This cohort study examines the implications of previous pregnancy and childbirth for the timing of clinically isolated syndrome among women of childbearing age.
Key Points
Question
Do pregnancies and childbirths delay the onset of clinically isolated syndrome (CIS)?
Findings
In this multicenter cohort study of 2557 women with CIS, women with previous pregnancies and childbirths had a later onset of CIS compared with women without pregnancies and childbirths. A higher number of pregnancies and childbirths was not associated with later CIS onset.
Meaning
Findings of this study suggest that future research is needed to explore the mechanisms underpinning the association between pregnancy and timing of the first presentation of multiple sclerosis.
Abstract
Importance
Multiple sclerosis (MS) is usually diagnosed in women during their childbearing years. Currently, no consensus exists on whether pregnancy can delay the first episode of demyelination or clinically isolated syndrome (CIS).
Objective
To investigate the association of pregnancy with time to CIS onset.
Design, Setting, and Participants
This multicenter cohort study collected reproductive history (duration of each pregnancy, date of delivery, length of breastfeeding) on all participants between September 1, 2016, and June 25, 2019. Adult women being treated at the MS outpatient clinics of 4 tertiary hospitals in 2 countries (Charles University and General University Hospital in Prague, Czech Republic; Royal Melbourne Hospital in Melbourne, Australia; Alfred Hospital in Melbourne, Australia; and John Hunter Hospital in Newcastle, Australia) were recruited to participate in the study. Preexisting data (date of CIS onset, date of birth, sex, date of clinical onset, and Expanded Disability Status Scale result) were collected from MSBase, an international registry of long-term prospectively collected data on patients with MS. Data analyses were performed from June 1, 2019, to February 3, 2020.
Exposures
Gravida (defined as any pregnancy, including pregnancy that ended in miscarriage and induced abortion) and parity (defined as childbirth after gestational age of more than 20 weeks, including livebirth and stillbirth) before CIS onset.
Main Outcomes and Measures
Time to CIS onset. The following were assessed: (1) whether women with previous pregnancies and childbirths had a delayed onset of CIS compared with those who had never been pregnant and those who had never given birth, and (2) whether a dose response existed, whereby a higher number of gravidity and parity was associated with a later onset of CIS.
Results
Of the 2557 women included in the study, the mean (SD) age at CIS onset was 31.5 (9.7) years. Of these women, before CIS onset, 1188 (46%) had at least 1 pregnancy and 1100 (43%) had at least 1 childbirth. The mean (SD) age at first pregnancy was 23.3 (4.5) years and at first childbirth was 23.8 (4.5) years. Women with previous pregnancies and childbirths had a later onset of CIS compared with those who had never been pregnant (HR, 0.68; 95% CI, 0.62-0.75; P < .001), with a median delay of 3.3 (95% CI, 2.5-4.1) years. Women who had given birth also had a later CIS onset compared with women who had never given birth (HR 0.68; 95% CI, 0.61-0.75; P < .001), with a similar median delay of 3.4 (95% CI, 1.6-5.2) years. A higher gravidity and parity number was not associated with delay in CIS onset.
Conclusions and Relevance
This study suggests an association between previous pregnancies and childbirths and timing of CIS onset, but having more pregnancies or childbirths did not appear to be associated with a later CIS onset. Further studies are needed to help explain the mechanisms behind the associations between pregnancy and onset of multiple sclerosis.
Introduction
A large burden of multiple sclerosis (MS) is found among women of childbearing age because MS is usually diagnosed between the ages of 20 and 50 years, and women are 3 times more likely than men to develop MS.1 Since the pivotal Pregnancy in Multiple Sclerosis study in 1998,2 it has been well documented that MS relapses are reduced during pregnancy, with a small proportion of women being at risk of rebound relapses in the postpartum period.3,4,5 Consensus is lacking, however, on the implications of pregnancy before the onset of MS. Approximately equal numbers of studies reported either the advantages of pregnancy6,7,8,9,10 or its net neutral outcome,11,12,13,14,15,16,17 with few studies identifying a heightened risk of developing MS associated with pregnancy.18,19
Some of the disparity in results may be explained by the use of various definitions for pregnancy and heterogenous cohort composition.20 Some studies reported childbirths,6,7,9,10,15,16,18 others included miscarriages and induced abortions,8,11,13,14,17 and a few did not specify the pregnancy duration.12,19 In addition, pregnancy was analyzed in different ways, either as a binary outcome (yes or no)6,7,10,15,16,18 or with the use of total number of pregnancies.8,9,10,12,13,14,15,16,17,19 The variation in approaches highlights some of the challenges of interpreting findings from previous studies.
Most past reports6,7,9,10,11,12,13,14,15,16,19 used MS, rather than clinically isolated syndrome (CIS), as the end point. Using CIS as an end point is advantageous in decreasing the inherent problem of reverse causality when exploring this association. In addition, most of the previous research6,8,9,10,12,13,14,15,16,17 focused on whether pregnancy reduced the risk of MS, and only a few studies7,18,19 explored whether pregnancy delayed MS onset.
In this multicenter cohort study, we investigated the association of pregnancy with time to CIS onset. We assessed (1) whether women who had previous pregnancies (gravida) and childbirths (parity) had a delayed onset of CIS compared with those who had never been pregnant (nulligravida) and never given birth (nulliparous), and (2) whether a dose response existed, whereby a higher number of gravidity and parity was associated with a later onset of CIS.
Methods
Ethics approval for this study was granted by the institutional review board of each of the 4 sites. All participants provided written or verbal consent. Data were collected between September 1, 2016, and June 25, 2019.
Participants and Study Design
Patients who were being treated at the MS outpatient clinics of 4 tertiary hospitals in 2 countries (Charles University and General University Hospital in Prague, Czech Republic; Royal Melbourne Hospital in Melbourne, Australia; Alfred Hospital in Melbourne, Australia; and John Hunter Hospital in Newcastle, Australia) were recruited to participate in the study. These MS centers participate in MSBase, an international registry of long-term, prospectively collected data on patients with MS.21 Established in 2004, MSBase was approved by the Melbourne Health Human Research Ethics Committee and by the local ethics committees in participating centers.
For inclusion in this study, women needed to be 18 years or older, have a date of CIS onset, and consent to providing access to their stored minimum data set in MSBase (date of birth, sex, date of clinical onset, and a minimum of 1 Expanded Disability Status Scale [EDSS] result).21 Exclusion criteria were a diagnosis of primary-progressive MS and a history of disease-modifying therapy (DMT) use during CIS.
Eligible participants were approached during clinic appointments or contacted by telephone at a single time point. Reproductive history was collected, including duration of each pregnancy, date of delivery, and length of breastfeeding. Patients were enrolled from September 1, 2016, until June 25, 2019, when data were extracted.
Outcomes and Definitions
The primary outcome was time to CIS onset, which was defined as the first clinical episode of central nervous system demyelination or symptom onset. Gravida was defined as any pregnancy, including pregnancy that ended in miscarriage and induced abortion. Parity was defined as childbirth after a gestational age of more than 20 weeks, including livebirth and stillbirth. Gravida and parity were evaluated as binary variables (yes or no) and ordinal variables (number of pregnancies and childbirths). In the binary variable, women with previous pregnancies and childbirths before CIS onset were compared with women who had never been pregnant, and women who gave birth before CIS onset were compared with women who had never given birth. For the ordinal variable, the total numbers of pregnancies and childbirths before CIS onset were grouped as 0, 1, 2, or 3 or more.
Statistical Analysis
All analyses were performed between June 1, 2019, and February 3, 2020. Mean (SD) or median (interquartile range [IQR]) was used to describe data distribution. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% CIs. The Schoenfeld global test was used to detect a violation of the Cox proportional hazards regression model assumption.22
The dependent or outcome variable was age at CIS onset. The explanatory variable was gravida or parity before CIS onset, which was modeled as a time-dependent variable in all analyses. A woman was considered nonexposed to pregnancy until the date of conception and exposed after conception until the date of CIS. Gravida and parity were assessed in 2 ways: as binary variables (yes or no) and ordinal variables (grouped as 0, 1, 2, or ≥3). The Cox models were adjusted for the site of each participant (Charles University and General University Hospital, Royal Melbourne Hospital, Alfred Hospital, or John Hunter Hospital).
The differences in median survival times were obtained for gravid vs nulligravid groups and parous vs nulliparous groups, and the 95% CIs were calculated from the pooled variance. Data were left censored at 16 years for age at CIS onset and age at pregnancy; that is, women who had CIS onset at 16 years or younger or those who were pregnant before age 16 years were excluded.
Three sensitivity analyses were performed. In the first sensitivity analysis, left censoring occurred at age 18 years instead of 16 years. In the second sensitivity analysis, analyses were stratified according to changing diagnostic criteria for clinically definite MS (CDMS): Poser criteria23 (MS diagnosed in 2001 or earlier), McDonald criteria 200124 (diagnosed 2002-2005), McDonald criteria 200525 (diagnosed 2006-2010), McDonald criteria 201026 (diagnosed 2011-2017), and McDonald criteria 201727 (diagnosed 2018 or later). In the third sensitivity analysis, patients treated with DMT during CIS (ie, before MS diagnosis) were included.
Adjusted Cox model curves were generated for the binary outcomes that allowed for a crude adjustment of the multivariable analysis. The discrete variable, site, was fixed to the level with the maximum participants (Charles University and General University Hospital). These Cox model curves were compared with unadjusted Kaplan-Meier curves using the Simon and Makuch method.28,29 The survival curves were all modeled using pregnancy as a time-dependent variable (ie, Mantel-Byar method).
All statistical analyses were performed with the survival package in R, version 3.6.0 (R Foundation for Statistical Computing). Analyses were 2-tailed, and a P < .05 was used to determine statistical significance.
Results
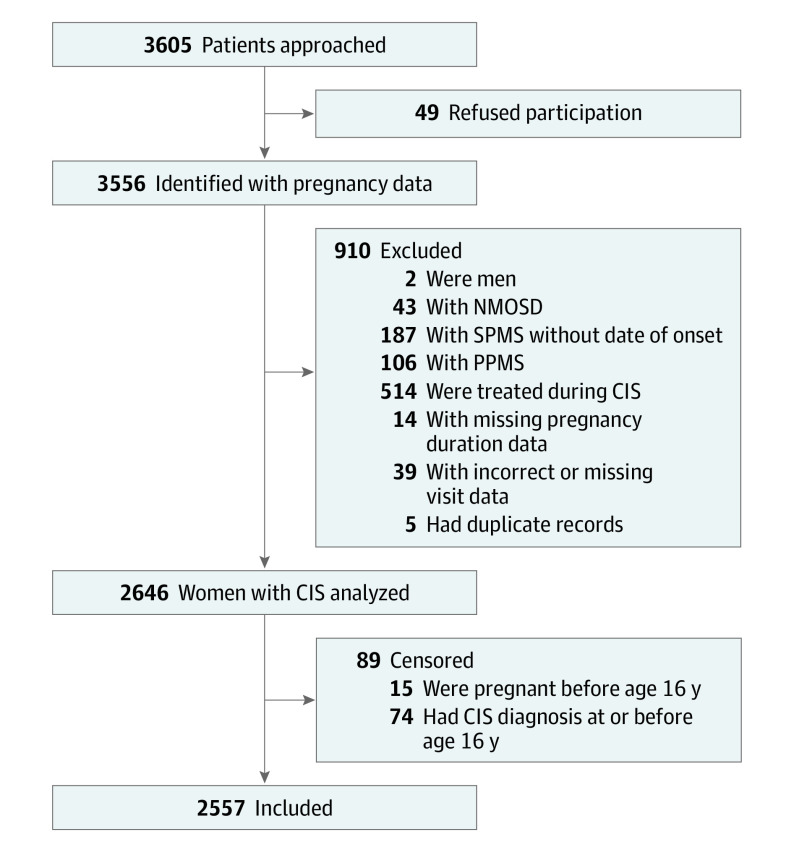
A total of 3605 patients were approached, and 49 refused participation. Of the 3556 patients from whom pregnancy data were collected, 2557 were included in the analysis, with a mean (SD) age at CIS onset of 31.5 (9.7) years (Figure 1 and Table 1). The demographic and clinical characteristics of the cohort are summarized in Table 1. The mean (SD) age at first pregnancy was 23.3 (4.5) years and at first childbirth was 23.8 (4.5) years. The mean (SD) maternal age at first childbirth was 25.6 (4.9) years in Australia (n = 317) and was 23.0 (4.2) years in Czech Republic (n = 783). The mean year of first childbirth was 1991 in Australia and 1987 in Czech Republic.
Figure 1. Flowchart for Study Inclusion.
Two men were included in initial enrollment by mistake. CIS indicates clinically isolated syndrome; NMOSD, neuromyelitis optica spectrum disorder; PPMS, primary progressive multiple sclerosis; and SPMS, secondary progressive multiple sclerosis.
Table 1. Patient Characteristics.
| Baseline characteristic | No. (%) |
|---|---|
| All patients | 2557 |
| Age at CIS onset, mean (SD), y | 31.5 (9.7) |
| Initial symptoms | |
| Supratentorial | 460 (18) |
| Optic neuritis | 599 (23) |
| Brainstem or cerebellum | 464 (18) |
| Spinal | 525 (21) |
| Unifocal onset | 1371 (54) |
| Multifocal onset | 301 (12) |
| No information | 885 (34) |
| Proportion of women with information on OCB status | |
| In Czech Republic (n = 1854) | 316 (17) |
| In Australia (n = 703) | 138 (20) |
| EDSS score at CIS onset, median (IQR) (n = 717)a | 1.5 (1.0-2.0) |
| No. of women with pregnancies before CIS onset | 1188 (46) |
| No. of women with childbirths before CIS onset | 1100 (43) |
| No. of women pregnant at time of CIS onsetb | 71 (3) |
| Age, mean (SD), y | |
| At first pregnancy before CIS onset (n = 1188) | 23.3 (4.5) |
| At first childbirth before CIS onset (n = 1100) | 23.8 (4.5) |
| Per woman, median (IQR) [range] | |
| No. of pregnancies before CIS onset | 0 (0-2) [0-11] |
| No. of childbirths before CIS onset | 0 (0-2) [0-6] |
| Duration, mean (SD), mo | |
| Pregnancies before CIS onset (n = 2586) | 7.2 (3.2) |
| Childbirths before CIS onset (n = 1961) | 9.0 (0.5) |
| Per woman, median (IQR) | |
| Total breastfeeding duration before CIS onset, median (IQR), mo | 0 (0-5) |
Abbreviations: CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale (score range: 0.0-6.5, with higher numbers indicating a greater degree of disability); IQR, interquartile range; OCB, oligoclonal bands.
An EDSS assessment was administered within 12 months of CIS onset. Mean (SD) time between CIS onset and EDSS score was 5.2 (3.5) months.
Seventy-one women were diagnosed with CIS during pregnancy: 66 had childbirths, 3 had spontaneous miscarriages, and 2 had induced abortions.
Almost half of the 2557 participants had at least 1 pregnancy (1176 [46%]) or childbirth (1099 [43%]) before CIS onset. Of the 2557 participants, 71 (3%) had their first demyelinating event during pregnancy. Of these women, 66 had childbirths, 3 had spontaneous miscarriages, and 2 had induced abortions. The median (IQR) cumulative duration of breastfeeding before CIS onset was 0 (0-5) months. Overall, 1371 participants (54%) had a unifocal onset compared with 301 participants (12%) with a multifocal onset, although information was not available for 885 patients (34%). The EDSS score (score range: 0.0-6.5, with higher numbers indicating a greater degree of disability) within 12 months of CIS onset was available for 717 participants (28%), and the median (IQR) EDSS score at this visit was 1.5 (1.0-2.0). No differences were observed between the nulligravid vs gravid groups and nulliparous vs parous groups on EDSS score at CIS onset or presentation of initial symptoms (unifocal/multifocal onset). Cerebrospinal fluid (CSF) oligoclonal band (OCB) positivity was present in 316 of 1854 patients (17%) in Czech Republic and 138 of 703 patients (20%) in Australia.
Primary Analysis
Women with previous pregnancies and childbirths had a later onset of CIS compared with women who had never been pregnant (HR, 0.68; 95% CI, 0.62-0.75; P < .001) (Table 2, Figure 2A, and Figure 3A). This later onset translated to a median delay to CIS onset of 3.3 years (95% CI, 2.5-4.1). Women who had given birth vs those who had not also had a later onset of CIS (HR, 0.68; 95% CI, 0.61-0.75; P < .001) and a similar median delay of 3.4 years (95% CI, 1.6-5.2) (Table 2, Figure 2B, and Figure 3B). Adjusted Cox model curves for time to CIS onset are displayed in Figure 2, and these results are similar to the Kaplan-Meier curves shown in the eFigure in the Supplement.
Table 2. Cox Proportional Hazards Regression Analysis of Clinically Isolated Syndrome Onseta.
| Primary analysis | HR (95% CI) | P value |
|---|---|---|
| Gravida | ||
| No | 1 [Reference] | |
| Yes | 0.68 (0.62-0.75) | <.001 |
| No. of pregnancies | ||
| 0 | 1 [Reference] | |
| 1 | 0.70 (0.61-0.79) | <.001 |
| 2 | 0.67 (0.60-0.76) | <.001 |
| ≥3 | 0.66 (0.58-0.75) | <.001 |
| Parity | ||
| No | 1 [Reference] | |
| Yes | 0.68 (0.61-0.75) | <.001 |
| No. of childbirths | ||
| 0 | 1 [Reference] | |
| 1 | 0.70 (0.62-0.79) | <.001 |
| 2 | 0.66 (0.58-0.74) | <.001 |
| ≥3 | 0.69 (0.58-0.81) | <.001 |
Abbreviation: HR, hazard ratio.
All analyses were adjusted for site.
Figure 2. Adjusted Cox Proportional Hazards Regression Model Curves for Time to Clinically Isolated Syndrome (CIS) Onset by Gravida and Parity.
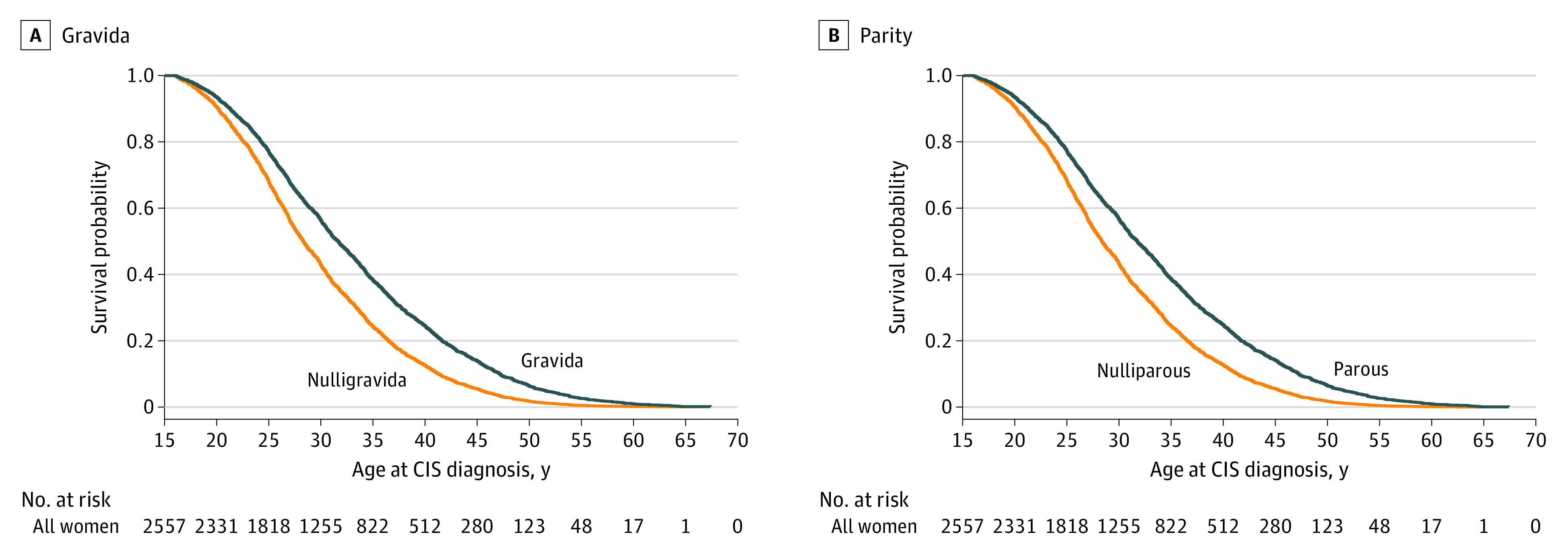
Cox model curves were adjusted for site, which was fixed to the level with the maximum participants (Charles University and General University Hospital). Kaplan-Meier curves using the Simon and Makuch method are shown in the eFigure in the Supplement.
Figure 3. Association of Gravida and Parity With Time to Clinically Isolated Syndrome (CIS) Onset.
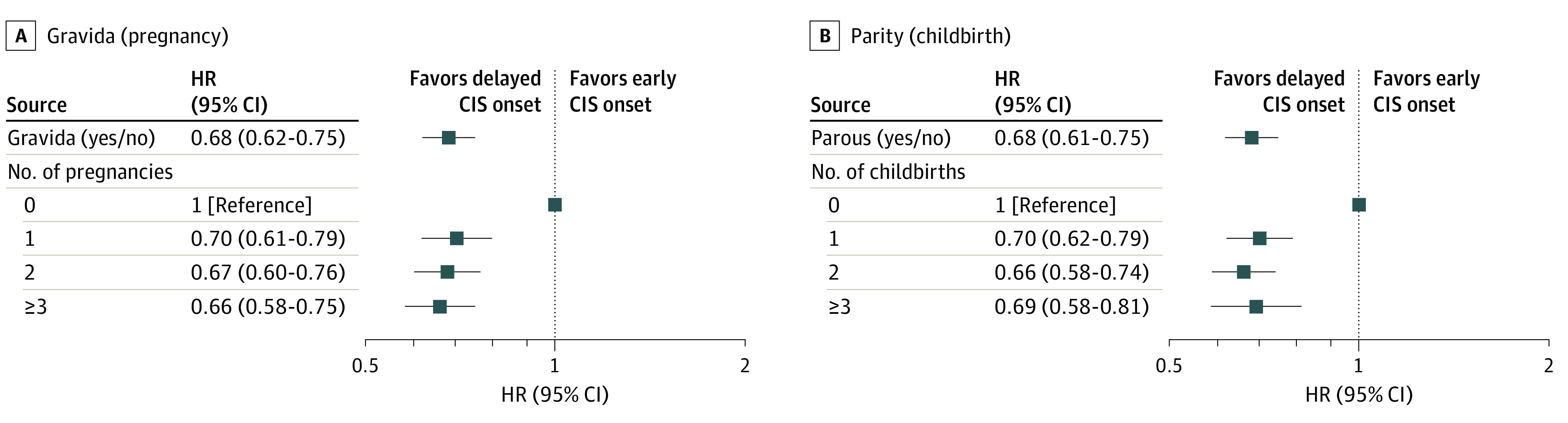
HR indicates hazard ratio. Analyses were adjusted by site.
A dose response of gravidity and parity number was not observed in the present cohort (Table 2 and Figure 3). Having 1 (HR, 0.70; 95% CI, 0.61-0.79), 2 (HR, 0.67; 95% CI, 0.60-0.76), or 3 or more (HR, 0.66; 95% CI, 0.58-0.75) pregnancies or 1 (HR, 0.70; 95% CI, 0.62-0.79), 2 (HR, 0.66; 95% CI, 0.58-0.74), or 3 or more (HR, 0.69; 95% CI, 0.58-0.81) childbirths all delayed the onset of CIS compared with 0 pregnancies (P < .001). The categories had overlapping CIs, and a higher gravidity and parity number was not associated with later CIS onset.
Sensitivity Analyses
All sensitivity analyses confirmed the results of the primary analysis. When participants were left censored at age 18 years rather than age 16 years, 173 women were excluded, leaving a total number of 2384 participants for analysis. Results from this first sensitivity analysis demonstrated that women with previous pregnancies (HR, 0.68; 95% CI, 0.62-0.75) and childbirths (HR, 0.69; 95% CI, 0.62-0.76) had a later onset of CIS, and a higher gravidity and parity number was not associated with a change in CIS onset (eTable in the Supplement).
In the second sensitivity analysis, 1046 patients were diagnosed with CIS before 2001 (inclusive), 439 between 2002 and 2005, 630 between 2006 and 2010, 434 between 2011 and 2017, and 8 in 2018 and after. An analysis stratified for diagnostic criteria showed that women with previous pregnancies and childbirths had a similar later CIS onset (gravid: HR, 0.62 [95% CI, 0.56-0.69; P < .001] vs parous: HR, 0.62 [95% CI, 0.56-0.68; P < .001]), compared with women who had never been pregnant and those who had never given birth.
In the third sensitivity analysis, inclusion of patients treated during CIS resulted in an additional 492 women, for a total of 3049 participants. Women with previous pregnancies and childbirths were found to have a delayed CIS onset (HR, 0.68; 95% CI, 0.62-0.74; P < .001).
Discussion
This retrospective cohort study found that women with previous pregnancies and childbirths had a later onset of CIS compared with women who had never been pregnant and those who had never given birth. The time to CIS onset was delayed by a median of 3.3 years in women with previous pregnancies and 3.4 years in women with previous childbirths. Having more pregnancies or childbirths was not associated with a later CIS onset.
Currently, no consensus exists on the association of pregnancy with CIS or MS onset. A recent review of the literature identified 14 studies6,7,8,9,10,11,12,13,14,15,16,17,18,19 that explored this association,20 in which the investigators of half of the studies argued against an association between pregnancy and CIS or MS onset.11,12,13,14,15,16,17 Other studies addressed different questions: a few explored whether pregnancy delayed symptom onset,7,18 but most studies focused on whether pregnancy changed the risk of developing CIS or MS.6,8,9,10,12,13,14,15,16,17 It can be argued that using risk rather than delay as an outcome is potentially problematic. Factors other than pregnancy may play a greater role in the risk of developing MS, such as genetic predisposition, low vitamin D levels, smoking, and Epstein-Barr virus infection.30,31 Therefore, it is not surprising that many historical reports11,12,13,14,15,16,17 did not find pregnancy to be associated with decreased risk of CIS or MS.
Of the 2 studies7,18 that did explore time to CIS or MS onset, 1 was a French prospective cohort study of 60 women with radiologically isolated syndrome (RIS); 7 of these 60 women became pregnant after a mean follow-up of 7 years.18 Briefly, RIS is a form of preclinical MS, in which magnetic resonance imaging findings suggestive of MS are present in the absence of any history or clinical signs of MS.27 Lebrun et al18 observed that the pregnant group had a shorter time from first magnetic resonance imaging to CIS conversion compared with the nonpregnant group (15 vs 36 months). The pregnant group vs the nonpregnant group was noted to have a higher proportion of CSF OCB positivity, a known poor prognostic marker for CDMS.32,33,34 Therefore, other factors were likely present that predisposed the pregnant group to develop CIS. This RIS cohort was small, and further studies are required to evaluate the role of pregnancy in women with RIS.
The second study that explored whether pregnancy delayed symptom onset was a Swedish retrospective cohort of 770 women with MS.7 This study found that women who had given birth had a later age at CIS onset compared with women who had not (31 years vs 23 years). The present study similarly found that women who had given birth had a delayed CIS onset and a smaller median delay of 3.4 years compared with the 8 years mean difference reported in the Swedish study. The present study incorporated a large cohort of more than 2500 patients, and survival analyses with pregnancy as a time-dependent variable were performed to address the issue of immortal time bias, which may account for some of the differences in results.
Only 1 previous study, the Barcelona MS and Gender project, used a time-dependent approach, although its main analysis was time to CDMS.35 The study did, however, similarly observe a younger mean age at CIS for women who had never given birth vs women who had given birth before CIS onset (28 years vs 37 years).
In this analysis, CIS rather than MS was used as an end point to reduce the risk of reverse causality. That is, women with more pronounced early symptoms may change their conceptive behavior and avoid pregnancy, or they may have subclinical disease that affects fertility.36 In this cohort, a well-balanced proportion of women reported at least 1 pregnancy (46%) or no pregnancy (54%) before their CIS diagnosis. We also performed an exploratory analysis using time to MS onset as the end point. We found a trend for women with previous pregnancies and childbirths to have a later onset of MS compared with women who had never been pregnant and those who had never given birth, although this association was not as strong as the association for CIS. These findings highlight the importance of using CIS as the outcome.
A Canadian matched cohort study found that patients with MS had more frequent health care use compared with control participants in the 5 years before CIS onset.37 This notion of an MS prodrome (ie, premorbid symptoms) in the years before CIS diagnosis suggests that the implications of reverse causality cannot be completely eliminated, even when CIS is used as an end point. To further reduce this potential bias, we excluded patients who received DMT during CIS. Treatment for CIS, as opposed to CDMS, is generally not approved in the countries in which this study was undertaken. A proportion of these treated patients may represent a biased group who had additional predisposing factors, such as prodromal symptoms, that prompted the clinician to commence early DMT.
Apart from assessing CIS diagnosis and excluding treated patients with CIS, we acknowledge that it is difficult to address the prodromal question given that none of the participating countries have national health registries to match patients with control participants on various prodromal symptoms such as depression and fatigue. We have, however, compared the mean maternal age at first childbirth in the cohort with that of the participating countries during a similar time frame. Historical reports indicated that the mean age of first childbirth in Czech Republic was 22.5 years in 199038 and in Australia was 25.8 years in 1991.39 Therefore, the cohort age at first childbirth was comparable with the national maternal age, which may indicate a lesser implication of an MS prodrome for conceptive behavior in the cohort that we studied.
In regard to gravidity and parity number, previous reports were inconclusive, with approximately half of the studies showing no association with gravidity or parity number12,13,14,16,17 and the other half demonstrating an advantage of lower CIS or MS risk associated with a higher gravidity or parity number.8,9,10,15 The Australian AusImmune Study was the only one to focus exclusively on patients with CIS.8 In that case-control study of 216 women with CIS and 542 control participants who were matched in age and postal code, a higher number of pregnancies and livebirths was found to reduce the risk of CIS.8
In the present study, we assessed timing (rather than risk) of CIS onset, with pregnancy modeled as a time-dependent variable, something that has not been done before. Using this method, we did not find an association between a higher gravidity or parity number and later CIS onset. One possible explanation for the lack of a dose response is that pregnancy may delay CIS onset through epigenetic mechanisms, such as DNA methylation changes or histone modifications. Although this area is understudied, recent data show, for example, that numerous DNA methylation changes occur by the 10th week of pregnancy.40 Therefore, the duration of the pregnancy (gravidity vs parity) may be less important than the occurrence of the pregnancy. In addition, a recent study of patients with relapsing-remitting MS found that estrogens promote a more tolerogenic T-cell repertoire in pregnancy through epigenetic mechanisms.41 Epigenetic changes that occur during pregnancy may persist beyond the pregnancy itself and for several years. For example, if a genetic locus is demethylated and remains so, then it will remain unchanged with a second or third pregnancy and may therefore explain the absence of a dose response and the absence of any difference between gravidity and parity in the present cohort. The biological substrate underlying this process is the subject of planned studies by our group.
Overall, findings of this study support the hypothesis that being pregnant before CIS onset is associated with a delay in CIS onset. However, the female to male ratio in relapsing-remitting MS has been increasing.42 Furthermore, consistent global trends show an increase in maternal age over the past 2 decades.43,44 In 2017, the mean age at first childbirth was 28.2 years in Czech Republic43 and 29.2 years in Australia,44 representing a 5.5- and 3.5-year increase, respectively, over 16 to 17 years. The delayed and reduced pregnancy rates reported are likely associated with the increasing burden of MS observed among women of childbearing age.
Limitations
This study has several limitations. First, we cannot completely eliminate the problem of reverse causality, given that evidence suggests that CIS and MS are associated with a prodrome or premorbid symptoms.37 We have tried to minimize reverse causality by using CIS rather than MS as the end point, excluding treated patients with CIS, and using pregnancy as a time-dependent variable.
Second, it is possible other confounders or mediators that were not accounted for played a role in time to CIS onset. Therefore, we do not purport to report the direct causal relationship between pregnancy and CIS onset because the study was designed only to investigate the overall implications of pregnancy. We did adjust the analyses for site, to account for its potential confounding role, and the results were comparable to the unadjusted analysis. The CSF OCB positivity was another potential confounder for which we were unable to adjust because (1) CSF analysis is not routinely performed in Australia and (2) the documentation of OCB is not compulsory in the MSBase registry. Although the numbers were likely to be an underestimate of their true value, we identified 316 women (17%) in Czech Republic and 138 women (20%) in Australia who had positive OCB information (Table 1). We did an exploratory analysis on these 454 OCB-positive patients only and found results that were comparable to the primary analysis for women with previous pregnancies and childbirths. Socioeconomic status is an additional confounder that could not be adjusted for, as this information is not currently collected in MSBase. However, because all 4 hospitals in the study are tertiary MS referral centers in their cities, they see a broad range of patients from various socioeconomic backgrounds, which may decrease this selection bias.
Third, recall bias was a possibility because of the retrospective design of this study, although the delivery dates and pregnancy durations were well recalled by most participants.
Conclusions
In women diagnosed with CIS, previous pregnancies and childbirths were associated with a later onset of CIS. The association of gravidity and parity with the timing of CIS onset was not dependent on the number of pregnancies. These findings support the notion that pregnancy is associated with the delay in CIS onset. Further research is needed to explore the mechanisms underpinning these associations of pregnancy with timing of MS onset.
eFigure. Kaplan-Meier Curves (Using the Simon and Makuch Method) for Time to CIS by Gravida and Parity
eTable. Cox Regression Analysis of the Hazard of CIS Onset: Sensitivity Analysis Left-Censoring at 18 Years Compared to 16 Years
References
- 1.Miller DH, Fazekas F, Montalban X, Reingold SC, Trojano M. Pregnancy, sex and hormonal factors in multiple sclerosis. Mult Scler. 2014;20(5):527-536. doi: 10.1177/1352458513519840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group . Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339(5):285-291. doi: 10.1056/NEJM199807303390501 [DOI] [PubMed] [Google Scholar]
- 3.Vukusic S, Hutchinson M, Hours M, et al. ; Pregnancy in Multiple Sclerosis Group . Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(pt 6):1353-1360. doi: 10.1093/brain/awh152 [DOI] [PubMed] [Google Scholar]
- 4.Finkelsztejn A, Brooks JB, Paschoal FM Jr, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? a systematic review and meta-analysis of the literature. BJOG. 2011;118(7):790-797. doi: 10.1111/j.1471-0528.2011.02931.x [DOI] [PubMed] [Google Scholar]
- 5.Hughes SE, Spelman T, Gray OM, et al. ; MSBase study group . Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler. 2014;20(6):739-746. doi: 10.1177/1352458513507816 [DOI] [PubMed] [Google Scholar]
- 6.Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain. 1995;118(pt 1):253-261. doi: 10.1093/brain/118.1.253 [DOI] [PubMed] [Google Scholar]
- 7.Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril. 2010;94(7):2835-2837. doi: 10.1016/j.fertnstert.2010.06.045 [DOI] [PubMed] [Google Scholar]
- 8.Ponsonby AL, Lucas RM, van der Mei IA, et al. . Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology. 2012;78(12):867-874. doi: 10.1212/WNL.0b013e31824c4648 [DOI] [PubMed] [Google Scholar]
- 9.Magyari M, Koch-Henriksen N, Pfleger CC, Sørensen PS. Reproduction and the risk of multiple sclerosis. Mult Scler. 2013;19(12):1604-1609. doi: 10.1177/1352458513481397 [DOI] [PubMed] [Google Scholar]
- 10.Salehi F, Abdollahpour I, Nedjat S, et al. . Uncovering the link between reproductive factors and multiple sclerosis: a case-control study on Iranian females. Mult Scler Relat Disord. 2018;20:164-168. doi: 10.1016/j.msard.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 11.Leibowitz U, Antonovsky A, Kats R, Alter M. Does pregnancy increase the risk of multiple sclerosis? J Neurol Neurosurg Psychiatry. 1967;30(4):354-357. doi: 10.1136/jnnp.30.4.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villard-Mackintosh L, Vessey MP. Oral contraceptives and reproductive factors in multiple sclerosis incidence. Contraception. 1993;47(2):161-168. doi: 10.1016/0010-7824(93)90088-O [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Hohol MJ, Olek MJ, Spiegelman D, Ascherio A. Oral contraceptives and the incidence of multiple sclerosis. Neurology. 2000;55(6):848-854. doi: 10.1212/WNL.55.6.848 [DOI] [PubMed] [Google Scholar]
- 14.Alonso A, Jick SS, Olek MJ, Ascherio A, Jick H, Hernán MA. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol. 2005;62(9):1362-1365. doi: 10.1001/archneur.62.9.1362 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen NM, Jørgensen KT, Stenager E, et al. . Reproductive history and risk of multiple sclerosis. Epidemiology. 2011;22(4):546-552. doi: 10.1097/EDE.0b013e31821c7adc [DOI] [PubMed] [Google Scholar]
- 16.Hedström AK, Hillert J, Olsson T, Alfredsson L. Reverse causality behind the association between reproductive history and MS. Mult Scler. 2014;20(4):406-411. doi: 10.1177/1352458513498126 [DOI] [PubMed] [Google Scholar]
- 17.Langer-Gould A, Smith JB, Hellwig K, et al. . Breastfeeding, ovulatory years, and risk of multiple sclerosis. Neurology. 2017;89(6):563-569. doi: 10.1212/WNL.0000000000004207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrun C, Le Page E, Kantarci O, Siva A, Pelletier D, Okuda DT; Club Francophone de Sclerose en Plaques (CFSEP); Radiologically Isolated Syndrome Consortium (RISC) Group . Impact of pregnancy on conversion to clinically isolated syndrome in a radiologically isolated syndrome cohort. Mult Scler. 2012;18(9):1297-1302. doi: 10.1177/1352458511435931 [DOI] [PubMed] [Google Scholar]
- 19.Mohammadbeigi A, Kazemitabaee M, Etemadifar M. Risk factors of early onset of MS in women in reproductive age period: survival analysis approach. Arch Womens Ment Health. 2016;19(4):681-686. doi: 10.1007/s00737-016-0600-1 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen AL, Eastaugh A, van der Walt A, Jokubaitis VG. Pregnancy and multiple sclerosis: clinical effects across the lifespan. Autoimmun Rev. 2019;18(10):102360. doi: 10.1016/j.autrev.2019.102360 [DOI] [PubMed] [Google Scholar]
- 21.Butzkueven H, Chapman J, Cristiano E, et al. . MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler. 2006;12(6):769-774. doi: 10.1177/1352458506070775 [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67(1):145-153. doi: 10.1093/biomet/67.1.145 [DOI] [Google Scholar]
- 23.Poser CM, Paty DW, Scheinberg L, et al. . New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231. doi: 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 24.McDonald WI, Compston A, Edan G, et al. . Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50(1):121-127. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Edan G, et al. . Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson AJ, Banwell BL, Barkhof F, et al. . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 28.Schultz LR, Peterson EL, Breslau N. Graphing survival curve estimates for time-dependent covariates. Int J Methods Psychiatr Res. 2002;11(2):68-74. doi: 10.1002/mpr.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35-44. doi: 10.1002/sim.4780030106 [DOI] [PubMed] [Google Scholar]
- 30.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25-36. doi: 10.1038/nrneurol.2016.187 [DOI] [PubMed] [Google Scholar]
- 31.van der Mei I, Lucas RM, Taylor BV, et al. . Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Mult Scler. 2016;22(4):461-469. doi: 10.1177/1352458515594040 [DOI] [PubMed] [Google Scholar]
- 32.Miller DH, Ormerod IE, Rudge P, Kendall BE, Moseley IF, McDonald WI. The early risk of multiple sclerosis following isolated acute syndromes of the brainstem and spinal cord. Ann Neurol. 1989;26(5):635-639. doi: 10.1002/ana.410260508 [DOI] [PubMed] [Google Scholar]
- 33.Tintoré M, Rovira A, Río J, et al. . Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology. 2008;70(13, pt 2):1079-1083. doi: 10.1212/01.wnl.0000280576.73609.c6 [DOI] [PubMed] [Google Scholar]
- 34.Skov AG, Skov T, Frederiksen JL. Oligoclonal bands predict multiple sclerosis after optic neuritis: a literature survey. Mult Scler. 2011;17(4):404-410. doi: 10.1177/1352458510391340 [DOI] [PubMed] [Google Scholar]
- 35.Zuluaga MI, Otero-Romero S, Rovira A, et al. . Menarche, pregnancies, and breastfeeding do not modify long-term prognosis in multiple sclerosis. Neurology. 2019;92(13):e1507-e1516. doi: 10.1212/WNL.0000000000007178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalla P, Rovei V, Masera S, et al. . Fertility in patients with multiple sclerosis: current knowledge and future perspectives. Neurol Sci. 2006;27(4):231-239. doi: 10.1007/s10072-006-0676-x [DOI] [PubMed] [Google Scholar]
- 37.Wijnands JMA, Kingwell E, Zhu F, et al. . Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451. doi: 10.1016/S1474-4422(17)30076-5 [DOI] [PubMed] [Google Scholar]
- 38.Sobotka T, Šťastná A, Zeman K, Hamplová D, Kantorová V.. Czech Republic: a rapid transformation of fertility and family behaviour after the collapse of state socialism. Demographic Research. 2008;19(14):403-454. doi: 10.4054/DemRes.2008.19.14 [DOI] [Google Scholar]
- 39.Australian Institute of Health and Welfare. Australia’s mothers and babies 1993. AIHW; 1996. Accessed May 4, 2020. https://www.aihw.gov.au/reports/mothers-babies/australias-mothers-babies-1993/contents/table-of-contents
- 40.Gruzieva O, Merid SK, Chen S, et al. . DNA methylation trajectories during pregnancy. Epigenet Insights. 2019;12:2516865719867090. doi: 10.1177/2516865719867090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannello A, Rolla S, Maglione A, et al. . Pregnancy epigenetic signature in T helper 17 and T regulatory cells in multiple sclerosis. Front Immunol. 2019;9:3075. doi: 10.3389/fimmu.2018.03075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trojano M, Lucchese G, Graziano G, et al. ; MSBase Study Group and the New Zealand MS Prevalence Study Group . Geographical variations in sex ratio trends over time in multiple sclerosis. PLoS One. 2012;7(10):e48078. doi: 10.1371/journal.pone.0048078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eurostat Mean age of women at childbirth and at birth of first child. Updated March 7, 2020. Accessed May 4, 2020. https://ec.europa.eu/eurostat/databrowser/view/tps00017/default/table?lang=en
- 44.Australian Institute of Health and Welfare Australia’s mothers and babies 2017—in brief. AIHW; 2019. Accessed May 4, 2020. https://www.aihw.gov.au/reports/mothers-babies/australias-mothers-and-babies-2017-in-brief/contents/table-of-contents
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Kaplan-Meier Curves (Using the Simon and Makuch Method) for Time to CIS by Gravida and Parity
eTable. Cox Regression Analysis of the Hazard of CIS Onset: Sensitivity Analysis Left-Censoring at 18 Years Compared to 16 Years