Abstract
Objective:
HIV disease and methamphetamine (METH) dependence share overlapping mechanisms of neurotoxicity that preferentially compromise monoamine-rich frontostriatal circuitry. However, norepinephrine (NE) function is poorly understood in HIV and METH dependence. We evaluated associations between cerebrospinal fluid (CSF) NE and HIV, METH dependence, and neurocognition.
Methods:
Participants included 125 adults, stratified by HIV serostatus (HIV+/HIV-) and recent METH dependence (METH+/METH-), who underwent comprehensive neurocognitive testing and lumbar puncture. CSF NE was assayed using high-performance liquid chromatography. Multivariable regression modelled NE as a function of HIV, METH, and their interaction, adjusting for demographic and clinical factors. Pearson’s correlations examined relationships between NE and demographically-adjusted neurocognitive domain scores.
Results:
HIV significantly interacted with METH (p<.001) such that compared to HIV-/METH-, CSF NE was markedly elevated in the single risk-groups (HIV+/METH- : d=0.96; HIV-/METH+: d=0.79) and modestly elevated in the dual-risk group (HIV+/METH+: d=0.48). This interaction remained significant after adjustment for lifetime depression, antidepressant use, and race/ethnicity. In the full sample, higher NE levels significantly correlated with worse global function (r=−0.19), learning (r=−0.23), and delayed recall (r=−0.18). Similar relationships between higher NE and worse neurocognition were detected in the METH- groups (i.e., HIV-/METH- and HIV+/METH-) and in the virally-suppressed persons HIV+ subgroup, but not in the METH+ groups (i.e., HIV-/METH+, HIV+/METH+).
Discussion:
HIV and METH independently, but not additively, relate to noradrenergic excess in the CNS, and perturbations to noradrenergic function may represent a pathophysiological mechanism of HIV-related neurocognitive dysfunction. Consistent with prior reports that noradrenergic excess compromises hippocampal and prefrontal function, higher NE related to worse neurocognition, even among successfully-treated PWH. Pharmacological and psychosocial interventions that stabilize NE function may improve neurocognition in PWH.
Keywords: HIV, methamphetamine, norepinephrine, cerebrospinal fluid, neurocognitive disorders, depression
INTRODUCTION
Chronic methamphetamine (METH) use is common among persons with HIV (PWH)1 and exacerbates HIV disease burden through enhancement of viral replication and disruption of antiretroviral therapy (ART) efficacy and adherence2,3. HIV and METH use independently compromise central nervous system (CNS) function, as evidenced by gray and white matter abnormalities, neuroinflammation, and cerebrovascular dysfunction4–7. The combination of HIV- and METH-related neural injury preferentially disrupts frontostriatal pathways that support multiple facets of neurobehavior, including higher-order neurocognition, emotional regulation, and reward sensitivity8. In addition to premorbid psychosocial factors (e.g., low socioeconomic status), the adverse CNS effects of HIV and METH amplify risk for neurocognitive deficits9, impairments in daily functioning10, and neuropsychiatric disorders11,12.
Alteration of monoamine neurotransmitter systems, which are highly active in frontostriatal circuitry, is a putative pathophysiological mechanism of HIV- and METH-related neurobehavioral dysfunction8,13. Chronic METH use repeatedly exposes the brain to excessive levels of monoamines, which can result in neurotoxicity14. Similarly, HIV is neurotoxic to dopaminergic neurons through the release of viral proteins and disrupts the metabolic pathway of serotonin by macrophage-induced inflammation13,15. Potentially additive effects of HIV and METH on dopaminergic dysregulation has received considerable attention, yet the combined effects of HIV and METH on norepinephrine (NE) in the CNS are poorly characterized16,17.
NE is primarily produced in the locus coeruleus, where it is diffusely projected across the brain to carry out its neuromodulatory actions18. Consequently, the NE system supports a host of cognitive processes, including attention, learning, memory, and decision-making14. Noradrenergic dysfunction contributes to neurocognitive impairment in several clinical disorders, including Parkinson’s and Alzheimer’s disease, schizophrenia, and attention-deficit/hyperactivity disorder19. NE also mediates the brain’s “fight-or-flight” response to threatening stimuli, which involves activation of the peripheral sympathetic nervous system and increased arousal20. Unsurprisingly, dysregulation of NE is implicated in depression and anxiety-related disorders, which are characterized by chronic stress and fluctuating levels of arousal and attention21,22.
Few studies, if any, have examined the effects of HIV or METH dependence on levels of NE in cerebrospinal fluid (CSF), as opposed to peripheral measurements in plasma or urine. Moreover, it is particularly unclear how the combination of HIV and METH impacts CSF NE levels. Therefore, the present study systematically evaluated CSF NE among a cohort of PWH and HIV-seronegative individuals with or without METH dependence. We hypothesized that HIV and METH dependence would independently be associated with altered NE levels relative to controls, and that comorbid HIV and METH dependence would synergistically contribute to the largest alterations in NE levels relative to controls and the single-risk conditions, even after accounting for neuropsychiatric factors that may also influence NE levels. Additionally, secondary analyses explored relationships between CSF NE and domain-specific neurocognition.
METHODS
Participants
Participants included 125 individuals enrolled in a National Institutes of Drug Abuse-funded, University of California, San Diego (UCSD) cohort study focusing on the CNS effects of HIV and METH. Participants were recruited from the San Diego area through several methods, including flyers, community outreach talks, HIV-focused clinics, and residential drug treatment programs. Participants were stratified into four groups based on HIV serostatus (HIV-/HIV+) and diagnoses of METH dependence (METH-/METH+): HIV-/METH- (n=23), HIV-/METH+ (n=35), HIV+/METH- (n=22), and HIV+/METH+ (n=45). All participants gave written informed consent and study procedures were approved by the UCSD Institutional Review Board. Exclusion criteria were: 1) DSM-IV diagnosis of other substance use dependence (except cannabis) within the last 5 years, or alcohol dependence within the last 12 months; 2) abuse of any substances (except alcohol and cannabis) other than METH within the last 12 months; 3) evidence of acute intoxication on the day of testing, which may confound neuropsychological test results, as determined by positive urine toxicology results; 4) history of psychotic or mood disorder with psychotic features, neurological, or medical condition that may also confound neuropsychological test results.
Neuropsychiatric Assessment
Participants were evaluated for METH dependence, other substance use dependence, and Major Depressive Disorder (MDD) diagnoses using the Composite International Diagnostic Interview (CIDI)23 or Structured Clinical Interview for DSM-IV (SCID-IV)24, as study methodology was developed prior to release of DSM-5. METH+ participants met DSM-IV diagnostic criteria for lifetime METH dependence and met criteria for METH dependence or abuse within the past 18 months. Lifetime METH use parameters were estimated using a timeline follow-back interview. Of the 45 METH- participants, 8 (4 HIV- and 4 HIV+) reported remote, recreational use of METH; however, such use was deemed to be modest and clinically inconsequential during clinical interview. Current depressive symptoms were assessed with the Beck Depression Inventory version two (BDI-II)25.
Neuromedical Assessment
Participants underwent a comprehensive neuromedical assessment, blood draw, and lumbar puncture. HIV disease was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. Among HIV+ participants, HIV RNA in plasma was measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN) and deemed undetectable at a lower limit of quantitation (LLQ) of 50 copies/ml. A detailed history of common and relevant medical conditions (see Table 1) as well as antiretroviral therapy (ART) use was collected via a structured, clinician-administered interview. Of the 51 participants on ART, 50 completed the AIDS Clinical Trials Group 4-day adherence self-report questionnaire to assess for nonadherence to any ART medications over the past four days26. Nonadherence was defined as report of any missed dose over the last four days. Hepatitis C virus (HCV) serostatus was diagnosed by standard clinical antibody detection.
Table 1.
Demographic and clinical characteristics by METH group
| Variable | HIV-/METH-(n=23) | HIV-/METH+(n=35) | HIV+/METH-(n=22) | HIV+/METH+(n=45) | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 40.8 (11.82) | 39.0 (9.34) | 44.1 (9.48) | 40.0 (7.44) | 0.225 |
| Sex (male), n (%) | 22 (95.7%) | 33 (94.3%) | 20 (90.9%) | 43 (95.6%) | 0.890 |
| Education (years), mean (SD)** | 12.8 (1.95) | 11.4 (2.35) | 12.6 (2.32) | 13.2 (1.73) | 0.002 |
| Estimated premorbid verbal IQ, mean (SD) | 97.6 (15.47) | 93.4 (12.96) | 96.9 (10.32) | 96.7 (10.97) | 0.542 |
| Race/Ethnicity | 0.062a | ||||
| Non-Hispanic White, n (%) | 12 (52.2%) | 21 (60.0%) | 11 (50.0%) | 35 (77.8%) | |
| Non-Hispanic Black, n (%) | 7 (30.4%) | 6 (17.1%) | 6 (27.3%) | 3 (6.7%) | |
| Hispanic, n (%) | 2 (8.7%) | 7 (20.0%) | 4 (18.2%) | 4 (8.9%) | |
| Other, n (%) | 2 (8.7%) | 1 (2.9%) | 1 (4.6%) | 3 (6.7%) | |
| Medical Comorbidities | |||||
| Hepatitis C virus, n (%)* | 2 (8.7%) | 8 (22.9%) | 8 (38.1%) | 17 (37.8%) | 0.037 |
| Hypertension, n (%)** | 6 (26.1%) | 1 (2.9%) | 6 (28.6%) | 3 (6.7%) | 0.005 |
| Hyperlipidemia, n (%)*** | 1 (4.3%) | 1 (2.9%) | 8 (38.1%) | 0 (0.0%) | <0.001 |
| Diabetes, n (%) | 2 (8.7%) | 1 (2.9%) | 2 (9.5%) | 2 (4.4%) | 0.590 |
| Body mass index (kg/m2), mean (SD)** | 27.9 (4.89) | 28.5 (4.75) | 24.8 (3.51) | 25.5 (3.11) | 0.001 |
| Neuropsychiatric Characteristics | |||||
| Lifetime Major Depressive Disorder, n (%)* | 6 (26.1%) | 13 (38.2%) | 7 (33.3%) | 28 (62.2%) | 0.014 |
| BDI score, median [IQR]*** | 1 [0, 6] | 10 [2.5, 18] | 8 [5, 15.5] | 13 [5.5, 19.5] | <0.001 |
| Antidepressant use, n (%)** | 6 (26.1%) | 4 (11.4%) | 11 (50%) | 20 (44.4%) | 0.002 |
| On SSRI, n (%)* | 4 (17.4%) | 1 (2.9%) | 6 (27.3%) | 10 (22.2%) | 0.029 |
| On SNRI, n (%) | 0 (0%) | 0 (0%) | 1 (4.6%) | 0 (0%) | 0.176 |
| On tricyclic, n (%) | 4 (17.4%) | 3 (8.6%) | 3 (13.6%) | 13 (28.9%) | 0.125 |
| On atypical, n (%)* | 0 (0%) | 0 (0%) | 4 (18.2%) | 2 (4.4%) | 0.011 |
| Lifetime alcohol use disorder, n (%) | 11 (47.8%) | 24 (70.6%) | 13 (61.9%) | 31 (68.9%) | 0.299 |
| Current alcohol use disorder, n (%) | 1 (4.3%) | 1 (3.3%) | 0 (0.0%) | 1 (2.2%) | 1.000 |
| Lifetime cannabis use disorder, n (%) | 8 (34.8%) | 19 (55.9%) | 9 (42.9%) | 20 (44.4%) | 0.451 |
| Current cannabis use disorder, n (%) | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0.808 |
| Lifetime cocaine use disorder, n (%) | 4 (17.4%) | 14 (41.2%) | 5 (23.8%) | 16 (35.6%) | 0.194 |
| Lifetime opioid use disorder, n (%) | 1 (4.3%) | 6 (17.6%) | 2 (9.5%) | 3 (6.7%) | 0.358 |
| Lifetime non-METH substance use disorder, n (%) | 17 (73.9%) | 29 (85.3%) | 14 (66.7%) | 38 (84.4%) | 0.288 |
| Current tobacco use, n (%)* | 7 (30.4%) | 18 (54.6%) | 5 (23.8%) | 25 (56.8%) | 0.021 |
| HIV Disease Characteristics | |||||
| AIDS diagnosis, n (%)* | - | - | 16 (76.2%) | 22 (48.9%) | 0.033 |
| Duration of HIV infection (years), median [IQR] | - | - | 9 [5, 12] | 5 [2, 14] | 0.222 |
| Current CD4 count (cells/mm3), median [IQR] | - | - | 435 [245, 589] | 410 [330, 601] | 0.554 |
| Nadir CD4 count (cells/mm3), median [IQR] | - | - | 138 [25, 251] | 212 [67, 396] | 0.165 |
| Detectable plasma HIV RNA, n (%)*** | - | - | 3 (14.3%) | 29 (67.4%) | <0.001 |
| On ART, n (%) | - | - | 18 (90.0%) | 33 (73.3%) | 0.111 |
| ART nonadherent, n (%)* | - | - | 0 (0.0%) | 6 (18.8%) | 0.016 |
| METH Use Parameters | |||||
| Lifetime days of use, median [IQR]* | 2780 [992, 4485] | 1340 [748, 2795] | 0.030 | ||
| Lifetime grams consumed, median [IQR] | 2542 [466, 5761] | 1060 [416, 2654] | 0.106 | ||
| Lifetime daily use (grams/day), median [IQR] | 0.91 [0.31, 1.46] | 0.61 [0.35, 1.59] | 0.885 | ||
| Days since last use, median [IQR] | 91 [23, 304] | 95 [39, 182] | 0.658 | ||
| Age of first use, mean (SD)* | 21.6 (7.93) | 26.0 (6.90) | 0.012 |
Note.
p<.001,
p<.01,
p<.05. ART= antiretroviral therapy; BDI-II= Beck Depression Inventory version two; SSRI= selective serotonin reuptake inhibitor; SNRI= serotonin-norepinephrine reuptake inhibitor
Represents group differences in percentage of non-Hispanic White
CSF Norepinephrine Assay
The CSF NE assay was performed using alumina extraction followed by high-performance liquid chromatography (HPLC; Waters, Milford, MA), as described in detail by Kumar et al.27–30. Briefly, NE was detected with electrochemical detectors and identified based on its retention time, determined with reference standards prepared from fresh stock solutions. Signal output was defined by the area under the curve that was proportional to the concentration of NE present in the standard or samples, calculated based on the ratio method by using the Waters Empower software. CSF NE levels were expressed as pg/ml and were log10-transformed in order to improve normality and facilitate parametric analysis. HPLC reliability was assessed by Kumar et al., who reported inter- and intra-assay coefficients of variance for NE that range from 3–10%28,29.
Neurocognitive Assessment
Participants completed a comprehensive and well-validated neuropsychological battery covering domains often impacted by HIV and METH9. Individual raw test scores were transformed to standard T scores (mean=50, standard deviation=10) that corrected for the expected effects of age, education, sex and race/ethnicity, as appropriate, based on normative samples of HIV- adults31–33. These demographically-corrected individual test T scores were averaged across the entire battery and within each domain to generate global and domain-specific summary scores.
2.3. Statistical Analysis
HIV/METH group differences on background characteristics (i.e., demographics, neuropsychiatric and neuromedical characteristics) were examined using analysis of variance (ANOVA), Wilcoxon/Kruskal-Wallis tests, and Chi-square statistics as appropriate. To determine the combined effects of HIV and METH on CSF NE levels, multivariable linear regression analyses modelled log10-transformed CSF NE as a function of HIV, METH, and their interaction. Follow-up comparisons examined pairwise HIV/METH group differences in NE levels, with correction for multiple comparisons using Tukey’s Honest Significant Difference (HSD) tests. To account for the potential influence of viral suppression, NE levels were compared between HIV+ individuals with undetectable (i.e., suppressed) and detectable plasma HIV RNA (i.e., unsuppressed), and pairwise HIV/METH group analyses were re-run such that the HIV+ groups were restricted to suppressed individuals. Beta coefficients derived from the full model and Cohen’s d statistics are presented for estimates of effect size for pairwise comparisons. To determine if the effects of HIV and METH on NE were attenuated by covariates, backward model selection guided by Akaike information criteria (AIC) was applied such that final models considered factors that differed by HIV or METH status at p<0.10 (i.e., education, race/ethnicity, lifetime MDD, BDI-II, antidepressant use, current tobacco use, HCV serostatus, hypertension, hyperlipidemia, and body mass index). Age and time of lumbar puncture were also considered as covariates given their potential to influence NE levels. Covariates were retained in the final model if they were deemed to improve overall model fit, based on the AIC metric. Last, Pearson’s r correlations explored the relationship between log10-transformed CSF NE and neurocognitive domain scores in the full study sample and within the subgroup of PWH on ART with undetectable plasma HIV RNA. Analyses were conducted using JMP Pro version 14.0.0 (JMP®, Version <12.0.1>. SAS Institute Inc., Cary, NC, 2018).
RESULTS
Participant Characteristics
The full study sample was 63% non-Hispanic White and 94% male with a mean age of 40.6 years (range: 19–59) and mean education of 12.5 years. Participant characteristics by HIV/METH group are presented in Table 1. Years of education were significantly lower in HIV-/METH+ individuals compared to the other three groups (ps<.030); however, estimates of premorbid verbal intelligence were comparable across groups. HIV-/METH- had lower BDI-II scores compared to the other three groups (ps<.013). Prevalence of lifetime MDD was higher in HIV+/METH+ compared to the other three groups (ps<.034) and rates of antidepressant use were higher in HIV+/METH- and HIV+/METH+ compared to HIV-/METH+ (ps<.002). Rates of remote non-METH substance use disorders were comparable across all four groups; however, current tobacco use was more prevalent in METH+ than METH- (p=.002), regardless of HIV serostatus. HIV-/METH+ individuals had an earlier age of onset of METH use and more estimated lifetime days of METH use than HIV+/METH+ individuals (ps<.031). HIV+/METH- and HIV+/METH+ individuals had higher rates of HCV than HIV-/METH- (ps<.023) and lower body mass index values than both HIV-/METH- and HIV-/METH+ (ps<.025). HIV-/METH- and HIV+/METH- had higher rates of hypertension than both HIV-/METH+ and HIV+/METH+ (ps<.031). Prevalence of hyperlipidemia was higher in HIV+/METH- compared to the other three groups (ps<.001).
In the HIV+ group, 78% were currently on ART and median current CD4 counts (411 cells/mm3) were higher compared to median nadir CD4 counts (190 cells/mm3). Although AIDS diagnoses were more prevalent in HIV+/METH- compared to HIV+/METH+ (p=.033), HIV+/METH+ individuals had higher rates of detectable HIV RNA in plasma compared to HIV+/METH- (p<.001). Self-reported ART adherence was 100% in HIV+/METH- individuals, yet 19% of HIV+/METH+ individuals failed to fully adhere to their ART regimens.
HIV, METH, and NE
In the full sample, NE in CSF ranged from 0.3 to 1032.1 pg/mL (median=33.5, interquartile range [IQR]=10.9–234.7). Table 2 presents raw and log10-transformed CSF NE values across the four HIV/METH groups as well as the HIV+ subgroups of virally suppressed and unsuppressed individuals. Figure 1 displays box and whisker plots for the log10-transformed CSF NE values by group. Regression analysis results indicated a significant HIV x METH interaction (beta=−0.88, t=3.41, p<.001) on log10-transformed CSF NE. Specifically, the effect of HIV on NE was moderated by METH such that HIV significantly related to higher NE in the absence of METH (HIV+/METH- vs. HIV-/METH-: beta=0.67, d=0.96, p=.002) but did not relate to NE in the presence of METH (HIV+/METH+ vs. HIV-/METH+: beta=−0.22, d=−0.31, p=.167). Similarly, the effect of METH on NE was moderated by HIV such that METH significantly related to higher NE in the absence of HIV (HIV-/METH+ vs. HIV-/METH-: beta=0.55, d=0.79, p=.004) but trended toward lower NE in the presence of HIV (HIV+/METH+ vs. HIV+/METH-: beta=−0.33, d=−0.48, p=.067). Relative to HIV-/METH- controls, the combination of HIV and METH trended toward higher NE (beta=0.34, d=0.48, p=.064). After correcting for multiple comparisons using Tukey’s HSD, NE levels remained significantly higher in HIV-/METH+ (adjusted-p=.020) and HIV+/METH- (adjusted-p=.009) compared to HIV-/METH- controls. Accounting for viral suppression did not alter findings; NE levels did not significantly differ between virally suppressed and unsuppressed HIV+ individuals (p=.50) and NE levels remained higher in HIV+/METH- compared to HIV-/METH- when restricting the HIV+/METH- group to virally suppressed individuals (beta=0.60, d=0.86, p=.008).
Table 2.
Raw and log10-transformed CSF norepinephrine levels by HIV and METH group
| HIV- | HIV+ | HIV+ | ||||
|---|---|---|---|---|---|---|
| Norepinephrine | METH- | METH+ | METH- | METH+ | Suppressed | Unsuppressed |
| pg/ml | 17 [9, 31] | 93 [16, 279] | 191 [20, 375] | 31 [10, 182] | 48 [10, 285] | 50 [23, 202] |
| log10(pg/ml)a | 1.26 (0.43) | 1.81 (0.78) | 1.93 (0.68) | 1.60 (0.74) | 1.67 (0.84) | 1.80 (0.59) |
Data presented as mean (SD) or median [IQR]
Log10-transformed values were used in statistical analyses
Figure 1. Cerebrospinal fluid (CSF) norepinephrine levels are elevated in methamphetamine (METH) dependence and HIV disease.
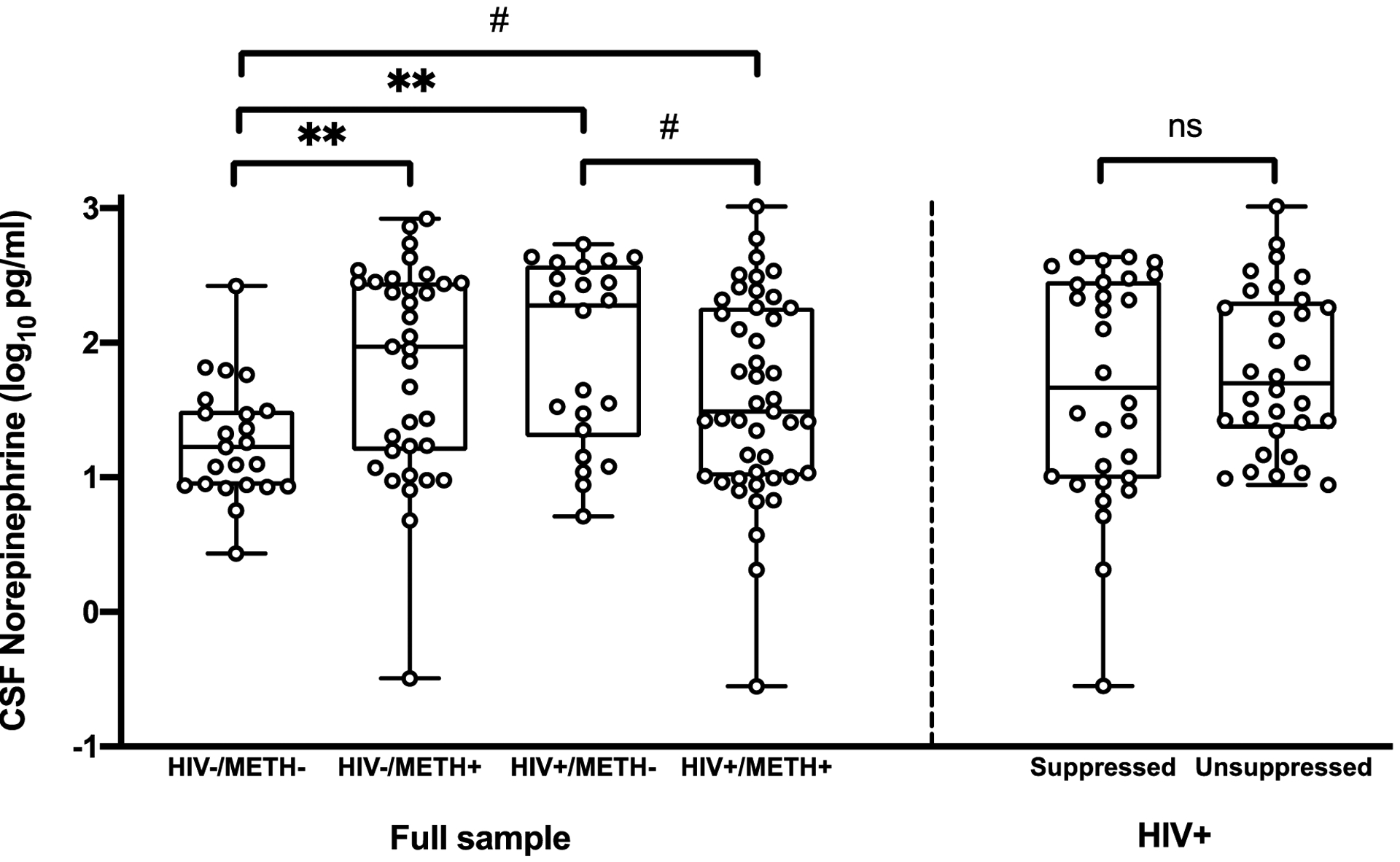
Compared to HIV-/METH-, significant and large elevations in CSF NE were detected in HIV-/METH+ (d=0.79, p=.004) and HIV+/METH- (d=0.96, p=.002), while a more modest, trend-level elevation in CSF NE occurred in HIV+/METH+ (d=0.48, p=.064). CSF NE remained higher in HIV-/METH+ and HIV+/METH- after correction for multiple comparisons. In the HIV+ group, CSF NE levels did not differ between virally suppressed and unsuppressed individuals (p=.50). **p<.01, #p<.10.
An AIC-guided multivariable linear regression model that employed backward selection of covariates was conducted to determine whether HIV/METH group differences on NE were better explained by clinical and demographic factors that differed across groups (see Table 3). In this covariate-adjusted model, the HIV x METH interaction was retained as a significant predictor of NE, with the difference in NE between HIV-/METH- and HIV+/METH- remaining significant in pairwise comparisons using Tukey’s HSD. Lifetime MDD and antidepressant use were also retained in the overall model. A lifetime diagnosis of MDD related to significantly higher levels of NE and antidepressant use related to significantly lower levels of NE. Race/ethnicity (White vs. not White) exhibited a trend-level association with NE (p=.099), but was retained in the model because its inclusion improved overall model fit (based on AIC criteria) compared to competing models.
Table 3.
Results of multiple linear regression examining the combined effects of HIV and METH on CSF norepinephrine
| Parameter | beta (SE) | 95% CI | p |
|---|---|---|---|
| HIVa | 0.75 (0.21) | 0.34, 1.16 | <0.001 |
| METHb | 0.45 (0.19) | 0.08, 0.81 | 0.017 |
| HIV x METH | −0.96 (0.26) | −1.47, –0.45 | <0.001 |
| Lifetime MDD | 0.28 (0.13) | 0.02, 0.54 | 0.035 |
| Antidepressant use | −0.34 (0.14) | −0.62, –0.06 | 0.017 |
| White (vs. not White) | 0.22 (0.13) | −0.04, 0.48 | 0.099 |
Represents effect of HIV+ vs. HIV- in METH- individuals only (reference group)
Represents effect of METH+ vs. METH- in HIV- individuals only (reference group)
NE and Neurocognition
Exploratory Pearson’s r correlations examined relationships between log10-transformed CSF NE and neurocognition in the full sample, within the four primary study groups, and within the HIV+ subgroups (i.e., virally suppressed and unsuppressed; Table 4). In the full sample, higher NE significantly correlated with lower (worse) global function (r=−0.19, p=.038). The negative association between NE and global function in the full sample was primarily driven by negative correlations with learning (r=−0.23, p=.012) and delayed recall (r=−0.18, p=.043). With respect to the four primary study groups, negative correlations between CSF NE and neurocognition were detected in the METH- (i.e., HIV-/METH- and HIV+/METH-) but not the METH+ groups (i.e., HIV-/METH+, HIV+/METH+). In the HIV-/METH- group, significant or trend-level negative correlations with medium-to-large effect sizes (r range: −0.39 to −0.60) were detected for global function, executive function, learning, and delayed recall. In the HIV+/METH- group, trend-level negative correlations with small-to-medium effect sizes (r range: −0.24 to −0.41) were detected for verbal fluency, delayed recall, and working memory. With respect to the HIV+ subgroups, only the virally suppressed group demonstrated significant negative correlations with medium effect sizes (r range: −0.39 to −0.42) for global function, verbal fluency, working memory, and motor skills.
Table 4.
Correlations between log10-transformed CSF norepinephrine and neurocognition
| HIV- | HIV+ | HIV+ | |||||
|---|---|---|---|---|---|---|---|
| Domain | All | METH- | METH+ | METH- | METH+ | Suppressed | Unsuppressed |
| Global | −0.19* | −0.39# | −0.08 | −0.30 | −0.04 | −0.42* | −0.10 |
| Verbal Fluency | −0.13 | −0.09 | −0.13 | −0.39# | 0.08 | −0.41* | 0.19 |
| Executive Function | −0.08 | −0.41# | 0.08 | −0.17 | 0.02 | −0.16 | −0.02 |
| Processing Speed | −0.11 | −0.24 | −0.12 | −0.06 | 0.03 | −0.15 | −0.16 |
| Learning | −0.23** | −0.60** | −0.10 | −0.17 | −0.20 | −0.18 | −0.20 |
| Delayed Recall | −0.18* | −0.44* | −0.09 | −0.24# | −0.09 | −0.10 | −0.12 |
| Working Memory | −0.15 | 0.03 | −0.04 | −0.41# | −0.13 | −0.42* | −0.13 |
| Motor Skills | −0.03 | −0.04 | 0.08 | −0.24 | 0.02 | −0.39* | −0.01 |
p<.01,
p<.05,
p<.10
DISCUSSION
The present study examined independent and combined effects of HIV infection and METH dependence on CNS noradrenergic tone, quantified as CSF NE concentrations. Compared to the HIV-/METH- group, CSF NE levels differed in the presence of HIV disease or METH-dependence such that the single-risk groups (i.e., HIV+/METH- and HIV-/METH+) exhibited notably large elevations in CSF NE. Surprisingly, the dual-risk HIV+/METH+ group exhibited a more modest elevation in CSF NE relative to controls and these levels did not exceed those observed in the single-risk conditions. This pattern suggests that HIV and METH both influence CNS noradrenergic tone, but that the combination of these risk-factors does not additively contribute to a hypernoradrenergic state in the CNS. Importantly, the effect of HIV on NE in the absence of METH-dependence was robust to neuropsychiatric factors with relevance to noradrenergic function and NE remained higher in the subset of HIV+/METH- participants on suppressive therapy. In turn, higher NE directly related to poorer neurocognitive function in the entire sample, in HIV- individuals and PWH without METH-dependence, and within the subgroup of PWH on suppressive therapy. These results suggest a pathophysiological link between noradrenergic excess and HIV-related neurocognitive dysfunction in the absence of METH, but also suggest that elevations in CSF NE in the presence of METH, either alone or in combination with HIV, are not similarly sensitive to neurocognitive dysfunction.
The few studies that have evaluated NE activity in PWH provide evidence of autonomic and hypothalamic pituitary–adrenal (HPA) axis dysfunction. In comparison to controls, PWH exhibited lower basal plasma levels of epinephrine and higher basal plasma levels of cortisol28. In response to physiological and cognitive challenge tasks, PWH also exhibited attenuated elevations of plasma NE and adrenocorticotropin hormone (ACTH) but higher cortisol responses28,29. These alterations to the biological substrates of stress in HIV are consistent with a model of chronic stress34, as chronic stress-related corticotropin releasing factor (CRF) signaling can facilitate neuronal adaptations in the locus coeruleus that sustain chronic elevations in forebrain NE levels35. Higher urine concentrations of NE independently predict a worse viral load response to protease inhibitors, mediate the effect of perceived stress on protease inhibitor efficacy, and predict longitudinal increases in plasma viral load and decreases in CD4 count36,37. The expression of adrenoreceptors on human monocytes and macrophages facilitates this noradrenergic influence on the peripheral immune response to infection, with cellular models indicating that higher NE accelerates viral replication in HIV-infected peripheral blood mononuclear cells through β-adrenoreceptor-dependent modulation of inflammatory cytokines and upregulation of HIV coreceptors CCR5 and CXCR417,38–40.
Our results expand on these peripherally-based studies by demonstrating the presence of HIV-associated hypernoradrenergic function in the CNS. The observation that CSF NE remained higher in HIV+/METH- individuals on suppressive ART may reflect continued crosstalk between noradrenergic signaling and microglial-mediated neuroinflammatory responses to CNS infection, rather than direct neuroendocrine modulation of infected CNS cells17. HIV gp120 rodent models show HIV-related disruption of β-adrenoreceptor function of microglia and astrocytes41 and stress-induced increases in neuroinflammatory cytokines is in part mediated by increased central noradrenergic activation of β-adrenoreceptors42. Notably, a recent study reported increased β−2 adrenoreceptor mRNA expression in post-mortem frontal lobe tissues in PWH with comorbid neurocognitive impairment and HIV encephalitis43. Nevertheless, the link between central noradrenergic function and the HIV neuroimmunological cascade, including region-specific (e.g., brainstem vs. cortex) and cell-specific (e.g., astrocytes vs. microglia) differences in cytokine release and chemotaxis, is poorly characterized and merits further investigation17.
Despite the critical involvement of the noradrenergic system in METH use, NE has largely been ignored in favor of dopamine and serotonin in human studies examining the effects of chronic METH dependence on monoaminergic biomarkers. The acute administration of METH results in an excessive release of NE through interference of the NE transporter (NET), vesicular monoamine transporter type-2, and monoamine oxidase enzyme. This extracellular release of NE, which is more potent than METH-induced release of dopamine and serotonin44, contributes to the psychoactive properties of METH (e.g., reward sensitivity, locomotor stimulation) directly through binding of α−1 adrenoreceptors and indirectly through mediation of dopaminergic activity45. In contrast to acute use, the current study examined CSF NE levels in METH users who met criteria for METH dependence or abuse within the last 18 months, but as a group had a median of three months since last use of METH and all tested negative for METH on urine toxicology screening. Our finding that CSF NE was higher in HIV-/METH+ individuals compared to HIV-/METH- controls is consistent with prior clinical studies demonstrating elevated NE (CSF, plasma, or urine) in substance-dependent patients following withdrawal from pro-noradrenergic substances46. Additionally, peripheral noradrenergic hyperactivity is linked to ecstasy use47 and METH-induced psychosis48, while medications that diminish noradrenergic activity show some efficacy in reducing withdrawal symptoms and promoting abstinence in cocaine users45. The mechanism underlying a sustained elevation of central NE after the cessation of METH use remains unclear, but may reflect a compensatory enhancement in neurotransmission following METH-related downregulation of NET49,50 and prolonged CRF signaling due to METH-induced HPA axis dysregulation51,52.
Although HIV+/METH+ individuals also exhibited modestly lower levels of CSF NE compared to HIV+/METH-, suggesting a potential antagonistic effect of METH dependence conditional on HIV status, this difference was marginal and substantially weakened after correction for multiple comparisons. Some salient differences that we did observe between groups were that the prevalence of an AIDS diagnosis and estimated duration of infection (nonsignificant) were higher in HIV+/METH- compared to HIV+/METH+ individuals, which may reflect greater historical disease severity that could exacerbate the progression of autonomic and HPA axis dysfunction. However, we did not observe significant associations between markers of HIV disease severity and NE levels (data not shown), including viral suppression, but there may be additional unmeasured HIV-related factors that influence NE and differ between the HIV+/METH+ and HIV+/METH- groups. Several neuroimaging studies have recently observed a similar antagonistic pattern of results, whereby METH related to smaller cortical area and reduced striatal activity during a motor sequencing task in HIV- individuals yet unexpectedly related to larger cortical area and greater striatal activity in PWH53,54. One proposed explanation for increased cortical area in HIV+/METH+ is the potential presence of cytotoxic edema due to enhanced neuroinflammation53. Under this hypothesis, a reduction in CSF NE is also plausible given that aquaporin 4, a membrane protein that contributes to cytoplasmic edema and is elevated in brain homogenates from patients with HIV-associated dementia55, has also been implicated in astrocytic-mediated reuptake of monoamines56. At the synaptic level, METH-induced spillover of dopamine into the extra-synaptic environment exposes neuroimmune cells to toxic levels of dopamine, which in turn exacerbates HIV-related neuroinflammation that reciprocally compromises dopaminergic tone and may promote dopaminergic neuronal loss in the striatum and midbrain57–60. It is unclear if METH-induced overstimulation of noradrenergic neurons in the locus coeruleus similarly contributes to depletion of CSF norepinephrine and neuronal loss in the setting of HIV, however recent studies have identified that the brainstem and pons are vulnerable to HIV infection and accompanying neuroinflammation and volume loss61,62.
Higher NE correlated with poorer global neurocognitive functioning in the entire sample, and particularly in the domains of learning and delayed recall. Subanalyses within study groups indicated additional adverse neurocognitive effects of NE on executive function, working memory, verbal fluency and motor skills in PWH and HIV- individuals without METH-dependence. Parallel to our findings, higher CSF NE has previously been associated with poorer processing speed, executive function, learning, and delayed recall in neurologically normal adults across the lifespan63. Similarly, steroid-induced reductions in CSF NE have been shown to correspond with improvements in free recall64. Overall, our observation that higher CSF NE levels relate to poorer neurocognition is supported by the inverted-U shaped model of catecholaminergic signaling and cortical function, whereby noradrenergic deficiency or excess results in neuronal dysfunction65,66. In the prefrontal cortex, low to moderate levels of synaptic NE stimulate postsynaptic α−2 adrenoreceptors that enhance working memory; however, as central concentrations of NE rise to abnormally high levels, such as in conditions of chronic stress, there is sufficient NE to stimulate low affinity α−1 adrenoreceptors that subsequently impair working memory67. In addition to the prefrontal cortex, intact noradrenergic signaling is critical for optimal hippocampal function. Degeneration of noradrenergic neurons in the locus coeruleus has emerged as a key pathophysiological mechanism of Alzheimer’s disease68. Although acute stress enhances memory for emotionally-salient stimuli, chronic stress can induce hippocampal excitotoxicity through the prolonged elevation of glucocorticoids, a process which is mediated by noradrenergic activity in the amygdala69. In the context of the present study, heightened noradrenergic activity related to HIV use may shift individuals to the right of the inverted-U curve, resulting in associations between higher CSF NE and worse neurocognition. Although HIV-/METH+ individuals also exhibited elevated CSF NE levels, variation in NE levels within this group, as well as the dual-risk HIV+/METH+ group, was not predictive of neurocognition. The majority of neuropsychological studies in METH+ populations support a link between METH-related neural injury and neurocognitive dysfunction, however, they also indicate remarkable heterogeneity in neurocognitive profiles among METH users70. Consistent with this variability, parameters of METH use are not predictive of neuropsychological performance and it has been suggested that substance use during adolescence or young adulthood can interfere with educational quality, which may obscure the detection of brain-behavior relationships in adulthood70,71. Other factors such as host genetics, including those involved in the metabolism of METH and catecholamines72,73, may also help clarify the role of NE in METH-associated neurobehavioral function.
In our sample, HIV and METH use were associated with greater levels of current depressive symptoms and the prevalence of lifetime MDD was highest in the dual-risk HIV+/METH+ group. In addition to HIV and METH, lifetime MDD and antidepressant use independently related to NE such that NE was highest in participants who were not currently taking antidepressant medication yet had a lifetime diagnosis of MDD. Hypernoradrenergic function has previously been implicated in the pathogenesis of depression due to its role in the activation of sympathetic and neuroendocrine reactions to stress and the inhibition of neurovegetative behaviors (e.g., eating, sleeping), particularly when depression is left untreated51. Persistent elevations in CSF NE have previously been reported in patients with melancholic depression51 as well as male combat veterans with chronic post-traumatic stress disorder74, raising the possibility of a sustained hypernoradrenergic state years after the onset of an initial traumatic stressor. Consistent with this notion, we observed a stronger relationship between CSF NE and lifetime MDD than current depressive symptoms (i.e., BDI-II). Although the specific psychosocial context of historical depression in our sample is unknown, the high rates of lifetime MDD may be influenced by the chronicity of METH use (average age of onset at 24 years) and HIV disease (median estimated duration of HIV disease of 6.4 years). Given that some antidepressants directly inhibit reuptake of NE, the association between antidepressant use and lower CSF NE levels was unexpected. However, it has been suggested that some antidepressants may dampen depression-related hyperactivation of noradrenergic activity in the locus coeruleus, potentially via reductions in inflammatory cytokines and stress75,76. Nevertheless, the present study was not designed to formally evaluate antidepressant effects on noradrenergic function and future studies should systematically examine how individual antidepressant agents impact CSF NE levels in the context of HIV- and METH-related factors.
The current study presents novel data that raises intriguing questions regarding the role of the noradrenergic system in HIV and METH-related neurobehavioral dysfunction, however, we acknowledge several limitations. Our assay of NE in CSF captures central noradrenergic function in vivo, yet it only reflects a global measurement of NE and cannot differentiate NE levels across different brain regions. Concentrations of NE in the brain greatly exceed levels detected in CSF, with brainstem regions lying adjacent to CSF thought to reflect the highest concentrations of NE77. The locus coeruleus has been reported to contain more than 70% of total NE in the brain78 and may be the strongest contributor to CSF NE levels obtained via lumbar puncture given that spinal cord NE is concentrated in spinal cord gray matter innervated by brainstem nuclei and the locus coeruleus77. The relationship between CSF NE and neurocognition is also likely influenced by brain regional differences in the rate of NE metabolism, which is primarily carried out by the monoamine oxidase A and catechol-o-methyltransferase (COMT) enzymes17. COMT, for example, is particularly active in clearing catecholamines from the prefrontal cortex and we have previously reported that the neurocognitive effects of HIV and METH are moderated by genetic variation in COMT73,79,80. The neurobehavioral effects of COMT are often attributed to the processing of dopamine, which may be increased or decreased in HIV and METH depending on disease stage and abstinence81–83. However, this is also complicated by the fact that COMT separately metabolizes NE and dopamine is a precursor to NE when metabolized by dopamine beta-hydroxylase. Investigations that integrate neuroimaging with CSF markers of dopamine, NE, and their metabolites, with consideration for the enzymatic factors that bridge these catecholaminergic systems, may help clarify the separate and joint contributions of NE and dopamine to neurobehavioral dysfunction and identify neuropharmacological targets for these deficits in HIV and METH.
The relatively small sample size of each HIV/METH group necessitates the replication of study methodology in larger samples. This would importantly increase the number of underrepresented participants (e.g., women) and facilitate analysis of potential moderators (e.g., depression) on the relationships between HIV, METH use, and CSF NE. In addition to CSF NE, future studies should examine the influence of HIV and METH on other central and peripheral biomarkers involved in stress, such as cortisol, CRF, and ACTH. Last, the cross-sectional and associative nature of our data limits our ability to make causal inferences about the relationships between HIV, METH, NE, and neurocognition. Although there is neurobiological evidence for HIV- and METH-related activation of noradrenergic activity, hypernoradrenergic function and neurocognitive dysfunction may also result in risky behaviors that precede the acquisition of HIV disease and onset of substance misuse.
Overall, our findings indicate that both PWH and METH users exhibit overactivation of the noradrenergic system, although the combination of these two risk factors did not enhance this hypernoradrenergic state. In PWH without METH-dependence, including those on effective ART, excess NE may confer risk for neurocognitive deficits characterized by prefrontal and hippocampal dysfunction and perturbations to noradrenergic function may therefore represent a pathophysiological mechanism of HIV-associated neurocognitive disorders. Higher NE did not similarly predict neurocognition in the METH+ groups, suggesting a more complex relationship between noradrenergic tone and neurocognition in the setting of METH use. Pharmacological treatments that stabilize NE function coupled with cognitive-behavioral interventions that buffer psychosocial stress may ameliorate neurocognitive dysfunction in the neurologically vulnerable population of PWH.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported by grants from the National Institute on Drug Abuse P50DA026306: Translational Methamphetamine AIDS Research Center (TMARC; PI: Grant, Igor) and P01DA012065: NeuroAIDS: Effects of Methamphetamine and HCV (PI: Grant, Igor). Stipend support to RS is funded by National Institute of Aging award F31AG064989. The authors declare no conflicts of interest. We thank Dr. Adarsh Kumar and her laboratory for conducting the norepinephrine assays.
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Discovery Institute (SBMDI), and the University of California, Irvine (UCI). The TMARC comprises: Administrative Coordinating Core (ACC) – Executive Unit: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Managers – Erin E. Morgan, Ph.D. and Jared Young, Ph.D.; Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Behavioral Assessment and Medical (BAM) Core – Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core – Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director).; Project 5: Marcus Kaul, Ph.D. (Project Director).
Footnotes
Conflicts of Interest and Source of Funding: This research was supported by grants from the National Institute on Drug Abuse: P50DA026306 (Translational Methamphetamine AIDS Research Center [TMARC]; PI: Grant, Igor) and P01DA012065 (NeuroAIDS: Effects of Methamphetamine and HCV; PI: Grant, Igor). Stipend support to RS is funded by National Institute of Aging award F31AG064989. The authors declare no conflicts of interest. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
REFERENCES
- 1.Degenhardt L, Mathers B, Guarinieri M, et al. Meth/amphetamine use and associated HIV: Implications for global policy and public health. The International journal on drug policy. 2010;21(5):347–358. [DOI] [PubMed] [Google Scholar]
- 2.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. The Journal of infectious diseases. 2003;188(12):1820–1826. [DOI] [PubMed] [Google Scholar]
- 3.Moore DJ, Blackstone K, Woods SP, et al. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS care. 2012;24(12):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masters MC, Ances BM. Role of Neuroimaging in HIV Associated Neurocognitive Disorders (HAND). Seminars in neurology. 2014;34(1):89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain research. 2015;1628(Pt A):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73(9):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polesskaya O, Silva J, Sanfilippo C, et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain research. 2011;1373:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soontornniyomkij V, Kesby JP, Morgan EE, et al. Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2016;11(3):495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society : JINS. 2004;10(1):1–14. [DOI] [PubMed] [Google Scholar]
- 10.Blackstone K, Iudicello JE, Morgan EE, et al. Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. Journal of addiction medicine. 2013;7(4):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM. Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clinical psychology review. 2017;51:164–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R. Depression among methamphetamine users: association with outcomes from the Methamphetamine Treatment Project at 3-year follow-up. The Journal of nervous and mental disease. 2009;197(4):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar AM, Fernandez J, Borodowsky I, Gonzalez L, Kumar M. HIV-1 infection and central monoamine neurotransmitters. Am J Infect dis. 2007;3:177–183. [Google Scholar]
- 14.Moszczynska A, Callan SP. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. The Journal of pharmacology and experimental therapeutics. 2017;362(3):474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gostner JM, Becker K, Kurz K, Fuchs D. Disturbed Amino Acid Metabolism in HIV: Association with Neuropsychiatric Symptoms. Frontiers in Psychiatry. 2015;6(97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein PS, Shah A, Gupte R, et al. Methamphetamine toxicity and its implications during HIV-1 infection. Journal of neurovirology. 2011;17(5):401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan R, Gaskill PJ. The role of catecholamines in HIV neuropathogenesis. Brain research. 2019;1702:54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Current biology : CB. 2015;25(21):R1051–r1056. [DOI] [PubMed] [Google Scholar]
- 19.Borodovitsyna O, Flamini M, Chandler D. Noradrenergic Modulation of Cognition in Health and Disease. Neural plasticity. 2017;2017:6031478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller J, Makara G, Kruk M. Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neuroscience & Biobehavioral Reviews. 1997;22(1):85–97. [DOI] [PubMed] [Google Scholar]
- 21.Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric disease and treatment. 2011;7(Suppl 1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blier P, Briley M. The noradrenergic symptom cluster: clinical expression and neuropharmacology. Neuropsychiatric disease and treatment. 2011;7(Suppl 1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Composite Diagnositic International Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 24.Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 25.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX, Psychology Corporation. 1996. [Google Scholar]
- 26.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–266. [DOI] [PubMed] [Google Scholar]
- 27.Kumar AM, Fernandez JB, Goodkin K, Schneiderman N, Eisdorfer C. An isocratic concurrent assay of free metabolites, 4-hydroxy-3-methoxy mandelic acid, 3-methoxy-4-hydroxy-phenylglycol, normetanephrine, metanephrine, and 5-hydroxy-indoleacetic acid in same sample of urine extract using HPLC-ECD. Journal of liquid chromatography & related technologies. 1997;20(12):1931–1943. [Google Scholar]
- 28.Kumar M, Morgan R, Szapocznik J, Eisdorfer C. Norepinephrine response in early HIV infection. J Acquir Immune Defic Syndr. 1991;4(8):782–786. [PubMed] [Google Scholar]
- 29.Chittiprol S, Kumar AM, Satishchandra P, et al. Progressive dysregulation of autonomic and HPA axis functions in HIV-1 clade C infection in South India. Psychoneuroendocrinology. 2008;33(1):30–40. [DOI] [PubMed] [Google Scholar]
- 30.Kumar AM, Kumar M, Fernandez JB, Mellman TA, Eisdorfer C. A simplified HPLC-ECD technique for measurement of urinary free catecholamines. Journal of liquid chromatography. 1991;14(19):3547–3557. [Google Scholar]
- 31.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 32.Heaton RK, Taylor MJ, Manly J. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III In: Clinical interpretation of the WAIS-III and WMS-III. San Diego, CA, US: Academic Press; 2003:181–210. [Google Scholar]
- 33.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33(7):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress (Amsterdam, Netherlands). 2003;6(3):167–172. [DOI] [PubMed] [Google Scholar]
- 35.Borodovitsyna O, Joshi N, Chandler D. Persistent Stress-Induced Neuroplastic Changes in the Locus Coeruleus/Norepinephrine System. Neural plasticity. 2018;2018:1892570–1892570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ironson G, Balbin E, Stieren E, et al. Perceived stress and norepinephrine predict the effectiveness of response to protease inhibitors in HIV. International journal of behavioral medicine. 2008;15(3):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ironson G, O’Cleirigh C, Kumar M, et al. Psychosocial and Neurohormonal Predictors of HIV Disease Progression (CD4 Cells and Viral Load): A 4 Year Prospective Study. AIDS Behav. 2015;19(8):1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. The Journal of Immunology. 1998;161(2):610–616. [PubMed] [Google Scholar]
- 39.Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12695–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole SW, Jamieson BD, Zack JA. cAMP up-regulates cell surface expression of lymphocyte CXCR4: implications for chemotaxis and HIV-1 infection. Journal of immunology (Baltimore, Md : 1950). 1999;162(3):1392–1400. [PubMed] [Google Scholar]
- 41.Levi G, Patrizio M, Bernardo A, Petrucci TC, Agresti C. Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(4):1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson JD, Campisi J, Sharkey CM, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–1307. [DOI] [PubMed] [Google Scholar]
- 43.Dever SM, Rodriguez M, El-Hage N. β-Adrenergic receptor gene expression in HIV-associated neurocognitive impairment and encephalitis: implications for MOR-1K subcellular localization. Journal of neurovirology. 2016;22(6):866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse (New York, NY). 2001;39(1):32–41. [DOI] [PubMed] [Google Scholar]
- 45.Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addiction biology. 2009;14(2):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgerald PJ. Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Substance abuse : research and treatment. 2013;7:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuerenburg HJ, Petersen K, Baumer T, Rosenkranz M, Buhmann C, Thomasius R. Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuro endocrinology letters. 2002;23(3):259–261. [PubMed] [Google Scholar]
- 48.Yui K, Goto K, Ikemoto S, Ishiguro T. Plasma monoamine metabolites and spontaneous recurrence of methamphetamine-induced paranoid-hallucinatory psychosis: relation of noradrenergic activity to the occurrence of flashbacks. Psychiatry research. 1996;63(2–3):93–107. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Z, Zhang HT, Bootzin E, Millan MJ, O’Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(6):1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu MY, Shamburger S, Li J, Ordway GA. Regulation of the human norepinephrine transporter by cocaine and amphetamine. The Journal of pharmacology and experimental therapeutics. 2000;295(3):951–959. [PubMed] [Google Scholar]
- 51.Wong ML, Kling MA, Munson PJ, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(1):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuloaga DG, Jacobskind JS, Raber J. Methamphetamine and the hypothalamic-pituitary-adrenal axis. Frontiers in neuroscience. 2015;9:178–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDuffie KE, Brown GG, McKenna BS, et al. Effects of HIV Infection, methamphetamine dependence and age on cortical thickness, area and volume. NeuroImage Clinical. 2018;20:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archibald SL, Jacobson MW, Fennema-Notestine C, et al. Functional interactions of HIV-infection and methamphetamine dependence during motor programming. Psychiatry research. 2012;202(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St Hillaire C, Vargas D, Pardo CA, et al. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. Journal of neurovirology. 2005;11(6):535–543. [DOI] [PubMed] [Google Scholar]
- 56.Ding JH, Sha LL, Chang J, Zhou XQ, Fan Y, Hu G. Alterations of striatal neurotransmitter release in aquaporin-4 deficient mice: An in vivo microdialysis study. Neuroscience letters. 2007;422(3):175–180. [DOI] [PubMed] [Google Scholar]
- 57.Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One. 2014;9(9):e108232–e108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28(22):5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain research. 2003;984(1–2):133–142. [DOI] [PubMed] [Google Scholar]
- 60.Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H. HIV, Tat and dopamine transmission. Neurobiol Dis. 2017;105:51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honeycutt JB, Wahl A, Baker C, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126(4):1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallianpur KJ, Valcour VG, Lerdlum S, et al. HIV DNA in CD14+ reservoirs is associated with regional brain atrophy in patients naive to combination antiretroviral therapy. AIDS. 2014;28(11):1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang LY, Murphy RR, Hanscom B, et al. Cerebrospinal fluid norepinephrine and cognition in subjects across the adult age span. Neurobiology of aging. 2013;34(10):2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolkowitz OM, Weingartner H, Rubinow DR, et al. Steroid modulation of human memory: biochemical correlates. Biological psychiatry. 1993;33(10):744–746. [DOI] [PubMed] [Google Scholar]
- 65.Baldi E, Bucherelli C. The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity in biology, toxicology, medicine. 2005;3(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological psychiatry. 2011;69(12):e89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacology & therapeutics. 2007;113(3):523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta neuropathologica communications. 2017;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osborne DM, Pearson-Leary J, McNay EC. The neuroenergetics of stress hormones in the hippocampus and implications for memory. Frontiers in neuroscience. 2015;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(2):259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cherner M, Suarez P, Casey C, et al. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and alcohol dependence. 2010;106(2–3):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherner M, Bousman C, Everall I, et al. Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. Journal of the International Neuropsychological Society : JINS. 2010;16(5):890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cherner M, Watson CW, Saloner R, et al. Adverse effect of catechol-O-methyltransferase (COMT) Val158Met met/met genotype in methamphetamine-related executive dysfunction. Addictive behaviors. 2019;98:106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geracioti TD Jr., Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. The American journal of psychiatry. 2001;158(8):1227–1230. [DOI] [PubMed] [Google Scholar]
- 75.Seki K, Yoshida S, Jaiswal MK. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural regeneration research. 2018;13(7):1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(12):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziegler MG, Raymond Lake C, Wood JH, Ebert MH. Norepinephrine in Cerebrospinal Fluid In: Wood JH, ed. Neurobiology of Cerebrospinal Fluid 1. Boston, MA: Springer US; 1980:141–152. [Google Scholar]
- 78.Lambert GW. Central nervous system norepinephrine metabolism in hypertension. Current Hypertension Reports. 2000;2(3):302–310. [DOI] [PubMed] [Google Scholar]
- 79.Bousman CA, Cherner M, Glatt SJ, et al. Impact of COMT Val158Met on executive functioning in the context of HIV and methamphetamine. Neurobehavioral HIV medicine. 2010;2010:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saloner R, Marquine MJ, Sundermann EE, et al. COMT Val158Met Polymorphism, Cardiometabolic Risk, and Nadir CD4 Synergistically Increase Risk of Neurocognitive Impairment in Men Living With HIV. Journal of acquired immune deficiency syndromes (1999). 2019;81(5):e148–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of neurovirology. 2011;17(1):26–40. [DOI] [PubMed] [Google Scholar]
- 82.Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA psychiatry. 2017;74(5):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scheller C, Arendt G, Nolting T, et al. Increased dopaminergic neurotransmission in therapy-naïve asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. Journal of neural transmission (Vienna, Austria : 1996). 2010;117(6):699–705. [DOI] [PubMed] [Google Scholar]


