Key Points
Question
Can a remote, algorithm-driven, navigator-administered medication optimization program enhance implementation of guideline-directed medical therapy in heart failure with reduced ejection fraction?
Findings
In this case-control study of 1028 patients, in 3 months, patients participating in the remote intervention experienced significant increases from baseline in the use and dosages of renin-angiotensin system antagonists and β-blockers but not mineralocorticoid receptor antagonists. Similar changes were not seen in a usual-care comparator group over the same period.
Meaning
Remote titration of guideline-directed medical therapy by navigators using encoded algorithms may represent an effective strategy for closing the gap between guidelines and clinical practice in patients with heart failure with reduced ejection fraction.
This case-control study assesses whether a remote, algorithm-driven, navigator-administered medication optimization program could enhance implementation of guideline-directed medical therapy for heart failure with reduced ejection fraction.
Abstract
Importance
Optimal treatment of heart failure with reduced ejection fraction (HFrEF) is scripted by treatment guidelines, but many eligible patients do not receive guideline-directed medical therapy (GDMT) in clinical practice.
Objective
To determine whether a remote, algorithm-driven, navigator-administered medication optimization program could enhance implementation of GDMT in HFrEF.
Design, Setting, and Participants
In this case-control study, a population-based sample of patients with HFrEF was offered participation in a quality improvement program directed at GDMT optimization. Treating clinicians in a tertiary academic medical center who were caring for patients with heart failure and an ejection fraction of 40% or less (identified through an electronic health record–based search) were approached for permission to adjust medical therapy according to a sequential titration algorithm modeled on the current American College of Cardiology/American Heart Association heart failure guidelines. Navigators contacted participants by telephone to direct medication adjustment and conduct longitudinal surveillance of laboratory tests, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure cardiologist. Patients and clinicians declining to participate served as a control group.
Exposures
Navigator-led remote optimization of GDMT compared with usual care.
Main Outcomes and Measures
Proportion of patients receiving GDMT in the intervention and control groups at 3 months.
Results
Of 1028 eligible patients (mean [SD] values: age, 68 [14] years; ejection fraction, 32% [8%]; and systolic blood pressure, 122 [18] mm Hg; 305 women (30.0%); 892 individuals [86.8%] in New York Heart Association class I and II), 197 (19.2%) participated in the medication optimization program, and 831 (80.8%) continued with usual care as directed by their treating clinicians (585 [56.9%] general cardiologists; 443 [43.1%] heart failure specialists). At 3 months, patients participating in the remote intervention experienced significant increases from baseline in use of renin-angiotensin system antagonists (138 [70.1%] to 170 [86.3%]; P < .001) and β-blockers (152 [77.2%] to 181 [91.9%]; P < .001) but not mineralocorticoid receptor antagonists (51 [25.9%] to 60 [30.5%]; P = .14). Doses for each category of GDMT also increased from baseline in the intervention group. Among the usual-care group, there were no changes from baseline in the proportion of patients receiving GDMT or the dose of GDMT in any category.
Conclusions and Relevance
Remote titration of GDMT by navigators using encoded algorithms may represent an efficient, population-level strategy for rapidly closing the gap between guidelines and clinical practice in patients with HFrEF.
Introduction
Optimal pharmacologic therapy of heart failure with reduced ejection fraction (HFrEF) is carefully scripted by evidence-based treatment guidelines,1,2 but a large proportion of patients with chronic HFrEF either do not receive guideline-directed medical therapy (GDMT) or are underdosed in clinical practice.3 Dose titration of GDMT is also uncommon, particularly when patients do not report progressive symptoms.4 We hypothesized that a remote medication optimization program managed by patient navigators, nurses, and pharmacists under the supervision of a heart failure (HF) specialist would improve use and dosing of GDMT in patients with HFrEF compared with routine, clinic-based care.
Methods
The remote optimization program was organized as a quality improvement initiative, with design details as previously reported.5 Patients with a chronic HF and left ventricular ejection fraction of 40% or less who were receiving longitudinal cardiovascular follow-up at our center were identified through an electronic health record–based search algorithm6,7 and contacted via telephone to verify eligibility and willingness to participate in the remote optimization program. For those whose clinicians granted permission, navigators (T.M., J.B.-H., and nonauthors) and pharmacists (J.D. and K.V.S.) adjusted medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines (eMethods in the Supplement), under supervision of a nurse practitioner (L.F.) and HF cardiologist (A.D.S.).1,8 A home blood pressure cuff was provided for surveillance of hypotension, and laboratory tests were arranged to assess electrolytes and kidney function within 1 week of any dosage change. Care was returned to primary clinicians after achievement of the maximally tolerated or guideline-directed dose of each medication class. Patients and clinicians who declined to participate in the remote optimization program served as a reference group. The initiative was approved by the institutional review board at Brigham and Women’s Hospital with a waiver of written informed consent because the study was a quality improvement intervention.
Data were collected between August 2017 and August 2019. The primary study outcomes were the change from baseline to 3 months in the proportion of patients receiving the guideline-directed medications in each category and the proportion receiving GDMT at 50% or more of target doses, analyzed using a binomial test. Outcomes in the intervention group were compared with those in the reference group using logistic regression adjusted for baseline medication use. Analyses were repeated for subgroups of patients followed up by HF specialists and general cardiologists. All analyses were conducted in Stata version 14.2 (StataCorp), with P < .05 considered statistically significant.
Results
Of 1131 patients who were initially eligible, 197 completed the 3-month follow-up in the remote intervention group (including 59 women [29.9%] and 34 Black individuals [17.3%]), and 831 patients (246 women [29.6%] and 88 Black individuals [10.6%]) whose physicians declined to participate in the intervention served as a usual-care reference group (eFigure in the Supplement). Patients in the intervention group were younger (mean [SD] age, 66 [12.7] years vs 68 [14.5] years; P = .01), had higher systolic blood pressure (mean [SD], 127 [15.1] mm Hg vs 121 [18.6] mm Hg; P < .001), had better kidney function (mean [SD] estimated glomerular filtration rate, 65 [18.7] mL/min/1.73 m2 vs 60 [19.1] mL/min/1.73 m2; P = .003), had less atrial fibrillation (64 [32.5%] vs 368 [44.3%]; P = .003), and were more likely to be followed up by specialty HF clinicians (66 [33.5%] vs 377 [45.4%]; P = .003). At baseline, fewer patients in the intervention group were treated with β-blockers (152 [77.2%] vs 702 [84.5%]; P = .02), angiotensin receptor–neprilysin inhibitors (ARNIs; 17 [8.6%] vs 133 [16.8%]; P = .007), and loop diuretics (62 [31.5%] vs 435 [52.3%]; P < .001) (Table).
Table. Baseline Characteristics, by Study Group.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| Remote guideline-directed medical therapy optimization (n = 197) | Usual care (n = 831) | ||
| Age, mean (SD), y | 66 (12.7) | 68 (14.5) | .01 |
| Ejection fraction, mean (SD), % | 33 (6.1) | 32 (6.7) | .33 |
| Female | 59 (29.9) | 246 (29.6) | .93 |
| Black racea | 34 (17.3) | 88 (10.6) | .01 |
| New York Heart Association functional class I or II | 177 (89.8) | 715 (86.0) | .20 |
| Systolic blood pressure, mean (SD), mm Hg | 127 (15.1) | 121(18.6) | <.001 |
| Heart rate, mean (SD), bpm | 73 (13.0) | 72 (13.2) | .25 |
| Serum test results, mean (SD) | |||
| Potassium, mEq/L | 4.4 (0.5) | 4.4 (0.5) | .82 |
| Creatinine, mg/dL | 1.1 (0.4) | 1.2 (0.5) | .001 |
| Estimated glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 65 (18.7) | 60 (19.1) | .003 |
| Diabetes | 60 (30.5) | 219 (26.4) | .25 |
| Atrial fibrillation | 64 (32.5) | 368 (44.3) | .003 |
| Asthma or chronic obstructive pulmonary disorder | 13 (6.6) | 30 (3.6) | .07 |
| Implantable defibrillator | 101 (51.3) | 426 (51.3) | .99 |
| Specialty heart failure clinician | 66 (33.5) | 377 (45.4) | .003 |
| Baseline medications | |||
| ACEis, ARBs, or ARNIs | 138 (70.1) | 603 (72.6) | .48 |
| β Blocker | 152 (77.2) | 702 (84.5) | .02 |
| Mineralocorticoid receptor antagonist | 51 (25.9) | 240 (28.9) | .43 |
| Loop diuretic | 62 (31.5) | 435 (52.3) | <.001 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; potassium to mmol/L, multiply by 1.0.
By patient self-report, as recorded in the electronic health record.
Over a mean (SE) follow-up of 140.6 (6.2) days (with 34 patients completing less than 90 days), navigators made a mean (SE) of 8.6 (0.4) telephone calls per patient (mean [SE] time between contacts, 16.2 [1.2] days), spent a mean (SE) of 4.1 (0.3) cumulative hours with each patient, and made 794 medication adjustments (mean [SE], 4.0 [0.2] per patient). Adjustments included 165 changes in the dosing of ACE inhibitors, 104 changes in angiotensin-receptor blockers (ARBs), 76 changes in ARNIs, 331 changes in β-blockers, and 47 changes in mineralocorticoid receptor antagonists (MRAs). Of the 831 patients in the usual-care group, 482 (58.0%) had at least 1 encounter with a cardiologist during the study period.
At 3 months, patients allocated to the remote intervention experienced significant increases from baseline in the use of renin-angiotensin system inhibitors (ACE inhibitors, ARBs, or ARNIs: 138 [70.1%] to 170 [86.3%]; P < .001) and β-blockers (152 [77.2%] to 181 [91.9%]; P < .001) but no change in the use of MRAs (51 [25.9%] to 60 [30.5%] P = .14). By comparison, no change from baseline was observed in the proportion of patients receiving any category of GDMT at 3 months in the usual-care group (Figure 1A). The increase in renin-angiotensin system inhibitor and β-blocker use in the intervention group was greatest among patients followed up by general cardiologists, with smaller changes seen in the group followed up by HF specialists (Figure 1B and C). The proportion of patients receiving GDMT at 50% or more of target dose at 3 months was also increased to a greater extent in patients allocated to the remote intervention, with little dose titration observed in the usual-care group (Figure 2). Key adverse events were similar between groups, except for worsening kidney function, which was more common in the remote intervention group (as measured by a rise in serum creatinine to >0.3 mg/dL [to convert to micromoles per liter, multiply by 88.4]: 18 [9.1%] vs 3 [0.4%]; P < .001) (eTable in the Supplement).
Figure 1. Change From Baseline in Use of Guideline-Directed Medical Therapy (GDMT) by Drug Category and Study Group.
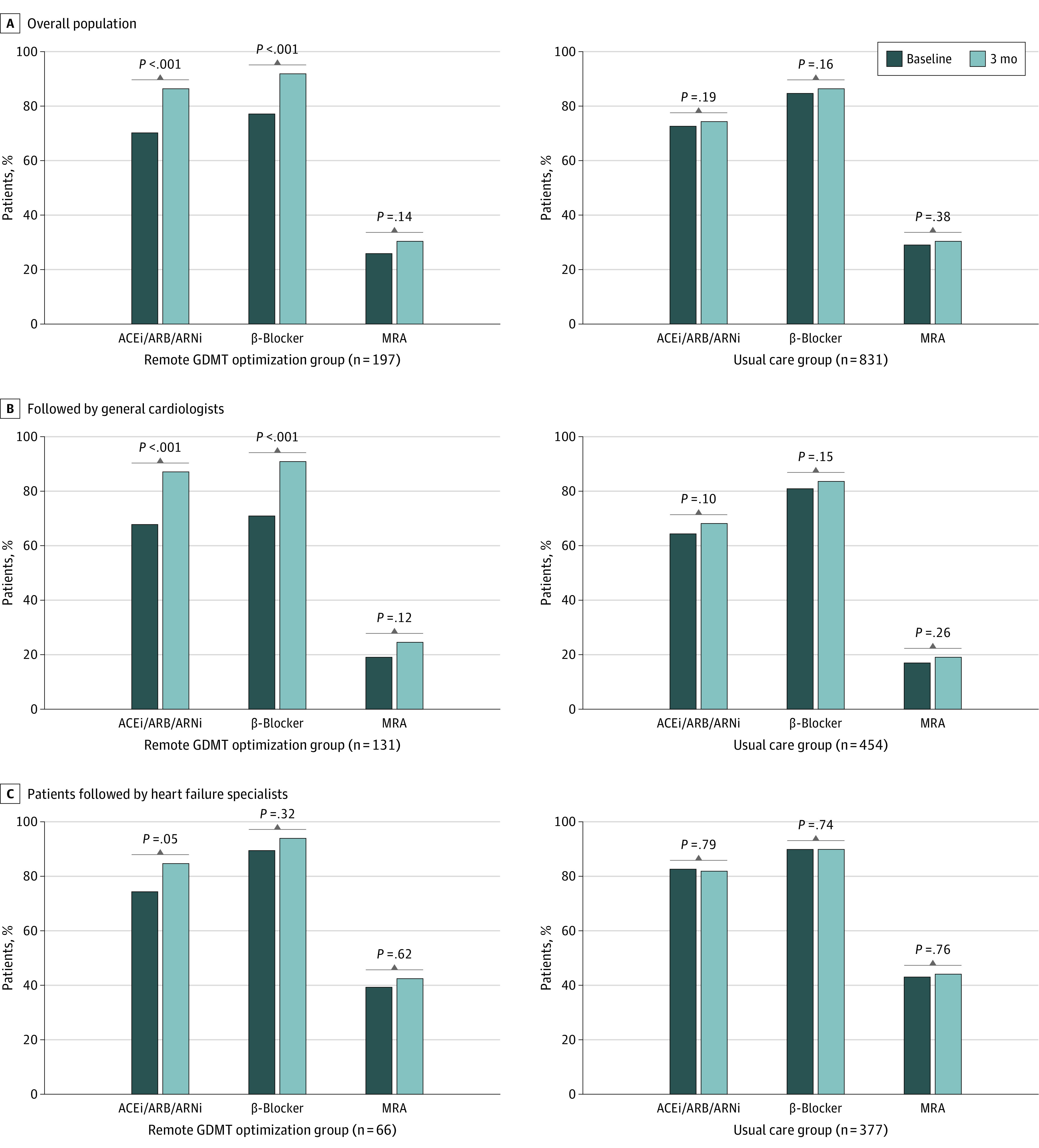
ACEi indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor–neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Figure 2. Dosing of Guideline-Directed Medical Therapy (GDMT) at 3 Months, by Treatment Group.
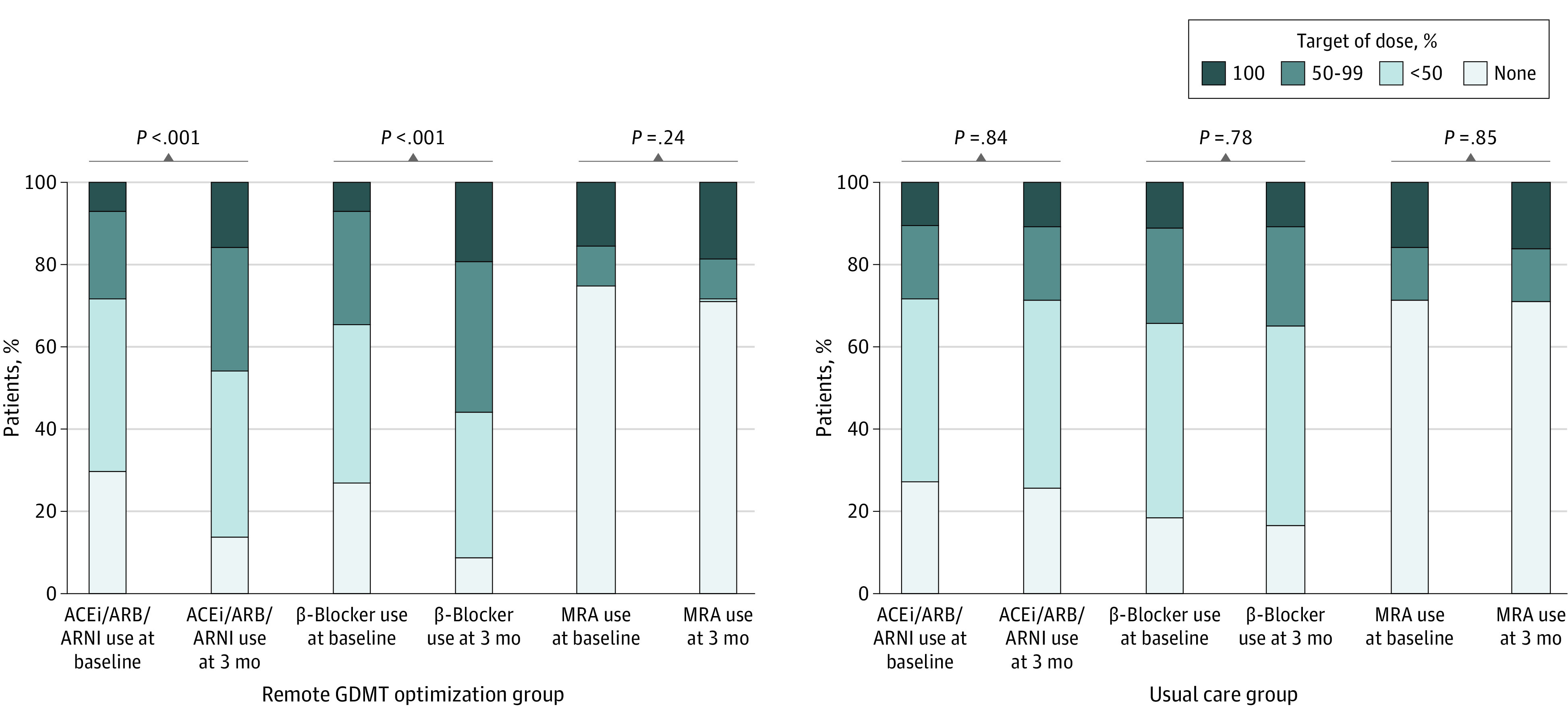
Colors indicate dosage as a percentage of guideline-directed targets for each medication category at each point. Statistical comparisons reflect the different proportions in those receiving 50% or more of target dose in each group. ACEi indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Discussion
In comparison with usual care over a 3-month period, an algorithm-driven, navigator-led remote medication optimization strategy improved both use of GDMT and titration of GDMT to target doses in patients with HFrEF. Remote drug titration was orchestrated with a low rate of adverse events without disruption of physician-patient relationships, with greater acceptance and efficacy among patients followed up by general cardiologists than in those followed up by HF specialists. This approach may represent a scalable, population-level strategy to close the gap between guidelines and implementation of GDMT in clinical practice.
Limitations
Key limitations of this study include the nonrandomized design and short duration of follow-up, which may have biased the results in favor of the intervention group. Patients with more advanced disease followed up by HF specialists had higher use of GDMT at baseline and were more likely than those followed up by general cardiologists to opt out of the remote titration approach, but were less likely to benefit from the intervention. Moreover, despite significant improvements in use of renin-angiotensin system antagonists and β-blockers, we did not observe similar improvements in the use of MRAs, perhaps as a consequence of the strict criteria used to assess MRA eligibility in this population, which had predominantly New York Heart Association I and II–level disease. Finally, the overall study duration was insufficient to examine an association of the intervention with clinical outcomes.
Conclusions
Our data suggest that many of the barriers to use of GDMT in clinical practice might be overcome at scale by engaging nonphysician personnel to supplement clinic-based follow-up and support the process of pharmacologic optimization. In the context of increasing emphasis on value-based care, costs associated with the personnel and infrastructure needed to support this approach must be considered against the potential long-term cost savings anticipated from reduced HF hospitalizations and increased life span as a consequence of greater use of appropriate pharmacologic therapy. Remote management by a centralized team might also help to expand access to specialty expertise, removing the constraints of resource limitations that may be prevalent in rural or underserved areas. In the context of a pandemic that has emphasized the vital importance of effective strategies for remote patient engagement, further study to assess the generalizability to community populations at scale seems warranted.
eFigure. Patient Selection
eTable. Adverse Events, by Study Group
eMethods. Physician-LIP Collaborative Drug Therapy Management Plan
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. . 2017 ACCF/ACC/HFSA focused update of the 2013 ACCF/AHA guidelines for the management of heart failure. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 3.Greene SJ, Butler J, Albert NM, et al. . Medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018;72(4):351-366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Fonarow GC, DeVore AD, et al. . Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blood AJ, Fischer CM, Fera LE, et al. . Rationale and design of a navigator-driven remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. Clin Cardiol. 2020;43(1):4-13. doi: 10.1002/clc.23291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagholikar KB, Fischer CM, Goodson A, et al. . Extraction of ejection fraction from echocardiography notes for constructing a cohort of patients having heart failure with reduced ejection fraction (HFrEF). J Med Syst. 2018;42(11):209. doi: 10.1007/s10916-018-1066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagholikar KB, Fischer CM, Goodson AP, et al. . Phenotyping to facilitate accrual for a cardiovascular intervention. J Clin Med Res. 2019;11(6):458-463. doi: 10.14740/jocmr3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Patient Selection
eTable. Adverse Events, by Study Group
eMethods. Physician-LIP Collaborative Drug Therapy Management Plan


