ABSTRACT
The adoption of a panel of Nutrient Reference Values (NRVs) in place of a single recommended intake allowed for assessment of nutritional adequacy and safe upper intake levels for nutrients on a population level and for individuals. The Average Requirement (AR) and Tolerable Upper Intake Level (UL) comprise 2 core NRVs needed to obtain accurate, comparable estimates of population-level nutrient intakes, which are necessary to plan and evaluate nutrition support programs globally. Harmonizing the derivation of NRVs, particularly the AR and UL, is essential to ensure inclusion of all countries, whether high-, middle-, or low-income, in the process and to improve access for all users to the tools and data needed to carry it out. The NRV process today is more rigorous and transparent than the first derivation of DRIs because of adoption of systematic reviews and bias assessment methodologies, updated food and nutrient databases, data on cultural and context-specific dietary patterns, and better metabolic markers of nutritional status. A proposed framework for the derivation of NRVs builds on available methodologies to support the NRV process; however, this is not sufficient to achieve harmonization of the process. Fundamental to moving forward toward harmonization is removing existing barriers, including limited access to resources and databases and variance in terminology used to identify specific NRVs; adoption of more rigorous and transparent methodologies, including chronic disease endpoints, in the review process; and creating a central repository for easily accessible evidence. Chief among the barriers to harmonization is a willingness of global bodies to support an agreed-upon approach to the derivation process. Improving access to tools and data resources and providing guidance and support to encourage their adoption are critical to achieving harmonization of the NRV process.
Keywords: harmonization, Nutrient Reference Values, Dietary Reference Intakes, Average Requirement, Tolerable Upper Intake Level
Introduction
Among the United States and Canada, the United Kingdom, and European Union countries, the adoption of a panel of Nutrient Reference Values (NRVs) in place of a single Recommended Intake (RI) or RDA occurred over the last 2.5 decades (1–4). Four basic values comprise the panel of NRVs—the Average Requirement (AR), the RI or RDA, the Adequate Intake (AI), and the Tolerable Upper Intake Level (UL), as defined in Table 1 (5). While many high-income countries around the world have adopted the NRV framework including the methodological approach to the derivation of the 2 core NRVs, the AR and the UL that are needed to assess the nutritional adequacy and safety of nutrient intakes by population groups, that is not the case for many countries, including low- and middle-income countries (LMICs), as well as global organizations such as the WHO and the FAO. The ability to carry out fully the process of deriving the AR and UL is often constrained by a lack of resources and access to data, particularly for conducting systematic reviews. Earlier iterations of the RDAs were not based on the concept of deriving an AR as a benchmark for setting an RDA as the AR plus 2 SDs and were not intended for planning and assessment of population groups. The RI or RDA is often used, however, even though it is not an appropriate benchmark to assess the prevalence of inadequate nutrient intakes for population groups (6). Because of the lack of consistency in the derivation and application of the AR and UL, particularly among LMICs, it is not possible to obtain accurate, comparable estimates of population-level nutrient gaps or excessive intakes, which are needed to plan and evaluate food-fortification and other nutrition-support programs globally (7).
TABLE 1.
Definitions of basic Nutrient Reference Values1
| Nutrient Reference Value | Definition |
|---|---|
| Estimated Average Requirement/Average Requirement (EAR/AR) | The average daily nutrient intake level that is estimated to meet the requirements of half of the healthy individuals in a particular life stage and gender group. |
| Recommended Dietary Allowance/Recommended Intake (RDA/RI) | The average daily dietary nutrient intake level that is sufficient to meet the nutrient requirements of nearly all (97–98%) healthy individuals in a particular life stage and gender group. |
| Adequate Intake (AI) | The recommended average daily intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate; used when an RDA cannot be determined. |
| Tolerable Upper Intake Level (UL) | The highest average daily nutrient intake level that is likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects may increase. |
1Adapted from reference 5 with permission.
It is therefore important to define a way for harmonizing, on a global scale, the methodological derivation of the core NRVs (i.e., the AR and the UL) in order to facilitate inclusion, particularly of LMICs, in the NRV process, as well as to improve access for all users to tools and data needed to carry out the process. Harmonizing the methodologies used to derive NRVs can benefit countries globally regardless of their economic status. As noted by King and Garza (8), important outcomes of harmonization include the following:
Improving the objectivity and transparency of NRVs that are derived in different contexts;
Providing a common basis for nutrient review panels to use throughout the NRV process;
Opening access to resources needed by LMICs to adapt existing NRVs to their population's specific requirements; and
Providing a common basis for establishing nutrition and health policies, such as feeding programs, food fortification, and population-level dietary guidance as well as for regulatory and trade purposes.
Status of Knowledge
A number of independent activities and reports have been produced over more than a decade, which, when considered collectively, provide strong justification for moving forward with harmonizing the methodologies for deriving NRVs, and in particular the AR and UL. The impetus for harmonization grew out of a convening held in Florence, Italy, in 2005, sponsored by the United Nations University's Food and Nutrition Program in collaboration with the FAO, the WHO, and UNICEF. The product was 10 commissioned reviews published collectively under the title International Harmonization of Approaches for Developing Nutrient-Based Dietary Standards (8). An important outcome of the convening was development of a Nutrient Intake Values (NIV) framework that conceptually defined the criteria needed for intake recommendations; defined the required values of the average nutrient requirement, individual nutrient level, and upper nutrient level; and identified methods and applications for assessing the adequacy of nutrient intakes in both individuals and populations (8).
The 2005 convening in Florence laid the groundwork for 2 subsequent activities of the National Academies of Sciences, Engineering, and Medicine (NASEM) to advance the effort toward achieving harmonization. The first NASEM activity was a workshop that explored the state of the evidence for achieving global harmonization of approaches to deriving NRVs (9). The range of issues addressed in the workshop included potential frameworks to enable methodological harmonization; approaches for evaluating evidence to facilitate harmonization; contextual factors across population groups, regions, or countries that could challenge harmonization of methodologies; ways to facilitate global sharing of resources; and identifying advantages, barriers, and challenges to achieving harmonization. Three strategic messages emerged at the close of the workshop. First, develop a standardized approach to harmonization, including nutrition-specific research tools, and develop a standardized terminology. Second, recognize that there may be differences in nutrient intake recommendations across countries and regions related to food composition, dietary surveys, nutrient bioavailability, and health status. Last, work to fill knowledge gaps and improve the evidence base for deriving nutrient requirements globally.
The workshop was followed by a consensus study that considered the implications of harmonizing NRV methodologies for 2 specific populations: young children from birth up to 5 y of age and women of reproductive age (10).
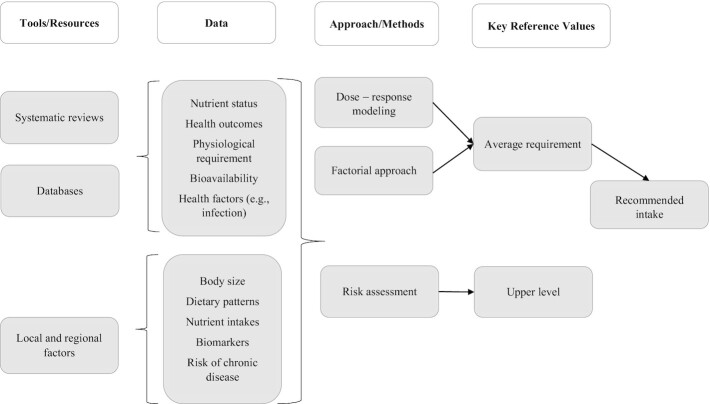
Overall, the NRV process today is more rigorous and transparent compared with the first derivation of the DRIs (11). For example, systematic reviews, updated food and nutrient databases, data on cultural and context-specific food choices and dietary patterns, bias assessment methodologies, and metabolic markers of nutritional status are now used routinely to support the process. Building on the work of the Florence meeting (8), the NASEM consensus committee re-envisioned the harmonization framework to take into account changes in the NRV process that have occurred since 2007. Figure 1 shows the proposed new NRV framework indicating the steps and resources needed to determine the AR and UL as the key or core reference values (10). This framework builds on the strengths and weaknesses in currently available methodologies used to support the NRV process. As described in the consensus committee's report (10), the framework entails 4 major steps:
FIGURE 1.
Risk-assessment framework. Reproduced from reference 9 with permission.
Choose the appropriate tools and data resources;
Collect data from those tools;
Identify the best approach for the nutrient under consideration; and
Derive the 2 key or core reference values, the AR and the UL.
While the steps illustrated in the framework are necessary, they are not sufficient to achieve harmonization of the NRV process. Among additional factors that need to be considered is the managing of uncertainties in the systematic review step of the nutrient review process in order to maintain the credibility of the overall process and its outcome. Uncertainties that can have an impact on the strength of a conclusion or recommendation include limitations in the study design, inconsistency in reported results, indirect evidence, imprecision, and reporting bias. The application of the “Grading of Recommendations, Assessment, Development, and Evaluation” (GRADE) system as a methodology for rating uncertainties has contributed greatly to understanding the overall certainty of evidence in evaluating critical outcomes in systematic reviews (12). Although GRADE is an important contribution to the NRV process, it does not supplant other components in the process, including consideration of the total range of evidence and interpretation of the evidence in consideration of the expertise represented on the nutrient review panel.
The consensus committee also identified core components of the NRV process that it considered critical to supporting harmonization. First is that NRVs must be updated on a regular basis. This is particularly important for applications such as defining the nutrient profile of a population group, and for generating data to support food and supplement programs. The process must also be transparent in selecting the nutrient review panel, include a robust systematic evidence review and public access to evidence used in the review, and it must exercise careful quality assessment throughout. Further, the methodologies used in the nutrient review step must be consistent, rigorous, and relevant to the context in which they are applied. Each step in the NRV process must be documented, including the methodologies used and the assumptions supporting them. Any limitations or uncertainties in the data and methods must be assessed and documented. Finally, the process must be complete and efficient, and able to facilitate access to existing NRVs, particularly for LMICs. The primary goal of including these core principles in the NRV process is to enhance the feasibility of harmonizing NRVs across diverse population groups. While these principles do not in themselves guarantee a harmonized NRV process, they do provide a foundation for moving toward harmonization of the process globally.
To illustrate, the NASEM consensus committee used a case-study approach to evaluate the application of the core components in the NRV process to exemplar nutrients of importance to women of childbearing age, infants, and young children (10). Zinc was selected as 1 exemplar nutrient because of the widespread vulnerability among both women and young children in LMICs to zinc deficiency. A number of factors were identified that contribute to the risk of zinc deficiency, and thus influence the zinc requirement for these population groups. Chief among them is the low bioavailability that results from diets high in phytate that binds zinc and reduces its absorption. Another important factor influencing zinc requirements is the presence of infection. Diarrheal infections, in particular, reduce zinc absorption, which contributes further to impaired immune function (13, 14).
The NASEM committee reviewed methodological approaches commonly used in deriving NRVs (10). For zinc, the committee identified the factorial approach (10) as the only feasible method, based on the absence of a zinc biomarker or a health outcome responsive to dietary variability. However, the committee also recognized the limitations in this approach and recommended further research to identify a reliable biomarker, research into genetic polymorphisms that may influence requirements, and development of comprehensive models to study inhibitors of zinc absorption (10).
An important outcome of the NASEM committee's work was a recommendation supporting an option (particularly for LMICs) to evaluate whether to utilize existing NRVs, based on their evaluation of the feasibility of keeping and updating, or adapting them to the specific populations’ needs. The pathways for accepting and revising or deriving new NRVs are illustrated in Figure 2. Having the ability to utilize or adapt existing NRVs provides a pathway to develop food and nutrition policies; evaluate nutrition intervention programs, including fortification, nutrition education, and monitoring; and assess the nutritional adequacy of population groups. Key to achieving these goals is that users understand the NRV process, particularly the derivation of the AR and UL as well as their application to public health.
FIGURE 2.
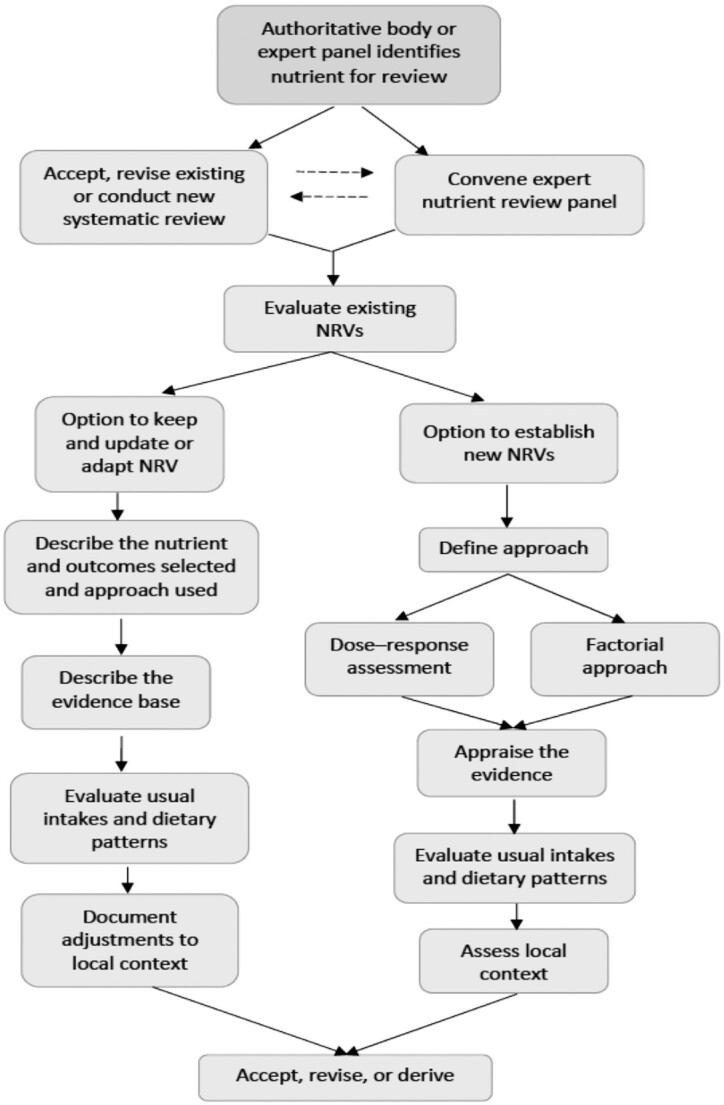
Flow diagram for deriving NRVs. NRV, Nutrient Reference Value. Reproduced from reference 10 with permission.
Subsequent to the 2 NASEM activities, Allen et al. (7) proposed an interim approach to harmonizing published AR and UL values for global application. While the interim derivation of harmonized NRVs (H-NRVs) utilizes assumptions about existing values, the proposed approach is consistent with the framework proposed by the NASEM committee in its report (10). The rationale for harmonizing these published reference values is that baseline physiologic requirements vary little across populations and, at the same time, there is always a level of uncertainty inherent in the NRV process.
In their perspective, Allen et al. (7) included corrections for factors affecting physiologic requirements, notably the bioavailability of some nutrients. Other contextual factors noted in the global harmonization workshop that could change physiological processes in a way that affects intake requirements include an array of genetic and epigenetic modifiers (e.g., single nucleotide polymorphisms and copy number variants). Additional potential modifiers include the microbiome, nutrient–nutrient or nutrient–food matrix interactions (e.g., phytate-binding zinc), and some pharmaceutical and toxin interactions (9). Allen et al. (7) noted, however, that requirements for absorbed nutrients would likely not differ greatly on a regional or global scale. Therefore, uniform and consistent core reference values could be applied across population groups. The authors’ primary objective in harmonizing the core values is to provide a uniform baseline for determining the prevalence of nutrient inadequacy or excessive intakes across population groups and to be able to apply that information to support food-fortification and other nutrition programs, particularly across LMICs globally. Additionally, because data were combined from >1 published set of recommendations, the harmonized reference values are more complete (7).
Harmonization of the methodology for deriving NRVs and adopting the goal of consistency in setting the AR and UL as core NRVs does not obviate the need for individual countries or regions to establish recommended intakes based on population needs. For example, local or regional expertise will still be needed to determine whether the harmonized values can be accepted as is or would need modification. Having harmonized core NRVs as a starting point could facilitate that discussion. Additionally, the process could provide a focus and scientific platform for discussing a country's or region's specific nutritional requirements. Finally, for those countries or regions that do not have extensive resources, an H-NRV process can contribute to the sharing of nutrition knowledge.
Fundamental to moving forward toward the goal of harmonization is removing existing barriers. These include limited access, particularly for LMICs, to resources such as databases and systematic reviews, as well as consistency in how these and other tools are utilized in the NRV process. Another barrier is the varied terminology used by different authoritative bodies to identify a specific NRV. Developing a common syntax for terms used in an H-NRV process is likely among the most difficult challenges to unifying the process. Although the method to establish an RDA, for example, may be consistent, the resulting values are given several different names. Establishing a common set of terms and definitions to be used by all countries and regions establishing NRVs, even though the absolute values will probably differ between various countries and regions, is nevertheless a crucial step toward achieving the goal of harmonization. Within the NRV process, additional challenges include assuring greater transparency, particularly in the systematic review process, in order to reduce uncertainties; identifying and developing standardized endpoints, including chronic disease endpoints, in the nutrient review process; and creating a central, easily accessible repository for evidence (9).
Perhaps the greatest barrier to harmonization is the willingness of global authoritative bodies to support an agreed-upon approach to deriving NRVs. In its report, the NASEM committee recommended a global body, such as the WHO, FAO, or International Union of Nutritional Sciences; or as an alternative, an international collaboration at the regional level to take a leadership role in adopting a harmonization process (10). Such a decision about leadership toward harmonization will require collaborative discussion among stakeholders and is beyond the scope of this review. However, while discussions about moving forward toward harmonization are ongoing, there are interim steps that can be taken to facilitate access to currently available resources and to encourage consistency in the approach used to derive the core NRVs, the AR and UL.
Conclusions
Although more work remains to be done to achieve a globally consistent and harmonized methodological approach to deriving NRVs, by guiding countries and organizations through the general steps involved in the process of adapting existing or establishing new NRVs the process can move forward. Additionally, the NASEM consensus committee identified several options for overcoming barriers to harmonization. First is to increase access to systematic reviews and other data resources, especially in resource-poor settings; second is to enable sharing of scientific expertise where needed; and last, to improve the consistency in nutrient content information in applications such as in product labeling, particularly among common trade partners, and in food-composition tables, used in intake assessment.
Contributing to the array of resources available to all stakeholders will ultimately help individual countries move toward achieving harmonization of the NRV process globally. Additionally, advances in technology have enhanced access to resources such as systematic reviews and cross-country data. A toolkit could serve as a bridge from the concept of harmonization to implementing the first steps needed to achieve a globally harmonized approach to deriving NRVs. Such a toolkit would facilitate authoritative bodies in their decision to accept or adapt existing NRVs that apply to their specific populations and circumstances, using currently available tools and resources. Development of a toolkit could be undertaken cooperatively or individually by authoritative bodies and could include former members of the NASEM committee.
Having the appropriate tools and data resources is critical to informing the nutrient review process. At a minimum, these should include the following:
Systematic reviews. These offer an organized and transparent mechanism for evaluating the strength and quality of the evidence for associations between nutrient intake and nutrition and health outcomes and other relevant endpoints, such as risk of chronic disease, and they strengthen the overall value of the evidence assessment.
Databases and other data resources. These provide information about the population of particular relevance to the NRV under consideration and inform how the evidence presented in the systematic review can be interpreted.
Local and regional factors. These provide information about the local context, such as body stature or risk of chronic disease, which is critical to making the most appropriate adjustments to an NRV so that it suits the population for which it is intended.
Collectively, these tools, data resources, and contextual information are essential to enhancing the rigor and integrity of the NRV process, and they must continuously be updated to meet the needs of users. Improving access to these tools and resources and providing guidance and support to encourage their adoption is a critical step on the pathway towards harmonization of the NRV process. Awareness of the advantages of harmonization of the NRV process along with improvements in access for all countries to data needed to derive NRVs could transform the way many countries approach the challenge of developing NRVs.
ACKNOWLEDGEMENTS
All authors read and approved the final manuscript.
Notes
The authors reported no funding received for this review.
Author disclosures: The authors report no conflicts of interest.
Any views that are not attributed to Health and Medicine Division reports are those of the authors and do not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine.
Abbreviations used: AI, Adequate Intake; AR, Average Requirement; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; H-NRV, harmonized Nutrient Reference Value; LMIC, low- and middle-income country; NASEM, National Academies of Sciences, Engineering, and Medicine; NRV, Nutrient Reference Value; RI, Recommended Intake; UL, Tolerable Upper Intake Level.
References
- 1. Murphy SP, Yates AA, Atkinson SA, Barr SI, Dwyer J. History of nutrition: the long road leading to the dietary reference intakes for the United States and Canada. Adv Nutr. 2016;7:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UK Department of Health. Dietary Reference Values for food energy and nutrients in the United Kingdom (Report on Health and Social Subjects; 41). [Internet]. London: Her Majesty's Stationery Office; 1991. Available from: https://www.nutrition.org.uk/attachments/article/234/Nutrition%20Requirements_Revised%20Oct%202016.pdf. Accessed on April 23, 2020. [PubMed] [Google Scholar]
- 3. European Food Safety Authority (EFSA). Dietary reference values for nutrients. Summary report. [Internet]. EFSA Supporting Publication; 2017:e15121; 2017. Available from: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121/epdf. Accessed on April 23, 2020. [Google Scholar]
- 4. EFSA Scientific Committee on Food and Scientific Panel on Dietetic Products, Nutrition and Allergies. Summary of Tolerable Upper Intake Levels, version 2; 2017; [Internet]. Available from: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf. Accessed on April 23, 2020. [Google Scholar]
- 5. Institute of Medicine. Dietary Reference Intakes: The essential guide to nutrient requirements. Washington (DC): The National Academies Press; 2006. [Google Scholar]
- 6. Institute of Medicine. Dietary Reference Intakes: applications in dietary assessment. Washington (DC): The National Academies Press; 2000. [PubMed] [Google Scholar]
- 7. Allen LH, Carriquiry AL, Murphy SP. Proposed harmonized nutrient reference values for populations. [Internet]. Adv Nutr. 2019. Available from: https://doi.org/10.1093/advances/nmz096. Accessed on April 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King JC, Garza C, editors. International harmonization of approaches for developing nutrient-based dietary standards. Food Nutr Bull. 2007;28(Suppl 1):S3–153. [DOI] [PubMed] [Google Scholar]
- 9. National Academies of Sciences, Engineering, and Medicine. Global harmonization of methodological approaches to nutrient intake recommendations. Proceedings of a Workshop. Washington (DC): The National Academies Press; 2018. [Google Scholar]
- 10. National Academies of Sciences, Engineering, and Medicine. Harmonization of approaches to Nutrient Reference Values: applications to young children and women of reproductive age. Washington (DC): The National Academies Press; 2018. [PubMed] [Google Scholar]
- 11. Institute of Medicine. The development of the DRIs 1994–2004: lessons learned and new challenges: workshop summary. Washington (DC): The National Academies Press; 2008.; [Google Scholar]
- 12. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberato SC, Singh G, Mulholland K. Zinc supplementation in young children: a review of the literature focusing on diarrhea prevention and treatment. Clin Nutr. 2015;34(2):181–8. [DOI] [PubMed] [Google Scholar]
- 14. Penny ME. Zinc supplementation in public health. Ann Nutr Metab. 2013;62(Suppl 1):31–42. [DOI] [PubMed] [Google Scholar]