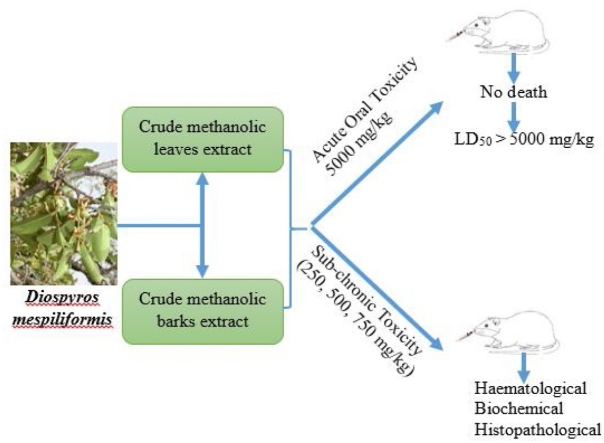
Graphical abstract
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; DW, Distilled water; EDTA, Ethylenediaminetetraacetic acid; HEL, H and E haematoxylin and eosin; K+, potassium; LD50, median lethal dose; MCH, Mean corpuscular haemoglobin; MCHC, Mean corpuscular haemoglobin concentration; MCV, Mean corpuscular volume; Na+, sodium; NC, normal control; OECD, Organisation for Economic Co-operation and Development; PCV, Packed cell volume; RBC, Red blood cells; SEM, standard error of mean; TB, Total bilirubin; TC, Total cholesterol; UDP, Up and Down” Procedure; UDU, Usmanu Danfodiyo University; WBC, White blood cells; WHO, world health organization
Keywords: Median lethal dose, Repeated dose toxicity, Diospyros mespiliformis, Ethyl acetate
Highlights
-
•
The oral toxicity effect of crude methanolic extract (CMX) of Diospyros mespiliformis hochst ex a. Dc (ebenaceae) was studied.
-
•
Crude methanolic extract of D. mespiliformis has an LD50 higher than 5 g/kg post oral treatment in rats, thus relatively safe.
-
•
The leaf and bark ethyl acetate fractions of D. mespiliformis were relatively safe post 28-days repeated treatment in rats.
Abstract
Diospyros mespiliformis, commonly called Jackal berry or African ebony, belongs to the plant family, Ebenaceae. The roots, barks and leaves have been used traditionally to treat wide varieties of conditions, however, there is limited information and literature reports concerning the toxicity and safety of this plant. The present study was conducted to evaluate the acute and sub-chronic toxicity of the crude methanolic extract of Diospyros mespiliformis and its fraction in Wistar rats. Diospyros mespiliformis was extracted by methanol 96 %. The crude methanolic extract was then fractionated into low, average and high polar compounds using hexane, ethyl acetate and butanol respectively. For the acute toxicity study, the revised limit Dose Test of “Up and Down” procedure according to the OECD guideline was used to determine the median lethal dose (LD50) of the crude methanolic leaf and bark extracts using a single fixed dose (5 g/kg) of the extracts administered by oral-gavage sequentially to 5 female Wistar rats. The rats were observed for instant death and toxicity signs for 24 h and then daily for 14 days. In the sub-chronic toxicity study, the bark and leaf ethyl acetate fractions (extract) was administered orally at doses of 250, 500 and 750 mg/kg bw /day respectively for 28 days to healthy Wistar rats. At the end of the experimental period, body weight, certain haematological, serum biochemical and histopathological parameters were evaluated. Results showed that acute oral administration of crude methanolic extract of Diospyros mespiliformis (5 g/kg bw) produced neither mortality nor visible changes in behavior or any other physiological activities and indicated that the LD50 of crude methanolic leaf and bark extract was greater than 5 g/kg bw in Wistar Rats. In the 28-days repeated dose oral toxicity study, no significant toxic effects was detected in any of the parameters evaluated. In conclusion, the crude methanolic extract was found safe in the acute toxicity study and the ethyl acetate fraction of Diospyros mespiliformis in the sub chronic study in rats could be safe for therapeutic purposes over a period not exceeding 28 days.
1. Introduction
For decades, humans and animals have depended on plant as means of food and medicines [1]. There is increase acceptance of crude herbal products in the developed countries [2]. Several cultures still rely on preserved information relating to traditional healing systems, and in countries like China and India, this has developed into a sophisticated system of diagnosis, medicinal preparations and treatment [3]. Natural products represent a considerable source of novel chemicals that could translate to drug development and discovery [4]. In the past, the increased interest in the use of herbal medicines in particular, has been powered by the pharmaceutical industry exploring newer compounds for the treatment of diseases. The World Health Organization (WHO) in recognition of the enormous importance of herbal medicine to primary health care delivery has recommended for actual identification, empirical utilization and development of medicinal herbs for their safety use and efficacy in medical care [4].
Diospyros mespiliformis, commonly called Jackal berry or African ebony, belongs to the plant family, Ebenaceae. In Nigeria, the plant is popularly known in Hausa as Kanya and in Yoruba as Igidudu. The leaves are simple and alternate in arrangement and dark green in coloration. The plant is dioecious and flowers in the months of April and May and matured fruits are large yellow berries [5]. Ethno botanical application of different parts of the plant has been reported.
The roots and barks are utilized in enhancing delivery and as remedy for pneumonia, malaria, leprosy syphilis and diarrhea [6]. The root bark is also used as an anthelmintic [7]. A decoction of the leaves is usually used against fever, whooping cough and to treat wounds [[8], [9], [10]]. Experimental studies have revealed that the leaf extract of D. mespiliformis was active against Plasmodium berghei, pain and fever [9,11]. The water, ethanol, and methanol extracts of the plant possess antibacterial activity [5,12]. Phytochemical investigations on D. mespiliformis have revealed a number of secondary metabolites including alkaloids, tannins, saponins, glycosides, anthraquinones, flavonoids and volatile oil [13]. Earlier studies [14] reported the effect of the plant extract in yankasa sheep while Jigam et al., [15] reported the acute toxicity of the plant extract in mice using intraperitoneally (i.p) route. However, there is limited published data about its safety and systemic evaluation at high doses of the plant using the oral route, thus its safety profile is needed. In this study, acute and sub-chronic evaluation of the different parts of Diospyros mespiliformis was conducted in Wistar rats
2. Material and methods
2.1. Plant materials, collection and identification
The fresh leaves, bark and root of the Diospyros mespiliformis (800 g) each were collected from Basawa area, Zaria, Nigeria. The samples were identified and authenticated by Namadi Sanusi at Herbarium of Biological Sciences Department, Ahmadu Bello University, Zaria. A voucher specimen (no. 938) of the samples was issued for future reference. The plant parts were air - dried in the laboratory under a stream of fast moving air and pulverized using mortar and pestle. The powdered plant parts were put in sealed plastic containers, labeled and kept at 4 °C until required to prepare the extracts.
2.2. Animal selection and care
Healthy Wistar rats of both sexes, with an average weight of 185 g were obtained from the Animal House, Department of Biological Sciences, Usmanu Danfodiyo University (UDU), Sokoto, Nigeria and were used in the present study. The animals were housed in cages in the Department of Veterinary Pharmacology and Toxicology Laboratory, UDU, Sokoto, Nigeria for a period of two weeks to acclimatize prior to commencement of the study. They were fed on pelletized growers feed (Vital feed®) and tap water provided ad libitum. All the experiments were approved by the Departmental Animal Ethics Committee of the Usmanu Danfodiyo University, Sokoto in line with the OECD 2001 standard guidelines on laboratory use of animals.
2.3. Experimental design
The rats were randomly divided into four groups, each consisting of 6 rats.
-
•
Group 1: Normal control (NC) group: These rats were orally (p.o) treated only with the equivalent volume of ultrapure water as used for the administration of Diospyros mespiliformis extract.
-
•
Groups 2,3 and 4: Diospyros mespiliformis extract was dissolve in ultrapure water to achieve doses of 250, 500 and 750 mg/kg respectively and administered orally once daily for 28 days.
2.4. Preparation of extracts and fractionation
Powdered form (500 g) of each plant part of Diospyros mespiliformis (DM) was mixed with two litres of methanol (Merck KGaA, Darmstadt, Germany), allowed to stand for 24 h with frequent shaking. Following 24 h the extracts were separately filtered using Whatman filter paper size 1 (Whatman No. 1, Fitchburg, WL, USA). The residues were again subjected to same treatment with methanol for complete extraction process. Thereafter the crude methanol extracts were combined for each plant parts and evaporated at room temperature under a fast stream of moving air [16] and stored in refrigerator. The percentage yield for each plant part extracts was then calculated as described [16].
The crude methanolic extract was subjected to fractionation using solvents with increasing polarity (hexane, ethyl acetate and n-butanol). For fractionation purpose, known volume of the crude extract was suspended in a separatory funnel using known volume of distilled water and the suspension was shaken with known volume of hexane, ethyl acetate and n-butanol (Sigma-Aldrich, Inc., St. Louis, MO, USA) successively to obtain the different fractions. All the fractions were kept separately in an amber glass bottle and maintained in a −20 °C refrigerator
2.5. Acute oral toxicity study of crude methanol extracts of the leaf and bark of Diospyros mespiliformis
The median lethal dose (LD50) as an indication of acute toxicity of the methanol extracts of the leaves and bark of Diospyros mespiliformis (DM) were determined using Limit Dose Test of the Revised “Up and Down” Procedure (UDP) according to OECD guideline [17]. Briefly, single limit dose of 5 g/kg each of the extracts was administered individually to 5 female nulliparous rats within 48 h interim depending on whether the earlier dosed rat survived or not. Each animal was observed for instantaneous death and then monitored for the first 48 h for the short-term effect and daily thereafter for the next 14 days for any delayed toxic effects.
2.6. Sub-chronic toxicity studies
Sub-chronic toxicity studies were carried out on forty rats divided at random into eight groups of 5 rats each using simple random sampling technique by balloting. The animals were first numbered and then randomly assigned into groups. Example: group A (1–5); group B (6–10) etc. Rats in groups A, B and C were orally administered ethyl acetate fraction of leaf of Diospyros mespiliformis at 250 (5% LD50), 500 (10 % LD50) and 750 (15 % LD50) mg/kg via gavage respectively while distilled water (5 mL/kg) was administered to rats in group D (untreated control) for 28 days. Similarly, the ethyl acetate fraction of bark of DM was orally administered to groups E, F and G (250, 500 and 750 mg/kg) respectively using gavage while group H serves as untreated control and was administered distilled water for 28 days. The choice of ethyl acetate fraction of the crude methanol extract was based on earlier preliminary pharmacological experiment conducted on wound healing. That's why we decided to further evaluate its toxicity for usage. All the treated rats were monitored for toxicity and weighed weekly for four weeks. The experiment was terminated at the end of the 28 day period. Following the last treatment (24 h later), the rats were euthanatized using a chloroform chamber. 2 mL of blood was collected from each experimental rat, by cardiac puncture; in sample bottles containing ethylenediaminetetraacetic acid (EDTA) for haematological evaluation. Additional 2 mL of blood (serum) was also obtained from individual rats for measurement of biochemical parameters.
2.7. Haematological analysis
Haematological parameters including Red blood cells (RBC), white blood cells (RBC), platelets, haemoglobin concentration and packed cell volume (PCV) were determined as described in the operational manual of Auto haemoanalizer - Erma(R) (PCE-210) Inc., Japan.
2.8. Determination of serum biochemical parameters
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activity were determined using Randox assay kit (Randox Laboratories Ltd, Ardmore, Antrim, UK) as described by [18]. Total bilirubin was assayed by the method earlier described by [19]. Albumin was assayed by the method described by [20]. Total protein was determined according to biuret method as described by [21]. Urea and uric acid were determined by the method of [22] while sodium (Na+) and potassium (K+) were estimated according to the method of [23].
2.9. Histological analysis
Liver, kidney, heart and skeletal muscle harvested from individual rat were immediately fixed in 10 % formalin (Sigma-Aldrich, Inc., St. Louis, MO, USA) solution and then processed for histological examination [24].
2.10. Statistical analysis
Results were expressed in mean ± SEM following analysis via one-way ANOVA to assess significant difference between groups followed by Tukey post-hoc test to examine significant difference (P ≤ 0.05).
3. Results
3.1. Acute oral toxicity of crude methanolic leaf and bark extracts of Diospyros mespiliformis
All the rats administered the leaf and bark extracts of Diospyros mespiliformis at 5 g/kg were calm but very alert. The rats continued feeding after 3 h of treatment. No death was recorded in all the rats treated with the extracts at the dose of up to 5 g/kg throughout the experimental period (Table 1). The oral LD50 of the crude methanol extracts of the leaf and bark of Diospyros. mespiliformis were found to be greater than 5 g/kg body weight /orally in rats respectively.
Table 1.
Result of limit dose test of methanolic leaf and bark extracts of Diospyros mespiliformis hochst ex a. Dc (ebenaceae) in rats.
| Test order | Animal Tag | Dose (g/kg) | Short-term effect (24 h) |
Delayed effect (14 days) |
|---|---|---|---|---|
| 01 | I | 5 | Survival | Survival |
| 02 | II | 5 | Survival | Survival |
| 03 | III | 5 | Survival | Survival |
| 04 | IV | 5 | Survival | Survival |
| 05 | V | 5 | Survival | Survival |
3.2. Effect of sub-chronic administration of the ethyl acetate fraction of the leaf and bark of Diospyros mespiliformis on body weighy of rats
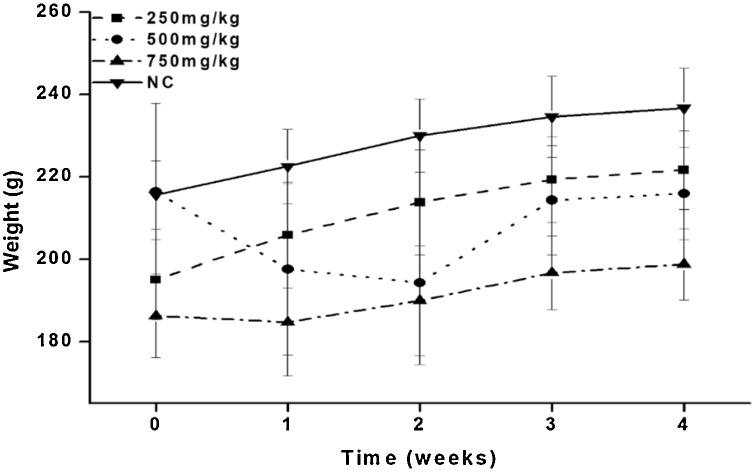
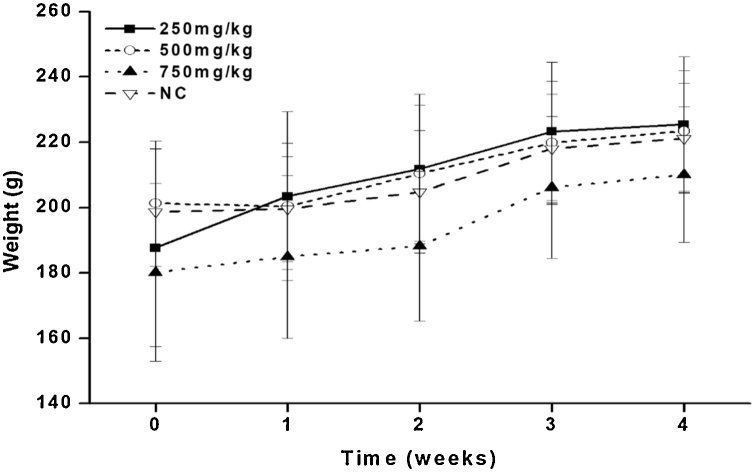
The effects of the leaf and bark ethyl acetate fractions of Diospyros mespiliformis on body weight changes of rats following sub-chronic treatment are presented in Fig. 1, Fig. 2. Rats treated with the ethyl acetate fraction of the leaf of DM at 250, 500 and 750 mg/kg had percentage increase in body weight of 15 %, 13 % and 10 %, respectively. Similarly, animals treated with the ethyl acetate fraction of the bark of the plant at 250, 500 and 750 mg/kg had their body weight increase by 23 %, 14 % and 15 %, respectively
Fig. 1.
Effect of the ethyl acetate leaf extract of Diospyros mespiliformis (DM) on the body weight changes of rats after sub-chronic administration.
Fig. 2.
Effect of the ethyl acetate bark extract of Diospyros mespiliformis (DM on the body weight changes of rats after sub-chronic administration.
3.3. Effect of sub-chronic administration of the bark of ethyl acetate fraction of Diospyros mespiliformis on haematological parameters of rats
The effect of the ethyl acetate fraction of the bark of Diospyros mespiliformis on some haematological parameters of Wistar rats after sub-chronic administration is shown on Table 2. There was a dose - dependent decrease in the WBC, lymphocytes, monocytes, and granulocytes levels, in rats treated with the bark ethyl acetate fraction of DM. Others, including RBC count and MCV, although had irregular patterns of increase and decrease These observations were not significant among various dose groups and even in comparison with the control group that were administered distilled water at 5 mL/kg.
Table 2.
Effects of daily treatment for 28 days with ethyl acetate bark extract of Diospyros mespilliformis on haematological parameters of rats.
| Ethyl acetate fraction bark (mg/kg) |
||||
|---|---|---|---|---|
| Parameters | 250 | 500 | 750 | DW |
| WBC (103/μl) | 22.78 ± 2.06 a | 14.60 ± 3.42 b | 10.76 ± 3.28 b | 14.56 ± 2.55 b |
| Lymphocyte (103/μl) | 8.76 ± 1.57 a | 9.74 ± 2.53 a | 14.62 ± 1.45 b | 9.92 ± 1.72 a |
| Monocyte (103/μl | 2.02 ± 0.32 a | 1.36 ± 0.31 b | 0.94 ± 0.27 a | 1.18 ± 0.26 a |
| Granulocytes (103/μl) | 6.12 ± 0.50 a | 3.50 ± 0.95 b | 2.06 ± 0.54 b | 3.40 ± 0.66 b |
| RBC (x106/μl) | 7.10 ± 0.33 a | 6.03 ± 1.56 a | 8.17 ± 0.53 a | 7.61 ± 0.34 b |
| Haemoglobin (g/dl) | 15.32 ± 0.83 a | 17.68 ± 1.74 a | 18.56 ± 1.78 a | 16.56 ± 0.74 a |
| PCV (%) | 39.94 ± 1.62 a | 33.64 ± 8.57 a | 46.08 ± 3.30 b | 42.46 ± 1.57 b |
| MCV (fl) | 56.30 ± 0.81 a | 54.08 ± 2.45 a | 56.26 ± 0.86 a | 55.84 ± 0.57 a |
| MCH (pg) | 21.52 ± 0.25 a | 21.76 ± 15.04 a | 22.48 ± 0.77 a | 21.74 ± 0.22 a |
| MCHC (g/dl) | 38.24 ± 0.65 a | 39.06 ± 16.24 a | 39.96 ± 1.05 a | 38.92 ± 0.33 a |
| Platelet (103/μl) | 891 ± 27 a | 907 ± 43 a | 966 ± 43 a | 926 ± 45 a |
Values are presented as mean ± standard error of mean. DW = Distilled water (5 mL/kg).
WBC = White blood cells; RBC = Red blood cells; PCV = Packed cell volume; MCV = Mean corpuscular volume; MCH = Mean corpuscular haemoglobin; MCHC = Mean corpuscular haemoglobin concentration.
Means in the same row compared to control with different superscript letters are significantly different (P<0.05).
3.4. Effect of sub-chronic administration of the leaf ethyl acetate fraction of Diospyros mespiliformis on haematological parameters of rats
The effects of the ethyl acetate extract of the leave of Diospyros mespiliformis on the haematological parameters of rats is shown in Table 3. The MCV increased with increase in dose. Other parameters like the RBC count, the MCH and the MCHC had no regular pattern of change and were not different across all the groups, including the control. In the cases of lymphocyte and monocyte counts, statistically (P < 0.05) lower value was observed in the control group compared with the highest dosed treatment group (750 mg/kg). However, there was no difference between the control group and the low dosed (250 mg/kg) and the mid dosed (500 mg/kg) treatment groups. Other parameters, including MCHC, had irregular change patterns, although not statistically (P > 0.05) different compared to the control group (distilled water, 5 mL/kg) and the highest treatment group (750 mg/kg).
Table 3.
Effect of daily treatment for 28 days with ethyl acetate leaf extract of Diospyros mespiliformis on the haematological parameters of Wistar rats.
| Ethyl acetate fraction leaf (mg/kg) |
||||
|---|---|---|---|---|
| Parameters | 250 | 500 | 750 | DW |
| WBC (103/μl) | 13.18 ± 1.53 a | 11.34 ± 2.50 b | 18.92 ± 1.84 c | 10.06 ± 1.89 b |
| Lymphocyte (103/μl) | 8.64 ± 1.14 a | 7.64 ± 1.65 a | 11.64 ± 1.08 b | 6.06 ± 1.25 c |
| Monocyte (103/μl | 1.20 ± 0.21 a | 0.90 ± 0.28 a | 1.90 ± 0.20 b | 0.90 ± 0.19 a |
| Granulocytes (103/μl) | 2.74 ± 0.57 a | 3.34 ± 0.56 a | 5.34 ± 0.77 b | 5.84 ± 5.07 b |
| RBC (x106/μl) | 6.78 ± 0.56 a | 7.40 ± 0.66 a | 8.19 ± 0.27 a | 7.61 ± 0.54 a |
| Haemoglobin (g/dl) | 14.18 ± 0.69 a | 15.84 ± 0.56 a | 17.92 ± 0.83 b | 16.24 ± 1.25 a |
| PCV (%) | 37.72 ± 1.79 a | 42.06 ± 1.34 a | 45.60 ± 3.06 a | 34.02 ± 2.77 b |
| MCV (fl) | 56.00 ± 1.70 a | 56.24 ± 1.97 a | 56.84 ± 0.89 a | 56.86 ± 0.64 a |
| MCH (pg) | 21.88 ± 0.56 a | 21.12 ± 0.77 a | 21.36 ± 0.21 a | 21.24 ± 0.22 a |
| MCHC (g/dl) | 39.10 ± 0.66 a | 37.56 ± 0.47 a | 37.60 ± 0.27 a | 37.42 ± 0.52 a |
| Platelet (103/μl) | 843 ± 27 a | 858 ± 43 a | 895 ± 32 a | 863 ± 27 a |
Values are presented as mean ± standard error of mean. DW = Distilled water (5 mL/kg).
WBC = White blood cells; RBC = Red blood cells; PCV = Packed cell volume; MCV = Mean corpuscular volume; MCH = Mean corpuscular haemoglobin; MCHC = Mean corpuscular haemoglobin concentration.
Means in the same row compared to control with different superscript letters are significantly different (P<0.05).
3.5. Effect of sub-chronic administration of the bark ethyl acetate fraction of Diospyros mespiliformis on biochemical parameters of rats
The effect of sub-chronic administration of ethyl acetate extract of bark of Diospyros mespiliformis on the biochemical parameters of rats is shown in Table 4. Although albumin fluctuated with increase in dose rate, there was no difference among all the groups, including the control. A similar observation was made in cases of TC, K+, urea and creatinine. On the other hand, a significant (P < 0.05) difference between the highest dose treatment group was observed when compared with the control group in respect to AST, ALT, and N+. However, there was no difference between the control group and the highest treatment group of 750 mg/kg, with respect to alkaline phosphatase, total bilirubin and total protein.
Table 4.
Effect of sub-chronic administration of ethyl acetate extract of the bark of Diospyros mespiliformis on some biochemical parameters of rats.
| Dose (mg/kg) |
||||
|---|---|---|---|---|
| Parameters | 250 | 500 | 750 | DW |
| AST (U/L) | 0.04 ± 0.0 a | 0.05 ± 0.0 b | 0.08 ± 0.0 b | 0.14 ± 0.1 c |
| ALT (U/L) | 0.05 ± 0.0 a | 0.05 ± 0.0 a | 0.16 ± 0.11 b | 0.16 ± 0.1 b |
| ALP (U/L) | 55.52 ± 3.4 a | 134.69 ± 8.04 b | 59.31 ± 5.50 a | 73.79 ± 7.70 a |
| Total bilirubin | 0.43 ± 0.1 a | 0.71 ± 0.08 a | 0.57 ± 0.18 a | 0.58 ± 0.07 a |
| Total cholesterol (mg/dl) | 0.29 ± 0.1 b | 0.32 ± 0.05 b | 0.23 ± 0.1 a | 0.32 ± 0.03 b |
| Total protein (g/dl) | 7.12 ± 0.3 a | 7.09 ± 0.2 a | 7.42 ± 0.2 a | 7.49 ± 0.4 a |
| Albumin (g/dl) | 3.64 ± 0.1 a | 3.50 ± 0.14 a | 3.95 ± 0.07 b | 3.72 ± 0.1 b |
| Sodium | 13076.0 ± 137.2 a | 13272.0 ± 92.9 a | 13692.0 ± 396.9 a | 13944.0 ± 104.8 a |
| Potassium | 3.70 ± 0.1 a | 3.94 ± 0.06 a | 4.40 ± 0.19 a | 4.50 ± 0.11 a |
| Urea (mg/dl) | 48.00 ± 5.4 a | 49.46 ± 0.13 a | 64.00 ± 4.5 b | 70.30 ± 3.2 b |
| Creatinine (mg/dl) | 0.67 ± 0.0 a | 0.80 ± 0.13 a | 0.80 ± 0.13 a | 0.67 ± 0.0 a |
Values are presented as mean ± standard error of mean. DW = Distilled water (5 mL/kg).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Means in the same row compared to control with different superscript letters are significantly different (P<0.05).
3.6. Effect of sub-chronic administration of the leaf ethyl acetate fraction of Diospyros mespiliformis on biocchemical parameters of rats
The effect of sub-chronic administration of ethyl acetate extract of leaf of Diospyros mespiliformis on the biochemical parameters of rats is shown in Table 5. Although both the AST and the albumin increased with increase in dose, there was no difference between the highest dose group and the control group. In both cases on the other hand, there was a significant (P < 0.05) difference between the highest dose group of 750 mg/kg and control in the case of AST.
Table 5.
Effect of sub-chronic administration of ethyl acetate extract of the leaf of Diospyros mespiliformis on some biochemical parameters of rats.
| Dose (mg/kg) |
||||
|---|---|---|---|---|
| Parameters | 250 | 500 | 750 | DW |
| AST (U/L) | 0.02 ± 0.0 | 0.05 ± 0.0 | 0.06 ± 0.0 | 0.06 ± 0.0 |
| ALT (U/L) | 0.05 ± 0.0 | 0.04 ± 0.0 | 0.04 ± 0.0 | 0.05 ± 0.0 |
| ALP (U/L) | 62.07 ± 8.14 a | 71.38 ± 9.70 a | 76.21 ± 10.1 b | 72.83 ± 14.4 a |
| Total bilirubin | 0.73 ± 0.11 | 0.63 ± 0.10 | 0.54 ± 0.14 | 1.17 ± 0.55 |
| Total cholesterol (mg/dl) | 0.37 ± 0.10 | 0.20 ± 0.04 | 0.29 ± 0.08 | 0.40 ± 0.12 |
| Total protein (g/dl) | 6.94 ± 0.16 | 7.45 ± 0.32 | 7.20 ± 0.44 | 6.87 ± 0.25 |
| Albumin (g/dl) | 3.64 ± 0.08 | 3.57 ± 0.08 | 3.59 ± 0.10 | 3.67 ± 0.07 |
| Sodium | 13328.0 ± 92.87 a | 13384.0 ± 84.00 a | 13776.0 ± 94.95 a | 14084.0 ± 56.00 a |
| Potassium | 3.88 ± 0.10 | 4.14 ± 0.10 | 4.20 ± 0.18 | 4.20 ± 0.06 |
| Urea (mg/dl) | 44.61 ± 0.97a | 54.30 ± 2.50 b | 39.27 ± 2.35 a | 48.00 ± 2.35 b |
| Creatinine (mg/dl) | 0.67 ± 0.0 a | 0.67 ± 0.0 a | 0.67 ± 0.0 a | 0.67 ± 0.0 a |
Values are presented as mean ± standard error of mean. DW = Distilled water (5 mL/kg).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Means in the same row compared to control with different superscript letters are significantly different (P<0.05).
3.7. Effect of sub-chronic administration of the leaf and bark ethyl acetate fraction of Diospyros mespiliformis on the histology of some organs of rats
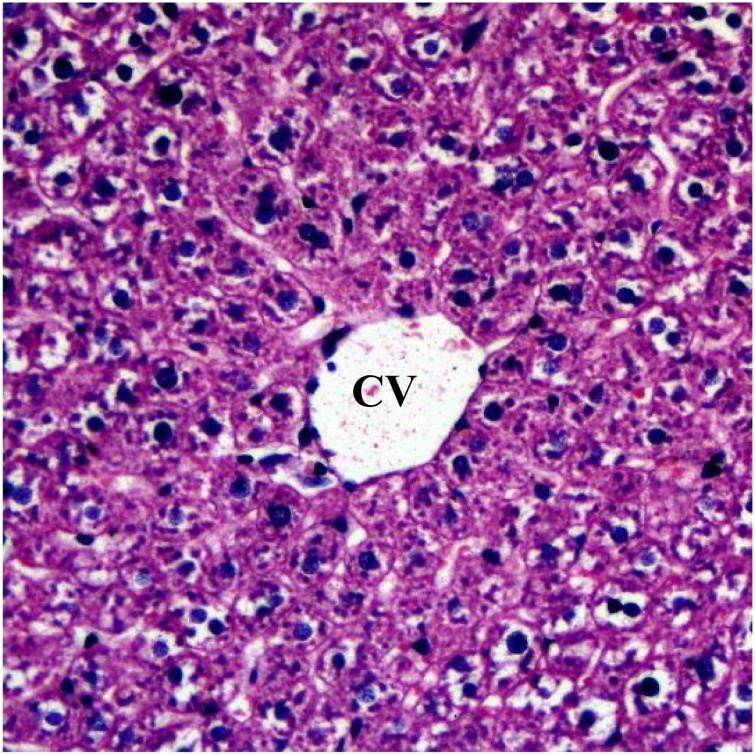
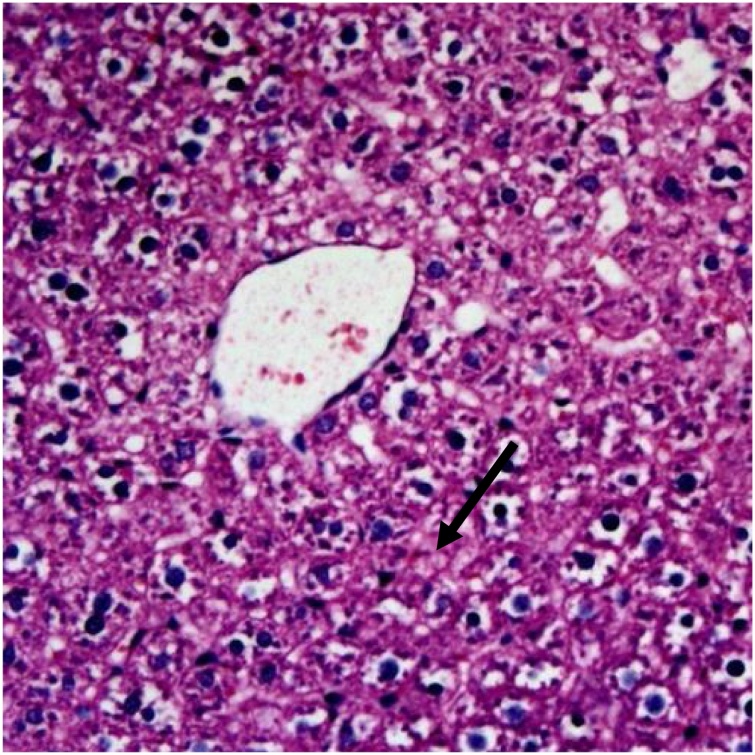
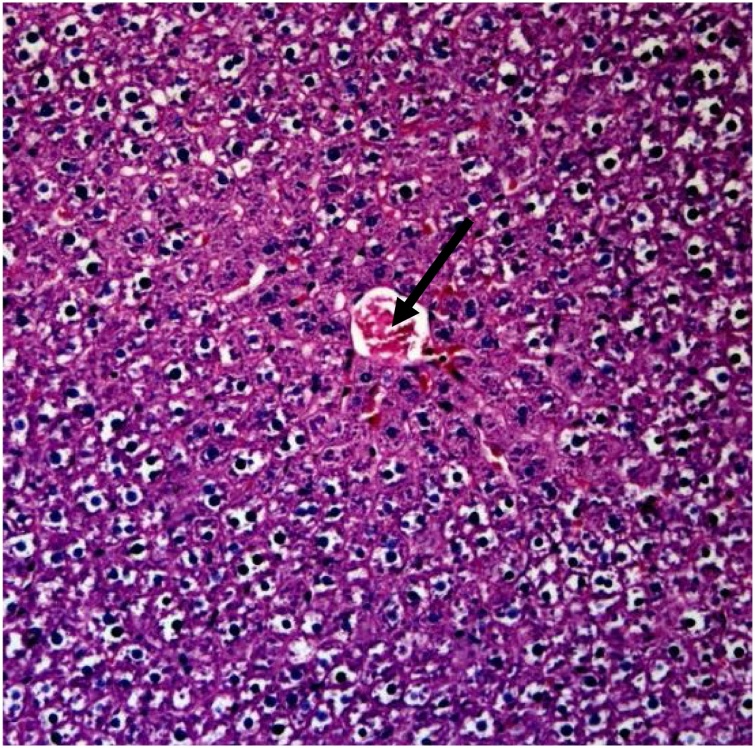
The effects of ethyl acetate fractions of the leaf and bark of Diospyros mespiliformis on the histology of some organs of rat are presented on Plate 1, Plate 2, Plate 3Plate 1 . The plates showed haematoxylin and eosin (H and E) stained sections of tissue samples from rats treated with leaf and bark ethyl acetate extracts of D. mespiliformis following sub-chronic administrations. The kidney, skeletal muscle and the heart of all the rats in the experiments showed no visible lesions, even at magnification of ×400. However, congestion of the central vein was observed in the liver of the group administered 750 mg/kg (Plate 3) of the leaf ethyl acetate fraction in the sub-chronic study. Focal area of hepatic degeneration was observed in the liver of the group of rats administered bark ethyl acetate fraction at 500 mg/kg (Plate II) in the sub-chronic study.
Plate 1.
Photomicrograph of the liver of a rat in control group (5 mL/kg, DW, 28 days) showing normal hepatocyte and normal central vein (CV). H and E × 400.
Plate 2.
Photomicrograph of liver of rat treated with 500 mg/kg ethyl acetate extract of the bark of D. mespiliformis in sub-chronic treatment, showing focal areas of hepatic degeneration (Arrow). H and E × 400.
Plate 3.
Photomicrograph of the liver of a rat treated with 750 mg/kg of the ethyl acetate extract of the leaf of D. mespiliformis in sub-chronic treatment, showing normal hepatocytes and congested central vein (arrow) H and E×200.
4. Discussion
Safety studies on herbal product have been assessed by performing acute toxicity test amongst other toxicity testing using laboratory animals [25]. Water, butanol and ethanol are all known to extract high polar compounds from plant materials. However, this present studies involve an activity guided experiment, thus methanol extraction was used. Methanol, a polar and an amphiphilic compound dissolves both polar and non-polar constituents of plant extracts [26]. In the acute toxicity study, no any sign of acute toxicity or instant mortality was seen in any of the rats tested following single oral administration of 5000 mg kg−1 body weight of Diospyros mespiliformis (Table 1). Thus, the oral LD50 of the extracts of the plant parts was considered to be greater than 5000 mg kg−1. Earlier report has observed substances having an LD50 value higher than 2000 mg/kg to be relatively safe for use [27]. This indicates that Diospyros mespiliformis crude methanolic extract has low toxicity and safe when administered orally. Similarly, in an earlier study, a low toxicity of the crude methanolic root extract of D. mespiliformis with LD50 of 620 mg/kg following intraperitoneal administration was reported in mice [14]. Measurement of haematological parameters plays is crucial in assessing the physiological and pathological status in man and animals. Thus alteration in normal values of these parameters could occur following ingestion of some toxic plants [28,29]. In the sub-chronic study for 28 days, no significant alteration was observed in all the haematological parameters of the treated rats when compared with the control group except for group treated with the fraction of leaf ethyl acetate of the plant at 750 mg/kg that had significantly (P < 0.05) higher values of neutrophil, basophil, eosinophil, monocyte and lymphocyte compares to control group rats.
The neutrophilia observed in this study could be connected with the daily administration of high dose of the extract, as a source of stress as noninfectious conditions including malignancy, chemical intoxication, and metabolic intoxication (uremia) usually stimulate stress reaction and could lead to neutrophilia. The increase but non-significant (P > 0.05) changes in RBC, PCV, Hb, WBC count and differential WBC count suggest that the extract could influences haematopoiesis pathway without cellular inflammatory process.
Antigen-antibody reactions, decomposition of tissues, parasitism or a wide variety of chronic diseases involving continuous degranulatiom of mast cells and causing release of histamine, mostly induce eosinophilia [30]. All the treated rats in the sub-chronic toxicity studies gained weight, although not dose dependently. This may be as a result of the nutritional components of the crude plant materials like carbohydrate, crude protein and lipid that are contained in the extracts of this plant [31]. Quantification of enzymes activities in body fluids is crucial in disease investigation and diagnosis. Tissue enzyme activities are frequently used as a 'marker' to establish toxic effects of administered compounds to experimental animals. Chalasani et al. [32] reported that regardless of the mechanism involved, reduce serum activities of aminotransferases have not been shown to correlate with the toxicologically significant outcome on the liver. Therefore, the decrease in the activity of AST as seen between the control group and 750 mg/kg bark ethyl acetate fraction treated group may not be attributed to toxic effect of the plant extract, as indicated by the result of histopathology of the liver. The insignificant difference observed in ALT, ALP, total bilirubin, conjugated bilirubin, total protein, urea and creatinine in the treated groups as compared with the control was an indication that the extract is relatively not toxic at the tested doses and period used in this study. One may conclude that based on the results of liver and kidney functions and histopathology of these organs, the extract had no overall significant toxicity in rats. Cellular morphological changes resulting from non-fatal injury are labeled degenerations and these are reversible disorders if the cause of the injury is removed [30]. Therefore the focal areas of hepatic degeneration that were observed in rats treated with leaf ethyl acetate extract at 500 mg/kg in the sub-chronic toxicity studies and congestion in rats treated with leaf ethyl acetate fraction at 750 mg/kg, were reversible pathological lesions, suggesting that the extracts, even at those doses are relatively safe. Body responses to this degenerative change include active hyperaemia and, consequently, congestion as seen in rats administered leaf ethyl acetate fraction at 750 mg/kg in the sub-chronic toxicity studies. The hyperaemia was as a result of increase in capillary blood to the liver. This was caused by increased physiological demand for blood, in response to challenges caused by the wound. This led to congestion as seen in the liver of rats administered the highest dose of 750 mg/kg in the sub-chronic study [30]. Lower doses of 250 mg/kg and 500 mg/kg of leaf ethyl acetate fraction gave no visible lesion in the sub-chronic toxicity studies. This is in agreement with earlier observation [33] who reported that toxicity of substances may be directly related to the doses exposed to, since every substance may be toxic if given in large enough doses, as the difference between medicine and poison is the dose, with toxicity increasing with increase in dose. The kidney, skeletal muscles and the heart tissues of all the rats in all experimental groups in the sub-chronic toxicity studies showed no visible lesions.
5. Conclusions
The crude methanolic extract of the plant parts of Diospyros mespiliformis has an LD50 ≥ 5 g/kg following oral administration in rats, thus safe based on the OECD standards. In the sub-chronic toxicity studies, the leaf and bark ethyl acetate fractions of the plant were relatively safe (P > 0.05). Further therapeutics studies are suggested to ascertain the extensive traditional use of the plant.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This study was supported by the Staff Training and Development Office (STDO), Usmanu Danfodiyo University, Sokoto, Nigeria.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.08.028.
Contributor Information
A.A. Ebbo, Email: aaliyuebbo@yahoo.co.uk.
D. Sani, Email: mdsani77@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Mcgaw L.J., Eloff J.N. Novel plant bioresources. In: Gurib-Fakim Ameenah., editor. Applications in Food, Medicine and Cosmetics. 1st ed. Johnwiley and Sons, Ltd; 2014. [Google Scholar]
- 2.Street R.A., Prinsloo G. Commercially important medicinal plants in South Africa: a review. J. Chem. 2013 Article ID 205048. [Google Scholar]
- 3.Houghton P. Foreword, in ethnoveterinary botanical medicine. In: Katerere D.R., editor. Herbal Medicines for Animal Health. 2010. pp. 121–132. [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Pro. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 5.Dangoggo S.M., Hassan L.G., Sadiq L.S., Manga S.B. Phytochemical analysis and antibacterial screening of leaves of Diospyros mespilifornis and Ziziphus spinachristi. J. Chem. Eng. 2012;1(1):13–17. [Google Scholar]
- 6.Mohamed I.E., Nur E.B.E.L., Choudhary M.I., Khan S.N. Bioactive natural products from two Sudanese Medicinal plants Diospyros mespiliformis and Croton zambesicus. Res. Nat. Prod. 2009;3(4):198–203. [Google Scholar]
- 7.Gyang S.N. University of Jos; 2001. Phytochemical Screenings of the Root Bark of Diospyros mespiliformis Hoechst ex.A.DC. Dissertation. [Google Scholar]
- 8.Watt J.M., Brandwijk M.G. 2nd edition. Livingstone; Edinburg: 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa; p. p 369. [Google Scholar]
- 9.Adzu B., Amos S., Dzarma S., Muazzam I., Gammaniel K.S. Pharmacological evidence favouring the folkloric use of Diospyros mespiliformis Hochst in the relief of pain and fever. J. Ethnopharmacol. 2002;3(82):191–195. doi: 10.1016/s0378-8741(02)00179-4. [DOI] [PubMed] [Google Scholar]
- 10.Abubakar M.S., Musa A.M., Ahmed A., Hussaini I.M. The perception and practice of traditional medicine in the treatment of cancers and inflammations by Hausa and Fulani tribes of Northern Nigeria. J. Ethnopharmacol. 2007;111:625–629. doi: 10.1016/j.jep.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Adzu B., Salawu O.A. Screening Diospyros mespiliformis extract for antimalarial potency. Intl. J. Biol. Chem. Sci. 2009;3(2):1232–1244. [Google Scholar]
- 12.Oluremi B.B., Osungunna M.O., Ogbole O.O. Phytochemical and Antimicrobial Screening of the Leaf Extract of Diospyros barteri. Gurke. Phcog. J. 2010;2(11):405–408. [Google Scholar]
- 13.Ebbo A.A., Sani D., Suleiman M.M., Ahmed A., Hassan A.Z. Phytochemical composition, proximate analysis and antimicrobial screening of the methanolic extract of Diospynos mespiliformis Hochst ex. Dc (Ebaenaceae. Phamacogn. J. 2019;11(2):362–368. [Google Scholar]
- 14.Luka J., Mbaya A.W., Biu A.A., Nwosu C.O. The effect of crude ethanolic extract of Diospyros mespiliformis (Ebenaceae) on the clinicopathological parameters of Yankasa sheep experimentally infected with Haemonchus contortus. Comp. Clin. Pathol. 2014:1925–1927. [Google Scholar]
- 15.Jigam A.A., Abdulrazaq U.T., Suleiman R.S., Kali P.S. Effects of sub-chronic administration of Diospyros Mespiliformis Hochst (Ebenaceae) root extracts on some biochemical parameters in mice. Int. J. Appl. Pharm. Sci. 2012;02(05):60–64. [Google Scholar]
- 16.Javid A., Munir R. Bioassay guided fractionation of withania somnifera for the management Ascochyta Rabiei. Int. J. Agric. Biol. 2012;14:797–800. [Google Scholar]
- 17.Acute Oral Toxicities, Up and Down Procedure. 2001. OECD guidelines for testing chemicals; pp. 1–26. pp. 425. [Google Scholar]
- 18.Mona B., Kenneth A.S. The De Ritis Ratio; the Test of Time. Clin. Biochem. Rev. 2013;34(3):117–130. [PMC free article] [PubMed] [Google Scholar]
- 19.Varley H., Gewenlock A.H., Bell M. CBS Publishers Abd Distributors; Delhi: 1991. Practical Clinical Biochemistry 5th Edn. pp. 741–742. 1. [Google Scholar]
- 20.Ueno T., Hirayama S., Nishioka E., Fikushima Y. Albumin concentration determined by the modified bromocresol purple method is superior to that by the bromocresol green method for assessing nutritional status in mal nourished patients with inflammation. J. Clin. Biochem. 2013;50(6):576–584. doi: 10.1177/0004563213480137. [DOI] [PubMed] [Google Scholar]
- 21.Morinko V., Peter K., Ivona Z. Concentration of proteins and protein fractions in blood plasma of chickens hatched from eggs irradiated with low dose gama radiation. Vet. Arhiv. 2014;84(4):401–409. [Google Scholar]
- 22.Wellington F.D., Camila B.M., Marcelo H.N. Establishing standards for studying renal function in mice through measurements of body size – adjusted creatinine and urea levels. Comput. Biomed. Res. 2014;2014:17–27. doi: 10.1155/2014/872827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nada K., Josiper K., Renata B. Mineral concentrations in plasma of young and adult red deer. Vet. Arhiv. 2013;83(4):425–434. [Google Scholar]
- 24.Guntupalli M., Chandana P., Pushpagadm P., Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia Cordifolia Linn. J. Ethnopharmacol. 2006;103:484–490. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 25.Fennell C.W., Lindsey K.L., McGaw L.J., Sparg S.G., Stafford G.I., Elgorashi E.E. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J. Ethnopharmacol. 2004;94:205–217. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Eloff J.N. Which extractant should be used for screening and isolation of antimicrobial components of plant. J. Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 27.United Nations Economic Commission for Europe. The Purple Book; New York, Geneva: 2005. Anonymous globally harmonized system of classification and labelling of chemicals (GHS) p. 60. [Google Scholar]
- 28.Hazarika I., Geetha K.M., Sundari P.S., Madhu D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in wister albino rats as per OECD 425 TG. Toxicol. Rep. 2019;1:321–328. doi: 10.1016/j.toxrep.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeneye A.A., Ajagbonna O.P., Adeleke T.I., Bello S.O. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J. Ethnopharmacol. 2006;105:374–379. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Akpavie S.O. 2nd ed. Stirling-Horden Publisher Ltd.; Lagos: 2014. General Veterinary Pathology; p. 12. [Google Scholar]
- 31.Hadi A., Abolfazl M., Hossein N. Essential oil analysis and antibacterial activities of some medicinal plants. Intern. J. Phytomed. 2012;4:212–219. [Google Scholar]
- 32.Chalasani N., Aljadhey H., Kesterson J., Murray M.D., Hall S.D. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Braide V.B., Anika S.M. Snaap, Press Ltd.; Enugu: 2010. Environmental Toxicology; pp. 1–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.