Abstract
Aims:
The effect of therapeutic lowering of apolipoprotein B (apoB) on mortality and major adverse cardiovascular events is uncertain. It is also unclear whether these potential effects vary by different lipid-lowering strategies.
Methods:
A total of 29 randomized controlled trials were selected using PubMed, Cochrane Library and EMBASE through 2018. We selected trials of therapies which ultimately clear apolipoprotein B particles by upregulating low-density lipoprotein receptor (LDL-R) expression (statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, bile acid sequestrants) or therapies which reduce apolipoprotein B independent of LDL-R (cholesteryl ester transfer protein inhibitor, fibrates, niacin, omega-3 fatty acids) with sample size of ≥1000 patients and follow-up of ≥1 year. The meta-regression and meta-analyses were constructed using a random effects model.
Results:
In 332,912 patients, meta-regression analyses showed relative risks of 0.95 for all-cause mortality (95% confidence interval 0.92–0.99) and 0.93 (0.88–0.98) for cardiovascular mortality for every 10 mg/dL decrease in apolipoprotein B by all interventions combined. Reduction in all-cause mortality was limited to statins (0.92 (0.86–0.98)). For MACE, the relative risk per 10 mg/dL reduction in apolipoprotein B was 0.93 (0.90–0.97) for all therapies combined, with both statin (0.88 (0.83–0.93)) and non-statin therapies (0.96 (0.94–0.99)). which clear apolipoprotein B by upregulating LDL-R showing significant reductions; whereas interventions which lower apolipoprotein B independent of LDL-R did not demonstrate this effect (1.02 (0.81–1.30)).
Conclusion:
While both statin and established non-statin therapies (PCSK9 inhibitor and ezetimibe) reduced cardiovascular risk per decrease in apolipoprotein B, interventions which reduce apolipoprotein B independently of LDL-R were not associated with cardiovascular benefit.
Keywords: Apolipoprotein B, cardiovascular outcomes, mortality, meta-regression analysis
Introduction
Low-density lipoprotein-cholesterol (LDL-C) is a primary therapeutic target for the management and prevention of atherosclerotic cardiovascular disease (ASCVD).1,2 However, there is accumulating evidence related to other lipid surrogate markers, such as non-high-density lipoprotein-cholesterol (non-HDL-C), total cholesterol/HDL-C ratio, lipoprotein (a), and apolipoprotein B (apoB), which can serve as additional risk factors for ASCVD.3–8 For the purposes of this review, we have focused on apoB. ApoB is a major structural protein embedded in very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL) and LDL particles, with one apoB protein per particle.4 Thus, measurement of apoB concentrations accounts for entire circulating concentrations of atherogenic lipoproteins and can provide a more comprehensive picture of a patient’s risk of ASCVD.4
Several investigators have identified apoB as an additional risk factor for ASCVD, independently of LDL-C levels, and suggest their potential role in management of ASCVD.4,5 The 2018 American Heart Association/American College of Cardiology/Multi-Society guidelines do not encourage routine testing of apoB levels due to extra cost and unreliable laboratory measurements.9 However, these guidelines suggest a relative indication of measuring apoB levels in patients with triglycerides ≥200 mg/dL and consider apoB levels ≥130 mg/dL as a “risk-enhancing factor” for ASCVD.
What remains uncertain is whether lowering apoB is associated with decrease in mortality and major adverse cardiovascular events (MACEs) and whether these potential clinical effects vary by different lipid-lowering strategies. Herein, we conducted meta-regression analyses across different lipid-lowering therapies to explore these questions.
Methods
This trial level meta-analysis followed the Cochrane Collaboration guidelines and Preferred Reporting Items for Systematic Reviews and Meta-Analysis.10,11
Data sources and searches
Two independent researchers (MUK and SV) performed the literature search using databases of PubMed, EMBASE, Cochrane library, and online resources (ClinicalTrials.gov, Clinical Trial Results (http://www.clinicaltrialresults.org), TCTMD (https://www.tctmd.com/)), and proceedings of major cardiovascular meetings from inception to 10 December 2018. A broad search strategy was used using relevant search terms: “lipid”, “apolipoprotein B”, “statin”, “proprotein convertase subtilisin/kexin type 9”, “ezetimibe”, “bile acid sequestrants”, “cholesteryl ester transfer protein (CETP) inhibitors”, “fibrates”, “niacin”, “diet”, “omega 3 fatty acids”, and “surgery” (Supplementary Material Table S1 online). After removal of duplicates, two authors (MUK and SV) scrutinized the records at the title and abstract level followed by full text screening based on the pre-determined study selection criteria.
Study selection
The a priori inclusion criteria were: (1) randomized controlled trials of lipid-lowering therapies (statins, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, ezetimibe, bile acid sequestrants, diet, ileal bypass surgery, CETP inhibitors, fibrates, niacin and omega-3 fatty acids (n-3 FAs));12 (2) studies with sample size of at least 1000 patients and follow-up duration of at least one year, to generate more reliable estimates;12,13 (3) studies must report lowering in apoB levels and mortality and cardiovascular outcomes of interest. Trials assessing the lipid-lowering therapies in patients with chronic kidney disease requiring hemodialysis or heart failure were excluded.12–14
Quality assessment and data extraction
Two authors (MUK and SV) independently extracted the data on pre-specified data extraction forms, adjudicated the data and resolved any disagreements related to data with mutual discussion or opinion of a third author (SUK). The following information was abstracted: baseline characteristics of the trials and participants, number of events, sample size, crude point estimates, follow-up duration, baseline and achieved lowering of apoB, LDL-C, triglycerides and non-HDL-C levels in each group, and difference between the groups (absolute reduction). Data were abstracted directly from the trial manuscript if available; otherwise, previous meta-analyses were examined for the required information.12–15 We contacted corresponding authors of the selected trials if baseline apoB values were not reported in the original study. In the case of the corresponding author not replying, we estimated baseline apoB values using the following equation: baseline apoB, mg/dL = −33.12+0.675 * baseline LDL-C+11.95 * ln (baseline triglycerides).16
The between-group differences were calculated as mean or median differences, whichever were available, over the course of follow-up duration of each trial.12,14 When possible, data acquisition was performed according to intention to treat principle. The trial level potential risk of bias assessment was performed using the Cochrane Risk of Bias Tool (Supplementary Table S2).17
Consistent with former reports,12,18 interventions were divided into the following groups: (1) statins and (2) specific non-statin therapies (diet, ileal bypass surgery, PCSK9 inhibitors, ezetimibe and bile acid sequestants), which all reduce apoB containing lipoproteins predominantly by decreasing intrahepatic cholesterol and result in upregulation of LDL receptor (LDL-R) expression or decreased LDL-R catabolism, and (3) non-statin therapies which reduce circulating apoB particles by mechanisms independent of LDL-R (CETP inhibitors, fibrates, niacin, and n-3 FA).
Outcome measures
The co-primary outcomes were all-cause mortality and cardiovascular mortality. The secondary endpoints were MACEs, myocardial infarction (MI), cerebrovascular events, and coronary revascularizations. MACEs and cerebrovascular events are defined in Supplementary Tables S3 and S4.
Statistical analysis
The analyses were performed according to the original randomization groups for which estimates were available.13,15 Data were pooled according to DerSimonian and Laird random effects models.19 Outcomes were reported as risk ratios (RRs) with 95% confidence intervals (CIs), which were derived from an analysis with adjusted models by person-years (a measure integrating study duration) to compensate for potential differences in study follow-up duration.13 Heterogeneity was evaluated via Q statistics with I2> 75% being consistent with a high degree of heterogeneity.20
For our primary analyses, the random effects meta-regression analyses were conducted to calculate change in RR per absolute reduction in apoB levels (each 10 mg/dL lowering) using the restricted maximum likelihood approach. In secondary analyses, we included a series of sequentially adjusted multivariable regression in the model to determine the impact of apoB lowering after accounting for baseline or change in other lipid parameters. First, we adjusted for baseline apoB. Second, we adjusted for baseline LDL-C. Third, we adjusted for absolute reduction in LDL-C levels. Fourth, we adjusted for baseline triglycerides and absolute reduction in triglyceride levels (mg/dL). Finally, we adjusted for age, state (fasting vs. non-fasting), diabetes mellitus, type of drug class, and setting (primary or secondary prevention).
In addition, we performed a series of supplemental exploratory analyses. First, we adjusted apoB values for different analytical methods employed for apoB testing and estimating LDL-C (Supplementary Tables S5 to S7). Second, we also adjusted for baseline and absolute reduction in non-HDL-C values. Because apoB and non-HDL-C are highly correlated, the addition of both parameters would be expected to render one (or both) non-significant due to collinearity (Supplementary Table S8). Third, for relative comparison with the apoB analyses, we also examined the association of baseline LDL-C and absolute LDL-C lowering (each 38.7 mg/dL (1 mmol/L)) with primary and secondary endpoints (Supplementary Table S9) after excluding n-3 FA trials since these drugs typically increase the LDL-C levels.21 In addition, we present the meta-analyses of the drug classes themselves with the primary and secondary outcomes (Supplementary Figures S3 to S20). Publication bias was assessed using funnel plots and Egger’s regression test (Supplementary Table S10 and Figure S21). The index R2 value was used to determine the proportion of variance accounted for by absolute reduction in apoB. We present Variance Inflation Factor for apoB and other lipid markers for each outcome in Supplementary Table S11.
For all analyses, statistical significance threshold was 0.05. Statistical analyses were conducted using the Metafor package version 3.30 (R Project for Statistical Computing)22 and Comprehensive Meta-Analysis Software 3.0 (Biostat, Englewood, New Jersey, USA).
Results
Of 25,119 identified records, 8200 were screened after removal of duplicates, 7620 citations were excluded at title and abstract level, and an additional 551 full text articles were removed based on a priori selection criteria. Finally, 29 trials (332,912 patients) met inclusion criteria (Figure 1). A total of 13 statin trials (115,144 patients),23–35 five trials (77,594 patients) testing non-statin therapies which ultimately reduce apoB containing lipoproteins by upregulating LDL-R (four PCSK9 inhibitor trials (59,450 patients)36–39 and one ezetimibe trial (18,144 patients)40) and 11 trials examining interventions which lower apoB levels via mechanisms independent of LDL-R (five trials of CETP inhibitors (75,102 patients),41–45 two trials of fibrates (12,326 patients),46,47 two trials of niacin therapy (29,087 patients),48,49 and two trials of n-3 FA (23,659 patients)21,50) were included. Five trials (55, 433 patients)25,29,33,35,50 were of primary prevention and the remaining 24 trials (277,479 patients)21,23,24,26–28,30–32,34,41–49 were of secondary prevention. The weighted mean age ± SD across the trials was 62.4 ± 2.3 years. The weighted mean baseline apoB was 99.8 ± 17.9 mg/dL. The weighted mean absolute reduction in apoB across all the interventions was 21.1 mg/dL (statins = 25.7 mg/dL; non-statin therapies (PCSK9 inhibitors (45.2 mg/dL) and ezetimibe (11 mg/dL) = 38.4 mg/dL; and other interventions (CETP inhibitors (11 mg/dL), fibrates (5.5 mg/dL), niacin (7.5 mg/dL) and n-3 FA (3.1 mg/dL) = 7.9 mg/dL). The weighted mean follow-up duration was 4.0 ± 1.7 years (Table 1).
Figure 1.
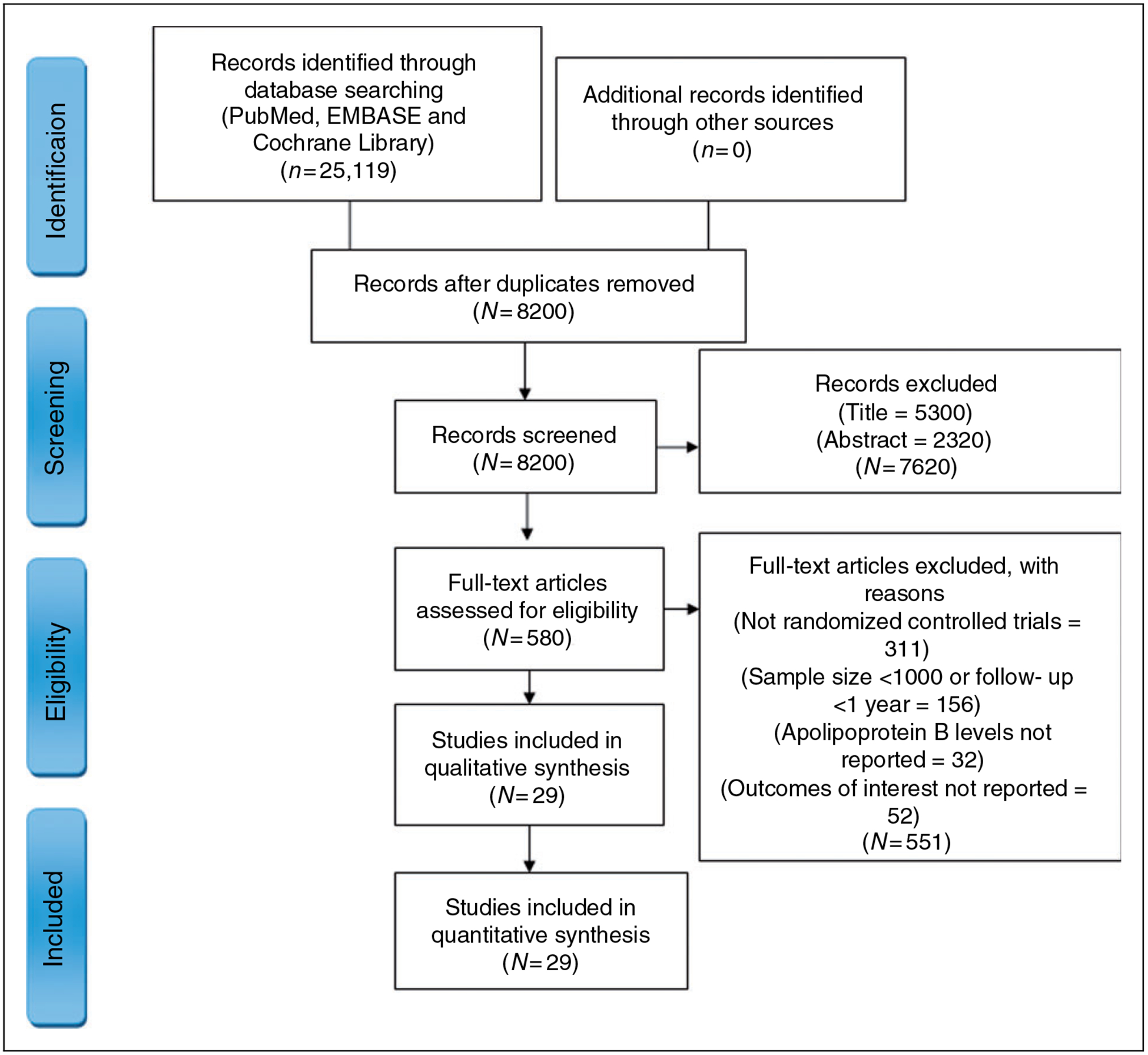
Study selection process according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Table 1.
Baseline characteristics of the trials reporting apolipoprotein B lowering and participants.
| No. (%) | Interventions | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (year) | N | Age | Women | CAD | Vascular disease | Diabetes | Active agent | No. | Control | No. | Baseline apoB, mg/dL | Between-group difference achieved apoB, mg/dL | Baseline LDL-C, mg/dL | Between-group difference achieved LDL-C, mg/dL | Follow-up, years | |||||||||||||||
| Statin | ||||||||||||||||||||||||||||||
| 4S (1994)24 | 4444 | 58.6 | 827 (19) | 4444 (100) | 4444 (100) | 202 (5) | Simvastatin 20–40 mg | 2221 | Placebo | 2223 | 116 | 28 | 188.3 | 67.6 | 5.4 | |||||||||||||||
| Post-CABG (I997)23 | 1351 | 61.5 | 105 (8) | 1351 (100) | 1351 (100) | 118 (9) | Lovastatin 40–80 mg | 676 | Lovastatin 2.5–5 mg | 675 | 117 | 17 | 155.5 | 42.9 | 4.3 | |||||||||||||||
| AFCAPS/TexCAPS | 6605 (1998)25 | 58.2 | 997 (15) | <1 | <1 | 239 (3.6) | Lovastatin 20–40 mg | 3304 | Placebo | 3301 | 120 | 28 | 150 | 41.7 | 5.2 | |||||||||||||||
| LIPID (1998)26 | 9014 | 62.0 | 1516 (17) | 9014 (100) | 9014 (100) | 782 (9) | Pravastatin 40 mg | 4512 | Placebo | 4502 | 133 | 32 | 150 | 37.5 | 6.1 | |||||||||||||||
| HPS (2002)27 | 20,536 | 63.4 | 5082 (25) | 13,386 (65) | 17,907 (87) | 5963 (29) | Simvastatin (40 mg) | 10,269 | Placebo | 10267 | 114 | 29 | 131.5 | 38.7 | 5 | |||||||||||||||
| PROVE IT (2004)28 | 4162 | 58.2 | 911 (22) | 4162 (100) | 4162 (100) | 734 (18) | Atorvastatin 80 mg | 2099 | Pravastatin 40 mg | 2063 | 102 | 24 | 106 | 32.9 | 2 | |||||||||||||||
| CARDS (2004)29 | 2841 | 61.6 | 909 (32) | <1 | <1 | 2838 (100) | Atorvastatin 10 mg | 1429 | Placebo | 1412 | 116 | 30 | 117 | 46.4 | 3.9 | |||||||||||||||
| IDEAL (2005)30 | 8888 | 61.7 | 1702 (19) | 8888 (100) | 8888 (100) | 1069 (12) | Atorvastatin 80 mg | 4439 | Simvastatin 20 mg | 4449 | 119 | 19 | 121.4 | 21.7 | 4.8 | |||||||||||||||
| TNT (2005)31 | 10,001 | 61.0 | 1902 (19) | 10,001 (100) | 10,001 (100) | 1501 (15) | Atorvastatin 80 mg | 4995 | Atorvastatin 10 mg | 5006 | 111 | 23 | 97.5 | 24.0 | 4.9 | |||||||||||||||
| SPARCL (2006)32 | 4731 | 62.7 | 1908 (40) | 0 | 4731 (100) | 794 (17) | Atorvastatin 80 mg | 2365 | Placebo | 2366 | 134 | 32 | 132.7 | 55.3 | 4.9 | |||||||||||||||
| JUPITER (2008)33 | 17,802 | 66.0 | 6801 (30) | 0 | 0 | 76 (<l) | Rosuvastatin 20 mg | 8901 | Placebo | 8901 | 109 | 39 | 108 | 54.9 | 1.9 | |||||||||||||||
| SEARCH (2010)34 | 12,064 | 64.2 | 2052 (17) | 12,064 (100) | 12,064 (100) | 1267 (11) | Simvastatin 80 mg | 6031 | Simvastatin 20 mg | 6033 | 90 | 10 | 96.7 | 13.5 | 6.7 | |||||||||||||||
| HOPE 3 (2016)35 | 12,705 | 65.7 | 5874 (46) | 0 | 0 | 731 (6) | Rosuvastatin 10 mg | 6361 | Placebo | 6344 | 103 | 23 | 127.8 | 34.4 | 5.6 | |||||||||||||||
| Ezetimibe | ||||||||||||||||||||||||||||||
| IMPROVE IT (2015)40 | 18,144 | 63.6 | 4416 (24) | 18,144 (100) | 18,144 (100) | 4933 (27) | Simvastatin 40 mg + ezetimibe 10 mg | 9067 | Simvastatin 40 mg | 9077 | 92.7 | 11 | 93.8 | 12.8 | 6.0 | |||||||||||||||
| PCSK9 inhibitor | ||||||||||||||||||||||||||||||
| ODYSSEY LONGTERM | 2341 | 60.5 | 884 (38) | 1607 (69) | 2341 (100) | 809 (35) | Alirocumab 150 mg every 2 weeks | 1553 | Placebo | 788 | 101.7 | 54.5 | 122.3 | 70.8 | 1.5 | |||||||||||||||
| (2015)36 | ||||||||||||||||||||||||||||||
| FOURIER (2017)37 | 27,564 | 62.5 | 6769 (25) | 22,351 (81) | 27,564 (100) | 10,081 (37) | Evolocumab 140 mg every two weeks or 420 mg every month | 13,784 | Placebo | 13,780 | 87.4a | 39.9 | 92 | 54.1 | 2.2 | |||||||||||||||
| SPIRE 2 (20I7)38 | 10,621 | 62.4 | 3675 (35) | NA | 8635 (81) | 4986 (47) | Bococizumab 150 mg every two weeks | 5312 | Placebo | 5309 | 105.8 | 62.7 | 133.6 | 58.0 | 1.0 | |||||||||||||||
| ODYSSEY | 18,924 | 58.5 | 4762 (25) | 18924 (100) | 759 (4) | 5444 (29) | 9462 | Placebo | 9462 | 83 | 24 | 92 | 38.7 | 2.8 | ||||||||||||||||
| OUTCOMES (2018)39 | Alirocumab 75–150 mg every two weeks | |||||||||||||||||||||||||||||
| Fibrate | ||||||||||||||||||||||||||||||
| VA-HIT (I999)46 | 2531 | 64.0 | NA | 1544 (61) | NA | 620 (25) | Gemfibrozil 1.2 g | 1264 | Placebo | 1267 | 96 | 5 | 111.5 | 0 | 5.1 | |||||||||||||||
| FIELD (2005)47 | 9795 | 62.2 | 3657 (37) | 1673 (17) | 1057 (13) | 9795 (100) | Fenofibrate 200 mg | 4895 | Placebo | 4900 | 97 | 6 | 119 | 13.9 | 5.0 | |||||||||||||||
| Niacin | ||||||||||||||||||||||||||||||
| AIM HIGH (2011)48 | 3414 | 63.7 | 504 (14.8) | 1923 (56.3) | 465 (13.6) | 1158 (34) | Simvastatin ± ezetimibe plus niacin 1.5—2g | 1718 | Simvastatin ± ezetimibe plus placebo (niacin 50 mg) | 1696 | 83 | 8 | 76 | 6.2 | 3.0 | |||||||||||||||
| HPS2- THRIVE (2014)49 | 25,673 | 64.9 | 4444 (17.3) | 16,770 (65.3) | 3214 (12.5) | 8299 (32.3) | Simvastatin ± ezetimibe plus niacin 2g /laropiprant 40 mg | 12,838 | Simvastatin ± ezetimibe plus placebo | 12,835 | 65a | 7 | 63 | 10.0 | 3.9 | |||||||||||||||
| CETP inhibitor | ||||||||||||||||||||||||||||||
| ILLUMINATE (2007)41 | 15,067 | 61.3 | 3352 (22.2) | 13,193 (87) | 1874 (12.6) | 6661 (44.2) | Torcetrapib 60 mg | 7533 | Placebo | 7534 | 73.4 | 11 | 79.8 | 22.4 | 4.5 | |||||||||||||||
| DEFINE (2010)42 | 1623 | 62.7 | 376 (23) | 888 (54.7) | NA | 862 (53) | Anacetrapib 100 mg | 811 | Placebo | 812 | 88.7 | 16.6 | 81.8 | 32.1 | 1.5 | |||||||||||||||
| Dal-OUTCOMES (2012)43 | 15,871 | 60.2 | 3070 (19) | 15,871 (100) | 1151 (7.2) | 3882 (24.5) | Dalcetrapib 600 mg | 7938 | Placebo | 7933 | 81.3 | 1 | 76.1 | 0 | 2.6 | |||||||||||||||
| ACCELERATE (2017)44 | 12,092 | 64.9 | 2784 (23) | NA | 1674 (13.8) | 8236 (68) | Evacetrapib 130 mg | 6038 | Placebo | 6054 | 78.0 | 14.6 | 81.3 | 29 | 2.1 | |||||||||||||||
| REVEAL (2017)45 | 30,449 | 67.0 | 4915 (16.1) | 26,679 (87.6) | 2435 (8.0) | 11320 (37.2) | Anacetrapib 100 mg | 15,225 | Placebo | 15224 | 68 | 12 | 61 | 26 | 3.8 | |||||||||||||||
| Omega-3 fatty acid | ||||||||||||||||||||||||||||||
| ASCEND (2018)50 | 15,480 | 63.3 | 5796 (37.4) | 0 | NA | 15,480 (100) | Marine n-3 FAb 1 g | 7740 | Placebo | 7740 | 80.5 | 1.2 | NA | NA | 7.4 | |||||||||||||||
| REDUCE IT (2018)21 | 8179 | 64.0 | 2357 (28.8) | 5785 (70.7) | 5785 (70.7) | 524 (6.4) | Icosapent ethyl 2g | 4089 | Placebo | 4090 | 82.5 | 5.0 | 75 | 5 | 4.9 | |||||||||||||||
Values are mean or median, whichever was available.
Estimated values.
One-gram capsules containing 840 mg of marine n-3 fatty acids (FA) (460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid).
LDL-C: low-density lipoprotein cholesterol; PCSK9: proprotein convertase subtilisin/kexin type 9; CETP: cholesteryl ester transfer protein; 4 S (SSSS): Scandinavian Simvastatin Survival Study; ACCELERATE: Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes; AFCAPS-TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study; AIM HIGH: the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; CARDS: Collaborative Atorvastatin Diabetes Study; ASCEND: Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus; Dal-OUTCOMES: a randomized, double-blind, placebo-controlled study assessing the effect of RO4607381 on cardiovascular mortality and morbidity in clinically stable patients with a recent acute coronary syndrome; DEFINE: Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib; FIELD: Fenofibrate Intervention and Event Lowering in Diabetes; FOURIER: Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk; HOPE-3: Heart Outcomes Prevention Evaluation; HPS: Heart Protection Study; HPS2-THRIVE: Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events; IDEAL: Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group; ILLUMINATE: Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events; IMPROVE-IT: Improved Reduction of Outcomes: Vytorin Efficacy International Trial; JUPITER: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin study group; LIPID: Long-term Intervention with Pravastatin in Ischaemic Disease; ODYSSEY LONG TERM: Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy; ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab; Post-CABG: Post Coronary Artery Bypass Graft Trial; PROVE IT: Pravastatin or Atorvastatin Evaluation and Infection Therapy; REVEAL: Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification; REDUCE IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; SEARCH: Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SPARCL: Stroke Prevention by Aggressive Reduction in Cholesterol Levels; SPIRE-2: Studies of PCSK9 Inhibition and the Reduction of Vascular Events 2; TNT: Treating to New Targets; VA-HIT: Veterans Affairs Cooperative Studies Program High-Density Lipoprotein Cholesterol Intervention Trial
Primary outcomes
All-cause mortality.
Among the total of 29 trials (332,901 patients) included, there was a total of 22,198 (6.6%) events of all-cause mortality. Of these, 13 statin trials (115,144 patients) reported 9686 (8.4%) mortality events. Among five trials (77,594 patients) that evaluated non-statin therapies that lead to upregulation of LDL-R, there were 4173 (5.3%) mortality events: (PCSK9 inhibitors (1727 (2.9%) events/59,450 patients); ezetimibe (2446 (13.4%) events/18,144 patients)).
Among 11 trials (140,163 patients) of other interventions that do not upregulate LDL-R, there were 8339 (5.9%) mortality events: (CETP inhibitors (3410 (4.5%) events/75,091 patients); fibrates (1097 (8.9%) events/12,326 patients), niacin (1708 (5.8%) events/29,087 patients) and n-3 FA (2124 (8.9%) events/23,659 patients).
For all therapies combined, each 10 mg/dL reduction in apoB was associated with a RR of 0.95 (95% CI, 0.92–0.99) in all-cause mortality (Table 2 and Figure 2). Statins showed a RR of 0.92 (95% CI, 0.86–0.98), established non-statin therapies which reduce apoB levels by upregulation of LDL-R showed a RR of 0.98 (95% CI, 0.91–1.06) and interventions which act independent of LDL-R showed a RR of 1.02 (95% CI, 0.79–1.32).
Table 2.
Multivariable meta-regression models for the associations of apolipoprotein B lowering with mortality and cardiovascular outcomes by lipid-lowering therapies.
| No. | Relative risk (95% confidence interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable models | |||||||||
| Outcome | Studies | Patients | Absolute reduction of apoB, mg/dL | Baseline apoB, mg/dL | apoB adjusted for baseline apoB | apoB adjusted for baseline LDL-C | apoB adjusted for absolute change in LDL-C | apoB adjusted for baseline and absolute reduction in triglycerides | apoB adjusted for statea setting, age, drug class and diabetes mellitus |
| Combined lipid-lowering therapies | |||||||||
| All-cause mortality 29 | 332,901 | 0.95 (0.92, 0.99) | 0.97 (0.95, 1.00) | 0.97 (0.92, 1.01) | 0.97 (0.93, 1.01) | 0.99 (0.92, 1.08) | 0.95 (0.92, 0.99) | 0.95 (0.87, 1.04) | |
| Cardiovascular mortality | 29 | 332,901 | 0.93 (0.88, 0.98) | 0.95 (0.92, 0.99) | 0.95 (0.89, 1.01) | 0.95 (0.90, 1.00) | 0.98 (0.88, 1.08) | 0.92 (0.88, 0.98) | 0.88 (0.81, 0.95) |
| Myocardial infarction | 29 | 332,901 | 0.92 (0.89, 0.96) | 0.94 (0.91, 0.97) | 0.95 (0.91, 0.99) | 0.95 (0.91, 0.99) | 0.97 (0.89, 1.05) | 0.92 (0.89, 0.96) | 0.92 (0.88, 0.96) |
| Revascularization | 29 | 332,901 | 0.94 (0.90, 0.99) | 0.94 (0.91, 0.97) | 0.98 (0.93, 1.03) | 0.97 (0.93, 0.99) | 1.02 (0.91, 1.12) | 0.93 (0.89, 0.98) | 0.95 (0.88, 1.03) |
| Cerebrovascular events | 29 | 332,901 | 0.93 (0.89, 0.97) | 0.96 (0.94, 0.99) | 0.94 (0.89, 0.99) | 0.94 (0.90, 0.99) | 0.91 (0.83, 1.00) | 0.93 (0.89, 0.97) | 0.94 (0.88, 1.02) |
| MACEs | 29 | 332,901 | 0.93 (0.90, 0.97) | 0.95 (0.92, 0.97) | 0.96 (0.92, 1.00) | 0.95 (0.92, 0.99) | 0.97 (0.90, 1.04) | 0.93 (0.90, 0.96) | 0.92 (0.88, 0.97) |
| Statin | |||||||||
| All-cause mortality | 13 | 115,144 | 0.92 (0.86, 0.98) | 0.98 (0.92, 1.03) | 0.90 (0.81, 0.99) | 0.95 (0.90, 1.01) | 0.97 (0.86, 1.08) | 0.90 (0.84, 0.95) | 0.92 (0.84, 1.01) |
| Cardiovascular mortality | 13 | 115,144 | 0.84 (0.77, 0.92) | 0.96 (0.88, 1.01) | 0.78 (0.69, 0.89) | 0.84 (0.75, 0.93) | 0.91 (0.81, 1.03) | 0.83 (0.74, 0.93) | 0.86 (0.78, 0.94) |
| Myocardial infarction | 13 | 1 15,144 | 0.89 (0.83, 0.95) | 0.95 (0.90, 0.99) | 0.85 (0.75, 0.96) | 0.91 (0.84,0.99) | 0.94 (0.86, 1.04) | 0.87 (0.79, 0.97) | 0.90 (0.84, 0.96) |
| Revascularization | 13 | 1 15,144 | 0.88 (0.81, 0.97) | 0.95 (0.89, 1.01) | 0.88 (0.77, 1.01) | 0.89 (0.80, 0.99) | 0.96 (0.84, 1.10) | 0.94 (0.87, 1.01) | 0.90 (0.81, 0.99) |
| Cerebrovascular events | 13 | 1 15,144 | 0.91 (0.84, 0.99) | 0.99 (0.95, 1.05) | 0.81 (0.70,0.93) | 0.92 (0.82, 1.03) | 0.93 (0.80, 1.08) | 0.89 (0.79, 1.01) | 0.93 (0.86, 1.02) |
| MACEs | 1 15,141 | 0.88 (0.83, 0.93) | 0.97 (0.91, 1.02) | 0.84 (0.77, 0.91) | 0.91 (0.86,0.96) | 0.93 (0.87, 0.99) | 0.87 (0.81, 0.94) | 0.90 (0.86, 0.94) | |
| Non-statin therapies – proprotein convertase subtilisin/kexin type 9 inhibitor, and ezetimibe | |||||||||
| All-cause mortality | 5 | 77,594 | 0.98 (0.91,1.06) | 0.99 (0.82,1.19) | 0.98 (0.86, l.l 1) | 1.01 (0.87, 1.16) | 1.03 (0.78, 1.35) | 0.95 (0.90, 1.00) | 0.99 (0.92, 1.06) |
| Cardiovascular mortality | 5 | 77,594 | 0.97 (0.90, 1.05) | 0.96 (0.78, 1.18) | 0.97 (0.85, 1.10) | 1.00 (0.90, l.l 1) | 0.97 (0.73, 1.29) | 0.93 (0.86, 1.01) | 0.97 (0.86, 1.07) |
| Myocardial infarction | 5 | 77,594 | 0.95 (0.91, 0.99) | 0.93 (0.79, 1.10) | 0.95 (0.90, 1.00) | 0.94 (0.89, 0.99) | 0.99 (0.85, 1.14) | 0.95 (0.91, 0.99) | 0.94 (0.90, 0.98) |
| Revascularization | 5 | 77,594 | 0.95 (0.91, 0.98) | 1.08 (0.92, 1.27) | 0.96 (0.91, 1.01) | 0.94 (0.90, 0.97) | 1.04 (0.88, 1.23) | 0.98 (0.93, 1.01) | 0.99 (0.83, 1.18) |
| Cerebrovascular events | 5 | 77,594 | 0.97 (0.90, 1.04) | 1.05 (0.86, 1.29) | 0.97 (0.90, 1.04) | 0.97 (0.89, 1.04) | 0.99 (0.99, 1.23) | 0.97 (0.89, 1.05) | 0.97 (0.72, 1.28) |
| MACEs | 5 | 77,594 | 0.96 (0.94, 0.99) | 0.96 (0.86, 1.08) | 0.96 (0.94, 0.99) | 0.96 (0.93, 0.99) | 0.99 (0.91, 1.08) | 0.95 (0.92, 0.98) | 0.90 (0.81, 0.99) |
| Other – cholesteryl ester transfer protein inhibitor, fibrate, niacin and omega-3 fatty acid | |||||||||
| All-cause mortality | 11 | 140,163 | 1.02 (0.79,1.32) | 0.99 (0.88,1.11) | 1.03 (0.72, 1.46) | 1.02 (0.77, 1.34) | 0.86 (0.42, 1.78) | 1.00 (0.67, 1.49) | 0.97 (0.55, 1.73) |
| Cardiovascular mortality | 11 | 140,163 | 1.02 (0.81, 1.27) | 1.01 (0.91, l.l 1) | 1.06 (0.77, 1.49) | 1.02 (0.78, 1.33) | 0.72 (0.35, 1.47) | 0.98 (0.68, 1.41) | 0.88 (0.67, 1.16) |
| Myocardial infarction | 11 | 140,163 | 1.09 (0.81, 1.46) | 0.92 (0.98, 1.04) | 0.99 (0.71, 1.40) | 1.06 (0.80, 1.41) | 1.10 (0.49, 2.53) | 0.97 (0.71, 1.33) | 0.94 (0.70, 1.27) |
| Revascularization | 11 | 140,163 | 0.97 (0.67, 1.37) | 0.91 (0.79, 1.05) | 0.82 (0.53, 1.27) | 0.95 (0.66, 1.36) | 0.87 (0.33, 2.29) | 0.84 (0.56, 1.28) | 0.72 (0.31, 1.68) |
| Cerebrovascular events | 11 | 140,163 | 1.02 (0.74, 1.40) | 0.92 (0.83, 1.07) | 0.92 (0.61, 1.39) | 0.99 (0.73, 1.34) | 0.77 (0.37, 1.61) | 0.81 (0.61, 1.08) | 0.78 (0.58, 1.05) |
| MACEs | 11 | 140,163 | 1.02 (0.81, 1.30) | 0.92 (0.83, 1.03) | 0.94 (0.71, 1.23) | 1.01 (0.79, 1.28) | 0.81 (0.42, 1.56) | 0.92 (0.71, 1.21) | 0.86 (0.60, 1.19) |
Absolute reduction in apolipoprotein B (apoB) quantifies to 10 mg/dL reduction and refers to between-group differences achieved in apoB. Magnitude of low-density lipoprotein cholesterol (LDL-C) refers to between-group differences achieved in LDL-C (each 38.7 mg/dL). Change in baseline apoB quantifies to 10 mg/dL increase in values. Setting refers to primary or secondary prevention. Drug class refers to proprotein convertase subtilisin/kexin type 9 inhibitor, and ezetimibe, cholesteryl ester transfer protein inhibitor, fibrate, niacin and omega-3 fatty acid. State refers to fasting versus non-fasting labs. apoB: apolipoprotein B; LDL-C: low-density lipoprotein cholesterol; MACE: major adverse cardiovascular event
Figure 2.

Meta-regression analyses demonstrating association of between-group difference in achieved apolipoprotein B levels (mg/dL) and all-cause mortality. (a) Combined therapies, (b) statins, (c) non-statin therapies, and (d) other therapies which reduce apolipoprotein B containing particles by mechanisms independent of low-density lipoprotein receptors. Change in risk ratios and 95% confidence intervals plotted against apolipoprotein B levels (mg/dL). The size of the data marker is proportional to the weight in the meta-regression. Data marker colors represent the classes of lipid-lowering agents used in the active treatment group as per trial randomization design. The solid line represents the meta-regression slope of the change in rate ratio for treatment across differences in achieved apolipoprotein B levels, which are either mean or median depending upon what was reported in each trial.
RR: risk ratio; CI: confidence interval; PCSK9: proprotein convertase subtilisin/kexin type 9; CETP: cholesteryl ester transfer protein.
In multivariable-adjusted meta-regression models, this benefit of apoB reduction (per 10 mg/dL) on all-cause mortality persisted after adjustment for baseline and absolute reduction in triglyceride levels (RR, 0.95, 95% CI, 0.92–0.99) (Table 2) but was no longer statistically significant after adjusting for baseline and change in LDL-C. Absolute reduction in apoB accounted for 26% between-study variability (R2).
Cardiovascular mortality.
Across a total of 29 trials (332,901 patients), there was a total of 11,102 (3.3%) events of cardiovascular mortality. Of these, 13 trials of statins (115,144 patients) reported 5623 (4.8%) events. Among the five trials (77,594 patients) of non-statin therapies that lead to upregulation of LDL-R, there were 2150 (2.7%) cardiovascular mortality events: (PCSK9 inhibitors (1075 (1.8%) events/59,450 patients); ezetimibe (1075 (5.9%) events/18,144 patients)). Among 11 trials (140,163 patients) of other interventions that do not upregulate LDL-R, there were 3329 (2.3%) cardiovascular mortality events: (CETP inhibitors (1438 (1.9%) events/75,091 patients); fibrates (414 (3.3%) events/12,326 patients), niacin (676 (2.3%) events/29,087 patients) and n-3 FA (801 (3.3%) events/23,659 patients).
For all interventions combined, each 10 mg/dL reduction in apoB was associated with a RR of 0.93 (95% CI, 0.88–0.98; R2 = 25%) for cardiovascular mortality (Table 2 and Figure 3). Statins showed a RR of 0.84 (95% CI, 0.77–0.92), non-statin therapies showed a RR of 0.97 (95% CI, 0.90–1.05), and drugs which act independently of LDL-R showed a RR of 1.02 (95% CI, 0.81–1.27). In multivariable meta-regression models, this benefit of apoB reduction (per 10 mg/dL) on cardiovascular mortality persisted after adjustment for baseline and absolute reduction in triglyceride levels (RR, 0.92, 95% CI, 0.88–0.98) and type of agent, setting, age, and diabetes mellitus (RR, 0.88, 95% CI, 0.81–0.95) (Table 2). The association of apoB reduction conferred by statin therapy on cardiovascular mortality remained statistically significant after adjustment for baseline LDL-C (RR 0.84, 95% CI, 0.75–0.93) but not absolute reduction in LDL-C levels.
Figure 3.

Meta-regression analyses demonstrating association of between-group difference in achieved apolipoprotein B levels (mg/dL) and cardiovascular mortality. (a) Combined therapies, (b) statins, (C) non-statin therapies, and (d) other therapies which reduce apolipoprotein B containing particles by mechanisms independent of low-density lipoprotein receptors. Change in risk ratios and 95% confidence intervals plotted against apolipoprotein B levels (mg/dL). The size of the data marker is proportional to the weight in the meta-regression. Data marker colors represent the classes of lipid-lowering agents used in the active treatment group as per trial randomization design. The solid line represents the meta-regression slope of the change in rate ratio for treatment across differences in achieved apolipoprotein B levels, which are either mean or median depending upon what was reported in each trial.
RR: risk ratio; CI: confidence interval; PCSK9: proprotein convertase subtilisin/kexin type 9; CETP: cholesteryl ester transfer protein
Secondary outcomes.
In terms of MACEs, combined therapies generated a RR of 0.93 (95% CI, 0.90–0.97; R− = 30%) (Table 2 and Figure 4). This benefit was consistent across statin (RR, 0.88, 95% CI, 0.83–0.93) and non-statin therapies (RR, 0.96, 95% CI, 0.94–0.99). For MACEs, the benefits of statin therapy per absolute reduction in apoB were independent of both baseline LDL-C (RR, 0.91, 95% CI, 0.86–0.96) and absolute reduction in LDL-C (RR, 0.93, 95% CI, 0.87–0.99). For MI, combined therapies resulted in a RR of 0.92 (95% CI, 0.89–0.96; R = 42%), with both statin (RR, 0.89, 95% CI, 0.83–0.95) and non-statin therapies (RR, 0.95, 95% CI, 0.91–0.99) demonstrating significant reduction in RR of MI per 10 mg/dL reduction in apoB levels (Figure S1). For revascularization, combined interventions produced a RR of 0.94 (95% CI, 0.90–0.99; R2 = 35%) with significant RR reductions by statin (RR, 0.88, 95% CI, 0.81–0.97) and non-statin therapies (RR, 0.95, 95% CI, 0.91–0.98) (Figure S2). In terms of cerebrovascular events, combined treatments generated a RR of 0.93 (95% CI, 0.89–0.97; R = 98%). This benefit was driven by statins (RR, 0.91, 95% CI, 0.84–0.99) only, while non-statin therapies (RR, 0.97, 95% CI, 0.90–1.04) did not demonstrate any benefit (Figure S3).
Figure 4.

Meta-regression analyses demonstrating association of between-group difference in achieved apolipoprotein B levels (mg/dL) and major adverse cardiovascular events. (a) Combined therapies, (b) statins, (c) non-statin therapies, and (d) other therapies which reduce apolipoprotein B containing particles by mechanisms independent of low-density lipoprotein receptors. Change in risk ratios and 95% confidence intervals plotted against apolipoprotein B levels (mg/dL). The size of the data marker is proportional to the weight in the meta-regression. Data marker colors represent the classes of lipid-lowering agents used in the active treatment group as per trial randomization design. The solid line represents the meta-regression slope of the change in rate ratio for treatment across differences in achieved apolipoprotein B levels, which are either mean or median depending upon what was reported in each trial.
RR: risk ratio; CI: confidence interval; PCSK9: proprotein convertase subtilisin/kexin type 9; CETP: cholesteryl ester transfer protein
Drugs which reduce apoB-containing particles by mechanisms independent of LDL-R did not show significant reductions in the RR of any of the secondary cardiovascular endpoints per decrease in apoB levels. The multivariate meta-regression models showed consistent benefit in secondary endpoints after adjustment for various covariates (Table 2).
Discussion
In these analyses, absolute reduction in apoB levels by different lipid-lowering therapies resulted in significant reduction in mortality and cardiovascular outcomes. The reduction in cardiovascular outcomes was driven by both statin and non-statin therapies which ultimately clear circulating apoB-containing lipoproteins by upregulating LDL-R expression. Conversely, therapies which reduce apoB levels by mechanisms independent of LDL-R did not show significant association between cardiovascular events corresponding to absolute change in apoB levels. These findings remained largely consistent after adjustment for various covariates. The benefits of statin therapy for MACE reduction conferred by absolute apoB lowering was independent of absolute reduction in LDL-C.
The weighted mean absolute reduction in apoB achieved with statins was expectedly less (25.7 mg/dL) compared with non-statin therapies (i.e. PCSK9 inhibitors and ezetimibe) (38.4 mg/dL); however, the RR reductions in mortality and cardiovascular outcomes were marginally higher with statin compared with magnitude of reductions achieved by non-statin therapies. This discrepancy can be explained by relatively short follow-up durations of the non-statin trials (weighted median follow-up 2.7 years) compared with the statin trials (weighted median follow-up of 4.7 years). A recent analysis comparing the results of the FOURIER and SPIRE trials with the Cholesterol Treatment Trialist Collaboration meta-analysis of statins concluded that the effect of PCSK9 inhibitors on MACE was exactly what would have been expected up to two years, suggesting that beneficial effect accumulates with extended duration of therapy.51 Hence, with longer treatment exposure, the intensified non-statin therapies are expected to generate more enhanced clinical benefits.
A mendelian randomization analysis of 102,837 patients evaluating the association between CETP and 3-hydroxy-3-methylglutaryl-CoA reductase scores and risk of cardiovascular outcomes, along with external validation analyses comprising a genetic score of 21 variants, showed that risk of cardiovascular outcomes was correlated to the change in apoB levels rather than LDL-C.5 Based on these observations, the authors concluded that cardiovascular benefits of LDL-C reductions may be regulated by the corresponding absolute lowering of apoB containing lipoproteins rather than the cholesterol mass carried by these lipoproteins (calculated as plasma LDL-C on lipid profile). Therefore, the authors concluded that apoB rather than the LDL-C levels might be a better predictor of ASCVD. On the same note, there are data suggesting that apoB/A-1 ratio (apoA-1: a component of antiatherogenic HDL-C) might be a better predictor of MACEs than LDL-C levels.52 In AMORIS (Apolipoprotein-related Mortality Risk) trial, the apoB/A-1 ratio was found to be a strong predictor of stroke. After adjustment for age, sex, total cholesterol and triglycerides, this ratio was stronger than LDL-C in predicting ASCVD (p < 0.025).53 In this current analysis, at least for MACEs, the benefits of absolute apoB reduction conferred by statins were independent of reductions in LDL-C and non-HDL-C. These findings were consistent with several other discordant analyses of apoB and LDL-C.3,54,55
The authors of these prior studies further argued that the mechanism by which LDL-C is lowered plays the key role in generating cardiovascular benefits. For instance, therapies which ultimately reduce LDL-C levels by upregulating LDL-R (statins, PCSK9 inhibitors and ezetimibe) reduce MACEs proportional to the absolute lowering in LDL-C or the concordant absolute reduction in apoB levels. Conversely, therapies which modify the lipid content of apoB containing particles instead of reducing the concentration of apoB containing particles and demonstrate discordant lowering in LDL-C and apoB levels are less likely to provide meaningful cardiovascular benefits. This current meta-analysis of clinical trials appears to validate this hypothesis. Another meta-regression analysis evaluating association of absolute LDL-C reduction and major vascular events reported a similar conclusion.12
One challenge across studies is defining LDL-C, as there are important methodologic differences in its measurement and estimation,56 although we adjusted for analytical methods to estimate apoB and LDL-C in a supplementary analysis. Drugs may also impact the concentrations of total LDL-C mass, specific lipoprotein particles (i.e. VLDL, IDL), and LDL particle subfractions differently, as noted for CETP inhibitors.56,57 However, we were unable to take into account LDL particle count or subfractions in our analyses.
The clinical implications of this analysis build upon a large body of evidence and reaffirm the efficacy of statin therapy as first line treatment for improving cardiovascular survival even when apoB is considered an independent therapeutic target.9,15 This report also supports the intensification of the lipid-lowering therapy by combining the drugs which share a common mechanism of clearing LDL-C and provide concordant reductions in LDL-C and apoB levels. Future outcome trials of new lipid-lowering drugs will further elaborate this concept. The CLEAR Outcomes trial (ClinicalTrials.gov identifier: NCT02993406) is evaluating bempedoic acid, a drug which inhibits ATP-citrate lyase and thus reduces cholesterol synthesis and upregulates LDL-R on liver cells.58 Inclisiran, a chemically modified small interfering RNA that blocks PCSK9 production by specifically targeting PCSK9 mRNA in hepatocytes, has shown dose dependent reductions in PCSK9, LDL-C and other atherogenic lipoproteins, including apoB in the phase 2 ORION-1 trial (ClinicalTrials.gov identifier: NCT02597127),59 and is being studied in larger phase 3 cardiovascular outcome trials.
Other lipid-mediated therapeutics that target apoB are also under study. LDL-R related protein 1 (LRP1) receptor works with LDL-R in facilitating the removal of LDL and VLDL remnants from circulation and regulates apoB levels. RNA inference of angiopoietin-like protein-3 gene (ANGPTL3) in mouse models and human hepatoma cells resulted in increased expression of LDL-R and LRP1, resulting in reduced apoB secretion and increased LDL/VLDL uptake.60 On-going trials evaluating the inhibition of ANGPTL3 protein using a monoclonal antibody (evinacumab)61 or messenger RNA via an antisense oligonucleotide62 will be important in this regard. Finally, the PROMINENT trial (ClinicalTrials.gov identifier: NCT03071692) evaluating treatment with the selective peroxisome proliferator-activated receptor-alpha modulator pemafibrate, in patients with type 2 diabetes who have high triglycerides and low HDL-C despite concomitant statin therapy, will provide further insights.
We compared our results with previous reports. Robinson and colleagues performed trial level meta-regressions of 25 trials and showed 9% decrease in coronary heart disease and 6% decrease in major cardiovascular disease risk per 10 mg/dL reduction in apoB.14 This association was more robust among statins; however, there was no reduction in stroke risk per absolute reduction in apoB. This meta-analysis did not assess the impact on mortality by therapeutic lowering of apoB. Moreover, the outcome data of PCSK9 inhibitors were not available at the time of analysis. Another patient level meta-analysis limited to statin trials (38,153 patients) showed that on-treatment levels of apoB were associated with increased risk of cardiovascular events, but this was similar to the risk conferred by on-treatment levels of LDL-C.63
Our meta-regression analysis has many strengths, including leveraging data across 29 trials, to provide insight into the role of apoB lowering as an important target for mortality and ASCVD reduction and comparing this benefit across specific drug classes. However, certain limitations of our study need consideration. First, the current analysis builds on trial level information and despite adjusting for various key study level variables, the study does not account for variability in patients’ clinical characteristics. Therefore, ideally, individual level data can provide more valuable information. Second, our study could not perform separate meta-regression analyses for ezetimibe, fibrates, niacin and n-3 FAs due to insufficient number of trials. Of mention, the REDUCE IT trial21 has shown a remarkable reduction in major cardiovascular outcomes with the use of higher doses of a purified eicosapentaenoic acid preparation (4 g/day) in patients with optimal levels of LDL-C but higher levels of triglycerides (150–500 mg/dL); thus a meta-regression analysis of n-3 FA trials would have provided important information. Third, a few studies did not report baseline apoB values and the estimated values might be different from those in original studies, which may under- or overestimate the associations. Moreover, since the equation for the apoB calculation was derived from Asian cohorts, the estimates might vary in other races, namely, Caucasians and Blacks, etc. Finally, we performed multiple statistical tests for outcomes that were often secondary endpoints and statistical significance may be due to chance; however, our findings are consistent with the results from the individual trials and consistent with biologic mechanisms.
In conclusion, current analyses suggested significant reductions in mortality and cardiovascular events proportional to absolute reduction in apoB. The clinical benefits of apoB lowering remained consistent after adjustments for various covariates, suggesting a potential role of apoB as a parallel therapeutic target beyond LDL-C. These findings suggest that therapies which ultimately act by upregulation of LDL-R and generate similar reductions in LDL-C and apoB levels can yield significant reductions in cardiovascular events. Although the current study suggests clinical benefits of apoB lowering, whether pharmacologic lowering of apoB has incremental benefit beyond LDL-C lowering can be further confirmed in the on-going randomized controlled trials evaluating apoB targeted therapeutics. Additional studies are also needed to determine whether measurement of apoB, at baseline and in response to drug therapy, provides more clinical information in a cost-effective manner compared with LDL-C for cardiovascular risk reduction.64
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EDM is funded by the Blumenthal Scholars Award at Johns Hopkins University.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MJB is on advisory Boards for Amgen, Sanofi, Regeneron, Novartis, MedImmune, Medicure and receives grants from Amgen Foundation.
References
- 1.Munkhaugen J, Sverre E, Otterstad JE, et al. Medical and psychosocial factors and unfavourable low-density lipoprotein cholesterol control in coronary patients. Eur J Prev Cardiol 2017; 24: 981–989. [DOI] [PubMed] [Google Scholar]
- 2.Sinning D and Landmesser U. Effective low-density lipoprotein-lowering therapy: Implementation in clinical practice. Eur J Prev Cardiol 2017; 24(3_Suppl): 71–76. [DOI] [PubMed] [Google Scholar]
- 3.Pencina MJ, D’Agostino RB, Zdrojewski T, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol 2015; 22: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 4.Harper CR and Jacobson TA. Using apolipoprotein B to manage dyslipidemic patients: time for a change? Mayo Clin Proc 2010; 85: 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 2017; 318: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018; 71: 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quispe R, Elshazly MB, Zhao D, et al. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: The ARIC study. Eur J Prev Cardiol. Epub ahead of print 10 July 2019; DOI: 10.1177/2047487319862401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 2005; 294: 326–333. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73: 3168–3209. [DOI] [PubMed] [Google Scholar]
- 10.Van Tulder M, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine (Phila Pa 1976) 2003; 28: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016; 316: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 13.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 2018; 319: 1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson JG, Wang S and Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol 2012; 110: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C, Blackwell L, et al. ; Cholesterol Treatment Trialists (CCT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho DS, Woo S, Kim S, et al. Estimation of plasma apolipoprotein B concentration using routinely measured lipid biochemical tests in apparently healthy Asian adults. Cardiovasc Diabetol 2012; 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur Heart J 2018; 39: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R and Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22. [DOI] [PubMed] [Google Scholar]
- 22.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 23.Campeau L, Knatterud GL, Domanski M, et al. ; Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med 1997; 336: 153–163. [DOI] [PubMed] [Google Scholar]
- 24.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389. [PubMed] [Google Scholar]
- 25.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 26.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 27.Pocock SJ, Assmann SE, Enos LE, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002; 21: 2917–2930. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 29.Colhoun HM, Betteridge DJ, Durrington PN, et al. ; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004; 364: 685–696. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomized controlled trial. JAMA 2005; 294: 2437–2445. [DOI] [PubMed] [Google Scholar]
- 31.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 32.Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359: 2195–2207. [DOI] [PubMed] [Google Scholar]
- 34.Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010; 376: 1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Bosch J, Dagenais G, et al. ; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374: 2021–2031. [DOI] [PubMed] [Google Scholar]
- 36.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 37.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Revkin J, Amarenco P, et al. ; SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097–2107. [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397. [DOI] [PubMed] [Google Scholar]
- 41.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 42.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010; 363: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012; 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 44.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017; 376: 1933–1942. [DOI] [PubMed] [Google Scholar]
- 45.Bowman L, Hopewell JC, Chen F, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017; 377: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 46.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999; 341: 410–418. [DOI] [PubMed] [Google Scholar]
- 47.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005; 366: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 48.AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 49.Group HTC, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014; 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 50.Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018; 379: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 51.Ference BA, Cannon CP, Landmesser U, et al. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: An analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2018; 39: 2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 53.Walldius G, Jungner I, Holme I, et al. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001; 358: 2026–2033. [DOI] [PubMed] [Google Scholar]
- 54.Mora S, Buring JE and Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation 2014; 129: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkins JT, Li RC, Sniderman A, et al. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: The CARDIA Study. J Am Coll Cardiol 2016; 67: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmes MV and Ala-Korpela M. What is ‘LDL cholesterol’? Nat Rev Cardiol 2019; 16: 197–198. [DOI] [PubMed] [Google Scholar]
- 57.Krauss RM, Pinto CA, Liu Y, et al. Changes in LDL particle concentrations after treatment with the cholesteryl ester transfer protein inhibitor anacetrapib alone or in combination with atorvastatin. J Clin Lipidol 2015; 9: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilen O and Ballantyne CM. Bempedoic acid (ETC-1002): An investigational inhibitor of ATP citrate lyase. Curr Atheroscler Rep 2016; 18: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins. Circulation 2018; 138: 1304–1316. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y-X, Redon V, Yu H, et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis 2018; 268: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017; 377: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graham MJ, Lee RG, Brandt TA, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017; 377: 222–232. [DOI] [PubMed] [Google Scholar]
- 63.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012; 307: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 64.Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying atherogenic lipoproteins: Current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A consensus statement from EAS and EFLM. Clin Chem 2018; 64: 1006–1033. [DOI] [PubMed] [Google Scholar]


