Abstract
There is increasing evidence regarding the importance of allergic sensitization through the skin. In this review, we provide an overview of the atopic march and immune mechanism underlying the sensitization and effector phase of food allergy. We present experimental models and human data that supports the concept of epicutaneous sensitization and how this forms one half of the dual allergen exposure hypothesis. We discuss specific important elements in the skin (FLG and other skin barrier gene mutations, Langerhans cells, Type 2 innate lymphoid cells, IL-33, TSLP) that have important roles in the development of allergic responses as well as the body of evidence on environmental allergen exposure and how this can sensitize an individual.
Given the link between skin barrier impairment, atopic dermatitis, food allergy, allergic asthma and allergic rhinitis, it is logical that restoring the skin barrier and prevention or treating atopic dermatitis, would have beneficial effects on prevention of related allergic diseases, particularly food allergy. We present the experimental and human studies that have evaluated this approach and discuss various factors which may influence the success of these approaches, such as the type of emollient chosen for the intervention, the role of managing skin inflammation and differences between primary and secondary prevention of atopic dermatitis to achieve the desired outcome.
Keywords: cutaneous sensitization, food allergy, FLG, emollient, atopic dermatitis
INTRODUCTION
Allergic diseases are the most common chronic diseases in Westernized countries and bear a substantial health and socio-economic burden. Atopic dermatitis (AD) affects approximately 20% to 30% of children.1–3 Asthma affects 9–18%4, 5 and allergic rhinitis (AR) affects up to 40% of children.6 Food allergy (FA), is an epidemic among children in westernized countries,7–9 and significantly impairs quality of life.10, 11 Between 15–40% of children with FA have experienced a severe, life-threatening allergic reaction and 30% report allergies to multiple foods.12, 13 The rate of confirmed FA is much higher in children with AD.14–16 FA is expensive - resulting in $24.8 billion dollars/year in expenditures to the healthcare system and US families,17 and in Europe mean annual household costs were found to be €791 higher amongst households with a food-allergic child than those without food-allergic children.18
PATHOGENESIS OF FOOD ALLERGY
The genesis of FA is a complex process, influenced by genes, host immune responses, epithelial function and environment factors. Increased antibiotic use, non-vaginal births, ultra-sanitary lifestyles, less time spent outdoors and the resulting “modern” microbial community structures of the gut and the skin, beginning at birth, have been implicated in aberrant immune system maturation, and development of atopy, including FA (Figure 1).19 For years, it was thought that children became sensitized to food allergens by exposure through the gut (via breastfeeding or early consumption);20 there is now increasing evidence that early life allergen exposure through the skin causes FA (Figure 2),21, 22 whereas, early oral exposure causes tolerance (known as the dual allergen exposure hypothesis: Figure 3). In the last 5 years, several studies have demonstrated allergen specific oral tolerance induction to allergenic foods in high risk children.23–27
Figure 1:
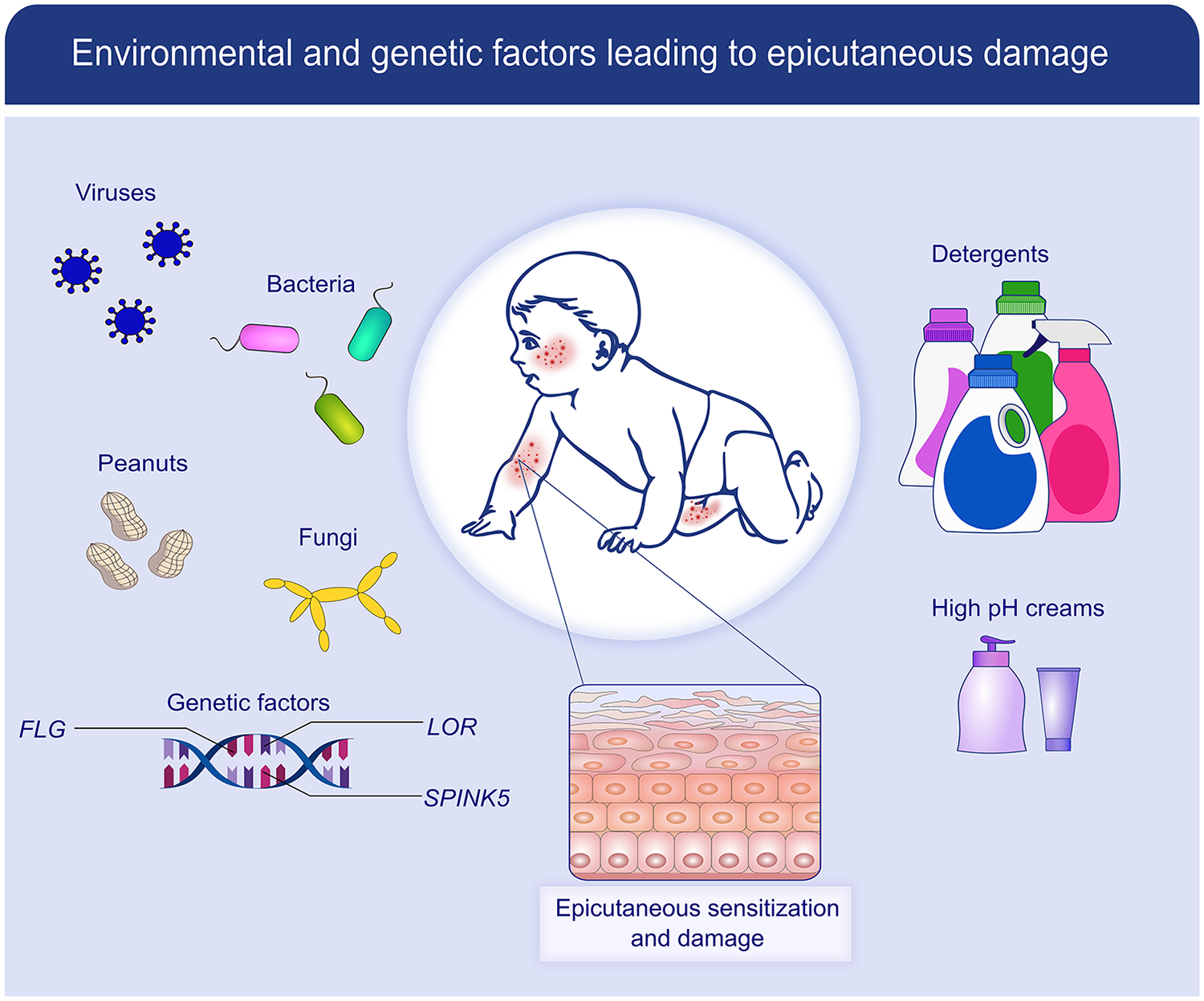
Factors leading to skin barrier dysfunction: The skin is constantly exposed to environmental factors, both natural (eg, bacteria, viruses, fungi, food and aero allergens) and man-made (e.g., detergents, high pH creams and lotions). In those genetically predisposed to allergic disease (e.g., filaggrin, SPINK5, and loricrin mutations), these factors lead to skin barrier dysfunction, epicutaneous damage, and allergic sensitization.
Figure 2:
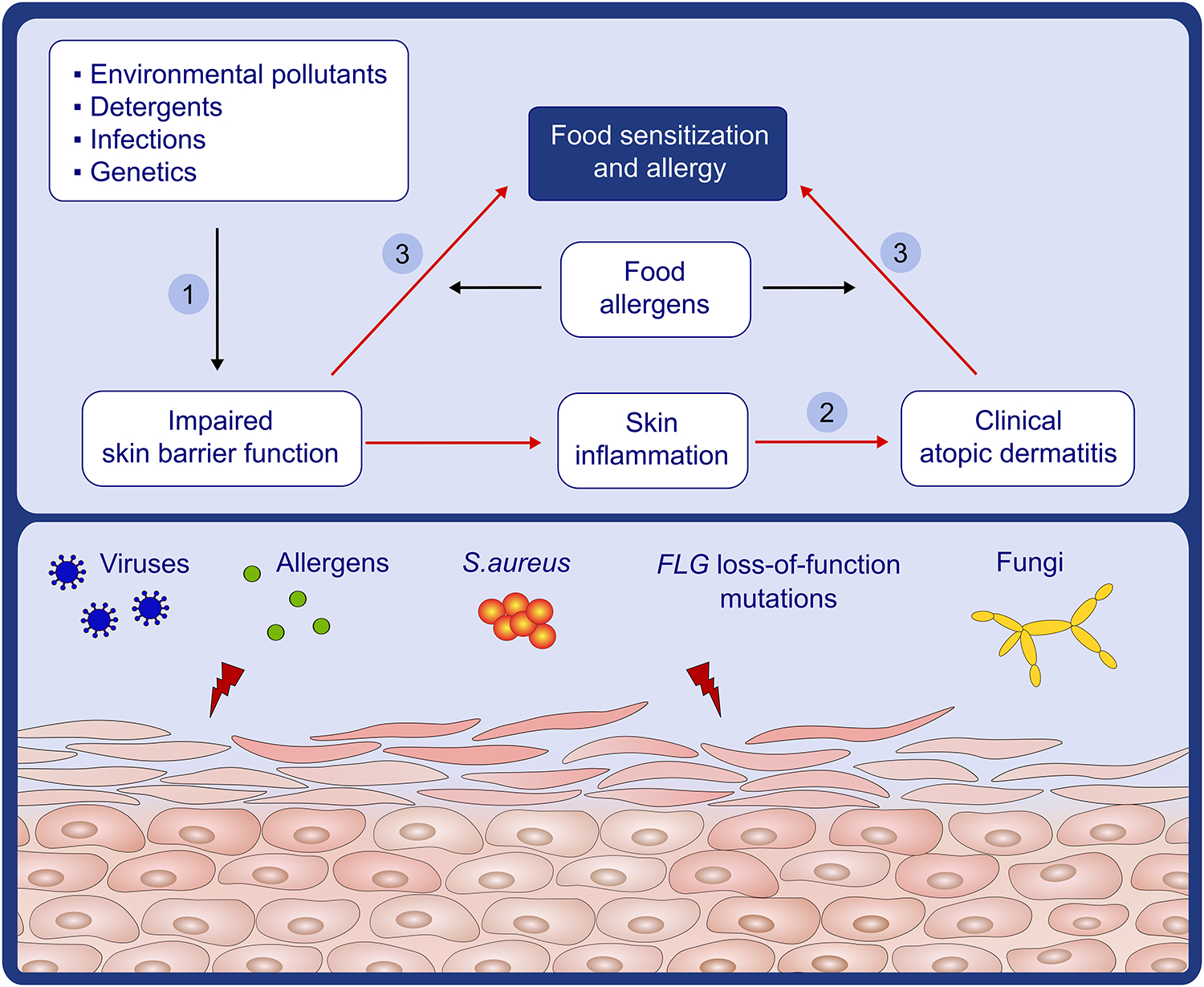
Pathways for skin barrier dysfunction and eczema leading to food allergy: Food allergy is manifested through the skin (1) the initial step towards food allergy is skin barrier impairment caused by environmental pollutants, detergents, infections, and genetics. (2) Skin barrier impairment leads to skin inflammation and clinical atopic dermatitis. (3) Exposure of allergens through skin that has an impaired barrier (dry) or has clinical atopic dermatitis leads to sensitization and food allergy.
Figure 3:
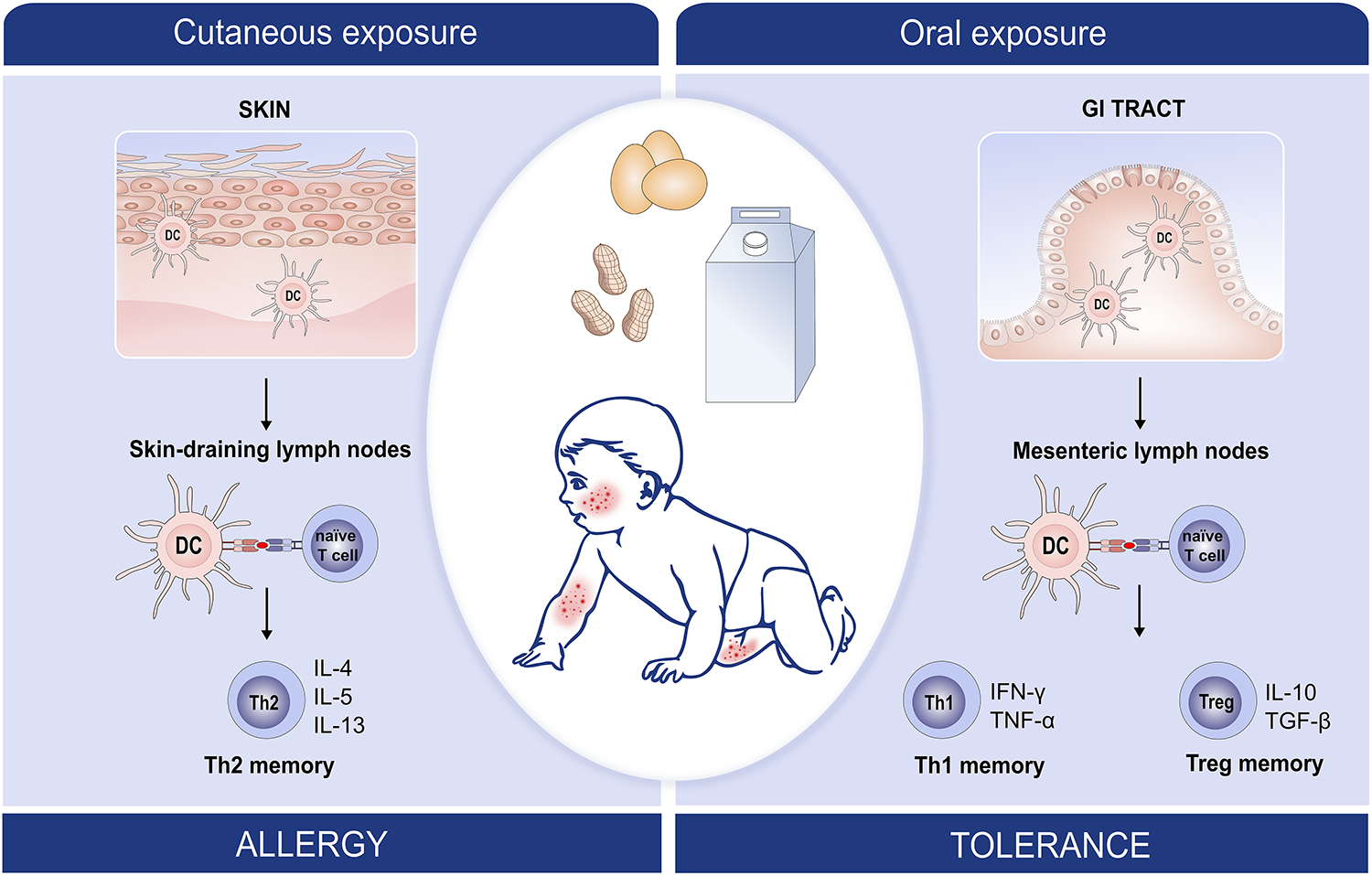
Dual-allergen Exposure: Increasing evidence suggests that early life allergen exposure through the skin causes T cell deviation towards a Th2 allergenic type and subsequent food allergy whereas early oral exposure causes T cell deviation towards tolerogenic Th1 and Treg subtypes (dual allergen exposure hypothesis).
LIMITATIONS OF ORAL TOLERANCE INDUCTION
The effect of oral tolerance induction appears to be allergen specific. For example, in the LEAP study, early peanut consumption did not lead to a prevention of tree nut or sesame seed allergy.28 Since FA develops early in life,29 there is a narrow window of opportunity to induce tolerance with oral introduction of multiple foods. In the HealthNuts population based study, 3.1% of children already had challenge proven peanut allergy (PA) by 12 months of age, and 9% were egg allergic.1 In addition, introduction of multiple allergenic foods into the diet of young infants is challenging. In the EAT study, the adherence rate for introducing 6 allergenic foods into the diet was only 42%.30 Thus, there is a need for an alternative approach to prevent FA.
There is evidence that diet diversity in infancy reduces food sensitization and allergic asthma (AA), but results for AD and AR are mixed.31 A systematic review found that breastfeeding was protective for AA; however, the evidence for AD and AR was weaker with no effect on FA.32 Other methods such as Vitamin D supplementation has produced mixed results.33, 34 There is little evidence for the role of prebiotics, and whilst meta-analyses and 2 individual studies show some benefit from probiotics, these studies have issues such as objective evaluation of outcomes, design and blinding.35, 36
ATOPIC MARCH
AD is often associated with the subsequent development of allergic disease, including FA, AA, or AR, as characterized by the concept of the ‘atopic march’.37–39 In the ALSPAC study, an oozing, crusting skin rash was an independent risk factor for the development of PA.40 Furthermore, early onset (particularly within the first 3 months), severe AD markedly increases the risk of FA.16, 41, 42 In the HealthNuts study, children with early onset severe AD had a 50% rate of challenge proven egg, peanut or sesame seed allergy by 12 months of age. In the LEAP screening study,43 there was a dose dependent increase in food sensitizations with increasing SCORing Atopic Dermatitis (SCORAD) levels in children between 4–11 months of age. Duration of infantile AD prior to inducing remission with proactive topical steroid treatment has also been shown to increase the risk of FA for each month that passes.44 This evidence suggests that shortening the duration of AD, by reducing inflammation, reduces the opportunity for epicutaneous exposure to environmental food allergens thereby preventing sensitization and subsequent FA.
THE IMMUNE RESPONSE BEHIND FOOD ALLERGY
A dysregulated immune system involving a Type 2 T helper (Th2) cell mediated inflammatory response is associated with FA.45, 46 This involves the formation of specific IgE antibodies against environmental and food allergens, as well as the production of several cytokines, including interleukin (IL)-3, IL-4, IL-5, IL-9, IL-13, IL-25, IL-31, IL-33 and Thymic stromal lymphopoietin (TSLP).47–50
The immune response in FA includes two phases.51, 52 The sensitization phase (Figure 4)53 is initiated when specific resident dendritic cell (DC) subsets capture allergens in the skin (also applicable to airways or gut) and transport the allergens to draining lymph nodes (LNs), where they are processed and presented to naïve CD4+ T cells. Within the LNs, in the presence of IL-4 and endothelial cell-derived Type 2 cytokines, the T cells differentiate into allergen-specific CD4+ T cells producing high levels of IL-4 and IL-13. IL-4 and IL-13 favor B cell isotype class switching to specific IgE and drives the production of IgE memory B cells (Figure 4).47 Through facilitated antigen presentation, a very low concentration of allergen can drive complex formation between sIgE, allergen, and low-affinity IgE receptor on antigen-presenting B cells (CD23+ cells);54 which then further drives Th2 cell proliferation, leading to further B cell isotype switching and IgE production.55 As the B cells mature, they differentiate into plasma cells and produce large amounts of allergen-specific IgE antibodies (sIgE) which bind to high-affinity FcεRI receptors on the surface of mast cells and basophils. During this phase, a memory pool of allergen-specific B cells and allergen-specific CD4 positive T helper 2 cells are generated. More recently, a subset of Th2 cells (Th2A cells) have been suggested to play an important role in the allergic immune response.56 Group 2 innate lymphoid cells (ILC2), which are found interspersed throughout barrier surfaces in the lung, gut, and skin, are also emerging as key regulators and effectors in type 2 immunity and tissue repair. They are enriched in human skin lesions, and ILC2 isolated from AD lesions, are activated by IL-33. They secrete proallergic cytokines, including IL-5 and IL-13 (Figure 5). IL-5 triggers the recruitment of eosinophils. IL-13 promotes recruitment of inflammatory cells, alterations in the skin microbiome, and decreases in epidermal skin barrier.57–59
Figure 4:
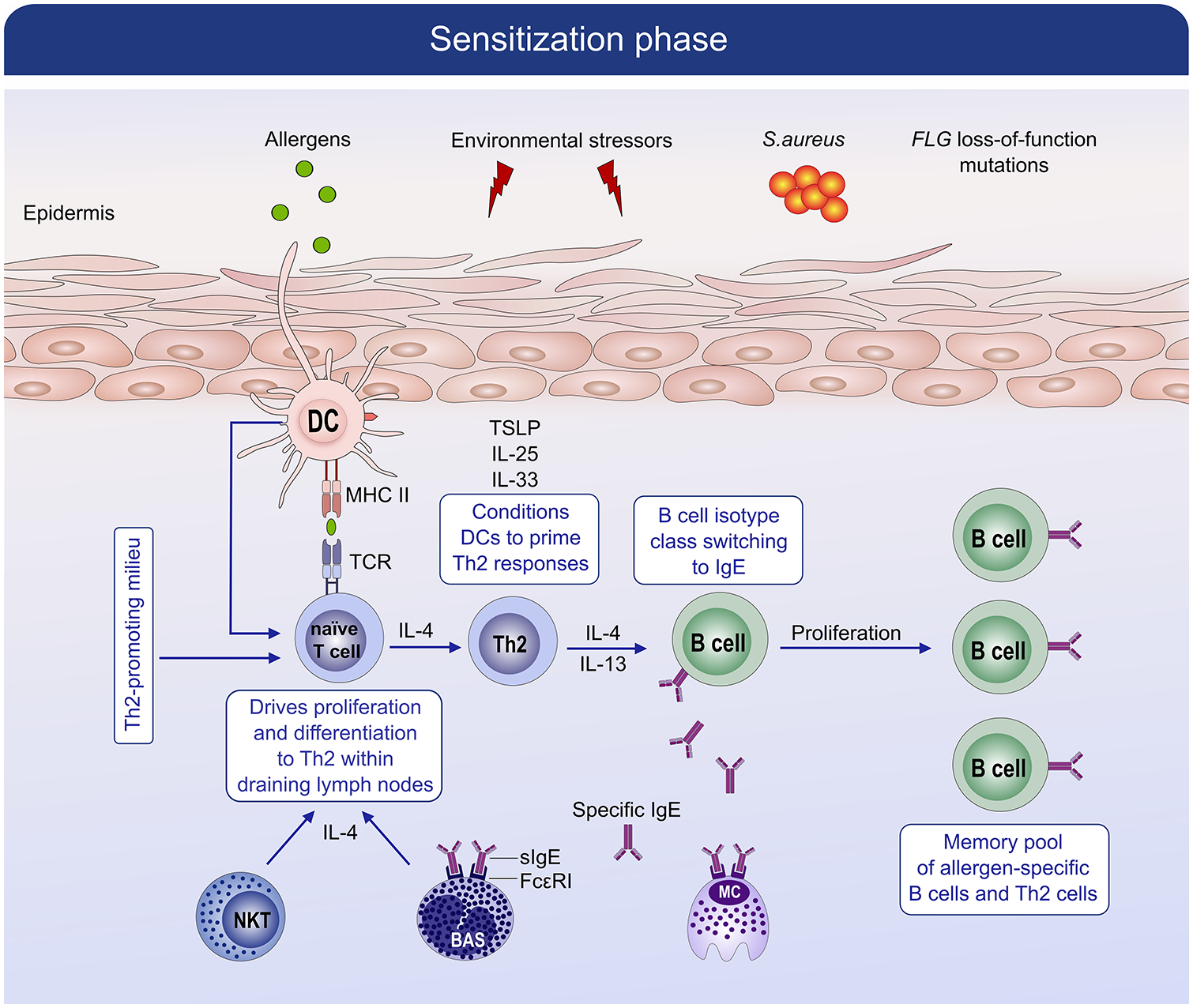
Mechanism of sensitization phase in allergic disease. During the allergic sensitization phase, in the setting of an impaired barrier, specific resident dendritic cell (DC) subsets capture allergens in the skin and transport the allergens to draining lymph nodes where they are processed and presented to naïve CD4+ T cells. Within the lymph nodes, the presence of IL-4 and IL-13 favors B cell isotype switching to specific IgE cells that differentiate into plasma cells and produce large amounts of allergen-specific IgE antibodies (sIgE). The sIgE bind to high-affinity FcεRI receptors on the surface of mast cells and basophils. During the sensitization phase, a memory pool of allergen-specific B cells and allergen-specific CD4 positive T helper 2 cells are generated.
Figure 5:
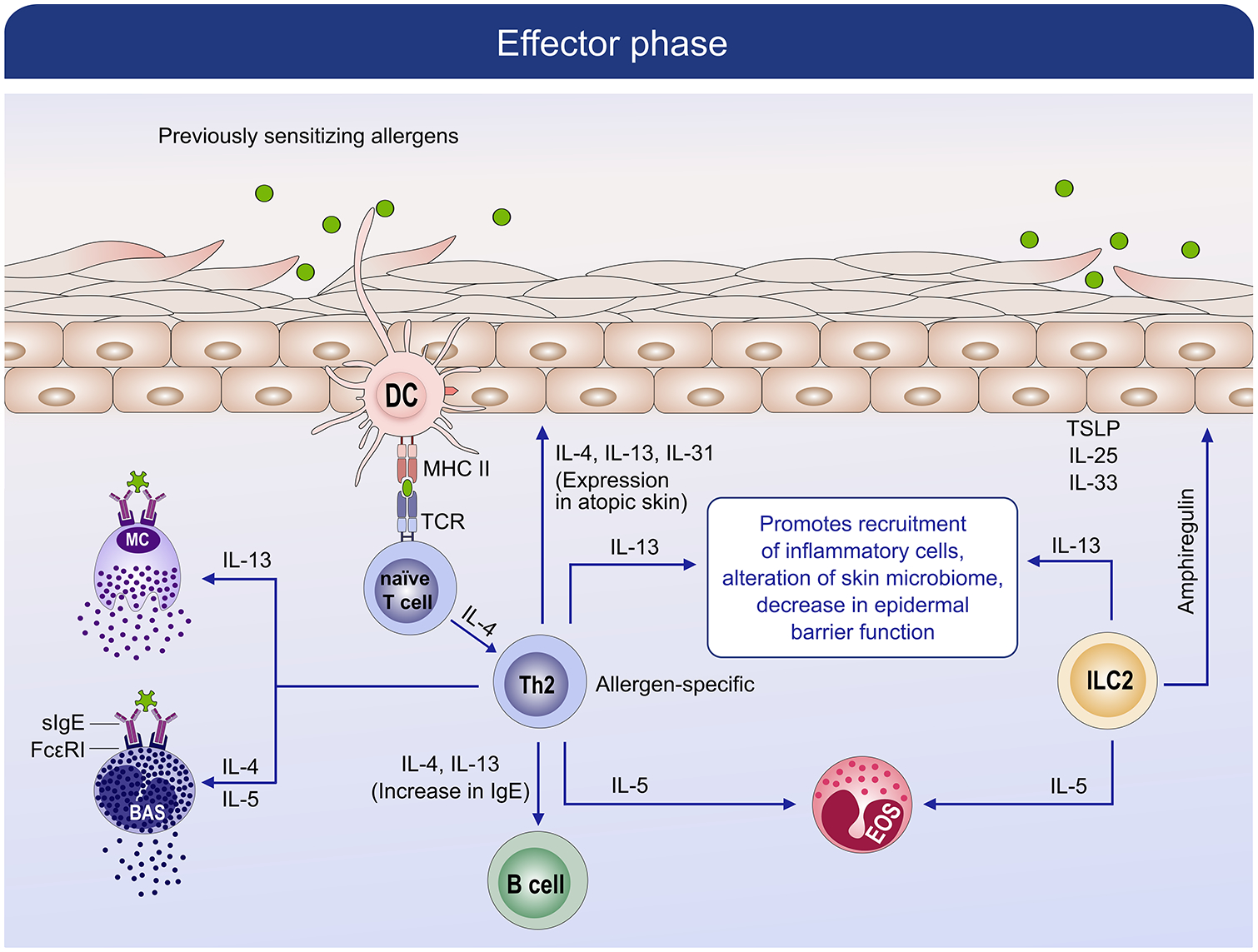
Mechanism of effector phase in allergic disease. During the effector, which follows the sensitization phase, subsequent encounters with a previously sensitized allergen leads to IgE-cross linking and activation of basophils and mast cells. Degranulation of mast cells and basophils leads to the release of preformed and de novo mediators which cause the symptoms of the immediate phase reaction as well as the subsequent late-phase reaction by activating memory allergen-specific Th2 cells.
The effector phase (Figure 5), follows the sensitization phase and is triggered when the host subsequently encounters a previously sensitizing allergen and causes cross-linking of FcεRI receptor-bound sIgE on sensitized mast cells and basophils leading to the release of preformed and de novo inflammatory mediators. These processes lead to the immediate phase reaction as well as subsequent late-phase allergic reaction through the activation of memory allergen-specific Th2 cells.47 As these cytokines accumulate, allergen specific Th2 cells are activated and produce IL-4, IL-5, and IL-13 among other cytokines. Recent evidence suggests that IL-13 is a key cytokine that drives peripheral inflammation in AD and is overexpressed locally, whereas IL-4 has a more central effect.60 The cytokines maintain allergen-specific IgE levels, eosinophilia, mucus production, and recruitment of inflammatory cells to inflamed tissues leading to tissue damage.
COMPONENTS OF IMMUNE SYSTEM LEADING TO EPICUTANEOUS SENSITIZATION
Thymic Stromal Lymphopoietin (TSLP)
An important cytokine believed to play a role in epicutaneous sensitization through an impaired skin barrier is TSLP.61 TSLP is increased in the stratum corneum (SC) of patients with AD and is positively correlated with AD severity (using SCORAD).62 TSLP is highly expressed by the keratinocytes of children with AD63 and by the epithelial cells of asthmatics.64 TSLP deficient mice are protected from developing allergic skin and airway inflammation following antigen exposure,65–67 as well as intestinal food allergy,68 which highlights the importance of this cytokine in allergic sensitization. Pro-inflammatory cytokines Tumour necrosis factor-alpha (TNF-α) and IL1-α, secreted in response to skin stripping,69 or scratching, induce TSLP secretion in vivo from human keratinocytes.70 TSLP levels increase following topical ovalbumin (OVA) application onto tape stripped skin.71 TSLP expression correlates with Langerhans cell (LC) maturation, upregulation of the TSLP receptor on LCs, migration of LCs to skin draining LNs where they promote the differentiation of naïve Th cells to Th2 cells and Th2 proliferation.63, 66, 72 TSLP induces migration of Th2 skewed antigen presenting cells (APCs) to the mesenteric LNs following tape stripping, providing evidence of a skin to gut migration.73 These findings link TSLP to the early stages of epicutaneous sensitization: increased secretion of TSLP by skin disruption or epicutaneous allergen exposure interaction with LCs to prime T helper cells.
Interleukin 33 (IL-33)
IL-33 is another epithelial cytokine that is released in response to defects in skin barrier surfaces characterized by AD.74 IL-33 is a member of the IL-1 cytokine family and is expressed in epithelial barrier tissues as well as lymphoid organs and plays an important role in triggering allergic inflammation after exposure to allergens.75 IL-33 levels are increased in the skin lesions and serum of individuals with AD,76, 77 and in mouse skin following epicutaneous sensitization with OVA.76 IL-33 polarizes skin-derived DCs and innate lymphoid cells to drive Type 2 cytokine production following epicutaneous sensitization to peanut extract,78 promotes increased secretion of IL-5 and IL-13 by in vitro polarized Th2 lymphocytes,79 and contributes to elevated serum IgE levels and eosinophilia when injected in vivo.80
A study exploring the role of IL-33 in epicutaneously sensitized FA, found IL-33 to be essential for inducing IgE-dependent anaphylaxis; IL-33 deficient mice and mice treated with soluble IL-33 receptor blocker were protected from oral-challenge induced anaphylaxis.81 Another study by Galand et al, demonstrated increased local and systemic release of IL-33 following mechanical skin injury induced by tape stripping in mice;82 IL-33R (ST2) deficiency or ST2 blockade prior to oral challenge, significantly reduced the severity of food induced oral anaphylaxis. The existing evidence suggests that IL-33 plays a key role in epicutaneous sensitization followed by subsequent food-induced anaphylaxis. Neutralization of IL-33 has also been shown as a promising strategy for the treatment for FA and AD.83, 84
EXPERIMENTAL MODELS OF EPICUTANEOUS SENSITISATION
Several animal models have shown that epicutaneous exposure to specific food allergens induce a potent allergic Type 2 immune response, associated with IL-4 secretion by T cells from draining LNs and high levels of allergen specific IgE by B cells.85–87 In addition, epicutaneous sensitization to food allergens has been shown to lead to systemic food allergic reactions, including anaphylaxis, on subsequent oral exposure.71 Bartnikas et al. demonstrated that epicutaneous OVA and/or peanut application onto tape stripped skin of BALB/c mice led to intestinal mast cell expansion, increased serum IL-4 and food-induced anaphylaxis.71 Following epicutaneous sensitization, systemic anaphylaxis was induced by a single oral challenge with allergen. Conversely, oral immunization with the cholera toxin (CT) adjuvant failed to induce anaphylaxis on subsequent oral challenge.71 In other experimental models of IgE-mediated anaphylaxis where mice were sensitized by intraperitoneal (IP) OVA injection with alum adjuvant, anaphylaxis was much harder to elicit on oral challenge, only after multiple oral challenges.88, 89 This demonstrated that epicutaneous sensitization primes the gut in a more effective manner for subsequent allergic reactions than oral or IP sensitization. When Bartnikas et al. performed epicutaneous sensitization in IgE-deficient mice, there was no intestinal expansion of mast cells, no rise in serum IL-4 and no features of anaphylaxis on intragastric challenge, which suggested that the effects arising from epicutaneous sensitization are IgE dependent.71 Th2 cytokines IL-4 and IL-13 have also been investigated in experimental food allergy models of epicutaneous sensitization; whereas mice deficient in IL-4 (genetically bred or in the presence of anti IL-4-blocking antibodies) were still able to mount Th2 responses following epicutaneous exposure,90, 91 however, IL-13 deficient mice (IL-13−/−) were not.92
In a more recent study by Geha et al.93 mechanical skin injury alone not only promoted cutaneous sensitization to foods, but also expanded and activated intestinal mast cells, thus promoting anaphylaxis in mouse models. Mechanical skin injury causes systemic release of IL-33, which interact directly with mast cells through the ST2 receptor,94 and leads to expansion of both mucosal and submucosal mast cells in the jejunum.93 Additionally, cutaneous injury led to increased intestinal permeability. The increase in intestinal mast cells as well as permeability that is elicited by mechanical skin injury or scratching, may play a significant role in promoting anaphylaxis in patients with FA and AD. In another study by Kawasaki et al.95 the authors demonstrated that inflammation from skin barrier disruption alone can worsen symptoms of FA even when the exposure to the allergen is removed. BALB/c mice were sensitized epicutaneously by repeated application of OVA through disrupted skin. After the first oral challenge, the skin barrier was further disrupted, and a second oral challenge was performed. Skin barrier disruption amplified the allergic reactions induced by the oral challenge, whereas topical pretreatment with dexamethasone reduced allergic reactions.95 These studies highlight the role that a disrupted skin barrier plays in the development of FA, and which may also contribute towards the severity of reactions.
Experimental models have carefully evaluated whether the skin barrier needs to be disrupted to achieve epicutaneous sensitization. In most models, mice only developed epicutaneous sensitization with the application of peanut to abraded skin.85–87, 90, 96 Conversely, application of peanut or OVA onto intact skin, or bypassing the SC (via subcutaneous skin layer) led to non-allergenic responses.85 In a recent experimental model, repeated applications of roasted peanut extract onto intact skin induced sensitization to the major peanut allergens Ara h 1 and Ara h 2 and anaphylaxis upon IP peanut re-challenge.78 The authors postulated that peanut protein was able to sensitize through the skin without the need for SC disruption or an adjuvant, because peanut protein itself had adjuvant properties. In support of this, they showed that peanut antigen led to bystander sensitization for the milk allergen α-lactalbumin. Having previously shown that application of milk allergen α-lactalbumin onto intact skin did not lead to sensitization or allergy on re-challenge, the authors then showed that concomitant application of peanut protein in addition to milk allergen α-lactalbumin onto intact skin led to anaphylaxis on subsequent oral exposure to milk allergen α-lactalbumin.78 In contrast, Walker et al. showed that, in filaggrin (FLG) deficient mice, epicutaneous sensitization to peanut or OVA required costimulation with Alternaria alternata or house dust mite extract.97
BARRIER DYSFUNCTION AND ITS ROLE IN AD AND FA
The discovery that FLG loss-of-function mutations, which have an important role in formation and integrity of the SC, are highly associated with AD and xerosis (dryness), was a major milestone in understanding AD as a ‘barrier’ disease.98, 99 Experimental models have shown that filaggrin protein deficiency in the skin leads to facilitated entry of irritants, pathogens and allergens, thereby eliciting an inflammatory response (Figure 1).100, 101 Similarly, decreased epithelial nasal barrier function has been shown to facilitate transepithelial allergen passage, allergic sensitization and allergen induced mast cell degranulation.102 Thus skin barrier impairment may itself lead to a maladaptive immune system with a predisposition towards atopy.
The local cytokine milieu has also been shown to affect FLG expression in the skin.103, 104 Filaggrin protein expression was assessed in the skin of 69 patients with at least one FLG loss-of-function mutation; filaggrin protein was reduced in non-inflamed skin, and even further in acute AD lesions.. Keratinocytes cultured in the presence of IL-4, IL-13 and /or IL-22 had reduced FLG expression, whereas those cultured in presence of IFNγ had increased FLG expression.105, 106 Taken together these studies show that skin barrier abnormality due to FLG loss-of-function mutations can enhance allergen penetration thus favoring Th2 inflammation. On the other hand, Th2 inflammation can impair skin barrier function via a reduction in filaggrin protein expression, leading to a vicious cycle of inflammation and skin barrier disruption.
There has been conflicting research as to whether FLG mutations alone increase the risk of FA, in the absence of AD or other factors. Brown et al. showed a significant association between FLG loss-of-function and challenge proven IgE-mediated PA, even after adjusting for AD in cohorts from the UK, Netherlands, Ireland and Canada.107 However, in infants, both the HealthNuts study and the EAT study showed that although FLG null mutations increased the odds of food sensitization, it did not significantly increase the odds of FA.108, 109 Venkataram et al.110 explored the longitudinal relationship between FLG mutations and FA; path analysis suggested an association between FLG mutations and AD in younger children and the progression to food allergic sensitization and FA in older children.
Beyond filaggrin, there are multiple other components of the skin barrier that may have a role in AD and FA.111 Mutations in the corneodesmosin gene leads to Peeling Skin Disease associated with severe skin barrier defect, pruritus and food allergies.112 Peeling Skin Disease shares clinical features with Netherton syndrome which is caused by SPINK5 mutations as well as patients with mutations in the transglutaminase 5 gene and desmoglein gene.113, 114 Importantly, Type 2 immune activation is associated with global downregulation of skin barrier genes including filaggrin, loricrin and involucrin thereby allowing food allergens to penetrate through the skin.115
Environmental factors, such as detergents and microplastics have an effect on epithelial barrier function. Anionic surfactants and commercial detergents have been shown to decrease tight junction barrier integrity in human keratinocytes116 and human bronchial epithelial cells,117 potentially increasing the risk of atopic disease. Surfactants have been shown to disrupt the lamellar architecture in the SC.118 In a mouse model, Ochi et al. showed that detergent-mediated skin damage promoted IgE/IgG1 responses and Th2 differentiation when epicutaneously sensitized to papain, a protease allergen. They also found that TSLP and IL-33 contributed to epicutaneous papain sensitization and the atopic march upon subsequent airway challenge with the protease allergen.119 In humans, application of traditional alkaline soaps increased transepidermal water loss (TEWL) and skin erythema, which are signs of prolonged damage to the skin barrier.120 The environmental damage caused by plastics are well documented; however, the role of microplastics, which have become ubiquitous in the environment, on human health is poorly understood. It is in our air, our water, and in the foods we eat. It is within us and has been detected in human lungs and feces. Ingestion of microplastics has been implicated in gut dysbiosis through mechanical disruption and microbiome alterations leading to inflammatory responses.121 An in vitro study of peripheral blood mononuclear cells found that high exposure to polypropylene microplastics (~20 μm and 25–200 μm) increased histamine levels.122 In an in vitro model of human epithelial colorectal cells, researchers found that exposure to microplastics upregulated transcriptional factors involved in cell inflammation and proliferation. The chronic exposure to low doses of microplastics has the potential to induce epithelial cell injury and alterations to intestinal barrier function.123 In a study of human lung epithelial cells, researchers found that exposure to inhaled polystyrene microplastics cause inflammatory and oxidative injury along with the disruption of intercellular junction proteins in the lung, potentially leading to pulmonary barrier dysfunction.124
IN VITRO MEASURES OF EPICUTANEOUS SENSITIZATION
In vitro evidence has shown that PA correlates with peanut-specific proliferation in the skin-homing memory T cell subset, suggesting that sensitization occurs though the skin.125, 126 The differential peanut-specific Th cell proliferation in peanut allergic versus peanut tolerant children was assessed using homing markers on CD4+ Th cells for the skin (Cutaneous Lymphocyte Antigen: CLA+) and gut-associated-lymphoid tissue (integrin α4β7+) as markers for the route of initial sensitization to peanut.125 Peanut specific Th cell proliferative responses were higher in CLA+ Th cells in peanut allergic children than peanut tolerant children. Cytokine responses from CLA+ peanut-specific Th cells in peanut allergic children showed a trend towards Th2-polarization (IL-13 and IL-4 secretion) whereas responses from α4β7+ peanut-specific Th cells in peanut tolerant children trended towards Th1 (IFNγ and TGFβ), suggesting that the origin of peanut sensitization occurs in the skin (Figure 3). Sensitization through the skin may be explained by the route of exposure to food allergens via the environment (dust and surfaces) or through transfer of the food allergen through hand-body contact, which is explored further below.
ENVIRONMENTAL EXPOSURE TO ALLERGENS IN HUMANS
In humans, several observational studies have shown an association between epicutaneous and environmental exposure to peanut allergens and the development of peanut sensitization,22, 127, 128 and challenge proven PA.40, 128, 129 One of the first studies to highlight the risk of epicutaneous peanut exposure was the ALSPAC birth cohort, where topical creams containing peanut (‘Arachis’) oil applied onto the skin of children with AD was an independent risk factor for the development of PA (peanut skin prick test (SPT) >8mm or sIgE >15kU/L).40 Among PA children with AD in the ALSPAC study, 90% had been topically exposed to creams containing Arachis oil in the first 6 months of life.40 In another study, household peanut consumption was used as an indirect marker of environmental peanut exposure.129 Peanut allergic children had a 10-fold higher level of household peanut protein consumption (18.8 grams/week) than high-risk egg allergic children without PA (1.9 grams/week) (p<0.0001) and 3-fold higher level than non-atopic children without PA (6.9 grams/week) (p<0.0001).129. Peanut butter consumption was more highly associated with PA than covered forms of peanut foods (e.g. chocolate peanuts); the authors postulated that environmental peanut exposure was exerting its’ impact through the skin, as peanut butter is sticky and therefore more likely to be transferred onto the skin of infants. In support of the dual allergen exposure hypothesis, infants who had eaten peanut in the first year of life were not affected by environmental peanut exposure. Subsequent studies showed that household peanut consumption is highly correlated with the concentration of peanut in the dust of an infants bed-sheet and play-area, even if the infant does not eat peanut.130
Further work analyzing peanut dust concentration in infants with AD, milk and/or egg allergy, showed that, for every unit increase in peanut dust concentration from the living room floor, there was a 1.97-fold increase in peanut sensitization and 2.34-fold increase in PA.22.In a population based study, children carrying at least one FLG loss-of-function mutation, were at increased risk of developing PA if they had high environmental peanut exposure in their bed-dust around the time of birth;128 for each unit increase in peanut dust concentration during infancy, there was a 6-fold increase in school-age peanut sensitization and a 3.3-fold increase in school-aged PA.128 In another population based Swedish study, environmental peanut exposure in high risk children (based on parental atopy or egg sensitization), increased risk of school-aged peanut sensitization.127 Based on this research, one could postulate that reducing environmental exposure to peanut (and potentially other allergenic foods), in high-risk children might reduce the risk of sensitization and allergy; however, this has not been proven. Additionally, peanut levels in areas outside the home are also high (for example in schools),131 thus an environmental allergen reduction approach may be difficult to implement. In summary, only high-risk children (those with an impaired skin barrier, egg allergy or predisposition towards atopy) seem to be at risk of peanut sensitization from high environmental peanut exposure, which has important ramifications for future research in this field. Interestingly, associations between impaired skin barrier and allergy may also be pertinent for inhalant allergies; environmental cat exposure around the time of birth was found to increase the risk of indoor allergen sensitization in children with at least one FLG loss-of-function mutations.132
THE ROLE OF THE MICROBIOME IN EPICUTANEOUS SENSITIZATION
With growing evidence that food sensitization can occur through the skin, more attention has been paid to dysbiosis of the skin microbiome as a factor in AD and FA.133 Skin commensals have been found to be essential for resident T cells and educate keratinocytes to become effective in combating skin pathogens. Conversely, commensals also appear to be required so that the host does not attack normal colonizing flora. Microbial dysbiosis has been observed in infants with AD,134, 135 particularly with S. aureus colonisation.136 In a study using minimally invasive skin tape stripping, shotgun metagenomic studies revealed that the skin of AD FA+ children was colonized with Staphylococcus aureus.137 In contrast, topical transplantation of commensal bacteria from healthy volunteers to patients with AD improved AD severity and reduced topical steroid use; this forms the basis for further work in this area.138, 139
Schwartz et al. identified a critical role for the microbiome in mice with an impaired skin barrier. The authors reported on their observation of skin inflammation in FLG-mutant (FLGft/ft) mice triggered by the interplay between the microbiome; specifically they reported a shift towards pathogenic staphylococci, IL-1β, and mast cells.140 Further experimental models have shown that applying Staphylococcus enterotoxin B (SEB) on skin enhances allergic responses to food antigens.141–144
In humans, S. aureus colonisation is significantly associated with AD severity, persistence and subsequent deterioration of AD.145 Tsilochristou et al. showed the importance of S. aureus in driving AD severity, and specific IgE to cow’s milk, hen’s egg and peanut, independent of AD severity in the LEAP study.145 Additionally, S. aureus colonization at any time was associated with prevention of acquisition of natural tolerance to hen’s egg, and the disruption of oral tolerance induction to peanut.145 Taken together, these studies highlight the role of S. aureus dysbiosis in children with AD, on AD severity and risk of AD deterioration, in addition to increasing risk of sensitization to food, enhanced allergic responses, persistence of FA and inhibition of oral tolerance induction.
PHENOTYPIC MEASURE OF SKIN BARRIER DYSFUNCTION
Functional assessment of the integrity of the epidermis using TEWL has been shown to predict subsequent development of AD regardless of filaggrin mutation.146, 147 It has also been associated with food sensitization (even after adjusting for AD and FLG mutation status) in young infants.148 Increased TEWL may reflect the spectrum of genetic and environmental factors leading to barrier impairment, such as water hardness,149, 150 frequency of washing and commercial detergents (known to decrease tight junction barrier integrity in human keratinocytes).116, 117 These findings are being assessed in randomized controlled trials (RCTs) to prevent AD such as the SOFTER (Softened Water for Eczema Prevention) trial.151
If the skin barrier is impaired, more water evaporates from the skin enabling allergens, irritants and bacteria to penetrate the skin.152 Carriage of a FLG loss-of-function mutation is associated with increased TEWL at three months, even in the absence of AD,148 suggesting this already shows a predilection towards skin barrier impairment which could subsequently lead to clinical AD. TEWL at 2 days and 2 months was shown to predict AD at 12 months,153 and subsequently the same group showed that raised TEWL predicts FA at 2 years of age.154 In a recently published study, it was shown that TEWL after skin tape stripping separated AD with FA vs AD without FA.137
IDENTIFYING SKIN BARRIER MARKERS TO PREDICT FOOD ALLERGY
A minimally invasive skin-tape-stripping measure of the SC in combination with a comprehensive multi-omics approach was used to determine whether AD with FA (AD FA+) children have skin biomarkers which distinguish them from AD without FA and non-atopic children.137 Despite similar skin disease severity, the SC integrity and FLG content were significantly lower in children who were AD FA+ vs. AD FA−. Lipidomics of the SC in the AD FA+ group revealed a relative reduction in esterified ω-hydroxy fatty acid (EO) sphingosine (S) ceramides (CER) which are ultra long chained ceramides (EOS CER) required for normal skin barrier function. At the same time, a significant increase in nonhydroxy fatty acid sphingosine ceramide levels was observed in AD FA+ skin samples, resulting in a disproportionate decrease in EOS CER in the skin. Transcriptome studies of the skin tape also revealed the AD FA+ skin samples expressed the highest Type 2 immune expression levels.
Interestingly, STS proteomics revealed an immature keratin profile consistent with keratinocyte hyperproliferation in the SC of AD FA+ participants. A network analysis demonstrated that keratin 5, 14 and 16 expression, and reduced filaggrin breakdown products (urocanic acid), were strongly correlated with AD FA+, with increased TEWL, and were the most important predictors of AD FA+.137 These data support the importance of skin barrier dysfunction in the pathogenesis of epicutaneous sensitization to environmental foods and may contribute to the persistence or severity of FA by chronically stimulating Type 2 immune responses in the skin.
THE ROLE OF EMOLLIENTS TO PREVENT THE DEVELOPMENT OF FOOD ALLERGY
Based on the body of evidence that sensitization occurrs through the skin, it would be logical to assume that reducing both severity and duration of AD could potentially reduce the incidence of FA. Pilot studies showed that standard moisturizers could reduce AD by up to 50%, primarily by enhancing skin hydration155, 156 The summary of the different pilot studies is presented in Supplementary Table A. These pilot studies were then followed up by larger RCTs trials; these are presented in Tables 1 (non-lipid emollients), 2 (emollients containing at least one ceramide) and 3 (emollients containing a combination of emollients and topical steroids). A more detailed version of the trials is presented in Supplementary Table B. Supplementary Table C lists emollients that have been tested in clinical trials. An RCT by Yonezawa et al. found statistically lower TEWL, diaper dermatitis, and risk of dry facial or body skin in the interventional group (1–2 applications per day for 12 weeks with a moisturizer containing either paraffin or alcohol/ceramide, n=114) than in the control group (n=113).157 The BEEP (Barrier Enhancement for Eczema Prevention) and PreventADALL (Preventing Atopic Dermatitis and ALLergies in Children) RCTs (Table 1) evaluated whether including petrolatum based oils in baths or applying petrolatum-based moisturizers from the first few weeks of life (and small ‘tastes’ of allergenic foods in PreventADALL) could prevent AD and FA. Both studies showed no significant reduction in the incidence of AD, and in the BEEP study, there was an increased rate of infections and a trend towards increased food allergy in the intervention group.158, 159 Similarly, an RCT by Dissanayake et al. compared the incidence of AD and FA in the first year of life in infants treated with synbiotics and an emollient (alone or in combination) and controls and found no statistical difference.160 The negative findings in these studies were surprising given the pilot study findings, and the authors discussed various reasons as to why this might have occurred including low adherence rate with the intervention (especially PreventADALL), contamination of the control arm (BEEP), and the type of emollient used. The PEBBLES (Prevention of Eczema By a Barrier Lipid Equilibrium Strategy) pilot study (Table 2) used a ceramide-containing trilipid cream (Supplementary Table A) for 6 months and demonstrated a reduction in investigator observed AD at 12 months, and food sensitization at 6 and 12 months of age (Figure 6).161 Per protocol analyses showed that in infants who received ≥5 days per week of treatment (twice daily trilipid cream), 0% (0 of 21) of children developed food sensitization compared to 19% (7 of 36) in the control group. Miyaji et al.’s retrospective cohort study44 (Supplementary Table A) showed that in infants with moderate-severe AD, proactive topical steroid treatment commenced by 4 months of age versus after 4 months of age was associated with an almost 2-fold reduction in FA by 24 months (46.3% in the delayed steroid treatment group versus 25.3% in the early steroid treatment group (p=0.01). A number of emollient studies for prevention of atopic diseases are ongoing (e.g., NCT02906475, NCT03871998, NCT03409367, and NCT03667651) and results have not been published (Tables 1 and 2).
Table 1.
Randomized Trials Assessing Non-Lipid based Emollients for Atopic Dermatitis
| Prevention type | RCT Author & Study Name | Participant Number & Study Arms | Population | Emollient | Duration, Frequency, & Location | Outcome measures | Results |
|---|---|---|---|---|---|---|---|
| 1ry | Blume-Peytavi et al. Atopic Dermatitis in Atopy Predispos ed Infants (ADAPI) NCT02906475 |
160 2 arms |
0–14 days of age Term newborns 18–45 year old moms Birthweight 2.5 to 4.5 Kg I, II, III, IV Fitzpatrick classification Parent or sibling with physician diagnosed AD, AR, or AA Must be breastfed and/or on cow-milk based formula |
Milk Lotion (no details about brand(s) or ingredients provided) | 12 months Applied once daily to entire body and face + Bathing addendum if needed |
1ry: Cumulative AD incidence at 12 months 2ndry: Cumulative AD incidence at 24 months EASI at 12 and 24 months IDQoL at 12 and 24 months TEWL, skin surface pH, and Stratum Corneum Hydration on mid-alveolar forearm at 14 days, 1, 3, 6, 12, and 24 months |
Study ongoing- no results published to date. |
| 1ry | Jonathan Hourihane et al. Short-term Topical Application to Prevent Atopic Dermatitis (STOP AD) NCT03871998 |
242 2 arms |
Full term healthy infant Up to 5 days of age 1st degree relative with AD, AR, AA, or FA |
Commercially available moisturizer (no details about brand(s) or ingredients provided) | 2 months 2x daily full-body application by intervention group standard skin care advice with no moisturizer application in control group |
1ry: Cumulative incidence of AD at 12 months Cumulative incidence of IgE-mediated food allergy at 24 months 2ndry: TEWL and Natural Moisturizing Factor measured at birth, 2, 4 and 8 weeks and at 6 and 12 months Microbial community and skin biomarker analysis in a subset of study participants, comparison of changes over 1st year between cheek and antecubital fossa and between intervention and control group |
Study ongoing- no results published to date. |
| 1ry | Chalmers, J.R. et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial.159 ISRCTN21528841 |
1394 Control group (skin care advice only):701 Intervention group (skin care advice+emollient): 693 |
Up to 3 weeks of age 1st degree relative with physician-diagnosed AD, FA, AR |
Choice between Doublebase Gel® (Dermal Laboratori es Ltd.) & Diprobase Cream® (Merck Sharp & Dohme Ltd.)-paraffin based without alcohol or lipids |
12 months >=1x daily |
1ry: Blinded assessment of AD at 24 months of age using UKWP diagnostic criteria 2ndry: Time to onset of AD Severity of AD based on EASI & POEM Confirmed diagnosis of FA at 24 months to milk, egg, and/or peanut (parental report, allergic sensitization, food challenge- if required) Parental report of clinical diagnosis of any FA between 12 and 24 months Safety & cost-effectiveness of prevention strategy Role of FLG genotyping as possible stratifier of response to emollient intervention |
No statistically significant difference in AD: present at age 2 (within the past year) in 150 (25%) of 612 in control group and in 139 (23%) of 598 in intervention group (adjusted RR 095 [95% CI 0·78 to 1·16]; p=061) Subgroup analyses (number of 1st-degree relatives with AD, AA, or AR, FLG genotype, season of birth, water hardness, and probiotic use) also showed no statistically significant differences Adherence was considered satisfactory in only 51% of intervention group (all of those who did not complete any questionnaires were assumed as not using emollient). Adherence was considered satisfactory at 70% (311 of 442) in those who completed all questionnaires Adherence in intervention group decreased with each time-point: 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months |
| 1ry | Skjerven HO et al. Skin emollient and early complement ary feeding to prevent infant atopic dermatitis (PreventAD ALL): a factorial, multicentre, cluster-randomised trial158 NCT02449850 |
2701 1 of 4 similar sized groups:
|
Infants General population |
Ceridal face cream (alcohol & polyethelyne glycol) in skin care group and dual intervention group | 9 months >= 5 days/week |
1ry: AD assessed first at 12 months FA to intervention food assessed first at 36 months 2ndry: Recurrent wheeze or AA, allergic sensitization, allergy to other foods, anaphylaxis, & AR |
Study closed to recruitment but ongoing No statistically significant difference in AD: present in 478 (8%) of 596 infants in control group, 64 (11%) of 575 in skin intervention group, 58 (9%) of 642 in food intervention group, and 31 (5%) of 583 in combined intervention group. Risk difference of 3·1% (95% CI – 0·3 to 6·5) for skin intervention versus control and 10% (−2·1 to 4·1) for food intervention versus control Reports of Itching, dry skin, and urticaria were similar in all groups |
POEM: Patient Oriented Eczema Measure; EASI: Eczema Area and Severity Index; IDQoL: Infants’ Dermatitis Quality of Life; RCT: Randomized controlled trial
Table 2.
Randomized Trials Assessing at Least One Ceramide-containing Emollient for Atopic Dermatitis
| Prevention type | RCT Author & Study Name | Participant Number & Study Arms | Population | Emollient | Duration, Frequency, & Location | Outcome measures | Results |
|---|---|---|---|---|---|---|---|
| 1ry | Yonezawa K, et al. Effects of moisturizing skincare on skin barrier function and the prevention of skin problems in 3-month-old infants: A randomized controlled trial157 |
227 Control group: 113 Intervention group: 114 |
1 week to 3 months of age | For bathing, Pigeon Baby Soap [Pigeon, Tokyo, Japan] or Kewpie Baby Body Soap, Cow Brand Soap [Kyoshinsh a, Tokyo, Japan] As moisturizer in intervention group: Pigeon Baby Milk Lotion [Pigeon] or Atopita Milky Lotion© [Tampei Pharmace utical, Tokyo, Japan] |
12 weeks Application 1–2x daily with bathing every 2 days in intervention group Vs Daily bathing without moisturizer in control group |
1 ry: TEWL, SCH, skin pH and sebum secretion at 3 months of age 2ndry: Incidence of skin problems (e.g. Diaper dermatitis) at 3 months of age |
Statically significant lower TEWL (mean ± SD, 14.69 ± 7.38 vs 17.08 ± 8.26 g/m2 per h, P = 0.033) and higher face SCH (60.38 ± 13.66 vs 53.52 ± 14.55, P = 0.001) and higher body SCH (58.89 ± 12.96 vs 53.02 ± 10.08, P < 0.001) in intervention vs. control group Diaper dermatitis at 1–3 months of age significantly lower in intervention vs. control group (6.3% vs 15.9%, P = 0.022; RRR= 0.399 [95% confidence interval [CI], 0.174–0.919]). Skin problems at 1–3 months of age significantly lower in intervention vs. control group (42.1% vs 55.2%, P = 0.064; relative risk ratio, 0.762 [95% CI, 0.569–1.021]). Risk of dry facial skin was reduced with intervention by 48.4% (relative risk ratio, 0.516; 95% CI, 0.333–0.799]) Risk of dry body skin was reduced with intervention by 39.7% (relative risk ratio, 0.603; 95% CI, 0.399–0.911) |
| 1ry | Eric Simpson A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE) NCT03409367 |
1250 2 arms |
Up to 2 months of age Born at > 25 weeks of gestation |
Choice of over the counter lipid-rich emollient (Vaseline/Vanicream/Cerave Healing Ointment/Cerave cream/Cetaphil cream) | 24 months Once daily full body application |
1ry: Cumulative incidence of AD at 24 months of age 2ndry: AD diagnosed by Children’s Eczema Questionnaire (CEQ) at 12 and 24 months Any prescription topical medication or over-the-counter hydrocortisone usage Parental report at 3, 6, 9, 12, 15, 18 and 24 months of provider-diagnosed AD and sleep loss of infant Parental report of immediate food allergy symptoms or a provider diagnosis of food allergy that was confirmed by prick testing or IgE blood test at 12 and 24 months Asthma risk using a modification of the asthma predictive index (API) at 12 and 24 months Global Health Status using one question from the PROMIS PGH-7 instrument at 12 and 24 months Atopic dermatitis severity as measured by parental report of eczema, age of onset Time to onset of AD as measured by provider-recorded date of first diagnosis retrieved from review of health record AD symptom severity as reported by POEM Atopic dermatitis severity by Parent-reported global severity of eczema assessment IDQoL instrument |
Study ongoing- no results published to date. |
| 1ry | Dissanayake et al. Skin care and synbiotics for prevention of AD or FA in newborn infants: A 2 × 2 factorial, randomized, non-treatment controlled trial160 |
605 pregnant women recruited - 549 infants enrolled Randomized into 4 treatment arms:
|
Infants | Locobase ® REPAIR cream (Daiichi Sankyo, Japan) | 6 months 2–3x daily |
1ry: Development of AD at 1 year of age 2ndry: Prevalence of FA, as reported in the questionnaires at 1 year Sensitization to any food or inhalant allergen at 9 months of age EASI score at 9 months of age TARC score at 9 months of age |
Cumulative incidence of AD (itchy skin condition lasting 2 or more months) at 12 months of age: 30.9% in group 1, 32.1% in group 2, 38.6% in group 3, and 25.6% in group 4 – No statistically significant difference Incidence of AD AS DEFINED BY UKWPC was 20.4% in group 1, 14.7% in group 2, 21.0% in group 3, and 18.8% in group 4 – No statistically significant difference No statistically significant difference in AD incidence after adjusting for emollient application rate (80% of the parents/guardians in the emollient groups applied emollient at least 2x/day) No statistically significant difference in AD rate when comparing group 1+3 (received emollients) to 2+4 (no emollients) TARC levels not significantly different between all infants of all groups TARC levels of infants who developed AD by 9 months of age also not significantly different between all groups EASI scores showed mild disease in most babies who developed AD by 9 months of age (only 5/49 babies with AD had moderate disease) Highest EASI score was 13.6. Median scores did not differ significantly among groups Prevalence of FA at 1 year of age, sensitization to any food or inhalant allergen at 9 months of age and allergen-specific IgE showed no difference in AD incidence between groups |
| 1ry | Lowe A, Su J, Tang M, et al. PEBBLES study protocol: RCT to prevent AD, FA and sensitization in infants with a family history of allergic disease using a skin barrier improvement strategy172 Phase III ACTRN12617001380381 NCT03667651 |
760 2 arms (380 in each) |
Up to 3 weeks of age 1st degree relative has a self-reported hx of AA, AD, hay fever/AR or FA |
EpiCeram® | 6 months 6 grams 2x daily |
1ry: Presence of AD in first 12 months of life assessed using UKWP criteria and/or visible AD at time of examination 2ndry: FA, based on skin prick tests, hx of reactions & OFC (if wheel ≥ 1 mm) at 12 months Adverse events (AEs) to EpiCeram® Skin barrier function as assessed by TEWL at 6 weeks & 12 months Food sensitization (positive skin prick test) at 12 months of age |
Study ongoing- no results published to date. |
OFC: oral food challenge; RCT: Randomized controlled trial; TARC: Thymus and activation-regulated chemokine
Table 3.
Randomized Trials Assessing Combination of Emollients and Topical Steroids for Atopic Dermatitis
| Prevention type | RCT Author & Study Name | Participant Number & Study Arms | Population | Emollient | Duration, Frequency, & Location | Outcome measures | Results |
|---|---|---|---|---|---|---|---|
| 2ndry | Yamamoto - Hanada et al. Early aggressive interventi on for infantile AD to prevent developm ent of FA: a multicenter, investigator-blinded, randomized, parallel group controlled trial (PACI Study)-protocol for a RCT168 UMIN000028043 |
650 Control group (standard guideline-based treatment): 325 Intervention group (early aggressive topical steroids & Heparinoid cream application): 325 |
7–13 weeks old Diagnosis of AD (UKWP criteria) with rash development within previous 28 days |
Alclometas one dipropionate (Almeta®) to face, Betametha sone Valerate (Rinderon®-V) and Mometasone Furoate (Fulumeta®) to body Heparinoid cream (Hirudoid® Soft ointment) as emollient |
28 weeks Whole body (except scalp) treatment Variable application depending on week in study & flares |
1ry: OFC-proven IgE-mediated hen’s egg allergy at 28 weeks of age 2ndry: OFC test scores at 28 weeks Total IgE antibody titer & Egg white, ovomucoid, milk, soy, wheat, & peanut-specific IgE & IgG4 antibody titers in serum at 28 weeks EASI scores at 2, 4, & 8 weeks after study entry & at the age of 28 weeks POEM scores weekly through study Percentage of disease-free days Dose of rescue medication (applications) used IDQoL and DFI questionnaires at 2, 4, & 8 weeks after study entry & at 28 weeks Cumulative incidence of IgE-mediated FA assessed by a doctor during study Cumulative incidence of wheezing assessed by a doctor during study (parental reports evaluated by doctor) |
Study ongoing- no results published to date. |
OFC: oral food challenge; POEM: Patient Oriented Eczema Measure; EASI: Eczema Area and Severity Index; IDQoL: Infants’ Dermatitis Quality of Life; DFI: Dermatitis Family Impact, RCT: Randomized controlled trial
Figure 6:
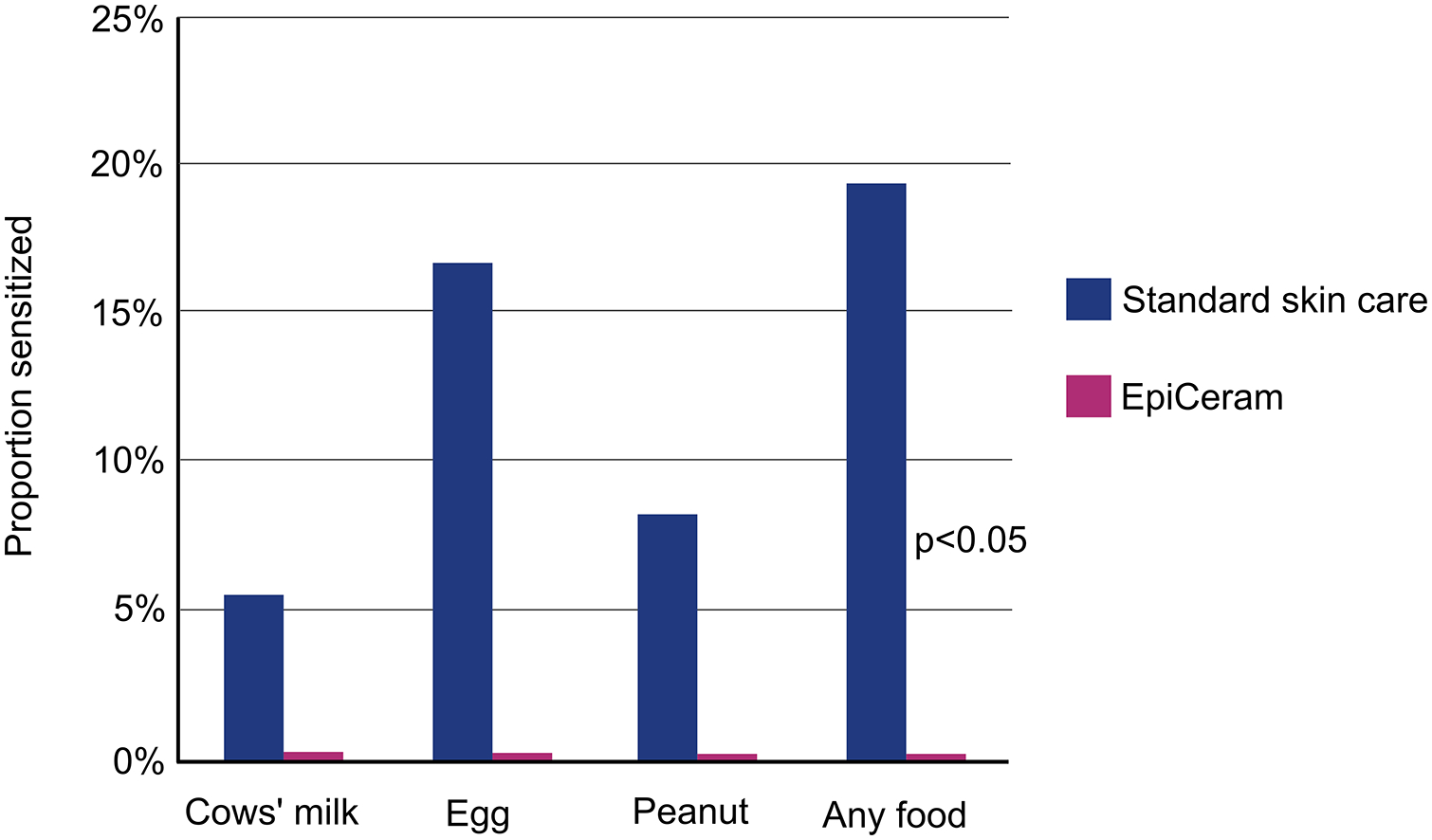
Effect of twice-daily EpiCeram® vs standard skin care for the first 6 months on skin prick test reactivity to food allergens at 12 months. Results were limited to intervention participants who were treated at least 5 times per week on average. Per protocol analyses revealed a significant reduction in food sensitization at 12 months in the treatment group (0%, 0 of 21) compared to the control group (19%, 7 of 36). Reproduced with permission from Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. Ann Allergy Asthma Immunol. 2018 Feb;120(2):145–151.
Although the experimental and mechanistic studies, to date, build a strong case towards targeting the skin to prevent AD to thereby FA, two large preventative emollient RCTs have been disappointingly negative (BEEP and PreventADALL); there are several reasons why they might not show a significant effect, as outlined below:
The age at which emollients were first applied: If emollients are applied after significant sensitization has progressed, it may difficult to prevent FA. In PEBBLES (Table 2), reduction in sensitization was only found in infants where application commenced before 3 weeks. Miyaji et al.44 (Supplementary Table A) showed that the time period until the of proactive topical steroid treatment in AD was a risk factor for FA at 24 months of age.
The rate of adherence with the study intervention: The PEBBLES study (Table 2) only showed a reduction in sensitization rates if emollients were applied twice daily for ≥5 days a week. In contrast, in the PreventADALL158 and BEEP159 study, adherence was defined as emollient application once a day, at least 3–4 times a week (less than the 5 days in PEBBLES study).
Emollient properties: The properties of emollients on skin hydration, skin pH, restoration of the skin barrier and immune function are all important properties to consider in the choice of emollient for the prevention of FA162 (Supplementary Table C). Emollients can have high pH levels, and high skin pH which can damage skin proteins and lipids, leading to dryness, irritation, itching and increased TEWL.163 BEEP and PreventADALL RCTs (Table 1) both used petrolatum-based emollients which have been shown to have lower benefits on TEWL compared to ceramide-containing trilipid creams used in PEBBLES (Table 2).164, 165
There may be a disconnect between AD and FA development depending on what needs to be reduced (e.g. dryness or inflammation or both). This disconnect was shown in other treatments for the prevention of AD that did not result in a reduction of IgE (with probiotics).166
AD is not just a barrier defect, but also an inflammatory process. Targeting inflammation early and aggressively with topical steroids has been shown to be associated with a significant reduction in food sensitization and allergy in infants with moderate to severe AD.44 Thus treating only the barrier defect in AD may not be enough to protect against the development of FA.
The role of environmental food exposure in these studies has not been assessed. It is not known if parents washed their hands prior to application of cream and whether increasing the contact of hands onto the skin of the child may have inadvertently increased topical food exposure.
SUMMARY
There is an increasing body of evidence to support the role of a disrupted, inflamed skin barrier being the root cause for the development of food sensitization and allergy.167 Interest in restoring the skin barrier to prevent AD and FA has gained ground in recent years. Oral tolerance induction has offered a completely new strategy for the prevention of FA; results are compelling for PA24 and moderate for egg allergy.25 If this strategy were to work for other food allergens, it would require the introduction of multiple food allergens very early on in life. Targeting the skin to prevent AD or reduce the duration and severity of AD could prevent the development of FA, by either preventing epicutaneous sensitization, or by increasing the window of opportunity to induce oral tolerance by early allergenic food introduction. However, results from two large RCTs using preventative emollient therapy have had initial negative results for the prevention of AD (BEEP159 and PreventADALL158) and FA (BEEP)159 and other RCTs are ongoing (CASCADE- A Community-based Assessment of Skin Care, Allergies, and Eczema, and PEBBLES) (Tables 1 and 2). There are also secondary prevention studies (Table 3) using both emollients and proactive topical steroid treatments ongoing or planned (PACI study: Prevention of Allergy via Cutaneous Intervention Study and SEAL-Stopping Atopic Dermatitis and Allergy Study).168 Regardless as to whether these studies are positive or negative in their findings, they will help inform this important area of research.
Supplementary Material
BOX 1: Major milestone discoveries.
1911: Oral tolerance induction is first described in an animal model.169
1994: The first report of epicutaneous sensitization leading to IgE mediated FA is followed by large body of evidence in experimental models.71
1996: The Dual Allergen Exposure Hypothesis is first proposed in 1996.170
2003: The ALSPAC birth cohort, shows an association between peanut oil containing creams applied to the skin of children with AD and the development of PA.40
2006: FLG loss-of-function mutations are confirmed to be major predisposing factor for AD,99 thus arguing that AD is a disease resulting from a defective skin barrier.
2008: FLG loss-of-function were shown to be associated with challenge proven IgE-mediated PA, even after adjusting for AD.107
2009: High household peanut consumption was associated with the development of PA.129
2014–2015: High environmental exposure to peanut (measured in dust) was shown to increases the risk of peanut sensitization and likely allergy in children with mutations in the FLG gene or with AD.129
2015: LEAP randomised controlled trial demonstrated that oral peanut ingestion from 4–11 months of age prevented PA in high-risk infants.23, 24
2013: HealthNuts study found that by 12 months of age, 3.1% of children had challenge proven FA and 9% were egg allergic.1 There is therefore a narrow window of opportunity to induce tolerance.
2014: Japanese,156US and UK171 preventative emollient therapy pilot studies showed that early emollient therapy in high risk children reduced the onset of AD by 33–50%.
2015–2016: TEWL at 2 days was shown to predict not only AD,153 but also FA at 2 years,154 providing an early marker of disrupted skin barrier predictive of later disease.
2017: PEBBLES pilot study demonstrated a trend towards a reduction in AD and food sensitization in infants treated with emollient therapy for 6 months.161
2019: Early (≤4 months) versus later (>4months) proactive use of topical steroids in infants with AD was shown to be associated with a 45% reduction in FA at 2 years (retrospective study).44
2019: A novel non-invasive technique using skin tape stripping identified skin barrier dysfunction in AD with FA subjects.137
2020: Two large preventative emollient RCTs using petrolatum-based creams/bath oils show no significant effect on the prevention of AD (BEEP159 and PreventADALL158) or FA (BEEP)159.
BOX 2: Future research perspectives.
To determine at what age interventions to prevent the development of food sensitization and allergy should commence, and the duration of these interventions.
The degree of adherence to therapies targeting the skin required to achieve a reduction in food sensitization and allergy.
The importance of different emollient properties (skin hydration, skin pH, restoration of the skin barrier and immune function) for preventing food sensitization and allergy.
The role of topical anti-inflammatory agents as part of the skin care regime to prevent food sensitization and allergy.
The role of reducing environmental food allergen exposure on reduction of sensitization and allergy.
Reduction in S. aureus colonisation in at risk children on the prevention of FA.
Acknowledgements
We thank the Levin Foundation, the Reinhard Foundation, the Sean N. Parker Center for Allergies and Asthma Research at Stanford University, and the NIH (grants R01AI140134 and U19AI070535) for their generous support.
ABBREVIATIONS:
- AA
Allergic asthma
- AD
Atopic dermatitis
- AEs
Adverse Events
- API
Asthma Predictive Index
- AR
Allergic Rhinitis
- BEEP
Barrier Enhancement for Eczema Prevention
- CER
Ceramides
- CEQ
Children’s Eczema Questionnaire
- CLA+
Cutaneous Lymphocyte Antigen
- DC
Dendritic cell
- DFI
Dermatitis Family Impact
- EASI
Eczema Area and Severity Index
- EOS CER
fatty acid ultra long chained ceramides
- FA
Food allergy
- FLG
Filaggrin gene
- TSLP
Thymic stromal lymphopoietin
- IDQoL
Infants’ Dermatitis Quality of Life
- IL
Interleukin
- ILC2
Type 2 innate lymphoid cells
- IP
Intraperitoneal
- LC
Langerhans cells
- LN
Lymph node
- OFC
Oral food challenge
- OVA
Ovalbumin
- PA
Peanut allergy
- PEBBLES
Prevention of Eczema By a Barrier Lipid Equilibrium Strategy
- PGH
Pediatric Global Health
- PreventADALL
Preventing Atopic Dermatitis and ALLergies in Children
- POEM
Patient Oriented Eczema Measure
- PROMIS
Patient Reported Outcome Measurement Information System
- RCT
Randomized controlled trial
- S. aureus
Staphylococcal aureus
- S
Sphingosine
- SC
Stratum corneum
- SCORAD
SCORing Atopic Dermatitis
- SDI
Shannon Diversity Index
- SEB
Staphylococcus enterotoxin B
- sIgE
Allergen-specific IgE antibodies
- SPT
Skin prick testing
- TARC
Thymus and activation-regulated chemokine
- TEWL
Transepidermal water loss
- Th
T helper cells
Footnotes
Conflict of Interest Statement
Dr. Brough reports personal fees from DBV Technologies, and non-financial support from ThermoScientific; Dr. Nadeau reports grants from National Institute of Allergy and Infectious Diseases (NIAID), Food Allergy Research & Education (FARE), End Allergies Together (EAT), Allergenis, and Ukko Pharma; Grant awardee at NIAID, National Institute of Environmental Health Sciences (NIEHS), National Heart, Lung, and Blood Institute (NHLBI), and the Environmental Protection Agency (EPA); is involved in Clinical trials with Regeneron, Genentech, AImmune Therapeutics, DBV Technologies, AnaptysBio, Adare Pharmaceuticals, and Stallergenes-Greer; Research Sponsorship by Novartis, Sanofi, Astellas, Nestle; Data and Safety Monitoring Board member at Novartis and NHLBI; Cofounded Before Brands, Alladapt, ForTra, and Iggenix; Chief Intellectual Office at FARE, Director of the World Allergy Organization (WAO) Center of Excellence at Stanford, Personal fees from Regeneron, Astrazeneca, ImmuneWorks, and Cour Pharmaceuticals; Consultant and Advisory Board Member at Ukko, Before Brands, Alladapt, IgGenix, Probio, Vedanta, Centecor, Seed, Novartis, NHBLI, EPA, National Scientific Committee of ITN and NIH Programs; US patents (patent numbers 62/647,389; 62/119,014; 12/610,940, 12/686,121, 10/064,936, 62/767,444; application numbers S10–392); Dr. Sindher reports grants from Aimmune, DBV Technologies, and Regeneron; Dr. Chan reports non-financial support from Novartis and grants from Aimmune; Dr. Bahnson reports personal fees from King’s College and DBV technologies; Dr. Lack reports grants from National Institute of Allergy and Infectious
Diseases (NIAID, NIH), Food Allergy & Research Education (FARE), MRC & Asthma UK Centre, UK Dept of Health through NIHR, National Peanut Board (NPB), UK Food Standards Agency (FSA), The Davis Foundation, Scientific Advisory Board member and Stock Options at DBV Technologies, Shareholder at Mighty Mission Me, personal fees/consultancy from Novartis, personal fees/consultancy from Sanofi-Genyzme, personal fees/consultancy from Regeneron, personal fees and consultancy from ALK-Abello; Drs. Alkotob and Leung have nothing to disclose
References
- 1.Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clin Exp Allergy 2013;43:642–651. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Haider S, Colicino S, Murray CS, Holloway J, Simpson A, et al. Different definitions of atopic dermatitis: impact on prevalence estimates and associated risk factors. Br J Dermatol 2019;181:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018;32:657–682. [DOI] [PubMed] [Google Scholar]
- 4.Borna E, Nwaru BI, Bjerg A, Mincheva R, Radinger M, Lundback B, et al. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019;74:1703–1715. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma. Global strategy for asthma management and prevention (2018 update). 2018. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed Feb 24, 2020
- 6.Meltzer EO. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol Allergy Clin North Am 2016;36:235–248. [DOI] [PubMed] [Google Scholar]
- 7.Scott LA, Jones BI, Berni TR, Berni ER, De Vries J, Currie CJ. Evaluation of the epidemiology of peanut allergy in the United Kingdom. Expert Rev Clin Immunol 2019;15:1333–1339. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Allen KJ, Suaini NHA, McWilliam V, Peters RL, Koplin JJ. The global incidence and prevalence of anaphylaxis in children in the general population: A systematic review. Allergy 2019;74:1063–1080. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaker MS, Schwartz J, Ferguson M. An update on the impact of food allergy on anxiety and quality of life. Curr Opin Pediatr 2017;29:497–502. [DOI] [PubMed] [Google Scholar]
- 11.Dufresne E, Poder TG, Begin P. The value of oral immunotherapy. Allergy 2019. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- 13.Upton J, Alvaro M, Nadeau K. A perspective on the pediatric death from oral food challenge reported from the Allergy Vigilance Network. Allergy 2019;74:1035–1036. [DOI] [PubMed] [Google Scholar]
- 14.Tran MM, Lefebvre DL, Dharma C, Dai D, Lou WYW, Subbarao P, et al. Predicting the atopic march: Results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol 2018;141:601–607 e608. [DOI] [PubMed] [Google Scholar]
- 15.Sicherer SH, Wood RA, Perry TT, Jones SM, Leung DYM, Henning AK, et al. Clinical factors associated with peanut allergy in a high-risk infant cohort. Allergy 2019;74:2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clinical and Experimental Allergy 2015;45:255–264. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013;167:1026–1031. [DOI] [PubMed] [Google Scholar]
- 18.Wai HM, Middelveld R, Thornqvist V, Ballardini N, Nilsson E, Stromquist J, et al. Pediatric food allergy-related household costs are influenced by age, but not disease severity. World Allergy Organ J 2019;12:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell-Smith R, Turner P. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health 2016;136:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346–349. [PubMed] [Google Scholar]
- 21.Tham EH, Rajakulendran M, Lee BW, Van Bever HPS. Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know? Pediatr Allergy Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 22.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015;135:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med 2016;374:1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N Engl J Med 2015;372:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkin MR, Logan K, Bahnson HT, Marrs T, Radulovic S, Craven J, et al. Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy. J Allergy Clin Immunol 2019;144:1606–1614 e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. JAMA 2016;316:1181–1192. [DOI] [PubMed] [Google Scholar]
- 27.Pitt TJ, Becker AB, Chan-Yeung M, Chan ES, Watson WTA, Chooniedass R, et al. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. J Allergy Clin Immunol 2018;141:620–625.e621. [DOI] [PubMed] [Google Scholar]
- 28.du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol 2018;141:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 30.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med 2016;374:1733–1743. [DOI] [PubMed] [Google Scholar]
- 31.Venter C, Greenhawt M, Meyer RW, Agostoni C, Reese I, du Toit G, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: Novel concepts and implications for studies in allergy and asthma. Allergy 2019. [DOI] [PubMed] [Google Scholar]
- 32.Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr 2015;104:38–53. [DOI] [PubMed] [Google Scholar]
- 33.Yepes-Nunez JJ, Brozek JL, Fiocchi A, Pawankar R, Cuello-Garcia C, Zhang Y, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy 2018;73:37–49. [DOI] [PubMed] [Google Scholar]
- 34.Hawrylowicz CM, Santos AF. Vitamin D: can the sun stop the atopic epidemic? Curr Opin Allergy Clin Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 35.Wang HT, Anvari S, Anagnostou K. The Role of Probiotics in Preventing Allergic Disease. Children (Basel) 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: Microbiome. Allergy 2018;73:2314–2327. [DOI] [PubMed] [Google Scholar]
- 37.Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol 2018;120:145–151. [DOI] [PubMed] [Google Scholar]
- 38.Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. [Review]. Allergy 2014;69:17–27. [DOI] [PubMed] [Google Scholar]
- 39.Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J Allergy Clin Immunol 2019;143:894–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lack G, Fox D, Northstone K, Golding J, Team ALSoPaCS. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003;348:977–985. [DOI] [PubMed] [Google Scholar]
- 41.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018;120:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill DJ, Hosking CS, Oranje AP, Bauchau V, Naspitz CK, Simons FER, et al. Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: An international study. Clin Exp Allergy 2008;38:161–168. [DOI] [PubMed] [Google Scholar]
- 43.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol 2013;131:135–143 e131–112. [DOI] [PubMed] [Google Scholar]
- 44.Miyaji Y, Yang L, Yamamoto-Hanada K, Narita M, Saito H, Ohya Y. Earlier aggressive treatment to shorten the duration of eczema in infants resulted in fewer food allergies at 2 years of age. J Allergy Clin Immunol Pract 2019. [DOI] [PubMed] [Google Scholar]
- 45.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol 2016;137:984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 2016;138:336–349. [DOI] [PubMed] [Google Scholar]
- 47.Palomares O, Akdis M, Martin-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev 2017;278:219–236. [DOI] [PubMed] [Google Scholar]
- 48.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int 2016;65:243–252. [DOI] [PubMed] [Google Scholar]
- 49.Zissler UM, Esser-von Bieren J, Jakwerth CA, Chaker AM, Schmidt-Weber CB. Current and future biomarkers in allergic asthma. Allergy 2016;71:475–494. [DOI] [PubMed] [Google Scholar]
- 50.Furue M, Yamamura K, Kido-Nakahara M, Nakahara T, Fukui Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018;73:29–36. [DOI] [PubMed] [Google Scholar]
- 51.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012;18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med 2012;18:736–749. [DOI] [PubMed] [Google Scholar]
- 53.Sindher SB, Long A, Acharya S, Sampath V, Nadeau KC. The Use of Biomarkers to Predict Aero-Allergen and Food Immunotherapy Responses. Clin Rev Allergy Immunol 2018;55:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holm J, Willumsen N, Wurtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol 2011;127:1029–1037. [DOI] [PubMed] [Google Scholar]
- 55.Turcanu V, Stephens AC, Chan SM, Rance F, Lack G. IgE-mediated facilitated antigen presentation underlies higher immune responses in peanut allergy. Allergy 2010;65:1274–1281. [DOI] [PubMed] [Google Scholar]
- 56.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen QA, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernink JH, Germar K, Spits H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr Opin Immunol 2014;31:115–120. [DOI] [PubMed] [Google Scholar]
- 58.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 2013;14:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pasha MA, Patel G, Hopp R, Yang Q. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc 2019;40:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bieber T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2019. [DOI] [PubMed] [Google Scholar]
- 61.Zhu TH, Zhu TR, Tran KA, Sivamani RK, Shi VY. Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation. Br J Dermatol 2018;179:570–581. [DOI] [PubMed] [Google Scholar]
- 62.Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol 2013;171:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–680. [DOI] [PubMed] [Google Scholar]
- 64.Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy 2002;32:866–871. [DOI] [PubMed] [Google Scholar]
- 65.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005;202:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Nat Acad Sci USA 2008;105:11875–11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou B, Comeau MR, De ST, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature Immunol 2005;6:1047–1053. [DOI] [PubMed] [Google Scholar]
- 68.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol 2014;133:1390–1399, 1399.e1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest 1992;90:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, et al. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol 2007;178:3373–3377. [DOI] [PubMed] [Google Scholar]
- 71.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 2013;131:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol 2007;178:1396–1404. [DOI] [PubMed] [Google Scholar]
- 73.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 984;126:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 2016;17:122–131. [DOI] [PubMed] [Google Scholar]
- 75.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 2014;31:31–37. [DOI] [PubMed] [Google Scholar]
- 76.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 2012;132:1392–1400. [DOI] [PubMed] [Google Scholar]
- 77.Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J Am Acad Dermatol 2014;70:882–888. [DOI] [PubMed] [Google Scholar]
- 78.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 2014;124:4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–490. [DOI] [PubMed] [Google Scholar]
- 80.Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy 2012;67:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol 2014;26:539–549. [DOI] [PubMed] [Google Scholar]
- 82.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol 2016;138:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen YL, Gutowska-Owsiak D, Hardman CS, Westmoreland M, MacKenzie T, Cifuentes L, et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med 2019;11. [DOI] [PubMed] [Google Scholar]
- 85.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol 2004;34:2100–2109. [DOI] [PubMed] [Google Scholar]
- 86.Saloga J, Renz H, Larsen GL, Gelfand EW. Increased airways responsiveness in mice depends on local challenge with antigen. Am J Resp Crit Care Med 1994;149:65–70. [DOI] [PubMed] [Google Scholar]
- 87.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest 1998;101:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Investig 2003;112:1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol 2010;125:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest 2000;105:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsieh KY, Tsai CC, Wu CH, Lin RH. Epicutaneous exposure to protein antigen and food allergy. Clin Exp Allergy 2003;33:1067–1075. [DOI] [PubMed] [Google Scholar]
- 92.Herrick CA, Xu L, McKenzie AN, Tigelaar RE, Bottomly K. IL-13 is necessary, not simply sufficient, for epicutaneously induced Th2 responses to soluble protein antigen. Journal of Immunology 2003;170:2488–2495. [DOI] [PubMed] [Google Scholar]
- 93.Leyva-Castillo JM, Galand C, Kam C, Burton O, Gurish M, Musser MA, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol 2018;142:381–393. [DOI] [PubMed] [Google Scholar]
- 95.Kawasaki A, Ito N, Murai H, Yasutomi M, Naiki H, Ohshima Y. Skin inflammation exacerbates food allergy symptoms in epicutaneously sensitized mice. Allergy 2018;73:1313–1321. [DOI] [PubMed] [Google Scholar]
- 96.Wang L-F, Lin J-Y, Hsieh K-H, Lin R-H. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol 1996:4079–4082. [PubMed] [Google Scholar]
- 97.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018;141:1711–1725 e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol 2012;132:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441–446. [DOI] [PubMed] [Google Scholar]
- 100.Hudson TJ. Skin barrier function and allergic risk. Nat Genet 2006;38:399–400. [DOI] [PubMed] [Google Scholar]
- 101.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 2009;124:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kortekaas Krohn I, Seys SF, Lund G, Jonckheere AC, Dierckx de Casterle I, Ceuppens JL, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2019. [DOI] [PubMed] [Google Scholar]
- 103.Gschwandtner M, Mildner M, Mlitz V, Gruber F, Eckhart L, Werfel T, et al. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy 2013;68:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014;134:792–799. [DOI] [PubMed] [Google Scholar]
- 105.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gutowska-Owsiak D, Schaupp AL, Salimi M, Taylor S, Ogg GS. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br J Dermatol 2011;165:492–498. [DOI] [PubMed] [Google Scholar]
- 107.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011;127:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan HT, Ellis JA, Koplin JJ, Matheson MC, Gurrin LC, Lowe AJ, et al. Filaggrin loss-of-function mutations do not predict food allergy over and above the risk of food sensitization among infants. J Allergy Clin Immunol 2012;130:1211–1213 e1213. [DOI] [PubMed] [Google Scholar]
- 109.Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol 2014;134:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol 2014;134:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marenholz I, Esparza-Gordillo J, Lee YA. The genetics of the skin barrier in eczema and other allergic disorders. Curr Opin Allergy Clin Immunol 2015;15:426–434. [DOI] [PubMed] [Google Scholar]
- 112.Oji V, Eckl KM, Aufenvenne K, Natebus M, Tarinski T, Ackermann K, et al. Loss of corneodesmosin leads to severe skin barrier defect, pruritus, and atopy: unraveling the peeling skin disease. Am J Hum Genet 2010;87:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashley SE, Tan HT, Vuillermin P, Dharmage SC, Tang MLK, Koplin J, et al. The skin barrier function gene SPINK5 is associated with challenge-proven IgE-mediated food allergy in infants. Allergy 2017;72:1356–1364. [DOI] [PubMed] [Google Scholar]
- 114.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 2013;45:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008;126:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xian M, Wawrzyniak P, Ruckert B, Duan S, Meng Y, Sokolowska M, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol 2016;138:890–893.e899. [DOI] [PubMed] [Google Scholar]
- 117.Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol 2019;143:1892–1903. [DOI] [PubMed] [Google Scholar]
- 118.Yanase K, Hatta I. Disruption of human stratum corneum lipid structure by sodium dodecyl sulphate. Int J Cosmet Sci 2018;40:44–49. [DOI] [PubMed] [Google Scholar]
- 119.Ochi H, Takai T, Shimura S, Maruyama N, Nishioka I, Kamijo S, et al. Skin Treatment with Detergent Promotes Protease Allergen-Dependent Epicutaneous Sensitization in a Manner Different from Tape Stripping in Mice. J Invest Dermatol 2017;137:1578–1582. [DOI] [PubMed] [Google Scholar]
- 120.Khosrowpour Z, Ahmad Nasrollahi S, Ayatollahi A, Samadi A, Firooz A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J Cosmet Dermatol 2019;18:857–861. [DOI] [PubMed] [Google Scholar]
- 121.Fackelmann G, Sommer S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar Pollut Bull 2019;143:193–203. [DOI] [PubMed] [Google Scholar]
- 122.Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ 2019;684:657–669. [DOI] [PubMed] [Google Scholar]
- 123.Wu S, Wu M, Tian D, Qiu L, Li T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ Toxicol 2019. [DOI] [PubMed] [Google Scholar]
- 124.Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater 2020;385:121575. [DOI] [PubMed] [Google Scholar]
- 125.Chan SM, Turcanu V, Stephens AC, Fox AT, Grieve AP, Lack G. Cutaneous lymphocyte antigen and alpha4beta7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy 2012;67:336–342. [DOI] [PubMed] [Google Scholar]
- 126.Weissler KA, Rasooly M, DiMaggio T, Bolan H, Cantave D, Martino D, et al. Identification and analysis of peanut-specific effector T and regulatory T cells in children allergic and tolerant to peanut. J Allergy Clin Immunol 2018;141:1699–1710 e1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brough HAJ-L ZK; Logan K; Richards K; Perkin M; Peacock J; Flohr C; Lack G Early exposure to environmental peanut in dust is associated with the development of peanut allergy but this effect is abrogated by early peanut consumption In: European Asthma, Allergy and Clinical Immunology Annual Conference Munich, Germany; 2018. [Google Scholar]
- 128.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol 2014;134:867–875 e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009;123:417–423. [DOI] [PubMed] [Google Scholar]
- 130.Brough HA, Santos A, Makinson K, Penagos M, Stephens AC, Fox AT, et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 2013;132:630–638. [DOI] [PubMed] [Google Scholar]
- 131.Sheehan WJ, Brough HA, Makinson K, Petty CR, Lack G, Phipatanakul W. Distribution of peanut protein in school and home environments of inner-city children. J Allergy Clin Immunol 2017;140:1724–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simpson A, Brough HA, Haider S, Belgrave D, Murray CS, Custovic A. Early-life inhalant allergen exposure, filaggrin genotype, and the development of sensitization from infancy to adolescence. J Allergy Clin Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alexander H, Paller AS, Traidl-Hoffmann C, Beck LA, De Benedetto A, Dhar S, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol 2013;23:R389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams MR, Gallo RL. The Role of the Skin Microbiome in Atopic Dermatitis. Curr Allergy Asthma Rep 2015;15:65. [DOI] [PubMed] [Google Scholar]
- 136.Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol 2016;175:687–695. [DOI] [PubMed] [Google Scholar]
- 137.Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gallo RL. Targeted Microbiome Transplant in Atopic Dermatitis. 2018. Available from: http://grantome.com/grant/NIH/U19-AI117673-04-8604. Accessed March 26, 2020.
- 140.Schwartz C, Moran T, Saunders SP, Kaszlikowska A, Floudas A, Bom J, et al. Spontaneous atopic dermatitis in mice with a defective skin barrier is independent of ILC2 and mediated by IL-1beta. Allergy 2019;74:1920–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saloga J, Lack G, Bradley K, Renz H, Larsen G, Leung DY, et al. Inhibition of the development of immediate hypersensitivity by staphylococcal enterotoxin B. Eur J Immunol 1994;24:3140–3147. [DOI] [PubMed] [Google Scholar]
- 142.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol 2009;123:231–238 e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Savinko T, Lauerma A, Lehtimaki S, Gombert M, Majuri ML, Fyhrquist-Vanni N, et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J Immunol 2005;175:8320–8326. [DOI] [PubMed] [Google Scholar]
- 144.Forbes-Blom E, Camberis M, Prout M, Tang SC, Le Gros G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin Exp Allergy 2012;42:305–314. [DOI] [PubMed] [Google Scholar]
- 145.Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Horimukai K, Morita K, Narita M, Kondo M, Kabashima S, Inoue E, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol Int 2016;65:103–108. [DOI] [PubMed] [Google Scholar]
- 147.Berents TL, Lodrup Carlsen KC, Mowinckel P, Skjerven HO, Rolfsjord LB, Bradley M, et al. Transepidermal water loss in infancy associated with atopic eczema at 2 years of age: a population-based cohort study. Br J Dermatol 2017;177:e35–e37. [DOI] [PubMed] [Google Scholar]
- 148.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Brit J Derm 2010;163:1333–1336. [DOI] [PubMed] [Google Scholar]
- 149.Perkin MR, Craven J, Logan K, Strachan D, Marrs T, Radulovic S, et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J Allergy Clin Immunol 2016;138:509–516. [DOI] [PubMed] [Google Scholar]
- 150.Engebretsen KA, Bager P, Wohlfahrt J, Skov L, Zachariae C, Nybo Andersen AM, et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: Cohort study. J Allergy Clin Immunol 2017;139:1568–1574.e1561. [DOI] [PubMed] [Google Scholar]
- 151.Jabbar-Lopez ZK, Gurung N, Greenblatt D, Briley A, Chalmers JR, Thomas KS, et al. Protocol for an outcome assessor-blinded pilot randomised controlled trial of an ion-exchange water softener for the prevention of atopic eczema in neonates, with an embedded mechanistic study: the Softened Water for Eczema Prevention (SOFTER) trial. BMJ Open 2019;9:e027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. [Review] [215 refs]. J Allergy Clin Immunol 2006;118:3–21. [DOI] [PubMed] [Google Scholar]
- 153.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol 2015;135:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol 2016;137:1111–1116 e1118. [DOI] [PubMed] [Google Scholar]
- 155.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134:824–830. [DOI] [PubMed] [Google Scholar]
- 157.Yonezawa K, Haruna M, Matsuzaki M, Shiraishi M, Kojima R. Effects of moisturizing skincare on skin barrier function and the prevention of skin problems in 3-month-old infants: A randomized controlled trial. J Dermatol 2018;45:24–30. [DOI] [PubMed] [Google Scholar]
- 158.Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020. [DOI] [PubMed] [Google Scholar]
- 159.Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dissanayake E, Tani Y, Nagai K, Sahara M, Mitsuishi C, Togawa Y, et al. Skin Care and Synbiotics for Prevention of Atopic Dermatitis or Food Allergy in Newborn Infants: A 2 × 2 Factorial, Randomized, Non-Treatment Controlled Trial. Int Arch Allergy Immunol 2019;180:202–211. [DOI] [PubMed] [Google Scholar]
- 161.Lowe AJ, Su JC, Allen KJ, Abramson MJ, Cranswick N, Robertson CF, et al. A randomised trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitisation: The PEBBLES Pilot Study. Br J Dermatol 2018;178:e19–e21. [DOI] [PubMed] [Google Scholar]
- 162.Elias PM, Sugarman J. Does moisturizing the skin equate with barrier repair therapy? Ann Allergy Asthma Immunol 2018;121:653–656 e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ananthapadmanabhan KP, Moore DJ, Subramanyan K, Misra M, Meyer F. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatologic Therapy 2004;17:Suppl-25. [DOI] [PubMed] [Google Scholar]
- 164.Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol 1995;131:809–816. [DOI] [PubMed] [Google Scholar]
- 165.Sindher SB, Alkotob SS, Shojinaga MN Brough HA Bahnson T, Chan S, Lack G, Leung DYM, Nadeau KC. Pilot study measuring transepidermal water loss in children suggests trilipid cream is more effective than a paraffin-based emollient. Allergy. Accepted Feb 2020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1604–1610. [DOI] [PubMed] [Google Scholar]
- 167.Tsakok T, Woolf R, Smith CH, Weidinger S, Flohr C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol 2019;180:464–474. [DOI] [PubMed] [Google Scholar]
- 168.Yamamoto-Hanada K, Kobayashi T, Williams HC, Mikami M, Saito-Abe M, Morita K, et al. Early aggressive intervention for infantile atopic dermatitis to prevent development of food allergy: a multicenter, investigator-blinded, randomized, parallel group controlled trial (PACI Study)-protocol for a randomized controlled trial. Clin Transl Allergy 2018;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wells HG, Osborne TB. The biological reactions of the vegetable proteins I. anaphylaxis. J Infect Dis 1911;8:66–124. [Google Scholar]
- 170.Lack G, Golding J. Peanut and nut allergy. Reduced exposure might increase allergic sensitisation. BMJ 1996;313:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lowe A, Su J, Tang M, Lodge CJ, Matheson M, Allen KJ, et al. PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ Open 2019;9:e024594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


