Abstract
BACKGROUND:
New randomized, controlled trials have become available on oral P2Y12 inhibitors in acute coronary syndrome. We aimed to evaluate current evidence comparing the efficacy and safety profile of prasugrel, ticagrelor, and clopidogrel in acute coronary syndrome by a meta-analysis of randomized controlled trials.
METHODS:
We performed a network meta-analysis and direct pairwise comparison analysis of efficacy and safety outcomes from 12 randomized controlled trials including a total of 52 816 patients with acute coronary syndrome.
RESULTS:
In comparison with clopidogrel, ticagrelor significantly reduced cardiovascular mortality (hazard ratio [HR], 0.82 [95% CI, 0.72–0.92]) and all-cause mortality (HR, 0.83 [95% CI, 0.75–0.92]), whereas there was no statistically significant mortality reduction with prasugrel (HR, 0.90 [95% CI, 0.80–1.01] and HR, 0.92 [95% CI, 0.84–1.02], respectively). In comparison with each other, there were no significant differences in mortality (HR prasugrel versus ticagrelor, 1.10 [95% CI, 0.94–1.29] and 1.12 [95% CI, 0.98–1.28]). In comparison with clopidogrel, prasugrel reduced myocardial infarction (HR, 0.81 [95% CI, 0.67–0.98]), whereas ticagrelor showed no risk reduction (HR, 0.97 [95% CI, 0.78–1.22]). Differences between prasugrel and ticagrelor were not statistically significant. Stent thrombosis risk was significantly reduced by both ticagrelor and prasugrel versus clopidogrel (28%–50% range of reduction). In comparison with clopidogrel, both prasugrel (HR, 1.26 [95% CI, 1.01–1.56]) and ticagrelor (HR, 1.27 [95% CI, 1.041–55]) significantly increased major bleeding. There were no significant differences between prasugrel and ticagrelor for all outcomes explored.
CONCLUSIONS:
Prasugrel and ticagrelor reduced ischemic events and increased bleeding in comparison with clopidogrel. A significant mortality reduction was observed with ticagrelor only. There was no efficacy and safety difference between prasugrel and ticagrelor.
REGISTRATION:
URL: https://www.crd.york.ac.uk/PROSPERO/; Unique identifier: CRD42019155648.
Keywords: acute coronary syndrome, meta-analysis, P2Y12 protein, human
Oral P2Y12 receptor inhibitors in combination with aspirin constitute the foundation of antiplatelet strategy after acute coronary syndrome (ACS).1,2 Current international guidelines recommend the P2Y12 inhibitors, ticagrelor or prasugrel over clopidogrel for patients with ACS3–5 because of the higher potency, faster onset of action, and lower interindividual variability that have in turn translated into improved clinical outcomes in the ACS setting.6–10
In a large double-blind randomized controlled trial (RCT) that compared prasugrel with clopidogrel in patients with ACS undergoing percutaneous coronary intervention, prasugrel was associated with a significant reduction of the composite end point of cardiovascular (CV) death, myocardial infarction (MI) and stroke, and stent thrombosis, but with an increased risk of major bleeding.10 In another pivotal large randomized double-blind trial, ticagrelor was compared with clopidogrel in patients with ACS with or without ST-segment elevation. Ticagrelor significantly reduced the composite rate of death from vascular causes, MI, or stroke and overall mortality with an increase in spontaneous major bleeding.9 Smaller randomized trials with potent oral P2Y12 inhibitors in comparison with clopidogrel have yielded conflicting results, and a recent randomized open-label trial comparing prasugrel and ticagrelor reported the greater efficacy of prasugrel over ticagrelor without any apparent trade-off in bleeding risk.11 Within this framework, we aimed to perform a meta-analysis of RCTs designed to assess the efficacy and safety of oral P2Y12 receptor inhibitors in the setting of ACS.
METHODS
The data, analytical methods, and study materials will be available to other researchers to reproduce the results or replicate the procedure from the corresponding author on reasonable request. Established methods recommended by the Cochrane Collaboration were used to conduct the meta-analysis.12 The findings were reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.13 The following databases were searched: Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, TCTMD (https://www.tctmd.com/), ClinicalTrials.gov, Clinical Trial Results (http://www.clinicaltrialresults.org), and major congress proceedings, from database inception date through October 19, 2019. The following keywords were used: ticagrelor, prasugrel, clopidogrel, P2Y12 inhibitor, randomized, controlled trial, ACS (Table I in the Data Supplement).
The main inclusion criteria were: (1) randomized trials investigating oral P2Y12 inhibitors (clopidogrel, prasugrel, or ticagrelor) administered in patients with ACS, (2) studies designed to investigate clinical outcomes, (3) studies reporting CV clinical outcomes of interest, and (4) studies investigating beyond a 30-day follow-up. Studies were excluded if they (1) investigated different regimens of the same P2Y12 inhibitor compared against one another; (2) were designed as pharmacokinetics/pharmacodynamics studies, primarily designed for analysis of platelet reactivity; (3) had a crossover design; and (4) had a nonrandomized design.
Quality Assessment and Data Extraction
Two investigators (M.K., S.K.) not involved in any of the selected trials independently abstracted the data using prespecified forms, appraised the accuracy of the abstractions, and resolved any discrepancies by consensus after discussion with a third investigator (E.P.N.). The data were abstracted on baseline characteristics of the trials and participants, outcomes, sample sizes, and follow-up duration. Two investigators (M.K., S.K.) independently appraised the potential risks of bias of the randomized clinical trials using the Cochrane Risk of Bias Tool.12
Outcome Measures
The prespecified efficacy and safety end points were analyzed. The efficacy end points were CV mortality, all-cause mortality, MI, stroke, definite or probable stent thrombosis (ST); the safety end point was major bleeding.
Statistical Analysis
We performed the frequentist network meta-analyses to generate direct and indirect evidence among interventions. Heterogeneity was interpreted by inconsistency factor (I2) with <25% considered low, 25% to 50% moderate, and >50% high.12 The model selection was based on I2; we selected the fixed-effects model if heterogeneity was low, and alternatively the random-effects model was selected for higher I2 values. We used network meta-analysis (NMA) methods on all available treatment comparisons to provide the most comprehensive evidence, incorporating direct comparisons within trials between 2 treatments (such as ticagrelor or prasugrel versus clopidogrel) and indirect comparisons from trials having 1 treatment in common (such as ticagrelor versus prasugrel using trials comparing ticagrelor versus clopidogrel and clopidogrel versus prasugrel). Estimates were reported as hazard ratio (HR) with 95% CIs to account for time-to-event data. The consistency between direct and indirect sources of evidence was examined by the node-splitting method (Table II in the Data Supplement). The P-score metric was used to assess comparative hierarchy of efficacy and safety of the treatments. The value of P-score ranges between 0 and 1, that is, the higher the value, the higher the likelihood that a therapy is highly effective or safe, and lower value demonstrates that a therapy is ineffective. The results were regarded as significant when the 95% CIs of the HRs did not include the unit value.
Direct pairwise meta-analyses were performed using the Mantel-Haenszel fixed-effects model or the DerSimonian and Laird random-effects model, where appropriate, reporting direct estimates. P<0.05 was considered statistically significant for statistical pairwise comparisons. Publication bias was estimated using the Egger regression test (Table III in the Data Supplement).
All analyses were performed using R Project for Statistical Computing-“netmeta” package and comprehensive Meta-Analysis version 3.1 (Biostat). The protocol was registered in https://www.crd.york.ac.uk/PROSPERO/; Unique identifier: CRD42019155648.
RESULTS
Of 7398 articles, 6057 were screened after removal of duplicates and screening at the title and abstract level, and an additional 29 full-text articles were removed based on a priori selection criteria. Ultimately, 12 trials (52816 patients) met inclusion criteria (Figure IA and IB in the Data Supplement).
Median follow-up of the included studies was 12 months. Most trials compared novel oral P2Y12 inhibitor (6 trials with ticagrelor9,14–18 and 4 with prasugrel10,19–21) against clopidogrel, 2 trials compared novel P2Y12 inhibitors against each other11,22 (Table). In 9 trials, all patients underwent invasive evaluation for their index ACS event.10,11,14–17,19,21–23 The mean age of study participants was 63.7 years; 29.9% and 54.8% were admitted with ST-segment-elevation MI and non-ST-segment-elevation MI, respectively; and 27.2% of patients had diabetes mellitus (Table IV in the Data Supplement).
Table.
Study Characteristics
| Study | Follow-Up | Recruitment Period | Arm (Maintenance Dose) | No. of Patients (Intention to Treat) | Study Setting Summary |
|---|---|---|---|---|---|
| [The Elderly ACS II trial]21 Savonitto et al 2018 Circulation [NCT01777503] | 12 mo | November 15, 2012 to January 25, 2017 | Prasugrel 5 mg od | 713 | Elderly ACS undergoing PCI |
| Clopidogrel 75 mg od | 730 | ||||
| [ISAR-REACT 5]11 Schupke et al 2019 NEJM [NCT01944800] | 12 mo | September 2013 to February 2018 | Ticagrelor 90 mg bid | 2012 | ACS with planned invasive evaluation |
| Prasugrel 5/10 mg od | 2006 | ||||
| [PHILO]15 Goto et al 2015 Circulation Journal [NCT01294462] | 12 mo | NA | Ticagrelor 90 mg bid | 401 | Japanese, Korean, and Taiwanese ACS patients with planned PCI |
| Clopidogrel 75 mg od | 400 | ||||
| [PLATO]9 Wallentin et al 2009 NEJM [NCT00391872] | 12 mo | October 2006 to July 2008 | Ticagrelor 90 mg bid | 9333 | ACS with or without STEMI |
| Clopidogrel 75 mg od | 9291 | ||||
| [POPular AGE trial]14 Marieke 2019 [NCT02317198] | 12 mo | June 2013 to October 2018 | Clopidogrel 75 mg od | 501 | 70 y or older with NSTEMI ACS |
| Ticagrelor 90 mg bid or Prasugrel 5/10 mg od* | 502 | ||||
| [PRAGUE-18]22 Motovska et al 2017 JACC [NCT02808767] | 12 mo | Completed May 2016 | Prasugrel 10 mg od | 634 | STEMI treated with primary PCI |
| Ticagrelor 90 mg bid | 596 | ||||
| [The PRASFIT-ACS]19 Saito et al 2013 Circulation Journal |
11 mo | December 2010 to June 2012 | Prasugrel 3.75 mg od | 685 | Japanese ACS patients undergoing PCI |
| Clopidogrel 75 mg od | 678 | ||||
| [TICAKOREA]16 Duk-Woo Park et al 2019 Circulation [NCT02094963] |
12 mo | July 5, 2014 to June 30, 2017 | Ticagrelor 90 mg bid | 400 | Korean ACS patients with or without STEMI |
| Clopidogrel 75 mg od | 400 | ||||
| [TRILOGY ACS]20 Roe et al 2012 NEJM [NCT00699998] | 17 mo | June 27, 2008 to September 12, 2011 | Prasugrel 10 mg od | 4663 | ACS without revascularization |
| Clopidogrel 75 mg od | 4663 | ||||
| [TRITON-TIMI 38]10 Wiviott et al 2007 NEJM [NCT00097591] | 15 mo | November 2004 to January 2007 | Prasugrel 10 mg od | 6813 | ACS with scheduled PCI |
| Clopidogrel 75 mg od | 6795 | ||||
| Tang et al17 2016 J Cardiovasc Pharmacol | 6 mo | January 1, 2013 to April 30, 2015 | Ticagrelor 90 mg bid | 200 | Chinese STEMI patients undergoing primary PCI |
| Clopidogrel 75 mg od | 200 | ||||
| Wang et al18 2016 TCRM | 12 mo | August 2013 to November 2014 | Ticagrelor 90 mg bid | 100 | Elderly Chinese patients with ACS |
| Clopidogrel 75 mg od | 100 |
ACS indicates acute coronary syndrome; bid, bis in die (twice a day); ISAR-REACT 5, Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes; JACC, Journal of the American College of Cardiology; NA, not available; NEJM, The New England Journal of Medicine; NSTEMI, non–ST-segment–elevation myocardial infarction; od, omni die (once daily); PCI, percutaneous coronary intervention; PHILO, Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients; PLATO, PLATelet inhibition and patient Outcomes; PRASFIT-ACS, PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI, TICAKOREA – Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management; STEMI, ST-segment–elevation myocardial infarction; TCRM, Therapeutics and Clinical Risk Management; TRILOGY ACS, The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes; TRITON–TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38; and UA, unstable angina.
2% of patients received prasugrel.
Five studies had an open-label design11,14,16,20–22 (Table V in the Data Supplement). With reference to other aspects of study conduction (selection, detection, attrition, reporting, and other bias), all studies were generally considered to be of a low bias risk.
Efficacy
CV Mortality
Twelve trials (52 816 patients) reported 2035 (3.8%) CV mortality events. In comparison with clopidogrel, ticagrelor was associated with a significant reduction in CV mortality (HR, 0.82 [95% CI, 0.72–0.92]), whereas CV mortality was not significantly reduced with prasugrel (HR, 0.90 [95% CI, 0.80–1.01]; Figure 1A). There were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 1.10 (95% CI, 0.94–1.29). There was low heterogeneity among treatments (I2=10.1%). Direct pairwise comparison analyses yielded consistent results with significant CV mortality reduction associated with ticagrelor versus clopidogrel (HR, 0.80 [95% CI, 0.71–0.92], P<0.001; Figure 1B).
Figure 1. Meta-analyses of randomized trials for cardiovascular mortality.
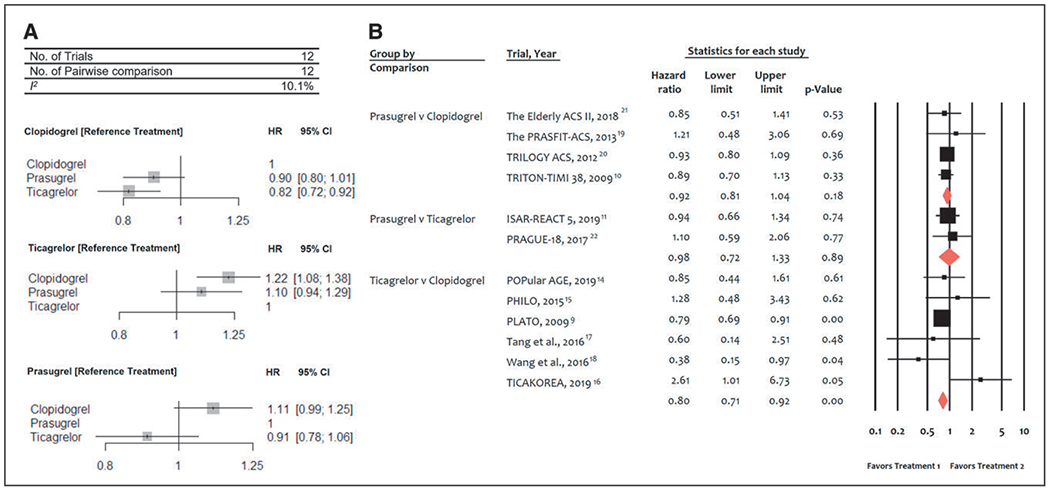
A, Network meta-analysis of randomized trials for cardiovascular mortality. Pooled hazard ratios (HRs) and 95% CIs determined by network meta-analysis. B, Pairwise meta-analysis of randomized trials for cardiovascular mortality. Individual and summary hazard ratios with 95% CIs. ISAR-REACT 5 indicates Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes, PHILO indicates Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients; PLATO, PLATelet inhibition and patient Outcomes; PRASFIT-ACS, PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI; TICAKOREA, Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management; TRILOGY ACS, The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes; and TRITON–TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38.
All-Cause Mortality
Twelve trials (52 816 patients) reported 2537 (4.8%) all-cause mortality events. In comparison with clopidogrel, ticagrelor was associated with a significant reduction in all-cause mortality (HR, 0.83 [95% CI, 0.75–0.92]), whereas prasugrel showed no significant difference (HR, 0.92 [95% CI, 0.84–1.02]; Figure IIA in the Data Supplement). There were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 1.12 (95% CI, 0.98–1.28). There was low heterogeneity among treatments (I2=21.7%). Similar results were obtained with the pairwise meta-analysis (Figure IIB in the Data Supplement).
Noncardiovascular Mortality
Eleven trials (51 373 patients) reported 475 (0.9%) noncardiovascular mortality events. In comparison with clopidogrel, there was no significant difference with ticagrelor (HR, 0.97 [95% CI, 0.80–1.16]) or prasugrel (HR, 0.88 [95% CI, 0.75–1.04]; Figure IIC in the Data Supplement). Similarly, there were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 0.89 (95% CI, 0.60–1.31). There was moderate heterogeneity among treatments (I2=35.5%). Similar results were obtained with the pairwise meta-analysis (Figure IID in the Data Supplement).
Myocardial Infarction
Twelve trials (52 816 patients) in total reported 3440 (6.5%) MI events. In comparison with clopidogrel, prasugrel was associated with a significant reduction in MI (HR, 0.81 [95% CI, 0.67–0.98]), whereas ticagrelor was not (HR, 0.97 [95% CI, 0.78–1.22]; Figure 2A). There were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 0.83 (95% CI, 0.64–1.07). There was high heterogeneity among treatments (I2=58.7%). Direct pairwise meta-analysis showed significant MI reduction with prasugrel in comparison with clopidogrel (HR, 0.84 [95% CI, 0.72–0.98], P=0.03), whereas no significant differences emerged between prasugrel and ticagrelor (Figure 2B).
Figure 2. Meta-analyses of randomized trials for myocardial infarction.
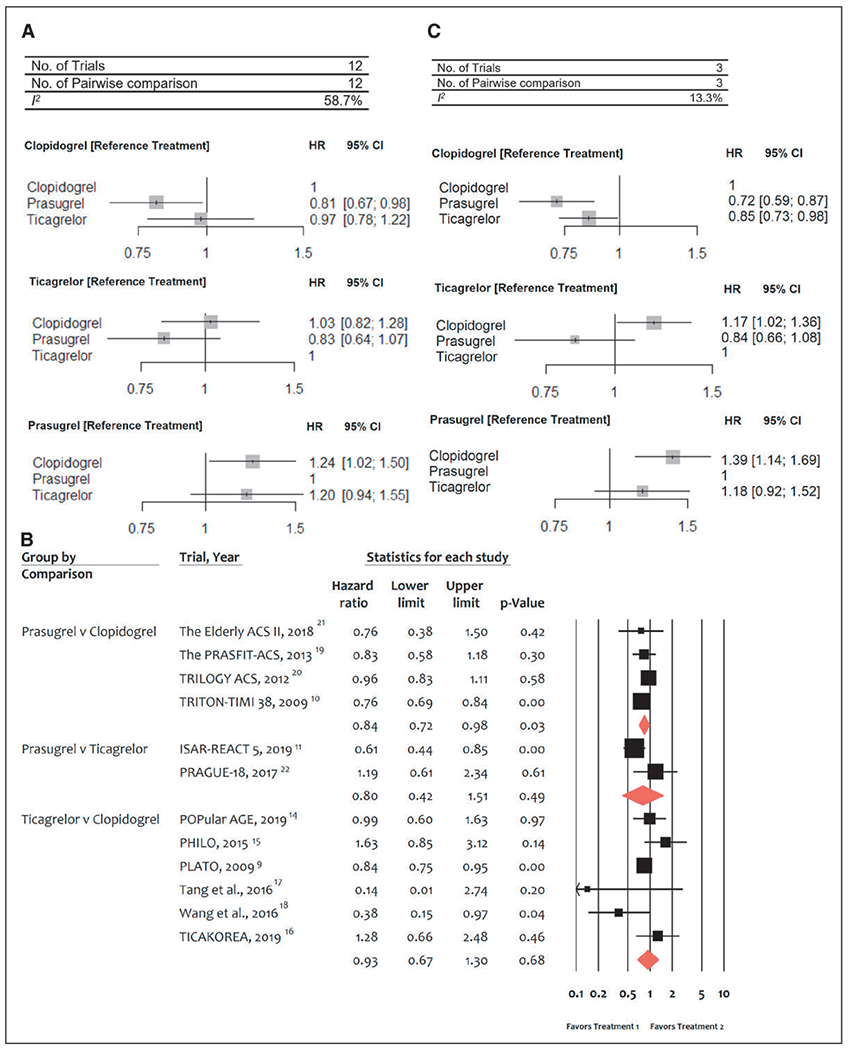
A, Network meta-analysis of randomized trials for myocardial infarction. Pooled hazard ratios (HRs) and 95% CIs determined by network meta-analysis. B, Pairwise meta-analysis of randomized trials for myocardial infarction. Individual and summary HRs with 95% CIs. C, Network meta-analysis of randomized trials for re–myocardial infarction. Pooled HRs and 95% CIs determined by network meta-analysis. ISAR-REACT 5 indicates Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes; PHILO, Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients; PLATO, PLATelet inhibition and patient Outcomes; PRASFIT-ACS, PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI; TICAKOREA, Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management; TRILOGY ACS, The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes; and TRITON–TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38.
In a NMA sensitivity analysis performed by excluding the periprocedural MI (type 4), and focused on re-MI only, in comparison with clopidogrel, a significant reduction of MI was observed with both prasugrel (HR, 0.72 [95% CI, 0.59–0.87]) and ticagrelor (HR, 0.85 [95% CI, 0.73–0.98]; Figure 2C).
Definite or Probable ST
Seven trials (40 459 patients) reported 568 (1.4%) definite or probable ST events. In comparison with clopidogrel, both ticagrelor (HR, 0.72 [95% CI, 0.58–0.90]) and prasugrel (HR, 0.50 [95% CI, 0.38–0.64]) were associated with a significant reduction of definite or probable ST (Figure 3A). Prasugrel was associated with a significantly lower ST risk than ticagrelor (HR, 0.68 [95% CI, 0.50–0.93]). There was no heterogeneity among treatments (I2=0%). In the pairwise analyses, results were consistent with both prasugrel and ticagrelor reducing ST risk significantly in comparison with clopidogrel (Figure 3B).
Figure 3. Meta-analysis of randomized trials for definite or probable stent thrombosis.
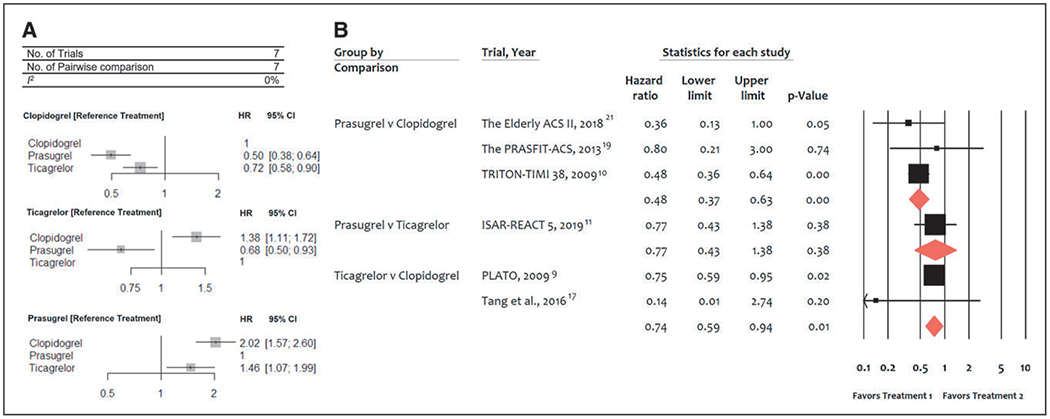
A, Network meta-analysis of randomized trials for definite or probable stent thrombosis. Pooled hazard ratios (HRs) and 95% CIs determined by network meta-analysis. B, Pairwise meta-analysis of randomized trials for definite or probable stent thrombosis. Individual and summary HRs with 95% CIs. ISAR-REACT 5 indicates Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes; PLATO, PLATelet inhibition and patient Outcomes; PRASFIT-ACS, PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI; and TRITON–TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38.
Definite ST
Four trials (24 672 patients) reported 233 (0.9%) cases of definite ST. In comparison with clopidogrel, both ticagrelor (HR, 0.67 [95% CI, 0.50–0.89]) and prasugrel (HR, 0.40 [95% CI, 0.21–0.77]) were associated with a significantly lower risk of definite ST (Figure IIIA in the Data Supplement). There were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 0.60 (95% CI, 0.34–1.08). There was no heterogeneity among treatments (I2=0%). Pairwise direct estimate results were consistent with the NMA analyses (Figure IIIB in the Data Supplement).
Stroke
Eleven trials (51 813 patients) reported 608 (1.1%) strokes. In comparison with clopidogrel, there was no significant difference with ticagrelor (HR, 1.12 [95% CI, 0.90–1.40]) or prasugrel (HR, 0.92 [95% CI, 0.74–1.15]; Figure IVA in the Data Supplement). Similarly, there were no significant differences between prasugrel and ticagrelor: HR prasugrel versus ticagrelor, 0.82 (95% CI, 0.62–1.10). There was no heterogeneity among treatments (I2=0%). Pairwise direct meta-analysis yielded consistent results (Figure IVB in the Data Supplement).
Hemorrhagic Stroke
Four trials (24 805 patients) reported 48 (0.2%) cases of hemorrhagic stroke. In comparison with clopidogrel, there was no significant difference with ticagrelor (HR, 1.59 [95% CI, 0.83–3.03]) or prasugrel (HR, 0.48 [95% CI, 0.10–2.21]) in terms of hemorrhagic stroke (Figure VA in the Data Supplement). Similarly, no significant differences were found between prasugrel and ticagrelor (HR prasugrel versus ticagrelor, 0.30 [95% CI, 0.07–1.28]). There was no heterogeneity among treatments (I2=0%).
Ischemic Stroke
Four trials (24 805 patients) reported 243 (0.9%) ischemic strokes. In comparison with clopidogrel, there was no significant difference with ticagrelor (HR, 1.04 [95% CI, 0.79–1.38]) or prasugrel (HR, 0.94 [95% CI, 0.52–1.72]), respectively (Figure VB in the Data Supplement). Similarly, there were no significant differences between prasugrel and ticagrelor (HR prasugrel versus ticagrelor, 0.90 [95% CI, 0.51–1.61]). There was no heterogeneity among treatments (I2=0%).
Safety
Major Bleeding
Twelve trials (52 816 patients) reported 2820 (5.3%) major bleeding events as classified by the individual trial definitions. In comparison with clopidogrel, both prasugrel (HR, 1.26 [95% CI, 1.01–1.56]) and ticagrelor (HR, 1.27 [95% CI, 1.04–1.55]) were associated with significantly more major bleeding events (Figure 4A). There were no statistically significant differences in bleeding risk between prasugrel and ticagrelor (HR prasugrel versus ticagrelor, 0.99 [95% CI, 0.79–1.24]). There was moderate heterogeneity among treatments (I2=35.3%). Direct pairwise analyses were consistent showing that, in comparison with clopidogrel, there was greater bleeding with prasugrel and ticagrelor, which was statistically significant with prasugrel only (HR, 1.29 [95% CI, 1.06–1.56], P=0.01; Figure 4B).
Figure 4. Meta-analysis of randomized trials for major bleeding.
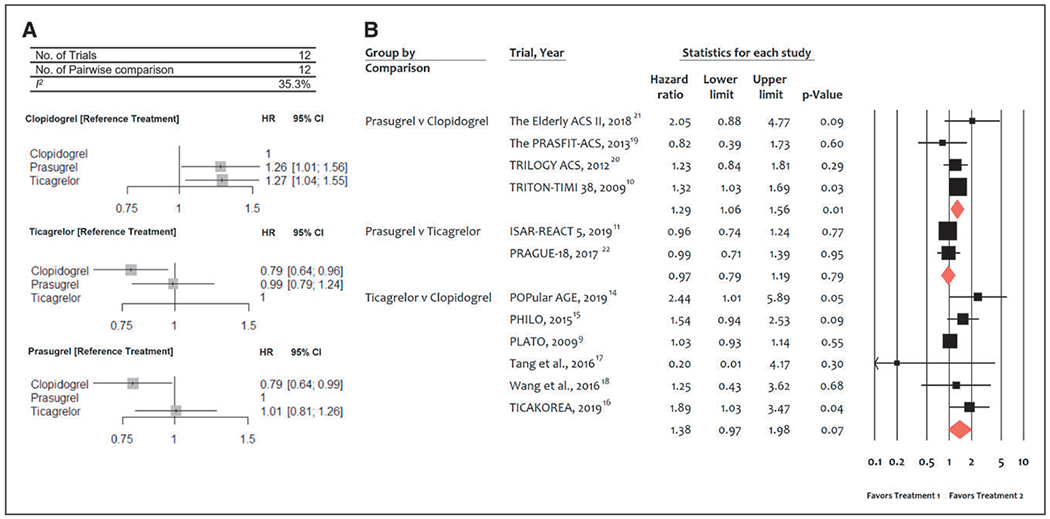
A, Network meta-analysis of randomized trials for major bleeding. Pooled hazard ratios (HRs) and 95% CIs determined by network meta-analysis. B, Pairwise meta-analysis of randomized trials for major bleeding. Individual and summary HRs with 95% CIs. ISAR-REACT 5 indicates Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes; PHILO, Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients; PLATO, PLATelet inhibition and patient Outcomes; PRASFIT-ACS, PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI; TICAKOREA, Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management; TRILOGY ACS, The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes; and TRITON–TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38.
Direct pairwise sensitivity analyses focused on major bleeding as per the criteria of the PLATO trial (Patients with Acute Coronary Syndromes), including 4 RCTs comparing ticagrelor versus clopidogrel, showed no significant difference between ticagrelor versus clopidogrel (HR, 1.04 [95% CI, 0.93–1.15]; Figure VIA in the Data Supplement), whereas no studies were available that compared prasugrel versus clopidogrel. Analyses focused on TIMI criteria including 5 RCTs (2 RCTs on prasugrel versus clopidogrel and 3 RCTs on ticagrelor versus clopidogrel) showed a significant bleeding increase with prasugrel but not ticagrelor, in comparison with clopidogrel (Figure VIB in the Data Supplement).
Sensitivity Analyses
In a NMA sensitivity conducted only in patients undergoing invasive evaluation, a significant reduction in MI with prasugrel in comparison with clopidogrel and in ST with ticagrelor and prasugrel in comparison with clopidogrel was found (Figure VIIA through VIIG in the Data Supplement). There were no statistically significant differences in bleeding risk between ticagrelor and clopidogrel (HR, 1.08 [95% CI, 0.99–1.19]), whereas bleeding was significantly greater with prasugrel versus clopidogrel (HR, 1.23 [95% CI 1.04–1.46]). The direction of the remaining estimates was in line with the main results.
By exclusion of the studies with open-label design, ticagrelor was associated with persistent significant risk reduction of CV and all-cause mortality in comparison with clopidogrel and significant risk reduction in all-cause mortality in comparison with prasugrel (Table VI in the Data Supplement). Ticagrelor significantly reduced definite or probable ST in comparison with clopidogrel. Prasugrel but not ticagrelor significantly increased the risk of major bleeding. There was no significant difference in terms of MI among all 3 drugs (Table VI in the Data Supplement).
Ranking of Treatment Strategies
Ticagrelor was ranked as possibly the most effective strategy for the prevention of CV mortality (P-score, 0.94; Figure VIII in the Data Supplement) or all-cause mortality (P-score, 0.97), whereas prasugrel ranked the most effective intervention for definite or probable ST (P-score, 0.99). Conversely, for major bleeding, clopidogrel was ranked as the safest strategy (P-score, 0.98).
Network Consistency
The node-splitting method did not detect significant disagreement between direct and indirect evidence (Table II in the Data Supplement). The Egger regression test did not detect significant publication bias (Table III in the Data Supplement).
DISCUSSION
The current network and direct pairwise meta-analysis, with 52 816 patients from randomized trials, examined the efficacy and safety profile of potent oral P2Y12 inhibitors in ACS in comparison with clopidogrel and with each other. The main findings (Figure 5) are as follows: (1) prasugrel and ticagrelor are more effective than clopidogrel in reducing the risk of MI and ST; (2) both agents carry a higher bleeding risk than clopidogrel; (3) in comparison with clopidogrel, a greater mortality reduction is observed with ticagrelor than with prasugrel; and (4) no significant difference is apparent between prasugrel and ticagrelor in the explored outcomes.
Figure 5. Summary pooled hazard ratios (HRs) and 95% CIs determined by network meta-analysis.
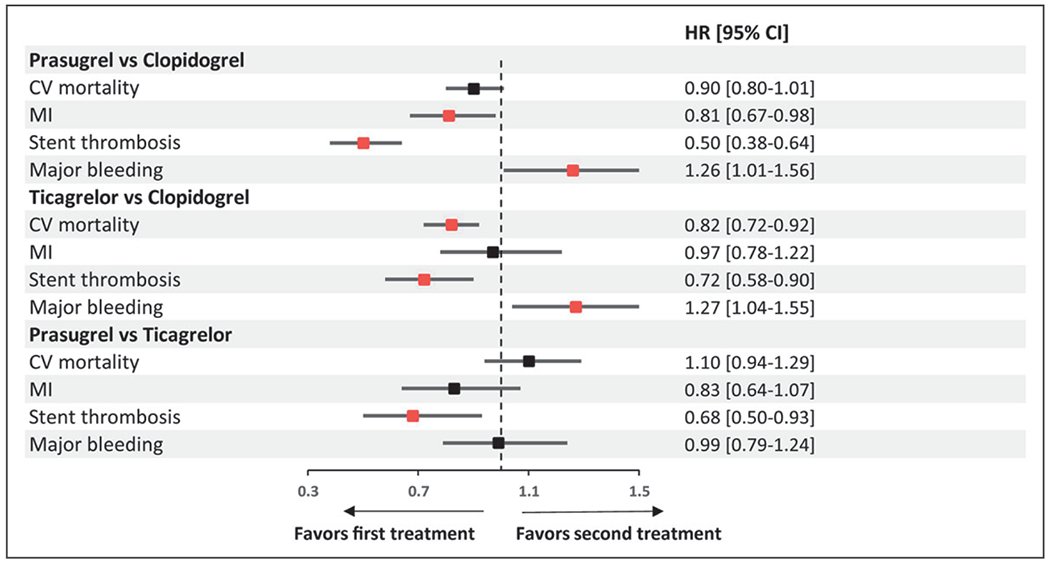
The red squares indicate significant results for the investigated outcome. CV indicates cardiovascular; and MI, myocardial infarction.
The current NMA, by simultaneously gathering direct and indirect evidence, aimed to provide clarification of inconsistent outcomes from studies on oral P2Y12 inhibitors in ACS9–11 and more precise effect estimation where limited direct comparisons between treatments are available.
This large-scale analysis allowed us to examine individual hard end points, which are less frequent than the composite outcomes assessed in the individual studies. Within this framework, a relevant finding of this NMA is that ticagrelor, in comparison with clopidogrel, was associated with a significant risk reduction of CV (−18%) and all-cause (−17%) mortality. Prasugrel did not significantly reduce mortality (−10% and −8%), with no significant difference in mortality between ticagrelor- and prasugrel-treated patients. This greater CV and all-cause mortality reduction of ticagrelor than prasugrel, in comparison with clopidogrel, needs confirmation in further investigations.
A potential mechanistic explanation for the greater mortality reduction is that ticagrelor is a reversible P2Y12 receptor inhibitor, which allows a quicker restoration of platelet function than thienopyridines. The reversibility of antiplatelet effect may be of clinical importance in patients experiencing bleeding, which is in turn associated with long-term mortality during follow-up.24 This property can further explain the effects observed for the P2Y12 inhibitors compared in this NMA. The mortality estimates of our NMA globally support the results from previous landmark studies. In the PLATO9 trial with ticagrelor versus clopidogrel, a significant reduction in death from vascular causes was observed, whereas no significant differences in CV death or all-cause death were observed with prasugrel versus clopidogrel in the TRITON-TIMI 38 trial10 (Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes).
The findings of this NMA should be put into perspective with current evidence from the most recent trials on oral P2Y12 inhibitors in ACS. The recently published ISAR-REACT 5 trial (Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes), an open-label study, tested 2 strategies of ticagrelor versus prasugrel administration in ACS.11
As per the ISAR-REACT 5 study design, ticagrelor administration was made as soon as possible after randomization, whereas in the prasugrel group, in patients with ST-segment elevation, prasugrel was to be administered as soon as possible after randomization; in patients with ACS without ST-segment elevation, the loading dose of prasugrel was postponed until the coronary anatomy was known.
The ISAR-REACT 5 study showed that prasugrel was superior to ticagrelor in preventing major adverse ischemic events, and this benefit was not paralleled by an increase in major bleeding.
In the ISAR-REACT 5 study, the investigators hypothesized a 22% relative risk reduction of the primary end point defined as composite of all-cause death, MI, or stroke at 1 year with ticagrelor over prasugrel; in contrast, a 36% significantly higher risk for the primary end point and numerically greater bleeding were observed with ticagrelor versus prasugrel in patients with ACS undergoing percutaneous coronary intervention. These figures appeared inconsistent with data from previous large blinded trials in terms of magnitude of efficacy and safety that had been found comparable for ticagrelor and prasugrel when tested versus clopidogrel. The results of the current NMA allow a better understanding of the contrasting findings between the ISAR-REACT 5 study and the evidence from clinical trials incorporated in the current large-scale analysis.
The primary composite end point of the ISAR-REACT 5 trial was mainly driven by differences in nonfatal MI and noncardiovascular death between ticagrelor and prasugrel. In our report, the overall analysis for MI, which included periprocedural and spontaneous infarctions, showed that prasugrel in comparison with clopidogrel was associated with a significant reduction in MI (−19%) whereas ticagrelor was not. There was no significant difference between prasugrel and ticagrelor with respect to the occurrence of MI. In a sensitivity analysis focused on re-MI excluding the periprocedural MI (type 4), both prasugrel and ticagrelor yielded a significant reduction of MI in comparison with clopidogrel. Both ticagrelor and prasugrel were associated with markedly reduced ST risk by 28% and 50%, respectively, in comparison with clopidogrel, and prasugrel was associated with significantly less ST risk than ticagrelor.
This NMA may assist in our understanding of the effects of oral antithrombotic therapy on MI end points based on MI definition. Our current results showed nonsignificant differences between prasugrel and ticagrelor by using an overall MI (type 1–4) definition across the included randomized trials; however, when excluding periprocedural MI, ticagrelor was associated with a significant reduction of spontaneous MI risk. Therefore, these findings trigger considerations on the role of periprocedural MI as a possible confounder in the ascertainment of the risk of MI. This bias may occur when the periprocedural MI is incorporated in the index event and might attenuate an observation of an effect on re-MI risk reduction attained with a novel P2Y12 inhibitor. In the ISAR-REACT 5 study, the rates of overall MIs appeared to be primarily driving the composite outcome in favor of prasugrel versus ticagrelor, with almost twice as many patients in the ticagrelor group experiencing a periprocedural MI as in the prasugrel group.11
The MI findings in our NMA are affected by the different design of the large pivotal trials. In the PLATO trial, randomization occurred before angiography and percutaneous coronary intervention, and there may have been a lower ability to ascertain periprocedural MI on top of the index MI. In TRITON-TIMI 38, where randomization was performed after coronary anatomy was known, the ascertainment of the differential effect of more potent platelet inhibition on periprocedural MI may have been facilitated.10 With the use of clopidogrel as a common comparator in the pivotal trials, both potent P2Y12 inhibitors significantly reduced MI, but there was a greater (24%) reduction of nonfatal MI with prasugrel in the TRITON-TIMI-38 than the 16% reduction with ticagrelor in the PLATO study. However, consistent with the results of our NMA, the effect on spontaneous MI was greater than periprocedural MI in the PLATO trial.25
Our analysis may provide an opportunity for further considerations on the comparative results for bleeding. In our NMA, in comparison with clopidogrel, both ticagrelor and prasugrel were associated with a significant increase in total bleeding risk; the direct pairwise meta-analysis confirmed directionally the estimates.
In summary, the current NMA showed that prasugrel and ticagrelor reduced ischemic events and increased bleeding in comparison with clopidogrel. A mortality reduction was observed with ticagrelor only. There was no efficacy and safety difference between prasugrel and ticagrelor.
Limitations
Our network meta-analysis has limitations that need to be considered when interpreting its findings. The results were analyzed on trial-level data and not on individual patient data and are affected by the design of the included major trials. In the included studies conducted in patients with ACS, there was a different proportion of patients undergoing invasive and noninvasive evaluation, although the invasive approach was the most frequently adopted; to this end, we conducted a prespecified analysis focused on patients treated with the invasive approach only, which confirmed directionally the overall findings. The majority of included studies did not report results for individual outcomes stratified by ACS; therefore, an analysis of outcomes according to the ACS presentation was not possible. Major bleeding events were classified by the individual trial definitions; however, pairwise sensitivity analyses have been conducted using the PLATO and TIMI definitions, where available, which confirmed directionally the overall results. Sample sizes per comparison varied, which might influence the summary results. Furthermore, the component trials of meta-analyses might not be powered for certain end points or lacked multiple adjustment in statistical hierarchy. However, there was consistency between direct and indirect estimates analyses, and the sensitivity analyses performed showed comparable results, suggesting that the overall effect is robust and justified.
Conclusions
This large-scale analysis on oral P2Y12 inhibitors in ACS provides consistent findings of comparable anti-ischemic efficacy with the different potent platelet inhibitors, ticagrelor and prasugrel, in comparison with clopidogrel. Greater CV mortality reduction is observed with ticagrelor than with clopidogrel, whereas both agents carry a higher bleeding risk. Further investigations should elucidate the potential underlying mechanisms for the greater mortality reduction with ticagrelor versus clopidogrel, which, if confirmed, might have a substantial impact on public health.
Supplementary Material
Clinical Perspective.
What Is New?
This large-scale analysis on oral P2Y12 inhibitors in acute coronary syndrome provides consistent findings of comparable anti-ischemic efficacy with ticagrelor and prasugrel, in comparison with clopidogrel.
In comparison with clopidogrel, only ticagrelor was associated with greater cardiovascular mortality reduction, whereas both ticagrelor and prasugrel were associated with a higher bleeding risk.
Sensitivity analyses without open-label studies confirmed consistent mortality reduction with ticagrelor therapy without a significant bleeding increase, at variance with prasugrel therapy.
What Are the Clinical Implications?
In patients with acute coronary syndrome, both ticagrelor and prasugrel carried superior efficacy to clopidogrel and should be the preferred antiplatelet agents as per guideline recommendations.
Our findings support the mortality benefit and reduction in ischemic outcomes with ticagrelor in high-risk patients.
Further investigations should elucidate the underlying mechanisms for the greater mortality reduction with ticagrelor that might reside in the reversibility of its antiplatelet effect in patients experiencing bleeding.
Acknowledgments
We thank Michal Siedlaczek, MD, for his contribution of further data checking to this project.
Sources of Funding
None.
Disclosures
Dr Navarese reports research grants from Amgen, Abbott and Medtronic, and lectures fees/honoraria from Amgen, Astra-Zeneca, Bayer, Sanofi-Regeneron and Pfizer, outside the submitted work. Dr Kubica reports personal fees from AstraZeneca, outside the submitted work. Dr Cannon served as a consultant for Alnylam, Amarin, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Janssen, Kowa, Merck, Pfizer, Regeneron, and Sanofi and received research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, and Merck. Dr Gurbel reports grants from Haemonetics, Duke Clinical Research Institute, National Institutes of Health, MedImmune, and Coramed; grants and personal fees from Merck; personal fees from AstraZeneca, Boehringer, Janssen, Bayer, and Medicure, outside the submitted work. In addition, Dr Gurbel has a patent Platelet Function Testing issued. Dr Budaj reports receiving grants and personal fees from AstraZeneca, Bristol-Myers Squibb/Pfizer, and GlaxoSmithKline; grants from Sanofi, Boehringer-Ingelheim, Novartis, and Eisai outside the submitted work. Dr Wallentin reports institutional research grant, consulting fee, lecture fee, and travel support from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Pfizer; institutional research grant, consulting fee, lecture fee, travel support, and honoraria from GlaxoSmithKline; institutional research grant from Roche Diagnostics and Merck & Co; consulting fees from Abbott; and patents EP2047275B1 and US8951742B2 licensed to Roche Diagnostics. Dr Ohman reports research/grant funding from Daiichi Sankyo, Gilead Sciences, Janssen Pharmaceuticals (Johnson & Johnson); consulting or honoraria from Abbott Vascular, Medscape, Faculty Connection, Abiomed, AstraZeneca, Biotie, Boehringer Ingelheim, Daiichi Sankyo, Merck, St. Jude Medical, Stealth Peptides, and The Medicines Company. Dr Roe reports receiving grants from Sanofi, Sanofi Aventis, Ferring Pharmaceuticals, Myokardia, American College of Cardiology, American Heart Association, Familial Hypercholesterolemia Foundation, and Patient-Centered Outcomes Research Institute; and personal fees from Janssen Pharmaceuticals, AstraZeneca, Amgen, Elsevier Publishers, Regeneron Pharmaceuticals, Roche-Genetech, Eli Lilly, Ardea Biosciences, Novo Nordisk, Flatiron, Novartis, and Merck. Dr James reports institutional research grants from AstraZeneca, Bayer, Jansen, The Medicines Company, Abbot Vascular, and Boston Scientific, and honoraria from AstraZeneca, Bayer, and Medtronic, as well. The other authors report no disclosures.
Footnotes
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.046786.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
This manuscript was sent to Prof. Harvey White, Guest Editor, for review by expert referees, editorial decision, and final disposition.
REFERENCES
- 1.Tantry US, Navarese EP, Myat A, Chaudhary R, Gurbel PA. Combination oral antithrombotic therapy for the treatment of myocardial infarction: recent developments. Expert Opin Pharmacother. 2018;19:653–665. doi: 10.1080/14656566.2018.1457649 [DOI] [PubMed] [Google Scholar]
- 2.Navarese EP, Buffon A, Kozinski M, Obonska K, Rychter M, Kunadian V, Austin D, De Servi S, Sukiennik A, Kubica J. A critical overview on ticagrelor in acute coronary syndromes. QJM. 2013;106:105–115. doi: 10.1093/qjmed/hcs187 [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. ; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 4.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. ; ESC Scientific Document Group. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 6.Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon CP, Becker RC, Wallentin L. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56:1456–1462. doi: 10.1016/j.jacc.2010.03.100 [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, Chandna H, Macias W, McCabe CH, Braunwald E. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51:2028–2033. doi: 10.1016/j.jacc.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 8.James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. doi: 10.1016/j.ahj.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. ; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, et al. ; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 11.Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, et al. ; ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. Updated March 2011. Cochrane Learning. http://training.cochrane.org/handbook. Accessed September 26, 2019. [Google Scholar]
- 13.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 14.Gimbel ME. Randomised comparison of clopidogrel versus ticagrelor or prasugrel in patients of 70 years or older with non-ST-elevation acute coronary syndrome. POPular AGE trial. Paper presented at: European Society of Cardiology Congress; August 31,2019; Paris, France. [Google Scholar]
- 15.Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome – randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–2460. doi: 10.1253/circj.CJ-15-0112 [DOI] [PubMed] [Google Scholar]
- 16.Park DW, Park SJ. Response by Park and Park to letter regarding article, “Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial.” Circulation. 2020;141:e741–e742. doi: 10.1161/CIRCULATIONAHA.120.045963 [DOI] [PubMed] [Google Scholar]
- 17.Tang X, Li R, Jing Q, Wang Q, Liu P Zhang P, Liu Y. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Cardiovasc Pharmacol. 2016;68:115–120. doi: 10.1097/FJC.0000000000000390 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag. 2016;12:1101–1105. doi: 10.2147/TCRM.S108965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684– 1692. doi: 10.1253/circj.cj-13-1482 [DOI] [PubMed] [Google Scholar]
- 20.Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, et al. ; TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. doi: 10.1056/NEJMoa1205512 [DOI] [PubMed] [Google Scholar]
- 21.Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, et al. ; Elderly ACS 2 Investigators. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. 2018;137:2435–2445. doi: 10.1161/CIRCULATIONAHA.117.032180 [DOI] [PubMed] [Google Scholar]
- 22.Motovska Z, Hlinomaz O, Kala P Hromadka M, Knot J, Varvarovsky I, Dusek J, Jarkovsky J, Miklik R, Rokyta R, et al. ; PRAGUE-18 Study Group. 1-Year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. J Am Coll Cardiol. 2018;71:371–381. doi: 10.1016/j.jacc.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 23.Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS, et al. ; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–293. doi: 10.1016/S0140-6736(09)62191-7 [DOI] [PubMed] [Google Scholar]
- 24.Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65:1411–1420. doi: 10.1016/j.jacc.2015.01.047 [DOI] [PubMed] [Google Scholar]
- 25.Mahaffey KW, Held C, Wojdyla DM, James SK, Katus HA, Husted S, Steg PG, Cannon CP, Becker RC, Storey RF, et al. ; PLATO Investigators. Ticagrelor effects on myocardial infarction and the impact of event adjudication in the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2014;63:1493–1499. doi: 10.1016/j.jacc.2014.01.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


