Abstract
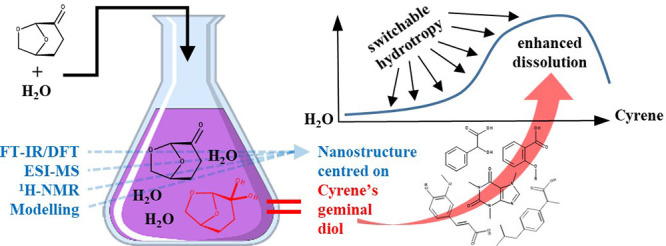
The addition of water to dihydrolevoglucosenone (Cyrene) creates a solvent mixture with highly unusual properties and the ability to specifically and efficiently solubilize a wide range of organic compounds, notably, aspirin, ibuprofen, salicylic acid, ferulic acid, caffeine, and mandelic acid. The observed solubility enhancement (up to 100-fold) can be explained only by the existence of microenvironments mainly centered on Cyrene’s geminal diol. Surprisingly, the latter acts as a reversible hydrotrope and regulates the polarity of the created complex mixture. The possibility to tune the polarity of the solvent mixture through the addition of water, and the subsequent generation of variable amounts of Cyrene’s geminal diol, creates a continuum of green solvents with controllable solubilization properties. The effective presence of microheterogenieties in the Cyrene/water mixture was adequately proven by (1) Fourier transform infrared/density functional theory showing Cyrene dimerization, (2) electrospray mass-spectrometry demonstrating the existence of dimers of Cyrene’s geminal diol, and (3) the variable presence of single or multiple tetramethylsilane peaks in the 1H NMR spectra of a range of Cyrene/water mixtures. The Cyrene–water solvent mixture is importantly not mutagenic, barely ecotoxic, bioderived, and endowed with tunable hydrophilic/hydrophobic properties.
Keywords: Solvents, Nanostructure, Hydrotrope, Biobased, Switchable, Sustainable
Short abstract
Cyrene−water generates a continuum of green solvents with controllable solubilization properties centered on Cyrene’s geminal diol, making the solvent nonmutagenic, barely ecotoxic, and bioderived.
Introduction
The dissolution of chemical substances is a key technology in the chemical industry, with over 20 megatonnes of solvent consumed per year.1−3 Presently, the use of many conventional solvents has come under increasing scrutiny because of their strongly negative environmental impacts and often high toxicities.4 In this respect, the “Registration, Evaluation, Authorisation and Restriction of Chemicals” (REACH) regulation is already beginning to lead to restrictions in the use of many common conventional solvents (e.g., nitrobenzene5 and 1,2-dichloroethane5,6), which has, in turn, reinforced the search for novel solvents with more benign characteristics. Ideally, these solvents would also be biobased, examples being methyltetrahydrofuran, glycerol, γ-valerolactone, ethyl acetate, and dihydrolevoglucosenone (Cyrene).7−10 The last of these is of particular importance as it is a rare biobased dipolar aprotic solvent displaying similar solvent characteristics to N-methylpyrrolidone (NMP) and dimethylformamide (DMF), but while both NMP and DMF are extremely versatile and important solvents ,they also display reproductive toxicity (reprotoxity).8 In contrast, Zhang et al. have reported that Cyrene is not mutagenic and is barely ecotoxic, showing an LD50 > 2000 mg L–1.9 Very recently, the Circa Group, as the sole manufacturer of Cyrene, has received REACH Annex VIII approval, allowing it to import and/or manufacture up to 100 tonnes/year of Cyrene in the European Union.11
Solubilization and extraction of solutes often require the use of (multiple) solvent mixtures and/or solubilizers added to the principal solvent.3 Besides biobased solvents, the use of aqueous solvent systems, switchable solvents, ionic liquids, deep eutectic solvents, CO2 tunable solvents, CO2 expanded liquids, and liquid polymers also often have solid green credentials.12−14 The most common solubilizers are surfactants and hydrotropes and these are most often amphiphilic compounds which can enhance the aqueous solubility of hydrophobic compounds markedly.15−18 On a molecular level, surfactants tend to feature longer C8–C20 alkyl chains while hydrotropes typically have shorter alkyl tails (≤C4) and/or aromatic rings.19 Hydrotropes are generally solids but they can also be liquids in which case they are known as “chameleonic solvents” or “solvo-surfactants”.15 Examples of chameleonic solvents are the short-chain ethers of mono-/di-/tripropylene glycols, which can aid dissolution of organic substances in water by a hydrotropic mechanism but also by forming monophasic microemulsions. However, as with NMP and DMF, glycol ethers have known or suspected toxicity including reprotoxicity.15 Only recently, the underlying, general principle of hydrotrope-based solubilization was shown to be the result of nonspecific association of hydrotropes with solutes, which more than compensates for the per hydrotrope solubilization inefficiency due to hydrotrope self-association.20 Also, the sudden onset of solubilization at critical hydrotrope concentrations, which is another characteristic signature of hydrotropy, has been linked to enhanced hydrotrope self-association around the solute.21
Here, we show that the addition of water to Cyrene, one of the new generations of biobased solvents, can significantly increase the solubility of a range of organic molecules, even for those with very low water solubility (Figure 1A, Table 1S_A,B). Furthermore, it is also apparent that the points of maximal solubilization are actually controlled by the nature of the substrate. Crucial to this behavior is the observation that Cyrene interacts chemically, and reversibly, with water, forming its geminal diol [(1S,5R)-6,8-dioxabicyclo[3.2.1]octan-4,4-diol] (Figure 1B). Consequently, marked amphiphilicity is created in the Cyrene–water solvent system. When taken together with the characteristic S-shape curve of the solubility profiles as viewed from the H2O side, we infer that Cyrene’s geminal diol is behaving as a hydrotrope. The occurrence of a controllable equilibrium between Cyrene, its geminal diol, and H2O is remarkable in that it imparts tunability of the properties of the Cyrene solvent. Indeed, while Cyrene has been classified as a dipolar aprotic solvent, the introduction of water and the consequent formation of Cyrene’s geminal diol introduces significant additional polarity from two hydroxyl groups. These two additional proton donor groups augment the existing proton-acceptor capacity of Cyrene and consequently enhance its overall hydrogen-bonding capacity.
Figure 1.
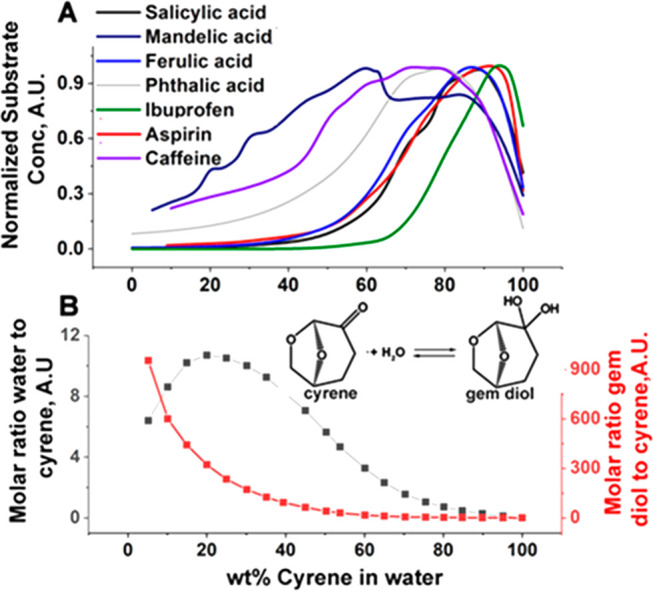
(A) Normalized solubility of a range of organic substrates; (B) molar composition of the Cyrene–H2O solution as a function of the initial Cyrene concentration (based on 1H/13C NMR data).
Results and Discussion
The composition of the ternary Cyrene/water/geminal
diol mixture
(hereafter abbreviated as TM-H2O) has been investigated
with 1H and quantitative 13C NMR. Figure 1B (and Table 2S_A) shows its composition as a function of the initial
amount of Cyrene added to water (in wt %) and with both the molar
amounts of geminal diol and excess H2O (i.e., water that
has not engaged in forming the geminal diol) normalized to 1 mol of
Cyrene. The composition of the ternary mixture can thus be adequately
described as [Cyrene normalized moles geminal diol; Cyrene normalized
moles H2O] couples (see Figure 1B). This data allows the investigation of
the equilibrium of the Cyrene hydration reaction (Table 2S_B). Two different models for the Cyrene–water
interaction/reaction have been considered: (i) one that follows the
intuitive reaction stoichiometry in which one molecule of Cyrene reacts
with one water molecule, yielding the geminal diol (see Figure 2A), and (ii) a reaction stoichiometry
in which two water molecules are involved, one reacting with the Cyrene
and one hydrogen bonding strongly to the geminal diol (Figure 2B). Model (i) is valid only
at initial cyrene concentrations >85 wt %, from which point it
displays
a constant 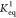 (Figure 2A, yellow-striped
zone). Alternatively, model (ii)
(Figure 2B, cyan zone)
yields a constant
(Figure 2A, yellow-striped
zone). Alternatively, model (ii)
(Figure 2B, cyan zone)
yields a constant 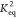 up to <50 wt % initial Cyrene concentration,
suggesting that this model provides a more realistic description of
the reaction within this concentration range. The zone between 50
and 85 wt % is not straightforwardly categorizable to either model
and may therefore require consideration of the involvement of other
species/complexes.
up to <50 wt % initial Cyrene concentration,
suggesting that this model provides a more realistic description of
the reaction within this concentration range. The zone between 50
and 85 wt % is not straightforwardly categorizable to either model
and may therefore require consideration of the involvement of other
species/complexes.
Figure 2.
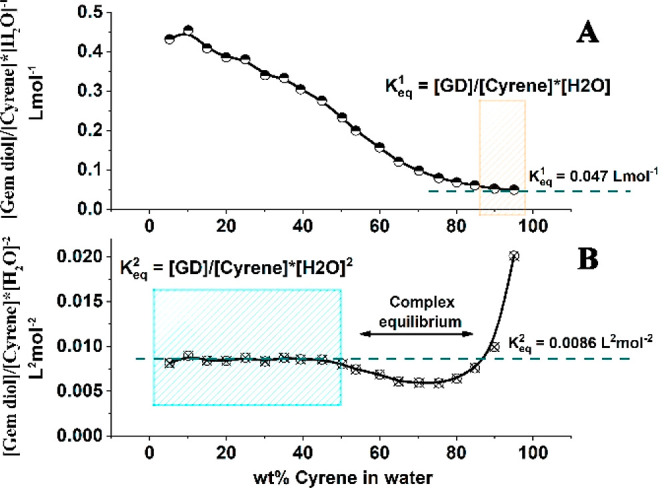
(A) Linearization of the Cyrene–H2O solution compositional data based on the natural Cyrene–geminal diol equilibrium involving one water molecule; (B) linearization of the Cyrene–H2O solution compositional data based on the involvement of two water molecules in the equilibrium.
To obtain more insight into the structure of Cyrene–water solutions, a systematic Fourier transform infrared (FT-IR) analysis of Cyrene and Cyrene–water solutions has been performed.
First, and most surprisingly, the FT-IR spectrum of pure Cyrene, with just a single carbonyl group, displays at least two different IR carbonyl absorption bands centered at about 1730 cm–1 (Figure 3A). Such an observation cannot relate to the opening of the acetal group because this occurs only in the presence of a strong acid and at temperatures >120 °C. The most plausible alternative explanation is that the carbonyl group sits in two or more different chemical environments. A Clausius–Clapeyron plot obtained from the variation of the vapor pressure with temperature determined the ΔHvap of Cyrene at 67 kJ mol–1. This value divided by Cyrene’s boiling point (476 K) gives a value for ΔSvap of 140 J mol–1 K–1. This is substantially higher than that predicted by Trouton’s rule which states that the entropy of vaporization for many (but not all) liquids is about the same at 85–88 J mol–1 K–1.22 Exceptions to this rule are the entropies of vaporization of water, ethanol, and formic acid, all of which form strong hydrogen-bonding interactions in the liquid phase. A reasonable conclusion therefore is that the positive deviation from Trouton’s rule for Cyrene is due to the existence of strong hydrogen-bonding interactions between Cyrene monomers. Consistent with this conclusion is the observation that the experimental FT-IR spectrum of Cyrene in excess CCl4, in which formation of higher order clusters of Cyrene molecules is likely to be impeded, reveals just a single carbonyl band (see Figure 3A).
Figure 3.
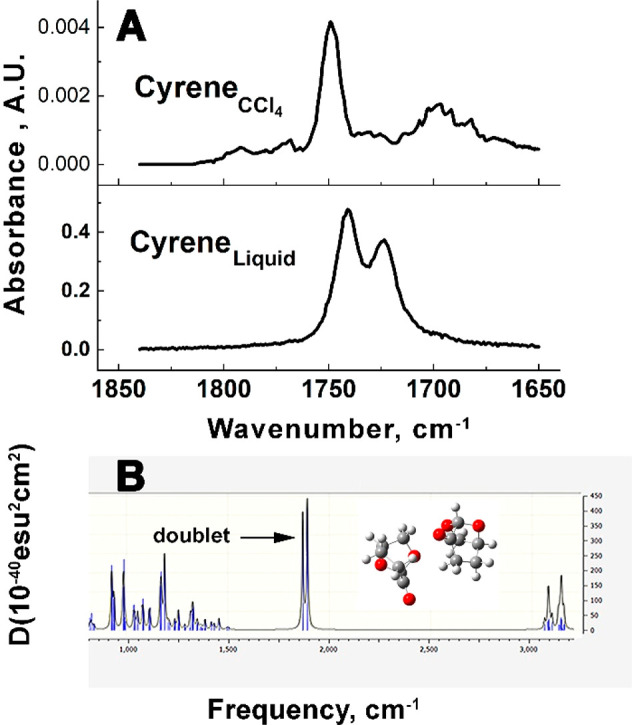
(A) A comparison of the FT-IR spectra of Cyrene in CCl4 (upper) with pure liquid Cyrene. (B) A simulation of the IR spectrum of one of the six stable conformations of the Cyrene dimer obtained from the DFT calculations. This structure is the second most stable (see Supporting Information for further examples).
To support this hypothesis, a series of density functional theory (DFT) calculations were carried out at the M062X/cc-pVDZ level on the Cyrene system. Geometry optimizations and vibrational frequency analysis allowed the simulation of IR spectra for each structure. As expected, the resulting simulated IR spectrum of the Cyrene monomer revealed just one single carbonyl stretching band (see Supporting Information). However, IR simulations of a number of different structural isomers of the Cyrene dimer revealed doublet carbonyl stretching bands where the structures of those dimers resulted in different chemical environments for the two carbonyl groups (see Figure 3B and the Supporting Information). Similarly, a simulated IR spectrum for one conformer of the Cyrene trimer revealed a triplet of carbonyl stretching bands (see Supporting Information).
Addition of water to Cyrene shows a progressive change in the relative intensities of the two carbonyl IR stretching bands, becoming equal at ∼24 wt % water. This suggests a persistent presence of the Cyrene dimer over a large range of concentrations (Figure 4A,B). Likewise, the symmetric and asymmetric geminal diol OH stretches at 1080 and 1063 cm–1 also show a progressive variation in relative intensities with increasing water content (Figure 4C,D). Of particular note, in the 25–65 wt % water range, rapid changes in the C–C skeletal vibrational bands of Cyrene at 906 and 917 cm–1 are observed, which suggests involvement of the acetal oxygens (1020 and 984 cm–1 bands) in hydrogen bonding to other Cyrene molecules, or to Cyrene’s geminal diol or indeed with H2O (Figure 4C,D).23
Figure 4.
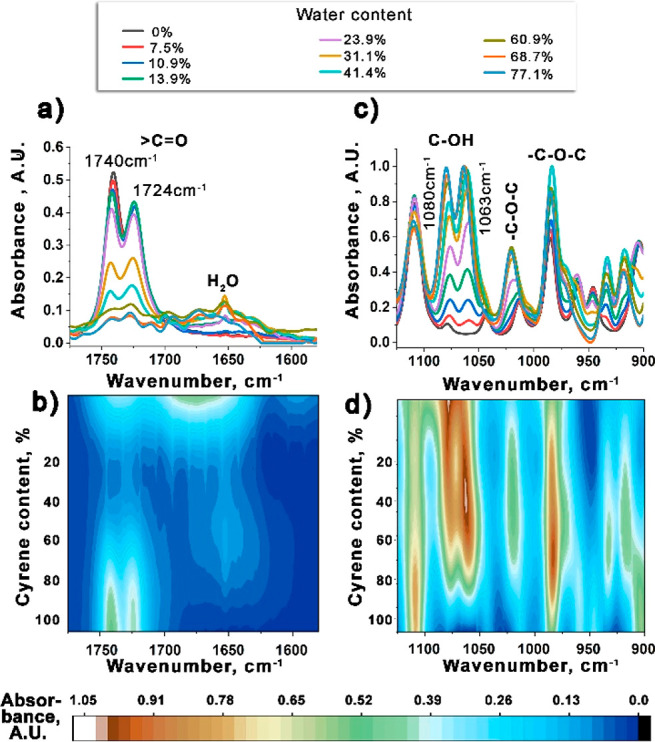
(a, b) Evolution of the carbonyl stretching bands of Cyrene with increasing water content presented as (a) a series of one-dimensional IR spectra and (b) a 2-D IR spectrum. (c, d) Evolution of the C–OH and C–O–C vibrational bands of Cyrene with increasing water content presented as (c) a series of one-dimensional IR spectra and (d) a 2-D IR spectrum.
To gain further insight, we evaluated the solubility profiles for a range of organic substrates (Figure 5 and Table 1S_A,B), through linear regression, as a function of the four main components [Cyrene (Cy), Cyrene dimer, geminal diol (GD), and H2O] and four possible molecular complexes “Cyrene–geminal diol”, “geminal diol–water”, “geminal diol dimer”, and “Cyrene–water” (see eq 1, Table 1 and Figure 5). These complexes then refer to the potential presence of microheterogeneities in the Cyrene–H2O solvent mixture. As can be seen from Figure 5, the concept of microheterogeneity proves valuable as the solubilities of all the tested solutes can be described adequately in this way. The amount to which the tested compounds dissolve in a certain microenvironment is thereby proportional to the probability of finding this microcluster in solution. The solubility of the organic substrates around maximum solubilization can always be described as a function of two to four main solvent components. The main contributors to dissolution are as follows:
-
(1)
Cyrene and the “Cyrene–geminal diol” complex for ibuprofen dissolution
-
(2)
Cyrene, Cyrene–water, Cyrene–geminal diol, and geminal diol dimer for aspirin dissolution
-
(3)
Cyrene, geminal diol, and the “Cyrene–geminal diol” complex for salicylic and ferulic acid dissolution
-
(4)
Geminal diol (major) and water (minor) for caffeine dissolution
-
(5)
“Cyrene–geminal diol” and water (minor) for mandelic acid dissolution
Figure 5.
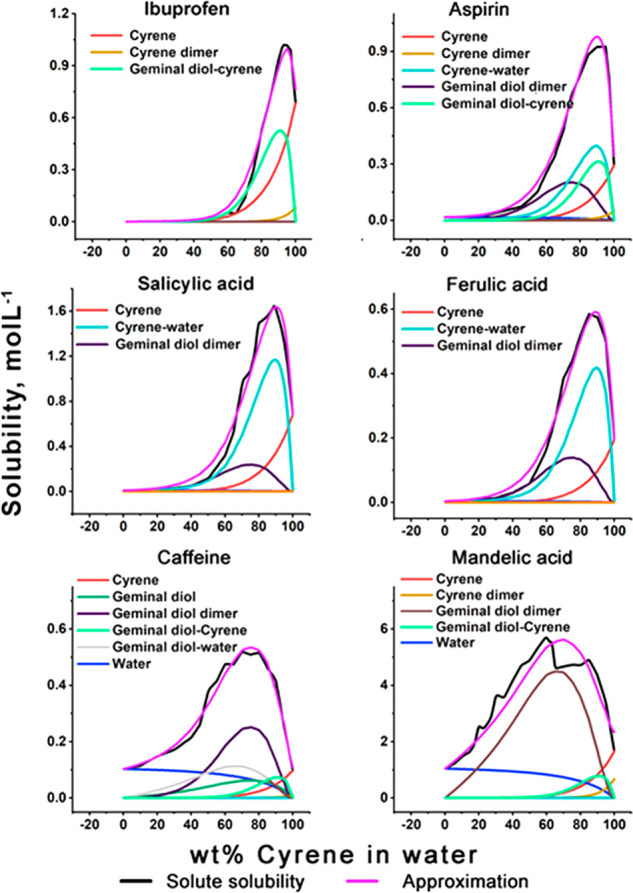
Impact of a range of potential solution components on the solubility of a series of organic compounds as identified by linear regression of the original solubility data.
Table 1. Constants Obtained for the Linear Regression Analysis of the Solubility Data.
| solutes |
||||||
|---|---|---|---|---|---|---|
| constants | ibuprofen | aspirin | salicylic acid | fenulic acid | caffeine | mandelic acid |
| K1 | 0.08 | 0.05 | 0 | 0 | 0.01 | 0.67 |
| K2 | 0.68 | 0.29 | 0.68 | 0.20 | 0.10 | 1.70 |
| K3 | 7.86 | 4.68 | 0 | 0 | 1.10 | 11.7 |
| K4 | 0 | 5.98 | 7.02 | 4.11 | 7.43 | 0 |
| K5 | 0 | 0 | 0 | 0 | 0.34 | 0 |
| K6 | 0 | 2.16 | 6.37 | 2.28 | 0 | 0 |
| K7 | 0.001 | 0.017 | 0.009 | 0.004 | 0.10 | 1.04 |
| 1 |
The existence of microheterogeneities in the Cyrene/water/geminal diol mixture was also revealed, somewhat serendipitously, through the behavior of the tetramethylsilane (TMS) reference peak in the 1H NMR spectra. It can be seen in Figure 6A that for 90–65 wt % Cyrene the TMS peak separates into multiplets. As all of the CH3 groups (and so all related protons) in TMS are chemically equivalent, this can be explained only if the TMS is experiencing different chemical environments on the molecular level. It is also noteworthy that the 1H NMR relaxation times of the TMS protons are different for all the observable TMS 1H NMR peaks, further supporting the presence of microheterogeneities in the Cyrene–water mixture (Figure 6B). To the best of our knowledge, this is unprecedented in the literature. Additionally, the explicit existence of dimeric geminal diol was proven by electrospray ionization mass spectrometry (ESI-MS) as shown in Figure S2. Interestingly, maximum solubility seems to always involve Cyrene’s geminal diol, irrespective of the identity of the solute. It is also noteworthy that we have presently not been able to establish a link between the ranges of maximum dissolution and any physical property of the solutes (e.g., density, viscosity, Kow of the substrates). It could thus be concluded that the observed hydrotropy is strongly linked to the presence of Cyrene’s geminal diol. Tables 3S and 4S show that the solubility of all tested compounds increases by a factor of between 4 and 100 compared to the solubility in water and between 1.5 and 9 times when compared to their solubility in Cyrene.
Figure 6.
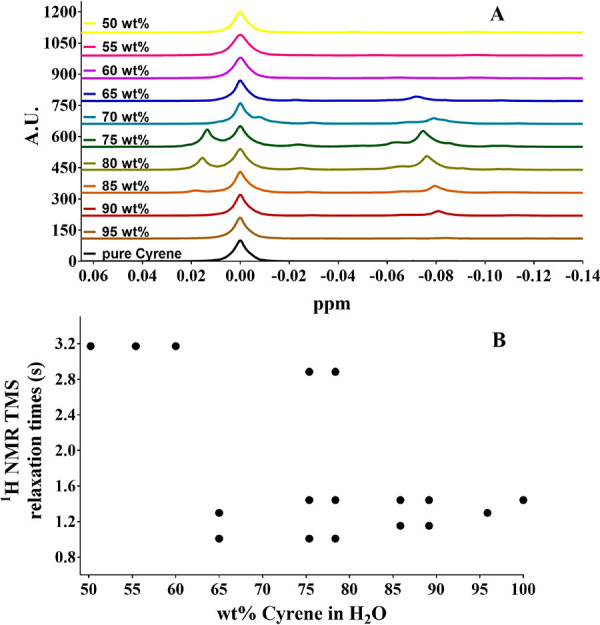
(A) appearance of the TMS peak(s) in the 1H NMR spectra of a range of different Cyrene/water mixtures; (B) 1H NMR relaxation times (in seconds) of the TMS protons for the different observable TMS peaks.
To date, hydrotropic solubilization, with the occurrence of strong solubility maxima, has been reported on very little. A noteworthy example is the dissolution of lignin monomeric model compounds and technical lignins using aqueous solutions of deep eutectic solvents (DESs).24 Also, ionic liquids (ILs) have been shown to function as “catanionic” hydrotropes when used in an aqueous medium.25 Very recently, Ma et al. published a comprehensive overview on how the addition of water to DESs and ILs affects their properties, behavior, and three-dimensional structure.26 Structural organization is long known to exist in urea/water mixtures.27
Importantly, for practical applications (e.g., extractions and isolations) recovery of the substrates can be achieved by shifting the TM-H2O equilibrium to a zone in which the solute is no longer soluble. This can be realized by adding the necessary amount of water to achieve, for example, a Cyrene concentration below 40 wt %, at which point most of the solutes discussed above are significantly less soluble (Figure 5). The drawback to this procedure is the need to distill out larger amounts of water to regain pure Cyrene, which evidently comes at a significant energetic cost. However, for this, and probably also many other applications, distillation basically needs to reform only a suitable technical grade of Cyrene such as 80 wt % Cyrene in water. In cases of poor solute solubility in Cyrene, the use of water as an antisolvent could be foresaken and the water could be directly distilled out of the mixture. Any recovered water could be reused to reform the required/desired Cyrene/water mixture and/or as the antisolvent without extensive purification. A full LCA analysis is currently in progress. Lowering or increasing the temperature may also aid the precipitation process as variable temperature NMR studies of the TM-H2O equilibrium show that a decrease/increase in temperature favors the geminal diol and Cyrene, respectively (Figure 3S_A,B), thus changing the polarity of the overall mixture.
Conclusion
In conclusion, this work describes an elegant way to tune the dissolution properties of Cyrene by the addition of water, thus generating a continuum of green solvents with controllable solubilization properties. Central to this is the unique ability of Cyrene to generate significant amounts of Cyrene’s geminal diol. In this respect, it is noteworthy that with most ketones in aqueous solutions the ketone/geminal diol equilibrium tends to lie dominantly on the ketone side. Additionally, many ketones are also insoluble in water, for example, cycloheptanone. Cyrene’s geminal diol is an amphiphilic molecule, which can act as a switchable and reversible hydrotrope. Solubility increases of up to 100-fold (over water solubility) can be achieved. Examination by linear regression of the solubility profiles of a range of compounds shows that the observed solubility profiles can be explained only by considering the existence of microenvironments in the TM-H2O system. The existence of such microenvironments is adequately, and uniquely, proven by FT-IR, DFT, ESI-MS, and the variable presence of single or multiple tetramethylsilane peaks in the 1H NMR spectra of a range of Cyrene/water mixtures. The Cyrene–water solvent mixture is importantly not mutagenic, barely ecotoxic, bioderived, and endowed with tunable hydrophilic/hydrophobic properties.
Acknowledgments
M.D.b. acknowledges the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 701028 (EU Marie Curie Global Fellowship). We also acknowledge the Circa Company, and especially Dr. Warwick Raverty and Mr. Tony Duncan for the kind and regular provision of double-distilled dihydrolevoglucosenone. We thank the UW-Madison Department of Chemistry for the use of a Bruker Avance 500 MHz NMR Spectrometer; a generous gift from Paul J. Bender enabled purchase of this spectrometer in 2012. A.J.H. thanks the Center of Excellence for Innovation in Chemistry (PERCH-CIC).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.9b00470.
1H and 13C NMR spectra of Cyrene–water mixtures (inclusive of variable T) and those including solutes; tabulated solubility values of a range of compounds in Cyrene–water mixtures; compositional data of the Cyrene/H2O/geminal diol ternary mixture inclusive of the calculated equilibrium constants; data on relative solubility increases vis-à-vis water/Cyrene and ranges of maximum solubility; ESI-MS of Cyrene–water; DFT calculations and IR simulations of single/dimeric/trimeric Cyrene (PDF)
Author Contributions
M.D.b., V.L.B., and A.M. conceived the different ideas/approaches and were the main experimental contributors; S.S. as an expert in hydrotropy and A.J.H. as an expert in solvents gave valuable background information, in addition to supervising and providing scientific input on the work of A.M.; H.F. and H.H. assisted with NMR experimentation; M.C. performed all of the DFT calculations and produced the simulated IR spectra; J.H.C., D.J.M., and B.M.W. gave valuable scientific, background, and redaction advice. The manuscript was written through contributions of all authors. All authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
The title was changed in the version published ASAP on March 26, 2019. The corrected version was published ASAP on March 29, 2019.
Supplementary Material
References
- Clark J. H.; Farmer T. J.; Hunt A. J.; Sherwood J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16 (8), 17101–17159. 10.3390/ijms160817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott S.; Booth J. J.; Shimizu S. Practical molecular thermodynamics for greener solution chemistry. Green Chem. 2017, 19 (1), 68–75. 10.1039/C6GC03002E. [DOI] [Google Scholar]
- Kunz W.; Holmberg K.; Zemb T. Hydrotropes. Curr. Opin. Colloid Interface Sci. 2016, 22, 99–107. 10.1016/j.cocis.2016.03.005. [DOI] [Google Scholar]
- Byrne F.P.; Paggiola S. J. G.; Petchey T. H. M.; Clark J.H.; Farmer T.J.; Hunt A.J.; McElroy C. R.; Sherwood J.; Jin S. Tools and techniques for solvent selection: green solvent selection guides. Sustainable Chem. Processes 2016, 4, 7. 10.1186/s40508-016-0051-z. [DOI] [Google Scholar]
- Candidate List of substances of very high concern for Authorisation; ECHA: Helsinki, Finland, https://echa.europa.eu/candidate-list-table (accessed 03/25/2019).
- Sherwood J. European Restrictions on 1,2-Dichloroethane: C-H Activation Research and Development Should Be Liberated and not Limited. Angew. Chem., Int. Ed. 2018, 57 (43), 14286–14290. 10.1002/anie.201800549. [DOI] [PubMed] [Google Scholar]
- Gu Y. L.; Jerome F. Bio-based solvents: an emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42 (24), 9550–9570. 10.1039/c3cs60241a. [DOI] [PubMed] [Google Scholar]
- Sherwood J.; De Bruyn M.; Constantinou A.; Moity L.; McElroy C. R.; Farmer T. J.; Duncan T.; Raverty W.; Hunt A. J.; Clark J. H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50 (68), 9650–9652. 10.1039/C4CC04133J. [DOI] [PubMed] [Google Scholar]
- Zhang J. F.; White G. B.; Ryan M. D.; Hunt A. J.; Katz M. J. Dihydrolevoglucosenone (Cyrene) As a Green Alternative to N,N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sustainable Chem. Eng. 2016, 4 (12), 7186–7192. 10.1021/acssuschemeng.6b02115. [DOI] [Google Scholar]
- Santoro S.; Ferlin F.; Luciani L.; Ackermann L.; Vaccaro L. Biomass-derived solvents as effective media for cross-coupling reactions and C-H functionalization processes. Green Chem. 2017, 19 (7), 1601–1612. 10.1039/C7GC00067G. [DOI] [Google Scholar]
- Mendes-Jorge B.Circa receives green light to sell non-toxic, bio-based and biodegradable solvent in EU. Press release. Sustainability Consult, Dec 10, 2018.
- Schuur B.; Smink D.; Sprakel L. M. J.; Brouwer T. Green solvents for sustainable separation processes. Current Opinion in Green and Sustainable Chemistry 2019, 18, 57–65. 10.1016/j.cogsc.2018.12.009. [DOI] [Google Scholar]
- Sheldon R. A. The greening of solvents: Towards sustainable organic synthesis. Current Opinion in Green and Sustainable Chemistry 2019, 18, 13–19. 10.1016/j.cogsc.2018.11.006. [DOI] [Google Scholar]
- Clarke C. J.; Tu W. C.; Levers O.; Brohl A.; Hallett J. P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118 (2), 747–800. 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]
- Molinier V.; Aubry J. M. Sugar-based hydrotropes: preparation, properties and applications. Spr Carb Ch 2014, 40, 51–72. 10.1039/9781849739986-00051. [DOI] [Google Scholar]
- Hodgdon T. K.; Kaler E. W. Hydrotropic solutions. Curr. Opin. Colloid Interface Sci. 2007, 12 (3), 121–128. 10.1016/j.cocis.2007.06.004. [DOI] [Google Scholar]
- Subbarao C. V.; Chakravarthy I. P. K.; Sai Bharadwaj A. V. S. L.; Prasad K. M. M. K. Functions of Hydrotropes in Solutions. Chem. Eng. Technol. 2012, 35 (2), 225–237. 10.1002/ceat.201100484. [DOI] [Google Scholar]
- Thakker M. R.; Parikh J. K.; Desai M. A. Ultrasound Assisted Hydrotropic Extraction: A Greener Approach for the Isolation of Geraniol from the Leaves of Cymbopogon martinii. ACS Sustainable Chem. Eng. 2018, 6 (3), 3215–3224. 10.1021/acssuschemeng.7b03374. [DOI] [Google Scholar]
- Hopkins Hatzopoulos M.; Eastoe J.; Dowding P. J.; Rogers S. E.; Heenan R.; Dyer R. Are Hydrotropes Distinct from Surfactants?. Langmuir 2011, 27 (20), 12346–12353. 10.1021/la2025846. [DOI] [PubMed] [Google Scholar]
- Booth J. J.; Omar M.; Abbott S.; Shimizu S. Hydrotrope accumulation around the drug: the driving force for solubilization and minimum hydrotrope concentration for nicotinamide and urea. Phys. Chem. Chem. Phys. 2015, 17 (12), 8028–8037. 10.1039/C4CP05414H. [DOI] [PubMed] [Google Scholar]
- Shimizu S.; Matubayasi N. The origin of cooperative solubilisation by hydrotropes. Phys. Chem. Chem. Phys. 2016, 18 (36), 25621–25628. 10.1039/C6CP04823D. [DOI] [PubMed] [Google Scholar]
- Rooney J. J. A Simple Statistical Mechanical Rationale of Trouton’s Rule. Proc. R. Irish Acad., B 1988, 88 (3), 39–43. [Google Scholar]
- Degen I. A.Tables of Characteristic Group Frequencies for the Interpretation of Infrared and Raman Spectra; Harrow: Acolyte Publishers, 1997. [Google Scholar]
- Soares B.; Tavares D. J. P.; Amaral J. L.; Silvestre A. J. D.; Freire C. S. R.; Coutinho J. A. P. Enhanced Solubility of Lignin Monomeric Model Compounds and Technical Lignins in Aqueous Solutions of Deep Eutectic Solvents. ACS Sustainable Chem. Eng. 2017, 5 (5), 4056–4065. 10.1021/acssuschemeng.7b00053. [DOI] [Google Scholar]
- Claudio A. F. M.; Neves M. C.; Shimizu K.; Canongia Lopes J. N.; Freire M. G.; Coutinho J. A. P. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17 (7), 3948–3963. 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. Y.; Laaksonen A.; Liu C.; Lu X. H.; Ji X. Y. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47 (23), 8685–8720. 10.1039/C8CS00325D. [DOI] [PubMed] [Google Scholar]
- Frank H. S.; Franks F. Structural Approach to Solvent Power of Water for Hydrocarbons - Urea as a Structure Breaker. J. Chem. Phys. 1968, 48 (10), 4746–4757. 10.1063/1.1668057. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


