Abstract
Flower malformation represented by phyllody is a common symptom of phytoplasma infection induced by a novel family of phytoplasma effectors called phyllogens. Despite the accumulation of functional and structural phyllogen information, the molecular mechanisms of phyllody have not yet been integrated with their evolutionary aspects due to the limited data on their homologs across diverse phytoplasma lineages. Here, we developed a novel universal PCR‐based approach to identify 25 phytoplasma phyllogens related to nine “Candidatus Phytoplasma” species, including four species whose phyllogens have not yet been identified. Phylogenetic analyses showed that the phyllogen family consists of four groups (phyl‐A, ‐B, ‐C, and ‐D) and that the evolutionary relationships of phyllogens were significantly distinct from those of phytoplasmas, suggesting that phyllogens were transferred horizontally among phytoplasma strains and species. Although phyllogens belonging to the phyl‐A, ‐C, and ‐D groups induced phyllody, the phyl‐B group lacked the ability to induce phyllody. Comparative functional analyses of phyllogens revealed that a single amino acid polymorphism in phyl‐B group phyllogens prevented interactions between phyllogens and A‐ and E‐class MADS domain transcription factors (MTFs), resulting in the inability to degrade several MTFs and induce phyllody. Our finding of natural variation in the function of phytoplasma effectors provides new insights into molecular mechanisms underlying the aetiology of phytoplasma diseases.
Keywords: horizontal gene transfer, MADS domain transcription factors, natural variation, phyllody, phyllogen, phytoplasma, α‐helix
Comparison of phyllogen, a phyllody‐inducing effector family, revealed its molecular evolution and functional variation attributed to a single amino acid polymorphism governing the phyllody symptoms of phytoplasma infection.
1. INTRODUCTION
Phytoplasmas (“Candidatus Phytoplasma” spp.) are obligate intracellular plant pathogens in the class Mollicutes. They are transmitted by insect vectors such as leafhoppers, planthoppers, and psyllids (Namba, 2019), and can infect more than 1,000 plant species worldwide (Marcone, 2014). Phytoplasma‐infected plants exhibit a wide range of unique symptoms, including flower malformation, yellowing, dwarfing, witches’ broom, purple tops, and phloem necrosis (Namba, 2019). Although the molecular mechanisms behind these symptoms are not fully understood, several secreted proteins of phytoplasmas, called effectors, can induce these symptoms (Hoshi et al., 2009; Sugio et al., 2011; Maejima et al., 2014; Minato et al., 2014).
Flower malformation, such as phyllody (floral organs turning into leaf‐like structures), virescence (green colouration of floral organs), and a loss of floral meristem determinacy (the production of stem‐like pistils), is a common symptom of phytoplasma infection (Chaturvedi et al., 2010; Musetti and Pagliari, 2019). Recently, it was reported that a novel gene family of phytoplasma effectors, designated phyllody‐inducing genes or the phyllogen family, induces flower malformation in several eudicots (MacLean et al., 2011; Maejima et al., 2014; Yang et al., 2015; Kitazawa et al., 2017). Phyllogens target the products of floral homeotic genes that constitute the floral quartet model (MacLean et al., 2014; Maejima et al., 2014), which in turn encode MADS domain transcription factors (MTFs) that are divided into four classes functionally: A, B, C, and E (Smaczniak et al., 2012). Phyllogens recognize A‐ and E‐class MTFs of angiosperms and degrade them in a proteasome‐dependent manner (MacLean et al., 2014; Maejima et al., 2014, 2015; Kitazawa et al., 2017). Additionally, SAP54, a phyllogen of “Ca. P. asteris” AY‐WB strain, interacts with two isoforms of the radiation sensitive 23 (RAD23) family, RAD23C/D (MacLean et al., 2014). RAD23C/D are substrate shuttle factors that can transfer ubiquitinated proteins to the 26S proteasome (Farmer et al., 2010) and are essential for phyllogen‐mediated phyllody induction (MacLean et al., 2014). Recent crystal structure analyses have revealed that the K domain of MTFs, a phyllogen‐binding region (MacLean et al., 2014), has two α‐helices with conserved hydrophobic residues that are important for the tetramerization of MTFs (Puranik et al., 2014). Two phyllogens (PHYL1OY and PHYL1PnWB, phyllogens of “Ca. P. asteris” OY and “Ca. P. aurantifolia” PnWB strains, respectively) have similar structures based on two α‐helices that are important for phyllody‐inducing‐activity (Iwabuchi et al., 2019; Liao et al., 2019). Thus, the function and structure of a couple of phyllogens have been reported; however, the molecular mechanisms of phyllody have not yet been integrated with evolutionary aspects due to the limited data on their homologs across diverse phytoplasma lineages.
The accumulation of phytoplasma genome information has enabled the identification of phyllogens from seven “Ca. Phytoplasma” species (Chung et al., 2013; Maejima et al., 2014; Mitrović et al., 2014; Wang et al., 2018a; Fernández et al., 2019), while phyllogens have not yet been found in several phytoplasmas that induce phyllody symptoms in their host plants, such as “Ca. P. japonicum” (Arashida et al., 2008b). Phyllogen genes have been identified using two approaches: PCR using specific primers targeting up‐ and downstream regions of phyllogens and whole‐ or draft‐genome sequencing. The former approach is based on the fact that several phyllogens have been found around the clusters of repeated gene sequences, namely, potential mobile units (PMUs) (Jomantiene et al., 2007; Arashida et al., 2008a; Sugio and Hogenhout, 2012). Phyllogens sharing highly conserved sequences with known phyllogen genes, such as PHYL1OY, were identified in 17 strains related to four different “Ca. Phytoplasma” species using a PCR‐based method (Maejima et al., 2014; Fernández et al., 2019). An alternative approach, whole‐ or draft‐genome sequencing, could identify phyllogens unable to be detected with PCR‐based methods. The phyllogen gene PHYL1PnWB, identified using this approach, was not located near PMUs (Chung et al., 2013). Nevertheless, no phyllogens have been found in draft‐genome sequences of “Ca. P. phoenicium”, “Ca. P. pruni”, or “Ca. P. oryzae” (Lee et al., 2015; Quaglino et al., 2015; Fischer et al., 2016), possibly due to the difficulty in determining the complete genome sequences of phytoplasmas. Therefore, more universal and efficient means to identify phyllogen genes from a variety of phytoplasmas are required.
Here, we report a novel approach to identifying diverse phyllogens by focusing on two amino acid regions conserved in the gene family. Phylogenetic analyses based on determined phyllogen gene sequences strongly suggest that phyllogen genes are horizontally transferred among phytoplasmas. By comparing variation in natural function among members of the phyllogen family, we found that a group of phyllogens lacks the ability to induce phyllody and identified a polymorphic residue that is essential for phyllody‐inducing activity as well as the degradation of A‐ and E‐class MTFs.
2. RESULTS
2.1. Identification of phyllogens with universal PCR and genome walking
Multiple sequence alignment of three phyllogen proteins (PHYL1OY, PHYL1PnWB, and SAP54) showed the presence of two conserved amino acid regions within the secreted part (Figure S2a). We designed a degenerate primer pair (PHYL‐F/R) on these conserved regions that also matched all other known phyllogens (Figure S3). PCR using the primer pair resulted in the amplification of DNA fragments ranging from 177 to 210 bp from the genomic DNA of 25 phytoplasma strains (Figure S2c; indicated as “this study” in Table S1). These strains were related to nine species, including four species (“Ca. P. fragariae”, “Ca. P. fraxini”, “Ca. P. japonicum”, and “Ca. P. oryzae”) whose phyllogen genes have not been reported (Table S1). Each amplicon shared >70% nucleotide sequence identity with at least one of the known phyllogen genes, which suggests that each of the amplified DNA fragments is part of a phyllogen (Table S3). We performed genome walking for nine strains related to seven species (“Ca. P. asteris” HP and PvWB, “Ca. P. aurantifolia” FBP and WBDL, “Ca. P. fragariae” SY, “Ca. P. fraxini” ASHy2, “Ca. P. japonicum” JHP, “Ca. P. oryzae” RYD, and “Ca. P. ziziphi” JWB strains) to determine the up‐ and downstream sequences of each PCR amplicon (Figure S2b). The 202–1,358 bp of upstream sequences and 464–1,508 bp of downstream sequences obtained were assembled with the corresponding sequences between primers PHYL‐F/R. The validity of each assembled sequence was examined with PCR amplification using a primer pair designed on the up‐ and downstream sequences (Figure S2b), which resulted in the amplification of a DNA fragment of appropriate size (Figure S2d). In each assembled sequence, a putative ribosome‐binding site (5′‐AAGGAG‐3′; Berg and Seemüller, 1999) and a start and in‐frame stop codon were found at almost the same positions as the known phyllogens. Thus, we identified nine new full‐length phyllogen genes. Several phyllogens, such as the one from the ASHy2 strain (PHYL1ASHy2), lost their α‐helix structure (indicated by the symbol Ψ in Table S1 and Figure S3) because of an additional premature stop codon.
A putative signal peptide cleavage site and two consensus α‐helices were predicted at the same location in each phyllogen (Figure 1a). Hydrophobic residues in the two α‐helical regions, which are important for phyllody‐inducing activity (Iwabuchi et al., 2019; Liao et al., 2019), were highly conserved among diverse phyllogens identified in this and previous studies (Figures 1a and S4; positions a, b, d, and e on helix 1 and positions a, d, and g on helix 2). The conserved hydrophobic residues at positions a and d on each helix (I20, I24, L27, I31, L58, I69, L76, and L83; numbering based on PHYL1OY excluding signal peptides) were oriented toward the interiors of both α‐helices (Figure S4). In contrast, the conserved hydrophobic residues at positions b and e on helix 1 (I25 and A35) and position g on helix 2 (L61, L68, and L82), which are suggested to be available to interact with host factors (Liao et al., 2019), are exposed on the protein surface (Figures 1b and S4). These results suggest that the conserved hydrophobic residues support protein structural integrity and the ability to interact with host factors of phyllogen.
FIGURE 1.
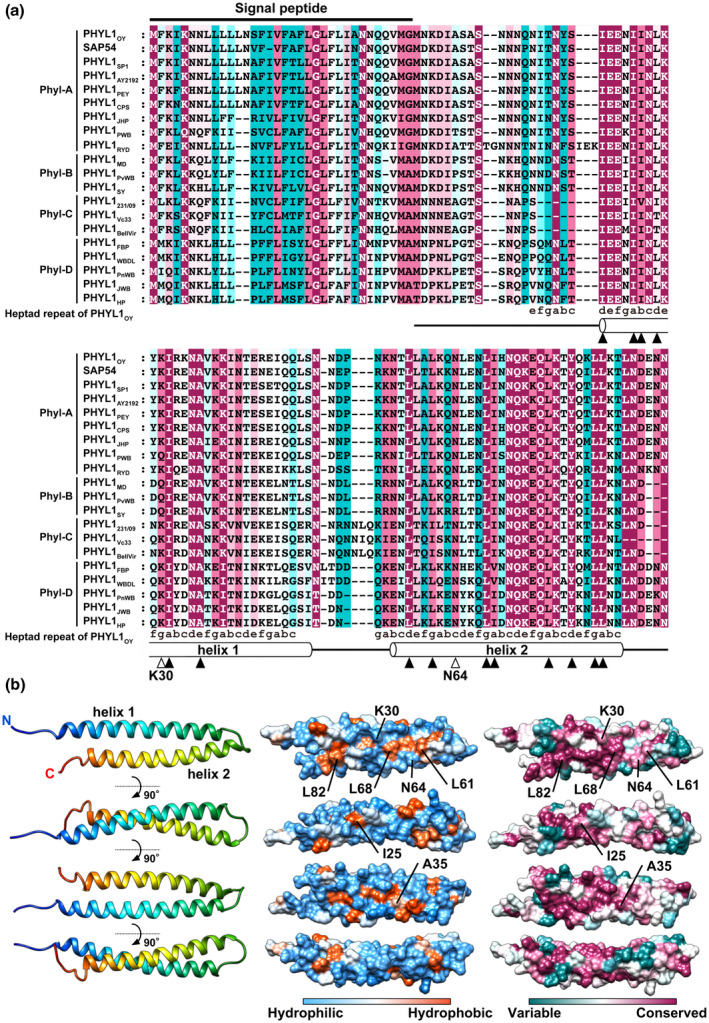
Amino acid conservation and structural properties of phyllogens. (a) Alignment of the full‐length protein sequences of the phyllogen family. Phyllogen protein sequences were aligned using the MUSCLE algorithm. Consensus secondary structure elements of phyllogens predicted by PROMALS3D are depicted below. Open boxes represent α‐helices. Filled and open arrowheads indicate conserved hydrophobic residues and polymorphic residues between phyl‐B and the other groups (K30 and N64, numbering based on PHYL1OY excluding signal peptides), respectively. Sequence conservation calculations were performed with ConSurf (Ashkenazy et al., 2016). Conservation scores range from cyan (not conserved) to white (average) and to magenta (highly conserved). (b) Surface structure properties of PHYL1OY. Overall view of the ribbon diagram (left), hydrophobicity surface (middle), and conserved structure surface (right) of PHYL1OY (PDB ID: 6JQA, residues 7–91 of subunit A without iodine atoms). Hydrophobicity scores range from blue (mostly hydrophilic) to white (average) and to orange red (mostly hydrophobic). Conservation scores range as (a)
2.2. Evolutionary and structural relationships in the phyllogen family
Pairwise sequence comparisons showed considerable sequence variation among the phyllogen family, with >66.4% and >40.9% sequence identity at the nucleotide and amino acid levels, respectively (Table S4). To investigate the evolutionary relationship within the gene family, we performed a phylogenetic comparison on phyllogen and the 16S rRNA gene representing the phylogeny of phytoplasmas. The neighbour‐joining tree using 43 full‐length phyllogen nucleotide sequences indicated that the phyllogen family can be divided into four distinct groups supported with high bootstrap values (78%, 99%, 99%, and 99% for phyl‐A, ‐B, ‐C, and ‐D, respectively; Figure 2b). Interestingly, the tree topology of the phyllogen gene was clearly different from that of the 16S rRNA gene; each phyllogen group consisted of diverse “Ca. Phytoplasma” species (Figure 2a). Phyllogens of “Ca. P. asteris” were included in three different groups—phyl‐B (MD and PvWB strains), phyl‐D (HP and SWB strains), and phyl‐A (the other strains)—and phyllogens of “Ca. P. pruni” were included in both phyl‐C (BellVir and Vc33 strains) and phyl‐A (the other strains; Figure 2b). Similar results were obtained from the tree topology of the phyllogen gene based on partial nucleotide sequences between the PHYL‐F/R regions of all strains (Figure S5). These results indicate that the phyllogen family has a different evolutionary history from the 16S rRNA gene.
FIGURE 2.
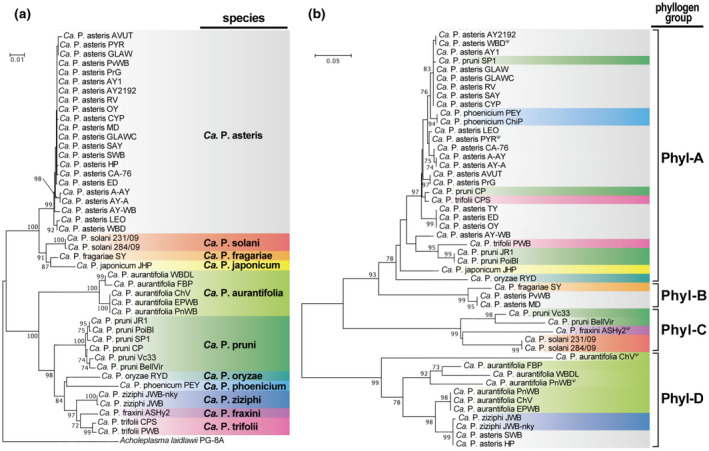
Phylogenetic comparison of the phyllogen family. (a) and (b) Neighbour‐joining phylogenetic tree based on 16S rRNA between primers SN910601/SN910502 (a) and full‐length phyllogen (b) gene nucleotide sequences. Sequences were aligned with the MUSCLE multiple alignment algorithm and analysed with a complete‐deletion option. Acholeplasma laidlawii strain PG‐8A was used to root the tree of 16S rRNA. Numbers at the nodes represent the percentage of bootstrap values obtained for 1,000 replicates (only values >70% are shown). Bars indicate the number of nucleotide substitutions per site. For the C‐terminus truncated phyllogens due to the premature stop codon (indicated by Ψ), the nucleotide regions after the premature stop codons were also included in the MUSCLE alignment. Full strain names and GenBank accession numbers are listed in Table S1. Background colours define related “Candidatus Phytoplasma” species
Structural modelling of PHYL1SY and PHYL1231/09 belonging to the phyl‐B and ‐C groups, respectively, was performed based on the PHYL1OY structure (PDB ID: 6JQA). The resulting model (C‐scores of 0.21 and −0.08 for PHYL1SY and PHYL1231/09, respectively) also contained a coiled‐coil structure with a hydrophobic surface similar to that of phyl‐A or ‐D groups (Figure S6), which also supports the functional importance of the conserved hydrophobic residues.
2.3. The phyl‐B group does not induce phyllody
Three phyllogens belonging to either phyl‐A (PHYL1OY and SAP54) or ‐D (PHYL1PnWB) induce phyllody in Arabidopsis thaliana (MacLean et al., 2011; Maejima et al., 2014; Yang et al., 2015). To test whether phyllody‐inducing activity is conserved across the gene family, we selected for further study a variety of phyllogens of each group (phyl‐A: PHYL1JHP and PHYL1PWB; phyl‐B: PHYL1MD, PHYL1PvWB, and PHYL1SY; phyl‐C: PHYL1231/09; phyl‐D: PHYL1FBP and PHYL1JWB) in addition to PHYL1OY and PHYL1PnWB. Each phyllogen was expressed in A. thaliana using the tobacco rattle virus (TRV) vector as described by Iwabuchi et al. (2019). Then, 20–30 days after virus inoculation, A. thaliana plants infected with TRV‐PHYL1JHP, ‐PHYL1PWB, ‐PHYL1231/09, ‐PHYL1JWB, ‐PHYL1FBP, or ‐PHYL1PnWB showed almost the same phyllody phenotype as described previously (Figure 3a; Iwabuchi et al., 2019). Sepals, petals, and stamens were converted into enlarged leaf‐like structures covered with stellate trichomes, and the pistil reverted to a stem‐like structure with secondary flowers at the top as well as a loss of floral meristem determinacy (Figure 3a). Furthermore, the transient co‐expression assay in Nicotiana benthamiana showed that PHYL1231/09 and PHYL1PnWB significantly decreased the amount of SEP1–4 or AP1 as in the case of PHYL1OY (Figure 4a,c,d; Maejima et al., 2014, 2015).
FIGURE 3.
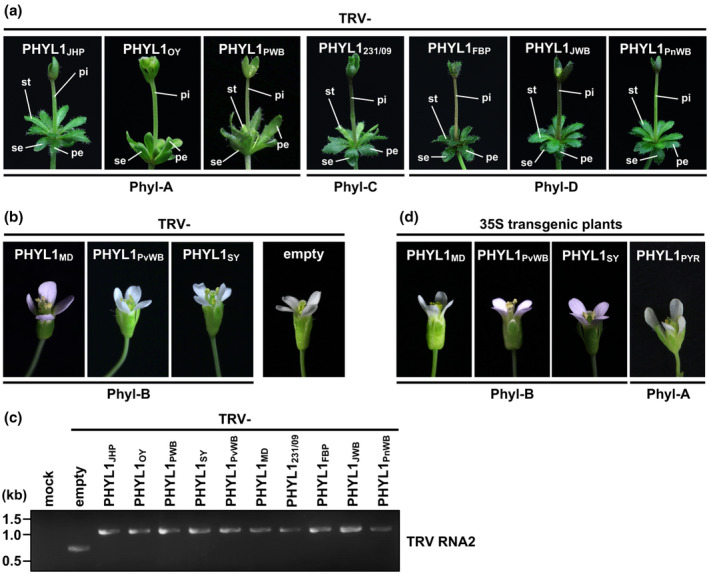
Phyllody‐inducing activity differs among phyllogen groups. (a) and (b) Floral phenotypes of Arabidopsis thaliana plants infected with the tobacco rattle virus (TRV) vector carrying phyllogens belonging to either the phyl‐A, ‐C, or ‐D group (a) or the phyl‐B group (b). Phyllody‐like phenotypes consisted of leaf‐like sepals (se), leaf‐like petals (pe), leaf‐like stamens (st), and a stem‐like pistil (pi). (c) Confirmation of the infection and insertion stability of the TRV vector by reverse transcription (RT)‐PCR. RT‐PCR was performed with primers flanking the site of insertion in RNA2 of the virus about 30 days after inoculation in A. thaliana plants. (d) Floral phenotypes of the phyl‐B group‐ and α‐helix truncated phyllogen (phyl‐A: PHYL1PYR)‐overexpressing transgenic A. thaliana plants
FIGURE 4.

PHYL1SY has little activity in the degradation of MADS domain transcription factors (MTFs). (a)–(d) Accumulation of transiently expressed Arabidopsis A‐ and E‐class MTFs upon co‐expression of phyllogens. Agrobacterium cultures (OD600 = 1.0) expressing P19, Myc‐fused MTFs (SEP1–4 and AP1), and either 3 × FLAG‐fused PHYL1s (phyl‐A: PHYL1OY [a], phyl‐B: PHYL1SY [b], phyl‐C: PHYL1231/09 [c], or phyl‐D: PHYL1PnWB [d]) were mixed at a ratio of 1:10:1 and infiltrated into Nicotiana benthamiana leaves. Accumulation of Myc‐ or FLAG‐fused proteins was evaluated 36 hr after infiltration by immunoblotting using an anti‐Myc (α‐myc) or anti‐FLAG (α‐flag) antibody. Coomassie brilliant blue‐stained membranes are shown as a loading control
In contrast, phyllogens belonging to phyl‐B (PHYL1MD, PHYL1PvWB, and PHYL1SY) induced no flower malformation in A. thaliana when expressed from the TRV vector (Figure 3b). Successful infection of the TRV vector and inserted sequence stability of each phyllogen gene therein were confirmed by reverse transcription (RT)‐PCR using pTRV2‐specific primers (Figure 3c). Floral organs in the 35S::PHYL1MD, 35S::PHYL1PvWB, and 35S::PHYL1SY transgenic A. thaliana also showed no malformation (Figure 3d). Similar results were obtained in flowers of N. benthamiana infected with TRV vector expressing PHYL1MD, PHYL1PvWB, or PHYL1SY (Figure S7). Because the non‐phyllody‐inducing phyllogens belonging to the phyl‐B group were almost identical (>92.4% at amino acid level), PHYL1SY was used to evaluate floral MTF‐degradation activity in planta. Compared to PHYL1OY, PHYL1SY did not significantly decrease the amount of SEP1–4 or AP1 (Figure 4b).
In addition, we tested the phyllody‐inducing activity of natural α‐helix‐truncated phyllogens due to early stop codons (Figure S3) using PHYL1PYR, which belongs to the phyl‐A group and does not induce degradation of SEP3 (Maejima et al., 2014). Floral organs in the 35S::PHYL1PYR transgenic A. thaliana showed no malformation (Figure 3d), in accordance with previous reports indicating the functional importance of the α‐helix (Iwabuchi et al., 2019; Liao et al., 2019).
2.4. Interaction specificity of the phyllogen family with A‐ and E‐class MTFs and RAD23
To determine the host factors involved in the loss of phyllody‐inducing activity in the phyl‐B group, we compared the interaction of phyllogens with Arabidopsis A‐ and E‐class MTFs and RAD23C/D using yeast two‐hybrid (Y2H) analyses (Table 1 and Figure S10). Phyllody‐inducing phyllogens (phyl‐A: PHYL1PWB, phyl‐C: PHYL1231/09, phyl‐D: PHYL1FBP, and PHYL1JWB) interacted with AP1 (A‐class), SEP1–4 (E‐class), and RAD23C/D to the same extent as PHYL1OY and PHYL1PnWB. Among non‐phyllody‐inducing phyllogens, PHYL1PYR failed to interact with AP1, SEP1, SEP2, SEP4, and RAD23C/D as well as SEP3 (Maejima et al., 2014). These results indicate that PHYL1PYR lost phyllody‐inducing activity because of the loss of interaction with these host factors. However, the phyl‐B group (PHYL1MD, PHYL1PvWB, and PHYL1SY) interacted with SEP1 and RAD23C/D to the same extent as the other phyllogens, although no interactions were observed with SEP4, and weak interactions were observed with AP1, SEP2, and SEP3. These results suggest that the phyl‐B group has a novel type of natural loss of phyllody‐inducing mutants due to changes in interaction specificity with MTFs.
TABLE 1.
Interaction specificity with floral MADS domain transcription factors and RAD23 protein in yeast cells
| Phyllogen group | DNA‐binding domain (bait) | Activation domain (prey) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Empty | SEP1 | SEP2 | SEP3 | SEP4 | AP1 | RAD23C | RAD23D | ||
| Empty | − | −a | −a | −b | −a | −b | −a | − | |
| Phyl‐A | PHYL1OY | −a | +++a | +++a | +++a | +++a | ++ | ++a | ++ |
| PHYL1PWB | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| PHYL1PYR Ψ | −b | − | − | −b | − | − | − | − | |
| Phyl‐B | PHYL1MD | − | ++ | + | + | − | + | ++ | ++ |
| PHYL1PvWB | − | ++ | + | + | − | + | ++ | ++ | |
| PHYL1SY | − | ++ | + | + | − | + | ++ | ++ | |
| PHYL1SY R64N | − | ++ | ++ | ++ | + | ++ | ++ | ++ | |
| PHYL1SY Q30K/R64N | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| Phyl‐C | PHYL1231/09 | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Phyl‐D | PHYL1FBP | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PHYL1JWB | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| PHYL1PnWB | − | ++ | ++ | ++c | ++ | ++ | ++ | ++ | |
+++ The yeast grew on all media; ++ the yeast grew on − LWH+3AT, −LWH, and − LW; + the yeast grew on − LWH and − LW; − the yeast grew only on − LW.
2.5. Polymorphic residues at position 64 in phyllogens responsible for phyllody induction
To elucidate the amino acid residue(s) involved in the functional variation among phyllogen groups, we compared their sequences. Two hydrophilic residues, lysine at position 30 (K30) and asparagine at position 64 (N64), were conserved among the phyl‐A, ‐C, and ‐D groups with one exception: PHYL1PWB, belonging to phyl‐A with glutamine at position 30 (Q30), but not in all members of phyl‐B (Q30 and arginine at position 64 [R64]; Figures 1a and S8; open arrowhead symbol). Mapping of both residues onto the PHYL1OY structure revealed that they were exposed on the same side of the PHYL1OY protein surface, unlike conserved hydrophobic residues at positions a and d, which are oriented toward the interiors of both α‐helices (Figures 1b, 5a and S4). To test whether K30 and N64 are important for phyllody‐inducing activity, we introduced reciprocal substitutions at positions 30 and 64 to those of PHYL1SY into PHYL1OY. PHYL1OY K30Q and PHYL1OY both induced severe flower malformation (Figures 3a and 5b). In contrast, PHYL1OY N64R induced moderate flower malformation, such as asymmetry of petals, but not phyllody. Moreover, PHYL1OY K30Q/N64R did not induce any flower malformation (Figure 5b), as in the case of the phyl‐B group. Similar results were obtained when mutations of corresponding K and N residues were introduced into PHYL1231/09 and PHYL1PnWB belonging to phyl‐C and phyl‐D, respectively (Figure 5b). Western blotting analyses showed that PHYL1OY K30Q/N64R did not decrease the amount of each Arabidopsis MTF compared to PHYL1OY (Figure 5c). These results suggest that N64 and K30 play critical and marginal roles, respectively, in phyllody induction.
FIGURE 5.
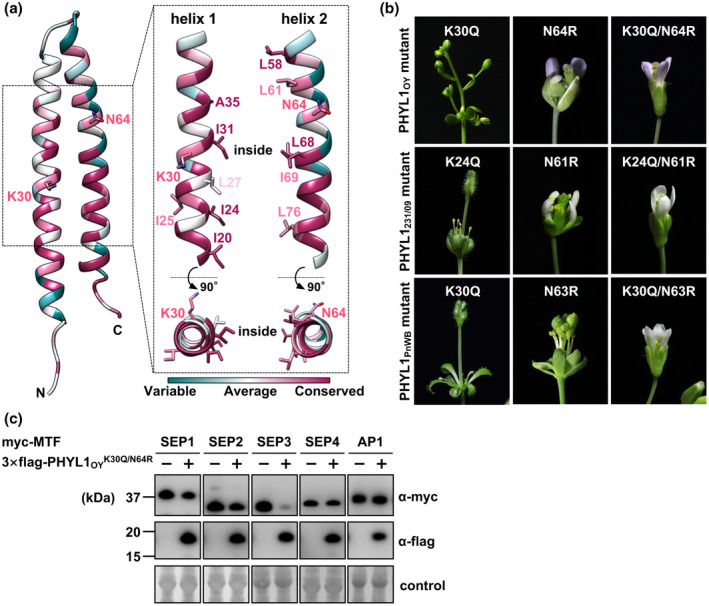
Substitution of conserved N64 residue abolishes the phyllody‐inducing activity of phyllogens. (a) Positions of polymorphic residues on ribbon representation of the crystal structure of PHYL1OY. Two hydrophilic residues (K30 and N64) that mutated to those of the phyl‐B group (Q30 and R64) and conserved hydrophobic residues such as leucine (L) and isoleucine (I) indicated in Figure 1a are shown as sticks. Conservation scores are mapped as in Figure 1b. (b) Floral phenotypes of Arabidopsis thaliana plants infected with tobacco rattle virus (TRV) vector carrying PHYL1OY K30Q and/or N64R mutants and PHYL1231/09 and PHYL1PnWB mutants of corresponding K and N residues (K24 and N61 for PHYL1231/09 and K30 and N63 for PHYL1PnWB, respectively). (c) Accumulation of transiently expressed Myc‐fused Arabidopsis floral MADS domain transcription factors (MTFs) upon co‐expression with 3 × FLAG‐fused PHYL1OY K30Q/N64R. In planta protein expression and detection were performed as described in Figure 4
To test whether these residues are involved in the loss of phyllody‐inducing activity in the phyl‐B group, and the mechanisms if they are, we introduced reciprocal substitutions at positions 30 and 64 to those of the other groups into PHYL1SY. Although PHYL1SY Q30K did not induce any flower malformation, PHYL1SY R64N and PHYL1SY Q30K/R64N induced similar phyllody phenotypes (Figure 6a), with slight differences in the morphology of stamens: PHYL1SY Q30K/R64N induced more greenish and enlarged leaf‐like stamens compared to PHYL1SY R64N. In contrast, PHYL1SY mutants at position 64 to other hydrophilic residues (asparagine acid [R64D], lysine [R64K], or glutamine [R64Q]) did not restore the phyllody‐inducing activity of PHYL1SY (Figure 6a). Western blotting analyses showed that PHYL1SY R64N decreased the amount of Arabidopsis AP1 and SEP1–4 MTFs compared to PHYL1SY (Figure 6b) and that PHYL1SY Q30K/R64N further enhanced activity (Figure 6c). These results indicate that N64R/K30Q substitutions also play critical and marginal roles, respectively, in the loss of phyllody‐inducing activity in the phyl‐B group. This supposition is supported by the fact that PHYL1PWB, which belongs to the phyl‐A group with Q30 (Figure 1a), also induced phyllody in A. thaliana (Figure 3a).
FIGURE 6.

R64N substitution is sufficient for conferring phyllody‐inducing activity on PHYL1SY. (a) Floral phenotypes of Arabidopsis thaliana plants infected with tobacco rattle virus (TRV) vector carrying PHYL1SY mutants at corresponding Q and R residues to K30 and N64 of PHYL1OY. Abbreviations of phyllody‐like phenotypes are described in Figure 3a. (b) and (c) Accumulation of transiently expressed Myc‐fused MADS domain transcription factors (MTFs; SEP1–4 and AP1) upon co‐expression with 3 × FLAG‐fused PHYL1SY R64N (b) or 3 × FLAG‐fused PHYL1SY Q30K/R64N (c). In planta protein expression and detection were performed as described in Figure 4
2.6. N64 residue regulates phyllogen–MTF interaction in cooperation with another polymorphic residue
To gain further insight into the role of N64 residue in phyllody induction, we examined the interaction of PHYL1SY mutants with host factors. Y2H assay showed that PHYL1SY R64N enhanced interactions with SEP2, SEP3, SEP4, and AP1 compared to PHYL1SY (Table 1 and Figure S10). PHYL1SY Q30K/R64N interacted with the MTFs to the same extent as the phyllody‐inducing phyllogens. To examine in planta interactions with the A‐ and E‐class MTFs of PHYL1SY R64N and PHYL1SY Q30K/R64N, we used co‐immunoprecipitation assays. SEP1‐, SEP2‐, SEP3‐, SEP4‐, and AP1–3 × Myc were coprecipitated with PHYL1SY R64N and PHYL1SY Q30K/R64N but not with PHYL1SY (Figure 7). Furthermore, each MTF was more efficiently coprecipitated with PHYL1SY Q30K/R64N compared to PHYL1SY R64N (Figure 7). These results suggest that N64 residue regulates the interaction of phyllogens with these MTFs in cooperation with K30.
FIGURE 7.
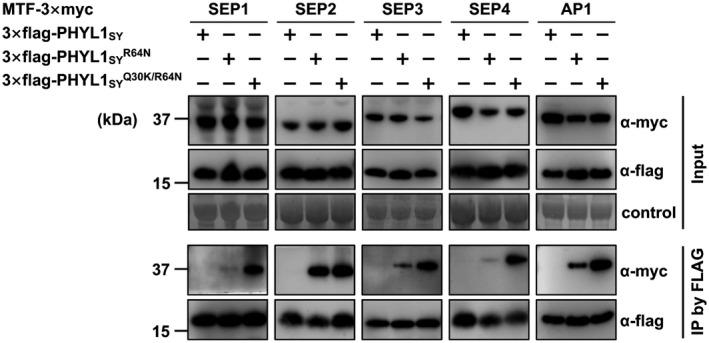
In planta interactions of PHYL1SY mutants with floral MADS domain transcription factors (MTFs). Agrobacterium cultures expressing 3 × Myc‐fused SEP1–4 and AP1 and either 3 × FLAG fused PHYL1SY or its mutants were mixed at a ratio of 1:1 and infiltrated into Nicotiana benthamiana leaves. Then, 36 hr after infiltration, total proteins were extracted and immunoprecipitation was performed with an α‐ FLAG antibody. The input and immunoprecipitated proteins (IP) were analysed by immunoblot analyses with α‐ FLAG and α‐Myc antibodies. Coomassie brilliant blue‐stained membranes are shown as a loading control
3. DISCUSSION
3.1. Biological implications of non‐phyllody‐inducing phyllogens
In this study, we identified new phyllogen genes of a wide variety of phytoplasmas (Table S1). The phyllogen family was composed of four distinct groups: phyl‐A, ‐B, ‐C, and ‐D (Figure 2). Phyllogens of phyl‐A, ‐C, and ‐D had phyllody‐inducing activity (Figure 3a), decreased the amount of SEP1–4 and AP1 in planta (Figure 4a,c,d; Maejima et al., 2014, 2015), and interacted with floral MTFs and RAD23C/D (Table 1), with the exception of α‐helix truncated phyllogens (Figures 3d, S3 and Table 1). In contrast, all members of the phyl‐B group failed to induce any flower malformation (Figures 3b,d and S7) or interact with MTFs while maintaining their interactions with SEP1 and RAD23C/D to the same extent as phyllody‐inducing phyllogens (Table 1). Furthermore, PHYL1SY, a representative of the phyl‐B group, did not decrease the amount of SEP1–4 and AP1 in planta (Figure 4b).
Although diverse phytoplasmas frequently induce phyllody symptoms (Chaturvedi et al., 2010; Musetti and Pagliari, 2019), there is little information on how functional diversity in effectors is involved in phytoplasma pathogenesis. In a variety of phyllogen families of diverse phytoplasmas, we identified phyllody‐inducing phyllogens from phyllody‐inducing phytoplasmas such as “Ca. P. japonicum” JHP and “Ca. P. aurantifolia” FBP (Figure 3a). In contrast, phyllody is not generally observed in diseased plants infected with phytoplasmas harbouring non‐phyllody‐inducing phyllogens, such as the phyl‐B group (Jung et al., 2003; Jiang et al., 2004; Tanaka et al., 2008). These results strongly suggest that natural functional variation in phyllogens is associated with the aetiology of phyllody in phytoplasma diseases. Considering that phytoplasmas harbouring the phyl‐B group derived from trees or vegetatively propagating plants (mulberry, porcelain vine, strawberry for PHYL1MD, PHYL1PvWB, and PHYL1SY, respectively), phyllody‐inducing activity could be dispensable for improvement of phytoplasma fitness in these perennial plants.
However, phyl‐B group phyllogens lacking the ability to induce phyllody still interacted with host factors, but the interaction specificity was different from that of phyllody‐inducing phyllogens—the former phyllogens interacted with RAD23C/D to the same extent as the latter phyllogens but not always with floral MTFs (Table 1). Reciprocal substitution analyses indicated that the phyl‐B‐group‐specific substitution of a polymorphic residue (R64) contributed to changes in interaction specificity with floral MTFs (Table 1 and Figure 7). These data may indicate that phyl‐B group phyllogens are not merely loss‐of‐function mutants but are a novel class of phyllogens that still have unresolved functions that are independent of phyllody induction. As an example of a phyllody‐independent function, SAP54, a phyllogen belonging to phyl‐A group that interacts with diverse non‐floral MTFs (MacLean et al., 2014), contributes to insect–vector attraction in a RAD23‐dependent but phyllody‐independent manner in A. thaliana (Orlovskis and Hogenhout, 2016). Although the mechanisms of the phyllody‐independent function of the phyllogen family are still unknown, further functional elucidation of the non‐phyllody‐inducing phyl‐B group may reveal unresolved roles of the effector family that improve phytoplasma fitness.
3.2. Phyllogen genes can be horizontally transferred among diverse phytoplasmas
To date, there is no universal and efficient method of identifying a variety of phyllogens from diverse phytoplasmas. To overcome this limitation, we developed a new approach based on PCR targeting of two conserved α‐helical regions of the protein family (Figure S2). Our identified phyllogens shared several conserved sequence characteristics with the well‐known phyllogens, such as the ribosome‐binding site, start and stop codons (Figure S3), signal peptide cleavage site, and two α‐helical secondary structures (Figure 1a), which indicates that each phyllogen is actually translated and secreted by the bacteria. As a result, this and previous studies have demonstrated that at least 59 strains related to 11 phytoplasma species (Table S1) have phyllogens with high sequence and evolutionary diversity (Table S4, Figures 2 and S5).
Although functional analyses of several effector homologs of phytoplasma have become areas of active research (Sugawara et al., 2013; Maejima et al., 2014; Chang et al., 2018; Wang et al., 2018b; Pecher et al., 2019), the evolutionary dynamics of these homologs have not yet been addressed. In the current study, phylogenetic analyses indicated that the phyllogen family evolved independently of “Ca. Phytoplasma” species, represented by the 16S rRNA phylogeny (Figures 2 and S5). Note that phyllogens found in closely related phytoplasmas (“Ca. P. asteris” and “Ca. P. pruni”) were separated into several different phylogenetic groups (Figure 2b). It is reasonable to hypothesize that phyllogens were transferred horizontally among phylogenetically distinct phytoplasmas. The acquisition of new genes via horizontal gene transfer contributes to the evolution of bacterial pathogens, particularly to the generation of new variants (Hacker et al., 1997; Pallen and Wren, 2007). Although the molecular mechanism of horizontal gene transfer among phytoplasmas remains poorly understood, PMUs are good candidates for the genetic mobile elements in diverse phytoplasma species because of their transposon‐like elements (Toruño et al., 2010; Chung et al., 2013; Ku et al., 2013). Most phyllogens belonging to phyl‐A are associated with PMUs (Jomantiene et al., 2007; Sugio and Hogenhout, 2012; Maejima et al., 2014). In one study, a phyllogen of the JWB‐nky strain (phyl‐D group) was located around a PMU (Wang et al., 2018a). These findings suggest that horizontal gene transfer of phyllogens by PMUs has contributed to the acquisition and sharing of phyllody‐inducing activity among phytoplasmas. Phytoplasmas have a wide plant host range and can naturally co‐infect the same host plants (Wei et al., 2016), whereas insect transmission is specific between different phytoplasmas and distinct insect vector taxa (Gonella et al., 2019). These results suggest that the co‐infection of phylogenetically diverse phytoplasma strains/species in their common plant hosts can facilitate horizontal gene transfer among phytoplasma genomes. Interestingly, however, it remains unclear how phyllogens originated due to the unavailability of potential ancestral protein sequences that are homologous with the phyllogen family, other than the phytoplasma genome, as previously mentioned (Rümpler et al., 2015). Further insights into the ancestral protein sequences of phyllogen genes are needed to make it possible to trace the comprehensive evolutionary trajectory of the phyllogen family during phytoplasma evolution.
3.3. Loss of phyllody‐inducing activity attributable to a polymorphic residue and α‐helix truncation
Mutational analyses of phyllogens revealed that the loss of phyllody‐inducing activity in the phyl‐B group was attributable to a polymorphic residue at position 64 (Figures 5b and 6a) that regulates the MTF‐binding and ‐degrading activity of the effector in cooperation with another polymorphic residue at position 30 (Figures 6b,c and 7 and Table 1). Thus, we identified a loss of phyllody‐inducing activity attributable to α‐helix truncation or a polymorphic residue. Several phytoplasma effectors (phyllogen, TENGU, and SAP11) induce the unique symptoms observed in phytoplasma‐infected plants (Namba, 2019). Although phyllogen and SAP11 homologs exhibit functional variation, including the ability to interact with and degrade their targets (Maejima et al., 2014; Chang et al., 2018; Pecher et al., 2019), it remains unclear how polymorphisms determine differences in effector function because of limited data on each effector family. Our finding of natural variation in the function of phytoplasma effectors attributable to a single amino acid provide the first molecular insights into polymorphisms in single effector‐mediated pathogenesis of phytoplasmas.
3.4. Contribution of N64 residue to α‐helix‐mediated interaction with MTFs of phyllogen
The phyllogen–MTF interaction is suggested to depend on hydrophobic interaction between the α‐helices based on two alanine insertions (Iwabuchi et al., 2019) or two amino acid substitutions (Liao et al., 2019) into hydrophobic residues within α‐helices of phyllogens. Further supporting this notion, we found that the two α‐helices with conserved hydrophobic residues were also found in the newly identified phyllogens (Figures 1 and S6), suggesting that two α‐helices are structural bases for the interactions of phyllogen with host factors as in the case of the K domain‐mediated tetramerization of MTFs (Puranik et al., 2014).
How then is the hydrophilic residue at position 64 involved in the putative hydrophobic interaction between phyllogens and MTFs? One hypothesis is that the N64 residue plays a key role in α‐helix formation and the N64R substitution disrupts the structural integrity; however, the interaction of the phyl‐B group with SEP1 and RAD23C/D in yeast cells (Table 1) indicates that N64R substitution does not disrupt the overall structure of phyllogens. Another hypothesis is that the phyllogen–MTF interaction is mediated by both hydrophilic and hydrophobic residues. Unlike conserved hydrophobic residues oriented toward the interiors of both α‐helices, N64 is exposed on the conserved surface of the phyllogen along with conserved external hydrophobic residues (Figures 1b and 5a). Particularly, two external hydrophobic residues in PHYL1PnWB (L61 and L65 based on PHYL1OY), which also contribute to the coordinated interaction with SEP3 (Liao et al., 2019), were exposed on the same side as and adjacent to the N64 residue (Figure S9). For SEP3 oligomerization, hydrophilic residues flanking hydrophobic residues could play a significant role in the stabilization of α‐helix‐mediated protein interaction (Puranik et al., 2014). These results suggest that the external surface structures of phyllogens formed by these hydrophobic and hydrophilic residues are important for phyllogen–MTF interactions. In addition, K30 exposed on the same side of N64 (Figures 1b, 5a and S9) can also contribute marginally to the interaction in a similar manner. The amino acid substitutions at position 64 showed that even glutamine, whose side chain has similar properties to asparagine, was not sufficient to confer phyllody‐inducing activity on PHYL1SY (Figure 6a). Because glutamine generates more steric hindrance due to its larger size compared to asparagine (Bogan and Thorn, 1998), the steric property of N64 may be crucial for the stabilization of phyllogen–MTF interaction. These results suggest that while the two α‐helices are the structural bases for the interaction with host factors, phyllogens have acquired functional variation via their amino acid polymorphisms on α‐helices, which may improve the fitness of each phytoplasma. Comprehensive interaction analyses among each phyllogen focusing on its polymorphisms will elucidate the structural mechanism underlying phyllody induction and contribute to controlling phyllody symptoms caused by phytoplasma.
4. EXPERIMENTAL PROCEDURES
4.1. DNA samples
Table S1 lists the DNA samples of the phytoplasmas used in this study with the full strain names and GenBank accession numbers. DNA samples of the FBP, SY, and PvWB strains were amplified before use for whole‐genome amplification using the REPLI‐g Mini Kit (Qiagen) according to the manufacturer's instructions. DNA samples of PoiBI and ChV strains were extracted from a PoiBI‐infected commercial poinsettia cultivar and ChV‐infected periwinkle, respectively, with the QIAamp DNA Mini Kit (Qiagen).
4.2. PCR analyses
Table S2 lists the primers used in this study. A degenerate primer pair, PHYL‐F/R (Figure S2a), was designed for two conserved regions in three phyllogen gene sequences (PHYL1OY, PHYL1PnWB, and SAP54). PCR was performed in 10 µl reaction volumes containing KOD FX (Toyobo) master mix, with 0.3 µM of each primer, and 10 ng of genomic DNA from each phytoplasma‐infected plant. The PCR conditions consisted of an initial denaturation at 94°C for 3 min; 35–45 cycles of 94°C for 15 s, 45°C for 30 s, and 68°C for 30 s; with a final 7 min extension at 68°C. 16S rRNA gene sequences of several phytoplasmas were determined using the methodology described in Iwabuchi et al. (2018). Phyllogens of MD, SWB, PWB, and PoiBI strains were identified in each genomic DNA sample using primers based on up‐ and downstream sequences of the known phyllogens indicated as “Target gene” in Table S2. The PCR amplicons were purified followed by direct sequencing with a BigDye Terminator kit (Applied Biosystems) and each of the primers used in PCR.
4.3. Genome walking
Up‐ and downstream sequences of the partially amplified phyllogens were determined with genome walking using APAgene Gold Genome Walking kit (Bio S&T) according to the manufacturer's instructions. A total of 100 ng of either genomic DNA or whole‐genome‐amplified DNA was used as a template. The purified PCR amplicons were TA‐cloned into pCR4‐TOPO vector (Invitrogen) and sequenced with gene‐specific primers (GSPs), M13F, and M13R primers. The sequence information obtained, a partial phyllogen gene sequence and its up‐ and downstream sequences, was assembled with ATGC v. 4.3.5 (GENETYX). To validate the assembled sequences, PCR analyses were performed using primers designed on the up‐ and downstream sequences of each phyllogen (Figure S2b).
4.4. Identification of ChV strain phyllogens using MiSeq sequencing
Phyllogens of the ChV strain were identified with draft‐genome sequencing using an Illumina MiSeq sequencer. A library was prepared with a Nextera XT DNA Sample Prep Kit (Illumina), according to the manufacturer's instructions. It was run on a MiSeq sequencer that provided paired reads 150 nt long. The CLC Genomic Workbench (CLC‐bio) was used to map the phytoplasma draft‐genome sequence of PnWB phytoplasma (GenBank assembly accession GCA_000364425.1). Two phyllogens of the ChV strain, PHYL1ChV and PHYL1ChVΨ, were identified with a homology search with tblastn (Camacho et al., 2009). An approximately 1.6 kb genomic region containing PHYL1ChV and an approximately 0.9 kb genomic region containing PHYL1ChVΨ were amplified by PCR with primers AUR‐1/AUR‐2 and AUR‐3/AUR‐4, respectively. These PCR products were sequenced as described previously.
4.5. Phyllogen sequence analyses and structural modelling
The nucleotide and amino acid sequences of the phyllogen family were aligned with the MUSCLE algorithm (Edgar, 2004). Consensus secondary structure elements were predicted with PROMALS3D (Pei et al., 2008). The web‐based SignalP v. 4.1 server (http://www.cbs.dtu.dk/services/SignalP/) was used to analyse the presence and location of signal peptide cleavage sites. The sequence identities were calculated with the Sequence Demarcation Tool (SDT) v. 1.2 (Muhire et al., 2014). Phylogenetic trees based on partial or complete nucleotide sequences of the phyllogen family and 16S rRNA gene sequences were constructed with MEGA v. 7.0 using the neighbour‐joining method, as detailed in Iwabuchi et al. (2018). Two identical copies of phyllogen at two locations in complete genome sequence of “Ca. P. ziziphi” JWB‐nky strain (Wang et al., 2018a) were treated as a single sequence. Structures were modelled with the I‐TASSER server (Zhang, 2008) and selected based on the C‐score, which is a confidence score used to estimate the quality of the predicted models. The C‐score is typically in the range of [−5,2], where a C‐score of higher value signifies a model with a high confidence and vice versa. Using a C‐score cutoff >−1.5 for the models of correct topology, both false positive and false negative rates are below 0.1 (Zhang, 2008). Structure visualization and hydrophobicity calculation based on the Kyte and Doolittle scale were performed with UCSF Chimera (Pettersen et al., 2004). Sequence conservation scores were calculated using the ConSurf server (Ashkenazy et al., 2016).
4.6. Cloning and site‐directed mutagenesis of phyllogens
The predicted coding regions of the phyllogens, excluding their signal peptides, were cloned. PHYL1PYR and plant codon‐optimized PHYL1OY and PHYL1PnWB were previously cloned into the pENTA vector (Maejima et al., 2014; Kitazawa et al., 2017; Iwabuchi et al., 2019). Seven other phyllogens were plant codon‐optimized and synthesized by Thermo Fisher Scientific (Figure S1). PHYL1231/09, PHYL1JHP, PHYL1JWB, PHYL1SY, and PHYL1PWB were cloned into the pENTA vector using the methodology described by Kitazawa et al. (2017). PHYL1FBP and PHYL1PvWB were cloned into the pTRV2 vector with additional sequences cloned into the pTRV2 vector (5′‐TCCAACCCTGGGCCC‐3′ at their 5′ end and 5′‐TAGGGATTTAAGGAC‐3′ at their 3′ end). Prior to cloning into the pENTA vector, the PHYL1FBP and PHYL1PvWB fragments were amplified from those synthesized genes. PHYL1MD was generated by adding site‐directed mutations into pENTA‐cloned PHYL1PvWB using the GeneArt site‐directed mutagenesis system (Invitrogen). Amino acid substitution mutants of phyllogens were generated in the same way.
4.7. Yeast two‐hybrid analyses
The Matchmaker GAL4 Two‐Hybrid System 3 kit (Clontech) was used. Activation domain (AD)‐fused AP1 and SEP1, SEP2 (aa 87–220), SEP3, and SEP4 (aa 57–257), RAD23C and BD‐fused PHYL1OY, PHYL1PnWB, and PHYL1PYR were constructed previously (Maejima et al., 2014; Kitazawa et al., 2017; Iwabuchi et al., 2019). To construct AD‐fused RAD23D, the RAD23D gene of A. thaliana (NM_001085211) was cloned into the pGADT7 vector (Clontech) using NdeI and EcoRI sites. To construct DNA‐binding domain (BD)‐fused PHYL1231/09, PHYL1FBP, PHYL1JWB, PHYL1MD, PHYL1PvWB, PHYL1PWB, PHYL1PnWB, and PHYL1SY and their mutants, these fragments were cloned into the pGBKT7 vector (Clontech) using the same restriction sites. Co‐transformation of yeast cells and evaluation of protein interaction were performed as described by Iwabuchi et al. (2019).
4.8. In planta protein expression and detection
A. thaliana was maintained in a growth chamber with 16 hr light/8 hr dark conditions at 22°C. N. benthamiana was grown under natural light conditions at 25°C. For transient in planta expression of Myc‐fused SEP1, SEP2, SEP4, and AP1, triple c‐Myc (3 × Myc)‐fused SEP1–4 and AP1, and triple FLAG (3 × FLAG)‐fused PHYL1231/09, PHYL1OY, PHYL1SY, PHYL1PnWB, and their mutants, these genes were subcloned into pEarleyGate203 (Earley et al., 2006), pEarleyGateC3myc (Okano et al., 2014), and pEarleyGateN3 × FLAG (Iwabuchi et al., 2019), respectively, using Gateway LR Clonase II enzyme mix (Invitrogen). Myc‐fused SEP3 was constructed previously (Iwabuchi et al., 2019). Agrobacterium‐mediated transient expression and protein detection were performed using the methodology described by Iwabuchi et al. (2019).
A modified TRV‐based gene expression vector system (Iwabuchi et al., 2019) was used for stable in planta gene expression of phyllogens and their mutants. Agrobacterium tumefaciens EHA105 cells containing pTRV1 and each of the pTRV2‐containing CP fused with each of the phyllogen genes via the FMDV 2A peptide were adjusted to an OD600 of 1.0, mixed at a ratio of 1:1, and co‐infiltrated into 3‐ or 4‐week‐old A. thaliana and 4‐ or 5‐week‐old N. benthamiana leaves. Virus infection and foreign gene retention were confirmed by RT‐PCR using the methodology described in Iwabuchi et al. (2019).
4.9. Transgenic plants
PHYL1MD, PHYL1PvWB, PHYL1SY, and PHYL1PYR were subcloned from pENTA into the binary plasmid vector pFAST‐G02 (Inplanta Innovations) under the control of the cauliflower mosiac virus 35S promoter using Gateway LR Clonase II enzyme mix (Invitrogen). A. tumefaciens was transformed with each of the constructs, and A. thaliana plants were transformed using the floral‐dip method using the methodology described in Hoshi et al. (2009). T1 seeds of the transgenic plants were selected under fluorescence stereomicroscopy. The expression of each phyllogen was confirmed by RT‐PCR.
4.10. In planta immunoprecipitation
Agrobacterium cultures expressing 3 × Myc‐fused SEP1–4, AP1, and either 3 × FLAG‐PHYL1SY or its mutants were mixed at a ratio of 1:1 and infiltrated into N. benthamiana leaves. The inoculated leaves were homogenized (500 mg/ml) in 1 × RIPA buffer (Yamaji et al., 2006) containing complete mini EDTA‐free protease inhibitors (Roche) and 0.1% 3‐mercapto‐1,2‐propanediol 36 hr after infiltration. The homogenate was centrifuged at 18,000 × g for 30 min at 4°C to remove debris. Then 60 µl of the supernatant was heat‐denatured in SDS sample buffer (Inputs). A total of 700–800 µl of the remaining supernatant was incubated for 90 min at 4°C with 30 µl of EZview Red ANTI‐FLAG M2 Affinity Gel (50% slurry in 1 × RIPA buffer; Sigma‐Aldrich). Following five washes with 800 µl of 1 × RIPA buffer, proteins bound to the beads were eluted with 30 µl of 1 × RIPA buffer containing 400 µg/ml 3 × FLAG peptide (Sigma‐Aldrich).
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We are grateful to Drs T. Shiomi (Department of Biological Safety, National Institute for Agro‐Environmental Sciences, Ibaraki, Japan), T. Usugi (Plant Protection Division, National Agricultural Research Center, Ibaraki, Japan), and N. Nishimura and T. Tsuchizaki (Department of Agribusiness, Koibuchi College of Agriculture and Nutrition, Ibaraki, Japan), M. Tanaka (Central Region Agricultural Research Center, NARO), the past and present members of the Chiba Prefectural Agriculture and Forestry Research Center, H.‐Y. Jung (Kyungpook National University, Daegu, Korea), and A. Bertaccini (DipSA, University of Bologna, Bologna, Italy) for providing phytoplasma DNA samples. This research was supported by funds from JSPS (nos. 17J09944 and 19K15840).
Iwabuchi N, Kitazawa Y, Maejima K, et al. Functional variation in phyllogen, a phyllody-inducing phytoplasma effector family, attributable to a single amino acid polymorphism. Molecular Plant Pathology. 2020;21:1322–1336. 10.1111/mpp.12981
DATA AVAILABILITY STATEMENT
The data supporting these findings are available in DDBJ/EMBL/GenBank at https://www.ncbi.nlm.nih.gov/ under the accession numbers listed in Table S1.
REFERENCES
- Arashida, R. , Kakizawa, S. , Hoshi, A. , Ishii, Y. , Jung, H.‐Y. , Kagiwada, S. et al (2008a) Heterogeneic dynamics of the structures of multiple gene clusters in two pathogenetically different lines originating from the same phytoplasma. DNA and Cell Biology, 27, 209–217. [DOI] [PubMed] [Google Scholar]
- Arashida, R. , Kakizawa, S. , Ishii, Y. , Hoshi, A. , Jung, H.‐Y. , Kagiwada, S. et al (2008b) Cloning and characterization of the antigenic membrane protein (Amp) gene and in situ detection of Amp from malformed flowers infected with Japanese hydrangea phyllody phytoplasma. Phytopathology, 98, 769–775. [DOI] [PubMed] [Google Scholar]
- Ashkenazy, H. , Abadi, S. , Martz, E. , Chay, O. , Mayrose, I. , Pupko, T. et al (2016) ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Research, 44, W344–W350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, M. and Seemüller, E. (1999) Chromosomal organization and nucleotide sequence of the genes coding for the elongation factors G and Tu of the apple proliferation phytoplasma. Gene, 226, 103–109. [DOI] [PubMed] [Google Scholar]
- Bogan, A.A. and Thorn, K.S. (1998) Anatomy of hot spots in protein interfaces. Journal of Molecular Biology, 280, 1–9. [DOI] [PubMed] [Google Scholar]
- Camacho, C. , Coulouris, G. , Avagyan, V. , Ma, N. , Papadopoulos, J. , Bealer, K. et al (2009) BLAST+: architecture and applications. BMC Bioinformatics, 10, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.H. , Tan, C.M. , Wu, C.T. , Lin, T.H. , Jiang, S.Y. , Liu, R.C. et al (2018) Alterations of plant architecture and phase transition by the phytoplasma virulence factor SAP11. Journal of Experimental Botany, 69, 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi, Y. , Rao, G.P. , Tiwari, A.K. , Duduk, B. and Bertaccini, A. (2010) Phytoplasma on ornamentals: detection, diversity and management. Acta Phytopathologica et Entomologica Hungarica, 45, 31–69. [Google Scholar]
- Chung, W.C. , Chen, L.L. , Lo, W.S. , Lin, C.P. and Kuo, C.H. (2013) Comparative analysis of the peanut witches'‐broom phytoplasma genome reveals horizontal transfer of potential mobile units and effectors. PLoS ONE, 8, e62770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, K.W. , Haag, J.R. , Pontes, O. , Opper, K. , Juehne, T. , Song, K. et al (2006) Gateway‐compatible vectors for plant functional genomics and proteomics. The Plant Journal, 45, 616–629. [DOI] [PubMed] [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, L.M. , Book, A.J. , Lee, K.H. , Lin, Y.L. , Fu, H. and Vierstra, R.D. (2010) The RAD23 family provides an essential connection between the 26S proteasome and ubiquitylated proteins in Arabidopsis . The Plant Cell, 22, 124–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, F.D. , Debat, H.J. and Conci, L.R. (2019) Molecular characterization of effector protein SAP54 in Bellis virescence phytoplasma (16SrIII‐J). Tropical Plant Pathology, 44, 392–397. [Google Scholar]
- Fischer, A. , Santana‐Cruz, I. , Wambua, L. , Olds, C. , Midega, C. , Dickinson, M. et al (2016) Draft genome sequence of “Candidatus Phytoplasma oryzae” strain Mbita1, the causative agent of Napier grass stunt disease in Kenya. Genome Announcements, 4, e00297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella, E. , Tedeschi, R. , Crotti, E. and Alma, A. (2019) Multiple guests in a single host: interactions across symbiotic and phytopathogenic bacteria in phloem‐feeding vectors—A review. Entomologia Experimentalis et Applicata, 167, 171–185. [Google Scholar]
- Hacker, J. , Blum‐Oehler, G. , Mühldorfer, I. and Tschäpe, H. (1997) Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Molecular Microbiology, 23, 1089–1097. [DOI] [PubMed] [Google Scholar]
- Hoshi, A. , Oshima, K. , Kakizawa, S. , Ishii, Y. , Ozeki, J. , Hashimoto, M. et al (2009) A unique virulence factor for proliferation and dwarfism in plants identified from a phytopathogenic bacterium. Proceedings of the National Academy of Sciences of the United States of America, 106, 6416–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi, N. , Endo, A. , Kameyama, N. , Satoh, M. , Miyazaki, A. , Koinuma, H. et al (2018) First report of “Candidatus Phytoplasma malaysianum” associated with Elaeocarpus yellows of Elaeocarpus zollingeri . Journal of General Plant Pathology, 84, 160–164. [Google Scholar]
- Iwabuchi, N. , Maejima, K. , Kitazawa, Y. , Miyatake, H. , Nishikawa, M. , Tokuda, R. et al (2019) Crystal structure of phyllogen, a phyllody‐inducing effector protein of phytoplasma. Biochemical and Biophysical Research Communications, 513, 952–957. [DOI] [PubMed] [Google Scholar]
- Jiang, H. , Wei, W. , Saiki, T. , Kawakita, H. , Watanabe, K. and Sato, M. (2004) Distribution patterns of mulberry dwarf phytoplasma in reproductive organs, winter buds, and roots of mulberry trees. Journal of General Plant Pathology, 70, 168–173. [Google Scholar]
- Jomantiene, R. , Zhao, Y. and Davis, R.E. (2007) Sequence‐variable mosaics: composites of recurrent transposition characterizing the genomes of phylogenetically diverse phytoplasmas. DNA and Cell Biology, 26, 557–564. [DOI] [PubMed] [Google Scholar]
- Jung, H.Y. , Yae, M.C. , Lee, J.T. , Hibi, T. and Namba, S. (2003) Aster yellows subgroup (Candidatus Phytoplasma sp. AY 16S‐group, AY‐sg) phytoplasma associated with porcelain vine showing witches’ broom symptoms in South Korea. Journal of General Plant Pathology, 69, 208–209. [Google Scholar]
- Kitazawa, Y. , Iwabuchi, N. , Himeno, M. , Sasano, M. , Koinuma, H. , Nijo, T. et al (2017) Phytoplasma‐conserved phyllogen proteins induce phyllody across the Plantae by degrading oral MADS domain proteins. Journal of Experimental Botany, 68, 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, C. , Lo, W.S. and Kuo, C.H. (2013) Horizontal transfer of potential mobile units in phytoplasmas. Mobile Genetic Elements, 3, e26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I.M. , Shao, J. , Bottner‐Parker, K.D. , Gundersen‐Rindal, D.E. , Zhao, Y. and Davis, R.E. (2015) Draft genome sequence of “Candidatus Phytoplasma pruni” strain CX, a plant‐pathogenic bacterium. Genome Announcements, 3, e01117–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, Y.T. , Lin, S.S. , Lin, S.J. , Sun, W.T. , Shen, B.N. , Cheng, H.P. et al (2019) Structural insights into the interaction between phytoplasmal effector causing phyllody 1 (PHYL1) and MADS transcription factor. The Plant Journal, 100, 706–719. [DOI] [PubMed] [Google Scholar]
- MacLean, A.M. , Sugio, A. , Makarova, O.V. , Findlay, K.C. , Grieve, V.M. , Toth, R. et al (2011) Phytoplasma effector SAP54 induces indeterminate leaf‐like flower development in Arabidopsis plants. Plant Physiology, 157, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, A.M. , Orlovskis, Z. , Kowitwanich, K. , Zdziarska, A.M. , Angenent, G.C. , Immink, R.G. et al (2014) Phytoplasma effector SAP54 hijacks plant reproduction by degrading MADS‐box proteins and promotes insect colonization in a RAD23‐dependent manner. PLoS Biology, 12, e1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima, K. , Iwai, R. , Himeno, M. , Komatsu, K. , Kitazawa, Y. , Fujita, N. et al (2014) Recognition of floral homeotic MADS domain transcription factors by a phytoplasmal effector, phyllogen, induces phyllody. The Plant Journal, 78, 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima, K. , Kitazawa, Y. , Tomomitsu, T. , Yusa, A. , Neriya, Y. , Himeno, M. et al (2015) Degradation of class E MADS‐domain transcription factors in Arabidopsis by a phytoplasmal effector, phyllogen. Plant Signaling & Behavior, 10, e1042635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcone, C. (2014) Molecular biology and pathogenicity of phytoplasmas. Annals of Applied Biology, 165, 199–221. [Google Scholar]
- Minato, N. , Himeno, M. , Hoshi, A. , Maejima, K. , Komatsu, K. , Takebayashi, Y. et al (2014) The phytoplasmal virulence factor TENGU causes plant sterility by downregulating of the jasmonic acid and auxin pathways. Scientific Reports, 4, 7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrović, J. , Siewert, C. , Duduk, B. , Hecht, J. , Mölling, K. , Broecker, F. et al (2014) Generation and analysis of draft sequences of “stolbur” phytoplasma from multiple displacement amplification templates. Journal of Molecular Microbiology and Biotechnology, 24, 1–11. [DOI] [PubMed] [Google Scholar]
- Muhire, B.M. , Varsani, A. and Martin, D.P. (2014) SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE, 9, e108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musetti R. and Pagliari L. (Eds.). (2019). Phytoplasmas: Methods and Protocols. Methods in Molecular Biology 1875 : Humana Press. [DOI] [PubMed] [Google Scholar]
- Namba, S. (2019) Molecular and biological properties of phytoplasmas. Proceedings of the Japan Academy, Series B, Physical and Biological Sciences, 95, 401–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano, Y. , Senshu, H. , Hashimoto, M. , Neriya, Y. , Netsu, O. , Minato, N. et al (2014) In planta recognition of a double‐stranded RNA synthesis protein complex by a potexviral RNA silencing suppressor. The Plant Cell, 26, 2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovskis, Z. and Hogenhout, S.A. (2016) A bacterial parasite effector mediates insect vector attraction in host plants independently of developmental changes. Frontiers in Plant Science, 7, 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen, M.J. and Wren, B.W. (2007) Bacterial pathogenomics. Nature, 449, 835–842. [DOI] [PubMed] [Google Scholar]
- Pecher, P. , Moro, G. , Canale, M.C. , Capdevielle, S. , Singh, A. , MacLean, A. et al (2019) Phytoplasma SAP11 effector destabilization of TCP transcription factors differentially impact development and defence of Arabidopsis versus maize. PLoS Pathogens, 15, e1008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, J. , Kim, B.H. and Grishin, N.V. (2008) PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Research, 36, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen, E.F. , Goddard, T.D. , Huang, C.C. , Couch, G.S. , Greenblatt, D.M. , Meng, E.C. et al (2004) UCSF Chimera—A visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Puranik, S. , Acajjaoui, S. , Conn, S. , Costa, L. , Conn, V. , Vial, A. et al (2014) Structural basis for the oligomerization of the MADS domain transcription factor SEPALLATA3 in Arabidopsis. The Plant Cell, 26, 3603–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino, F. , Kube, M. , Jawhari, M. , Abou‐Jawdah, Y. , Siewert, C. , Choueiri, E. et al (2015) “Candidatus Phytoplasma phoenicium” associated with almond witches'‐broom disease: from draft genome to genetic diversity among strain populations. BMC Microbiology, 15, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümpler, F. , Gramzow, L. , Theißen, G. and Melzer, R. (2015) Did convergent protein evolution enable phytoplasmas to generate “zombie plants”? Trends in Plant Science, 20, 798–806. [DOI] [PubMed] [Google Scholar]
- Smaczniak, C. , Immink, R.G. , Angenent, G.C. and Kaufmann, K. (2012) Developmental and evolutionary diversity of plant MADS‐domain factors: insights from recent studies. Development, 139, 3081–3098. [DOI] [PubMed] [Google Scholar]
- Sugawara, K. , Honma, Y. , Komatsu, K. , Himeno, M. , Oshima, K. and Namba, S. (2013) The alteration of plant morphology by small peptides released from the proteolytic processing of the bacterial peptide TENGU. Plant Physiology, 162, 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio, A. and Hogenhout, S.A. (2012) The genome biology of phytoplasma: modulators of plants and insects. Current Opinion in Microbiology, 15, 247–254. [DOI] [PubMed] [Google Scholar]
- Sugio, A. , Kingdom, H.N. , MacLean, A.M. , Grieve, V.M. and Hogenhout, S.A. (2011) Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 108, E1254–E1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, M. , Tanaka, C. , Usugi, T. , Uematsu, S. and Ebihara, Y. (2008) Occurrence of strawberry yellows caused by “Candidatus Phytoplasma fragariae”. Japanese Journal of Phytopathology, 74, 258. [Google Scholar]
- Toruño, T.Y. , Musić, M.S. , Simi, S. , Nicolaisen, M. and Hogenhout, S.A. (2010) Phytoplasma PMU1 exists as linear chromosomal and circular extrachromosomal elements and has enhanced expression in insect vectors compared with plant hosts. Molecular Microbiology, 77, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Song, L. , Jiao, Q. , Yang, S. , Gao, R. , Lu, X. et al (2018a) Comparative genome analysis of jujube witches’‐broom Phytoplasma, an obligate pathogen that causes jujube witches’‐broom disease. BMC Genomics, 19, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. , Yang, H. , Yin, Z. , Liu, W. , Sun, L. and Wu, Y. (2018b) Phytoplasma effector SWP1 induces witches' broom symptom by destabilizing the TCP transcription factor BRANCHED1. Molecular Plant Pathology, 19, 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, W. , Wu, W. , Davis, R.E. , Lee, I.M. and Zhao, Y. (2016) Development of molecular markers and a diagnostic tool for investigation of coinfections by and interactions between potato purple top and potato witches’‐broom phytoplasmas in tomato. Annals of Applied Biology, 168, 133–141. [Google Scholar]
- Yamaji, Y. , Kobayashi, T. , Hamada, K. , Sakurai, K. , Yoshii, A. , Suzuki, M. et al (2006) In vivo interaction between Tobacco mosaic virus RNA‐dependent RNA polymerase and host translation elongation factor 1A. Virology, 347, 100–108. [DOI] [PubMed] [Google Scholar]
- Yang, C.Y. , Huang, Y.H. , Lin, C.P. , Lin, Y.Y. , Hsu, H.C. , Wang, C.N. et al (2015) MicroRNA396‐targeted SHORT VEGETATIVE PHASE is required to repress flowering and is related to the development of abnormal flower symptoms by the phyllody symptoms1 effector. Plant Physiology, 168, 1702–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. (2008) I‐TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The data supporting these findings are available in DDBJ/EMBL/GenBank at https://www.ncbi.nlm.nih.gov/ under the accession numbers listed in Table S1.


