Abstract
Suaeda rigida is a lignified, true haplotype that predominantly grows in the Tarim basin, China. It has significant economic and ecological value. Herein, with aim to determine the genes associated with salt tolerance, transcriptome sequencing was performed on its stem, leaves and root over three set NaCl gradients regimens at treatment intervals of 3 h and 5 days. From our findings, we identified 829,095 unigenes, with 331,394 being successfully matched to at least one annotation database. In roots, under 3 h treatment, no up-regulated DEGs were identified in 100 and 500 mM NaCl treated samples. Under 5 days treatment, 97, 60 and 242 up-regulated DEGs were identified in 100, 300, 500 mM NaCl treated samples, respectively. We identified 50, 22 and 255 down-regulated DEGs in 100, 300, 500 mM NaCl treated samples, respectively. GO biological process enrichment analysis established that down-regulated DEGs were associated with nitrogen compound transport, organic substance transport and intracellular protein transport while the up-regulated genes were enriched in cell wall biogenesis, such as plant-type cell wall biogenesis, cell wall assembly, extracellular matrix organization and plant-type cell wall organization. These findings provide valuable knowledge on genes associated with salt tolerance of Suaeda rigida, and can be applied in other downstream haplotype studies.
Subject terms: Genome informatics, Plant molecular biology, Plant stress responses
Introduction
The growth and development of plants is highly affected by several abiotic stress factors. These factors may include salinity, temperature, drought and heavy metals. Of these many abiotic factors, salinity is a significant environmental factor that affects plant productivity1. Globally, it is estimated that salinity affects almost 1 billion hectares of land2, a situation that has continued to be more critical due to the rising changes in climatic conditions3.
Congruently, plants possess various intricate adaptive responses to these stress factors4. In plants with ability to survive high salinity (halophytes), their response and adaptation to high saline levels typically relies on succulence buildup in their cells and tissues5–9. These high salt tolerance plants include representatives such as Sonneratia acida, Pentatropis sianshoides, S. nudiflra, S. nudiflra, Limnitzera racemosa, S. maritima, Salvadora persica, S. salsa and S. rigida10–13. These halophyte plants have the ability to compartmentalize the disproportionate Na+ and Cl− ions that are present in the cell vacuole, a mechanism that aids them in reducing the cells’ water potential and thereafter enhance their water absorbance ability from the saline soil, thus providing optimum ion concentration conditions in the cell cytoplasm for enzymatic and other biological activities14–16.
A previous study had shown that even though the Arabidopsis thaliana has a weak salt tolerance, and its response mechanisms is centered on the action of SALT OVERLY SENSITIVE3 [SOS3], a sensor, and SOS2, a protein kinase. These two protein complexes are implicated in detecting and responding to Na+ influx17–20, involving activation of SOS1, a plasma membrane-located sodium/proton antiporter. The SOS1 activation mediates the transfer and redistribution of the Na+ ions all over the plant21–23. Equally, SOS complex has been found to exist in other plant species, hence it may be a general feature of plants24. Equally, a study on the effect of salinity on Suaeda fruticosa linked its salt tolerance to its capability to uptake K+ so as to retain a higher K+/Na+ balance in the shoots25. Further, halophytes have mechanisms capable of regulating several biological pathways like signal transduction, energy metabolism and photosynthesis26.Other studies on plants’ salt and drought tolerance have also recognized several inducible stress regulators, with up-regulation of catalase, aquaporins, peroxidase and proline accumulation27. Moreover, plant abscisic acid (ABA) signaling pathways have been shown to be stimulated via stress factors28. The Sodium and chloride build-up in plant’s cytoplasm leads to cell cytotoxicity induced by accumulation of reactive oxygen species (ROS)29; a situation that can cause protein and lipid degradation, which may disrupts the cell functions30.
Suaeda rigida (HW Kung et GL Chu) is a lignified plant under genus Polygonaceae. It is a true haplotype plant that is endemic in the Tarim basin in Xinjiang, North west China. It is a nutrient-rich wild vegetable that is also an excellent local forage resource with important economic and ecological value in the region31. Notably, nearly one-third of the cultivated land in Xinjiang province is salinized, and the development and utilization of salt-tolerant plants form a key focal point in plant research in this region. Overall, study of plants’ salt tolerance mechanisms and the mining of related genes have important theoretical and practical significance in the development and utilization of excellent germplasm resources, enhancement of crop stress resistance and improvement of agricultural production. However, presently there is a lack of detailed knowledge on the saline stress and associated signaling pathways of Suaeda rigida plant. Therefore this study was aimed at applying transcriptomic sequencing in identifying and analyzing the putative genes implicated in salt response and tolerance mechanisms of Suaeda rigida plants over a set gradient of NaCl treatment regimens.
Results
Sequencing summary for all the libraries
A total of 343.88 GB of raw reads were obtained from the 42 libraries of the roots, leafs and stem samples (Supplementary Data 1). The average sequencing error rate was 0.029% while the average GC content was 42.76%. The Q20 and Q30 data ratio of sequencing quality was 97.10% and 92.35%, respectively (Supplementary Data 1). Since S. rigida does not have a reference genome yet, Trinity software was utilized to de novo assemble the obtained clean reads. In total, 2,212 million high-quality clean reads were de novo assembled into 829,095 transcripts with an N50 length 1453 bp and an N90 length of 269 bp (Table 1).
Table 1.
Summary of transcriptome assembly.
| Category | Number | Total | Mean length (bp) | N50 (bp) | N90 (bp) | |||
|---|---|---|---|---|---|---|---|---|
| 200–500 bp | 500–1 kbp | 1 k–2 kbp | > 2 kbp | |||||
| Transcripts | 518,152 | 140,336 | 97,377 | 73,230 | 829,095 | 359 | 1,453 | 269 |
| Unigenes | 135,845 | 127,207 | 96,747 | 73,154 | 432,953 | 759 | 1,885 | 531 |
Expression analysis, functional annotation, gene ontology assignments and analysis
After mapping of all the filtered reads to the de novo assembled contigs, we obtained all the expression data for them in all libraries. Thereafter, DESeq was utilized in identifying the differentially expressed genes. Comparison of gene expression analysis for each different experimental condition are as illustrated in Fig. 1.
Figure 1.

Comparison of gene expression levels under different experimental conditions.
Gene function annotation was attained by searching the obtained transcripts against the Nr, Nt, KO, Swiss-Prot, Pfam, GO and KOG databases. In sum, 331,394 unigenes successfully matched with at least one database, with 253,490 (58.54%), 241,135 (55.69%), 116,108 (26.81%), 224,239 (51.79%), 219,455 (50.68%), 221,659 (51.19%), and 113,379 (26.18%) annotated transcripts showing a significant hit against the Nr, Nt, KO, Swiss-Prot, Pfam, GO and KOG, respectively (Table 2).
Table 2.
Gene annotation statistics.
| Number of genes | Percentage (%) | |
|---|---|---|
| Annotated in NR | 253,490 | 58.54 |
| Annotated in NT | 241,135 | 55.69 |
| Annotated in KO | 116,108 | 26.81 |
| Annotated in SwissProt | 224,239 | 51.79 |
| Annotated in PFAM | 219,455 | 50.68 |
| Annotated in GO | 221,659 | 51.19 |
| Annotated in KOG | 113,379 | 26.18 |
| Annotated in all databases | 57,824 | 13.35 |
| Annotated in at least one database | 331,394 | 76.54 |
| Total unigenes | 432,953 | 100 |
GO categorization established that cellular process, metabolic process and single-organism processes were the highly enriched biological process whereas, the genes associated with cell, cell part and organelle as the most significant among the cellular component terms. Three molecular function GO terms; binding, catalytic activity and transporter activity were significantly enriched (Fig. 2).
Figure 2.
GO classification. The figure comprises of biological processes, cellular component and molecular function.
The KEGG classification analysis revealed that Transport and catabolism, signal transduction, membrane transport, Translation, Carbohydrate metabolism, Energy metabolism and Environmental adaptation were among the few important pathways that were significantly enriched under Cell processes, Environmental information processing, Genetic information Processing, Information processing, Metabolism and Organic Systems of Suaeda rigida plant in response to salt stress (Fig. 3).
Figure 3.
KEGG classification. The figure is divided into five branches based on the corresponding KEGG metabolic pathways: cell processes (A), environmental information processing (B), genetic information processing (C) information processing, metabolism (D) and organic systems (E).
Differential expression pattern for salt tolerance in root
Differential gene cluster analysis for all the treatments was conducted (Fig. 4). Generally, roots are the main plant tissue that are directly in contact with high salt concentration in the soil. Thus, gene expression profiles patterns in Suaeda rigida roots were important. Under 3 h of treatment, no up-regulated DEGs were recorded in 100 mM NaCl and 500 mM NaCl treated samples, whereas there were only 26 up-regulated DEGs in 300 mM NaCl treated samples. Meanwhile, there were 3, 37 and 0 down-regulated DEGs in 100 mM NaCl, 300 mM NaCl, 500 mM NaCl treated samples, respectively. However, in the 5 days’ treatment group, 97, 60 and 242 up-regulated DEGs were identified in 100, 300, 500 mM NaCl treated samples, respectively. Further, we identified 50, 22 and 255 down-regulated DEGs in 100, 300, 500 mM NaCl treated samples, respectively. Therefore, based on these findings, we focused on these DEGs in 5 days’ treatment group. Overall, 43 up-regulated DEGs and 24 down-regulated DEGs were present in more than two samples. For the down-regulated genes, GO biological process (BP) enrichment analysis indicated that they were enriched in nitrogen compound transport (GO: 0071705, p = 5.61E−06), organic substance transport (GO: 0071702, p = 0.010173) and intracellular protein transport (GO: 0006886, p = 0.015322) while for the up-regulated genes, they were found enriched in plant-type cell wall biogenesis related GO entries, such as plant-type cell wall biogenesis (GO: 0009832, p = 0.0088759), cell wall assembly (GO: 0070726, p = 0.0088759), extracellular matrix organization (GO: 0030198, p = 0.0088938) and plant-type cell wall organization (GO: 0009664, p = 0.027692).
Figure 4.
Differential gene cluster Heatmap.
qPCR validation
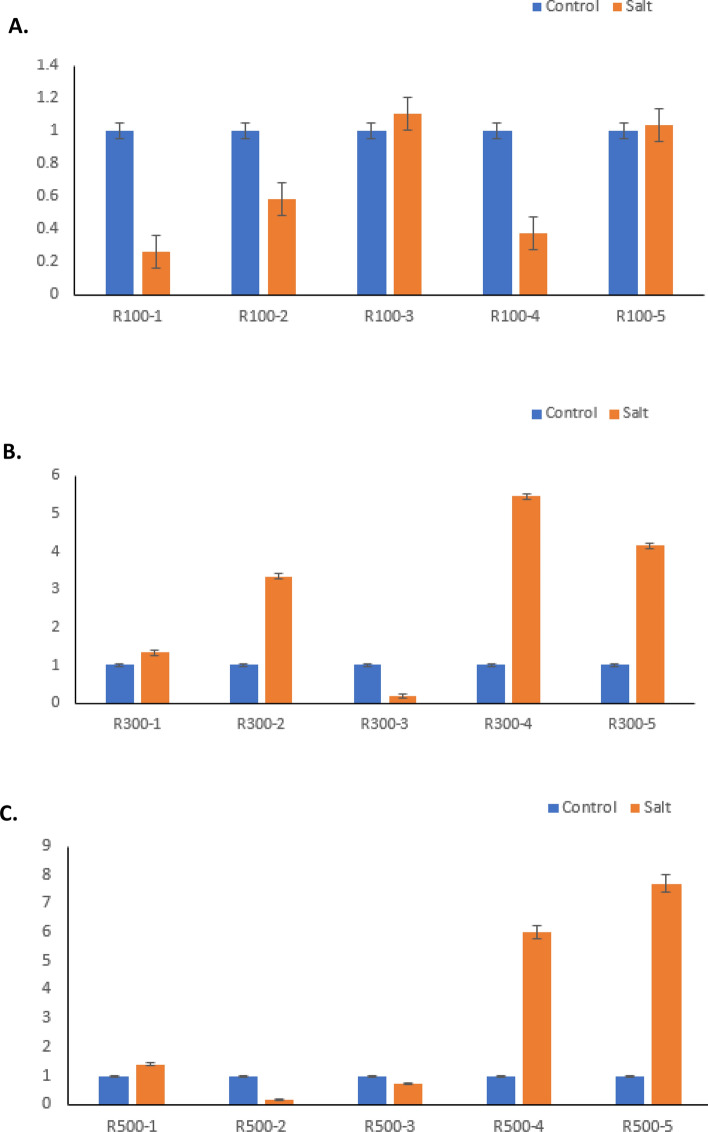
A set of differentially genes were randomly selected for detection and subsequent validation via qPCR, Fig. 5.
Figure 5.
Real time-PCR validation of DE transcripts. The figure represents root treatments at three different timepoints (A: R100, B: R300 and C: R500). Bars represent mean ± SE (n = 3).
Discussion
Herein, we conducted a transcriptomic profile analysis of Suaeda rigida response to saline stress conditions. In this, sequencing of the leaves, stems and roots of the plant were conducted so as to identify the salt-responsive genes. In spite of the recent development in genome sequence methods, genomic data for various non-model plants is absent. Though transcriptomic mRNA-sequencing, it has been made possible to identify and quantify transcripts even in circumstances where the reference genome sequence is not yet available32. Although transcriptome studies have been conducted in closely related species like Suaeda fruticosa, such information on S. rigida is still lacking.
In this study, we applied transcriptomic sequencing in identifying and analyzing the putative genes related to salt response and tolerance mechanisms in Suaeda rigida plants over a set gradient of NaCl treatment regimens. Suaeda rigida (HW Kung et GL Chu) is a lignified plant under genus Polygonaceae31. Hence, it is highly suitable in examining salt tolerant mechanisms in plants. Therefore, Suaeda rigida may offer a significant basis for validating salt tolerance associated genes.
In haplotypes, roots are the main plant tissue that are in direct interaction with high concentration of salt in the soil. Thus, we focused on the gene expression profiles in Suaeda rigida roots. From the transcriptome analysis, 28,555 DEGs were identified. A significant majority of these DEG’s clustered to four functional groups involving signal transduction, transporters, cell wall metabolism and transcription factors; that might have typical biological functions in Suaeda rigida salt-tolerant mechanisms.
For plants under abiotic or biotic stress, signal transduction is very crucial for adjustment in such unfavorable conditions33–36. From our study findings, genes associated with signal transduction processes were differentially expressed when under saline stress. Differentially expressed genes associated with control of ABA signaling pathway were highly up-regulated in 500 mM NaCl treated samples (Supplementary Data 2). The genes encoding for probable protein phosphatase 2C 25 (Cluster-47345.139659) and protein phosphatase 2C 22 (Cluster-47345.93406) were up-regulated in response to high salt levels. Phosphatase genes are considered to be important regulators of the ABA signaling in plants37,38. In a study conducted by Liu et al., they established that over-expression of AtPP2CG1 in Arabidopsis thaliana led to improved salt tolerance, while its depletion led to reduced salt tolerance levels39. Further, ZmPP2C2 over-expression in tobacco has been established to enhance tolerance to cold stress40. Similarly, previously conducted studies on other haplotype such as Suaeda fruticose and Suaeda glauca demonstrated phosphatase 2C family proteins were up-regulated at high salt treatment regimens25,41. Thus, protein phosphatase 2C might have a crucial regulatory function in salt tolerance of Suaeda rigida.
Abscisic acid (ABA) is a key endogenous indicator. Abiotic factors like salinity stimulates ABA biosynthesis, which thereafter initiates the signaling pathways leading to several downstream responses42. Cytochrome P450 is elucidated to play a major part in ABA catabolism43, while other cytochrome P450s might facilitate growth and stress responses26,27. From our dataset, three unigenes encoding for cytochrome P450 76AD1 (Cluster-47345.95314), cytochrome P450 84A1 (Cluster-47345.105167) and cytochrome P450 CYP73A100 were up-regulated. However, the precise roles of each cytochrome P450 in Suaeda rigida is yet to be known. Notably, these genes might be linked to ABA signal transduction, implying ABA signal transduction may have a significant function and show prompt response of Suaeda rigida in the earlier stage during saline stress.
In plants, Oligopeptide transporters (OPTs) are membrane-confined proteins that have numerous transportation roles44–46. From our study findings, we identified one gene that encodes for an oligopeptide transporter 6 (Cluster-47345.229496), and it was up-regulated, demonstrating that it may be associated with metal transport and homeostasis during salt stress. Further, ABC proteins are actively involved in transportation of various molecules like hormones, secondary metabolites, lipids, metals and modulators of ion channels across membranes47,48. Thus, these ABC transporters augment drought and salt resistance48. Herein, three gene associated with ABC transporter families, Cluster-47345.112862, Cluster-47345.140732 and Cluster-47345.152644, were upregulated in the roots of Suaeda rigida. Our study findings are consistent with previously reported studies on Suaeda glauca and Suaeda fruticosa29,41.
As a result of saline stress, normal plant growth and development is highly affected. Cell wall modification is a response measure in plants when under biotic or abiotic stress. herein, genes related to cell wall responses were enriched. Specifically, plant-type cell wall biogenesis related GO entries, such as plant-type cell wall biogenesis (GO: 0009832), cell wall assembly (GO: 0070726), extracellular matrix organization (GO: 0030198) and plant-type cell wall organization (GO: 0009664) were up regulated in response to high salt concentration (Fig. 2). Further, genes associated with cell wall and growth were up-regulated due to salt stress. Expansin-like A2 (Cluster-47345.195511) and expansin-like B1 (Cluster-47345.119414), which are linked to cell elongation and cell wall modification49, were up-regulated.
Several transcription factors (TFs) take part in various significant roles in plant response mechanisms to abiotic and biotic stress. This is usually achieved through their regulation of genes associated with stress responses, such as ERFs and WRKY transcription factors50–53. ERFs perform various significant roles in plants’ abiotic stress tolerance by regulating stress-responsive genes54–57. Three genes, Cluster-47345.149382, Cluster-47345.137633 and Cluster-47345.166382, that encode for ERFs were up-regulated in Suaeda rigida roots under salt stress. Equally, WRKY transcription factors have been implicated in salt as well as drought tolerance in Gossypium hirsutum, Arabidopsis thaliana, Jatropha curcas and Jatropha curcas58–61. From our study findings, we identified two genes, Cluster-47345.231608 and Cluster-47345.62923, encoding WRKY’s were up-regulated under salt stress. Consistent with previously conducted studies in other plants41,62–64, high salinity levels led to up-regulation of bHLH transcription factor. In our study, four bHLH transcription factors, Cluster-47345.43143, Cluster-47345.120470, Cluster-47345.67446 and Cluster-47345.180519 were highly upregulated in the Suaeda rigida roots than in its leaves, implying they might be playing a significant role in plants’ salt tolerance and regulation in the roots.
In conclusion, 42 Suaeda rigida RNA-seq libraries representing various treated Sodium Chloride (NaCl) treatment regimens (100 mM NaCl, 300 mM NaCl and 500 mM NaCl), were sequenced in this study. From these sequenced libraries, a total of 2,212 M high-quality clean reads were assembled into 829,095 transcripts, from which 28,555 DEGs were identified. These DEGs contained up-regulated and down-regulated genes in Suaeda rigida. They comprised of genes implicated in four functional groups namely, signal transduction, transporters, cell wall metabolism and transcription factors; which might have typical biological functions in salt-tolerant mechanism of haplotype Suaeda rigida. These findings can be further applied in downstream genetic-based studies that aim to study the salt tolerance mechanisms and regulation in haplotype plants.
Materials and methods
Plant materials and RNA isolation
Suaeda rigida plants were grown at Tarim university, Xinjiang, China as per a previously published article of a related species29, but with slight adjustments. In duplicates, plants were exposed to established NaCl treatments as described in Supplementary Data 3. Sodium Chloride (NaCl) treatment regimens were 100 mM NaCl, 300 mM NaCl and 500 mM NaCl. Control group (CK) was not exposed to any treatment plan. In duplicates, the leaves (L), stem (S) and roots (R) were collected at set intervals of 3 h and 5 days post NaCl treatment (Supplementary Table 2). The collected samples were triturated to powder form in liquid nitrogen using TRIzol, total RNA was isolated using Spin Column Plant total RNA Purification Kit following the manufacturer's protocol (Sangon Biotech, Shanghai, China). The RNA quality, integrity and concentration were evaluated through the NanoPhotometer spectrophotometer (IMPLEN, CA, USA), Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA) and Qubit RNA Assay Kit in Qubit 2.0 Flurometer (Life Technologies, CA, USA). High quality total RNA samples were utilized for downstream library preparation.
Library preparation for Transcriptome sequencing
From each sample, 1.5 µg RNA was expended for use in library preparation. NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) was used to generate the sequencing libraries, as per the manufacturer’s guidelines. Index tags were added to each sample to serve as identification points. In brief, mRNA was purified from total RNA by means of poly-T oligo-attached magnetic beads. Fragmentation was done through divalent cations under high temperature in NEBNext First Strand Synthesis Reaction Buffer (5×). First strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-). Next, second strand cDNA synthesis done using DNA Polymerase I and RNase H. Residual overhangs were converted into blunt ends through exonuclease/polymerase activity. After adenylation of 3′ DNA fragments ends, NEBNext Adaptor with hairpin loop structure were ligated in preparation for hybridization. Thereafter, 250 ~ 300 base pairs long cDNA fragments were selected through purification of the library segments using AMPure XP system (Beckman Coulter, Beverly, USA). Afterwards, 3 µl USER Enzyme (NEB, USA) was utilized with size-selected, adaptor-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C before PCR. Next, PCR was conducted using Phusion High-Fidelity DNA polymerase, universal primers and Index (X) Primer. Lastly, the obtained PCR products were purified (AMPure XP system) and library quality evaluated on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was done on a cBot Cluster Generation System by TruSeq PE Cluster Kit v3-cBot-HS (Ilumina), as per the manufacturer’s guidelines. Thereafter, the prepared libraries were sequenced on an Illumina Hi-seq 4000 platform.
Bioinformatics analysis
Raw fastq reads were initially assessed using trimmomatic v0.38 with parameters “PE-phred33 ILLUMINACLIP:TruSeq3-PE.fa:2:30:10 LEADING:20 TRAILING:20 HEADCROP:10 SLIDINGWINDOW:4:20 MINLEN:60”65. Briefly, clean reads were attained through filtering off reads with adapters, poly-N residuals and low-quality reads from original raw fastq reads. Simultaneously, Q20, Q30, GC-content and sequence duplication level of the clean reads were determined. For the obtained clean data, all read1 files and read2 files were pooled into single independent reads and transcriptome assembly performed using Trinity66, with min_kmer_cov set to 2 by default and all other parameters set as default. Gene function annotation was done using the Nr (NCBI non-redundant protein sequences); Nt (NCBI non-redundant nucleotide sequences); Pfam (Protein family); KOG/COG (Clusters of Orthologous Groups of proteins); Swiss-Prot; KO (KEGG Ortholog database)67; GO (Gene Ontology) databases, with an E-value threshold of 1e − 5. Picard—tools v1.41 and samtools v0.1.18 were used to sort, remove duplicated reads and merge the bam alignment results of each sample. GATK3 software68 was used to perform SNP calling. Raw vcf files were filtered with GATK standard filter method and other parameters (cluster: 3; WindowSize: 35; QD < 2.0 or FS > 60.0 or MQ < 40.0 or SOR > 4.0 or MQRankSum < − 12.5 or ReadPosRankSum < − 8.0 or DP < 10). SSR of the transcriptome were identified using MISA (https://pgrc.ipk-gatersleben.de/misa/misa.html), and primer for each SSR was designed using Primer3 (https://primer3.sourceforge.net/releases.php). For quantification of gene expression levels, gene expression levels were estimated by RSEM69 for each sample. Clean data was mapped back onto the assembled transcriptome and Read count for each gene was obtained from the mapping results. Differential expression analysis of treated versus control conditions was made via the DESeq R package (1.10.1)70. Benjamini and Hochberg’s filtering approach was applied on the resulting P values so as to control the false discovery rate. Genes with a P-value < 0.05 were dispensed as differentially expressed. GO enrichment analysis of the DEGs was analyzed by use of the GOseq R packages71, as per Wallenius non-central hyper-geometric distribution72. KOBAS software73 was utilized in evaluating the statistical enrichment of differential expression genes in KEGG pathways (https://www.genome.jp/kegg/). DEGs sequences were blast (blastx) to the genome of a closely linked species in the STRING database (https://string-db.org/); so as to obtain the predicted protein interaction (PPI) of them. Cytoscape was used to visualize these DEGs PPI74.
qPCR validation
To validate the RNA-Seq data, some DEG’s were randomly selected from our constructed libraries and their levels of expression under saline conditions was assessed through qPCR analysis. In brief, 3 μg of total RNA was utilized to construct the cDNA. Reverse transcription was conducted using oligo (dT) primer as per the manufacturer’s guidelines (Fermentas, Burlington, Ontario, Canada). Primer 5 software (version 5.2.0) was utilized in designing the specific primers for the selected genes. The qPCR was conducted in a total volume of 20 μL, with each assay comprising 2 μM of the forward and reverse primers, 2 μL cDNA, and 10 μL of 2 × SYBR Green qPCR Mix (Takara, Otsu, Shiga, Japan). Thermal cycling conditions were 35 cycles of fast denaturation at 94 °C for 5 s, followed by annealing and extension at 52–55 °C for 20 s. To test the Amplicon specificity was tested through generation of a melting curve by gradually increasing the temperature to 95 °C. To establish the relative fold changes for each test, the 2 − ΔΔCT method was applied based on standardization with the reference gene. The qPCR analysis was done in triplicates, and the mean value utilized.
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Sciences Foundation of China (31360055), Open Project of Xinjiang Production & Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin (BRZD1902).
Author contributions
Z.-J.H. conceived and designed the experiments; Z.-J.H., Y.S. and M.Z. performed the experiments; Z.-J.H., Y.S., M.Z. and J.-T.Z. analyzed the data; Z.-J.H. contributed reagents/materials/analysis tools; Z.-J.H., Y.S., M.Z. and J.-T.Z. wrote the paper.
Data availability
All genetic data have been submitted to the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) PRJNA644132.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71529-2.
References
- 1.Zörb C, Geilfus CM, Dietz KJ. Salinity and crop yield. Plant Biol. 2019;21:31–38. doi: 10.1111/plb.12884. [DOI] [PubMed] [Google Scholar]
- 2.Shrivastava P, Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J, Shi J, Zeng L, Xu J, Wu L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. Int. 2015;22:2976–2986. doi: 10.1007/s11356-014-3542-z. [DOI] [PubMed] [Google Scholar]
- 4.Zhu JK. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001;4:401–406. doi: 10.1016/s1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Li Y, Han G, Song J, Wang B. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 2018;45:350. doi: 10.1071/FP17181. [DOI] [PubMed] [Google Scholar]
- 6.Aslam R, Bostan N, Nabgha A, Maria M, Safdar W. A critical review on halophytes: Salt tolerant plants. J. Med. Plant Res. 2011;5:7108–7118. [Google Scholar]
- 7.Song J, et al. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017;32:107–114. [Google Scholar]
- 8.Guo J, Suo S, Wang BS. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci. Res. 2015;25:335–344. [Google Scholar]
- 9.Han N, et al. Expression of a Suaeda salsa Vacuolar H +/Ca2+ transporter gene in Arabidopsis contributes to physiological changes in salinity. Plant Mol. Biol. Rep. 2012;30:470–477. [Google Scholar]
- 10.Liu Q, Liu R, Ma Y, Song J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat. Bot. 2018;146:1–7. [Google Scholar]
- 11.Duan H, et al. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol. Biochem. 2018;127:231–237. doi: 10.1016/j.plaphy.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Yuan F, Guo J, Shabala S, Wang B. Reproductive physiology of halophytes: Current standing. Front. Plant Sci. 2019;9:1954. doi: 10.3389/fpls.2018.01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza H, et al. Potential use of halophytes to remediate saline soils. Biomed Res. Int. 2014;2014:1–12. doi: 10.1155/2014/589341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu JK. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 15.Shao Q, Han N, Ding T, Zhou F, Wang B. SsHKT1;1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct. Plant Biol. 2014;41:790. doi: 10.1071/FP13265. [DOI] [PubMed] [Google Scholar]
- 16.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 17.Halfter U. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. 2000;97:3735–3740. doi: 10.1073/pnas.040577697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng NH, Pittman JK, Zhu JK, Hirschi KD. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004;279:2922–2926. doi: 10.1074/jbc.M309084200. [DOI] [PubMed] [Google Scholar]
- 19.Quan R, et al. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell. 2007;19:1415–1431. doi: 10.1105/tpc.106.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, et al. Phosphorylation of SOS3-like calcium binding protein8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in arabidopsis. Plant Cell. 2009;21:1607–1619. doi: 10.1105/tpc.109.066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane NA+/H+ antiporter SOS1 controls long-distance NA+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh DH, et al. Loss of Halophytism by interference with SOS1 expression. Plant Physiol. 2009;151:210–222. doi: 10.1104/pp.109.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diray-Arce J, Clement M, Gul B, Khan MA, Nielsen BL. Transcriptome assembly, profiling and differential gene expression analysis of the halophyte Suaeda fruticosa provides insights into salt tolerance. BMC Genom. 2015;16:353. doi: 10.1186/s12864-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liska AJ, Shevchenko A, Pick U, Katz A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol. 2004;136:2806–2817. doi: 10.1104/pp.104.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jithesh MN, Prashanth SR, Sivaprakash KR, Parida A. Monitoring expression profiles of antioxidant genes to salinity, iron, oxidative, light and hyperosmotic stresses in the highly salt tolerant grey mangrove, Avicennia marina (Forsk.) Vierh. by mRNA analysis. Plant Cell Rep. 2006;25:865–876. doi: 10.1007/s00299-006-0127-4. [DOI] [PubMed] [Google Scholar]
- 28.Barrero JM, et al. Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 2006;29:2000–2008. doi: 10.1111/j.1365-3040.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 29.Hameed A, et al. Salt tolerance of a cash crop halophyte Suaeda fruticosa: Biochemical responses to salt and exogenous chemical treatments. Acta Physiol. Plant. 2014;36:2331–2340. [Google Scholar]
- 30.Howell SH. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013;64:477–499. doi: 10.1146/annurev-arplant-050312-120053. [DOI] [PubMed] [Google Scholar]
- 31.Zhao K, Song J, Feng G, Zhao M, Liu J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil. 2011;342:495–509. [Google Scholar]
- 32.Ziemann M, et al. Analysis of the barley leaf transcriptome under salinity stress using mRNA-Seq. Acta Physiol. Plant. 2013;35:1915–1924. [Google Scholar]
- 33.Zhang H, Zhou C. Signal transduction in leaf senescence. Plant Mol. Biol. 2013;82:539–545. doi: 10.1007/s11103-012-9980-4. [DOI] [PubMed] [Google Scholar]
- 34.Strohm AK, Baldwin KL, Masson PH. Molecular mechanisms of root gravity sensing and signal transduction. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:276–285. doi: 10.1002/wdev.14. [DOI] [PubMed] [Google Scholar]
- 35.Seyfferth C, Tsuda K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014;5:697. doi: 10.3389/fpls.2014.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang GT, et al. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012;39:969–987. doi: 10.1007/s11033-011-0823-1. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Jha SK, Bagri J, Pandey GK. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in arabidopsis. PLoS ONE. 2015;10:e0125168. doi: 10.1371/journal.pone.0125168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, et al. Molecular character of a phosphatase 2C (PP2C) gene relation to stress tolerance in Arabidopsis thaliana. Mol. Biol. Rep. 2013;40:2633–2644. doi: 10.1007/s11033-012-2350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, et al. AtPP2CG1, a protein phosphatase 2C, positively regulates salt tolerance of Arabidopsis in abscisic acid-dependent manner. Biochem. Biophys. Res. Commun. 2012;422:710–715. doi: 10.1016/j.bbrc.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, et al. Enhanced tolerance to low temperature in tobacco by over-expression of a new maize protein phosphatase 2C, ZmPP2C2. J. Plant Physiol. 2010;167:1307–1315. doi: 10.1016/j.jplph.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Jin H, Dong D, Yang Q, Zhu D. Salt-responsive transcriptome profiling of suaeda glauca via RNA Sequencing. PLoS ONE. 2016;11:e0150504. doi: 10.1371/journal.pone.0150504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SC, Luan S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012;35:53–60. doi: 10.1111/j.1365-3040.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- 43.Kushiro T, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendoza-Cózatl DG, et al. OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Mol. Plant. 2014;7:1455–1469. doi: 10.1093/mp/ssu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki A, Yamaji N, Xia J, Ma JF. OsYSl6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011;157:1832–1840. doi: 10.1104/pp.111.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pike S, Patel A, Stacey G, Gassmann W. Arabidopsis OPT6 is an oligopeptide transporter with exceptionally broad substrate specificity. Plant Cell Physiol. 2009;50:1923–1932. doi: 10.1093/pcp/pcp136. [DOI] [PubMed] [Google Scholar]
- 47.Perlin MH, Andrews J, Toh SS. Essential letters in the fungal alphabet: ABC and MFS transporters and their roles in survival and pathogenicity. Adv. Genet. 2014;85:201–253. doi: 10.1016/B978-0-12-800271-1.00004-4. [DOI] [PubMed] [Google Scholar]
- 48.Hellsberg E, Montanari F, Ecker GF. The ABC of phytohormone translocation. Planta Med. 2015;81:474–487. doi: 10.1055/s-0035-1545880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An C, et al. Transcriptome profiling, sequence characterization, and SNP-based chromosomal assignment of the EXPANSIN genes in cotton. Mol. Genet. Genom. 2007;278:539–553. doi: 10.1007/s00438-007-0270-9. [DOI] [PubMed] [Google Scholar]
- 50.Shi H, et al. The cysteine2/histidine2-type transcription factor zinc finger of arabidopsis thaliana6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and c-repeat-binding factor genes in arabidopsis. Plant Physiol. 2014;165:1367–1379. doi: 10.1104/pp.114.242404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jisha V, et al. Overexpression of an AP2/ERF type transcription factor OsEREBP1 confers biotic and abiotic stress tolerance in rice. PLoS ONE. 2015;10:e0127831. doi: 10.1371/journal.pone.0127831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai C, et al. Soybean transcription factor ORFeome associated with drought resistance: A valuable resource to accelerate research on abiotic stress resistance. BMC Genom. 2015;16:596. doi: 10.1186/s12864-015-1743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L, et al. Multiple NUCLEAR FACTOR Y transcription factors respond to abiotic stress in Brassica napus L. PLoS ONE. 2014;9:e111354. doi: 10.1371/journal.pone.0111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu D, Ji J, Wang G, Guan C, Jin C. LchERF, a novel ethylene-responsive transcription factor from Lycium chinense, confers salt tolerance in transgenic tobacco. Plant Cell Rep. 2014;33:2033–2045. doi: 10.1007/s00299-014-1678-4. [DOI] [PubMed] [Google Scholar]
- 55.Klay I, et al. Ethylene response factor Sl-ERF.B.3 is responsive to abiotic stresses and mediates salt and cold stress response regulation in tomato. Sci. World J. 2014;2014:167681. doi: 10.1155/2014/167681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan Y, et al. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012;31:349–360. doi: 10.1007/s00299-011-1170-3. [DOI] [PubMed] [Google Scholar]
- 57.Dong W, et al. Isolation and characterization of a bread wheat salinity responsive ERF transcription factor. Gene. 2012;511:38–45. doi: 10.1016/j.gene.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 58.Scarpeci TE, Zanor MI, Mueller-Roeber B, Valle EM. Overexpression of AtWRKY30 enhances abiotic stress tolerance during early growth stages in Arabidopsis thaliana. Plant Mol. Biol. 2013;83:265–277. doi: 10.1007/s11103-013-0090-8. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal P, Dabi M, Agarwal PK. Molecular cloning and characterization of a group II WRKY transcription factor from Jatropha curcas, an important biofuel crop. DNA Cell Biol. 2014;33:503–513. doi: 10.1089/dna.2014.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye S, et al. Constitutive expression of the poplar WRKY transcription factor PtoWRKY60 enhances resistance to Dothiorella gregaria Sacc. in transgenic plants. Tree Physiol. 2014;34:1118–1129. doi: 10.1093/treephys/tpu079. [DOI] [PubMed] [Google Scholar]
- 61.Yan H, et al. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic nicotiana benthamiana through aba signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014;55:2060–2076. doi: 10.1093/pcp/pcu133. [DOI] [PubMed] [Google Scholar]
- 62.Sharma R, et al. De novo assembly and characterization of stress transcriptome in a salinity-tolerant variety CS52 of Brassica juncea. PLoS ONE. 2015;10:e0126783. doi: 10.1371/journal.pone.0126783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg R, et al. Deep transcriptome sequencing of wild halophyte rice, Porteresia coarctata, provides novel insights into the salinity and submergence tolerance factors. DNA Res. 2014;21:69–84. doi: 10.1093/dnares/dst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu L, et al. RNA-seq for gene identification and transcript profiling in relation to root growth of bermudagrass (Cynodon dactylon) under salinity stress. BMC Genom. 2015;16:575. doi: 10.1186/s12864-015-1799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minoru K, Yoko S, Masayuki K, Miho F, Mao T. KEGG as a reference resource for gene and protein annotation—PubMed. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Auwera GA, et al. From fastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013;11:11.10.1. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B, Dewey CN. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria (2018).
- 72.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 74.Shannon P, et al. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genetic data have been submitted to the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) PRJNA644132.