Abstract
The first trans-selective cyanoboration reaction of an alkyne, specifically a 1,3-enyne, is described. The reported palladium-catalyzed cyanoboration of 1,3-enynes is site-, regio-, and diastereoselective, and is uniquely enabled by the 1,4-azaborine-based Senphos ligand structure. Tetra-substituted alkenyl nitriles are obtained providing useful boron-dienenitrile building blocks that can be further functionalized. The utility of our method has been demonstrated with the synthesis of Satigrel, an anti-platelet aggregating agent
Keywords: trans-cyanoboration, azaborine, enyne
Graphical Abstract
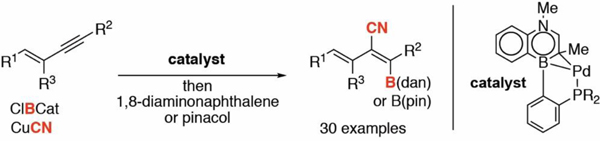
A trans-selective alkyne cyanoboration reaction debuts! A Pd complex supported by an 1,4-azaborine-derived phosphine ligand is uniquely capable in transforming 1,3-enynes into tetra-substituted borylated dienenitriles with high site-, regio- and trans-diastereoselectivity.
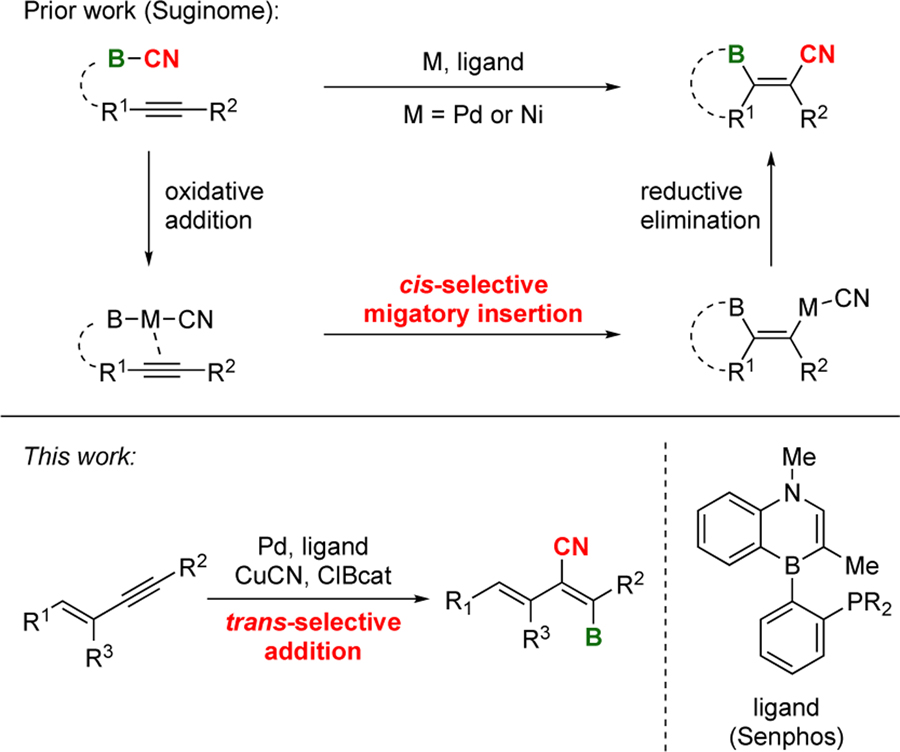
The alkenyl nitrile motif plays an important role in the field of polymers,1 pharmaceutics,2 and agrochemistry.3 Thus, versatile and stereoselective synthetic approaches to substituted alkenyl nitriles has attracted significant attention. To date, a number of methods have been reported that involve alkyne X-CN difunctionalization (X = B,4 C,5 N,6 O,7 halogen,5d, 8 Ge,9 S,10 Se,10e Si11 and Sn12). The difunctionalization approach is attractive because the additionally installed functional group X provides a handle for possible structural diversification. Among the difunctionalization methods, the cyanoboration of alkynes4 is particularly appealing due to the rich functionalization chemistry of organoboron derivatives.13 Suginome reported the first intra- and intermolecular cyanoboration of alkynes catalyzed by Pd and Ni complexes (Scheme 1, top).4 The working mechanistic hypothesis suggests initial oxidative addition of the B–CN bond to the metal followed by cis-selective β-migratory insertion into the alkyne and C–C reductive elimination, furnishing the cis-cyanoboration product.14 The trans-selective X-CN difunctionalization of alkynes has significantly less precedent. 5b–d, 10b,12 For example, no trans-selective cyanoboration reaction of an alkyne has been reported to date. Herein we describe the trans-cyanoboration of 1,3-enynes catalyzed by a Pd complex supported by a 1,4-azaborine-based biaryl phosphine (Senphos) ligand (Scheme 1, bottom). Highly substituted alkenyl nitriles, including tetra-substituted derivatives are obtained in a site-, regio- and diastereoselective fashion, providing boron-dienenitrile building blocks that cannot be readily accessed by other synthetic methods.
Scheme 1.
Cyanoboration of alkynes.
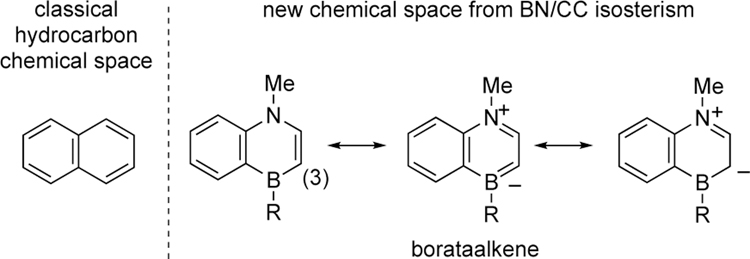
Our laboratory has been investigating BN/CC isosterism15 (substitution of a CC bond unit with a BN bond unit) as a strategy to create structural, and as a consequence, functional diversity. To date, BN/CC isosterism has been successfully applied to create new properties and functions in biomedical research,16 materials science,17 and organic synthesis.18 Somewhat surprisingly, the application of BN/CC isosterism to the ligand space has attracted less attention.19 To this end, we recently reported a ligand family based on the biaryl 1,4-azaborine scaffold.20 Electronic structure elucidation revealed a strong borataalkene character (Scheme 2) which renders the C(3) carbon significantly more nucleophilic/electron rich than the corresponding carbonaceous arene. The access to the new ligand space from BN/CC isosterism has resulted in new reaction selectivity, specifically the trans-selective hydroboration of 1,3-enynes.21 In our continued efforts to expand the utility of Senphos-type ligands in metal-catalyzed transformations we are addressing in this work the outstanding problem of trans-selective cyanoboration reaction.
Scheme 2.
Electronic structure of 1,4-azaborines.
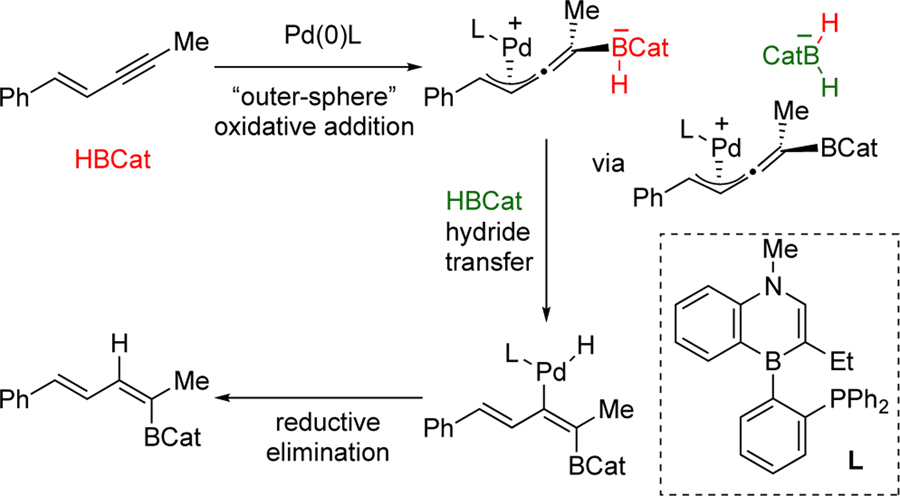
DFT calculations for the reported trans-hydroboration reaction predict a reaction pathway that involves an outer-sphere oxidative addition with catecholborane that is then followed by hydride transfer and reductive elimination (Scheme 3).22 We envisioned that chlorocatecholborane (Cl-BCat) could serve as a potential boron source capable of facilitating the outersphere oxidative addition step and that a cyanide anion23 (instead of a hydride) would attack the resulting Pd complex to furnish the cyanoboration product.
Scheme 3.
Mechanism of the trans-hydroboration of 1, 3-enynes predicted by DFT calculations.
Thus, with Cl-BCat as the boron source,24 copper(I) cyanide as the cyanide source,25 Pd complex 3 as the precatalyst, and enyne 1a as the initial substrate, we evaluated the effects of the ligand structure on the cyanoboration reactivity and selectivity. As can be seen from Table 1, the absence of a supporting ligand does not lead to an efficient productive reaction (entry 1). The presence of monodentate phosphine ligands promotes the product formation, albeit in low yield (entries 2 and 3). On the other hand, the bidentate phosphine ligand dppe is completely ineffective (entry 4). Gratifyingly, the use of Senphos-type ligands results in a substantial increase in the product yield while also achieving greater than 95:5 trans addition selectivity (entries 5–9). The substituent R at the C(3) position of the ligand has a profound influence on the product yield but not on the diastereoselectivity. For example, when L1, which bears the methyl group at the C(3) position, is used as the ligand, the reaction gives the product in superior yield (Table 1, entry 5) compared to those with bigger R substituents (entries 6–8). Switching the boron substituent from the o-dicyclohexyl-phosphinophenyl to o-diphenyl-phosphino-phenyl group results in diminished reactivity for the model substrate 1a (Table 1, entry 5 vs. 9). Lastly, we determined that CC-L1, the carbonaceous analogue of the best performing Senphos ligand, is inferior to L1 with regard to reaction efficiency and selectivity (entry 10 vs. entry 5), highlighting the importance of the unique electronic structure of the 1,4-azaborine motif in promoting the reaction.26
Table 1.
Pd-catalyzed trans-cyanoboration as a function of the ligand structure
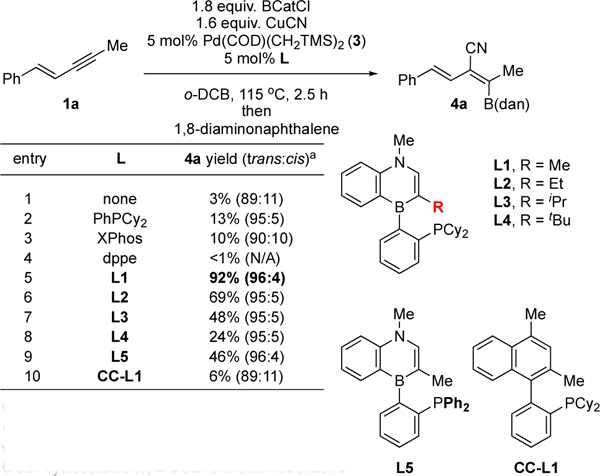 |
Determined by 1H NMR of the crude mixture vs. a calibrated internal standard after quenching with dan. dan: 1,8-diaminonaphthalene, COD: cyclooctadiene, o-DCB: orthodichlobenzene.
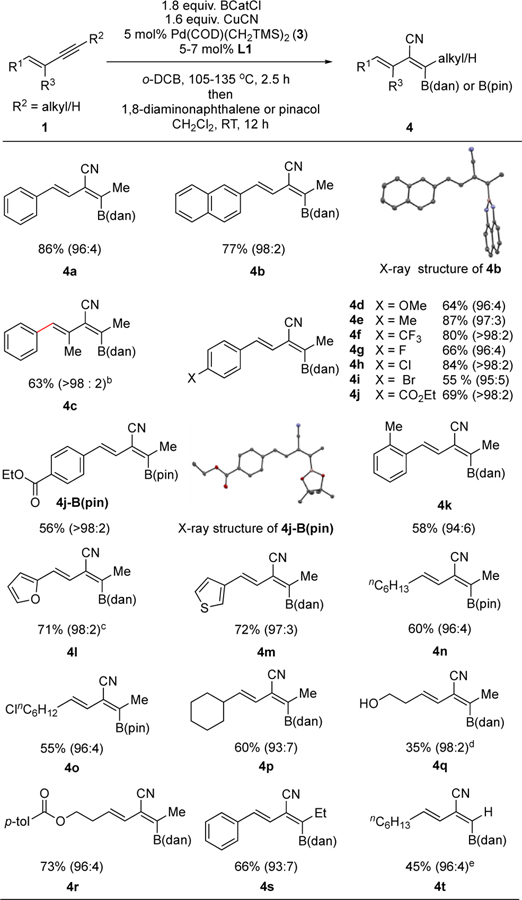
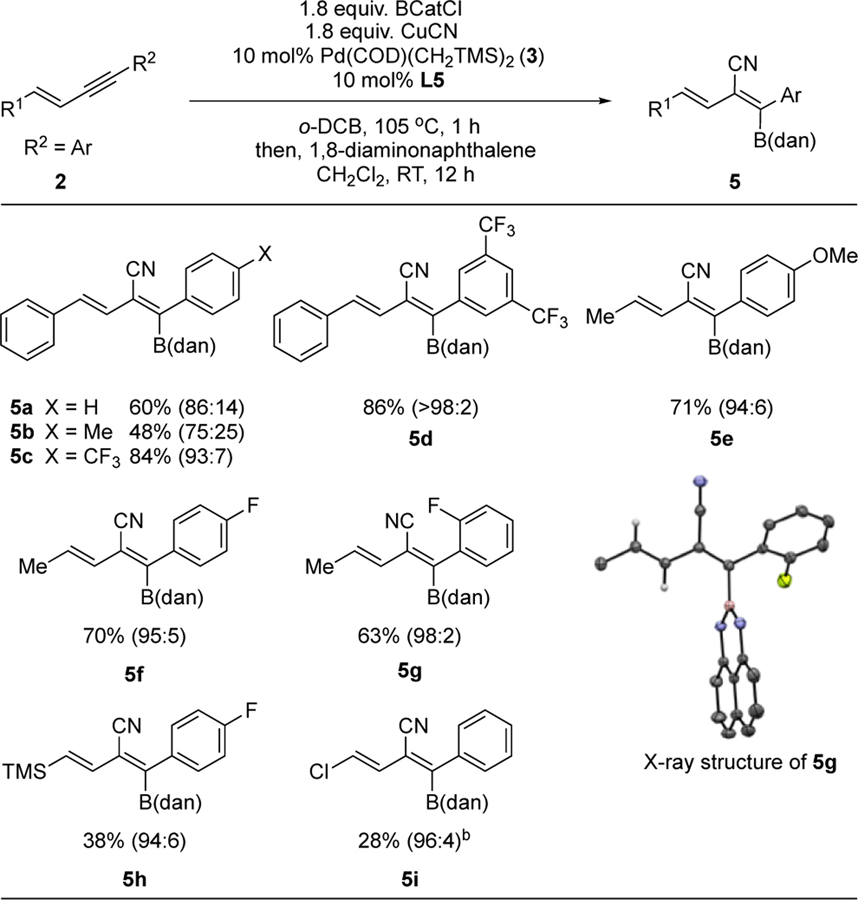
Under optimized reaction conditions, various alkyl/terminal (E)-1,3-enynes 1 were subjected to the trans-selective cyanoboration followed by quench with 1,8-diaminonaphthalene (dan)27 or pinacol (pin), and the results are summarized in Table 2. High trans-selectivity was observed consistently with an array of electronically (e.g., entries 4d–4j) and sterically different (e.g., entries 4c and 4k) substituents on the alkene. In addition to arenes, the R1 position also tolerates heteroarenes (entries 4l and 4m) and alkyl groups (entries 4n-4r). Functional groups such as aryl-halide (entries 4g–4i), alkyl chloride (entry 4o), esters (entries 4j and 4r), methoxy (entry 4d), and alcohol (with pre-treatment with H-BCat; entry 4q) are also tolerated. When the steric demand of the R2 substituent is increased from Me to Et, a slight decrease in diastereoselectivity was observed (4s vs. 4a). For the furyl substrate 1l, L5 was a superior ligand compared to L1 with regard to reaction selectivity.28 The terminal enyne substrate 1t required higher catalyst loading at a lower reaction temperature and reaction time (entry 4t). The bond connectivity and stereochemistry of two trans-cyanoboration products, 4b and 4j-B(pin) was confirmed by single crystal X-ray diffraction analysis (Table 2).
Table 2.
Pd-catalyzed trans-cyanoboration of alkyl/terminal 1,3-enynes (R2 = alkyl/H)a
Yields of isolated isomerically pure trans product (average of 2 runs), based on 1. The diastereomeric ratio in parenthesis (trans:cis) was determined by 1H NMR of the crude material after addition of 1,8-diaminonaphthalene or pinacol.
Isomerization occured at the highlighted position. E/Z ratio of the crude material is 5:1. Yield is of isolated pure E isomer.
L5 was used instead of L1.
The substrate was first pre-treated with HBCat before subjecting it to the reaction conditions.
10 mol% catalyst loading, 90 °C, 40 min reaction time.
For aryl (E)-1,3-enynes (R2 = Ar) 2, we determined that L5 was a superior ligand compared to L1.29 The diastereoselectivity of the reaction for diaryl 1,3-enynes (R1 = Ar, R2 = Ar) substrates 2a-d is dependent on the electronic nature of the R2 substituent, with electron-deficient R2 groups resulting in higher trans-cyanoboration selectivity (entry 5c and 5d vs. 5b and 5a). On the other hand, for monoaryl 1,3-enynes (R2 = Ar, R1 ≠ Ar) the observed trans-cyanoboration selectivity remains excellent (>94:6) regardless of the electronic nature of the R2 substituent (entries 5e-i). Good diastereoselectivity was also observed for the alkenyl silane and alkenyl chloride substrates 2h and 2i, albeit with diminished yields. We have obtained the X-ray crystal structure of product 5g, thus unambiguously establishing connectivity and diastereoselectivity.
Table 3.
Pd-catalyzed trans-cyanoboration of aryl 1,3-enynes (R2 = Ar)a
Yields of isolated isomerically pure trans product (average of 2 runs), based on 2. The diastereomeric ratio in parenthesis (trans:cis) was determined by 1H NMR of the crude material after addition of 1,8-diaminonaphthalene.
15 mol% catalyst loading, 1.2 equiv. CuCN, 1.6 equiv. BCatCl, 90 °C, 25 min.
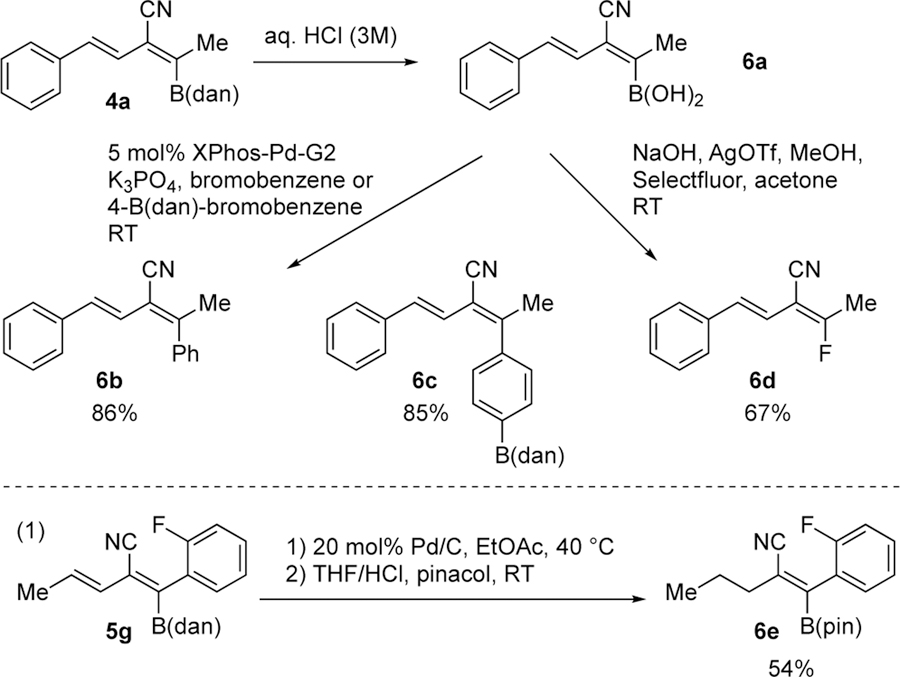
Vicinal boron-substituted alkenylnitrile derivatives are versatile synthetic building blocks. For example, Scheme 4 illustrates that cyanoboration product 4a undergoes hydrolysis (to form boronic acid derivative 6a) and subsequent Pd-catalyzed Suzuki-Miyaura coupling with bromobenzene or 4-B(dan)-bromobenzene to furnish 6b and 6c in 86% and 85% yield, respectively, with complete retention of olefin stereochemistry. Furthermore, fluorination of 6a with Selectfluor30 produces a novel (E)-2-nitrile-fluorodiene motif 6d. We also determined that our borylated dienylnitriles can be hydrogenated regioselectively. For example, when diene 5g is subjected to Pd/C catalyzed hydrogenation, tetra-substituted borylated alkenylnitrile 6e is obtained after transesterification with pinacol (eq 1).
Scheme 4.
Functionalization of cyanoboration products.
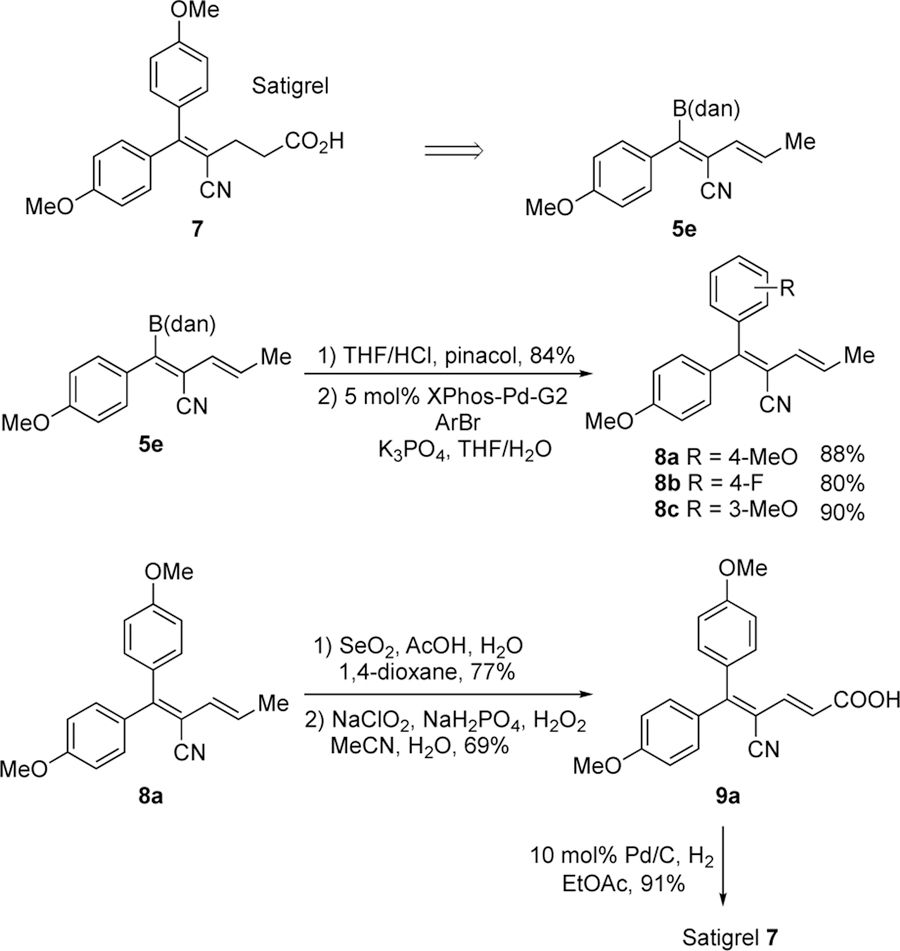
Finally, we applied our trans-selective cyanoboration reaction to the synthesis of Satigrel 7, an anti-platelet aggregating agent that contains a tetra-substituted acrylonitrile core.31 With a stereoselective method for the construction of tetra-substituted acrylonitrile now at our disposal, we reasoned that Satigrel could be synthesized from 5e in a straight forward fashion (Scheme 5). We commenced first with the transesterification of 5e followed by Suzuki-Miyaura coupling to produce a variety of bis-aryl substituted dienenitriles 8a-c in a stereospecific manner. The bis-4-methoxy-phenyl derivative 8a was then subjected to oxidation32 to yield the carboxylic acid 9a. Finally, catalytic hydrogenation33 selectively reduced the more accessible alkene to furnish Satigrel. The synthesis described in Scheme 5 offers a modular and stereoselective synthetic approach toward bis-aryl substituted dienenitriles, taking advantage of the versatile boron functional handle.
Scheme 5.
Synthesis of Satigrel.
In summary, we have developed the first trans-selective cyanoboration reaction of an alkyne. The described palladium-catalyzed cyanoboration of 1,3-enynes is site-, regio-, and diastereoselective, and we have determined that our 1,4-azaborine-based Senphos ligand structure is uniquely suited to support the Pd catalysis. The described method provides access to the important tetra-substituted alkenyl nitrile motif in a straightforward fashion, and we demonstrated the utility of our method with the synthesis of Satigrel. Future efforts will be directed at developing additional stereoselective difunctionalization reactions of alkynes employing the Senphos ligand framework.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM094541 and by Boston College start-up funds.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Brazdil JF, Acrylonitrile in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. [Google Scholar]
- [2].a) Fleming FF, Nat. Prod. Rep 1999, 16, 597–606; [Google Scholar]; b) Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC, J. Med. Chem 2010, 53, 7902–7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Van Boven M, Blaton N, Cokelaere M, Daenens P, J. Agric. Food. Chem 1993, 41, 1605–1607. [Google Scholar]
- [4].a) Suginome M, Yamamoto A, Murakami M, J. Am. Chem. Soc 2003, 125, 6358–6359; [DOI] [PubMed] [Google Scholar]; b) Suginome M, Yamamoto A, Murakami M, Angew. Chem 2005, 117, 2432–2434; Angew. Chem. Int. Ed. 2005, 44, 2380–2382; [Google Scholar]; c) Suginome M, Yamamoto A, Murakami M, J. Organomet. Chem 2005, 690, 5300–5308; [Google Scholar]; d) Ohmura T, Awano T, Suginome M, Yorimitsu H, Oshima K, Synlett 2008, 423–427.
- [5].a) Liu B, Wang Y, Chen Y, Wu Q, Zhao J, Sun J, Org. Lett 2018, 20, 3465–3468; [DOI] [PubMed] [Google Scholar]; b) Murayama H, Nagao K, Ohmiya H, Sawamura M, Org. Lett 2016, 18, 1706–1709; [DOI] [PubMed] [Google Scholar]; c) He Y-T, Li L-H, Wang Q, Wu W, Liang Y-M, Org. Lett 2016, 18, 5158–5161; [DOI] [PubMed] [Google Scholar]; d) Sakata N, Sasakura K, Matsushita G, Okamoto K, Ohe K, Org. Lett 2017, 19, 3422–3425; [DOI] [PubMed] [Google Scholar]; e) Arai S, Sato T, Koike Y, Hayashi M, Nishida A, Angew. Chem 2009, 121, 4598–4601; Angew. Chem. Int. Ed. 2009, 48, 4528–4531; [DOI] [PubMed] [Google Scholar]; f) Arai S, Koike Y, Nishida A, Adv. Synth. Catal 2010, 352, 893–900; [Google Scholar]; g) Arai S, Sato T, Nishida A, Adv. Synth. Catal 2009, 351, 1897–1904; [Google Scholar]; h) Igarashi T, Arai S, Nishida A, J. Org. Chem 2013, 78, 4366–4372. [DOI] [PubMed] [Google Scholar]; i) For reviews, see:Chen P-H, Billett BA, Tsukamoto T, Dong G, ACS Catal 2017, 7, 1340–1360; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Wen Q, Lu P, Wang Y, RSC Adv 2014, 4, 47806–47826; [Google Scholar]; k) Kou X, Fan J, Tong X, Shen Z, Chin. J. Org. Chem 2013, 33, 1407–1422; [Google Scholar]; l) Tobisu M, Chatani N, Chem. Soc. Rev 2008, 37, 300–307. [DOI] [PubMed] [Google Scholar]
- [6].a) Chen L, Cao S, Zhang J, Wang Z, Tetrahedron Lett 2019, 60, 1678–1681, [Google Scholar]; b) Liao Z-Y, Liao P-Y, Chien T-C, Chem. Commun 2016, 52, 14404–14407; [DOI] [PubMed] [Google Scholar]; c) Qiu G, Qiu X, Liu J, Wu J, Adv. Synth. Catal 2013, 355, 2441–2446. [Google Scholar]
- [7].a) Wang X, Studer A, J. Am. Chem. Soc 2016, 138, 2977–2980; [DOI] [PubMed] [Google Scholar]; b) Zhu Y, Shen Z, Adv. Synth. Catal 2017, 359, 3515–3519. [Google Scholar]
- [8].a) Moreau P, Commeyras A, J. Chem. Soc., Chem. Commun 1985, 817–818;; b) Murai M, Hatano R, Kitabata S, Ohe K, Chem. Commun 2011, 47, 2375–2377; [DOI] [PubMed] [Google Scholar]; c) Barrado AG, Zielinski A, Goddard R, Alcarazo M, Angew. Chem 2017, 129, 13586–13590; Angew. Chem. Int. Ed. 2017, 56, 13401–13405; [DOI] [PubMed] [Google Scholar]; e) Lukashev NV, Kazantsev AV, Borisenko AA, Beletskaya IP, Tetrahedron 2001, 57, 10309–10317. [Google Scholar]
- [9].Chatani N, Horiuchi N, Hanafusa T, J. Org. Chem 1990, 55, 3393–3395. [Google Scholar]
- [10].a) Kamiya I, Kawakami J, Yano S, Nomoto A, Ogawa A, Organometallics 2006, 25, 3562–3564; [Google Scholar]; b) Higashimae S, Kurata D, Kawaguchi SI, Kodama S, Sonoda M, Nomoto A, Ogawa A, J. Org. Chem 2018, 83, 5267–5273; [DOI] [PubMed] [Google Scholar]; c) Lee YT, Choi SY, Chung YK, Tetrahedron Lett 2007, 48, 5673–5677; [Google Scholar]; d) Bürger M, Loch MN, Jones PG, Werz B, Chem. Sci 2020, 11, 1912–1917; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ozaki T, Nomoto A, Kamiya I, Kawakami J, Ogawa A, Bull. Chem. Soc. Jpn 2011, 84, 155–163. [Google Scholar]
- [11].a) Chatani N, Hanafusa T, J. Chem. Soc., Chem. Commun 1985, 838–839;; b) Chatani N, Takeyasu T, Horiuchi N, Hanafusa T, J. Org. Chem 1988, 53, 3539–3548; [Google Scholar]; c) Suginome M, Kinugasa H, Ito Y Tetrahedron Lett 1994, 35, 8635–8638. [Google Scholar]
- [12].Obora Y, Baleta AS, Tokunaga M, Tsuji Y, J. Organomet. Chem 2002, 660, 173–177. [Google Scholar]
- [13].a) Fyfe JWB, Watson AJB, Chem 2017, 3, 31–55; [Google Scholar]; b) Pattison G, Org. Biomol. Chem 2019, 17, 5651–5660; [DOI] [PubMed] [Google Scholar]; c) Yang X, Kalita SJ, Maheshuni S, Huang Y-Y, Chem. Rev 2019, 392, 35–48. [Google Scholar]
- [14].Suginome M, Yamamoto A, Sasaki T, Murakami M, Organometallics 2006, 25, 2911–2913. [Google Scholar]
- [15].a) Liu Z, Marder TB, Angew. Chem 2007, 120, 248–250; Angew. Chem. Int. Ed. 2008, 47, 242–244; [Google Scholar]; b) Bosdet MJD, Piers WE, Can. J. Chem 2009, 87, 8–29; [Google Scholar]; c) Campbell PG, Marwitz AJ, Liu S-Y, Angew. Chem 2012, 124, 6178–6197; Angew. Chem. Int. Ed. 2012, 51, 6074–6092; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Giustra ZX, Liu S-Y, J. Am. Chem. Soc 2018, 140, 1184–1194; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) McConnell c. R., Liu S-Y, Chem. Soc. Rev 2019, 48, 3436–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Knack DH, Marshall JL, Harlow GP, Dudzik A, Szaleniec M, Liu S-Y, Heider J, Angew. Chem 2013, 125, 2660–2662; Angew. Chem. Int. Ed. 2013, 52, 2599–2601; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee H, Fischer M, Shoichet BK, Liu S-Y, J. Am. Chem. Soc 2016, 138, 12021–12024; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhao P, Nettleton DO, Karki RG, Zecri FJ, Liu S-Y, ChemMedChem 2017, 12, 358–361; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Boknevitz K, Italia JS, Li B, Chatterjee A, Liu S-Y, Chem. Sci 2019, 10, 4994–4998; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Liu Y, Liu S-Y, Org. Biomol. Chem 2019, 17, 7002–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Wang X-Y, Wang J-Y, Pei J, Chem. Eur. J 2015, 21, 3528–3539; [DOI] [PubMed] [Google Scholar]; b) Liu Z, Ishibashi JSA, Darrigan C, Dargelos A, Chrostowska A, Li B, Vasiliu M, Dixon DA, Liu S-Y, J. Am. Chem. Soc 2017, 139, 6082–6085. [DOI] [PubMed] [Google Scholar]
- [18].a) Baggett AW, Vasiliu M, Li B, Dixon DA, Liu S-Y, J. Am. Chem. Soc 2015, 137, 5536–5541; [DOI] [PubMed] [Google Scholar]; b) Brown AN, Li B, Liu S-Y, J. Am. Chem. Soc 2015, 137, 8932–8935; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Burford RJ, Li B, Vasiliu M, Dixon DA, Liu S-Y, Angew. Chem 2015, 127, 7934–7938; Angew. Chem. Int. Ed. 2015, 54, 7823–7827; [DOI] [PubMed] [Google Scholar]; d) Giustra ZX, Yang X, Chen M, Bettinger HF, Liu S-Y, Angew. Chem 2019, 131, 19094–19098; Angew. Chem. Int. Ed. 2019, 58, 18918–18922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Bailey JA, Haddow MF, Pringle PG, Chem. Commun 2014, 50, 1432–1434; [DOI] [PubMed] [Google Scholar]; b) Gorman AD, Bailey JA, Fey N, Young TA, Sparkes HA, Pringle PG, Angew. Chem 2018, 130, 16028–16032; Angew. Chem. Int. Ed. 2018, 57, 15802–15806. [DOI] [PubMed] [Google Scholar]
- [20].Xu S, Haeffner F, Li B, Zakharov LN, Liu S-Y, Angew. Chem 2014, 126, 6913–6917; Angew. Chem. Int. Ed. 2014, 53, 6795–6799. [DOI] [PubMed] [Google Scholar]
- [21].Xu S, Zhang Y, Li B, Liu S-Y, J. Am. Chem. Soc 2016, 138, 14566–14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang Y, Jiang J, Yu H, Shi J, Chem. Eur. J 2018, 24, 178–186. [DOI] [PubMed] [Google Scholar]
- [23].a) Ellis GP, Romney-Alexander TM, Chem. Rev 1987, 87, 779–794; [Google Scholar]; b) Sakamoto T, Ohsawa K, J. Chem. Soc., Perkin Trans 1 1999, 2323–2326. [Google Scholar]
- [24]. The use of Cl-BCat is essential for the reaction. For example, the use of Cl-B(pin) under otherwise identical reaction conditions led to an intractable mixture.
- [25]. To the best of our knowledge, NC-BCat is an unknown compound. CuCN may react with Cl-BCat to form NC-BCat in situ during the cyanoboration reaction. Independent treatment of CuCN with Cl-BCat at room temperature results in slow formation of a new boron-containing species with a 11B NMR chemical shift of 22 ppm in CDCl3. For the synthesis of the related 2-cyano-2,3-dihydro-1,3-dimethyl-1H-1,3,2-benzodiazabolole, see reference 14.
- [26]. Under the optimized conditions, about 1% of chloroboration of substrate 1a was observed in 78:22 trans:cis ratio.
- [27].Noguchi H, Hojo K, Suginome M, J. Am. Chem. Soc 2007, 129, 758–759. [DOI] [PubMed] [Google Scholar]
- [28]. When the reaction was performed with L1 as the ligand, the product 4l was obtained in 81% yield 94:6 (trans/cis) ratio.
- [29]. When the reaction was performed with 2f as the substrate and L1 as the ligand, the product 5f was obtained in 46% yield with (91:9) trans/cis ratio.
- [30].Furuya T, Ritter T, Org. Lett 2009, 11, 2860–2863. [DOI] [PubMed] [Google Scholar]
- [31].a) Fujimori T, Harada K, Saeki T, Kogushi M, Akasaka K, Yamagishi Y, Yamatsu I, Arzneim. Forsch 1987, 37, 1143–1148; [PubMed] [Google Scholar]; b) Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H, J. Biol. Chem 2000, 275, 883–889; [DOI] [PubMed] [Google Scholar]; c) Nakajima T, Kitajima I, Shin H, Matsumoto HW, Soejima Y, Maruyama I, Biochem. Biophys. Res. Commun 1994, 203, 1181–1187; [DOI] [PubMed] [Google Scholar]; d) Fujimori T, Harada K, Saeki T, Kogushi M, Katayama K, Satoh M, Cardiovascular Drug Reviews 1991, 9, 264–284. [Google Scholar]
- [32].a) Moazami Y, Gulledge TV, Laster SM, Pierce JG, Bioorg. Med. Chem. Lett 2015, 25, 3091–3094; [DOI] [PubMed] [Google Scholar]; b) Huang Q, Pennington JD, Williams HJ, Scott AI, Synth. Commun 2006, 36, 2577–2585; [Google Scholar]; c) Dalcanale E, Montanari F, J. Org. Chem 1986, 51, 567–569. [Google Scholar]
- [33].Majetich G, Yu J, Can. J. Chem 2012, 90, 75–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.