Highlights
-
•
Differential distributions of vitamin D were observed in the Indian population.
-
•
Vitamin D levels was inversely correlated with SARS-CoV-2 infection rate.
-
•
COVID-19 mortality rate was negatively associated with mean vitamin D levels.
Keywords: Vitamin D, SARS-CoV-2, COVID-19, Indian population, Infection, Mortality
Abstract
Background
The role of vitamin D in the susceptibility and severity of various viral diseases has been well documented. Recently, some reports highlighted the possible importance of vitamin D in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although India receives adequate sunlight throughout the year, the majority of Indians are deficient in vitamin D levels. In the present study, we hypothesized that vitamin D deficiency would be associated with the SARS-CoV-2 infection rate and mortality in the Indian population.
Materials and methods
SARS-CoV-2 infection and mortality data were obtained from the Government of India's official website (accessed on 16th August 2020). Various literature databases like PubMed and Google Scholar were searched to find the mean of 25-hydroxyvitamin D [25(OH)D] levels in different states and union territories of India, Pearson correlation was carried out to investigate the possible link between mean 25(OH)D levels and SARS-CoV-2 infection and mortality per million of the population.
Results
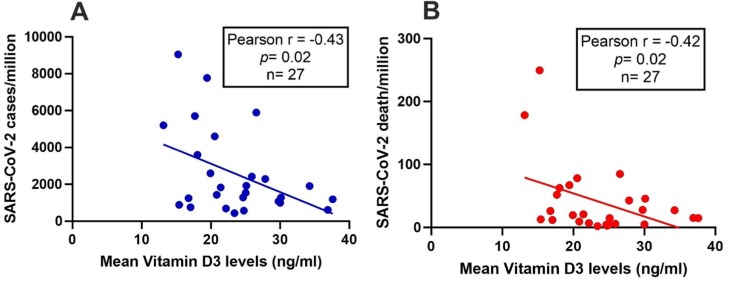
An inverse correlation was observed between the mean level of 25(OH)D and SARS-CoV-2 infection rate (r = −0.43, p = 0.02) and mortality rate (r = −0.42, p = 0.02).
Conclusions
The present observational study revealed an association of vitamin D with SARS-CoV-2 infection and related mortality. Further studies are required to validate our observations.
1. Introduction
Novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, in December 2019 [1] and has spread worldwide to 215 countries till date (https://www.worldometers.info/coronavirus/). On 11th March 2020, COVID-19 disease was declared a global pandemic by the World Health Organization (WHO)(https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020). India reported the first case of COVID-19 in a student who returned from Wuhan University China in Thrissur district of Kerala state on 30th January 2020 (https://www.cnbc.com/2020/01/30/india-confirms-first-case-of-the-coronavirus.html). As on 16th August 2020, 2.59 million of SARS-CoV-2 infected cases has been reported in India, and a total of 50,431 death has been encountered. Maharashtra and Tamil Nadu states contributed the maximum number of infected cases and posed a high mortality of SARS-CoV-2 infected patients in India.
Individual immune systems, which are determined by several factors, play a major role in the susceptibility and pathogenesis of viral diseases. The importance of vitamin D in the modulation of both innate and adaptive immune systems has been demonstrated [2]. In the skin, provitamin D3 (7-dehydrocholesterol) is converted to previtamin D3 after exposure to Ultraviolet-B (UVB) radition. The previtamin D3 is transported to the liver and hydroxylated at 25th carbon atom. The active metabolites 1,25(OH) vitamin D3 is hydroxylated for the second time at C-1 at the kidneys[3]. Furthermore, 1-hydroxylation of vitamin D3 is also reported in macrophages, monocytes, B and T lymphocytes[4].Vitamin D deficiency has been associated with susceptibility to a wide range of viral infections, such as Human Immunodeficiency Virus, Influenza Virus, Epstein - Barr Virus, Hepatitis B, Human Respiratory Syncytial Virus [5]. However, the role of vitamin D in SARS-CoV-2 infection is not well understood. Some recent investigations have highlighted the beneficial role of vitamin D against SARS-CoV-2 infection and related clinical severity [6], [7], [8], [9], [10], [11], [12], [13], by modulating the immune system [14]. In addition, a recent study in UK population reported the worst morbidity outcome in older patients with vitamin D deficiency[13].
Although India is close to the equator and receives a large amount of sunlight throughout the year, most of the Indian population (50–90%) is deficient in vitamin D[15]. In addition, differential levels of 25(OH)D have been observed in different geographical areas of India. Furthermore, unusual rates of SARS-CoV-2 infection and mortality have been observed in different states and Union territories of India. Based on these observations, we hypothesized that the mean 25(OH)D levels in the cohort would be correlated with the infection and mortality rate of COVID-19 in the Indian population.
2. Materials and methods
2.1. Vitamin D data
We investigated the correlation between vitamin D and COVID-19 disease in the Indian population to minimize the effect of various confounding factors such as ethnicity, latitude, health facility, etc. India is comprised of twenty-eight states and eight union territories (UTs) and estimated inhabitants of 1380 million equivalent to 17.7% of the total world population. Vitamin D levels in healthy adults were searched through PubMed and Google Scholar in the Indian population. All relevant articles were screened, and data such as the author's name, year of publication, number of healthy subjects enrolled for the analysis, mean ± standard deviation or median, interquartile range of vitamin D were extracted.
2.2. COVID-19 data
SARS-CoV-2 data such as the number of cases, death figures, and the number of recovered persons were obtained from the official website of the Ministry of Health and Family Welfare, Govt. of India (www.mohfw.gov.in accessed on 16/08/2020). The population of each state and UTs were gathered from data of census performed in the year 2011 (https://censusindia.gov.in/2011-common/censusdata2011.html). SARS-CoV-2 cases per million and death per million were calculated by using the census-2011 data. Based on suggestions how to present numerical data appropriately [16], the infection rate per million were converted into multiples of ten. Death rate and mean vitamin D levels were represented in whole numbers.
2.3. Statistical analysis
Data on levels of 25(OH)D in the median and interquartile range were converted into mean ± standard deviation format as described earlier [17]. The mean 25(OH)D levels in a state or UT with more than one report were pooled (https://home.ubalt.edu/ntsbarsh/Business-stat/otherapplets/Pooled.htm). The Pearson test performed a correlation of mean 25(OH)D levels with SARS-CoV-2 infection and mortality rate. All statistical analysis was carried out by GraphPad Prism 8.3.0. A p-value < 0.05 was considered as statistically significant.
3. Results
Out of 28 states and 8 UTs, mean 25(OH)D data was available for 23 states and 4 UTs (Andhra Pradesh, Assam, Chandigarh, Bihar, Delhi, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Meghalaya, Odisha, Puducherry, Punjab, Rajasthan, Tamil Nadu, Telangana, Tripura, Uttar Pradesh, Uttarakhand, West Bengal) (Table 1 ). However, there are eight states viz. Bihar, Goa, Himachal Pradesh, Jharkhand, Madhya Pradesh, Manipur, Meghalaya, Uttarakhand have only one report containing 25(OH)D levels in healthy adults (Table 1).
Table 1.
SARS-CoV-2 cases, mortality rate and the mean 25(OH)D levels in different states and union territories of India.
| State/ union territories | Case/million | Death/million | Total no. of healthy subjects | Mean 25(OH)D levels (ng/ml) | References |
|---|---|---|---|---|---|
| Andhra Pradesh | 5530 | 50 | 1936 | 18 | [32], [33], [34], [35], [36] |
| Assam | 2390 | 6 | 768 | 26 | [37], [38], [39] |
| Bihar | 940 | 4 | 50 | 30 | [40] |
| Chandigarh | 1830 | 27 | 878 | 34 | [41], [42], [43], [44], [45], [46] |
| Delhi | 8970 | 249 | 2919 | 15 | [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68] |
| Goa | 7520 | 64 | 640 | 19 | [69] |
| Gujarat | 1270 | 45 | 160 | 30 | [70], [71] |
| Haryana | 1800 | 20 | 26,460 | 21 | [72], [73], [74] |
| Himachal Pradesh | 560 | 3 | 105 | 25 | [75] |
| Jammu and Kashmir | 2240 | 42 | 841 | 28 | [76], [77], [78], [79], [80] |
| Jharkhand | 650 | 7 | 72 | 22 | [81] |
| Karnataka | 3460 | 61 | 263 | 18 | [82], [83] |
| Kerala | 1240 | 4 | 206 | 25 | [84], [85], [86], [87], [88] |
| Madhya Pradesh | 600 | 15 | 50 | 37 | [89] |
| Maharashtra | 5100 | 173 | 4471 | 13 | [90], [91], [92], [93], [94], [95], [96], [97] |
| Manipur | 1540 | 5 | 108 | 25 | [98] |
| Meghalaya | 410 | 2 | 109 | 23 | [99] |
| Odisha | 1300 | 8 | 413 | 21 | [100], [101], [102], [103], [104] |
| Puducherry | 5610 | 85 | 265 | 27 | [105], [106], [107], [108], [109] |
| Punjab | 1045 | 26 | 152 | 30 | [110], [111] |
| Rajasthan | 860 | 12 | 175 | 15 | [112], [113] |
| Tamil Nadu | 4520 | 76 | 991 | 21 | [107], [114], [115], [116], [117], [118] |
| Telangana | 2560 | 19 | 715 | 20 | [119], [120], [121] |
| Tripura | 1890 | 14 | 224 | 25 | [122], [123] |
| Uttar Pradesh | 730 | 12 | 389 | 17 | [124], [125], [126], [127], [128], [129], [130] |
| Uttarakhand | 1150 | 15 | 100 | 38 | [131] |
| West Bengal | 1210 | 25 | 2029 | 17 | [132], [133], [134], [135], [136], [137], [138] |
Note: SARS-CoV-2 data was accessed on 16.08.2020.
A possible correlation between the mean levels of 25(OH)D with SARS CoV-2 infection/million and mortality/million by Pearson correlation test and results are shown in Fig. 1 . An inverse correlation was observed among mean 25(OH)D and SARS-CoV-2 infection rate/million in the Indian population (r = −0.43, p = 0.02) (Fig. 1A). Interestingly, the mortality rate due to SARS-CoV-2 infection was also negatively correlated with vitamin D status of healthy Indian subjects (r = −0.42, p = 0.02) (Fig. 1B).
Fig. 1.
Correlation of 25(OH)D with SARS-CoV-2 infection and death rate in the Indian population. Data on the number of subjects infected with SARS-CoV-2 and the number of death due to SARS-CoV-2, were obtained from the Government of India website (https://www.mohfw.gov.in/) (accessed on 16.08.2020). Mean 25(OH)D levels of different states and union territories populations were obtained from earlier published literature (n = 27). Pearson correlation analysis was performed to investigate the correlation of mean 25(OH)D levels with SARS-CoV-2 infection/million (A: r = −0.43, p = 0.02) and the mortality rate per million (B: r = −0.42, p = 0.02). A p-value of less than 0.05 was taken as significant.
In the Indian population, higher prevalence of SARS-CoV-2 infections has been recorded in the younger age group, while the elderly have the highest death rate (https://www.ha-asia.com/63-of-covid-19-deaths-in-india-are-of-60-age-group/). Based on this observation, we hypothesized a potential link between SARS-CoV-2 infection or mortality rate in different age profile of the Indian population. We obtained age group data from census 2011 (https://censusindia.gov.in/2011-common/censusdata2011.html). Correlation analysis showed no substantial relationship between the mean vitamin D levels and the risk of infection in the younger population (r = −0.16, p = 0.42) or the rate of mortality in the older age group (r = −0.24, p = 0.23).
4. Discussion
This study is the first of its kind to investigate the association of vitamin D with SARS-CoV-2 infection and mortality rates in the Indian population. The current epidemiological investigation revealed a significant association of lower 25(OH)D with SARS-CoV-2 related deaths and susceptibility to infection.
Several studies have recently been performed in different populations to decipher the possible role of vitamin D in SARS-CoV-2 infection. A retrospective analysis of 3,48,598 UK biobank participants showed a higher chance of SARS-CoV-2 in subjects with lower levels of 25(OH)D [18]. However, the statistical significance levels had vanished after adjustment of other confounding factors. In addition, infected patients with SARS-CoV-2 had lower levels of 25(OH)D compared to those without a virus infection in Switzerland[5] and the Israeli population[7]. We observed a negative correlation in the Indian population between mean levels of 25(OH)D and the rate of infection with SARS-CoV-2 per million (r = −0.43, p = 0.02). Corroborating with the results of the present study, an analysis including data from 20 European continent countries also found a marginal inverse correlation between the mean 25(OH)D level and the rate of SARS-CoV-2 infection (r = −0.44, p = 0.05, estimated on 8th April 2020)[19]. Another independent analysis in the same population showed a stronger correlation [12], after reaching the post-COVID-19 infection peak (accessed on 12th May 2020). Lower levels of 25(OH)D have been linked to an increased risk of viral infections. Vitamin D induces the production of antiviral molecules such as cathelicidins and defensins, which decrease the rate of viral replication, decline the concentration of pro-inflammatory cytokines, and increase the production of anti-inflammatory molecules [20].
We examined the possible correlation in the Indian population between mean 25(OH)D level and COVID 19 mortality and found an inverse relationship (r = −0.42, p = 0.02). Recent reports including COVID-19 mortality data and mean 25(OH)D from 12 countries of European origin, also showed similar observations (p = 0.04). Similarly, another independent report involving records from 20 European countries revealed a marginal correlation (r = −0.44, p = 0.05, assed on 8th April 2020)[19]. However, after updating data on 12th May 2020, the relationship was disappeared[21]. Further, a recent report highlighted a higher chance of SARS-CoV-2 infected patients being admitted to an intensive care unit compared to those with insufficient or sufficient levels of vitamin D in the UK population[9]. The risk of hospitalization in SARS-CoV-2 infected subjects with lower levels of 25(OH)D was higher in the Israeli cohort [7]. A recent analysis revealed lower mortality rates of COVID-19 in the equatorial region compared to geographical areas away from the equatorial line[22], further reinforcing the importance of vitamin D against SARS-CoV-2 related death, as subjects residing at the equatorial line receive ample sunlight and possibly have higher levels of 25(OH)D. A meta-analysis of 61 independent studies including more than 10,000 COVID-19 cases demonstrated chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), cerebrovascular disease and coronary heart disease (CHD) as confounding factors for SARS-CoV-2 related death[23]. Severe vitamin D deficiencies have been associated with those confounding diseases such as CKD [24], COPD [25], cerebrovascular disease [26], and CHD [27].
Although several clinical trials have been registered to investigate the role of vitamin D supplementation in the treatment of SARS-CoV-2 patients (https:/clinicaltrials.gov/ct2/results?cond=Covid19&term=vitamin+d&cntry=&state=&city=&dist=) majority of these trials are in the enrolment phase or have yet to be started. A recent study has shown that the administration of 1000 IU of vitamin D per day to four patients infected with SARS-CoV-2 leads to normalization of vitamin D levels, improved clinical condition, decreased oxygen requirements, reduced inflammatory markers, and shorter hospital stays [8]. In addition, another hospital-based randomised clinical study found that the use of calcifediol in infected SARS-CoV-2 subjects minimised the need for intensive care and reduced disease severity [28]. These observations further support the important role of vitamin D supplementation in the management of COVID-19 and necessitate completion of related clinical trials on an urgent basis to minimize the mortality rate worldwide.
Our study has several limitations, and those need to be disclosed. First, the investigation was not done in SARS-CoV-2 infected patients and healthy controls. Mean vitamin D levels and COVID-19 data of Indian states and union territories were obtained from the online database and earlier published reports. Second, seasonal fluctuations in mean vitamin D levels have been demonstrated [29], [30], and the timing of the collection of the vitamin D data considered in this analysis is not known. Third, due to data unavailability, other confounding factors such as age, gender, comorbidity status, economic status, physicians, and nursing staff density have not been included in this analysis. Fourth, vitamin D exerts its cellular effect through vitamin D receptor (VDR) and transportation of vitamin D carried out through vitamin D binding protein (DBP)[31]. Various functional genetic variants believed to affect levels of VDR and DBP have not been included in the present study.
In conclusion, we observed an inverse correlation of mean 25(OH)D levels with SARS CoV-2 infection and mortality in the Indian population. The SARS-CoV-2 infection and mortality rate in India is increasing. For robust observations, it is advisable to re-analyze the correlation between 25(OH)D and COVID-19 once the infection rate has reached its peak. Besides, case-control studies, including a large number of subjects and randomized control trials in the Indian population, are required to validate our observations.
CRediT authorship contribution statement
Sunali Padhi: Investigation, Formal analysis. Subham Suvankar: Investigation, Formal analysis. Venketesh K. Panda: Investigation, Formal analysis. Abhijit Pati: Investigation, Formal analysis. Aditya K. Panda: Conceptualization, Supervision, Writing - original draft, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranow C. Vitamin D and the immune system. J. Invest. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Rosa M., Malaguarnera M., Nicoletti F., Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., Keller F., Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5) doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020 doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am. J. Therap. 2020 doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panagiotou G., Tee S.A., Ihsan Y., Athar W., Marchitelli G., Kelly D., Boot C.S., Stock N., Macfarlane J., Martineau A.R., Burns G., Quinton R. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020 doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J.K., Zhang W.H., Zou L., Liu Y., Li J.J., Kan X.H., Dai L., Shi Q.K., Yuan S.T., Yu W.K., Xu H.Y., Gu W., Qi J.W. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging. 2020;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teymoori-Rad M., Marashi S.M. Vitamin D and Covid-19: From potential therapeutic effects to unanswered questions. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2159. [DOI] [PubMed] [Google Scholar]
- 12.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2020:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Network Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y., Baylink D.J., Chen C.S., Reeves M.E., Xiao J., Lacy C., Lau E., Cao H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Trans. Med. 2020;18(1):322. doi: 10.1186/s12967-020-02488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aparna P., Muthathal S., Nongkynrih B., Gupta S.K. Vitamin D deficiency in India. J. Family Med. Prim. Care. 2018;7(2):324–330. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole T.J. Too many digits: the presentation of numerical data. Arch. Dis. Child. 2015;100(7):608–609. doi: 10.1136/archdischild-2014-307149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Method. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., Jani B.D., Welsh P., Mair F.S., Gray S.R., O'Donnell C.A., Gill J.M., Sattar N., Pell J.P. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metabolic Syndrome. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S., Kaur R., Singh R.K. Revisiting the role of vitamin D levels in the prevention of COVID-19 infection and mortality in European countries post infections peak. Aging Clin. Exp. Res. 2020:1–4. doi: 10.1007/s40520-020-01619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittemore P.B. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang X., Li S., Yu H., Wang P., Zhang Y., Chen Z., Li Y., Cheng L., Li W., Jia H., Ma X. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams S., Malatesta K., Norris K. Vitamin D and chronic kidney disease. Ethn. Dis. 2009;19(4 Suppl 5):S5–S11. [PMC free article] [PubMed] [Google Scholar]
- 25.García de Tena J., El Hachem Debek A., Hernández Gutiérrez C., Izquierdo Alonso J.L. The role of vitamin D in chronic obstructive pulmonary disease, asthma and other respiratory diseases. Arch. Bronconeumol. 2014;50(5):179–184. doi: 10.1016/j.arbres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Kienreich K., Grubler M., Tomaschitz A., Schmid J., Verheyen N., Rutters F., Dekker J.M., Pilz S. Vitamin D, arterial hypertension & cerebrovascular disease. Indian J. Med. Res. 2013;137(4):669–679. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K., Benjamin E.J., D’Agostino R.B., Wolf M., Vasan R.S. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M.E. Castillo, L.M. Entrenas Costa, J.M. Vaquero Barrios, J.F. Alcalá Díaz, J.L. Miranda, R. Bouillon, J.M. Quesada Gomez, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol. (2020) 105751. [DOI] [PMC free article] [PubMed]
- 29.Klingberg E., Oleröd G., Konar J., Petzold M., Hammarsten O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49(3):800–808. doi: 10.1007/s12020-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M. Seasonal, ethnic and gender variations in serum vitamin D3 levels in the local population of Peterborough. Biosci. Horizons: Int. J. Student Res. 2010;3(2):124–131. [Google Scholar]
- 31.Speeckaert M.M., Delanghe J.R. Association between low vitamin D and COVID-19: don't forget the vitamin D binding protein. Aging Clin. Exp. Res. 2020;32(7):1207–1208. doi: 10.1007/s40520-020-01607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harinarayan C.V., Ramalakshmi T., Prasad U.V., Sudhakar D., Srinivasarao P.V., Sarma K.V., Kumar E.G. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am. J. Clin. Nutrit. 2007;85(4):1062–1067. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 33.Harinarayan C.V., Gupta N., Kochupillai N. Vitamin D status in primary hyperparathyroidism in India. Clin. Endocrinol. 1995;43(3):351–358. doi: 10.1111/j.1365-2265.1995.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 34.Harinarayan C.V., Sachan A., Reddy P.A., Satish K.M., Prasad U.V., Srivani P. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: a cross-sectional study from south India. J. Assoc. Phys. India. 2011;59:698–704. [PubMed] [Google Scholar]
- 35.Harinarayan C.V., Ramalakshmi T., Venkataprasad U. High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pacific J. Clin. Nutrit. 2004;13(4):359–364. [PubMed] [Google Scholar]
- 36.Nagarjunakonda S., Amalakanti S., Uppala V., Rajanala L., Athina S. Vitamin D in epilepsy: vitamin D levels in epilepsy patients, patients on antiepileptic drug polytherapy and drug-resistant epilepsy sufferers. Eur. J. Clin. Nutr. 2016;70(1):140–142. doi: 10.1038/ejcn.2015.127. [DOI] [PubMed] [Google Scholar]
- 37.Barman M., Mattack N. A Study Of Serum Vitamin D In Psoriatic Patients In A Tertiary Care Hospital Of Assam. Paripex Indian J. Res. 2019;8(9) [Google Scholar]
- 38.Dasgupta A., Saikia U., Sarma D. Status of 25(OH)D levels in pregnancy: A study from the North Eastern part of India. Indian J. Endocrinol. Metab. 2012;16(Suppl 2):S405–S407. doi: 10.4103/2230-8210.104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D. J, P. D, M. N, J.E. T, A Study on the Biological Reference Interval of Vitamin D in North-East India, Nat. J. Lab. Med. 8(2) (2019) BO01-BO04.
- 40.Usmani F., Poonam M., Sinha S., Haque R. Kumari. Evaluation of Calcium, Phosphorus and Vitamin D Level in Different Stages of Pregnancy in East Indian Population. Int. J. Dental Med. Specialty. 2016;3:16. [Google Scholar]
- 41.Aggarwal A., Yadav A.K., Ramachandran R., Kumar V., Kumar V., Sachdeva N., Khandelwal N., Jha V. Bioavailable vitamin D levels are reduced and correlate with bone mineral density and markers of mineral metabolism in adults with nephrotic syndrome. Nephrology. 2016;21(6):483–489. doi: 10.1111/nep.12638. [DOI] [PubMed] [Google Scholar]
- 42.Z. Jabbar, P.K. Aggarwal, N. Chandel, H.S. Kohli, K.L. Gupta, V. Sakhuja, V. Jha, High prevalence of vitamin D deficiency in north Indian adults is exacerbated in those with chronic kidney disease 14(3) (2009) 345-349. [DOI] [PubMed]
- 43.M. Daroach, T. Narang, U.N. Saikia, N. Sachdeva, M. Sendhil Kumaran, Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: a clinical paradigm 57(2) (2018) 217-222. [DOI] [PubMed]
- 44.Gupta A., Prabhakar S., Modi M., Bhadada S.K., Lal V., Khurana D. Vitamin D status and risk of ischemic stroke in North Indian patients. Indian J. Endocrinol Metab. 2014;18(5):721–725. doi: 10.4103/2230-8210.139241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramakrishnan S., Bhansali A., Bhadada S.K., Sharma R., Walia R., Ravikiran M., Shanmugasundar G., Ravikumar P. Vitamin D status and its seasonal variability in healthy young adults in an Asian Indian urban population. Endocrine Practice: Off. J. Am. College Endocrinol. Am. Assoc. Clin. Endocrinol. 2011;17(2):185–191. doi: 10.4158/EP10155.OR. [DOI] [PubMed] [Google Scholar]
- 46.Singla R., Gurung P., Aggarwal N., Dutta U., Kochhar R. Relationship between preeclampsia and vitamin D deficiency: a case control study. Arch. Gynecol. Obstet. 2015;291(6):1247–1251. doi: 10.1007/s00404-014-3550-8. [DOI] [PubMed] [Google Scholar]
- 47.Himani R., Kumar J.A., Ansari A.A., Mahdi D., Sharma B., Karunanand S.K. Datta, Blood Lead Levels in Occupationally Exposed Workers Involved in Battery Factories of Delhi-NCR Region: Effect on Vitamin D and Calcium Metabolism. Indian J. Clin. Biochem. 2020;35(1):80–87. doi: 10.1007/s12291-018-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda S., Tiwari A., Luthra K., Sharma S.K., Singh A. Status of vitamin D and the associated host factors in pulmonary tuberculosis patients and their household contacts: A cross sectional study. J. Steroid Biochem. Mol. Biol. 2019;193 doi: 10.1016/j.jsbmb.2019.105419. [DOI] [PubMed] [Google Scholar]
- 49.Marwaha R.K., Tandon N., Shivaprasad C., Kanwar R., Mani K., Aggarwal R., Bhadra K., Singh S., Sharma B., Tripathi R.P. Peak bone mineral density of physically active healthy Indian men with adequate nutrition and no known current constraints to bone mineralization. J. Clin. Densitometry: Off. J. Int. Soc. Clin. Densitometry. 2009;12(3):314–321. doi: 10.1016/j.jocd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Garg D., Patidar R., Dhamija R.K. Letter by Garg et al Regarding Article, “Vitamin D Status and Risk of Stroke: The Rotterdam Study”. Stroke. 2019;50(12) doi: 10.1161/STROKEAHA.119.027523. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt S.P., Misra A., Sharma M., Guleria R., Pandey R.M., Luthra K., Vikram N.K. Vitamin D insufficiency is associated with abdominal obesity in urban Asian Indians without diabetes in North India. Diabetes Technol. Ther. 2014;16(6):392–397. doi: 10.1089/dia.2013.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vupputuri M.R., Goswami R., Gupta N., Ray D., Tandon N., Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am. J. Clin. Nutrit. 2006;83(6):1411–1419. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]
- 53.Goswami R., Gupta N., Goswami D., Marwaha R.K., Tandon N., Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am. J. Clin. Nutrit. 2000;72(2):472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 54.Goswami R., Kochupillai N., Gupta N., Goswami D., Singh N., Dudha A. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J. Assoc. Phys. India. 2008;56:755–757. [PubMed] [Google Scholar]
- 55.Tandon N., Marwaha R.K., Kalra S., Gupta N., Dudha A., Kochupillai N. Bone mineral parameters in healthy young Indian adults with optimal vitamin D availability. Nat. Med. J. India. 2003;16(6):298–302. [PubMed] [Google Scholar]
- 56.Agarwal N., Mithal A., Dhingra V., Kaur P., Godbole M.M., Shukla M. Effect of two different doses of oral cholecalciferol supplementation on serum 25-hydroxy-vitamin D levels in healthy Indian postmenopausal women: A randomized controlled trial. Indian J. Endocrinol. Metab. 2013;17(5):883–889. doi: 10.4103/2230-8210.117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal N., Mithal A., Kaur P., Dhingra V., Godbole M.M., Shukla M. Vitamin D and insulin resistance in postmenopausal Indian women. Indian J. Endocrinol. Metab. 2014;18(1):89–93. doi: 10.4103/2230-8210.126583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagpal J., Pande J.N., Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabetic Med.: J. Brit. Diabetic Assoc. 2009;26(1):19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 59.Goswami R., Marwaha R.K., Gupta N., Tandon N., Sreenivas V., Tomar N., Ray D., Kanwar R., Agarwal R. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Brit. J. Nutrit. 2009;102(3):382–386. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 60.Chakravarti A., Bharara T., Kapoor N., Ashraf A. Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi. Trop. Med. Infect. Dis. 2020;5(2):72. doi: 10.3390/tropicalmed5020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rathored J., Sharma S.K., Singh B., Banavaliker J.N., Sreenivas V., Srivastava A.K., Mohan A., Sachan A., Harinarayan C.V., Goswami R. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int. J. Tuberculosis Lung Disease: Off. J. Int. Union against Tuberculosis Lung Disease. 2012;16(11):1522–1528. doi: 10.5588/ijtld.12.0122. [DOI] [PubMed] [Google Scholar]
- 62.Malik S., Giri S., Madhu S.V., Rathi V., Banerjee B.D., Gupta N. Relationship of levels of Vitamin D with flow-mediated dilatation of brachial artery in patients of myocardial infarction and healthy control: A case-control study. Indian J. Endocrinol. Metab. 2016;20(5):684–689. doi: 10.4103/2230-8210.190558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agrawal P., Mehta S., Verma N. Level of Vitamin D in hypothyroid subjects: a study on the suburban population of North-West Delhi. Int. J. Clin. Biochem. Res. 2020;7:116–119. [Google Scholar]
- 64.Dhanwal D.K., Sahoo S., Gautam V.K., Saha R. Hip fracture patients in India have vitamin D deficiency and secondary hyperparathyroidism. Osteoporosis Int.: J. Established Result Cooperation Between Europ. Found. Osteoporosis National Osteoporosis Found. USA. 2013;24(2):553–557. doi: 10.1007/s00198-012-1993-y. [DOI] [PubMed] [Google Scholar]
- 65.Dharmshaktu P., Saha S., Kar P., Sreenivas V., Ramakrishnan L., Goswami R. Absence of vitamin D deficiency among common outdoor workers in Delhi. Clin. Endocrinol. 2019;91(2):356–362. doi: 10.1111/cen.14012. [DOI] [PubMed] [Google Scholar]
- 66.Roy A., Lakshmy R., Tarik M., Tandon N., Reddy K.S., Prabhakaran D. Independent association of severe vitamin D deficiency as a risk of acute myocardial infarction in Indians. Indian Heart J. 2015;67(1):27–32. doi: 10.1016/j.ihj.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh I., Lavania M., Pathak V.K., Ahuja M., Turankar R.P., Singh V., Sengupta U. VDR polymorphism, gene expression and vitamin D levels in leprosy patients from North Indian population. PLoS Negl.Trop. Dis. 2018;12(11) doi: 10.1371/journal.pntd.0006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.N. Tyagi, A. Mohanty, C. Kaur, A. Kabi, S. Kumari, Mamta, S. Sharma, S. Rani, D. Sahu, J. Prasad, C. Bhaskar, Kabi, A study of vitamin D levels in patients of coronary artery disease, World J. Pharm. Pharm. Sci. 8 (2019) 1179-1186.
- 69.Bawaskar P.H., Bawaskar H.S., Bawaskar P.H., Pakhare A.P. Profile of Vitamin D in patients attending at general hospital Mahad India. Indian J. Endocrinol. Metab. 2017;21(1):125–130. doi: 10.4103/2230-8210.196004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaikh M.N., Malapati B.R., Gokani R., Patel B., Chatriwala M. Serum Magnesium and Vitamin D Levels as Indicators of Asthma Severity. Pulmonary Med. 2016;2016:1643717. doi: 10.1155/2016/1643717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prakash S., Rathore C., Makwana P., Dave A., Joshi H., Parekh H. Vitamin D Deficiency in Patients With Chronic Tension-Type Headache: A Case-Control Study. Headache. 2017;57(7):1096–1108. doi: 10.1111/head.13096. [DOI] [PubMed] [Google Scholar]
- 72.S. Kalra, B. Kalra, S. Khandelwal, Vitamin D deficiency in Healthy Postmenopausal Women in Haryana, 2011.
- 73.Sehrawat M., Arora T.C., Chauhan A., Kar H.K., Poonia A., Jairath V. Correlation of Vitamin D Levels with Pigmentation in Vitiligo Patients Treated with NBUVB Therapy. ISRN Dermatol. 2014;2014 doi: 10.1155/2014/493213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shukla K., Sharma S., Gupta A., Raizada A., Vinayak K. Current Scenario of Prevalence of Vitamin D Deficiency in Ostensibly Healthy Indian Population: A Hospital Based Retrospective Study. Indian J. Clin. Biochem.: IJCB. 2016;31(4):452–457. doi: 10.1007/s12291-016-0552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma N., Sharma B., Singh G., Gupta A., Sharma R., Kapil U. Vitamin D Status in Cold Trans-Himalayan Deserts at Altitude of 4000 meter and above in India. Indian J. Community Health. 2018;30(4):400–402. [Google Scholar]
- 76.Laway B.A., Kotwal S.K., Shah Z.A. Pattern of 25 hydroxy vitamin D status in North Indian people with newly detected type 2 diabetes: A prospective case control study. Indian J. Endocrinol. Metab. 2014;18(5):726–730. doi: 10.4103/2230-8210.139242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rehman F., Dogra N., Wani M.A. Serum Vitamin D Levels and Alopecia Areata- A Hospital Based Case-Control Study from North-India. Int J Trichology. 2019;11(2):49–57. doi: 10.4103/ijt.ijt_3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zargar A.H., Ahmad S., Masoodi S.R., Wani A.I., Bashir M.I., Laway B.A., Shah Z.A. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad. Med. J. 2007;83(985):713–716. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tandon V.R., Sharma S., Mahajan S., Raina K., Mahajan A., Khajuria V., Gillani Z. Prevalence of vitamin d deficiency among Indian menopausal women and its correlation with diabetes: A first Indian cross sectional data. J. Mid-Life Health. 2014;5(3):121–125. doi: 10.4103/0976-7800.141188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Din I., Majid S., Rashid F., Hussain I., Koul R.K., Qadir J., Farooq R. Combinatorial effect of leptin, tumor necrosis factor-Αlpha, and vitamin d in progression of type 2 diabetes in kashmiri population. Asian J. Pharm. Clin. Res. 2018;11:477–481. [Google Scholar]
- 81.Singh V., Barik A., Imam N. Vitamin D(3) Level in Women with Uterine Fibroid: An Observational Study in Eastern Indian Population. J. Obstetrics Gynaecol. India. 2019;69(2):161–165. doi: 10.1007/s13224-018-1195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Majumdar V., Prabhakar P., Kulkarni G.B., Christopher R. Vitamin D status, hypertension and ischemic stroke: a clinical perspective. J. Hum. Hypertens. 2015;29(11):669–674. doi: 10.1038/jhh.2015.10. [DOI] [PubMed] [Google Scholar]
- 83.Nayak K., Garg A., Mithra P., Manjrekar P. Serum Vitamin D(3) Levels and Diffuse Hair Fall among the Student Population in South India: A Case-Control Study. Int. J. Trichol. 2016;8(4):160–164. doi: 10.4103/ijt.ijt_57_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph R., Nagrale A.V., Joseraj M.G., Pradeep Kumar K.M., Kaziyarakath J.A., Chandini R. Low levels of serum Vitamin D in chronic periodontitis patients with type 2 diabetes mellitus: A hospital-based cross-sectional clinical study. J. Indian Soc. Periodontol. 2015;19(5):501–506. doi: 10.4103/0972-124X.167162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sasidharan P.K., Rajeev E., Vijayakumari V. Tuberculosis and vitamin D deficiency. J. Assoc. Physicians India. 2002;50:554–558. [PubMed] [Google Scholar]
- 86.V. G, S. PK, Vitamin D Status In Hypertension, Am. Int. J. Res. Formal, Appl. Nat. Sci. 8(1) (2014) 28-30.
- 87.Angel Abraham, Sajitha Krishnan, Kuzhikandathil Narayanan Subhakumari, G.G. Chakkalakkudy, Vitamin D Levels In Depressive Disorder, 3(6) (2019) 862-865.
- 88.S. Samuel, Association of Vitamin D deficiency and Hypothyroidism in a tertiary teaching hospital in Kerala, J. Med. Sci. Clin. Res. 7 (2019).
- 89.Jain V, Shaikh MKS, J. S, M. M, Comparative study of serum vitamin d levels and other biomarkers in patients attending tertiary cardiac care center, Int. J. Bioassays 4(4) (2015) 3812-3814.
- 90.V. Patwardhan, Z. Mughal, S. Chiplonkar, A. Webb, R. Kift, V. Khadilkar, R. Padidela, A. Khadilkar, Duration of casual sunlight exposure necessary for adequate Vitamin D status in Indian Men 22(2) (2018) 249-255. [DOI] [PMC free article] [PubMed]
- 91.Muthukrishnan J., Dhruv G. Vitamin D status and gestational diabetes mellitus. Indian J. Endocrinol. Metab. 2015;19(5):616–619. doi: 10.4103/2230-8210.163175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garg M.K., Marwaha R.K., Tandon N., Bhadra K., Mahalle N. Relationship of lipid parameters with bone mineral density in Indian population. Indian J. Endocrinol. Metab. 2014;18(3):325–332. doi: 10.4103/2230-8210.131165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kadam N., Chiplonkar S., Khadilkar A., Divate U., Khadilkar V. Low bone mass in urban Indian women above 40 years of age: prevalence and risk factors, Gynecological endocrinology: the official journal of the International Society of. Gynecol. Endocrinol. 2010;26(12):909–917. doi: 10.3109/09513590.2010.487604. [DOI] [PubMed] [Google Scholar]
- 94.Multani S.K., Sarathi V., Shivane V., Bandgar T.R., Menon P.S., Shah N.S. Study of bone mineral density in resident doctors working at a teaching hospital. J. Postgrad. Med. 2010;56(2):65–70. doi: 10.4103/0022-3859.65272. [DOI] [PubMed] [Google Scholar]
- 95.Shivane V.K., Sarathi V., Lila A.R., Bandgar T., Joshi S.R., Menon P.S., Shah N.S. Peak bone mineral density and its determinants in an Asian Indian population. J. Clin. Densitometry: Off. J. Int. Soc. Clin. Densitometry. 2012;15(2):152–158. doi: 10.1016/j.jocd.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Sahasrabuddhe A., Pitale S., Gupta M., Chari S., Sagdeo M. Study of vitamin D levels and its correlation with insulin resistance. Nat. J. Physiol., Pharm. Pharmacol. 2017;7:1. [Google Scholar]
- 97.Vedak T.K., Ganwir V., Shah A.B., Pinto C., Lele V.R., Subramanyam A., Shah H., Deo S.S. Vitamin D as a marker of cognitive decline in elderly Indian population. Ann. Indian Acad. Neurol. 2015;18(3):314–319. doi: 10.4103/0972-2327.160052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.S.K. Shylla, G. Devi, C. Wann, B. Khongwir, G. Longmei, Vitamin D Deficiency : Highly Prevalent Among Apparently Healthy Female Adolescents In Both Urban And Rural Population Of Manipur, India, 2018.
- 99.Dutta C., Kakati S., Barman B., Bora K. Vitamin D status and its relationship with systemic lupus erythematosus as a determinant and outcome of disease activity. Hormone Mol. Biol. Clin. Invest. 2019;38(3) doi: 10.1515/hmbci-2018-0064. [DOI] [PubMed] [Google Scholar]
- 100.Mangaraj S., Choudhury A.K., Swain B.M., Sarangi P.K., Mohanty B.K., Baliarsinha A.K. Evaluation of Vitamin D Status and its Impact on Thyroid Related Parameters in New Onset Graves' Disease- A Cross-sectional Observational Study. Indian J. Endocrinol. Metab. 2019;23(1):35–39. doi: 10.4103/ijem.IJEM_183_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.R. Padhi, B. Panda, S. Jagati, S. Patra, Vitamin D status in adult critically ill patients in Eastern India: An observational retrospective study, 31(3) (2014) 212-216. [DOI] [PMC free article] [PubMed]
- 102.Mukherjee B., Patra S., Sibasish S. Evaluation of Vitamin D Status in Urban Population Employed In Office Jobs. IOSR J. Dental Med. Sci. 2015;14:18–20. [Google Scholar]
- 103.Rath D., Nanda R., Mishra P.K., Patra P. Evaluation of Serum 25 Hydroxy Vitamin D level in acute myocardial infarction patients in a tertiary care hospital. Asian J. Med. Sci. 2016;7:11. [Google Scholar]
- 104.Mukherjee B., Patra S. Prevalence of vitamin D deficiency in type-2 diabetes mellitus patients and its correlation with glycemic control. Int. J. Bioassays. 2014;03:3313–3317. [Google Scholar]
- 105.Akshay Kumar, Nanda SK, Bharathy N, Ravichandran K, Dinakaran A, R. L, Evaluation of vitamin D status and its correlation with glycated haemoglobin in type 2 diabetes mellitus, Biomed. Res. 28(1) (2017).
- 106.Chandrashekar L., Kumari G.R.K., Rajappa M., Revathy G., Munisamy M., Thappa D.M. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br. J. Biomed. Sci. 2015;72(2):56–60. doi: 10.1080/09674845.2015.11666797. [DOI] [PubMed] [Google Scholar]
- 107.Karthikayan A., Sureshkumar S., Kadambari D., Vijayakumar C. Low serum 25-hydroxy vitamin D levels are associated with aggressive breast cancer variants and poor prognostic factors in patients with breast carcinoma. Arch. Endocrinol. Metabolism. 2018;62(4):452–459. doi: 10.20945/2359-3997000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gandhe M., Velu K., Shyamini S., Saha S., Ramesh R., Murugaiyan S. Circulating 25-hydroxyvitamin D status in apparently healthy adolescents and its association with body mass index in Puducherry population. Indian J. Child Health. 2017;110 [Google Scholar]
- 109.Gade V.K.V., Mony A., Munisamy M., Chandrashekar L., Rajappa M. An investigation of vitamin D status in alopecia areata. Clin. Exp. Med. 2018;18(4):577–584. doi: 10.1007/s10238-018-0511-8. [DOI] [PubMed] [Google Scholar]
- 110.B. Chopra, S. Singh, K. Singh, Is there A Need to Reasses Reference Levels of Vitamin D for India ?-A Preliminary Survey of Vitamin D Levels in the Normal Population of Punjab, 2015.
- 111.Gupta A, Soin D, Garg R, G. G., Prevalence of vitamin d levels in patients of diabetes mallitus in rural and urban population of Malwa region of Punjab., J. Adv. Med. Dent. Sci. Res. 5(2) (2017) 111-115.
- 112.J. S, S.J. C, S. M, Association of Deficiency of Maternal Vitamin D Levels with Severity of Preeclampsia, Epidem. Int. 4(3) (2019) 2455-7048.
- 113.D.M. N, P. U, Role of vitamin d and risk of prostate cancer, Int. J. Sci. Res. 8(8) (2019) 76-77.
- 114.P.A. Kiran B., Thilagavathi R., J.R. R., Serum 25- Hydroxy Vitamin D, Calcium,Phosphorus and Alkaline Phosphatase Levels In Healthy Adults Above the age of 20 Living in Potheri Village of Kancheepuram District, Tamilnadu, J. App. Pharm. Sci. 4 (12) (2014) 030-034.
- 115.Shetty S., Kapoor N., Naik D., Asha H.S., Prabu S., Thomas N., Seshadri M.S., Paul T.V. Osteoporosis in healthy South Indian males and the influence of life style factors and vitamin d status on bone mineral density. J. Osteoporosis. 2014;2014 doi: 10.1155/2014/723238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paul T.V., Thomas N., Seshadri M.S., Oommen R., Jose A., Mahendri N.V. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: relationship to calcium nutrition and vitamin D status. Endocrine Practice: Off. J. Am. College Endocrinol. Am. Assoc. Clin. Endocrinol. 2008;14(6):665–671. doi: 10.4158/EP.14.6.665. [DOI] [PubMed] [Google Scholar]
- 117.Bachali S., Dasu K., Ramalingam K., Naidu J.N. Vitamin d deficiency and insulin resistance in normal and type 2 diabetes subjects. Indian J. Clin. Biochem.: IJCB. 2013;28(1):74–78. doi: 10.1007/s12291-012-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.A.J. Joseph, Biju George, A.B. Pulimood, M.S. Seshadri, A. Chacko, 25 (OH) vitamin D level in Crohn's disease: association with sun exposure & disease activity, Indian J. Med. Res. (2009) 133-137. [PubMed]
- 119.N.K. S, N.K. M, Pruthvi, V. B, Correlation of Vitamin-D Levels With Blood Sugar Levels in Diabetes Mellitus, Galore Int. J. Health Sci. Res. 3(3) (2018) 8-13.
- 120.Amarendra Reddy G., Kulkarni B., Shatrugna V., Thilak Ravindra Reddy P., Nagalla B., Ajeya Kumar P., Usha Rani K. Bone mass of overweight affluent Indian youth and its sex-specific association with body composition. Arch. Osteoporosis. 2009;4(1–2):31–39. doi: 10.1007/s11657-009-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yasovanthi J., Venkata Karunakar K., Sri Manjari K., Pulla Reddy B., Ajeya Kumar P., Sesha Charyulu M., Aruna P., Narasimulu G., Jyothy A. Association of vitamin D receptor gene polymorphisms with BMD and their effect on 1, 25-dihydroxy vitamin D3 levels in pre- and postmenopausal South Indian women from Andhra Pradesh. Clin. Chim. Acta; Int. J. Clin. Chem. 2011;412(7–8):541–544. doi: 10.1016/j.cca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 122.Debbarma M., Dasgupta A., Biswas C. Study of Vitamin D levels in patients of acute myocardial infarction. J. Evidence Based Med. Healthcare. 2018;5:1788–1791. [Google Scholar]
- 123.Chakraborty A., Choudhury A., Saha A. Development of Non-alcoholic Fatty Liver Disease (NAFLD) in Young Obese Tribal Subjects of Tripura: Link between Low 25 (OH) Vitamin-D Levels and Immune Modulators. J. Assoc. Phys. India. 2019;67(8):52–56. [PubMed] [Google Scholar]
- 124.Sharma D., Saxena R., Saxena R., Sharma M., Lal A. Systemic inflammation and alteration in vitamin D levels in pregnancy induced hypertension. Asian J. Med. Sci. 2014;5 [Google Scholar]
- 125.Agrawal N.K., Sharma B. Prevalence of osteoporosis in otherwise healthy Indian males aged 50 years and above. Arch. Osteoporosis. 2013;8:116. doi: 10.1007/s11657-012-0116-x. [DOI] [PubMed] [Google Scholar]
- 126.Malhotra N., Mithal A., Gupta S., Shukla M., Godbole M. Effect of vitamin D supplementation on bone health parameters of healthy young Indian women. Arch. Osteoporosis. 2009;4(1–2):47–53. doi: 10.1007/s11657-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arya V., Bhambri R., Godbole M.M., Mithal A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporosis Int.: J. Established Result Cooperation Europ. Found. Osteoporosis National Osteoporosis Found. USA. 2004;15(1):56–61. doi: 10.1007/s00198-003-1491-3. [DOI] [PubMed] [Google Scholar]
- 128.Soam S., Singh T., Chaturvedi S., Sarkar G. A Study on Association of Degree of Physical Exercise and Plasma 25-(OH) Vitamin D Levels. Indian J. Med. Biochem. 2018;22:90–93. [Google Scholar]
- 129.Priyambada L., Bhatia V., Singh N., Bhatia E. Serum 25 hydroxyvitamin D profile after single large oral doses of cholecalciferol (vitamin D3) in medical staff in North India: a pilot study. J. Postgrad. Med. 2014;60(1):52–56. doi: 10.4103/0022-3859.128812. [DOI] [PubMed] [Google Scholar]
- 130.B. M, Gupta BK, N. O, C. N, G. A, Comparison of Calcium, Phosphorous and 25 (OH)D2 Levels in Sedentary and Labourer Females, Ann. Clin. Lab. Res. 7(1) (2019) 289.
- 131.Oberoi D., Mehrotra V., Rawat A. “Vitamin D” as a profile marker for cardiovascular diseases. Ann. Cardiac Anaesthesia. 2019;22(1):47–50. doi: 10.4103/aca.ACA_66_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pan T., Banerjee R., Dasgupta A., Paul B. Vitamin D status among women aged 40 years and above in a rural area of West Bengal: A community-based study. J. Family Med. Prim. Care. 2018;7(6):1263–1267. doi: 10.4103/jfmpc.jfmpc_130_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.S. Garg, A. Dasgupta, S. Maharana, B. Paul, L. Bandyopadhyay, A. Bhattacharya, Sun exposure and Vitamin D in rural India: A cross-sectional study, 62(3) (2018) 175-181. [DOI] [PubMed]
- 134.Baidya A., Chowdhury S., Mukhopadhyay S., Ghosh S. Profile of vitamin D in a cohort of physicians and diabetologists in Kolkata. Indian J. Endocrinol. Metab. 2012;16(Suppl 2):S416–S417. doi: 10.4103/2230-8210.104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dutta D., Maisnam I., Shrivastava A., Sinha A., Ghosh S., Mukhopadhyay P., Mukhopadhyay S., Chowdhury S. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J. Med. Res. 2013;138(6):853–860. [PMC free article] [PubMed] [Google Scholar]
- 136.Maisnam I., Dutta D., Mukhopadhyay S., Chowdhury S. Lean mass is the strongest predictor of bone mineral content in type-2 diabetes and normal individuals: an eastern India perspective. J. Diabetes Metabolic Disorders. 2014;13(1):90. doi: 10.1186/s40200-014-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lodh M., Goswami B., Mahajan R.D., Sen D., Jajodia N., Roy A. Assessment of Vitamin D status In Patients of Chronic Low Back Pain of Unknown Etiology. Indian J. Clin. Biochem.: IJCB. 2015;30(2):174–179. doi: 10.1007/s12291-014-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Basu S., Gupta R., Mitra M., Ghosh A. Prevalence of vitamin d deficiency in a pediatric hospital of eastern India. Indian J. Clin. Biochem.: IJCB. 2015;30(2):167–173. doi: 10.1007/s12291-014-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.