Key Points
Question
Does intraoperative low tidal volume, compared with conventional tidal volume, decrease postoperative pulmonary complications in patients undergoing major surgery?
Findings
In this randomized clinical trial of 1236 adults, the rate of pulmonary complications within the first 7 postoperative days was 38% among those randomized to a strategy of mechanical ventilation with low tidal volume compared with 39% among those randomized to a strategy with conventional tidal volume, a difference that was not statistically significant.
Meaning
Among adults undergoing major surgery, an intraoperative mechanical ventilation strategy with low tidal volume did not significantly reduce postoperative pulmonary complications within the first 7 postoperative days.
Abstract
Importance
In patients who undergo mechanical ventilation during surgery, the ideal tidal volume is unclear.
Objective
To determine whether low-tidal-volume ventilation compared with conventional ventilation during major surgery decreases postoperative pulmonary complications.
Design, Setting, and Participants
Single-center, assessor-blinded, randomized clinical trial of 1236 patients older than 40 years undergoing major noncardiothoracic, nonintracranial surgery under general anesthesia lasting more than 2 hours in a tertiary hospital in Melbourne, Australia, from February 2015 to February 2019. The last date of follow-up was February 17, 2019.
Interventions
Patients were randomized to receive a tidal volume of 6 mL/kg predicted body weight (n = 614; low tidal volume group) or a tidal volume of 10 mL/kg predicted body weight (n = 592; conventional tidal volume group). All patients received positive end-expiratory pressure (PEEP) at 5 cm H2O.
Main Outcomes and Measures
The primary outcome was a composite of postoperative pulmonary complications within the first 7 postoperative days, including pneumonia, bronchospasm, atelectasis, pulmonary congestion, respiratory failure, pleural effusion, pneumothorax, or unplanned requirement for postoperative invasive or noninvasive ventilation. Secondary outcomes were postoperative pulmonary complications including development of pulmonary embolism, acute respiratory distress syndrome, systemic inflammatory response syndrome, sepsis, acute kidney injury, wound infection (superficial and deep), rate of intraoperative need for vasopressor, incidence of unplanned intensive care unit admission, rate of need for rapid response team call, intensive care unit length of stay, hospital length of stay, and in-hospital mortality.
Results
Among 1236 patients who were randomized, 1206 (98.9%) completed the trial (mean age, 63.5 years; 494 [40.9%] women; 681 [56.4%] undergoing abdominal surgery). The primary outcome occurred in 231 of 608 patients (38%) in the low tidal volume group compared with 232 of 590 patients (39%) in the conventional tidal volume group (difference, −1.3% [95% CI, −6.8% to 4.2%]; risk ratio, 0.97 [95% CI, 0.84-1.11]; P = .64). There were no significant differences in any of the secondary outcomes.
Conclusions and Relevance
Among adult patients undergoing major surgery, intraoperative ventilation with low tidal volume compared with conventional tidal volume, with PEEP applied equally between groups, did not significantly reduce pulmonary complications within the first 7 postoperative days.
Trial Registration
ANZCTR Identifier: ACTRN12614000790640
This randomized clinical trial compares the effect of intraoperative ventilation with low vs conventional tidal volume (6 vs 10 mL/kg predicted body weight) on pulmonary complications in the first 7 postoperative days among patients undergoing major surgery lasting longer than 2 hours.
Introduction
More than an estimated 300 million surgical procedures are performed worldwide every year.1 More than 30% of patients undergoing surgery lasting at least 2 hours with general anesthesia and mechanical ventilation may experience postoperative pulmonary complications.2 According to an international expert panel–based consensus from 2019, prevention of complications is an important therapeutic and economic goal, which may in part be achieved by the optimization of mechanical ventilation.2,3,4
Intraoperative mechanical ventilation with supraphysiologic tidal volumes has traditionally been applied to prevent hypoxia and atelectasis.5 However, there has been a growing concern that such large tidal volumes may be injurious and contribute to postoperative morbidity when compared with low-tidal-volume ventilation.4,6,7,8,9,10 In 2013, a multicenter randomized clinical trial compared a lung-protective ventilation strategy (6-8 mL/kg predicted body weight [PBW] of tidal volume, 6-8 cm H2O of positive end-expiratory pressure [PEEP], and periodic recruitment maneuvers) with a nonprotective strategy. The results showed a significant reduction in postoperative pulmonary and extrapulmonary complications with the use of lung-protective ventilation.11 However, due to the multiple elements of the strategy, it was not possible to identify which component of the intervention was most important. Moreover, the control group underwent ventilation with no PEEP, an uncommon practice, making the external validity of such a trial uncertain.12,13,14
Therefore, the present assessor-blinded randomized clinical trial was conducted to determine whether a strategy of low-tidal-volume ventilation reduces the incidence of postoperative pulmonary complications within the first 7 postoperative days compared with conventional-tidal-volume ventilation in adult patients undergoing major surgery and receiving the same level of PEEP.
Methods
Study Design and Oversight
This was an investigator-initiated, assessor-blinded, single-center, randomized clinical trial conducted in a tertiary hospital in Melbourne, Australia. The protocol and statistical analysis plan have been published and are available in Supplement 1.15 The local ethics committee approved the study (HREC approval number HREC/14/Austin260). Written informed consent was obtained from all participating patients.
Patients
Patients were included if they were older than 40 years, scheduled to have major surgery with an expected duration of more than 2 hours, and expected to have invasive arterial pressure monitoring as part of their routine care. Patients were excluded if they were pregnant, were scheduled to have cardiac, thoracic, or intracranial neurological surgery, or had been previously enrolled in the trial. The rationale for the inclusion and exclusion criteria is outlined in the eAppendix in Supplement 2.
Randomization and Interventions
A computer-generated randomization list was prepared by an independent investigator. Randomization was conducted using sealed, sequentially numbered, and opaque envelopes placed in the operating room and without any stratification factor. Patients who satisfied all inclusion criteria and had no exclusion criteria were randomly assigned in a 1:1 ratio to either low-tidal-volume ventilation or conventional-tidal-volume ventilation using a permuted-block method with random sizes of 4, 6, or 10.
General management, including fraction of inspired oxygen, respiratory rate, anesthesia technique, fluid management, use of vasoactive drugs, analgesia plan, and use of prophylactic antibiotics and antiemetic agents, was at the discretion of the treating anesthesiologists and in accordance with existing protocols for patients undergoing major surgery. Predicted body weight was calculated as 50 + 0.91 × (height [cm] – 152.4) for men and 45.5 + 0.91 × (height [cm] – 152.4) for women. Aligned with usual practice in Australia and the United States, all patients were managed with volume-controlled ventilation with a PEEP of 5 cm H2O during the entire procedure.12,13,14
Patients were randomly assigned to receive ventilation at either low tidal volume (6 mL/kg PBW) or conventional tidal volume (10 mL/kg PBW). The tidal volume and PEEP of the conventional ventilation group were chosen to reflect current practice at the time of the study design.11,12,13 The tidal volume setting of 6 mL/kg PBW was chosen for the low-tidal-volume ventilation group consistent with several other major trials of low-tidal-volume ventilation in the literature.6,8,9,10,11 The PEEP and tidal volume were maintained for the duration of the surgical procedure.
Three arterial blood gas samples were taken for analysis during the study. The first was taken after induction and at least 15 minutes after the institution of the trial ventilation protocol (ie, early maintenance phase). A second sample was taken at least 15 minutes before emergence from anesthesia and before preparation for extubation (ie, late maintenance phase). A third arterial blood gas sample was taken approximately 15 minutes after arrival in the postanesthesia care unit. Chest x-ray was performed in the postoperative period according to clinical need, as determined by the attending clinical staff.
Data Collection
A standardized case report form was used for data collection. The research staff collected all data directly from the clinical chart source data. Until postoperative day 7 or hospital discharge (whichever came first), all patients were assessed daily by the trial’s research team. Patients discharged to home before day 7 without complications were considered free of complications at day 7.
Research nurses blinded to the intraoperative intervention collected information regarding clinical outcomes. After the first 7 days (if a patient was still hospitalized), additional data were retrieved from the electronic chart. In the intraoperative period, for all ventilatory data and vital signs, the lowest and/or highest values during the procedure were recorded. In addition, the use and type of regional anesthesia and use and dose of vasopressors and opioids were recorded. Arterial blood gas measurements were collected as described above and all results are reported herein, including pH, arterial partial pressure of oxygen, arterial partial pressure of carbon dioxide, bicarbonate, and lactate. After completion of data collection, the database was locked, and only the principal investigator and the statistician responsible for the analyses had access to it.
Blinding
The investigators who were responsible for assessing all outcomes were blinded to study group assignment. However, the attending anesthesiologists, intraoperative and postanesthesia care nursing personnel, and intraoperative assessors were not blinded to study group allocation.
Outcomes
Primary Outcome
The primary outcome was the incidence of a composite of postoperative pulmonary complications, defined as positive if any component developed within the first 7 days after surgery. These complications included pneumonia, bronchospasm, atelectasis, pulmonary congestion, respiratory failure, pleural effusion, pneumothorax, or unplanned requirement for postoperative mechanical ventilation, continuous positive airway pressure, or noninvasive or invasive ventilation (see eTable 1 in Supplement 2 for definitions). The diagnoses of atelectasis, pleural effusion, and pneumothorax were based on chest x-rays and were adjudicated by assessors blinded to study group allocation.
Secondary Outcomes
Thirteen prespecified secondary outcomes were assessed (see eTable 2 in Supplement 2 for definitions): (1) incidence of postoperative pulmonary complications during hospital stay, (2) incidence of pulmonary embolism, (3) incidence of acute respiratory distress syndrome, (4) incidence of systemic inflammatory response syndrome, (5) incidence of sepsis, (6) incidence of acute kidney injury, (7) incidence of wound infection (superficial and deep), (8) rate of intraoperative need for vasopressor; (9) incidence of unplanned intensive care unit (ICU) admission, (10) rate of need for rapid response team call, (11) ICU length of stay, (12) hospital length of stay, and (13) incidence of in-hospital mortality.
Statistical Analysis
Based on a previous study in Australia, a rate of postoperative pulmonary complications of 10.8% in the conventional tidal volume group was anticipated.16 Assuming a dropout rate of 3%, a study population of 1240 patients was estimated to provide an 80% power at a 2-sided significance level of P = .05 to detect an absolute reduction in primary outcome of 3.4%. In the absence of a consensus definition of a minimally important difference in primary outcome for these patients, we estimated on clinical grounds that a greater than 30% relative reduction in postoperative pulmonary complications would be necessary to change clinical practice. Patients were analyzed according to their randomization group, and the analysis data set included all patients who were randomized and had general anesthesia for eligible surgery. Because the amount of missing data for the primary outcome was negligible, only a complete-case analysis was carried out. Also, for all other data, no imputation method was considered because the amount of missing data was also negligible (rate of missing data available in eTable 3 in Supplement 2).
Absolute differences of intraoperative variables between the groups are reported with respective 95% confidence intervals, calculated as mean differences from an independent t test for continuous variables or risk differences derived from a generalized linear model considering a binomial distribution with an identity link for categorical variables. The effect of the intervention on the primary outcome is reported as numbers and percentages with risk differences and 95% confidence intervals, calculated using a generalized linear model with binomial distribution and an identity link function. In a sensitivity analysis, the effect of the intervention on the primary outcome was reestimated using a generalized linear model with binomial distribution with an additional adjustment for age, sex, baseline oxygen saturation measured by pulse oximetry, body mass index (BMI), and the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score, plus any variables with imbalance across treatment groups at baseline (defined as P < .05). Because the primary outcome was a composite outcome, other prespecified sensitivity analyses were performed (described in the eAppendix in Supplement 2).
The effects of the intervention on binary secondary outcomes were estimated as risk differences and 95% confidence intervals, calculated using a generalized linear model with binomial distribution and an identity link function. The effect of the intervention on the length of ICU stay and hospital stay was estimated with generalized linear models considering an inverse Gaussian distribution. Length of hospital stay was further compared using Kaplan-Meier survival curves and is reported as hazard ratios calculated from a Cox proportional hazard model. The Schoenfeld residuals against the transformed time were used to test the proportional hazard assumptions (P = .66 for the Schoenfeld residuals).
In addition, a Holm-Bonferroni correction to control for the family-wide error rate to the P values for all 13 secondary outcomes was applied.
Treatment effects were analyzed according to the following prespecified subgroups: (1) abdominal vs nonabdominal surgery; (2) open vs laparoscopic surgery; (3) BMI greater than 35 vs 35 or lower(calculated as weight in kilograms divided by height in meters squared); and (4) higher (ARISCAT score ≥26) vs lower (ARISCAT score <26) risk of postoperative pulmonary complications. Analyses of heterogeneity of effects across subgroups used generalized linear models considering a binomial distribution with logit link with an interaction between each subgroup and study group as a fixed effect. In a post hoc sensitivity analysis, the effect of low tidal volume on the primary and secondary outcomes was assessed in a subgroup of patients undergoing laparoscopic surgery.
Three additional post hoc analyses were conducted. First, we assessed an interaction between the duration of surgery and type of surgery (grouped as nonabdominal, laparoscopic abdominal, and nonlaparoscopic abdominal) and the effect of the intervention on the primary outcome.
Second, an additional sensitivity analysis was conducted to assess whether the different number of patients randomized to each group affected the primary outcome. In this analysis, 22 additional patients were added to the conventional group (to achieve the same sample size as the low tidal volume group) and the allocated incidence of the primary outcome in these patients was varied with different scenarios to test the fragility of the findings.
Third, the number of chest x-rays performed was compared between the groups to assess if this could have affected the primary outcome.
Baseline characteristics were reported as counts and percentages, means and standard deviations, or medians and interquartile ranges, as appropriate. Hypothesis tests were 2-sided at an α = .05. Because of the potential for type I error due to lack of adjustment for multiple comparisons, findings of subgroup analyses, sensitivity analyses, and post hoc analyses should be interpreted as exploratory. All analyses were performed using R version 3.6.0 (R Foundation).
Results
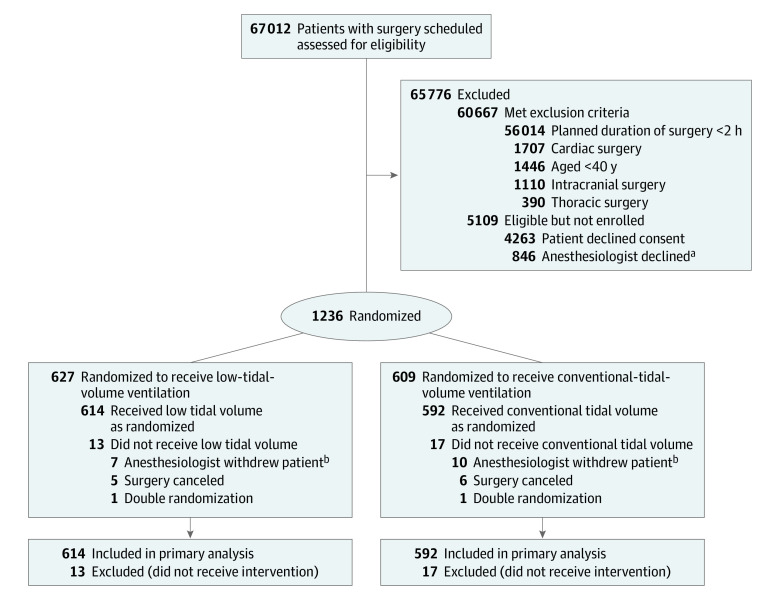
From February 2015 to February 2019, a total of 1236 patients were randomized. Of these, 627 were randomly assigned to receive low-tidal-volume ventilation and 609 patients to receive conventional-tidal-volume ventilation (Figure 1). In 30 patients, either the surgery did not proceed or the anesthesiologist in charge declined to participate in the trial (declined trial protocol ventilation or no arterial line was planned to be placed). These 30 patients were not included, leaving data from 1206 patients in the primary analysis. The amount of missing data is reported in eTable 3 in Supplement 2, but overall it was low (eg, 0.7% missing data for components of the primary outcome).
Figure 1. Participant Flow Through the Randomized Clinical Trial.
Discrepancy exists in the number of patients between groups because the study was undertaken in 2 phases, and in both phases, a randomization permuted by blocks of random size ranging from 4, 6, and 10 was used. In the first pilot phase of the study, recruitment was undertaken by a group of 10 anesthesiologists, each with an allocated permuted randomization sequence to follow. The second phase of the study occurred after securing additional funding support, which led to expansion of recruitment to all anesthesiologists. This also involved a combined permuted randomization sequence.
aReasons for anesthesiologist decline were not collected.
bAnesthesiologist withdrew patient after randomization (lack of clinical equipoise or when an arterial line was not inserted).
Baseline characteristics of the 2 groups were well balanced at randomization (Table 1). Overall mean age was 63.5 years, and the majority of the patients (n = 712 [59.0%]) were male. Patients were considered at a moderate or high risk of postoperative pulmonary complications in 57% of cases. The most common type of surgery was abdominal surgery, of which 48.2% were laparoscopic. The next most common surgical groups were spinal surgery and major peripheral orthopedic surgery. A more detailed description of the procedures is in eTable 4 in Supplement 2.
Table 1. Baseline Participant Characteristics.
| Characteristics | Low tidal volume (n = 614) | Conventional tidal volume (n = 592) |
|---|---|---|
| Age, mean (SD), y | 63.5 (11.8) | 63.8 (12.1) |
| Sex, No. (%) | ||
| Male | 366 (59.6) | 346 (58.4) |
| Female | 248 (40.4) | 246 (41.6) |
| Body weight, mean (SD), kg | ||
| Actual | 82.6 (20.1) | 82.9 (18.5) |
| Predicted | 63.0 (10.4) | 63.2 (10.4) |
| Body mass index, mean (SD)a | 29.0 (6.5) | 29.1 (6.2) |
| ARISCAT score, mean (SD)b | 27.7 (11.9) | 27.4 (12.1) |
| No. (%) | n = 554 | n = 518 |
| Low | 193 (34.8) | 196 (37.8) |
| Moderate | 324 (58.5) | 282 (54.4) |
| High | 37 (6.7) | 40 (7.7) |
| American Society of Anesthesiology physical status, No. (%)c | n = 606 | n = 584 |
| 1 (Healthy) | 65 (8.7) | 51 (10.7) |
| 2 (Mild systemic disease) | 225 (38.0) | 222 (37.1) |
| 3 (Severe systemic disease) | 282 (48.6) | 284 (46.5) |
| 4 (Severe systemic disease that is a constant threat to life) | 34 (4.6) | 27 (5.6) |
| Preoperative Spo2, mean (SD), % | 96.8 (1.4) | 96.8 (1.4) |
| <96%, No./total (%) | 107/608 (17.6) | 101/585 (17.3) |
| Preoperative laboratory measurements, mean (SD) | ||
| Bicarbonate, mmol/L | 25.8 (2.6) | 25.8 (2.5) |
| Hemoglobin, g/dL | 13.7 (1.8) | 13.6 (1.8) |
| Creatinine, mg/dL | 1.04 (0.85) | 1.09 (1.01) |
| >1.70, No./total (%) | 20/442 (4.5) | 23/408 (5.6) |
| Comorbidities, No./total (%) | ||
| Hypertension | 301/613 (49.1) | 327/591 (55.3) |
| Obesityd | 225/595 (37.8) | 207/562 (36.8) |
| Diabetes | 119/613 (19.4) | 126/591 (21.3) |
| Current smoker | 100 (16.3) | 109 (18.4) |
| Coronary artery disease | 93/613 (15.2) | 100/591 (16.9) |
| Asthma | 66 (10.7) | 68 (11.5) |
| Chronic obstructive pulmonary disease | 62 (10.1) | 65 (11.0) |
| Obstructive sleep apnea | 59 (9.6) | 63 (10.6) |
| Chronic renal disease | 56/613 (9.1) | 67/591 (11.3) |
| Chronic liver disease | 48/613 (7.8) | 52/591 (8.8) |
| Recent lower respiratory tract infection | 8 (1.3) | 8 (1.4) |
| Interstitial lung disease | 8 (1.3) | 2 (0.3) |
| Bronchiectasis | 1 (0.2) | 1 (0.2) |
| Type of surgery, No./total (%) | ||
| Abdominal | 348 (56.7) | 333/591 (56.3) |
| Laparoscopic | 158/348 (45.4) | 170/333 (51.1) |
| Spine | 125 (20.4) | 120/591 (20.3) |
| Orthopedic | 43 (7.0) | 46/591 (7.8) |
| Plastic | 31 (5.0) | 36/591 (6.1) |
| Vascular | 29 (4.7) | 28/591 (4.7) |
| Ear, nose, and throat | 17 (2.8) | 13/591 (2.2) |
| General | 6 (1.0) | 2/591 (0.3) |
| Other | 15 (2.4) | 13/591 (2.2) |
| Emergency surgery, No./total (%) | 29/613 (4.7) | 17/591 (2.9) |
Abbreviation: Spo2, arterial oxygen saturation measured by pulse oximetry.
SI conversion: To convert creatinine to micromoles per liter, multiply by 88.4.
Calculated as weight in kilograms divided by height in meters squared.
The Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score ranges from 0 to 123; higher scores indicate a higher risk of postoperative pulmonary complications. Patients with scores of 26 or greater are considered at intermediate risk; those with a score greater than 44 are considered at high risk.
Scores in the American Society of Anesthesiologists physical status classification system range from 1 to 6, with higher scores indicating a more severe condition.
Defined as body mass index greater than 30.
Intraoperative Procedures
As shown in Table 2, there was a significant difference in mean tidal volume between the 2 study groups (6.3 [SD, 1.0] mL/kg PBW vs 9.7 [SD, 1.0] mL/kg PBW; P < .001). A PEEP of 5 cm H2O was applied equally in both groups with no significant difference (mean difference, 0.0 cm H2O [95% CI, −0.0 to 0.0 cm H2O]; P = .33). Patients receiving low-tidal-volume ventilation had statistically significantly lower intraoperative peak airway pressures (mean difference, −2.4 cm H2O [95% CI, −3.1 to −1.7 cm H2O]; P < .001), lower recorded nadir arterial oxygen saturation measured by pulse oximetry (mean difference, −0.4% [95% CI, −0.6% to −0.1%]; P = .009), higher respiratory rates (mean difference, 4.6/min [95% CI, 4.2/min-4.9/min]; P < .001), higher peak end-tidal carbon dioxide (mean difference, 3.9 mm Hg [95% CI, 3.3-4.5 mm Hg]; P < .001), higher arterial partial pressure of carbon dioxide (mean difference, 5.6 mm Hg [95% CI, 4.8-6.5 mm Hg]; P < .001), a greater rate of severe hypercapnia (difference, 3.2% [95% CI, 1.4%-5.2%]; P = .001), higher bicarbonate concentration (mean difference, 0.4 mmol/L [95% CI, 0.1-0.7 mmol/L]; P = .009), lower lactate concentration (mean difference, −0.2 mmol/L [95% CI, −0.4 to −0.1 mmol/L]; P = .001), lower pH (difference, −0.04 [95% CI, −0.05 to −0.04]; P < .001), and a greater rate of acidemia (difference, 12.6% [95% CI, 8.6%-16.7%]; P < .001). However, there was no significant difference in the arterial partial pressure of oxygen intraoperatively (mean difference, −4.9 mm Hg [95% CI, −13.3 to 3.4 mm Hg]; P = .25) or in the fraction of inspired oxygen (mean difference, 0.7% [95% CI, −1.9% to 3.2%]; P = .60) between the 2 groups. An analysis of arterial blood gases in the postanesthesia care unit revealed that none of these intraoperative differences persisted into the postoperative period (eTables 5 and 6 and eFigure 1 in Supplement 2). After randomization, ventilation protocol violations occurred in 75 patients, 41 receiving low-tidal-volume ventilation and 34 receiving conventional ventilation. The reasons for protocol violations are described in eTable 7 in Supplement 2. The mean duration of surgery was not significantly different between the groups (223.6 [SD, 127.1] minutes vs 212.8 [SD, 121.0] minutes; difference, 10.7 [95% CI, −3.4 to 24.9] minutes; P = .14).
Table 2. Intraoperative Characteristics, Ventilation, and Oxygenation.
| Low tidal volume (n = 614) | Conventional tidal volume (n = 592) | Absolute difference (95% CI) | P value | |
|---|---|---|---|---|
| Tidal volume | ||||
| Absolute, mL | ||||
| Mean (SD) | 396.6 (83.5) | 611.1 (111.9) | −214.5 (−225.7 to −203.2)a | <.001 |
| Median (IQR) | 395 (340-446) | 620 (525-700) | ||
| Adjusted, mL/kg PBWb | ||||
| Mean (SD) | 6.3 (1.0) | 9.7 (1.0) | −3.4 (−3.5 to −3.3)a | <.001 |
| Median (IQR) | 6.0 (6.0-6.1) | 10.0 (9.8-10.0) | ||
| PEEP, cm H2O | ||||
| Mean (SD) | 5.0 (1.0) | 5.0 (1.0) | 0.0 (−0.0 to 0.0)a | .33 |
| Median (IQR) | 5 (5-5) | 5 (5-5) | ||
| Highest peak pressure, mean (SD), cm H2O | 22.7 (6.2) | 25.1 (6.3) | −2.4 (−3.1 to −1.7)a | <.001 |
| Respiratory rate, mean (SD), /min | ||||
| Lowest | 12.4 (2.6) | 9.2 (2.0) | 3.2 (2.9 to 3.4)a | <.001 |
| Highest | 16.2 (3.4) | 11.6 (2.5) | 4.6 (4.2 to 4.9)a | <.001 |
| Lowest Spo2, mean (SD), % | 96.5 (2.4) | 96.9 (2.3) | −0.4 (−0.6 to −0.1)a | <.01 |
| Fio2, mean (SD), % | ||||
| Lowest | 47.6 (11.9) | 47.4 (12.2) | 0.2 (−1.2 to 1.6)a | .78 |
| Highest | 71.1 (22.0) | 70.4 (22.6) | 0.7 (−1.9 to 3.2)a | .60 |
| Highest end-tidal carbon dioxide, mean (SD), mm Hg | 41.3 (5.5) | 37.4 (4.9) | 3.9 (3.3 to 4.5)a | <.001 |
| Arterial blood gas measurements after induction | ||||
| pH, mean (SD) | 7.37 (0.05) | 7.41 (0.05) | −0.04 (−0.05 to −0.04)a | <.001 |
| Pao2, mean (SD), mm Hg | 231.0 (94.1) | 237.8 (88.2) | −6.8 (−17.5 to 3.8)a | .21 |
| Paco2, mean (SD), mm Hg | 44.0 (5.8) | 39.1 (5.1) | 4.9 (4.3 to 5.5)a | <.001 |
| Bicarbonate, mean (SD), mmol/L | 25.0 (2.1) | 24.5 (2.5) | 0.5 (0.2 to 0.7)a | <.01 |
| Pao2/Fio2, mean (SD) | 412.3 (143.1) | 426.8 (130.0) | −14.5 (−30.7 to 1.6)a | .08 |
| Base excess, mean (SD) | 0.2 (2.5) | 0.5 (2.4) | −0.2 (−0.5 to 0.0)a | .08 |
| Hemoglobin, mean (SD), g/dL | 12.5 (1.8) | 12.4 (1.7) | 0.3 (−1.7 to 2.4)a | .73 |
| Lactate, mean (SD), mmol/L | 1.2 (0.5) | 1.3 (0.7) | −0.1 (−0.2 to −0.0)a | <.01 |
| Hypoxemia, No. (%)c | 0 | 0 | ||
| Acidosis, No. (%)d | 36 (6.3) | 5 (0.9) | 5.3 (3.3 to 7.6)e | <.001 |
| Hypercapnia, No. (%)f | 6 (1.0) | 1 (0.2) | 0.8 (−0.0 to 1.9)e | .06 |
| Arterial blood gas measurements prior to closure | ||||
| pH, mean (SD) | 7.34 (0.06) | 7.38 (0.06) | −0.04 (−0.05 to −0.04)a | <.001 |
| Pao2, mean (SD), mm Hg | 192.2 (71.2) | 197.1 (68.9) | −4.9 (−13.3 to 3.4)a | .25 |
| Paco2, mean (SD), mm Hg | 46.1 (7.8) | 40.4 (6.3) | 5.6 (4.8 to 6.5)a | <.001 |
| Bicarbonate, mean (SD), mmol/L | 24.0 (2.2) | 23.6 (2.6) | 0.4 (0.1 to 0.7)a | <.01 |
| Pao2/Fio2, mean (SD) | 387.3 (138.7) | 400.0 (127.7) | −12.7 (−28.7 to 3.4)a | .12 |
| Base excess, mean (SD) | −1.2 (2.7) | −0.9 (2.4) | −0.2 (−0.5 to 0.1)a | .12 |
| Hemoglobin, mean (SD), g/dL | 12.1 (1.8) | 12.0 (1.8) | 0.5 (−1.6 to 2.7)a | .63 |
| Lactate, mean (SD), mmol/L | 1.4 (0.7) | 1.6 (1.4) | −0.2 (−0.4 to −0.1)a | <.01 |
| Hypoxemia, No. (%)c | 2 (0.4) | 0 | ||
| Acidosis, No. (%)d | 113 (20.4) | 42 (7.8) | 12.6 (8.6 to 16.7)e | <.001 |
| Hypercapnia, No. (%)f | 24 (4.3) | 6 (1.1) | 3.2 (1.4 to 5.2)e | <.01 |
| Duration of surgery, min | ||||
| Mean (SD) | 223.6 (127.1) | 212.8 (121.0) | 10.7 (−3.4 to 24.9)a | .14 |
| Median (IQR) | 189 (135-267) | 185 (140-249) | ||
| Use of regional anesthesia, No. (%)g | 180 (30.0) | 164 (28.0) | 2.0 (−3.2 to 7.1)e | .46 |
| Epidural | 16 (2.7) | 9 (1.5) | 1.1 (−0.5 to 2.8)e | .17 |
| Spinal opioid | 106 (17.7) | 93 (15.9) | 1.8 (−2.5 to 6.0)e | .41 |
| TAP/abdominal blockh | 33 (5.5) | 25 (4.3) | 1.2 (−1.2 to 3.7)e | .33 |
| Otheri | 84 (14.0) | 78 (13.4) | 0.6 (−3.3 to 4.6)e | .75 |
Abbreviations: Fio2, fraction of inspired oxygen; IQR, interquartile range; Pao2, partial pressure of oxygen; Paco2, partial pressure of carbon dioxide; PEEP, positive end-expiratory pressure; Spo2, arterial oxygen saturation measured by pulse oximetry.
Mean difference from a generalized linear model considering a Gaussian distribution.
Predicted body weight (PBW) was calculated as 50 + 0.91 × (height [cm] – 152.4) for men and 45.5 + 0.91 × (height [cm] – 152.4) for women.
Defined as Pao2 <60 mm Hg.
Defined as pH <7.30.
Risk difference from a generalized linear model considering a binomial distribution with an identity link.
Defined as Paco2 >60 mm Hg.
In addition to general anesthesia.
Transversus abdominis plane block (TAP) is defined as block of a peripheral nerve designed to anesthetize the nerves supplying the anterior abdominal wall (T6 to L1).
Other: brachial plexus block, femoral nerve block, fascia iliaca block, sciatic nerve block, intercostal nerve block, interpleural catheter, wound catheter.
Primary Outcome
Postoperative pulmonary complications within the first 7 days occurred in 231 patients (38%) receiving low tidal volume and in 232 patients (39%) receiving conventional tidal volume (difference, −1.3% [95% CI, −6.8% to 4.2%]; P = .64) (Table 3). The most common postoperative pulmonary complication was atelectasis, which occurred in 150 patients (24.7%) in the low tidal volume group and in 147 patients (24.9%) in the conventional tidal volume group.
Table 3. Primary and Secondary Outcomes.
| Outcomes | No. of events/total (%) | Absolute difference (95% CI) | P valuea | |
|---|---|---|---|---|
| Low tidal volume (n = 614) | Conventional tidal volume (n = 592) | |||
| Primary outcome | ||||
| Composite respiratory complications within 7 d | 231/608 (38.0) | 232/590 (39.3) | −1.3 (−6.8 to 4.2)b | .64 |
| Components of the primary outcome | ||||
| Atelectasisc | 150/608 (24.7) | 147/591 (24.9) | −0.2 (−5.1 to 4.7)b | .93 |
| Respiratory failured | 103/612 (16.8) | 109/590 (18.5) | −1.6 (−6.0 to 2.7)b | .45 |
| Pleural effusion | 67/611 (11.0) | 69/590 (11.7) | −0.7 (−4.3 to 2.9)b | .69 |
| Pneumonia | 19/610 (3.1) | 22/591 (3.7) | −0.6 (−2.7 to 1.5)b | .56 |
| Unplanned noninvasive or invasive ventilation | 15/612 (2.5) | 14/591 (2.4) | 0.1 (−1.7 to 1.9)b | .92 |
| Pulmonary congestion | 10/612 (1.6) | 14/591 (2.4) | −0.7 (−2.4 to 0.9)b | .36 |
| Bronchospasm | 5/612 (0.8) | 5/590 (0.8) | −0.0 (−1.1 to 1.1)b | .95 |
| Pneumothorax | 2/611 (0.3) | 0/591 (0.0) | 0.3 (−0.1 to 0.8)b | .26 |
| Secondary outcomes | ||||
| Composite respiratory complications during hospital stay | 237/598 (39.6) | 239/579 (41.3) | −1.6 (−7.2 to 4.0)b | .56 |
| Pulmonary embolism | 5/595 (0.8) | 6/573 (1.0) | −0.2 (−1.4 to 1.0)b | .71 |
| Acute respiratory distress syndrome | 3/594 (0.5) | 0/574 (0.0) | 0.5 (−0.1 to 1.1)b | |
| Systemic inflammatory response syndrome | 6/612 (1.0) | 13/591 (2.2) | −1.2 (−2.8 to 0.2)b | .09 |
| Sepsis | 20/612 (3.3) | 13/591 (2.2) | 1.1 (−0.8 to 3.0)b | .26 |
| Acute kidney injurye | 36/420 (8.6) | 42/394 (10.7) | −2.1 (−6.2 to 2.0)b | .31 |
| Risk | 24/36 (66.7) | 32/42 (76.2) | ||
| Injury | 8/36 (22.2) | 4/42 (9.5) | ||
| Failure | 4/36 (11.1) | 6/42 (14.3) | ||
| Wound infection | 11/597 (1.8) | 10/575 (1.7) | 0.1 (−1.5 to 1.7)b | .89 |
| Intraoperative need of vasopressor | 518/600 (86.3) | 511/584 (87.5) | −1.2 (−5.0 to 2.7)b | .55 |
| Unplanned ICU admission | 30/595 (5.0) | 25/577 (4.3) | 0.7 (−1.7 to 3.2)b | .56 |
| Need for rapid response team call | 67/612 (10.9) | 63/591 (10.7) | 0.3 (−3.2 to 3.8)f | .87 |
| Length of stay, d | ||||
| ICU, mean (SD) | 0.5 (1.3) | 0.6 (3.5) | −0.1 (−0.4 to 0.2)f | .84 |
| Hospital, mean (SD) | 8.3 (8.6) | 7.9 (10.6) | 0.4 (−0.6 to 1.6)f | .40 |
| In-hospital mortality, No. (%) | 8 (1.3) | 7 (1.2) | 0.1 (−1.2 to 1.4)f | .85 |
Abbreviation: ICU, intensive care unit.
Calculated using the χ2 test for categorical variables or from a generalized linear model considering an inverse Gaussian distribution for the continuous variables.
Risk difference from a generalized linear model considering a binomial distribution with an identity link.
Defined as lung opacification with a shift of the mediastinum, hilum, or hemidiaphragm toward the affected area and compensatory overinflation in the adjacent nonatelectatic lung.
Defined as postoperative partial pressure of oxygen <60 mm Hg on room air, a partial pressure of oxygen/fraction of inspired oxygen ratio <300 mm Hg, or arterial oxygen saturation measured by pulse oximetry <90% and requiring oxygen therapy.
Classified in 3 categories according to stage of kidney injury. Risk is defined as an increase in creatinine 1.5 times baseline level; injury, an increase in creatinine 2 times baseline level; and failure, an increase in creatinine 3 times baseline level or an increase in creatinine of ≥0.5 mg/dL if baseline creatinine was ≥4 mg/dL.
Mean difference from a generalized linear model considering a Gaussian distribution.
The effect of tidal volume on the frequency of postoperative pulmonary complications was consistent across all subgroups, including patients with a moderate to high preoperative risk of postoperative pulmonary complications, patients undergoing abdominal surgery, and patients with high BMI (Figure 2).
Figure 2. Absolute Differences of Postoperative Respiratory Complications in Prespecified Subgroups.
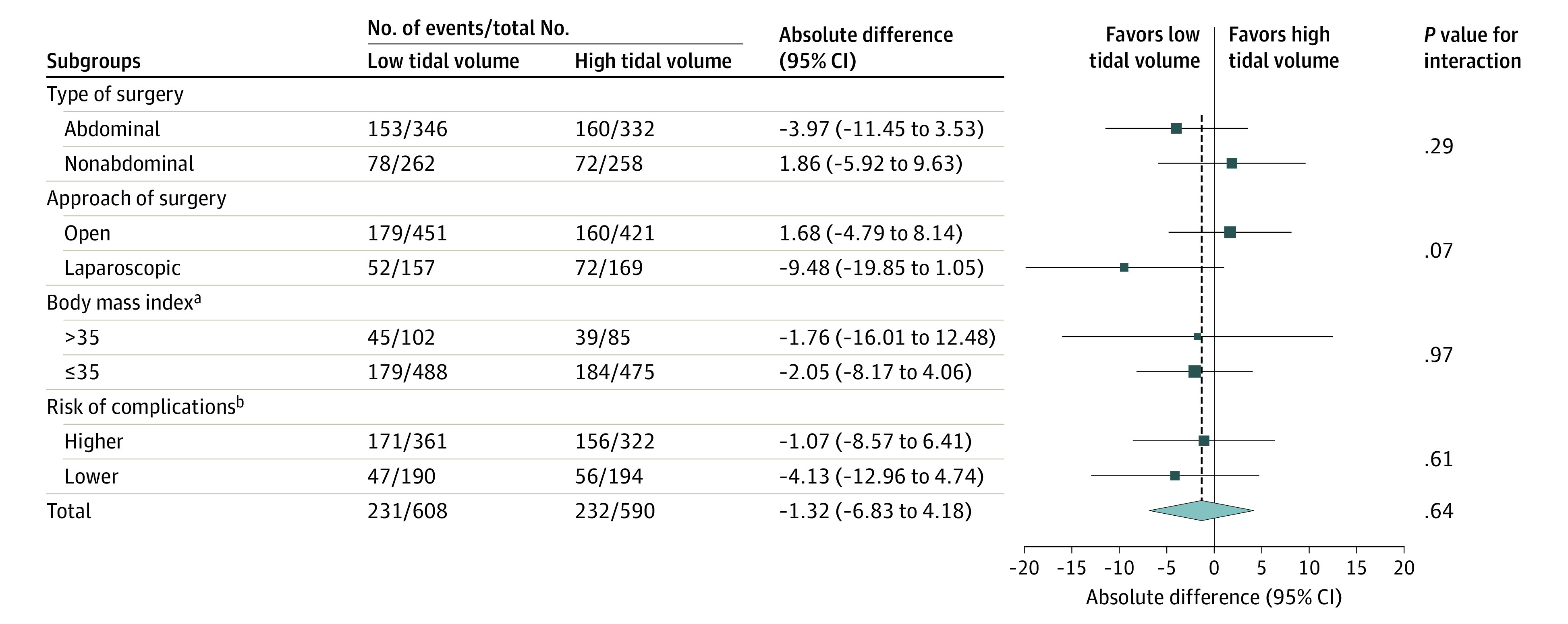
Sizes of data markers are proportional to the numbers of patients entering the analysis. P values are for the interaction between the subgroup and the treatment group. Lack of a significant interaction implies that the results are consistent across subgroups and that the overall effects estimated are the most appropriate estimates of treatment effect within each subgroup.
aCalculated as weight in kilograms divided by height in meters squared.
bRisk of complications is defined according to the Assess Respiratory Risk in Surgical Patients in Catalonia score as higher (≥26) or lower (<26).
Secondary Outcomes
The postoperative secondary outcomes are presented in Table 3 and, after adjustment for multiplicity, in eTable 8 in Supplement 2. Postoperatively, there was no significant difference in extrapulmonary complications, need for attendance by the rapid response team, or need for unexpected admission to the ICU. Duration of hospital stay (eFigure 2 in Supplement 2) and in-hospital mortality were not significantly different between groups.
Additional Analyses
Additional sensitivity analyses using different statistical assumptions yielded similar results (eTable 9 and eFigure 3 in Supplement 2). Results of the post hoc sensitivity analysis are shown in eTable 10 in Supplement 2. In patients undergoing laparoscopic surgery, postoperative pulmonary complications within the first 7 days occurred in 52 patients (33.1%) in the low tidal volume group and in 72 patients (42.6%) in the conventional tidal volume group (difference, −9.5% [95% CI, −19.9% to 1.0%]; P = .08). Results of the post hoc sensitivity analyses of the secondary outcomes found a significantly lower incidence of postoperative pulmonary complications during the entire hospital stay only in patients in the low tidal volume group (33.8% vs 44.9%; difference, −11.1% [95% CI, −21.6% to −0.5%]; P = .04). There was no interaction between the effect of tidal volume on the primary outcome and either type of surgery or duration of surgery (eFigure 4 in Supplement 2). The sensitivity analysis allocating 22 additional patients to the conventional tidal volume group did not materially change the study findings (eFigure 5 in Supplement 2). Chest x-rays were undertaken in 281 patients (47.5%) in the low tidal volume group and in 276 (47.9%) in the conventional tidal volume group (difference, −0.45% [95% CI, −6.17% to 5.27%]; P = .88).
Discussion
In this randomized clinical trial of adult patients undergoing major surgery, intraoperative mechanical ventilation with low tidal volume did not significantly reduce postoperative pulmonary complications when compared with ventilation with conventional tidal volume when PEEP was applied equally between groups.
Preliminary studies of the use of low-tidal-volume ventilation during surgery have been small, underpowered, and focused on physiological rather than clinical outcomes.8,9,10,11,12,13 Despite such limitations, however, a biologic plausibility for a benefit with low-tidal-volume ventilation was strongly suggested. In keeping with such proposed benefits, a randomized clinical trial by French investigators provided the first evidence in favor of protective lung ventilation.11 However, in that study of 400 patients, PEEP was used in only one group and in combination with periodic recruitment maneuvers and low tidal volume, while the control group received no PEEP, no recruitment maneuver, and high tidal volume. This strategy makes it difficult to identify the benefits of low tidal volume or PEEP per se. Some of these issues were addressed by the multicenter PROVHILO trial undertaken in Europe, the United States, and South America, which compared intraoperative ventilation with a tidal volume of 8 mL/kg PBW and a PEEP of less than 2 cm H2O vs a tidal volume of 8 mL/kg PBW and a PEEP of 12 cm H2O combined with scheduled recruitment maneuvers (performed after intubation, before extubation, and after any ventilator circuit disconnection) in 900 moderate- to high-risk patients receiving open intra-abdominal surgery.17 That study found no effect of the intervention on postoperative pulmonary complications but reported a greater use of vasopressors in the intervention group. More recently, a multicenter trial in 77 hospitals randomized 2013 severely obese patients (mean BMI, 44; only patients with BMI >35 were included) to a tidal volume of 7 mL/kg PBW with a PEEP of 12 cm H2O and hourly recruitment maneuvers compared with a tidal volume of 7 mL/kg PBW and a PEEP of 2 cm H2O without recruitment maneuvers.18 That study found no difference in postoperative pulmonary complications but greater hemodynamic instability in the intervention group.18 The present study did not include recruitment maneuvers because of their unproven benefit and known hemodynamic and barotrauma risks.17,18,19 Moreover, as the prevailing practice is to deliver a PEEP of 5 cm H2O,11,12,13,14,20 such background management was applied in the present study to both groups.
To our knowledge, this is the only randomized clinical trial of the effect of low-tidal-volume ventilation during major surgery to date. It is the largest study, to our knowledge, to assess arterial blood gas analysis both intraoperatively and in the immediate postoperative period, thus permitting a detailed analysis of the effects of the intervention on gas exchange, acid-base status, and arterial partial pressure of carbon dioxide. The assessment of outcomes was blinded to treatment allocation, and there was no loss to follow-up, attenuating ascertainment bias. Protocol violations were uncommon, thus minimizing performance bias. A permuted-block randomization was applied to decrease selection bias. Patients who underwent surgery lasting for more than 2 hours were selected to increase the putative effect of the intervention. Furthermore, multiple types of surgeries were assessed to increase the generalizability of the findings.
Limitations
This study has several limitations. First, this was a single-center study and has all the limitations inherent in that study design. However, it did include a diverse range of patients and surgeries. Moreover, anesthesia was administered by more than 140 different anesthesiologists. Second, because of the nature of the intervention, blinding of treatment was not possible. However, scoring of clinical outcomes was made by blinded assessors. Third, respiratory management during emergence from anesthesia and during the immediate postoperative period was not standardized. However, no consensus guidelines exist to inform such management. Accordingly, a pragmatic approach was chosen. Fourth, the primary composite outcome implies equivalence of each of its components. This may not be correct. However, this approach had been used in the largest trial of a similar intervention published at the time of this study design.18,19 Such complications were included regardless of severity. Nevertheless, even minor postoperative pulmonary complications would be clinically relevant and lead to undesirable outcomes. Moreover, the rate of the composite primary outcome is similar to that reported in the previous largest study of intraoperative ventilation management, which used a similar primary outcome measure.18 Fifth, there was a small (<4%) imbalance in the number of patients treated with low vs conventional tidal volume. This imbalance resulted in part from anesthesiologist-based postrandomization patient withdrawal and in part from the play of chance within the randomization blocks. However, when sensitivity analyses were conducted assuming best- or worst-case scenarios for such imbalance, the study findings were not materially altered. Sixth, chest x-rays were performed only when clinically indicated by the team in charge of patient care and were not systematically evaluated; therefore, all diagnoses based on radiological reports could have being underestimated. However, this limitation affected both groups equally. Seventh, there is no clinical or objective definition of a minimal clinically important difference in studies of mechanical ventilation during surgery. In the present study, the minimal clinically important difference was defined based on clinical consensus among a team of anesthesiologists. Nevertheless, the relative risk reduction of around 30% proposed in the study is consistent with the effect estimate used in several other trials of mechanical ventilation in the ICU and operating room.
Conclusions
Among adult patients undergoing major surgery, intraoperative ventilation with low tidal volume compared with conventional tidal volume, with PEEP applied equally between groups, did not significantly reduce pulmonary complications within the first 7 postoperative days.
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplemental Methods
eTable 1. Definition of the Primary Outcome
eTable 2. Definitions of the Secondary Outcomes
eTable 3. Missing Values for Study Variables
eTable 4. Detailed Description of Surgical Procedures
eTable 5. Additional Intraoperative Data
eTable 6. Postoperative Characteristics
eTable 7. Protocol Violations and Reason for Violations
eTable 8. Adjusted P Values for the Secondary Outcomes
eTable 9. Additional Sensitivity Analyses for the Primary Outcome
eTable 10. Primary and Secondary Outcomes Only in Patients Undergoing Laparoscopic Surgery
eFigure 1. Trend Over Time for Arterial Blood Gases
eFigure 2. Kaplan-Meier Estimates for Patients in the Low Tidal Volume and Conventional Tidal Volume Groups
eFigure 3. Results of the Sensitivity Analyses for the Primary Outcome
eFigure 4. Risk Ratio for Postoperative Respiratory Complications in Post Hoc Subgroups
eFigure 5. Results of the Sensitivity Analysis Allocating 22 Additional Patients in the Conventional Arm With Different Incidences of the Primary Outcomes
Data Sharing Statement
References
- 1.Meara JG, Leather AJM, Hagander L, et al. . Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569-624. doi: 10.1016/S0140-6736(15)60160-X [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Bustamante A, Frendl G, Sprung J, et al. . Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157-166. doi: 10.1001/jamasurg.2016.4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317-334. doi: 10.1093/bja/aex002 [DOI] [PubMed] [Google Scholar]
- 4.Young CC, Harris EM, Vacchiano C, et al. . Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth. 2019;123(6):898-913. doi: 10.1016/j.bja.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 5.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation: a concept of atelectasis. N Engl J Med. 1963;269:991-996. doi: 10.1056/NEJM196311072691901 [DOI] [PubMed] [Google Scholar]
- 6.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 7.Serpa Neto A, Cardoso SO, Manetta JA, et al. . Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651-1659. doi: 10.1001/jama.2012.13730 [DOI] [PubMed] [Google Scholar]
- 8.Zupancich E, Paparella D, Turani F, et al. . Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130(2):378-383. doi: 10.1016/j.jtcvs.2004.11.061 [DOI] [PubMed] [Google Scholar]
- 9.Wrigge H, Uhlig U, Baumgarten G, et al. . Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Med. 2005;31(10):1379-1387. doi: 10.1007/s00134-005-2767-1 [DOI] [PubMed] [Google Scholar]
- 10.Reis Miranda D, Gommers D, Struijs A, et al. . Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg. 2005;28(6):889-895. doi: 10.1016/j.ejcts.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 11.Futier E, Constantin JM, Paugam-Burtz C, et al. ; IMPROVE Study Group . A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428-437. doi: 10.1056/NEJMoa1301082 [DOI] [PubMed] [Google Scholar]
- 12.Wanderer JP, Blum JM, Ehrenfeld JM. Intraoperative low-tidal-volume ventilation. N Engl J Med. 2013;369(19):1861. doi: 10.1056/NEJMc1311316 [DOI] [PubMed] [Google Scholar]
- 13.Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113(1):97-108. doi: 10.1093/bja/aeu054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karalapillai D, Weinberg L, Galtieri J, et al. . Current ventilation practice during general anaesthesia: a prospective audit in Melbourne, Australia. BMC Anesthesiol. 2014;14:85. doi: 10.1186/1471-2253-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karalapillai D, Weinberg L, Peyton P, et al. . Low tidal volume ventilation during anaesthesia for major surgery: protocol and statistical analysis plan. Crit Care Resusc. 2019;21(4):243-250. [PubMed] [Google Scholar]
- 16.Weinberg L, Li M, Churilov L, et al. . Associations of fluid amount, type, and balance and acute kidney injury in patients undergoing major surgery. Anaesth Intensive Care. 2018;46(1):79-87. doi: 10.1177/0310057X1804600112 [DOI] [PubMed] [Google Scholar]
- 17.Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ; PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology . High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384(9942):495-503. doi: 10.1016/S0140-6736(14)60416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluth T, Serpa Neto A, Schultz MJ, et al. ; Writing Committee for the PROBESE Collaborative Group of the Protective Ventilation Network (PROVEnet) for the Clinical Trial Network of the European Society of Anaesthesiology; PROBESE Collaborative Group . Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA. 2019;321(23):2292-2305. doi: 10.1001/jama.2019.7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. ; Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial Investigators . Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335-1345. doi: 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LAS VEGAS Investigators Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS—an observational study in 29 countries. Eur J Anaesthesiol. 2017;34(8):492-507. doi: 10.1097/EJA.0000000000000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplemental Methods
eTable 1. Definition of the Primary Outcome
eTable 2. Definitions of the Secondary Outcomes
eTable 3. Missing Values for Study Variables
eTable 4. Detailed Description of Surgical Procedures
eTable 5. Additional Intraoperative Data
eTable 6. Postoperative Characteristics
eTable 7. Protocol Violations and Reason for Violations
eTable 8. Adjusted P Values for the Secondary Outcomes
eTable 9. Additional Sensitivity Analyses for the Primary Outcome
eTable 10. Primary and Secondary Outcomes Only in Patients Undergoing Laparoscopic Surgery
eFigure 1. Trend Over Time for Arterial Blood Gases
eFigure 2. Kaplan-Meier Estimates for Patients in the Low Tidal Volume and Conventional Tidal Volume Groups
eFigure 3. Results of the Sensitivity Analyses for the Primary Outcome
eFigure 4. Risk Ratio for Postoperative Respiratory Complications in Post Hoc Subgroups
eFigure 5. Results of the Sensitivity Analysis Allocating 22 Additional Patients in the Conventional Arm With Different Incidences of the Primary Outcomes
Data Sharing Statement