ABSTRACT
The association between FokI polymorphism in the vitamin D receptor (VDR) gene and susceptibility to arterial hypertension (HT) is controversial. Thus, we evaluated the relation between FokI and HT according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using MEDLINE® (Medical Literature Analysis and Retrieval System Online)/PubMed, Scopus, and Cochrane Library CENTRAL databases. Data from case-control studies, including the number of participants, age, 25-hydroxyvitamin D concentrations, systolic and diastolic blood pressure values, FokI allele, and genotype frequency were extracted by 2 independent authors and OR was calculated with the 95% CI to assess the strength of the association between the FokI variant and odds of HT. In general and subgroup analyses, we used allelic (f compared with F), common (ff compared with FF + Ff), risk (ff + Ff compared with FF), and additive (ff compared with FF) models. Six case-control studies including 3140 cases and 3882 controls were reviewed in the meta-analysis. Global assessment revealed a correlation between FokI and reduced odds of HT in the additive/homozygote model (ff compared with FF; OR: 0.65; 95% CI: 0.45–0.94) and common/recessive model (ff compared with FF + Ff; OR: 0.75; 95% CI: 0.57–0.99). In Asian subjects, there was a significant reduction in the odds of HT in additive (ff compared with FF; OR: 0.84; 95% CI: 0.73–0.98) and risk models (ff + Ff compared with FF; OR: 0.87, 95% CI: 0.78–0.97), in particular, for Indians (South). In Africans, the statistically significant association occurred in the additive and common models. Allele f in the FokI polymorphism of the VDR gene was associated with reduced odds of HT in the general population based on the risk model. Thus, nutritional genomics can help understand the influence of nutrition on metabolic homeostasis pathways and the clinical consequences of hypertension. This study shows the need for healthy, anti-inflammatory, and antioxidant compounds to prevent or treat chronic complications.
Keywords: rs10735810; 25(OH)D; 1,25(OH)2D; renin; cardiovascular disease
Introduction
Arterial hypertension (HT) is a prevalent disease worldwide. This complex and multifactorial disease, for which the incidence rate increases progressively with increased age (1), represents a significant threat to the health and wellbeing of several population groups as a risk factor for cardiovascular illness (2). The impact of lifestyle changes (smoking, sedentarism, obesity, and excessive consumption of alcoholic drinks, calories, and sodium), age, sex, and heredity affect arterial pressure (3). Healthy food intake plays a central role in regulating chronic inflammation, a condition that in response to HT damage leads to atherosclerotic plaque rupture and thrombosis (4). Thus, the organism is altered by diet, nutritional genomics, the interaction between diet and genetics, the relation between diet and disease, and the individual contribution of genotype to HT (5). Genetic factors influence the development of HT in 30–50% of cases (6), especially polymorphisms that cause changes in vitamin D metabolism (7, 8).
Therefore, vitamin D, by binding to its receptor (VDR), classically acts on calcium, phosphorus, and bone metabolism homeostasis, and extraskeletal functions such as cell proliferation, immunomodulation, oncogenesis (9), endothelial function, inflammation, and modulation of renin-angiotensin-aldosterone system (RAAS) activity, which in particular regulates blood pressure (10). Studies have revealed an augmented risk of HT in people exposed to low serum concentrations of 25-hydroxyvitamin D (25 [OH] D), a biomarker of vitamin D status (11–17).
In addition, in cardiovascular metabolism, the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25 [OH]2D), exerts its biological effects on myocardiocytes (18), binds to the VDR, a transcription factor in the aortic endothelium (19), and smooth muscle vascular cells (20).
Human VDR is the product of a single gene on chromosome 12 at position 12q12–14. This protein mediates the pleiotropic actions of 1,25(OH)2D by modulating the expression of target genes (21). 1,25(OH)2D is a negative endocrine-regulating hormone in the RAAS that acts by inhibiting the expression of renin mRNA, regardless of calcium metabolism, which is involved in bone function. These biological activities of vitamin D are mediated by binding to the VDR, in which single nucleotide polymorphisms (SNPs) can cause changes in arterial blood pressure and contribute to the onset of hypertension (22, 23).
One of the most widely studied SNPs in the VDR gene is the polymorphism known as the FokI restriction fragment (rs228570 or rs10735810), characterized as the replacement of thymine with cytosine in the translation initiation codon (AGT) on chromosome 12q13.1 of exon 2. Studies reporting hypertension data have shown controversial results, with some studies detecting a significant statistical association between FokI and high blood pressure (24, 25) and others showing opposite results (26).
FokI is the only VDR polymorphism resulting in an altered protein length, which likely has functional consequences such as vitamin D deficiency or excess formation and/or denaturation of the active form of vitamin D (27). The variant A or T (allele f) in FokI results in the production of the complete protein (427 amino acids) with lower biological activity compared with the polymorphic form containing the variant G or C (allele F); variants G and C generate a shorter protein of 424 amino acids. VDR with the FokI genotype GG or CC (FF) results in increased VDR protein activity compared to that with GA or CT (Ff) or AA or TT (ff) genotypes (23, 28–31). Thus, dominant homozygotes (FF) and heterozygotes (Ff) have a 2.2-fold higher risk of hypertension than recessive homozygotes (ff) (25).
We performed a meta-analysis of case-control studies to resolve the controversial results reported in previous studies, aiming to assess the role of the FokI polymorphism in VDR gene function and the odds of HT.
Methods
Search strategy
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (32). Two independent researchers reviewed published studies in the MEDLINE® (Medical Literature Analysis and Retrieval System Online)/PubMed, Scopus, and Cochrane Library CENTRAL databases between 17 January and 24 February, 2019, using combinations of 3 keyword sets as follows: “Genetic and Hypertension and Polymorphism VDR,” “Vitamin D and Hypertension and Polymorphism,” and “VDR and Hypertension and FokI.”
Inclusion and exclusion criteria
Study selection was not limited by the time of year or by sex, age, or ethnicity of the investigated individuals. We selected studies that used a case-control design conducted on humans, reporting the genotype frequency of hypertensive (cases) and normotensive (control) individuals, which enabled us to perform the meta-analysis. All studies analyzed were published in English. We excluded studies on pregnant women and animals, those conducted in vitro, transversal studies, revisions, letters to the editor, and those that did not report any results of the genotype and allele frequency for the 2 population groups compared. For studies reporting the same data more than once, we analyzed the publication found in the first search and ignored the duplicate results.
Study analysis
After reading the complete text and subsequent discussion between authors regarding this meta-analysis, the articles were analyzed to determine the design type, population characteristics, and main observed results. Study quality was assessed using the Newcastle–Ottawa scale (NOS) (33), which uses the “star” classification system and ranges from 0 (worst) to 9 (best). Studies with a score equal to or >7 were considered high-quality studies and those with values equal to or <6 were considered medium quality. Two investigators independently assessed the quality of the included studies, and the results were revised by a third investigator. Any disagreements were resolved through discussion.
Statistical analysis
The software STATA (version 12.1; Stata Corp.) was used for all statistical analyses. The analyses and comparison completed were considered exploratory. Genotype frequencies were assessed using the χ2 test for the control group in Hardy–Weinberg equilibrium (HWE; P < 0.05 was considered to indicate significant disequilibrium). Four genetic models were used for the analyses; allelic (f compared with F), common (ff compared with FF + Ff), risk (ff + Ff compared with FF), and additive (ff compared with FF). The results were expressed using the OR for dichotomic data with the 95% CI. A P value of 0.05 for the grouped OR was considered statistically significant. I2 was used to test heterogeneity between studies and assess the global association between the FokI polymorphism in the VDR gene and odds of HT. Due to the varying participant characteristics and genotyping methods used throughout these studies, we considered using random effects models for all the analyses. For sensitivity analysis, a single study was omitted at a time to determine if the omission influenced the effects and heterogeneity among studies. Stratification analyses were conducted to identify possible sources of heterogeneity between variables such as ethnicity, age groups, sample size, genotyping methods, and control types. A funnel plot was used to estimate publication bias among the studies included, with Egger's test and Begg's funnel plot used in all comparison models.
Results
Study characteristics
A scheme of the study selection process is shown in Figure 1. The initial literary search revealed 583 publications. After reading the titles and abstracts of the studies, 12 potential studies were included, and their complete text was read. According to the inclusion criteria, 6 case-control studies comprising 3140 HT patients and 3882 normotensive controls were selected for final analysis.
FIGURE 1.
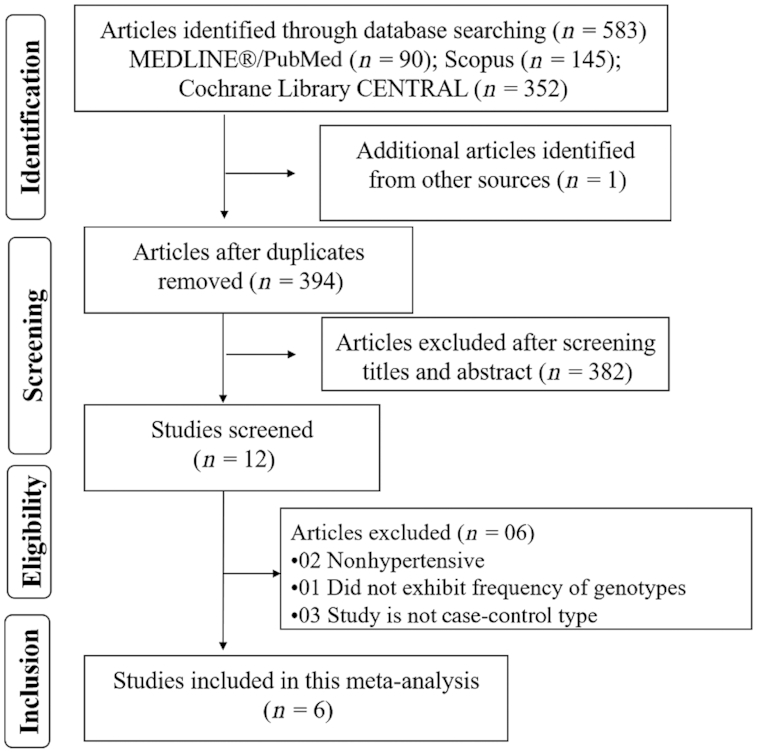
Research flow chart.
The analyzed studies included both male and female subjects from several continents including Europe (n = 3) (27, 34, 35), Asia (n = 2) (36, 37), and Africa (n = 1) (38). The main characteristics of the selected studies are summarized in Table 1, including the following: first author, year of publication, original country of the researched individuals, genotyping method of the FokI polymorphism, the number of participants, average values and age SE, 25(OH)D, and systolic (SBP) and diastolic blood pressure (DBP). Notably, all studies (100%) scored 5 stars or more according to the NOS, supporting the quality of the publications. Cottone et al. (34) used the following exclusion criteria for study participants; secondary or malignant hypertension, diabetes or fasting glucose ≥126 mg/dL, heart failure, positive history or clinical signs of ischemic heart disease, cerebrovascular disease, renal disease, and major noncardiovascular diseases. Glocke et al. (39) evaluated 2 groups of volunteers; over and aged under 90 y. Gussago et al. (27) evaluated those aged over 100 y and those aged 70–79 y. Jia et al. (36) evaluated individuals with a self-reported history of chronic kidney disease, liver disease, or cancer. Swapna et al. (35) did not include individuals with diabetes and cardiovascular disease. Errouagui et al. (37) included sex-matched healthy controls. The distribution of genotypes and P values for HWE of the controls are shown in Table 2. Among the controls, the genotype distribution of FokI in all 6 studies was included in HWE.
TABLE 1.
Studies evaluated in the meta-analysis
| First author (reference) | Genotyping method | n 1 | Age,2 y | Circulating 25(OH)D, ng/mL | SBP, mmHg | DBP, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Country | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | NOS | ||
| Swapna (35) | 2011 | India | PCR-RFLP | 280 | 200 | 55.6±0.6 | 47.6±0.7 | NA | NA | 161.3±1.3 | 119.0±0.2 | 98.7±0.8 | 80.4±1.3 | 6 |
| Glocke (39) | 2013 | Germany | Sequencing | 101 | 208 | 92.0 | 49.0 | NA | NA | NA | NA | NA | NA | 5 |
| Cottone (34) | 2014 | Italy | PCR-RFLP | 71 | 72 | 45.0 | NA | 20.0** | NA | 140.0 | NA | 90.0 | NA | 8 |
| Jia (36) | 2014 | China | RT-PCR | 2409 | 3063 | 60.7±11.2 | 58.1±11.0 | NA | NA | 141.0±14.0 | 121.0±12.0 | 88.0±8.0 | 77.0±7.0 | 6 |
| Errouagui (37) | 2014 | Morocco | PCR-RFLP | 177 | 176 | 49.6±12.0 | 56.9±1.5 | 25.2±9.5 | 30.3±13.0 | 147.8±17.8 | 127.9±6.6 | 84.5±10.1 | 73.2±4.1 | 5 |
| Gussago (27) | 2016 | Italy | PCR-RFLP | 102 | 163 | 102.3 ± 0.3 | 73.0±0.6 | NA | NA | NA | NA | NA | NA | 7 |
n- number of observations.
Values are means ± SDs.
DBP, diastolic blood pressure; NA, not available; NOS, Newcastle-Ottawa scale; RFLP, restriction fragment length polymorphism; RT, real-time; SBP, systolic blood pressure. *Values are means ± SDs; **median; 25(OH)D, 25- hydroxyvitamin D.
TABLE 2.
Genotypic and allelic frequencies of VDR FokI polymorphisms in the studies included in the meta-analysis
| Genotypic frequencies (FF/Ff/ff) | Allele frequencies (F/f) | ||||
|---|---|---|---|---|---|
| Author | Case | Control | Case | Control | HWE (P) |
| Swapna (35) | 150/100/30 | 68/102/30 | 400/160 | 238/162 | 0.41 |
| Glocke (39) | 36/52/13 | 75/102/31 | 124/78 | 252/164 | 0.70 |
| Cottone (34) | 36/30/5 | 29/36/7 | 102/40 | 94/50 | 0.38 |
| Jia (36) | 756/1180/472 | 910/1500/648 | 1936/1652 | 2410/2148 | 0.52 |
| Errouagui (37) | 120/91/9 | 79/74/21 | 211/100 | 153/95 | 0.57 |
| Gussago (27) | 45/40/10 | 79/63/21 | 130/60 | 221/105 | 0.14 |
HWE, Hardy–Weinberg equilibrium; VDR, vitamin D receptor.
Meta-analysis
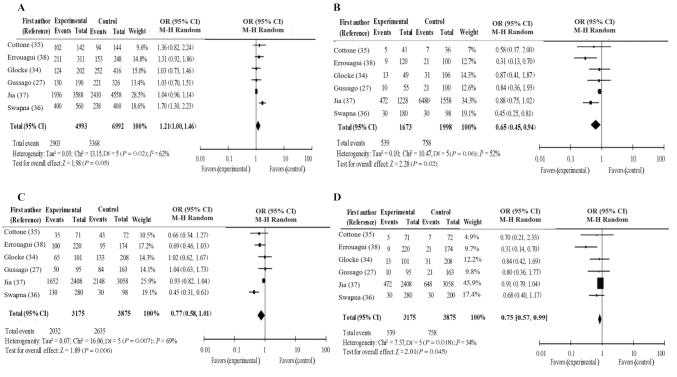
Pooled ORs were calculated to determine the global association between the FokI polymorphisms in the VDR gene and the odds of HT. Our analysis revealed no odds of HT based on the FokI polymorphism in the VDR gene in the model considering the presence of the variant allele (f compared with F: P = 0.05; OR: 1.21; 95% CI: 1.00, 1.46) and the risk/dominant model (ff + Ff compared with FF: P = 0.06; OR: 0.77; 95% CI: 0.58, 1.01). In contrast, the additive/homozygote model (ff compared with FF: P = 0.02; OR: 0.65; 95% CI: 0.45, 0.94) and common/recessive model (ff compared with FF + Ff: P = 0.045; OR: 0.75; 95% CI: 0.57, 0.99) revealed an association between a decreased odds of HT and FokI polymorphism in the VDR gene (Figure 2A–D).
FIGURE 2.
A–D. Forest plot of genetic models; A: f compared with F, B: ff compared with FF, C: ff + Ff compared with FF, and D: ff compared with FF + Ff.
To broaden the heterogeneity investigation, we conducted subgroup analyses (Table 3). To evaluate the effect of geographic differences on the global estimates, studies were classified as Asian, comprising 2 groups, namely Eastern (China) and Southern (India), European (subdivided as Italians and Germans), and Africans. Regarding ethnicity, we found an increased odds of HT in the Asian (f compared with F; OR: 1.09; 95% CI: 1.01, 1.19; P-heterogeneity <0.0001) and Southern Asian (f compared with F; OR: 1.70; 95% CI: 1.30, 2.23) populations under the allelic model. However, in Asians, an inverse association was observed with the additive model (ff compared with FF; OR: 0.84; 95% CI: 0.73, 0.98; P-heterogeneity = 0.003) and risk model (ff + Ff compared with FF; OR: 0.87; 95% CI: 0.78, 0.97; P-heterogeneity <0.0001). In parallel, this was the same for Indians (South) in the additive and risky genetic models. In Africans, the statistically significant association occurred in the additive and common models.
TABLE 3.
Meta-analysis of the VDR gene FokI polymorphism and hypertension
| Characteristics | n 1 | f vs. F | ff vs. FF | ff + Ff vs. FF | ff vs. FF + Ff | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR2 (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Total | 6 | 1.21 (1.00, 1.46) | 0.02 | 0.65 (0.45, 0.94)*,3 | 0.06 | 0.77 (0.58, 1.01) | 0.007 | 0.75 (0.57, 0.99)* | 0.18 |
| I 2, % | 62 | 52 | 69 | 34 | |||||
| Ethnicities | |||||||||
| Asians | 2 | 1.09 (1.01, 1.19)* | 0.000 | 0.84 (0.73, 0.98)* | 0.03 | 0.87 (0.78, 0.97)* | 0.000 | 0.89 (0.78, 1.02) | 0.31 |
| Han Chinese (East) | 1 | 1.04 (0.96, 1.14) | 0.88 (0.75, 1.02) | 0.93 (0.82, 1.04) | 0.91 (0.79, 1.04) | ||||
| Indians (South) | 1 | 1.70 (1.30, 2.23)* | 0.45 (0.25, 0.81)* | 0.45 (0.31, 0.65)* | 0.68 (0.40, 1.17) | ||||
| Europeans | 3 | 1.09 (0.87, 1.37) | 0.64 | 0.80 (0.48, 1.34) | 0.85 | 0.93 (0.68, 1.27) | 0.50 | 0.80 (0.50, 1.30) | 0.97 |
| Italians | 2 | 1.14 (0.84, 1.55) | 0.63 | 0.74 (0.37, 1.49) | 0.84 | 0.88 (0.59, 1.31) | 0.53 | 0.77 (0.39, 1.49) | 0.92 |
| Germany | 1 | 1.03 (0.73, 1.46) | 0.87 (0.41, 1.87) | 1.02 (0.62, 1.67) | 0.84 (0.42, 1.69) | ||||
| Africans | 1 | 1.31 (0.92, 1.86) | 0.31 (0.13, 0.70)* | 0.69 (0.46, 1.03) | 0.31 (0.14, 0.70)* | ||||
| Subtotal | 1.15 (1.04, 1.28)* | 0.05 | 0.74 (0.61, 0.89)* | 0.08 | 0.81 (0.70, 0.95)* | 0.020 | 0.84 (0.74, 0.96)* | 0.243 | |
| I2, % | 52.2 | 47 | 60.2 | 24.4 | |||||
| Age group | |||||||||
| Adults | 3 | 1.10 (1.02, 1.20)* | 0.002 | 0.81 (0.70, 0.94)* | 0.006 | 0.85 (0.77, 0.95)* | 0.000 | 0.87 (0.76, 0.98)* | 0.02 |
| Elderly | 2 | 1.03 (0.80, 1.33) | 0.98 | 0.86 (0.49, 1.54) | 0.94 | 1.03 (0.72, 1.47) | 0.94 | 0.82 (0.49, 1.39) | 0.91 |
| Adults + elderly | 1 | 1.36 (0.82, 2.24) | 0.58 (0.17, 2.00) | 0.66 (0.34, 1.27) | 0.70 (0.21, 2.33) | ||||
| Subtotal | 1.18 (0.93, 1.49) | 0.004 | 0.69 (0.44, 1.09) | 0.012 | 0.80 (0.57, 1.13) | 0.001 | 0.77 (0.57, 1.05) | 0.089 | |
| I2, % | 82.2 | 77.4 | 84.8 | 58.6 | |||||
| Sample size | |||||||||
| Large (>500) | 1 | 1.04 (0.96, 1.14) | 0.88 (0.75, 1.02) | 0.93 (0.82, 1.04) | 0.91 (0.79, 1.04) | ||||
| Small (<500) | 5 | 1.31 (1.12, 1.53)* | 0.14 | 0.55 (0.39, 0.78)* | 0.31 | 0.69 (0.56, 0.85)* | 0.04 | 0.64 (0.46, 0.89)* | 0.40 |
| Subtotal | 1.16 (0.92, 1.47) | 0.008 | 0.69 (0.41, 1.15) | 0.006 | 0.79 (0.57, 1.09) | 0.005 | 0.76 (0.53, 1.12) | 0.029 | |
| I2, (%) | 85.9 | 87.0 | 87.4 | 79.1 | |||||
| Genotyping methods | |||||||||
| PCR-RFLP | 4 | 1.39 (1.17, 1.66)* | 0.65 | 0.49 (0.33, 0.72)* | 0.40 | 0.64 (0.51, 0.80)* | 0.06 | 0.59 (0.41, 0.86)* | 0.35 |
| Others | 2 | 1.04 (0.96, 1.14) | 0.96 | 0.88 (0.75, 1.02) | 0.99 | 0.93 (0.83, 1.04) | 0.71 | 0.90 (0.79, 1.03) | 0.84 |
| Subtotal | 1.17 (0.89, 1.54) | 0.005 | 0.68 (0.38, 1.20) | 0.005 | 0.78 (0.54, 1.13) | 0.003 | 0.76 (0.50, 1.15) | 0.028 | |
| I2, (%) | 87.5 | 87.1 | 88.3 | 79.2 | |||||
| Source of control | |||||||||
| Healthy | 5 | 1.31 (1.12, 1.53)* | 0.65 | 0.55 (0.39, 0.78)* | 0.31 | 0.69 (0.56, 0.85)* | 0.04 | 0.64 (0.46, 0.89)* | 0.40 |
| Healthy and diabetic | 1 | 1.04 (0.96, 1.14) | 0.88 (0.75, 1.02) | 0.93 (0.82, 1.04) | 0.91 (0.79, 1.04) | ||||
| Subtotal | 1.16 (0.92, 1.47) | 0.008 | 0.69 (0.41, 1.15) | 0.006 | 0.79 (0.57, 1.09) | 0.005 | 0.77 (0.52, 1.11) | 0.029 | |
| I2, (%) | 85.9 | 87.0 | 87.4 | 79.1 | |||||
Adults (aged 35–60 y); Adults + elderly (aged 18–75 y); Elderly (aged 90–102 y); Healthy: studies, with controls comprising healthy persons; Healthy and diabetic: studies, with controls comprising healthy persons and those with illnesses; HT, hypertension; P-heterogeneity; RLFP, restriction fragment length polymorphism; VDR, vitamin D receptor.
n, number of studies.
α, 0.05; used to determine statistical significance of the OR.
*OR with statistical significance.
An analysis of studies divided according to the subjects’ age revealed an association with HT in adults based on all genetic models as follows: allelic (f compared with F; OR: 1.10; 95% CI: 1.01, 1.20; P-heterogeneity = 0.002), additive (ff compared with FF; OR: 0.81; 95% CI: 0.70, 0.94; P-heterogeneity = 0.001), risk (ff + Ff compared with FF; OR: 0.85; 95% CI: 0.77, 0.95; P-heterogeneity <0.0001), and common model (ff compared with FF + Ff; OR: 0.87; 95% CI: 0.76, 0.98; P-heterogeneity = 0.02). In addition, when categorized by both sample size (>500 or <500), with a cut-off point of 500, PCR-restriction fragment length polymorphism (RFLP) genotyping, and health condition of the control group, an association between FokI polymorphism and HT in all genetic models was revealed.
Sensitivity analysis
Sensitivity analysis to test the data quality revealed no altered outcomes in overall and subgroup comparisons, indicating the statistical stability of the findings.
Publication bias
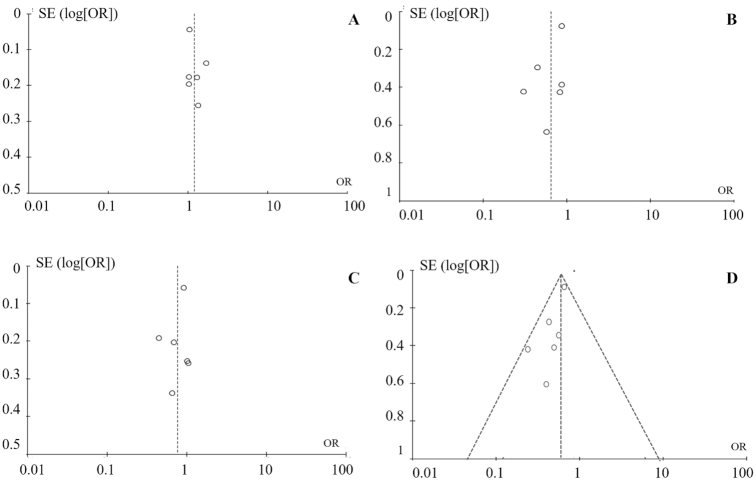
A funnel plot was generated to estimate the publication bias among the studies included in this meta-analysis (Figure 3A–D). The shape of the funnel plots revealed no evidence of publication bias in the genetic models used for the following comparisons: f compared with F, ff compared with FF, ff + Ff compared with FF, and ff compared with FF + Ff.
FIGURE 3.
Analysis of the funnel plot to detect publication bias from 6 eligible studies. A: f compared with F, B: ff compared with FF, C: ff + Ff compared with FF, and D: ff compared with FF + Ff. Circles represent the weight of individual study. log, logarithm.
Conclusions
The VDR gene is a good candidate to study arterial HT, which is affected by the complex interactions among genetic and environmental factors (36, 37). Several genetic variants are involved in this multifactorial disease, such as SNPs, showing phenotypic effects and the ability to alter gene expression. In this context, FokI is considered a functional polymorphism of VDR, as it produces an altered protein. Considering that this SNP is not in linkage disequilibrium with other SNPs on the VDR gene, its associations with genotypes are independent markers (23).
The short version of the protein (genotype FF) likely has an elevated transcriptional activity compared with the longer protein, as it is more responsive to 1,25(OH)2D, supporting differences in VDR functionality and the effect of vitamin D on cells and tissues (38). In 2000, an in vitro study confirmed that the SNP FokI-f results in a lower transcriptional activity than the specific RNA polymerase II transcription factor, having a protective effect against vitamin D deficiency, because of its influence on the circulating vitamin D concentrations and cardiovascular disease risk (29).
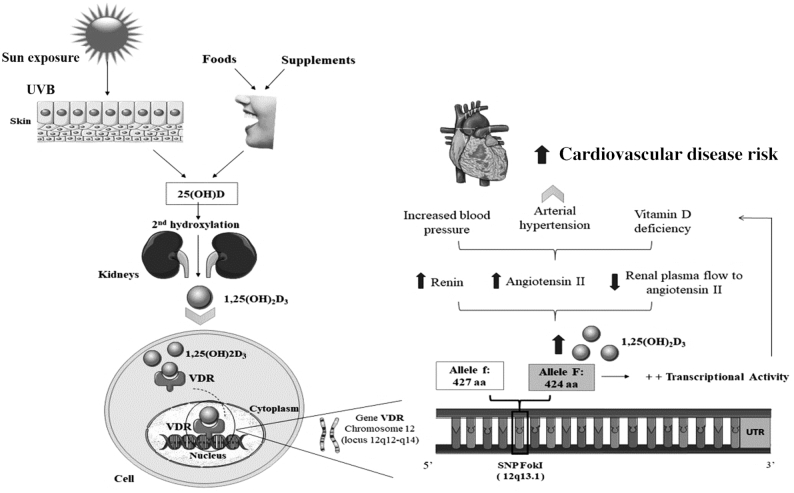
Low concentrations of 25(OH)D combined with FokI have been associated with increased plasmatic renin activity and RAAS (35), supporting the biological role of 1,25(OH)2D in inhibiting renin expression in humans. This might increase cardiovascular and metabolic risks (40). A previous meta-analysis detected an inverse linear association between 1,25(OH)2D and cardiovascular disease risk (41), indicating that the genomic effects of 1,25(OH)2D are mediated in a wide variety of tissues by VDR (42) (Figure 4).
FIGURE 4.
Metabolic interaction among vitamin D, FokI, and cardiovascular disease risk. SNP, single nucleotide polymorphism; UVB, UV radiation B; UTR, untranslated region; VDR, vitamin D receptor; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3.
The present meta-analysis tested the strength of the association between FokI and the odds of HT based on 6 case-control studies, as by grouping data from individual studies, the probability of random errors is reduced. Additionally, the surveys included in the meta-analysis were scored with 5 or more stars according to the NOS criteria, suggesting the excellent quality of the publications, considering that the sample size, genotype, inclusion criteria, and patient and control subject characteristics were successfully obtained.
Data analysis has consistently demonstrated the influence of FokI on HT. Allele f was found to be associated with a significantly lower odds of HT in global analysis under the additive/homozygote and common/recessive genetic models (Figure 2B, D), particularly in the Asian population and specifically in the southern population, under the risk/dominant, additive/homozygote, and common/recessive models. However, the results were not significant for Europeans (Table 3).
These data might be explained by ethnic differences, as the SNP FokI plays a multifunctional role in HT and varies among ethnic populations, indicating the influence of genetic and environmental factors. The ethnic populations evaluated in this study were well-defined. Studies on mixed populations are needed, such as the previous study on Brazilians (43), to assess the impact of ethnicity more accurately.
Notably, the expression and role of VDR in transactivating target genes are determined not only by genetics but also by ethnicity and the environment and involve complex interactions that can alter associations with the disease. The impact of debilitating variants on VDR function can be exacerbated by vitamin D deficiency or the reduced cutaneous production or inadequate ingestion of vitamin D (44). To the best of our knowledge, this is the first meta-analysis to evaluate the association between HT and the SNP FokI; however, unique nucleotide polymorphisms of VDR have been widely studied in other diseases such as tuberculosis, multiple sclerosis, systemic lupus erythematosus, asthma, cirrhosis, and cancer (45). This meta-analysis included only specific cases of HT from relevant studies. In addition, the results have been corroborated by other experimental studies (46–48), which could not be included in this meta-analysis because they did not meet the eligibility criteria.
Regarding the role of SNP FokI in other chronic diseases, Liu et al. (49) analyzed its association with psoriasis but the results were not significant for Caucasians and East Asians. In contrast, Alizadeh et al. (50) found no significant association between SNP FokI and coronary artery disease (CAD) based on a general analysis in Caucasians and East Asians. Recently, Shi et al. (51) observed no relation between this SNP and susceptibility to polycystic ovary syndrome. However, Liu et al. (52) showed that FokI is a susceptibility factor for ovarian cancer. Further, Zhao et al. (53) found an increased risk of intervertebral disc degeneration in Hispanics and Asians with the f allele and Lu et al. (54) suggested that FokI protects against CAD. Cao et al. (55) found that homozygosis is associated with an increased risk of tuberculosis, particularly in East and Southeast Asia, and Jiao et al. (56) observed that the F allele is associated with a decreased risk of diabetic retinopathy in Chinese subjects.
Whether FokI increases disease susceptibility remains unclear. Our meta-analysis demonstrated that the FokI polymorphism in the VDR gene is significantly associated with HT; particularly the f allele, which is considered protective. Our results appear to be reliable based on the statistical power of the 6 studies and the funnel chart, which did not indicate publication bias, as the data were analyzed based on ethnicity. Additionally, heterogeneity was significant in the general analysis.
One limitation of this meta-analysis is the limited sample size used in some of the subgroup assessments, which might not represent the whole population. The publications were restricted to those in English, which could have also introduced bias in the data analysis. Moreover, a limited number of electronic databases were examined.
Another limitation of this study is the risk of making a type I error due to multiple test results, considering that all analyses were exploratory. To reduce this risk, the α-value of 5% was adopted, the value which is most commonly used in research.
Additional studies are needed to understand the mechanisms underlying the association between SNPs and VDR, such as 25(OH)D and 1,25(OH)2D concentrations, and SBP and DBP. Thus, our results should be considered with caution, and large-scale case-controlled studies are still needed to validate our findings.
Studies are also needed to evaluate the functional activity of the normal VDR protein (ff) compared with that of the truncated protein (FF) (57). Moreover, the possible interactions among epistatic genes should be evaluated to identify combinations of genes that synergistically influence the regulation of blood pressure and HT (6) and reveal the physiopathological mechanisms underlying hypertension, as SBP and DBP are associated with FokI (58).
In addition, nutritionally balanced food consumption is important, from birth to old age, for the control of blood pressure throughout the life of an individual (59). Adequate supplementation with vitamin D, dietary intake of vitamin D nutrient sources, fortified foods and supplements, and/or exposure to sunlight to reach adequate concentrations of this vitamin might be useful to prevent cardiovascular diseases (60). Long-term clinical and random control studies are needed to confirm these associations, which might enable the correction of vitamin D deficiency in individuals with and without the FokI polymorphism (61).
Thus, a genetic variant could influence the response to dietary factors and modulate the risk of disease development. In addition, the epigenome responds to environmental changes and depends on the individual's lifestyle. Adequate vitamin D intake and/or supplementation response rate, categorized into low, medium, and high responders, might change due to aging or the onset of diseases such as high blood pressure (62). Thus, the evaluation of gene-nutrient interactions is vital for public health policies worldwide to identify prevention strategies, such as the practice of healthy eating, in each country (63) (Figure 5).
FIGURE 5.
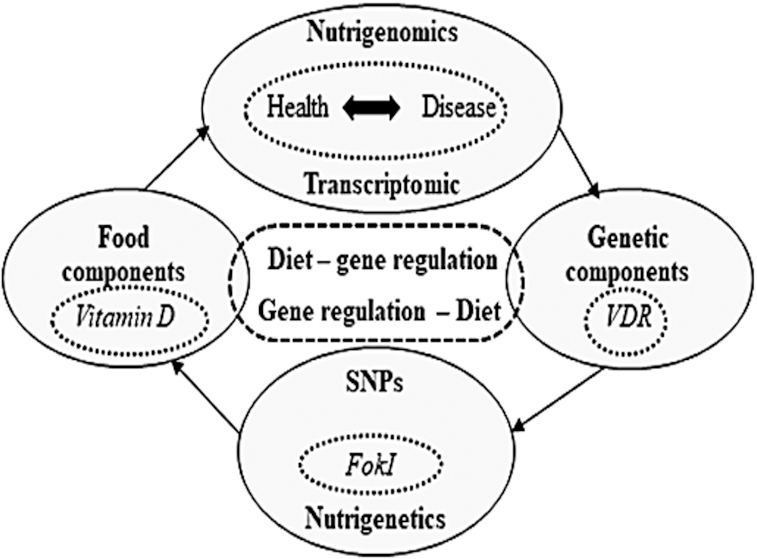
Cycle of nutritional genomics. SNP, single nucleotide polymorphism; VDR, vitamin D receptor.
However, most studies do not analyze genetic and environmental factors together, limiting the broad analysis of this theme. The studies selected for the present meta-analysis did not reveal data on participants’ usual dietary intake, vitamin D supplement use, the nutritional content of macronutrients or micronutrients, and especially vitamin D, or energy. The authors also did not try to evaluate such variables associated with diet, which could also be associated with FokI polymorphism, and reveal more information on its influence on hypertension.
ACKNOWLEDGEMENTS
We acknowledge the Post-Graduation Program in Food and Nutrition of the Federal University of Piauí. The authors’ responsibilities were as follows—JLRS, MSPC, and ACBL, substantial contributions to conception and design; NSCCAT, AAP, and LFMN, data acquisition; AACMC and MVOBC, analysis; IFOCN, MDFCarvalho, LRC, MMR, and CMRGC, interpretation, drafting the article and revising it critically for important intellectual content; and all authors: read and approved the final manuscript.
Notes
Supported by the Federal University of Piauí.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CAD, coronary artery disease; DBP, diastolic blood pressure; HWE, Hardy–Weinberg equilibrium; HT, arterial hypertension; MEDLINE®, Medical Literature Analysis and Retrieval System Online; NOS, Newcastle–Ottawa scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; SNP, single nucleotide polymorphism; VDR, vitamin D receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Ivone F O C Nunes, Post-Graduate Program in Food and Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
Ana A C M Cavalcante, Post-Graduate Program in Pharmaceutical Sciences, Federal University of Piauí, Teresina, Piauí, Brazil.
Marcus V O B Alencar, Post-Graduate Program in Pharmaceutical Sciences, Federal University of Piauí, Teresina, Piauí, Brazil.
Marcos D F Carvalho, Department of Animal Science, Federal University of Piauí, Teresina, Piauí, Brazil.
José L R Sarmento, Post-Graduate Program in Animal Science, Federal University of Piauí, Teresina, Piauí, Brazil.
Nayra S C C A Teixeira, Post-Graduate Program in Food and Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
Adriana A Paiva, Post-Graduate Program in Food and Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
Lídia R Carvalho, Post-Graduate Program in Food and Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
Leopoldo F M Nascimento, Post-Graduate Program in Animal Science, Federal University of Piauí, Teresina, Piauí, Brazil.
Maria S P Cruz, Post-Graduate Program in Animal Science, Federal University of Piauí, Teresina, Piauí, Brazil.
Marcelo M Rogero, Faculty of Public Health, University of São Paulo, São Paulo, São Paulo, Brazil.
Andréia C B Lima, Department of Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
Cecilia M R G Carvalho, Post-Graduate Program in Food and Nutrition, Federal University of Piauí, Teresina, Piauí, Brazil.
References
- 1. Wu L, Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. 2017;31(9):547–54. [DOI] [PubMed] [Google Scholar]
- 2. Kretchy IA, Acheampong F, Laryea J, Osafo J, Asampong E, Dickson E. Personality traits, clinical characteristics, and health-related quality of life of patients with hypertension in a primary hospital in Ghana. Int J Hypertens. 2019;2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Almeida MMS, Guimarães RA, Jardim PCBV, Sousa ALL, de Souza MM. Association between arterial hypertension and nutritional status in adolescents from Goiânia, Goiás, Brazil. PLoS One. 2017;12(12):e0188782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139(12):2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rana S, Kumar S, Rathore N, Padwad Y, Bhushana S. Nutrigenomics and its impact on life style associated metabolic diseases. Curr Genomics. 2016;17(3):261–78./bib> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler MG. Genetics of hypertension. Current status. J Med Liban. 2010;58(3):175–8. [PMC free article] [PubMed] [Google Scholar]
- 7. Bosu WK, Reilly ST, Aheto JMK, Zucchelli E. Hypertension in older adults in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0214934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens. 2018;24:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franczyk A, Stolarz-Skrzypek K, Wesołowska A, Czarnecka D. Vitamin D and vitamin D receptor activators in treatment of hypertension and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets. 2014;14(1):34–44. [DOI] [PubMed] [Google Scholar]
- 10. Torres YC, Pérez AF, Puerta RC, Valdez MV, Castillo IS. Vitamin D deficiency and hypertension. Supporting evidence. Rev Colomb Cardiol. 2016;23(1):42–8. [Google Scholar]
- 11. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–9. [DOI] [PubMed] [Google Scholar]
- 12. Milovanovic M, Pesic G, Nikolic V, Jevtovic-Stoimenov T, Vasic K, Jovic Z, Deljanin-Ilic M, Pesic S. Vitamin D deficiency is associated with increased IL-17 and TNFα levels in patients with chronic heart failure. Arq Bras Cardiol. 2012;98(3):259–65. [DOI] [PubMed] [Google Scholar]
- 13. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am J Hypertens. 2007;20(7):713–9. [DOI] [PubMed] [Google Scholar]
- 14. Dorjgochoo T, Shu XO, Xiang Y-B, Yang G, Cai Q, Li H, Ji BT, Cai H, Gao YT, Zheng W. Circulating 25-hydroxyvitamin D levels in relation to blood pressure parameters and hypertension in the Shanghai Women's and Men's Health Studies. Br J Nutr. 2012;108(3):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lategan R, van den Berg VL, Ilich JZ, Walsh CM. Vitamin D status, hypertension and body mass index in an urban black community in Mangaung, South Africa. Afr J Prim Health Care Fam Med. 2016;8(1):1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meehan M, Penckofer S.. The role of vitamin D in the aging adult. J Aging Gerontol. 2014;2(2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akbari R, Adelani B, Ghadimi R. Serum vitamin D in hypertensive patients versus healthy controls is there an association? Caspian J Intern Med. 2016;7(3):168–72. [PMC free article] [PubMed] [Google Scholar]
- 18. Saponaro F, Marcocci C, Zucchi R. Vitamin D status and cardiovascular outcome. J Endocrinol Invest. 2019;1–6. (doi: 10.1007/s40618-019-01057-y). [DOI] [PubMed] [Google Scholar]
- 19. Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83(6):1903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merke J, Hofmann W, Goldschmidt D, Ritz E. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int. 1987;41(2):112–4. [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, Inoue Y, Morita K, Takeda E, Pike JW. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol. 1997;11(8):1165–79. [DOI] [PubMed] [Google Scholar]
- 22. Zattar LC, Boing AF, Giehl MWC, d'Orsi E. Prevalence and factors associated with high blood pressure, awareness, and treatment among elderly in Southern Brazil. Cad Saude Publica. 2013;29(3):507–21. [DOI] [PubMed] [Google Scholar]
- 23. Uitterlinden AG, Fang, van Meurs Y, Pols JBJ, Van Leeuwen JPTM HAP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–56. [DOI] [PubMed] [Google Scholar]
- 24. Kulah E, Dursun A, Acikgoz S, Can M, Kargi S, Ilikhan S, Bozdogan S. The relationship of target organ damage and 24-hour ambulatory blood pressure monitoring with vitamin D receptor gene FokI polymorphism in essential hypertension. Kidney Blood Press Res. 2006;29(6):344–50. [DOI] [PubMed] [Google Scholar]
- 25. El Gawad S, Samee E, Metwali A, El Gawad M. Vitamin D receptor gene polymorphism and its association with 1,25-dihydroxyvitamin D 3 in patients with Graves disease in an Egyptian population: a pilot study. Endocr Pract. 2012;18(2):132–9. [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Chu A, Buring JE, Ridker PM, Chasman DI, Sesso HD. Common genetic variations in the vitamin D pathway in relation to blood pressure. Am J Hypertens. 2014;27(11):1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gussago C, Arosio B, Guerini FR, Ferri E, Costa AS, Casati M, Bollini EM, Ronchetti F, Colombo E, Bernardelli G et al.. Impact of vitamin D receptor polymorphisms in centenarians. Endocrine. 2016;53(2):558–64. [DOI] [PubMed] [Google Scholar]
- 28. Arai H, Miyamoto K-I, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12(6):915–21. [DOI] [PubMed] [Google Scholar]
- 29. Colin EM, Weel AEAM, Uitterlinden AG, Buurman CJ, Birkenhager JC, Pols HAP, van Leeuwen JPTM. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin Endocrinol. 2000;52(2):211–6. [DOI] [PubMed] [Google Scholar]
- 30. Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh J-C, Zitzer H, Tavakkoli P, Galligan MA, Dang HTL, Haussler CA et al.. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14(3):401–20. [DOI] [PubMed] [Google Scholar]
- 31. McGrath JJ, Saha S, Burne THJ, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121(1–2):471–7. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle-Ottawa Quality assessment Scale Case Control Studies. 2000. [Google Scholar]
- 34. Cottone S, Guarino L, Arsena R, Scazzone C, Tornese F, Guarneri M, Guglielmo C, Bono A, Mulè G. Vitamin D receptor gene polymorphisms and plasma renin activity in essential hypertensive individuals. J Hum Hypertens. 2015;29(8):483–7. [DOI] [PubMed] [Google Scholar]
- 35. Swapna N, Vamsi UM, Usha G, Padma T. Risk conferred by FokI polymorphism of vitamin D receptor (VDR) gene for essential hypertension. Indian J Hum Genet. 2011;17(3):201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia J, Shen C, Mao L, Yang K, Men C, Zhan Y. Vitamin D receptor genetic polymorphism is significantly associated with decreased risk of hypertension in a Chinese Han population. J Clin Hypertens. 2014;16(9):634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Errouagui A, Charoute H, Ghalim N, Barakat A, Kandil M, Rouba H. Relationship with vitamin D receptor (RVD) gene and essential arterial hypertension in Moroccan population. Int J Innov Appl Stud. 2014;8(2):556–66. [Google Scholar]
- 38. Legarth C, Grimm D, Wehland M, Bauer J, Krüger M. The impact of vitamin D in the treatment of essential hypertension. Int J Mol Sci. 2018;19(2):455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glocke M, Lang F, Schaeffeler E, Lang T, Schwab M, Lang UE. Impact of vitamin D receptor VDR rs2228570 polymorphism in oldest old. Kidney Blood Press Res. 2013;37(4–5):311–22. [DOI] [PubMed] [Google Scholar]
- 40. Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, Haussler CA, Galligan MA, Thatcher ML, Encinas Dominguez C et al.. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177(1–2):145–59. [DOI] [PubMed] [Google Scholar]
- 41. Vaidya A, Sun B, Forman JP, Hopkins PN, Brown NJ, Kolatkar NS, Williams GH, Williams JS. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin Endocrinol. 2011;74(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C et al.. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. 2000;22(2):203–17. [DOI] [PubMed] [Google Scholar]
- 44. Rezende VB, Barbosa F, Montenegro MF, Sandrim VC, Gerlach RF, Tanus-Santos JE. An interethnic comparison of the distribution of vitamin D receptor genotypes and haplotypes. Clin Chim Acta. 2007;384(1–2):155–9. [DOI] [PubMed] [Google Scholar]
- 45. O'Neill V, Asani FF, Jeffery TJ, Saccone DS, Bornman L. Vitamin D receptor gene expression and function in a South African population: ethnicity, vitamin D and FokI. PLoS One. 2013;8(6):e67663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 47. Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12(3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L, Ma J, Manson JE, Buring JE, Gaziano JM, Sesso HD. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur J Nutr. 2013;52(7):1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu JL, Zhang SQ, Zeng HM. ApaI, BsmI, FokI and TaqI polymorphisms in the vitamin D receptor (VDR) gene and the risk of psoriasis: a meta-analysis. J Eur Acad Dermatol Venereol. 2013;27(6):739–46. [DOI] [PubMed] [Google Scholar]
- 50. Alizadeh S, Djafarian K, Alizadeh H, Mohseni R, Shab-Bidar S. Common variants of vitamin D receptor gene polymorphisms and susceptibility to coronary artery disease: a systematic review and meta-analysis. J Nutrigenet Nutrigenomics. 2017;10(1–2):9–18. [DOI] [PubMed] [Google Scholar]
- 51. Shi X-Y, Huang A-P, Xie D-W, Yu X-L. Association of vitamin D receptor gene variants with polycystic ovary syndrome: a meta-analysis. BMC Med Genet. 2019;20(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Li C, Chen P, Li X, Li M, Guo H, Li J, Chu R, Wang H. Polymorphisms in the vitamin D receptor (VDR) and the risk of ovarian cancer: a meta-analysis. PLoS One. 2013;8(6):e66716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao J, Yang M, Shao J, Bai Y, Li M. Association between VDR FokI polymorphism and intervertebral disk degeneration. Genomics Proteomics Bioinformatics. 2015;13(6):371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu S, Guo S, Hu F, Guo Y, Yan L, Ma W, Wang Y, Wei Y, Zhang Z, Wang Z. The associations between the polymorphisms of vitamin D receptor and coronary artery disease: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(21):e3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao Y, Wang X, Cao Z, Cheng X. Vitamin D receptor gene FokI polymorphisms and tuberculosis susceptibility: a meta-analysis. Arch Med Sci. 2016;12(5):1118–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiao J, Li Y, Xu S, Wu J, Yue S, Liu L. Association of FokI, TaqI, BsmI and ApaI polymorphisms with diabetic retinopathy: a pooled analysis of case-control studies. Afr H Sci. 2018;18(4):891–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gross C, Krishnan AV, Malloy PJ, Eccleshall TR, Zhao XY, Feldman D. The vitamin D receptor gene start codon polymorphism: a functional analysis of FokI variants. J Bone Miner Res. 1998;13(11):1691–9. [DOI] [PubMed] [Google Scholar]
- 58. Ndiaye NC, Said ES, Stathopoulou MG, Siest G, Tsai MY, Visvikis-Siest S. Epistatic study reveals two genetic interactions in blood pressure regulation. BMC Med Genet. 2013;14(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fantin F, Macchi F, Giani A, Bissoli L. The importance of nutrition in hypertension. Nutrients. 2019;11(10):2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wimalawansa SJ. Vitamin D and cardiovascular diseases: causality. J Steroid Biochem Mol Biol. 2018;175:29–43. [DOI] [PubMed] [Google Scholar]
- 61. Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol. 2018;175:12–7. [DOI] [PubMed] [Google Scholar]
- 62. Barnes S. Nutritional genomics, polyphenols, diets, and their impact on dietetics. J Am Diet Assoc. 2008;108(11):1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ordovas JM, Corella D. Nutritional genomics. Annu Rev Genom Hum Genet. 2004;5(1):71–118. [DOI] [PubMed] [Google Scholar]