Abstract
Purpose
Our purpose was to identify human ovarian extracellular matrix (ECM) components that would support in vitro culture of human ovarian tissue and be compatible with possible future clinical applications. We characterized ovarian expression of laminins and selected three laminin tripeptides for culture experiments to be compared with Matrigel, an undefined and animal-based mixture of ECM components.
Methods
Expression of the 12 laminin genes was determined on transcript and protein levels using cortical tissue samples (n = 6), commercial ovary RNA (n = 1), follicular fluid granulosa cells (n = 20), and single-cell RNA-sequencing data. Laminin 221 (LN221), LN521, LN511, and their mixture were chosen for a 7-day culture experiment along with Matrigel using tissue from 17 patients. At the end of the culture, follicles were evaluated by scoring and counting from serial tissue sections, apoptosis measured using in situ TUNEL assay, proliferation by Ki67 staining, and endocrine function by quantifying steroids in culture media using UPLC-MS/MS.
Results
Approximately half of the cells in ovarian cortex expressed at least one laminin gene. The overall most expressed laminin α-chains were LAMA2 and LAMA5, β-chains LAMB1 and LAMB2, and γ-chain LAMC1. In culture experiments, LN221 enhanced follicular survival compared with Matrigel (p < 0.001), whereas tissue cultured on LN521 had higher proportion of secondary follicles (p < 0.001). LN511 and mixture of laminins did not support the cultures leading to lower follicle densities and higher apoptosis. All cultures produced steroids and contained proliferating cells.
Conclusions
LN221 and LN521 show promise in providing xeno-free growth substrates for human ovarian tissue cultures, which may help in further development of folliculogenesis in vitro for clinical practices. The system could also be used for identification of adverse effects of chemicals in ovaries.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01886-4) contains supplementary material, which is available to authorized users.
Keywords: Human ovary, Tissue culture, Laminins, Xeno-free, Matrigel
Introduction
In vitro growth and maturation of human ovarian follicles has been attempted since the 1990s. Successful production of developmentally competent oocytes in vitro would revolutionize the field of assisted reproductive technologies and fertility preservation, as well as provide completely new opportunities for studying folliculogenesis, factors affecting oocyte quality and competence, reproductive toxicity of chemicals, reproductive failure, and germline epigenetics, for example. From the start, two main approaches have been presented including the culture of isolated secondary follicles [1] and ovarian cortical tissue strip culture [2]. Since isolated primordial follicles survive poorly in culture, techniques combining initial growth within strips followed by isolation of secondary follicles have been developed [3, 4], in addition to techniques involving the encapsulation of isolated secondary follicles to provide them with 3D structural support [5, 6]. The newest culture approaches involve tissue-engineered artificial ovaries, into which primordial follicles can be seeded for possible future transplantation into patients [7–9]. It is important to continue developing culture conditions for primordial follicles because tissue cryopreserved in fertility preservation programs is ovarian cortex that mainly contains primordial follicles. Not all patients can receive the tissue back as a transplant due to cancer cell contamination, and for these patients, in vitro growth of follicles represents the only opportunity for fertility restoration.
Only two reports to date provide proof-of-concept for full growth and maturation of human pre-antral follicles in vitro. Xiao et al. used ovarian cortical tissue from 44 women to isolate secondary follicles for culture and successfully stimulated the growth of four mature oocytes [10]. A few years later, McLaughlin et al. reported in vitro growth and maturation of oocytes starting from ovarian cortical strip culture followed by secondary follicle isolation for further growth and maturation of isolated oocytes [4]. Using cortical tissue from 10 cesarean section (c-sec) patients containing thousands of primordial follicles, nine mature oocytes were produced [4]. In both cases, substantial amount of ovarian tissue was required for production of rather few oocytes. Hence, despite significant advances in the field, the progress in human in vitro folliculogenesis is still modest compared with mice. The first live mouse pups derived from in vitro matured follicles were born already in 1996 [11], and the newest protocols describe full mouse gametogenesis in vitro starting from pluripotent stem cells and leading to developmentally competent oocytes that give rise to live pups when fertilized and transferred to surrogate dams [12].
Ovarian extracellular matrix (ECM) is known to affect folliculogenesis [13], and ECM components are utilized in some culture systems. For example, Matrigel has been shown to help human follicles grow and survive in vitro [2, 14] and in xenotransplants in mice [7]. Matrigel has also been shown to assist in the development of baboon follicles in 3D culture [15]. However, Matrigel is a mouse-derived, undefined mixture of ECM components and it is therefore not compatible with clinical applications [16]. In addition, variability in composition could hamper studies aiming at understanding mechanisms of folliculogenesis or mechanisms of chemical toxicity in ovaries. Our aim was to identify components of human ovarian ECM that could support follicle survival and growth at least as to the same extent as Matrigel. We focused on laminins because these ECM components provide essential pro-survival support and phenotypic stability for tissues [17], and we successfully earlier developed protocols for xeno-free derivation and culture of human embryonic stem cells with the help of laminins that are naturally expressed by pre-implantation embryos [18].
Laminins are heterotrimeric proteins formed of α-, β-, and γ-chains that come in several isoforms and splice variants [17]. There are altogether 12 separate laminin genes in humans that are known to give rise to 16 different mature laminin tripeptides combinations [17]. Mature laminins are essential components of basal lamina, and together with collagens, they provide both structure and function to all tissues. The expression profile of laminin genes is tissue specific and changes during differentiation processes, leading to specific mature laminins tripeptides being expressed in different tissues and cell types. Laminins affect the functionality of tissues by signaling via cell surface receptors such as integrins to affect cell shape, polarization, function, and survival [17]. Immunostaining studies have localized at least laminins α2, β1, β2, and γ1 to theca and granulosa cell layers of growing bovine ovarian follicles [19]. In mice, laminins α1, β1, and γ1 are expressed in follicles of all developmental stages [20]. In humans, α2, α5, β1, β2, and γ1 have been localized to the corpora lutea with changing expression pattern along with luteal regression [21]. Laminins have significant effects on human granulosa cell proliferation, survival, and steroidogenesis in vitro [13], suggesting that laminins may play a role in folliculogenesis. However, to our knowledge, there are no systematic studies of laminin expression profiles in human ovary or reports of ovarian tissue culture on specific laminins in vitro. Here, our objective was to determine the laminin expression profile in human ovarian cortex and test human recombinant laminin tripeptides naturally expressed by ovaries along with stem cell–grade Matrigel as growth support for ovarian follicles in vitro. Our ambition was to identify robust culture conditions for in vitro growth of pre-antral follicle, which could be applied both for the study of reproductive toxicants and to further development of in vitro folliculogenesis.
Materials and methods
Study participants and ethical approval
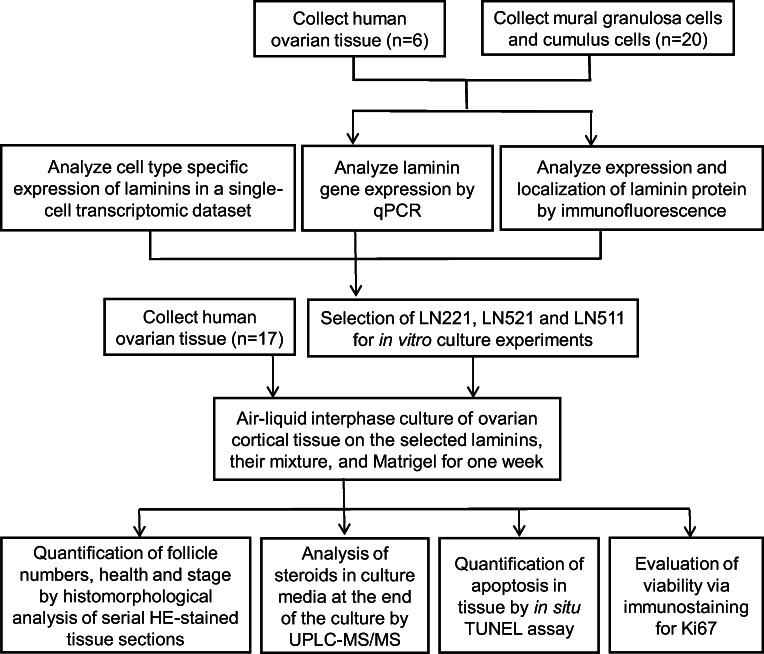
The study design is demonstrated in Fig. 1. Women undergoing elective c-sec at the Karolinska University Hospital were recruited to the study with no formal exclusion criteria. All participants received oral and written information on the project and gave a written consent. A thin piece of the ovarian cortex (5 mm × 1 mm) was biopsied, collected in warm phosphate buffered saline supplemented with calcium, magnesium, glucose, and pyruvate (PBS, Thermo Fisher Scientific, MA, USA) in the operating theater, and transported to the research laboratory within 10 min. Follicular fluid was obtained from women undergoing assisted reproductive technology at the Reproductive Medicine Unit of the Karolinska University Hospital. All participants received oral and written information on the project and gave a written consent. This study was approved by the Stockholm Region Ethics Board (license numbers 2010/549-31/2 and 2015/798-31/12). An overview of the clinical samples used in the different parts of the study is described in Online Resource Table 1.
Fig. 1.
Flow chart of study design. The expressions of laminins in human ovarian cortex and follicular fluid granulosa cells were determined by qPCR, immunofluorescence, and single-cell sequencing. Based on these expression profiles, three laminin tripeptides were selected for testing in an air-liquid interface culture system. The development of follicles and tissue viability were explored in order to assess laminins as growth substrates in comparison to Matrigel
Human granulosa cell collection
Follicular fluid obtained from women undergoing assisted reproductive technology was centrifuged at 400g for 5 min. Subsequently, the pellet was resuspended in DMEM/F12 (Gibco, MA, USA) and Percoll (Sigma-Aldrich, Darmstadt, Germany) density gradient centrifugation was performed to enrich granulosa cells. Mural granulosa cells were collected at the interface of the cell suspension and Percoll. Red blood cells were further depleted by red blood cell lysis buffer (Sigma-Aldrich, Darmstadt, Germany). Dynabeads CD45 (Thermo Fisher Scientific, MA, USA) were used to immunomagnetically deplete CD45-positive leukocytes from the granulosa cell suspension according to the manufacturer’s instructions for negative cell selection. The identity of the mural granulosa cells was controlled by immunostaining for FSHR and INH-α using antibodies listed in Online Resource Table 2 and the same staining protocols as described for laminins below. Positive staining for both markers confirmed the identity of the cells (Online Resource Fig. 1). Cumulus cells were collected manually from cumulus-oocyte complexes during oocyte denudation process of patients undergoing intracytoplasmic sperm injection (ICSI).
Laminin gene expression analyses
Total RNA was extracted from ovarian cortical strips from 6 consecutively consenting patients using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and from granulosa cells obtained from 20 consecutively consenting patients using Arcturus PicoPure Kit (Thermo Fisher Scientific, MA, USA) with on-column DNase treatment following the manufacturer’s instructions. A total amount of 0.4–1 μg RNA was reverse transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kits with RNase Inhibitor (Life Technologies, Grand Island, NY, USA). Quantitative polymerase chain reaction (qPCR) analysis was carried out using Fast SYBR Green Master Mix (Life Technologies, Grand Island, NY, USA) on an Applied Biosystems 7500 Real-Time PCR System equipped with 96-well optical reaction plates. Gene expression was normalized to house-keeping gene RPLP0 and HPRT1 via the ΔCt method. Primers were designed using Primer-BLAST [22] and their specificity and efficiency were validated by template dilution experiments, melt curve analysis, and agarose gel electrophoresis of the qPCR products. The sequences are provided in Online Resource Table 3. The analysis was carried out using the standard fast qPCR program in the Applied Biosystems 7500 Fast Real-Time PCR system. Human Ovary Poly A+ RNA (Clontech, Mountain View, CA, USA) was used as a reference. This commercially available RNA sample is a pool of normal human ovary samples collected from 15 Caucasian women aged 20–60 years who died of sudden death or trauma.
Immunofluorescent staining of ovarian tissue and follicular fluid granulosa cells
Laminin expression analysis by immunostaining in tissues was carried out using the same sample set as in qPCR analyses (n = 6). A piece of every tissue sample was fixed freshly upon collection in formalin over night at room temperature, transferred to 70% ethanol, embedded in paraffin, and cut to 4-μm sections. Laminin expression was analyzed in 2 to 4 sections per patient. The sections were dewaxed in xylene, rehydrated in increasing ethanol concentrations of 70–100%, and rinsed in Tris-buffered saline (TBS). Antigen retrieval was performed by 30-min incubation in citrate buffer (pH 6) at 96 °C water bath. After cooling, tissue sections were treated with 3% H2O2 in methanol for 30 min. Sections were then treated with blocking buffer for 15 min before incubation with primary antibodies overnight at 4 °C. After washing three times in TBS, sections were treated with horse radish peroxidase–conjugated secondary antibodies for 30 min and incubated in fluorophore tyramide working solution in order to amplify the signal (TSA Fluorescein System, Perkin Elmer Life Sciences, Waltham, USA) for 8 min. After washing in TBS, sections were placed in mounting solution with DAPI nuclear stain. Granulosa cells were cultured in DMEM/F12 (Life Technologies, Grand Island, NY, USA) with 15% FBS (Life Technologies) and 1% penicillin-streptomycin (Life Technologies) in chamber slides (Nunc, Roskilde, Denmark) overnight to allow the cells to attach. The cells then were washed in PBS and fixed with 4% paraformaldehyde (PFA) for 10 min. After 10-min permeabilization with 0.3% Triton X-100, cells were washed three times in PBS and blocked with blocking solution (0.1% Tween20 and 4% FBS in PBS) 1 h at room temperature. Subsequently, the cells were incubated with primary antibodies diluted in blocking solution overnight at 4 °C followed by three times wash in blocking solution and incubation with fluorochrome-conjugated secondary antibodies diluted in blocking solution for 1 h at room temperature. After washing in blocking solution, chambers were removed from the slides and granulosa cells were mounted in mounting medium with DAPI nuclear stain (VECTASHEILD Antifade Mounting Medium, Vector laboratories, Peterborough, UK). In negative controls, primary antibodies were replaced by IgGs. All antibodies and dilutions are listed in Online Resource Table 2. The excitation and emission wave lengths were 360 nm and 460 nm for DAPI and 494 nm and 517 nm for fluorescein. Images were taken using an Olympus IX-81 microscope and were compiled for publication in Image J (NIH, USA) and Adobe Photoshop (Adobe, California, USA).
Analysis of single-cell transcriptomic data
We retrieved the Seurat-normalized expression data of laminin genes as well as cell identities from the 10 × Genomics single-cell RNA-sequencing data set previously described [23]. All data processing and formatting were done in R/Bioconductor. The plotting was done using the ggplot2 and ggbeeswarm libraries. Average laminin expression levels within a cell type were compared with each other using Tukey HSD test in base R. The full ovarian single-cell transcriptomic data set is available in the ArrayExpress database at EMBL-EBI under the accession code E-MTAB-8381.
Ovarian tissue culture
Ovarian cortical tissues were biopsied from 17 consecutively consenting patients, and each biopsy was cut into six strips (3 × 1 × 1 mm) with one strip immediately fixed in Bouin’s solution as fresh control. Remaining five tissue fragments from each biopsy were randomly assigned to different study groups including LN521, LN511, LN221, mix of the three, and Matrigel. Millicell cell culture plate inserts (Merck Millipore, Darmstadt, Germany) were coated with a total concentration of 10 μg/ml of LN521, LN511, LN221, or their mixture (3.3 μg/ml each, tot concentration 10 μg/ml) (BioLamina, Sundbyberg, Sweden) over night at + 4 °C, and washed with PBS before the plates were used in tissue culture. Matrigel (hESC qualified, Corning, NY, USA) diluted 1:3 into a culture medium was used as a control matrix. Tissue was cultured on coated inserts in 24-well plates in air-liquid interphase in normoxia for 7 days. Culture media consisted of high glucose DMEM with glutamax (Life Technologies, Grand Island, NY, USA) supplemented with 10% human serum albumin solution (Vitrolife, Göteborg, Sweden), 1% insulin-transferrin-selenium (Life Technologies, Grand Island, NY, USA), 1% antibiotic-antimycotic (Life Technologies, Grand Island, NY, USA), and 0.5 IU/ml human recombinant follicle-stimulating hormone (FSH, Puregon, MSD, Stockholm, Sweden). Culture media were changed to fresh every 48 h. On the last day, all tissue strips were fixed in Bouin’s solution and culture media were collected and stored in − 80 °C for later steroid hormone analyses.
Histomorphometric analysis of tissue
Ovarian tissue was fixed in Bouin’s solution at + 4 °C overnight, rinsed thoroughly with 70% ethanol, and embedded in paraffin. Serial tissue sections at a thickness of 4 μm were prepared through the tissue block. Every 7th and 8th sections were stained with hematoxylin-eosin (HE) and the sections were scanned with a Mirax Slide Scanner (Zeiss, Göttingen, Germany) at × 20 objective. Remaining sections were spared for TUNEL and Ki67 analysis as described below. Follicles with a visible oocyte were counted and scored using Panoramic Viewer software (3DHistech, Budapest, Hungary) as follows. Follicles with mostly flattened granulosa cells surrounding the oocyte were considered primordial follicles; follicles with one layer of mainly cuboidal granulosa cells were classified as primary follicles; and follicles with at least two full layers of cuboidal granulosa cells were classified as secondary follicles. Follicles were considered atretic if the oocyte was eosinophilic, of irregular shape or had crumped chromatin, or if the follicle had > 5% pyknotic granulosa cells. Tissue section surface area was measured manually.
Hormone measurements
Medium samples collected at termination of cultures from five patients were chosen for steroid hormone analysis based on the presence of follicles because resources did not allow analysis of media from all 17 patients. Samples used in these analyses are indicated in Online Resource Data 1. Progesterone, androstenedione, testosterone, and estradiol were measured using our UPLC-MS/MS method as described earlier [24].
In situ apoptosis and proliferation assays
To evaluate apoptosis in situ, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was used following the manufacturer’s instructions (in situ cell death detection kit with fluorescein, Roche, Penzberg, Germany). Samples from nine patients were selected for analysis based on the presence of follicles because resources did not allow analysis of all 17 patients. Samples used in these analyses are indicated in Online Resource Data 1. Paraffin sections were deparaffinized, dehydrated, and rehydrated in xylene, ethanol, distilled water, and TBS pH 7. Sections were incubated for 20 min at 37 °C with 100 μL of proteinase K (1:1333 dilution in PBS) to permeabilize the cells and rinsed with PBS. Positive controls were prepared by incubating sections with DNase I (1:50 dilution in PBS) and negative controls by omitting the terminal transferase from the reactions. TUNEL reaction mixture (1:10 enzyme:label) was added to the samples, incubated for 1 h at 37 °C in the dark, and then rinsed with PBS. Glasses were mounted using DAPI-containing mounting medium (VECTASHEILD Antifade Mounting Medium, Vector laboratories, Peterborough, UK). Images were taken with Olympus IX-81 microscope fitted with Olympus XM10 camera. The TUNEL label (fluorescein) and DAPI were excited with 470-–495-nm and 360-–370-nm filters and emission was collected using 510-–550-nm and 420-–460-nm filters, respectively. Same imaging parameters were used for all samples. Four to five fields-of-view from one to two sections per patient were imaged for quantification. Quantification was carried out using Image J software (NIH, University of Wisconsin, USA) as described [24]. Briefly, DAPI images were used to count the nuclei; the images were converted into 8-bit grayscale, the threshold was adjusted, and binary watershed operation used to highlight individual nuclei that were counted using the “analyze particles” function. The TUNEL images were converted into 8-bit images, inverted, and calibrated using blank background as reference. Signal was quantified using integrated optical density function. The signal was adjusted to cell number in the same region, and the 3–4 regions quantified per sample were averaged for statistical analyses. Ki67 staining was carried out using Bouin-fixed tissue sections. Samples from three patients were selected for analyses as indicated in Online Resource Data 1. The sections were deparaffinized and rehydrated as described above, and antigens were retrieved using Tris-EDTA buffer pH 9 in 96 °C for 30 min. Primary antibodies (Online Resource Table 2) were added at 1:500 dilution and incubated over night at + 4 °C followed by exposure to secondary antibodies for 1 h at room temperature and mounting with Fluorescent Mounting Medium (VECTASHEILD Antifade Mounting Medium, Vector laboratories, Peterborough, UK).
Statistical analyses
Statistical analyses were performed using R [25] with RStudio program [26]. Pearson correlation was used to test association between follicle density and patient age. Repeated measures ANOVA was used for comparing tissue volumes, follicle densities, steroid concentrations, and TUNEL data. All data was LOG transformed to conform to normality assumptions. In case of significant findings (p < 0.05), the groups were compared with each other using paired t tests and Bonferroni-Holm adjusted two-tailed p values are shown in figures. Chi-square test was used to analyze follicles in different categories (healthy vs. degenerate; primordial, primary vs. secondary). All p values are two tailed and corrected for multiple comparisons. Results were considered significant if the corrected p < 0.05. Results 0.05 < p < 0.1 are also indicated in figures.
Results
Laminin expression in human ovary
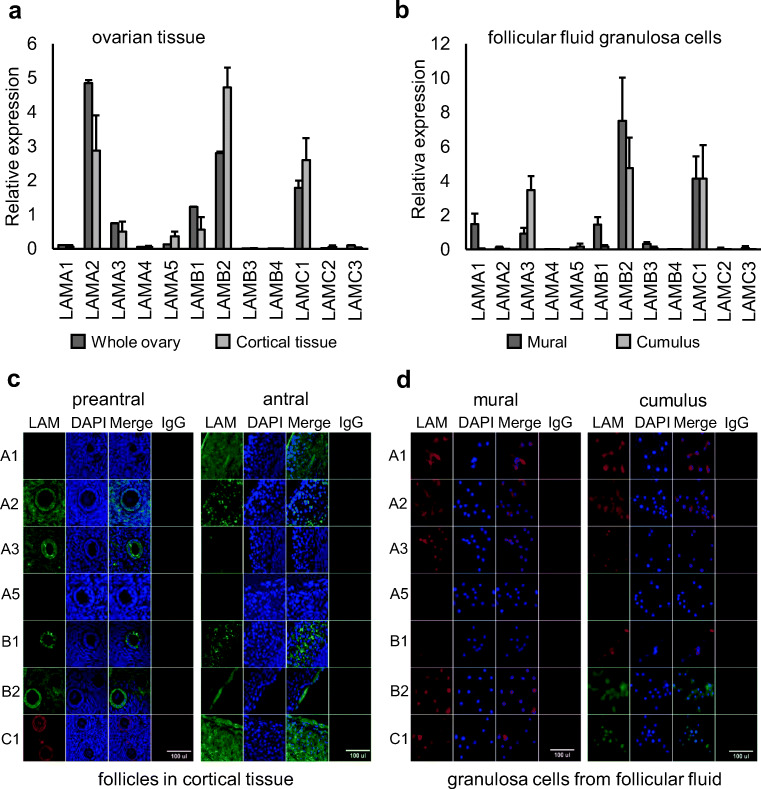
We studied the expression of the 12 human laminin genes in human ovarian samples. The expression on RNA level was measured using six individual human ovarian cortex samples and a commercially available pooled whole ovary RNA sample. Genes found to be robustly expressed in both sample types were LAMA2, LAMB2, and LAMC1 (Fig. 2a). We further studied the expression in mural and cumulus granulosa cells isolated from ovarian follicular fluid samples obtained from women undergoing in vitro fertilization (IVF) treatments. The expression profile of β- and γ-chains was very similar to tissue with LAMB2 and LAMC1 being the most abundant isoforms present (Fig. 2b). However, α-chain expression differed. Instead of LAMA2, cumulus granulosa cells mainly expressed LAMA3 and mural granulosa cells both LAMA1 and LAMA3 (Fig. 2b).
Fig. 2.
Laminin expression in human ovaries. The expression of the 12 laminin genes was analyzed by qPCR in human ovarian cortex (n = 6) and commercially available pooled whole ovary RNA sample from 15 women (n = 1) (a), and in mural (n = 10) and cumulus (n = 10) granulosa cells isolated from ovarian follicular fluid (b). The results were normalized to house-keeping gene RLPL0 and HPRT1 and are shown as average relative expression (+ SEM). The same commercial reference “whole ovary” sample was included in every run. Main laminin chains expressed were LAMA2, LAMB2, and LAMC1 in ovarian tissue. Follicular fluid granulosa cells expressed LAMA1, LAMA3, LAMB2, and LAMC1. The expression was further studied on protein level by immunostaining of ovarian cortical tissue sections (c) and mural and cumulus granulosa cells isolated from ovarian follicular fluid (d). DAPI was used as nuclear counter stain, and IgG as negative control. Tissue from 6 patients was used in stainings and representative images are shown. In pre-antral follicles, LAMA2, LAMA3, LAMB1, LAMB2, and LAMC1 showed robust staining, whereas LAMA1, LAMA2, LAMB1, and LAMC1 dominated in walls of small antral follicles. In isolated granulosa cells, LAMA1, LAMA2, and LAMA3 showed positive staining in addition to LAMB2 and LAMC1
Immunostainings were then used to study laminin protein expression. LAMA4, LAMB3, LAMB4, LAMC2, and LAMC3 were excluded from these analyses due to non-detectable RNA expression (Fig. 2a, b). Formalin-fixed ovarian cortical tissue sections containing pre-antral and occasional small antral follicles were used in the analyses. In pre-antral follicles and cortical stroma, abundant expression of LAMA2 and LAMB2 was found in stroma and follicles (Fig. 2c). LAMA3, LAMB1, and LAMC1 were detected mainly in the granulosa cells of follicles. In walls of small antral follicles, LAMA1, LAMA2, LAMB1, and LAMC1 showed most abundant staining (Fig. 2c). Isolated mural and cumulus granulosa cells mainly expressed LAMA1, LAMA2, LAMA3, LAMB2, and LAMC1 (Fig. 2d).
Cell type–specific expression of laminins in ovarian cortex
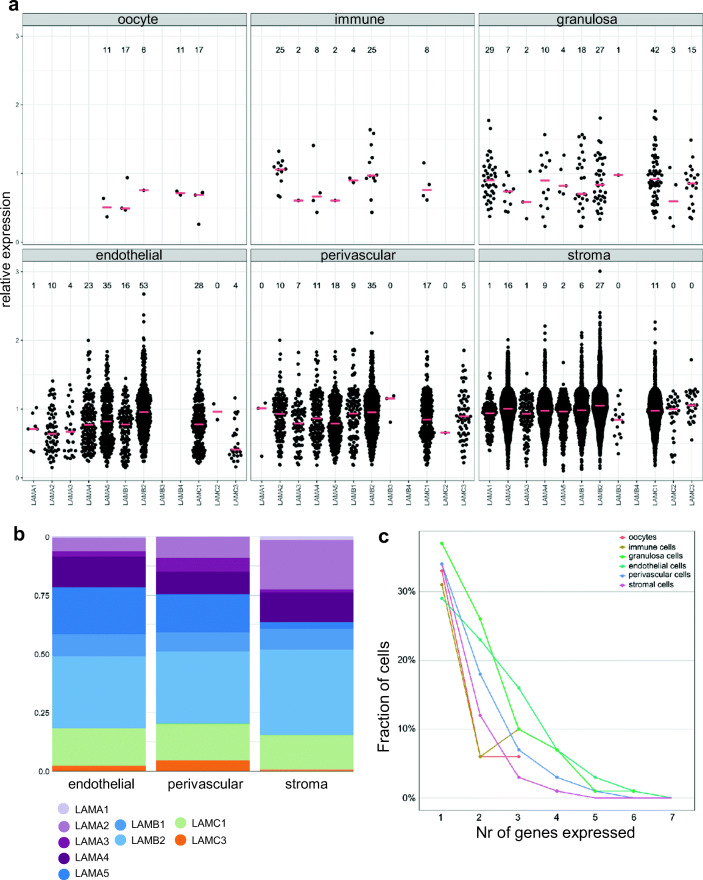
We recently published a single-cell transcriptomic map of human ovarian cortex [23]. We used this dataset to study cell type–specific expression and co-expression of laminin isoforms in 12,160 ovarian cells. Only cells expressing at least one laminin gene were included, leaving 6514 cells available for analysis. This data set consisted of 8 oocytes, 23 immune cells, 112 granulosa cells, 501 endothelial cells, 772 perivascular cells, and 5098 stromal cells.
Expression level of each laminin gene was plotted by cell type (Fig. 3a). Most laminin genes were expressed in at least one cell type, except for LAMB4 that was not detected. In agreement with the qPCR and immunostaining data, the expression of laminin α-chains was more varied than that of β- and γ-chains. (Fig. 3a). The expression of all laminins was compared with each other within each cell type. The dataset contained only few oocytes, immune cells, and granulosa cells, and they were excluded from statistical analyses. In endothelial cells, LAMB2 was expressed on a significantly higher level (p < 0.001) and in a bigger fraction of cells (53%) than LAMB1 (16%), and LAMC1 was expressed on a significantly higher level (p < 0.05) and in more cells (28%) than LAMC3 (4%) (Fig. 3a, Online Resource Table 4). Of the α-chains, LAMA5 was expressed by most endothelial cells (35%), but the median expression level did not differ from other α-isoforms. In perivascular cells, median expression levels did not differ between laminin isoforms; however, the cells were found to most often express LAMA5 (18%), LAMB2 (35%), and LAMC1 (17%). Within stromal cells, the most often expressed isoforms were LAMA2 (16%), LAMB2 (27%), and LAMC1 (11%). The median expression level of LAMA2 was significantly higher than that of LAMA3 (p < 0.05) and LAMA5 (p < 0.001), and the expression of LAMAB2 was significantly higher than that of LAMB1 (p < 0.001) (Fig. 3a, Online Resource Table 4). To visualize the relative abundance of the different laminin isoforms per cell type, the percentage of cells expressing a specific laminin was plotted (Fig. 3b). The blood vessel cells (endothelial and perivascular) expressed most often LAMA5, LAMB2, and LAMC1, while the stromal cells preferably expressed LAMA2, LAMB2, and LAMC1 (Fig. 3b).
Fig. 3.
Cell type–specific expression of laminin isoforms in ovarian cortex. Cell type–specific laminin expression was evaluated using our single-cell RNA-sequencing data set of human ovarian cortex. a Relative expression of the 12 laminin genes in individual oocytes, immune cells, granulosa cells, endothelial cells, perivascular cells, and stromal cells is shown as swarm plots where median is marked with horizontal red lines. The numbers above the plots indicate the percentage of cells expressing the laminin isoform in each cluster in the full set of 12,160 cells, whereas the swarm plots show the expression in those 6514 cells that express any laminin. b The proportion (%) of cells within each cell type expressing a certain laminin gene. The majority of cells in ovarian cortex are stromal cells and blood vessel cells. The most commonly expressed α-isoform in blood vessels was LAMA5, β-chain was mainly expressed from LAMB2, and γ-chain from LAMC1. In stromal cells, LAMA2, LAMB2, and LAMC1 were most commonly expressed. c Co-expression of laminin genes in cells. The fraction of cells (y-axis) expressing 1–7 different laminin genes (x-axis) is shown by cell type. Approximately 30% of the cells in each cluster expressed only one laminin gene, 7–26% of the cells co-expressed two laminins, and 4–16% co-expressed three laminins. Most common co-expression profiles are shown in Online Resources Data 2
We next evaluated co-expression of laminin genes by single ovarian cells. In total, 54% of ovarian cells expressed at least one laminin (6514 of 12,160 cells). Most commonly, only one laminin gene was expressed per cell within each cell type (Fig. 3c). We listed the most common forms of (co-)expression by cell type ranging from only one laminin gene being expressed to up to seven laminin genes expressed by the same cell (Fig. 3c, Online Resource Data 2). Cells expressing at least three different laminins made up 2–25% of the cells within each category (Fig. 3c).
Collectively, these data suggest that laminins have a distinct expression pattern in human ovaries. While variable expression of several different isoforms of α-chain were detected on a transcript and protein level in different ovarian cell types, the β-chain and γ-chain were mainly expressed from LAMB1, LAMB2, and LAMC1 genes. We summarize the laminin expression analysis results in Online Resource Table 5. Based on collective evaluation of the results, we selected laminin tripeptides composed of α2, β2, and γ1 (LN221); α5, β2, and γ1 (LN521); and α5, β1, and γ1 for in vitro culture studies to mimic the laminin composition of human ovarian stroma and blood vessels, which make up the majority of the cortex. Stem cell–grade Matrigel was used as an established control ECM.
In vitro culture of ovarian cortical tissue on laminins
Ovarian cortical tissue from 17 c-sec patients was used in culture experiments (Online Resource Table 1). Each tissue sample was divided between the six experimental groups: immediately fixed fresh control, and tissue cultured for 7 days on Matrigel, LN221, LN521, LN511, or a mix of LN221, LN521, and LN511. The fresh samples were used as a control for assessing tissue and follicle health before culture. Total follicle density in the samples negatively correlated with patient age, as expected (Online Resource Fig. 2a). The volume of tissue available for analysis was not significantly different between the groups, indicating an even distribution of the study material (data not shown).
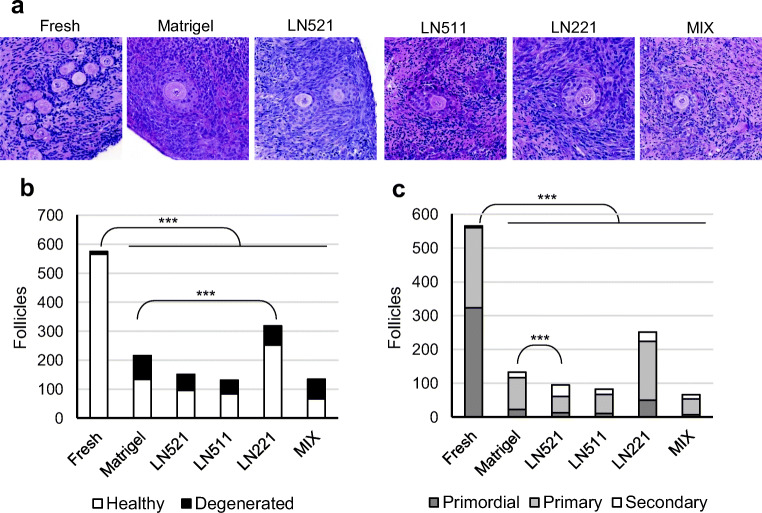
Follicles of healthy morphology were found in all experimental groups (Fig. 4a). Fresh control tissue was characterized by high numbers of primordial follicles and very few atretic follicles, whereas tissue exposed to culture had generally more growing, less primordial, and more degenerated follicles. All follicles with a visible oocyte were evaluated and counted for statistical analysis using the entire study material prepared to serial sections. A total of 1525 follicles were identified and analyzed (Online Resource Data 1). Total follicle counts and follicle densities were found to decrease during culture in all groups (Fig. 4b, Online Resource Fig. 2b). Tissue cultured on LN511 and mixture of laminins had significantly lower follicle density after 7 days of culture compared with fresh control (p < 0.05, Online Resource Fig. 2b). In addition, the proportion of degenerated follicles increased in all groups during culture compared with fresh control (p < 0.001 for all, Fig. 4b). Compared with Matrigel, follicular survival was significantly increased in tissue cultured on LN221 (p < 0.001, Fig. 4b). Among all culture groups, tissue cultured on LN221 had the overall highest total number of detectable follicles (n = 318) and the largest fraction of healthy follicles (79%). Conversely, tissue cultured on the mixture of laminins had the poorest overall follicle recovery (n = 120) and the smallest fraction of healthy follicles (46%) (Fig. 4b).
Fig. 4.
Follicle survival and development in culture. Ovarian cortical tissue was processed to thin strips and placed on filters coated with laminins or Matrigel in an air-liquid interphase culture system. Medium was changed every 48 h, and after 7 days the tissue was collected for histological analysis. a Growing follicles of healthy morphology were present in all culture groups after 7 days of in vitro culture. b Follicles were counted from serially sectioned tissue strips through the block and the number of unique follicles is shown by treatment group. Proportion of poor morphology follicles increased during culture in all groups compared with fresh control (p < 0.001). Compared with Matrigel, tissue cultured on LN221 had a higher proportion of surviving follicles (p < 0.001). c Healthy follicles were further divided into primordial, primary, and secondary. All culture groups were significantly different from the fresh control; after culture, the proportion of primordial follicles was smaller and that of secondary follicles higher in all cultured groups compared with fresh tissue (p < 0.001). Among culture groups, tissue cultured on LN521 was significantly different from Matrigel having a larger proportion of secondary follicles (p < 0.001). Chi-squared test, Bonferroni-corrected two-tailed p values
Follicle development was evaluated by comparing fractions of healthy follicles in different developmental stages: primordial, primary, and secondary before and after culture. The proportions significantly changed in culture compared with fresh tissue; primordial follicles dominated in fresh tissue, whereas the cultured tissue mainly contained growing follicles (Fig. 4c). Similar proportions of follicles in different developmental categories were found in all culture groups except for LN521, which significantly differed from Matrigel. Tissue cultured on LN521 had the overall largest proportion (36%) and number (n = 34) of secondary follicles. In all other groups, the fraction of secondary follicles was 10–20%, that of primary 70–80%, and that of primordial 10–20%, respectively (Fig. 4c).
We noticed clear differences in the abundance of follicles between tissue samples (Online Resource Data 1). Follicle densities were higher in younger patients (Online Resource Fig. 2) and consequently the majority of the follicles in this study were derived from the youngest patients. The entire cohort of 17 consecutively consenting patients had an age span of 21–40 years (Online Resource Table 1) and contributed 1525 follicles to the study. If we restricted the analyses to patients 21–30 years of age (n = 8), 1323 follicles were available, and the same statistical results were obtained as with the full cohort (data not shown). This encourages to focus on donors younger than 30 years of age in cortical tissue culture studies based on c-sec patients.
Endocrine function of tissue in culture
Endocrine function of the tissue in culture was evaluated by measuring steroid hormones in conditioned media collected at the end of the culture. As the media were changed every other day, the measured concentrations reflected cumulative steroid production during the last 24 h of culture (from day 6 to day 7). Blank samples, composed of culture medium only, did not contain any steroids. The main ovarian steroids such progesterone, androstenedione, testosterone, and estradiol were detected in all culture media samples (Online Resource Fig. 3). Progesterone was present in 10-fold higher median concentrations (10–30 nM) compared with androstenedione, testosterone, and estradiol (0.5–1 nM). There were no significant differences in steroid production between the groups (Online Resource Fig. 3).
Tissue viability
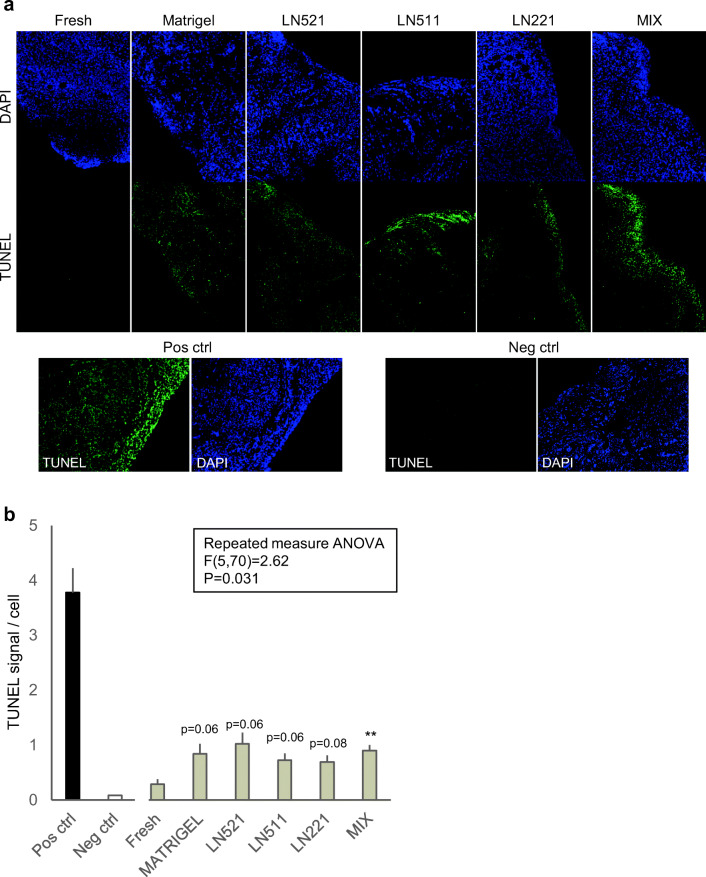
We evaluated the general tissue survival in culture by carrying out in situ TUNEL assay that detects single-strand DNA breaks as a proxy for apoptosis, and immunostaining of Ki67 that labels proliferating cells. In the TUNEL assay, all cell nuclei in the positive control sample, which was prepared by treating the sections with DNase I, showed bright labeling while negative controls remained negative (Fig. 5a). Study samples showed varying degrees of labeling. Fresh tissue was mainly negative. Scattered positive nuclei were found across all cultured samples with the signal being most intense towards the edges of the sections (Fig. 5a). The TUNEL signal was quantified and normalized to the number of cells in the measured area. The average TUNEL signal increased in all culture conditions compared with fresh control tissue reaching statistical significance in tissue cultured on mixture of laminins (p < 0.05) (Fig. 5b). In agreement with the relatively low levels of apoptosis, Ki67 staining showed scattered positive nuclear staining across stroma and follicles confirming the presence of proliferating cells at the end of the culture in all groups (Online Resource Fig. 4).
Fig. 5.
Tissue viability. Tissue viability was evaluated in nine samples per group by using in situ TUNEL assay that detects fragmented DNA as a sign of apoptosis. a Representative images of stained tissue sections. TUNEL signal is shown in green and the nuclear counter stain DAPI in blue. Positive control was prepared by treating tissue sections with DNase I, and negative control by omitting the terminal transferase enzyme. b Signal intensity was quantified and normalized to cell count in the evaluated area. Apoptosis significantly increased in tissue cultured on mixture of laminins (**p < 0.01). There was a tendency to increased signals in other groups as well (p = 0.06–0.08). Repeated measures ANOVA followed by Benjamin-Holm post hoc test. P values are two tailed
Discussion
In this study, we tested human recombinant laminins as growth substrates for in vitro culture of human ovarian tissue in our search for xeno-free ECM that could replace Matrigel. Culture protocols that support ovarian tissue survival and follicle growth in vitro without animal-derived components would be compatible with clinical applications. In addition, supportive culture conditions with minimal variation would allow studies on subtle changes in follicle biology for instance in response to chemotherapy agents or environmental chemicals. We found that human ovarian cells have a distinct laminin expression profile, and that some commercially available laminin tripeptides might provide at least equally good support to cortical tissue in culture as Matrigel.
We characterized laminin isoform expression profile of reproductive age human ovarian tissue samples and ovarian follicular fluid granulosa cells on transcript and protein levels. As expected, we found selective expression of laminin isoforms. The most variable chain was the α-chain, which was found to be expressed at varying levels from all genes except for LAMA4. In all samples, β-chain was consistently expressed from LAMB1 or LAMB2, and γ-chain only from LAMC1. These observations get support from bovine and mouse studies, where LAMA1, LAMB1, LAMB2, LAMC1 protein expressions have been detected by immunostaining in ovaries [19, 20]. Because analysis of homogenized tissue samples does not allow conclusions of cell type–specific expression, we further studied laminin expression with the help of our single-cell transcriptomic dataset of human ovarian cortex [23]. We could clearly see specific expression patterns. Blood vessel cells mainly expressed LAMA5, LAMB2, and LAMC1 and stromal cells LAMA2, LAMB2, and LAMB1. As stroma cells and blood vessels make up the majority of cells in ovarian cortex [23], these laminin types can be assumed to be the most abundant in this region of the ovary. Interestingly, only a fraction of the cells within each cell type had detectable laminin expression, suggesting that laminin-expressing sub-populations exist. Since laminins are essential components of basal laminas, it could be the cells closest to basal lamina that express most laminins. We found that co-expression of laminin chains by the same cell was not common, which may suggest that mature laminin tripeptides are assembled of proteins provided by different cells.
Expression of LAMA4 was clear in the single-cell data, although it was not detected by qPCR despite primers that were designed to amplify several annotated LAMA4 transcript variants. This might suggest that there are additional splice variants expressed in the ovary. A recent proteomic map of human ovarian cortex also suggests expression of α4 [27]. Other laminin isoforms detected by this mass spectrometric analysis were α2, α5, β1, β2, and γ1, which agrees with our data [27]. The laminin profile in the ovary is even similar to that in human testis, where variable α-chains are expressed (α1 and α5) in combination with β2 and γ1 [28].
Interestingly, the laminin expression profile in small antral follicles differed from stromal cells. The general strong LAMA2 expression in cortical stroma was replaced by LAMA1 and LAMA3 expressions in granulosa cells of antral follicles. Laminin expression profile is known to change during developmental processes [17]. For example, the pattern of laminin chain expression changes during human corpus luteum development and regression [21]. Therefore, changes in expression patterns during follicle growth are not surprising. This suggests that multi-step culture systems aiming at full maturation of follicles in vitro cold benefit from choosing specific laminins for each step of the culture protocol.
We chose LN221, LN521, and LN511 for our one-step, 7-day culture experiment based on the robust and high expression of α2-, α5-, β1-, β2-, and γ1-chains in multiple cell types of the ovarian cortex. In addition, LN521 and LN511 are generally the most widely expressed laminins in the body and have wide applicability domain in cell culture being used in the culture of human and mouse stem cells, epithelial cells, hepatic cells, and kidney cells, for example [18, 29–32]. As a reference ECM, we used Matrigel because it is commonly used in tissue culture and shown to enhance follicle survival in culture systems ranging from cortical strip culture to transplantable ovarian scaffolds in humans and animals [2, 7, 15]. The main laminin isoform in Matrigel is mouse LN111.
Previously, mouse laminin was shown to support human ovarian tissue in culture equally well as Matrigel [14]. Our data suggest that the use of human laminins naturally expressed by ovarian cortex may be advantageous. Follicle survival was significantly higher in the LN221 group compared with Matrigel. On the other hand, tissue cultured on LN521 had a higher proportion of growing secondary follicles than Matrigel. Interestingly, in human embryonic stem cells, LN521 activates PI3K/Akt signaling [18], which is also recognized as an important pathway in activation of follicle growth [33]. It should be addressed in follow-up studies if LN521 could bring an advantage to multi-step culture systems where isolation of highest possible number of secondary follicles would be beneficial. LN511 and the mixture of the three laminins provided the least consistent support to ovarian tissue. In both groups, the follicle densities decreased significantly during culture, and apoptosis was significantly increased in LN511 tissue. Although the laminin-mixture group did not provide further improvement, maybe due to lower concentrations of the individual laminins in the mixture, future studies should test other combinations and higher concentrations of relevant laminins.
We detected steroids in all culture conditions but did not find significant differences between groups. The media samples were collected at the termination of cultures and reflected steroid production over the last 24 h only. This short time together with the fact that the production of steroids is typically highly variable between patient samples [24] may have contributed to difficulties in detecting differences. In future studies, samples should be collected over the whole culture period and from a larger number of patients. Nevertheless, the results confirm that the tissue maintained its endocrine activity in culture.
Human ovarian follicle culture systems have been developed for decades, and up to date, there is no consensus on optimal culture condition. The field has moved towards multi-step culture systems [4, 10], 3D-solutions [6], and advanced tissue engineering [8, 34] to enable follicle growth to maturity in vitro, and to even create transplantable ovarian constructs [7, 35, 36]. The newest culture systems seldom include ECM to support the growth of the follicles. It should be investigated whether defined ECM components such as laminins could enhance follicle survival and growth in these systems. Likewise, possibilities to replace Matrigel with specific laminins in decellularized transplantable scaffolds should be studied [7].
Conclusions
In summary, our results indicate that LN221 and LN521 provide certain advantages over Matrigel in human ovarian tissue culture providing the first evidence that a defined ECM substrate can be used to support the development of pre-antral follicles in vitro. Specifically, LN221 enabled higher survival of follicles, and LN521 stimulated the development of secondary follicles. The often used ECM preparation Matrigel has several disadvantages, which are particularly critical for any applications in human fertility preservation in a clinic setting, including exposure to animal-derived components, undefined composition, batch-to-batch variability, the potential risk for pathogen transmission, and the potential to contain tumor-derived growth factors. The use of laminins would be appropriate in clinical settings for fertility preservation and could also provide a system for toxicological applications. Follow-up studies should focus on further development of the laminin-based ovarian tissue cultures towards multi-step systems that allow full folliculogenesis.
Electronic supplementary material
(XLSX 16 kb)
(XLSX 16 kb)
(PDF 384 kb)
(PDF 154 kb)
Acknowledgments
The authors would like to thank all patients who donated their ovarian tissue and follicular fluid, without which this study would not have been possible. We would also like to thank the midwives and clinicians who assisted with patient recruitment and logistics of tissue collection. We acknowledge Richelle Duque Björvang and Magdalena Wagner for their valuable input in writing of this manuscript. We thank Raoul Kuiper and Tarja Schröder from the Morphological Phenotype Analysis facility, Karolinska Institutet, Sweden, for H&E stains and tissue scans.
Authors’ contributions
JH: preparation of samples, experimental design, data collection and analysis, and drafting of the manuscript; AT: data analysis and drafting of the manuscript; CH: TUNEL experiments and data analysis; AD: bioinformatic analyses; MODS and JL: steroid analysis; BN and KP: coordination of sample collection; OH: experimental design and interpretation of data; PD: preparation of samples, experimental design, data analysis, and writing of the manuscript. All authors participated in the writing of the manuscript and approved the final article.
Funding information
This study was funded by research grants from Swedish Research Council (#2017-02316), Swedish Research Council FORMAS (#2015-00623 and #2016-02031), and Swedish Childhood Cancer Foundation (#PR2017-0044).
Availability of data and material
Data related to patient samples cannot be shared due to ethical and personal data protection restrictions. All materials (except human ovarian tissue) are commercially available.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Stockholm Region Ethics Board (license numbers 2010/549-31/2 and 2015/798-31/12).
Consent to participate
Informed consent was obtained from all individuals included in the study.
Consent for publication
Not applicable.
Code availability
The code used to plot laminin expression in single-cell RNA-seq data is available upon request. The original RNA-seq data is described in Wagner et al. Nat Comm 2020 and has been deposited in the ArrayExpress database at EMBL-EBI under the accession code E-MTAb-8381.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59(4):783–790. doi: 10.1016/S0015-0282(16)55860-9. [DOI] [PubMed] [Google Scholar]
- 2.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12(5):1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 3.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24(3):135–142. doi: 10.1093/molehr/gay002. [DOI] [PubMed] [Google Scholar]
- 5.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31(8):1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin H, Kristensen SG, Jiang H, Rasmussen A, Andersen CY. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum Reprod. 2016;31(7):1531–1539. doi: 10.1093/humrep/dew049. [DOI] [PubMed] [Google Scholar]
- 7.Pors SE, Ramlose M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34(8):1523–1535. doi: 10.1093/humrep/dez077. [DOI] [PubMed] [Google Scholar]
- 8.Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulini F, Vilela JM, Chiti MC, Donnez J, Jadoul P, Dolmans MM, et al. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod BioMed Online. 2016;33(3):425–432. doi: 10.1016/j.rbmo.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 12.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 13.Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24(4):262–269. doi: 10.1055/s-2006-948555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod BioMed Online. 2004;9(3):287–293. doi: 10.1016/S1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84(4):689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- 18.Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M, Inzunza J, Nilsson AS, Baker D, Kuiper R, Sun Y, Blennow E, Nordenskjöld M, Grinnemo KH, Kere J, Betsholtz C, Hovatta O, Tryggvason K. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 19.Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24(4):195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 20.Irving-Rodgers HF, Hummitzsch K, Murdiyarso LS, Bonner WM, Sado Y, Ninomiya Y, Couchman JR, Sorokin LM, Rodgers RJ. Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice. Cell Tissue Res. 2010;339(3):613–624. doi: 10.1007/s00441-009-0905-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irving-Rodgers HF, Friden BE, Morris SE, Mason HD, Brannstrom M, Sekiguchi K, Sanzen N, Sorokin LM, Sado Y, Ninomiya Y, Rodgers RJ. Extracellular matrix of the human cyclic corpus luteum. Mol Hum Reprod. 2006;12(9):525–534. doi: 10.1093/molehr/gal060. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, Hovatta O, Kere J, Lanner F, Damdimopoulou P. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao J, Tuck AR, Sjodin MOD, Lindberg J, Sand A, Niklasson B, et al. Resveratrol supports and alpha-naphthoflavone disrupts growth of human ovarian follicles in an in vitro tissue culture model. Toxicol Appl Pharmacol. 2018;338:73–82. doi: 10.1016/j.taap.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 26.team R. RStudio . Integrated Development for R. Boston: RStudio, Inc; 2019. [Google Scholar]
- 27.Ouni E, Vertommen D, Chiti MC, Dolmans MM, Amorim CA. A draft map of the human ovarian proteome for tissue engineering and clinical applications. Mol Cell Proteomics. 2019;18(Suppl 1):S159–SS73. doi: 10.1074/mcp.RA117.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baert Y, Stukenborg JB, Landreh M, De Kock J, Jornvall H, Soder O, et al. Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod. 2015;30(2):256–267. doi: 10.1093/humrep/deu330. [DOI] [PubMed] [Google Scholar]
- 29.Kanninen LK, Harjumaki R, Peltoniemi P, Bogacheva MS, Salmi T, Porola P, et al. Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials. 2016;103:86–100. doi: 10.1016/j.biomaterials.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Tjin MS, Chua AWC, Moreno-Moral A, Chong LY, Tang PY, Harmston NP, Cai Z, Petretto E, Tan BK, Tryggvason K. Biologically relevant laminin as chemically defined and fully human platform for human epidermal keratinocyte culture. Nat Commun. 2018;9(1):4432. doi: 10.1038/s41467-018-06934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc. 2018;13(7):1662–1685. doi: 10.1038/s41596-018-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not −332, −111, or −411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26(11):2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356(1–2):24–30. doi: 10.1016/j.mce.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, van Ruymbeke E, Champagne SD, Donnez J, Amorim CA. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35(1):41–48. doi: 10.1007/s10815-017-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manavella DD, Cacciottola L, Pomme S, Desmet CM, Jordan BF, Donnez J, et al. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum Reprod. 2018;33(6):1107–1116. doi: 10.1093/humrep/dey080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 16 kb)
(XLSX 16 kb)
(PDF 384 kb)
(PDF 154 kb)
Data Availability Statement
Data related to patient samples cannot be shared due to ethical and personal data protection restrictions. All materials (except human ovarian tissue) are commercially available.