Abstract
Fibroblast activation protein-α (FAP) is a type-II transmembrane serine protease expressed almost exclusively to pathological conditions including fibrosis, arthritis and cancer. Across most cancer types, elevated FAP is associated with worse clinical outcomes. Despite the clear association between FAP and disease severity, the biological reasons underlying these clinical observations remain unclear. Here we review basic FAP biology and FAP’s role in non-oncologic and oncologic disease. We further explore how FAP may worsen clinical outcomes via its effects on extracellular matrix remodeling, intracellular signaling regulation, angiogenesis, epithelial-to-mesenchymal transition and immunosuppression. Lastly, we discuss the potential to exploit FAP biology to improve clinical outcomes.
Keywords: Fibroblast activation protein (FAP), fibroblasts, stroma, invasion
Introduction
Fibroblast activation protein-α (FAP) was first described in 1986 by Wolfgang Rettig as a cell surface antigen expressed on the reactive stromal fibroblasts of epithelial cancers, most soft tissue sarcomas, granulation tissue of wound healing and certain fetal mesenchymal fibroblasts. Conversely, it was not expressed in normal fibroblasts, normal or malignant epithelial cells or the stroma of benign epithelial tumors. Hence, it was named “fibroblast activation protein”. In 1990, Atsuko Aoyama identified a dimeric 170 kDa membrane-bound gelatinase on the invadopodia of the human melanoma cell line LOX, which was subsequently named “seprase” for surface expressed protease [1, 2]. Cloning and sequence analysis later revealed FAP and seprase to be identical [3, 4]. Unfortunately, both names are only partially accurate. Subsequent research went on to demonstrate that FAP could be expressed in non-fibroblast cell types, such as epithelial tumors [5–7], melanocytes and melanoma [2, 8], and recently macrophages [9]. Seprase also is not entirely accurate as FAP can be shed from the plasma membrane forming a soluble FAP, which is referred to as α2-antiplamin cleaving enzyme (APCE) [10]. Hence, it is not restricted to the cell surface. In any event, the name “fibroblast activation protein-a” and the symbol “FAP” predominate in the literature and are the official name and symbol listed in NCBI Gene and, consequently will be used throughout this review.
FAP Protein Structure and Function
Enzymatic Activity
FAP is a 97-kDa type II transmembrane serine protease. FAP is a member of the proplyl peptidase family, which also contains dipeptidyl peptidase IV (DPPIV, CD26), DPP7 (DPP II, quiescent cell proline dipeptidase), DPP8, DPP9, and prolyl carboxypeptidase (PCP, angiotensinase C). Within this family FAP is most like DPPIV, sharing 70% amino acid sequence homology [11]. These proteins contain a catalytic triad of serine, aspartic acid and histidine. [12] The serine acts as a nucleophile, cleaving N-terminal Pro-X peptide bonds, where X is any amino acid except proline or hydroxyproline. FAP contains dipeptidyl peptidase enzymatic activity and endopeptidase activity, sometimes referred to as gelatinase activity. Both FAP and PDDIV have dipeptidylpetidase activity, but endopeptidase activity is specific to FAP. Hence, endopeptidase activity is the basis for FAP specific detection methods and FAP specific inhibitory molecules. FAP’s endopeptidase activity prefers amino acid sequences of Gly-Pro-X, is most effective where X is Phe or Met, and least effective when X is His or Glu [13]. Furthermore, FAP is ineffective with large charged amino acids at position P4 and P2’ [14–16].
Substrates
While FAP’s substrate repertoire is largely unknown, some substrates were identified by a study that screened known DPPIV substrates for cleavage by FAP. This study demonstrated FAP’s dipeptidyl peptidase activity enables it to cleave neuropeptide Y, peptide YY, substance P and brain natriuretic peptide 32 [17]. Known substrates of FAP’s endopeptidase activity include denatured collagen type I and III (the components of gelatin) [18, 19], α−2 antiplasmin cleaving enzyme, and recently discovered fibroblast growth factor 21 [20]. Of note, FAPs ability to cleave collagen is dependent on prior collagen degradation by matrix metalloproteases or heat.
FAP’s ability to cleave α−2 anti-plasmin has been extensively detailed. During tissue repair, fibrin is deposited to form a fibrin clot. Fibrinolysis is the natural process in which a fibrin clot is dissolved by plasmin leading to scar resolution. A−2 anti-plasmin is an inhibitor of plasmin and therefore reduces the rate of lysis of the fibrin clot. Cleavage of α2-antiplasmin by FAP converts α2-antiplasmin into a more potent inhibitor of plasmin [21]. Therefore, soluble FAP, referred to as APCE, functions to enhance clotting.
Non-enzymatic activity
Research with a catalytically mutant FAP (in which the Ser 642 is mutated to Ala) has suggested that FAP can have functional impacts independent from its enzymatic activity. Mouse melanoma lines transfected to express FAP had reduced tumorigenicity. This effect was enhanced when the same cells were transfected with catalytically inactive FAP. While this study contradicts many reports of FAP being oncogenic, it suggests that catalytically inactive FAP can still induce biological effects [22]. In a similar study, breast cancer lines transfected either FAP or catalytically inactive FAP grew more rapidly in vivo, were more invasive on collagen gels, and had greater degradation of extracellular matrix in comparison to untransfeced cell lines [23], suggesting enzymatic activity was unnecessary for the observed phenotype. Another study demonstrated that breast cancer cell lines transfected with FAP and catalytically mutant FAP both had increased cellular growth and motility and both proteins activated signaling molecules PI3K and MMP2/9 [24].
Structure
FAP is 760 amino acids long with residues 1–4 composing the intracellular domain, 5–25 composing the transmembrane domain and 26–760 composing the extracellular domain. APCE results from post translational cleavage and is thus the extracellular portion of FAP, residues 24–760 [10].
Katheen Aertgeerst [25] was the first to obtain a high resolution crystalline structure of FAP. FAP’s secondary structure consists of two domains (Figure 1).
Fig. 1. Ribbon model of FAP structure.
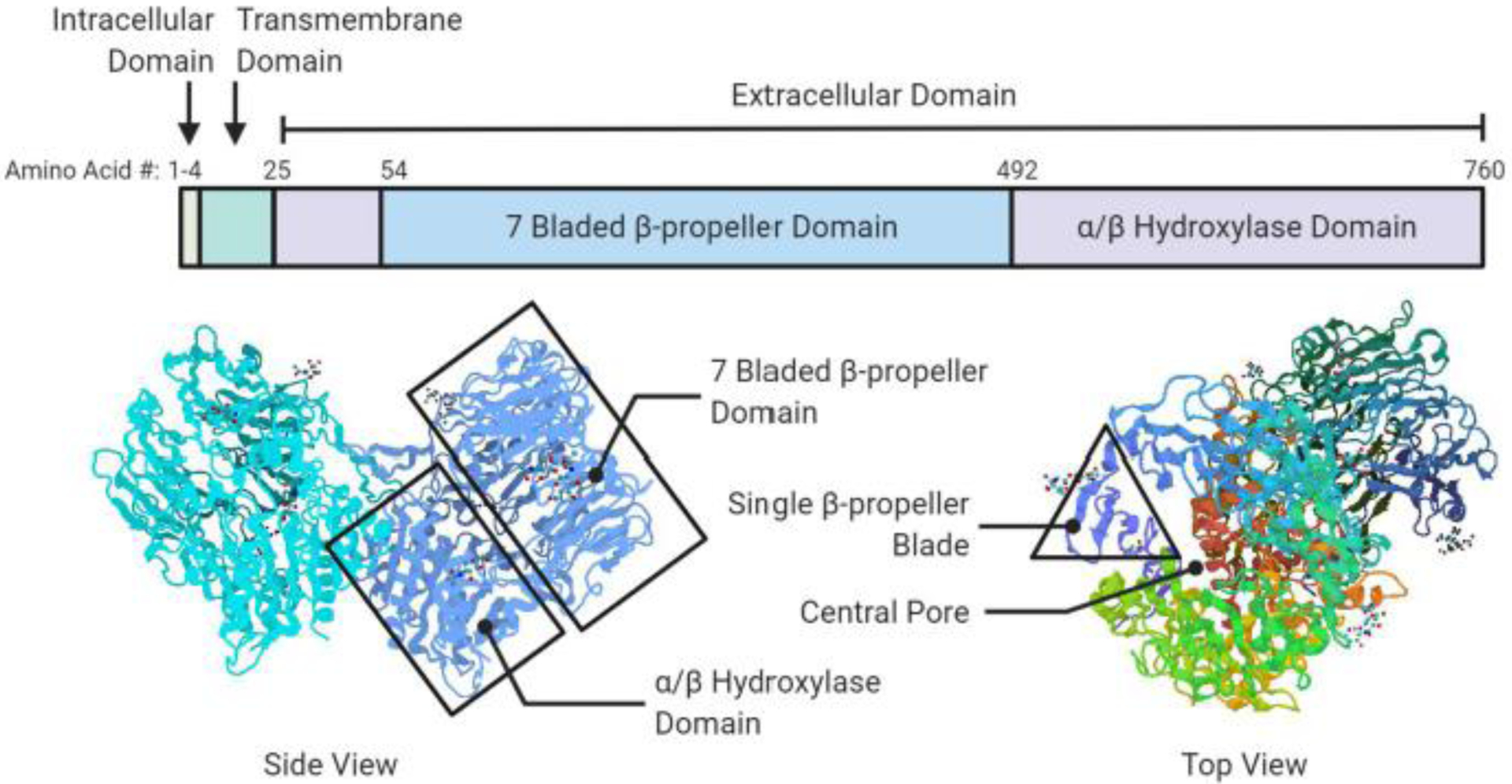
A schematic diagram of FAP domain structure (top) and ribbon models (bottom) depicting the FAP dimer. The seven-bladed β-propeller domain, αβ hydroxylase domain and β-propeller blade are highlighted. Figure created in biorender.com, PDB ID# 1Z68 [25].
Residues 54–492 comprise the β-propeller domain while residues 27–53 and 493–760 comprise the α/β-hydrolase domain. The β-propeller domain can be further broken down into eight blades surrounding a central pore of approximately 27 angstroms in length and 14 angstroms in width. Each blade is comprised of three or four anti-parallel β-sheets. The hydroxylase domain contains the catalytic triad while the β-propellar domain is believed to serve as filter so selectively permit peptides into the catalytic domain. The β-propeller domain is also thought to serve as the scaffolding region of FAP as certain β-sheets are the site for homodimerization, heterodimerization with DPPIV or interaction with other cell surface molecules such as integrins.
FAP’s catalytic triad is located at the interface of the β-propeller domain and the α/β-hydroxylase domain. The catalytic triad is accessible via the pore formed by the β-propeller domain or via the cavity between FAP’s two domains. The cavity offers greater access to substrates as its 24-angstrom width makes it wider than the pore.
DPPIV vs. FAP Catalytic Activity
Since FAP shares such sequence homology with DPPIV, attempts have been made to identify the structural differences that allot FAP its additional endopeptidase activity. Both enzymes’ dipeptidyl peptidase activities are dependent on conserved amino acids Glu205, Glu206 and Tyrosine662, which render the catalytic binding site negatively charged and allow for binding of the positively charged amino group at the N-terminus of peptides. Two more conserved peptides, Arg125 and Asn710 (numbering based on DPPIV) are required for DPPIV activity because they bind to and stabilize the carbonyl oxygen of the P2 amino acid in the substrate [25]. Aertgeerts et al. discovered that where DPPIV contains an Asp (663) FAP contains Ala (657) and this difference is responsible for FAP’s endopeptidase activity. The Asp in DPPIV is gives the catalytic site a greater negative charge, enhancing its dipeptidyl peptidase activity. Meadows et al. expanded on this observation and demonstrated that the Ala in FAP endows it with endopeptidase activity by transition state stabilization [26].
Regulation of FAP activity
FAP requires both dimerization and glycosylation to be functionally active [3, 27] FAP is can homodimerize or heterodimerize with DPPIV [28]. Hence, original work identified FAP as having two subunits, α and β, until further studies revealed FAP β was in fact DPPIV. FAP can also bind to β-integrins. It is believed integrins provide localization to invadopodia in cells grown on a collagenous matrix. Thus it was assumed that this heterodimer functions to enhance extra cellular matrix degradation and invasion [29]. Furthermore, since FAP has a short cytoplasmic domain, integrins may serve as the liaison for FAP’s effects on intracellular signaling. FRET data also suggests FAP can colocalize with urokinase plasminogen activator receptor (uPAR) [30]. Given that uPAR and FAP both play a role in tissue organization, their biological association seems reasonable.
FAP has five potential N-linked glycosylation sites on asparagine residues 49, 92, 227, 314 and 679. Four are in the β-propeller domain and one is in the hydroxylase domain. Sun et al. found that glycosylation was necessary for FAP endopeptidase activity [27].
FAP Genetics
Gene
The human FAP gene is located on chromosome 2q23. It spans approximately 73 kb and contains 26 exons. FAP continues to share remarkable homogeneity with DPPIV even at the gene level. DPPIV is located on chromosome 2q24.3, spans 70 kb and contains 26 exons. Hence some believe FAP arose from a DPPIV duplication. FAP has been identified in several other species including mouse [31, 32] and xenopus [33]. The mouse FAP gene is highly similar to human, located on chromosome 2, spanning 60 kb and containing 26 exons. Thus, mouse models can offer useful preclinical models to study FAP.
In 2010 Jiping Zhang identified the human and mouse promoter region of FAP. It is a 245-bp fragment surrounding the transcription start site. It contains early growth response-1 (EGR1), HOXA4, and E2F1 transcription binding sites. Of these three binding sites, EGR1 appeared to be the most important transcription factor for driving FAP expression [34].
Splice Variants
Like many proteins, FAP is known to have splice variants. Leslie Goldstein identified alternatively spliced FAP that forms a truncated protein in the melanoma cell line LOX. This variant is generated by an out-of-frame deletion of exonic region spanning 1223 bps. This region encodes part of the cytoplasmic tail, transmembrane and portions of the proximal and central extracellular domains. Sequence analysis of this alternatively spliced FAP variant predicts it to be entirely cytoplasmic. It is currently unknown if this splice variant has catalytic activity [35]. Additionally, three FAP splice variants have been identified in mouse embryonic tissues. All three variants encode the entire protein, including the catalytic triad, but lack part of the extracellular domain near the transmembrane domain [36]. Interestingly, there are no reports of DPPIV alternative splicing events.
Induction
Little is known about the physiologic regulators of FAP expression, however in vitro studies have offered some insights. In vitro, FAP can be induced in leptomeningeal fibroblasts by TGFβ, TPA (tetradecanoyl phorbol-13-acetate), retinol or retinoic acid [37]. TGF-β and IL1-β alone and synergistically induce FAP expression in mouse fibroblasts [38]. UVA and UVB can induce FAP expression in fibroblasts, melanocytes and primary melanoma cells. Furthermore, primary melanoma cell line media, but not metastatic melanoma media, can induce FAP expression in fibroblasts without UVR exposure [39]. In human aortic smooth muscle cells FAP is induced by TNFα. This study also demonstrated conditioned media from peripheral blood-derived macrophages induced FAP expression in aortic smooth muscle cells and that this effect was abolished upon addition of TNFα inhibitors. Thus, they infer that TNFα released from immune cells, in this instance macrophages, is responsible for induction of FAP.
In vitro studies investing the role of FAP expression in ovarian cancer found that FAP is induced in ovarian fibroblasts by exposure to conditioned media from an ovarian cell line HO-8910PM or upon adhesion to type I collagen [38, 40]. It was proposed that collagen induces FAP expression through binding of α3β1 integrin. This concept was supported by the observation that application of a β1 integrin binding antibody induced the same upregulation of FAP as collagen did [40]. Once elevated, FAP promotes proliferation, adhesion and migration of metastatic ovarian cancer cell and ovarian cancer associated fibroblasts [38, 40, 41].
One study in glial tumors demonstrates that FAP is increased upon cellular differentiation. In this study glioma stem-like cells from glioblastoma were isolated, then differentiation was induced in vitro by long term culture with basic fibroblast growth factor and epidermal growth factor. After differentiation, FAP was upregulated 40-fold, yet DPPIV remained unchanged.
A recent study points to micro-RNAs as regulators of FAP expression. Peng Ruan demonstrated that miR-30a-5p downregulated FAP expression in oral cavity cancer cells, resulting in decreased cell propagation, migration and invasion, consistent with previous reports of FAP function [42]. Many other factors have been shown to influence FAP expression in a context-dependent manner and will be addressed throughout the review.
FAP in Development and Health
Most of what is known about FAP’s role during development is from studies on frogs and mice. Amphibian metamorphosis, the transformation of the larva to a miniature adult, involves complex developmental programs that requires physiologic and morphological changes regulated by thyroid hormone. Most of the thyroid hormone regulated tissue remodeling, including tail resorption, involves cell death. Donald Brown’s group conducted a time course gene expression screen to identified thyroid hormone upregulated and downregulated genes responsible for tail resorption. They identified a set of “direct response genes” that are activated 2–4 hours after exposure to thyroid hormone and peak at 12 hours, and a set of “delayed response genes” that were maximally upregulated 24 hours after thyroid hormone induction. They proposed that the direct response genes were responsible for inducing the delayed response genes. One of the eight genes identified in the delayed response genes was FAP, in addition to two other proteases, collagenase-3 and peptidase R. Expression of FAP at this stage of metamorphosis was not exclusive to the tail, and it was proposed that this is because tissue remodeling is not limited to the tail but is essential for many other organs during metamorphosis [33, 43]. From here we can presume that FAP is expressed in addition with collagenase-3 and peptidase E to remodel the extracellular matrix to allow for tissue remodeling.
FAP deficient mice (FAP−/−) are viable and display no overt developmental defects [44]. Joachim Neidermeyer et al replaced the FAP gene with a β-galactosidase that was under regulation of the FAP promoter. After 11.5 days post conception, they found β-galactosidase expression in somites, myotubes and perichondral mesenchyme from the cartilage primordia. At day 16.5 post conception scattered developing intercostal muscle fibers expressed β-galactosidase but β-galactosidase subsequently repressed after birth. The replacement of FAP with β-galactosidase resulted in no obvious phenotypes, suggesting that FAP is associated with tissue remodeling but not necessary in embryonic development. The upregulation of compensatory proteolytic enzymes may contribute to normal development in FAP deficient models [45].
While FAP has been traditionally considered absent from adult tissues, a more systemic approach to FAP expression profiling in mice with extra-chromosomal luciferase under the control of the FAP promoter suggests that low basal levels of FAP expression might be found in many tissues, including muscle, bone marrow, adipose, skin, and pancreas [46]. FAP has also been identified in human plasma from non-diseased individuals, although the source of this circulating FAP is unknown [47]. There is one context in which FAP expression in adult tissues is universally accepted — wound healing. Consistent with FAP’s tissue remodeling role in embryologic development, FAP is known to be strongly induced in the process of scar formation. Immunohistological evaluation of six human surgical incision wounds demonstrated all six had extensive FAP expression [48].
FAP in Non-Oncologic Diseases
FAP has been linked to multiple human pathologies including fibrosis, arthritis, atherosclerosis, autoimmune diseases, metabolic diseases and cancer. In most instances, FAP is associated with progression and heightened severity of the disease, but there are some conflicting reports.
Fibrosis
Given FAP’s role in tissue remodeling and expression on activated fibroblasts of scarring tissue, it is unsurprising that FAP expression is associated with diseases of uncontrolled scarring, known as fibrosis. FAP has been reported elevated in fibrotic conditions involving the liver, lung and colon.
Liver fibrosis can ultimately lead to liver failure, a condition termed cirrhosis. Initiation of liver fibrosis is believed to be chronic injury from etiologies such as a viral hepatitis infection, non-alcoholic fatty-liver disease or alcoholism. With chronic liver injury, hepatic stellate cells, which are normally quiescent and function to store vitamin A, become activated and begin producing the extracellular matrix responsible for hepatic scarring. Activated hepatic stellate cells take on a more myofibroblast like phenotype and express α smooth muscle actin (αSMA), glial fibrillary acidic protein (GFAP), and FAP [19]. Intrahepatic expression of FAP, but not GFAP or αSMA, correlated with degree of liver fibrosis in patients with viral hepatitis C infections [49]. FAP activity was 14–18 fold greater in cirrhotic livers compared to healthy livers and circulating FAP was almost doubled in the presence of alcoholic cirrhosis [47]. Shirley Uitte de Willige showed that the concentration and activity of circulating FAP was significantly increased in patients with liver cirrhosis and that these increased levels correlated with increased cleavage of α−2 anti-plasmin. N-terminal cleaved α−2 anti-plasmin is a more potent inhibitor of fibrinolysis than its uncleaved protein and thus they propose that increased circulating FAP may be responsible for the hemostasis related bleeding and thrombotic events associated with liver cirrhosis. Interestingly, FAP levels normalized with successful liver transplant [50, 51]. A study by KH Williams demonstrates that low levels of circulating FAP can be used clinically to rule out clinically significant liver fibrosis in patients with non-alcoholic fatty liver disease [52].
Idiopathic pulmonary fibrosis (IPF) is another disease of uncontrolled fibrosis, this time affecting the lung. This chronic lung disease is characterized by excessive fibrosis of the lung interstitium with no clear etiology or successful treatments. FAP is specifically upregulated in fibroblastic foci and the fibroblastic interstitium of patients with IPF but not in adjacent normal tissue, lung tissue from healthy individuals or lung tissues from patients with centri-acinar emphysema [53]. FAP is also upregulated in mouse models of IPF and levels of FAP expression in the lungs correlate to the severity of IPF [54]. Interestingly, IPF is exacerbated in FAP deficient mice, and restoration of FAP to FAP deficient mice significantly reduced lung collagen content. This finding therefore suggests that FAP plays a protective role in the lung and functions to combat fibrosis by promoting collagen clearance and matrix degradation [55]. However, these surprising findings are contradicted by a study demonstrating that a nonspecific FAP inhibitor (PT-100, Val-boro-pro, talabostat, BXCL-701) had anti-fibrotic effects. In in vivo models of IPF, an FAP inhibitor slowed disease and reduced fibrosis [56]. While the specific roles of FAP in IPF remain uncertain, its involvement in the disease is undisputed.
Other pathologies in which extensive fibrosis is correlated with upregulated FAP expression include keloid formation and Crohn’s disease. Keloid scars are benign, fibroproliferative dermal lesions of unknown etiology and commonly occur following surgical resection. Keloids progress in a manner dependent on increased deposition of extracellular matrix and invasion into surrounding healthy skin. One study demonstrated that fibroblasts derived from keloid skin samples had elevated expression of FAP, increased invasiveness and enhanced extracellular matrix deposition when compared to fibroblasts derived from control skin samples. Selective inhibition of FAP/DPPIV resulted in decreased invasion but had no effect on other phenotypes such as increased extracellular matrix deposition or expression of pro-inflammatory cytokines [57].
Crohn’s disease is an autoimmune condition resulting in chronic gut inflammation that can be complicated by intestinal fibrosis and stricture formation. One study identified FAP to be overexpressed in uninflamed strictures compared to non-strictured colonic regions in biopsies taken from Crohn’s Disease patients. FAP was not overexpressed in colonic biopsies taken from healthy individuals or individuals with ulcerative colitis, a different inflammatory bowel disease. FAP expression was increased in myofibroblasts derived from strictured lesions upon exposure to TNFα and TGF-β, but that this was not true for myofibroblasts derived from nonstrictured lesions [58]. These results imply that FAP cannot be induced in any fibroblast upon exposure to inducing factors, but some reprogramming of cells prior to pro-FAP factors is required.
Arthritis
Arthritis is a term used to mean any disorder that affects the joints. The two most common forms of arthritis are osteoarthritis and rheumatoid arthritis. Osteoarthritis is also known as degenerative joint disease and occurs with aging. Rheumatoid arthritis is an autoimmune condition. The investigation of FAP in arthritis was sparked when a phase I clinical trial of radiolabeled anti-FAP antibody demonstrated minor antibody uptake in the knees and shoulders of patients who lacked clinical symptoms of arthritis [59].
Osteoarthritis is characterized by degradation of joint cartilage. Joint cartilage is largely composed of proteoglycans, collagen and chondrocytes, the cells responsible for cartilage maintenance. Milner et al. were the first to demonstrate that chondrocytes expressed FAP and that chondrocyte FAP expression was elevated in patients with osteoarthritis. They demonstrated that chondrocytes increased FAP expression in response to cartilage resorption signaling cytokines, IL-1 and oncostatin M, and that this induction of FAP correlated with increased collagen breakdown in vitro. FAP expression was elevated in mRNA extracted from collagen derived from osteoarthritis patients compared to cartilage of normal patients. All osteoarthritis patients expressed FAP in the superficial zone of cartilage and on chondrocyte membranes by immunohistochemistry [60]. Thus, this paper suggests FAP is involved in cartilage degradation associated with osteoarthritis.
Rheumatoid arthritis is an autoimmune chronic inflammatory disease of unknown etiology and is characterized by chronic inflammation of the joint capsule’s synovial membrane. This chronic inflammation ultimately destroys the underlying cartilage and bone. Activated fibroblast-like synoviocytes (FLS) line the synovial membrane and are a prominent cell type responsible for inflammation and joint destruction. One study identified FAP expression in synovial samples taken from both rheumatoid arthritis and osteoarthritis patients. However, FAP expression was greater in samples taken from refractory rheumatoid arthritis patients in comparison to end stage osteoarthritis patients [61]. While the association of FAP and arthritis was clear, the role of FAP in arthritic diseases remained elusive. Ospelt et al. showed that inhibition of FAP/DPPIV worsened arthritic lesions in vivo models. Treatment of animals with a FAP/DPPIV inhibitor increased synovial expression of MMP-1 and MMP-3 and increased collagen destruction [62]. However this group also demonstrated that DPPIV knockout mice had worsened arthritic lesions [63] and as such the pro-arthritic effects of this inhibitor can be attributed to its effects on DPPIV. In 2015, Waldele et al. developed a transgenic mouse model of chronic inflammatory arthritis that lacked FAP. In this model, FAP deficiency led to decreased cartilage degradation, even though the amount of inflammation and bone degradation was unchanged. They demonstrated that synovial fibroblasts derived from FAP deficient mice had decreased ability to adhere to cartilage [64]. Laverman et al demonstrated that the use of radiolabeled anti-FAP antibodies accurately represented synovial inflammation severity in mouse models of rheumatoid arthritis [65], suggesting the association between FAP and arthritis could be exploited for clinical benefit.
Cardiovascular Disease
Many pathologies fall under the term cardiovascular disease, including atherosclerosis and myocardial infarction. Atherosclerosis is characterized by subendothelial accumulation of fatty substances, called plaques, that lead to inflammation and tissue remodeling. These atheromatous plaques can rupture and cause myocardial infarction, stroke or sudden cardiac death. There are two types of atheromatous plaques- thin cap and thick cap. One study identified overexpression of FAP in human aortic smooth muscle cells of thin cap atheromas in human biopsies. FAP was induced by TNFα released from macrophages and FAP levels correlated with macrophage infiltration. In vitro studies then demonstrated that once FAP is expressed, it cleaves the type I collagen present in the cap and renders the plaque rupture-prone. Treatment with an anti-FAP antibody resulted in decreased collagen cleavage [66].
Several studies investigated the levels of soluble FAP in the plasma of patients with various atherosclerosis related diseases. These studies showed levels of soluble FAP were unaffected by conditions such as ischemic stroke and peripheral artery disease, but that FAP levels were decreased in patients with coronary heart disease and acute coronary syndrome. In acute coronary syndrome, decreased soluble FAP levels correlated with worse clinical outcomes, as patients with FAP levels in the first quartile had a 3-fold higher risk of death. Furthermore, investigators found that fluctuations in FAP levels were not permanent and that over time, levels returned to that of the control population [67, 68].
One study demonstrated that in rats, cardiac expression of FAP increased after induction of a myocardial infarction (MI). This was especially true for the myofibroblasts in the peri-infarct area. Peak FAP expression was seen 7 days post MI. These findings were confirmed in human cardiac specimens, with FAP+ fibroblasts being abundant in ischemic tissue post-MI but absent in healthy control cardiac specimens [69]. In plasma samples obtained from patients post ST-elevation myocardial infarction, FAP concentrations were inversely related to established cardiac enzymes, CK and CPR. Greater declines of FAP from admission to 5 days post admissions were associated with increased myocardial damage and inflammation [70].
Metabolic Disease
Given the recent discovery that FAP cleaves and inactivates the hormone FGF21 [20, 71], the role of FAP in metabolic diseases has just started to be investigated. FGF21 is a stress-induced hormone with potent anti-obesity, insulin-sensitizing and hepatoprotective properties. One study demonstrated that administration of talabostat, a nonspecific inhibitor of FAP, to mice with diet induced obesity had significantly reduced body weight, food consumption, adiposity and cholesterol with simultaneously increased energy expenditure, glucose tolerance and insulin sensitivity [72]. This affect was abrogated in FGF21 deficient mice, thus confirming that the metabolic benefits of FAP inhibition can be attributed to increased circulating FGF21.
FAP in Cancer
While FAP expression in normal tissues is usually low or undetectable, it is overexpressed in many cancers, including 90% of carcinomas. FAP is known to be overexpressed in breast, colorectal, pancreatic, lung, bladder, ovarian and other cancers. In these cancers, FAP is usually heavily expressed in the stroma, and has thus become a universal marker of cancer-associated fibroblasts (CAFs). While the presence of FAP in malignant tissues is undisputed, the role of FAP biologically and its impact on disease prognosis has been inconsistent throughout the literature.
Breast Cancer
One of the earliest publications about FAP identified FAP overexpression in the stroma of breast epithelial tumors and focal expression in some of the samples of fibrocystic disease while FAP was absent from normal breast tissue or benign breast tumors [48]. One study identified increased FAP expression in ductal carcinoma in situ that would progress to ductal carcinoma versus DCIS that would not progress. This suggests pathologists could utilize FAP to improve clinical prediction of progression and fine tune treatment recommendations [73]. While most studies confirmed the existence of FAP in the stroma surrounding breast cancer cells, one study identified FAP expression in the breast cancer cell lines themselves [74]. Reports on the impact of FAP expression on disease prognosis are inconsistent. FAP expression in stromal tumor components is greater in invasive lobular carcinoma than invasive carcinoma of no special type [75]. In invasive ductal carcinoma, elevated FAP was associated with high histological tumor grade as well as an inflammatory- and adipose- type stroma but not desmoplastic, sclerotic or normal-like stroma [76, 77]. In phyllodes tumors, a benign breast tumor that has rare malignant transformation, increased FAP mRNA levels were associated with malignant transformation, suggesting that FAP can be utilized to determine the malignant potential of these tumors [78], similar to its prognostic value for DCIS. The prognostic value of FAP in breast cancers of all subtypes is controversial, with some studies demonstrating that elevated FAP is associated with worse survival [79, 80], and others associating elevated FAP is associated with improved survival [81].
Colorectal Cancer
In human colon cancer specimens, FAP expression has been identified in both cancer cells and in adjacent stromal cells, including myofiboblasts, fibroblasts and endothelial cells [5]. FAP staining intensity was inversely correlated with patient tumor stage and xenograft tumor size. Elevated FAP expression noted early in tumor development [82]. These data suggested that stromal FAP may play a role in the development of colorectal tumors. Perhaps in accordance with this finding, human colorectal specimens were noted to have elevated FAP at the tumor front versus the tumor center, suggesting the role of FAP in tumor invasion. This study also found that FAP was more likely to be expressed in the center of tumors post-radiotherapy, perhaps due to the tissue remodeling required after radiation inflicted damage [83]. In human samples, high FAP was associated with increased depth of invasion, lymph node metastasis, higher grade and stage and worse overall survival. [5, 82–84]. Tumoral FAP expression also correlated with a shift in immune cell populations. Elevated FAP was associated with reduced CD3+ cells but increased CD11b+ cells [84].
Pancreatic Cancer
Ninety percent of pancreatic ductal adenocarcinomas (PDAC) demonstrate FAP staining. FAP expression has been identified in both the tumor stromal compartment as well as PDAC tumor cells and pancreatic cancer cell lines [85]. FAP expression in stromal tissue is greatest at the tumor front. Low FAP expression is associated with increased pancreatic fibrosis while high FAP expression is associated with increased risk of lymph node metastasis, tumor recurrence and death [86]. In vivo studies utilizing an endogenous KPC PDAC tumor mouse model in FAP knockout mice demonstrated that FAP deficiency delays tumor onset and prolongs survival, increases tumor necrosis and impedes distant metastasis [8]. FAP expression was identified in both the malignant lesions as well as the pre-malignant lesions, termed PanINs, of KPC mice [87]. Many more studies have confirmed the association between elevated FAP and worse clinical outcomes [8, 85]. Elevated FAP expression was positively correlated with patient age, tumor size, fibrotic foci, perineural invasion and pore survival [85]. However, some studies have found that FAP expression was correlated with improved clinical outcomes [88, 89].
Gastric Cancer
Gastric cancer consists primarily of two types: intestinal-type and diffuse-type. Both types express FAP, however intestinal-type does so to a larger degree. Unlike other cancers, in gastric cancer the majority of FAP expression is localized to the gastric carcinoma cells and is only weakly expressed in stromal and endothelial cells [7, 90]. In human tissues high FAP expression is correlated with high grade, lymph node metastasis, peritoneal invasion and worse overall survival [91, 92]. Models of gastric cancer demonstrated that co-culture of gastric cancer cells with FAP expressing fibroblasts resulting in increased proliferation and migration in vitro and increased tumor growth and resistance to anti-PD-1 therapy in vivo [92]. One gastric cancer model study showed that administration of polyphyllin, a plant derived compound, decreased CAF proliferation in vitro and decreased tumor growth in vivo via downregulation of FAP [93].
Brain Cancer
Original work studying FAP suggested primary brain tumors did not express FAP but metastatic carcinoma lesions did [48, 94]. Future work would go on to challenge this concept and demonstrate that FAP is expressed in high grade lesions. Grade III and IV human astrocytic tumors express FAP mRNA, while Grade II and nonmalignant lesions do not [95]. In glial tumors, there is increasing FAP mRNA expression as grade increases and within the grade IV subtypes, glial sarcomas have significantly more FAP expression than glioblastomas [96–98]. FAP expression in gliomas is correlated with worse overall survival, however this can be attributed to the fact that the most malignant gliomas are associated with increase FAP expression [99].
Ovarian Cancer
FAP expression was detected in 97% of ovarian cancers, but not in normal ovarian tissue, benign ovarian tumors or ovarian tumors of low malignant potential [48, 94, 100]. While FAP is not believed to be expressed in ovarian epithelial cancer cells, one study demonstrated FAP knock down in SKOV3 ovarian cancer cells lines resulted in decrease decreased FAP expression in surrounding fibroblasts, decreased tumor growth, volume and proliferation [41]. In a complementary experiments, SKOV3 lines transfected with FAP to over-express FAP stably had increased tumor growth, proliferation and invasion in vitro [101]. In human studies, an elevated level of FAP in peritoneal or pleural effusions from epithelial ovarian cancer patients correlated with decreased survival rates [102]. Strong stromal staining for FAP and DPPIV by IHC and mRNA levels by in-situ hybridization were associated with higher stage and increased metastasis to the lymph nodes and the omentum. By contrast, no significant correlation was detected among FAP/DPPIV protein/mRNA levels and patient age, histological grade or tumor type. Furthermore, elevated FAP levels, but not DPPIV levels, were associated with shorter disease-free survival [100, 103].
Myeloma
Multiple myeloma is a hematologic malignancy that affects plasma cells. Unique to myeloma is the clinical feature of osteolytic bone disease whereby increased osteoclast activity and decreased osteoblast numbers results in bone break down, which has been hypothesized as a means for myeloma cell expansion within the bone marrow. While FAP is not expressed in myeloma cells, it was identified as one of 28 genes selectively upregulated in osteoclasts upon coculture with myeloma cells, while the other related serine protease levels were unchanged. In multiple myeloma patient bone marrow biopsies, FAP was expressed by osteoclasts, osteoblasts and osteocytes along the bone surface and in fibrotic regions. In the same study FAP knockdown in osteoclasts led to decreased myeloma cell survival in coculture. In vivo myeloma studies demonstrated FAP mRNA was upregulated more than 40-fold in the bones of mice inoculated with myeloma cell lines compared to uninoculated mice [104]. Further work by this group demonstrated that the addition of talabostat to cocultures of patient-derived osteoclast and myeloma cells resulted in talabostat concentration-dependent decreased myeloma cell proliferation. In vivo application of talabostat in SCID myeloma models reduced osteoclast activity, bone resorption and tumor burden [105].
Melanoma
Even though the earliest descriptions of FAP were within the context of melanoma, the role of FAP in melanoma is still controversial. Huber et al. systematically determined the expression pattern and enzymatic activity of FAP in both stromal cells and melanocytes in a series of melanocytic lesions ranging from benign melanocytic nevi, commonly referred to as moles, to metastatic melanoma. FAP is expressed in the stromal fibroblasts of all melanocytic tumors, including benign, premalignant and malignant, however, FAP expression was absent in fibroblasts from normal adult skin. While FAP is expressed in the stroma of benign melanocytic tumors, its expression increases in the stroma of malignant and metastatic lesions. This study identified FAP expression on the surface of melanocytes in 30% of benign melanocytic nevi, while melanocytes from primary and metastatic melanoma lesions had no detectable levels of FAP expression [106]. However, Aoyama et al. demonstrated FAP expression by melanoma cell lines correlated with an increasingly invasive phenotype [1]. In these melanoma cell lines, FAP was found to be localized to invadopodia, thus promoting matrix degradation and cellular invasion [2, 3].
In summary, FAP expression’s impact on clinical factors such as tumor type and clinical outcomes is highly variable and depends on cancer type, histological type, tumor localization and specific cellular expression (stromal vs. malignant cells). A recent meta-analysis assessed the prognostic value of FAP in solid tumors by performing a global analysis of 15 studies and concluded that FAP overexpression in tumor tissues displayed significant associations with poor overall survival and tumor progression. Subgroup analysis revealed the correlation between FAP overexpression and poor overall survival and lymph node metastasis was more pronounced in patients with FAP expression in tumor cells [107].
Functional Roles of FAP in Cancer
Given the extensive expression of FAP in many cancer types, the pro-tumorigenic or anti-tumorigenic role of FAP has been thoroughly investigated. To date, FAP has been reported to influence tumor growth via multiple mechanisms including promoting proliferation, invasion, angiogenesis, epithelial-to-mesenchymal transition, stem cell promotion, immunosuppression and drug resistance.
Proliferation, Migration and Invasion
Perhaps the most consistent finding in the literature is the effect of FAP on cell proliferation, migration and invasion, all of which promote tumor growth. It has been demonstrated FAP can promote invasion of endothelial cells, melanoma cells, ovarian cancer cell lines, oral cancer cells, and fibroblasts [2, 28, 39, 40, 42]. How FAP promotes proliferation and migration is still contested. There are two main hypotheses. The first is the indirect hypothesis: FAP regulates extracellular matrix remodeling and the changes to the matrix are then responsible for increased capability of cell growth. Even proponents of this hypothesis, however, dispute if FAPs regulation of the extracellular matrix can be attributed to its enzymatic activity or if it is due to FAP independent of its enzymatic activity. The second hypothesis is a direct hypothesis: FAP expression alters intracellular signaling pathways, which in turn affect cell cycle and proliferation pathways to promote cell growth.
The indirect hypothesis has been supported by many studies. Some of the earliest work on FAP demonstrated its localization to the tips of invadopodia in melanoma cells and associated increased extracellular matrix degradation and invasion [2, 108]. It is believed that α3β1 integrin is necessary for appropriate localization of FAP to invadopodia [29]. The role of α3β1 integrin in FAP induced proliferation and migration was further investigated in a study where inhibition of α3β1 integrin attenuated the FAP induced proliferation invasion and migration in ovarian cancer cell lines [109]. This then implies that it is not the enzymatic activity of FAP that is causing these phenotypic changes but rather the association of FAP with α3β1 integrin. These findings are further supported by evidence that breast cancer cell overexpressing wild type and catalytically inactive FAP display increased extracellular matrix degradation and invasion on type I collagen gels [23]. One study generated doxycycline-inducible FAP overexpressing fibroblasts and cocultured them with pancreatic ductal adenocarcinoma cells to assess the effects of FAP on extracellular matrix and malignant cell phenotype. The authors found that FAP expressing fibroblasts induced architectural and compositional changes to the extracellular matrix that allowed for enhanced velocity of pancreatic cancer cell migration. In agreement with previous literature, this study concluded that enhanced migratory phenotype is mediated by β1 integrin as addition of an integrin inhibitor reversed the phenotypic changes [110]. However, in the same study the addition of an FAP inhibitor led to extracellular matrix disorganization that impeded pancreatic cancer cell invasion, thus implying that the enzymatic activity is also required for extracellular matrix remodeling. The role of FAP’s enzymatic activity in extracellular matrix remodeling has been investigated in other studies as well. FAP knock out mice had accumulation of intermediate-sized collagen fragments in lung tissue in compared to wild type mice. This observation was recapitulated when wild type mice were treated with an FAP inhibitor. In another study focusing on melanoma, ultraviolet radiation-induced FAP expression in fibroblasts and these fibroblasts displayed greater migratory capacity that was associated with increased collagenase I activity [39].
The hypothesis that FAP has direct effects on intracellular proliferation and cell cycle signaling pathways is also supported by many studies. Alterations of FAP expression induces changes in common cell signaling pathways or gene expression. SiRNA knockdown of FAP in tumor-associated fibroblasts derived from ovarian cancers inhibited cell proliferation, induced cell cycle arrest and decreased the expression of stem cell associated genes. [41]. In a squamous cell lung carcinoma cell line, FAP overexpression promoted proliferation, motility and invasion while simultaneously upregulating PI3K/Akt and SHH/Gli1 signaling [111]. The importance of these signaling pathways in promoting cellular proliferation and invasion was confirmed when inhibition of SHH and PI3K abrogated the phenotype. This same group studied the effects of FAP on cell signaling in breast cancer lines. Interestingly, the overexpression of FAP in breast cancer lines resulted in decreased motility. Overexpression of FAP reduced FAK phosphorylation, and the reduction in FAK activity caused the decreased motility phenotype [80]. In oral squamous cell carcinoma, knockdown of FAP resulted in decreased growth and metastasis in vitro and in vivo. Silencing FAP expression reduced the activation of pRb and oncogenic cell-cycle regulators including CCNE1, E2F1, and c-Myc, but elevated the expression of tumor suppressors such as p27 and p21. Furthermore, FAP silencing significantly decreased the expression of phosphorylated PI3K, AKT, MEK1/2, ERK1/2, and GSK3b, whereas total levels remained unchanged. These results suggested that FAP is an upstream regulator of the PTEN/PI3K/Akt and Ras-ERK signaling pathways in oral squamous cell carcinoma [112]. One study focused on the effects of FAP expressing fibroblasts on pancreatic ductal adenocarcinoma cell lines, showing that coculture of PDAC lines with FAP+ fibroblasts resulted in increased phosphorylation of Rb in the cancer cells, leading to cell cycle progression and increased proliferation [88].
Both hypotheses have merit and are supported by the available evidence. FAP’s effects on proliferation, motility and invasion could be a consequence of its extracellular matrix remodeling as well as its intracellular signaling, and could depend on both the enzymatic and non-enzymatic activities of FAP. Yang et al. demonstrated that in ovarian cancer cell lines, FAP− integrin dimer formation and FAP induced intracellular activation of Rac1 induced increased proliferation and migration; inhibition of either integrin or Rac1 reversed the phenotype [109]. One can imagine a situation in which the docking of FAP to invadopodia by integrins serves two purposes. The first is to localize FAP to the leading edge of cellular invasion to allow to matrix remodeling and easier migration. The second is so that FAP can trigger intracellular signaling through integrins to promote invasion, migration and proliferation gene signaling. This complementary perspective of FAP signaling also implicates the need for FAP’s enzymatic function and non-enzymatic function to promote the pro-tumorigenic phenotype.
Angiogenesis
In 2003, Aimes et al. discovered that human endothelial cells are capable of producing FAP and that FAP, like other serine proteases, has regulatory roles in microvascular endothelial cell reorganization and capillary morphogenesis [113]. In in vivo models, inoculation of SCID mice with FAP+ breast cancer cell lines resulted in faster growing, highly vascularized tumors even though these FAP+ cells did not have any proliferative advantage in vitro. Histological analysis of gastric cancer biopsies demonstrated that gastric cancers with high FAP expression also had increased micro-vessel density compared to gastric cancers with lower FAP expression [114]. These findings were further validated by a study demonstrating that FAP knock out or pharmacologic inhibition of FAP resulted in decreased tumor growth and decreased tumor microvascular density in in vivo models of lung cancer and colon cancer [115]. These data suggest that the enzymatic activity of FAP is responsible for increased angiogenesis. While FAP is not believed to be expressed by ovarian epithelial cancer cells, one study demonstrated that FAP knockdown in SKOV3 ovarian cancer cells lines led to decreased expression of VEGF and EGF, suggesting FAP’s role in tumor angiogenesis [41]. A recent study aimed at elucidating the differential functions of the endopeptidase and dipeptidyl peptidase activates of FAP demonstrated that FAP expression by human endothelial cells early in the stages of capillary tube formation, followed by subsequent abrogation of FAP expression once tubes had formed [116]. These findings are further validated by a study that demonstrated FAP expression by the endothelial cells of capillaries, but not large blood vessels, in invasive ductal carcinoma in vivo. FAP expression localized to the invadopodia of endothelial cells [28]. This observation suggests FAP promotes capillary growth and invasion into the extracellular matrix. FAP expressing stromal cells have been seen to localize around dysplastic blood vessels in glioblastoma [99]. Additional studies have identified FAP expression on endothelial cells in the developing microvasculature in malignancies such as multiple myeloma, gastric carcinoma and breast cancer [90, 104, 117].
It has been hypothesized that the proangiogenic qualities of FAP can be attributed to the dipeptidyl peptidase activity that it shares with DPPIV. One of the known substrates of FAP and DPPIV is neuropeptide Y, which, upon cleavage, becomes proangiogenic, promoting endothelial cell migration and tube formation on Matrigel [118]. Another theory is that MMP-9, often co-expressed with FAP, is responsible for the angiogenic phenotypes of FAP expressing tumors, since MMP-9 is a known pro-angiogenic signaler [119]. Interestingly, studies with catalytically inactive and active FAP demonstrate equal upregulation of MMP-9; therefore, this means of angiogenesis would not require FAP enzymatic activity [23]. The final way FAP may be involved in angiogenesis is indirectly, via its effect on extracellular matrix reorganization that may promote endothelial cell migration and neovascularization.
Epithelial-to-Mesenchymal Transition
Epithelial-to-mesenchymal transition (EMT) is defined as the acquisition of mesenchymal phenotype by malignant epithelial cells to allow for increased migration and invasion ultimately required for metastasis. In a technical paper warning against the use of anti-FAP antibodies as a means of isolating fibroblasts, it was demonstrated that many cell lines of epithelial origin expressed FAP in response to TGB-β induced EMT [120]. Oral squamous cell carcinoma cell lines with stable FAP knock down had decreased expression EMT-marker genes such as Snail, Slug, N-cadherin and Vimentin with E-cadherin expression increased [112].
While EMT is typically associated with invasive phenotypes of epithelial derived cancers, similar acquisition of mesenchymal phenotype has recently been observed in glial tumors, where the mesenchymal phenotype is associated with increased clinically aggressive tumors. TCGA analysis of glioblastomas demonstrated that 70% of mesenchymal glioblastomas had a 2-fold increase in FAP expression compared to other subtypes [99]. A well-known regulator of EMT is the transcription factor TWIST1. In vitro glioma studies showed upregulation of TWIST1 in malignant glioma lines and association between TWIST1 and invasion. Subsequent studies demonstrated TWIST1 had pro-tumorigenic effects by inducing mesenchymal changes in glioma cell lines, including upregulation of FAP. This study went on to confirm TWIST1 and FAP were jointly upregulated in biopsies from the most aggressive glioblastoma tumors [96].
Immunological Regulation
The effects of FAP on the immune system began to be investigated fairly recently. In 2009, Douglas Fearon’s group published a study in Science that detailed the ability of FAP+ cells to suppress antitumor immunity. They generated transgenic murine models in which the fap gene contained a cassette encoding either GFP or diphtheria toxin receptor (DTR). Using GFP strains, they demonstrated FAP expression in both CD45+ and CD45− cells. Further sub-phenotyping of these cells revealed the CD45+ population to resemble the CD11b+/classII+/Col1+/αSMA+ fibrocyte and the CD45− population to resemble mesenchymal stem cells. Using the DTR strain they could ablate cells that express FAP by injecting diphtheria toxin. They then created immunogenic tumors by transfecting tumor cell lines with ovalbumin and vaccinated the mice with vaccinia virus expressing OVA. Prophylactic treatment of non-transgenic mice with the OVA vaccine successfully reduced tumor growth, demonstrating the efficacy of the vaccine. They then investigated the efficacy of OVA vaccine treatment with vaccine administration after tumor inoculation and found immediate tumor growth arrest upon FAP ablation for immunogenic tumors but not nonimmunogenic tumors. Surprisingly, they found no changes in T cell populations between FAP depleted and nondepleted mice, suggesting that the immunological impact of FAP is not T cell-mediated. Furthermore, reduction in tumor growth upon FAP ablation was reversed with anti-TNFα/anti-IFNγ treatment. Therefore, this paper proposed that FAP suppresses production of TNFα and IFNγ, or attenuates cellular responses to these cytokines. The relatively unchanged levels of these cytokines after FAP ablation would suggest the latter [121]. The same group utilized the DTR transgenic mice to investigate the role of FAP in PDAC. The found significantly reduced tumor growth upon ablation of FAP+ cells. However, contradictory to their previous findings, they found the reduced tumor growth was dependent on CD4+/CD8+ T cell activity and that FAP ablation enhanced the therapeutic benefits of anti-PD-1 and to a lesser extend anti-CTLA4 [87]. This suggests that FAP contributes to the resistance of PDA to these immune checkpoints, at least in murine models. This is not the only study to imply that FAP serves as a resistance mechanism to immune checkpoints. In vivo models of colorectal cancer demonstrated that co-injection of CRC cell lines with FAP+CAFs led to anti-PD-1 resistance [122]. In vivo models of gastric cancer demonstrated a synergistic reduction in tumor growth of anti-PD-1 and an FAP inhibitor [92].
These findings stimulated investigations of the mechanism by which FAP may alter the intratumor immune milieu. One study demonstrated that FAP expressing cancer associated fibroblasts (CAFs) had a uniquely inflammatory gene expression signature in comparison to FAP− CAFs. Of the inflammatory genes upregulated by the FAP+CAFs, Ccl2 was most highly expressed [84]. Furthermore, this study demonstrated that FAP’s induction of CCL2 was independent of its enzymatic activity as addition of talabostat did not change the levels of these proteins. This group went onto to investigate the function of FAP+CAFs by co-injecting them with Hepa1–6 fibroma tumor lines. Tumors resulting from FAP+CAF containing mixtures had increased levels of PMN-MDSCs, M-MDSCs and macrophages, yet decreased IFNγ+CD8+ T cells when compared to FAP-CAF cell mixtures. The showed that FAP+CAFs release CCL2, which in turn is recognized by the CCL2 receptor, CCR2, on circulating MDSCs, leading to their recruitment to tumor tissues. In Ccl2 knock out mice, tumor inoculation with FAP+CAFs lost their growth advantage over FAP-CAF tumors, and the resultant tumors had comparable levels of MDSCs. The ability of FAP+CAFs to produce CCL2, and its effects on MDSCs was also seen in a study investigating colorectal cancer [122]. Other studies argue that a different cytokine, CXCL12, is responsible for the immunosuppressive environments associated with FAP+ fibroblasts. Feig et al. identified the primary source of tumor CXCL12 to be from FAP+CAFs. They then demonstrated that addition of an inhibitor to the CXCL12 receptor, CXCR4, reduced tumor growth in a T-cell dependent manner and enhanced the efficacy of anti-PD-1 but not anti-CTLA-4 [87]. The ability of FAP+CAFs to secrete CXCL12 was confirmed by a study demonstrating that FAP+CAFs recognition of adenosine by the adenosine receptor A2B induces CXCL12 [123].
The role of FAP in the immune system extends beyond its expression in cancer associated fibroblasts. There have been recent observations that FAP can be expressed by various immunological cells, including myeloid derived suppressor cells (MDSCs) and macrophages. Both healthy donor MDSCs and MDSCs derived from multiple myeloma patients expressed FAP on their cell membranes. When cultured in conditioned media from myeloma cell lines, the level of FAP expressed by multiple myeloma-derived MDSCs significantly increased. In vitro studies went on to demonstrate that when CD4+ T cells were cocultured with multiple myeloma derived-MDSCs, the CD4+ T cells exhibited decreased proliferation, increased senescence and increased differentiation into Th17 T cells. These changes were then reversed upon the addition of an FAP inhibitor. The phenotypic changes in the CD4+ T cells upon exposure to FAP were caused by activation of AKT; an AKT inhibitor rescued abnormal T cell differentiation and senescence. Another study detailed the presence of intra-tumoral FAP expressing F4/80hi/CCR2+/CD206+ M2 macrophages that induced immunosuppression via release of heme oxygenase-1. Heme oxygenase creates carbon monoxide, which suppresses the pro-apoptotic effects of TNFα on endothelial cells [9].
Not every study suggests FAP has an immunosuppressive role. One study in non-small cell lung cancer used tissue microarray to identify correlations between CAF subtypes and immune markers. They demonstrated that in tumors with high CD3+/CD8+ T cell infiltration, high FAP expression was correlated with increased patient survival [124]. This study proposed a beneficial prognostic role of FAP+CAFs and warned that targeting FAP as a therapeutic approach should be done cautiously.
Tumor Suppression
With the amounting evidence to suggest FAP’s role in tumor promotion, its potential as a tumor suppressor must be addressed. As previously discussed, FAP expression is specifically silenced in proliferating melanocytes undergoing malignant transformation. Melanocytes engineered to overexpress FAP or a catalytically inactive form of FAP regained contact inhibition, cell cycle arrest and increased susceptibility to stress-induced apoptosis. Furthermore, implantation of these FAP expressing melanocytes abrogated tumorgenicity in vivo [22].
Signaling
Several signaling pathways affected by FAP result in the phenotype witnessed in FAP expressing cells. Downstream signaling targets of FAP include PI3K/AKT, RAS/ERK, SHH/GLI, FAK and many others (Figure 2).
Fig. 2.
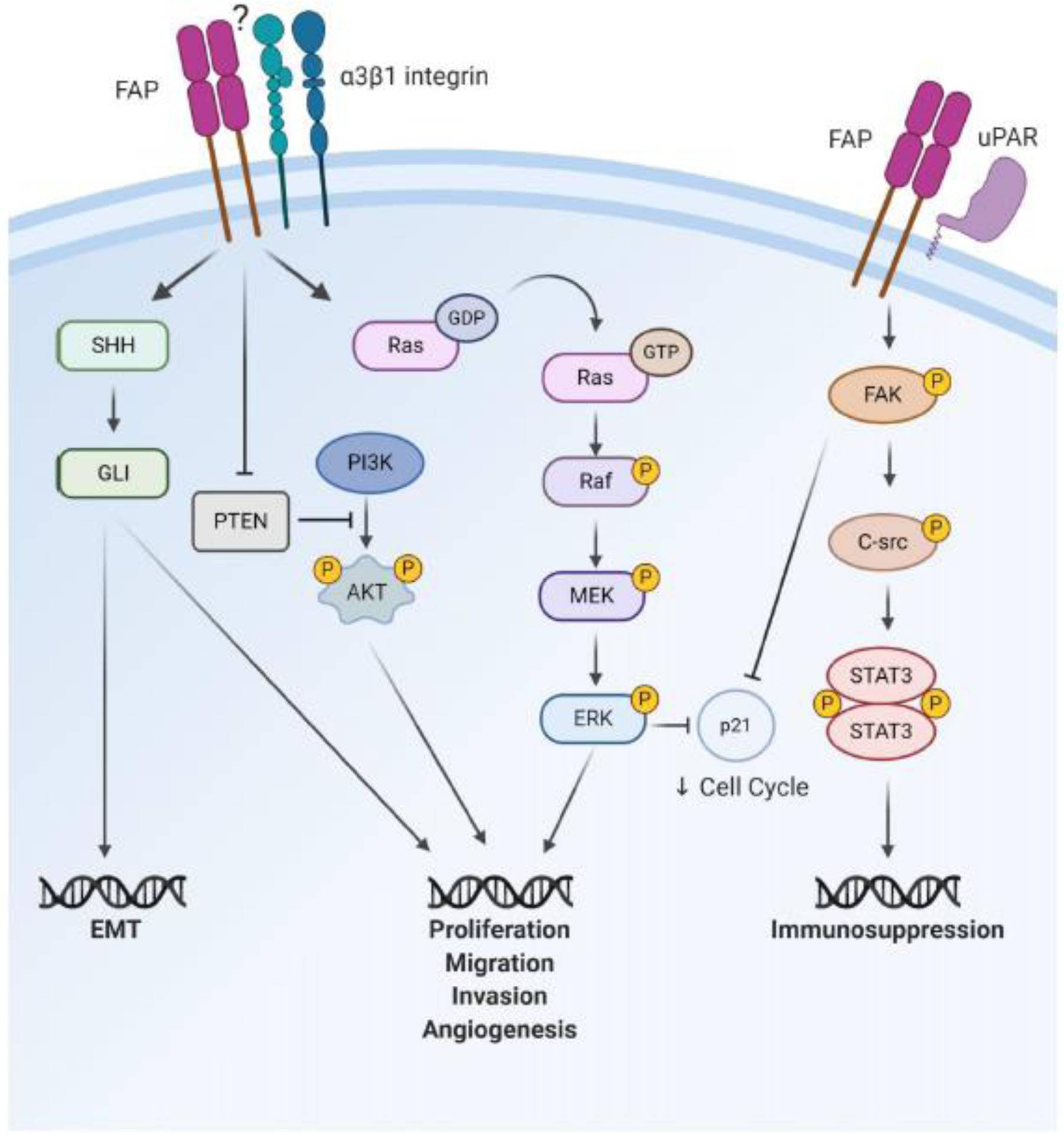
Potential signaling pathways affected by FAP that are responsible for the tumor promoting phenotypes associated with FAP expression
PI3K/AKT:
Cells engineered to overexpress FAP have increased proliferation and migration due to activation of the PI3K and the Sonic Hedgehog (SHH) pathways, which are intracellular signaling pathways required for cell cycle and differentiation, respectively. Exposure to inhibitors of PI3K and SHH abrogated the FAP induced phenotypic changes [80]. In oral SCC cells, it has been reported that the knockdown of FAP resulted in suppressed proliferation, migration and invasion via inactivation the PTEN/PI3K/AKT and Ras-ERK signaling pathways [112].
FAK:
Focal adhesion kinase (FAK), an intracellular tyrosine kinase recruited to the sites of integrin clustering or focal adhesions, functions as a major mediator of signal transduction by cell surface receptors, including integrins, growth factor and cytokine receptors. FAK partially regulates cell adhesion, migration, and invasion. Overexpression of FAP was associated with a decrease in phosphorylated FAK protein. One study suggested that FAP might form a complex with the FAK protein, and in doing so reduce its phosphorylation, which thus results in reduction of adhesion and motility ability [80]. Furthermore, in FAP knockout mice, deletion of FAP increased p21 via ECM-mediated signaling through FAK and ERK [125]. p21 is known to arrest the cell cycle. Therefore, FAP may inhibit the inhibitor, allowing for cell cycle progression and increased growth. In another study, FAP overexpression promoted proliferation in breast cancer cells in vitro. The addition of a FAK inhibitor reversed the proliferative ability of these cells, while inhibitors to PI3K, ERK and ROCK had no effect [80].
uPAR:
FAP’s association with uPAR has been implicated in both the cellular migration and immunosuppression phenotypes associated with FAP. In ovarian cancer cells, FAP complex with integrin α3β1 and the uPAR signaling complex mediated cellular migration via the small GTPase Rac1 pathway [126]. In murine liver models, the expression of immunosuppressive cytokine CCL2 is mediated through a uPAR-dependent FAK-Src-STAT3 pathway, with STAT3 being the transcription factor responsible for Ccl2 expression. This paper validated these results in intrahepatic cholangiocarcinoma human specimens by tissue microarray, demonstrating that expression of FAP positively correlated with CCL2 and p-STAT3 levels [84].
SHH/GLI:
In addition to SHH/GLI pathways’ roles in promoting proliferation, invasion and migration as previously mentioned, FAP’s effect on EMT may also be due to its activation of the SHH/GLI pathway. The expression of GLI1 was associated with changes in the expression of EMT markers E-cadherin and β-catenin in lung SCC specimens. Inhibition of the SHH/GLI pathway suppressed the migration of and upregulated E-cadherin in lung SCC cells. Conversely, stimulation of the SHH pathway increased migration and downregulated the expression of E-cadherin in the lung SCC cells [127]. Since FAP overexpression activates the SHH a [111], FAP may be indirectly involved in the EMT process by regulating SHH. SHH has also been shown to promote the desmoplasia associated with pancreatic cancer [128].
Therapeutic Targeting of FAP
While the function of FAP within malignancies remains poorly understood, there have been many efforts to exploit FAP biology clinically. Approaches that target FAP clinically include: inhibiting FAP’s proteinase activity with small molecules or antibodies, using FAP proteinase activity to cleave oncologic drugs attached to peptides targeted to FAP, vaccination against FAP, and most recently, FAP CAR T cells.
Inhibition of Enzymatic Activity
Talabostat (Val-Boro-Pro, PT-100, BXCL-701) is one of the first small molecules designed to inhibit the dipeptidyl peptidase activity shared by DPPIV and FAP. Original preclinical work with the molecule was promising. Oral administration of talabostat slowed growth of syngeneic tumors derived from fibrosarcoma, lymphoma, melanoma, mastocytoma, rhabdomyosarcoma and bladder cancer cell lines in mice, in some instances causing complete regression and rejection of tumors [129, 130]. Talabostat also enhanced the efficacy of oxaliplatin in murine models of colon carcinoma [131]. Talabostat’s effects seemed immunologic in nature, as the anti-tumor effects were attenuated in immunodeficient mice. Talabostat enhanced cytotoxic lymphocyte anti-tumor effects, as CD8+ T cells from talabostat-treated mice had greater cytotoxic capabilities compared to untreated controls. This was further supported by data showing that talabostat enhanced the efficacy of tumor specific antibodies [130]. Further studies suggested that talabostat enhanced dendritic cell trafficking, resulting in acceleration of T-cell priming. Interestingly, this study demonstrated that inhibition of extracellular FAP alone is insufficient to reduce tumor volume, thus suggesting that inhibition of intracellular dipeptidyl peptidases may be responsible [129]. To this point, one study suggested talabostat’s mechanism of action was independent of its effects on FAP but rather depended on inhibition of DPP8/9, which induced pyropotosis in monocytes and macrophages that in turn activated the immune system [132].
Despite the lack of consensus on talabostat’s mechanism of action, it was further investigated in clinical trials. A phase I clinical trial of talabostat in relapsed or refractory pediatric solid tumors used maximal target inhibition to identify the appropriate dose of talabostat. At a dose of 600 μg/m2, there was serum DPPIV inhibition of 85% at 24 hours. No dose-limiting toxicities were observed, however the impact of talabostat on patient tumor growth could not be determined, since clinical development of talabostat was discontinued during the trial [133]. A phase II clinical trial investigated talabostat as a single agent for advanced metastatic colorectal cancer. While the study identified no complete or partial responses, there were cases of prolonged stable disease in previously progressing tumors, suggesting possible anti-cancer activity. The patients enrolled in the study were heavily pre-treated and thus the lack of clinical response could have been attributed to the refractory patient population. An idea that is supported by the finding that FAP exerts greater biological effects at earlier stages in colorectal cancers [82]. Other phase II trials investigated talabostat in combination with standard of care chemotherapeutics. A phase II trial assessing talabostat with cisplatin as second-line therapy in stage IV melanoma identified 8.1% of patients with partial response and 62.5% with stable disease. Of the patients who responded, the duration of response ranged from 62 to 287 days [134]. A phase II trial of talabostat and docetaxel for advanced non-small cell lung cancer yielded two durable complete responses and three partial responses, for an overall response rate of 9.1% and a stable disease rate of 54% [135].
Talabostat has also been noted to have several side effects, most of which are related to cytokine release. The most common adverse events that could definitely be attributed to talabostat was edema. In the single agent trial there was one Grade 5 adverse event, a patient who died seven days after treatment due to acute renal failure due to cytokine storm. In the melanoma trial 56% of patients experienced grade 3 or 4 adverse events with 18% discontinuing talabostat due to the side effects. In the non-small cell lung cancer trial eight patients experienced adverse events resulting in death. However, none of these events were considered definitely or probably related to talabostat. The cytokine stimulation effects of talabostat may be clinically beneficial in cases of blood cell deficiencies. One study demonstrated that talabostat promoted growth of primitive hematopoietic progenitor cells by increasing G-CSF, IL-6, and IL-11 production from bone marrow stromal cells. Therefore, talabostat may be utilized to treat neutropenia or anemia [136].
Talabostat’s nonspecific targeting of FAP complicates the ability to assess the effects of FAP inhibition on tumor growth. There has been an ongoing effort to develop an FAP-specific inhibitor to allow for better understanding of FAP biology as well as potentially improve FAP targeting clinically. Of note, Pieter Van der Veken’s group has developed a compound, termed “compound 60” that selectively and completely inhibits FAP in murine models [137]. It should also be mentioned that DPPIV inhibitors are already an FDA approved class of drugs commonly utilized to treat Type II diabetes, because of their ability to enhance concentrations of incretins such as GLP-1.
Inhibition of FAP activity has also been attempted using antibodies. Early work on FAP− targeting monoclonal antibodies focused on clinical utility of the antibody originally used to identify FAP, F19. These studies did not investigate or expect improved clinical outcomes. Instead, they hoped that the elevated expression of FAP in both primary tumors and metastasis would mean that radioactively labeled F19 could improve imaging modalities in patients with hepatic metastases from colorectal carcinoma. In fact it did, with 131Iodine labeled F19 showing specific enrichment of the antibody in tumor areas and detection of metastasis. [138, 139]. These studies indicated potential diagnostic and therapeutic applications of FAP targeting antibodies. The first evidence that an anti-FAP antibody could suppress tumor growth came in 2002 from Louis Weiner’s group. In this study, rabbits were immunized with recombinant murine FAP to obtain anti-FAP antisera. The anti-FAP antisera significantly attenuated tumor growth in colorectal carcinoma cell lines xenografted into nude mice [31]. Since then, specific anti-FAP antibodies and single-chain variable fragments (scFv) targeting FAP have been developed [140, 141].
ScFv are fusion proteins consisting of the variable regions of heavy and light chains of an immunoglobulin. These constructs have been further modulated to form bispecific antibodies capable of targeting both FAP and CD3 to target effector T cells to FAP expressing tumor tissue. In vitro studies demonstrated this FAP-CD3 bispecific antibody had enhanced cytotoxic activity against FAP expressing tumor cells [142, 143].
Then, sibrotuzumab, a humanized monoclonal anti-FAP antibody was produced. In a phase I dose escalation study in patients with advanced or metastatic FAP+ cancer, sibrotuzumab was proven safe as there was only one dose limiting toxicity during this trial. Unfortunately, there were no clinical responses and only 2/26 patients had stable disease [59]. A phase II clinical trial of sibrotuzumab in metastatic colorectal cancer was suspended because of lack of clinical activity, although sibrotuzumab was well tolerated. [144].
Despite the disappointing results, the study of more efficient FAP antibodies continues. Radiolabeled human-mouse cross-reactive anti-FAP antibodies selectively accumulated in FAP expressing melanoma cell lines in vitro and in vivo. The uptake of radiolabeled antibody led to decreased tumor growth and improved survival murine models of melanoma [145]. While these studies show promise, more preclinical and clinical experiments are needed to explore the diagnostic and therapeutic effects FAP targeting molecules.
Prodrugs Utilizing FAP Proteinase Activity
Since FAP is overexpressed in the tumor microenvironment and is generally absent from other tissues in a healthy adult, some groups have focused efforts on utilizing FAP protease activity to selectively activate prodrugs at tumor sites to enhance drug efficacy and reduce toxicity. So far, these prodrugs have yet to make it to clinical trials but pre-clinical trials show promise. In a murine model of breast carcinoma, FAP overexpressing cancers showed equal sensitivity to epirubicin compared to compound that was an FAP substrate conjugated to epirubicin. Mice receiving the conjugated compound experienced less weight loss and less cardiotoxicity [146]. A study of another anthracycline, doxorubicin, showed similar results with FAP substrate conjugated doxorubicin eliciting reduced toxicity to the heart, liver, kidney, spleen and peripheral white blood cells in both murine and canine models. The improved safety profile of this compound allowed for a two-fold increase in the dose of doxycycline administered in vivo [147]. This technique was also applied to vascular disrupting agents. Administration of a vinblastine pro-drug conjugated to an FAP substrate markedly reduced tumor growth in tumors derived from HepG2, A549, HeLa, CNE-2 xenografts as well as ductal carcinoma and hepatocellular carcinoma patient-derived xenografts [148].
FAP Vaccination
Vaccines targeting FAP provide another therapeutic strategy that takes advantage of the restricted distribution of FAP in tumor sites. Prophylactic vaccination with a DNA vaccine directed against FAP in mice inoculated with colon or breast carcinoma cells resulted in decreased tumor growth, suppressed pulmonary metastasis, increased chemotherapy uptake and increased survival in a CD8+ T cell dependent manner [149, 150]. Another group engineered tumor cells to express murine FAP and then used the resulting whole cell vaccine with success. This FAP-expressing whole cell vaccine reduced tumor growth and improved survival in a CD8+ T cell dependent manner in both the prophylactic and post tumor inoculation settings [151]. FAP vaccination has also been attempted with dendritic cell vaccines. A dendritic cell vaccine was developed to co-express FAP and tumor antigen tyrosine-related protein 2 had potent antitumor activity in murine models of melanoma [152].
FAP CAR T Cells
Chimeric antigen receptor (CAR) T cells represent an exciting new class of immunotherapy strategies where cytotoxic T cells are engineered to recognize specific cancer antigens resulting in cancer cell elimination. CAR T cell therapy has already been approved by the FDA for some forms of leukemia and lymphoma [153]. The potential to use FAP CAR T cells to clear FAP expressing tumor cells was first demonstrated by Schuberth et al. In this study they demonstrated FAP CAR T cells successfully killer FAP expressing malignant pleural mesothelioma (MPM) lines and improved overall survival in murine models of MPM [154]. However, expression of FAP by malignant cells is restricted to a few cancer types. Targeting FAP+ stromal cells with CAR Ts could greatly broaden FAP CAR T cell use. Further, given the pro-tumorgenic roles of FAP expressing CAFs, it is reasonable to hypothesize that using CAR T cells to selectively ablate FAP expressing cells could improve patient outcomes. Kakarla et al where the first to test if FAP CAR T cells could improve outcomes when used to deplete stomal cells. They showed that FAP CAR T cells effectively lyse FAP expressing target cell in vitro and improve mouse overall survival in murine models of lung adenocarcinoma [155]. Subsequent studies demonstrated FAP CAR T cells reduced tumor growth in murine models of lymphoma, mesothelioma and breast, colon and lung adenocarcinoma [156]. In this study they demonstrated FAP CAR T cells were ineffective in immunodeficient mice and showed FAP CAR T treatment enhanced endogenous tumoral T cell activity and infiltration. However, the clinical use of FAP CAR T cells should proceed with caution. One study showed that FAP CAR T cells failed to regulate tumor growth, and induced lethal bone toxicity and cachexia, potentially through the lysis of multipotent bone marrow stromal cells [157]. The reason for the discrepancy in outcomes remains unclear, however it could be related to differences in FAP construct design and specificity, warranting further investigation into FAP CAR T cell optimization. Along these lines, one study demonstrated that the costimulatory domains expressed by FAP CAR T cells impacted their efficacy. In this study, the Δ-CD28 (which lacks the lck binding moiety) costimulatory domain resulted in superior tumor clearance when combined with anti-PD-1 than CD28 or 4–1BB costimulatory domains [158]. They also performed the first-in-human trial of FAP CAR T cells and demonstrated that a FAP CAR T cells therapy induced stable disease for one year in a patient with malignant pleural mesothelioma. Of note, this patient did not experience any treatment terminating toxicities. Lastly, FAP CAR T cells are might be efficacious in other diseases as well. Aghajanian et al demonstrated that FAP CAR T cells reduce cardiac fibrosis in murine models of cardiac fibrosis [159].
Conclusion
Since the discovery of FAP, great strides have been made to better understand FAP biology. We now appreciate that its expression is not limited to activated fibroblasts, but includes endothelial, malignant epithelial, embryologic and immunologic tissues. Our understanding of its physiological role has expanded from simple collagen degradation to functions including activation of tumorigenic signaling cascades, angiogenesis, EMT and even immunosuppression. We also have learned that its physiologic functions may be independent of its peptidase activity and is instead dependent upon association with other molecules such as integrins and uPAR. Despite the apparent lack of FAP-targeting therapeutics clinical success, the striking occurrence of FAP in many pathologies continues to suggest it can provide some clinically targetable value.
Acknowledgements
This work was supported by grants from the NIH (R01 CA050633 (LMW), F30 CA239441 (AAF) and P30 CA051008 (LMW)). Figures were created with Biorender.com.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: All other authors declare no potential conflicts of interest to disclose.
References
- 1.Aoyama A, Chen WT (1990) A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc Natl Acad Sci U S A 87:8296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monsky WL, Lin CY, Aoyama A, et al. (1994) A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res 54:5702–10 [PubMed] [Google Scholar]
- 3.Piñeiro-Sánchez ML, Goldstein LA, Dodt J, et al. (1997) Identification of the 170-kDa melanoma membrane-bound gelatinase (seprase) as a serine integral membrane protease. J Biol Chem 272:7595–601. 10.1074/JBC.272.12.7595 [DOI] [PubMed] [Google Scholar]
- 4.Mathew S, Scanlan MJ, Mohan Raj BK, et al. (1995) The gene for fibroblast activation protein α (FAP), a putative cell surface-bound serine protease expressed in cancer stroma and wound healing, maps to chromosome band 2q23. Genomics 25:335–337. 10.1016/0888-7543(95)80157-H [DOI] [PubMed] [Google Scholar]
- 5.Iwasa S, Jin X, Okada K, et al. (2003) Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colorectal cancer. Cancer Lett 199:91–8. 10.1016/S0304-3835(03)00315-X [DOI] [PubMed] [Google Scholar]
- 6.Kelly T, Kechelava S, Rozypal TL, et al. (1998) Seprase, a membrane-bound protease, is overexpressed by invasive ductal carcinoma cells of human breast cancers. Mod Pathol 11:855–63 [PubMed] [Google Scholar]
- 7.Mori Y, Kono K, Matsumoto Y, et al. (2004) The expression of a type II transmembrane serine protease (Seprase) in human gastric carcinoma. Oncology 67:411–9. 10.1159/000082926 [DOI] [PubMed] [Google Scholar]
- 8.Lo A, Li C-P, Buza EL, et al. (2017) Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. J Clin Invest 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold JN, Magiera L, Kraman M, Fearon DT (2014) Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1. Cancer Immunol Res 2:121–6. 10.1158/2326-6066.CIR-13-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KN, Jackson KW, Christiansen VJ, et al. (2006) Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood 107:1397–404. 10.1182/blood-2005-08-3452 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LA, Ghersi G, Pineiro-Sanchez ML, et al. (1997) Molecular cloning of seprase: a serine integral membrane protease from human melanoma. Biochim Biophys Acta 1361:11–19 [DOI] [PubMed] [Google Scholar]
- 12.Rosenblum JS, Kozarich JW (2003) Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol 7:496–504. 10.1016/S1367-5931(03)00084-X [DOI] [PubMed] [Google Scholar]
- 13.Collins PJ, McMahon G, O’Brien P, O’Connor B (2004) Purification, identification and characterisation of seprase from bovine serum. Int J Biochem Cell Biol 36:2320–2333. 10.1016/J.BIOCEL.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Edosada CY, Quan C, Tran T, et al. (2006) Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly 2 -Pro 1 -cleaving specificity. FEBS Lett 580:1581–1586. 10.1016/j.febslet.2006.01.087 [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Brennen WN, Kole TP, et al. (2008) Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 47:1076–86. 10.1021/bi701921b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C-H, Suen C-S, Lin C-T, et al. (2011) Cleavage-site specificity of prolyl endopeptidase FAP investigated with a full-length protein substrate. J Biochem 149:685–692. 10.1093/jb/mvr017 [DOI] [PubMed] [Google Scholar]
- 17.Keane FM, Nadvi NA, Yao T-W, Gorrell MD (2011) Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-α. FEBS J 278:1316–1332. 10.1111/j.1742-4658.2011.08051.x [DOI] [PubMed] [Google Scholar]
- 18.Christiansen VJ, Jackson KW, Lee KN, McKee PA (2007) Effect of fibroblast activation protein and alpha2-antiplasmin cleaving enzyme on collagen types I, III, and IV. Arch Biochem Biophys 457:177–86. 10.1016/j.abb.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MT, McCaughan GW, Abbott CA, et al. (1999) Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 29:1768–1778. 10.1002/hep.510290631 [DOI] [PubMed] [Google Scholar]
- 20.Dunshee DR, Bainbridge TW, Kljavin NM, et al. (2016) Fibroblast Activation Protein Cleaves and Inactivates Fibroblast Growth Factor 21. J Biol Chem 291:5986–96. 10.1074/jbc.M115.710582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KN, Jackson KW, Christiansen VJ, et al. (2004) A novel plasma proteinase potentiates alpha2-antiplasmin inhibition of fibrin digestion. Blood 103:3783–8. 10.1182/blood-2003-12-4240 [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Montagut T, Blachere NE, Sviderskaya EV, et al. (2004) FAPα, a surface peptidase expressed during wound healing, is a tumor suppressor. Oncogene 23:5435–5446. 10.1038/sj.onc.1207730 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Simms AE, Mazur A, et al. (2011) Fibroblast activation protein-α promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin Exp Metastasis 28:567–579. 10.1007/s10585-011-9392-x [DOI] [PubMed] [Google Scholar]
- 24.Lv B, Xie F, Zhao P, et al. (2016) Promotion of Cellular Growth and Motility Is Independent of Enzymatic Activity of Fibroblast Activation Protein-α. Cancer Genomics Proteomics 13:201–8 [PubMed] [Google Scholar]
- 25.Aertgeerts K, Levin I, Shi L, et al. (2005) Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J Biol Chem 280:19441–4. 10.1074/jbc.C500092200 [DOI] [PubMed] [Google Scholar]
- 26.Meadows SA, Edosada CY, Mayeda M, et al. (2007) Ala657 and Conserved Active Site Residues Promote Fibroblast Activation Protein Endopeptidase Activity via Distinct Mechanisms of Transition State Stabilization. Biochemistry 46:4598–4605. 10.1021/BI062227Y [DOI] [PubMed] [Google Scholar]
- 27.Sun S, Albright CF, Fish BH, et al. (2002) Expression, Purification, and Kinetic Characterization of Full-Length Human Fibroblast Activation Protein. Protein Expr Purif 24:274–281. 10.1006/PREP.2001.1572 [DOI] [PubMed] [Google Scholar]
- 28.Ghersi G, Zhao Q, Salamone M, et al. (2006) The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res 66:4652–61. 10.1158/0008-5472.CAN-05-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SC, Ghersi G, Akiyama SK, et al. (1999) A novel protease-docking function of integrin at invadopodia. J Biol Chem 274:24947–52. 10.1074/JBC.274.35.24947 [DOI] [PubMed] [Google Scholar]
- 30.Artym VV, Kindzelskii AL, Chen W-T, Petty HR (2002) Molecular proximity of seprase and the urokinase-type plasminogen activator receptor on malignant melanoma cell membranes: dependence on beta1 integrins and the cytoskeleton. Carcinogenesis 23:1593–601 [DOI] [PubMed] [Google Scholar]
- 31.Cheng JD, Dunbrack RL, Valianou M, et al. (2002) Promotion of Tumor Growth by Murine Fibroblast Activation Protein, a Serine Protease, in an Animal Model. CANCER Res 62:4767–4772 [PubMed] [Google Scholar]
- 32.Niedermeyer J, Enenkel B, Park JE, et al. (1998) Mouse fibroblast-activation protein. Conserved Fap gene organization and biochemical function as a serine protease. Eur J Biochem 254:650–654. 10.1046/j.1432-1327.1998.2540650.x [DOI] [PubMed] [Google Scholar]
- 33.Brown DD, Wang Z, Furlow JD, et al. (1996) The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Dev Biol 93:1924–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Valianou M, Cheng JD (2010) Identification and characterization of the promoter of fibroblast activation protein. Front Biosci 1154–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein LA, Chen WT (2000) Identification of an alternatively spliced seprase mRNA that encodes a novel intracellular isoform. J Biol Chem 275:2554–9. 10.1074/JBC.275.4.2554 [DOI] [PubMed] [Google Scholar]
- 36.Niedermeyer J, Scanlan MJ, Garin-Chesa P, et al. (1997) Mouse fibroblast activation protein: Molecular cloning, alternative splicing and expression in the reactive stroma of epithelial cancers. Int J Cancer 71:383–389. [DOI] [PubMed] [Google Scholar]
- 37.Rettig WJ, Su SL, Fortunato SR, et al. (1994) Fibroblast activation protein: Purification, epitope mapping and induction by growth factors. Int J Cancer 58:385–392. 10.1002/ijc.2910580314 [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Yang W-W, Wen Q-T, et al. (2009) TGF-β-induced fibroblast activation protein expression, fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM. Exp Mol Pathol 87:189–194. 10.1016/J.YEXMP.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 39.Wäster P, Rosdahl I, Gilmore BF, Seifert O (2011) Ultraviolet exposure of melanoma cells induces fibroblast activation protein-α in fibroblasts: Implications for melanoma invasion. University of Crete, Faculty of Medicine, Laboratory of Clinical Virology [DOI] [PubMed] [Google Scholar]
- 40.Kennedy A, Dong H, Chen D, Chen W-T (2009) Elevation of seprase expression and promotion of an invasive phenotype by collagenous matrices in ovarian tumor cells. Int J Cancer 124:27–35. 10.1002/ijc.23871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LAI D, MA L, WANG F (2012) Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol 41:541–550. 10.3892/ijo.2012.1475 [DOI] [PubMed] [Google Scholar]
- 42.Ruan P, Tao Z, Tan A (2018) Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. Biosci Rep 38:BSR20171027 10.1042/BSR20171027 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Kanamori A, Brown DD (1996) The analysis of complex developmental programmes: amphibian metamorphosis. Genes to Cells 1:429–435. 10.1046/j.1365-2443.1996.d01-251.x [DOI] [PubMed] [Google Scholar]
- 44.Niedermeyer J, Kriz M, Hilberg F, et al. (2000) Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol 20:1089–94. 10.1128/MCB.20.3.1089-1094.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedermeyer J, Garin-Chesa P, Kriz M, et al. (2001) Expression of the fibroblast activation protein during mouse embryo development. Int J Dev Biol 45:445–7. 10.1387/IJDB.11330865 [DOI] [PubMed] [Google Scholar]
- 46.Roberts EW, Deonarine A, Jones JO, et al. (2013) Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 210:1137–51. 10.1084/jem.20122344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keane FM, Yao T-W, Seelk S, et al. (2014) Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio 4:43–54. 10.1016/j.fob.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garin-Chesa P, Oldt LJ, Rettigt WJ (1990) Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Immunology 87:7235–7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy M, McCaughan G, Marinos G, Gorrell M (2002) Intrahepatic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepatitis C virus infection. Liver Int 22:93–101. 10.1034/j.1600-0676.2002.01503.x [DOI] [PubMed] [Google Scholar]
- 50.Uitte de Willige S, Malfliet JJMC, Janssen HLA, et al. (2013) Increased N-terminal cleavage of alpha-2-antiplasmin in patients with liver cirrhosis. J Thromb Haemost 11:2029–2036. 10.1111/jth.12396 [DOI] [PubMed] [Google Scholar]
- 51.Uitte de Willige S, Keane FM, Bowen DG, et al. (2017) Circulating fibroblast activation protein activity and antigen levels correlate strongly when measured in liver disease and coronary heart disease. PLoS One 12:e0178987 10.1371/journal.pone.0178987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams KH, Viera de Ribeiro AJ, Prakoso E, et al. (2015) Lower serum fibroblast activation protein shows promise in the exclusion of clinically significant liver fibrosis due to non-alcoholic fatty liver disease in diabetes and obesity. Diabetes Res Clin Pract 108:466–472. 10.1016/J.DIABRES.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 53.Acharya PS, Zukas A, Chandan V, et al. (2006) Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol 37:352–60 [DOI] [PubMed] [Google Scholar]
- 54.Wenlong L, Leilei Y, Wei F, et al. (2015) Luciferase expression is driven by the promoter of fibroblast activation protein-α in murine pulmonary fibrosis. Biotechnol Lett 37:1757–1763. 10.1007/s10529-015-1855-8 [DOI] [PubMed] [Google Scholar]
- 55.Fan M-H, Zhu Q, Li H-H, et al. (2016) Fibroblast Activation Protein (FAP) Accelerates Collagen Degradation and Clearance from Lungs in Mice. J Biol Chem 291:8070–89. 10.1074/jbc.M115.701433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egger C, Cannet C, Gérard C, et al. (2017) Effects of the fibroblast activation protein inhibitor, PT100, in a murine model of pulmonary fibrosis. Eur J Pharmacol 809:64–72. 10.1016/j.ejphar.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 57.Dienus K, Bayat A, Gilmore BF, Seifert O (2010) Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res 302:725–731. 10.1007/s00403-010-1084-x [DOI] [PubMed] [Google Scholar]
- 58.Rovedatti L, Di Sabatino A, Knowles CH, et al. (2011) Fibroblast activation protein expression in Crohnʼs disease strictures. Inflamm Bowel Dis 17:1251–1253. 10.1002/ibd.21446 [DOI] [PubMed] [Google Scholar]
- 59.Scott AM, Wiseman G, Welt S, et al. (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 9:1639–47 [PubMed] [Google Scholar]
- 60.Milner JM, Kevorkian L, Young DA, et al. (2006) Fibroblast activation protein alpha is expressed by chondrocytes following a pro-inflammatory stimulus and is elevated in osteoarthritis. Arthritis Res Ther 8:R23 10.1186/ar1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bauer S, Jendro MC, Wadle A, et al. (2006) Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther 8:. 10.1186/ar2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ospelt C, Mertens JC, Jüngel A, et al. (2010) Inhibition of fibroblast activation protein and dipeptidylpeptidase 4 increases cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 62:1224–1235. 10.1002/art.27395 [DOI] [PubMed] [Google Scholar]
- 63.Busso N, Wagtmann N, Herling C, et al. (2005) Circulating CD26 Is Negatively Associated with Inflammation in Human and Experimental Arthritis. Immunopathol Infect Dis 166:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wäldele S, Koers-Wunrau C, Beckmann D, et al. (2015) Deficiency of fibroblast activation protein alpha ameliorates cartilage destruction in inflammatory destructive arthritis. Arthritis Res Ther 17:12 10.1186/s13075-015-0524-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laverman P, van der Geest T, Terry SYA, et al. (2015) Immuno-PET and Immuno-SPECT of Rheumatoid Arthritis with Radiolabeled Anti-Fibroblast Activation Protein Antibody Correlates with Severity of Arthritis. J Nucl Med 56:778–83. 10.2967/jnumed.114.152959 [DOI] [PubMed] [Google Scholar]
- 66.Brokopp CE, Schoenauer R, Richards P, et al. (2011) Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J 32:2713–2722. 10.1093/eurheartj/ehq519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tillmanns J, Widera C, Habbaba Y, et al. (2013) Circulating concentrations of fibroblast activation protein α in apparently healthy individuals and patients with acute coronary syndrome as assessed by sandwich ELISA ☆. Int J Cardiol 168:3926–3931. 10.1016/j.ijcard.2013.06.061 [DOI] [PubMed] [Google Scholar]
- 68.Uitte De Willige S, Malfliet JJMC, Deckers JW, et al. (2015) Plasma levels of soluble fibroblast activation protein in arterial thrombosis; determinants and cleavage of its substrate alpha-2-antiplasmin ☆. Int J Cardiol 178:105–110. 10.1016/j.ijcard.2014.10.091 [DOI] [PubMed] [Google Scholar]
- 69.Tillmanns J, Hoffmann D, Habbaba Y, et al. (2015) Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol 87:194–203. 10.1016/J.YJMCC.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 70.Tillmanns J, Fraccarollo D, Galuppo P, et al. (2017) Changes in concentrations of circulating fibroblast activation protein alpha are associated with myocardial damage in patients with acute ST-elevation MI. Int J Cardiol 232:155–159. 10.1016/j.ijcard.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 71.Zhen EY, Jin Z, Ackermann BL, et al. (2016) Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem J 473:605–614. 10.1042/BJ20151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sánchez-Garrido MA, Habegger KM, Clemmensen C, et al. (2016) Fibroblast activation protein (FAP) as a novel metabolic target. Mol Metab 5:1015–1024. 10.1016/j.molmet.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hua X, Yu L, Huang X, et al. (2011) Expression and role of fibroblast activation protein-alpha in microinvasive breast carcinoma. Diagn Pathol 6:111 10.1186/1746-1596-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodman JD, Rozypal TL, Kelly T (2003) Seprase, a membrane-bound protease, alleviates the serum growth requirement of human breast cancer cells. Clin Exp Metastasis 20:459–70 [DOI] [PubMed] [Google Scholar]
- 75.Park CK, Jung WH, Koo JS (2016) Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer Res Treat 159:55–69. 10.1007/s10549-016-3929-2 [DOI] [PubMed] [Google Scholar]
- 76.Park SY, Kim HM, Koo JS (2015) Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat 149:727–741. 10.1007/s10549-015-3291-9 [DOI] [PubMed] [Google Scholar]
- 77.Jung YY, Lee YK, Koo JS (2015) Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. Tumor Biol 36:8685–8695. 10.1007/s13277-015-3594-9 [DOI] [PubMed] [Google Scholar]
- 78.Gong C, Nie Y, Qu S, et al. (2014) Molecular and Cellular Pathobiology miR-21 Induces Myofibroblast Differentiation and Promotes the Malignant Progression of Breast Phyllodes Tumors. Cancer Res 74:4341–4352. 10.1158/0008-5472.CAN-14-0125 [DOI] [PubMed] [Google Scholar]
- 79.Yu H, Yang J, Li Y, Jiao S (2015) [The expression of fibroblast activation protein-α in primary breast cancer is associated with poor prognosis]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 31:370–4 [PubMed] [Google Scholar]
- 80.Jia J, Martin TA, Ye L, Jiang WG (2014) FAP-α (Fibroblast activation protein-α) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway. BMC Cell Biol 15:16 10.1186/1471-2121-15-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ariga N, Sato E, Ohuchi N, et al. (2001) Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int J cancer 95:67–72 [DOI] [PubMed] [Google Scholar]
- 82.Henry LR, Lee H-O, Lee JS, et al. (2007) Clinical Implications of Fibroblast Activation Protein in Patients with Colon Cancer. Clin Cancer Res 13:1736–1741. 10.1158/1078-0432.CCR-06-1746 [DOI] [PubMed] [Google Scholar]
- 83.Wikberg ML, Edin S, Lundberg IV, et al. (2013) High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol 34:1013–1020. 10.1007/s13277-012-0638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang X, Lin Y, Shi Y, et al. (2016) FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res 76:4124–35. 10.1158/0008-5472.CAN-15-2973 [DOI] [PubMed] [Google Scholar]
- 85.Shi M, Yu D-H, Chen Y, et al. (2012) Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol 18:840–6. 10.3748/wjg.v18.i8.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen SJ, Alpaugh RK, Palazzo I, et al. (2008) Fibroblast Activation Protein and Its Relationship to Clinical Outcome in Pancreatic Adenocarcinoma. Pancreas 37:154–158. 10.1097/MPA.0b013e31816618ce [DOI] [PubMed] [Google Scholar]
- 87.Feig C, Jones JO, Kraman M, et al. (2013) Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 110:20212–7. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawase T, Yasui Y, Nishina S, et al. (2015) Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol 15:109 10.1186/s12876-015-0340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park H, Lee Y, Lee H, et al. (2017) The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumor Biol 1–9. 10.1177/1010428317718403 [DOI] [PubMed] [Google Scholar]
- 90.Okada K, Chen W-T, Iwasa S, et al. (2003) Seprase, a membrane-type serine protease, has different expression patterns in intestinal- and diffuse-type gastric cancer. Oncology 65:363–70. 10.1159/000074650 [DOI] [PubMed] [Google Scholar]
- 91.Hu M, Qian C, Hu Z, et al. (2017) Biomarkers in Tumor Microenvironment? Upregulation of Fibroblast Activation Protein-α Correlates with Gastric Cancer Progression and Poor Prognosis. Omi A J Integr Biol 21:38–44. 10.1089/omi.2016.0159 [DOI] [PubMed] [Google Scholar]
- 92.Wen X, He X, Jiao F, et al. (2017) Fibroblast Activation Protein-α-Positive Fibroblasts Promote Gastric Cancer Progression and Resistance to Immune Checkpoint Blockade. Oncol Res Featur Preclin Clin Cancer Ther 25:629–640. 10.3727/096504016X14768383625385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong R, Guo J, Zhang Z, et al. (2018) Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expression of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem Biophys Res Commun 497:1129–1134. 10.1016/j.bbrc.2018.02.193 [DOI] [PubMed] [Google Scholar]
- 94.Rettig WJ, Chesa PG, Beresford HR, et al. (1986) Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res 46:6406–12 [PubMed] [Google Scholar]
- 95.Stremenova J, Krepela E, Mares V, et al. (2007) Expression and enzymatic activity of dipeptidyl peptidase-IV in human astrocytic tumours are associated with tumour grade. Int J Oncol 31:785–792. 10.3892/ijo.31.4.785 [DOI] [PubMed] [Google Scholar]
- 96.Mikheeva SA, Mikheev AM, Petit A, et al. (2010) TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol Cancer 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matrasova I, Busek P, Balaziova E, Sedo A (2017) Heterogeneity of molecular forms of dipeptidyl peptidase-IV and fibroblast activation protein in human glioblastomas. Biomed Pap 161:252–260. 10.5507/bp.2017.010 [DOI] [PubMed] [Google Scholar]
- 98.Mentlein R, Hattermann K, Hemion C, et al. (2011) Expression and role of the cell surface protease seprase/fibroblast activation protein-a (FAP-a) in astroglial tumors. Biol Chem 392:199–207. 10.1515/BC.2010.119 [DOI] [PubMed] [Google Scholar]
- 99.Busek P, Balaziova E, Matrasova I, et al. (2016) Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumor Biol 37:13961–13971. 10.1007/s13277-016-5274-9 [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Tang H, Cai J, et al. (2011) Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 303:47–55. 10.1016/J.CANLET.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 101.Yang L, Ma L, Lai D (2013) Over-expression of fibroblast activation protein alpha increases tumor growth in xenografts of ovarian cancer cells. Acta Biochim Biophys Sin 45:928 10.1093/abbs/gmt095 [DOI] [PubMed] [Google Scholar]
- 102.Zhang M-Z, Qiao Y-H, Nesland JM, et al. (2007) Expression of seprase in effusions from patients with epithelial ovarian carcinoma. Chin Med J (Engl) 120:663–8 [PubMed] [Google Scholar]
- 103.Zhang M, Xu L, Wang X, et al. (2015) Expression levels of seprase/FAPα and DPPIV/CD26 in epithelial ovarian carcinoma. Oncol Lett 10:34–42. 10.3892/ol.2015.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ge Y, Zhan F, Barlogie B, et al. (2006) Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol 133:83–92. 10.1111/j.1365-2141.2006.05976.x [DOI] [PubMed] [Google Scholar]
- 105.Pennisi A, Li X, Ling W, et al. (2009) Inhibitor of DASH proteases affects expression of adhesion molecules in osteoclasts and reduces myeloma growth and bone disease. Br J Haematol 145:775–87. 10.1111/j.1365-2141.2009.07696.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huber MA, Schubert RD, Peter RU, et al. (2003) Fibroblast Activation Protein: Differential Expression and Serine Protease Activity in Reactive Stromal Fibroblasts of Melanocytic Skin Tumors. J Invest Dermatol 120:182–188. 10.1046/J.1523-1747.2003.12035.X [DOI] [PubMed] [Google Scholar]
- 107.Liu F, Qi L, Liu B, et al. (2015) Fibroblast Activation Protein Overexpression and Clinical Implications in Solid Tumors: A Meta-Analysis. PLoS One 10:e0116683 10.1371/journal.pone.0116683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakahara H, Nomizu M, Akiyama SK, et al. (1996) A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem 271:27221–4. 10.1074/JBC.271.44.27221 [DOI] [PubMed] [Google Scholar]
- 109.Yang W, Han W, Ye S, et al. (2013) Fibroblast activation protein-α promotes ovarian cancer cell proliferation and invasion via extracellular and intracellular signaling mechanisms. Exp Mol Pathol 95:105–110. 10.1016/j.yexmp.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 110.Lee H-O, Mullins SR, Franco-Barraza J, et al. (2011) FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11:245 10.1186/1471-2407-11-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jia J, Martin T, Ye L, et al. (2017) Fibroblast activation protein-α promotes the growth and migration of lung cancer cells via the PI3K and sonic hedgehog pathways. Int J Mol Med 41:275–283. 10.3892/ijmm.2017.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H, Wu Q, Liu Z, et al. (2014) Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis 122:. 10.1038/cddis.2014.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aimes RT, Zijlstra A, Hooper JD, et al. (2003) Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost 89:561–72 [PubMed] [Google Scholar]
- 114.Gao L-M, Wang F, Zheng Y, et al. (2017) Roles of Fibroblast Activation Protein and Hepatocyte Growth Factor Expressions in Angiogenesis and Metastasis of Gastric Cancer. Pathol Oncol Res 1–8. 10.1007/s12253-017-0359-3 [DOI] [PubMed] [Google Scholar]
- 115.Santos AM, Jung J, Aziz N, et al. (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119:3613–25. 10.1172/JCI38988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christiansen VJ, Jackson KW, Lee KN, et al. (2013) Targeting Inhibition of Fibroblast Activation Protein-α and Prolyl Oligopeptidase Activities on Cells Common to Metastatic Tumor Microenvironments. Neoplasia 15:348–358. 10.1593/NEO.121850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhati R, Patterson C, Livasy CA, et al. (2008) Molecular characterization of human breast tumor vascular cells. Am J Pathol 172:1381–90. 10.2353/ajpath.2008.070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zukowska Z, Grant DS, Lee EW (2003) Neuropeptide Y: A Novel Mechanism for Ischemic Angiogenesis. Trends Cardiovasc Med 13:86–92. 10.1016/S1050-1738(02)00232-3 [DOI] [PubMed] [Google Scholar]
- 119.Vu TH, Shipley JM, Bergers G, et al. (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kahounová Z, Kurfürstová D, Bouchal J, et al. (2017) The fibroblast surface markers FAP, anti-fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial-to-mesenchymal transition. Cytom Part A. 10.1002/cyto.a.23101 [DOI] [PubMed] [Google Scholar]
- 121.Kraman M, Bambrough PJ, Arnold JN, et al. (2010) Suppression of Antitumor Immunity by Stromal Cells Expressing Fibroblast Activation Protein. Science (80- ) 330:827–830. 10.1126/science.1195300 [DOI] [PubMed] [Google Scholar]
- 122.Chen L, Qiu X, Wang X, He J (2017) FAP positive fibroblasts induce immune checkpoint blockade resistance in colorectal cancer via promoting immunosuppression. Biochem Biophys Res Commun 487:8–14. 10.1016/j.bbrc.2017.03.039 [DOI] [PubMed] [Google Scholar]
- 123.Sorrentino C, Miele L, Porta A, et al. (2016) Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression. Oncotarget 7:64274–64288. 10.18632/oncotarget.11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kilvaer TK, Rakaee M, Hellevik T, et al. (2018) Tissue analyses reveal a potential immune-adjuvant function of FAP-1 positive fibroblasts in non-small cell lung cancer. PLoS One 13:e0192157 10.1371/journal.pone.0192157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Santos AM, Jung J, Aziz N, et al. (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119:3613–3625. 10.1172/JCI38988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chung K-M, Hsu S-C, Chu Y-R, et al. (2014) Fibroblast Activation Protein (FAP) Is Essential for the Migration of Bone Marrow Mesenchymal Stem Cells through RhoA Activation. PLoS One 9:e88772 10.1371/journal.pone.0088772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yue D, Li H, Che J, et al. (2014) Hedgehog/Gli promotes epithelial-mesenchymal transition in lung squamous cell carcinomas. J Exp Clin Cancer Res 33:34 10.1186/1756-9966-33-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bailey JM, Swanson BJ, Hamada T, et al. (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004. 10.1158/1078-0432.CCR-08-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walsh MP, Duncan B, Larabee S, et al. (2013) Val-BoroPro Accelerates T Cell Priming via Modulation of Dendritic Cell Trafficking Resulting in Complete Regression of Established Murine Tumors. PLoS One 8:. 10.1371/journal.pone.0058860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Adams S, Miller GT, Jesson MI, et al. (2004) PT-100, a Small Molecule Dipeptidyl Peptidase Inhibitor, Has Potent Antitumor Effects and Augments Antibody-Mediated Cytotoxicity via a Novel Immune Mechanism. CANCER Res 64:5471–5480 [DOI] [PubMed] [Google Scholar]
- 131.Li M, Li M, Yin T, et al. (2016) Targeting of cancer-associated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Mol Med Rep 13:2476–84. 10.3892/mmr.2016.4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okondo MC, Johnson DC, Sridharan R, et al. (2017) DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol 13:46–53. 10.1038/nchembio.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meany H, Balis FM, Aikin A, et al. (2010) Pediatric Phase i trial Design using maximum target inhibition as the Primary endpoint. J Natl Cancer Inst 102:. 10.1093/jnci/djq174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eager RM, Cunningham CC, Senzer NN, et al. (2009) Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. BMC Cancer 9:263 10.1186/1471-2407-9-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eager RM, Cunningham CC, Senzer N, et al. (2009) Phase II Trial of Talabostat and Docetaxel in Advanced Non-small Cell Lung Cancer. Clin Oncol 21:464–472. 10.1016/J.CLON.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 136.Jones B, Adams S, Miller GT, et al. (2003) Hematopoietic stimulation by a dipeptidyl peptidase inhibitor reveals a novel regulatory mechanism and therapeutic treatment for blood cell deficiencies. Blood 102:1641–8. 10.1182/blood-2003-01-0208 [DOI] [PubMed] [Google Scholar]
- 137.Jansen K, Heirbaut L, Verkerk R, et al. (2014) Extended Structure–Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)glycyl-2-cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP). J Med Chem 57:3053–3074. 10.1021/jm500031w [DOI] [PubMed] [Google Scholar]
- 138.Tanswell P, Garin-Chesa P, Rettig WJ, et al. (2001) Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. Br J Clin Pharmacol 51:177–180. 10.1111/j.1365-2125.2001.01335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Welt S, Divgi CR, Scott AM, et al. (1994) Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 12:1193–203. 10.1200/JCO.1994.12.6.1193 [DOI] [PubMed] [Google Scholar]
- 140.Zhang J, Valianou M, Simmons H, et al. (2013) Identification of inhibitory scFv antibodies targeting fibroblast activation protein utilizing phage display functional screens. FASEB J 27:581–589. 10.1096/fj.12-210377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmidt A, Müller D, Mersmann M, et al. (2001) Generation of human high-affinity antibodies specific for the fibroblast activation protein by guided selection. Eur J Biochem 268:1730–8 [PubMed] [Google Scholar]
- 142.Wüest T, Moosmayer D, Pfizenmaier K (2001) Construction of a bispecific single chain antibody for recruitment of cytotoxic T cells to the tumour stroma associated antigen fibroblast activation protein. J Biotechnol 92:159–168 [DOI] [PubMed] [Google Scholar]
- 143.Hornig N, Kermer V, Frey K, et al. (2012) Combination of a Bispecific Antibody and Costimulatory Antibody-Ligand Fusion Proteins for Targeted Cancer Immunotherapy. J Immunother 35:418–429. 10.1097/CJI.0b013e3182594387 [DOI] [PubMed] [Google Scholar]
- 144.Hofheinz R-D, al-Batran S-E, Hartmann F, et al. (2003) Stromal Antigen Targeting by a Humanised Monoclonal Antibody: An Early Phase II Trial of Sibrotuzumab in Patients with Metastatic Colorectal Cancer. Oncol Res Treat 26:44–48. 10.1159/000069863 [DOI] [PubMed] [Google Scholar]
- 145.Fischer E, Chaitanya K, Wüest T, et al. (2012) Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin Cancer Res 18:6208–18. 10.1158/1078-0432.CCR-12-0644 [DOI] [PubMed] [Google Scholar]
- 146.Wang J, Li Q, Li X, et al. (2017) A novel FAPα-based Z-Gly-Pro epirubicin prodrug for improving tumor- targeting chemotherapy. Eur J Pharmacol 815:166–172. 10.1016/j.ejphar.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 147.Huang S, Zhang Y, Zhong J, et al. (2018) Toxicological profile and safety pharmacology of a single dose of fibroblast activation protein-α-based doxorubicin prodrug. Anticancer Drugs 29:1 10.1097/CAD.0000000000000593 [DOI] [PubMed] [Google Scholar]
- 148.Chen M, Lei X, Shi C, et al. (2017) Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J Clin Invest 127:3689–3701. 10.1172/JCI94258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA (2006) Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 116:1955–62. 10.1172/JCI26532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wen Y, Wang C-T, Ma T-T, et al. (2010) Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci 101:2325–2332. 10.1111/j.1349-7006.2010.01695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen M, Xiang R, Wen Y, et al. (2015) A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts. Sci Rep 5:14421 10.1038/srep14421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gottschalk S, Yu F, Ji M, et al. (2013) A Vaccine That Co-Targets Tumor Cells and Cancer Associated Fibroblasts Results in Enhanced Antitumor Activity by Inducing Antigen Spreading. PLoS One 8:. 10.1371/journal.pone.0082658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghobadi A (2018) Chimeric antigen receptor T cell therapy for non-Hodgkin lymphoma. Curr Res Transl Med 66:43–49. 10.1016/j.retram.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schuberth PC, Hagedorn C, Jensen SM, et al. (2013) Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med 11:1–11. 10.1186/1479-5876-11-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kakarla S, Chow KKH, Mata M, et al. (2013) Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 21:1611–1620. 10.1038/mt.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L-CS, Lo A, Scholler J, et al. (2014) Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2:154–166. 10.1158/2326-6066.CIR-13-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tran E, Chinnasamy D, Yu Z, et al. (2013) Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med 210:1125–35. 10.1084/jem.20130110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gulati P, Ruhl J, Kannan A, et al. (2018) Aberrant Lck signal via CD28 Costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin Cancer Res 24:3981–3993. 10.1158/1078-0432.CCR-17-1788 [DOI] [PubMed] [Google Scholar]
- 159.Aghajanian H, Kimura T, Rurik JG, et al. (2019) Targeting cardiac fibrosis with engineered T cells. Nature 573:430–433. 10.1038/s41586-019-1546-z [DOI] [PMC free article] [PubMed] [Google Scholar]


