemistry & Cell Biology, 77, 91–101, doi: 10.1016/j.biocel.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 548.Liu J, Shi W, Wu C, Ju J, & Jiang J (2014). miR-181b as a key regulator of the oncogenic process and its clinical implications in cancer (Review). Biomed Rep, 2(1), 7–11, doi: 10.3892/br.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, et al. (2010). MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. Journal of Experimental and Clinical Cancer Research, 29(1), 29, doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Gan R, Yang Y, Yang X, Zhao L, Lu J, & Meng QH (2014). Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. [Original Article]. Cancer Gene Therapy, 21(7), 290–296, doi: 10.1038/cgt.2014.29. [DOI] [PubMed] [Google Scholar]
- 551.Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, et al. (1999). Methylation-associated Silencing of the Tissue Inhibitor of Metalloproteinase-3 Gene Suggests a Suppressor Role in Kidney, Brain, and Other Human Cancers. Cancer Research, 59(4), 798–802. [PubMed] [Google Scholar]
- 552.Janiak M, Paskal W, Rak B, Garbicz F, Jarema R, Sikora K, et al. (2017). TIMP4 expression is regulated by miR-200b-3p in prostate cancer cells. APMIS, 125(2), 101–105, doi: 10.1111/apm.12638. [DOI] [PubMed] [Google Scholar]
- 553.Boufraqech M, Zhang L, Nilubol N, Sadowski SM, Kotian S, Quezado M, et al. (2016). Lysyl Oxidase (LOX) Transcriptionally Regulates SNAI2 Expression and TIMP4 Secretion in Human Cancers. Clinical Cancer Research, 22(17), 4491–4504, doi: 10.1158/1078-0432.Ccr-15-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Sato M, Kadota M, Tang B, Yang HH, Yang Y. a., Shan M, et al. (2014). An integrated genomic approach identifies persistent tumor suppressive effects of transforming growth factor-β in human breast cancer. Breast Cancer Research, 16(3), R57, doi: 10.1186/bcr3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Gökmen-Polar Y, Toroni RA, Hocevar BA, Badve S, Zhao Q, Shen C, et al. (2011). Dual targeting of EphA2 and ER restores tamoxifen sensitivity in ER/EphA2-positive breast cancer. [journal article]. Breast Cancer Research and Treatment, 127(2), 375–384, doi: 10.1007/s10549-010-1004-y. [DOI] [PubMed] [Google Scholar]
- 556.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, et al. (2012). Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol, 14(2), 159–167, doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, & Ding WQ (2015). Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer, 14, 133, doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Lindemann RK, Ballschmieter P, Nordheim A, & Dittmer J (2001). Transforming Growth Factor β Regulates Parathyroid Hormone-related Protein Expression in MDA-MB-231 Breast Cancer Cells through a Novel Smad/Ets Synergism. Journal of Biological Chemistry, 276(49), 46661–46670, doi: 10.1074/jbc.M105816200. [DOI] [PubMed] [Google Scholar]
- 559.Liu F, Sang M, Meng L, Gu L, Liu S, Li J, et al. (2018). miR-92b promotes autophagy and suppresses viability and invasion in breast cancer by targeting EZH2. International Journal of Oncology, 53(4), 1505–1515, doi: 10.3892/ijo.2018.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TFE, et al. (2008). MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood, 112(10), 4202–4212, doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 561.Valencia AM, & Kadoch C (2019). Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nature Cell Biology, 21(2), 152–161, doi: 10.1038/s41556-018-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, & Kleer CG (2006). Identification of EZH2 as a Molecular Marker for a Precancerous State in Morphologically Normal Breast Tissues. Cancer Research, 66(8), 4095–4099, doi: 10.1158/0008-5472.can-05-4300. [DOI] [PubMed] [Google Scholar]
- 563.Yang WS, Chadalapaka G, Cho SG, Lee SO, Jin UH, Jutooru I, et al. (2014). The transcriptional repressor ZBTB4 regulates EZH2 through a MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia, 16(12), 1059–1069, doi: 10.1016/j.neo.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Myatt SS, Wang J, Monteiro LJ, Christian M, Ho K-K, Fusi L, et al. (2010). Definition of microRNAs That Repress Expression of the Tumor Suppressor Gene FOXO1 in Endometrial Cancer. Cancer Research, 70(1), 367–377, doi: 10.1158/0008-5472.can-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Coomans de Brachène A, & Demoulin J-B (2016). FOXO transcription factors in cancer development and therapy. [journal article]. Cellular and Molecular Life Sciences, 73(6), 1159–1172, doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Zhao L, & Zheng XY (2016). MicroRNA-490 inhibits tumorigenesis and progression in breast cancer. Onco Targets Ther, 9, 4505–4516, doi: 10.2147/ott.S100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Chandra Mangalhara K, Manvati S, Saini SK, Ponnusamy K, Agarwal G, Abraham SK, et al. (2017). ERK2-ZEB1-miR-101–1 axis contributes to epithelial-mesenchymal transition and cell migration in cancer. Cancer Letters, 391, 59–73, doi: 10.1016/j.canlet.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 568.Mohammadi-Yeganeh S, Hosseini V, & Paryan M (2019). Wnt pathway targeting reduces triple-negative breast cancer aggressiveness through miRNA regulation in vitro and in vivo. Journal of Cellular Physiology, 234(10), 18317–18328, doi: 10.1002/jcp.28465. [DOI] [PubMed] [Google Scholar]
- 569.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, & Derynck R (2012). TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. Journal of Cell Science, 125(Pt 5), 1259–1273, doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Pronina IV, Loginov VI, Burdennyy AM, Fridman MV, Kazubskaya TP, Dmitriev AA, et al. (2016). Expression and DNA methylation alterations of seven cancer-associated 3p genes and their predicted regulator miRNAs (miR-129–2, miR-9–1) in breast and ovarian cancers. Gene, 576(1, Part 3), 483–491, doi: 10.1016/j.gene.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 571.Gao J, Wang G, Wu J, Zuo Y, Zhang J, & Jin X (2019). Skp2 Expression Is Inhibited by Arsenic Trioxide through the Upregulation of miRNA-330–5p in Pancreatic Cancer Cells. Molecular Therapy - Oncolytics, 12, 214–223, doi: 10.1016/j.omto.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Duan X, Bai J, Wei J, Li Z, Liu X, & Xu G (2017). MicroRNA-508–5p suppresses metastasis in human gastric cancer by targeting S-phase kinaseassociated protein 2. Mol Med Rep, 16(2), 2163–2171, doi: 10.3892/mmr.2017.6793. [DOI] [PubMed] [Google Scholar]
- 573.Men X, Ma J, Wu T, Pu J, Wen S, Shen J, et al. (2018). Transcriptome profiling identified differentially expressed genes and pathways associated with tamoxifen resistance in human breast cancer. Oncotarget, 9(3), 4074–4089, doi: 10.18632/oncotarget.23694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, et al. (2007). FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. Journal of Clinical Investigation, 117(12), 3765–3773, doi: 10.1172/jci32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Dobson JR, Taipaleenmäki H, Hu Y-J, Hong D, van Wijnen AJ, Stein JL, et al. (2014). hsa-mir-30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell International, 14(1), 73, doi: 10.1186/s12935-014-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Ouellet V, Tiedemann K, Mourskaia A, Fong JE, Tran-Thanh D, Amir E, et al. (2011). CCN3 Impairs Osteoblast and Stimulates Osteoclast Differentiation to Favor Breast Cancer Metastasis to Bone. The American journal of pathology, 178(5), 2377–2388, doi: 10.1016/j.ajpath.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Brigstock DR (2003). The CCN family: a new stimulus package. Journal of Endocrinology, 178(2), 169–175, doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 578.Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, & Mansel RE (2004). Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocrine-Related Cancer, 11(4), 781, doi: 10.1677/erc.1.00825. [DOI] [PubMed] [Google Scholar]
- 579.Ghayad SE, Vendrell JA, Bieche I, Spyratos F, Dumontet C, Treilleux I, et al. (2009). Identification of TACC1, NOV, and PTTG1 as new candidate genes associated with endocrine therapy resistance in breast cancer. Journal of Molecular Endocrinology, 42(2), 87–103, doi: 10.1677/jme-08-0076. [DOI] [PubMed] [Google Scholar]
- 580.Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, et al. (2013). Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. [Original Article]. Oncogene, 32(20), 2555–2564, doi: 10.1038/onc.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Garofalo M, & Croce CM (2015). Role of microRNAs in maintaining cancer stem cells. Advanced Drug Delivery Reviews, 81, 53–61, doi: 10.1016/j.addr.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Jia Y, Zhou J, Luo X, Chen M, Chen Y, Wang J, et al. (2018). KLF4 overcomes tamoxifen resistance by suppressing MAPK signaling pathway and predicts good prognosis in breast cancer. Cellular Signalling, 42, 165–175, doi: 10.1016/j.cellsig.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 583.Tong D, Czerwenka K, Heinze G, Ryffel M, Schuster E, Witt A, et al. (2006). Expression of KLF5 is a Prognostic Factor for Disease-Free Survival and Overall Survival in Patients with Breast Cancer. Clinical Cancer Research, 12(8), 2442, doi: 10.1158/1078-0432.CCR-05-0964. [DOI] [PubMed] [Google Scholar]
- 584.Takagi K, Miki Y, Onodera Y, Nakamura Y, Ishida T, Watanabe M, et al. (2012). Kruppel-like factor 5 in human breast carcinoma: a potent prognostic factor induced by androgens. Endocr Relat Cancer, 19(6), 741–750, doi: 10.1530/erc-12-0017. [DOI] [PubMed] [Google Scholar]
- 585.Gu C, Xu Y, Zhang S, Guan H, Song S, Wang X, et al. (2016). miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARγ and GREM1. Sci Rep, 6(1), 38491, doi: 10.1038/srep38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Ren J, Smid M, Iaria J, Salvatori DCF, van Dam H, Zhu HJ, et al. (2019). Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Research, 21(1), 109, doi: 10.1186/s13058-019-1194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Neckmann U, Wolowczyk C, Hall M, Almaas E, Ren J, Zhao S, et al. (2019). GREM1 is associated with metastasis and predicts poor prognosis in ER-negative breast cancer patients. Cell Communication and Signaling, 17(1), 140, doi: 10.1186/s12964-019-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Shi P, Chen C, Li X, Wei Z, Liu Z, & Liu Y (2019). MicroRNA124 suppresses cell proliferation and invasion of triple negative breast cancer cells by targeting STAT3. Mol Med Rep, 19(5), 3667–3675, doi: 10.3892/mmr.2019.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang C, et al. (2015). MicroRNA-33b Inhibits Breast Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci Rep, 5(1), 9995, doi: 10.1038/srep09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Wang M, Li C, Nie H, Lv X, Qu Y, Yu B, et al. (2012). Down-regulated miR-625 suppresses invasion and metastasis of gastric cancer by targeting ILK. FEBS Letters, 586(16), 2382–2388, doi: 10.1016/j.febslet.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 591.Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S, et al. (2013). Replacement treatment with microRNA-143 and −145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Letters, 328(2), 353–361, doi: 10.1016/j.canlet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 592.Cortez V, Nair BC, Chakravarty D, & Vadlamudi RK (2011). Integrin-linked kinase 1: role in hormonal cancer progression. Front Biosci (Schol Ed), 3, 788–796, doi: 10.2741/s187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Wendt MK, Allington TM, & Schiemann WP (2009). Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol, 5(8), 1145–1168, doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Pang M-F, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, & Nelson CM (2016). Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells. Cancer Research, 76(18), 5277, doi: 10.1158/0008-5472.CAN-16-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Zhao D, & Cui Z (2019). MicroRNA-361–3p regulates retinoblastoma cell proliferation and stemness by targeting hedgehog signaling. Exp Ther Med, 17(2), 1154–1162, doi: 10.3892/etm.2018.7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Katoh Y, & Katoh M (2009). Integrative genomic analyses on GLI1: positive regulation of GLI1 by Hedgehog-GLI, TGFbeta-Smads, and RTK-PI3K-AKT signals, and negative regulation of GLI1 by Notch-CSL-HES/HEY, and GPCR-Gs-PKA signals. International Journal of Oncology, 35(1), 187–192, doi: 10.3892/ijo_00000328. [DOI] [PubMed] [Google Scholar]
- 597.Kurebayashi J, Kanomata N, Koike Y, Ohta Y, Saitoh W, & Kishino E (2018). Comprehensive immunohistochemical analyses on expression levels of hedgehog signaling molecules in breast cancers. [journal article]. Breast Cancer, 25(6), 759–767, doi: 10.1007/s12282-018-0884-2. [DOI] [PubMed] [Google Scholar]
- 598.Rudolph M, Sizemore ST, Lu Y, Teng KY, Basree MM, Reinbolt R, et al. (2018). A hedgehog pathway-dependent gene signature is associated with poor clinical outcomes in Luminal A breast cancer. [journal article]. Breast Cancer Research and Treatment, 169(3), 457–467, doi: 10.1007/s10549-018-4718-x. [DOI] [PubMed] [Google Scholar]
- 599.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, et al. (2011). miR-22 represses cancer progression by inducing cellular senescence. The Journal of Cell Biology, 193(2), 409–424, doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, & Zhou Q (2011). miR-200a Regulates SIRT1 Expression and Epithelial to Mesenchymal Transition (EMT)-like Transformation in Mammary Epithelial Cells. Journal of Biological Chemistry, 286(29), 25992–26002, doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Yamakuchi M, Ferlito M, & Lowenstein CJ (2008). miR-34a repression of SIRT1 regulates apoptosis. Proceedings of the National Academy of Sciences, 105(36), 13421–13426, doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Yarahmadi S, Abdolvahabi Z, Hesari Z, Tavakoli-Yaraki M, Yousefi Z, Seiri P, et al. (2019). Inhibition of sirtuin 1 deacetylase by miR-211–5p provides a mechanism for the induction of cell death in breast cancer cells. Gene, 711, 143939, doi: 10.1016/j.gene.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 603.Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Lüscher B, et al. (2012). The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proceedings of the National Academy of Sciences, 109(4), E187–E196, doi: 10.1073/pnas.1105304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Gollavilli PN, Kanugula AK, Koyyada R, Karnewar S, Neeli PK, & Kotamraju S (2015). AMPK inhibits MTDH expression via GSK3β and SIRT1 activation: potential role in triple negative breast cancer cell proliferation. The FEBS Journal, 282(20), 3971–3985, doi: 10.1111/febs.13391. [DOI] [PubMed] [Google Scholar]
- 605.Santolla MF, Avino S, Pellegrino M, De Francesco EM, De Marco P, Lappano R, et al. (2015). SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. [Original Article]. Cell Death Dis, 6, e1834, doi: 10.1038/cddis.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Wang C, Yang W, Dong F, Guo Y, Tan J, Ruan S, et al. (2017). The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis. Oncotarget, 8(39), 66343–66351, doi: 10.18632/oncotarget.18494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Lipson KE, Wong C, Teng Y, & Spong S (2012). CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair, 5(1), S24, doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Wu W, Zaal EA, Berkers CR, Lemeer S, & Heck AJR (2018). CTGF/VEGFA-activated Fibroblasts Promote Tumor Migration Through Micro-environmental Modulation. Molecular & Cellular Proteomics, 17(8), 1502–1514, doi: 10.1074/mcp.RA118.000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Jain K, & Basu A (2014). The Multifunctional Protein Kinase C-epsilon in Cancer Development and Progression. Cancers (Basel), 6(2), 860–878, doi: 10.3390/cancers6020860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Wang H, Gutierrez-Uzquiza A, Garg R, Barrio-Real L, Abera MB, Lopez-Haber C, et al. (2014). Transcriptional Regulation of Oncogenic Protein Kinase Cϵ (PKCϵ) by STAT1 and Sp1 Proteins. Journal of Biological Chemistry, 289(28), 19823–19838, doi: 10.1074/jbc.M114.548446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Ørom UA, Lim MK, Savage JE, Jin L, Saleh AD, Lisanti MP, et al. (2012). MicroRNA-203 regulates caveolin-1 in breast tissue during caloric restriction. Cell Cycle, 11(7), 1291–1295, doi: 10.4161/cc.19704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Matos I, Dufloth R, Alvarenga M, Zeferino LC, & Schmitt F (2005). p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Archiv, 447(4), 688–694, doi: 10.1007/s00428-005-0010-7. [DOI] [PubMed] [Google Scholar]
- 613.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, & Struhl K (2010). Loss of miR-200 Inhibition of Suz12 Leads to Polycomb-Mediated Repression Required for the Formation and Maintenance of Cancer Stem Cells. Molecular Cell, 39(5), 761–772, doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Comet I, Riising EM, Leblanc B, & Helin K (2016). Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat Rev Cancer, 16(12), 803–810, doi: 10.1038/nrc.2016.83. [DOI] [PubMed] [Google Scholar]
- 615.Hu J, Su P, Jiao M, Bai X, Qi M, Liu H, et al. (2018). TRPS1 Suppresses Breast Cancer Epithelial-mesenchymal Transition Program as a Negative Regulator of SUZ12. Transl Oncol, 11(2), 416–425, doi: 10.1016/j.tranon.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Xin Y, Wang X, Meng K, Ni C, Lv Z, & Guan D (2019). Identification of exosomal miR-455–5p and miR-1255a as therapeutic targets for breast cancer. Bioscience Reports, doi: 10.1042/bsr20190303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Schöndorf T, Göhring UJ, Becker M, Hoopmann M, Schmidt T, Rützel S, et al. (2004). High Apoptotic Index Correlates to p21 and p27 Expression Indicating a Favorable Outcome of Primary Breast Cancer Patients, but Lacking Prognostic Significance in Multivariate Analysis. Pathobiology, 71(4), 217–222, doi: 10.1159/000078676. [DOI] [PubMed] [Google Scholar]
- 618.Kurozumi S, Inoue K, Takei H, Matsumoto H, Kurosumi M, Horiguchi J, et al. (2015). ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer, 15, 622, doi: 10.1186/s12885-015-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Silberstein GB, Van Horn K, Strickland P, Roberts CT Jr., & Daniel CW (1997). Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 94(15), 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, et al. (2002). High Expression of Wilms’ Tumor Suppressor Gene Predicts Poor Prognosis in Breast Cancer Patients. Clinical Cancer Research, 8(5), 1167–1171. [PubMed] [Google Scholar]
- 621.Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y, Liang Y, et al. (2012). High Wilms’ tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer. Oncology Reports, 28(4), 1231–1236, doi: 10.3892/or.2012.1906. [DOI] [PubMed] [Google Scholar]
- 622.McGregor RJ, Chau Y-Y, Kendall TJ, Artibani M, Hastie N, & Hadoke PWF (2018). WT1 expression in vessels varies with histopathological grade in tumour-bearing and control tissue from patients with breast cancer. British Journal of Cancer, 119(12), 1508–1517, doi: 10.1038/s41416-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Wu M-Y, Fu J, Xiao X, Wu J, & Wu R-C (2014). MiR-34a regulates therapy resistance by targeting HDAC1 and HDAC7 in breast cancer. Cancer Letters, 354(2), 311–319, doi: 10.1016/j.canlet.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 624.Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, et al. (2005). Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Research and Treatment, 94(1), 11–16, doi: 10.1007/s10549-005-6001-1. [DOI] [PubMed] [Google Scholar]
- 625.Browne G, Dragon JA, Hong D, Messier TL, Gordon JA, Farina NH, et al. (2016). MicroRNA-378-mediated suppression of Runx1 alleviates the aggressive phenotype of triple-negative MDA-MB-231 human breast cancer cells. Tumour Biology, 37(7), 8825–8839, doi: 10.1007/s13277-015-4710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Teng H, Wang P, Xue Y, Liu X, Ma J, Cai H, et al. (2016). Role of HCP5-miR-139-RUNX1 Feedback Loop in Regulating Malignant Behavior of Glioma Cells. Molecular Therapy, 24(10), 1806–1822, doi: 10.1038/mt.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 627.Chimge NO, & Frenkel B (2013). The RUNX family in breast cancer: relationships with estrogen signaling. [Review]. Oncogene, 32(17), 2121–2130, doi: 10.1038/onc.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Ramaswamy S, Ross KN, Lander ES, & Golub TR (2003). A molecular signature of metastasis in primary solid tumors. Nature Genetics, 33(1), 49–54, doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 629.Zhang N, Zhang H, Liu Y, Su P, Zhang J, Wang X, et al. (2019). SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death and Differentiation, 26(5), 843–859, doi: 10.1038/s41418-018-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Bertolio R, Napoletano F, Mano M, Maurer-Stroh S, Fantuz M, Zannini A, et al. (2019). Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nature Communications, 10(1), 1326, doi: 10.1038/s41467-019-09152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Perone Y, Farrugia AJ, Meira AR, Győrffy B, Ion C, Uggetti A, et al. (2019). SREBP1 drives Keratin-80-dependent cytoskeletal changes and invasive behavior in endocrine-resistant ERα breast cancer. Nature Communications, 10(1), 2115, doi: 10.1038/s41467-019-09676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Bao J, Zhu L, Zhu Q, Su J, Liu M, & Huang W (2016). SREBP-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol Lett, 12(4), 2409–2416, doi: 10.3892/ol.2016.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Puts GS, Leonard MK, Pamidimukkala NV, Snyder DE, & Kaetzel DM (2018). Nuclear functions of NME proteins. Laboratory Investigation, 98(2), 211–218, doi: 10.1038/labinvest.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.McCorkle JR, Leonard MK, Kraner SD, Blalock EM, Ma D, Zimmer SG, et al. (2014). The metastasis suppressor NME1 regulates expression of genes linked to metastasis and patient outcome in melanoma and breast carcinoma. Cancer Genomics Proteomics, 11(4), 175–194. [PMC free article] [PubMed] [Google Scholar]
- 635.Toulas C, Mihura J, de Balincourt C, Marques B, Marek E, Soula G, et al. (1996). Potential prognostic value in human breast cancer of cytosolic Nme1 protein detection using an original hen specific antibody. British Journal of Cancer, 73(5), 630–635, doi: 10.1038/bjc.1996.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, et al. (2008). MicroRNAs in the miR-106b Family Regulate p21/CDKN1A and Promote Cell Cycle Progression. Molecular and Cellular Biology, 28(7), 2167–2174, doi: 10.1128/mcb.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Zohny SF, Al-Malki AL, Zamzami MA, & Choudhry H (2019). p21Waf1/Cip1: its paradoxical effect in the regulation of breast cancer. Breast Cancer, 26(2), 131–137, doi: 10.1007/s12282-018-0913-1. [DOI] [PubMed] [Google Scholar]
- 638.Pellikainen MJ, Pekola TT, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, et al. (2003). p21WAF1 expression in invasive breast cancer and its association with p53, AP-2, cell proliferation, and prognosis. Journal of Clinical Pathology, 56(3), 214–220, doi: 10.1136/jcp.56.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, et al. (2001). Subcellular localisation of cyclin B, Cdc2 and p21WAF1/CIP1 in breast cancer: association with prognosis. European Journal of Cancer, 37(18), 2405–2412, doi: 10.1016/S0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 640.Lee JJ, Drakaki A, Iliopoulos D, & Struhl K (2012). MiR-27b targets PPARgamma to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene, 31(33), 3818–3825, doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Asukai K, Kawamoto K, Eguchi H, Konno M, Asai A, Iwagami Y, et al. (2017). Micro-RNA-130a-3p Regulates Gemcitabine Resistance via PPARG in Cholangiocarcinoma. Annals of Surgical Oncology, 24(8), 2344–2352, doi: 10.1245/s10434-017-5871-x. [DOI] [PubMed] [Google Scholar]
- 642.Grygiel-Górniak B (2014). Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutrition Journal, 13(1), 17, doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. (1998). Terminal Differentiation of Human Breast Cancer through PPARγ. Molecular Cell, 1(3), 465–470, doi: 10.1016/S1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 644.Rubin GL, Zhao Y, Kalus AM, & Simpson ER (2000). Peroxisome proliferator-activated receptor gamma ligands inhibit estrogen biosynthesis in human breast adipose tissue: possible implications for breast cancer therapy. Cancer Research, 60(6), 1604–1608. [PubMed] [Google Scholar]
- 645.Qin C, Burghardt R, Smith R, Wormke M, Stewart J, & Safe S (2003). Peroxisome proliferator-activated receptor gamma agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor alpha in MCF-7 breast cancer cells. Cancer Research, 63(5), 958–964. [PubMed] [Google Scholar]
- 646.Jiang WG, Douglas-Jones A, & Mansel RE (2003). Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. International Journal of Cancer, 106(5), 752–757. [DOI] [PubMed] [Google Scholar]
- 647.Roberts TC (2014). The MicroRNA Biology of the Mammalian Nucleus. [Review]. Mol Ther Nucleic Acids, 3, e188, doi: 10.1038/mtna.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Christiansen JJ, & Rajasekaran AK (2006). Reassessing Epithelial to Mesenchymal Transition as a Prerequisite for Carcinoma Invasion and Metastasis. Cancer Research, 66(17), 8319–8326, doi: 10.1158/0008-5472.can-06-0410. [DOI] [PubMed] [Google Scholar]
- 649.Charpin C, Garcia S, Bonnier P, Martini F, Andrac L, Choux R, et al. (1998). Reduced E-Cadherin Immunohistochemical Expression in Node-Negative Breast Carcinomas Correlates With 10-Year Survival. American Journal of Clinical Pathology, 109(4), 431–438, doi: 10.1093/ajcp/109.4.431. [DOI] [PubMed] [Google Scholar]
- 650.Gould Rothberg BE, & Bracken MB (2006). E-cadherin Immunohistochemical Expression as a Prognostic Factor in Infiltrating Ductal Carcinoma of the Breast: a Systematic Review and Meta-Analysis. Breast Cancer Research and Treatment, 100(2), 139–148, doi: 10.1007/s10549-006-9248-2. [DOI] [PubMed] [Google Scholar]
- 651.Yamasaki T, Seki N, Yamada Y, Yoshino H, Hidaka H, Chiyomaru T, et al. (2012). Tumor suppressive microRNA138 contributes to cell migration and invasion through its targeting of vimentin in renal cell carcinoma. International Journal of Oncology, 41(3), 805–817, doi: 10.3892/ijo.2012.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Cattoretti G, Andreola S, Clemente C, D’Amato L, & Rilke F (1988). Vimentin and p53 expression on epidermal growth factor receptor-positive, oestrogen receptor-negative breast carcinomas. British Journal of Cancer, 57(4), 353–357, doi: 10.1038/bjc.1988.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Sethi S, Sarkar FH, Ahmed Q, Bandyopadhyay S, Nahleh ZA, Semaan A, et al. (2011). Molecular markers of epithelial-to-mesenchymal transition are associated with tumor aggressiveness in breast carcinoma. Transl Oncol, 4(4), 222–226, doi: 10.1593/tlo.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature, 436(7050), 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Nagai MA, Gerhard R, Fregnani JHTG, Nonogaki S, Rierger RB, Netto MM, et al. (2011). Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Research and Treatment, 126(1), 1–14, doi: 10.1007/s10549-010-0867-2. [DOI] [PubMed] [Google Scholar]
- 656.Lin RJ, Lin YC, & Yu AL (2010). miR-149* induces apoptosis by inhibiting Akt1 and E2F1 in human cancer cells. Molecular Carcinogenesis, 49(8), 719–727, doi: 10.1002/mc.20647. [DOI] [PubMed] [Google Scholar]
- 657.Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li YQ, et al. (2014). Estrogen receptor α mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS Journal, 281(3), 927–942, doi: 10.1111/febs.12658. [DOI] [PubMed] [Google Scholar]
- 658.Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian K, et al. (2016). miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget, 7(13), 16205–16216, doi: 10.18632/oncotarget.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, et al. (2009). miR-205 Exerts Tumor-Suppressive Functions in Human Prostate through Down-regulation of Protein Kinase C. Cancer Research, 69(6), 2287–2295, doi: 10.1158/0008-5472.can-08-2894. [DOI] [PubMed] [Google Scholar]
- 660.Piovan C, Palmieri D, Di Leva G, Braccioli L, Casalini P, Nuovo G, et al. (2012). Oncosuppressive role of p53-induced miR-205 in triple negative breast cancer. Mol Oncol, 6(4), 458–472, doi: 10.1016/j.molonc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Bell LA, & Ryan KM (2004). Life and death decisions by E2F-1. Cell Death and Differentiation, 11(2), 137–142, doi: 10.1038/sj.cdd.4401324. [DOI] [PubMed] [Google Scholar]
- 662.Ofir M, Hacohen D, & Ginsberg D (2011). miR-15 and miR-16 Are Direct Transcriptional Targets of E2F1 that Limit E2F-Induced Proliferation by Targeting Cyclin E. Molecular Cancer Research, 9(4), 440–447, doi: 10.1158/1541-7786.mcr-10-0344. [DOI] [PubMed] [Google Scholar]
- 663.Vuaroqueaux V, Urban P, Labuhn M, Delorenzi M, Wirapati P, Benz CC, et al. (2007). Low E2F1 transcript levels are a strong determinant of favorable breast cancer outcome. Breast Cancer Research, 9(3), R33, doi: 10.1186/bcr1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Hudson J, Duncavage E, Tamburrino A, Salerno P, Xi L, Raffeld M, et al. (2013). Overexpression of miR-10a and miR-375 and downregulation of YAP1 in medullary thyroid carcinoma. Experimental and Molecular Pathology, 95(1), 62–67, doi: 10.1016/j.yexmp.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Kang W, Tong JH, Lung RW, Dong Y, Zhao J, Liang Q, et al. (2015). Targeting of YAP1 by microRNA-15a and microRNA-16–1 exerts tumor suppressor function in gastric adenocarcinoma. Mol Cancer, 14, 52, doi: 10.1186/s12943-015-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Totaro A, Panciera T, & Piccolo S (2018). YAP/TAZ upstream signals and downstream responses. Nature Cell Biology, 20(8), 888–899, doi: 10.1038/s41556-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, et al. (2016). ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nature Communications, 7(1), 10498, doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Wu Y, Hou Y, Xu P, Deng Y, Liu K, Wang M, et al. (2019). The prognostic value of YAP1 on clinical outcomes in human cancers. Aging (Albany NY), 11(19), 8681–8700, doi: 10.18632/aging.102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Tian F, Shen Y, Chen Z, Li R, Lu J, & Ge Q (2016). Aberrant miR-181b-5p and miR-486–5p expression in serum and tissue of non-small cell lung cancer. Gene, 591(2), 338–343, doi: 10.1016/j.gene.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 670.Burbee DG, Forgacs E, Zöchbauer-Müller S, Shivakumar L, Fong K, Gao B, et al. (2001). Epigenetic Inactivation of RASSF1A in Lung and Breast Cancers and Malignant Phenotype Suppression. JNCI: Journal of the National Cancer Institute, 93(9), 691–699, doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, et al. (2007). RASSF1A Elicits Apoptosis through an MST2 Pathway Directing Proapoptotic Transcription by the p73 Tumor Suppressor Protein. Molecular Cell, 27(6), 962–975, doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Chatzifrangkeskou M, Pefani D-E, Eyres M, Vendrell I, Fischer R, Pankova D, et al. (2019). RASSF1A is required for the maintenance of nuclear actin levels. The EMBO Journal, 38(16), e101168, doi: 10.15252/embj.2018101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Pan Q, Luo X, & Chegini N (2008). Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. Journal of Cellular and Molecular Medicine, 12(1), 227–240, doi:doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 674.Yu G, Wan L, Li Y, Xiang Y, Song Y, & Tan L (2019). Dysregulated miR-142, −33b, and −423 in granulosa cells target TGFBR1 and SMAD7: a possible role in polycystic ovary syndrome. Molecular Human Reproduction, 25(10), 368–346, doi: 10.1093/molehr/gaz014. [DOI] [PubMed] [Google Scholar]
- 675.Ghellal A, Li C, Hayes M, Byrne G, Bundred N, & Kumar S (2000). Prognostic significance of TGF beta 1 and TGF beta 3 in human breast carcinoma. Anticancer Research, 20(6b), 4413–4418. [PubMed] [Google Scholar]
- 676.Zhang Y, Jiang F, He H, Ye J, Mao X, Guo Q, et al. (2018). Identification of a novel microRNA-mRNA regulatory biomodule in human prostate cancer. Cell Death & Disease, 9(3), 301, doi: 10.1038/s41419-018-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Westfall MD, & Pietenpol JA (2004). p63: molecular complexity in development and cancer. Carcinogenesis, 25(6), 857–864, doi: 10.1093/carcin/bgh148. [DOI] [PubMed] [Google Scholar]
- 678.Gomez LC, Sottile ML, Guerrero-Gimenez ME, Zoppino FCM, Redondo AL, Gago FE, et al. (2018). TP73DNA methylation and upregulation of ΔNp73 are associated with an adverse prognosis in breast cancer. Journal of Clinical Pathology, 71(1), 52–58, doi: 10.1136/jclinpath-2017-204499. [DOI] [PubMed] [Google Scholar]
- 679.Dominguez G, Silva JM, Silva J, Garcia JM, Sanchez A, Navarro A, et al. (2001). Wild Type p73 Overexpression and High-Grade Malignancy in Breast Cancer. Breast Cancer Research and Treatment, 66(3), 183–190, doi: 10.1023/A:1010624717311. [DOI] [PubMed] [Google Scholar]
- 680.Wang X, Tong Z, & Liu H (2019). MiR-223–3p targeting epithelial cell transforming sequence 2 oncogene inhibits the activity, apoptosis, invasion and migration of MDA-MB-468 breast cancer cells. Onco Targets Ther, 12, 7675–7684, doi: 10.2147/ott.S217019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Li M, Bian C, & Yu X (2014). Poly(ADP-ribosyl)ation is recognized by ECT2 during mitosis. Cell Cycle, 13(18), 2944–2951, doi: 10.4161/15384101.2014.947197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Wang HK, Liang JF, Zheng HX, & Xiao H (2018). Expression and prognostic significance of ECT2 in invasive breast cancer. Journal of Clinical Pathology, 71(5), 442–445, doi: 10.1136/jclinpath-2017-204569. [DOI] [PubMed] [Google Scholar]
- 683.Wang Y, Jiaqi C, Zhaoying C, & Huimin C (2016). MicroRNA-506–3p regulates neural stem cell proliferation and differentiation through targeting TCF3. Gene, 593(1), 193–200, doi: 10.1016/j.gene.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 684.Slyper M, Shahar A, Bar-Ziv A, Granit RZ, Hamburger T, Maly B, et al. (2012). Control of Breast Cancer Growth and Initiation by the Stem Cell-Associated Transcription Factor TCF3. Cancer Research, 72(21), 5613–5624, doi: 10.1158/0008-5472.can-12-0119. [DOI] [PubMed] [Google Scholar]
- 685.Buffa FM, Camps C, Winchester L, Snell CE, Gee HE, Sheldon H, et al. (2011). microRNA-Associated Progression Pathways and Potential Therapeutic Targets Identified by Integrated mRNA and microRNA Expression Profiling in Breast Cancer. Cancer Research, 71(17), 5635–5645, doi: 10.1158/0008-5472.can-11-0489. [DOI] [PubMed] [Google Scholar]
- 686.Kwak HJ, Kim YJ, Chun KR, Woo YM, Park SJ, Jeong JA, et al. (2011). Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene, 30(21), 2433–2442, doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 687.Lo TL, Yusoff P, Fong CW, Guo K, McCaw BJ, Phillips WA, et al. (2004). The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Research, 64(17), 6127–6136. [DOI] [PubMed] [Google Scholar]
- 688.Faratian D, Sims AH, Mullen P, Kay C, Um I, Langdon SP, et al. (2011). Sprouty 2 Is an Independent Prognostic Factor in Breast Cancer and May Be Useful in Stratifying Patients for Trastuzumab Therapy. PLoS ONE, 6(8), e23772, doi: 10.1371/journal.pone.0023772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Zhang P, Hong H, Sun X, Jiang H, Ma S, Zhao S, et al. (2016). MicroRNA-10b regulates epithelial-mesenchymal transition by modulating KLF4/Notch1/E-cadherin in cisplatin-resistant nasopharyngeal carcinoma cells. Am J Cancer Res, 6(2), 141–156. [PMC free article] [PubMed] [Google Scholar]
- 690.Yang X, Ni W, & Lei K (2013). miR-200b suppresses cell growth, migration and invasion by targeting Notch1 in nasopharyngeal carcinoma. Cellular Physiology and Biochemistry, 32(5), 1288–1298, doi: 10.1159/000354527. [DOI] [PubMed] [Google Scholar]
- 691.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, et al. (2012). CCL2 Mediates Cross-talk between Cancer Cells and Stromal Fibroblasts That Regulates Breast Cancer Stem Cells. Cancer Research, 72(11), 2768–2779, doi: 10.1158/0008-5472.can-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Zhou W, He L, Dai Y, Zhang Y, Wang J, & Liu B (2018). MicroRNA-124 inhibits cell proliferation, invasion and migration by targeting CAV1 in bladder cancer. Exp Ther Med, 16(4), 2811–2820, doi: 10.3892/etm.2018.6537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 693.Bouras T, Lisanti MP, & Pestell RG (2004). Caveolin in breast cancer. Cancer Biology & Therapy, 3(10), 931–941, doi: 10.4161/cbt.3.10.1147. [DOI] [PubMed] [Google Scholar]
- 694.Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J, et al. (2013). Prognostic role of caveolin in breast cancer: A meta-analysis. The Breast, 22(4), 462–469, doi: 10.1016/j.breast.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 695.Eliyatkin N, Aktas S, Diniz G, Ozgur HH, Ekin ZY, & Kupelioglu A (2018). Expression of Stromal Caveolin- 1 May Be a Predictor for Aggressive Behaviour of Breast Cancer. Pathology Oncology Research, 24(1), 59–65, doi: 10.1007/s12253-017-0212-8. [DOI] [PubMed] [Google Scholar]
- 696.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, et al. (2013). MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. Journal of Clinical Investigation, 123(2), 566–579, doi: 10.1172/jci65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Serra R, Easter SL, Jiang W, & Baxley SE (2011). Wnt5a as an effector of TGFbeta in mammary development and cancer. Journal of Mammary Gland Biology and Neoplasia, 16(2), 157–167, doi: 10.1007/s10911-011-9205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Prasad CP, Manchanda M, Mohapatra P, & Andersson T (2018). WNT5A as a therapeutic target in breast cancer. Cancer and Metastasis Reviews, 37(4), 767–778, doi: 10.1007/s10555-018-9760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Lejeune S, Huguet EL, Hamby A, Poulsom R, & Harris AL (1995). Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clinical Cancer Research, 1(2), 215. [PubMed] [Google Scholar]
- 700.Leris ACA, Roberts TR, Jiang WG, Newbold RF, & Mokbel K (2005). WNT5A Expression in Human Breast Cancer. Anticancer Research, 25(2A), 731–734. [PubMed] [Google Scholar]
- 701.Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, et al. (2005). Expression and Signaling Activity of Wnt-5a/Discoidin Domain Receptor-1 and Syk Plays Distinct but Decisive Roles in Breast Cancer Patient Survival. Clinical Cancer Research, 11(2), 520. [PubMed] [Google Scholar]
- 702.Zhong Z, Shan M, Wang J, Liu T, Shi Q, & Pang D (2016). Decreased Wnt5a Expression is a Poor Prognostic Factor in Triple-Negative Breast Cancer. Med Sci Monit, 22, 1–7, doi: 10.12659/msm.894821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Cascio S, D’Andrea A, Ferla R, Surmacz E, Gulotta E, Amodeo V, et al. (2010). miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. Journal of Cellular Physiology, 224(1), 242–249, doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 704.Hu Y, Qiu Y, Yague E, Ji W, Liu J, & Zhang J (2016). miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis, 7(6), e2291, doi: 10.1038/cddis.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Wang R, Tian S, Wang HB, Chu DP, Cao JL, Xia HF, et al. (2014). MiR-185 is involved in human breast carcinogenesis by targeting Vegfa. FEBS Letters, 588(23), 4438–4447, doi: 10.1016/j.febslet.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 706.Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. (2011). Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Molecular and Cellular Biochemistry, 351(1–2), 157–164, doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 707.Lu Y, Qin T, Li J, Wang L, Zhang Q, Jiang Z, et al. (2017). MicroRNA-140–5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Therapy, 24(9), 386–392, doi: 10.1038/cgt.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Favaro E, Lord S, Harris AL, & Buffa FM (2011). Gene expression and hypoxia in breast cancer. Genome Med, 3(8), 55, doi: 10.1186/gm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Kim M, Jang K, Miller P, Picon-Ruiz M, Yeasky TM, El-Ashry D, et al. (2017). VEGFA links self-renewal and metastasis by inducing Sox2 to repress miR-452, driving Slug. [Original Article]. Oncogene, 36(36), 5199–5211, doi: 10.1038/onc.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Linderholm BK, Lindahl T, Holmberg L, Klaar S, Lennerstrand J, Henriksson R, et al. (2001). The expression of vascular endothelial growth factor correlates with mutant p53 and poor prognosis in human breast cancer. Cancer Research, 61(5), 2256–2260. [PubMed] [Google Scholar]
- 711.Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, et al. (2009). Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Annals of Oncology, 20(10), 1639–1646, doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- 712.Zou Q, Wu H, Fu F, Yi W, Pei L, & Zhou M (2016). RKIP suppresses the proliferation and metastasis of breast cancer cell lines through up-regulation of miR-185 targeting HMGA2. Archives of Biochemistry and Biophysics, 610(Supplement C), 25–32, doi: 10.1016/j.abb.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 713.Yoo J-O, Kwak S-Y, An H-J, Bae I-H, Park M-J, & Han Y-H (2016). miR-181b-3p promotes epithelial-mesenchymal transition in breast cancer cells through Snail stabilization by directly targeting YWHAG. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1863(7, Part A), 1601–1611, doi: 10.1016/j.bbamcr.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 714.Hiraoka E, Mimae T, Ito M, Kadoya T, Miyata Y, Ito A, et al. (2019). Breast cancer cell motility is promoted by 14-3-3γ. Breast Cancer, 26(5), 581–593, doi: 10.1007/s12282-019-00957-4. [DOI] [PubMed] [Google Scholar]
- 715.Song Y, Yang Z, Ke Z, Yao Y, Hu X, Sun Y, et al. (2012). Expression of 14-3-3γ in patients with breast cancer: Correlation with clinicopathological features and prognosis. Cancer Epidemiology, 36(6), 533–536, doi: 10.1016/j.canep.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 716.Nairismagi ML, Vislovukh A, Meng Q, Kratassiouk G, Beldiman C, Petretich M, et al. (2012). Translational control of TWIST1 expression in MCF-10A cell lines recapitulating breast cancer progression. Oncogene, 31(47), 4960–4966, doi: 10.1038/onc.2011.650. [DOI] [PubMed] [Google Scholar]
- 717.Haga CL, & Phinney DG (2012). MicroRNAs in the Imprinted DLK1-DIO3 Region Repress the Epithelial-to-Mesenchymal Transition by Targeting the TWIST1 Protein Signaling Network. Journal of Biological Chemistry, 287(51), 42695–42707, doi: 10.1074/jbc.M112.387761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Li LZ, Zhang CZ, Liu LL, Yi C, Lu SX, Zhou X, et al. (2014). miR-720 inhibits tumor invasion and migration in breast cancer by targeting TWIST1. Carcinogenesis, 35(2), 469–478, doi: 10.1093/carcin/bgt330. [DOI] [PubMed] [Google Scholar]
- 719.Yeh TC, Huang TT, Yeh TS, Chen YR, Hsu KW, Yin PH, et al. (2016). miR-151–3p Targets TWIST1 to Repress Migration of Human Breast Cancer Cells. PLoS ONE, 11(12), e0168171, doi: 10.1371/journal.pone.0168171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.De S, Das S, Mukherjee S, Das S, & Sengupta S (2017). Establishment of twist-1 and TGFBR2 as direct targets of microRNA-20a in mesenchymal to epithelial transition of breast cancer cell-line MDA-MB-231. Experimental Cell Research, 361(1), 85–92, doi: 10.1016/j.yexcr.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 721.Tao W, Sun W, Zhu H, & Zhang J (2018). Knockdown of long non-coding RNA TP73-AS1 suppresses triple negative breast cancer cell vasculogenic mimicry by targeting miR-490–3p/TWIST1 axis. Biochemical and Biophysical Research Communications, 504(4), 629–634, doi: 10.1016/j.bbrc.2018.08.122. [DOI] [PubMed] [Google Scholar]
- 722.Tsai JH, Donaher JL, Murphy DA, Chau S, & Yang J (2012). Spatiotemporal Regulation of Epithelial-Mesenchymal Transition Is Essential for Squamous Cell Carcinoma Metastasis. Cancer Cell, 22(6), 725–736, doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Drasin DJ, Guarnieri AL, Neelakantan D, Kim J, Cabrera JH, Wang C-A, et al. (2015). TWIST1-Induced miR-424 Reversibly Drives Mesenchymal Programming while Inhibiting Tumor Initiation. Cancer Research, 75(9), 1908, doi: 10.1158/0008-5472.CAN-14-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Riaz M, Sieuwerts AM, Look MP, Timmermans MA, Smid M, Foekens JA, et al. (2012). High TWIST1 mRNA expression is associated with poor prognosis in lymph node-negative and estrogen receptor-positive human breast cancer and is co-expressed with stromal as well as ECM related genes. Breast Cancer Research, 14(5), R123, doi: 10.1186/bcr3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Martin TA, Goyal A, Watkins G, & Jiang WG (2005). Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Annals of Surgical Oncology, 12(6), 488–496. [DOI] [PubMed] [Google Scholar]
- 726.Moes M, Le Béchec A, Crespo I, Laurini C, Halavatyi A, Vetter G, et al. (2012). A Novel Network Integrating a miRNA-203/SNAI1 Feedback Loop which Regulates Epithelial to Mesenchymal Transition. PLoS ONE, 7(4), e35440, doi: 10.1371/journal.pone.0035440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Yu Y, Zhao Y, Sun XH, Ge J, Zhang B, Wang X, et al. (2015). Down-regulation of miR-129–5p via the Twist1-Snail feedback loop stimulates the epithelial-mesenchymal transition and is associated with poor prognosis in breast cancer. Oncotarget, 6(33), 34423–34436, doi: 10.18632/oncotarget.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, et al. (2005). The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochemical Journal, 394, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Dong C, Yuan T, Wu Y, Wang Y, Fan Teresa W. M., Miriyala S, et al. (2013). Loss of FBP1 by Snail-Mediated Repression Provides Metabolic Advantages in Basal-like Breast Cancer. Cancer Cell, 23(3), 316–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Chang CW, Yu JC, Hsieh YH, Yao CC, Chao JI, Chen PM, et al. (2016). MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer. Oncotarget, 7(13), 16462–16478, doi: 10.18632/oncotarget.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Wu Z, Li X, Cai X, Huang C, & Zheng M (2016). miR-497 inhibits epithelial mesenchymal transition in breast carcinoma by targeting Slug. Tumour Biology, 37(6), 7939–7950, doi: 10.1007/s13277-015-4665-7. [DOI] [PubMed] [Google Scholar]
- 732.Liang Y-J, Wang Q-Y, Zhou C-X, Yin Q-Q, He M, Yu X-T, et al. (2012). MiR-124 targets Slug to regulate epithelial-mesenchymal transition and metastasis of breast cancer. Carcinogenesis, doi: 10.1093/carcin/bgs383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX, & Barsky SH (2008). ERalpha suppresses slug expression directly by transcriptional repression. Biochemical Journal, 416(2), 179–187, doi: 10.1042/bj20080328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Liu T, Zhang X, Shang M, Zhang Y, Xia B, Niu M, et al. (2013). Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. Journal of Surgical Oncology, 107(2), 188–194, doi: 10.1002/jso.23240. [DOI] [PubMed] [Google Scholar]
- 735.Rajabi H, Jin C, Ahmad R, McClary C, Joshi MD, & Kufe D (2010). MUCIN 1 oncoprotein expression is suppressed by the miR-125b oncomir. Genes Cancer, 1(1), 62–68, doi: 10.1177/1947601909357933 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Ma J, Yang Y, Huo D, Wang Z, Zhai X, Chen J, et al. (2018). LincRNA-RoR/miR-145 promote invasion and metastasis in triple-negative breast cancer via targeting MUC1. Biochemical and Biophysical Research Communications, 500(3), 614–620, doi: 10.1016/j.bbrc.2018.04.119. [DOI] [PubMed] [Google Scholar]
- 737.Jin C, Rajabi H, & Kufe D (2010). miR-1226 targets expression of the mucin 1 oncoprotein and induces cell death. International Journal of Oncology, 37(1), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Li XY, Zhou LY, Luo H, Zhu Q, Zuo L, Liu GY, et al. (2019). The long noncoding RNA MIR210HG promotes tumor metastasis by acting as a ceRNA of miR-1226–3p to regulate mucin-1c expression in invasive breast cancer. Aging (Albany NY), 11(15), 5646–5665, doi: 10.18632/aging.102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Rajabi H, & Kufe D (2017). MUC1-C Oncoprotein Integrates a Program of EMT, Epigenetic Reprogramming and Immune Evasion in Human Carcinomas. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1868(1), 117–122, doi: 10.1016/j.bbcan.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Park S-M, Gaur AB, Lengyel E, & Peter ME (2008). The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & Development, 22(7), 894–907, doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Deng S, Li X, Niu Y, Zhu S, Jin Y, Deng S, et al. (2015). MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1. Oncotarget, 6(37), 39661–39675, doi: 10.18632/oncotarget.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Chen B, Chen B, Zhu Z, Ye W, Zeng J, Liu G, et al. (2019). Prognostic value of ZEB-1 in solid tumors: a meta-analysis. BMC Cancer, 19(1), 635, doi: 10.1186/s12885-019-5830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Katsura A, Tamura Y, Hokari S, Harada M, Morikawa M, Sakurai T, et al. (2017). ZEB1-regulated inflammatory phenotype in breast cancer cells. Mol Oncol, 11(9), 1241–1262, doi: 10.1002/1878-0261.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Soini Y, Tuhkanen H, Sironen R, Virtanen I, Kataja V, Auvinen P, et al. (2011). Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer, 11(1), 73, doi: 10.1186/1471-2407-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Alkatout I, Wiedermann M, Bauer M, Wenners A, Jonat W, & Klapper W (2013). Transcription factors associated with epithelial-mesenchymal transition and cancer stem cells in the tumor centre and margin of invasive breast cancer. Experimental and Molecular Pathology, 94(1), 168–173, doi: 10.1016/j.yexmp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 746.Jang MH, Kim HJ, Kim EJ, Chung YR, & Park SY (2015). Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Human Pathology, 46(9), 1267–1274, doi: 10.1016/j.humpath.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 747.Brown CY, Dayan S, Wong SW, Kaczmarek A, Hope CM, Pederson SM, et al. (2018). FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by down regulating ZEB2 independently of ZEB1. Oncotarget, 9(45), 27708–27727, doi: 10.18632/oncotarget.25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Ji H, Sang M, Liu F, Ai N, & Geng C (2019). miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathology - Research and Practice, 215(4), 697–704, doi: 10.1016/j.prp.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 749.di Gennaro A, Damiano V, Brisotto G, Armellin M, Perin T, Zucchetto A, et al. (2018). A p53/miR-30a/ZEB2 axis controls triple negative breast cancer aggressiveness. Cell Death and Differentiation, 25(12), 2165–2180, doi: 10.1038/s41418-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Dimitrova Y, Gruber AJ, Mittal N, Ghosh S, Dimitriades B, Mathow D, et al. (2017). TFAP2A is a component of the ZEB1/2 network that regulates TGFB1-induced epithelial to mesenchymal transition. Biol Direct, 12(1), 8, doi: 10.1186/s13062-017-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Wang H, An X, Yu H, Zhang S, Tang B, Zhang X, et al. (2017). MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget, 8(60), 102119–102133, doi: 10.18632/oncotarget.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Almeida MI, Reis RM, & Calin GA (2010). MYC-microRNA-9-metastasis connection in breast cancer. Cell Research, 20(6), 603–604. [DOI] [PubMed] [Google Scholar]


