Abstract
Gene therapy is rapidly emerging as a powerful therapeutic strategy for a wide range of neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD). Some early clinical trials have failed to achieve satisfactory therapeutic effects. Efforts to enhance effectiveness are now concentrating on three major fields: identification of new vectors, novel therapeutic targets, and reliable of delivery routes for transgenes. These approaches are being assessed closely in preclinical and clinical trials, which may ultimately provide powerful treatments for patients. Here, we discuss advances and challenges of gene therapy for neurodegenerative disorders, highlighting promising technologies, targets, and future prospects.
Key words: Gene therapy, Neurodegenerative disorders, Adeno-associated viruses, Central nervous system, Delivery routes
Abbreviations: AADC, aromatic-l-amino-acid; AAVs, adeno-associated viruses; AD, Alzheimer's disease; Adv, adenovirus; ARSA, arylsulfatase A; ASOs, antisense oligonucleotides; ASPA, aspartoacylase; BBB, blood–brain barrier; BCSFB, blood–cerebrospinal fluid barrier; Bip, glucose regulated protein 78; BRB, blood–retina barrier; CHOP, CCAAT/enhancer binding homologous protein; CLN6, ceroidlipofuscinosis neuronal protein 6; CNS, central nervous system; CSF, cerebrospinal fluid; ER, endoplasmic reticulum; FDA, U.S. Food and Drug Administration; GAA, lysosomal acid α-glucosidase; GAD, glutamic acid decarboxylase; GDNF, glial derived neurotrophic factor; HD, Huntington's disease; HSPGs, heparin sulfate proteoglycans; HTT, mutant huntingtin; IDS, iduronate 2-sulfatase; Lamp2a, lysosomal-associated membrane protein 2a; LVs, retrovirus/lentivirus; mTOR, mammalian target of rapamycin; NGF, nerve growth factor; PD, Parkinson's disease; PGRN, Progranulin; PINK1, putative kinase 1; PTEN, phosphatase and tensin homolog; RGCs, retinal ganglion cells; RNAi, RNA interference; RPE, retinal pigmented epithelial; SGSH, lysosomal heparan-N-sulfamidase gene; siRNA, small interfering RNA; SMN, survival motor neuron; SOD, superoxide dismutase; SUMF, sulfatase-modifying factor; TFEB, transcription factor EB; TPP1, tripeptidyl peptidase 1; TREM2, triggering receptor expressed on myeloid cells 2; UPR, unfolded protein response; ZFPs, zinc finger proteins
Graphical abstract
Gene therapy for neurodegenerative disorders has made the unprecedented progress. This review summarizes advances and challenges of gene therapy for neurodegenerative disorders, highlighting promising technologies, targets, and future prospects with the authors’ insights and experience.
1. Introduction
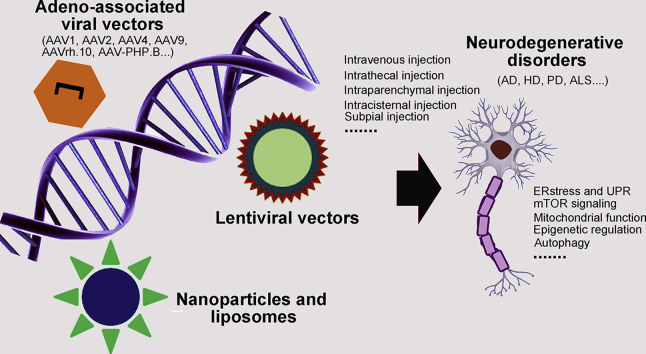
Over the past few decades, gene therapy for neurodegenerative disorders has made straightforward progress. Growing understanding of the pathogenetic mechanisms of these diseases has enabled numerous advances in key technologies to converge including identification of novel therapeutic targets and new vectors1. This increased knowledge has led to remarkable targeting by multiple genetic interventions of the root causes of neurodegenerative disorders with both single-gene and complex etiologies. The sustained, even permanent therapeutic effects of gene therapy are especially appealing for compartmentalized organs such as eye, cochlea or central nervous system (CNS), which structures are difficult to treat because most agents cannot breach the physiological barriers such as the blood–cerebrospinal fluid barrier (BCSFB), blood–retina barrier (BRB), and blood–brain barrier (BBB)2, 3, 4. Furthermore, some genetic targets that are refractory to treatment with traditional agents are potentially manageable by gene therapy, which is capable of both gene silencing to handle gain of function mutations and gene overexpression to handle loss of function mutations.
Viral and non-viral vectors can successfully direct transgenes that express therapeutic proteins, antibodies, Cas9/gRNA for gene editing, microRNAs, and small interfering RNA (siRNA) to diseased tissues in human and animals. For neurodegenerative disorders, the most commonly applied vector is one of the adeno-associated viruses (AAVs)5. Additionally, a large number of capsids can be employed across species to favorably target multiple tissues and cells within the CNS, including oligodendrocytes, astrocytes and neurons6, 7, 8, 9, 10, 11, 12, 13.
It is critical that there have been significant advances in developing effective delivery routes, especially for the CNS and eye. Preclinical studies have demonstrated that numerous routes of gene delivery, including subpial, intracerebroventricular, intrathecal, intraparenchymal, intravitreal, and subretinal injection, can attain sufficient gene quantities in diseased tissues14, 15, 16, 17. Intravenous injection has the advantage of being noninvasive, but the BBB and BRB are troublesome obstacles to the passage of agents into CNS or optic nerve18. Intramuscular injection provides an effective route for the delivery and production of vaccines and antibodies, and has the theoretical capacity to supply a source of antibodies that permit treatments to cross these physiological barriers in sufficient levels for clinical benefits19. It is noteworthy that recently reported vectors have an unequaled capability to transfer genes to the CNS after systemic injection. These vectors are an obvious improvement over AAV9 and potentially enhance our ability to treat additional neurological disorders20,21.
There have been a considerable number of clinical trials of gene therapy for neurodegenerative disorders (Table 1). Some early clinical trials failed to achieve satisfactory therapeutic effects, perhaps due to insufficient biodistribution within their intended tissues. With improvement in AAVs and non-viral delivery systems, gene therapies have shown wider transgene expression and therapeutic safety. Importantly, there have been recent reports of excellent functional outcomes in experimental models of numerous neurodegenerative disorders, including Alzheimer's disease (AD), Huntington's disease (HD), aromatic-l-amino-acid decarboxylase (AADC) deficiency, and Parkinson's disease (PD)5,22, 23, 24, 25. Here we review clinical and preclinical studies to describe recent advances in gene therapy for neurodegenerative disorders. We focus on the critical properties that efficient treatment by gene therapy requires, including vector design and selection of transgene strategy, target, and delivery route. We also discuss the challenges and future prospects of gene therapy, and share our own insights and experience.
Table 1.
Ongoing gene therapy clinical trials for neurodegenerative disorders.
| Disorders | Trial code | Delivery route | Gene therapy | Phase |
|---|---|---|---|---|
| Alzheimer's disease | NCT00876863 | Direct basal forebrain injection | AAV2-NGF | Phase Ⅱ |
| Huntington's disease | NCT02519036 | Intrathecal injection | ASOs to HTT messenger RNA | Phase Ⅲ |
| Huntington's disease |
NCT03225833 NCT03225846 |
Intrathecal injection | ASOs to HTT mutant pre-messenger RNA | Phase Ⅰ |
| Pompe's disease | NCT02240407 | Intramuscular injection | AAV9-GAA | No results |
| Pompe's disease | NCT00976352 | Intramuscular injection | AAV1-GAA | Phase Ⅱ |
| Parkinson's disease |
NCT03065192 NCT01793543 |
Intraputaminal injection | AAV2-AADC | Phase Ⅰ |
| Parkinson's disease | NCT01621581 | Intraputaminal injection | AAV2-GDNF | Phase Ⅰ |
| Parkinson's disease | NCT02418598 | Intraputaminal injection | AAV2-AADC | Phase Ⅱ |
| Parkinson's disease |
NCT00400634 NCT00985517 |
Intraputaminal injection | AAV2-neurturin | Phase Ⅱ |
| Parkinson's disease | NCT00627588 | Intraputaminal injection | Lentivirus-AADC | Phase Ⅰ |
| Parkinson's disease | NCT00643890 | Injection into the subthalamic nucleus | AAV2-GAD | Phase Ⅱ |
| Metachromic leukodystrophy | NCT01801709 | Intracerebral injection | AAVrh10-ARSA | No results |
| Spinal muscular atrophy | NCT02122952 | Intravenous injection | AAV9-SMN | Phase Ⅰ |
| Spinal muscular atrophy | NCT02292537 | Intrathecal injection | ASOs targeting SMN2 splicing | Phase Ⅲ |
| Amyotrophic lateral sclerosis | NCT01041222 | Intrathecal injection | ASOs to SOD1 | Phase Ⅰ |
| Mucopolysaccharidosis type III A | NCT01474343 | Intracerebral injection | AAVrh10-SGSH | Phase Ⅰ |
| Mucopolysaccharidosis type III A | NCT02053064 | Intracerebral injection | AAVrh10-SUMF1 | Phase Ⅱ |
| Mucopolysaccharidosis type II | NCT03041324 | Intravenous injection | AAV6-IDS | Phase Ⅱ |
| Batten | NCT02725580 | Intrathecal injection | AAV9-CLN6 | Phase Ⅰ/Ⅱ |
| Batten | NCT01414985 | Intracranial injection | AAVrh10-TPP1 | Phase Ⅰ/Ⅱ |
| Batten | NCT01161576 | Intracranial injection | AAVrh10-TPP1 | Phase Ⅰ |
| Canavan | NA | Intraparenchymal injection | AAV2-ASPA | Phase Ⅰ |
NA, not applicable.
2. Transgene strategies
Transgene strategies have been designed to deliver any nucleic acid as a genomic cargo, including siRNA, cDNA (gene addition or augmentation), microRNA, guide RNA (gene editing), RNA or DNA editing enzyme, docking site for a DNA binding protein, antisense oligonucleotide, or shRNA2,20,26, 27, 28. Importantly, none of these genomic cargos should be larger than 4.7 kb for AAV-based gene therapy, the size of the AAV genome2. Gene addition or augmentation has been assessed as a treatment strategy for several neurodegenerative disorders, including PD, Canavan disease, spinal muscular atrophy, and AD. This approach has been evaluated for targeted delivery of cDNA for AADC, survival motor neuron (SMN), human aspartoacylase (ASPA), and nerve growth factor (NGF), and has been reported to be effective and well tolerated with reduced clinical stabilization over a long-term follow-up research2,29,30.
Engineered transcriptional regulators and gene editing targeted to specific genes are also being investigated as novel therapeutic applications for neurodegenerative diseases. Zinc finger proteins (ZFPs) are appealing experimental substances from a clinical perspective due to the similarity of rodent and human proteins and their relatively short genomes. However, although a clinical trial of inserting the iduronate 2-sulfatase (IDS) gene into albumin loci to treat mucopolysaccharidosis II has been carried out, successful gene editing will not be easy to achieve because of the off-target effects31. The recent suggestion that CRISPR/Cas9 nanocomplexes targeting BACE1 could suppress cognitive deficits and amyloid β-associated pathologies in AD highlights the huge application potential of non-viral vectors or viral vectors based CRISPR/Cas9 gene editing for neurodegenerative disorders. However, there are still many hurdlers need to be overcome when applying this approach to treat human neurodegenerative diseases, especially safety concerns31,32.
Another promising transgene strategy is gene silencing. RNA interference (RNAi) is a widespread biological process in which siRNAs decrease synthesis of specific targeted proteins by degrading their corresponding mRNAs. Multiple clinical trials have suggested that artificial siRNAs can be utilized in humans to inhibit targeted proteins or genes and are commonly well tolerated (e.g., NCT01559077 and NCT01437059)33, 34, 35. For example, recent clinical study demonstrated that HD patients showed dose-dependent reductions in concentrations of mutant huntingtin (HTT) after intrathecal injection of an antisense oligonucleotide (IONIS-HTTRx), suggesting this agent maybe a promising therapeutic36. Notably, because synthetic shRNAs or microRNA produced from a single injection of AAV can generate a more lasting gene silencing than artificial siRNAs, these substances provide superior gene therapy approaches for neurodegenerative disorders. For instance, the preclinical research indicated that one-dose administration of gene therapy candidate VY-HTT01 (Voyager Therapeutics) could effectively reduce the levels of HTT responsible for HD in critical brain areas of nonhuman primates. Moreover, studies demonstrating that synthetic primary-microRNA cassettes or AAV5 expressing a microRNA targeting HTT (AAV5-miHTT, UniQure) can generate efficacious, safe production of mature-microRNAs targeting ataxin-1 and HTT in mouse models of spinocerebellar ataxia type-1 and HD, respectively, provide proof-of-concept support for these strategies to utilize RNAi36, 37, 38, 39. Overall, the rational for transgene strategies selection would be determined by multiple factors including safety concerns, insertional mutagenesis and genotoxicity as well as different pathological conditions2.
3. Vectors: viral and non-viral based gene therapy
3.1. Viral vectors
AAV based vectors have been applied almost exclusively in clinical trials of gene therapy for neurodegenerative diseases. AAV serotypes are the major determinant of several crucial characteristics of successful AAV-based gene therapy, including biodistribution, tissue tropism, and susceptibility to neutralizing antibody generated in vivo. Discovering how the specific serotypes distribute gene cargos to their intended tissues for vector delivery is vital for developing a reliable and predictable gene therapy strategy6,40, 41, 42, 43. More than one hundred AAV variants consisting of 13 serotypes (AAV1–13) have been identified from humans and nonhuman primates44, 45, 46, 47, 48. Because of its relative safety profile and its sustained expression in neurons, AAV2 has been used in numerous clinical trials and is currently considered a satisfactory vector for gene therapy of neurodegenerative disorders48, 49, 50, 51. Specifically, researchers indicated that intracerebral administration of AAV2-NGF is well tolerated and shows evidences of therapeutic effect on cognitive decline in AD-related dementia50. Interestingly, after administration near or into cerebral ventricles, AAV4 has a predilection to transfect ependymal cells, which constitute the epithelial lining of neuroblasts and the lateral ventricles52,53. Because the BBB is an important barrier hindering delivery of most vectors to the CNS, the ability of AAV9 and AAVrh.10 to penetrate this obstacle is also consequential21,54,55. Interestingly, reports that AAV-PHP.B, a recently engineered AAV capsid, can transduce more than 50% of astrocytes and neurons and deliver far more AAV genomes (forty fold greater) into the CNS after intravenous injection than other capsids, highlight the critical importance of capsid engineering20,56. Indeed cell-type specific screening of different AAV capsid libraries has identified increasing numbers of bioengineered AAV capsids with specific tropisms57.
Adenovirus (Adv) is an icosahedral capsid virus with size ranging from 70 to 100 nm. Adv cannot insert its gene into the host genome, which leads to relative transient transgene expression but an excellent safety profile. The innate immune responses against Adv restricts Adv's therapeutic potential efficacy for CNS gene therapy58,59. Although few studies use Adv as gene therapy vectors to treat neurodegenerative disorders, it should be pointed out, however, that Adv is well tolerated with little side effects in these researches59. Unlike AAV and Adv capsids, retrovirus/lentivirus (LVs) could fully integrate DNA into the host genome through reverse transcription, thus providing more stable and longer transgene expression in vivo. Of note, these insertions should be controlled under strict conditions to avoid genotoxicity and insertional mutagenesis. The one important clinical trial to date is the use of a lentiviral vector, which can deliver larger DNA cargos for PD60. Their data indicated that ProSavin, a lentiviral vector-based gene therapy aimed at restoring dopamine production, improved motor behavior and demonstrated safe in all patients with advanced PD60. Further investigations into Adv and LVs-mediated gene therapy of neurodegenerative disorders are desperately needed given the limited clinical data thus far.
3.2. Non-viral vectors
Although most clinical trials have used viral vectors such as AAVs, lentivirus, Adv, and retroviruses to carry therapeutic genes, these vectors have numerous drawbacks, including broad tropism, limited loading capacity, difficulty in vector production, and host inflammatory responses61, 62, 63, 64, 65, 66. Gene therapies based on non-viral vectors have the potential to avoid several of these drawbacks, especially those related to safety67, 68, 69. Moreover, although few of these strategies have been used in the clinic, it is extremely important to exploit novel kinds of vectors, particularly nanoparticles and liposomes. Based on the composition of the carriers' material, non-viral delivery vectors can be sorted into lipid-based vectors and polymeric vectors. The most extensively applied non-viral gene carriers are lipid-based vectors70. Neutral lipids, like cholesterol, DOPE, and DSPE, have served as the ‘helper lipid’ among liposomal components to improve liposome stability and transfection capacity71. The prominent features of cationic lipids, such as DOTAP, DODAP, DOTMA, and DC-cholesterol, which have been used for gene therapy, include three major domains: hydrophobic tails, linking groups, and cationic cap groups69,72. The main shortcomings of cationic lipids are their unsatisfactory pharmacokinetic biodistribution due to nonspecific binding and rapid clearance, and their cytotoxicity69,73. To overcome these drawbacks, optimized cationic lipids with appropriate pKa values have been developed68,70. Lipidoids (lipid-like materials), magnetic nanoparticles, and exosomes have also shown promise as gene delivery carriers for neurodegenerative disorders74, 75, 76. For instance, recent studies indicated that magnetic Fe3O4 nanoparticles coated with N-isopropylacrylamide derivatives and oleic acid molecules carrying shRNA-α-syn can significantly alleviate PD in mice74. Cationic polymers provide another kind of non-viral vector that is extremely attractive for gene therapy due to their capacity for endosomal/lysosome escape, which is the result of their sponge-proton effect, fine spherical architecture, and tremendous chemical diversity70,77, 78, 79. Overall, therefore, non-viral gene therapy has improved substantially in recent decades. Additional insights into the relationship between structure and function of gene delivery material and fuller understanding of the critical factors that restrict effective gene delivery are likely to advance the clinical treatment of neurodegenerative disorders. We summarize the types, specific characteristics, advantages and disadvantages of viral and non-viral vectors in this section in Table 2.
Table 2.
Comparison of the different gene vectors for neurodegenerative disorders.
| Vector type | Specific characteristics | Advantage | Disadvantage |
|---|---|---|---|
| AAVs | Numerous AAV serotypes; lack of targeting and site-specific; relative safety profile | Relative stable transgene expression; nonpathogenic; various serotypes available | Immune responses; limited gene packaging capacity |
| Adv | Adv cannot introduce its gene into the host genome | Lower genotoxicity and insertional mutagenesis | Immune responses; relative transient transgene expression; re-administration; requires receptors for cell uptake |
| LVs | Retroviruses can integrate DNA payloads into the host genome | Lower frequency of administration; stable and long-term transgene expression | Immune responses; genotoxicity; insertional mutagenesis |
| Polymer- and lipid-based vectors | Non-viral vectors can be altered to impart desired functionalities | Large-scale production; controlled release; large gene packaging capacity; lower immunogenicity | Cytotoxicity; nonspecific binding and rapid clearance |
4. Target selection for neurodegenerative disorders
Neurodegenerative disorders are characterized by progressive dysfunction of neurons in specific regions of CNS, eventually leading to disability and death. The growing number of recently identified targets enlarges the range of potential clinical applications. However, as shown in Table 1, many therapeutic agents and their related targets offer nothing beyond symptomatic relief and do not address the underlying pathology80. It is therefore urgently necessary to identify promising pathogenic targets for gene therapy of neurodegenerative disorders, as indicated in Table 381, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100.
Table 3.
Representative promising therapeutic targets for gene therapy of neurodegenerative disorders.
| Target selection | Disorder | Gene therapy | Delivery route | Ref. |
|---|---|---|---|---|
| ER stress and UPR | Traumatic optic nerve injury | AAV2-XBP-1 | Intravitreal injection | 81 |
| Parkinson's disease | AAV2-XBP-1 | Unilateral brain injection | 82 | |
| Optic neuritis and encephalomyelitis | AAV2-CHOP shRNA | Intravitreal injection | 83 | |
| Parkinson's disease | AAV5-Bip | Intracerebral injection | 84 | |
| Amyotrophic lateral sclerosis | AAV6-SIL1 | Unilateral brain injection | 85 | |
| Huntington's disease | AAV2-XBP-1 | Intrastriatal injection | 86 | |
| mTOR signaling | Optic nerve injury | AAV2-AKT | Intravitreal injection | 87 |
| Optic nerve injury | AAV2-S6K1 | Intravitreal injection | 88 | |
| Optic nerve injury | AAV2-PTEN | Intravitreal injection | 89 | |
| Parkinson's disease | AAV1-AKT | Intrastriatal injection | 90 | |
| Alzheimer's disease and Parkinson's disease | AAV1-AKT | Intrastriatal injection | 91 | |
| Huntington's disease | AAV1-caRheb | Unilateral brain injection | 92 | |
| Mitochondrial function | Parkinson's disease | AAV2-HSP70 | Substantial nigra dense area injection | 93 |
| Alzheimer's disease | AAV2-PINK1 | Intrahippocampal injection | 94 | |
| Epigenetic regulation | Alzheimer's disease | AAV2-PSD95-6ZF-VP64 | Intrahippocampal injection | 95 |
| Autophagy | Alzheimer's disease | AAV2-PINK1 | Intrahippocampal injection | 94 |
| Parkinson's disease | AAV6-Lamp2a | Intrastriatal injection | 96 | |
| Parkinson's disease | AAV2-TFEB | Unilateral brain injection | 97 | |
| Amyotrophic lateral sclerosis | AAV9-snapin | Intravenous injection | 98 | |
| Microglial and astrocyte function | Alzheimer's disease | AAV2/8-sTREM2 | Intracerebral injection | 99 |
| Alzheimer's disease | Lentivirus-PGRN | Unilateral brain injection | 100 |
4.1. Endoplasmic reticulum stress and unfolded protein response
Almost all neurodegenerative disorders share the same pathological characteristic: abnormal accumulation of misfolded proteins101,102. The negative consequences of aggregating misfolded proteins include generation of endoplasmic reticulum (ER) stress and ER-associated degradation103. Misfolded proteins, such as amyloid β oligomers and α-synuclein, which aggregate in the ER-lumen, destabilize ER calcium homeostasis and distort unfolded protein response (UPR) signaling intended to restore cellular proteostasis, but instead resulting in proapoptotic responses and neuron death104, 105, 106. Importantly, investigators, including ourselves, have suggested that gene therapies to reduce ER stress by targeting UPR signaling to enhance protein folding are more likely to provide long-term, local therapeutic effects than antibodies and small molecules. AAV delivered to the mouse retina to downregulate CCAAT/enhancer binding homologous protein (CHOP) or activate XBP-1 prevents the optic nerve degeneration and apoptotic death of retinal ganglion cells (RGCs) that is triggered by glaucoma, optic neuritis, and traumatic optic nerve injury81,83,107. Similarly, AAVs-XBP-1 administered locally to the striatum or substantia nigra block neurodegeneration induced by neurotoxins that experimentally model HD and PD82,86,108,109. Moreover, gene therapy consisting of overexpression of BiP (glucose regulated protein 78) to treat experimental PD has been reported to reduce dopaminergic neuron apoptosis, enhance motor performance, and delay disease progression84. The same strategy has excellent effects in a mouse model of amyotrophic lateral sclerosis (ALS); intracerebral delivery of AAV6-SIL1 restores ER homeostasis and prolongs survival85. Because upregulation of UPR signaling has been reported to sustain the proliferation and invasion of glioblastoma, however, safety assessment in long-term follow-up studies will be required before this approach can be considered for the clinic110,111.
4.2. mTOR signaling
Signaling transductions of mammalian target of rapamycin (mTOR) have been reported to play a pathogenic role in neurodegenerative disorders with diverse clinical characteristics, such as AD, PD, HD, and traumatic brain and optic nerve injury112,113. Abnormal mTOR signaling is likely to have distinct effects in different neural cells, such as those in the substantial nigra, caudate nucleus, retina, and entorhinal cortex, but degeneration is the common fate of all of these cells because they are unable to clear toxic protein accumulation114. Our work has previously demonstrated that delivery to retina by AAV of positive regulators or effectors of mTOR signaling (such as AAV2-AKT and AAV2-S6K1) or AAV-mediated deletion from retina of negative regulators of mTOR signaling (such as PTEN) can prevent death of RGCs and promote CNS axon regeneration following traumatic optic nerve injury87, 88, 89,115. Other investigators report that AAV-based overexpression of S6K1 or AKT also has therapeutic effects in a mouse model of PD, indicating that activation of mTOR signaling may provide a new treatment option for PD and traumatic nerve injury90,91. Of note, multiple studies have suggested that mTOR signaling is hyperactivated in HD and AD, and that reinstating aberrant mTORC1 activity can rescue neurodegeneration. Future studies should therefore focus on the cellular and molecular mechanisms that relate mTOR signaling and neurodegenerative disorders92,116, 117, 118, 119.
4.3. Mitochondrial function
Mitochondrial respiratory dysfunction has been shown to contribute to numerous neurodegeneration disorders, such as AD, PD, HD, glaucoma, ALS, and lysosomal storage diseases120, 121, 122. These disorders exhibit many characteristics of mitochondrial respiratory dysfunction, including limited regulation of mitochondrial quality, oxidative damage, NAD+ depletion, disrupted ATP synthesis, protein aggregates, and unbalanced mitochondrial calcium homeostasis120, 121, 122, 123, 124. Therapeutic agents that inhibit mitochondrial damage or promote mitochondrial biogenesis, among them CoQ10, Bendavia, MitoQ, and NAM, mitigate neurodegeneration in mouse models121,125, 126, 127, 128. Moreover, gene therapy that overexpresses regulators of mitochondrial oxidative stress and dynamics, such as PGC-1α, HSP70, TFEB, can reduce neurotoxicity in experimental PD and HD, suggesting that these strategies may be significant therapeutic approaches for other neurodegeneration diseases93,129. However, clinical translations of mitochondrial treatments have been unsatisfactory, which may be because patients enter clinical trials when their neurodegenerative disorders are too advanced for effective intervention. Optimism about mitochondrial-based gene therapy for neurodegenerative disorders continues to be warranted, particularly for those with obvious mitochondrial dysfunctions130.
4.4. Epigenetic regulation
Epigenetic regulatory mechanisms, such as chromatin remodeling, DNA methylation, histone variant, and histone post-translational modification, have been suggested to regulate numerous aspects of axonal development and neuronal survival131. One study presented evidence that changes in H3K27ac or H3K4me3 occurred in connection with genetic variants in AD, suggesting an important function for immune-associated enhancers and promoter proteins in determining AD susceptibility132. Another study demonstrated that H4K16ac, a histone associated with DNA repair and neurodegenerative disorders, is significantly reduced in the cortex of AD patients, suggesting that the aged brain of these individuals is incapable of upregulating H4K16ac133. It is also noteworthy that multiple reports have associated loss of H3K4me3, a protein related to gene activation, with the deterioration found in PD and HD, but that overexpression of H3K4me3 can accelerate A–T mutation that mitigates behavioral impairments and neurodegeneration134, 135, 136. Additionally, HDAC inhibitors can prevent neurodegeneration in models of glaucoma, AD, and HD, despite the differences in pathogenesis137, 138, 139, 140. These reports demonstrate that epigenetic profiles are regulated in neurodegenerative diseases and suggest that better understanding of these mechanisms could provide the foundation for developing more precisely targeted epigenome therapies. For example, recent work suggesting that epigenetic editing of the post-synaptic density protein 95 gene can improve cognition in AD highlights the potential of epigenetic regulation-based gene therapy for neurodegenerative disorders95.
4.5. Autophagy
Autophagy, the process by which evolutionarily-conserved intracellular machinery degrades dysfunctional organelles and denatured proteins in lysosomes, has been demonstrated to be associated with the severity of such neurodegenerative disorders as AD, PD, HD, glaucoma and ALS141,142. Neuroprotection by autophagy is mainly due to its elimination of misfolded proteins, including tau, HTT, and α-synuclein142,143. Previous work has indicated that PTEN-induced overexpression of putative kinase 1 (PINK1) mediated by AAV2 promotes autophagy that facilitates clearance of dysfunctional mitochondria, which in turn ameliorates the loss of mitochondrial functions, cognitive decline and synapses induced by amyloid β oligomers in experimental AD94. Similarly, overexpression of the transcription factor EB (TFEB) or lysosome-associated membrane protein 2a via intracerebral injection of AAV vectors can effectively alleviate α-synuclein-induced neurodegeneration in PD by enhancing axon regeneration and neuron survival; these benefits have been attributed to induction of lysosome biogenesis and chaperone-mediated autophagy96,97. Additionally, overexpressing snapin (AAV9-snapin) rescues defects in retrograde transport, which reverse impairments of autophagy/lysosomes, improve mitochondrial fitness, enhance motor neuron survival, and mitigate disease phenotypes in mouse ALS98. Although multiple lines of evidence therefore support the potential of gene therapies to treat neurodegenerative disorders by regulating autophagy, the approach is challenged by difficulties in target selection and limited understanding of underlying mechanisms.
4.6. Microglial and astrocyte function
Microglia, as the major neuro-immune cells, execute numerous critical tasks: housekeeping functions that maintain neuronal wellbeing and neuronal networks, sentinel functions associated with constant perception of environmental changes, and defensive functions essential for neuroprotection144. Neuronal damage in AD, PD, HD, ALS, glaucoma, and the degeneration associated with chronic and acute trauma stems from disruption of these microglial functions and neuroinflammation. Preventing dysregulation of these functions therefore represents a potential mode of treatment. Specifically, variants of microglial surface innate-immune receptors, such as complement receptor 1 (CR1), CD33, and triggering receptor expressed on myeloid cells 2 (TREM2), have been genetically associated with the risk for AD145, 146, 147. Moreover, overexpression of soluble TREM2 mediated by AAVs improves microglial migration, proliferation, and degradation of amyloid β protein, which reduces amyloid plaque deposition and rescues dysfunctional spatial memory in a model of AD99. Additionally, by modulating microglial function, lentivirus-mediated haploinsufficiency of progranulin overexpression inhibits neuronal loss and spatial memory deficits in AD mice100.
Astrocytes fulfill lots of interactive and homeostatic functions in the CNS: regulating extracellular neurotransmitters and ions; providing energy metabolites; promoting neurogenesis; and controlling synaptic activity148. The complexity and diversity of these performances clearly suggest that the correct activities of astrocytes are very important to physiological functioning of the CNS, and their dysfunction can promote the progression of multiple neurodegenerative diseases149. A precise and effective approach to modulate astrocyte signaling pathways involves boosting or inhibiting genes in specific manners. Importantly, studies have demonstrated that AAV capsids AAV9P1 and Anc80L65 are promising tools for gene delivery to astrocytes, which could facilitate activation or inactivation of persisting dysfunction genes150. Moreover, pseudotyping lentiviruses with glycoproteins were found to selectively transfect astrocytes after intraparenchymal administration150,151. Nanoparticles functionalized with bradykinin B2 receptor antibodies, transferrin receptor or apolipoprotein E have also been indicated to successfully deliver siRNA and mRNA to astrocytes152,153. For example, optimized branched poly (β-amino ester)s are applied to deliver NGF expression DNA to astrocytes, and high transfection efficiency is achieved, which provides a viable gene therapy approach for neurodegenerative disorders154. Collectively, these studies indicate that targeting microglia or astrocyte may be a promising therapeutic strategy for neurodegenerative diseases.
4.7. Neuronal progenitors or stem cell therapy
The therapeutic strategies for neurodegenerative diseases may have a revolutionized change via transplanting neuronal progenitors or stem cells. However, better controlling of their proliferation and ameliorating their engraftment as well as improving their differentiation and survival are very important. To modulate stem cell function, delicate regulations of gene expression via gene therapy approaches are emerging as safe methods155. For example, Jakobsson et al.155 have used LVs-based CRISPR-Cas9 tool to knockout DNMT1 in neural progenitor cells which results in feasible, proliferating cells and further implicating a novel gene therapy in human brain disease and development156. As another example, Biffi et al.157 have applied LVs to introduce functional genes into hematopoietic stem cells ex vivo and indicated that transplantation of these engineered HSCs inhibited and alleviated the symptoms of metachromatic leukodystrophy. Although neuronal progenitors or stem cell therapy have previously demonstrated clinical benefit, these therapeutic strategies are often restricted, especially for disorder conditions owing to cell autonomous defects.
5. Delivery routes: a major determinant of efficacy and safety
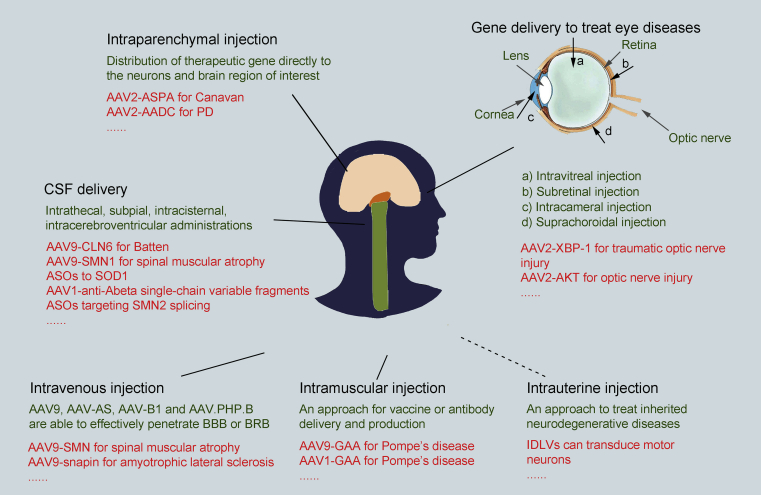
Gene delivery to sensory organs or CNS, including eye, spinal cord, and brain, is a challenging undertaking. Attaining a proper balance between treatment efficiency and compliance and safety is largely decided by the judicious combination of delivery routes and vectors. Fig. 1 lists many of the preclinical studies and clinical trials and their multiple delivery routes and vectors that will be mentioned in the following section.
Figure 1.
Delivery routes of gene therapy for neurodegenerative disorders. Although intravenous or cerebrospinal fluid (intrathecal, intracerebroventricular, and subpial routes) administration can effectively treat multifocal disorders, intraparenchymal injection is the most frequently applied delivery route for brain diseases. Local gene delivery is preferable for diseases of the eye, because of its relatively straightforward surgical and instrumental accessibility. Intramuscular injection provides a strategy for vaccine and antibody delivery and production, and intrauterine injection may provide an approach to treat inherited neurodegenerative diseases.
5.1. Intravenous injection
The physiological barriers that compartmentalize sensory and CNS tissues, such as BBB, present serious obstacles to therapeutic gene access. AAV9 is one agent that effectively penetrates these barriers after intravenous injection. This feature enables widespread expression in CNS to treat multifocal disorders and acts as a stimulus for further technological exploration and development158, 159, 160. Moreover, capsid engineering research has discovered AAV variants that seem to be superior to AAV9, such as AAV-AS, AAV-B1, and AAV.PHP.B, which represents a significant breakthrough for intravenous delivery20,161,162. This delivery route could also result in delivery of the gene cargos to most body tissues which has potential drawbacks but in some cases also potential advantages. Even though intravenous delivery of AAVs is noninvasive and technically feasible, major obstacles continue to complicate clinical applications, including the large doses necessary, generation of antibodies against AAVs, and ongoing safety concerns.
5.2. Intraparenchymal injection
Local delivery of vectors has obvious advantages over systemic administration. Intraparenchymal injection is tolerated well and delivers therapeutic genes directly to the neurons and brain region of interest, with little biodistribution to peripheral organs5,24,163. Vectors that do not bind heparin sulfate proteoglycans (HSPGs), like AAV1, AAV8, AAV9, and AAVrh.10, diffuse over larger regions after intraparenchymal injection than vectors that bind HSPGs, such as AAV2, AAV-DJ88, and AAV6164. For disorders such as Canavan disease, which has been treated with AAV2-ASPA, and PD, which has been treated with AAV2-AADC, AAV2 provides an appropriate vehicle to limit diffusion while obtaining satisfactory delivery29.
5.3. Intrathecal, subpial, intracisternal, intracerebroventricular and intrauterine injection
Other delivery routes include administration into various cerebrospinal fluid (CSF) compartments. Intrathecal injection of AAVs is especially suitable for delivering vectors to sensory neurons in dorsal root ganglia or motor neurons and has been well tolerated in numerous preclinical studies12,165,166. Interestingly, AAVs, such as AAVrh.10 and AAV9, primarily target spinal cord motor neurons following intrathecal injection in nonhuman primates167. Because subpial administration has only been investigated in the laboratory to date, translation of this dosing method for gene therapy of neurodegenerative disorders requires additional studies15. Preclinical studies have shown that intracisternal and intracerebroventricular administration also produces effective expression of transgenes in spinal cord and cerebral tissues that has alleviated symptoms in models of numerous neurodegenerative disorders, including AD, ALS, and spinal muscular atrophy168, 169, 170. Additionally, integration-deficient lentiviral vectors (IDLVs) have recently been reported to transduce motor neurons efficiently and permanently after intrauterine injection, indicating the potential for IDLVs to become effective tools to treat inherited neurodegenerative diseases171.
5.4. Gene therapy in eye diseases
The eye is especially suitable for local injection of AAVs. U.S. Food and Drug Administration (FDA) approval of Luxturna (AAV2-RPE65) to treat Leber's hereditary optic neuropathy signals the arrival of the gene therapy era172. Local administration presents multiple advantages for treating ophthalmic diseases, largely because the relatively easy surgical and instrumental accessibility enables practical interventions and rapid examinations, and because the compartmental characteristics of the eye prevent systemic dispersion of the vectors. Intracameral administration provides delivery to the anterior chamber of the eye, which can be targeted to the cornea or the trabecular meshwork173. Notably, AAV-based gene therapies for ophthalmic diseases have mostly concentrated on retinal diseases, including the neurodegenerative retinal disorders, and have efficiently targeted the inner retina through intravitreal administration and the outer retina through subretinal administration2. These AAVs accumulate between the neural retina and the retinal pigmented epithelial (RPE) cell layer after subretinal administration174. Clinically, intravitreal administration has been used to produce AAV-based therapeutic protein expression or to target retinal ganglion cells175. However, this delivery route needs high doses of AAVs, which can increase the risk of inflammatory responses remarkably in comparison with subretinal administration176,177.
Taken together, the selection of delivery routes is a key one yet needs a balancing of numerous factors along a risk/benefit equation. Local delivery route of administration has been acknowledged to be the preferential choice for neurodegenerative disorders as it maximizes delivery while minimizing the safety concerns. In order to target more broadly than what local administration routes can achieve and avoid the invasiveness of the local injection procedures, new techniques were urgently required that achieve meaningful levels of gene transfer through these strategies. These delivery routes almost always need higher doses, putting burden on drug manufacturing and raising the risk of toxicity. In some circumstances, a variety of routes of injection are combined when certain disorders require many organs to be treated178.
6. Clinical challenges
Numerous preclinical and clinical studies of gene therapy strategies for preventing or treating a wide range of neurodegenerative diseases have been carried out in recent decades2. However, safety concerns remain one of the biggest barriers to successful clinical application. Gene therapy may cause severe toxicity due to overexpression of the transgene in targeted tissues or expression in off target cells. Toxic effects have included impaired ambulation, ataxia, damaged dorsal root ganglia, elevated transaminases, and proprioceptive deficits2,179. Host responses can also affect the duration and safety of every gene therapy strategy. Patients with adaptive immune responses can produce corresponding neutralizing antibodies, which may prevent the vectors from reaching their intended tissues or cells180,181. Insertional mutagenesis and genotoxicity are probably also concerns when certain transgenes are injected with high-dose vectors182, 183, 184. Potential gene-based therapeutic strategies to treat neurodegenerative disorders should therefore be carefully scrutinized for clinical development, including evaluation of available safety profiles and pharmacological effects, and identification of individuals who can benefit.
7. Concluding remarks and future prospects
Gene therapy is an important emerging strategy for treating neurodegenerative disorders, which is especially suited for well-validated genetic targets that are not amenable to traditional therapies. It has been well tolerated and shown long-lasting efficacy in clinical trials for various human neurodegenerative diseases, including PD, AD, HD, and AADC deficiency5,65. Moreover, improvements in delivery, such as direct administration into the CNS, and carriers, such as AAV9 and liposomes, are being vigorously investigated and refined. Although non-viral vectors-based gene therapy has yet to be approved as therapeutics for neurodegenerative disorders, recent advances in the clinical trials have generated great excitement32, 33, 34, 35, 36. Better understanding of the onset and progression of the neurodegenerative disorders will facilitate prompt diagnosis and target selection, which should allow early treatment for certain of these diseases. As progress continues in optimizing transgene design, delivery, and vectors, the prospects of gene therapy for neurodegenerative disorders will undoubtedly become even brighter.
Acknowledgments
Our work is supported by National Natural Science Foundation of China (Nos. 81773620 and 81573332) and National Key Basic Research Program of China (No. 2015CB931800) to Dianwen Ju and NIH NEI (EY024932, EY023295 and EY028106, USA) to Yang Hu.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yang Hu, Email: huyang@stanford.edu.
Dianwen Ju, Email: dianwenju@fudan.edu.cn.
Author contributions
Wei Chen and Yang Hu wrote the manuscript. Yang Hu and Dianwen Ju designed structures and supervised the work. The final version of the paper has been approved by all authors.
Conflicts of interest
All authors declare no competing interests.
References
- 1.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;59 doi: 10.1126/science.aan4672. eaan4672. [DOI] [PubMed] [Google Scholar]
- 2.Hudry E., Vandenberghe L.H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101:839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.H., Wang J.H., Chen J., Li F., Edwards T.L., Hewitt A.W. Gene therapy for visual loss: opportunities and concerns. Prog Retin Eye Res. 2019;68:31–53. doi: 10.1016/j.preteyeres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg M.S., Samulski R.J., McCown T.J. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology. 2013;69:82–88. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaranch L., Salegio E.A., San Sebastian W., Kells A.P., Bringas J.R., Forsayeth J. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the CSF of non-human primates. Hum Gene Ther. 2013;24:526–532. doi: 10.1089/hum.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang C., Zhang Y., Guo W., Liang X.J. Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm Sin B. 2020;10:239–248. doi: 10.1016/j.apsb.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cearley C.N., Vandenberghe L.H., Parente M.K., Carnish E.R., Wilson J.M., Wolfe J.H. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett J.S., Samulski R.J., McCown T.J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 10.Hutson T.H., Verhaagen J., Yáñez-Muñoz R.J., Moon L.D. Corticospinal tract transduction: a comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Ther. 2012;19:49–60. doi: 10.1038/gt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz M.L., Tecedor L., Chen Y., Williamson B.G., Lysenko E., Wininger F.A. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aac6191. 313ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federici T., Taub J.S., Baum G.R., Gray S.J., Grieger J.C., Matthews K.A. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 13.Passini M.A., Watson D.J., Vite C.H., Landsburg D.J., Feigenbaum A.L., Wolfe J.H. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of β-glucuronidase deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson R.M., Gimenez F., Salegio E.A., Su X., Bringas J., Berger M.S. T2 imaging in monitoring of intraparenchymal real-time convection-enhanced delivery. Neurosurgery. 2011;69:154–163. doi: 10.1227/NEU.0b013e318217217e. [DOI] [PubMed] [Google Scholar]
- 15.Miyanohara A., Kamizato K., Juhas S., Juhasova J., Navarro M., Marsala S. Potent spinal parenchymal AAV9-mediated gene delivery by subpial injection in adult rats and pigs. Mol Ther Methods Clin Dev. 2016;3:16046. doi: 10.1038/mtm.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morabito G., Giannelli S.G., Ordazzo G., Bido S., Castoldi V., Indrigo M. AAV-PHP.B-mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol Ther. 2017;25:2727–2742. doi: 10.1016/j.ymthe.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passini M.A., Bu J., Richards A.M., Treleaven C.M., Sullivan J.A., O'Riordan C.R. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther. 2014;25:619–630. doi: 10.1089/hum.2014.011. [DOI] [PubMed] [Google Scholar]
- 18.Challis R.C., Ravindra Kumar S., Chan K.Y., Challis C., Beadle K., Jang M.J. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat Protoc. 2019;14:379–414. doi: 10.1038/s41596-018-0097-3. [DOI] [PubMed] [Google Scholar]
- 19.Balazs A.B., Bloom J.D., Hong C.M., Rao D.S., Baltimore D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat Biotechnol. 2013;31:647–652. doi: 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwu W.L., Muramatsu S., Tseng S.H., Tzen K.Y., Lee N.C., Chien Y.H. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003640. 134ra61. [DOI] [PubMed] [Google Scholar]
- 23.Sehara Y., Fujimoto K.I., Ikeguchi K., Katakai Y., Ono F., Takino N. Persistent expression of dopamine-synthesizing enzymes 15 years after gene transfer in a primate model of Parkinson's disease. Hum Gene Ther Clin Dev. 2017;28:74–79. doi: 10.1089/humc.2017.010. [DOI] [PubMed] [Google Scholar]
- 24.Mittermeyer G., Christine C.W., Rosenbluth K.H., Baker S.L., Starr P., Larson P. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy S.R., Chang C.C., Dogbevia G., Bryleva E.Y., Bowen Z., Hasan M.T. Acat1 knockdown gene therapy decreases amyloid-beta in a mouse model of Alzheimer's disease. Mol Ther. 2013;21:1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han C.L., Ge M., Liu Y.P., Zhao X.M., Wang K.L., Chen N. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J Neuroinflammation. 2018;15:103. doi: 10.1186/s12974-018-1139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garanto A., Chung D.C., Duijkers L., Corral-Serrano J.C., Messchaert M., Xiao R. In vitro and in vivo rescue of aberrant splicing in CEP290-associated LCA by antisense oligonucleotide delivery. Hum Mol Genet. 2016;25:2552–2563. doi: 10.1093/hmg/ddw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone P., Shera D., McPhee S.W., Francis J.S., Kolodny E.H., Bilaniuk L.T. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003454. 165ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 30.Harmatz P., Muenzer J., Burton B.K., Ficicioglu C., Lau H., Leslie N.D. Update on phase 1/2 clinical trials for MPS I and MPS II using ZFN-mediated in vivo genome editing. Mol Genet Metabol. 2018;123:S59–S60. [Google Scholar]
- 31.Park H., Oh J., Shim G., Cho B., Chang Y., Kim S. In vivo neuronal gene editing via CRISPR-Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer's disease. Nat Neurosci. 2019;22:524–528. doi: 10.1038/s41593-019-0352-0. [DOI] [PubMed] [Google Scholar]
- 32.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald K., Frank-Kamenetsky M., Shulga-Morskaya S., Liebow A., Bettencourt B.R., Sutherland J.E. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 35.Spronck E.A., Brouwers C.C., Vallès A., de Haan M., Petry H., van Deventer S.J. AAV5-miHTT gene therapy demonstrates sustained huntingtin lowering and functional improvement in Huntington disease mouse models. Mol Ther Methods Clin Dev. 2019;13:334–343. doi: 10.1016/j.omtm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabrizi S.J., Leavitt B.R., Landwehrmeyer G.B., Wild E.J., Saft C., Barker R.A. Targeting huntingtin expression in patients with Huntington's disease. N Engl J Med. 2019;380:2307–2316. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
- 37.Her L.S., Mao S.H., Chang C.Y., Cheng P.H., Chang Y.F., Yang H.I. MiR-196a enhances neuronal morphology through suppressing RANBP10 to provide neuroprotection in Huntington's disease. Theranostics. 2017;7:2452–2462. doi: 10.7150/thno.18813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan S., Selvan S.T., Archunan G., Gulyas B., Padmanabhan P. MicroRNAs—the next generation therapeutic targets in human diseases. Theranostics. 2013;3:930–942. doi: 10.7150/thno.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudhakar V., Richardson R.M. Gene therapy for neurodegenerative diseases. Neurotherapeutics. 2019;16:166–175. doi: 10.1007/s13311-018-00694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciesielska A., Mittermeyer G., Hadaczek P., Kells A.P., Forsayeth J., Bankiewicz K.S. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol Ther. 2011;19:922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kells A.P., Hadaczek P., Yin D., Bringas J., Varenika V., Forsayeth J. Efficient gene therapy based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci U S A. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green F., Samaranch L., Zhang H.S., Manning-Bog A., Meyer K., Forsayeth J. Axonal transport of AAV9 in nonhuman primate brain. Gene Ther. 2016;23:520–526. doi: 10.1038/gt.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samaranch L., Blits B., San Sebastian W., Hadaczek P., Bringas J., Sudhakar V. MR-guided parenchymal delivery of adeno-associated viral vector serotype 5 in non-human primate brain. Gene Ther. 2017;24:253–261. doi: 10.1038/gt.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbetman A.E., Lochrie M., Zhou S., Wellman J., Scallan C., Doroudchi M.M. Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties. J Virol. 2005;79:15238–15245. doi: 10.1128/JVI.79.24.15238-15245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabatino D.E., Mingozzi F., Hui D.J., Chen H., Colosi P., Ertl H.C. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol Ther. 2005;12:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao G., Alvira M.R., Somanathan S., Lu Y., Vandenberghe L.H., Rux J.J. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeWitt P.A., Rezai A.R., Leehey M.A., Ojemann S.G., Flaherty A.W., Eskandar E.N. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 49.Marks W.J., Ostrem J.L., Verhagen L., Starr P.A., Larson P.S., Bakay R.A. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 50.Rafii M.S., Tuszynski M.H., Thomas R.G., Barba D., Brewer J.B., Rissman R.A. Adeno associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75:834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marks W.J., Bartus R.T., Siffert J., Davis C.S., Lozano A., Boulis N. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 52.Liu G., Martins I.H., Chiorini J.A., Davidson B.L. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 53.Davidson B.L., Stein C.S., Heth J.A., Martins I., Kotin R.M., Derksen T.A. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adachi K., Enoki T., Kawano Y., Veraz M., Nakai H. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nat Commun. 2014;5:3075. doi: 10.1038/ncomms4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albright B.H., Storey C.M., Murlidharan G., Castellanos Rivera R.M., Berry G.E., Madigan V.J. Mapping the structural determinants required for AAVrh.10 transport across the blood–brain barrier. Mol Ther. 2018;26:510–523. doi: 10.1016/j.ymthe.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen W.E., Kauvar I.V., Chen M.Z., Richman E.B., Yang S.J., Chan K. Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron. 2017;94:891–907. doi: 10.1016/j.neuron.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalkara D., Byrne L.C., Klimczak R.R., Visel M., Yin L., Merigan W.H. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005708. 189ra76. [DOI] [PubMed] [Google Scholar]
- 58.Kritzinger A., Ferger B., Gillardon F., Stierstorfer B., Birk G., Kochanek S. Age-related pathology after adenoviral overexpression of the leucine-rich repeat kinase 2 in the mouse striatum. Neurobiol Aging. 2018;66:97–111. doi: 10.1016/j.neurobiolaging.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Barkats M., Bilang-Bleuel A., Buc-Caron M.H., Castel-Barthe M.N., Corti O., Finiels F. Adenovirus in the brain: recent advances of gene therapy for neurodegenerative diseases. Prog Neurobiol. 1998;55:333–341. doi: 10.1016/s0301-0082(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 60.Palfi S., Gurruchaga J.M., Ralph G.S., Lepetit H., Lavisse S., Buttery P.C. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 61.Waehler R., Russell S.J., Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 63.Baum C., Kustikova O., Modlich U., Li Z., Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 64.Bouard D., Alazard-Dany D., Cosset F.L. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 66.Deverman B.E., Ravina B.M., Bankiewicz K.S., Paul S.M., Sah D.W. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018;17:641–659. doi: 10.1038/nrd.2018.110. [DOI] [PubMed] [Google Scholar]
- 67.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 68.Mintzer M.A., Simanek E.E. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 69.Buck J., Grossen P., Cullis P.R., Huwyler J., Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13:3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 70.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 71.Li W., Szoka F.C. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res (N Y) 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 72.Conceição M., Mendonça L., Nóbrega C., Gomes C., Costa P., Hirai H. Intravenous administration of brain-targeted stable nucleic acid lipid particles alleviates Machado-Joseph disease neurological phenotype. Biomaterials. 2016;82:124–137. doi: 10.1016/j.biomaterials.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 73.Carradori D., Eyer J., Saulnier P., Préat V., des Rieux A. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials. 2017;123:77–91. doi: 10.1016/j.biomaterials.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 74.Niu S., Zhang L.K., Zhang L., Zhuang S., Zhan X., Chen W.Y. Inhibition by multifunctional magnetic nanoparticles loaded with α-synuclein RNAi plasmid in a Parkinson's disease model. Theranostics. 2017;7:344–356. doi: 10.7150/thno.16562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 76.Kojima R., Bojar D., Rizzi G., Hamri G.C., El-Baba M.D., Saxena P. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment. Nat Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen W., Luan J., Wei G., Zhang X., Fan J., Zai W. In vivo hepatocellular expression of interleukin-22 using penetratin-based hybrid nanoparticles as potential anti-hepatitis therapeutics. Biomaterials. 2018;187:66–80. doi: 10.1016/j.biomaterials.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 78.Malhotra M., Tomaro-Duchesneau C., Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34:1270–1280. doi: 10.1016/j.biomaterials.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Morris V.B., Labhasetwar V. Arginine-rich polyplexes for gene delivery to neuronal cells. Biomaterials. 2015;60:151–160. doi: 10.1016/j.biomaterials.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borel F., Kay M.A., Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol Ther. 2014;22:692–701. doi: 10.1038/mt.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aguzzi A., O'Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9:237–248. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 82.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 83.Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 84.Hetz C., Russelakis-Carneiro M., Maundrell K., Castilla J., Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abisambra J.F., Jinwal U.K., Blair L.J., O'Leary J.C., Li Q., Brady S. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33:9498–9507. doi: 10.1523/JNEUROSCI.5397-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B. α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu Y., Park K.K., Yang L., Wei X., Yang Q., Cho K.S. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang L., Li S., Miao L., Huang H., Liang F., Teng X. Rescue of glaucomatous neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J Neurosci. 2016;36:5891–5903. doi: 10.1523/JNEUROSCI.3709-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang H., Miao L., Liang F., Liu X., Xu L., Teng X. Neuroprotection by eIF2α-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valenzuela V., Martinez G., Duran-Aniotz C., Hetz C. Gene therapy to target ER stress in brain diseases. Brain Res. 2016;1648:561–570. doi: 10.1016/j.brainres.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 91.Zuleta A., Vidal R.L., Armentano D., Parsons G., Hetz C. AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington's disease. Biochem Biophys Res Commun. 2012;420:558–563. doi: 10.1016/j.bbrc.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 92.Sado M., Yamasaki Y., Iwanaga T., Onaka Y., Ibuki T., Nishihara S. Protective effect against Parkinson's disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 93.Valdés P., Mercado G., Vidal R.L., Molina C., Parsons G., Court F.A. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci U S A. 2014;111:6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorbatyuk M.S., Shabashvili A., Chen W., Meyers C., Sullivan L.F., Salganik M. Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol Ther. 2012;20:1327–1337. doi: 10.1038/mt.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filézac de L'Etang A., Maharjan N., Cordeiro Braña M., Ruegsegger C., Rehmann R., Goswami A. Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat Neurosci. 2015;18:227–238. doi: 10.1038/nn.3903. [DOI] [PubMed] [Google Scholar]
- 96.Obacz J., Avril T., Le Reste P.J., Urra H., Quillien V., Hetz C. Endoplasmic reticulum proteostasis in glioblastomas—from molecular mechanisms to therapeutic perspectives. Sci Signal. 2017;10 doi: 10.1126/scisignal.aal2323. eaal2323. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Chen W., Fan J., Wang S., Xian Z., Luan J. Disrupting CD47-SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. 2018;39:689–699. doi: 10.1093/carcin/bgy041. [DOI] [PubMed] [Google Scholar]
- 98.Crino P.B. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol Med. 2011;17:734–742. doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Bockaert J., Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- 100.Lipton J.O., Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang H., Miao L., Yang L., Liang F., Wang Q., Zhuang P. AKT-dependent and -independent pathways mediate PTEN deletion-induced CNS axon regeneration. Cell Death Dis. 2019;10:203. doi: 10.1038/s41419-018-1289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang L., Miao L., Liang F., Huang H., Teng X., Li S. The mTORC1 effectors S6K1 and 4E-BP play different roles in CNS axon regeneration. Nat Commun. 2014;5:5416. doi: 10.1038/ncomms6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miao L., Yang L., Huang H., Liang F., Ling C., Hu Y. mTORC1 is necessary but mTORC2 and GSK3β are inhibitory for AKT3-induced axon regeneration in the central nervous system. Elife. 2016;5 doi: 10.7554/eLife.14908. e14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ries V., Henchcliffe C., Kareva T., Rzhetskaya M., Bland R., During M.J. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson's disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng H.C., Kim S.R., Oo T.F., Kareva T., Yarygina O., Rzhetskaya M. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oddo S. The role of mTOR signaling in Alzheimer's disease. Front Biosci. 2012;4:941–952. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morita T., Sobue K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J Biol Chem. 2009;284:27734–27745. doi: 10.1074/jbc.M109.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee J.H., Tecedor L., Chen Y.H., Monteys A.M., Sowada M.J., Thompson L.M. Reinstating aberrant mTORC1 activity in Huntington's disease mice improves disease phenotypes. Neuron. 2015;85:303–315. doi: 10.1016/j.neuron.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ravikumar B., Vacher C., Berger Z., Davies J.E., Luo S., Oroz L.G. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 111.Pryor W.M., Biagioli M., Shahani N., Swarnkar S., Huang W.C., Page D.T. Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington's disease. Sci Signal. 2014;7:ra103. doi: 10.1126/scisignal.2005633. [DOI] [PubMed] [Google Scholar]
- 112.Murphy M.P., Hartley R.C. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 113.Johri A., Beal M.F. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Therapeut. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moreira P.I., Zhu X., Wang X., Lee H.G., Nunomura A., Petersen R.B. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W., Zhang X., Fan J., Zai W., Luan J., Li Y. Tethering interleukin-22 to apolipoprotein A-I ameliorates mice from acetaminophen-induced liver injury. Theranostics. 2017;7:4135–4148. doi: 10.7150/thno.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dawson T.M., Dawson V.L. Mitochondrial mechanisms of neuronal cell death: potential therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:437–454. doi: 10.1146/annurev-pharmtox-010716-105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McManus M.J., Murphy M.P., Franklin J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams P.A., Harder J.M., Foxworth N.E., Cochran K.E., Philip V.M., Porciatti V. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Long A.N., Owens K., Schlappal A.E., Kristian T., Fishman P.S., Schuh R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol. 2015;15:19. doi: 10.1186/s12883-015-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bingol B., Tea J.S., Phu L., Reichelt M., Bakalarski C.E., Song Q. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510:370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 121.Dong Z., Wolfer D.P., Lipp H.P., Büeler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Tsunemi T., Ashe T.D., Morrison B.E., Soriano K.R., Au J., Roque R.A. PGC-1α rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003799. 142ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Onyango I.G., Dennis J., Khan S.M. Mitochondrial dysfunction in Alzheimer's disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201–214. doi: 10.14336/AD.2015.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berson A., Nativio R., Berger S.L., Bonini N.M. Epigenetic regulation in neurodegenerative diseases. Trends Neurosci. 2018;41:587–598. doi: 10.1016/j.tins.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gjoneska E., Pfenning A.R., Mathys H., Quon G., Kundaje A., Tsai L.H. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nativio R., Donahue G., Berson A., Lan Y., Amlie-Wolf A., Tuzer F. Dysregulation of the epigenetic landscape of normal aging in Alzheimer's disease. Nat Neurosci. 2018;21:497–505. doi: 10.1038/s41593-018-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pavlou M.A.S., Pinho R., Paiva I., Outeiro T.F. The yin and yang of α-synuclein-associated epigenetics in Parkinson's disease. Brain. 2017;140:878–886. doi: 10.1093/brain/aww227. [DOI] [PubMed] [Google Scholar]
- 128.Vashishtha M., Ng C.W., Yildirim F., Gipson T.A., Kratter I.H., Bodai L. Targeting H3K4 trimethylation in Huntington disease. Proc Natl Acad Sci U S A. 2013;110:E3027–E3036. doi: 10.1073/pnas.1311323110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J., Hart R.P., Mallimo E.M., Swerdel M.R., Kusnecov A.W., Herrup K. EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci. 2013;16:1745–1753. doi: 10.1038/nn.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hockly E., Richon V.M., Woodman B., Smith D.L., Zhou X., Rosa E. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steffan J.S., Bodai L., Pallos J., Poelman M., McCampbell A., Apostol B.L. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 132.Ferrante R.J., Kubilus J.K., Lee J., Ryu H., Beesen A., Zucker B. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen W., Dong G., Wu Y., Zhang W., Miao C., Sheng C. Dual NAMPT/HDAC inhibitors as a new strategy for multitargeting antitumor drug discovery. ACS Med Chem Lett. 2017;9:34–38. doi: 10.1021/acsmedchemlett.7b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bustos F.J., Ampuero E., Jury N., Aguilar R., Falahi F., Toledo J. Epigenetic editing of the Dlg4/PSD95 gene improves cognition in aged and Alzheimer's disease mice. Brain. 2017;140:3252–3268. doi: 10.1093/brain/awx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kacher R., Lamazière A., Heck N., Kappes V., Mounier C., Despres G. CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington's disease. Brain. 2019;142:2432–2450. doi: 10.1093/brain/awz174. [DOI] [PubMed] [Google Scholar]
- 136.Scrivo A., Bourdenx M., Pampliega O., Cuervo A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018;17:802–815. doi: 10.1016/S1474-4422(18)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiang H.G., Zhang J.F., Lin C.C., Zhang L., Liu B., Ouyang L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. 2020;10:569–581. doi: 10.1016/j.apsb.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du F., Yu Q., Yan S., Hu G., Lue L.F., Walker D.G. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain. 2017;140:3233–3251. doi: 10.1093/brain/awx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xilouri M., Brekk O.R., Landeck N., Pitychoutis P.M., Papasilekas T., Papadopoulou-Daifoti Z. Boosting chaperone-mediated autophagy in vivo mitigates α-synuclein-induced neurodegeneration. Brain. 2013;136:2130–2146. doi: 10.1093/brain/awt131. [DOI] [PubMed] [Google Scholar]
- 140.Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., Björklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y., Zhou B., Lin M.Y., Wang S., Foust K.D., Sheng Z.H. Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron. 2015;87:355–370. doi: 10.1016/j.neuron.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 145.Bertram L., Lange C., Mullin K., Parkinson M., Hsiao M., Hogan M.F. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong L., Xu Y., Zhuo R., Wang T., Wang K., Huang R. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer's disease model. Nat Commun. 2019;10:1365. doi: 10.1038/s41467-019-09118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Minami S.S., Min S.W., Krabbe G., Wang C., Zhou Y., Asgarov R. Progranulin protects against amyloid β deposition and toxicity in Alzheimer's disease mouse models. Nat Med. 2014;20:1157–1164. doi: 10.1038/nm.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allen N.J., Eroglu C. Cell biology of astrocytesynapse interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Valori C.F., Guidotti G., Brambilla L., Rossi D. Astrocytes: emerging therapeutic targets in neurological disorders. Trends Mol Med. 2019;25:750–759. doi: 10.1016/j.molmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 150.Kunze C., Börner K., Kienle E., Orschmann T., Rusha E., Schneider M. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia. 2018;66:413–427. doi: 10.1002/glia.23254. [DOI] [PubMed] [Google Scholar]
- 151.Cannon J.R., Sew T., Montero L., Burton E.A., Greenamyre J.T. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp Neurol. 2011;228:41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gu J., Al-Bayati K., Ho E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood–brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv Transl Res. 2017;7:497–506. doi: 10.1007/s13346-017-0368-5. [DOI] [PubMed] [Google Scholar]
- 153.Tanaka H., Nakatani T., Furihata T., Tange K., Nakai Y., Yoshioka H. In vivo introduction of mRNA encapsulated in lipid nanoparticles to brain neuronal cells and astrocytes via intracerebroventricular administration. Mol Pharm. 2018;15:2060–2067. doi: 10.1021/acs.molpharmaceut.7b01084. [DOI] [PubMed] [Google Scholar]
- 154.Liu S., Gao Y., Zhou D., Zeng M., Alshehri F., Newland B. Highly branched poly(β-amino ester) delivery of minicircle DNA for transfection of neurodegenerative disease related cells. Nat Commun. 2019;10:3307. doi: 10.1038/s41467-019-11190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.André E.M., Passirani C., Seijo B., Sanchez A., Montero-Menei C.N. Nano and microcarriers to improve stem cell behaviour for neuroregenerative medicine strategies: application to Huntington's disease. Biomaterials. 2016;83:347–362. doi: 10.1016/j.biomaterials.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 156.Jönsson M.E., Ludvik Brattas P., Gustafsson C., Petri R., Yudovich D., Pircs K. Activation of neuronal genes via LINE-1 elements upon global DNA demethylation in human neural progenitors. Nat Commun. 2019;10:3182. doi: 10.1038/s41467-019-11150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 158.Foust K.D., Wang X., McGovern V.L., Braun L., Bevan A.K., Haidet A.M. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Murrey D.A., Naughton B.J., Duncan F.J., Meadows A.S., Ware T.A., Campbell K.J. Feasibility and safety of systemic rAAV9-hNAGLU delivery for treating mucopolysaccharidosis IIIB: toxicology, biodistribution, and immunological assessments in primates. Hum Gene Ther Clin Dev. 2014;25:72–84. doi: 10.1089/humc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mattar C.N., Waddington S.N., Biswas A., Johana N., Ng X.W., Fisk A.S. Systemic delivery of scAAV9 in fetal macaques facilitates neuronal transduction of the central and peripheral nervous systems. Gene Ther. 2013;20:69–83. doi: 10.1038/gt.2011.216. [DOI] [PubMed] [Google Scholar]
- 161.Choudhury S.R., Fitzpatrick Z., Harris A.F., Maitland S.A., Ferreira J.S., Zhang Y. In vivo selection yields AAV-B1 capsid for central nervous system and muscle gene therapy. Mol Ther. 2016;24:1247–1257. doi: 10.1038/mt.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choudhury S.R., Harris A.F., Cabral D.J., Keeler A.M., Sapp E., Ferreira J.S. Widespread central nervous system gene transfer and silencing after systemic delivery of novel AAV-AS vector. Mol Ther. 2016;24:726–735. doi: 10.1038/mt.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tardieu M., Zérah M., Gougeon M.L., Ausseil J., de Bournonville S., Husson B. Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial. Lancet Neurol. 2017;16:712–720. doi: 10.1016/S1474-4422(17)30169-2. [DOI] [PubMed] [Google Scholar]
- 164.Cearley C.N., Wolfe J.H. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hinderer C., Bell P., Katz N., Vite C.H., Louboutin J.P., Bote E. Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum Gene Ther. 2018;29:15–24. doi: 10.1089/hum.2017.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hinderer C., Bell P., Gurda B.L., Wang Q., Louboutin J.P., Zhu Y. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther. 2014;22:2018–2027. doi: 10.1038/mt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Samaranch L., Salegio E.A., San Sebastian W., Kells A.P., Foust K.D., Bringas J.R. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Levites Y., Jansen K., Smithson L.A., Dakin R., Holloway V.M., Das P. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid β, amyloid β40, and amyloid β42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Armbruster N., Lattanzi A., Jeavons M., Van Wittenberghe L., Gjata B., Marais T. Efficacy and biodistribution analysis of intracerebroventricular administration of an optimized scAAV9-SMN1 vector in a mouse model of spinal muscular atrophy. Mol Ther Methods Clin Dev. 2016;3:16060. doi: 10.1038/mtm.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dirren E., Aebischer J., Rochat C., Towne C., Schneider B.L., Aebischer P. SOD1 silencing in motoneurons or glia rescues neuromuscular function in ALS mice. Ann Clin Transl Neurol. 2015;2:167–184. doi: 10.1002/acn3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ahmed S.G., Waddington S.N., Boza-Morán M.G., Yáñez-Muñoz R.J. High-efficiency transduction of spinal cord motor neurons by intrauterine delivery of integration-deficient lentiviral vectors. J Contr Release. 2018;273:99–107. doi: 10.1016/j.jconrel.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang L., Xiao R., Andres-Mateos E., Vandenberghe L.H. Single stranded adeno-associated virus achieves efficient gene transfer to anterior segment in the mouse eye. PloS One. 2017;12 doi: 10.1371/journal.pone.0182473. e0182473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vandenberghe L.H., Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–168. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- 175.Trapani I., Auricchio A. Seeing the light after 25 years of retinal gene therapy. Trends Mol Med. 2018;24:669–681. doi: 10.1016/j.molmed.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 176.Miller J.W., Vandenberghe L.H. Breaking and sealing barriers in retinal gene therapy. Mol Ther. 2018;26:2081–2082. doi: 10.1016/j.ymthe.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takahashi K., Igarashi T., Miyake K., Kobayashi M., Yaguchi C., Iijima O. Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Mol Ther. 2017;25:296–302. doi: 10.1016/j.ymthe.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Biferi M.G., Cohen-Tannoudji M., Cappelletto A., Giroux B., Roda M., Astord S. A newAAV10-U7-mediated gene therapy prolongs survival and restores function in an ALS mouse model. Mol Ther. 2017;25:2038–2052. doi: 10.1016/j.ymthe.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Amado D., Mingozzi F., Hui D., Bennicelli J.L., Wei Z., Chen Y. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000659. 21ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vandamme C., Adjali O., Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chandler R.J., Sands M.S., Venditti C.P. Recombinant adenoassociated viral integration and genotoxicity: insights from animal models. Hum Gene Ther. 2017;28:314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nault J.C., Datta S., Imbeaud S., Franconi A., Mallet M., Couchy G. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 184.Gil-Farina I., Fronza R., Kaeppel C., Lopez-Franco E., Ferreira V., D'Avola D. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol Ther. 2016;24:1100–1105. doi: 10.1038/mt.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]