Abstract
Due to the demographic shift complex care management of older multimorbid patients with changing functional capacities has become core clinical business for many stakeholders in western health care systems. It is the aim of the mini‐review to summarise evidence to be translated into clinical practice for pharmacists and medical doctors and interested readers. The review is based upon a comprehensive literature review in PubMed and EMBASE from 2000 to 2018 and grey literature. Interprofessional exchange and discussion among stakeholders from geriatric medicine and the International Association for Pharmaceutical Technology during a meeting in Graz, Austria 2018, led to the narrowing of the review addressing complex care needs of geriatric patients. In this mini‐review, attention is drawn to a comprehensive therapeutic goal setting according to evidence‐based guidelines: clinical, disease‐related care aspects, functional capacities, evaluated by comprehensive geriatric assessment, and patient's wishes and perspectives as main drivers for personalised complex care of geriatric patients.
Keywords: complex care, comprehensive geriatric assessment, drug treatment, older patient, prescription
1. INTRODUCTION INTO THE CLINICAL CHARACTERISTICS OF THE OLDER PATIENT POPULATION
The age demographic of the European population is projected to change dramatically over the coming decades. The percentage of citizens older than 65 years is predicted to rise from 18 to 28% by 2060; the percentage of over‐80s will increase from 5 to 12% during the same period, becoming as numerous as young people are now in 2016.1 Beyond simple life expectancy the importance of disability‐free life expectancy has come into focus of many stakeholders. Disability‐free life expectancy remained almost unchanged over the whole period, stagnating, at around 9.6 years and 8.8 years, respectively, for females and males at age 65 years. It is interesting to see, however, in subanalysis of these data sets, that the level of overall severity or clinical, individual burden of disability seems to decrease.2
This decreasing burden of disability in Europe is aligned with data from the USA. An analysis of 19 064 data sets from adults from 65 years that participated in the US Health and Retirement Study in 1998 and 2006 provides evidence that people are getting older in a very favourable way seen from the viewpoint of health‐related quality of life. Seven percent of people in this analysis showed successful ageing, which is identified by 4 operational definitions without any limitations, 6–8% did not exhibit any cognitive decline, but 35–42% had no chronic disease and 48–50% no disability during their ageing process.3 This means that, when looking closer at older persons, we will not find a homogeneous population of older adults: some of them will be found to be healthy and physically active, some of them will be observed as coping well with good quality of life, despite illness and co‐ or multimorbidity and some of them to be ill and care dependent.
The current mini‐review is based upon a comprehensive literature review in PubMed and EMBASE from 2000–2018 and grey literature, using the search terms “pharmacological care” and “older patient”. Interprofessional exchange and discussion among stakeholders from geriatric medicine and the International Association for Pharmaceutical Technology during a meeting in Graz, Austria 2018, led to the narrowing of the review addressing complex care needs of geriatric patients, aiming to provide support for daily interprofessional, complex care of geriatric patients.
2. COMPLEXITY OF OLDER, GERIATRIC PATIENTS
2.1. Physiology and pathophysiology of ageing
Due to the ageing process per se, the spectrum from organ integrity to organ pathology, multiple changes occur. These changes affect intra‐ and interindividually different anatomic regions, display different mechanisms, and therefore convey into unpredictable clinical consequences.4 As a result, complexity of ageing and geriatrics warrants a comprehensive and systematic consideration. Incorporation of a variety of clinical pictures, mortality risk as well as the functional performance in decision‐making is essential to care for this population following a personalised clinical care approach.
Biology studies highlighting ageing mechanisms on molecular level are vital to understand physiological ageing processes and to distinguish normal ageing from disease‐related and/or accelerated ageing. On the biological level, ageing is characterised by continuous accumulation of molecular and cellular damage that results in a progressive, generalised impairment in many body functions, an increased vulnerability to environmental challenges and a growing risk of disease and death.5 Different ageing theories favour changes on genetic, and/or cellular level, all of them impacting physiological organ capacities and reserves.6
On a clinical level, senescence is characterised by reduced organ capacity. Trigger systems for the ageing process are endocrine and autoimmunologic processes. Chronic proinflammatory processes, better described as inflammageing,7 affect organ integrity. By tipping homeostasis to proinflammation, diseases may develop. In this context, it seems important to establish potential biomarkers to measure the extent of inflammageing. The extent of inflammageing can then be added to clinical decision‐making and risk prediction. From the current evidence in literature, possible candidates for biomarkers include immune cell markers such as shortage of naive CD8+ T lymphocytes, increased CD8+ T cells and decreased CD4+ T and CD19+ B cells, and inhibited T cells (all predictors of inflammageing), serum cytokine markers such as interleukin (IL)‐10 and tumour necrosis factor‐α, and microRNAs such as molecules involved in the regulation of gene expression and modulators of biological pathways (including nuclear factor‐κB, mammalian target of rapamycin, sirtuins, transforming growth factor‐β, and Wnt). Changes in levels of factors described have been clinically interrelated with diseases such as Alzheimer's disease, Parkinson's disease and other neurological disorders, atherosclerosis, heart disease, age‐related macular degeneration, type II diabetes, osteoporosis and insulin resistance, cancer, and others.8 Inflammageing has also been shown to significantly increase the risk of mortality and functional decline in vivo. The clinical picture based upon proinflammatory changes is called frailty. Frailty is the result of the interaction of various systems finally resulting in loss of resilience and functional capacity.9 As a result, functional capacity declines and people become care dependent. Different markers for activation of pathways involving components such as ILs, C‐reactive protein and others are independently associated with frailty.10 Frailty is also associated with decreased levels of anti‐inflammatory cytokines, such as IL‐10.11 A role for insulin resistance also seems to be crucial in many of the mechanisms involved in frailty (low‐grade inflammation, oxidative stress, vascular dysfunction, among others).12 These observations seem to bridge phenomena of early onset of ageing and senescence in the context of chronic diseases and unhealthy lifestyle. Major examples of chronic diseases and premature ageing are chronic kidney disease, chronic obstructive pulmonary disease, chronic heart failure, human immunodeficiency virus infection and rheumatoid arthritis, all of which share important phenotypic similarities that reflect premature ageing, such as vascular disease, muscle wasting, osteoporosis and frailty.13
2.2. Co‐ and multimorbidity
Multiple chronic diseases, and resulting polypharmacy, as well as geriatric syndromes including diminished cognitive capacity, socio‐economic deprivation, such as loneliness and/or missing informal assistance have a strong impact on peoples' quality of life as well as the quality of health care offered to this group of patients. In a world of disease‐centred medical care the needs for integrated care and advanced care planning procedures for those patients are often not addressed properly.
A complex patient is defined as someone for whom clinical decision‐making and required care processes are not foreseen in routine or standard procedures. They are mostly characterised by chronic comorbidity or multimorbidity and diminished functional capacity.14
Comorbidity is characterised as combination of several diseases beyond an indexed major disease. According to this model, the major treatment focus is on the disease primary listed. It is assumed that comorbidities impact the prognosis and trajectory of the disease primary listed. Multimorbidity, in contrast, is defined as co‐occurrence of several diseases in 1 patient regardless of 1 main disease.15, 16 It has been estimated that more than 60% of persons aged 65 years or older present with multimorbidity and this prevalence increases with increasing age.17 In a cross‐sectional analysis of a national dataset of patients aged from 0 to over 85 years (stratified age groups) from 314 medical practices in Scotland, a multimorbidity pattern across the screened older population could be elaborated.18 Men were less likely to have a mental health disorder than were women, and those in the most deprived social background were more than twice as likely to have a mental health disorder than were those in the most affluent background (adjusted odds ratio 2.28, 95% confidence interval 2.21–2.32). The presence of a mental health disorder was strongly associated with the number of physical disorders that an individual had—e.g. people with 5 or more disorders had an odds ratio of 6.74 (95% confidence interval 6.59–6.90) compared with those with none. Morbidities of people diagnosed with coronary heart disease, diabetes, chronic obstructive pulmonary disease, or cancer were more common in people living in deprived areas.18 These data strongly suggest that in highly deprived areas, one should expect a greater number of both physical and mental health disorders to manage simultaneously in clinical practice. However, the effect of multimorbidity on individuals' quality of life and functional capacity will also vary with the combination and severity of disorders. People with multimorbidity have poorer functional status, quality of life and health outcomes, and are higher users of ambulatory and inpatient care than are those without multimorbidity.19, 20 People with multimorbidity have more difficulties with fragmentation of care and medical error because much specialist care is focused on treatment of 1 disease.21
2.3. Ageing and functionality
In its report on Ageing 2015, the World Health Organisation outlines the concept of functional capacity closely related to health‐related quality of life.1 Thus, maintaining function has become the supreme aim in the care of the older person. To reach the aim of maintaining functional capacity, the best strategy is to prevent disability. Once disability is established, it is very unlikely that full autonomy will be recovered. In this effort, frailty has gained increasing attention in routine clinical care in older people.
Frailty is usually the result of the interaction of multiple changes in several systems, resulting in a global process.22 This multifactorial aetiology also explains the varying clinical presentation of people showing up functional decline. Despite varying clinical presentation of frailty, there is an inevitable evidence for the clinical usefulness of this concept. Frailty has been shown to be a predictive factor of undesirable outcomes independent of chronic diseases in populations of older adults. This predictive value has been confirmed irrespective of the assessment instruments used to diagnose frailty, the target populations and the settings tested. The increased risk of negative health‐related events includes falls, hospitalisations, incident disability, institutionalisation, and mortality.23 Frailty, however, when treated in a comprehensive care approach and when detected at an early stage of trajectory, may be reversible. Data from the Survey of Health, Aging and Retirement in Europe (SHARE) demonstrate trajectories for frailty in a large European real‐life, observational cohort: 31.7% of robust participants became pre‐ frail and 2.6% became frail, while 32.4% of pre‐frail participants recovered to a robust condition after 2 years of follow‐up. The percentage of frail participants returning to a robust state restored at the end of the follow‐up in SHARE was only 7.0%.24 Functionality furthermore seems to be related to co‐ and multimorbidity. In a publication of the Joint Action on Frailty Prevention the group could demonstrate correlations between multimorbidity and frailty. However, according to this metanalysis, a group of older people presents with frailty also independent of multimorbidity. Authors state that “the causal association between the 2 conditions still remains unclear according to the current scientific evidence”.25 However, the clinical concept of co‐occurring multimorbidity and frailty remains unattached. In this context, frailty and polypharmacy are also co‐occurring and common entities in geriatric patients, although little is known about the impact they may have on each other.26 In a recent systematic review in the British Journal of Clinical Pharmacology,27 18 cross‐sectional analyses were finally assessed and 16 of those studies demonstrated a significant association between frailty and polypharmacy. Several studies show that the mean drug consumption by frail patients is higher than that of robust patients. However, data for hospitalised patients remain inconsistent.27 The association between frailty and polypharmacy seems so evident from clinical point of view, that even some scales to measure frailty, including the Edmonton Frail Scale, the Groningen Frailty Indicator or some versions of Frailty Index include the consumption of drugs.27
2.4. Ageing and geriatric syndromes
The term geriatric syndromes is used to describe clinical phenomena in older patients that do not address classical disease pattern. While heterogeneous from the clinical presentation, geriatric syndromes share many common features. Geriatric syndromes are prevalent in older adults, especially in frail older people. Impacting health‐related quality of life, they also promote and cause decline of functional capacity and disability. Among the most prevalent geriatric syndromes are recurrent falls, syncopes, frailty, dementia, delirium and incontinence. Despite the high prevalence in the older population and the high impact for health care systems, the underlying concept for geriatric syndromes is still under debate. Multiple underlying factors and organ systems contribute to the development of geriatric syndromes.28 Geriatric syndromes may also result in the development of more risk factors and more geriatric syndromes thereby further reducing intrinsic functional capacity. These pathways lead to the final outcomes of disability, dependence and death.
This conceptual model provides a unifying framework for comprehensive management strategies for older, vulnerable patients.29 Geriatric syndromes, multimorbidity and disability are closely interrelated.30 Data from China provide evidence that these entities often overlap, increasingly with advancing age.31 It could also be demonstrated that geriatric syndromes, especially when co‐occurring with male sex, institutional care and underweight or depressive symptoms predispose patients for unfavourable outcome and increased risk of mortality.32 To facilitate a tailored and person‐centred medical care, also geriatric syndromes should be also considered as clinical outcome parameters, reflecting factors of successful treatment and clinical care. Remodelling healthcare services in order to privilege and facilitate integrated and multidisciplinary models of care seems mandatory in this context.30
3. CHALLENGES OF DIAGNOSIS, TREATMENT AND MANAGEMENT OF OLD AND MULTIMORBID PATIENTS
3.1. Atypical presentation in acute diseases and overlap with geriatric syndromes
Vital adults have sufficient reserve in the functioning of organs and systems. The body can easily deal with minor stress. A single disease presentation will be classical with no involvement of other organs in those people. In frail older people, the intrinsic capacity to react to minor stress is reduced. Minor stressors overcome trigger points and acute detoriation of connected organ structures occurs. The result is an acute geriatric syndrome, with acute loss of functional capacity. People then present with reduced functional performance, reduced mobility or cognition as leading symptoms.33 In frail older patients, atypical disease presentations such as a sudden mobility problem or behavioural changes are often the only signal that a new disease episode of different organ origin occurred. Classical signals of disease may be lacking completely. Atypical disease presentations are much more prevalent in older patients, although they may also occur in younger individuals. Atypical presentation implies that these symptoms involve organs that at first sight do not seem to be involved in the pathophysiology of the symptoms. Atypical disease presentation is often connected clinically to acute and chronic geriatric syndromes. Loss of stability, involuntary loss of urine, loss of or variable attention, loss of consciousness or behavioural changes often precede syndromes such as falls, incontinence, delirium, syncope or functional decline.34
3.2. Diagnostic challenges in older patients with complex care needs
According to the described ageing process and multimorbidity patterns older patients desire an individualised care and management approach. Patients often present with complex histories. Careful history taking in the context of functional decline is the first step to tailor a diagnostic approach for a new complaint or a symptom actually leading to care consultation.
Religious beliefs, cultural values, level of education and socio‐economic situation influence people's ability and openness to be actively involved in a diagnostic and therapeutic process. Geriatric patients often present with factors that add to the complexity of clinical decision‐making during history: cognitive impairment may influence capacity of judgement of actual situations and options offered to people. Impaired hearing and vision offer the chance of misinterpretation and misunderstanding. Well‐being is highly important, but the individual perception of what well‐being means to a person varies in older patients. Thoroughly discussing individual goals moves the decision‐making process away from the focus on disease and technological possibilities. Therefore, the frame for further diagnostic procedures is specified in a tailored approach already at an early phase of consultation.35
Goal setting in complex care patients orients at personal patient‐oriented preferences and is highly attuned with individual functional capacity at the time of consultation. The management of geriatric patients should, in any care setting, include evaluation of some domains of functional capacity. Comprehensive geriatric assessment (CGA) has been proven effective in deterring functional capacity of geriatric patients for complex care planning. The CGA is a multidimensional and interdisciplinary diagnostic process adapted for identifying the medical, psychological and functional capabilities of frail older patients with the purpose to develop a coordinated and integrated plan for treatment and follow up. A main attribute of the CGA is its multidisciplinary approach that enables geriatric care corresponding with the patients' individuality, complexity and priorities.36 Literature provides evidence that CGA improves diagnostic accuracy for comprehensive care planning, prevents the development of geriatric syndromes, ameliorates discharge trajectories, and increases the chance to living at home after hospitalisation. Furthermore, CGA‐based predictive tools give information on prognosis and life expectancy of the older subject in various care settings.37, 38 There is especially strong evidence for the efficacy of implementation of CGA in hospital care of geriatric patients. Tailoring diagnostic and therapeutic care pathways in an interprofessional approach using CGA may reduce in‐hospital mortality by up to 39%. The chance for discharge back to home‐care setting is raised by 25% and reduces re‐admission rates by 12% when implementing CGA in the daily care process. Cognitive capacity preservation has also been shown to be affected favourably when implementing complex care management based upon CGA.37 In this context, it could be shown that, within the interprofessional health care teams, pharmacists are making a positive impact on various health outcomes among diverse patient populations, including older adults.39
3.3. Key considerations in planning drug interventions
Given the aspect of functional capacity in the diagnostic care pathway, another important issue to consider in daily clinical practice is organ specific capacity. Pharmacokinetics is highly influenced by biological age. Reduced hepatic metabolism, especially through CYP450, in older age is well documented.40, 41 Renal and hepatic excretion of substances is a key issue to consider in drug prescription in older patients. However, estimation of organ function by routine laboratory tests may cause substantial bias. Estimation of kidney function needs thorough evaluation. Due to sarcopenia and currently missing validation studies for glomerular filtration rate (GFR) in older patients with changing anthropometry, creatinine‐based GFR measurements may systematically misinterpret GFR. Current evidence suggests that other filtration markers that are not affected by muscle loss (i.e. cystatin C, β‐trace protein or β2 microglobulin) may better predict negative outcomes and facilitate drug management42 in older adults. Recommendations have been published by the European Geriatric Medicine Society and European Renal Association/European Dialysis and Transplantation Association for the management of older patients with chronic kidney disease stage 3b and above.43 This aspect of diagnostics and care is important as it was demonstrated that chronic kidney disease increases the risk of adverse drug reactions. When renal function declines many drugs or their active metabolites that depend on renal excretion may accumulate.44 Seen from this point, the challenge to determine actual renal capacity to tailor pharmaceutical interventions is crucial, although challenging.
A third issue to consider in the management of older patients is distribution of substances due to changed anthropometry. It has been shown that decreased body water increases serum concentration of water‐soluble drugs. In contrast, increased body fat (which happens to accumulate due to the ageing process and also in alliance with chronic diseases such as diabetes mellitus and others) prolongs half‐life of fat‐soluble drugs.45 This evidence‐based knowledge implies the need to assess status of hydration and also individual anthropometry of older complex care patients on a regular basis together with laboratory measures of liver, kidney, and fluid and electrolyte balance.
Finally, it seems essential to check pre‐existing medication records. This process includes obtaining and verifying a complete and accurate list of all current medications taken by a patient. A complete check‐up includes names, dosages, frequency and route of application.46 This evaluation is also the first step for a clinical medication review. Each medication record should be aligned with a given indication and history taking should include questions addressing possible symptoms pointing towards drug–drug or drug–disease interactions or even adverse drug reactions.47
4. WAYS TO OPTIMISE MEDICATION PRESCRIBING AND DESPRESCRIBING IN DAILY PRACTICE
When prescribing drugs in older patients with complex care needs, there are several points to consider.
A careful check of current medication should detect possible adverse drug reactions causal for symptoms or patient's complaints. This first step is essential to avoid starting a prescribing cascade, adding a new drug due to misinterpretation of a new clinical condition or symptom not detecting adverse drug reactions as cause of a complaint. The prescribing cascade places the patient at risk for developing new adverse events related to this potentially unnecessary medication.48
Whenever adding new drugs into medication regimens of older patients with complex care needs the first attempt in clinical care is not to harm. Benefit–risk estimation in terms of drug–drug and drug–disease interactions, tolerability and formulation, dosage regimens according to anthropometry, and organ capacity are vital. Special attention should be drawn to keep medication records updated according to guidelines such as the ones from the National Institute for Health and Care Excellence or Scottish Intercollegiate Guidelines Network. This management has to be reflected with multimorbidity and frailty patterns as well as changing individual therapeutic goal setting as discussed before.
In this context also deprescribing is in the focus of health carers and medical doctors. Initiatives like the EU funded projects SENATOR (Development and clinical trials of a new Software ENgine for the Assessment & optimisation of drug and non‐drug Therapy in Older peRsons) and EPHOR (Expertisecentre PHarmacotherapy in Old peRsons) have developed and validated tools to manage appropriate and person‐centred deprescribing in older patients with polypharmacy.49, 50
For patient safety, the use of electronic recording systems, where available, is recommended. Finally, relatives should also be trained and informed about medication regimens, to reassure adherence and compliance for patients whenever needed.51
In any medical treatment, the drug regimens recommended shall be evidence‐based. For geriatric patients presenting with multimorbidity, geriatric syndromes and varying functional capacities, evidence is not always easy to achieve in daily clinical practice. Complexity is the core clinical challenge when dealing with geriatric patients. As may be seen from Figure 1, their clinical problems interact dynamically in a system of ageing organ, environmental factors, diseases, drugs and other contributing factors.
Figure 1.
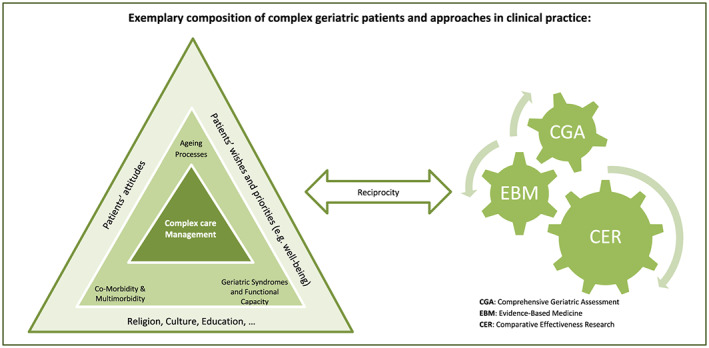
Complexity of care of multimorbid older patients. The inter‐relationship between intrinsic and extrinsic factors determining the quality of care for patients with complex care needs
1This complexity and missing linearity between cause and disease presentation is the main reason why complex patients have often been excluded from randomised, controlled clinical trails and traditional scientific research with mortality or secondary morbidity as major endpoints of the trial.
4.1. Current evidence for complex care management
For many years, evidence‐based medicine has worked with the paradigm that evidence from high‐quality randomised controlled trials would make clinical care more scientific and empirically grounded and thereby safer. Geriatric patients presenting with multimorbidity have often not been included in trials. Therefore, an evidence‐based medicine approach strictly based on scientific literature is not useful for the growing number of older patients with complex care needs. As a consequence, guidelines therefore often lack recommendations for patients with multimorbidity or frailty.
When looking for the evidence we should, however, not forget to look beyond survival as the relevant outcome. Older people prefer more often psycho‐social well‐being over morbidity and mortality, especially when they are frail and have a limited life expectancy. In this respect, it is very helpful that the National Institute of Clinical Excellence in the UK issued a guideline on multimorbidity, which state that disease‐oriented guidelines cannot directly be applied in case of older patients with frailty or repeated falls, polypharmacy, serious functional or cognitive dependency and frequent unplanned use of health care services, also because the relevant outcomes mostly are not taken into account.52
When, having checked evidence for treatment of a certain situation, the evidence has to be translated to the individual patient's situation. Adapting medications to the benefit of an individualised therapeutic goal and professional experience, avoiding adverse drug effects and complications from interventions is key for high quality patient care management of older patients. Including patient's preferences of the possible outcomes of an intervention and critical reflection of uncertainty of outcomes due to the complex and varying characteristics of older patients helps to prevent failures in clinical geriatric care. If interprofessional and patient‐guided decision‐making is carried out well, the overall quality of geriatric care is acceptable.
A key element of proven efficacy is the implementation of CGA and interprofessional management of those patients with complex care needs.
5. ADDRESSING THE GAPS IN MEDICINES DEVELOPMENT FOR IMPROVED PRESCRIBING AND BETTER THERAPEUTIC OUTCOMES
Evidence of efficacy and safety of new drug products is based primarily on results of 1 or more randomised, placebo‐controlled clinical trials.53 To account for the increasing number of older adults and their special pharmacotherapeutic needs, the International Conference on Harmonization has recommended the inclusion of a meaningful number of geriatric patients (age 65 years and older) in phase 3 or phase 2/3 studies. The International Conference on Harmonization further recommends trials exclusively dedicated to geriatric patients in order to appropriately account for co‐ and multimorbidities and investigate the influence of polymedication in those patients. The methodological option to address this issue is comparative effectiveness research (CER), which has been defined as “the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies.” The aim is to mimic real‐world settings and to learn how to prevent, diagnose, treat and monitor health conditions.54 CER includes a variety of research methodologies such as systematic reviews of existing evidence and meta‐analyses (statistical pooling or other syntheses of the results of multiple studies), experimental studies, such as randomised controlled trials and pragmatic clinical trials that randomly assign interventions, but also include nonexperimental studies, such as retrospective and prospective observational studies that do not assign interventions but leave the choice of treatments up to patients and their health care providers. Observational studies are best used to evaluate the real‐world applicability of evidence that has been derived through randomised trials for defined, clustered cohorts. Observational CER includes patients and conditions that are not typically included or studied in randomised controlled trials. It aims at better understanding current treatment practices and how patients are assessed and to provide information that can be derived only through larger studies or long‐term follow‐up for patient groups with similar patterns of multimorbidity and/or functionality.55 Large‐scale databases can build the source to gather this knowledge and are therefore best fit to address the need to better understand risks and benefits of interventions applied in complex care of geriatric patients. CER in geriatric patients can help to build evidence for future integrated and personalised care pathways. CER has the potential to accelerate the movement towards an outcome‐driven, evidence‐based clinical decision‐making and quality assessment in daily routine also for geriatric patients.56 This evidence can then be translated into political action and healthcare planning.
COMPETING INTERESTS
There are no competing interests to declare.
Roller‐Wirnsberger R, Thurner B, Pucher C, Lindner S, Wirnsberger GH. The clinical and therapeutic challenge of treating older patients in clinical practice. Br J Clin Pharmacol. 2020;86:1904–1911. 10.1111/bcp.14074
REFERENCES
- 1. World Health Organization . World report on aging and health. Geneva: World Health Organization; 2015. [Google Scholar]
- 2. Robine J‐M, Jagger C. Health expectancies In: Michel J‐P, ed. Oxford textbook of geriatric medicine. 3rd ed. Oxford: Oxford University Press; 2018. [Google Scholar]
- 3. McLaughlin SJ, Jette AM, Connell CM. An examination of healthy aging across a conceptual continuum: prevalence estimates, demographic patterns, and validity. J Gerontol A Biol Sci Med Sci. 2012;67(7):783‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craig T, Smelick C, Tacutu R, et al. The digital ageing atlas: integrating the diversity of age‐related changes into a unified resource. Nucleic Acids Res. 2015;43(Database issue):D873‐D878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkwood TB. A systematic look at an old problem. Nature. 2008;451(7179):644‐647. [DOI] [PubMed] [Google Scholar]
- 6. Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226(1):73‐82. [DOI] [PubMed] [Google Scholar]
- 7. Franceschi C, Bonafe M, Valensin S, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244‐254. [DOI] [PubMed] [Google Scholar]
- 8. Xia S, Zhang X, Zheng S, et al. An update on Inflamm‐aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;2016:8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Junius‐Walker U, Onder G, Soleymani D, et al. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3‐10. [DOI] [PubMed] [Google Scholar]
- 10. Heuberger RA. The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr. 2011;30(4):315‐368. [DOI] [PubMed] [Google Scholar]
- 11. Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63(4):391‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angulo J, El Assar M, Rodriguez‐Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med. 2016;50:1‐32. [DOI] [PubMed] [Google Scholar]
- 13. Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10(12):732‐742. [DOI] [PubMed] [Google Scholar]
- 14. Weiss KB. Managing complexity in chronic care: an overview of the VA state‐of‐the‐art (SOTA) conference. J Gen Intern Med. 2007;22(Suppl 3):374‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430‐439. [DOI] [PubMed] [Google Scholar]
- 16. Prados‐Torres A, Calderon‐Larranaga A, Hancco‐Saavedra J, Poblador‐Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254‐266. [DOI] [PubMed] [Google Scholar]
- 17. Calderon‐Larranaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72(10):1417‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet. 2012;380(9836):37‐43. [DOI] [PubMed] [Google Scholar]
- 19. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269‐2276. [DOI] [PubMed] [Google Scholar]
- 20. Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . Multimorbidity: Technical Series on Safer Primary Care. Geneva: World Health Organization; 2016. [Google Scholar]
- 22. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54(6):991‐1001. [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146‐M156. [DOI] [PubMed] [Google Scholar]
- 24. Borrat‐Besson C, Ryser V‐A, Wernli B. 15 Transitions between frailty states–a European comparison In: Börsch‐Supan A, Brandt M, Litwin H, Weber G, eds. Active ageing and solidarity between generations in Europe: First results from SHARE after the economic crisis. Berlin, Germany: De Gruyter; 2013:175‐185. [Google Scholar]
- 25. Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta‐analysis. J Gerontol A Biol Sci Med Sci. 2018;74(5):659‐666. [DOI] [PubMed] [Google Scholar]
- 26. Palmer K, Marengoni A, Russo P, Mammarella F, Onder G. Frailty and drug use. J Frailty Aging. 2016;5(2):100‐103. [DOI] [PubMed] [Google Scholar]
- 27. Gutierrez‐Valencia M, Izquierdo M, Cesari M, Casas‐Herrero A, Inzitari M, Martinez‐Velilla N. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84(7):1432‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fried LP, Storer DJ, King DE, Lodder F. Diagnosis of illness presentation in the elderly. J am Geriatr Soc. 1991;39(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 29. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J am Geriatr Soc. 2007;55(5):780‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marzetti E, Sanna T, Calvani R, Bernabei R, Landi F, Cesari M. Brand new medicine for an older society. J Am Med Dir Assoc. 2016;17(6):558‐559. [DOI] [PubMed] [Google Scholar]
- 31. Cheung JTK, Yu R, Wu Z, Wong SYS, Woo J. Geriatric syndromes, multimorbidity, and disability overlap and increase healthcare use among older Chinese. BMC Geriatr. 2018;18(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang CC, Lee JD, Yang DC, Shih HI, Sun CY, Chang CM. Associations between geriatric syndromes and mortality in community‐dwelling elderly: results of a National Longitudinal Study in Taiwan. J Am Med Dir Assoc. 2017;18(3):246‐251. [DOI] [PubMed] [Google Scholar]
- 33. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olde Rikkert MG, Rigaud AS, van Hoeyweghen RJ, de Graaf J. Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61(3):83‐87. [PubMed] [Google Scholar]
- 35. van de Pol MHJ, Fluit C, Lagro J, Slaats Y, Olde Rikkert MGM, Lagro‐Janssen ALM. Shared decision making with frail older patients: proposed teaching framework and practice recommendations. Gerontol Geriatr Educ. 2017;38(4):482‐495. [DOI] [PubMed] [Google Scholar]
- 36. Pilotto A, Polidori M. The Comprehensive Geriatric Assessment: Goal‐Oriented, Patient‐Centered Care In: Roller‐Wirnsberger R, Singler K, Polidori M, eds. Learning Geriatric Medicine. Switzerland: Springer; 2018. [Google Scholar]
- 37. Ellis G, Whitehead MA, Robinson D, O'Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343(1):d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pilotto A, Cella A, Pilotto A, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J am Med Dir Assoc. 2017;18(2):192.e1‐192.e11. [DOI] [PubMed] [Google Scholar]
- 39. Lee JK, Slack MK, Martin J, Ehrman C, Chisholm‐Burns M. Geriatric patient care by U.S. pharmacists in healthcare teams: systematic review and meta‐analyses. J am Geriatr Soc. 2013;61(7):1119‐1127. [DOI] [PubMed] [Google Scholar]
- 40. Page RL 2nd, Linnebur SA, Bryant LL, Ruscin JM. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clin Interv Aging. 2010;5:75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kinirons MT, Crome P. Clinical pharmacokinetic considerations in the elderly. An update. Clin Pharmacokinet. 1997;33(4):302‐312. [DOI] [PubMed] [Google Scholar]
- 42. Foster MC, Inker LA, Levey AS, et al. Novel filtration markers as predictors of all‐cause and cardiovascular mortality in US adults. Am J Kidney Dis. 2013;62(1):42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farrington K, Covic A, Nistor I, et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European renal best practice group. Nephrol Dial Transplant. 2017;32(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 44. Doogue MP, Polasek TM. Drug dosing in renal disease. Clin Biochem Rev. 2011;32(2):69‐73. [PMC free article] [PubMed] [Google Scholar]
- 45. Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55(2):347‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agency for Healthcare Research and Quality U.S. Department of Health and Human Services . Medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. https://innovations.ahrq.gov/qualitytools/medications‐transitions‐and‐clinical‐handoffs‐match‐toolkit‐medication‐reconciliation. Accessed November 11, 2018.
- 47. Somers A, Mallet L, van der Cammen T, Robays H, Petrovic M. Applicability of an adapted medication appropriateness index for detection of drug‐related problems in geriatric inpatients. Am J Geriatr Pharmacother. 2012;10(2):101‐109. [DOI] [PubMed] [Google Scholar]
- 48. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Mahony D, O'Connor MN, Eustace J, Byrne S, Petrovic M, Gallagher P. The adverse drug reaction risk in older persons (ADRROP) prediction scale: derivation and prospective validation of an ADR risk assessment tool in older multi‐morbid patients. Eur Geriatr Med. 2018;9(2):191‐199. [DOI] [PubMed] [Google Scholar]
- 50. Ephor . Polypharmacy optimization method 2011. https://ephor.nl/wp‐content/uploads/2018/12/polypharmacy‐optimization‐method‐may‐2011‐ephor.pdf. Accessed June 18, 2019.
- 51. General Medical Council . Good practice in prescribing and managing medicines and devices. https://www.gmc‐uk.org/‐/media/documents/prescribing‐guidance_pdf‐59055247.pdf. Accessed November 29, 2018.
- 52. National Guideline Centre (UK) . Multimorbidity: assessment, prioritisation and Management of Care for people with commonly occurring multimorbidity (NICE guideline, no. 56). London: National Institute for Health and Care Excellence (UK); 2016. https://www.ncbi.nlm.nih.gov/pubmed/27683922 [PubMed]
- 53. Lesko LJ, Schmidt S. Individualization of drug therapy: history, present state, and opportunities for the future. Clin Pharmacol Ther. 2012;92(4):458‐466. [DOI] [PubMed] [Google Scholar]
- 54. US Department of Health and Human Services FCCfCER . Report to the president and the congress 2009. https://osp.od.nih.gov/wp‐content/uploads/FCCCER‐Report‐to‐the‐President‐and‐Congress‐2009.pdf. Accessed December 1, 2018.
- 55. Dreyer NA, Tunis SR, Berger M, Ollendorf D, Mattox P, Gliklich R. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood). 2010;29(10):1818‐1825. [DOI] [PubMed] [Google Scholar]
- 56. Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364(26):2478‐2481. [DOI] [PubMed] [Google Scholar]


