Abstract
Repeated invasive and painful procedures are often necessary components of pediatric cancer treatment. Adequate pain control during procedures is essential; however, procedure-related pain may be underestimated and undertreated. Currently, there is not a standard approach for the appropriate level of sedation to manage procedure-related pain in children with cancer. A team was assembled to review the evidence and develop recommendations to determine the appropriate level of sedation necessary for pain control in patients undergoing pediatric oncology procedures. After a systematic search of the literature, 15 research-based articles were synthesized and critically appraised. A recommendation was made related to the level of sedation utilized for bone marrow aspirates and bone marrow biopsies. There is a need for further research related to the necessary level of sedation for patients undergoing pediatric oncology procedures.
Keywords: pain, procedure, sedation, pediatric, cancer
Background
Repeated invasive and painful procedures are often necessary components of pediatric cancer treatment (Hockenberry et al., 2011; Traivaree, Jindakam, Monsereenusom, Rujkijyanont, & Lumkul, 2014). Lumbar puncture (LP), bone marrow aspiration (BMA), and bone marrow biopsy (BMB), are essential for the evaluation and management of many childhood cancers (Siegel, Miller, & Jemal, 2018). Results of laboratory testing, performed on specimens obtained through these procedures in the setting of collaborative clinical trials, including those conducted through Children’s Oncology Group (COG; 2010), have contributed to a combined overall 5-year survival rate for pediatric cancers of more than 80% (Howlander et al., 2013). Adequate pain control during procedures is essential; however, procedure-related pain may be underestimated and undertreated (Ljungman, Gordh, Sorensen, & Kreuger, 2001; McGrath et al., 1990; Schechter, 1989; Schechter, Altman, & Weiss, 1990).
Pain is a unique experience for each individual. Numerous factors influence an individual’s perception of pain. Previous experiences, anxiety, fear, or other behavioral components may directly influence an individual’s report of pain (Cohen et al., 2008). Decades ago, procedure-related pain was identified as the most feared symptom by children with cancer, and still persists as a predominant concern (Enskär, Carlsson, Golsäter, Hamrin, & Kreuger, 1997).
A core value in pediatric oncology nursing is the provision of atraumatic care (Hockenberry-Eaton, Barerra, Bown, Bottomley, & O’Neill, 1999). Nursing practice may include the use of several nonpharmacologic or psychosocial approaches to procedure-related pain such as distraction, imagery, relaxation, positive incentives or reinforcement, or hand holding, humor, and music. The benefits to the use of nonpharmacologic approaches to decrease procedure-related pain have been previously reviewed (Landier & Tse, 2010).
Although there are multiple approaches to adequately managing procedure-related pain in children with cancer, and some children may benefit from psychosocial strategies alone or in combination with pharmacologic approaches, the focus of this literature review is on pharmacologic sedation in managing procedure-related pain in pediatric oncology. Currently, there is not a standard approach for the appropriate level of sedation to manage procedure-related pain in children with cancer. Children who receive inadequate analgesia for their initial procedure during cancer diagnosis/treatment may experience diminished efficacy of interventions for pain in later procedures (Weisman, Bernstein, & Schecter, 1998). The child’s developmental stage may also affect their ability to comprehend the intention of the health care team to provide improved pain control during subsequent procedures. Memories of inadequate pain control during initial procedures may lead to anticipatory anxiety for subsequent procedures (Iannalfi et al., 2005). Parental perception of the threat of danger and possible distress his or her child may experience also influences the child’s pain perception before, during, and after a procedure (Caes et al., 2014). Bhatnagar et al. (2007) suggest that inadequate pain control during procedures in children with cancer may lead to depression, affecting the patient’s entire treatment experience and long-term quality of life. The multiple compounding variables contributing to the patient experience must be integrated into each patient’s procedural plan of care.
Understanding the effect of pharmacologic sedation for pain control is paramount to the development of each patient’s plan of care for procedural pain management. There are multiple tools available for the measurement of pain (Bieri, Reeve, Champion, Addicoat, & Ziegler, 1990; Cella et al., 2015; Cohen et al., 2008; Keck, Gerkensmeyer, Joyce, & Schade, 1996; McGrath et al., 1990; Merkel, Voepel-Lewis, Shayevitz, & Malviya, 1997; Wong & Baker, 1988). To ensure that optimal pain assessment is achieved, it is important to choose a pain scale that takes into account the patient’s developmental level and verbal status (Cohen et al., 2008). Patients who are young or non-verbal may have difficulty effectively and accurately communicating pain, reporting may rely on parent, nurse, or clinician observations to evaluate patient pain. As children develop and mature, self-assessment of pain becomes possible with the use of pain scales incorporating images or numbers to allow the child to rank their level of pain. Utilization of a self-reported pain scale is optimal, as it allows the patient’s perspective to be integrated into their own plan of care (Cella et al., 2015). Regardless of which pain scale is utilized for patient assessment, providers must evaluate the pain level to provide appropriate sedation and analgesia for their patients.
When choosing the appropriate medication regimen, it is important to be aware that patients may have varied responses for both efficacy and toxicity. Some medications effective in providing sedation and managing pain may result in significant side effects, requiring evaluation of both risks and benefits of sedation. Thus, customization of care for the patient is paramount for optimal outcomes.
The American Society of Anesthesiologists (ASA) advocates for the use of sedation for prolonged and/or painful procedures to relieve anxiety, discomfort, and pain. They describe sedation as a continuum that can be classified as mild, moderate, and deep sedation/general anesthesia (GA; ASA, 2018; Table 1). The ASA also warns that combinations of medications can potentially result in unintended outcomes, including oversedation.
Table 1.
American Society of Anesthesiology Levels of Sedation.
| Level of sedation | Description |
|---|---|
| Mild Sedation (anxiolysis) | Intent is anxiolysis with maintenance of consciousness. Patient responds to verbal commands. |
| Moderate Sedation/Analgesia (formerly known as conscious sedation) | A controlled state of depressed consciousness. Airway patency is maintained. Patient responds appropriately to developmentally appropriate commands. |
| Deep Sedation/Analgesia | A controlled state of depressed consciousness. Airway reflexes and airway patency may not be maintained. The ability to independently maintain ventilation may be impaired. The patient cannot be easily aroused but does respond purposefully to painful stimulation. |
| General Anesthesia | Loss of consciousness occurs. The patient will have impaired airway reflexes, airway patency, and ventilation. Patients are not arousable even to painful stimulus. |
Note. From the American Society of Anesthesiologists (2018).
The Institute of Medicine (2001) has identified that the provision of high-quality health care is a necessity. A major barrier to providing high-quality cancer care is unnecessary variation in clinical practice because of lack of standardization (Bowles et al., 2008). Given the lack of standardization for procedure-related pharmacologic pain control, decision making regarding the type of sedation utilized for the management of procedural pain varies both within and across institutions (Holdsworth et al., 2003). The goal of this literature review was to determine recommendations for the level of sedation necessary to provide adequate pain control for the pediatric oncology patient undergoing invasive procedures.
Significance
Invasive procedures are integral to the provision of treatment necessary to achieve long-term disease-free survival for many children with cancer. For example, a child diagnosed with standard risk B-cell acute lymphoblastic leukemia without disease in the central nervous system may undergo between 17 to 22 LPs over the course of their treatment (Matloub et al., 2011). Comparatively, a child with high risk B-cell acute lymphoblastic leukemia with central nervous system disease may undergo more than 30 LPs over the duration of treatment (Larsen et al., 2016). While the number and timing of invasive procedures is often dictated by study protocol, the use of sedation and pain control methods for these procedures varies not only across institutions but also within institutions, and have evolved over time. Both the parent and child may experience high levels of stress during procedures, and their anxiety typically does not decrease throughout treatment. Parental and patient anxiety can be minimized with adequate preparation and pain management (Cline et al., 2006; Crock et al., 2003; Hockenberry et al., 2011; Kasak, Penati, Brophy, & Himelstein, 1998).
Methods Used for Development of Evidence-Based Recommendations
Evidence-Based Practice Review Team
A multidisciplinary team was formed to complete a systematic review in response to a call from the COG Nursing Discipline Evidence-Based Practice Subcommittee to address the topic of the necessary level of sedation for pain control in children with cancer requiring invasive procedures. The team was composed of three pediatric oncology nurse practitioners, an oncology/bone marrow transplant pharmacist, and a pediatric oncology nurse. A doctorally prepared pediatric nurse practitioner with experience in evidence-based reviews mentored the team.
Question Development
The topic was chosen to align with the COG Nursing Discipline Group’s blueprint and organizing framework (Kelly, Hooke, Ruccione, Landier, & Hasse, 2014; Landier, Leonard, & Ruccione, 2013). Addressing illness-related distress is a current focus within the COG Nursing Discipline, and guided the development of the following clinical question that identified a population (P), intervention (I), comparison (C), and outcome (O, PICO; Melnyk & Fineout-Overholt, 2011) for the systematic review: Among patients with pediatric cancer requiring procedures (P), what level (C) of sedation (I) is necessary for pain control (O)? In this review, “pain control” is defined as “minimal pain or discomfort,” and was determined for each individual study included in this review based on the pain scale that was used.
Literature Search Strategies
A systematic search of literature was performed according to the PRISMA criteria (Moher, Liberati, Tetzlaff, & Altman, 2009) with guidance from a medical librarian. PubMed, CINAHL, and the Cochrane Library were searched; key words and MeSH terms included the following: pediatrics, child, adolescent, neoplasms, medical oncology, anesthesia, analgesics, non-narcotic, deep sedation, pain management, biopsy, biopsy-needle, catheterization-peripheral, spinal puncture, and bone marrow examination. Limits were set for the English language, human studies, and publication dates within the past 20 years to retrieve the most recent evidence. Research studies and meta-analyses were included. The full search strategy is included in the appendix. In addition, websites of professional organizations applicable to the topic were also searched for relevant clinical guidelines. The organizations included the American Society of Clinical Oncology, Oncology Nursing Society, Association of Pediatric Hematology Oncology Nurses, and American Academy of Pediatrics. In addition, the Agency for Healthcare Research and Quality, National Center for Complimentary and Integrative Health, National Guideline Clearinghouse, and the National Comprehensive Cancer Network were searched for clinical practice guidelines.
Inclusion/Exclusion Criteria
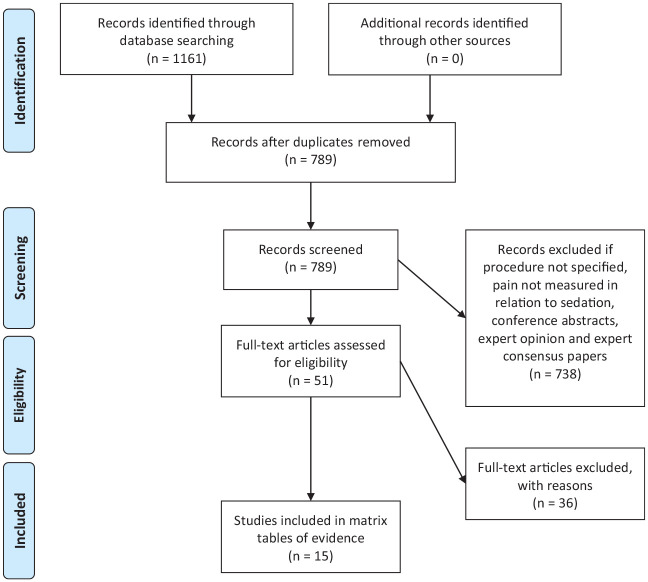
Initially 1,161 articles were identified (Figure 1). Inclusion criteria included survivors of pediatric cancer, patients undergoing active pediatric cancer treatment, and procedures involving patients with pediatric cancer. Articles were excluded if they did not have a form of pain measurement (objective, subjective, parent report, and/or health care provider report). Conference abstracts, expert opinion, and expert consensus papers were excluded from the review.
Figure 1.
PRISMA diagram presenting the process of sample determination (Moher et al., 2009).
Evidence Review
The team met once in-person at Duke University School of Nursing, and also met monthly via conference call to evaluate the evidence. Each of the 15 articles had a primary and secondary reviewer that summarized the evidence in a matrix table. The level of sedation used in each of the 15 included studies was classified according to the ASA classification system for sedation as mild, moderate, or deep sedation/GA (ASA, 2018; Table 1). More than one sedation classification could be assigned per study, if applicable.
The primary reviewer presented the information and the team evaluated whether the evidence met the inclusion criteria. The evidence quality was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system (Guyatt et al., 2011). This included evaluation for methodologic issues, effect size, publication bias, inconsistency, and indirectness. An overall rating was determined for the quality of the body of evidence (high to very low), and a recommendation statement (strong vs. weak) was developed (Guyatt et al., 2008).
Results
Fifteen studies met the criteria for inclusion in this review, including 1 retrospective and 14 prospective studies. Studies were classified according to the analgesia/type of sedation employed, as follows: (a) mild sedation, (b) moderate sedation, and (c) deep sedation/GA. Four studies were included in more than one analgesia/sedation category. The 15 identified studies used a variety of scales for pain assessment. The pain scales used in the identified studies are summarized in Table 2. There were no applicable clinical practice guidelines discovered in the search.
Table 2.
Pain Scales Used in the 15 Studies Included as Evidence in This Review.
| Pain scale | Age range | Pain scale defined | Pain scale ratings |
|---|---|---|---|
| Bieri Faces Scale | Children 4-16 years old | Self-reported pain intensity scale; uses adapted facial expressions correlated to numerical scale (0-6) | 0 = no pain to 6 = maximum pain |
| Children’s Hospital of Eastern Ontario Pain Scale | Children 0-7 years old | Observational behavioral scale for evaluating pain in children; scores range is from 0-2 or 0-3 in all categories except crying which is 1-3. Total numerical score calculated (1-13) | Cry: 1: No cry; 2: Moaning; 2: Crying; 3: Screaming |
| Facial: 0: Smiling; 1: Composed; 2: Grimaced | |||
| Child Verbal: 0: Positive; 1: None; 1: Complaints, other; 2: Complaints, pain; 2: Complaints, pain, and other | |||
| Torso: 1: Neutral; 2: Shifting; 2: Tense; 2: Shivering; 2: Upright; 2: Restrained | |||
| Touch: 1: Not touching; 2: Reach; 2: Touch; 2: Grab; 2: Restrained | |||
| Legs: 1: Neutral; 2: Squirming; 2: Drawn up; 2: Standing; 2: Restrained | |||
| Child Perception Questionnaire | >6 Years old | Self-report behavioral scale; pain and distress assessed in the five categories: Fear and anxiety, needle pain, discomfort from anesthesia, postprocedural pain, problems associated with overall distress. Of note: Children are given this questionnaire one hour postprocedure; total numerical score (0-20) | Fear anxiety 0-4; needle pain 0-4; discomfort from anesthesia 0-4; postprocedural pain 0-4; problems associated with overall distress 0-4; sum of all categories added together for overall pain score ranging 0-20 |
| Faces Scale–Revised | Children 4-16 years old | Self-report pain intensity scale; uses revised facial expressions correlated to numerical scale (0-10) | Six faces are used with the lowest score of 0 correlated with no pain to 10 correlated with very much pain |
| Face, Legs, Activity, Cry, Consolability (FLACC) Pain Scale | Children 2 months to 7 years old or unable to communicate pain scale | Observational behavioral scale; observer notes facial expression, movement of legs, generalized activity, crying and consolability of the patient; scores assigned to behaviors noted | Score of 0 = No particular facial expression or smile, normal or relaxed legs, quiet activity, no crying, content, and relaxed mood; score of 1 = Occasional grimace or frown, uneasy, restless legs, squirming or shifting activity, moans, whimpers, and can be reassured by touch or distraction; score of 2 = Frequent to constant frown or clenched jaw, kicking of drawing up legs, arched, rigid or jerking activity, and difficult to comfort; sum of scores from each category of added together for an overall pain intensity rating range of 0-10 |
| Modified Visual Analog Scale (VAS) | Children greater than 12 years old | Self-report pain intensity scale; uses numbers ranged 0-100 | 0-100 scale; 0 = No discomfort or pain to 100 = Worst discomfort or pain |
| Oucher Pain Scale | Children 3-12 years old | Self-report scale pain intensity; children view either pictures or numbers correlated to pain intensity on numerical scale (0-10) | 0: No pain; 2: Mild pain; 4: Discomforting; 6: Distressing; 8: Intense; 10: Excruciating |
| Parent Perception Questionnaire | No age specified | Parent observational behavioral scale—parents assess pain and distress in the categories: fear and anxiety, needle pain, discomfort from anesthesia, postprocedural pain, problems associated with overall distress; total numerical score (0-20) | Fear anxiety 0-4; needle pain 0-4; discomfort from anesthesia 0-4; postprocedural pain 0-4; problems associated with overall distress 0-4; sum of all categories added together for overall pain score ranging 0-20 |
| Procedure Behavior Checklist | No age specified | Professional observational behavioral scale; behaviors assessed:
muscle tension; screaming, crying, restraint needed, physical
resistance, verbalized pain, verbalized anxiety, verbalized
stalling Numerical total score applied (0-32) |
Muscle Tension 0-4; Screaming 0-4; Crying 0-4; Restraint needed 0-4 Physical; Resistance 0-4; Verbalized pain 0-4; Verbalized anxiety 0-4; Verbalized stalling 0-4; Sum of all categories added together for overall pain score ranging 0-32 |
| VAS | Children greater than 12 years old | Self-report pain intensity scale; uses numbers ranged 0-10 | 0: No pain; 1-4: Mild pain; 5-6: Moderate pain; 7-10: Severe pain |
| Wong–Baker Faces Scale | Children 3-18 years old | Self-report pain intensity scale; uses facial expressions correlated to numerical scale (0-10) | 0: No hurt; 2: Hurts little bit; 4: Hurts little more; 6: Hurts even more; 8: Hurts whole lot more; 10: Hurts worst |
Mild Sedation
One retrospective cohort crossover study (Holdsworth et al., 2003) and one prospective cohort study (Crock et al., 2003) evaluated the severity of pain during initial and subsequent BMAs and LPs using topical analgesia and an anxiolytic (Table 3). Crock et al. evaluated pain scores utilizing the Bieri Faces pain scale (0 = no pain to 6 = maximum pain). Mixed results for the topical anesthetic, Eutectic Mixture of Local Anesthetics (EMLA®), and midazolam (oral/intranasal) were demonstrated between the two studies. One study demonstrated that children experienced considerable pain during initial procedures with sedation, median pain score of 6—maximum pain (Crock et al., 2003), while the other study reported better pain control with a mean pain score of 2.5/5 utilizing the Faces pain scale (0 = none to 5 = severe; Holdsworth et al., 2003). Considerable pain was reported during subsequent procedures by children (or for children unable to self-report by their parent/proxy) with median pain scores of 6/6 in one study (Crock et al., 2003) and a mean pain score of 2.5/6 for LP and 3.5/6 for BMA in the other study (Holdsworth et al., 2003).
Table 3.
Studies With Mild Sedation for Procedure Pain Management.
| First author (year) | Sample | Procedures, pain scales | Medications | Findings |
|---|---|---|---|---|
| Crock et al. (2003) | N = 96 children, ages 1-17 | N = 59 First procedure, n = 22 BMA only, n = 2 LP only, n = 35 BMA +LP; N = 21 Under sedation, n = 3 BMA only, n = 11 LP only, n = 7 BMA + LP; Bieri Faces pain scale (0 = no pain; 6 = maximum pain) | SED: (a) Local anesthetic AnGel; (b) Oral or intranasal midazolam; (c) Lidocaine 1% subcutaneously and subperiosteally | Median Pain Scores: First procedure: Sedation: 6; Current procedure: Parents reported children with SED: 6; Children reported: Children with SED: 6 |
| Holdsworth et al. (2003) | N = 73 children, ages 1-18 years undergoing BMA; N = 105 children, ages 1-18 receiving an LP | BMA and LP: Faces pain scale (0 = none to 5 = severe); Parent-proxy reporting for children <5 years | (a) EMLA cream; (b) Oral midazolam; (c) GA: Propofol and fentanyl | SD Pain ratings of LPs: First Procedure: EMLA vs. midazolam/EMLA, n = 33; 2.4 ± 1.8; Repeated Procedure: EMLA vs. midazolam/EMLA, n = 31; 2.4 ± 1.5; Midazolam/EMLA vs. propofol/fentanyl, n = 22; 2.6 ± 1.6, p < .001; SD Pain ratings of BMA; First Procedure: Midazolam/EMLA, n = 23, 2.4 ± 1.8; Repeated Procedure: EMLA vs. midazolam/EMLA, n = 10; 2.8 ± 2.1, p = NS; Midazolam/EMLA vs. propofol/fentanyl, n = 8; 4.1 ± 1.1, p = .011 |
| Ljungman et al. (2001) | N = 25 children, ages 1-15 years; n = 22 CS; n = 19 both CS and GA | LP performed more than once; Self-report 10 modified questions using a 100 point VAS (1-100) children 7 years and older completed, younger than 7 did not. IQR from the 25% to the 75% | (a) EMLA; (b) Meperidine; (c) Midazolam; (d) Lidocaine SC | Assessed 1 hour after procedure: 1. Local anesthesia discomfort: Parents: n = 18 (Mdn = 18, IQR = 10-50); Nurses: n = 16 (Mdn = 30, IQR = 10-64); 2. Pain at LP; Parents: n = 17 (Mdn = 11, IQR = 2-22); Nurses: n = 17 (Mdn = 22, IQR = 6-44) |
Note. EMLA = Eutectic Mixture of Local Anesthetics; BMA = bone marrow aspirate; CS = conscious sedation; GA = general anesthesia; IQR = interquartile range; LP = lumbar puncture; SC = subcutaneous; SD = standard deviation; SED = sedation; VAS = visual analog scale.
Ljungman et al. (2001) evaluated the addition of a narcotic (i.e., meperidine) to the anxiolytic and topical anesthetic regimen for pain control during LPs. Pain was assessed using a modified Visual Analog Scale (VAS; 1-100). The narcotic-containing regimen seemed equivalent concerning pain to GA in this procedural setting with parents and nurses rating median pain scores of 11/100 and 22/100, respectively (Ljungman et al., 2001).
Moderate Sedation
One prospective observational study (Abdelkefi et al., 2004) and one randomized control trial (Iannalfi et al., 2005) demonstrated effective pain control with VAS scores indicating mild pain, utilizing a topical anesthetic in combination with an inhaled nitrous oxide mixture alone or in combination with an anxiolytic (Table 4). Topical EMLA cream followed by an inhaled 50% nitrous oxide mixture (Equimolar Mixture of Oxygen and Nitrous Oxide), provided satisfactory pain control during central venous catheter (CVC) placement, as children reported a median VAS pain score of 30/100 (Abdelkefi et al., 2004). Similarly, topical EMLA cream followed by an inhaled 50% nitrous oxide mixture and intravenous midazolam in combination or alone provided effective pain control during LP and BMA based upon the Perception Evaluation Questionnaire (0-4 scale). Parents (as proxies for their children) reported mean needle pain of 0.4 and discomfort from anesthetic as 0.55. Children self-reported mean needle pain of 0.27 and discomfort from anesthetic of 0.33 (Iannalfi et al., 2005).
Table 4.
Studies With Moderate Sedation for Procedure Pain Management.
| First author (year) | Sample | Procedures, pain scales | Medications | Findings |
|---|---|---|---|---|
| Abdelkefi et al. (2004) | N = 50 children, ages 4-13 | N = 50 CVC placements; 100 point VAS (1-100) completed by children 6-13 years | 1. EMLA Cream | Median pain intensity was 10 out of 100 (range 0-30) |
| 2. 50% nitrous oxide mixture | ||||
| Bhatnagar et al. (2007) | N = 60 children, ages 1-10 | Group 1: n = 12 LP, n = 18 BMA/BMB; Group 2: n = 11 LP, n = 19 BMA/BMB; 100 point VAS (1-100) completed by observer | Group 1: n = 30 | Group 1: 8.33 ± 15.99 |
| 1. Ketamine | ||||
| 2. Midazolam | Group 2: 9.33 ± 16.3 (p = .892) | |||
| 3. Atropine IM | ||||
| Group 2: n = 30 | ||||
| 1. Ketamine | ||||
| 2. Midazolam | ||||
| 3. Atropine PO | ||||
| Dufresne (2010) | N = 14 children, ages 6-17 | n = 9 LP; n = 5 BMA; n = 4 LP followed by BMA; FPS-R (scored 0-5); 10 point VAS (0-10) | 1. EMLA Lidocaine | T1: Baseline assessment: Entry to procedure room |
| • Child VAS 0.71 ± 0.83 | ||||
| • Child FPS-R 0.71 ± 0.83 | ||||
| • Relative VAS 1.89 ± 3.38 | ||||
| • Physician VAS 0.29 ± 0.80 | ||||
| 2. Midazolam | T2: Entry to procedure room to administration of sedation | |||
| • Child VAS 1.35 ± 2.18 | ||||
| • Child FPS-R 0.75 ± 1.06 | ||||
| • Relative VAS 1.11 ± 1.04 | ||||
| • Physician VAS 0.31 ± 0.48 | ||||
| 3. Ketamine | T3: Sedation administration to completion of first needle insertion | |||
| • Child VAS 1.71 ± 2.74 | ||||
| • Child FPS-R 1.17 ± 1.03 | ||||
| • Relative VAS 4.01 ± 2.23 | ||||
| • Physician VAS 1.83 ± 2.32 | ||||
| T4: Recovery and assessment prior to departure from procedure room | ||||
| • Child VAS 1.13 ± 1.22 | ||||
| • Child FPS-R 1.00 ± 0.95 | ||||
| • Relative VAS 1.13 ± 1.19 | ||||
| • Physician VAS 0.25 + 0.60 | ||||
| Evans et al. (2005) | N = 58 children, ages 1-13 years | N = 121 sedations studied | 1. EMLA | Observer median VAS pain score was 0 (range 0-3); Caregiver median VAS pain score was 0 (range 0-4) |
| • 73% LP | 2. Ketamine | |||
| • 13% BMBx/A | ||||
| • 13% LP/BMBx/A | ||||
| VAS (0-10) recorded by observer and caregiver independently | ||||
| Iannalfi et al. (2005) | N = 31 children, ages 1-16 years; Moderate sedation (MS): n = 30; general anesthesia (GA): n = 35 | N = 65 procedures; LP and/or BMA; MS: n = 16 LP; n = 10 BMA; n = 4 BMA/LP; GA: n = 8 LP; n = 15 BMA; n = 12 BMA/LP; PBCL—recorded by neutral observer (0-4); Perception Evaluation Questionnaire (PEQ): Given to Children >6 years; Results of Perception’s Evaluation Questionnaire (0-4) | EMLA and 2% lidocaine | PBCL—no significant difference in mean assessed pain between MS and GA; Parent perception: Needle pain mean GA 0.36/MS 0.40; Discomfort by anesthetics mean GA 0.79/ MS 0.55; Child’s perception: Needle pain mean GA 0.18/MS 0.27; Discomfort by anesthetics mean GA 0.45/MS 0.33 |
| MS | ||||
| 1. Premixed nitrous oxide 50% and oxygen 50% | ||||
| 2. Midazolam IV; n = 24 nitrous oxide and midazolam; n = 3 nitrous oxide; n = 3 Midazolam | ||||
| GA | ||||
| 1. Premixed nitrous oxide 50% and oxygen 50% | ||||
| 2. Midazolam IV | ||||
| 3. Fentanyl IV | ||||
| 4. Ketamine IV | ||||
| 5. Sevoflurane in oxygen | ||||
| Monsereenusorn, Rujkijyanont, and Traivaree (2015) | N = 55 children, ages 1-18 | N = 110 procedures, IT, and/or BMA/BMB; Children <8 years —observed pain scores by parent; Children ≥8 years—children self-completed pain scales; n = 9; FLACC scale to assess pain in those unable to communicate age 3 months-4 years. n = 24; FACES pain rating 4-8 years. N = 22; 10 point VAS (0-10) for 8 years and older | Fentanyl IV or ketamine IV | Fentanyl: 1.55 ± 1.65 (Mdn = 2, range = 0-8); ketamine 2.44 + 1.66 (Mdn = 2, range = 0-8; p = .002); Patients ages 4-8 years; Fentanyl: 1.33 + 1.27 (Mdn = 2, range = 0-4); Ketamine: 2.83 + 1.86 (Mdn = 2, range = 0-8; p = .002) |
| Pellier et al. (1999) | N = 92 children, ages 3 days to 18 years | N = 226 procedures; BMA, BMB, CVC, LP, lymph node biopsy; Patients older than 5 years reported pain based on 10-point VAS (1-10) following procedure | Midazolam IV and Ketamine IV; Additional doses of Ketamine as needed | 24% of children had response to painful stimuli; Children younger than 5 years had good score of analgesia in 96% of cases; No patient reported pain at the time of discharge, VAS was below 2 in all children |
| Tamminga and Faber-Nijholt (2000) | N = 16 children, ages 4-16 | N = 32 Bone Marrow punctures (BMP) total, 2 consecutive BMPs; 100-point Oucher scale (1-100) | Procedure 1 | Patient pain score 0, n = 20 |
| 1. PO diazepam or PO placebo | Patient pain score 10, n = 3 | |||
| 2. Atropine | Patient pain score 20, n = 2 | |||
| 3. Ketamine | Patient pain score 30, n = 2 | |||
| Procedure 2 | Patient pain score not obtained, n = 5 | |||
| Same variables as above but patient received diazepam or placebo the opposite from Procedure 1 | ||||
| Traivaree et al. (2014) | N = 46 children, ages 6 months to 15 years | N = 46 procedures; n = 23 LP; n = 10 BMA ± biopsy; n = 13; Combination of the two procedures; 10-point VAS (0-10) | Ketamine IV for sedation, no other sedative agent given | Median VAS 2 hours after procedure 3 (0-8 range) per parent or guardian. Median for all ages: 3; 6 months-7 years: 4 (0-8 range); 7 years-15 years: 2 (0-6 range); Statistical difference between the two groups of age (p = .001) |
Note. BMA = bone marrow aspirate; BMB = bone marrow biopsy; BMBx/A = bone marrow biopsy/aspirate; BMP = bone marrow puncture; CVC = central venous catheter; FLACC = Face, Legs, Activity, Cry, Consolability; FPS-R = Faces Pain Scale–Revised; GA = general anesthesia; IT = intrathecal; IV = intravenous; kg = kilogram; LP = lumbar puncture; mcg = microgram; mg = milligram; MS = moderate sedation; PO = by mouth; VAS = Visual Analog Scale; EMLA = Eutectic Mixture of Local Anesthetics.
Multiple studies evaluated the role of ketamine for control of procedure-associated pain. Two prospective observational studies utilized ketamine as a single sedative agent and demonstrated VAS (1-10 scale) scores of 3 or less during LP, BMB, and BMA (Evans et al., 2005; Traivaree et al., 2014). The addition of midazolam was associated with VAS (1-10 scale) scores ranging from 1 to 9 during LP, BMB, BMA, CVC placement, and lymph node biopsy (Bhatnagar et al., 2007; Dufrense et al., 2010; Pellier et al., 1999). Ketamine in combination with diazepam produced similar findings, assessed with OUCHER scores ranging from 0 to 30 (1-100 scale) during LP, BMA, and BMB (Tamminga & Faber-Nijholt, 2000). Monsereenusorn et al. (2015) compared fentanyl and ketamine for procedural pain during LP, BMA, and BMA. A statistically significant decrease in median VAS (1-10 scale) pain scores 2/10 (p = .002) was seen with fentanyl, as compared with ketamine.
Deep Sedation/General Anesthesia
Three studies compared the use of GA with mild sedation (Crock et al., 2003; Holdsworth et al., 2003; Ljungman et al., 2001; Table 5). A statistically significant reduction in mean and median Bieri Faces pain scale and Faces pain scale scores (p < .001) were noted for children undergoing both first procedures and repeated procedures under GA (LP, BMA, and BMB; Crock et al., 2003; Holdsworth et al., 2003). Children, parents, and nurses found that the use of mild sedation during LPs provided equivalent procedural pain control when compared with GA (Ljungman et al., 2001). Furthermore, most children, parents, and nurses preferred LPs performed with mild sedation (Ljungman et al., 2001). One study compared GA with moderate sedation in the setting of LP or BMA. There was no statistically significant difference between mean pain scores observed in the GA versus moderate sedation regimens (Iannalfi et al., 2005).
Table 5.
Studies With Deep Sedation/General Anesthesia for Procedure Pain Management.
| First author (year) | Sample | Procedures, pain scales | Mediations | Findings |
|---|---|---|---|---|
| Anghelescu et al. (2013) | N = 110 children, ages 2-17 years | N = 316 procedures; BMA, LP; Faces pain scale (0-11) or numerical rating system as appropriate for age and cognitive ability | 1. Fentanyl 1 mcg/kg, 0.5 mcg/kg, or placebo | No significant difference in the frequency of pain after treatment with fentanyl 1 mcg/kg vs. placebo (p = .53); Group A comparison pairs (n = 74); Frequency of pain >0, 5 (6.8%); Frequency of pain 5-10, 7 (9.5%); Group B comparison pairs (n = 71); Frequency of pain >0, 7 (9.9%); Frequency of pain 5-10, 10 (14.1%); Group C comparison pairs (n = 76); Frequency of pain >0, 14 (17.1%); Frequency of pain 5-10, 11 (13.9%) |
| 2. Propofol and topical anesthetic (EMLA and SQ lidocaine) | ||||
| Group A: (n = 111) Fentanyl 1 mcg/kg | ||||
| Group B: (n = 129); Fentanyl 0.5 mcg/kg | ||||
| Group C: (n = 115); Placebo (NS) | ||||
| All patients reporting pain on waking received 0.5 mcg/kg IV Fentanyl as needed (max 3 doses) | ||||
| Chiaretti et al. (2010) | N = 20 children, ages 2-15 years | N = 40 LP; n = 20 Protocol A; n = 20 Protocol B; Mean CHEOPS scores Children’s Hospital Eastern Ontario Pain Scale | Protocol A | Mean Pain ratings: A: 5.25 ± 0.6; B: 5.5 ± 0.5 (p > .05); All patients had satisfactory sedation and analgesia with both Protocol A and B: Protocol A: 15 propofol boluses; Protocol B: 5 propofol boluses |
| 1. Propofol | ||||
| 2. Alfentanil IV through Central Line | ||||
| Protocol B | ||||
| 1. Propofol | ||||
| 2. Ketamine IV through central line | ||||
| Both Protocols if sedation not adequate repeat propofol | ||||
| Crock et al. (2003) | N = 96 children, ages 1-17 | N = 27 first procedures; n = 6 BMA only; n = 5 LP only; n = 16 BMA + LP; N = 75 current procedure; n = 16 BMA only; n = 28 LP only; n = 31 BMA + LP | GA: Sevoflurane in 30% nitrous oxide and oxygen | First procedure: Parents reported median pain scores 6 as with SED as compared with median pain scores of 0 with GA (p < .001) |
| Current procedure; Parents reported: Children that received GA median pain score = 0; Children reported: Children that received GA median pain score = 0 | ||||
| Holdsworth et al. (2003) | N = 73 children ages 1-18 years undergoing BMA; N = 105 children ages 1-18 years receiving an LP | BMA and LP; Faces pain scale (0 = none to 5 = severe); Parent-proxy reporting for children <5 years | 1. EMLA cream | SD Pain ratings of LPs: |
| 2. Oral midazolam | Repeated LP: EMLA vs. propofol/fentanyl; n = 16; 2.8 ± 1.5, p = .001; Midazolam/EMLA vs. propofol/fentanyl; n = 22; 2.6 ± 1.6, p < .001; SD Pain ratings of BMA | |||
| 3. GA: Propofol and fentanyl (sedation chosen by child or parent) | First LP: Propofol/fentanyl; n = 43; 0.4 ± 1.0; First BMA: Propofol/fentanyl n = 29; 0.5 ± 1.0; Repeated BMA: EMLA vs. propofol/fentanyl; n = 7; 4.1 ± 0.9, p = .017; Midazolam/EMLA vs. propofol/fentanyl; n = 8; 4.1 ± 1.1, p = .011 | |||
| Iannalfi et al. (2005) | N = 31 children, ages 1-16 years; Moderate sedation (MS): n = 30; GA: n = 35 | N = 65 procedures; LP and/or BMA; MS: n = 16 LP; n = 10 BMA; n = 4 BMA/LP; GA: n = 8 LP; n = 15 BMA; n = 12 BMA/LP; PBCL—recorded by neutral observer; PEQ: Given to Children >6 years; Results of Perception’s Evaluation Questionnaire (0-4) | EMLA and 2% lidocaine | PBCL—no significant difference in mean assessed pain between MS and GA; Parent perception: Needle pain mean GA 0.36/MS 0.40; Discomfort by anesthetics mean GA 0.79/MS 0.55; Child’s perception: Needle pain mean GA 0.18/MS 0.27; Discomfort by anesthetics mean GA 0.45/MS 0.33 |
| MS | ||||
| 1. Premixed nitrous oxide 50% and oxygen 50% | ||||
| 2. Midazolam IV; n = 24 nitrous oxide and midazolam; n = 3 nitrous oxide; n = 3 Midazolam | ||||
| GA | ||||
| 1. Premixed nitrous oxide 50% and oxygen 50% | ||||
| 2. Midazolam IV | ||||
| 3. Fentanyl IV | ||||
| 4. Ketamine IV | ||||
| 5. Sevoflurane in oxygen | ||||
| Ljungman et al. (2001) | N = 25 children, ages 1-15 years | LP performed more than once; n = 22 GA; n = 19 both CS and GA; Self Report 100 VAS (1-100) children 7 years and older completed, younger than 7 did not; Inter quartile range from the 25% to the 75% | 1. Atropine IV | Not able to obtain results due to GA |
| 2. Fentanyl IV | ||||
| 3. Propofol IV and additional doses given as required | ||||
| Nagel et al. (2008) | N = 25 children, ages 4-17 years | BMA and LP with IT chemo; Faces pain scale (0 = no hurt to 6 = hurts worse) | Prior to procedure | During the first 12 hours post procedure |
| 1. Ondansetron IV or Placebo | Fentanyl group | |||
| 2. Midazolam + fentanyl or Placebo | • Reduction of pain 1.65, p < .0001 if they were receiving placebo ondansetron | |||
| 3. Propofol to achieve sufficient anesthesia to permit procedure | • Reduction of pain 2.09, p = .0048 if they were receiving ondansetron (p = .0048). | |||
| Each child was to receive in random order for 4 interactions: placebo + placebo, placebo + fentanyl, placebo + ondansetron, fentanyl + ondansetron | • In the second 12-hour period postprocedure: | |||
| • Fentanyl group: significantly lower pain scores while receiving fentanyl compared with not receiving fentanyl if they were also receiving placebo ondansetron. (p = .013), but not if they were receiving ondansetron (p = .033) | ||||
| Whitlow, Saboda, Roe, Bazzell, and Wilson (2014) | 25 children, ages 3-21 | LP | EMLA and propofol; Placebo and propofol | EMLA 4 mg/kg propofol needed (95% CI [3.5, 4.4]); With placebo 4.9 mg/kg propofol needed (95% CI [4.3, 5.6]; p = .008.) |
Note. BMA = bone marrow aspirate; CHEOPS = Children’s Hospital Eastern Ontario Pain Scale; CI = confidence interval; CSF = cerebral spinal fluid; GA = general anesthesia; IQR = interquartile range; IT = intrathecal; IV = intravenous; kg = kilogram; LP = lumbar puncture; mcg = microgram, mg = milligram; MS = moderate sedation; NO = nitrous oxide; PBCL = Procedure Behavior Check List; PEQ = Perception Evaluation Questionnaire; SD = standard deviation; SED = sedation; SQ = subcutaneous; VAS = Visual Analog Scale.
Four studies included the use of the anesthetic, propofol, for GA. Whitlow et al. (2014) examined the contribution of the local anesthetic, EMLA with propofol-containing regimens. Use of EMLA cream was associated with a reduction in propofol dosing required for desired procedural sedation. Three studies evaluated the addition of fentanyl or alfentanil to propofol-containing regimens for GA and its effect on pain control. Anghelescu et al. (2013) did not demonstrate a statistically significant effect on the frequency of postprocedure pain following LP and BMA with the addition of fentanyl, as assessed with the FLACC, Faces Scale, and numerical reporting of pain (p = .53). Chiaretti et al. (2010) compared alfentanil plus propofol with ketamine plus propofol for postprocedure LP pain reduction. All children after awakening reported that they did not feel pain during the procedure. Analgesia scores and mean Children’s Hospital of Eastern Ontario Pain Scale scores did not show superior efficacy of either medication (p > .05). Propofol boluses (1 mg/kg) if sedation was inadequate were significantly less in the ketamine group compared with the alfentanil group (5-15, respectively) indicating better pain control with ketamine, but children reported no difference after waking.
Nagel et al. (2008) evaluated GA with propofol and midazolam plus (a) fentanyl or placebo, (b) ondansetron or placebo, (c) fentanyl plus ondansetron, or (d) placebo plus placebo, on pain scores following LP and BMA. Patients reported significantly lower pain scores utilizing the Faces Pain Rating Scale (p = .013) while receiving fentanyl, compared with not receiving fentanyl, if they were also receiving placebo ondansetron. Patients did not report lower pain scores while receiving fentanyl, compared with no fentanyl, if they were receiving ondansetron (p = .033).
Quality of the Evidence
Fifteen articles were used as evidence to answer the PICO question addressed in this systematic review. The overall quality of the evidence was moderate. The evidence related to mild sedation consisted of one retrospective cohort crossover study and two prospective studies. The moderate sedation evidence consisted of five prospective observational studies and four randomized control trials. The evidence for deep sedation/GA, consisted of one retrospective cohort study and seven prospective studies (two prospective crossover design, one prospective randomized crossover design, one prospective cohort study, and three randomized control trials). Issues related to the quality of the evidence include lack of reported power analyses (or insufficient power analyses when reported), small sample size (two studies), potential prescriber bias (two studies), inconsistency between comparison groups, and potential publication bias due to length of time from study to publication (two studies).
Overall Summary of Recommendations
There was insufficient evidence on which to base an overarching recommendation regarding the level of sedation necessary for pain control among children with cancer requiring procedures; however, the following recommendation was formulated based upon the body of synthesized evidence. There is a strong recommendation based on moderate level of evidence that moderate sedation or GA should be utilized for bone marrow aspirates and bone marrow biopsies in pediatric oncology care.
Discussion and Summary
There was insufficient evidence to answer the main PICO question. The review of the literature provides a guide to assist in the decision making regarding pharmacological agents that are effective when utilized together to achieve a necessary a level of sedation for procedural pain control. The use of a topical analgesic and an anxiolytic for children undergoing initial or subsequent BMAs and LPs demonstrated mixed results in pain control. Multiple studies examined the use of moderate sedation. Topical analgesia in conjunction with inhaled nitrous oxide with or without an anxiolytic demonstrated effective pain control in CVC placement, BMAs, and LPs (Abdelkefi et al., 2004; Iannalfi et al., 2005). The use of ketamine with or without the addition of an anxiolytic demonstrated mixed findings in pain control during BMA, BMB, LP, CVC placement, and lymph node biopsy (Bhatnagar et al., 2007; Dufresne et al., 2010; Evans et al., 2005; Pellier et al., 1999; Tamminga & Faber-Nijholt, 2000; Traivaree et al., 2014). Fentanyl when compared with ketamine demonstrated lower pain scores in BMA, BMB, and LP (Monsereenusorn et al., 2015).
GA when compared with mild sedation demonstrated mixed results in pain control; however, there was some preference by children, parents, and nurses for mild sedation during LPs (Crock et al., 2003; Holdsworth et al., 2003; Ljungman et al., 2001). GA compared with moderate sedation did not demonstrate differences in mean pain scores (Iannalfi et al., 2005). The use of GA with or without the use of topical analgesia was also evaluated in many studies. One study noting a reduction of propofol dosing when local anesthesia was utilized (Whitlow et al., 2014).
Limitations for identifying the necessary level of sedation for procedural pain control in pediatric oncology patients include the ability to adequately measure pain scores during moderate and deep sedation and GA (Ljungman et al., 2001). Retrospective reporting of children’s pain scores did not consistently correlate with the parent’s or physician’s observational reporting during the procedure (Dufresne et al., 2010). Proxy reporting by parents yielded higher scores than the patient’s retrospective reporting of pain. Nurses must rely on other factors to interpret and predict the patient’s pain.
Conclusion
Appropriate analgesia and sedation are essential in the effective management of painful procedures for children with cancer. The overarching goal for the management of children undergoing painful procedures is the reduction or elimination of pain. Nurses must act as advocates for the implementation of best practices to minimize procedural pain. Pharmacological choices related to the level of sedation are based upon many variables such as patient status, type of procedure, available resources, and institutional policies/procedures. There is limited research evaluating the safety and efficacy of providing the adequate level of sedation during procedures in the pediatric oncology patient population. Prospective or retrospective single institution studies provide an opportunity to evaluate current pharmacological interventions to alleviate pain during procedures. In the future, multisite collaborative studies that standardize procedural sedation and evaluate pain control have the potential to positively influence patient outcomes for children with cancer undergoing procedures.
Author Biographies
Elizabeth A. Duffy, DNP, RN, CPNP, is a clinical assistant professor at the University of Michigan School of Nursing. The focus of her scholarship is preventing central line-associated bloodstream infection (CLABSI) in pediatric oncology patients.
Tara Adams, BSN, RN, CPON, is a clinical program coordinator in Nursing Administration at Dell Children’s Medical Center in Austin, Texas. She has 17 years of combined nursing experience in inpatient and outpatient hematology oncology, bone marrow transplant, clinical education, and nursing leadership. During her career, Tara has held positions at Children’s Hospital of Austin in Austin, Texas; Loma Linda University Children’s Hospital in Loma Linda, CA; Driscoll Children’s Hospital in Corpus Christi, Texas; and Dell Children’s Medical Center, also in Austin.
Clifton P. Thornton, MSN, BS, RN, CNMT, CPNP, is a pediatric hematology/oncology nurse practitioner at the Herman and Walter Samuelson Children’s Hospital at Sinai and a PhD student at the Johns Hopkins School of Nursing in Baltimore, MD.
Beth Fisher, MSN, CPNP, CPON, is a lecturer and clinical instructor at Clemson University, Clemson, South Carolina. She also practices as a nurse practitioner in pediatric hematology/oncology at Children’s Healthcare of Atlanta, Atlanta, GA and at BILO Charities Children’s Cancer Center at Prisma Health, Greenville, South Carolina.
Jennifer Misasi, BSN, MS, CPNP, CPON, CPHPN, is a pediatric nurse practitioner at Rush University Medical Center in Chicago, Ilinois.
Sally McCollum, PharmD, is the clinical program manager for the Marcus Center for Cellular Cures within the Duke University School of Medicine. At the time of this project, Sally was the clinical pharmacist practitioner in the Pediatric Blood and Marrow Transplant Program within Duke University Health Systems.
Appendix
Appendix.
| PubMed Database | |
| Date last searched | April 27, 2017 |
| No. of results: 1 | |
| Final search strategy | (“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields]) AND (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields]) AND (“anaesthesia”[All Fields] OR “anesthesia”[MeSH Terms] OR “anesthesia”[All Fields]) AND (“pain management”[MeSH Terms] OR (“pain”[All Fields] AND “management”[All Fields]) OR “pain management”[All Fields]) AND (“pathology”[Subheading] OR “pathology”[All Fields] OR “biopsy”[All Fields] OR “biopsy”[MeSH Terms]) |
| # of results: 5 | |
| Final search strategy | (“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields]) AND (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields]) AND (“pain management”[MeSH Terms] OR (“pain”[All Fields] AND “management”[All Fields]) OR “pain management”[All Fields]) AND (“anaesthesia”[All Fields] OR “anesthesia”[MeSH Terms] OR “anesthesia”[All Fields]) |
| No. of results: 57 | |
| Final search strategy | ((“pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “pediatric”[All Fields]) AND (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “oncology”[All Fields]) AND (“patients”[MeSH Terms] OR “patients”[All Fields])) AND ((“bone marrow examination”[MeSH Terms] OR (“bone”[All Fields] AND “marrow”[All Fields] AND “examination”[All Fields]) OR “bone marrow examination”[All Fields]) OR (medical procedure[All Fields] OR medical procedures[All Fields]) OR (“spinal puncture”[MeSH Terms] OR (“spinal”[All Fields] AND “puncture”[All Fields]) OR “spinal puncture”[All Fields])) AND ((“anaesthesia”[All Fields] OR “anesthesia”[MeSH Terms] OR “anesthesia”[All Fields]) OR (“pain management”[MeSH Terms] OR (“pain”[All Fields] AND “management”[All Fields]) OR “pain management”[All Fields] OR (“pain”[All Fields] AND “control”[All Fields]) OR “pain control”[All Fields])) |
| No. of results: 893 | |
| Final search strategy | ((“child”[MeSH Terms] OR “child”[All Fields]) OR (“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields]) OR (“adolescent”[MeSH Terms] OR “adolescent”[All Fields])) AND ((“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “oncology”[All Fields]) OR (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “cancer”[All Fields]) OR (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields])) AND ((“anaesthesia”[All Fields] OR “anesthesia”[MeSH Terms] OR “anesthesia”[All Fields]) OR (“analgesics”[Pharmacological Action] OR “analgesics”[MeSH Terms] OR “analgesics”[All Fields]) OR sedation[All Fields]) AND ((“pain”[MeSH Terms] OR “pain”[All Fields]) OR “pain measurement”[All Fields] OR “pain management”[All Fields]) AND ((“pathology”[Subheading] OR “pathology”[All Fields] OR “biopsy”[All Fields] OR “biopsy”[MeSH Terms]) OR “bone marrow”[All Fields] OR “spinal puncture”[All Fields] OR “lumbar puncture”[All Fields] OR “clinical assessment tools”[All Fields] OR (“methods”[Subheading] OR “methods”[All Fields] OR “methods”[MeSH Terms])) |
| Medline Database | |
| Date last searched | April 27, 2017 |
| No. of results: 16 | |
| Final search strategy | (pediatrics OR child*) AND (neoplasms OR “medical oncology”) AND (anesthesia or analgesics or sedation) AND (“pain management” or “pain measurement”) AND (biopsy or catheterization or “spinal puncture”) |
| No. of results: 20 | |
| Final search strategy | (pediatrics OR child*) AND oncology AND (anesthesia or sedation) AND (“pain management” or “pain measurement”) AND (biopsy or catheterization or “spinal puncture”) |
| No. of results: 70 | |
| Final search strategy | ((paediatrics OR pediatrics) OR child OR adolescent) AND ((neoplasms OR cancer) OR oncology)) AND (anesthesia OR analgesics, non-narcotic OR “deep sedation”) AND (“pain management” OR “pain measurement”) AND (pathology OR biopsy OR “catheterization, peripheral” OR “spinal puncture” OR methods) |
| No. of results: 78 | |
| Final search strategy | ((paediatrics OR pediatrics) OR child OR adolescent) AND ((neoplasms OR cancer) OR oncology)) AND (anesthesia OR analgesics, non-narcotic OR “deep sedation”) AND (“pain management” OR “pain measurement” OR “pain control”) AND (pathology OR biopsy OR “catheterization, peripheral” OR “spinal puncture” OR methods) |
| No. of results: 446 | |
| Final search strategy | ( child* OR pediatric* OR adolescent* ) AND ( oncology OR cancer OR neoplasms ) AND ( anesthesia OR analgesics OR sedation ) AND ( pain OR “pain measurement” OR “pain management” OR “pain control” ) AND ( biopsy or “bone marrow” OR “spinal puncture” OR “lumbar puncture” OR “clinical assessment tools” OR methods ) |
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Cancer Institute/National Clinical Trials Network Group Operations Center Grant (U10CA180886; PI-Adamson).
ORCID iD: Elizabeth A. Duffy  https://orcid.org/0000-0001-5038-803X
https://orcid.org/0000-0001-5038-803X
References
- Abdelkefi A., Abdennebi Y. B., Mellouli F., Othman T. B., Torjman L., Ladeb S., . . . Abdeladhim A. B. (2004). Effectiveness of fixed 50% nitrous oxide oxygen mixture and EMLA® cream for insertion of central venous catheters in children. Pediatric Blood & Cancer, 43, 777-779. doi: 10.1002/pbc.20186 [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists. (2018). Practice guidelines for moderate procedural sedation and analgesia 2018: A report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association for Oral and Maxillofacial surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology, 128, 437-479. doi: 10.1097/ALN.0000000000002043 [DOI] [PubMed] [Google Scholar]
- Anghelescu D. L., Burgoyne L. L., Faughnan L. G., Hankins G. M., Smeltzer M. P., Pui C. H. (2013). Prospective randomized crossover evaluation of three anesthetic regimens for painful procedures in children with cancer. Journal of Pediatrics, 162, 137-141. doi: 10.1016/jpeds.2012.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S., Mishra S., Gupta M., Srikanti M., Mondol A., Diwedi A. (2007). Efficacy and safety of a mixture of ketamine, midazolam and atropine for procedural sedation in paediatric oncology: A randomised study of oral versus intramuscular route. Journal of Paediatrics and Child Health, 44, 201-204. doi: 10.1111/j.1440-1754.2007.01233.x [DOI] [PubMed] [Google Scholar]
- Bieri D., Reeve R., Champion G., Addicoat L., Ziegler J. (1990). The faces pair scale for the self assessment of the severity of pain experienced by children: development, initial validation and preliminary investigation for ratio scale properties. Pain, 41, 139-150. doi: 10.1016/0304-3959(90)90018-9 [DOI] [PubMed] [Google Scholar]
- Bowles E. J., Tuzzio C. J., Kirlin B., Greene S. M., Clauser S. B., Wagner E. H. (2008). Understanding high-quality cancer care: A summary of expert perspectives. Cancer, 112, 934-942. doi: 10.1002/cncr.23250 [DOI] [PubMed] [Google Scholar]
- Caes L., Vervoort T., Devos P., Verlooy J., Benoit Y., Goubert L. (2014). Parental distress and catastrophic thoughts about child pain: Implications for parental protective behavior in the context of child leukemia-related medical procedures. Clinical Journal of Pain, 30, 787-799. doi: 10.1097/AJP.0000000000000028 [DOI] [PubMed] [Google Scholar]
- Cella D., Hahn E. A., Jensen S. E., Butt Z., Nowinski C. J., Rothrock N., Lohr K. (2015). Patient-reported outcomes in performance measurement (RTI Press Publication No. BK-0014-1509). Research Triangle Park, NC: RTI Press. doi: 10.3768/rtipress.2015.bk.0014.1509 [DOI] [PubMed] [Google Scholar]
- Chiaretti A., Ruggiero A., Barone G., Antonelli A., Lazzareschi I., Genovese O., . . . Riccardi R. (2010). Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: Safety, efficacy and their correlation with pain neuromediator expression. European Journal of Cancer Care, 19, 212-220. doi: 10.1111/j.1365-2354.2008.01006.x [DOI] [PubMed] [Google Scholar]
- Children’s Oncology Group. (2010). Risk adapted chemotherapy in treating younger patients with newly diagnosed standard-risk acute lymphoblastic leukemia or localized b-lineage lymphoblastic lymphoma (ClinicalTrials.gov Identifier NCT01190930). Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT01190930?term=AALL0932&cond=Pediatric+Leukemia%2C+Acute+Lymphocytic&rank=1
- Cline R., Harper F., Penner L., Peterson A. M., Taub J. W., Albreecht T. L. (2006). Parent communication and child pain and distress during painful pediatric cancer treatments. Social Science & Medicine, 63, 883-898. doi: 10.1016/j.socscimed.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Cohen L., Lemanek K., Blount R., Dahlequist L., Lim C., Palermo T., . . . Weiss K. (2008). Evidence-based assessment of pediatric pain. Journal of Pediatric Psychology, 33, 939-955. doi: 10.1093/jpepsy/jsm103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crock C., Olsson C., Phillips R., Chalkiadis G., Sawyer S., Ashley D., . . . Monagle P. (2003). General anaesthesia or conscious sedation for painful procedures in childhood cancer: The family’s perspective. Archives of Disease in Childhood, 88, 253-257. doi: 10.1136/adc.88.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A., Dugas M., Samson Y., Barre P., Turcot L., Marc I. (2010). Do children undergoing cancer procedures under pharmacological sedation still report pain and anxiety: A preliminary report. Pain Medicine, 11, 215-223. doi: 10.1111/j.1526-4637.2009.00701.x [DOI] [PubMed] [Google Scholar]
- Enskär K., Carlsson M., Golsäter M., Hamrin E., Kreuger A. (1997). Life situation and problems as reported by children with cancer and their parents. Journal of Pediatric Oncology Nursing, 14, 18-26. doi: 10.1016/S1043-4542(97)90061-8 [DOI] [PubMed] [Google Scholar]
- Evans D., Turnham L., Barbour K., Kobe J., Wilson L., Vandebeek C., . . . Rogers P. (2005). Intravenous ketamine sedation for painful oncology procedures. Pediatric Anesthesia, 15, 131-138. doi: 10.1111/j.1460-9592.2005.01407.x [DOI] [PubMed] [Google Scholar]
- Guyatt G., Oxman A. D., Akl E. A., Kunz R., Vist G., Brozek J., . . . Schünemann H. J. (2011). GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 64, 383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H. J. (2008). Rating quality of evidence and strength of recommendations GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal, 336, 924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry-Eaton M. J., Barerra P., Brown M., Bottomley S. J., O’Neill J. B. (1999). Pain management in children with cancer. Houston: Texas Cancer Council. [Google Scholar]
- Hockenberry M. J., McCarthy K., Taylor O., Scarberry M., Franklin Q., Louis C. U., Torres L. (2011). Managing painful procedures in children with cancer. Journal of Pediatric Hematology/Oncology, 33, 119-127. doi: 10.1097/MPH.0b013e3181f46a65 [DOI] [PubMed] [Google Scholar]
- Holdsworth M. T., Raisch D. W., Winter S. S., Frost J. D., Moro M. A., Doran N. H., . . . Mathew P. (2003). Pain and distress from bone marrow aspirations and lumbar punctures. Annals of Pharmacotherapy, 37, 17-22. doi: 10.1345/aph.1C088 [DOI] [PubMed] [Google Scholar]
- Howlander N., Noone A. M., Krapcho M., Garshell J., Neymen N., Altekruse S. F., Cronin K. A. (Eds.). (2013). SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; Retrieved from https://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- Iannalfi A., Bernini G., Caprilli S., Lippi A., Tucci F., Messeri A. (2005). Painful procedures in children with cancer: Comparison of moderate sedation with general anesthesia for lumbar puncture and bone marrow aspiration. Pediatric Blood & Cancer, 45, 933-938. doi: 10.1002/pbc.20567 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2001). Crossing the quality chasm: A new health system for the 21st century (Committee on Quality of Health Care in America). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Kasak A. E., Penati B., Brophy P., Himelstein B. (1998). Pharmacologic and psychologic interventions for procedural pain. Pediatrics, 102, 59-66. doi: 10.1542/peds.102.1.59 [DOI] [PubMed] [Google Scholar]
- Keck J. F., Gerkensmeyer J. E., Joyce B. A., Schade J. G. (1996). Reliability and validity of the FACES and word descriptor scales to measure procedural pain. Journal of Pediatric Nursing, 11, 368-374. doi: 10.1016/S0882-5963(96)80081-9 [DOI] [PubMed] [Google Scholar]
- Kelly K. P., Hooke M. C., Ruccione K., Landier W., Hasse J. (2014). Developing an organizing framework to guide nursing research in the Children’s Oncology Group (COG). Seminars in Oncology Nursing, 30, 17-25. doi: 10.1016/j.soncn.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier W., Leonard M., Ruccione K. S. (2013). Children’s Oncology Group’s 2013 blueprint for research: Nursing discipline. Pediatric Blood & Cancer, 60, 1031-1036. doi: 10.1002/pbc.24415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier W., Tse A. M. (2010). Use of complementary and alternative medical interventions for the management of procedure-related pain, anxiety, and distress in pediatric oncology: An integrative review. Journal of Pediatric Nursing, 25, 566-579. doi: 10.1016/j.pedn.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E. C., Devidas M., Chen S., Salzer W. L., Raetz E. A., Loh M. L., . . . Carroll W. L. (2016). Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk b-acute lymphoblastic leukemia: A report from children’s oncology group study AALL0232. Journal of Clinical Oncology, 34, 2380-2388. doi: 10.1200/JCO.2015.62.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman G., Gordh T., Sorensen S., Kreuger A. (2001). Lumbar puncture in pediatric oncology: conscious sedation vs. general anesthesia. Medical and Pediatric Oncology, 36, 372-379. doi: 10.1002/mpo.1088 [DOI] [PubMed] [Google Scholar]
- Matloub Y., Bostrom B. C., Hunger S. P., Stork L. C., Angiolillo A., Sather H., . . . Gaynon P. S. (2011). Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood, 118, 243-251. doi: 10.1182/blood-2010-12-322909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath P. J., Beyer J., Cleeland C., Eland J., McGrath P. A., Portenoy R. (1990). Report of the subcommittee on assessment and methodological issues in management of pain in childhood cancer. Pediatrics, 86, 814-817. [PubMed] [Google Scholar]
- Merkel S. I., Voepel-Lewis T., Shayevitz J. R., Malviya S. (1997). The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing, 23, 293-297. [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med, 6, e1000097. doi: 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsereenusorn C., Rujkijyanont P., Traivaree C. (2015). The clinical effect of fentanyl in comparison with ketamine analgesic effect for oncology procedures in children: A randomized, double-blinded, crossover. Journal of the Medical Association of Thailand, 98, 358-363. [PubMed] [Google Scholar]
- Nagel K., Willan A., Lappan J., Korz L., Buckley N., Barr R. D. (2008). Pediatric oncology sedation trial (POST): A double-blinded randomized study. Pediatric Blood & Cancer, 51, 634-638. doi: 10.1002/pbc.21669 [DOI] [PubMed] [Google Scholar]
- Pellier I., Monrigal J. P., Le Moine P., Rod B., Rialland X., Granry J. C. (1999). Use of intravenous ketamine-midazolam association for pain procedures in children with cancer: A prospective study. Paediatric Anaesthesia, 9, 61-68. doi: 10.1046/j.1460-9592.1999.9120280.x [DOI] [PubMed] [Google Scholar]
- Schechter N. (1989). The undertreatment of pain in children: An overview. Pediatric Clinics of North America, 36, 781-794. [DOI] [PubMed] [Google Scholar]
- Schechter N., Altman A., Weisman S. (1990). Report of the consensus conference on the management of pain in childhood cancer. Pediatrics, 86, 816-834. [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68, 7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Tamminga R.Y.J., Noordhoek M., Kroon J., Faber-Nijholt R. (2000). Ketamine anesthesia with or without diazapam premedication for bone marrow punctures in children with acute lymphoblastic leukemia. Pediatric Hematology Oncology, 17, 383-388. [DOI] [PubMed] [Google Scholar]
- Traivaree C., Jindakam W., Monsereenusom C., Rujkijyanont P., Lumkul R. (2014). The factors of ketamine that affect sedation in children with oncology procedures: Parent satisfaction perspective. Journal of the Medical Association of Thailand, 97, 19-24. [PubMed] [Google Scholar]
- Weisman S., Bernstein B., Schecter N. (1998). Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatric and Adolescent Medicine, 152, 147-149. doi: 10.1001/archpedi.152.2.147 [DOI] [PubMed] [Google Scholar]
- Whitlow P. G., Saboda K., Roe D. J., Bazzell S., Wilson C. (2014). Topical analgesia treats pain and decreases propofol use during lumbar punctures in a randomized pediatric leukemia trial. Pediatric Blood & Cancer, 62, 85-90. doi: 10.1002/pbc.25236 [DOI] [PubMed] [Google Scholar]
- Wong D., Baker C. (1988). Pain in children: Comparison of assessment scales. Pediatric Nursing, 14, 9-17. doi: 10.1007/978-1-4613-1677-0_2 [DOI] [PubMed] [Google Scholar]