Abstract
Although the survival of metastatic colorectal cancer (mCRC) patients has improved five-fold over the last century, CRC remains a significant global health burden. Impressive strides have been made in identifying new regimens, employing maintenance strategies to limit treatment toxicities, and combining multidisciplinary approaches to achieve cure in oligometastatic disease. Attempts at personalized integration of targeted agents have been limited by the ability to identify molecularly enriched patient populations most likely to benefit. In this review, we discuss novel therapeutics and regimens recently approved and in development for mCRC. In addition, we discuss using older agents in novel combination and maintenance strategies, and highlight evidence for implementing pharmacogenomic data and non-invasive monitoring into the personalized management of mCRC patients.
Keywords: Angiogenesis, biomarker, EGFR, fluoropyrimidines, immunotherapy, metastatic colorectal cancer, microsatellite instability, pharmacogenetics, RAF, RAS
Introduction
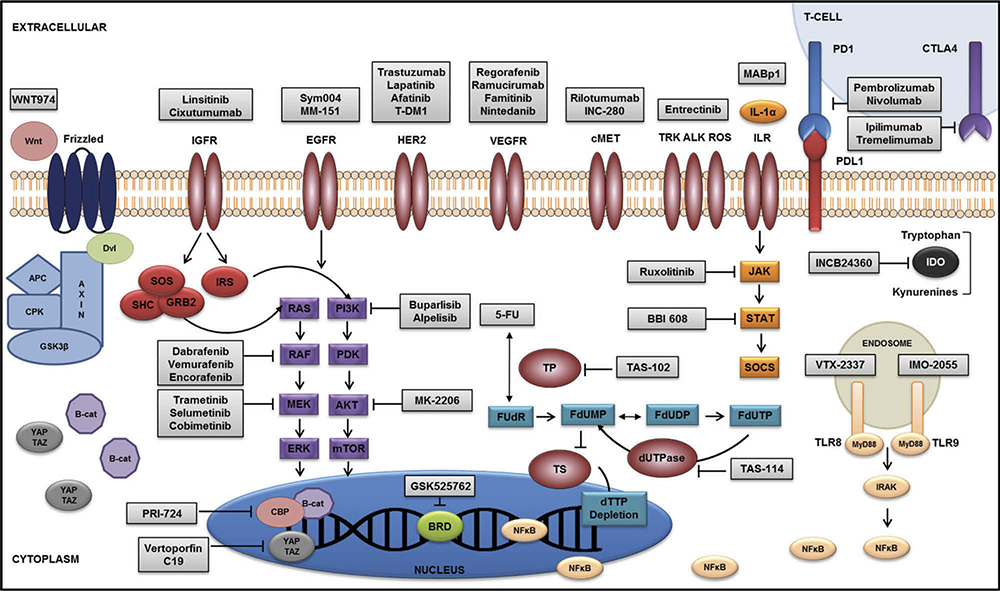
The survival of patients with metastatic colorectal cancer (mCRC) has improved fivefold over the last century. Nonetheless, CRC remains a significant global health burden, with an incidence of 1.4 million cases and 693,000 deaths worldwide [1]. While 5-fluorouracil (5-FU) remains the most active single agent, agents targeting the vascular endothelial growth factor receptor (VEGFR; bevacizumab, aflibercept, and ramucirumab), epidermal growth factor receptor (EGFR; cetuximab and panitumumab), and the multitargeted tyrosine kinase (regorafenib) have further improved outcomes for mCRC patients. However, the incremental survival benefit achieved with each new therapeutic is modest, tempered by the fact that only a fraction of patients demonstrates a measurable or durable response. Attempts at personalized integration of targeted agents have been limited by the ability to identify molecularly enriched patient populations most likely to benefit. Herein, we review, in a pathway-based approach (Figure 1), new therapeutics and regimens recently approved (Table 1) and in development (Table 2) for mCRC. In addition, we discuss using older agents in new combination and maintenance strategies and summarize evidence for implementing pharmacogenomic data into a personalized management approach for mCRC patients.
Figure 1.
Molecular landscape of novel therapeutics in metastatic colorectal cancer.
Table 1.
Pivotal trials of recently approved regimens for metastatic colorectal cancer.
| Study | Patient population | Treatment arms | Main findings | Reference |
|---|---|---|---|---|
| RECOURSE | Refractory mCRC | TAS-102 vs. placebo | OS: 7.1 vs. 5.3 months, HR 0.68 (p < 0.001) PFS: 2.0 vs. 1.7 months, HR 0.48 (p < 0.001) |
[2] |
| RAISE | Second-line after progression on fluoropyrimidine, oxaliplatin, and bevacizumab | FOLFIRI-ramucirumab vs. FOLFIRI-placebo | OS: 13.3 vs. 11.7 months, HR 0.84 (p = 0.0219) PFS: 5.7 vs. 4.7 months, HR 0.79 (p = 0.0005) |
[3] |
| TRIBE | First-line mCRC | FOLFOXIRI-bevacizumab vs. FOLFIRI-bevacizumab | OS: 31.0 vs. 25.8 months, HR 0.79 (p = 0.054) PFS: 12.1 vs. 9.7 months, HR 0.75 (p = 0.003) ORR: 65% vs. 53% (p = 0.006) |
[4] |
| CAIRO3 | Maintenance after disease control on first-line CAPOX-B | Capecitabine-bevacizumab vs. observation | PFS2: 11.7 vs. 8.5 months HR 0.67 (p < 0.0001) | [5] |
Table 2.
Key ongoing studies of new therapeutics in metastatic colorectal cancer.
| Phase | Treatment arms (therapeutic target) | Primary end points | Trial identifier |
|---|---|---|---|
| III | Nintedanib (VEGFR, FGFR, PDGFR) vs. placebo | PFS, OS | LUME-Colon1 NCT02149108 |
| II | Sym004 (EGFR) vs. investigator’s choice therapy | OS | NCT02083653 |
| II | Irinotecan-cetuximab ± vemurafenib (BRAF) | PFS | SWOG 1406 NCT02164916 |
| II | Panitumumab + dabrafenib (BRAF) + trametinib (MEK) | RR, PFS | NCT01750918 |
| II | Cetuximab + aleplisib (PI3K) + encorafenib (BRAF) | PFS | NCT01719380 |
| II | T-DM1 (HER2) after progression on trastuzumab-lapatinib | RR | HERACLES-RESCUE EudraCT 2015–003275–30 |
| II | Pembrolizumab (PD-1) | RR | KEYNOTE-164 NCT02460198 |
| II | Ipilimumab (CTLA-4) + nivolumab (PD-1) | RR | CheckMate-142 NCT02060188 |
| III | MABp1 (Xilonix™; IL-1α) vs. placebo | OS | XCITE NCT01767857 |
| III | BBI-608 (STAT3, β-catenin, Nanog) + BSC vs. placebo +BSC | OS | NCT01830621 |
| II | FOLFOXIRI-bevacizumab (concurrent vs. sequential) vs. FOLFOX-bevacizumab | ORR1 | STEAM NCT01765582 |
| PFS1 |
Pyrimidine pathway modulation
Since the advent of fluorouracil in 1957 [6,7], pyrimidine pathway inhibition remains a cornerstone of CRC treatment [8]. The antimetabolite, 5-FU, an integral component of first and subsequent treatment lines in mCRC, binds 2′-deoxy-5-fluorouridine-5′-monophosphate (FdUMP), thereby inhibiting thymidylate synthase (TS) and leading to the thymidine-5′-triphosphate (dTTP) depletion and cancer cell death. This leads to accumulation of 2′-deoxyuridine-5′-monophosphate (dUMP) and FdUMP, which are then incorporated in their triphosphate forms into DNA. The enzyme deoxyuridine 5′-triphosphate (dUTPase) degrades dUMP and FdUMP and prevents their incorporation into DNA, serving as a mechanism of resistance toward 5-FU [9]. Two new agents, TAS-102 and TAS-114, aim to circumvent such resistance and/or enhance TS-directed therapy.
TAS-102 (Lonsurf™)
TAS-102 (Lonsurf™), the latest therapeutic to achieve a survival benefit and US FDA approval in refractory mCRC [2], consists of an oral thymidine analog, trifluridine, coupled to the thymidine phosphorylase inhibitor, tipiracil hydrochloride. Although trifluridine inhibits TS when administered as a continuous infusion, its primary mode of action as an oral agent is incorporation into tumor DNA, which leads to strand breaks and dysfunction. By inhibiting thymidine phosphorylase, tipiracil hydrochloride prevents trifluridine degradation, allowing for sustained plasma levels of active drug, more stable pharmacokinetics, and an improved toxicity profile. In the pivotal double-blind phase III RECOURSE trial [2], 800 patients were randomized in 2 : 1 manner to TAS-102 versus placebo along with best supportive care, after progressing on a fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and an anti-EGFR agent in patients with KRAS-wildtype cancers. Among patients receiving TAS-102, the median overall survival (OS) was significantly improved to 7.1 months as compared to 5.3 months in patients assigned placebo (HR 0.68; 95% CI 0.58–0.81; p < 0.001)[2]. Importantly, TAS-102 also delayed decline in performance status by almost 2 months (5.7 vs. 4.0 months; HR 0.66; 95% CI 0.56–0.78; p < 0.001). The most commonly reported adverse effects of any grade with TAS-102 in this study included nausea/vomiting, appetite suppression, fatigue, and diarrhea, all of which occurred in at least 30% but were typically grade 1–2 and manageable. In addition, neutropenia (38%), leukopenia (21%), and anemia (18%) were the most common grade ≥3 laboratory abnormalities. Altogether, the clinical benefit of TAS-102 was demonstrated in prespecified subgroups defined by KRAS mutation status, performance status, recent disease progression on fluoropyrimidines, prior regorafenib exposure, primary tumor site, and geographic location.
Preclinical evidence from colorectal xenograft models suggests synergistic activity of TAS-102 with oxaliplatin in both treatment-naïve and 5-FU refractory disease [10]. An ongoing phase I/II study is determining the maximum tolerated dose (MTD) of irinotecan in combination with the DNA hypomethylating agent, SGI-110, followed by randomization to the addition of TAS-102 versus regorafenib in refractory mCRC patients (NCT01896856). Other studies aim to examine the combination of TAS-102 with panitumumab (NCT02613221; APOLLON) and yttrium-90 microsphere radioembolization for the treatment of colorectal liver metastases (NCT02602327).
TAS-114
Another pyrimidine pathway modulator in development, TAS-114, is an oral first-in-class dUTPase inhibitor. By inhibiting dUTPase, TAS-114 allows for the incorporation of dUTP and FdUTP into tumor cells. TAS-114 only exhibits antitumor activity in conjunction with a TS inhibitor, such as 5-FU or capecitabine [9]. In the first-in-human phase I study, TAS-114 also demonstrated inhibition of dihydropyrimidine dehydrogenase, the enzyme that causes 5-FU degradation [9]. TAS-114 is currently in phase I development in combination with S-1 (NCT02454062, NCT01610479) and capecitabine (NCT02025803).
Angiogenesis inhibition
Angiogenesis is integral to cancer development, growth, and survival, and antiangiogenic agents have advanced clinical outcomes in CRC. Bevacizumab, a monoclonal antibody inhibiting the interaction between VEGFA and VEGFR1 and VEGFR2, was the first targeted agent to receive FDA approval for mCRC based on its ability to improve progression-free survival (PFS) and OS when added to platinum- [11] or irinotecan-based [12] regimens in the first-line setting. Data from the TML [13] study also support the use of bevacizumab in the second-line setting.
Evidence from VELOUR [14] demonstrated an OS benefit with the use of ziv-aflibercept in the second-line mCRC setting. Ziv-aflibercept is a recombinant decoy receptor fusion protein, targeting VEGFA and VEGFB, placental growth factor 1, 2, and their interaction with VEGFR 1, 2 [15]. The phase III VELOUR study [11] evaluated patients with metastatic CRC who had progressed on oxaliplatin-based therapy and randomized them to FOLFIRI with or without ziv-aflibercept. Patients who received ziv-aflibercept had superior median OS, relative to those receiving FOLFIRI alone (13.5 vs. 12.1 months; p = 0.0032). Based on this data, ziv-aflibercept was approved in combination with irinotecan-based second-line therapy [14].
Ramucirumab (Cyramza™)
Ramucirumab, a humanized IgG1 monoclonal antibody directed against VEGFR2, is the latest antiangiogenic agent to gain approval for second-line therapy in mCRC. In the double-blind, phase III RAISE trial [16], 1072 patients were randomized to receive ramucirumab plus FOLFIRI or placebo plus FOLFIRI. Those receiving ramucirumab achieved a significantly longer median OS of 13.3 months compared to 11.7 months in those receiving placebo (HR 0.84; 95% CI 0.73–0.98; log-rank p = 0.0219). The most common grade 3 or worse AEs seen more frequently in the experimental group included neutropenia (38%), fatigue (12%), hypertension (11%), diarrhea (11%), and febrile neutropenia (3%).
Although antiangiogenic therapies have become standard of care in both the first- and second-line mCRC settings, resistance develops and results from compensatory signaling through pathways of the fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) family. FGF and PDGF have been shown to control the tumor cell migration and promote blood vessel wall stability, mediated by pericytes and smooth muscle cells [3,17]. Therefore, antiangiogenic agents in development target these collateral pathways as a means of overcoming treatment resistance.
Nintedanib
Nintedanib is an oral tyrosine angiokinase inhibitor targeting VEGFR 1, 2, 3, as well as FGFR 1, 2, 3 and the PDGFRα and β receptors. Specifically, it has been shown to reduce autophosphorylation of VEGFR2, downregulate PDGFRβ-expressing perivascular cells, as well as inhibit MAPK/Akt pathways in pericytes and smooth muscle cells [3,18]. One feature that distinguishes it from other multitargeted angiokinase drugs is its more balanced inhibition of its targets: VEGFR 1, 2, 3 (IC50 13–34 nmol/l); FGFRs 1, 2, and 3 (IC50 37–108 nmol/l); and PDGFRα and β (IC50 59–65 nmol/l) [19].
Based on initial phase I studies, the MTD for nintedanib as a single agent has been determined to be 200 mg p.o. b.i.d. [20–23]. In CRC, a clinically relevant antiangiogenic effect was observed in 67% patients, with one confirmed partial response (PR) [22,24]. In a phase II feasibility study of alternating nintedanib and afatinib as second-line therapy in 46 CRC patients, stable disease was achieved in 43.5% of patients, with a median PFS of 1.9 months and OS of 5.5 months [25]. Another randomized, phase I/II study established the optimal dose of nintedanib with mFOLFOX6 to be 200 mg p.o. b.i.d. and further demonstrated similar efficacy and PFS of mFOLFOX6 with nintedanib compared to mFOLFOX6 with bevacizumab in the first-line setting [26]. Additional single-arm studies have shown promising disease control rates of nintedanib in combination with FOLFOX [27] and FOLFIRI [28] in the first- [27] and second-line [28] settings. The ongoing LUME-Colon1 study [24] (NCT02149108) is an international, double-blind, randomized, phase III study of nintedanib versus placebo plus best supportive care in refractory mCRC patients. The co-primary end points are PFS and OS, and secondary end points are objective tumor response and disease control. Thus far, approximately 200 patients have been enrolled, and initial results are forthcoming in 2016.
The most common adverse events seen in patients receiving nintedanib as either monotherapy or combined with cytotoxic regimens include diarrhea, liver enzyme elevations, nausea/vomiting, and fatigue [22,23]. Notably, unlike other antiangiogenics, nintedanib has not been shown to cause significant hypertension [3].
Famitinib
Famitinib, a structural analog of sunitinib, is another multitargeted antiangiogenic tyrosine kinase inhibitor of VEGFR2, VEGFR3, PDGFR, as well as c-Kit, RET, and Flt3 [29]. Phase I data revealed a recommended dose of 25 mg q.d. and a 41% disease control rate [29]. In a randomized, multicenter, phase II study [30], 154 mCRC patients refractory to second or later line therapy were randomized in a 2 : 1 ratio to receive famitinib or placebo. Preliminary results show the median PFS to be significantly longer in the famitinib group at 2.8 compared to 1.5 months (p = 0.0034; HR = 0.58). Disease control was also superior in the treatment group (57.6% vs. 30.9%; p = 0.0023). The most frequently reported adverse events included neutropenia, thrombocytopenia, hypertension, proteinuria, and hand-foot syndrome, most of which were grade 1–2 severity. Further survival results are forthcoming (NCT01762293), and a phase III study is also underway (NCT02390947).
EGFR/RAS/BRAF/MEK inhibition
EGFR is a transmembrane protein within the ErbB receptor superfamily, which also includes ErbB2 (human epidermal growth factor receptor 2; HER2), ErbB3 (HER3), and ErbB4 (HER4). ErbB dimerization stimulates tyrosine kinase activity and downstream signaling cascades, including BRAF/KRAS/MEK/ERK and mitogen-activated protein kinase (MAPK)/phosphatidylinositol-3-kinase-AKT (PI3K-AKT)/mammalian target of rapamycin (mTOR) pathways, which promote tumor cell survival and proliferation. Cetuximab and panitumumab target the extracellular domain (ECD) of EGFR and block ligand binding and subsequent downstream signaling. These drugs have demonstrated a survival benefit in mCRC patients with RAS-wildtype tumors, as single agents [31,32] and in combination with fluoropyrimidine doublets [33–37].
Downstream of EGFR, BRAF-mediated signaling may contribute to resistance toward anti-EGFR antibodies [38]. Approximately 8–10% of mCRC patients have tumors harboring the BRAF V600E mutation, which leads to a constitutively active protein, resulting in increased MAPK signaling, cancer cell growth, and proliferation. BRAF mutations are more likely to be seen in right-sided primary cancers, are associated with the microsatellite rather than chromosomal instability, and portend a poor prognosis with median OS of 12–18 months [39].
Sym004
Approximately 20% of mCRC develop acquired resistance to cetuximab or panitumumab through ECD EGFR mutations [40]. These mutations primarily prohibit cetuximab binding, though a proportion also inhibits binding of panitumumab to EGFR [40]. Sym004, a new antibody combination targeting two distinct EGFR epitopes, is effective against cancer harboring EGFR ECD mutations [41]. In preclinical studies, this agent demonstrated significant EGFR downregulation [42] and tumor growth suppression relative to approved EGFR antibodies [43]. In cetuximab-refractory cell lines, Sym004 resulted in EGFR degradation, and in cetuximab-refractory xenograft models, Sym004 resulted in suppressed EGFR expression and delayed cancer growth. In a phase I study of mCRC patients whose tumors were resistant to cetuximab or panitumumab, Sym004 demonstrated a 13% response rate [44], albeit with potentially significant toxicities [45]. It is currently being tested in a dose-optimization, phase II study in refractory CRC patients (NCT02083653), as well as in combination with FOLFIRI (NCT02568046).
MM-151
MM-151 is a human monoclonal IgG1 antibody directed toward three distinct EGFR epitopes [46]. Similar to Sym004, MM-151 has demonstrated efficacy against EGFR ECD mutations [47], as it binds multiple regions of the EGFR ectodomain [46], leading to inhibit EGFR and downstream signaling pathways. In cetuximab-treated mCRC patients whose cancers were found to have EGFR ECD mutations, tumor response to MM-151 was achieved with concomitant decrease in circulating mutant EGFR [40]. In a phase I study examining MM-151 as monotherapy or in combination with irinotecan, three mCRC patients achieved PRs, and eight patients had stable disease for more than 4 months on single-agent MM-151 [48]. An additional patient treated with MM-151 plus irinotecan had a PR. The most common AEs of MM-151 include rash, hypomagnesemia, fatigue, and diarrhea (NCT01520389). Much remains to be determined regarding the integration of MM-151 and Sym004 into the treatment algorithm of mCRC patients with RAS-wildtype tumors and the role of monitoring circulating tumor DNA as a means of monitoring therapeutic response and predicting secondary resistance to these agents [49].
Vemurafenib
In contrast to the success seen in melanoma and despite preclinical rationale, the use of vemurafenib monotherapy in mCRC has yielded unexpectedly limited clinical benefit thus far. In an open-label, phase II study [50], 21 patients with previously treated BRAF-mutant mCRC were administered vemurafenib. Among this cohort, the median PFS was 2.1 months, with only one patient achieving a PR and one-third having stable disease. Notably, 56% of patients’ tumors were found to have a low-level frequency (0.21% allele frequency) of KRAS/NRAS mutations, which were associated with therapeutic resistance in patient-derived xenograft (PDX) models. Furthermore, there was no association between EGFR or PTEN expression and microsatellite instability (MSI) or CpG island methylator phenotype (CIMP) status and treatment response. With regards to EGFR expression, only baseline values were measured, which limits the conclusions that can be drawn, as acquired feedback EGFR activation with subsequent MAPK upregulation is a proposed resistance mechanism toward BRAF inhibition [50–52]. In BRAF-mutant PDX mCRC models treated with vemurafenib plus cetuximab, upregulation of the MAPK pathway has been observed and associated with the emergence of KRAS mutations [53]. In these BRAF/KRAS-mutant models, subsequent treatment with the MEK inhibitor, trametinib, along with cetuximab was found to be effective. Likewise, the addition of trametinib was able to restore tumor sensitivity upon resistance to vemurafenib and cetuximab.
In line with these findings, more encouraging clinical results have been demonstrated by combining vemurafenib with EGFR, MET, and MEK inhibition. For example, in a pilot study combining panitumumab with vemurafenib in BRAF-mutant mCRC, 10 of 12 evaluable patients showed durable tumor regression, two achieved a PR, and two maintained stable disease for over 6 months [54]. In a phase I study of irinotecan plus cetuximab and vemurafenib, 35% of mCRC patients with BRAF-mutant tumors achieved a PR, with a median PFS of 7.7 months [55]. Expanding on these findings, the ongoing SWOG 1406 trial is examining the combination of irinotecan plus cetuximab with or without vemurafenib in patients with BRAF-mutant mCRC (NCT02164916). Results are also forthcoming from a pilot study of the cMET inhibitor, onartuzumab, in combination with vemurafenib and/or cobimetinib, another MEK antagonist, in patients with BRAF or KRAS-mutant CRC (NCT01974258).
Dabrafenib plus trametinib
Likewise, studies combining dabrafenib with MEK inhibition have demonstrated clinical benefit in patients with BRAF-mutant mCRC. In a recent study [56], 43 such patients were treated with dabrafenib and trametinib, an MEK inhibitor. This cohort demonstrated a 10% PR rate, with one patient achieving a complete response for more than 36 months and 56% of patients with stable disease. Evaluation of pretreatment and post-treatment biopsies revealed therapy-induced reduction of MAPK activation, as evidenced by decreased phosphorylated ERK levels. Also noteworthy is that treatment response was associated with the presence of PIK3CA mutations. As with prior studies, PTEN loss and microsatellite instability were not associated with outcomes.
The addition of panitumumab to dabrafenib and trametinib has shown more remarkable activity, with acceptable tolerability. In a cohort of 35 BRAF-mutant mCRC patients treated with this triplet regimen, there was a 26% response rate (including one patient who achieved a complete response), with 57% maintaining disease stability [57]. As with the dabrafenib plus trametinib doublet, pretreatment and post-treatment biopsies revealed a reduction in ERK phosphorylation. A phase II study combining panitumumab, dabrafenib, and trametinib is currently recruiting mCRC patients with either BRAF-mutant tumors or acquired resistance to anti-EGFR therapy (NCT01750918).
Selumetinib
Selumetinib (AZD6244), an oral MEK 1/2 inhibitor, has been investigated in patients with KRAS-mutant CRC as a means of overcoming resistance to cetuximab. However, combinations of selumetinib with EGFR and AKT inhibition have shown modest activity, both clinically and at the molecular level. In a phase I study that incorporated a dose expansion cohort in KRAS-mutant CRC [58], a total of 33 patients were treated with weekly cetuximab and daily selumetinib. One CRC patient was found to have an unconfirmed PR in the dose escalation phase, and five of 14 evaluable patients achieved stable disease as the best response in the dose expansion phase. Expectedly, the most common adverse events with this combination were rash, nausea/vomiting, diarrhea, and fatigue. In another phase I study [59], selumetnib was combined with MK-2206, an AKT 1/2/3 inhibitor, in a cohort of 21 patients with both KRAS-wildtype and mutant refractory mCRC. Unfortunately, there were no objective responses seen, and examination of paired tumor biopsies did not demonstrate sufficient inhibition of MEK/ERK or AKT pathways.
In contrast, selumetinib has shown encouraging results in combination with cytotoxic chemotherapy. In a phase II trial by Hochster et al. [60], selumetinib plus irinotecan was administered as second-line treatment in a cohort of 31 patients with KRAS-mutant tumors. Although the study was terminated before full accrual, three patients achieved a PR, and 56% had stable disease, with three of those patients maintaining disease stability for over 1 year.
Furthermore, dual inhibition of the MEK and insulin-like growth factor receptor (IGFR) pathways has been promising, with cell line data demonstrating synergistic efficacy [61]. IGFR signaling intersects both RAS/RAF/MEK and PI3K/AKT/mTOR pathways. Specifically, upon ligand binding, IGFR activation results in RAS-mediated signaling, as well as recruitment of insulin receptor substrate that upregulates the PI3K pathway [62] (Figure 1). In a phase I study of selumetinib and the IGFR inhibitor, cixutumumab, six of 30 patients achieved stable disease for greater than 6 months [63]. Similarly, linsitinib, a dual inhibitor of IGFR and insulin receptors, demonstrated clinical activity in a cohort of 36 refractory CRC patients, of which 47% achieved stable disease [62].
Ongoing studies are examining the efficacy of selumetinib in combination with afatinib (NCT02450656), cyclosporine (NCT02188264), and the PDL1 inhibitor, MEDI4376 (NCT02586987).
PI3K/AKT/mTOR inhibition
Concomitant to signaling downstream of EGFR, the PI3K/AKT/mTOR axis provides another means of resistance to EGFR inhibition in both KRAS-wildtype and mutant CRC. Preclinical studies have yielded data to support inhibition of both of these pathways, though the clinical benefit of this approach remains to be determined.
Buparlisib (BKM120)
Buparlisib is an oral PI3K inhibitor. In a study by Hong et al., buparlisib was found to suppress the proliferation of KRAS-mutant CRC cell lines, independent of PI3K mutation status [64]. However, decreased ERK activity was seen in PI3K-wildtype and not PI3K-mutant cell lines. Furthermore, cetuximab combined with buparlisib was found to result in superior tumor regression in KRAS-mutant xenograft models, when compared to cetuximab alone. In the first-in-man study of buparlisib, in which a significant number of patients had CRC (31 of 83), no tumor responses were seen in the CRC cohort [65]. In a subsequent phase I study, 17 patients with gastrointestinal cancers, including CRC, were treated with BKM120 plus mFOLFOX6. This regimen had significant toxicity, with 76% of patients experiencing treatment-related grade 3/4 adverse events, including neutropenia, fatigue, leukopenia, hyperglycemia, and thrombocytopenia [66].
Alpelisib (BYL719)
As alluded to above, the combination of BRAF and EGFR inhibition has demonstrated synergy in BRAF-mutant CRC. Additional evidence suggests that PI3K inhibition may further enhance such synergy in this subgroup of patients. To this end, an ongoing phase I/II study combining encorafenib (LGX818), a BRAF inhibitor, and cetuximab, with or without alpelisib, an α-specific oral PI3K inhibitor, has shown encouraging results thus far [67]. Among the 26 patients enrolled in the dual treatment arm, 23% had a PR, as did 32% (n = 28) in the triple arm. The median PFS was 3.7 and 4.3 months in the dual and triple arms, respectively [68]. The most frequently observed adverse events were fatigue and vomiting. Enrollment onto the phase II portion is ongoing (NCT01719380).
HER2 inhibition
The reported frequency of HER2/ERBB2 mutations or amplifications in advanced CRC varies within the literature and ranges from 2% to 15% [69], a reflection of an evolving classification system [70]. The prognostic role of HER2 amplification is controversial. HER2/neu derangements have been associated with more advanced stage, distal primary tumor location, KRAS/BRAF-wildtype status, [71] and worse prognosis [72]. Conversely, in an analysis of the FOCUS and PICCOLO trials, HER2 status was not prognostic for PFS or OS [71]. Initial studies examining the use of trastuzumab in mCRC patients with HER2-amplified tumors demonstrated modest activity [73]. Whether HER2-directed drugs provide clinical benefit in mCRC is controversial, though the recent approval of trastuzumab in advanced gastric cancer has renewed interest in defining the role of these agents in mCRC and other gastrointestinal malignancies. In addition to cytotoxics, anti-HER2 therapy may be optimized in combination with monoclonal antibodies directed against EGFR and/or HER3. Data from xenograft models have shown HER2 activation as a resistance mechanism against anti-EGFR therapy in KRAS-wildtype mCRC [74]. More recent preclinical work further supports the requirement of concomitant EGFR and HER3 inhibition, or the use of the irreversible HER2 inhibitor, afatinib, in order to achieve tumor regression [75]. Clinically, the phase II HERACLES trial (EudraCT 2012–002128–33) screened 646 refractory mCRC patients and treated 18 with lapatinib and trastuzumab, demonstrating a 33% response rate (median response duration 8.5 months), with an additional 22% achieving stable disease for over 4 months. The follow-up HERACLES-RESCUE trial is an ongoing endeavor treating those who have progressed on HERACLES with trastuzumab-emtansine (TDM1) (EudraCT 2015–003275–30). Furthermore, there is evidence to suggest that patients with Lynch syndrome or BRAF-wildtype tumors with sporadic microsatellite instability may be especially vulnerable to irreversible HER2 and pan-HER inhibition [69], though this hypothesis has yet to be tested in clinical trials.
MET inhibition
In line with the notion of augmenting EGFR inhibition by blocking collateral pathways in RAS-wildtype tumors, the hepatocyte growth factor (HGF)/MET axis provides another promising therapeutic approach. MET is a tyrosine kinase, whose dysregulation is implicated in cancer development, progression, and metastases formation [76–78].
Rilotumumab
Rilotumumab is an IgG2, fully humanized antibody directed against HGF, the sole ligand for MET, which neutralizes HGF/MET signaling [76]. In a three-part phase Ib/II study, Van Cutsem et al. compared the efficacy of panitumumab with rilotumumab or ganitumumab, an IGFR inhibitor [76]. Prior exposure to an anti-EGFR drug was allowed in the adjuvant setting at least 6 months prior to enrollment. In the phase Ib portion, the MTD of rilotumumab combined with panitumumab was identified as 10 mg/kg. In the subsequent randomized, phase II study, 142 patients with previously treated KRAS-wildtype mCRC were randomized to panitumumab plus either rilotumumab, ganitumumab, or placebo. The panitumumab/rilotumumab regimen demonstrated a superior response rate (31%, 21%, and 22%, respectively), with a 71% disease control rate. Although this combination showed a favorable PFS (5.2, 5.2, and 3.7 months, respectively) and OS (13.8, 10.6, and 11.6 months, respectively), these differences did not meet statistical significance. No predictive markers emerged from this study, though there was a trend toward improved response in patients receiving panitumumab/rilotumumab whose tumors had high cytoplasmic cMET expression. The last part of the study randomizing patients in the placebo arm upon disease progression, to either rilotumumab or ganitumumab monotherapy is ongoing.
Studies are also currently ongoing with INC280, an orally available cMET inhibitor, in combination with cetuximab in cetuximab-refractory mCRC patients (NCT02205398), as well as bevacizumab (NCT02386826). The MErCuRIC1 trial, examining the combination of a cMET inhibitor (PF-03241066) and MEK inhibitor (PD-0325901), is also ongoing (NCT02510001).
ALK/ROS/TRK inhibition
Entrectinib
Gene fusions and re-arrangements within the tropomysin receptor kinase (TRK) family have recently been identified as actionable targets in advanced CRC. The TRK receptor kinases consist of three primary receptor isoforms, TrkA (encoded by NTRK1), TrkB (encoded by NTRK2), and TrkC (encoded by NTRK3) [79]. Autophosphorylation and transphosphorylation of TrkA lead to downstream crosstalk of multiple pathways, including RAS/RAF/MEK and PI3K/AKT signaling [80]. In particular, the TPM3-TRK1 re-arrangement has been repeatedly isolated in CRC and renders tumors sensitive to TRK inhibition [81]. More recently, a new LMNA-NTRK1 fusion has been identified, which also proved susceptible to TRK blockade [82]. Entrectinib is a pan-TRK, ALK, and ROS inhibitor with demonstrated activity in mCRC patients harboring either TPM3-TRK1 or LMNA-NTRK1 fusions as well as ALK rearrangements [83,84]. Two ongoing studies, STARTRK-1 and STARTRK-2, are testing entrectinib in patients with ALK, ROS, and NTRK alterations (NCT02097810, NCT02568267). Importantly, further translational studies are needed to identify modes of resistance to these agents. In a recent report by Russo et al., a unique TRKA mutation was identified as an acquired resistance mechanism toward entrectinib [85]. In addition to revealing a clinically relevant genetic variant, this study illustrates the value of real-time noninvasive monitoring (e.g. circulating tumor DNA) and patient xenograft models (avatars) in the elucidation of treatment resistance and potential to inform the development of next-generation targeted drugs.
Immune-based therapies
Given its association with inflammatory bowel disease and the intestinal microbiome, CRC would appear especially vulnerable to immunologics. Indeed, histologic evidence of peritumoral immune infiltrates has been associated with improved survival in CRC patients [86], especially in those with microsatellite unstable cancers [87]. Effector T cells, antigen-presenting dendritic cells, and myeloid-derived suppressor cells orchestrate the antitumor immune response and help form the basis for immune-targeted therapy. Equally important are the immunogenetic profile of the patient and antigenicity specific to a particular tumor. The presence of a functional host immune system and increased tumor antigen load correlate with therapeutic response, offering further rationale for immunotherapy in CRC. Inhibitors blocking the PD1 checkpoint have shown the most promise to date, in particular subsets of CRC patients [88].
Immune checkpoint inhibition
The role of immune checkpoint inhibition in the management of CRC is actively being defined. CTLA4 and PD1 are the primary checkpoints that tumors exploit to suppress the immune system. PD1 is an inhibitory co-receptor expressed on the surface of T-cells NK cells, B-cells, and monocytes and is bound by either the PDL1 or PDL2 ligands. Tumors upregulate PDL1 expression, thereby evading the antitumor immune response, and PDL1 also directly inhibits T-cell function and tumor cell apoptosis, independent of its binding to PD1 [89].
In a phase II study of the CTLA-4 blocking agent, tremelimumab, only 1 of 45 patients had a confirmed PR [90]. Likewise, initial studies with anti-PDL1 [91] and anti-PD1 [92] therapy have yielded limited to no activity in unselected refractory CRC patients. In contrast, anti-PD1 therapy has shown encouraging results in patients with microsatellite unstable tumors. In a phase II trial of pembrolizumab monotherapy, refractory metastatic CRC patients with mismatch repair-deficient tumors achieved a superior immune-related overall response rate (40% vs. 0%), 20-week PFS (70% vs. 11%), and OS (not reached vs. 5 months) relative to those with mismatch repair-proficient cancers [88]. These findings underscore the importance of tumor mutational load on the efficacy of PD1-directed therapy and provide rationale for the ongoing KEYNOTE-164 trial, which is limited to CRC patients with MSI-high cancers (NCT02460198). In addition to mutational load, the importance of intratumoral PDL1 expression as a predictive marker has been demonstrated in other cancers [93] and is expected to be an important part of patient selection for checkpoint inhibition in CRC as well. Urelumab, currently in phase I development (NCT02110082), is a monoclonal agonistic antibody toward CD137, which is a co-stimulatory molecule expressed on activated NK and memory T cells [94].
Ensituximab
Ensituximab (NPC-1C), a chimeric antibody that promotes ADCC, is directed against the MUC5AC-related antigen that mediates mucosal immunity. Initial results of a phase I/II trial have been encouraging, with a median OS of 10.2 months in CRC patients who have progressed on at least two lines of therapy (NCT01040000) [95].
MGD007
In addition to monoclonal antibodies, another promising immune-targeting strategy is the dual-affinity re-targeting protein. These constructs engage immune cells with cancer cells using their bispecific binding properties. One such drug, MGD007, re-directs CD3-expressing T cells to cancer cells bearing the glycoprotein A33 antigen, which is almost uniformly present in CRC [96]. A phase I study of MGD007 in refractory metastatic CRC is underway (NCT02248805).
Synergistic immunotherapeutic strategies
Pairing different immunologics with chemotherapy, antibodies, and epigenetic agents can further enhance their therapeutic efficacy in several ways. For example, in preclinical models, the exposure of CRC cell lines to urelumab provided synergy with cetuximab to enhance CD137-expressing NK cell activity and increase the proportion of EGFR-specific cytotoxic T cells [94]. Another promising cetuximab-based regimen uses Imprime PGG®, a yeast-derived beta glucan that binds anti-beta-glucan antibodies to trigger both the complement pathway and adaptive immune responses [97,98]. Imprime PGG® is currently being tested in a phase III study with or without cetuximab in patients with RAS-wildtype tumors (NCT01309126). Preclinical evidence also suggests enhanced efficacy of bevacizumab with Imprime PGG® [99].
Evidence from myelodysplastic syndromes reveals that hypomethylating agents augment PDL1, PDL2, PD1, and CTLA4 expression [100], thereby sensitizing cancer cells to immune checkpoint inhibition. This effect may be especially relevant in patients with MSI-high or CIMP-positive tumors. Furthermore, as response to platinum agents is affected by PDL1 expression [101,102], and hypomethylators have been shown to reverse platinum resistance [103,104], the potential for combining these agents with immune checkpoint inhibition certainly merits further investigation. To this end, a phase I/II study combining romidepsin and/or oral azacitidine with pembrolizumab is ongoing (NCT02512172), and results from a study evaluating azacitidine with capecitabine and oxaliplatin (CapeOx) are forthcoming (NCT01193517).
Lastly, dual checkpoint inhibition has been recently approved in other malignancies [105], and a phase I/II study of ipilimumab and nivolumab in CRC (CheckMate-142) is underway (NCT02060188), with other potential combinations on the horizon [106].
Adoptive T-cell therapy
The progress of autologous T-cell infusion in CRC patients has been hampered by short-lived clinical improvement and excessive autoimmune toxicity in those who respond. In one instance, a patient received T-cells containing a chimeric antigen receptor toward HER2. Within minutes of infusion, the patient suffered from a cytokine release syndrome with resulting respiratory failure and death 5 days later [107]. In a subsequent series, three metastatic CRC patients received T-cells harboring a receptor against carcinoembryonic antigen (CEA) [108]. While one patient had an objective response and all patients demonstrated significant CEA suppression, dose-limiting colitis was also a uniform toxicity. The discord between serum CEA and radiographic responses, as well as the marked toxicities associated with therapy, remains considerable challenges. Using antigens that are specific to an individual tumor and accounting for potential immunosuppressive properties of the tumor microenvironment will be necessary to optimize this type of therapy.
Several studies employing adoptive T-cell strategies are ongoing (NCT01174121), including those that target CEA systemically (NCT02349724) and by hepatic artery infusion (NCT02416466), as well as EGFR (NCT01869166), VEGFR2 (NCT01218867), and MAGEA4 and survivin (NCT02239861). Another trial is coupling bortezomib in an attempt to enhance NK cell cytotoxicity (NCT00720785).
Oncolytic vaccines
Vaccines were among the first form of immune therapy to be explored in CRC [109–111]. Formulations differ with respect to vector (e.g. bacterial/viral, peptide, autologous tumor cell, dendritic cells, etc.), genetic modifications, adjuvants, and antigen specificity. Regardless of preparation, a key limitation of vaccine therapy in CRC has been striking a balance between achieving tumor specificity with inducing a clinically relevant and durable antitumor response, which largely hinges on identifying the appropriate antigen.
With regards to dendritic cell vaccinations, several potential tumor-derived antigens have been identified, including CEA (NCT01890213), MUC1 (NCT00103142), and NY-ESO-1 (NCT01697527). In addition to dendritic cell preparations specific to certain antigens, vaccines targeting pathways such as RAS or HER2 deranged tumors are showing promise. For example, a phase II trial of Reolysin, a virus that replicates in RAS-activated cancer cells, is ongoing in mCRC patients (NCT01622543). A phase I study is currently examining a combination vaccine of two chimeric HER2 B-cell epitopes in patients with HER2-overexpressing solid tumors (NCT01376505).
Cytokine-based therapy and JAK/STAT inhibition
Xilonix
Developed by XBiotech, Xilonix™ is a recombinant IgG1 monoclonal antibody (MABp1) targeting interleukin-1α (IL-1α). IL-1α is constitutively expressed on the surface of platelets and monocytes, and cancer cells, and plays a pivotal role in tumor growth, angiogenesis, metastasis, as well as cancer cachexia, which is an established negative prognostic factor [112–114]. Furthermore, IL-1α is released upon tissue injury and necrotic cell death, which leads to release of other ILs, VEGF, macrophages, and neutrophils, as a means of facilitating physiologic wound healing [115]. However, tumors exploit this response to promote malignant vascular development and block the antitumor immune response through myeloid-derived suppressor cells [116]. Neutralizing IL-1α activity is proposed to not only reverse metabolic dysregulation associated with tumor progression and increase lean body mass, but also improve survival in mCRC patients. Results from a phase I study in non-small cell lung cancer also suggest potential synergy with anti-EGFR-directed therapy [116]. In the ongoing, phase III XCITE trial, refractory mCRC patients are being randomized to Xilonix versus placebo (NCT01767857), with a primary end point of OS and secondary outcomes to include change in lean body mass, PFS, response rate, and quality of life.
Ruxolitinib, INCB039110
An important mechanism of antiangiogenic and chemotherapeutic resistance [117] is the JAK-STAT signaling cascade, which has been implicated in autoimmune disease and inflammation-driven solid tumorigenesis [118]. JAK1 is one among four cytoplasmic receptor tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) within the JAK family, which forms heterodimers with JAK2 or TYK2 and JAK3 to mediate inflammatory responses through interferon and IL-2 signaling, respectively. Preclinical data support JAK1 inhibition as a therapeutic target in CRC. In cell line studies, JAK1/TYK2 has been shown to promote CRC cell growth, migration, invasion, and drug resistance [119,120]. In vitro and in vivo data demonstrate that JAK1/JAK2 inhibition reduces STAT3 activation, resulting in reduced intestinal tumor cell proliferation and increased apoptosis, as well as tumor growth suppression in a colitis-associated colon cancer mouse model [121]. Furthermore, JAK1/2 inhibition has been shown to overcome MEK resistance in KRAS-mutant colorectal tumor models [122]. Notably, caspase-mediated CRC cell death has been shown to be more heavily reliant on JAK1 as compared to JAK2 inhibition [123]. Ruxolitinib, a JAK1/JAK2 inhibitor, approved for the treatment of myelofibrosis and polycythemia vera, is currently being combined with regorafenib in refractory mCRC, though accrual is currently on hold (NCT02119676). INCB039110, an oral selective JAK1 inhibitor, with >20- and 200-fold selective inhibition over JAK2 and JAK3, is being combined with pembrolizumab in MSI-high mCRC patients (NCT02646748).
Stem cell inhibiton
BBI-608
Among the many downstream effects of JAK/STAT signaling are activation and maintenance of stem cell genes. BBI-608 is an oral, first-in-class inhibitor of STAT3, β-catenin, and Nanog-induced transcription of cancer stem cell genes [124]. An ongoing phase III, randomized, double-blind study is examining the efficacy of BBI-608 versus placebo plus best supportive care in refractory CRC (NCT01830621). In addition, encouraging results have been presented from combination studies. In a phase Ib study, BBI-608 was added to FOLFIRI with or without bevacizumab in 18 mCRC patients with at least three prior therapies (including FOLFIRI), of which 94% had stable disease (including two patients with a PR; NCT02024607) [125]. BBI-608 has also shown acceptable toxicity and clinical benefit when combined with panitumumab [126]. In a phase Ib/II study, initial results from 24 mCRC patients revealed a disease control rate of 44%in anti-EGFR naïve patients and 53% of patients who had progressed on cetuximab, suggesting a potential role for resensitization to anti-EGFR agents (NCT01776307).
PRI-724
The canonical Wnt pathway plays a critical role in colon stem cell biology and CRC development. APC and Wnt/β-catenin activation are essential steps in both familial and sporadic colorectal tumor initiation [127–129]. Upon activation, the Wnt cascade promotes transcription of several genes that mediate cell cycle progression and facilitate stem cell proliferation and differentiation during embryogenesis, organ homeostasis, and tumorigenesis [130]. β-catenin, the main regulator of the Wnt pathway, is normally suppressed by a complex comprised of APC, CK1, GSK3β, and Axin2 [130]. Importantly, transcription activation is dependent on β-catenin binding to CBP, which stimulates stem cell maintenance and proliferation [130,131]. Left unchecked, β-catenin leads to constitutive Wnt pathway activation and allows cancer stem cells to proliferate and differentiate to form primary tumors and metastases [132]. Downstream of Wnt, activated KRAS phosphorylates β-catenin to increase its nuclear concentration and the TCF/β-catenin-mediated transcription of Wnt target genes, an effect that is amplified by KRAS mutations [133].
PRI-724 is a new small-molecule inhibitor that specifically binds to the N-terminus of CBP, thereby disrupting the interaction between CBP and β-catenin and suppressing survivin expression [131,133] in vitro and in vivo [131,135]. PRI-724 increases p300/β-catenin binding to promote stem cell differentiation and increase sensitivity to cytotoxic or targeted drugs but also prevents transcription of S100A4, which is involved in CRC metastasis [136–138]. PRI-724 is currently in phase Ib development, and preliminary results from the first-in-human study demonstrated an acceptable toxicity profile [139].
Wnt974 (LKG974)
Stimulation of the Wnt pathway relies upon intact porcupine activity [140]. Porcupine (PORCN) is a membrane-bound O-acyltransferase that enables the palmitoylation, a necessary post-translation modification, of Wnt ligands [141]. In the absence of PORCN activity, Wnt ligand secretion is suppressed. Recent preclinical evidence suggests that resistance of BRAF-mutant CRC to BRAF inhibition may be partly due to Wnt activation and dependency [142]. Specifically, BRAF-mutant tumors are often found to harbor mutations within RNF43, which codes for an ubiquitin ligase that degrades the Wnt receptors, Frizzled and LRP6 [142]. RNF43 inactivating mutations therefore lead to Wnt upregulation. The cooperation between these BRAF and Wnt derangements, along with EGFR signaling, has prompted efforts to simultaneously block these pathways in BRAF-mutant CRC. The oral agent, WNT974, binds and inhibits PORCN within the endoplasmic reticulum of cancer cells, thereby blocking Wnt signaling. A phase I/II study of WNT974, combined with cetuximab and encorafenib (LGX818) in BRAF-mutant mCRC patients whose tumors harbor RNF43 mutations, is in progress (NCT02278133).
Bromodomain inhibition
GSK525762
Regulation of epigenetic modifications (acetylation, methylation, etc.) is increasingly becoming recognized as a therapeutic target in gastrointestinal cancers. Among these modifications, bromodomains (BRD) are evolutionary conserved amino acid domains that identify and bind acetyl lysine residues, leading to transcriptional regulation of oncogenes [143]. Proteins containing BRDs and an extraterminal domain belong to the BET family and include BRD2, BRD3, BRD4, and BRDT. BRD4, in particular, has been shown to be essential to CRC development [144]. BET inhibitors are currently in development and have shown activity in preclinical advanced CRC models, particularly in tumors with CIMP [144]. A phase I/II study evaluating the GSK525762 in patients with advanced CRC is ongoing (NCT01587703).
IDO1 inhibition
INCB24360
IDO1 is an enzyme released by tumor and myeloid suppressor cells, which catalyzes the rate-limiting step of tryptophan catabolism within the kynurenine pathway [145] and leads to tryptophan depletion within T-cells, thereby attenuating their activity [146]. IDO1 also directly activates Wnt-β-catenin signaling to promote colitis-associated tumor development and progression by decreasing the tryptophan/kynurenine ratio [147]. In preclinical models, IDO1 inhibition has been shown to suppress expression of proinflammatory cytokines and decrease the number of suppressor T cells within the tumor microenvironment [148]. A phase I/II study investigating the IDO1 inhibitor, INCB24360, plus nivolumab in CRC is currently recruiting patients (NCT02327078).
New combinations of approved agents
First-line triplet chemotherapy: FOLFOXIRI plus bevacizumab
Selection of first-line therapy is among the most important decisions in mCRC management. The choice of first-line therapy ultimately dictates the sequence of drugs and number of therapeutic lines administered (which may be limited in certain patients) and influences sensitivity to subsequent therapies. Moreover, independent of treatment choice and other traditional prognostic factors, it is evident that inducing early and deep tumor shrinkage leads to improved PFS and OS [149,150], partly as it pertains to permitting curative resection in patients with oligometastatic (e.g. liver-limited) disease. To this end, optimizing first-line therapy is of paramount clinical relevance.
The Triplet plus Bevacizumab (TRIBE) study [151] was a randomized, open-label, multicenter, phase III trial, comparing bevacizumab with either FOLFOXIRI or FOLFIRI as initial therapy for mCRC patients. Patients treated with FOLFOXIRI-bevacizumab achieved a superior response rate (65% vs. 53%; p = 0.006) and PFS (12.1 vs. 9.7 months; HR 0.75; p = 0.003) and had a favorable trend for OS (31.0 vs. 25.8 months; HR 0.79; p = 0.054). The ongoing STEAM study is further examining the superior sequence of triplet versus dual cytotoxic chemotherapy paired with bevacizumab, with the primary objectives being first PFS and response rate and secondary objectives including rate of hepatic metastatectomy and conversion from unresectable to resectable disease (NCT01765582).
First-line triplet chemotherapy: FOLFOXIRI plus anti-EGFR antibodies
Though not yet standard of care, the use of triplet cytotoxic plus anti-EGFR therapy has shown encouraging results thus far in RAS-wildtype mCRC patients. In a phase II study of FOLFOXIRI with cetuximab, Sardiaki et al. reported a response rate of 70%, PFS of 10.2 months, OS of 30.0 months, and R0 resection rate of 62% in patients with liver-limited metastases [4]. In a phase II study of FOLFOXIRI with panitumumab, there was an 89% response rate, with PFS of 11.3 months, and 35% of all patients underwent R0 resection [152]. Increased toxicities, notably diarrhea and neutropenia, continue to be concerns. Various dosing schedules have been used across trials, and it is likely that omitting the bolus administration of 5-FU and using a modified irinotecan dose (i.e. 150 mg/m2) can achieve a favorable therapeutic index.
Metronomic maintenance therapy
The daily continuous administration of lower doses of chemotherapy, also referred to as metronomic dosing, aims to strike a balance between constant treatment pressure and reduced toxicity [153]. Metronomic therapy has been evaluated and used for almost two decades, but recent evidence supports its use in mCRC patients who attain stable or responding disease on first-line chemotherapy along with bevacizumab. In the multicenter, phase III CAIRO3 study [154], 558 mCRC patients were randomly assigned to observation versus maintenance therapy with capecitabine (administered at a continuous twice-daily dose of 625 mg/m2) and bevacizumab after achieving stable disease or better upon completion of six cycles of capecitabine, oxaliplatin, and bevacizumab (CAPOX-B). On first progression, patients in both groups were treated with CAPOX-B until second progression (PFS2). Patients receiving maintenance therapy had a significantly improved PFS2 (the primary end point) compared to those who underwent initial observation (11.7 vs. 8.5 months; HR 0.67; p < 0.0001). This difference remained significant when any treatment after first progression free survival (PFS1) was considered. In addition, patients receiving maintenance therapy had a longer PFS1 (8.5 vs. 4.1 months; HR 0.39; p < 0.001) and time to second progression (TTP2; 13.9 vs. 11.1 months; HR 0.68; p < 0.001) relative to those on observation. Although not powered to demonstrate an OS benefit, there was a 3.5-month OS difference favoring patients receiving maintenance therapy.
Primary tumor site directed therapy
The location of a colon tumor influences its molecular makeup [5,155] and clinical behavior [156–159]. The right side of the colon (cecum, ascending colon, and proximal two-thirds of the transverse colon) is derived from the midgut, whereas the left side (distal third of the transverse colon, descending colon, sigmoid colon, and rectum) originates from the hindgut [160]. The left and right sides of the colon also have distinct vascular supplies [160], metabolic pathways, and bacterial microflora [161]. Left-sided tumors often present with wildtype BRAF (BRAF-wt), KRAS point mutations (codons 12, 13, and 61; KRAS-mut), extensive copy number alterations and chromosomal instability, loss of heterozygosity, and TP53 alterations, as well as activated HER1/2 pathways. In contrast, right-sided tumors are enriched for BRAF V600E point mutation (BRAF-mut), wildtype KRAS (KRAS-wt) diploid copy number, MSI, DNA hypermutation, and extensive DNA hypermethylation associated with CIMP. Primary tumor site is increasingly becoming recognized as a prognostic factor in mCRC [158], and its predictive value for different therapeutics is currently being investigated.
For instance, correlative analyses from pivotal phase III trials support the notion that patients with left-sided, but much fewer right-sided, RAS-wt cancers derive benefit from EGFR inhibition [162]. The NCIC CTG CO.17 trial randomized refractory mCRC patients to best supportive care or cetuximab, and those with KRAS-wt, left-sided tumors treated with cetuximab had improved PFS, whereas those with right-sided tumors showed no PFS difference [163]. In addition, similar effects have been seen in the CRYSTAL [164] and FIRE-3 [33] trials. In FIRE-3, patients with left-sided tumors treated with FOLFIRI plus cetuximab showed longer OS than those receiving FOLFIRI plus bevacizumab (38 vs. 18 months) [165]. These consistent findings across multiple patient cohorts provide substantial evidence that mCRC patients with right-sided tumors derive less benefit from EGFR inhibition. In addition, other studies suggest that bevacizumab appears less beneficial for patients with right-sided tumors compared to those with left-sided tumors [33,158,166]. Altogether, prospective validation is warranted before the use of primary tumor site becomes integrated into clinical decision-making as a standard of care.
Pharmacogenomic profiling
Interpatient differences, clonal diversity, stromal heterogeneity, and treatment-imposed selective pressures all contribute to tumor complexity and limit the clinical impact of new targeted therapeutics. As a result, the influx of targeted agents into research trials and clinical practice has been associated with modest clinical and survival benefits overall. Moreover, an actionable alteration identified in preclinical studies may only be present in a small subset of patients, making large-scale clinical testing in single tumor types more cumbersome and less feasible. Appreciating new connections between signaling pathways and defining more global tumor phenotypes, such as the consensus molecular subtypes [167], will inform both drug development strategies and tailored patient selection. In parallel with comprehensive tumor characterization, integration of patient profiling is necessary to advance precision oncology. Accumulating evidence suggests that pharmacogenomics [168] has the potential to identify drug susceptibility to certain tumor types, determine prognosis upon initial diagnosis, and predict response and toxicity to individual therapeutics. Moreover, preclinical, translational, and cost–effectiveness data support the practice of pre-emptive rather than reactive pharmacogenomic-guided drug dosing and selection.
On that note, we conclude this review where it began – with fluoropyrimidines, for which the preponderance of evidence exists in using pharmacogenetic testing to guide treatment decisions, particularly for the dihydropyrimidine dehydrogenase (DPYD) gene. In a recent study, Deenen et al. examined the cost and efficacy of testing for the DPYD*2A variant, which is associated with severe and life-threatening toxicity [169]. Over 2000 patients were screened, of whom 1.1% carried a DPYD*2A variant allele and accordingly received an initial ≥50% dose reduction. Using genotype-guided dosing, grade ≥3 toxicities (28% vs. 73%; p < 0.001) and treatment-related deaths (0% vs. 10%) were reduced in DPYD*2A variant patients as compared to historical controls. The incidence of grade ≥3 toxicities was similar between variant carriers and wildtype patients receiving standard dose therapy. Moreover, the cost of care was less in those receiving genotyping, relative to those who did not undergo screening. Additional evidence suggests predictive and/or prognostic roles for polymorphisms within the methylenetetrahydrofolate reductase (MTHFR) [170], TS [171], and cytidine deaminase (CDA) [172] genes in patients undergoing fluoropyrimidine therapy.
In addition, genetic variants implicated in cancer cell growth, EMT activation, DNA repair (e.g. ERCC1), angiogenesis, inflammatory pathways, and stem cell homeostasis have been shown to affect PFS and OS in mCRC patients, with evidence for gender-based and tumor-location-specific differences [173–177]. For example, polymorphisms within the EGF, VEGF, VEGFR, CD133, and CXCR genes have been associated with response and survival in mCRC being treated with bevacizumab-based regimens [178,179]. Circulating plasma levels of VEGF-A and VEGFR isoforms as well as neuropilin have also shown potential as predictive markers [180]. EGFR and FCGR [173,181,182] genetic variants have also demonstrated prognostic [182] and predictive value in mCRC patients treated with cetuximab [173,182]. To date, these markers have yet to be validated and incorporated into common practice. It is expected that pharmacogenetic haplotyping (rather than a single marker), in conjunction with tumor-specific profiling, will best inform the individualized management of mCRC patients.
Conclusions
Impressive strides have been made in identifying new regimens, employing maintenance strategies to limit treatment toxicities, and combining multidisciplinary approaches to achieve cure in oligometastatic disease, all of which have improved outcomes for mCRC patients. However, significant progress remains to be gained in translating molecular and pharmacogenomic insights into personalized therapy. While several new biologics have been integrated into the treatment schema of advanced CRC, the optimal combination and sequence of therapies (EGFR vs. VEGF, regorafenib vs. TAS-102, etc.) continue to evolve. A more comprehensive understanding of tumor biology, utilization of noninvasive predictive biomarkers (e.g. circulating tumor cells, liquid biopsies, etc.), and pharmacogenetic testing will hasten the therapeutic advances destined for this disease.
Expert commentary
Several new agents have been recently approved for the treatment of mCRC, though the optimal sequence and combination of cytotoxic and biologic agents continue to be redefined. For instance, continuing antiangiogenic therapy in the maintenance and second-line settings improves survival, though the best such agent, chemotherapy backbone and ordering with other antiangiogenics and anti-EGFR agents, warrants further investigation. Agents such as regorafenib and TAS-102 are both options for patients with refractory mCRC, though no head-to-head comparison exists to suggest superiority of one drug over another, and their distinct adverse event profiles should guide clinical decision-making. Identifying new predictive biomarkers and molecular phenotypes comprised of multiple rather than single molecular alterations will be essential to optimizing individualized CRC treatment and clinical outcomes.
Five-year view
Over the next 5 years, we expect the integration of new predictive markers, such as intratumoral gene expression, and circulating tumor cell count and genetic characterization, into the management of advanced CRC. With regards to new agents, we anticipate that checkpoint inhibitors will be approved for the treatment of microsatellite unstable CRC. Additional evidence regarding the role of pharmacogenetic profiling in individualizing drug selection and dosing is forthcoming and may influence patient management.
Key issues.
TAS-102 (Lonsurf™) is the most recent pyrimidine modulator to be approved for refractory mCRC, though optimal sequencing with regorafenib remains to be determined and is currently best guided by selection based on adverse effects.
Ramucirumab (Cyramza™) is the latest anti-angiogenic to achieve regulatory approval in combination with irinotecan-based second-line chemotherapy of mCRC.
Sym004 and MM-151, novel antibody combinations which target distinct EGFR epitopes, have demonstrated activity in mCRC patients whose tumors are resistant to cetuximab or panitumumab.
In BRAF mutant tumors, BRAF directed monotherapy has not demonstrated significant efficacy, though combination regimens with MEK, cMET and/or EGFR inhibitors are more efficacious and under active investigation.
Similarly, HER2 directed monotherapy has not yielded clinical benefit, though combinations using trastuzumab and lapatinib, as well as trastuzumab-emtansine, appear more encouraging in ongoing studies.
Immune based drugs, notably PD1/PDL1 inhibitors, are effective in mCRC patients with microsatellite unstable tumors and are being assessed in combination strategies.
Promising targets with drugs in early development include ALK/ROS, cMET, IDO1, stem cell, and bromodomain inhibition.
Triplet combination cytotoxic therapy with FOLFOXIRI and bevacizumab may enable patients with borderline resectable oligometastatic CRC to achieve a curative resection.
Metronomic maintenance therapy with capecitabine and bevacizumab has been shown to prolong survival in mCRC patients who have stable or responding disease to first-line oxaliplatin-based regimens.
Independent of molecular profiling, primary tumor location may inform treatment response to approved agents (e.g. cetuximab) and is increasingly being incorporated as a stratification factor in clinical trials.
Acknowledgments
Financial & competing interests disclosure
The authors were supported by University of Southern California. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest.
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325.• Pivotal phase III trial leading to approval of TAS-102.
- 3.Capdevila J, Carrato A, Tabernero J, et al. What could nintedanib (BIBF 1120), a triple inhibitor of VEGFR, PDGFR, and FGFR, add to the current treatment options for patients with metastatic colorectal cancer? Crit Rev Oncol Hematol. 2014. doi: 10.1016/j.critre-vonc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Saridaki Z, Androulakis N, Vardakis N, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer. 2012;107(12):1932–1937. doi: 10.1038/bjc.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487 (7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duschinsky RPE, Heidelberger C. The synthesis of 5-fluoropyrimidines. J Am Chem Soc. 1957;79(16):4559–4560. [Google Scholar]
- 7.Heidelberger C, Chaudhuri NK, Danneberg P, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179(4561):663–666. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PM, Danenberg PV, Johnston PG, et al. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11(5):282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 9.Saito K, Nagashima H, Noguchi K, et al. First-in-human, phase I dose-escalation study of single and multiple doses of a first-in-class enhancer of fluoropyrimidines, a dUTPase inhibitor (TAS-114) in healthy male volunteers. Cancer Chemother Pharmacol. 2014;73 (3):577–583. doi: 10.1007/s00280-014-2383-2. [DOI] [PubMed] [Google Scholar]
- 10.Nukatsuka M, Nakagawa F, Takechi T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with oxaliplatin on human colorectal and gastric cancer xenografts. Anticancer Res. 2015;35(9):4605–4615. [PubMed] [Google Scholar]
- 11.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(16):3706–3712. Epub 2005/05/04. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. Epub 2004/06/04. doi: 10.1056/NEJMoa032691350/23/2335[pii]. [DOI] [PubMed] [Google Scholar]
- 13.Arnold DAT, Bennouna J, et al. Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized phase III intergroup study (TML study). J Clin Oncol. 2012;30(suppl):abstr CRA3503. [Google Scholar]
- 14.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clinl Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 15.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99 (17):11393–11398. Epub 2002/08/15. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0.• Pivotal phase III trial leading to approval of ramucirumab.
- 17.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 19.Kutluk Cenik B, Ostapoff KT, Gerber DE, et al. BIBF 1120 (nintedanib), a triple angiokinase inhibitor, induces hypoxia but not EMT and blocks progression of preclinical models of lung and pancreatic cancer. Mol Cancer Ther. 2013;12(6):992–1001. doi: 10.1158/1535-7163.MCT-12-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Bois A, Huober J, Stopfer P, et al. A phase I open-label dose-escalation study of oral BIBF 1120 combined with standard paclitaxel and carboplatin in patients with advanced gynecological malignancies. Ann Oncol. 2010;21(2):370–375. doi: 10.1093/annonc/mdp506. [DOI] [PubMed] [Google Scholar]
- 21.Ellis PM, Kaiser R, Zhao Y, et al. Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients. Clin Cancer Res. 2010;16(10):2881–2889. doi: 10.1158/1078-0432.CCR-09-2944. [DOI] [PubMed] [Google Scholar]
- 22.Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res. 2010;16(1):311–319. doi: 10.1158/1078-0432.CCR-09-0694. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto I, Kaneda H, Satoh T, et al. Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors. Mol Cancer Ther. 2010;9(10):2825–2833. doi: 10.1158/1535-7163.MCT-10-0379. [DOI] [PubMed] [Google Scholar]
- 24.Lenz HJTJ, Yoshino T, Oum’Hamed Z, et al. LUME-Colon 1: a double-blind, randomized phase III study of nintedanib plus best supportive care (BSC) versus placebo plus BSC in patients with colorectal cancer (CRC) refractory to standard therapies. J Clin Oncol. 2015;33(suppl):abstr TPS3625. [DOI] [PubMed] [Google Scholar]
- 25.Bouche O, Maindrault-Goebel F, Ducreux M, et al. Phase II trial of weekly alternating sequential BIBF 1120 and afatinib for advanced colorectal cancer. Anticancer Res. 2011;31(6):2271–2281. [PubMed] [Google Scholar]
- 26.Van Cutsem EA. Phase l/lI, open-label, randomised study of BIBF 1120 plus mFOLFOX6 compared to bevacizumab plus mFOLFOX6 in patients with metastatic colorectal cancer. Eur J Cancer. 2011;47 (Supplement 2):8–9.21095116 [Google Scholar]
- 27.Garcia-Carbonero R, Rivera F, Maurel J, et al. An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist. 2014;19(4):350–351. doi: 10.1634/theoncologist.2014-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshino T, Yamazaki K, Gotoh M, et al. Safety and pharmacokinetics of second-line ramucirumab plus FOLFIRI in Japanese patients with metastatic colorectal carcinoma. Anticancer Res. 2015;35(7):4003–4007. [PubMed] [Google Scholar]
- 29.Zhou A, Zhang W, Chang C, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol. 2013;72(5):1043–1053. doi: 10.1007/s00280-013-2282-y. [DOI] [PubMed] [Google Scholar]
- 30.Xu RSL, Wang K, Wu G, et al. A randomized, double-blind, parallelgroup, placebo-controlled, multicenter, phase II clinical study of famitinib in the treatment of advanced metastatic colorectal cancer. J Clin Oncol. 2015;33(suppl 3):abstr 513. [Google Scholar]
- 31.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 32.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15 (6):569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 33.Bokemeyer C, Bondarenko I, Makhson A, et al. Cetuximab plus 5-FU/FA/oxaliplatin (FOLFOX-4) versus FOLFOX-4 in the first-line treatment of metastatic colorectal cancer (mCRC): OPUS, a randomized phase II study. J Clin Oncol. 2007;25(suppl; abstr 4035):172s. [Google Scholar]
- 34.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 35.Heinemann V, Von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clinl Oncol. 2011;29 (15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 37.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clinl Oncol. 2010;28 (31):4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 38.Siena S, Sartore-Bianchi A, Di Nicolantonio F, et al. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101(19):1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clinl Oncol. 2008;26(35):5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 40.Arena S, Bellosillo B, Siravegna G, et al. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin Cancer Res. 2015;21(9):2157–2166. doi: 10.1158/1078-0432.CCR-14-2821.• Elegant account highlighting the emergence of resistance to cetuximab in CRC cell lines and patient-derived tumor tissue.
- 41.Sanchez-Martin FJ, Bellosillo B, Gelabert M, et al. The first-in-class anti-EGFR antibody mixture Sym004 overcomes cetuximab-resistance mediated by EGFR extracellular domain mutations in colorectal cancer. Clin Cancer Res. 2016. doi: 10.1158/1078-0432.CCR-15-2400.• Preclinical and clinical study demonstrating the efficacy of Sym004 in cetuximab-refractory CRC.
- 42.Skartved NJ, Jacobsen HJ, Pedersen MW, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res. 2011;17(18):5962–5972. doi: 10.1158/1078-0432.CCR-11-1209. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70(2):588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 44.Dienstmann R, Patnaik A, Garcia-Carbonero R, et al. Safety and activity of the first-in-class Sym004 anti-EGFR antibody mixture in patients with refractory colorectal cancer. Cancer Discov. 2015;5 (6):598–609. doi: 10.1158/2159-8290.CD-14-1432. [DOI] [PubMed] [Google Scholar]
- 45.Stintzing S, Heinemann V. Sym004: truly a new level of anti-EGFR treatment? Cancer Discov. 2015;5(6):578–580. doi: 10.1158/2159-8290.CD-15-0441. [DOI] [PubMed] [Google Scholar]
- 46.Kearns JD, Bukhalid R, Sevecka M, et al. Enhanced targeting of the EGFR network with MM-151, an oligoclonal anti-EGFR antibody therapeutic. Mol Cancer Ther. 2015;14(7):1625–1636. doi: 10.1158/1535-7163.MCT-14-0772. [DOI] [PubMed] [Google Scholar]
- 47.Arena S, Siravegna G, Mussolin B, et al. MM-151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med. 2016;8(324):324ra14. doi: 10.1126/scitranslmed.aad5640. [DOI] [PubMed] [Google Scholar]
- 48.Lieu CHBM, Harb WA, Kearns JD, et al. Safety, pharmacology, and preliminary clinical activity of MM-151: an oligocolnal anti-EGFR theraputic in patients with cetuximab-resistant CRC and other refractory solid tumors. J Clin Oncol. 2015;33(suppl 3):abstr 647. [Google Scholar]
- 49.Schirripa M, Lenz HJ. Colorectal cancer: overcoming resistance to anti-EGFR therapy - where do we stand? Nat Rev Gastroenterol Hepatol. 2016. doi: 10.1038/nrgastro.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clinl Oncol. 2015;33(34):4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 52.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris MKWJ, Adam L, Tian F, et al. Role of MEK inhibition in improving anti-tumor responses in xenograft models of BRAF-mutated metastatic colorectal cancer. J Clin Oncol. 2016;34(suppl 4S):abstr 265. [Google Scholar]
- 54.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21(6):1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong DSMV, Osta BE, Fu S, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers. J Clin Oncol. 2015;33(suppl):abstr 3511. [Google Scholar]
- 56.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clinl Oncol. 2015;33(34):4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Cutsem CA, André T, Bendell J, et al. Updated results of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC). Ann Onc. 2015;26(suppl4):iv119LBA–07. [Google Scholar]
- 58.Deming DA, Cavalcante LL, Lubner SJ, et al. A phase I study of selumetinib (AZD6244/ARRY-142866), a MEK1/2 inhibitor, in combination with cetuximab in refractory solid tumors and KRAS mutant colorectal cancer: for submission to investigational new drugs. Invest New Drugs. 2015. doi: 10.1007/s10637-015-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do K, Speranza G, Bishop R, et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs. 2015;33 (3):720–728. doi: 10.1007/s10637-015-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hochster HS, Uboha N, Messersmith W, et al. Phase II study of selumetinib (AZD6244, ARRY-142886) plus irinotecan as second-line therapy in patients with K-RAS mutated colorectal cancer. Cancer Chemother Pharmacol. 2015;75(1):17–23. doi: 10.1007/s00280-014-2609-3. [DOI] [PubMed] [Google Scholar]
- 61.Flanigan SA, Pitts TM, Newton TP, et al. Overcoming IGF1R/IR resistance through inhibition of MEK signaling in colorectal cancer models. Clin Cancer Res. 2013;19(22):6219–6229. doi: 10.1158/1078-0432.CCR-13-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puzanov I, Lindsay CR, Goff L, et al. A phase I study of continuous oral dosing of OSI-906, a dual inhibitor of insulin-like growth factor-1 and insulin receptors, in patients with advanced solid tumors. Clin Cancer Res. 2015;21(4):701–711. doi: 10.1158/1078-0432.CCR-14-0303. [DOI] [PubMed] [Google Scholar]
- 63.Wilky BA, Rudek MA, Ahmed S, et al. A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours. Br J Cancer. 2015;112(1):24–31. doi: 10.1038/bjc.2014.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong S, Kim S, Kim HY, et al. Targeting the PI3K signaling pathway in KRAS mutant colon cancer. Cancer Med. 2015. doi: 10.1002/cam4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodon J, Brana I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32 (4):670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 66.McRee AJ, Sanoff HK, Carlson C, et al. A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest New Drugs. 2015. doi: 10.1007/s10637-015-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geel RVEE, Bendell JC, Faris JE, et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer. J Clin Oncol. 2014;32(suppl; abstr 3514):5s. [Google Scholar]
- 68.Elez E, Van Geel R, Bendell J, et al. Results of a phase 1b study of the selective BRAF V600 inhibitor encorafenib in combination with cetuximab alone or cetuximab + alpelisib for treatment of patients with advanced BRAF-mutant metastatic colorectal cancer. Ann Onc. 2015;26(suppl 4):iv120. [Google Scholar]
- 69.Kloth M, Ruesseler V, Engel C, et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in Lynch and Lynch-like colorectal cancer. Gut. 2015. doi: 10.1136/gutjnl-2014-309026. [DOI] [PubMed] [Google Scholar]
- 70.Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Modern Pathol. 2015;28(11):1481–1491. doi: 10.1038/mod-pathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 71.Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2015. doi: 10.1002/path.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ingold Heppner B, Behrens HM, Balschun K, et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111(10):1977–1984. doi: 10.1038/bjc.2014.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramanathan RK, Hwang JJ, Zamboni WC, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22(6):858–865. [DOI] [PubMed] [Google Scholar]
- 74.Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–523. doi: 10.1158/2159-8290.CD-11-0109.•• An elegant account of capturing tumoral heterogeneity over time to identify clinically relevant mechanisms of resistance toward cetuximab and identify promising therapeutic targets.
- 75.Leto SM, Sassi F, Catalano I, et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res. 2015;21(24):5519–5531. doi: 10.1158/1078-0432.CCR-14-3066. [DOI] [PubMed] [Google Scholar]
- 76.Van Cutsem E, Eng C, Nowara E, et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res. 2014;20(16):4240–4250. doi: 10.1158/1078-0432.CCR-13-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 78.Kammula US, Kuntz EJ, Francone TD, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248(2):219–228. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 80.Pulciani S, Santos E, Lauver AV, et al. Oncogenes in solid human tumours. Nature. 1982;300(5892):539–542. [DOI] [PubMed] [Google Scholar]
- 81.Ardini E, Bosotti R, Borgia AL, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol Oncol. 2014;8 (8):1495–1507. doi: 10.1016/j.molonc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sartore-Bianchi A, Ardini E, Bosotti R, et al. Sensitivity to entrectinib associated with a novel LMNA-NTRK1 gene fusion in metastatic colorectal cancer. J Natl Cancer Inst. 2016;108(1). doi: 10.1093/jnci/djv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amatu A, Somaschini A, Cerea G, et al. Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer. Br J Cancer. 2015;113(12):1730–1734. doi: 10.1038/bjc.2015.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Kim HC, Hong JY, et al. Detection of novel and potentially actionable anaplastic lymphoma kinase (ALK) rearrangement in colorectal adenocarcinoma by immunohistochemistry screening. Oncotarget. 2015;6(27):24320–24332. doi: 10.18632/oncotarget.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Russo M, Misale S, Wei G, et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 2016;6 (1):36–44. doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- 86.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 87.Guidoboni M, Gafa R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159(1):297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clinl Oncol. 2010;28(21):3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 91.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366 (26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohrt HE, Colevas AD, Houot R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124(6):2668–2682. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Beg MSMM, Tan BR, Kim RD, et al. A phase II multicenter study of the chimeric monoclonal antibody NEO102 (N) in adults with refractory colorectal cancer (CC). J Clin Oncol. 2015;33(suppl):abstr e14013. [Google Scholar]
- 96.Moore PAAR, Shah K, Yang Y, et al. Development of MGD007, a gpA33 x CD3 bi-specific DART for T-cell immunotherapy of metastatic colorectal cancer. Cancer Res. 2014;74(19Suppl):Abstractnr 669. [Google Scholar]
- 97.Hong F, Hansen RD, Yan J, et al. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63 (24):9023–9031. [PubMed] [Google Scholar]
- 98.Qi C, Cai Y, Gunn L, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117(25):6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salvador C, Li B, Hansen R, et al. Yeast-derived beta-glucan augments the therapeutic efficacy mediated by anti-vascular endothelial growth factor monoclonal antibody in human carcinoma xenograft models. Clin Cancer Res. 2008;14(4):1239–1247. doi: 10.1158/1078-0432.CCR-07-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PDL2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28 (6):1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tel J, Hato SV, Torensma R, et al. The chemotherapeutic drug oxaliplatin differentially affects blood DC function dependent on environmental cues. Cancer Immunol Immunother. 2012;61 (7):1101–1111. doi: 10.1007/s00262-011-1189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu S, Hu W, Iyer R, et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117(8):1661–1669. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Hu W, Shen D-Y, et al. Azacitidine enhances sensitivity of platinum-resistant ovarian cancer cells to carboplatin through induction of apoptosis. Am J Obstet Gynecol. 2009;200(2):177.e1–9. doi: 10.1016/j.ajog.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 105.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanmamed MF, Rodriguez I, Schalper KA, et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2−/−IL2R null immunodeficient mice. Cancer Res. 2015;75(17):3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 107.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19 (3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80(1):30–36. [DOI] [PubMed] [Google Scholar]
- 110.Gutterman JU, Mavligit GM, Blumenshein G, et al. Immunotherapy of human solid tumors with bacillus Calmette-Guerin: prolongation of disease-free interval and survival in malignant melanoma, breast, and colorectal cancer. Ann N Y Acad Sci. 1976;277(00):135–159. [DOI] [PubMed] [Google Scholar]
- 111.Mavligit GM, Gutterman JU, Malahy MA, et al. Adjuvant immunotherapy and chemoimmunotherapy in colorectal cancer (Dukes’ class C): prolongation of disease-free interval and survival. Cancer. 1977;40(5Suppl):2726–2730. [DOI] [PubMed] [Google Scholar]
- 112.Dinarello CA. Interleukin-1alpha neutralisation in patients with cancer. Lancet Oncol. 2014;15(6):552–553. doi: 10.1016/S1470-2045(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 113.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1alpha and liver metastasis in gastric carcinoma. Cancer. 2001;91(7):1272–1276. [DOI] [PubMed] [Google Scholar]
- 115.Rock KL, Latz E, Ontiveros F, et al. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hong DS, Janku F, Naing A, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33(3):621–631. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moon SU, Kang MH, Sung JH, et al. Effect of Smad3/4 on chemotherapeutic drug sensitivity in colorectal cancer cells. Oncol Rep. 2015;33 (1):185–192. doi: 10.3892/or.2014.3582. [DOI] [PubMed] [Google Scholar]
- 118.Meyer SC, Levine RL. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014;20(8):2051–2059. doi: 10.1158/1078-0432.CCR-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malilas W, Koh SS, Kim S, et al. Cancer upregulated gene 2, a novel oncogene, enhances migration and drug resistance of colon cancer cells via STAT1 activation. Int J Oncol. 2013;43(4):1111–1116. doi: 10.3892/ijo.2013.2049. [DOI] [PubMed] [Google Scholar]
- 120.Overman MJ, Kopetz S, Varadhachary G, et al. Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest. 2008;26(8):794–799. doi: 10.1080/07357900802087242. [DOI] [PubMed] [Google Scholar]
- 121.Stuart E, Buchert M, Putoczki T, et al. Therapeutic inhibition of Jak activity inhibits progression of gastrointestinal tumors in mice. Mol Cancer Ther. 2014;13(2):468–474. doi: 10.1158/1535-7163.MCT-13-0583-T. [DOI] [PubMed] [Google Scholar]
- 122.Van Schaeybroeck S, Kalimutho M, Dunne PD, et al. ADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep. 2014;7 (6):1940–1955. doi: 10.1016/j.celrep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 123.An HJ, Choi EK, Kim JS, et al. INCB018424 induces apoptotic cell death through the suppression of pJAK1 in human colon cancer cells. Neoplasma. 2014;61(1):56–62. [PubMed] [Google Scholar]
- 124.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112(6):1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hubbard JMONB, Jonker DJ, Halfdanarson TR, et al. Phase Ib study of cancer stem cell (CSC) pathway inhibitor BBI-608 administered in combination with FOLFIRI with and without bevacizumab (Bev) in patients (pts) with advanced colorectal cancer (CRC). J Clin Oncol. 2016;34(suppl 4S):abstr 569. [Google Scholar]
- 126.Ciombor KKEW, Hubbard JM, O’Dwyer PJ, et al. A phase Ib/II study of cancer stem cell inhibitor BBI608 administered with panitumumab in KRAS wild-type (wt) patients (pts) with metastatic colorectal cancer (mCRC) following progression on anti-EGFR therapy. J Clin Oncol. 2015;33(suppl):abstr 3617. [Google Scholar]
- 127.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1(4):229–233. [DOI] [PubMed] [Google Scholar]
- 128.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 129.Clevers H Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 130.de Sousa EM, Vermeulen L, Richel D, et al. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res. 2011;17(4):647–653. doi: 10.1158/1078-0432.CCR-10-1204. [DOI] [PubMed] [Google Scholar]
- 131.Ma H, Nguyen C, Lee KS, et al. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24(22):3619–3631. Epub 2005/03/23. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 132.Varnat F, Siegl-Cachedenier I, Malerba M, et al. Loss of WNT-TCF addiction and enhancement of HH-GLI1 signalling define the metastatic transition of human colon carcinomas. EMBO Mol Med. 2010;2(11):440–457. doi: 10.1002/emmm.201000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Janssen KP, Alberici P, Fsihi H, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131(4):1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 134.Eguchi M, Nguyen C, Lee SC, et al. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem. 2005;1 (5):467–472. [DOI] [PubMed] [Google Scholar]
- 135.Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A. 2004;101(34):12682–12687. Epub 2004/08/18. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sack U, Walther W, Scudiero D, et al. S100A4-induced cell motility and metastasis is restricted by the Wnt/beta-catenin pathway inhibitor calcimycin in colon cancer cells. Mol Biol Cell. 2011;22 (18):3344–3354. doi: 10.1091/mbc.E10-09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stein U, Arlt F, Smith J, et al. Intervening in beta-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13(2):131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stein U, Arlt F, Walther W, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131(5):1486–1500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 139.El-Khoueiry AB, Ning Y, Yang D, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors [abstract]. J Clin Oncol. 2013;31(suppl):abstr 2501. [Google Scholar]
- 140.Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287 (41):34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Madan B, Ke Z, Harmston N, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2015. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang YPM, Jaeger S, Bagdasarian L, et al. Dual Wnt and EGFRMAPK dependency of BRAFV600E-mutant colorectal cancer. Cancer Res. 2015;75(15Suppl):Abstractnr 2140. doi: 10.1158/1538-7445AM2015-2140. [DOI] [Google Scholar]
- 143.Fu LL, Tian M, Li X, et al. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget. 2015;6 (8):5501–5516. doi: 10.18632/oncotarget.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McCleland ML, Mesh K, Lorenzana E, et al. CCAT1 is an enhancer-templated RNA that predicts BET sensitivity in colorectal cancer. J Clin Invest. 2016;126(2):639–652. doi: 10.1172/JCI83265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forouhar F, Anderson JL, Mowat CG, et al. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2007;104(2):473–478. doi: 10.1073/pnas.0610007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thaker AI, Rao MS, Bishnupuri KS, et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145(2):416–25e1-4. doi: 10.1053/j.gastro.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takamatsu M, Hirata A, Ohtaki H, et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci. 2015;106(8):1008–1015. doi: 10.1111/cas.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188–1194. doi: 10.1093/annonc/mdv112.• Seminal study underlining the importance of the timing and depth of response as clinically relevant biomarkers in CRC.
- 150.Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clinl Oncol. 2013;31(30):3764–3775. doi: 10.1200/JCO.2012.42.8532. [DOI] [PubMed] [Google Scholar]
- 151.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 152.Fornaro L, Lonardi S, Masi G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013;24(8):2062–2067. doi: 10.1093/annonc/mdt165. [DOI] [PubMed] [Google Scholar]
- 153.Kerbel RS, Grothey A. Gastrointestinal cancer: rationale for metronomic chemotherapy in phase III trials. Nat Rev Clin Oncol. 2015;12 (6):313–314. doi: 10.1038/nrclinonc.2015.89. [DOI] [PubMed] [Google Scholar]
- 154.Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. doi: 10.1016/S0140-6736(14)62004-3.• Pivotal study illustrating the clinical benefit of maintenance therapy in CRC.
- 155.Triff K, Konganti K, Gaddis S, et al. Genome-wide analysis of the rat colon reveals proximal-distal differences in histone modifications and proto-oncogene expression. Physiol Genomics. 2013;45 (24):1229–1243. doi: 10.1152/physiolgenomics.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benedix F, Kube R, Meyer F, et al. , Colon/Rectum Carcinomas Study G. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 157.Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clinl Oncol. 2013;31(29):3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3). doi: 10.1093/jnci/dju427.• Pooled analysis of over 2000 patients demonstrating the heterogeneity of CRC and importance of primary tumor location as a stratification factor in clinical trials.
- 159.Lynch PM, Lynch HT, Harris RE. Hereditary proximal colonic cancer. Dis Colon Rectum. 1977;20(8):661–668. [DOI] [PubMed] [Google Scholar]
- 160.Sadler TW, Langman J. Langman’s medical embryology. 11th ed. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 161.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomark Prev. 2003;12(8):755–762. [PubMed] [Google Scholar]
- 162.Oikonomou E, Koustas E, Goulielmaki M, et al. BRAF vs RAS oncogenes: are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget. 2014;5 (23):11752–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brule SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51 (11):1405–1414. doi: 10.1016/j.ejca.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 164.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 165.Tejpar S, Shen L, Wang X, et al. Integrating biomarkers in colorectal cancer trials in the West and China. Nat Rev Clin Oncol. 2015;12 (9):553–560. doi: 10.1038/nrclinonc.2015.88. [DOI] [PubMed] [Google Scholar]
- 166.Wang Y, Poulin EJ, Coffey RJ. LRIG1 is a triple threat: ERBB negative regulator, intestinal stem cell marker and tumour suppressor. Br J Cancer. 2013;108(9):1765–1770. doi: 10.1038/bjc.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gillis NK, Patel JN, Innocenti F. Clinical implementation of germ line cancer pharmacogenetic variants during the next-generation sequencing era. Clin Pharmacol Ther. 2014;95(3):269–280. doi: 10.1038/clpt.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deenen MJ, Meulendijks D, Cats A, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clinl Oncol. 2016;34(3):227–234. doi: 10.1200/JCO.2015.63.1325.•• A population-based analysis illustrating the clinical utility of pharmacogenomic-based fluoropyrimidine dosing in cancer patients.
- 170.Zhang W, Press OA, Haiman CA, et al. Association of methylenetetrahydrofolate reductase gene polymorphisms and sex-specific survival in patients with metastatic colon cancer. J Clinl Oncol. 2007;25(24):3726–3731. doi: 10.1200/JCO.2007.11.4710. [DOI] [PubMed] [Google Scholar]
- 171.Pullarkat ST, Stoehlmacher J, Ghaderi V, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001;1(1):65–70. [DOI] [PubMed] [Google Scholar]
- 172.Caronia D, Martin M, Sastre J, et al. A polymorphism in the cytidine deaminase promoter predicts severe capecitabine-induced hand-foot syndrome. Clin Cancer Res. 2011;17(7):2006–2013. doi: 10.1158/1078-0432.CCR-10-1741. [DOI] [PubMed] [Google Scholar]
- 173.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clinl Oncol. 2007;25(24):3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 174.Volz NB, Stintzing S, Zhang W, et al. Genes involved in pericytedriven tumor maturation predict treatment benefit of first-line FOLFIRI plus bevacizumab in patients with metastatic colorectal cancer. Pharmacogenomics J. 2015;15(1):69–76. doi: 10.1038/tpj.2014.40. [DOI] [PubMed] [Google Scholar]
- 175.Sebio A, Gerger A, Matsusaka S, et al. Genetic variants within obesity-related genes are associated with tumor recurrence in patients with stages II/III colon cancer. Pharmacogenet Genomics. 2015;25(1):30–37. doi: 10.1097/FPC.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sebio A, Matsusaka S, Zhang W, et al. Germline polymorphisms in genes involved in the Hippo pathway as recurrence biomarkers in stages II/III colon cancer. Pharmacogenomics J. 2015. doi: 10.1038/tpj.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ning Y, Gerger A, Zhang W, et al. Plastin polymorphisms predict gender- and stage-specific colon cancer recurrence after adjuvant chemotherapy. Mol Cancer Ther. 2014;13(2):528–539. doi: 10.1158/1535-7163.MCT-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gerger A, El-Khoueiry A, Zhang W, et al. Pharmacogenetic angiogenesis profiling for first-line bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2011;17(17):5783–5792. doi: 10.1158/1078-0432.CCR-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pohl A, El-Khoueiry A, Yang D, et al. Pharmacogenetic profiling of CD133 is associated with response rate (RR) and progression-free survival (PFS) in patients with metastatic colorectal cancer (mCRC), treated with bevacizumab-based chemotherapy. Pharmacogenomics J. 2013;13(2):173–180. doi: 10.1038/tpj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lambrechts D, Lenz HJ, De Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clinl Oncol. 2013;31 (9):1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 181.Loupakis F, Antoniotti C, Cremolini C, et al. Prospective study of EGFR intron 1 (CA)n repeats variants as predictors of benefit from cetuximab and irinotecan in chemo-refractory metastatic colorectal cancer (mCRC) patients. Pharmacogenomics J. 2014;14(4):322–327. doi: 10.1038/tpj.2014.1. [DOI] [PubMed] [Google Scholar]
- 182.Wang WS, Chen PM, Chiou TJ, et al. Epidermal growth factor receptor R497K polymorphism is a favorable prognostic factor for patients with colorectal carcinoma. Clin Cancer Res. 2007;13 (12):3597–3604. doi: 10.1158/1078-0432.CCR-06-2601. [DOI] [PubMed] [Google Scholar]