Summary Statement
We discuss minimally-invasive and non-invasive cardiac output monitoring technologies available in the clinical practice and how to evaluate these systems objectively.
Introduction
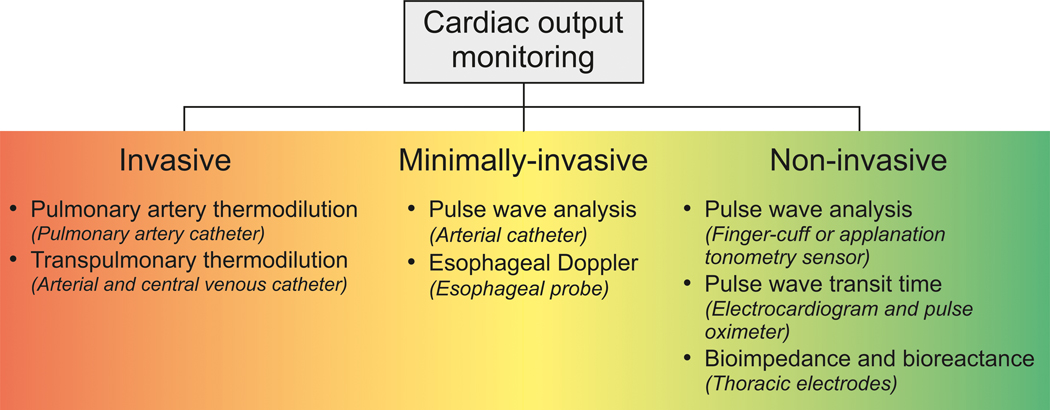
Cardiac output (CO) is a main determinant of oxygen delivery. Maintenance of adequate CO is thus a mainstay of hemodynamic management in perioperative and intensive care medicine. Methods to measure CO can be classified as invasive, minimally-invasive, or non-invasive methods (Figure 1).1 While invasive indicator dilution methods (i.e., pulmonary artery and transpulmonary thermodilution) remain the clinical reference methods for CO measurement,2 numerous minimally-invasive and non-invasive methods to estimate CO have been proposed in recent years.1,3–5 Understanding the principles of these systems and their limitations is crucial to be able to select the appropriate method for the individual patient and clinical setting.6 In this manuscript, we describe minimally-invasive and non-invasive CO monitoring technologies available in the clinical practice and we discuss how to evaluate these systems objectively. After reading the manuscript, the readers will be able to understand how these new monitoring systems work and how to evaluate their measurement performance.
Figure 1.
Cardiac Output Monitoring Methods
Minimally-invasive CO monitoring methods
Minimally-invasive CO monitoring methods include arterial catheter-based pulse wave analysis and the esophageal Doppler (Table 1).1
Table 1:
Minimally-Invasive and Non-Invasive Cardiac Output Monitoring
| Method | Device name | Pitfalls | |
|---|---|---|---|
| Minimally-invasive cardiac output monitoring methods | |||
| Minimally-invasive pulse wave analysis | FloTrac (Edwards Lifesciences) ProAQT/Pulsioflex (Pulsion) LiDCOrapid (LiDCO) Argos CO monitor (Retia Medical) Mostcare UP (Vygon) |
• Invasive • Estimation of stroke volume relies on theoretical assumptions • Measurement performance essentially depends on blood pressure waveform quality • Rapid changes in vasomotor tone make CO estimations less reliable (e.g., patients with liver disease or liver surgery and septic patients) |
|
| Esophageal Doppler | CardioQ-ODM (Deltex Medical) | • (Invasive) • Operator-dependent • Prone to motion artifacts, not easily usable in awake and alert patients • Assumption of constant distribution of arterial blood flow between the upper and lower parts of the body does not hold in all pathophysiologic circumstances • Estimation of blood flow depends on the correct estimation of the diameter of the aorta |
|
| Non-invasive cardiac output monitoring methods | |||
| Non-invasive pulse wave analysis | Finger cuff method | CNAP (CNSystems) ClearSight (Edwards Lifesciences) |
• Same general limitations as minimally-invasive pulse wave analysis • Limited in patients with peripheral vasoconstriction, impaired finger perfusion, and severe peripheral edema |
| Radial artery applanation tonometry | T-Line (Shanshi International Medical Group) DMP Life (DAEYOMEDI) |
• Same general limitations as minimally-invasive pulse wave analysis • Prone to motion artifacts |
|
| Pulse wave transit time | esCCO (Nihon Kohden) | • Does not work when patients have cardiac arrhythmias or rapid changes in peripheral vascular tone | |
| Thoracic bioimpedance and bioreactance | Thoracic bioimpedance | BioZ (Cardiodynamics) CSM3000 (Cheers Sails Medical) ICG (Philips Medical Systems) ICON (Osypka Cardiotronic) NCCOM (Bomed Medical) NICOMON (Larsen and Toubro) Physioflow (Manatec Biomedical) |
• Prone to motion artifacts and electrical interference • Limited in patients with arrhythmias and mechanical ventilation • Erroneous stroke volume estimations in patients with obesity, pleural effusion, and pulmonary edema |
| Thoracic bioreactance | NICOM (Cheetah Medical) Starling (Cheetah Medical) |
||
Minimally-invasive pulse wave analysis
CO can be estimated by pulse wave analysis, i.e., by mathematically analyzing the shape and characteristics of the arterial pressure waveform.7–9 Minimally-invasive pulse wave analysis systems analyze an arterial pressure waveform recorded with an arterial catheter (most systems are optimized to analyze radial arterial pressure waveforms).
In contrast to externally calibrated invasive pulse wave analysis systems that use a reference indicator dilution method to calibrate CO estimations (e.g., VolumeView, Edwards Lifesciences, Irvine, CA, USA; PiCCO; Pulsion Medical Systems, Feldkirchen, Germany; LiDCOplus, LiDCO, Cambridge, UK)7 minimally-invasive pulse wave analysis only requires an arterial catheter and uses the waveform characteristics as well as biometric and demographic data to estimate stroke volume. Different minimally-invasive pulse wave analysis methods use different physiologic assumptions and apply different mathematical models to estimate stroke volume.7,9,10
The FloTrac System (Edwards Lifesciences) empirically estimates stroke volume using a proprietary hemodynamic database from pulse pressure and vascular tone, with the latter being estimated from mean arterial pressure and numerous arterial pressure waveform features.9 The ProAQT/Pulsioflex System (Pulsion) derives stroke volume from the area of the systolic portion of arterial pressure waveform and uses patient data to internally calibrate stroke volume estimations and account for compliance of the aorta. The LiDCOrapid system (LiDCO) estimates stroke volume using pulse power analysis and a nomogram including age, weight, height, body surface area, and aortic volume. The Argos CO monitor (Retia Medical, Valhalla, NY, USA) uses so-called multi-beat analysis to estimate CO after analyzing the arterial pressure waveform over periods of several heart beats and scaling CO estimations to biometric data.11–14 The MostCare system (Vygon, Écouen, France) uses the pressure recording analytical method; it analyzes the systolic and diastolic part of the arterial pressure waveform with a frequency of 1,000Hz and estimates CO considering the arterial impedance and the impact of reflected pulse waves on the forward traveling pulse wave.15,16
The main advantage of pulse wave analysis is that CO can be estimated continuously, with rapid response time. Continuous CO monitoring is considered the optimal way to monitor the response to fluid responsiveness tests, such as a fluid challenge maneuver17 or a passive leg raising test.18 In addition, pulse wave analysis allows for the determination of dynamic variables of cardiac preload – i.e., pulse pressure variation and stroke volume variation19,20 – that allow predicting fluid responsiveness. Minimally-invasive pulse wave analysis can thus be used for perioperative goal-directed hemodynamic therapy21 and to track CO changes during functional tests of fluid responsiveness in critically ill patients.1,5 Because the blood pressure waveform characteristics are not only influenced by stroke volume but by numerous cardiovascular variables, the estimation of stroke volume using pulse wave analysis relies on theoretical assumptions and the measurement performance – in terms of trueness and precision of agreement22,23 – compared to invasive reference methods can be impaired under certain clinical circumstances. The measurement performance of uncalibrated invasive pulse wave analysis essentially depends on blood pressure waveform quality. To ensure an impeccable waveform quality, the damping properties of the measurement system need to be optimal.24 Rapid changes in vasomotor tone make CO estimations using minimally-invasive pulse wave analysis less reliable. Minimally-invasive pulse wave analysis shows good agreement with indicator dilution reference methods in general critically ill and (cardiac) surgical patients, but not in patients with liver disease or liver surgery and septic patients.25 In particular, pulse contour devices may struggle to adapt to changes in vascular tone induced by vasopressors.26 Stroke volume can theoretically be estimated beat-by-beat using pulse wave analysis in patients with cardiac arrhythmias, but pulse pressure variation and stroke volume variation cannot be used in patients with arrhythmia.
Esophageal Doppler
The esophageal Doppler method (CardioQ-ODM, Deltex Medical, Chichester, UK) can be used to estimate blood flow in the descending aorta using the blood velocity time-integral and the aortic cross-sectional area.27 From the blood flow in the descending aorta, CO can be inferred assuming a constant distribution of blood flow between the upper and lower parts of the arterial system. While the esophageal Doppler method allows estimating CO continuously and in real-time, the main limitations include that the method is operator-dependent, prone to motion artifacts, and not easily used in awake and alert patients; in addition, there are inherent limitations to the basic underlying assumptions. First, the assumption of a constant distribution of arterial blood flow between the upper and lower parts of the body does not hold in all pathophysiologic circumstances. Second, the estimation of blood flow depends on the correct estimation of the diameter of the aorta and –because the cross-sectional area is dependent on the square of the radius– even slight errors in the estimation of the aortic diameter can result in erroneous estimations of blood flow. Esophageal Doppler monitoring can be used in patients having surgery to guide hemodynamic and fluid therapy and to monitor short term CO-changes in sedated critically ill patients.1,5
Non-Invasive CO Monitoring Methods
Methods for non-invasive CO estimation include non-invasive pulse wave analysis (using non-invasive sensors for arterial pressure waveform recording), pulse wave transit time, and thoracic electrical bioimpedance and bioreactance (Table 1).1,4,28–30
Non-invasive pulse wave analysis
Based on the same principles as with invasive pulse wave analysis, CO can be estimated from a non-invasively recorded arterial pressure waveform.3–5,30 Several sensors for non-invasive pulse wave analysis are available. The two main technologies for non-invasive pulse wave analysis are the finger cuff method (also known as vascular unloading technique or volume clamp method) and automated radial artery applanation tonometry.3–5,30,31
The finger cuff technology is based on a physical measurement principle that was first described in the 1970s.32 Using an inflatable high-frequently adjusting finger cuff that houses an infrared light source and light detector the blood volume in the finger is kept constant.4,5,30,31 The blood pressure waveform is calculated from the changes in finger cuff pressure that are needed to keep finger blood volume constant. Changes in cardiovascular dynamics influence the point of “unloaded volume” that constitutes the state of optimal measurement conditions (zero transmural pressure). Therefore, measurement systems using the finger cuff technology check and account for arterial compliance and resistance using proprietary algorithms.33–35
The two main commercially available systems – the ClearSight system (Edwards Lifesciences) and the CNAP system (CNSystems Medizintechnik, Graz, Austria) – use different approaches to transfer the blood pressure signal obtained with the finger cuff to a brachial blood pressure signal;30 the ClearSight system adjusts for height differences between the level of the right atrium and the finger and the CNAP system is calibrated to oscillometric upper-arm cuff measurements.
Another technology for non-invasive pulse wave analysis is automated radial artery applanation tonometry.4,5,30,31,36 It uses a single sensor (T-Line system; Shanshi Medical, Shangqiu, China; formerly, Tensys Medical, San Diego, California, USA) or arrays of multiple sensors (DMP-Life; DAEYOMEDI, Ansan, South Korea) placed over the radial artery;36,37 the sensor compresses the radial artery until the transmural pressure across the arterial wall is zero and the blood pressure measurement can be performed at the optimal applanation position, i.e. the point of maximal pulse pressure.30,36 Similar to finger cuff technologies, the radial artery blood pressure signal recorded using applanation tonometry needs to be scaled to match brachial pressure.36
The finger cuff technology and automated radial artery applanation tonometry allow for the estimation of CO and assessment of dynamic cardiac preload variables using pulse wave analysis without the need for arterial cannulation. The general limitations discussed previously for invasive pulse wave analysis also apply for non-invasive pulse wave analysis. In addition, both methods have technical limitations. While the measurement performance of the finger cuff technology is limited in patients with peripheral vasoconstriction, impaired finger perfusion, and severe peripheral edema, automated radial artery applanation tonometry is prone to motion artifacts. Both methods are currently not recommended for the use in high-risk surgical or critically ill patients who are equipped with an arterial catheter anyway, but may become valuable tools for perioperative monitoring in surgical patients given that technical limitations can be improved.1,5
Pulse wave transit time
The pulse wave transit time is the time the pulse wave takes to propagate from the heart to the peripheral arteries. The pulse wave transit time can be used to estimate stroke volume under the assumption that there is an inverse relationship between the two.29 In clinical practice, the time between the R-wave in the electrocardiogram and the pulse wave in the periphery (using a pulse oximeter) reflects the pulse wave transit time. A CO monitoring system based on pulse wave transit time is the esCCO system (Nihon Kohden, Tokyo, Japan). To estimate stroke volume, blood pressure and biometric patient data are needed. Considering the underlying measurement principle, it becomes clear that pulse wave transit time-based CO estimation cannot work when patients have cardiac arrhythmias or rapid changes in peripheral vascular tone. Additionally, preliminary studies suggest the esCCO technique is not ready for clinical use.38–40
Thoracic bioimpedance and bioreactance
Thoracic bioimpedance and bioreactance estimate CO using thoracic electrodes that record the amplitude and frequency of alternating current (AC) applied across the chest.4,28,29 AC has both an amplitude and frequency component, and the resistance to AC (known as “impedance”) has both a frequency and phase component. Changes in blood volume in the intrathoracic compartment (mainly induced by changes in aortic blood volume) induce changes in the electrical impedance of the thorax, which can be used to estimate the volume of electrically conducting blood moving in and out of the chest (stroke volume).4,28–30 Bioimpedance measures changes in amplitude, and bioreactance measures phase shifts. Commercially available systems for thoracic bioimpedance include BioZ (Cardiodynamics, San Diego, CA, USA), CSM3000 (Cheers Sails Medical, Shenzhen, China), ICG (Philips Medical Systems, Andover, MA, USA), ICON (Osypka Cardiotronic, Berlin, Germany), NCCOM (Bomed Medical, Irvine, CA, USA), Niccomo (Medis, Ilmenau, Germany), NICOMON (Larsen and Toubro, Mumbai, India), Physioflow (Manatec Biomedical, Paris, France). The NICOM and Starling systems (both Cheetah Medical, Portland, OR, USA) are available for bioreactance. Both techniques present some practical limitations at the bedside. Limitations include motion artifacts, electrical interference, arrhythmias, and mechanical ventilation and erroneous stroke volume estimations in patients with obesity, pleural effusion, and pulmonary edema.4,28–30
Objective Evaluation of CO Monitoring Systems
Evolution of the field
CO method comparison studies differ from studies measuring other (hemodynamic) variables in several respects. CO is a highly dynamic variable that changes rapidly from one heartbeat to another within a wide normal range (in contrast to – for example –many laboratory variables that change slowly or to arterial pressure that is closely regulated within narrow normal ranges). Additionally, numerous methods to measure CO have been developed over time, with changes in reference standards as well as statistical methods which complicate comparisons between devices.
Original studies on CO monitoring devices were performed in animals, used invasive reference standards (electromagnetic flowmeters), and data were analyzed using linear regression.41 Over time, as the measurement performance of thermodilution methods (intermittent pulmonary artery thermodilution or transpulmonary thermodilution) was increasingly accepted, clinicians began using it as the “gold standard” for CO monitoring, when in fact it is really a “clinical” standard, not a laboratory or “reference” standard.41 Some recent studies have used the aortic flow probe as the gold standard to assess new CO monitors but these studies were conducted in very specific settings such as pediatric cardiac surgery.42,43 Additionally, increased appreciation of the shortcomings of linear regression (primarily the impact of outliers44), combined with the development of the Bland-Altman analysis technique led to a change in the presentation of comparison data. The Bland-Altman analysis has its own shortcomings, e.g. dependence on a wide range of tested values (two devices tested over a narrow range of values might be misconstrued as producing “acceptable” agreement despite having no mathematical correlation whatsoever44), and should be used in conjunction with, not instead of, linear regression.
What should readers be looking for in a method comparison study on CO monitoring to assess the technology fairly?
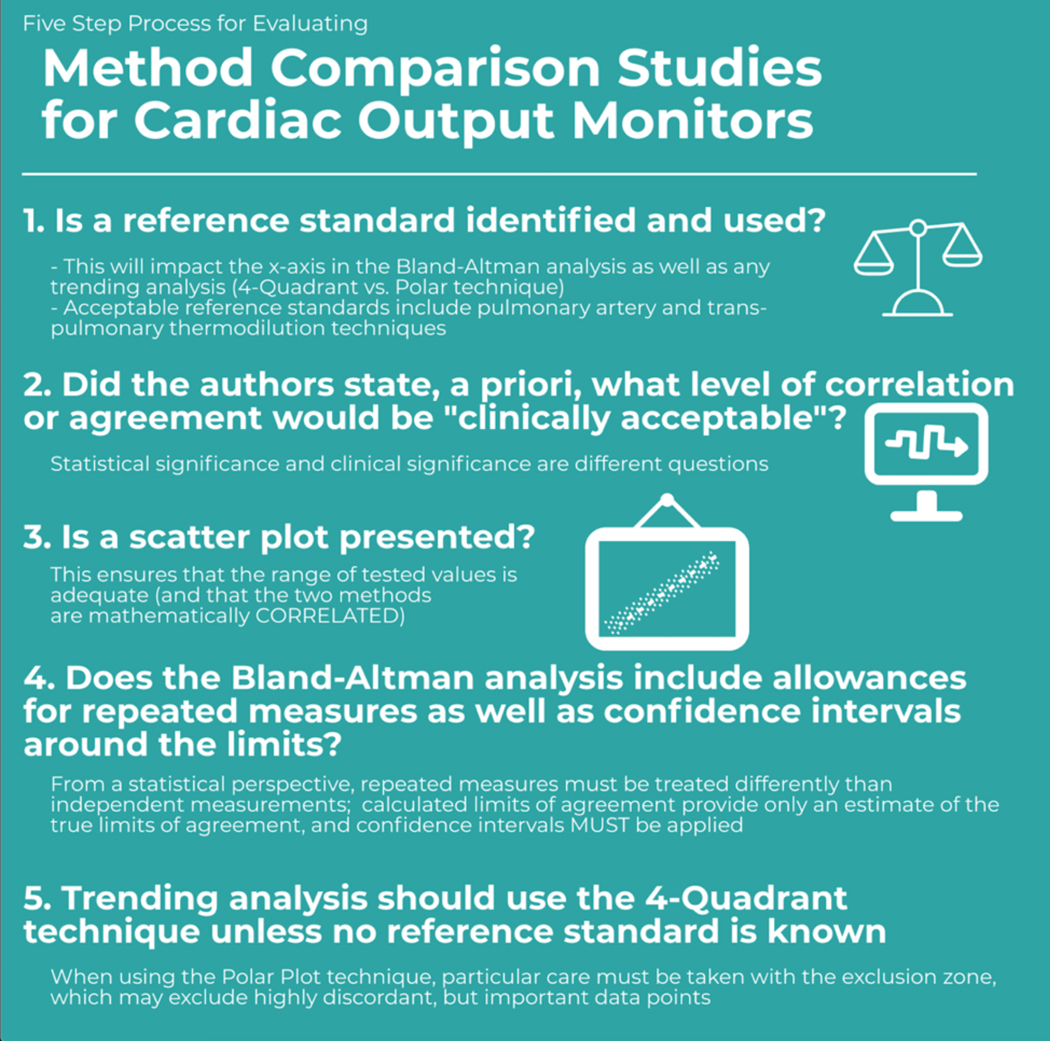
High quality CO method comparison studies share several features - they utilize a reliable standard/reference method (either a laboratory reference standard such as a flowmeter or a clinical reference standard, i.e., intermittent pulmonary artery thermodilution or transpulmonary thermodilution), they test over a wide range of values and conditions, they analyze the data using a combination of statistical approaches, and they are adequately powered.44
The method agreement is generally examined through a version of Bland-Altman analysis that allows multiple measurements per subject.45 Therefore, the agreement is visualized by a plot of the differences of two paired measurements, which were each made by one of the investigated methods, against the average of the paired measurements. There are three related statistics to assess agreement. First, the mean of the observed differences (often called bias) serves as a measure of a systematic deviation. Second, a 95% prediction interval of the differences, referred to as the 95% limits of agreement (LoA), describes the deviation of methods on the measurement level that has to be expected for most, that is about 95% of the measurements. Importantly, computation of the limits of agreement only takes the sample size into account if a t-distribution is assumed for the differences of measurements.44 Third, the so-called percentage error expresses the deviation of methods in terms of a percentage of the average level of measurements. It is therefore computed from the one-sided width of the LoA divided by the average CO. This statistic is used for a very general classification of agreement and for comparison across different studies as it is unit-free.
All of these statistics are estimated from a limited sample of observations and it has been recommended to provide the respective 95% confidence intervals to demonstrate the precision of estimation.46–49 The mean of the differences and LoA are assumed to be constant across the range of the observed CO values and Bland-Altman analysis can be used to explore deviations of the data distribution from this assumption. Transformation of the data, e.g. a log-transformation, and use of regression models have been proposed to estimate non-constant bias in such a case.45
With multiple measurements per subject, there are two sources of variance that contribute to the assessment of agreement by LoA and the percentage error. One of them is the between-subjects variance, which is often referred to as a random between-subjects effect, a method-subject interaction or the trueness. The further one is the within-subjects variance, which is often called the random error or precision of a method.45,50–52 As better or worse agreement results from these components it has been recommended to present both of them.47,48,50,52 A related argument is that even a very well performing new method can hardly agree with a standard method if the latter is very imprecise.45,50 This problem translates to the percentage error which could indicate poor agreement, potentially leading to the rejection of a new method, although the disagreement may be caused by the imprecision of the standard method. Comparisons of the percentage error to supposedly universal thresholds (e.g., 30%) and comparisons across studies can be misleading in such a case.51,53
Sample Size
Sample size estimation is rarely seen but is highly recommended for CO method comparison studies.54 Early work on method comparison studies by Bland and Altman recommend construction of 95% confidence intervals around the LOA, to ensure that the study is adequately powered.55 Unfortunately, the standard approach are to setting confidence intervals around LoA is not applicable in case of repeated measurements per subject. However, it has been suggested that this limitation can be ignored under certain circumstances, e.g., when the number of replicates is less than the number of subjects.45 Sample sizes obtained through the calculations mentioned above can serve as a rough guide in such a case. A more sophisticated framework, which is based on linear mixed-effects regression models, focuses on the precision of the estimation of the variance components that are used to compute the LoA and therefore provides recommendations on two components of the sample size, that is the number of subjects and the number of repeated measurements per subject. A recent publication motivates sample size calculation by power analysis but is restricted to single measurements per subject.56 A very general approach is to compute the sample size through simulation studies. Historical data may also be used to guide decisions on sample size.47
Methodology of CO method comparison studies
Performing a CO method comparison study starts with the study design and the sample size calculation. After data acquisition, all data should be presented as a scatter plot.44 The Bland-Altman plot should then be drawn and explored for a trend in the relation between observed differences and the magnitude of measurements. Depending on this, a suitable method to compute the mean of the differences, LoA and respective 95% confidence intervals should be chosen. The results of this computation should be presented as plain numbers and as lines or graphs within the Bland-Altman plot, including confidence intervals. The method used to perform the computations need to be described. For example it has to be stated whether the original approach suggested by Bland and Altman55 has been followed or if mixed-effects models have been applied, whether a t-distribution or normal distribution has been assumed for the computation of the LoA, etc. Use of formulas may facilitate this task and avoids misunderstandings caused by definitions, terms, and notations that are not uniquely specified.
Finally, all available methods describe statistical agreement rather than the effect of measurement differences on clinical decision-making. Critchley et al. have suggested that when using limits of agreement analysis, a percentage error of up to 30% is “acceptable,”57 but this must be taken into clinical context and clinicians who rely purely on this metric may be misled if the range of CO tested is inadequate.44
Conclusion
While the Swan-Ganz catheter remains the clinical standard for CO monitoring, its use has declined and today several minimally-invasive and non-invasive CO monitoring devices are available. Knowing the basic measurement principles of these new monitoring systems is important to understand their inherent limitations regarding the measurement performance and clinical applicability. In addition, as new CO monitoring devices are being introduced, clinicians should understand the basis of how to assess a new monitoring method against a reference method in a method comparison study.
Figure 2.
Five Steps for Evaluating Method Comparison Studies for Cardiac Output Monitors
Acknowledgments
Funding: This work is supported by NIH R01 HL144692 and R01 EB029751.
Conflicts of interest: BS has received honoraria for consulting, honoraria for giving lectures, and refunds of travel expenses from Edwards Lifesciences Inc. (Irvine, CA, USA). BS has received honoraria for consulting, institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems SE (Feldkirchen, Germany). BS has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik GmbH (Graz, Austria). BS has received institutional restricted research grants from Retia Medical LLC. (Valhalla, NY, USA). BS has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). BS has received honoraria for consulting, institutional restricted research grants, and refunds of travel expenses from Tensys Medical Inc. (San Diego, CA, USA). RHT has no conflicts of interest. AH has no conflicts of interest. MC is a consultant for Edwards Lifesciences (Irvine, CA) and Masimo Corp (Irvine, CA), and has funded research from Edwards Lifesciences and Masimo Corp. He is also the founder of Sironis and he owns patents and receives royalties for closed loop hemodynamic management technologies that have been licensed to Edwards Lifesciences.
References
- 1.Saugel B, Vincent JL: Cardiac output monitoring: how to choose the optimal method for the individual patient. Curr Opin Crit Care 2018; 24:165–172 [DOI] [PubMed] [Google Scholar]
- 2.Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K: Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013:CD003408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Backer D, Bakker J, Cecconi M, Hajjar L, Liu DW, Lobo S, Monnet X, Morelli A, Myatra SN, Perel A, Pinsky MR, Saugel B, Teboul JL, Vieillard-Baron A, Vincent JL: Alternatives to the Swan-Ganz catheter. Intensive Care Med 2018; 44:730–741 [DOI] [PubMed] [Google Scholar]
- 4.Saugel B, Cecconi M, Wagner JY, Reuter DA: Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth 2015; 114:562–75 [DOI] [PubMed] [Google Scholar]
- 5.Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, Perel A, Pinsky MR, Reuter DA, Rhodes A, Squara P, Vincent JL, Scheeren TW: Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016; 42:1350–9 [DOI] [PubMed] [Google Scholar]
- 6.Cannesson M, Manach YL: Noninvasive hemodynamic monitoring: no high heels on the farm; no clogs to the opera. Anesthesiology 2012; 117:937–9 [DOI] [PubMed] [Google Scholar]
- 7.Esper SA, Pinsky MR: Arterial waveform analysis. Best Pract Res Clin Anaesthesiol 2014; 28:363–80 [DOI] [PubMed] [Google Scholar]
- 8.Jozwiak M, Monnet X, Teboul JL: Pressure Waveform Analysis. Anesth Analg 2018; 126:1930–1933 [DOI] [PubMed] [Google Scholar]
- 9.Thiele RH, Durieux ME: Arterial waveform analysis for the anesthesiologist: past, present, and future concepts. Anesth Analg 2011; 113:766–76 [DOI] [PubMed] [Google Scholar]
- 10.Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H: Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth 2016; 30:461–80 [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Mukkamala R: Continuous cardiac output monitoring in humans by invasive and noninvasive peripheral blood pressure waveform analysis. J Appl Physiol 2006; 101:598–608 [DOI] [PubMed] [Google Scholar]
- 12.Mukkamala R, Reisner AT, Hojman HM, Mark RG, Cohen RJ: Continuous cardiac output monitoring by peripheral blood pressure waveform analysis. IEEE Trans Biomed Eng 2006; 53:459–67 [DOI] [PubMed] [Google Scholar]
- 13.Saugel B, Heeschen J, Hapfelmeier A, Romagnoli S, Greiwe G: Cardiac output estimation using multi-beat analysis of the radial arterial blood pressure waveform: a method comparison study in patients having off-pump coronary artery bypass surgery using intermittent pulmonary artery thermodilution as the reference method. J Clin Monit Comput 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greiwe G, Peters V, Hapfelmeier A, Romagnoli S, Kubik M, Saugel B: Cardiac output estimation by multi-beat analysis of the radial arterial blood pressure waveform versus intermittent pulmonary artery thermodilution: a method comparison study in patients treated in the intensive care unit after off-pump coronary artery bypass surgery. J Clin Monit Comput 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romagnoli S, Franchi F, Ricci Z, Scolletta S, Payen D: The Pressure Recording Analytical Method (PRAM): Technical Concepts and Literature Review. J Cardiothorac Vasc Anesth 2017; 31:1460–1470 [DOI] [PubMed] [Google Scholar]
- 16.Romano SM, Pistolesi M: Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 2002; 30:1834–41 [DOI] [PubMed] [Google Scholar]
- 17.Cecconi M, Parsons AK, Rhodes A: What is a fluid challenge? Curr Opin Crit Care 2011; 17:290–5 [DOI] [PubMed] [Google Scholar]
- 18.Monnet X, Teboul JL: Passive leg raising: five rules, not a drop of fluid! Crit Care 2015; 19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire S, Rinehart J, Vakharia S, Cannesson M: Technical communication: respiratory variation in pulse pressure and plethysmographic waveforms: intraoperative applicability in a North American academic center. Anesth Analg 2011; 112:94–6 [DOI] [PubMed] [Google Scholar]
- 20.Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ: The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg 2009; 108:513–7 [DOI] [PubMed] [Google Scholar]
- 21.Chong MA, Wang Y, Berbenetz NM, McConachie I: Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol 2018; 35:469–483 [DOI] [PubMed] [Google Scholar]
- 22.Squara P, Scheeren TWL, Aya HD, Bakker J, Cecconi M, Einav S, Malbrain M, Monnet X, Reuter DA, van der Horst ICC, Saugel B: Metrology part 1: definition of quality criteria. J Clin Monit Comput 2020 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squara P, Scheeren TWL, Aya HD, Bakker J, Cecconi M, Einav S, Malbrain M, Monnet X, Reuter DA, van der Horst ICC, Saugel B: Metrology part 2: Procedures for the validation of major measurement quality criteria and measuring instrument properties. J Clin Monit Comput 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saugel B, Kouz K, Meidert AS, Schulte-Uentrop L, Romagnoli S: How to measure blood pressure using an arterial catheter: a systematic 5-step approach. Crit Care 2020; 24:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slagt C, Malagon I, Groeneveld AB: Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth 2014; 112:626–37 [DOI] [PubMed] [Google Scholar]
- 26.Meng L, Tran NP, Alexander BS, Laning K, Chen G, Kain ZN, Cannesson M: The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth Analg 2011; 113:751–7 [DOI] [PubMed] [Google Scholar]
- 27.Singer M: Oesophageal Doppler. Curr Opin Crit Care 2009; 15:244–8 [DOI] [PubMed] [Google Scholar]
- 28.Marik PE: Noninvasive cardiac output monitors: a state-of the-art review. J Cardiothorac Vasc Anesth 2013; 27:121–34 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LS, Squara P: Non-Invasive Monitoring of Cardiac Output in Critical Care Medicine. Front Med 2017; 4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saugel B, Cecconi M, Hajjar LA: Noninvasive Cardiac Output Monitoring in Cardiothoracic Surgery Patients: Available Methods and Future Directions. J Cardiothorac Vasc Anesth 2019; 33:1742–1752 [DOI] [PubMed] [Google Scholar]
- 31.Saugel B, Dueck R, Wagner JY: Measurement of blood pressure. Best Pract Res Clin Anaesthesiol 2014; 28:309–22 [DOI] [PubMed] [Google Scholar]
- 32.Penaz J, Voigt A, Teichmann W: [Contribution to the continuous indirect blood pressure measurement]. Z Gesamte Inn Med 1976; 31:1030–3 [PubMed] [Google Scholar]
- 33.Fortin J, Marte W, Grullenberger R, Hacker A, Habenbacher W, Heller A, Wagner C, Wach P, Skrabal F: Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med 2006; 36:941–57 [DOI] [PubMed] [Google Scholar]
- 34.Fortin J, Wellisch A, Maier K: CNAP - Evolution of Continuous Non-Invasive Arterial Blood Pressure Monitoring. Biomed Tech 2013; 58 Suppl 1 [DOI] [PubMed] [Google Scholar]
- 35.Truijen J, van Lieshout JJ, Wesselink WA, Westerhof BE: Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput 2012; 26:267–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dueck R, Goedje O, Clopton P : Noninvasive continuous beat-to-beat radial artery pressure via TL-200 applanation tonometry. J Clin Monit Comput 2012; 26:75–83 [DOI] [PubMed] [Google Scholar]
- 37.Zayat R, Goetzenich A, Lee JY, Kang H, Jansen-Park SH, Schmitz-Rode T, Musetti G, Schnoering H, Autschbach R, Hatam N, Aljalloud A: Comparison between radial artery tonometry pulse analyzer and pulsed-Doppler echocardiography derived hemodynamic parameters in cardiac surgery patients: a pilot study. PeerJ 2017; 5:e4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball TR, Tricinella AP, Kimbrough BA, Luna S, Gloyna DF, Villamaria FJ, Culp WC Jr., : Accuracy of noninvasive estimated continuous cardiac output (esCCO) compared to thermodilution cardiac output: a pilot study in cardiac patients. J Cardiothorac Vasc Anesth 2013; 27:1128–32 [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui M, Araki Y, Masui K, Kazama T, Sugo Y, Archer TL, Manecke GR Jr., : Pulse wave transit time measurements of cardiac output in patients undergoing partial hepatectomy: a comparison of the esCCO system with thermodilution. Anesth Analg 2013; 117:1307–12 [DOI] [PubMed] [Google Scholar]
- 40.Magliocca A, Rezoagli E, Anderson TA, Burns SM, Ichinose F, Chitilian HV: Cardiac Output Measurements Based on the Pulse Wave Transit Time and Thoracic Impedance Exhibit Limited Agreement With Thermodilution Method During Orthotopic Liver Transplantation. Anesth Analg 2018; 126:85–92 [DOI] [PubMed] [Google Scholar]
- 41.Thiele RH, Bartels K, Gan TJ: Cardiac output monitoring: a contemporary assessment and review. Crit Care Med 2015; 43:177–85 [DOI] [PubMed] [Google Scholar]
- 42.Sigurdsson TS, Aronsson A, Lindberg L: Extracorporeal Arteriovenous Ultrasound Measurement of Cardiac Output in Small Children. Anesthesiology 2019; 130:712–718 [DOI] [PubMed] [Google Scholar]
- 43.Trieu CT, Williams TM, Cannesson M, Marijic J: Babies and Children at Last: Pediatric Cardiac Output Monitoring in the Twenty-first Century. Anesthesiology 2019; 130:671–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiele RH, McMurry TL: Data Agnosticism and Implications on Method Comparison Studies. Anesth Analg 2015; 121:264–6 [DOI] [PubMed] [Google Scholar]
- 45.Bland JM, Altman DG: Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17:571–82 [DOI] [PubMed] [Google Scholar]
- 46.Chhapola V, Kanwal SK, Brar R: Reporting standards for Bland-Altman agreement analysis in laboratory research: a cross-sectional survey of current practice. Ann Clin Biochem 2015; 52:382–6 [DOI] [PubMed] [Google Scholar]
- 47.Montenij LJ, Buhre WF, Jansen JR, Kruitwagen CL, de Waal EE: Methodology of method comparison studies evaluating the validity of cardiac output monitors: a stepwise approach and checklist. Br J Anaesth 2016; 116:750–8 [DOI] [PubMed] [Google Scholar]
- 48.Olofsen E, Dahan A, Borsboom G, Drummond G: Improvements in the application and reporting of advanced Bland-Altman methods of comparison. J Clin Monit Comput 2015; 29:127–39 [DOI] [PubMed] [Google Scholar]
- 49.Zou GY: Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat Methods Med Res 2013; 22:630–42 [DOI] [PubMed] [Google Scholar]
- 50.Bland JM, Altman DG: Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8:135–60 [DOI] [PubMed] [Google Scholar]
- 51.Hapfelmeier A, Cecconi M, Saugel B: Cardiac output method comparison studies: the relation of the precision of agreement and the precision of method. J Clin Monit Comput 2016; 30:149–55 [DOI] [PubMed] [Google Scholar]
- 52.Carstensen B: Comparing and predicting between several methods of measurement. Biostatistics 2004; 5:399–413 [DOI] [PubMed] [Google Scholar]
- 53.Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM: Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies--with specific reference to the measurement of cardiac output. Crit Care 2009; 13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riou B: Continuous measurement of hemoglobin: methodological approach and lessons for the future. Anesthesiology 2013; 118:497–9 [DOI] [PubMed] [Google Scholar]
- 55.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–10 [PubMed] [Google Scholar]
- 56.Lu MJ, Zhong WH, Liu YX, Miao HZ, Li YC, Ji MH: Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. Int J Biostat 2016; 12 [DOI] [PubMed] [Google Scholar]
- 57.Critchley LA, Critchley JA: A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999; 15:85–91 [DOI] [PubMed] [Google Scholar]