Abstract
Background
Although TP53 co-mutation with KRAS/ATM/EGFR/STK11 have been proved to have predictive value for response to immune checkpoint inhibitors (ICIs), not all TP53 mutations are equal in this context. As the main part of TP53 mutant types, Missense and Nonsense alternations in TP53 as independent factors to predict the response to ICIs within Lung Adenocarcinoma (LUAD) patients have not yet been reported.
Methods
An integrated analysis based on multiple-dimensional data types including genomic, transcriptomic, proteomic and clinical data from published lung adenocarcinoma data and local database of LUAD taking immune checkpoint inhibitors. Gene set enrichment analysis (GSEA) was used to determine potentially relevant gene expression signatures between specific subgroups. Single-sample GSEA (GSVA) is conducted to calculate the score for enrichment of a set of genes regulating DNA damage repair (DDR) pathway.
Findings
The TP53-missense-mutation group showed increased PD-L1 (CD274) level and enriched IFN-γ signatures compared with the TP53-wild-type subgroup, but no differences were noted in patients with nonsense-mutant vs wild-type p53. Furthermore, a group of suppressor Immune cells like M2 Macrophage and Neutrophils are found enriched in nonsense group. On the other-side, both TP53 missense and nonsense mutations are associated with elevated TMB and neoantigen levels and contribute equally to DNA damage repair deficiency. The distribution regarding to multi-dimensional factors determining the efficacy of ICIs finally transformed into diverse clinical benefits for LUAD. TP53 missense but not -nonsense Mutants are associated with better clinical benefits taking antiPD-1/1L. However, all such TP53 subgroups responds well to nivolumab (antiPD-L1) plus ipilimumab (antiCTLA-4) therapy.
Interpretation
Our study demonstrated that not all TP53 mutations are equal in predicting efficacy in patients with LUAD treated with ICIs. Multi-center data showed that TP53 missense and nonsense mutations were significantly different in terms of associations with PD-L1 expression, IFN-γ signatures and TME composition. Special attention should be paid to potential TP53 mutation heterogeneity when evaluating TP53 status as biomarker for ICIs.
Funding
The study was supported by Key Lab System Project of Guangdong Science and Technology Department – Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (Grant No. 2017B030314120, to Yi-Long WU)
Keywords: TP53, Lung adenocarcinoma, Immunotherapy, Subtypes
Research in context.
Evidence before this study
Several studies evaluating TP53 mutations as biomarkers for ICIs among LUAD have produced controversial outcomes. Besides, a series of studies demonstrate that distinct combinations of STK11, EGFR, KRAS, ATM and TP53 were associated with different tumor microenvironment in terms of immune cell composition and of PD-L1 expression by tumor cells. Study proved that mutant P53 could increase PD-L1 level by loss function in trans-activating Mir-34 family. On the contrary, p53 stabilization caused by Nutlin-3 are proved to increase PD-L1 expression in tumor cell. The role of p53 as biomarker for ICIs treatment is not fully evaluated.
Added value of this study
Our study demonstrate that not all TP53 mutations are equal in predicting efficacy of ICIs in patients with LUAD. Multi-center data showed that TP53 missense and nonsense mutations were significantly different in terms of associations with PD-L1 expression, IFN-γ signatures and TME composition. On the contrary, both TP53 missense and Nonsense mutation contribute equally to DNA damage repair deficiency. Our study showed therapy options for special TP53 Mutation Groups.
Implications of all the available evidence
Our study demonstrate that not all TP53 mutations are equal in predicting efficacy in patients with LUAD treated with ICIs. Multi-center data showed that TP53 missense and nonsense mutations were significantly different in terms of associations with PD-L1 expression, IFN-γ signatures and TME composition. TP53 missense but not nonsense mutants showed superior clinical outcomes compared with wild-type TP53 taking antiPD-1/L1.
Alt-text: Unlabelled box
1. Introduction
Immune checkpoint inhibitors (ICIs) have successfully transformed the therapy landscape for lung adenocarcinoma and can bring promising durable clinical benefits [1,2]. However, only a small group of patients achieve a long-lasting response to ICIs, and effective biomarkers are needed. Signatures based on PD-L1, TMB and IFN-γ have performed well as reliable biomarkers for ICIs, but the detection of TMB and immune signatures relies on expensive panels. In addition, the internal heterogeneity of PD-L1 expression also limits its value for predicting response 3, 4, 5. As such, efforts have been made to identify which patients will respond in a more feasible and accessible way, such as by detecting associated genomic alterations via next-generation sequencing (NGS) [1].
Several genomic alternations, such as alterations in EGFR, KRAS, STK11, ALK, JAK2, TP53, and ATM, have been proven to be correlated with ICI efficacy. Among these mutations, co-occurring mutations in TP53 and other genes (EGFR, STK11, or KRAS) have been proven to have predictive value for immune checkpoint inhibitors 2, 3, 4. However, growing evidence demonstrates that different p53 mutants differ substantially in form and function, and not all p53 mutants have equivalent cellular effects [5]. But no researches have been set up to address the question whether all TP53 alternations could be equally taken as a biomarker for ICIs till now, as several studies have produced controversial outcomes in evaluating TP53 as biomarkers for ICIs which overlooked its heterogeneity [6].
TP53 missense mutations account for most TP53 mutations in lung adenocarcinomas and can result in the accumulation of mutant p53 due to increased protein stability. In addition, many mutant p53 proteins have been proven to acquire gain-of-function (GOF) activities rather than being merely deficient in primary activity. On the other hand, nonsense mutations are less frequent than missense mutations in TP53 but nonetheless constitute 10% of all TP53 mutations [7,8]. Previous research indicated that nonsense mutations lead to premature termination (stop) codons and expression of a truncated inactive p53 protein, which could result in p53 deficiency through the nonsense-mediated decay (NMD) pathway [9]. Regarding the notable difference in such properties between TP53 subtypes, special attention should be paid to potential TP53 mutation heterogeneity when evaluating TP53 mutations.
The use of TP53 mutation subtypes, including TP53 missense or nonsense mutations, as independent factors for predicting the response to ICIs has not yet been reported. After all the evidences mentioned before, TP53 mutant forms differ in functions, we hypothesized that all TP53 mutations are also not completely equal in predicting the efficacy of ICIs and thus systematic functional categorization of the various mutant p53 forms is essential for ensuring their optimal use as biomarkers for ICIs.
2. Method
2.1. mRNA expression profiling and reverse phase protein array (RPPA) analysis
Level 3 and 4 transcriptomic and reverse-phase protein array data of cancer patients were obtained from The Cancer Genome Atlas (TCGA) cBioPortal and The Cancer Proteome Atlas (TCPA) [10]. RNA-seq data was available in 563 LUAD patients in TCGA, and among of which 354 subjects both have the RPPA data. The gene expression extracted for correlation analysis using the corresponding packages in R. For GSEA, the java GSEA Desktop Application was downloaded from http://software.broadinstitute.org/gsea/index.jsp. GSEA was used to associate the gene signature with the TP53 missense or TP53 nonsense mutation. Dotplots of enriched functional pathways were plotted by ClusterProfiler package in R. Micro-RNA data is downloaded from the NCI Genomic Data Commons (GDC, https://portal.gdc.cancer.gov/). The IFN-γ score was calculated based on a previously published method [11]. The cellular heterogeneity of tumor immune microenvironment was analyzed through xCELL platform with bulk gene expression from TCGA [12].
2.2. Assessment of TMB, HDR score and DDR pathway distribution
TMB was defined as the number of somatic nonsynonymous variations which including insertions, and deletions in examined coding regions detected in tumor tissues by whole-exon sequencing in TCGA and NGS in the MSKCC and Geneplus databases. HDR score is directly obtained from published data [13].
Single-sample GSEA (ssGSEA) was conducted to calculate the score for enrichment of a set of genes regulating DNA damage repair (DDR) pathway using the GSVA Bioconductor package. DNA damage repair (DDR) gene list including 276 genes was assembled from relevant gene lists including MSigDB v5.0 an online catalog of DDR genes from recently published resources [14]. Hierarchical clustering of DDR pathway enrichment score was performed using the R package pheatmap using canberra as clustering distance and ward.D2 as linkage.
2.3. Local clinical cohorts
Local Cohort including 44 patients taking antiPD-L1/1 therapy between 2016/11 to 2019/11 were enrolled in Guang Dong Lung Cancer Institute (GDLCI), TP53 status is confirmed by NGS detection. More characteristics of the patients treated with mono ICIs are presented in eTable in the Supplement.
2.4. Published multi-clinical cohorts
Published clinical data were collected to verify the clinical efficacy of ICIs within LUAD patients. 147 LUAD patients taking anti-PD-L1 mono-therapy were involved in MSK cohort [15]. 59 non-squamous non-small cell lung cancer patients taking nivolumab plus ipilimumab as first-line therapy are enrolled in Checkmate-012 cohort [16]. Both clinical and somatic mutation data were obtained from previous studies. More characteristics of patients are presented in eTable in the Supplement.
2.5. Statistical analysis
Data analysis was performed by GraphPad Prism 8 (GraphPad Software, Inc.) and in the R version 3.60. For comparison of continuous variables, such as gene expression value, RPPA, TMB and Neoantigen, Wilcox test was applied. For categorical variables between groups, such as sex, lines of therapy, PD-L1 IHC portion, Response rate, DCB rate. We used Fisher's exact test. We performed the log-rank test to compare the progression-free survival, using GraphPad Prism 8, and Kaplan–Meier survival curves were plotted. In the present study, all tests of significance were two sided, and p <0.05 was considered statistically significant. P<0.1 was considered marginally significant.
2.6. Role of funders
Funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
3. Results
3.1. TP53 missense but not nonsense mutations are associated with increased PD-L1 levels
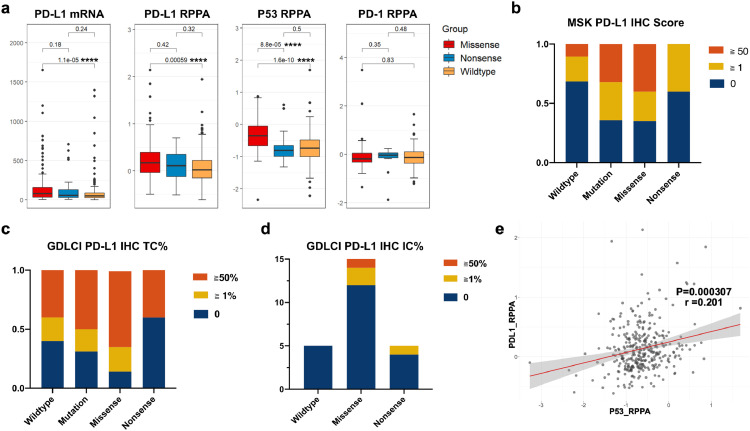
PDL1 expression have been widely taken as a reliable biomarker in guiding ICIs administration, and TP53 mutant status in LUAD was reported to be associated with higher PD-L1 expression level. To address the different role of TP53 subtypes in determining ICIs response, then several analyses were conducted to determine the association between different TP53 mutations and PD-L1 expression. RNA sequencing (RNA-Seq) data and reverse phase protein array (RPPA) data were collected and analyzed for LUAD from The Cancer Genome Atlas (TCGA). The TP53-missense-mutation group showed significantly increased PD-L1 (CD274) mRNA expression compared with the TP53-wild-type subgroup (P<0.05, Wilcox test), but no differences were noted in patients with nonsense-mutant vs wild-type p53 (P = 0.24, Wilcox test). Further analysis confirmed this result at the PD-L1 protein level with RPPA data, which indicated that TP53 missense mutation was associated with higher PD-L1 protein levels than wild-type TP53 (P<0.01, Wilcox test). We also conducted a comparison between each TP53 group and the PD-1 expression level, but no significant difference between groups was evident (Fig. 1a).
Fig. 1.
Correlation of TP53 Missense and nonsense status with PD-L1 in lung adenocarcinoma patients.
a) Correlations between TP53 status and PD-L1 mRNA or RPPA level in lung adenocarcinoma patients based on the analysis of the TCGA database (Wilcox test).
b) IHC score obtained from MSK cohort showed that PD-L1 level in TP53 missense group is significantly higher than TP53 wildtype. But no difference is generated between TP53 nonsense and wildtype group.
c) PD-L1 IHC tumor cells (TC) from local database showed larger portion of patients with PD-L1 ≧50% (7/14) or ≧1% (12/14) were shown in the TP53 missense group
d) PD-L1 IHC immune cells (IC) from local database, positive IC PD-L1expression was shown within 3 patients (3/14) in TP53 missense group, 1(1/5) in TP53 nonsense group.
e) Correlation between p53 and PDL1 (CD274) RPPA level in samples of LUAD data from TCGA (p < 0.001, spearman).
****, p < 0.0001; ***, p < 0.001; **, p < 0.01, * p < 0.05; NS, not significant.
Sixty-six LUAD patients from the MSK cohort with PD-L1 IHC data were enrolled, and the proportion of patients with a PD-L1 IHC score ≥1 was prominently higher in the TP53-missense-mutant group than in the TP53-wild-type group (13/20 vs 12/38, P = 0.028, Fisher's exact test). No evidence showed that TP53 nonsense mutation was associated with a higher PD-L1 IHC score than other TP53 statuses. Stratification as regard to PD-L1 expression analysis was conducted either, the portion of LUAD patients with PD-L1 score more than 50 was also higher in TP53 missense groups (Fig. 1b). Notably, the distribution of each level of PD-L1 expression on tumor cells (TC) in local GDLCI cohort is consistent with MSK cohort, larger portion of patients with PD-L1 ≧50% (7/14) or ≧1% (12/14) were shown in the TP53 missense group both than TP53 wildtype and nonsense group (Fig. 1c). The PD-L1 level as regard to immune cells were shown based on local data. Few subjects showed positive PD-L1 expression on immune cells, 3 patients (3/14) in the TP53 missense group, 1(1/5) in TP53 nonsense group, and none was shown in the TP53 wildtype group (0/5). (Fig. 1d).
Previous research showed that mutation in TP53 would hinder its downregulation of PD-L1 expression via miR-34. We thus compared the expression of miR-34 family members between different TP53 statuses, but no significant difference was found (Figure S1a, b). This demonstrates that loss of miR-34 regulation via TP53 loss of function does not fully explain the elevated PD-L1 level [17].
It is well known that TP53 missense mutation but not TP53 nonsense mutation results in the accumulation of mutant p53 protein. Consistent with this conclusion, a significantly increased P53 protein level was observed in the TP53-missense-mutant group (P<0.05, Wilcox test, between both missense and wild type, and missense and nonsense) (Fig. 1a). Furthermore, we found a statistically positive correlation between p53 and PDL1 at the protein level (r = 0.29, P < 0.001, Spearman), consistent with a previous report that the increased P53 protein levels caused by Nutlin-3 are associated with increased PD-L1 expression (Fig. 1d) [18]. But no significantly difference was generated in the RNA level between groups. (Fig. S1c) We conducted another correlation analysis among TP53 wildtype patients, which also showed a statistically positive correlation (r = 0.22, P < 0.001, Spearman). (Fig. S1d)
The TP53 missense mutation is associated with accumulation of mutant P53 protein, which may result in p53 gaining a new function as regard to the regulation of PD-L1. Our data revealed that TP53 missense mutation had the potential to alter PD-L1 expression through mechanisms other than loss of p53 caused by nonsense mutation.
3.2. TP53 missense mutation is associated with enriched JAK-stat pathway signatures compared with TP53 nonsense mutation
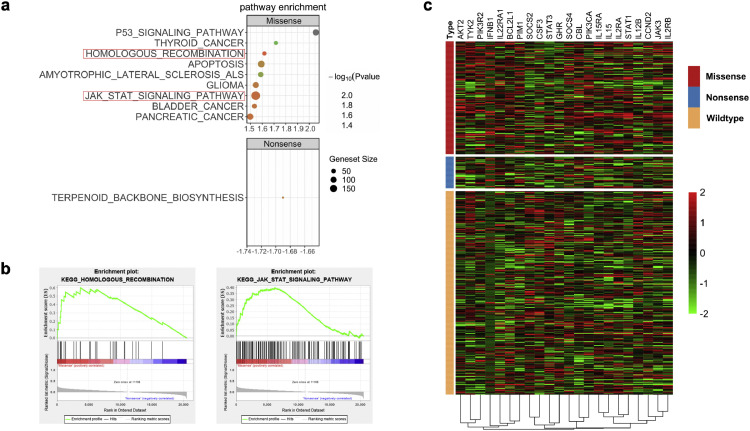
To further explore the difference between TP53 missense and nonsense mutation subtypes, we performed GSEA based on RNA-seq data from TCGA, which revealed a prominent enrichment in TP53-missense-mutant group of signatures related to P53 signaling, apoptosis, homologous recombination (P = 0.047), and the JAK-STAT signaling pathway (P = 0.032) (Fig. 2a, 2b).
Fig. 2.
GSEA analysis and Heatmap representation of relative mRNA expression of JAK-STAT signaling pathway enriched in TP53 missense compared with nonsense group.
a) GSEA analysis showed enriched functional pathway including the p53 signaling pathway, homologous recombination and JAK-STAT signaling pathway.
b) The enrichment plot regarding to homologous recombination and JAK-STAT signaling pathway.
c) Heatmap representation of relative mRNA expression of JAK-STAT pathway showed significant hot-zone in TP53 missense group but not in -nonsense LUAD patients.
A previous report showed that the activity of the JAK-signal transducer and activator of transcription (STAT) signaling pathway increased PD-L1 expression levels [19,20]. We generated a heatmap to depict the distribution of RNA levels in the JAK-STAT pathway between the TP53-wild-type, -missense-mutant and -nonsense-mutant groups. The JAK-STAT pathway was highly enriched in LUAD samples with TP53 missense mutations, but STAT3, which can cause enhanced PD-L1 expression as a result of hyperactivation of the ALK signaling pathway, was not affected [21]. (Fig. 2c).
We next sought to identify statistically altered protein levels within the JAK-STAT pathway. Significantly higher levels of BCL2L1, AKT2, IL2RA and TYK2, which are involved in the JAK-STAT pathway, were confirmed in TP53-missense-mutant tumors compared to TP53-nonsense-mutant tumors. (Fig. S2). Interestingly, our analysis also displayed a significant increase in BCL2L1 expression (missense-mutant tumors vs nonsense-mutant tumors, P = 0.017, Wilcox test) (Fig. S2), which may serve as a bridge between missense-mutant p53 and the JAK-STAT pathway.
In addition, our data showed a marginally significant difference in JAK3 (P = 0.08, Wilcox test) and STAT1 levels between the TP53-missense-mutant and nonsense-mutant groups (P = 0.066, Wilcox test). Cancer cells can activate the IFN-γ/JAK/STAT pathway to increase PD-L1 mRNA expression [19,22]. TP53 mutations were previously shown to be associated with an enriched IFN-γ signature [23]. We tried to determine whether both TP53 missense and nonsense mutants showed higher IFN-γ expression than wild-type TP53 (Fig. S3a).
IFN-γ score was calculated based on a previously published method [11]. The TP53-missense-mutant group displayed significantly higher IFN-γ score than the TP53-wild-type group (P<0.001, Wilcox test), but no difference was evident between the TP53-nonsense-mutant and TP53-wild-type groups or the TP53-nonsense-mutant and TP53-missense-mutant groups (Fig. S3a). The heatmap further showed the diverse distribution of IFN-γ-associated signatures between missense and wildtype groups (Fig. S3b). However, the diverse distribution should be interpreted with caution as regarding to the limited subjects in Nonsense mutation group.
The enrichment of JAK-STAT and IFN-γ signatures agrees with the higher PD-L1 expression in TP53-missense-mutant LUAD patients. Furthermore, differences in JAK-STAT pathway-associated protein expression levels, including JAK3, STAT1, and BCL2L1, between the missense-mutant and nonsense-mutant groups further suggest that the role of TP53 mutations in PD-L1 regulation is not equal.
3.3. Both TP53 missense and nonsense mutations are associated with elevated TMB and neoantigen levels and contribute equally to DNA damage repair deficiency
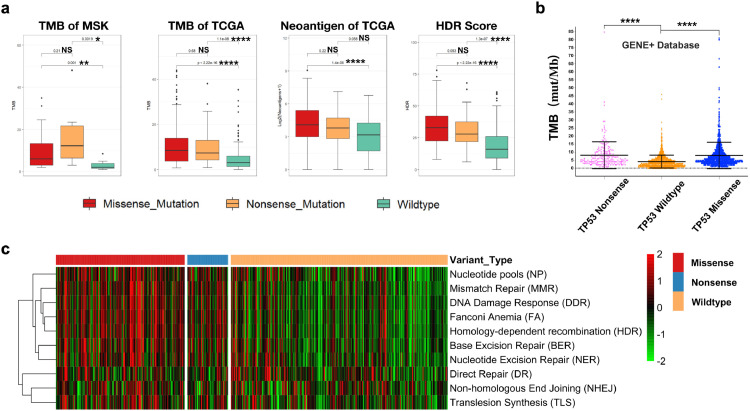
P53 is a central tumor suppressor that enforces genomic stability by orchestrating a variety of DNA damage response (DDR) mechanisms. Our GSEA data showed enrichment in the homologous recombination (HR) (P = 0.047, Wilcox test) pathway within the TP53-missense-mutant group. We next sought to verify which DDR pathway was related to the TP53 mutation status and whether deficiencies in DDR would lead to a significant increase in TMB and neoantigen levels among different TP53 statuses.
We first compared the TMB data gathered from public databases (MSK and TCGA). Both the TP53-missense-mutant and -nonsense-mutant groups displayed higher TMB levels than the TP53-wild-type group (Fig. 3a). Neoantigen and HDR scores were also calculated based on TCGA data according to established methods. The TP53-missense-mutant and nonsense-mutant groups showed higher neoantigen levels and HR scores than the TP53-wild-type group (Fig. 3a). Next, an analysis based on NGS data from 3040 LUAD samples in the GENE+ database further verified the elevated TMB level in both the TP53-missense-mutant and -nonsense-mutant groups (P<0.001, Wilcox test) (Fig. 3b). However, no difference was evident between the TP53-missense-mutant and -nonsense-mutant groups.
Fig. 3.
Both TP53 missense and nonsense mutations are associated with elevated TMB and neoantigen level and contribute equally to DNA damage repair deficiency.
a) TMB data from MSK and TCGA cohort, Neoantigen and HDR score were directly obtained from published data both showed significantly increased in TP53 missense and nonsense group compared with LUAD harboring wildtype TP53(Wilcox test).
b) TMB calculated based on NGS data from 3040 LUAD samples in the GENE+ database elevated in both the TP53-missense-mutant and -nonsense-mutant groups(Wilcox test).
c) Single-sample GSEA (ssGSEA) was conducted to calculate the score for enrichment of a set of genes regulating DNA damage repair (DDR) pathway, and based on which a heatmap was plotted to demonstrate the distribution between TP53 subgroups.
****, p < 0.0001; ***, p < 0.001; **, p < 0.01, * p < 0.05; NS, not significant.
DNA damage repair includes diverse mechanisms to protect genomic stability. To determine the influence of TP53 status on DDR, we further calculated the GSEA score for all DDR-related pathways and created a heatmap. The heatmap clearly showed that both TP53 missense and nonsense mutations were associated with enriched signatures in HR, MMR, BER, and NER but not in direct repair (DR) and non-homologous end joining (NHEJ) (Fig. 3c). The data from multiple databases were consistent, which supports that both TP53 missense and nonsense mutations can significantly increase the TMB and neoantigen levels by disrupting the HR and MMR pathways.
3.4. Tumor-associated M2 macrophages and neutrophils are enriched in TP53-nonsense-mutant LUAD patients compared with patients with TP53 missense mutations and patients with wild-type TP53
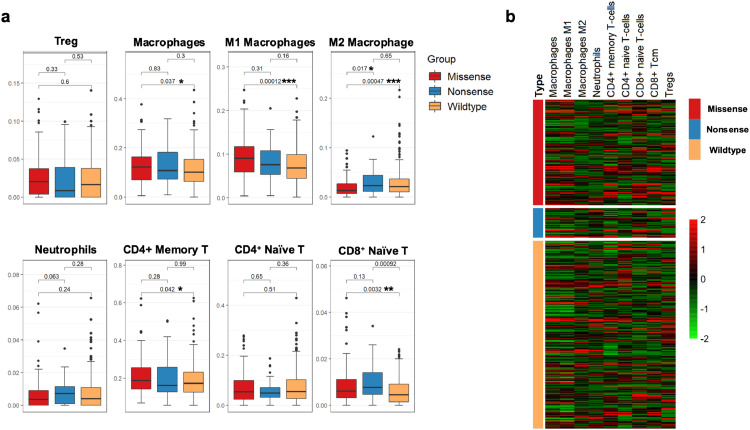
Tumor-infiltrating lymphocytes (TILs) can demonstrate either tumor-suppressive or tumor-promoting effects. Regulatory T cells (Tregs), neutrophils, and tumor-associated macrophages (TAMs; M2) have been associated with pro-tumor functions and inferior responses to immune therapy 24, 25, 26. As TILs have been proven to be enriched in tumors with TP53 alterations, we sought to further explore the distribution of immune cells within LUAD tumors between TP53 mutation groups [2].
The immune cell level was calculated based on an established method that was designed for cell type enrichment analysis based on bulk RNA expression data [12]. In agreement with a previous report, CD8+ T naïve cell levels were significantly increased in both the TP53-missense-mutant and -nonsense-mutant groups compared with the wild-type-TP53 group (P<0.05, Wilcox test). Besides, CD4+ memory T cells which were defined as the collection of CD4+ Tcm and Tem cells were only enriched in LUAD samples harboring TP53 missense mutations (P = 0.042, Wilcox test) (Fig. 4a). Further details as regard to the compositions of T cells subtypes were also shown (supplementary Fig. 4S).
Fig. 4.
TME compositions correlation with different TP53 status
a) TME composition is analyzed based on Bulk RNA-seq data from TCGA and generated through X-cell method. Suppressive immune cells including M2 macrophage and Neutrophils infiltrated in TP53 nonsense mutation, which is associated with inferior clinical benefits for ICIs (Wilcox test).
b) A heatmap is plotted to describe the key composition of TME in LUAD.
Regarding the negative regulation of immune cells, there was no difference in Tregs between groups (P>0.05, Wilcox test). Considering the presence of M2 macrophages or neutrophils proved to be associated with an inferior response to immune therapy. We also evaluated the M2 macrophage and neutrophil levels. The results revealed a significantly higher M2 macrophage level in the TP53-nonsense-mutant group than in the TP53-missense-mutant group (P = 0.017, Wilcox test). Interesting, activated JAK-STAT1 pathway was previously proved to be played an important role in polarization of macrophages toward an M1 phenotype instead M2. [27,28] And then the enriched JAK-STAT1 signal in TP53 missense group showed above in our study could help to explain the diverse distribution regarding to macrophage phenotypes. In addition, a marginally significant increase in neutrophils was also observed in the TP53-nonsense-mutant group (P = 0.063, Wilcox test) (Fig. 4a, b).
Taken together, patients harboring TP53 missense mutants showed enrichment T cells and less M2 macrophage than WT group, which both contribute to a superior response to ICIs. Furthermore, enriched M2 Macrophage and neutrophils were showed in nonsense group than TP53 Missense group, which would hinder the immune anti-tumor effect.
3.5. LUAD harboring TP53 missense mutation is associated with a better anti-PD-1/L1 response than LUAD harboring TP53 nonsense mutation
The data have revealed similarities and differences between TP53 subgroups regarding PD-L1 expression, TMB, neoantigen level and the TME, which all can influence the efficacy of immune checkpoint blockade. We then sought to verify the role of TP53 missense and nonsense mutations in LUAD patients as biomarkers for anti-PD-1/L1 therapy.
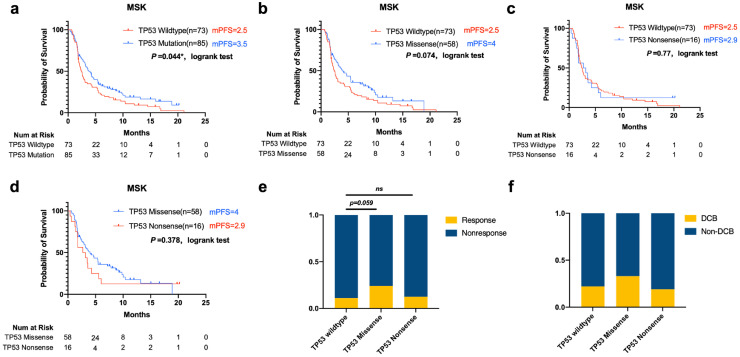
A total of 147 LUAD patients receiving anti-PD-L1 mono-therapy were included in the published MSK cohort. The data revealed that progression-free survival was significantly longer in the TP53-mutant group than in the TP53-wild-type group (mPFS=3.5 vs 2.5 months, log-rank P = 0.043) (Fig. 5a). This retrospective analysis also showed a longer PFS in TP53-missense-mutant vs TP53-wild-type tumours, and a marginally significant difference was established (median PFS = 4 vs 2.5 months, log-rank P = 0.074) (Fig. 5b). We further compared the TP53-nonsense-mutant group with the TP53-missense-mutant or -wild-type LUAD group. The TP53 missense group displayed a longer median PFS, but no significant difference was evident, which may be due to the smaller number of patients with TP53 nonsense mutations (Fig. 5c, d). We also evaluated the presence of co-occurring mutations involving EGFR, ALK, STK11, and KRAS between groups in the MSK cohort, the different TP53 statuses showed a similar presence of co-occurring mutations. Furthermore, the portion of LUAD patients taking ICIs at 1st line, 2nd line or following therapy was evenly distributed between groups. Patients taking ICIs as third line of following therapies accounts each for 22% and 38% in TP53 missense and nonsense group, and no significant difference was generated (Table 1 and Fig. S5b).
Fig. 5.
Association of TP53 status With Prognosis in Patients Treated with Immune Checkpoint Inhibitors in the Memorial Sloan Kettering Cancer Center Cohort
a, b, c, d: Kaplan–Meier (KM) survival curves estimates of PFS compared TP53 missense with the wild-type group in patients treated with mono-ICI treatment (log-rank)
e, f Response Rate and percentage of LUAD with durable clinical benefits (Fisher's exact test).
Table 1.
Characters between TP53 subgroups of LUAD patients in MSK and GDLCI cohort.
| Characters | TP53 wildtype | TP53 Missense | TP53 Nonsense | Fisher's Exact Test | |||
|---|---|---|---|---|---|---|---|
| GDLCI | P | ||||||
| Total | 12 | 17 | 5 | ||||
| Sex | |||||||
| Female | 4 | 33% | 3 | 18% | 2 | 40% | 0.5024 |
| Male | 8 | 67% | 14 | 82% | 3 | 60% | |
| Line | |||||||
| Line 1st | 4 | 33% | 5 | 29% | 1 | 20% | 1 |
| Line 2nd | 4 | 33% | 6 | 35% | 2 | 40% | |
| Line 3rd or more | 4 | 33% | 6 | 35% | 2 | 40% | |
| MSK | |||||||
| Total | 73 | 58 | 16 | P | |||
| Sex | |||||||
| Female | 44 | 60% | 29 | 50% | 6 | 38% | 0.2033 |
| Male | 29 | 40% | 29 | 50% | 10 | 63% | |
| Line | |||||||
| Line 1st | 8 | 11% | 13 | 22% | 2 | 13% | 0.1184 |
| Line 2nd | 34 | 47% | 32 | 55% | 8 | 50% | |
| Line 3rd or more | 31 | 42% | 13 | 22% | 6 | 38% | |
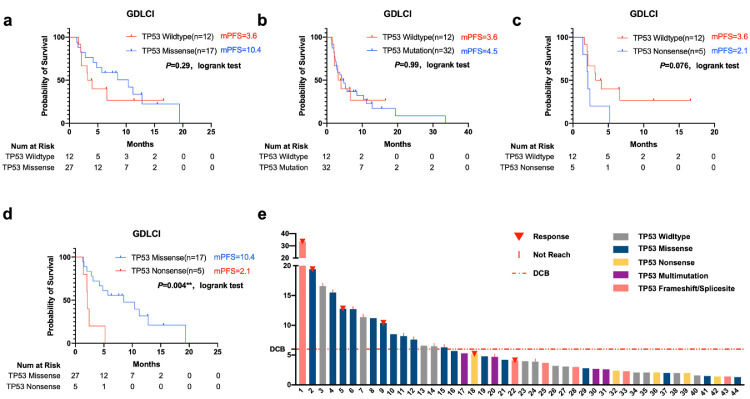
Then we next sought to verify this result in local cohort data, a retrospective analysis with LUAD patients receiving anti-PD-1/L1 monotherapy in GDLCI cohort was conducted. Forty-four patients receiving ICI therapy between 2016 and 2019 were enrolled, and the TP53 status was confirmed by NGS detection. The characteristics as regard to therapy lines of included patients were well balanced. 35% of patients in TP53 missense group taken ICIs as third or following line therapy which is similar with the portion (40%) in TP53 nonsense group (P = 0.1184, Fisher's exact test). (Table 1)
No difference between the TP53-mutant and TP53 wild-type group was detected (TP53-wild-type group vs TP53-mutant group, mPFS= 3.6 vs 4.5 months, log-rank P = 0.99) (Fig. 6a). We then compared the TP53-missense-mutant and -nonsense-mutant groups and found significantly prolonged PFS in the TP53-missense-mutant group (mPFS=10.4 vs 2.1 months, log-rank P = 0.004) (Fig. 6d). Moreover, patients with LUAD without TP53 mutation also showed a marginally significant prolonged mPFS compared with patients with TP53 nonsense mutations (mPFS=3.6 vs 2.1, log-rank P = 0.076) (Fig. 6b). Although the median PFS was substantially prolonged in the TP53-missense-mutant group compared with the wild-type group, the difference was not significant (mPFS= 10.4 vs 3.78 months, log-rank P = 0.36) (Fig. 6c).
Fig. 6.
Association of TP53 status with prognosis in patients treated with antiPD-1/L1 monotherapy in local Guangdong Lung Cancer Institute cohort (GDLCI)
a, b, c, d Kaplan–Meier estimates of progression-free survival between groups harboring different TP53 status (log-rank).
e. The swimming plot described the PFS of each LUAD patient harboring different TP53 status involved in GDLCI. TP53 missense mutation groups is marked with dark blue, and nonsense mutation is marked with yellow.
Furthermore, the swimmer plot regarding to local GDLCI cohort indicated that 5 LUAD patients harboring TP53 nonsense mutations manifested inferior PFS, and all failed to gain durable clinical benefits (Fig. 6e). On the other hand, TP53-missense-mutant LUAD patients (highlighted in blue) showed significantly prolonged PFS. We also evaluated other TP53 mutation statuses, and patients with multiple tp53 mutations were inclined to gain inferior clinical benefits from immune checkpoint blockade. We also noticed that not all TP53 missense mutations demonstrated a longer PFS in this cohort, which revealed that not all TP53 missense mutations had equal potential in this context. Further analysis is needed to verify the locations and alternations of the different TP53 missense mutations.
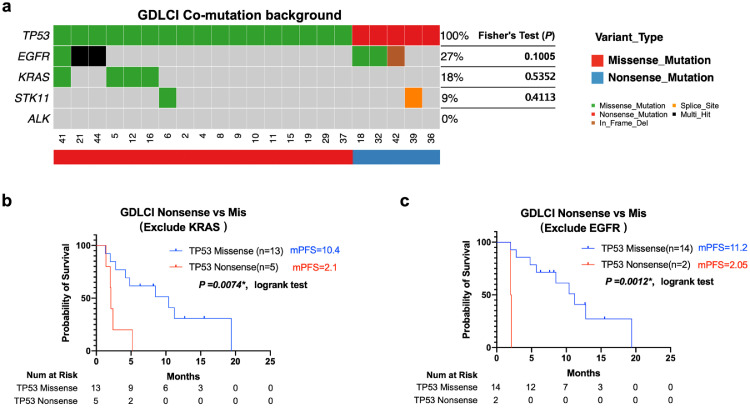
As emerging studies proved co-mutation background matters when evaluating efficacy of ICIs, thus assessment of co-mutation such KRAS, STK11, EGFR, ALK in each group was essential to avoid associated bias. Then all such genetic alterations were eventually distributed between Missense and Nonsense group and no significant differences were generated (Fig. 7a). Nevertheless, as KRAS mutation only occurred in TP53 missense group among Local cohort., and former study demonstrates that KRAS co-mutation with TP53 showed superior efficacy of ICIs treatment [2]. All patients harbored KRAS in TP53 missense group were exclude. The median PFS was still significantly prolonged in the TP53-missense-mutant group. (mPFS= 10.4 vs 2.1 months, log-rank P = 0.0074*) (Fig. 7b). Besides, EGFR mutation showed association with inferior clinical benefits to ICIs treatment [29]. We exclude patients harboring EGFR mutation and significant difference was still shown between TP53 missense and nonsense group. (m PFS= 10.4 vs 2.1 months, log-rank P = 0.0074*). (Fig. 7c)
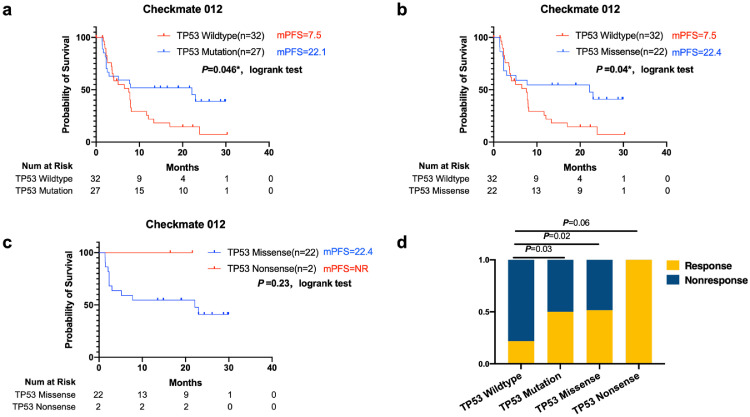
Fig. 8.
Association of TP53 status With Prognosis in Patients Treated with nivolumab plus ipilimumab therapy in the Checkmate 012 cohort.
Progression-free survival (PFS) and overall survival (OS) by TP53 status, a, b, c showed Kaplan-Meier estimates of progression-free survival between groups harboring different TP53 status(log-rank).
d. The response rate was significantly increased in the TP53-missense-mutant group compared with that in the TP53-wild-type group. All two patients harboring TP53 nonsense mutation responded to combine ICIs treatment (Fisher's exact test).
Fig. 7.
The commutation background did not showed bias among TP53 mutation subgroups in GDLCI cohort.
a) The commutation background as regard to TP53 missense and nonsense group in GDLCI cohort (Fisher's exact test).
b) Kaplan–Meier estimates of progression-free survival between groups harboring different TP53 groups which exclude patients harboring KRAS mutation(log-rank).
c) Kaplan–Meier estimates of progression-free survival between groups harboring different TP53 groups which exclude patients harboring EGFR mutation(log-rank).
In summary, results from local GDLCI and published MSK cohort both demonstrate that TP53 missense and nonsense statues have different values in predicting ICI response. LUADs harboring TP53 nonsense mutations failed to gain more clinical benefits from ICI treatment.
3.6. TP53-missense-mutant and nonsense-mutant nonsquamous non-small-cell lung cancer responds well to nivolumab plus ipilimumab therapy
LUADs with TP53 nonsense and missense mutations were associated with higher levels of TMB and neoantigens than LUADs with wild-type TP53. Likewise, a series of studies have demonstrated that TMB is an independent biomarker that correlates with the efficacy of PD-1 plus CTLA-4 blockade in NSCLC. Therefore, we wondered whether anti-CTLA-4 combined with anti-PD-L1 therapy would bring clinical benefits in both LUADs with TP53 missense mutations or TP53 nonsense mutations.
Fifty-nine no squamous non-small-cell lung cancer patients receiving nivolumab plus ipilimumab as first-line therapy were enrolled in the Checkmate-012 cohort [16]. The data showed significantly prolonged PFS in the TP53-mutant group (median PFS, TP53 wild-type vs TP53 mutant, 22.1 vs 7.5 months, logrank, P = 0.046) (Fig. 7a). The median PFS in the TP53-missense-mutant group was 22.4 months, which was significantly prolonged compared with that in the TP53-wild-type group (logrank, P<0.04) (Fig. 7b). In addition, the response rate was also significantly increased in the TP53-missense-mutant group compared with that in the TP53-wild-type group (47% vs 21%, p = 0.02, Fisher's exact test) (Fig. 7d). Two patients with TP53 nonsense mutations receiving nivolumab plus ipilimumab were included in this cohort, and both responded well to the combination therapy (one achieved a complete response) (Fig. 7c).
Taken together, these data suggest that TP53 mutations are associated with superior clinical benefits in non-squamous non-small-cell lung cancer patients receiving combined ICI therapy, and two patients harboring TP53 nonsense mutations also gained durable clinical benefits. However, more data are needed to further verify whether TP53 nonsense mutations are associated with more clinical benefit from ipilimumab plus nivolumab.
4. Discussion
Several studies have evaluated the utility of TP53 status as a biomarker for immune checkpoint inhibitors and demonstrated that TP53 mutations in combination with mutations in KRAS, STK11, ATM or EGFR could influence the efficacy of ICIs. However, an increased understanding of the effects of distinct mutations on p53 activity has led to the recognition of the different properties of specific mutations. Furthermore, several studies have produced controversial outcomes in evaluating TP53 as biomarkers for ICIs which overlooked its heterogeneity till now [6,30].
Considering the differences between mutant p53 forms, a systematic analysis is essential to ensure their optimal use as biomarkers for ICIs. Our study is the first systematic evaluation of the association of TP53 missense and nonsense mutations with ICI response in LUAD. More importantly, TME composition, PD-L1 expression and IFN-γ score, which have been proven to be the best predictive biomarkers for ICIs, showed different distributions between samples with TP53 missense and nonsense mutations in our analysis.
Firstly, tumor PD-L1 expression has been proven to be a potential efficacy biomarker for anti-PD-L1 therapy, but the complex mechanisms underlying its regulation by p53 are not completely understood. Contrary to a previous report suggesting that loss of p53 function regulating miR-34 could enhance PD-L1 expression, no positive correlation between miR-34 and TP53 status was established [17]. In our study, LUAD harboring TP53 missense mutations showed significantly higher PD-L1 levels than the wildtype Group and the results were consistence cross multi-database (IHC, RNA-seq, RPPA), but Nonsense patients failed to generate increased PD-L1 both in RNA and protein level than TP53 wildtype (Fig. 1). The diverse distribution of JAK/STAT pathway between TP53 missense and Nonsense shown in our article provide a hypothesis that Missense mutant P53 might enhance PD-L1 expression through new gained function in activating BCL2L1/JAK3/STAT1 signal. It worth noting that we observed a higher level of PD-L1 in Missense than Nonsense group (both in IHC, RNA-seq and RPPA level), but the difference was not statistically significant. This may due to the limited samples in Nonsense group, or that the loss of function in p53 caused by nonsense mutation may enhance the PD-L1 level to some extent, which would narrow its gap between Missense and Nonsense group. More studies are needed to further explore the underlying mechanism about different p53 mutant forms in regulating PD-L1 expression.
In addition, the composition of the TME, including TILs, Tregs and TAMs, is crucial for the immune response. Furthermore, our data showed a higher ratio of M2-type macrophages in TP53-nonsense-mutant than in TP53-missense-mutant samples, which is associated with a suppressive immune environment for ICI therapy. Interestingly, several studies have demonstrated that activity of JAK/STAT1 pathway played an important role in polarization of macrophages toward an M1 phenotype. And the balance between activation of STAT1 and STAT3/STAT6 finally regulates macrophage polarization and activity [31,32]. Which is consistence with our results regarding to the diverse distribution of JAK/STAT pathway and Macrophage phenotype compositions between groups.
Finally, in agreement with the distributions of biomarkers cross groups (PD-L1, TME compositions eta.), our retrospective clinical analysis based on multi-center data showed patients harboring TP53 missense but not nonsense mutation could generate more efficacy benefits than wildtype status. (Table 2) It is contrary to previously published data which proved all mutant TP53 could serve as independent biomarker for ICIs. Furthermore, in our local data analysis, TP53 nonsense mutations were associated with a significantly inferior response to anti-PD1 therapy compared with TP53 missense mutations. Furthermore, the comparation between TP53 nonsense and Wildtype group was also marginally significant different regarding to PFS. Besides, co-occurring mutations in EGFR, STK11, KRAS and ALK were well balanced in each group. Taken together, not all mutant p53 were equal in predicting the efficacy of ICIs, furthermore, mono antiPD-1/L1 therapy were not recommended to patients harboring TP53 nonsense alternations.
Table 2.
Distribution of multi-dimension characters and the clinical differences between each group.
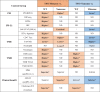 |
* significantly difference, P<0.05; †marginally significant, P<0.1; ND, No difference. Column with Yellow color showed results favored ICIs treatment. Column with Blue Color showed results associated with inferior clinical benefits of ICIs.
Multiple recent studies have shown that TMB can serve as a surrogate for overall neoantigen load, and disruption of the DNA damage repair pathway can result in an elevated TMB level [6,27]. P53 plays an important role in guarding genomic stability by orchestrating a variety of DNA damage response (DDR) mechanisms, and p53-null tumor cells are defective in certain DNA repair activities [31]. Therefore, we wondered whether TP53 missense and nonsense mutants had different impacts on the activity of various DNA repair systems. Data collected from multiple databases showed that TMB, and the neoantigen level were significantly higher in TP53-mutant than in TP53-wild-type LUAD patients. We also performed GSEA for different DNA repair systems and created a heatmap to show the distribution. Pathways related to HR, mismatch repair, base excision repair and nucleotide excision repair were all significantly enriched in TP53-missense-mutant and -nonsense-mutant samples. However, no differences were found in the pathways of direct repair, nonhomologous end joining and translesion synthesis. In summary, the distribution of DDR-related signatures is similar between TP53-missense-mutant and -nonsense-mutant groups, which indicates that the role in altering DNA damage repair is consistent between these groups. Totally, we believed that TP53 missense makes widely differences of mRNA and protein expression from nonsense mutation regarding to the new gained function such as overactive JAK-STAT pathway which only enriched in TP53 missense group. But key functions such as apoptosis or cell cycle arrest which required complete transactive function of p53 relied on the intact core domain (DNA binding domain), thus DNA damage repair role associated with cell cycle arrest regulated by p53 were abolished both in missense and nonsense groups.
As an independent variable, high TMB correlates with the efficacy of PD-1 plus CTLA-4 combination therapy for LUAD patients [16]. Consistent with this, our data showed that TP53 mutation was significantly associated with the improved efficacy of PD-1 plus CTLA-4 therapy. In addition, both patients harboring nonsense mutations responded well to the combination immune therapy.
Our research has several limitations. As previously published data showed the distinctly different relationship between TME and mutation signatures among LUSC and LUAD patients [32], and the prevalent of TP53 mutation is significantly higher in LUSC group (80 vs 50%). so we excluded LUSC patients to make our analysis more rigorous; Our analysis only proved that the relationship between TP53 status and the efficacy of ICI therapy is significant, and more studies should be conducted to explore the potential mechanism; Furthermore, published data with regard to mRNA expression profiling and reverse phase protein array (RPPA) were collected from different database and thus involved subjects were various between different analysis, even though all such database shares the same TCGA patients ID; At the same time, the heterogeneous of subjects involved in different database is inevitable. But results within Multiple dimensions showed the same tendency across different database, which make our conclusion more convinced; As only two patients with TP53 nonsense mutations were included in the Checkmate 012 trial analyzing the response to nivolumab plus ipilimumab, more data are needed to prove that LUADs harboring TP53 nonsense mutations should receive combination therapy instead of anti-PD1/L1 mono-therapy.
In summary, we demonstrate that not all TP53 mutations are equal in predicting efficacy in patients with LUAD treated with ICIs. Multi-center data showed that TP53 missense and nonsense mutations were significantly different in terms of associations with PD-L1 expression, IFN-γ score and TME composition. Our study showed that patients harboring TP53 nonsense mutation were not recommended for mono antiPD-1/L1 therapy, combine therapy with CTLA-4 might be a more ideal therapy option which need further verified.
Data sharing statement
Publicly data were all available in the supplementary tables. Associated analysis method was based on published articles which was cited in the references.
Contributors
Conception and design: Hao Sun, Yi-Long Wu
Development of methodology: Hao Sun, Jin-Tian Xu, Huang-Kai Zhang, Hong-Hong Yan, Jiao-Jiao Huan, Ping-Ping Dai, Yan-Fang Guan, Xin Yi, Rong-Shan Yu
Acquisition of data: Hao Sun, Jin-Tian Xu, Huang-Kai Zhang, Si-Yang Liu, Jia-Ying Zhou
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Hao Sun, Jin-Tian Xu, Huang-Kai Zhang, Hong-Hong Yan, Jiao-Jiao Huan, Ping-Ping Dai, Yan-Fang Guan, Xin Yi, Rong-Shan Yu
Writing, review, and/or revision of the manuscript: Hao Sun, Si-Yang Liu, Jia-Ying Zhou
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Hao Sun, Jin-Tian Xu, Huang-Kai Zhang, Hong-Hong Yan, Jiao-Jiao Huan, Ping-Ping Dai, Yan-Fang Guan, Xin Yi, Rong-Shan Yu
Study supervision: Hao Sun, Jian Su, Chong-Rui Xu, Wen-Zhao Zhong, Yi-Long Wu
Declaration of Competing Interest
There are no interests to declare for Hao Sun, Si-Yang Liu, Jia-Ying Zhou, Hong-Hong Yan, Chong-Rui Xu, Jian Su and Wen-Zhao Zhong.
Jin-Tian Xu, Huang-Kai Zhang and Rong-Shan Yu are full-time employees of Aginome Scientific Co., Ltd, Xiamen, China
Jiao-Jiao Huan, Ping-Ping Dai, Yan-Fang Guan, and Xin Yi are full-time employees of Geneplus-Beijing Institute, Beijing, China
Yi-Long Wu need to declare the personal financial interests - Consulting and advisory services, speaking engagements: Roche, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Sanofi, MSD, BMS
Acknowledgments
The study was supported by Key Lab System Project of Guangdong Science and Technology Department – Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (Grant No. 2017B030314120, to Yi-Long WU).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102990.
Appendix. Supplementary materials
Reference
- 1.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015 Apr 2;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Z.-.Y., Zhong W.-.Z., Zhang X.-.C., Su J., Xie Z., Liu S.-.Y. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017 Jun 15;23(12):3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 3.Biton J., Mansuet-Lupo A., Pécuchet N., Alifano M., Ouakrim H., Arrondeau J. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin Cancer Res. 2018 Nov 15;24(22):5710–5723. doi: 10.1158/1078-0432.CCR-18-0163. [DOI] [PubMed] [Google Scholar]
- 4.Skoulidis F., Byers L.A., Diao L., Papadimitrakopoulou V.A., Tong P., Izzo J. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015 Aug 3;5(8):860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabapathy K., Lane D.P. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol. 2018 Jan;15(1):13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 6.Assoun S., Theou-Anton N., Nguenang M., Cazes A., Danel C., Abbar B. Association of TP53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer. Lung Cancer. 2019 Jun;132:65–71. doi: 10.1016/j.lungcan.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Leroy B., Anderson M., Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Soussi T, editor. TP53 mutations in human cancer: database reassessment and prospects for the next decadeHum Mutat. 2014 May 20;35(6):672–688. doi: 10.1002/humu.22552. editor. [DOI] [PubMed] [Google Scholar]
- 8.Bouaoun L., Sonkin D., Ardin M., Hollstein M., Byrnes G., Zavadil J. TP53variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016 Jul 8;37(9):865–876. doi: 10.1002/humu.23035. [DOI] [PubMed] [Google Scholar]
- 9.Lykke-Andersen S., Jensen T.H. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 2015 Nov;16(11):665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Lu Y., Akbani R., Ju Z., Roebuck P.L., Liu W., et al. TCPA: a resource for cancer functional proteomics data. 2013 Sep 15;10(11):1046–7. [DOI] [PMC free article] [PubMed]
- 11.Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. 2017 Aug 1;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aran D., Hu Z., Butte A.J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017 Nov 15;18(1):1960. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.-.H. The immune landscape of cancer. Immunity. 2018 Apr 17;48(4):812–814. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knijnenburg T.A., Wang L., Zimmermann M.T., Way G.P., Greene C.S., Liu Y. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. CellReports. 2018 Apr 3;23(1):239–254.e6. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi H., Sanchez-Vega F., La K., Chatila W., Jonsson P., Halpenny D. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. JCO. 2018 Mar;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellmann M.D., Nathanson T., Rizvi H., Creelan B.C., Sanchez-Vega F., Ahuja A. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018 May;33(5):843–844. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortez M.A., Ivan C., Valdecanas D., Wang X., Peltier H.J., Ye Y. PDL1 Regulation by p53 via miR-34. JNCI: J Natl Cancer Inst. 2015 Nov 17;108(1):M1. doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi Y., Goyama S., Liu X., Tamura M., Asada S., Tanaka Y. Antitumor immunity augments the therapeutic effects of p53 activation on acute myeloid leukemia. Nat Commun. 2019 Oct 25;10(1):359. doi: 10.1038/s41467-019-12555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. CellReports. 2017 May 9;19(6):1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prestipino A., Emhardt A.J., Aumann K., O'Sullivan D., Gorantla S.P., Duquesne S. Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. 2018 Feb 21;10(429):eaam7729. doi: 10.1126/scitranslmed.aam7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzec M., Zhang Q., Goradia A., Raghunath P.N., Liu X., Paessler M. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008 Dec 30;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002 Jun 24;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 23.Rooney M.S., Shukla S.A., Wu C.J., Getz G., Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. 2015 Jan 15;160(1–2):48–61. [DOI] [PMC free article] [PubMed]
- 24.Nishikawa H., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014 Apr;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014 Jul 17;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma M., Lewis C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013 Mar 18;23(3):277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Investig. 2007 May;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012 Mar;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai Y., Chen X., Hou L., Qian J., Jiang T., Zhou C. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol Med. 2018 Nov 1;15(4):434–442. doi: 10.20892/j.issn.2095-3941.2018.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlisle J.W., Nho N.T., Kim C., Chen Z., Li S., Hill C. Impact of TP53 mutations on efficacy of PD-1 targeted immunotherapy in non-small cell lung cancer (NSCLC) JCO. 2018 May 20;36(15_suppl) e21090–0. [Google Scholar]
- 31.Williams A.B., Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016 May 2;6(5) doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.-.C., Wang J., Shao G.-.G., Wang Q., Qu X., Wang B. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. 2019 Apr 10:1–12. doi: 10.1038/s41467-019-09762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.