Abstract
Selective activation of the cannabinoid receptor subtype 2 (CB2) shows promise for treating pain, inflammation, multiple sclerosis, cancer, ischemic/reperfusion injury, and osteoporosis. Target selectivity and off-target side effects are two major limiting factors for orthosteric ligands, and, therefore, the search for allosteric modulators (AMs) is a widely used drug discovery approach. To date, only a limited number of CB2 AMs have been identified, possessing only micromolar activity at best, and the CB2 receptor’s allosteric site(s) are not well characterized. Herein, we used computational approaches including receptor modeling, site mapping, docking, molecular dynamics (MD) simulations and binding free-energy calculations to predict, characterize and validate sites within the complex of the CB2 receptor with bound orthosteric agonist CP55,940. After docking of known negative CB2 allosteric modulators (NAMs), dihydro-gambogic acid (DHGA) and trans-β-caryophyllene (TBC) (note that TBC also shows agonist activity) at the predicted allosteric sites, the best total complex with CB2, CP55,940 and NAM was embedded into a hydrated lipid-bilayer and subjected to a 200 ns MD simulation. The presence of an AM affected the CB2–CP55,940 complex, altering the relative positioning of the toggle switch residues and promoting a strong π-π interaction between Phe1173.36 and Trp2586.48. Binding of either TBC or DHGA to a putative allosteric pocket directly adjacent to the orthosteric ligand reduced the binding free energy of CP55,940, which is consistent with the expected effect of a negative AM. The identified allosteric sites present immense scope for the discovery of novel classes of CB2 AMs.
Keywords: CB2 receptor, CB2 allosteric modulator, Docking, G-protein-coupled receptor, Molecular dynamics
Graphical Abstract
Introduction
Most of the existing drugs that act on G-protein-coupled receptors (GPCRs) bind to their orthosteric binding sites (Christopoulos, 2002). The cannabinoid receptor subtype 2 (CB2) is predominantly expressed throughout the periphery (Galiegue et al., 1995; Marino and Idris, 2017) and is also found in lesser amounts in the brain (Shang and Tang, 2017; Van Sickle et al., 2005). Orthosteric CB2 agonists may be useful for treating pain, inflammation, amyotrophic lateral sclerosis (ALS), cancer, and ischemic/reperfusion injury (Han, Thatte, Buzard, & Jones, 2013; Pressly et al., 2018; Smith, 2002; Xu et al., 2007; M. Zhang et al., 2007). Despite the therapeutic potential of CB2 agonists, prolonged activation of the CB2 receptor may cause immunosuppression (Rieder, Chauhan, Singh, Nagarkatti, & Nagarkatti, 2010), cancer growth (Zhu et al., 2000) and bone loss (Idris, Sophocleous, Landao-Bassonga, van’t Hof, & Ralston, 2008). Meanwhile, CB2 inverse agonists/antagonists are also emerging as potential drugs to treat diseases such as post-traumatic brain injuries (Presley et al., 2015), acute kidney injuries and osteoporosis (Idris, 2010). Target selectivity and off-target side effects are the two major limiting factors for orthosteric ligands, and, therefore, the search for allosteric modulators (AMs) is one of the widely used drug discovery approaches to avoid such limitations. All GPCRs analyzed to date for allostery have been shown to have allosteric binding sites for endogenous/synthetic ligands that are discrete from the orthosteric site (Christopoulos, 2002; Foster and Conn, 2017; May, Leach, Sexton, & Christopoulos, 2007; Saleh et al., 2018). The binding of AMs typically leads to a conformational change of the receptor, which affects the affinity and/or efficacy of the orthosteric ligands, thereby fine-tuning the biological activities of the orthosteric ligands (May, et al., 2007). Altogether, AMs exhibit distinct therapeutic advantages over orthosteric ligands in terms of enhanced target specificity and reduced or null off-target side effects (Christopoulos, 2002; May, et al., 2007).
There have been more studies of allosteric modulators for the CB2 homolog cannabinoid receptor subtype 1 (CB1) than for CB2. To date, the well-known AMs of CB1 are: the endogenous lipid lipoxin A4 (Pamplona et al., 2012), the selective dopamine transporter inhibitor RTI-371 (Navarro, Howard, Pollard, & Carroll, 2009), ZCZ011 (Ignatowska-Jankowska et al., 2015) (CB1 agonist/positive AM (PAM) at CB1), cannabidiol-dimethylheptyl (CBD-DMH) (agonist/positive allosteric modulator at CB1) (Tham et al., 2018) and GAT211 (Laprairie et al., 2017) are CB1 PAMs; and fenofibrate (NAM of CB1 receptor at higher concentrations and agonist at lower concentrations and CB2 receptor agonist) (Priestley, Nickolls, Alexander, & Kendall, 2015), pregnenolone (NAM of CB1R-mediated ERK1/2 phosphorylation with no effect on orthosteric agonist binding affinity or cAMP-mediated signaling) (Vallee et al., 2014), cannabidiol (Tham, et al., 2018), Org27569, Org29647, Org27759, PSNCBAM-1, and RTICBM-74, all negative AMs (NAMs) (Horswill et al., 2007; Khurana et al., 2017; Laprairie, Bagher, Kelly, & Denovan-Wright, 2015; Martínez-Pinilla et al., 2017; Nguyen et al., 2017; Price et al., 2005). GAT100 has been studied as the first CB1 covalent allosteric probe (Abood, 2016; Alaverdashvili and Laprairie, 2018; Laprairie et al., 2016). However, only a few AMs of the CB2 receptor have been identified so far and they have micromolar activity. For example, trans-β-caryophyllene (TBC) and dihydro-gambogic acid (DHGA) are the two reported NAMs (Maheswari, 2011) of the CB2 receptor (Fig 1). In addition, Maheswari et al. (2011) (Maheswari, 2011) had also reported gambogic analogs as positive allosteric modulators. Petrucci et al. (2017) (Petrucci et al., 2017) identified pepcan-12 (RVD-hemopressin, an endogenous peptide) as a PAM of CB2 receptor. Recently, Gado et al. (2018) (Gado et al., 2018) reported a first synthetic (C2) PAMs of CB2s.
Figure 1.
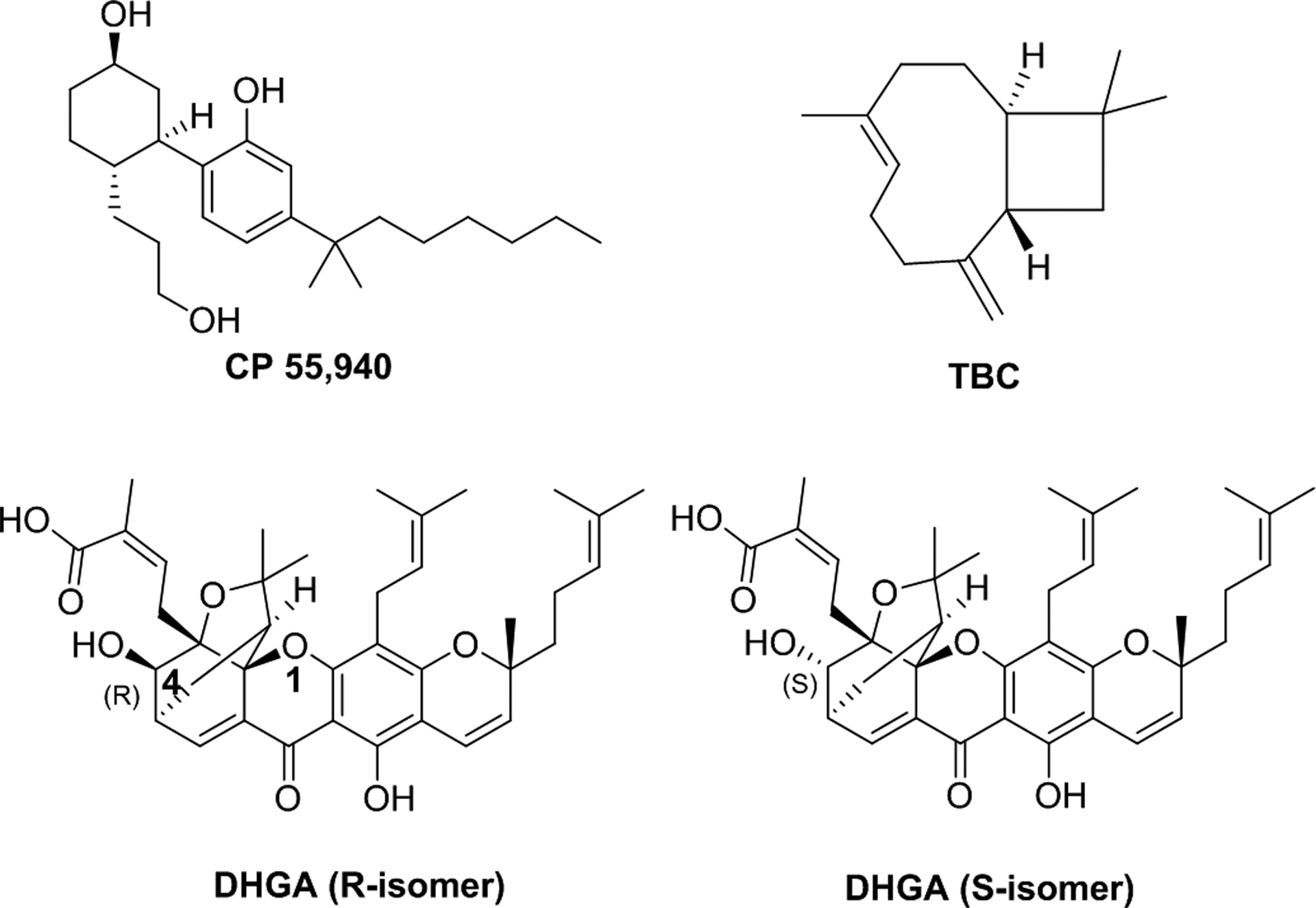
Chemical structures of selected CB compounds, including CP55,940, trans-β-caryophyllene (TBC), and two diastereomers of dihydro-gambogic acid (DHGA) which differ at the 4-hydroxy position.
Known negative CB2 allosteric modulators
Trans-β-caryophyllene (TBC)
TBC (Fig 1) is a natural bicyclic sesquiterpene plant volatile oil found in the essential oils from various plants including rosemary (Rosmarinus officinalis), cinnamon (Cinnamomum zeylanicum), hops (Humulus lupulus), clove (Syzygium aromaticum), black pepper (Piper nigrum), basil (Ocimum gratissimum and Ocimum micranthum), and cannabis (Cannabis sativa) (Bernardes et al., 2010; Hudaib, Speroni, Di Pietra, & Cavrini, 2002; Jayaprakasha, Jagan Mohan Rao, & Sakariah, 2003; Mockute, Bernotiene, & Judzentiene, 2001; Orav, Stulova, Kailas, & Muurisepp, 2004; Zheng, Kenney, & Lam, 1992). It is usually found in a mixture with its isomers, isocaryophyllene and α-humulene (a ring-opened isomer), or its oxidation product trans-β-caryophyllene oxide (Ghelardini, Galeotti, Di Cesare Mannelli, Mazzanti, & Bartolini, 2001). TBC, a dietary compound, is considered a cannabinoid due to its CB2 agonistic effect. This compound has shown anti-inflammatory effects mediated by the CB2 receptor in mice (Klauke et al., 2014). TBC constitutes 3.8–37.5% of the essential oil of Cannabis sativa, and is a selective CB2 agonist with a Ki (binding affinity) of 780 ± 12 nM (Gertsch et al., 2008). Due to its effect as a CB2 selective agonist, it has a beneficial therapeutic potential for treating arthritis, pain, metabolic disorders and other disease/disorders (Gertsch, et al., 2008; Ghelardini, et al., 2001; Horvath et al., 2012; Klauke, et al., 2014). Although it acts as an agonist, TBC has also been shown to act as an allosteric ligand. Maheshwari et al. (Maheswari, 2011) reported TBC as a highly selective dietary CB2 cannabinoid with unique ago-allosteric properties, based on in vitro experiments. From their studies, they found TBC to be a negative ago-allosteric modulator with a Ki of 2.37 μM (Maheswari, 2011).
Dihydro-gambogic acid (DHGA)
Gambogic acid is a xanthonoid derived from the resins isolated from the Garcinia hanburyi tree. It exhibits pro-apoptotic, anticancer and anti-inflammatory properties (Cascao et al., 2014; Davenport et al., 2011; H. Z. Zhang et al., 2004; Zhao et al., 2010). DHGA (Fig 1), a derivative of gambogic acid, has shown negative AM effects on the CB2 receptor in a probe-dependent manner (Maheswari, 2011).
Plan of this work
One of the reasons behind the limited reports of AMs of CB2 could be the lack of knowledge about the allosteric site(s) within the CB2 receptor. An exploration and validation of such potential allosteric sites within CB2 could accelerate targeted searches for new potent AMs. Therefore, in the present study, we aimed to identify, characterize and validate allosteric site(s) within the CB2 receptor using the two known CB2 NAMs, TBC and DHGA (Maheswari, 2011). To accomplish this aim, a multi-template-based active-state model of the human CB2 receptor was prepared, validated, and then used for detection and characterization of sites suitable for allosteric modulator binding. Next, making use of the most promising of the identified sites, we have carried out MD simulations of the CB2 receptor together with bound allosteric and orthosteric ligands in an explicitly-hydrated lipid-bilayer environment, and report insights that could be useful for design of optimized NAMs.
Results and Discussion
Homology modeling, refinement, quality assessments and flexible docking
We used four X-ray crystal structures of three GPCRs as templates (β1-AR, β2-AR and rhodopsin) to construct 100 homology models of the human CB2 receptor, which resulted from simulated annealing sampling of the structure with constraints. The multiple sequence alignment used in the homology modeling is shown in Figure 2. Out of the generated models (Fig 3A), an optimal model was chosen according to molpdf and DOPE scores (Fig 3B). For the best identified model (Fig 4), all amino acids were present in the allowed/favored region in the Ramachandran plot (Fig 4C), thus supporting its good quality.
Figure 2.
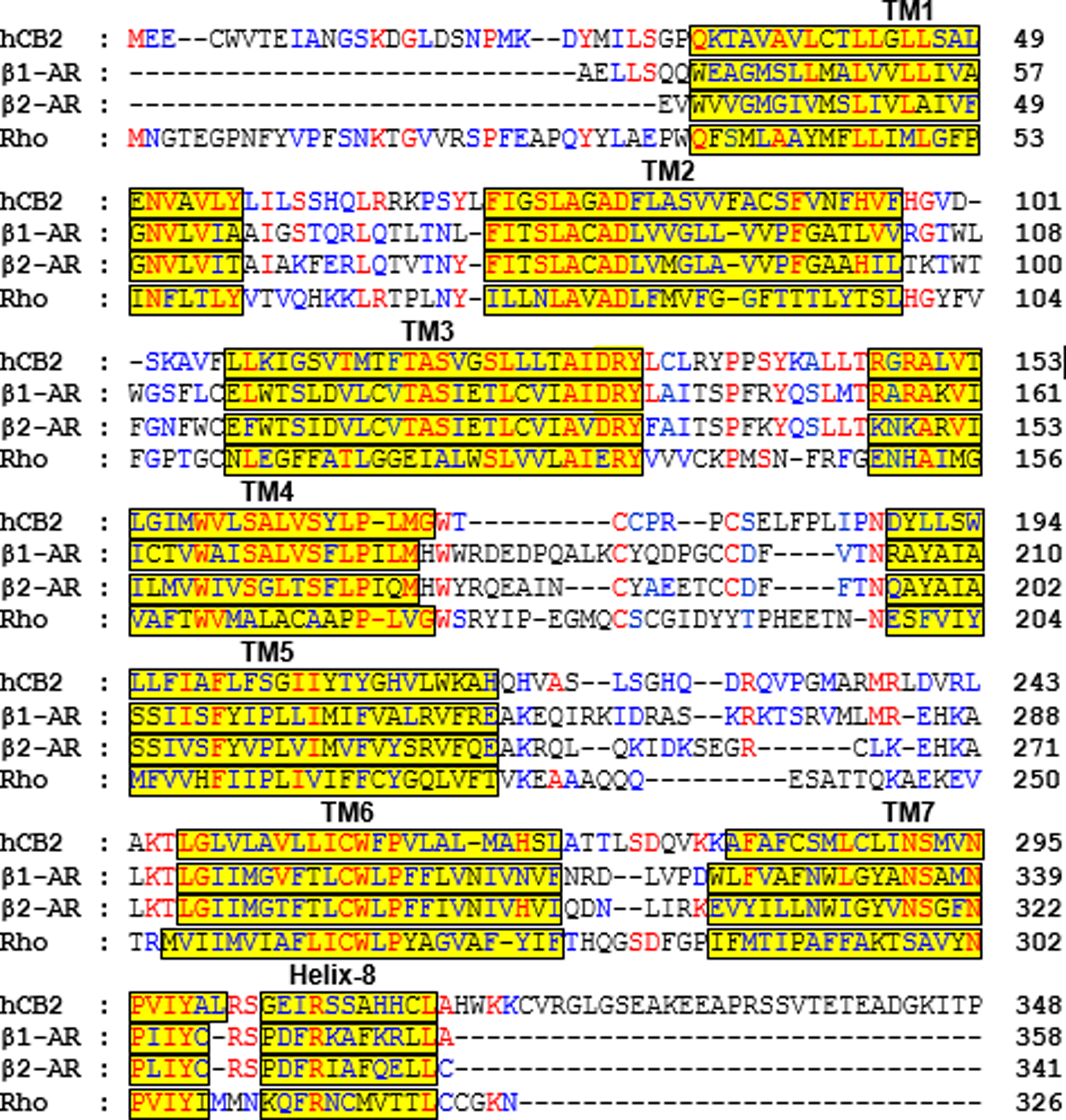
Multiple sequence alignment of human CB2 (hCB2) with turkey β1 adrenergic receptor (β1-AR) (PDB-ID: 2Y02), human β2 adrenergic receptor (β2-AR) (PDB-IDs: 3SN6); and bovine rhodopsin (Rho) (PDB-ID: 3PQR). Identical residues are colored red while similar residues are colored blue. Transmembrane (TM) domains are highlighted with yellow background.
Figure 3.
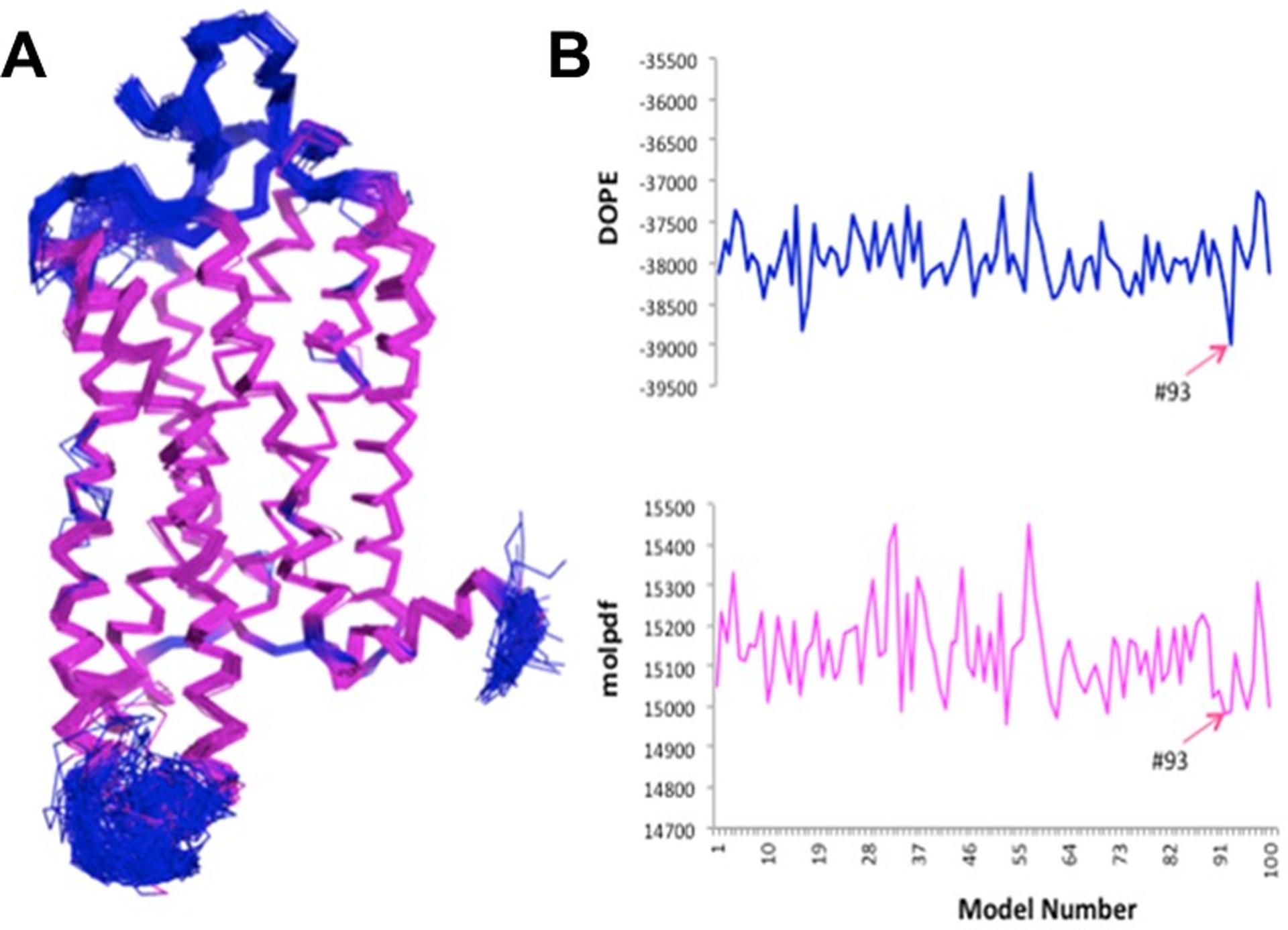
(A) Cα-atom based overlay of the 100 generated models of the hCB2 receptor. Helices and loops are shown in magenta and blue, respectively. (B) Plots of DOPE (top) and molpdf (bottom) scores of the 100 generated models of the hCB2 receptor.
Figure 4.
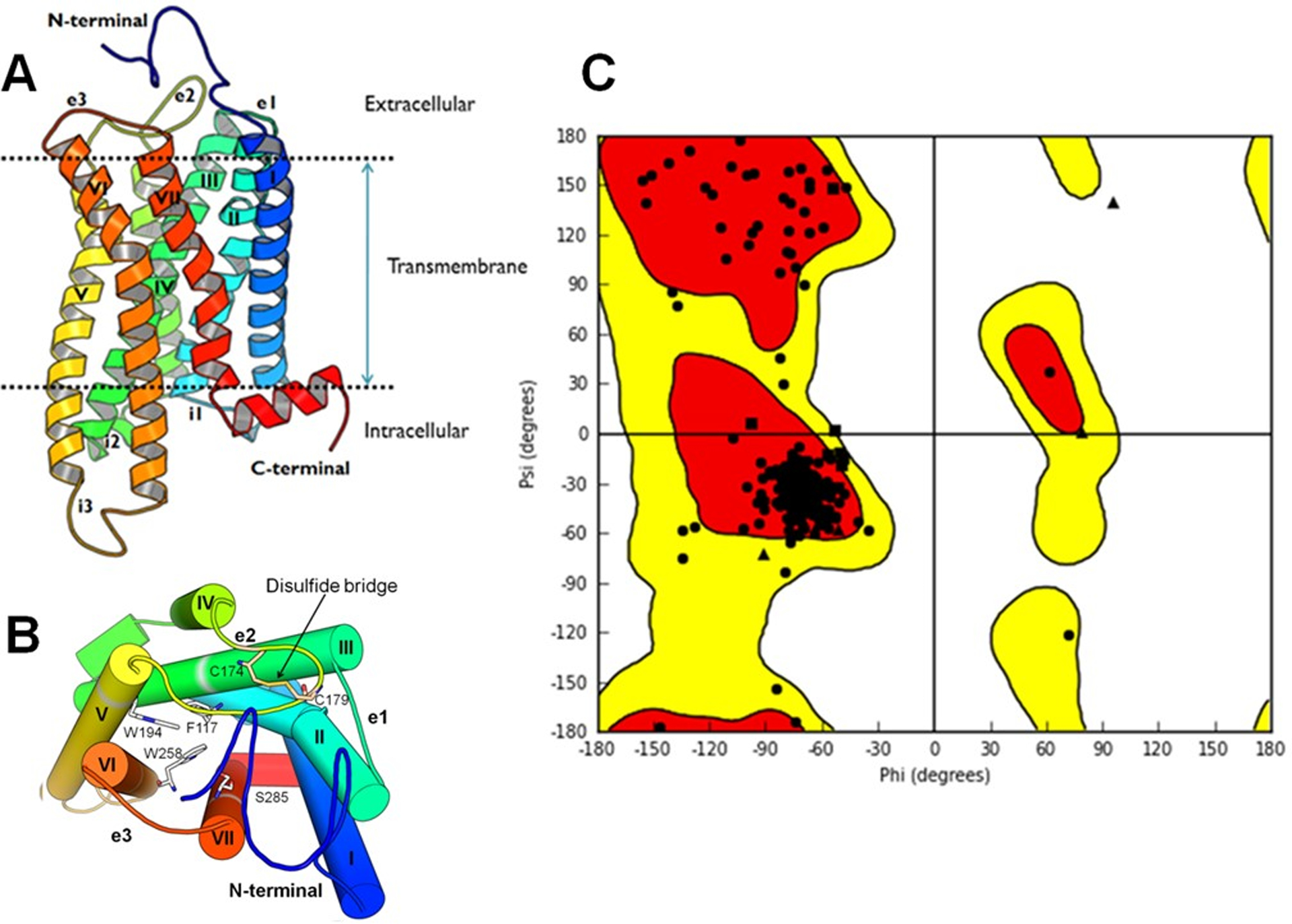
(A) Overall cartoon view of the selected hCB2 receptor model, with helices labeled with Roman numerals and loops labeled as e (extracellular) or i (intracellular). (B) An alternative view of the hCB2 receptor model from the extracellular side depicting some key residues of hCB2 and a disulfide bridge in the second extracellular loop (EC2). (C). Ramachandran plot of the CB2 3D model showing the backbone angles (phi and psi) of the amino acids. Most-favorable region in red and favored region in yellow. Triangles represent glycines and squares represent prolines, which are not expected to match the typical phi/psi ratio of the other amino acids.
Induced Fit Docking of CP55,940 with the CB2 receptor
The known non-selective CB agonist CP55,940 was docked into the orthosteric site of the CB2 receptor using the Induced Fit Docking (Sherman, Day, Jacobson, Friesner, & Farid, 2006) protocol of the Schrödinger software. The docking study identified a total of 13 poses of CP55,940 which were further used for binding free energy calculations using the Prime MM-GBSA module (Table 1). The binding pose of CP55,940 that showed the best binding as reflected by the calculated binding free energy, Pose number 6 (Fig 5), was used in the next stage of studies. The observed interaction pattern between CP55,940 and hCB2 in the best docked pose (Fig 5) agreed well with previous mutagenesis and modeling studies (Hurst et al., 2010; Y. Zhang et al., 2011).
Table 1.
Docking scores and binding free-energies for all of the docked poses of CP55,940 with the CB2 homology model.
| Pose number | Docking score (kcal/mol) | Emodel value | Binding free-energy (kcal/mol) |
|---|---|---|---|
| 1 | −10.814 | −89.252 | −131.54 |
| 2 | −10.432 | −77.002 | −118.56 |
| 3 | −10.831 | −90.182 | −125.79 |
| 4 | −10.098 | −84.849 | −127.41 |
| 5 | −10.045 | −77.207 | −120.43 |
| 6 | −9.242 | −74.521 | −135.61 |
| 7 | −9.203 | −75.927 | −119.57 |
| 8 | −9.167 | −75.676 | −103.80 |
| 9 | −10.304 | −74.382 | −102.37 |
| 10 | −9.489 | −71.529 | −116.85 |
| 11 | −10.388 | −70.913 | −103.69 |
| 12 | −10.108 | −79.800 | −113.50 |
| 13 | −10.300 | −82.124 | −104.45 |
Figure 5.
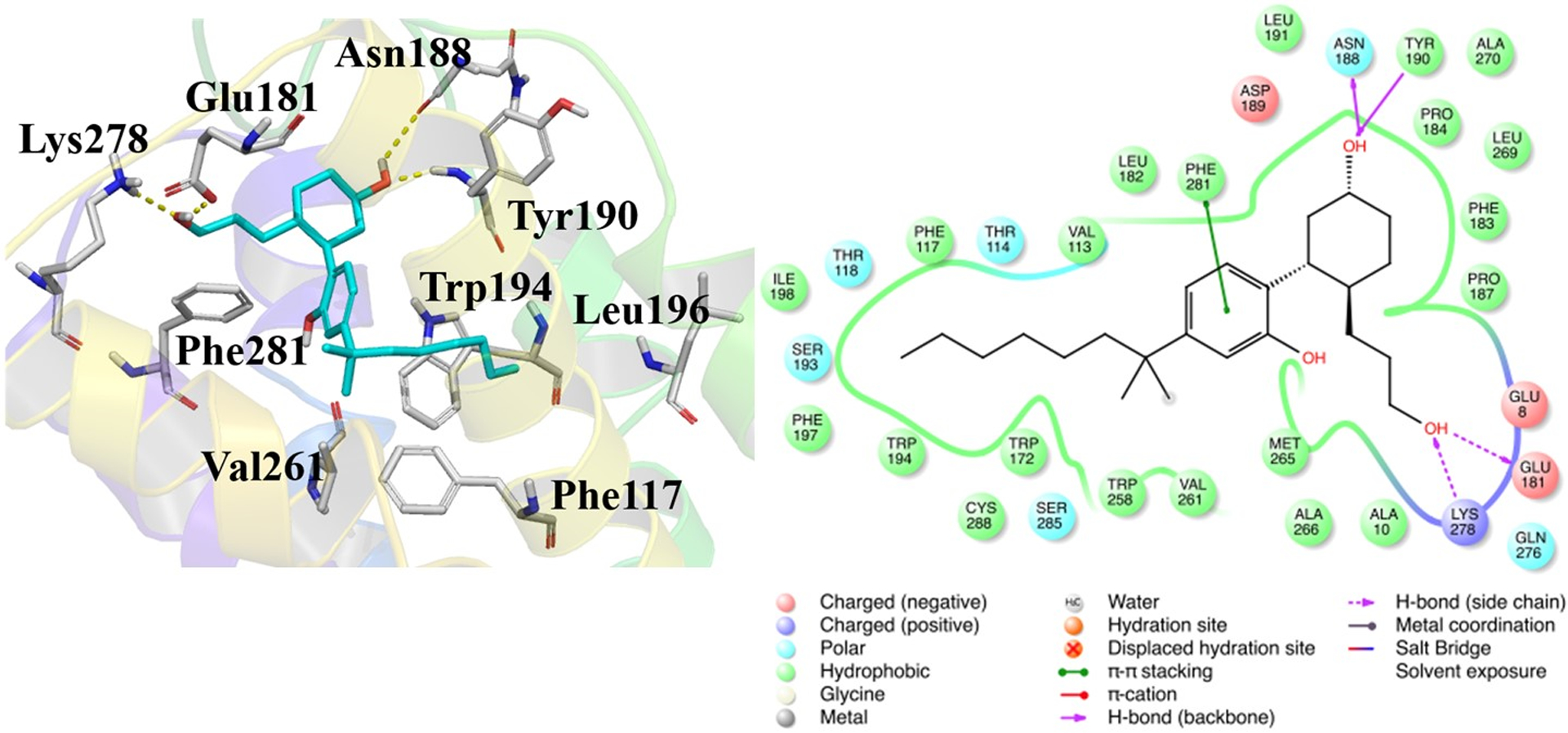
The interaction profile of CP55,940 with the hCB2 receptor, from the best docking pose. (A) 3D representation, with CP55,940 (cyan carbons) and key residues of CB2 (gray carbons); H-bonds are shown as yellow dashed lines. (B) 2D representation.
Identification and Characterization of Potential Binding Pockets
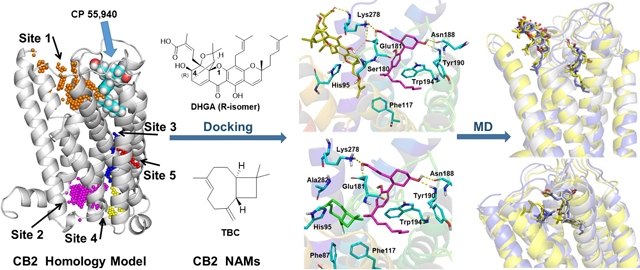
Five potential allosteric binding pockets (represented as spheres of unique color for each pocket) (shown in Fig 6) were found within the CB2 receptor complexed with agonist CP55,940, using the SiteMap (Halgren, 2009) tool.
Figure 6.
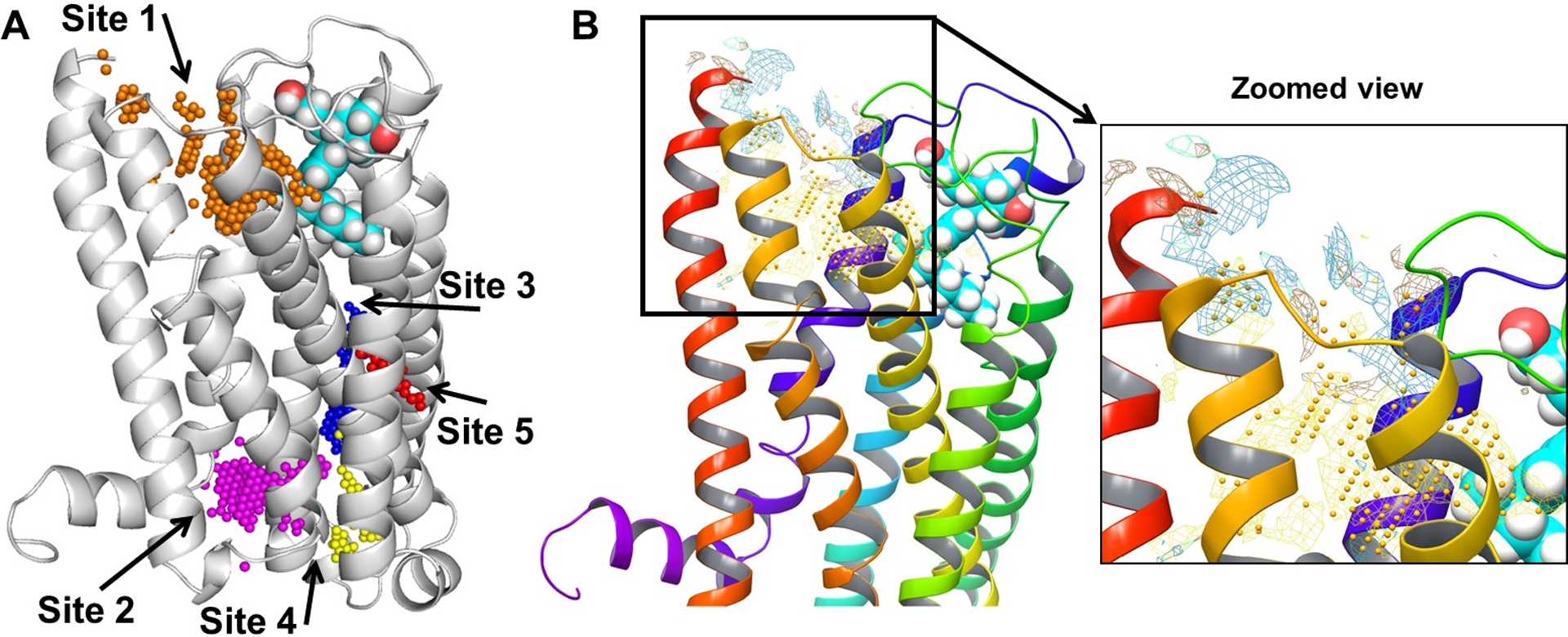
SiteMap-detected allosteric binding pockets. (A) Five potential allosteric binding pockets (represented as spheres with unique color for each pocket) found for the complex of the CB2 receptor (gray ribbon representation) and CP55,940 (cyan carbon CPK representation): Site 1 (gold); Site 2 (magenta); Site 3 (blue); Site 4 (yellow); and Site 5 (red). (B) Binding map of Site 1 within the complex of the CB2 receptor (multi-colored ribbon representation) and CP55,940 (blue carbon CPK representation); the appearance of the map types is as follows: Hydrophobic map (yellow mesh), Hydrophilic map (green mesh), Hydrogen-bond donor map (blue mesh), and Hydrogen-bond acceptor map (red mesh).
We selected the top-ranked pockets based on SiteScore, Site Size, Dscore (Druggability score), and Site Volume (Table 2). If SiteScore and Dscore are greater than 1, then the predicted site/pocket is considered to be more promising, based on the training set used in the SiteMap software. By considering these parameters, only the two best-scoring pockets (Site 1 and Site 3), both of which were near the extracellular regions of the CB2 receptor, were chosen as potential allosteric binding sites.
Table 2.
Numerical scores of the SiteMap-predicted binding sites in the CB2–CP55,940 complex.
| Predicted Site | SiteScore | Number of Site Points | Dscore | Site Volume (Å3) |
|---|---|---|---|---|
| Site 1 | 1.159 | 179 | 1.230 | 475.741 |
| Site 2 | 1.079 | 104 | 1.114 | 283.318 |
| Site 3 | 0.973 | 60 | 1.071 | 157.780 |
| Site 4 | 0.810 | 46 | 0.776 | 179.389 |
| Site 5 | 0.858 | 32 | 0.866 | 72.030 |
While the site scores (Site 1: 1.159 and Site 2: 1.079) reported are both greater than 1, indicating that both sites are promising as ligand binding sites, the difference in the scores are not very significant. However, Site 2 was not considered for further studies because of other considerations such as the docking scores of known CB2 allosteric modulators, which indicate that Site 1 is a more probable site for these compounds. We did not get any results in attempts to dock TBC into Site 2. However, DHGA had a good docking score (Glide score = −4.68 kcal/mol), but the major part of the molecule was situated outside the pocket and had only had significant H-bond interactions between the lower end of Helix 6 (Arg242 and Arg238) and DHGA’s carbonyl and carboxylic acid moieties. In the current study, we did not consider predicted Site 2 as a potential allosteric binding site because it is located between the C-terminal and intracellular loops IC1 and IC2, known to be a site of G-protein binding; however, this site may have potential for future investigation.
Conformational search of TBC and DHGA
We performed careful conformational searches to identify the low-energy conformers of TBC and DHGA. Since DHGA has been reported as a mixture of two diastereomers, differing only at the 4-OH position, we considered separately R and S stereoisomers of this molecule (cf. Fig 1) for the conformational searches. A total of eight low energy conformers were identified for TBC, while 252 and 278 conformers were identified for R- and S-isomers, respectively, for DHGA. These conformers were used in the docking analysis as discussed below.
Docking analysis of TBC and DHGA to the CB2–CP55,940 receptor complex
The low energy conformers of TBC and DHGA were docked into the two best-scoring pockets of the CB2–CP55,940 complex. In each case, the best docking scores were found with Site 1, and hence the poses of the NAMs in Site 1 were used for the further analysis and MD simulations as discussed below. The best docking scores were −6.251, −6.517, and −4.861 kcal/mol, for TBC, 4R-DHGA, and 4S-DHGA, respectively. The docked poses of TBC and 4R–DHGA are shown in Figure 7. Docking results revealed that DHGA was engaged in hydrogen bonding with Ser180 (EC2) and Lys2787.32 and exhibited π-π stacking with His952.65. The alkyl chain surrounded and hydrophobically engaged with several hydrophobic residues of the CB2 receptor, including Phe872.57, Ala882.58, Val922.62, and Phe1173.36. Along with those interactions, TBC also exhibited strong hydrophobic interactions with residues Ile1103.29 and Phe2817.35 (Fig 7).
Figure 7.
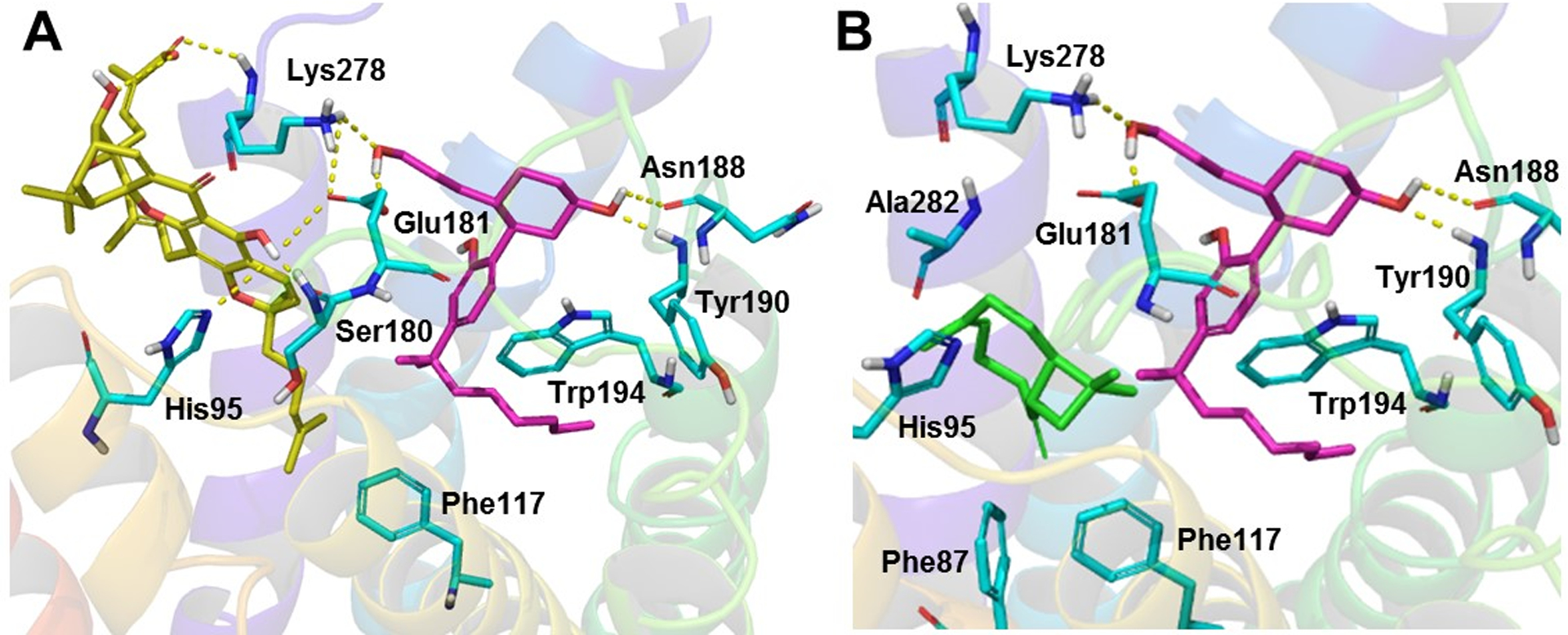
The 3D representation of putative binding modes of the allosteric inhibitors into the CB2–CP55,940 (magenta carbon) complex: (A) 4R-DHGA (yellow carbon); (B) TBC (green carbon).
We compared the orthosteric-docking pose of TBC reported by Gertsch et al. (2008) (Gertsch, et al., 2008) and our docking pose of TBC. We found that some of the residues reported to be present within their orthosteric binding site are in close proximity to our docked NAM site (Site 1), e.g., Val1133.32, Ile1103.29 and Phe872.57 (all residues are within 3 Å) and Phe1173.36 (4 Å). Our orthosteric docking site overlaps with the reported orthosteric site for TBC and covers Ring B and part of the alkyl chain of CP55,940. We found that, in the presence of CP55,940, TBC binds in a site slightly further up (Site 1) compared to the site into which it binds in the absence of an orthosteric ligand, within the CB2 receptor.
Molecular dynamics simulation studies
In order to examine the stability and dynamics of the CB2 receptor complexed with allosteric and orthosteric ligands, MD simulations were performed in an explicitly-hydrated lipid-bilayer environment. We performed 200 ns simulations on each of three protein–ligand complexes: 1) the CB2 receptor complexed with the orthosteric ligand (CP55,940) only; 2) the CB2 receptor complexed with TBC and CP55,940; and 3) the CB2 receptor complexed with 4R-DHGA and CP55,940. The root-mean-square deviation (RMSD) plot of atom locations vs. simulation time (Fig 8) indicated that after the initial 20 ns in each of the three simulations, the protein and ligands attained a significantly-stable state that they maintained well throughout the remainder of the 200 ns simulations. The major fluctuations in terms of RMSD were observed at the beginning of the simulations, during which the bound ligands adjusted their positions to attain stable poses. Figure 9 shows the overlay of the first, 50,000th (100 ns), and last frames of the simulations, depicting the geometrical variations in ligand structure as well as in protein structure over the course of the simulations.
Figure 8.
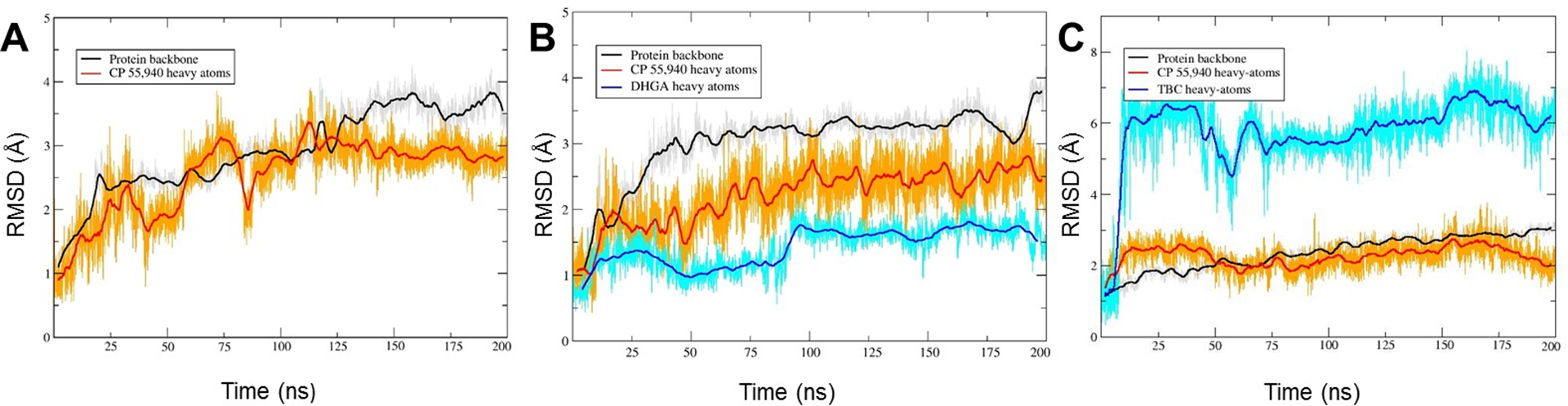
RMSD for protein backbone atoms (gray line; running average in a window of 200 ps is indicated in black), CP55,940 heavy atoms (gold line; running average in a window of 200 ps is indicated in red), and NAM heavy atoms (cyan line; running average in a window of 200 ps is indicated in blue) for MD simulations of the CB2 receptor and the orthosteric ligand (CP55,940), without or with NAM: (A) CP55,940 only; (B) CP55,940 and TBC; and (C) CP55,940 and DHGA.
Fig 9.
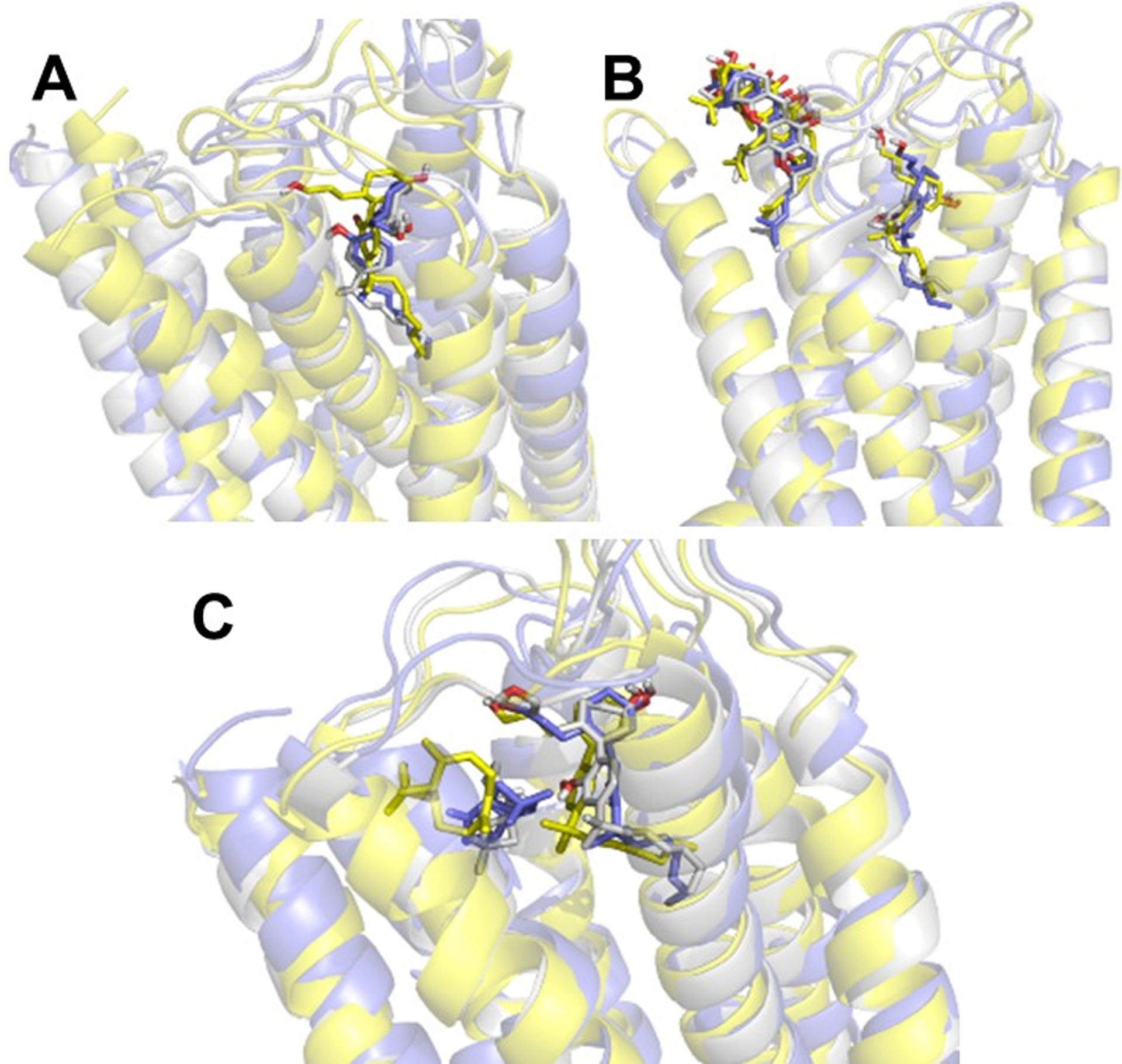
MD simulation of the CB2 receptor with: (A) CP55,940 (the orthosteric ligand) only; (B) CP55,940 and DHGA; and (C) CP55,940 and TBC. An overlay of the first (yellow), middle (50000th) (gray), and last (blue) frames of each of the three MD simulations is presented. The protein is represented in cartoon form, while the ligands are represented in stick form with matching colors.
We performed MD trajectory clustering using the dihedral principal components analysis (dPCA) method implemented in Grcarma software (Koukos and Glykos, 2013). The dPCA clustering groups MD snapshots into distinct clusters, with the members of each cluster being similar to each other. We allowed a maximum of 10 clusters for each simulation; however, we only obtained 6, 4 and 2 clusters, respectively, for the CB2–CP55,940, CB2–CP55,940–TBC and CB2–CP55,940–DHGA simulations. We used the most representative structures of each cluster for further analysis (Table 3).
Table 3.
Binding free energies (for all representative conformations from each of the three MD simulations) of CP55,940 with the CB2 receptors in the presence or absence of AMs.
| Complex with CB2 Receptor | dPCA-Based Cluster | Binding Free-Energies of CP55,940 with or without AM within CB2 Receptor (kcal/mol) |
|---|---|---|
| CB2–CP55,940 | I | −149.13 |
| II | −143.92 | |
| III | −141.75 | |
| IV | −128.83 | |
| V | −133.24 | |
| VI | −126.75 | |
| CB2–CP55,940–TBC | I | −125.78 |
| II | −135.00 | |
| III | −123.94 | |
| IV | −135.59 | |
| CB2–CP55,940–DHGA | I | −122.76 |
| II | −129.45 |
The interaction patterns between the CB2 receptor and the allosteric ligands (TBC and DHGA), in representative structures extracted from the MD trajectories via dPCA analysis, are shown in Figures 10 and 11. In the representative structure of the CB2–CP55,940–TBC complex, TBC exhibited strong hydrophobic interactions with several amino acids such as Val361.35, Phe872.57, Phe912.61, Val922.62, Val962.66, Phe1063.25, Leu182 (EC2), Phe2817.35, and Ala2827.36. Superimposition of the starting structure with this conformation (Fig 9C) revealed that the ligands penetrated slightly deeper into the binding pocket during the simulation and showed stronger hydrophobic interactions compared to those in the starting structure. In the case of the representative conformation of the CB2–CP55,940–DHGA complex, DHGA showed H-bonding with Lys2797.33 and had interactions with a set of water molecules on the extracellular region. In addition, DHGA also showed strong hydrophobic interactions with Val361.35, Ala371.36, Cys401.40, Val922.62, Phe942.64, Phe2817.35, Ala2827.36, Phe2837.37, Met2867.40, and Leu2897.43.
Figure 10.
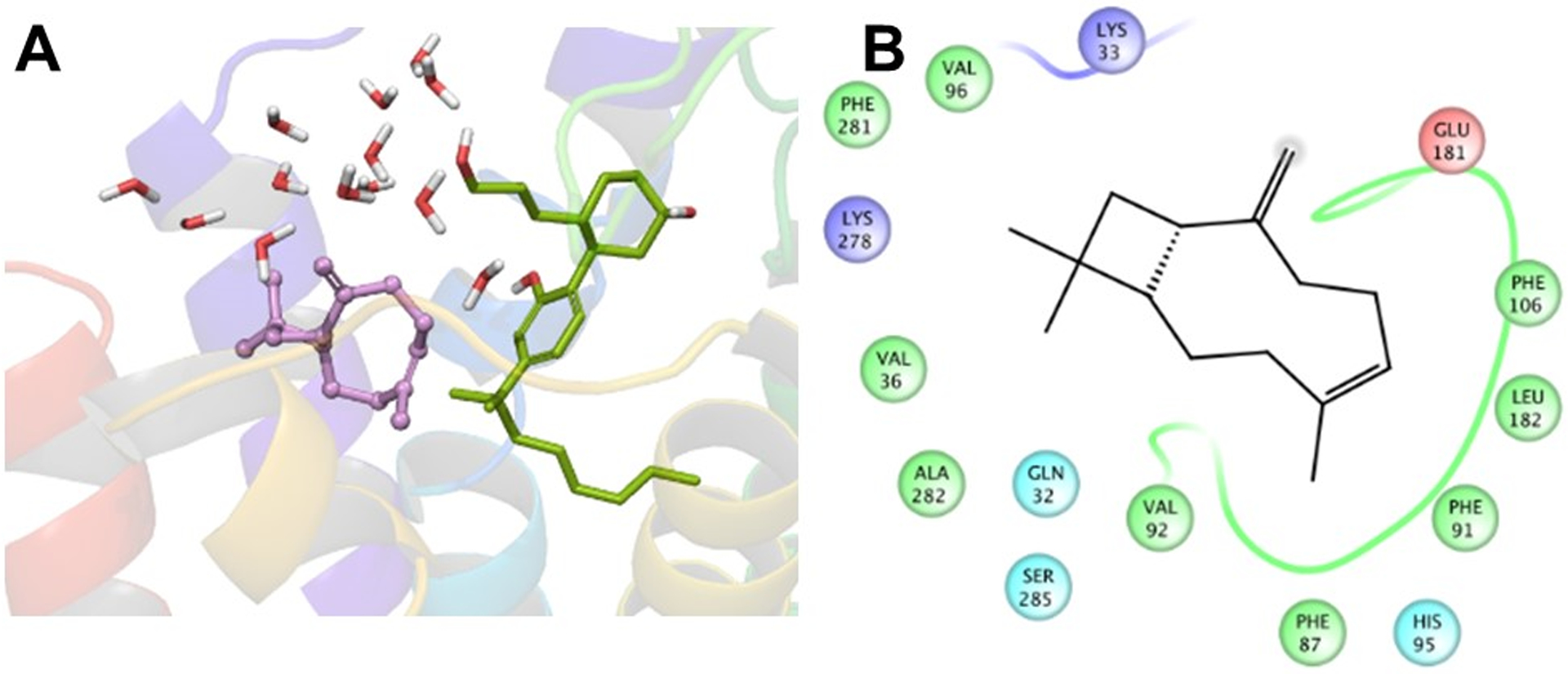
(A) 3D view of a representative structure of TBC (plum carbon) with the CB2 receptor in the presence of CP55,940 (green carbon) (nearby waters are also shown as stick model) and (B) the corresponding 2D interaction diagram of TBC interacting with the CB2 receptor in the presence of CP55,940.
Figure 11.
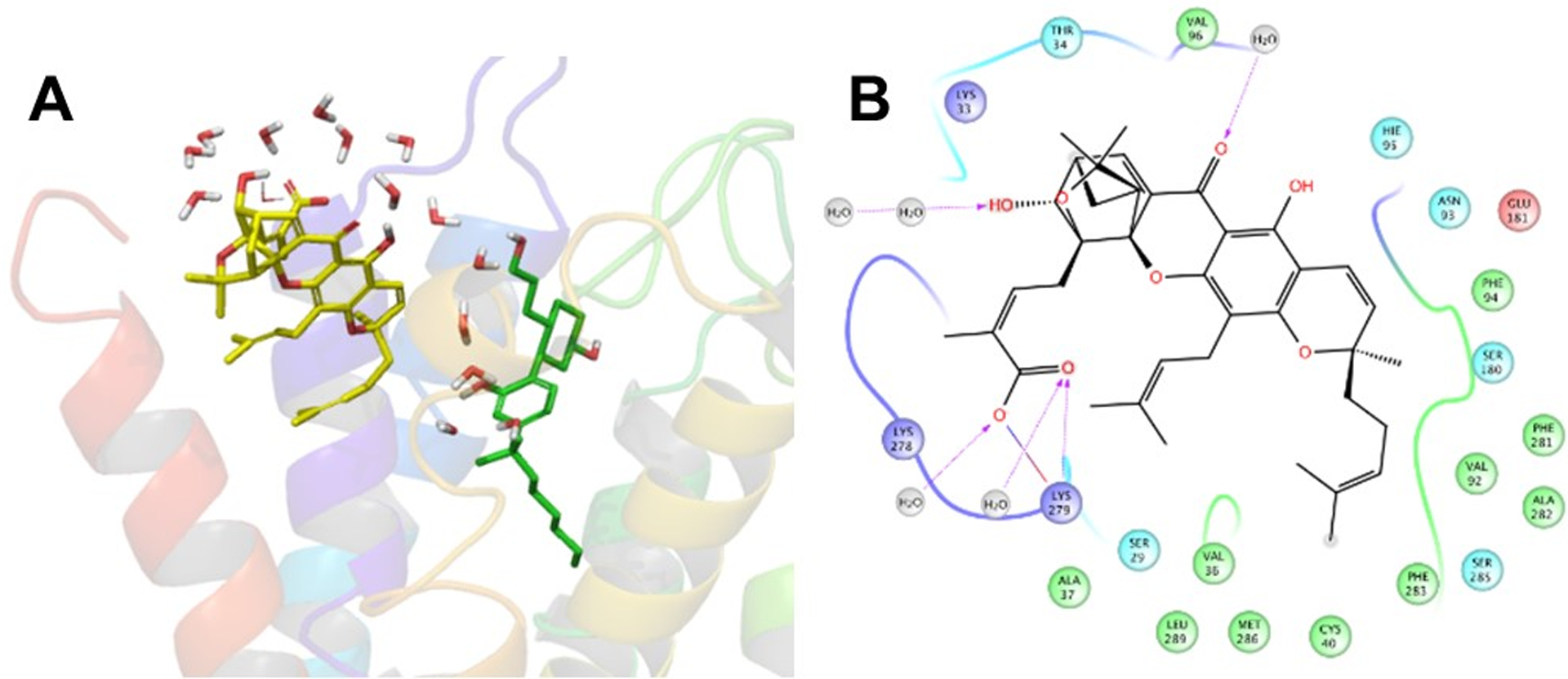
(A) 3D view of a representative structure of DHGA (yellow carbon) with the CB2 receptor in the presence of CP55,940 (green carbon) (nearby waters are also shown as stick model) and (B) the corresponding 2D interaction diagram of DHGA interacting with the CB2 receptor.
In order to understand further the interactions of the NAMs with CB2, we used the representative structures of CB2–CP55,940, CB2–CP55,940–TBC, and CB2–CP55,940–DHGA, retrieved from the MD trajectories via dPCA, for calculating their binding free energies using the Prime MM-GBSA module of the Schrödinger software. In principle, negative allosteric modulators weaken (decrease the absolute value of the negative quantity) the binding affinity of endogenous or synthetic ligands while positive allosteric modulators strengthen (increase the absolute value of the negative quantity) the binding affinity of endogenous or synthetic ligands; however, exceptions exist to these defined rules for cannabinoid ligands. For example, Org27569 acts as a positive allosteric modulator by enhancing agonist binding, whereas in terms of the functional activity of CB1, Org27569 is studied as a NAM. PSNCBAM-1 (Horswill, et al., 2007) increased agonist binding to the CB1 receptor but it behaved as a NAM in [35S]-GTPγS binding and cAMP assays. ZCZ011 (Ignatowska-Jankowska, et al., 2015) increased [3H]-CP55,940 binding to the CB1 receptor and enhanced anandamide (AEA)-stimulated signaling in the [35S]-GTPγS binding, β-arrestin pathway, and extracellular signal-regulated kinase (ERK) phosphorylation assays. GAT228, a R-(+)-enantiomer of GAT211, showed no effect on binding to the CB1 receptor of the CB1 agonist [3H]CP55,940 up to 1 μM but exhibited a weak PAM effect at a 10 μM concentration. GAT228 did not compete with CP55,940 and exhibited allosteric agonism by itself at the CB1 receptor through increasing basal arrestin2 recruitment and cAMP inhibition (Laprairie, et al., 2017). In this study, the binding free energy of CP55,940 was weakened when either TBC or DHGA was bound within the allosteric site of the CB2 receptor. The relative amount of decrease in the magnitude of the binding free energy of CP55,940 in the presence of TBC or DHGA signifies the druggability of the identified allosteric pocket within the hCB2 receptor model (Table 3).
Molecular dynamics study on the CB2–CP55,940 complex showed three salient changes in CP55,940 conformation during the 200 ns simulation. The first major change was that the aliphatic side chain containing the southern hydroxyl group moved from between TM3 and TM7 (close to Glu181 in EC2) towards the end of the EC2 loop at TM4, after only 60 ns of simulation; this change remained stable for the rest of the 200 ns simulation. This is clearly visible in Figure 9, when comparing the initial to the middle and final conformations. After the shift towards the EC2 loop end of TM4, CP55,940 exhibited strong H-bond interactions between its southern hydroxyl group and Trp1724.64 (located in TM4, close to the start of EC2) (Fig 12). The importance of Trp1724.64 in CB2 ligand binding was previously supported by Rhee et al (Rhee, Nevo, Bayewitch, Zagoory, & Vogel, 2000). Secondly, the CP55,940 molecule shifted closer to the toggle switch residues (Phe1173.36 and Trp2586.48) during the first 30 ns of the simulation. This movement (of ~2 Å) of CP55,940 towards the two toggle switch residues, indicates the activation of the CB2 receptor because the distance between toggle switch residue Trp2586.48 and CP55,940 (to the terminal carbon of dimethyl heptyl side chain of CP55,940) remained short, ~3.8 Å, throughout the remainder of the MD simulation after t = 40 ns (Fig 13). A modeling study which compared the relative position of the toggle switch residues reported for the CB1 receptor (McAllister et al., 2004; McAllister et al., 2003; Singh et al., 2002), proposed that a similar toggle switch mechanism could exist between Phe1173.36 and Trp2586.48 within the CB2 receptor (Hurst, et al., 2010; Latek et al., 2011). In addition, the reports on the CB1 active-state crystal structure also described the synergistic movement of two residues, Phe2003.36 and Trp3566.48 known as a toggle switch (Hua et al., 2017). No π–π stacking of the side chains of Phe2003.36 and Trp3566.48 is observed in their active-state crystal structures and it is speculated that this twin toggle switch is related to CB1 receptor activation. Similar observations were found in the CB2 receptor where Phe1173.36 and Trp2586.48 can act as a toggle switch. This is in agreement with the previous modeling studies (Hurst, et al., 2010; Latek, et al., 2011). During activation, the π–π stacking of the side chains of Phe1173.36 and Trp2586.48 is disrupted which is a distinguishing feature for the active-state CB2 receptor compared to the inactive-state CB2 receptor. Thirdly, we observed an indirect H-bond interaction mediated by two water molecules in the active site between the phenolic OH of CP55,940 and Ser2857.39 (OH) of the CB2 receptor throughout the 200 ns simulation (Fig 14A). Rhee et al (Rhee, 2002) and Alqarni et al. (Alqarni et al., 2014) reported the importance of Ser2857.39 in their studies. However, Alqarni et al. [59] inaccurately reported the interaction of Ser2857.39 with CP55,940 as a hydrophobic interaction. The distance between Ser2857.39 (OH) and the phenolic OH of CP55,940 was ~4.8 Å during the MD simulation (Fig 14B). Other key residues that showed polar interactions were Glu181 (EC2), Leu182 (EC2), Trp1945.43, and Val2616.51; while Val1133.32, Phe1173.36, Trp1945.43, Phe1975.46, Trp2586.48, Leu2626.52, Met2656.55, and Phe2817.35 showed hydrophobic interactions with CP55,940.
Figure 12.
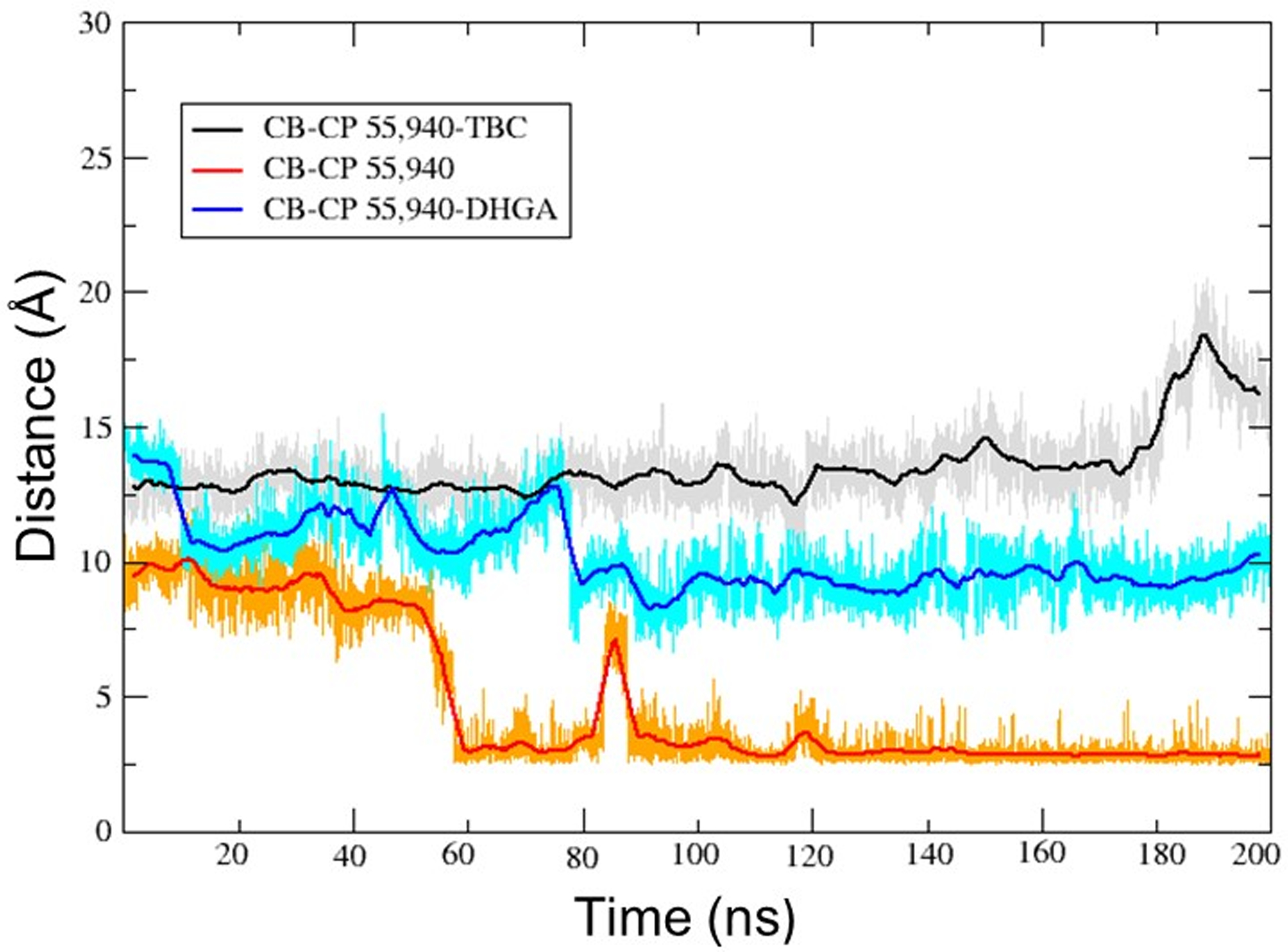
The distance between Trp1724.64 and CP55,940 (southern hydroxyl group) in: CB2–CP55,940–TBC complex (gray line; running average in a window of 200 ps is indicated in black); CB2–CP55,940 complex (gold line; running average in a window of 200 ps is indicated in red); and CB2–CP55,940–DHGA complex (cyan line; running average in a window of 200 ps is indicated in blue).
Figure 13.
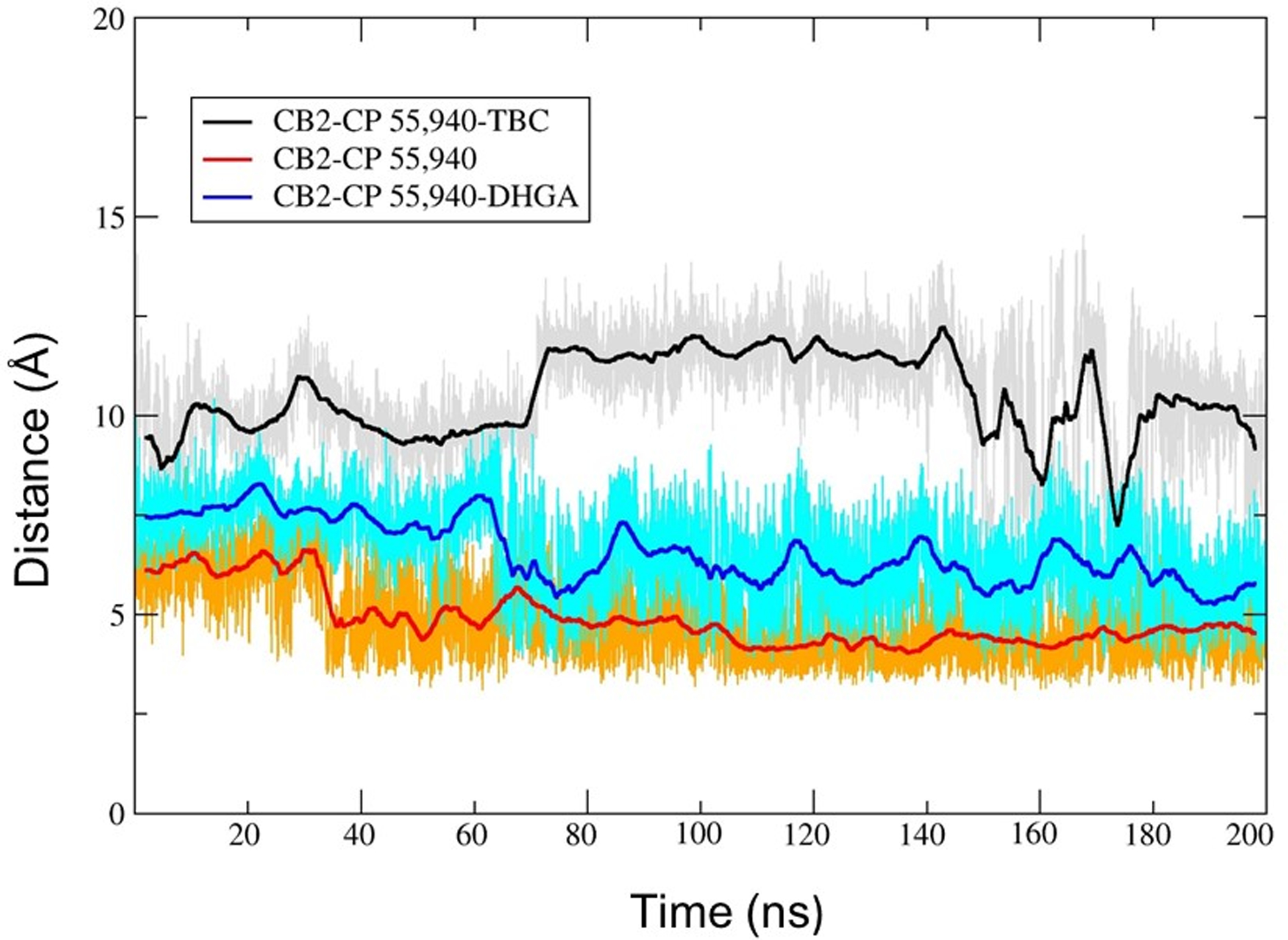
The distance between Trp2586.48 and CP55,940 (terminal carbon of dimethyl heptyl side chain) in: CB2–CP55,940–TBC complex (gray line; running average in a window of 200 ps is indicated in black); CB2–CP55,940 complex (gold line; running average in a window of 200 ps is indicated in red); and CB2–CP55,940–DHGA complex (cyan line; running average in a window of 200 ps is indicated in blue).
Fig 14.
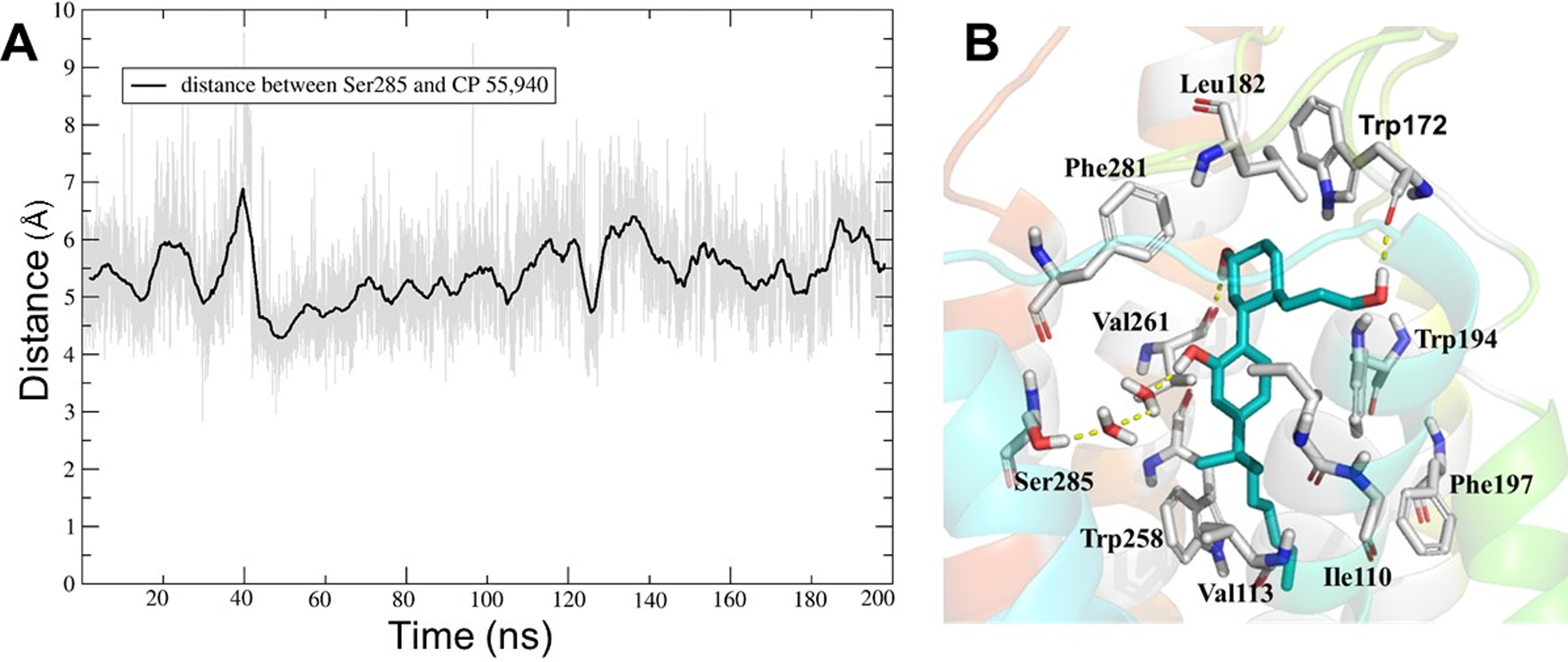
(A) The distance between Ser2857.39 (OH) and CP55,940 (phenolic OH) in the CB2–CP55,940 complex (gray line; running average in a window of 200 ps is indicated in black). (B) The putative binding mode of CP55,940 with the CB2 receptor after MD simulation.
In contrast to the CB2–CP55,940 complex, the above-mentioned major changes did not occur when either of the two AMs (TBC and DHGA) were present at Site 1 of the CB2 receptor. The presence of the AM in the CB2–CP55,940 complexes restricted the degree of flexibility of CP55,940, which is essential for receptor activation. The aliphatic side chain containing the southern hydroxyl group showed very limited flexibility during the 200 ns simulations and did not move close to Trp1724.64, in contrast to what happened for the CB2–CP55,940 complex only. The distance between the southern hydroxyl group and Trp1724.64 during the 200 ns simulation was found to be ~12–14 Å (in the presence of TBC) and ~8–10 Å (in the presence of DHGA) (Fig 12). In the presence of TBC, CP55,940 showed H-bond interactions with Leu182 (EC2), Pro187 (EC2), and Tyr190 (EC2) (Fig 15A), while in the presence of DHGA, CP55,940 interacted with residues such as Glu181 (EC2), Leu182 (EC2), Tyr190 (EC2), Trp1945.43, Glu2767.30, and Lys2787.32 (Fig 15B). Another important feature observed in our simulations was that the presence of the AMs in the bound CB2–CP55,940 complex changed the CB2 conformation in such a way as to alter the toggle switch residues so that they had a strong π-π interaction between Phe1173.36 and Trp2586.48. The distance between the terminal carbon of the dimethyl heptyl side chain of CP55,940 and Trp2586.48 was >~6.5 Å (in the presence of TBC) and >~5 Å (for DHGA) during the 200 ns MD simulations (Fig 13), in contrast to the short <3.0 Å distance found in the simulation carried out without a NAM. Furthermore, we also observed that in the NAM-containing simulations, CP55,940 interacted significantly with the EC2 loop region, TM3, and TM5-TM7, and showed hydrophobic interactions with the toggle switch residues, which allowed the receptor to be in a conformation close to its inactive state.
Figure 15.
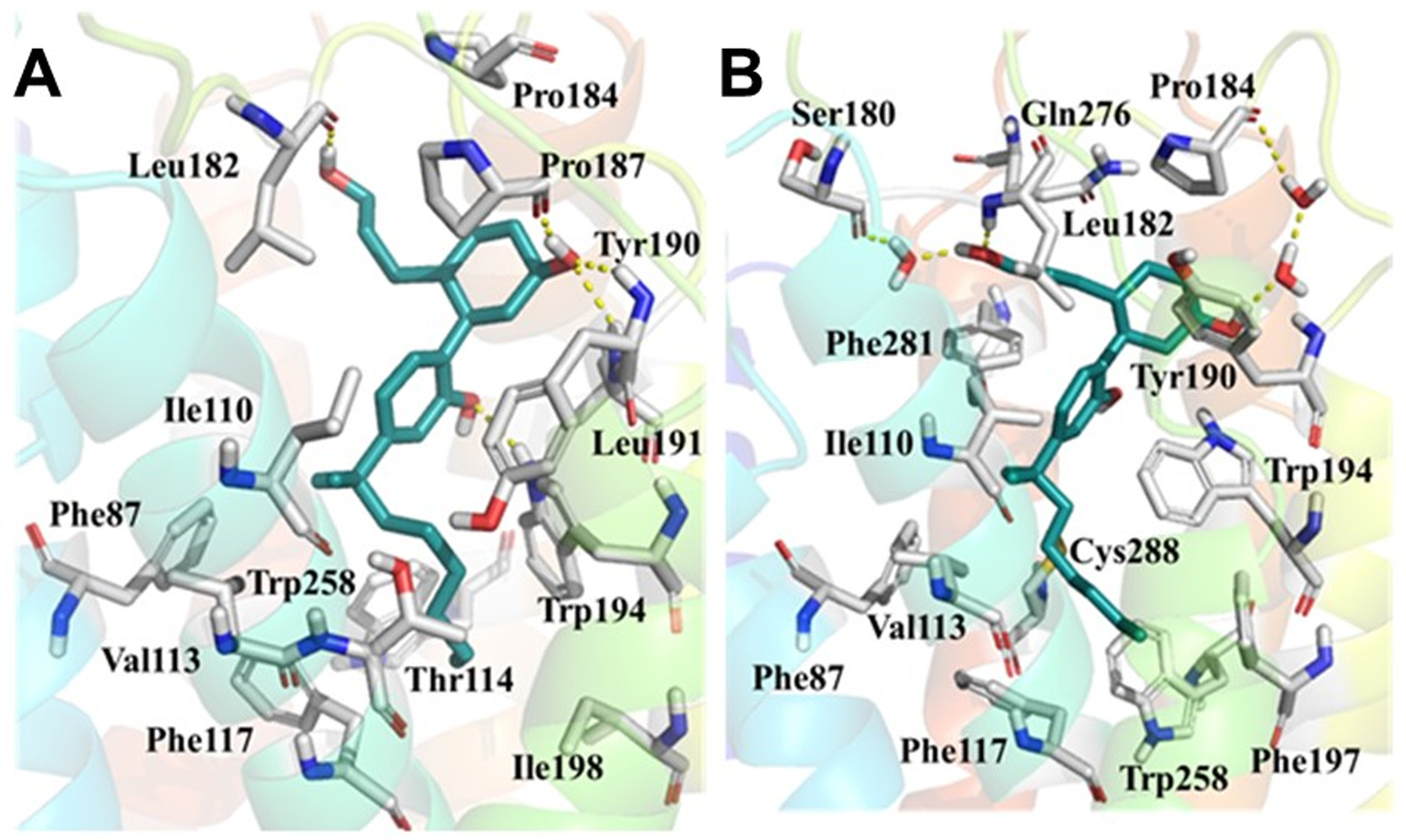
The putative binding mode after MD simulations of: (A) CP55,940 with CB2–TBC complex and (B) CP55,940 with CB2–DHGA complex.
Overall, these results indicated that CP55,940 has a degree of flexibility to interact with the CB2 receptor including different H-bonds and/or hydrophobic interactions. This flexibility of CP55,940 may be key in its capability to allow CB2 activation. However, the presence of a negative AM helps to restrict the flexibility of this CP55,940–CB2 interaction, not allowing the major conformational changes needed in CP55,940 and CB2 receptor for receptor activation.
Conclusions
We have prepared and validated homology models of the CB2 receptor in its active state using a multi-template approach. The best model was used to dock the CB1/2 non-selective agonist CP55,940 into the CB2 receptor. Potential allosteric sites were mapped within the CB2 receptor while CP55,940 was already bound in the orthosteric site. The identified potential allosteric binding sites within CB2 were used to dock the known negative allosteric modulators, TBC and DHGA. The best-optimized CB2–CP55,940–AM complexes were further used as starting points for MD simulations. The representative structures from dPCA analysis were used to calculate binding free energies of CP55,940 with or without the NAMs. Binding of either TBC or DHGA (known NAMs) decreased the absolute value of the binding free energy of CP55,940, and thus demonstrated the appropriateness of the identified allosteric sites for these NAMs. Our dynamics simulations showed that the degree of flexibility of CP55,940 is restricted in the presence of a NAM, which also supports the nature of these AMs as NAMs. It is important to mention that the binding free-energies were obtained from a single trajectory. Running multiple trajectories with different initial velocity seeds would be a good approach to use in future work in order to obtain more reliable binding free-energies. The identified allosteric sites—in particular, Site 1—may be useful in the search for new selective and potent CB2 allosteric modulators using the protein structure-based virtual screening approach. Meanwhile, the identified allosteric site can further be used to guide the design and optimization of already identified or new AMs of CB2. In future, we aim to use these identified allosteric sites for the identification of new selective and potent CB2 allosteric modulators using the protein structure-based virtual screening approach.
Experimental Section
CB2 receptor model building
With notable advancements made in X-ray crystallization techniques as well as structural biology, there has been impressive progress in X-ray structure determination of GPCRs. Today, more than 30 GPCRs have medium to high-resolution crystal structures available with small molecule ligands or modulators. These GPCRs include CB1, rhodopsin, β1- and β2-adrenergic receptors, adenosine A2A receptor, chemokine CXCR4 receptor, dopamine D3 receptor, histamine H1 receptor, sphingosine-1-phosphate receptor, and kappa (κ)-, mu (μ)- and delta (δ)-opioid receptors (Cong, Topin, & Golebiowsk, 2017; Topiol, 2013). These X-ray structures, bound to different classes of modulators including agonists, inverse-agonists, and antagonists, are helpful for understanding some common mechanisms of GPCR activation and inactivation. Meanwhile, continual improvement has been made in protein structure prediction methods for providing good-to-high quality models based on the available X-ray structure templates. These advances have opened up new perspectives for homology modeling of GPCRs whose structures so far have not been solved and for protein structure-based drug discovery.
When we commenced this research, none of the available GPCR structures shared ≥ 30% sequence identity with the human CB2 (hCB2), and in that circumstance use of multiple templates is a wise approach for homology modeling. In order to build an active-state model of hCB2, promising active-state templates were selected: (i) human β2-AR–Gs complex (PDB-ID: 3SN6); (Rasmussen et al., 2011) (ii) nanobody stabilized human β2-AR–agonist complex (PDB-ID: 3P0G); (Rasmussen, et al., 2011) (iii) turkey β1-AR–agonist complex (PDB-ID: 2Y02); (Warne et al., 2011) and (iv) bovine rhodopsin (PDB-ID: 3PQR) (Choe et al., 2011). The full sequence of the human CB2 receptor [Sequence ID - P34972 (CNR2_HUMAN), 360 residues] was downloaded from the UniProt website (www.uniprot.org). Figure 2 shows the multiple sequence alignment of the CB2 receptor and the template proteins (β1-AR, β2-AR, and rhodopsin) used for homology modeling.
Multiple homology models of the hCB2 receptor were generated using the MODELLER (v9.12) program (Sali and Blundell, 1993) (Fig 3A). The first 28 residues at the CB2 N-terminus (Fig 4A) were not included in the protein model because they are thought to be insignificant in ligand binding (Cichero et al., 2011; Kusakabe et al., 2013; Xie, Chen, & Billings, 2003). A reported disulfide bridge (Fay, Dunham, & Farrens, 2005) between second extracellular loop (EC2) residues C174 and C179 was included in the hCB2 models (Fig 4B). During the alignment step, we used conserved motifs (Topiol and Sabio, 2009) present in most of the GPCRs such as the E/DRY motif on the intracellular side of TM3, CWxP in TM6 and NPxxY in TM7, in order to get a reasonable sequence alignment (Fig 2). The 100 CB2 models, which were selected from simulated annealing sampling of the initial structure with constraints, were subjected to preliminary quality assessments such as molpdf (the standard Modeller scoring function, with the optimal value being the lowest positive value) and Discrete Optimized Protein Energy (DOPE) scores (Fig 3B) as well as Ramachandran plot analysis. Model #93, which had the lowest DOPE and molpdf scores (Fig 3B) and had no outliers in the Ramachandran plot (Fig 4C), was used in the rest of this work.
Induced Fit Docking
We began with flexible docking of the widely used non-selective CB agonist CP55,940 into our best optimized CB2 receptor active state model (Fig 4). The molecular model of CP55,940 was generated, checked for any alternate ionization states, and energy minimized in Maestro. To retrieve the optimized binding mode and the docking score, we used the Induced Fit Docking (IFD) algorithm (Sherman, et al., 2006) with standard-precision (SP) docking mode in order to allow optimization of the side chains of amino acids in close proximity to the docked ligand. The docking site was chosen to be the centroid of amino acids Lys1093.28, Ser1123.31, Phe1173.36, Trp1945.43, Trp2586.48, Lys2787.32, and Ser2857.39 of the CB2 model (Hurst, et al., 2010; Y. Zhang, et al., 2011). As part of IFD protocol, the residues having any atoms within 5 Å of the initial ligand pose were automatically refined using the Prime tool. After docking, the binding free energies of the CB2–CP55,940 complexes were calculated using the Prime molecular mechanics generalized Born and surface area (MM-GBSA) solvation module of the Schrödinger software (Sherman, et al., 2006).
Prediction of allosteric binding pocket(s) within CB2 receptor
SiteMap (Halgren, 2009) from the Schrödinger suite was used to detect the most favorable binding pockets available to accommodate the allosteric ligands (e.g., DHGA and TBC) within the agonist CP55,940 bound CB2 receptor model. In principle, this tool identifies sites in a manner analogous to Goodford’s GRID algorithm (Goodford, 1985), using its particular definitions to map regions of hydrophobicity and hydrophilicity, including hydrogen bond acceptor and donor regions.
Conformational search for TBC and DHGA
The CB2 allosteric modulators TBC and DHGA were sketched in Maestro (Suite 2012: Maestro, 2012) and prepared using the LigPrep (Suite 2012: LigPrep, 2012) module of the Schrödinger software. The conformational search of the prepared allosteric modulators was performed using the Macrocycle Conformational Analysis tool (Suite 2012: MacroModel, 2012) of the Schrödinger suite. Redundant conformers were removed using a RMSD cutoff of 1.0 Å.
Docking of DHGA and TBC in the predicted allosteric sites
Grids for docking of DHGA and TBC were generated based on pockets detected using SiteMap. The following residues were considered as active site residues of Site 1: Ser29NTER, Gly30 NTER, Glu32 NTER, Lys331.32, Val361.35, Leu391.38, Cys401.39, Leu431.42, Phe872.57, Ala882.58, Cys892.59, Val922.62, His952.65, Val962.66, Phe1063.25, Ile1103.29, Val1133.32, Thr1143.33, Phe1173.36, Ser180 (EC2), Glu181 (EC2), Leu182 (EC2), Lys2787.32, Lys2797.33, Phe2817.35, Ala2827.36, Phe2837.37, Ser2857.39, Leu2897.43, and Ile2907.44. Meanwhile Thr1183.37, Ala1193.38, Gly1223.41, Leu1253.44, Leu1263.45, Ile1564.48, Met1574.49, Leu1604.52, Ser1614.53, Val1644.56, Leu1965.45, Phe1975.46, and Leu2015.50 were considered as active site residues of Site 3. Rigid docking was performed with standard precision using Glide (Halgren et al., 2004) software.
Molecular dynamics simulation studies
The best docked complexes of TBC and DHGA into the allosteric binding pockets of the CB2–CP55,940 receptor complex were further used to prepare input files using the CHARMM-GUI webserver (Jo, Kim, & Im, 2007) for MD simulation in an explicit hydrated lipid-bilayer environment utilizing the NAMD software (Phillips et al., 2005). The CHARMM parameter and topology files for DHGA and TBC were generated using the Paramchem server (Vanommeslaeghe, Raman, & MacKerell, 2012). Each CB2–CP55,940–AM complex was embedded into a pre-equilibrated POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) lipid-bilayer and solvated with TIP3P water on the extracellular and intracellular sides, and the whole system was neutralized using potassium chloride (KCl) and set to an ionic strength of 0.15 M. The system was subjected to an initial minimization for 10,000 steps (conjugate-gradient minimization) keeping the protein backbone fixed, followed by 10,000 steps of minimization without any constraint (to allow the system to relax freely). Thereafter, six consecutive CHARMM input files were used for the equilibration steps. During the equilibration steps, various restraints were applied to the protein, water, ions, and lipid molecules to assure gradual equilibration of the assembled system as described by Sunhwan, Jo. et al (2007) (Jo, et al., 2007). The equilibration steps included the following constraints: 1) harmonic restraints to ions and heavy atoms of the protein, (2) repulsive planar restraints to prevent water from entering into the membrane hydrophobic region, and (3) planar restraints to hold the positions of head groups of membranes to be along the Z-axis. As the equilibration progressed, these restraint forces were slowly reduced (Jo, et al., 2007). We used the NVT dynamics (constant volume and temperature) for the first and second steps to avoid instability of dynamics integrations during equilibration, and NPAT (constant pressure, area, and temperature) dynamics for the remainder at 303.15 K (POPC). The total time of the six equilibration steps was 600 ps. An NPT simulation was run on the equilibrated system for 10 ns keeping the temperature at 303.15 K and pressure at 1 bar using the Nosé–Hoover Langevin piston-coupling algorithm. An integration time step of 2 fs was set for the simulations, the SHAKE algorithm was used to constrain lengths of all chemical bonds involving hydrogen atoms at their equilibrium values, and the SETTLE algorithm was used to restrain water geometries. Non-bonded van der Waals (vdW) interactions were treated by using a switching function at 10 Å which reached 0 at a distance of 12 Å. The particle-mesh Ewald algorithm was used to handle long-range electrostatic forces. A production run was carried out for 200 ns on each of the equilibrated systems. The MD trajectory processing and analysis were done using VMD software (Humphrey, Dalke, & Schulten, 1996).
Grcarma, a graphical user interface to the Carma program, was also used in the MD trajectory analysis (Koukos and Glykos, 2013). MD snapshots were clustered using dihedral principal component analysis, the top three principal components were used to generate distinct clusters of structures/conformations, and the binding free energy of the most-representative structure of each cluster (the natural structure with the smallest deviation from the average structure) was calculated.
Supplementary Material
Acknowledgement
This study was supported by an Institutional Development Award (IDeA) Grant Number P20GM104932 and by R15GM119061 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. This study was also supported by a National Science Foundation (NSF) Major Research Infrastructure Grant entitled “MRI: Acquisition of a GPU Cluster for Computational Science in Mississippi”, Award #CHE-1338056, and an Extreme Science and Engineering Discovery Environment (XSEDE) grant of supercomputer time, MCB140248. It was conducted in part in a facility constructed with support from the Research Facilities Improvements Program (C06 RR-14503) from NIH National Center for Research Resources.
Abbreviations Used
- AMs
Allosteric modulators
- CB1
Cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- DHGA
Dihydro-gambogic acid
- DOPE
Discrete Optimized Protein Energy
- dPCA
Dihedral principal component analysis
- EC
Extracellular or extracellular loop
- GPCR
G-protein-coupled receptor
- IC
Intracellular or intracellular loop
- IFD
Induced Fit Docking
- MD
Molecular dynamics
- MM-GBSA
Molecular Mechanics/Generalized Born Surface Area
- NAM
negative allosteric modulator
- NAMD
Nanoscale molecular dynamics
- OPLS2005
Optimized potentials for liquid simulations 2005 force field
- PDB
Protein data bank
- POPC
1-palmitoyl-2-oleoylphosphatidylcholine
- PPW
Protein Preparation Wizard
- SP
Standard precision
- TBC
Trans β-caryophyllene
- VMD
Visual molecular dynamics
- XP
Extra precision
Footnotes
Supporting Information
Models of the protein-ligand interaction poses.
Homology Models
Models have been deposited in the Protein Model DataBase and are available for download from https://bioinformatics.cineca.it/PMDB/main.php. Authors will release the atomic coordinates upon article publication.
Competing interests
The authors declare that they have no competing interests.
References
- Abood ME (2016). Allosteric Modulators: A Side Door. J Med Chem, 59(1), pp. 42–43. doi:10.1021/acs.jmedchem.5b01824 Retrieved from 10.1021/acs.jmedchem.5b01824https://www.ncbi.nlm.nih.gov/pubmed/26645411 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26645411 [DOI] [PubMed] [Google Scholar]
- Alaverdashvili M, & Laprairie RB (2018). The future of type 1 cannabinoid receptor allosteric ligands. [Review]. Drug Metabolism Reviews, 50(1), pp. 14–25. doi:10.1080/03602532.2018.1428341 Retrieved from 10.1080/03602532.2018.1428341https://www.scopus.com/inward/record.uri?eid=2-s2.0-85043599300&doi=10.1080%2f03602532.2018.1428341&partnerID=40&md5=be3e00d51ce447166f488e2e7df031fe Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85043599300&doi=10.1080%2f03602532.2018.1428341&partnerID=40&md5=be3e00d51ce447166f488e2e7df031fe [DOI] [PubMed] [Google Scholar]
- Alqarni M, Myint KZ, Tong Q, Yang P, Bartlow P, Wang L, … Xie XQ (2014). Examining the critical roles of human CB2 receptor residues Valine 3.32 (113) and Leucine 5.41 (192) in ligand recognition and downstream signaling activities. Biochem Biophys Res Commun, 452(3), pp. 334–339. doi:10.1016/j.bbrc.2014.08.048 Retrieved from 10.1016/j.bbrc.2014.08.048http://www.ncbi.nlm.nih.gov/pubmed/25148941 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25148941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes WA, Lucarini R, Tozatti MG, Flauzino LG, Souza MG, Turatti IC, … Cunha WR (2010). Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Z Naturforsch C, 65(9–10), pp. 588–593. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21138060 [DOI] [PubMed] [Google Scholar]
- Cascao R, Vidal B, Raquel H, Neves-Costa A, Figueiredo N, Gupta V, … Moita LF (2014). Potent anti-inflammatory and antiproliferative effects of gambogic acid in a rat model of antigen-induced arthritis. Mediators Inflamm, 2014, p 195327. doi:10.1155/2014/195327 Retrieved from 10.1155/2014/195327http://www.ncbi.nlm.nih.gov/pubmed/24623960 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24623960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, … Ernst OP (2011). Crystal structure of metarhodopsin II. Nature, 471(7340), pp. 651–655. doi:10.1038/nature09789 Retrieved from 10.1038/nature09789http://www.ncbi.nlm.nih.gov/pubmed/21389988 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21389988 [DOI] [PubMed] [Google Scholar]
- Christopoulos A (2002). Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov, 1(3), pp. 198–210. doi:10.1038/nrd746 Retrieved from 10.1038/nrd746http://www.ncbi.nlm.nih.gov/pubmed/12120504 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12120504 [DOI] [PubMed] [Google Scholar]
- Cichero E, Ligresti A, Allara M, di Marzo V, Lazzati Z, D’Ursi P, … Fossa P (2011). Homology modeling in tandem with 3D-QSAR analyses: a computational approach to depict the agonist binding site of the human CB2 receptor. Eur J Med Chem, 46(9), pp. 4489–4505. doi:10.1016/j.ejmech.2011.07.023 Retrieved from 10.1016/j.ejmech.2011.07.023https://www.ncbi.nlm.nih.gov/pubmed/21835510 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21835510 [DOI] [PubMed] [Google Scholar]
- Cong X, Topin J, & Golebiowsk J (2017). Class a GPCRs: Structure, function, modeling and structure-based ligand design. [Review]. Current Pharmaceutical Design, 23(29), pp. 4390–4409. doi:10.2174/1381612823666170710151255 Retrieved from 10.2174/1381612823666170710151255https://www.scopus.com/inward/record.uri?eid=2-s2.0-85038830749&doi=10.2174%2f1381612823666170710151255&partnerID=40&md5=8a58e5ed5348e9eb7fc8383251aaa30d Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85038830749&doi=10.2174%2f1381612823666170710151255&partnerID=40&md5=8a58e5ed5348e9eb7fc8383251aaa30d [DOI] [PubMed] [Google Scholar]
- Davenport J, Manjarrez JR, Peterson L, Krumm B, Blagg BS, & Matts RL (2011). Gambogic acid, a natural product inhibitor of Hsp90. J Nat Prod, 74(5), pp. 1085–1092. doi:10.1021/np200029q Retrieved from 10.1021/np200029qhttp://www.ncbi.nlm.nih.gov/pubmed/21486005 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21486005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay JF, Dunham TD, & Farrens DL (2005). Cysteine residues in the human cannabinoid receptor: only C257 and C264 are required for a functional receptor, and steric bulk at C386 impairs antagonist SR141716A binding. Biochemistry, 44(24), pp. 8757–8769. doi:10.1021/bi0472651 Retrieved from 10.1021/bi0472651http://www.ncbi.nlm.nih.gov/pubmed/15952782 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15952782 [DOI] [PubMed] [Google Scholar]
- Foster DJ, & Conn PJ (2017). Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron, 94(3), pp. 431–446. doi:10.1016/j.neuron.2017.03.016 Retrieved from 10.1016/j.neuron.2017.03.016https://www.ncbi.nlm.nih.gov/pubmed/28472649 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28472649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gado F, Di Cesare Mannelli L, Lucarini E, Bertini S, Cappelli E, Digiacomo M, … Manera C (2018). Identification of the First Synthetic Allosteric Modulator of the CB2 Receptors and Evidence of Its Efficacy for Neuropathic Pain Relief. J Med Chem doi :10.1021/acs.jmedchem.8b00368 Retrieved from 10.1021/acs.jmedchem.8b00368http://www.ncbi.nlm.nih.gov/pubmed/29990428 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29990428 [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, … Casellas P (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem, 232(1), pp. 54–61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7556170 [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, … Zimmer A (2008). Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A, 105(26), pp. 9099–9104. doi:10.1073/pnas.0803601105 Retrieved from 10.1073/pnas.0803601105http://www.ncbi.nlm.nih.gov/pubmed/18574142 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18574142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Di Cesare Mannelli L, Mazzanti G, & Bartolini A (2001). Local anaesthetic activity of beta-caryophyllene. Farmaco, 56(5–7), pp. 387–389. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11482764 [DOI] [PubMed] [Google Scholar]
- Goodford PJ (1985). A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. [Article]. J Med Chem, 28(7), pp. 849–857. Retrieved from https://www.scopus.com/inward/record.url?eid=2-s2.0-0021871375&partnerID=40&md5=8e91ea81fbadb3f0da262be01db4f38d [DOI] [PubMed] [Google Scholar]
- Halgren TA (2009). Identifying and characterizing binding sites and assessing druggability. J Chem Inf Model, 49(2), pp. 377–389. doi:10.1021/ci800324m Retrieved from 10.1021/ci800324mhttp://www.ncbi.nlm.nih.gov/pubmed/19434839 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19434839 [DOI] [PubMed] [Google Scholar]
- Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, & Banks JL (2004). Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem, 47(7), pp. 1750–1759. doi:10.1021/jm030644s Retrieved from 10.1021/jm030644shttp://www.ncbi.nlm.nih.gov/pubmed/15027866 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15027866 [DOI] [PubMed] [Google Scholar]
- Han S, Thatte J, Buzard DJ, & Jones RM (2013). Therapeutic utility of cannabinoid receptor type 2 (CB(2)) selective agonists. J Med Chem, 56(21), pp. 8224–8256. doi:10.1021/jm4005626 Retrieved from 10.1021/jm4005626http://www.ncbi.nlm.nih.gov/pubmed/23865723 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23865723 [DOI] [PubMed] [Google Scholar]
- Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, … Wong Kai In P (2007). PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol, 152(5), pp. 805–814. doi:10.1038/sj.bjp.0707347 Retrieved from 10.1038/sj.bjp.0707347http://www.ncbi.nlm.nih.gov/pubmed/17592509 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17592509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B, Mukhopadhyay P, Kechrid M, Patel V, Tanchian G, Wink DA, … Pacher P (2012). beta-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic Biol Med, 52(8), pp. 1325–1333. doi:10.1016/j.freeradbiomed.2012.01.014 Retrieved from 10.1016/j.freeradbiomed.2012.01.014http://www.ncbi.nlm.nih.gov/pubmed/22326488 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22326488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, … Liu ZJ (2017). Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature, 547(7664), pp. 468–471. doi:10.1038/nature23272 Retrieved from 10.1038/nature23272http://www.ncbi.nlm.nih.gov/pubmed/28678776 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28678776 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hudaib M, Speroni E, Di Pietra AM, & Cavrini V (2002). GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J Pharm Biomed Anal, 29(4), pp. 691–700. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12093498 [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, & Schulten K (1996). VMD: visual molecular dynamics. J Mol Graph, 14(1), pp. 33–38, 27–38. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8744570 [DOI] [PubMed] [Google Scholar]
- Hurst DP, Grossfield A, Lynch DL, Feller S, Romo TD, Gawrisch K, … Reggio PH (2010). A lipid pathway for ligand binding is necessary for a cannabinoid G protein-coupled receptor. J Biol Chem, 285(23), pp. 17954–17964. doi:10.1074/jbc.M109.041590 Retrieved from 10.1074/jbc.M109.041590http://www.ncbi.nlm.nih.gov/pubmed/20220143 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20220143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AI (2010). Cannabinoid receptors as target for treatment of osteoporosis: a tale of two therapies. Curr Neuropharmacol, 8(3), pp. 243–253. doi:10.2174/157015910792246173 Retrieved from 10.2174/157015910792246173http://www.ncbi.nlm.nih.gov/pubmed/21358974 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21358974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AI, Sophocleous A, Landao-Bassonga E, van’t Hof RJ, & Ralston SH (2008). Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology, 149(11), pp. 5619–5626. doi:10.1210/en.2008-0150 Retrieved from 10.1210/en.2008-0150http://www.ncbi.nlm.nih.gov/pubmed/18635663 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18635663 [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, … Ross RA (2015). A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology, 40(13), pp. 2948–2959. doi:10.1038/npp.2015.148 Retrieved from 10.1038/npp.2015.148http://www.ncbi.nlm.nih.gov/pubmed/26052038 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26052038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasha GK, Jagan Mohan Rao L, & Sakariah KK (2003). Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem, 51(15), pp. 4344–4348. doi:10.1021/jf034169i Retrieved from 10.1021/jf034169ihttp://www.ncbi.nlm.nih.gov/pubmed/12848508 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12848508 [DOI] [PubMed] [Google Scholar]
- Jo S, Kim T, & Im W (2007). Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS One, 2(9), p e880. doi:10.1371/journal.pone.0000880 Retrieved from 10.1371/journal.pone.0000880http://www.ncbi.nlm.nih.gov/pubmed/17849009 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17849009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana L, Fu BQ, Duddupudi AL, Liao YH, Immadi SS, Kendall DA, & Lu D (2017). Pyrimidinyl Biphenylureas: Identification of New Lead Compounds as Allosteric Modulators of the Cannabinoid Receptor CB1. J Med Chem, 60(3), pp. 1089–1104. doi:10.1021/acs.jmedchem.6b01448 Retrieved from 10.1021/acs.jmedchem.6b01448https://www.ncbi.nlm.nih.gov/pubmed/28059509 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28059509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauke AL, Racz I, Pradier B, Markert A, Zimmer AM, Gertsch J, & Zimmer A (2014). The cannabinoid CB(2) receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol, 24(4), pp. 608–620. doi:10.1016/j.euroneuro.2013.10.008 Retrieved from 10.1016/j.euroneuro.2013.10.008http://www.ncbi.nlm.nih.gov/pubmed/24210682 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24210682 [DOI] [PubMed] [Google Scholar]
- Koukos PI, & Glykos NM (2013). Grcarma: A fully automated task-oriented interface for the analysis of molecular dynamics trajectories. J Comput Chem, 34(26), pp. 2310–2312. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24159629 [DOI] [PubMed] [Google Scholar]
- Kusakabe K, Tada Y, Iso Y, Sakagami M, Morioka Y, Chomei N, … Hanasaki K (2013). Design, synthesis, and binding mode prediction of 2-pyridone-based selective CB2 receptor agonists. Bioorg Med Chem, 21(7), pp. 2045–2055. doi:10.1016/j.bmc.2013.01.006 Retrieved from 10.1016/j.bmc.2013.01.006https://www.ncbi.nlm.nih.gov/pubmed/23395112 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23395112 [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, & Denovan-Wright EM (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol, 172(20), pp. 4790–4805. doi:10.1111/bph.13250 Retrieved from 10.1111/bph.13250http://www.ncbi.nlm.nih.gov/pubmed/26218440 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26218440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Kulkarni AR, Kulkarni PM, Hurst DP, Lynch D, Reggio PH, … Thakur GA (2016). Mapping Cannabinoid 1 Receptor Allosteric Site(s): Critical Molecular Determinant and Signaling Profile of GAT100, a Novel, Potent, and Irreversibly Binding Probe. [Article]. ACS Chemical Neuroscience, 7(6), pp. 776–798. doi:10.1021/acschemneuro.6b00041 Retrieved from 10.1021/acschemneuro.6b00041https://www.scopus.com/inward/record.uri?eid=2-s2.0-84975034058&doi=10.1021%2facschemneuro.6b00041&partnerID=40&md5=96dad4add6cffb31a04a1bd04757b56e Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-84975034058&doi=10.1021%2facschemneuro.6b00041&partnerID=40&md5=96dad4add6cffb31a04a1bd04757b56e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Kulkarni PM, Deschamps JR, Kelly MEM, Janero DR, Cascio MG, … Thakur GA (2017). Enantiospecific Allosteric Modulation of Cannabinoid 1 Receptor. ACS Chemical Neuroscience, 8(6), pp. 1188–1203. doi:10.1021/acschemneuro.6b00310 Retrieved from 10.1021/acschemneuro.6b00310http://www.ncbi.nlm.nih.gov/pubmed/28103441 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28103441 [DOI] [PubMed] [Google Scholar]
- Latek D, Kolinski M, Ghoshdastider U, Debinski A, Bombolewski R, Plazinska A, … Filipek S (2011). Modeling of ligand binding to G protein coupled receptors: cannabinoid CB1, CB2 and adrenergic beta 2 AR. J Mol Model, 17(9), pp. 2353–2366. doi:10.1007/s00894-011-0986-7 Retrieved from 10.1007/s00894-011-0986-7http://www.ncbi.nlm.nih.gov/pubmed/21365223 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21365223 [DOI] [PubMed] [Google Scholar]
- Maheswari R (2011). Characterization of allosteric modulators of CB2 receptors as novel therapeutics for inflammatory diseases. University of Arkansas for Medical Sciences, p 198. [Google Scholar]
- Marino S, & Idris AI (2017). Emerging therapeutic targets in cancer induced bone disease: A focus on the peripheral type 2 cannabinoid receptor. [Review]. Pharmacological Research, 119, pp. 391–403. doi:10.1016/j.phrs.2017.02.023 Retrieved from 10.1016/j.phrs.2017.02.023https://www.scopus.com/inward/record.uri?eid=2-s2.0-85015013920&doi=10.1016%2fj.phrs.2017.02.023&partnerID=40&md5=eed20ea1ec9dea242fb6d4dfd44f806a Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85015013920&doi=10.1016%2fj.phrs.2017.02.023&partnerID=40&md5=eed20ea1ec9dea242fb6d4dfd44f806a [DOI] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, … Franco R (2017). Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. [Article]. Frontiers in Pharmacology, 8(OCT)doi:10.3389/fphar.2017.00744 Retrieved from 10.3389/fphar.2017.00744https://www.scopus.com/inward/record.uri?eid=2-s2.0-85032187574&doi=10.3389%2ffphar.2017.00744&partnerID=40&md5=941f1b4029347ca2664eaec8922c1a09 Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85032187574&doi=10.3389%2ffphar.2017.00744&partnerID=40&md5=941f1b4029347ca2664eaec8922c1a09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, & Christopoulos A (2007). Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol, 47, pp. 1–51. doi:10.1146/annurev.pharmtox.47.120505.105159 Retrieved from 10.1146/annurev.pharmtox.47.120505.105159http://www.ncbi.nlm.nih.gov/pubmed/17009927 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17009927 [DOI] [PubMed] [Google Scholar]
- McAllister SD, Hurst DP, Barnett-Norris J, Lynch D, Reggio PH, & Abood ME (2004). Structural mimicry in class A G protein-coupled receptor rotamer toggle switches: the importance of the F3.36(201)/W6.48(357) interaction in cannabinoid CB1 receptor activation. J Biol Chem, 279(46), pp. 48024–48037. doi:10.1074/jbc.M406648200 Retrieved from 10.1074/jbc.M406648200http://www.ncbi.nlm.nih.gov/pubmed/15326174 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15326174 [DOI] [PubMed] [Google Scholar]
- McAllister SD, Rizvi G, Anavi-Goffer S, Hurst DP, Barnett-Norris J, Lynch DL, … Abood ME (2003). An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J Med Chem, 46(24), pp. 5139–5152. doi:10.1021/jm0302647 Retrieved from 10.1021/jm0302647http://www.ncbi.nlm.nih.gov/pubmed/14613317 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14613317 [DOI] [PubMed] [Google Scholar]
- Mockute D, Bernotiene G, & Judzentiene A (2001). The essential oil of Origanum vulgare L. ssp. vulgare growing wild in vilnius district (Lithuania). Phytochemistry, 57(1), pp. 65–69. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11336262 [DOI] [PubMed] [Google Scholar]
- Navarro HA, Howard JL, Pollard GT, & Carroll FI (2009). Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol, 156(7), pp. 1178–1184. doi:10.1111/j.1476-5381.2009.00124.x Retrieved from 10.1111/j.1476-5381.2009.00124.xhttp://www.ncbi.nlm.nih.gov/pubmed/19226282 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19226282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, German N, Decker AM, Langston TL, Gamage TF, Farquhar CE, … Zhang Y (2017). Novel Diarylurea Based Allosteric Modulators of the Cannabinoid CB1 Receptor: Evaluation of Importance of 6-Pyrrolidinylpyridinyl Substitution. J Med Chem, 60(17), pp. 7410–7424. doi:10.1021/acs.jmedchem.7b00707 Retrieved from 10.1021/acs.jmedchem.7b00707https://www.ncbi.nlm.nih.gov/pubmed/28792219 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28792219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orav A, Stulova I, Kailas T, & Muurisepp M (2004). Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J Agric Food Chem, 52(9), pp. 2582–2586. doi:10.1021/jf030635s Retrieved from 10.1021/jf030635shttp://www.ncbi.nlm.nih.gov/pubmed/15113161 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15113161 [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O Jr., Duarte FS, Bento AF, Forner S, … Takahashi RN (2012). Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A, 109(51), pp. 21134–21139. doi:10.1073/pnas.1202906109 Retrieved from 10.1073/pnas.1202906109http://www.ncbi.nlm.nih.gov/pubmed/23150578 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23150578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci V, Chicca A, Glasmacher S, Paloczi J, Cao Z, Pacher P, & Gertsch J (2017). Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage. Sci Rep, 7(1), p 9560. doi:10.1038/s41598-017-09808-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2884261910.1038/s41598-017-09808-8https://www.ncbi.nlm.nih.gov/pubmed/28842619https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573408/pdf/41598_2017_Article_9808.pdf Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28842619 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573408/pdf/41598_2017_Article_9808.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, … Schulten K (2005). Scalable molecular dynamics with NAMD. J Comput Chem, 26(16), pp. 1781–1802. doi:10.1002/jcc.20289 Retrieved from 10.1002/jcc.20289http://www.ncbi.nlm.nih.gov/pubmed/16222654 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16222654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley C, Abidi A, Suryawanshi S, Mustafa S, Meibohm B, & Moore BM (2015). Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist. Pharmacol Res Perspect, 3(4), p e00159. doi:10.1002/prp2.159 Retrieved from 10.1002/prp2.159http://www.ncbi.nlm.nih.gov/pubmed/26196013 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26196013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressly JD, Mustafa SM, Adibi AH, Alghamdi S, Pandey P, Roy KK, … Park F (2018). Selective Cannabinoid 2 Receptor Stimulation Reduces Tubular Epithelial Cell Damage after Renal Ischemia-Reperfusion Injury. J Pharmacol Exp Ther, 364(2), pp. 287–299. doi:10.1124/jpet.117.245522 Retrieved from 10.1124/jpet.117.245522https://www.ncbi.nlm.nih.gov/pubmed/29187590 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29187590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, … Ross RA (2005). Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol, 68(5), pp. 1484–1495. doi:10.1124/mol.105.016162 Retrieved from 10.1124/mol.105.016162http://www.ncbi.nlm.nih.gov/pubmed/16113085 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16113085 [DOI] [PubMed] [Google Scholar]
- Priestley RS, Nickolls SA, Alexander SP, & Kendall DA (2015). A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB J, 29(4), pp. 1446–1455. doi:10.1096/fj.14-263053 Retrieved from 10.1096/fj.14-263053http://www.ncbi.nlm.nih.gov/pubmed/25550466 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25550466 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, … Kobilka BK (2011). Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature, 469(7329), pp. 175–180. doi:10.1038/nature09648 Retrieved from 10.1038/nature09648http://www.ncbi.nlm.nih.gov/pubmed/21228869 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21228869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee MH (2002). Functional role of serine residues of transmembrane dopamin VII in signal transduction of CB2 cannabinoid receptor. J Vet Sci, 3(3), pp. 185–191. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12514330 [PubMed] [Google Scholar]
- Rhee MH, Nevo I, Bayewitch ML, Zagoory O, & Vogel Z (2000). Functional role of tryptophan residues in the fourth transmembrane domain of the CB(2) cannabinoid receptor. J Neurochem, 75(6), pp. 2485–2491. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11080201 [DOI] [PubMed] [Google Scholar]
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, & Nagarkatti P (2010). Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology, 215(8), pp. 598–605. doi:10.1016/j.imbio.2009.04.001 Retrieved from 10.1016/j.imbio.2009.04.001http://www.ncbi.nlm.nih.gov/pubmed/19457575 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19457575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh N, Hucke O, Kramer G, Schmidt E, Montel F, Lipinski R, … Tautermann CS (2018). Multiple Binding Sites Contribute to the Mechanism of Mixed Agonistic and Positive Allosteric Modulators of the Cannabinoid CB1 Receptor. [Article]. Angewandte Chemie - International Edition, 57(10), pp. 2580–2585. doi:10.1002/anie.201708764 Retrieved from 10.1002/anie.201708764https://www.scopus.com/inward/record.uri?eid=2-s2.0-85041234548&doi=10.1002%2fanie.201708764&partnerID=40&md5=13c63eb5a3ffd25c0b0af6f0fd592be5 Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85041234548&doi=10.1002%2fanie.201708764&partnerID=40&md5=13c63eb5a3ffd25c0b0af6f0fd592be5 [DOI] [PubMed] [Google Scholar]
- Sali A, & Blundell TL (1993). Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol, 234(3), pp. 779–815. doi:10.1006/jmbi.1993.1626 Retrieved from 10.1006/jmbi.1993.1626http://www.ncbi.nlm.nih.gov/pubmed/8254673 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8254673 [DOI] [PubMed] [Google Scholar]
- Shang Y, & Tang Y (2017). The central cannabinoid receptor type-2 (CB2) and chronic pain. [Review]. International Journal of Neuroscience, 127(9), pp. 812–823. doi:10.1080/00207454.2016.1257992 Retrieved from 10.1080/00207454.2016.1257992https://www.scopus.com/inward/record.uri?eid=2-s2.0-85021710781&doi=10.1080%2f00207454.2016.1257992&partnerID=40&md5=8c5e4cacdc2ae0c346cc0538ee9cf6f3 Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85021710781&doi=10.1080%2f00207454.2016.1257992&partnerID=40&md5=8c5e4cacdc2ae0c346cc0538ee9cf6f3 [DOI] [PubMed] [Google Scholar]
- Sherman W, Day T, Jacobson MP, Friesner RA, & Farid R (2006). Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem, 49(2), pp. 534–553. doi:10.1021/jm050540c Retrieved from 10.1021/jm050540chttp://www.ncbi.nlm.nih.gov/pubmed/16420040 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16420040 [DOI] [PubMed] [Google Scholar]
- Singh R, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, & Guarnieri F (2002). Activation of the cannabinoid CB1 receptor may involve a W6 48/F3 36 rotamer toggle switch. J Pept Res, 60(6), pp. 357–370. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12464114 [DOI] [PubMed] [Google Scholar]
- Smith PF (2002). Cannabinoids in the treatment of pain and spasticity in multiple sclerosis. Curr Opin Investig Drugs, 3(6), pp. 859–864. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12137404 [PubMed] [Google Scholar]
- Suite 2012: LigPrep, v., Schrödinger, LLC, New York, NY,. (2012). [Google Scholar]
- Suite 2012: MacroModel, v., Schrödinger, LLC, New York, NY, . (2012). [Google Scholar]
- Suite 2012: Maestro, v., Schrödinger, LLC, New York, NY, . (2012). [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, & Laprairie RB (2018). Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol doi:10.1111/bph.14440 Retrieved from 10.1111/bph.14440http://www.ncbi.nlm.nih.gov/pubmed/29981240 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29981240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiol S (2013). X-ray structural information of GPCRs in drug design: What are the limitations and where do we go? [Review]. Expert Opinion on Drug Discovery, 8(6), pp. 607–620. doi:10.1517/17460441.2013.783815 Retrieved from 10.1517/17460441.2013.783815https://www.scopus.com/inward/record.uri?eid=2-s2.0-84877964373&doi=10.1517%2f17460441.2013.783815&partnerID=40&md5=20c8587b5cd1ac8ceebe9484844dd807 Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-84877964373&doi=10.1517%2f17460441.2013.783815&partnerID=40&md5=20c8587b5cd1ac8ceebe9484844dd807 [DOI] [PubMed] [Google Scholar]
- Topiol S, & Sabio M (2009). X-ray structure breakthroughs in the GPCR transmembrane region. [Review]. Biochem Pharmacol, 78(1), pp. 11–20. doi:10.1016/j.bcp.2009.02.012 Retrieved from 10.1016/j.bcp.2009.02.012http://www.ncbi.nlm.nih.gov/pubmed/19447219 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19447219 [DOI] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, … Piazza PV (2014). Pregnenolone can protect the brain from cannabis intoxication. Science, 343(6166), pp. 94–98. doi:10.1126/science.1243985 Retrieved from 10.1126/science.1243985http://www.ncbi.nlm.nih.gov/pubmed/24385629 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24385629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, … Sharkey KA (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310(5746), pp. 329–332. doi:10.1126/science.1115740 Retrieved from 10.1126/science.1115740http://www.ncbi.nlm.nih.gov/pubmed/16224028 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16224028 [DOI] [PubMed] [Google Scholar]
- Vanommeslaeghe K, Raman EP, & MacKerell AD Jr. (2012). Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J Chem Inf Model, 52(12), pp. 3155–3168. doi:10.1021/ci3003649 Retrieved from 10.1021/ci3003649http://www.ncbi.nlm.nih.gov/pubmed/23145473 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23145473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, … Tate CG (2011). The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature, 469(7329), pp. 241–244. doi:10.1038/nature09746 Retrieved from 10.1038/nature09746http://www.ncbi.nlm.nih.gov/pubmed/21228877 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21228877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XQ, Chen JZ, & Billings EM (2003). 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins, 53(2), pp. 307–319. doi:10.1002/prot.10511 Retrieved from 10.1002/prot.10511https://www.ncbi.nlm.nih.gov/pubmed/14517981 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14517981 [DOI] [PubMed] [Google Scholar]
- Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, … Forrester JV (2007). Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J Leukoc Biol, 82(3), pp. 532–541. doi:10.1189/jlb.0307159 Retrieved from 10.1189/jlb.0307159http://www.ncbi.nlm.nih.gov/pubmed/17537989 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17537989 [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, … Cai SX (2004). Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem, 12(2), pp. 309–317. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14723951 [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, & Tuma RF (2007). Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab, 27(7), pp. 1387–1396. doi:10.1038/sj.jcbfm.9600447 Retrieved from 10.1038/sj.jcbfm.9600447http://www.ncbi.nlm.nih.gov/pubmed/17245417 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17245417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie Z, Wang L, Schreiter B, Lazo JS, Gertsch J, & Xie XQ (2011). Mutagenesis and computer modeling studies of a GPCR conserved residue W5.43(194) in ligand recognition and signal transduction for CB2 receptor. Int Immunopharmacol, 11(9), pp. 1303–1310. doi:10.1016/j.intimp.2011.04.013 Retrieved from 10.1016/j.intimp.2011.04.013http://www.ncbi.nlm.nih.gov/pubmed/21539938 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21539938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhen C, Wu Z, Hu R, Zhou C, & Guo Q (2010). General pharmacological properties, developmental toxicity, and analgesic activity of gambogic acid, a novel natural anticancer agent. Drug Chem Toxicol, 33(1), pp. 88–96. doi:10.3109/01480540903173534 Retrieved from 10.3109/01480540903173534http://www.ncbi.nlm.nih.gov/pubmed/20001662 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20001662 [DOI] [PubMed] [Google Scholar]
- Zheng GQ, Kenney PM, & Lam LK (1992). Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J Nat Prod, 55(7), pp. 999–1003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1402962 [DOI] [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, & Dubinett SM (2000). Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol, 165(1), pp. 373–380. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10861074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


