ABSTRACT
This systematic review assessed outcomes after using human milk–derived fortifier (HMF) compared with bovine milk–derived fortifier (BMF) in preterm infants. Six randomized controlled trials (RCTs) were included. Meta-analysis using a random-effects model showed the following results: 1) lower risk of necrotizing enterocolitis (NEC; ≥Stage II) (RR: 0.38; 95% CI: 0.15, 0.95; P = 0.04, I2 = 9%; n = 334, 4 RCTs) and surgical NEC (RR: 0.13; 95% CI: 0.02, 0.67; P = 0.02, I2 = 0%; n = 209, 3 RCTs) in the HMF group; 2) no significant difference in mortality (RR: 0.40; 95% CI: 0.14, 1.15; P = 0.09, I2 = 0%; n = 334, 4 RCTs); 3) lower weight gain in the HMF group [mean difference (MD) = −1.08 g · kg−1 · d−1; 95% CI: −1.96, −0.21 g · kg−1 · d−1; P = 0.02, I2 = 0%; n = 241, 4 RCTs]; 4) no differences for length (MD = −0.11 cm/wk; 95% CI: −0.26, 0.04 cm/wk; P = 0.14, I2 = 68%) and head circumference (MD = −0.02 cm/wk; 95% CI: −0.08, 0.05 cm/wk; P = 0.59, I2 = 23%); and 5) no significant difference in late-onset sepsis (RR: 0.96; 95% CI: 0.56, 1.67; P = 0.90, I2 = 63%; n = 334, 4 RCTs). The beneficial effects of HMF for NEC were no longer significant in sensitivity analyses after excluding studies with high risk of bias. Quality of evidence as per Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis was low to very low, and hence the confidence in these results is low. In summary, fortification of milk in preterm infants with HMF compared with BMF decreased the risk of NEC but was associated with lower weight gain. Given the low quality of evidence, adequately powered and well-designed RCTs without the influence of industry are required in this field.
Keywords: bovine, human milk fortifier, preterm, necrotizing enterocolitis, breast milk
Introduction
Advances in neonatal intensive care have resulted in increasing survival of very preterm and extremely preterm infants. Optimization of nutrition in survivors of extreme prematurity is a priority considering that undernutrition and extrauterine growth restriction are significant morbidities in this population (1–4).
Breast milk alone, even at an intake of 150 mL · kg−1 · d−1, is not sufficient to meet the high requirements of essential nutrients in extremely preterm infants. Considering their high content of essential nutrients such as protein, calcium, and phosphate, the use of human milk–derived fortifiers (HMFs) has become the standard of care in the management of this cohort (5–8).
A systematic review and meta-analysis (14 randomized trials, 1071 participants) provided low-quality evidence that multinutrient fortification of breast milk, when compared with breast milk alone, improved growth rates during initial hospitalization without increasing the risk of necrotizing enterocolitis (NEC). Very limited available data showed no effects of fortification on growth and development beyond infancy (6, 9).
Most of the fortification studies have involved bovine milk–derived fortifiers (BMFs), which are rich in proteins and have varying amounts of other nutrients for meeting the high demand of these nutrients in preterm infants. Administration of new BMFs with higher protein content has been shown to improve short-term weight gain in preterm infants (10, 11). Protein content of the currently available BMFs and HMFs ranges from 0.3 to 0.8 g and 1.2 to 3 g per gram of the fortifier, respectively (5). In the past, there was concern that early exposure to cow milk protein (CMP) may increase the risk of allergy and atopy. However, recent evidence indicates that early exposure to CMPs is not associated with an increased risk of allergic outcomes (12). In fact, it may reduce the risk (13).
On the contrary, emerging evidence suggests that an exclusive human milk diet is associated with reduced risk of feed intolerance, NEC, retinopathy of prematurity, and sepsis in preterm infants compared with bovine-derived formulas or fortifiers (14). Hence, HMFs are potentially more suitable for preterm infants. However, only a few randomized controlled trials (RCTs) have evaluated their effects in preterm infants. Given the current emphasis on an exclusive human milk diet for preterm infants (14), it is important to assess whether HMFs have beneficial effects compared with BMFs in this high-risk population. We therefore conducted a systematic review to assess the effects of HMFs compared with BMFs on mortality, morbidity, growth, and development in preterm infants.
Methods
We followed the Cochrane methodology (15), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (16) for conducting and reporting this systematic review. Ethics approval was not required.
Study eligibility criteria
Types of studies
Only RCTs were eligible for inclusion in the review.
Participants
Preterm infants born at <37 weeks of gestation.
Type of intervention and control
Fortification of milk for preterm infants using HMF (intervention) compared with BMF (control).
Primary outcomes
1) All-cause mortality; 2) late-onset sepsis (LOS; blood culture positive sepsis ≥48 h after birth); 3) NEC ≥Stage II (17); and 4) growth: weight, length, head circumference, skinfold thickness, BMI, and growth restriction (weight, length, or head circumference measurements <10th percentile for the index population).
Secondary outcomes
1) Feed intolerance; 2) time to reach full enteral feeds; 3) duration of hospital stay; and 4) measures of bone mineralization such as serum calcium, phosphorus, and alkaline phosphatase, bone mineral content on DXA, or X-ray findings of osteopenia/rickets upon long-term follow-up.
Data sources and searches
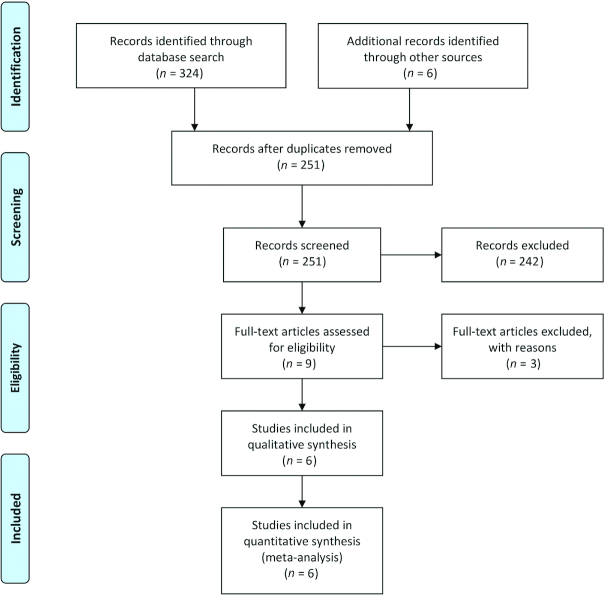
We searched the Cochrane central register of controlled trials (www.thecochranelibrary.com, through December 2019), PubMed (www.ncbi.nlm.nih.gov, 1966–December 2019), EMBASE (ExcerptaMedica database via Ovid—http://ovidsp.tx.ovid.com, 1980–December 2019), and CINAHL (Cumulative Index of Nursing and Allied Health Literature, via Ovid—http://ovidsp.tx.ovid.com, 1980–December 2019) databases, using the following keywords: 1) population: “infant, newborn” OR “infant, premature” OR “infant, low birth weight” OR “infant, extremely low birth weight” OR “infant, very low birth weight” OR “infant, small for gestational age” AND 2) intervention: “human milk fortifier” OR “fortifier” OR “bovine fortifier” OR “HMF” AND 3) randomized controlled trial (publication type). Other databases were searched using similar terminologies. Animal studies were excluded. Gray literature was searched through the National Technical Information Service (http://www.ntis.gov/), Open Grey (http://www.opengrey.eu/), and Trove (http://trove.nla.gov.au/) for articles that might not have been cited in the standard medical databases. The international trial registries and Australian Clinical Trials Registry were checked for ongoing/registered trials in this area. All reviewers (AA, HB, SR, and SP) conducted the literature search independently. Figure 1 summarizes the search strategy.
FIGURE 1.
Flow diagram showing the study selection process.
Study selection process
Reviewers AA and HB independently assessed the eligibility for selection of all studies identified using the prespecified inclusion criteria and search strategy. We obtained the full-text articles of studies that potentially met the inclusion criteria (RCTs which compared fortification with HMF or BMF in preterm infants), and independently assessed them for inclusion. We excluded studies which were non-RCTs, which included term infants, and which did not compare HMF with BMF in preterm infants. Any disagreements were resolved by group discussion among all reviewers. We recorded the selection process to complete a PRISMA flow diagram (16).
Data extraction
Reviewers AA and HB independently extracted the data from included studies using a prepiloted data collection form. For dichotomous outcomes such as ≥Stage II NEC, surgical NEC, mortality, and sepsis, the number of participants with the event and the number of participants analyzed in each treatment group of each study were entered into the form. For continuous outcomes such as gain in weight, length, and head circumference, we entered their mean and SD. The information on study design and outcomes was verified by all reviewers. We used the method suggested by Wan et al. (18) to calculate mean and SD from median and IQR. The formula suggested by Hozo et al. (19) was used to calculate mean and SD from median and range. Any disagreements were discussed until consensus was achieved. If required, we planned to contact the investigators for clarification and/or additional data for analysis.
Risk of bias assessment
We used the Cochrane guidelines to assess the methodological quality of the included RCTs. For each trial, information was sought regarding the method of random assignment, allocation concealment, and blinding, and reporting of all outcomes of all enrolled participants was collected. Reviewers AA and HB separately assessed each study. Any disagreement was resolved by discussion among all reviewers.
Assessment of publication bias
This was planned to be assessed by a funnel plot.
Data synthesis
Meta-analysis was conducted using a random-effects model (DerSimonian and Laird) using Review Manager version 5.3 (Cochrane Collaboration, Nordic Cochrane Centre).
Summary effect sizes were expressed as RRs and 95% CIs for dichotomous outcomes. Continuous outcomes were expressed as mean differences (MDs) and 95% CIs. Statistical heterogeneity was assessed with the χ2 test, I2 statistic, and by visual inspection of the forest plot (overlap of CIs). A P value < 0.1 on the χ2 statistic was considered to indicate heterogeneity. I2 values were interpreted according to the Cochrane handbook guidelines (15).
Sensitivity analysis
1) Considering the importance of random sequence generation and allocation concealment in RCTs, sensitivity analyses were conducted by excluding studies with high risk of bias (ROB) in these 2 domains separately; 2) we conducted further sensitivity analyses by excluding studies that had the potential for “industry bias.”
Preplanned subgroup analyses
These involved analyses of studies exclusively enrolling 1) extremely-low-birth-weight (ELBW; birth weight <1000 g) or 2) very-low-birth-weight (VLBW; birth weight <1500 g) infants, considering they are at high risk of NEC.
Quality and strength of evidence
These interpretations were based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system (20).
Summary of findings table
The key information about the quality of evidence, the magnitude of effect of the intervention, and the sum of the available data on the main outcome is presented in the summary of findings table according to the GRADE guidelines (20).
Results
The literature search retrieved 330 potential relevant citations, of which 6 studies (n = 389) were finally included in the systematic review. The study selection process is shown in the PRISMA flow diagram (Figure 1).
During the selection process, 9 studies comparing HMF with BMF were identified, of which 3 were excluded due to different primary outcomes—primary outcome as phenylalanine concentrations, bactericidal action of human milk, and likelihood of requiring parenteral nutrition: 1 study each (21–23).
Supplemental Table 1 gives the characteristics of the included studies (24–29).
Three out of the 6 included studies started fortification when the infant reached a feed volume of 150 mL · kg−1 · d−1 (25–27); the remaining 3 started it at 100 mL · kg−1 · d−1 (24, 28, 29). The duration of fortification was 3 wk in 2 studies (25, 26), 2 wk in 1 study (27), or until discharge in 3 studies (24, 28, 29). Four of the 6 included studies focused on preterm VLBW infants (24, 27–29). The remaining 2 studies included infants with birth weight ≤1800 g (25, 26). Three studies reported outcomes in infants with birth weight <1250 g (24, 28, 29), but the outcomes of ELBW infants were not reported separately in any of the studies.
ROB of included studies
Two studies were judged to have low ROB for the domain of random sequence generation (24, 28), 2 had low ROB for allocation concealment (25, 28), 2 had low ROB for blinding of interventions (28, 29), and 3 had low ROB for blinding of outcome assessors (24, 28, 29) (Table 1).
TABLE 1.
Risk of bias of the included randomized controlled trials1
| Study ID | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Boehm et al. (27) | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Cristofalo et al. (29) | Unclear | Unclear | Low | Low | Low | Low | High |
| Hagelberg et al. (26) | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| O'Connor et al. (28) | Low | Low | Low | Low | Low | Low | Low |
| Polberger et al. (25) | Unclear | Low | High | High | Low | Low | Unclear |
| Sullivan et al. (24) | Low | Unclear | High | Low | Unclear | Low | High |
Low risk: clearly defined random sequence generation, allocation concealment, blinding of participants, and outcome assessment, no incomplete data, no selective reporting. Unclear risk: information about any of the risk assessors not defined. High risk: random sequence generation, allocation concealment, blinding, and data outcome not done, selective reporting possible.
Primary outcomes
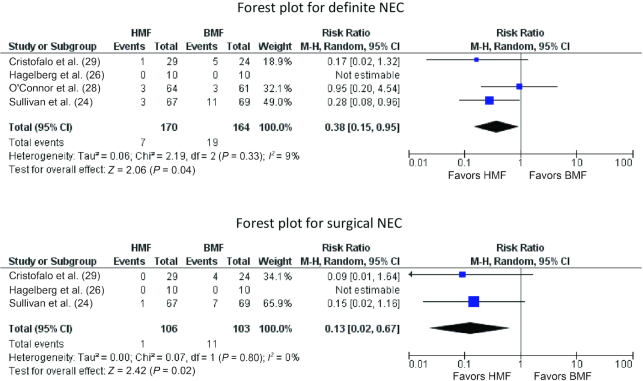
NEC ≥Stage II
Four studies (n = 334) (24, 26, 28, 29) reported on this outcome. A lower proportion of infants in the HMF group developed definite NEC than in the BMF group: 7 of 170 (4.11%) compared with 19 of 164 (11.58%). Meta-analysis showed significantly reduced risk (RR: 0.38; 95% CI: 0.15, 0.95; P = 0.04, I2 = 9%) of NEC ≥Stage II in the HMF group (Figure 2). Surgical NEC developed less frequently in HMF than in BMF group infants: 1 of 106 (0.94%) compared with 11 of 103 (10.68%). Meta-analysis estimated significantly lower risk (RR: 0.13; 95% CI: 0.02, 0.67; P = 0.02, I2 = 0%) of surgical NEC in the HMF group (Figure 2).
FIGURE 2.
Forest plots for definite NEC and surgical NEC comparing HMF with BMF. BMF, bovine milk–derived fortifier; HMF, human milk–derived fortifier; M-H, Mantel-Haenszel method; NEC, necrotizing enterocolitis.
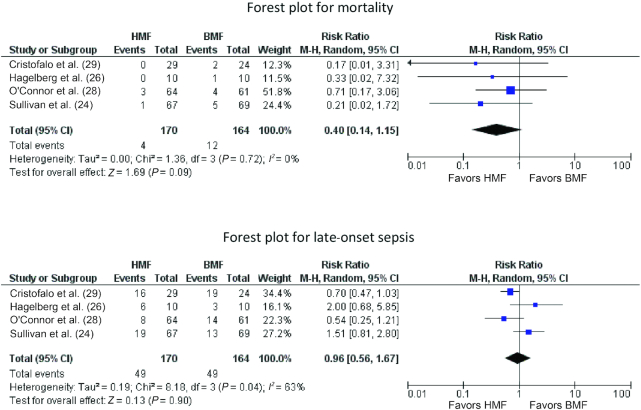
Mortality
Four trials reported data on this outcome (n = 334) (24, 26, 28, 29). Meta-analysis showed no significant difference in mortality in the HMF group compared with the BMF group (RR: 0.40; 95% CI: 0.14, 1.15; P = 0.09). There was no significant heterogeneity (I2 = 0%) between the trials (Figure 3).
FIGURE 3.
Forest plots for mortality and late-onset sepsis comparing HMF with BMF. BMF, bovine milk–derived fortifier; HMF, human milk–derived fortifier; M-H, Mantel-Haenszel method.
Sepsis
Pooling of data from 4 trials (n = 334) (24, 26, 28, 29) showed no significant difference in LOS between the HMF and BMF groups (RR: 0.96; 95% CI: 0.56, 1.67; P = 0.90) (Figure 3). There was significant heterogeneity between the trials (I2 = 63%).
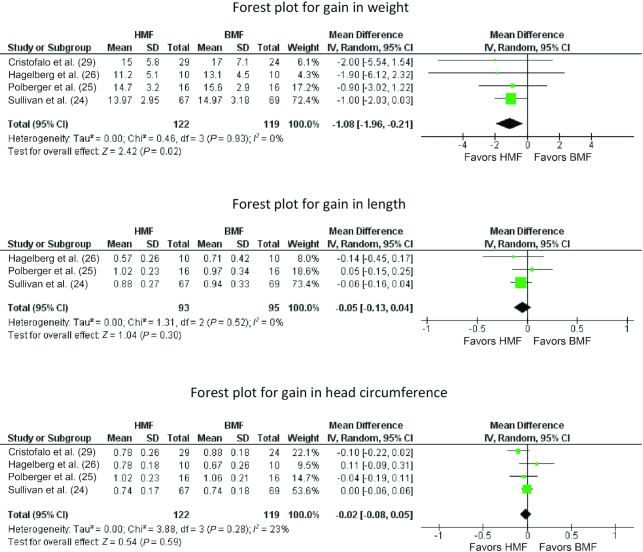
Weight
Meta-analysis of data from 4 trials (24–26, 29) (n = 241) showed significantly lower weight gain in the HMF than in the BMF group (MD = −1.08 g · kg−1 · d−1; 95% CI: −1.96 to −0.21 g · kg−1 · d−1; P = 0.02, I2 = 0%) (Figure 4).
FIGURE 4.
Forest plots for gains in weight, length, and head circumference comparing HMF with BMF. BMF, bovine milk–derived fortifier; HMF, human milk–derived fortifier; IV, inverse variance.
Length
Meta-analysis of data from 4 trials (24–26, 29) (n = 241) showed no significant difference in length gain in the HMF compared with the BMF group (MD = −0.11 cm/wk; 95% CI: −0.26, 0.04 cm/wk; P = 0.14, I2 = 68%) (Figure 4).
Head circumference
Meta-analysis of data from 4 trials (24–26, 29) (n = 241) showed no significant difference between the HMF and BMF groups on head circumference gain per week (MD = −0.02 cm/wk; 95% CI: −0.08, 0.05 cm/wk; P = 0.59, I2 = 23%) (Figure 4).
Markers of bone mineralization
Only Hagelberg et al. (26) reported on serum calcium and phosphorus concentrations before and after 3 wk supplementation with the study fortifiers. There were no significant differences between the groups at these 2 time points [serum calcium after 3 wk supplementation: mean (range) HMF: 2.7 (2.1–5.1) mmol/L; BMF: 2.3 (2.1–2.6) mmol/L, P = nonsignificant; serum phosphorus after 3 wk supplementation: HMF: 2.0 (1.5–2.6) mmol/L; BMF: 1.9 (1.0–2.6) mmol/L, P = nonsignificant]. None of the included studies reported on DXA and X-ray findings of rickets.
Secondary outcomes
None of the secondary outcomes of interest for this review were reported in the 6 included RCTs.
Publication bias
Assessment of publication bias by a funnel plot or statistical tests was not attempted considering the small number of included studies (30).
Sensitivity analyses
The beneficial effects of HMF on NEC and surgical NEC were no more significant after excluding studies with high ROB (Supplemental Table 2, Supplemental Figures 1–3).
Quality of evidence and summary of findings
The overall quality of evidence was deemed “low to very low” in view of the few RCTs, small sample size, wide CIs around the effect size estimate, inability to rule out publication bias, and high ROB in important domains in the included studies. Table 2 provides the overall evidence as per GRADE guidelines and a summary of findings.
TABLE 2.
Summary of findings as per GRADE guidelines1
| Outcome | Estimate with BMF supplementation | Corresponding risk estimate with HMF supplementation | Relative effect (RR) (95% CI) | Participants, n | Quality of evidence as per GRADE |
|---|---|---|---|---|---|
| Definite NEC | 19 of 164 (11.58%) | 7 of 170 (4.11%) | 0.38 (0.15, 0.95), P = 0.04, I2 = 9% | 334 | Low |
| Surgical NEC | 11 of 103 (10.68%) | 1 of 106 (0.94%) | 0.13 (0.02, 0.67), P = 0.02, I2 = 0% | 209 | Low |
| Mortality | 12 of 164 (7.31%) | 4 of 170 (2.35%) | 0.40 (0.14, 1.15), P = 0.09, I2 = 0% | 334 | Very low |
| Sepsis | 49 of 164 (29.88%) | 49 of 170 (28.82%) | 0.96 (0.56, 1.67), P = 0.90, I2 = 63% | 334 | Very low |
| Weight gain, g/kg·d | 15.16 | 13.72 | −1.08 (−1.96, −0.21), P = 0.02, I2 = 0% | 241 | Very low |
| Length gain, cm/w | 0.93 | 0.83 | −0.11 (−0.26, 0.04), P = 0.14, I2 = 68% | 241 | Very low |
| Head circumference gain, cm/w | 0.84 | 0.83 | −0.02 (−0.08, 0.05), P = 0.59, I2 = 23% | 241 | Very low |
1The overall quality of evidence was deemed “low to very low” in view of the few RCTs, small sample size, wide CIs around the effect size estimate, inability to rule out publication bias, and high risk of bias in important domains in the included studies. BMF, bovine milk–derived fortifier; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; HMF, human milk–derived fortifier; NEC, necrotizing enterocolitis.
Discussion
The results of our systematic review found that compared with BMF, fortification with HMF decreased the risk of NEC ≥Stage II and surgical NEC, but resulted in lower weight gain in preterm infants. However, our confidence in these findings is limited because the GRADE quality of evidence was “low to very low” for the majority of the outcomes assessed. The validity of the results is also compromised by the results of the sensitivity analyses after excluding studies with high ROB including industry influence.
The heterogeneity in the fortification protocols of the included trials needs to be discussed. The milk intake volume at which fortification was started in the included RCTs ranged from 100 to 150 mL · kg−1 · d−1. Recent reviews indicate that currently fortification is started at milk volumes of 50–100 mL · kg−1 · d−1 (5). Evidence from RCTs and experimental studies suggests that gut development and maturity are achieved after reaching feeding volumes of 50–60 mL · kg−1 · d−1 (31, 32). Starting fortification with BMF too early, before reasonable gut development and maturation (<50 mL · kg−1 · d−1) (31, 32), may increase the risk of CMP allergy, feed intolerance, and NEC (33, 34).
The optimal duration of fortification of milk in preterm infants is another important issue. In our review, fortification was continued for 2 wk in 1 RCT (27), 3 wk in 3 RCTs (25, 26, 29), and for 12 wk/until discharge in 2 RCTs (24, 28). Standardization of the timing of commencement and duration of fortification is important in future trials to minimize heterogeneity.
Our systematic review found that HMF decreased the risk of NEC, but resulted in slower weight gain. Suboptimal weight gain is known to be associated with worse long-term neurodevelopmental outcomes (35, 36). On the contrary, newer BMFs have lower osmolality and improved essential fatty acid and protein content compared with the older BMFs (5). Hence it is possible that newer BMFs may help in achieving optimal growth without increasing the risk of NEC in preterm infants. They are also easily available at a relatively low cost compared with HMFs. Hence, future trials should include assessment of clinical outcomes as well as economic evaluation of newer BMFs compared with HMFs in this population. This is particularly important considering that NEC carries significant mortality and morbidity, including long-term neurodevelopmental disability, and a cost of ≤$1 billion/y in countries such as the United States (37).
At the time of reporting these results, a Cochrane systematic review assessing HMF compared with BMF for preventing mortality and morbidity in preterm infants has been published (38). Only 1 RCT (n = 127) was included. HMF did not decrease the risk of NEC in exclusively breast milk–fed preterm infants (RR: 0.95; 95% CI: 0.20, 4.54; low certainty of evidence) (28). HMF had no effect on growth, feeding intolerance, LOS, or mortality. The Cochrane reviewers emphasized the need for well-designed large RCTs in this field (38). There are differences between this systematic review and the Cochrane review. We included 6 RCTs, whereas the Cochrane review included 1 and they mentioned Sullivan et al. (24) and Cristofalo et al. (29) as among those excluded. The studies by Hagelberg et al. (26), Boehm et al. (27), and Polberger et al. (25) were not considered in the Cochrane review. Keeping a pragmatic view, we included RCTs comparing fortification of any type of milk (breast milk or donor milk, or formula) for preterm infants with either HMF or BMF. Hence we included the Sullivan et al. (24) and Cristofalo et al. (29) RCTs, whereas the Cochrane review excluded them because they selected only studies involving exclusively breast milk–fed preterm infants.
The strength of our review includes its comprehensive nature and robust methodology. Its limitations include the small number and sample sizes of the included RCTs, heterogeneity in the fortification protocols, and lack of data specifically on extremely preterm (<28 weeks of gestation) or ELBW infants. The very high rates of NEC and surgical NEC in control groups in 2 studies (24, 29) warrant a note of caution in interpreting the results. Except for Hagelberg et al. (26), none of the included studies addressed the issue of bone mineralization in detail. Furthermore, some trials reported funding and employment of the researchers by manufacturers of HMFs. This has the potential for introducing “industry bias” into such trials. A recent Cochrane review found that industry-sponsored studies more often had efficacy results that were favorable to the sponsors’ products (RR: 1.27; 95% CI: 1.17, 1.37) than did non-industry-sponsored drug and device studies (39). Hence, it may be prudent to avoid or minimize industry bias in future trials.
In summary, the results of our systematic review found very-low-quality evidence that, when compared with BMF, fortification with HMF decreased NEC ≥Stage II and surgical NEC in preterm infants, but resulted in slower weight gain. Adequately powered RCTs free from the influence of industry are required to address this issue definitively. The sample size required for such a trial would be 1498 (749 infants in each arm) to detect a statistically significant reduction of 50% in the risk of NEC ≥Stage II from a baseline incidence of 6% with 80% power, and significance <0.05. Standardization of the protocol (optimal postnatal age/milk intake at start, and duration) for fortification, and considering the influence of formula feeding—a major risk factor for NEC—will be critical issues in designing such a trial. Conducting a large RCT involving only exclusively breast milk–fed infants randomly assigned to HMF or BMF is ideal but difficult, because many units are not supported by a breast milk bank. Pragmatic trials of HMF compared with BMF in which the use of formula milk is allowed when breast milk or donor milk is not available may be more generalizable. Assessment of short-term outcomes (NEC, sepsis, mortality, physical growth, bone mineralization) as well as long-term outcomes (growth, bone mineralization, and neurodevelopment) and economic analysis are essential in future trials.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SP: conceived and designed the study; SP and SR: rechecked and analyzed the data and provided input for the initial and final drafts of the manuscript; AA: performed the independent literature search and analyzed the data; AA and HB: selected the studies, extracted the data, and wrote the first and final drafts of the manuscript with guidance from SR and SP; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BMF, bovine milk–derived fortifier; CMP, cow milk protein; ELBW, extremely low birth weight; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; HMF, human milk–derived fortifier; LOS, late-onset sepsis; MD, mean difference; NEC, necrotizing enterocolitis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; ROB, risk of bias.
References
- 1. Cole TJ, Statnikov Y, Santhakumaran S, Pan H, Modi N. Birth weight and longitudinal growth in infants born below 32 weeks’ gestation: a UK population study. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffin IJ, Tancredi DJ, Bertino E, Lee HC, Profit J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):F50–5. [DOI] [PubMed] [Google Scholar]
- 3. Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, Buzas JS, Bertino E, Gagliardi L, Bellu R. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics. 2015;136(1):e84–92. [DOI] [PubMed] [Google Scholar]
- 4. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA et al.. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D, Bertino E, Gaya A, Gebauer C, Grovslien A et al.. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front Pediatr. 2019;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;(5):CD000343. [DOI] [PubMed] [Google Scholar]
- 7. Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Ann Nutr Metab. 2011;58(Suppl 1):8–18. [DOI] [PubMed] [Google Scholar]
- 8. Koo W, Tice H. Human milk fortifiers do not meet the current recommendation for nutrients in very low birth weight infants. JPEN J Parenter Enteral Nutr. 2018;42(4):813–20. [DOI] [PubMed] [Google Scholar]
- 9. Hortensius LM, van Elburg RM, Nijboer CH, Benders M, de Theije CGM. Postnatal nutrition to improve brain development in the preterm infant: a systematic review from bench to bedside. Front Physiol. 2019;10:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rigo J, Hascoet JM, Billeaud C, Picaud JC, Mosca F, Rubio A, Saliba E, Radke M, Simeoni U, Guillois B et al.. Growth and nutritional biomarkers of preterm infants fed a new powdered human milk fortifier: a randomized trial. J Pediatr Gastroenterol Nutr. 2017;65(4):e83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu TT, Dang D, Lv XM, Wang TF, Du JF, Wu H. Human milk fortifier with high versus standard protein content for promoting growth of preterm infants: a meta-analysis. J Int Med Res. 2015;43(3):279–89. [DOI] [PubMed] [Google Scholar]
- 12. Zachariassen G, Faerk J, Esberg BH, Fenger-Gron J, Mortensen S, Christesen HT, Halken S. Allergic diseases among very preterm infants according to nutrition after hospital discharge. Pediatr Allergy Immunol. 2011;22(5):515–20. [DOI] [PubMed] [Google Scholar]
- 13. Peters RL, Koplin JJ, Dharmage SC, Tang MLK, McWilliam VL, Gurrin LC, Neeland MR, Lowe AJ, Ponsonby AL, Allen KJ. Early exposure to cow's milk protein is associated with a reduced risk of cow's milk allergic outcomes. J Allergy Clin Immunol Pract. 2019;7(2):462–70.e1. [DOI] [PubMed] [Google Scholar]
- 14. Taylor SN. Solely human milk diets for preterm infants. Semin Perinatol. 2019;43(7):151158. [DOI] [PubMed] [Google Scholar]
- 15. Higgins J, Thomas J, editors. Cochrane handbook for systematic reviews of interventions, version 6. [Internet] The Cochrane Collaboration; 2019; [cited December 2019]. Available from: https://training.cochrane.org/handbook/current. [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 17. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17(4):213–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B et al.. GRADE guidelines: 12. Preparing summary of findings tables—binary outcomes. J Clin Epidemiol. 2013;66(2):158–72. [DOI] [PubMed] [Google Scholar]
- 21. Thomaz DM, Serafin PO, Palhares DB, Tavares LV, Grance TR. Serum phenylalanine in preterm newborns fed different diets of human milk. J Pediatr (Rio J). 2014;90(5):518–22. [DOI] [PubMed] [Google Scholar]
- 22. Chan GM, Lee ML, Rechtman DJ. Effects of a human milk-derived human milk fortifier on the antibacterial actions of human milk. Breastfeed Med. 2007;2(4):205–8. [DOI] [PubMed] [Google Scholar]
- 23. Ghandehari H, Lee ML, Rechtman DJ; for the H2MF Study Group. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Res Notes. 2012;5:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM et al.. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–7..e1. [DOI] [PubMed] [Google Scholar]
- 25. Polberger S, Raiha NC, Juvonen P, Moro GE, Minoli I, Warm A. Individualized protein fortification of human milk for preterm infants: comparison of ultrafiltrated human milk protein and a bovine whey fortifier. J Pediatr Gastroenterol Nutr. 1999;29(3):332–8. [DOI] [PubMed] [Google Scholar]
- 26. Hagelberg S, Lindblad BS, Persson B. Amino acid levels in the critically ill preterm infant given mother's milk fortified with protein from human or cow's milk. Acta Paediatr Scand. 1990;79(12):1163–74. [DOI] [PubMed] [Google Scholar]
- 27. Boehm G, Müller DM, Senger H, Borte M, Moro G. Nitrogen and fat balances in very low birth weight infants fed human milk fortified with human milk or bovine milk protein. Eur J Pediatr. 1993;152(3):236–9. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, Daneman A, Francis J, Kotsopoulos K, Shah PS et al.. Nutrient enrichment of human milk with human and bovine milk–based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108(1):108–16. [DOI] [PubMed] [Google Scholar]
- 29. Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, Dudell G, Rechtman DJ, Lee ML, Lucas A et al.. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–5..e1. [DOI] [PubMed] [Google Scholar]
- 30. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111(3):529–34. [DOI] [PubMed] [Google Scholar]
- 32. Owens L, Burrin DG, Berseth CL. Minimal enteral feeding induces maturation of intestinal motor function but not mucosal growth in neonatal dogs. J Nutr. 2002;132(9):2717–22. [DOI] [PubMed] [Google Scholar]
- 33. Cordova J, Sriram S, Patton T, Jericho H, Gokhale R, Weinstein D, Sentongo T. Manifestations of cow's-milk protein intolerance in preterm infants. J Pediatr Gastroenterol Nutr. 2016;62(1):140–4. [DOI] [PubMed] [Google Scholar]
- 34. Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. 2012;7(1):29–37. [DOI] [PubMed] [Google Scholar]
- 35. Vinall J, Grunau RE, Brant R, Chau V, Poskitt KJ, Synnes AR, Miller SP. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med. 2013;5(168):168ra8. [DOI] [PubMed] [Google Scholar]
- 36. Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr. 2003;143(2):163–70. [DOI] [PubMed] [Google Scholar]
- 37. Ganapathy V, Hay JW, Kim JH, Lee ML, Rechtman DJ. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Premkumar MH, Pammi M, Suresh G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst Rev. 2019;(11):CD013145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2:MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.