Abstract
Objective
To determine performance and repair kinetics of the ChonDux hydrogel scaffold for treating focal articular cartilage defects in the knee over 24 months.
Design
This assessor-blinded trial evaluates ChonDux hydrogel scaffold implantation in combination with microfracture in 18 patients across 6 sites. Male and female patients 18 to 65 years of age with full-thickness femoral condyle defects 2 to 4 cm2 in area were enrolled. Eligible patients received ChonDux treatment followed by rehabilitation. Defect volume fill was evaluated after 3, 6 (primary outcome), 12, 18, and 24 months by assessor blinded magnetic resonance imaging (MRI) analysis. Secondary outcomes were T2-weighted MRI relaxation time and patient surveys via visual analogue scale (VAS) pain and International Knee Documentation Committee (IKDC) knee function scoring.
Results
ChonDux maintained durable tissue restoration over 24 months with final defect percent fill of 94.2% ± 16.3% and no significant loss of fill volume at any time points. Tissues treated with ChonDux maintained T2 relaxation times similar to uninjured cartilage between 12 and 24 months. VAS pain scoring decreased between 1 and 6 weeks, and IKDC knee function scores improved by approximately 30.1 with ChonDux over 24 months.
Conclusion
ChonDux treatment is a safe adjunct to microfracture therapy and promotes stable restoration of full thickness articular cartilage defects for at least 24 months.
Keywords: cartilage repair, scaffold, microfracture, tissue engineering, biomaterials
Introduction
Articular cartilage damage is observed in up to 66% of patients that have knee injuries requiring arthroscopy and 20% of these patients present full-thickness cartilage defects1 (also known as lesions). Acute traumatic injury, torsional overloading, and/or repetitive impact, as encountered in high impact sports participation and occupational hazards, may lead to cartilage degeneration and the formation of an articular defect exposing the subchondral bone. Sequelae of such defects include osteoarthritis, osteonecrosis, and chronic pain concomitant with loss of function. Despite these morbidities and associated cost, there remains a dearth of therapeutic options for long-lasting repair of cartilage defects. The primary challenge arises in cartilage’s extremely limited healing capacity because of its aneural, avascular, and hypocellular nature.2 Consequently, conservative management with rehabilitation and nonsteroidal anti-inflammatory drugs is palliative rather than therapeutic. This accentuates the need for novel approaches to regenerate lost cartilage and restore healthy knee function.
Several surgical procedures have been investigated for cartilage defect repair, though with limited success or severe technical limitation. Surgical resurfacing typically involves debridement or abrasion arthroplasty in order to induce an acute injury response that promotes a fibrocartilage biased healing response, though is insufficient to reverse disease progression alone or encourage hyaline cartilage formation.3 Cell transplantation into the defect region have shown efficacy in de novo cartilage tissue regeneration and include procedures such as autologous cell implantation or cartilage grafts, either autologous or allogeneic.4-7 Although cell-based therapy has shown promising results for cartilage repair, it suffers several limitations. Autologous chondrocyte implantation involves cell isolation and ex vivo expansion from healthy non-loadbearing cartilage, and reimplantation during an additional surgical procedure weeks later. As a result of these multiple cell manipulation steps, chondrocyte implantation is cumbersome and expensive to implement.8 Autologous cartilage tissue transplantation is especially prone to donor site morbidity, and both autologous and donor-derived cartilage transplants are poorly integrated into existing tissues.9 Alternatively, autologous cells released from the bone marrow in microfracture surgery is effective in alleviating symptoms in the short term, but the repair is not stable over time.10-13 We address these challenges in cell-/tissue-based therapy with a tissue engineered biomaterials system that (1) incorporates and retains autologous marrow cells within the cartilage defect, (2) is well integrated with surrounding tissues, and (3) can be accomplished within a single procedure. Our goal was to create a simple cartilage repair technology that uses biomaterials to guide endogenous healing and new cartilage formation.
We previously described ChonDux, a hydrogel biomaterial scaffold/tissue adhesive system designed to provide a chondrogenic microenvironment for autologous bone marrow cells released via microfracture.14,15 ChonDux employs a functionalized chondroitin-sulfate adhesive that bonds tissue surfaces to a polyethylene glycol (PEG) hydrogel that is polymerized with long wave ultraviolet light in situ. The end result is a biocompatible hydrogel scaffold that takes the shape of an irregular tissue defect. Preclinical studies showed that the ChonDux hydrogel system alone is conducive to chrondrogenesis in knee cartilage defects, encouraging formation of glycosaminoglycan rich tissues that are histologically similar to nearby uninjured cartilage.14,15 When used in combination with microfracture in a goat model, ChonDux is still able to completely fill the defect space and is infiltrated with blood and marrow components within hours.14
A 6-month pilot clinical study demonstrated decreased pain and increased defect fill with ChonDux treatment following microfracture.14,15 This study further evaluates safety and efficacy of ChonDux in treating full-thickness articular defects over a 24-month period using frequent and detailed imaging in conjunction with patient evaluation.
Materials and Methods
Experimental Overview
The purpose of this phase II clinical trial was to determine the kinetic remodeling characteristics of a photoreactive chondroitin-sulfate/PEG hydrogel (ChonDux) in conjunction with microfracture surgery for repairing full thickness cartilage defects in the femoral condyle of the knee. The safety of the device, efficacy in filling the defect, and effect on pain and function were characterized by using magnetic resonance imaging and patient surveys over a 24-month time course.
Ethics and Regulatory Approvals
The Clinical Investigation Plan (CIP), patient information letter, and informed consent form were submitted to the structured institutional review boards (ethics committees) of each investigational center and a positive vote of the Board of Directors was obtained prior to start of the enrollment, in accordance with local law. This investigation was conducted in accordance with the Good Clinical Practice Guidelines, with the Declaration of Helsinki, ISO 14155, and all relevant national guidelines.
Patient Recruitment
This assessor single blind study was conducted between March 18, 2009 and December 5, 2010 as described at https://clinicaltrials.gov (Identifier NCT01110070) across 6 sites: Baarn, the Netherlands; Hilversum, the Netherlands; Altentreptow, Germany; Freiburg, Germany; Mannheim, Germany; Linz, Austria. Eligible male and female patients 18 to 65 years of age (inclusive) were enrolled according to the following inclusion criteria: body mass index (BMI) ⩽33 kg/m2, having a prior radiograph of the knee showing a Kellgren score of 0 to 2, and were candidates for arthroscopy based on a previous magnetic resonance imaging (MRI), arthroscopy, or failure of conservative treatment to address the problem. Patients were ineligible for enrollment according to the following exclusion criteria: moderate or severe osteoarthritis, passive motion deficits of >5° of extension and >15° of flexion, patellofemoral instability, malalignment with 5° valgus or varus compared with contralateral knee, active osteomyelitis, pregnant or nursing mothers, active inflammatory disease such as rheumatoid arthritis or gout, autoimmune disease, type I diabetes, chronic steroid intake, or a history of drug or alcohol use. Additional inclusion criteria were applied at surgery: An Outerbridge score of III or IV without need for bone graft (International Cartilage Repair Society grade 3, B-C), confirmation of a single, full-thickness, femoral condyle defect with an estimated surface area of 2 to 4 cm2 following debridement, a meniscus with no more than partial resection in the affected knee, and confirmation that patient is suitable for microfracture. A total of 18 patients were treated using microfracture with ChonDux implantation. Additionally, 3 patients were treated with arthroscopic microfracture alone. This group was neither powered for a direct comparison with ChonDux nor was it an open surgical procedure like ChonDux implantation, and instead provides a reference to demonstrate consistency with microfracture responses in the literature. ChonDux patient demographic information is provided in Table 1 . A total of 28 patients were enrolled in the study with 21 satisfying inclusion criteria. Eighteen (18) of these patients were treated with ChonDux (3 were allocated to the arthroscopic microfracture reference group), of which 12 completed the 24-month study. Early termination with ChonDux treatment was due to the following: protocol violation (n = 1), unscheduled arthroscopy (n = 1), an adverse event (n = 2), lost during follow-up (n = 1), and medically unrelated reasons (n = 1).
Table 1.
Patient Demographics and Safety.
| Characteristic | ChondDux |
|---|---|
| Participants | |
| Total, n | 18 |
| Age, years | |
| Mean | 37.8 |
| Standard deviation | 9.5 |
| Median | 35.5 |
| Range | 24-57 |
| Sex, n (%) | |
| Male | 8 (44.4) |
| Female | 10 (55.6) |
| Subjects reporting any adverse events, n (%) | |
| Mild | 3 (16.7) |
| Moderate | 10 (55.6) |
| Severe | 1 (5.6) |
| Total | 14 (77.8) |
| Total number of adverse events, n (%) | |
| Mild | 24 (61.5) |
| Moderate | 14 (35.9) |
| Severe | 1 (2.6) |
| Total | 39 (100.0) |
Surgical Procedures
Eligible patients with full thickness articular cartilage defects 2 to 4 cm2 in size were treated with ChonDux, a chondroitin-sulfate/PEG hydrogel, as previously described. In brief, microfracture was performed prior to ChonDux hydrogel implantation. A small open incision was made to access the cartilage defect, which was then debrided of damaged tissue along the defect border. Multiple holes (fractures) were created in the subchondral bone within the cartilage defect area using an arthroscopic awl.16 All fractures were created perpendicular to the condyle surface and were spaced approximately 3 to 4 mm apart to avoid damage to the subchondral plate between holes. The microfracture only reference employed these methods arthroscopically.
ChonDux implantation occurred immediately following microfracture. First, a chondroitin sulfate tissue adhesive base was applied to the entire surface of the defect using a polyvinyl alcohol spear applicator. Excessive blood accumulation was removed with sterile gauze before proceeding. The entire defect volume was then completely filled with PEG diacrylate pre-gel mixed with hyaluronic acid and a photoinitiator compound. A solid hydrogel formed within the defect from the pre-gel mixture by exposure to ultraviolet light for 240 seconds, which simultaneously polymerized the PEG and linked it to the defect tissue via the adhesive linker. The capsule, subcutaneous tissues, and skin were then closed. Patients received 12 weeks of rehabilitation postoperation. Patients were treated with continuous passive motion beginning at 1 day postoperation and continuing daily for 1 to 2 weeks or until 90° to 100° range of motion was reached. Cold therapy was used to manage pain and swelling. Patients remained non-weightbearing for 6 weeks postoperation and transitioned to protected weightbearing for an additional 5 weeks, followed by low-impact resistance exercise. Running, jumping, and any sharp movements were restricted until 12 months pos-operation.
Safety
Patients were monitored for adverse events and serious adverse events. Adverse events were characterized as mild (aware of sign or symptom, but easily tolerated), moderate (enough discomfort to cause interference with usual activity), or severe (incapacitating with inability to work or do usual activity). Serious adverse events were defined as an adverse event that led to a death, resulted in a life-threatening illness or injury, resulted in a permanent impairment of a body structure or a body function, results in medical or surgical intervention to prevent permanent impairment to body structure or body function, required in-patient hospitalization or prolongation of existing hospitalization, or led to fetal distress, fetal death, or a congenital abnormality or birth defect. Serious adverse events were characterized for likelihood of being related to the device. All adverse events were recorded by the investigators beginning at enrollment until termination of study at the 24-month visit.
Quantitative Magnetic Resonance Imaging Analysis
Patients were evaluated for percent fill of the cartilage defect by comparing defect volume at baseline (3 weeks postoperation) to 3, 6, 12, 18, and 24 months postoperation by MRI. All patients were imaged using 1.5 T MRI scanners, and uniformity and linearity phantom scans were conducted to ensure linearity of the magnetic field and gradients to enable quantification of patient images. Table 2 lists the N-value for MRI imaging at each time point. Patients were imaged in a saggital and coronal 2-dimensional (2D) fast spin echo (FSE) sequence, a sagittal and coronal 3D FLASH/SPGR sequence, a 3D gradient recalled echo sequence (GRE), and a sagittal multiecho spin sequence for T2 mapping.
Table 2.
Magnetic Resonance Imaging Participation.
| Time Point | ChondDux (N) |
|---|---|
| Baseline | 18 |
| 3 months | 17 |
| 6 months | 17 |
| 12 months | 13 |
| 18 months | 12 |
| 24 months | 10 |
MRI image analysis was conducted by blinded radiologists (VirtualScopics Inc., Rochester, NY). The volume and depth of the cartilage defect and surrounding cartilage was determined at baseline by the following procedure: (1) fusing the FLASH and GRE sequences, (2) identify bones and apply to an articulated registration algorithm, (3) identify cartilage and segment the defect region from adjacent cartilage. Manual identification and segmentation were performed by a blinded radiologist. Subsequent time points were then compared to baseline using an automated algorithm to track to initial morphology with adjustment by the reader. Percent fill of the cartilage defect was calculated as
T2 relaxation was calculated for each time point to determine the similarity of the repaired tissue to native cartilage tissue. The previously defined cartilage defect regions were automatically tracked and adjusted as described above to the 4 echo sequences for T2 mapping. The first echo was only included in the calculation when T2 was less than twice the first echo time to prevent underestimation. The average T2 relaxation time was calculated within repaired tissue and compared to native cartilage. All MRI data is reported as the mean ± standard error of the mean unless otherwise indicated.
Qualitative observations of the presence of osseous overgrowth and cartilage delamination were scored. Cartilage delamination was defined as fluid between the cartilage tissue and subchondral bone.
Pain and Function Surveys
Patients were administered questionnaires to assess pain and overall function following treatment. Patients were asked to characterize their pain severity and frequency using a visual analogue scale (VAS) between 4 and 7 days postoperation and after 6 weeks postoperation. The pain severity scale ranged between “no pain” and “worst pain imaginable” and the frequency scale between “never” and “continuously.” The 2000 International Knee Documentation Committee (IKDC) Patientive Knee Evaluation Form and SF-36 health survey was completed at enrollment, and at 3, 6, 12, 18, and 24 months postoperation to evaluate knee function and overall health/function, respectively. All survey forms were scored according to established guidelines.
Statistical Analysis
The stability of ChonDux repair over time was determined by 1-way analysis of variance with post hoc Tukey testing for differences between individual time points for the following outcomes: defect thickness, defect % fill, and IKDC score. T2 relaxation time was analyzed by 2-way analysis of variance with post hoc Tukey testing for differences between individual time points and between native cartilage/ChonDux. Paired t tests were performed to compare VAS frequency and VAS severity between 4 to 7 days and 6 weeks postoperation. Pearson correlation coefficients were calculated to characterize the relationship between initial defect size and % defect fill, T2 relaxation time, and IKDC scores at 24 months for ChonDux-treated defects. P < 0.05 is considered statistically significant. Patients treated with microfracture only are included in the present study as a reference and statistical comparisons with ChonDux treatment are not provided due to disparity in group size. All graphs display the mean ± standard deviation (SD). Statistical analysis was performed using Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
Structural Analysis via MRI
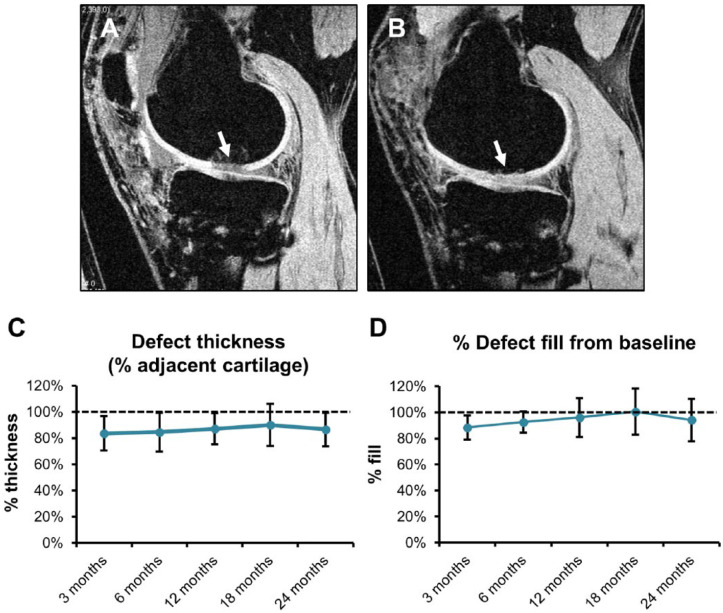
MRI analysis showed that ChonDux treatment provided durable repair of focal articular defects. Articular defects ( Fig. 1A and B , arrows), which was quantified by blinded assessment of defect thickness and % volume fill ( Fig. 1C and D ). Repair tissue thickness following ChonDux treatment was maintained between 83.5% and 90.0% of uninjured cartilage between the full 3- to 24-month time course postoperation. The primary outcome of percent defect fill with ChonDux was 92.5% ± 8.2% defect fill at the short-term 6-month time point and well maintained with 94.2% ± 16.3% fill at the final 24-month time point. ChonDux-mediated defect fill was consistent over the full 24-month time course, with no statistically significant differences between any time points, including between 6 and 24 months (P > 0.9).
Figure 1.
Magnetic resonance imaging (MRI) analysis of articular cartilage defect structural remodeling with ChonDux treatment. Full MRI image processing workflow to quantify defect fill are provided in the Materials and Methods section. (A) Representative image of a fully processed MRI scan of the articular defect (white arrow) at baseline before ChonDux implantation compared with (B) the same defect 6 months following ChonDux treatment. (C) Quantified defect thickness normalized to adjacent uninjured cartilage, and (D) percent defect fill normalized to initial defect size at baseline over the full 24-month time course with ChonDux treatment (mean ± SD). Dashed lines reference 100% defect thickness and fill. One-way analysis of variance with post hoc Tukey testing was performed to compare time points following ChonDux treatment, with no significant differences observed between any time points.
T2 relaxation times of the repaired tissues were calculated to compare the similarity of the remodeled tissue to normal cartilage composition between 3 and 24 months ( Fig. 2 ). While ChonDux-treated defects initially showed abnormally high T2 times (113 ± 88.3 ms) relative to uninjured adjacent cartilage (67.7 ± 11.4 ms) at 3 months (P < 0.01), these decreased to normal times by 12 months, ranging between 57.7 and 65.5 ms. T2 relaxation times remained stable for the remainder of the study (no significant differences from adjacent cartilage between 6 and 24 months). Cartilage delamination was observed in 5 of 18 ChonDux patients. Osseus overgrowth was found in 4 of 18 ChonDux patients.
Figure 2.
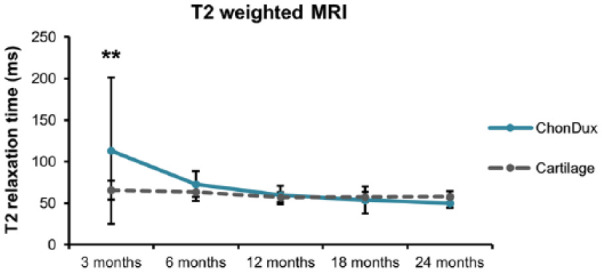
T2-weighted magnetic resonance imaging analysis of remodeled tissue within ChonDux-treated defects compared with adjacent uninjured cartilage. T2 relaxation times for adjacent uninjured cartilage were pooled from all patients (mean ± SD). Two-way analysis of variance with post hoc Tukey testing was performed to compare ChonDux time points and to uninjured cartilage. **P < 0.01 for ChonDux versus cartilage at 3 months.
Pain and Function Surveys
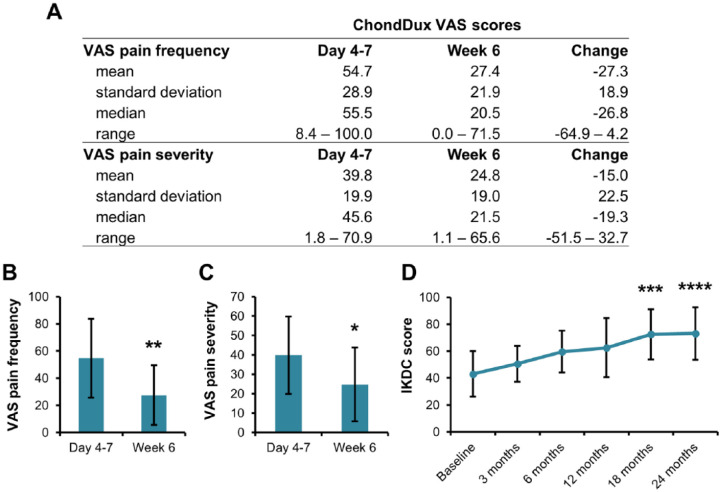
Patient surveys were conducted over the initial 6 weeks of treatment using the VAS to determine short-term pain ( Fig. 3 ). ChonDux treatment scored an initial pain frequency of 54.7 ± 28.9 and decreased to 27.4 ± 22.0 at 6 weeks, a reduction of 27.3 ( Fig. 3A and B ). VAS pain severity ( Fig. 3A and C ) followed a similar trend as frequency, decreasing by 15.0 with ChonDux treatment (39.8 ± 19.9 at 4-7 days and 24.8 ± 19.0 at 6 weeks).
Figure 3.
Patient visual analogue scale (VAS) pain and International Knee Documentation Committee (IDKC) knee function scoring. (A) Statistics of the pain frequency and severity using the VAS at 4 to 7 days and at week 6 postoperation. The change in score was determined for each patient by subtracting the score at 4 to 7 days from the score at week 6. Graphical comparison of (B) VAS pain frequency score and (C) VAS pain severity score for microfracture and ChonDux treatment (mean ± SE). (D) IKDC knee function scores tracked over the course of the study from baseline to 24 months (mean ± SE).
IKDC surveys were administered to evaluate knee pain and function over time ( Fig. 3D ). All patients showed consistent improvements in IKDC score following ChonDux treatment with mean increases over baseline of 29.5 and 30.1 at 18 and 24 months, respectively. The SF-36 survey showed minimal change to overall health in both treatment groups over the course of the study (Supplemental Figure 1, available in the online version of the article). Scores for physical function, physical role limitations, and pain showed incremental improvements for ChonDux between baseline and 24 months. Scores for social functioning, emotional well-being and limitations, and general health did not change during the study period and were consistently high.
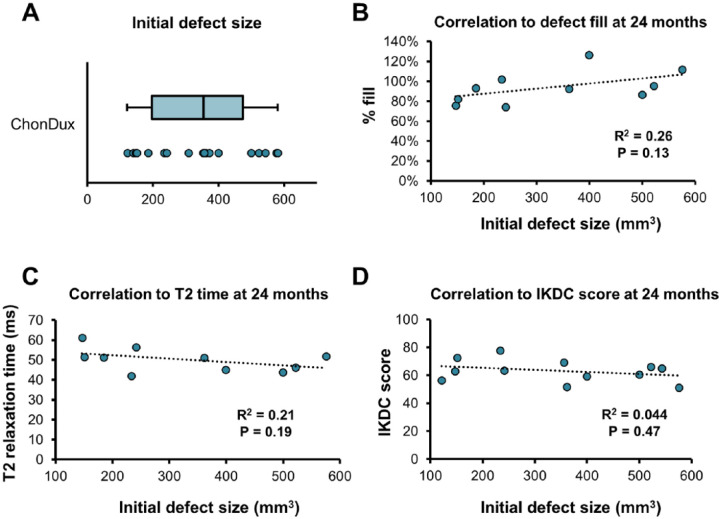
Correlation to Initial Defect Size
Baseline defect volumes ranged between 121 and 579 mm3, with mean initial defect volumes of 337.9 ± 146.2 mm3 prior to ChonDux implantation ( Fig. 4A ). Correlation analysis was conducted to determine whether the efficacy of ChonDux repair at 24 months was related to the initial size of the articular cartilage defect. Initial defect volume was not significantly correlated to percent defect fill ( Fig. 4B ), T2 relaxation time ( Fig. 4C ), or IKDC knee function score ( Fig. 4D ).
Figure 4.
Correlation analysis of initial defect size to 24-month outcomes with ChonDux treatment. (A) Initial defect volumes for patients receiving ChonDux determined by magnetic resonance imaging analysis (Box plot indicates median volume, and the first and third quartiles. Whiskers extend to the minimum/maximum values). Pearson correlation analysis of initial defect size to (B) % defect fill, (C) T2 relaxation time, and (D) International Knee Documentation Committee (IKDC) scores at 24 months with ChonDux treatment. Linear regression (dotted line), with associated R2 and P values.
Safety
ChonDux treatments were well tolerated during the course of this study; 77.8% of the patients reported 39 adverse events, and most adverse events were classified as either mild or moderate as summarized in Table 1 . One patient in the ChonDux group (5.6%, hemarthrosis) reported an adverse event classified as severe. The most common adverse events were related to joint pain (50%) and general pain/swelling (44.4%). A full listing and classification of adverse events are provided in Supplemental Table 1 (available in the online version of the article). There were 2 knee-related adverse events classified as likely or definitely device related to ChonDux, both of which were mild in severity (joint pain and a patient fall). Three serious adverse events were reported in the ChonDuxTM group, none of which were device related. Two of the serious adverse events involved implantation of a knee prosthesis to treat pain resulting from progression of secondary arthritis.
Microfracture Reference Control
Arthroscopic microfracture was performed on 3 patients as a reference to previous studies. The arthroscopic nature of this procedure (ChonDux implantation used open surgery) and low patient number in this group precludes direct comparison with ChonDux. Microfracture patient demographics and safety (Supplemental Table 2), MRI participation (Supplemental Table 3), VAS pain scoring (Supplemental Table 4), and a detailed list/classification of adverse events (Supplemental Table 5) are provided. Structural MRI analysis with arthroscopic microfracture shows the % thickness (Supplemental Figure 2A), % defect fill (Supplemental Figure 2B), and T2 relaxation time (Supplemental Figure 2C) over 24 months.
Discussion
Focal cartilage defects present a difficult treatment challenge for clinicians. Once damaged, hyaline cartilage at the articular surface not only possesses very limited ability to heal but also a compromised ability to function within the dynamic mechanical environment of the joint.2,17 Even very localized damage will eventually affect nearby tissues, such as adjacent cartilage and subchondral bone, and ultimately lead to chronic pain and loss of function. There are few options for treating focal cartilage defects and thus several tissue engineering strategies have been investigated. Multipotent bone marrow–derived mesenchymal stem cells have established chondrogenic differentiation potential, though their isolation and expansion are expensive and requires multiple surgeries. Likewise, autologous chondrocytes have these same limitations in addition to the unknown aspect of passing aged and potentially abnormal cells through multiple generations of ex vivo cell culture. Juvenile allogeneic cells have been used for this reason,5 but limitations of supply will likely limit availability.
Because of the dearth of economical options, microfracture has become a common if flawed treatment for focal cartilage lesions. Microfracture does not require expensive ex vivo cell manipulation and is a comparatively simple and affordable technique.8,18 While microfracture has shown promise in alleviating symptoms in the short term, the repair is not durable. The defect is replaced with scar-like tissue rather than hyaline cartilage, with recurrence of symptoms becoming increasing prevalent within 2 years,10 especially in patients that are less active, have large defects (>2 cm), have BMI greater than 30 kg/m2, and/or middle-aged or older.11,19,20 Microfracture procedures are also associated with negative bone changes, including bruising and cyst formation.21
Biomaterial scaffolds offer an off-the-shelf alternative to cell therapies that involve ex vivo manipulation or cartilage transplant. A foundational tenet of tissue engineering, biomaterial scaffolds provide the 3D substrate that cells need to adhere and organize into complex tissues. Cells within a compatible scaffold can assemble, proliferate, and survive more effectively than cells in scaffold-free 2D environment in cartilage tissue engineering applications.22 While cell-biomaterial constructs may be developed outside of the body, endogenous cells can also effectively populate a scaffold in situ provided the scaffold provides a conducive microenvironment and access. In other words, an appropriate scaffold can recruit autologous progenitor cells without the need for additional isolation and purification steps, greatly simplifying the repair technology.
Marrow-derived cells released during microfracture are an appealing cell source for biomaterial scaffolds in cartilage defect repair due to the ease of the technique.23 Microfracture connects the subchondral bone marrow to the defect, resulting in an influx of marrow elements such as stem cells and immune cells to the defect region. These cells may populate an implanted scaffold, which in turn improve the organization and integration of these stem cells with the defect tissue. A biomaterial may also impart the benefit of cartilage-mimetic mechanical environment. Although early weight bearing can be protected, shear forces are not eliminated and patient compliance during the early phase of repair may be less than optimal. Such forces may be responsible for the extreme variability in microfracture results. An appropriate biomaterial may mitigate this variability by providing a more natural mechanical and structural environment.24
Several biomaterials have been investigated in preclinical and clinical studies that use microfracture as a cell source. Solid, preformed scaffolds composed of porous polyglycolic acid/hyaluronic acid25 can be trimmed to the defect size and implanted. Hydrogel materials offer additional benefits to solid scaffolds such as possessing a high, biomimetic water content similar to hyaline cartilage. Hydrogels can also be polymerized in situ to conform to the microscopic imperfections of the defect tissue surfaces thereby improving integration compared with a preformed solid scaffold. Polyethylene glycol (PEG)/diacrylate hydrogels are one such example and can be quickly polymerized within the defect by brief exposure with low intensity ultraviolet light and a photoinitiator compound. Various PEG formulations have been investigated experimentally,26 and we have previously described ChonDux as a next-generation hydrogel system for cartilage repair.14,15
ChonDux consists of a multifunctional chondroitin sulfate adhesive and a PEG/hyaluronic acid (HA) hydrogel that rapidly adheres, fills, and polymerizes (solidifies) to the defect surface.15 Functionalized chondroitin sulfate (CS) is applied to the defect surface where one functional group chemically immobilizes the CS to amine groups present on the tissue while the other functional group crosslinks to the PEG hydrogel during photo-polymerization. The result is suture-free fixation to the tissue, which is advantageous in a mechanically dynamic environment such as the knee where the implant will experience repeated deformations. The glycosaminoglycans CS and HA aid in cell adhesion and population within the scaffold by mimicking the native composition of native cartilage.27 Indeed, we have shown that the ChonDux material is infiltrated by bone marrow cells and supports chondrogenic differentiation in vitro and hyaline cartilage formation in a goat model in vivo.14,15 A clinical study of 15 patients demonstrated safety, decreased pain, and improved defect fill by MRI analysis after 6 months compared with microfracture alone.14
This trial expands the clinical evaluation of ChonDux to 18 additional patients with regular 3- to 6-month follow-ups over a 24-month period to determine the safety and maintenance of defect fill. Blinded MRI analysis showed that the ChonDux treatment maintained defect fill to greater than 88% over the entire 24-month time course, in contrast to microfracture alone wherein deterioration begins 18 to 24 months following surgery.11,13,28 There were no significant differences in defect fill between any time points, suggesting a stable repair ability of ChonDux. T2-weighted MRI relaxation time is primarily influenced by the high water content of hyaline cartilage and thus can be used as an indicator of cartilage health during the remodeling process. ChonDux-treated defects initially have long T2 relaxation times suggesting abnormally low matrix density, but subsequently remodels into tissue with T2 relaxation times similar to uninjured cartilage by 6 to 12 months. This hyaline cartilage-like T2 signal in ChonDux-treated defects is preserved for the remaining 24-month duration of the study. The kinetics of defect fill correlates strongly with T2 times for ChonDux at later time points. Therefore, ChonDux maintains the defect fill persistently and preserves the quality of the regenerated cartilage tissue. Patient surveys indicate a substantial pain decrease between 4 to 7 days and 6 weeks postoperation with ChonDux treatment. The high initial pain is likely the result of the procedure using open surgery. Structural cartilage integrity visualized by MRI is correlated to clinical outcome29 and agrees with the results of the present study. The low incidence of adverse events, especially those likely related to the device, indicates that ChonDux is safe for further clinical investigation in a larger patient pool.
Other hydrogel-based systems that use microfracture as an autologous cell source have also shown encouraging results for cartilage repair, supporting the notion that biomaterials can be a clinically effective treatment option. BST-CarGel, a chitosan-based scaffold used to treat full thickness cartilage defects with microfracture, maintained defect fill and delayed symptom recurrence after a 5-year follow-up.30,31 The sustained improvement with a chitosan hydrogel supports the assertion that a conformal, defect filling hydrogel provides an ideal scaffold environment for cartilage repair. The ChonDux system provides the addition of a tissue adhesive component with a PEG hydrogel, creating a mechanically stabilized environment and unique physiochemical properties. Additional studies are necessary to determine whether these characteristics convey a clinical benefit.
There were several limitations in this study that warrant additional investigation. Although this trial improved follow-up for ChonDux from 6 to 24 months, and showed excellent structural imaging, long-term outcomes remain unknown. Likewise, only 12 of the initial 18 patients were evaluated at 24 months due to patient dropout. Another area of further investigation is the physical therapy procedure. While it is known that physical therapy is beneficial for improved healing and functional outcomes, clinical data demonstrating an optimal rehabilitation protocol is lacking. The present study incorporated standard rehabilitation elements such as early motion and non-weightbearing, though it is unknown whether these parameters are optimal for a biomaterial implant that recapitulates the cartilage mechanical environment compared with standard microfracture.14 Additional research into the role of rehabilitation and biomaterials-mediated cartilage repair is required.
In conclusion, the ChonDux treatment was shown to provide a suitable environment for stable cartilage repair of a full thickness defect over a 2-year time course. The improved structural remodeling and low pain scores in this report justifies further clinical study with a larger patient population and longer follow-up period. ChonDux implantation in combination with microfracture is a promising therapy for the treatment of focal articular cartilage defects with the potential for long-term symptom relief.
Supplemental Material
Supplemental material, Supplementary_Information_Only_(1) for Two-Year Follow-Up and Remodeling Kinetics of ChonDux Hydrogel for Full-Thickness Cartilage Defect Repair in the Knee by Matthew T. Wolf, Hong Zhang, Blanka Sharma, Norman A. Marcus, Uwe Pietzner, Stefan Fickert, Achim Lueth, G. H. Robert Albers and Jennifer H. Elisseeff in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The clinical study was supported and conducted by Biomet, Inc. J.H.E. is supported by the Morton Goldberg Professorship. M.T.W. was supported by the Hartwell Foundation Postdoctoral Fellowship. The authors would like to acknowledge Jennifer Woodell-May of Zimmer Biomet for assistance with data acquisition. The authors thank Dr. Otto Stibbe and Dr. Nikolaus Böhler for their surgical expertise.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Clinical Investigation Plan (CIP), patient information letter, and informed consent form were submitted to the structured institutional review boards (ethics committees) of each investigational center and a positive vote of the Board of Directors was obtained prior to start of the enrollment, in accordance with local law. This investigation was conducted in accordance with the Good Clinical Practice Guidelines, with the Declaration of Helsinki, ISO 14155, and all relevant national guidelines.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: This assessor single blind study was conducted between March 18, 2009 and December 5, 2010 as described at https://clinicaltrials.gov (Identifier NCT01110070)
ORCID iD: Matthew T. Wolf  https://orcid.org/0000-0002-0328-2475
https://orcid.org/0000-0002-0328-2475
Supplemental Material: Supplemental material is available for this article online.
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 2. Fox AJS, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson LL. Arthroscopic abrasion arthroplasty: a review. Clin Orthop Relat Res. 2001;(391 Suppl):S306-S317. [PubMed] [Google Scholar]
- 4. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466(4):952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2(4):346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;(360):159-68. [PubMed] [Google Scholar]
- 7. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-30. [DOI] [PubMed] [Google Scholar]
- 8. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 10. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 11. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. [DOI] [PubMed] [Google Scholar]
- 12. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-7 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826-31. [DOI] [PubMed] [Google Scholar]
- 13. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 14. Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):167ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):294-304. [DOI] [PubMed] [Google Scholar]
- 17. Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32(6):1451-8. [DOI] [PubMed] [Google Scholar]
- 18. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001(391 Suppl):S362-S369. [DOI] [PubMed] [Google Scholar]
- 19. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180-6. [DOI] [PubMed] [Google Scholar]
- 20. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 21. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cools P, Mota C, Lorenzo-Moldero I, Ghobeira R, De Geyter N, Moroni L, et al. Acrylic acid plasma coated 3D scaffolds for cartilage tissue engineering applications. Sci Rep. 2018;8(1):3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buda R, Castagnini F, Cavallo M, Ramponi L, Vannini F, Giannini S. “One-step” bone marrow-derived cells transplantation and joint debridement for osteochondral lesions of the talus in ankle osteoarthritis: clinical and radiological outcomes at 36 months. Arch Orthop Trauma Surg. 2016;136(1):107-16. [DOI] [PubMed] [Google Scholar]
- 24. Petters O, Schmidt C, Thuemmler C, Peinemann F, Zscharnack M, Somerson JS, et al. Point-of-care treatment of focal cartilage defects with selected chondrogenic mesenchymal stromal cells—an in vitro proof-of-concept study. J Tissue Eng Regen Med. 2018;12(7):1717-27. [DOI] [PubMed] [Google Scholar]
- 25. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res. 2009;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16(3):305-29. [DOI] [PubMed] [Google Scholar]
- 27. Wang T, Lai JH, Yang F. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng Part A. 2016;22(23-24):1348-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579-88. [DOI] [PubMed] [Google Scholar]
- 29. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41(6):1426-34. [DOI] [PubMed] [Google Scholar]
- 30. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6(2):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671-86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Information_Only_(1) for Two-Year Follow-Up and Remodeling Kinetics of ChonDux Hydrogel for Full-Thickness Cartilage Defect Repair in the Knee by Matthew T. Wolf, Hong Zhang, Blanka Sharma, Norman A. Marcus, Uwe Pietzner, Stefan Fickert, Achim Lueth, G. H. Robert Albers and Jennifer H. Elisseeff in CARTILAGE