Abstract
Oxytocin is a pleiotropic, peptide hormone with broad implications for general health, adaptation, development, reproduction, and social behavior. Endogenous oxytocin and stimulation of the oxytocin receptor support patterns of growth, resilience, and healing. Oxytocin can function as a stress-coping molecule, an anti-inflammatory, and an antioxidant, with protective effects especially in the face of adversity or trauma. Oxytocin influences the autonomic nervous system and the immune system. These properties of oxytocin may help explain the benefits of positive social experiences and have drawn attention to this molecule as a possible therapeutic in a host of disorders. However, as detailed here, the unique chemical properties of oxytocin, including active disulfide bonds, and its capacity to shift chemical forms and bind to other molecules make this molecule difficult to work with and to measure. The effects of oxytocin also are context-dependent, sexually dimorphic, and altered by experience. In part, this is because many of the actions of oxytocin rely on its capacity to interact with the more ancient peptide molecule, vasopressin, and the vasopressin receptors. In addition, oxytocin receptor(s) are epigenetically tuned by experience, especially in early life. Stimulation of G-protein–coupled receptors triggers subcellular cascades allowing these neuropeptides to have multiple functions. The adaptive properties of oxytocin make this ancient molecule of special importance to human evolution as well as modern medicine and health; these same characteristics also present challenges to the use of oxytocin-like molecules as drugs that are only now being recognized.
Significance Statement
Oxytocin is an ancient molecule with a major role in mammalian behavior and health. Although oxytocin has the capacity to act as a “natural medicine” protecting against stress and illness, the unique characteristics of the oxytocin molecule and its receptors and its relationship to a related hormone, vasopressin, have created challenges for its use as a therapeutic drug.
I. Introduction
Oxytocin was the first peptide hormone to be biochemically described and synthesized. This comparatively simple nine–amino acid molecule has been called the “best understood neuropeptide” (Jurek and Neumann, 2018). There is abundant evidence for diverse functions and health benefits for the molecule known as oxytocin. Here we examine the hypothesis that oxytocin and oxytocin-like molecules are components of a “natural medicine” capable of both preventing and treating various disorders and influencing a broad range of diseases.
As background for the current review, it is helpful to recall that the word “medicine” is commonly used in at least two ways, including “the science or practice of the diagnosis, prevention and treatment of disease” or a “compound or drug used for the prevention or treatment of disease.” This review addresses the potential of both endogenous and exogenous oxytocin to serve as medicine in both senses of the word.
Oxytocin has been tested in the treatment of conditions as apparently diverse as autism spectrum disorders, schizophrenia, postpartum depression, anxiety, post-traumatic stress disorders, borderline personality, addiction, pain, metabolic and digestive disorders, diabetes, cardiovascular diseases, cancer, and infectious diseases (to name only a few) (Hurlemann and Grinevich, 2018). Our intent is not to duplicate the massive literature in this field. Rather, from this literature we have attempted to extract basic mechanisms of action of the endogenous oxytocin system and to highlight gaps in knowledge. Awareness of the basic biology of oxytocin actions can help guide the future development of drugs based on this system as well as behavioral interventions for regulating the production and release of endogenous peptides.
II. Overview and Challenges
In June of 2020, a Pubmed search for “oxytocin” yielded over 27,700 articles, most of which were published within the last two decades. Among these articles are thousands of empirical studies and hundreds of recent reviews (Grinevich et al., 2016; Carter, 2017; Carter and Perkeybile, 2018; Hurlemann and Grinevich, 2018; Jurek and Neumann, 2018), with many examining the therapeutic properties of oxytocin or oxytocin-like molecules for physical and behavioral disorders. In theory, with so much information it should have been easy to create effective drugs based on the oxytocin system. However, as summarized here, attempts to use oxytocin or oxytocin-like molecules as drugs have faced a variety of obstacles.
A. Oxytocin and Vasopressin: an Integrated System
As summarized below, at least part of the confusion associated with understanding oxytocin arises because this molecule is a component of an ancient and evolved system that includes the related peptide, vasopressin, and its receptors (Carter, 2014, 2017; Carter and Perkeybile, 2018) (Fig. 1; Table 1). Awareness of the characteristics of oxytocin-vasopressin interactions is necessary to address the challenges associated with creating therapeutics based on this system. The oxytocin-vasopressin system interacts with the hypothalamic-pituitary-adrenal (HPA) axis as well as acetylcholine, GABA, glutamate, opioids, cannabinoids, catecholamines, indoleamines, and steroids (Hurlemann and Grinevich, 2018; Jurek and Neumann, 2018). Although they are beyond the scope of this review, these interactions have broad functional consequences that can affect the usefulness of oxytocin-based molecules as “drugs.”
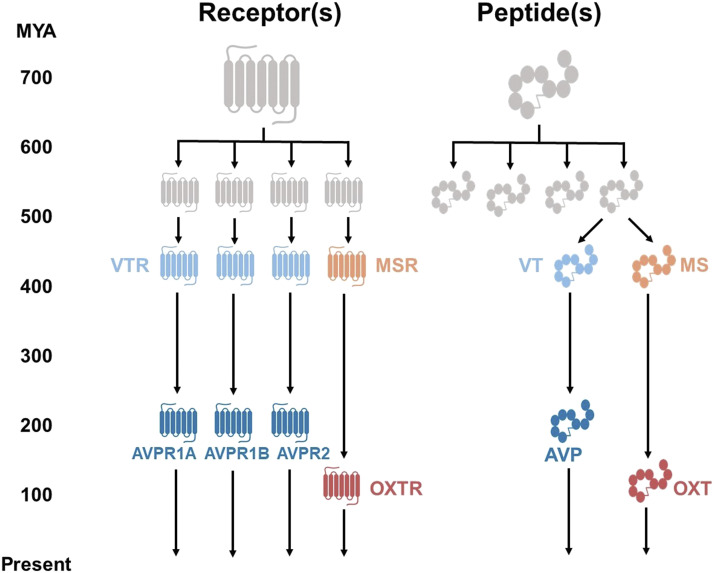
Fig. 1.
The descent of the neuropeptide ligand and receptor systems through evolutionary time, adapted from Grinevich et al. (2016). Beginning approximately 700 million years ago (MYA), the ancestral peptide and its receptor predate mammals and indeed vertebrates. Both peptide and receptor underwent two rounds of genome duplication approximately 550 MYA. These systems eventually consisted of mesotocin (MS), the mesotocin receptor (MSR), vasotocin (VT), and three vasotocin receptors (VTRs). At the emergence of mammals approximately 200 MYA, the vasotocin peptide and receptor evolved into their modern forms, consisting of AVP and its three receptors (AVPR1A, AVPR1B, and AVPR2). Modern mammalian oxytocin (OXT) and its receptor (OXTR) first evolved approximately 100 MYA.
TABLE 1.
Comparative adaptive functions of vasopressin and oxytocin
Individual and species differences are commonly observed. These differences are adaptive and context-dependent. Early life experiences can epigenetically tune these systems (see text and Fig. 3).
| Hypothesized Functions | Vasopressin – or AVPR Activation | Oxytocin – or OXTR Activation |
|---|---|---|
| Life history strategies and reproductive investment | More primitive – faster | More modern – slower |
| Lower parental investment and more offspring | Greater parental investment and fewer offspring | |
| Responses to challenges defense strategies | Mobilization (fight-flight) | Immobilization without fear |
| Amplifying stress and fear | Stress coping and resilience | |
| Reduced cooperation | Social cooperation | |
| Mobilization | Activation | Approach |
| Reactive aggression | Positive social behaviors | |
| Immobility with fear | Immobilization without fear | |
| Freezing or subordination? | Parental and sexual behavior | |
| Anxiety | Anxiogenic | Anxiolytic |
| Mild stress | Release of vasopressin | Inhibition of oxytocin? |
| Extreme acute stress | Release of vasopressin | Release of oxytocin |
| Chronic stress | Release of vasopressin? | Inhibition (males?); release of oxytocin (females) |
| Autonomic nervous systema | Sympathoadrenal | Parasympathetic (vagalb) |
| Inflammation | Proinflammatory (primarily) | Anti-inflammatory |
| Pain | Increasing or reducing? | Prevention or reducing |
Interactions between AVP and OXT and the autonomic nervous system support flexibility in behavioral, emotional states and allow different strategies for dealing with challenges with effects that may differ between males and females.
The vagus nerve has more than one branch arising from different source nuclei in the brainstem. The more modern branch arises in the ventral-vagal complex and supports social behaviors and features that are unique to mammals, such as facial expression, social engagement, and language. The more primitive branch arises in DMX (i.e., 10th cranial nerve) and is associated with conservation of energy in response to extreme stressors or trauma (Porges, 2011).
Oxytocin and vasopressin have acute and lasting effects on both the central (Carter, 2014) and autonomic nervous system (Porges, 2012; Quintana et al., 2019). This, in turn, creates another source of complexity, since peptide receptors in the nervous system influence the entire body but also often differ in abundance and localization among individuals and species (Witt et al., 1991; Freeman and Young, 2016; Freeman et al., 2018).
Of particular importance to oxytocin’s capacity to promote health and influence behavior is the growing evidence for actions of oxytocin throughout the immune system and its major role as an anti-inflammatory and antioxidant (Szeto et al., 2011; Bordt et al., 2019; Kingsbury and Bilbo, 2019). Effects on immunity and inflammation suggest another source of apparently “non-specific” effects of oxytocin. Components of the immune system also control the production (CD38) and act as a binding protein regulating the capacity of oxytocin to cross membranes (Yamamoto and Higashida, 2020).
The oxytocin-vasopressin system is “plastic,” and its receptors are sensitive to epigenetic modification across the lifespan, but especially in early life (Kenkel et al., 2019; Perkeybile et al., 2019). Functions of this system are context-dependent and changed by experience, including a history of stress and trauma (Fig. 2). Furthermore, there is evidence, especially in the face of psychologic or physical challenges, that males and females may differ in their response to oxytocin and vasopressin (Carter and Perkeybile, 2018; Jirikowski et al., 2018).
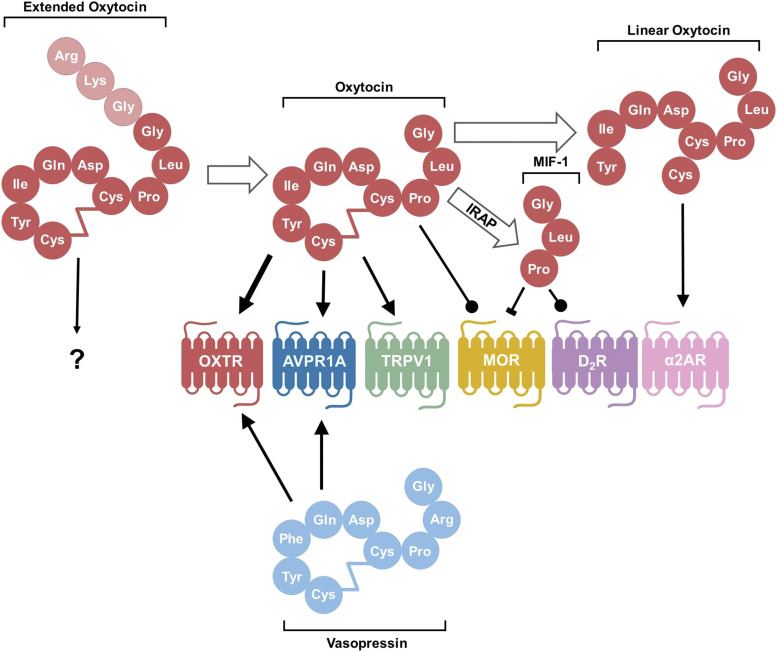
Fig. 2.
Processing of the oxytocin molecule and its targets. After conversion from the prohormone form, oxytocin exists in an extended form with three extra amino acids. The conversion from extended OXT to oxytocin consisting of nine amino acids occurs with maturation of the hypothalamus in neurotypical individuals. It is not known how this extended form interacts with receptors. In their canonical nine–amino acid forms, both oxytocin and vasopressin bind and act as agonists to both OXTR and AVPR1A, although oxytocin has a higher affinity for OXTR than the AVPR1A, as denoted by the thicker arrow. Oxytocin also acts as an agonist to the pain-sensing transient receptor potential vanilloid-1 (TRPV1) receptor and as a positive allosteric modulator at the MOR. After degradation by IRAP, the C-terminal tail is cleaved from oxytocin to form MIF-1, which can both inhibit MOR and act as an allosteric modulator on the D2 subtype of dopamine receptors (D2R). Oxytocin can also be degraded by other as of yet unspecified peptidase activity into a linear form that stimulates activity of the α-2 type adrenoreceptors (α2ARs). Conventional arrow = agonist; circle-tipped arrow = positive allosteric modulator; block-tipped arrow = antagonist.
Taken together these factors help to explain why responses to oxytocin-vasopressin pathways may vary among species, among individuals, and by sex. To truly appreciate oxytocin’s potential as a medicine for a broad range of human disorders, it is useful to recognize these sources of diversity and to briefly review the history of knowledge in this field and the evolution of the oxytocin-vasopressin system.
III. Discovery of Oxytocin and Vasopressin
The pressor function of the pituitary gland was discovered in the late 19th century. In the early 1900s, it was shown that pituitary gland extracts could be used to induce uterine contractions (Dale, 1906), and that milk ejection could be stimulated by pituitary extracts (Mackenzie, 1911). In 1928, Ernst and Berta Scharrer identified the largest cells in the hypothalamus, calling these the “magnocellular neurons” (Scharrer and Scharrer, 1939). However, the behaviorally active chemicals that were secreted by the magnocellular neurons were not identified until Vincent du Vigneaud synthesized oxytocin in 1953 (du Vigneaud et al., 1954) and vasopressin in 1954 (du Vigneaud et al., 1958). Du Vigneaud received the 1955 Nobel Prize in Chemistry for the “first synthesis of a polypeptide hormone.” His Nobel lecture titled “A Trail of Sulfa Research: From Insulin to Oxytocin” set the stage for the understanding that physiologically active hormones were produced not just in the pituitary or peripheral endocrine organs but also in the nervous system. The field of neuroendocrinology arose in part from these discoveries. However, early investigations focused on oxytocin as a “female reproductive hormone” that was involved mainly in birth and lactation. The mistaken notion that oxytocin was only important to one sex (female) and in one context (female reproduction) with no known consequences for males may have slowed initial interest in this molecule.
Studies by de Wied in the 1970s (Kovacs et al., 1979) showed that learning behavior, especially active avoidance, could be affected by oxytocin and vasopressin. In 1979, Pedersen and Prange (1979) demonstrated the ability of oxytocin to facilitate maternal behavior in reproductively naïve female rats. In the 1980s, Keverne and Kendrick (Kendrick et al., 1987) showed that maternal bonding in sheep also depended on oxytocin. A decade later, our own research using the highly social prairie vole revealed a role for oxytocin in adult social behaviors (Witt et al., 1990) in the formation of adult social bonds in both sexes (Williams et al., 1994) and showed for the first time that prairie voles had a novel pattern of distribution of the oxytocin receptors (Witt et al., 1991). Since that time, socially monogamous voles, with many behavioral and physiologic parallels to humans, have become particularly useful for the analysis of the neural, genetic, and epigenetic actions of oxytocin and its receptors (Gobrogge and Wang, 2016; Carter and Perkeybile, 2018; Perkeybile et al., 2019).
IV. Evolution as an Organizing Concept
Understanding the functional versatility of oxytocin requires recognition of the long evolutionary history of the genes that code for vasopressin, oxytocin, and their receptors. More than 600 million years ago, oxytocin-vasopressin–like molecules appeared in the shared ancestors of modern vertebrates and invertebrates (Acher et al., 1995; Knobloch and Grinevich, 2014). Vertebrates emerged approximately 500 million years ago. The signaling properties of the class of molecules to which the oxytocin and vasopressin receptors belong appeared in this period, and the genes for these receptors are associated with behavioral phenotypes that are consistent among divergent species (Yamashita and Kitano, 2013).
It is interesting to note that aspects of the behavioral patterns elicited by oxytocin-like peptides in modern-day nematodes appear remarkably similar to those found in mammals, suggesting that these peptides are both genetically and functionally conserved (Lockard et al., 2017). Even in nematodes, oxytocin-like molecules regulate a series of behavioral patterns and social interactions necessary for successful mating (Garrison et al., 2012). Moreover, the peptide-stimulated behaviors described in nematodes appeared strikingly similar to those seen in vertebrates.
Vasopressin is more ancient than oxytocin, and vasopressin receptors are sensitive to and regulated by both peptides (Carter, 2017). Both oxytocin and vasopressin arose through duplication from a common ancestral molecule presumed to be vasotocin (Acher et al., 1995; Goodson, 2013). The vasopressin gene currently found in vertebrates appeared over 200 million years ago, and the oxytocin gene appeared over 100 million years ago (Knobloch and Grinevich, 2014; Grinevich et al., 2016). It is estimated that mammalian evolution began around 200 million years ago. Three separate modern receptors for arginine vasopressin (AVP) [AVP receptor (AVPR) 1A, AVPR1B, and AVPR2] and the receptor for oxytocin (OXTR) evolved during the period of mammalian global radiation and expansion (Fig. 1) (Ocampo Daza et al., 2012). The original genes responsible for oxytocin- and vasopressin-like peptides and their receptors are associated with the evolution of behavioral adaptations in modern mammals, including lactation and maternal behavior.
Evolutionarily, more modern functions, including social behavior patterns and reproductive strategies found in mammals, may be especially dependent on the effects of oxytocin or related molecules on the oxytocin receptor rather than through actions on the vasopressin receptor (Table 1). Among these is the uniquely mammalian function of milk ejection (Caldwell, 2017). Behaviors associated with selective social memories, high levels of sociality, and social reward necessary for mammalian as well as avian parenting emerged in conjunction with the evolutionary appearance of the oxytocin receptor (Goodson et al., 2012; Kelly and Goodson, 2014). Taken together, the appearance of oxytocin and its receptor set the stage for human evolution (Carter, 2014).
V. Oxytocin-Like Molecules
Oxytocin and vasopressin are small peptides with similar chemical structures (Fig. 2). Classic vertebrate versions of these peptides consist of nine amino acids in a six–amino acid ring formed by cysteine bonds and a three–amino acid tail with a terminal amine. Vasopressin differs in structure from oxytocin by two amino acids. Both oxytocin and vasopressin are synthesized in conjunction with carrier proteins (neurophysin 1 and 2, respectively). The gene for oxytocin is located on human chromosome 20, adjacent to the gene for vasopressin. These genes lie on opposite strands and have opposite transcriptional orientations (toward one another) (Gainer, 2012).
Oxytocin and vasopressin are synthesized in brain regions that are critical to behavioral and physiologic homeostasis. Different cells in specific brain regions produce these two peptides, including the supraoptic nucleus and paraventricular nucleus (PVN) of the hypothalamus (Sannino et al., 2017). The neurons of the PVN, termed magnocellular neurons, which synthesize oxytocin and vasopressin, extend processes to the posterior pituitary gland where these peptides are released into the systemic circulation.
The primary cells of origin of oxytocin also produce networks of neural projections reaching throughout the brain and spinal cord (Grinevich et al., 2016). For example, oxytocin from the PVN can reach the central amygdala with the capacity to quickly modulate emotional functions of the amygdala and brainstem (Stoop, 2012). The PVN is a major site of convergence and integration for neural communication relating to stress with effects on the HPA axis and autonomic functions (Ulrich-Lai and Herman, 2009). Oxytocin is colocalized in at least some of its cells of origin with corticotropin-releasing hormone (CRH), which in turn regulates the HPA axis (Sawchenko and Swanson, 1985). CRH or CRH-vasopressin actions also have been implicated in some of the detrimental effects of chronic stress (Elkabir et al., 1990; Aguilera et al., 2008). Oxytocin may be coreleased with CRH or vasopressin as an adaptive response to a variety of challenges (Neumann and Slattery, 2016) possibly facilitating coping.
Early work on oxytocin and vasopressin suggested that high concentrations of the receptors for these peptides were located in brain regions that were distant from the source nuclei. This led to the hypothesis that within the nervous system, oxytocin and vasopressin were transported by passive diffusion or “volume transmission” (Landgraf and Neumann, 2004). More recently, fine peptide-containing processes have been described throughout the nervous system; these findings support the hypothesis that the peptides are released locally, but peptide also is detected within fibers that reach some distance from the cells of origin (Grinevich et al., 2016; Chini et al., 2017).
VI. Precursors and Fragments of Oxytocin
A. Precursors and Extended Forms of Oxytocin
Precursors, from which the nine–amino acid form of oxytocin is produced, have been shown to be biologically active. The precursors or extended forms of oxytocin, sometimes called OT-X (Fig. 2), consist of 10, 11, or 12 amino acids. Cleavage of the extended form of oxytocin creates the nine–amino acid molecule classically known as oxytocin (Fig. 2). The extended forms of oxytocin likely have many unidentified functions both in adults and during development (de Wied et al., 1991; Gainer, 2012). For example, the extended form of oxytocin is found in mammalian fetuses (Gainer, 2012). Studies in mice also suggest that both oxytocin and the extended form have a critical role in tissue differentiation in the heart and in survival or restoration of heart tissue after damage (such as hypoxia) or disease (such as diabetes) (Gutkowska et al., 2014; Jankowski et al., 2016).
There is one report that the extended form of oxytocin may be elevated in autistic individuals in conjunction with lower-than-normal amounts of the nine–amino acid form of oxytocin (Green et al., 2001), suggesting that a failure to modify the oxytocin precursor could produce a less-active form of the molecule. However, the characteristics and functions of extended forms of oxytocin remain at present poorly understood (Uvnäs Moberg et al., 2019).
B. Fragments of Oxytocin May Be Biologically Active
The nine–amino acid oxytocin molecule also can be further cleaved into other biologically active forms (Fig. 2). As one example, in mouse models of autism, the oxytocin metabolite OT 4–9 has been shown to produce a dose-dependent increase in prosocial behaviors without a change in measures of anxiety (Moy et al., 2019). This effect was seen 24 hours after the termination of a subchronic regimen (2.0 mg/kg of OT 4–9) and persisted for 12 days, suggesting an effect that outlives the presence of the OT 4–9 molecule. Interestingly, OT 5–9 did not affect social behavior.
As originally identified by Kastin (Kheterpal et al., 2009), the N-terminal fragment of oxytocin, proline-leucine-glycine-NH2 (OT 7-9), is also known as melanocyte-inhibiting factor-1 (MIF-1). Among the functions of MIF-1 is the regulation of pain through interactions with the opioid receptors (Khan et al., 2010). MIF-1 differs from oxytocin in that it is more resistant to degradation and easily passes through the blood-brain barrier. Animal and human research supported the notion that MIF-1 could function as an antidepressant and “endogenous naloxone.” Specifically, MIF-1 decreases the analgesic effect of morphine (Galina and Kastin, 1986; Bocheva and Dzambazova-Maximova, 2004) and acts as a positive allosteric modulator of the D2 and D4 dopamine receptor subtypes (Vartak et al., 2007; Mann et al., 2010). Oxytocin also can be degraded by aminopeptidase activity into a so-called “linear” form (Fig. 2) (Burbach et al., 1980, 1983), and this linear form acts to stimulate α-2 adrenoreceptors (Uvnäs Moberg et al., 2019). The oxytocin system and adrenoreceptors interact in a variety of ways with potential functions that are at present only partially understood (Saniger et al., 2011; Wrzal et al., 2012a,b).
The original use patent for MIF-1 was filed by Abbott Laboratories in the late 1960s, and in 1991, a patentable analog was developed by another company. Although these efforts did not result in medications for reasons described by Ehrensing (2015), in conjunction with more recent animal research, they do offer proof of concept for the functions of oxytocin fragments (Uvnäs Moberg et al., 2019). The creation of MIF-1 from oxytocin is accomplished by insulin-regulated aminopeptidase (IRAP), which thereby regulates the persistence of extracellular oxytocin levels. Thus, when conceiving of oxytocin-signaling equilibria, we must account for not only the relative contributions of ligand and receptor but also degradation via peptidases, such as IRAP. For example, when IRAP is knocked out in male mice, approach to stranger conspecifics is more frequent, suggesting increased oxytocin action (Burns et al., 2019).
VII. Processes Regulating Oxytocin and Vasopressin
A. Oxytocin and Vasopressin Are an Integrated System
The effect of endogenous oxytocin signaling as well as vasopressin and related molecules depends on many factors. These include variation in genetics and epigenetics of the peptides and their various receptors, which may differ across and within species (reviewed by Feldman et al. (2016), Carter and Perkeybile (2018)). Although oxytocin and vasopressin are often studied separately, they function as an integrated system, creating additional levels of complexity (Caldwell and Albers, 2016; Carter, 2017).
B. The Structure of These Peptides Identifies Some of the Challenges They Present
The disulfide bonds in oxytocin and vasopressin are important in explaining the broad biologic consequences of these molecules. These bonds also help to explain the challenges and apparent discrepancies in the measurement of oxytocin and vasopressin (MacLean et al., 2019). Another consideration for understanding and interpreting oxytocin’s effects is the fact that acute versus chronic patterns of oxytocin release can have biologic consequences that also differ. The speed at which these peptides degrade or bind to other molecules is critical to their function. Moreover, the pharmacodynamics of these peptides at their receptors is another essential element in determining their availability (Jurek and Neumann, 2018). In addition, oxytocin and vasopressin differ in their effects on receptor-transduced subcellular signaling cascades (Gulliver et al., 2019).
C. Feedback Loops
Oxytocin-producing cells and OXTR also are sensitive to the oxytocin peptide. Under some conditions, a form of autocrine feedback regulates the functions of oxytocin-producing cells. Stimulation of the oxytocin system, especially in early life, can “feed forward” to release more oxytocin (Bowen et al., 2011; Suraev et al., 2014; Kenkel et al., 2019). Genetic deletion of Oxtr results in lifelong, region-specific changes in oxytocin peptide expression, which speaks to oxytocin’s autoregulation (Vaidyanathan and Hammock, 2020). In some cases, the administration of oxytocin enhances the synthesis of endogenous oxytocin in the central nervous system (Grippo et al., 2012). As described below, the expression of Oxtr in adulthood also is regulated by oxytocin’s actions in early life (Kenkel et al., 2019; Perkeybile et al., 2019). Moreover, availability of OXTRs can be dynamically modulated by increasing exogenous oxytocin administration (Plested and Bernal, 2001; Robinson et al., 2003).
D. Patterns of Peptide Release Matter
The availability and actions of endogenous oxytocin and vasopressin differ by patterns of release or administration. The hypothalamic neurons synthesizing oxytocin exhibit the capacity for pulsatile release. This may be related to a unique morphologic and functional plasticity of these cells, which can change in minutes (Theodosis, 2002; Hatton and Wang, 2008). In adult rats, oxytocin-synthesizing neurons undergo physical transformations in response to hormonal and social stimulation. During pregnancy, birth, and lactation and under other physiologic conditions (such as dehydration or sexual stimulation), glial processes extending from pituicytes (the resident astrocytes of the posterior pituitary) that normally separate the oxytocin-containing neurons may be retracted, allowing electrical coupling and subsequent stimulus-driven or endogenous pulsatile release of oxytocin. These dynamic neuronal-glial interactions allow for remarkable plasticity that is intricately tuned to hormonal demand for oxytocin (Hatton et al., 1984). Interestingly, vasopressin-containing neurons typically do not show this form of dramatic plasticity and pulsatile release. In the context of labor, this pulsatile release of oxytocin coordinates the intermittent smooth muscle contractions of the uterus that occur by oxytocin binding to OXTRs and the subsequent influx of extracellular calcium (Batra, 1986). Pretreatment with oxytocin or the clinical use of Pitocin (synthetic oxytocin) can cause uterine OXTR desensitization, receptor internalization, and a decreased responsiveness to further oxytocin exposure (Robinson et al., 2003; Gottlieb, 2016). Moreover, compared with continuous exogenous oxytocin exposure, intermittent exposure has been shown to maintain oxytocin-induced uterine contractility and uterine responsiveness, highlighting the importance of oxytocin’s pulsatile release (Talati et al., 2019). It seems likely that oxytocin’s evolutionarily conserved role in smooth muscle contractility during labor (Batra, 1986), milk lactation (Hatton and Wang, 2008), and ejaculation (Filippi et al., 2003; Viveiros et al., 2003) was coadapted for social behavioral functions, such as enhanced mother-infant bonding during birth (Nissen et al., 1995) and lactation as well as selective partner preference formation during sexual intercourse (Carter, 2017). It is possible that the capacity for pulsatile release allows oxytocin to stimulate different receptors and even override some of the actions of vasopressin. Thus, dynamic interactions of oxytocin and vasopressin are important for both the acute and chronic effects of these molecules.
E. Individual Differences Are to Be Expected
In describing individual differences in the actions of oxytocin in humans, it will be important to keep in mind that oxytocin and vasopressin and their receptors are genetically variable, epigenetically regulated, and sensitive to stressors and diet across the lifespan. As one example, salt releases vasopressin and also oxytocin (Leng and Russell, 2019). In addition, nicotine is a potent regulator of vasopressin; thus, smoking, including prenatal exposure of a fetus, holds the potential to adjust this system with effects that likely differ between males and females and that may be transgenerational.
F. Effects of Other Molecules on the Oxytocin-Vasopressin System
Beyond the scope of this review but important to understanding oxytocin and vasopressin is the fact that many other molecules, including dopamine (Wang and Aragona, 2004; Gobrogge and Wang, 2016), serotonin (Dolen, 2015), GABA (Ben-Ari, 2018), and opioids (Meguro et al., 2018), interact with these peptides to influence behavior and other functions. Use of drugs, such as opiates, stimulants, and selective serotonin reuptake inhibitors, and diet, can affect both endogenous oxytocin and its receptors with effects that are largely unknown. In addition, sex differences in oxytocin and vasopressin are common, and it is likely that steroids, including estrogens, androgens (Carter and Perkeybile, 2018; Jirikowski et al., 2018), and glucocorticoids (Liberzon and Young, 1997), play a major role in the regulation of the actions of oxytocin and availability of receptors for oxytocin across the lifecycle. As described below, stress and experiences across the life cycle also have major consequences for the release and actions of oxytocin and vasopressin.
VIII. Measurement of Oxytocin
A. Oxytocin Is Difficult to Assay
Important to the study of oxytocin, even when considering only the classic form, is the capacity to measure this molecule. Initial measurements of oxytocin-using bioassays and radioimmunoassay suggested that oxytocin was typically present in human blood at low concentrations of approximately 5 pg/ml (Leng and Ludwig, 2016), with small pulses in oxytocin release (∼15 pg/ml) being sufficient to induce lactation and facilitate uterine contractions in mammals (Saameli, 1963; Leng and Ludwig, 2016). Studies using immunoassay or mass spectrometry (MS) preceded by an extraction procedure continued to detect concentrations in the 5–30 pg/ml range (Szeto et al., 2011; Kagerbauer et al., 2013). However, other methods, including MS, have indicated that endogenous oxytocin may occur in blood at much higher concentrations greater than 500–1000 pg/ml (Kramer et al., 2004; Brandtzaeg et al., 2016; MacLean et al., 2017b).
In addition, local tissue concentrations of oxytocin may give results of particular relevance to understanding the beneficial effects of this peptide. For example, within the microenvironment of ovarian tumors, oxytocin levels were measured at 200 times higher than those in plasma (Cuneo et al., 2019). These high levels of oxytocin may reflect a response to the tumor with apparent benefits to reducing inflammation. But they also could be related to the absence within the tumor (compared with blood) of peptide binding molecules, which, in turn, might affect the concentrations of oxytocin detected by antibody-based methods (MacLean et al., 2019; Yamamoto and Higashida, 2020).
B. Controversy Was Generated by Divergent Findings
It was initially suggested that high levels detected by immunoassay were measurement artifacts due to the binding of antibodies to nonhormonal components of blood (Szeto et al., 2011; McCullough et al., 2013). However, recent work indicates that oxytocin readily engages in complexes at its disulfide bridge (Avanti et al., 2013) and that the vast majority of oxytocin in biosamples evades detection using conventional approaches to measurement (Brandtzaeg et al., 2016). Importantly, these findings have been confirmed using MS—which is highly specific compared with immunoassay—suggesting that earlier critiques of the high oxytocin concentrations detected by immunoassay (which were within concentration ranges deemed implausible) may have been misguided. However, despite its advantages regarding specificity, MS-based methods for measuring oxytocin are still in active development, and many researchers have faced substantial challenges for reliable detection of oxytocin using this approach. For example, Brandtzaeg et al. (2016) struggled to reproduce earlier MS-based methods for measurement of unbound oxytocin. Furthermore, Franke et al. (2019) have argued that Brandtzæg et al.’s method may have been significantly affected by the lower resolution MS instrument used. Hence, it is important to keep in mind that although MS is a very sophisticated approach, the verdict on the optimal measurement method is still being debated. Additional areas in which MS measurement of oxytocin can be improved include the removal of interferents (e.g., phospholipids, which can suppress signals, resulting in vast underestimation of oxytocin concentrations), automation (including sample preparation) for higher throughput, and further development/maturation of high-sensitivity microfluidic separation systems (e.g., nano liquid chromatography).
The dynamic factors that affect the measurement of oxytocin and vasopressin also can influence attempts to create a pharmacology based on oxytocin. At present, measurements of peptides have suffered from poor specification regarding the forms in which researchers intend to detect oxytocin; this is, in part, because the biologic significance of different states of oxytocin remains poorly understood. Current work suggests that oxytocin and vasopressin are dynamically changing in biologic matrices, such as blood. In addition, detecting the presence of binding molecules will be critical to accurate representations of the availability of these elusive molecules (Yamamoto and Higashida, 2020).
C. Disulfide Bonds
Disulfide bonds in the cysteines of both oxytocin and vasopressin allow these molecules to be highly reactive, forming relatively temporary unions with other molecules. Furthermore, oxytocin may be quickly sequestered by binding to other abundant thiol-containing molecules, such as glutathione as well as phospholipids and cellular elements, such as blood cells that are discarded in the creation of plasma. This binding of nonapeptides to other molecules could be influenced by temperature, pH, availability of oxygen, other factors in the medium being studied, or methods in use (MacLean et al., 2019). These dynamics may explain at least some of the unique properties of oxytocin and vasopressin but also account for problems associated with in vivo measurement of oxytocin and vasopressin. For example, common approaches to sample preparation, such as solid-phase extraction, likely discard the majority of oxytocin in blood (Brandtzaeg et al., 2016; MacLean et al., 2019). Thus, measurements in blood in particular introduce many other factors that can affect peptide determinations.
D. Salivary Oxytocin
Salivary measurements, possibly with fewer sources of variation, offer an alternative for the assessment of changes in oxytocin (Carter et al., 2007; White-Traut et al., 2009; Bernhard et al., 2018; MacLean et al., 2018). Several studies have revealed reliable variations in oxytocin concentration, especially in rapid response to specific experiences, such as anticipation of breast feeding (White-Traut et al., 2009), sexual stimulation, exercise, affiliative social contact, and psychologic stress (Jong et al., 2015; MacLean et al., 2017a). However, the relationships between concentrations of peptides in saliva, blood, and peptide concentrations in the source brain nuclei are not yet well-understood (Quintana et al., 2018).
E. Can Endogenous Hormone Levels in Bodily Fluids Be Useful in Predicting Responses to Exogenous Oxytocin Treatments?
Knowledge of endogenous oxytocin or vasopressin concentrations is important to predicting the consequences of exogenous oxytocin exposure. Such assessments are rarely made, in part because of the difficulties associated with the measurement of oxytocin (MacLean et al., 2019). However, at least in autism spectrum disorders, measurements of endogenous oxytocin (Parker et al., 2017) and especially vasopressin (Carson et al., 2015; Oztan et al., 2018a) did predict behavioral sensitivity to exogenous hormone treatments. Comparatively low levels of vasopressin (but not oxytocin) measured in neonatal cerebrospinal fluid have been associated with a subsequent diagnosis of autism (Oztan et al., 2020). In another recent example, relative concentrations of endogenous oxytocin and vasopressin in plasma were associated with sex, age, anxiety, attachment behaviors, and cognition (Plasencia et al., 2019). These studies support the usefulness of measurements of both oxytocin and vasopressin but leave many empirical questions unresolved.
F. Exogenous Peptides
One of the most remarkable aspects of pharmacological studies of the effects of exogenous oxytocin or related drugs is the fact that these compounds are being given to humans—with a few notable exceptions—without attempts to measure either endogenous oxytocin or vasopressin. The measurement of endogenous oxytocin has indeed proven difficult and contentious but is not impossible (MacLean et al., 2019). Doses of exogenous oxytocin have the potential to either be ineffectively low or nonspecifically high (see oxytocin’s ability to act on the AVPR1A, below), particularly when these doses are not informed by endogenous concentrations and release patterns.
G. Other Measures May Index Receptor Sensitivity
Most studies of receptors are limited to genetic markers or in rare circumstances made in postmortem tissues (Bao and Swaab, 2018). For example, age-related reductions in receptors for oxytocin have been detected in postmortem brain tissues from individuals with autism (Freeman et al., 2018). However, as described below, proxies for the status of oxytocin and vasopressin receptors, including measures of methylation levels in receptor genes, also may predict reactions to endogenous or exogenous oxytocin (Lancaster et al., 2015; Ebner et al., 2019). Thus, there is usefulness in measuring both the availability of oxytocin and the sensitivity of receptors that are capable of responding to this molecule (see below).
IX. Receptors for Oxytocin and Vasopressin
A. The Oxytocin Receptor
Only one oxytocin receptor has been described (OXTR), the gene for which is located in humans on chromosome 3p24–26 (Gimpl and Fahrenholz, 2001; Jurek and Neumann, 2018). The OXTR gene encodes a G-protein–coupled receptor (GPCR) with a seven-transmembrane domain. The same oxytocin receptor is present in neural tissue and in other parts of the body, such as the uterus, breast, and gastrointestinal tract.
Although there is technically only one OXTR, it is now understood that oxytocin affects numerous different GPCRs (Fig. 2). Oxytocin can bind to receptors that were identified in vitro, such as OXTR, but also to vasopressin receptors (AVPR1A, AVPR1B, AVPR2) (de Wied et al., 1991; Peter et al., 1995; Ragnauth et al., 2004; Manning et al., 2008; Ocampo Daza et al., 2012). In general, oxytocin (vs. vasopressin) has its highest affinity for OXTR. However, oxytocin also readily binds to AVPR1A (reviewed in Song and Albers (2018)). Outside these two neuropeptide systems, recent research has found several other targets for oxytocin’s effects. For example, oxytocin acts as an agonist on the pain-sensing transient receptor potential vanilloid-1 receptor, where it attenuates nociception and drives nocifensive behavior (Nersesyan et al., 2017; Wee et al., 2019). Oxytocin also acts as a positive allosteric modulator of the μ-opioid receptor (MOR) (Meguro et al., 2018), where it likely furthers antinociception.
B. Measuring the Oxytocin Receptor
The measurement of peptide receptors is problematic because the pharmacological tools that are available for identifying, stimulating, or blocking receptors for oxytocin and vasopressin may not be sufficiently selective to allow easy identification or manipulations of these receptors, especially in vivo (Busnelli and Chini, 2018). Cell culture or other in vitro methods do not necessarily reflect the functional availability of these peptides, especially since the binding that occurs in vivo, including in blood or tissues, could be influenced by competing molecules that differ from one test condition to another (Yamamoto et al., 2019). For example, as described below, microglial OXTR has been difficult to detect in vivo but appears to be significantly upregulated after immune challenges/stressors, especially when microglia and macrophages are challenged in vitro (Yuan et al., 2016; Szeto et al., 2017).
C. Vasopressin Receptors
The three vasopressin receptor subtypes respond to oxytocin to varying degrees. These are expressed in different tissues, and their genes are located on separate chromosomes. AVPR1A is found in the nervous system and throughout the cardiovascular system with a broad set of behavioral functions. AVPR1B is found in the pituitary but also in brain areas with a role in the management of stress and aggression (Vaccari et al., 1998; Stevenson and Caldwell, 2012). AVPR2 is localized primarily to the kidney with a classic role in fluid balance. Functional effects of oxytocin and vasopressin depend on interactions with both OXTR and AVPR1A (Song et al., 2014; Hicks et al., 2015; Carter, 2017; Chini et al., 2017; Song and Albers, 2018).
D. Receptor Distributions
Receptors for both oxytocin and vasopressin are localized in areas of the nervous system that regulate social, emotional, and adaptive behaviors, including the amygdala, the HPA axis, and the autonomic nervous system (Quintana and Guastella, 2020). Based on work primarily in rodents and birds, among the regions with especially high levels of OXTR are various parts of the amygdala, the bed nucleus of the stria terminalis, the nucleus accumbens, brainstem source nuclei for the autonomic nervous system, and in systems that regulate the HPA axis as well as in brainstem tissues involved in pain and social attention (Stoop et al., 2015; Freeman and Young, 2016; Wilson et al., 2016; Gobrogge et al., 2017; Poisbeau et al., 2018). OXTR is also found in various cortical areas and the hippocampus, brain areas that are especially variable among species and individuals, with possible consequences for behavioral plasticity (Witt et al., 1991; Grunewald et al., 2012; Seelke et al., 2016a,b; Duchemin et al., 2017).
Both individual and species differences in OXTR and AVPR1A distributions are commonly identified and presumably adaptive (Witt et al., 1991; Hammock and Young, 2005; Freeman and Young, 2016; Okhovat et al., 2017; Ophir, 2017; Carter and Perkeybile, 2018; Okhovat et al., 2018). Species variation is likely important in the evolution of different patterns of sociality, including those that differentiate socially monogamous from nonmonogamous rodent species (Carter and Perkeybile, 2018) and affiliative versus nonaffiliative avian species (Goodson et al., 2006; Wilson et al., 2016). However, regional distributions of CNS OXTR in primates, including humans, have proven challenging to identify and interpret (Freeman and Young, 2016).
X. Subcellular Signaling Specifies the Functions of Oxytocin and Vasopressin
Oxytocin acts via GPCRs to activate subcellular cascades with eventual effects on transcription factors (Busnelli and Chini, 2018; Jurek and Neumann, 2018). Knowledge of the subcellular signaling pathways that respond to the stimulation of oxytocin or vasopressin receptors are among the next generation of approaches to the creation of medicines based on oxytocin pathways (Lerman et al., 2018; Gulliver et al., 2019; Kingsbury and Bilbo, 2019). Genes for OXTR and the three vasopressin receptors code for separate GPCRs, each with a seven-transmembrane domain. The subcellular signaling pathways vary among receptors. In addition, the capacity of oxytocin and vasopressin to activate a given receptor subcellular signaling pathway may differ according to the concentrations of the peptides and based on variations in the regional location of receptors in the nervous system (Stoop et al., 2015; Chini et al., 2017; Busnelli and Chini, 2018; Song and Albers, 2018). Differences in subcellular signaling could help to explain the capacity of oxytocin and vasopressin to have different functions in various tissues, such as those regulating birth (Arrowsmith and Wray, 2014), social behavior (Caldwell and Albers, 2016; Song et al., 2016), reactivity to stressors (Murgatroyd and Nephew, 2013; Stoop et al., 2015; Neumann and Slattery, 2016), cancer (Lerman et al., 2018), and cardiovascular disease (Reiss et al., 2019).
When given as an exogenous treatment, the behavioral consequences of oxytocin are dose-dependent but not typically linear (Ceanga et al., 2010; Klein et al., 2011, 2013; Borland et al., 2019). As with other peptide-based treatments acting on GPCRs, more is rarely better. This may explain several of the unexpected or paradoxical findings reported when oxytocin is given as an exogenous treatment (Carter, 2017; Song and Albers, 2018). The lack of a full understanding of the subcellular pathways through which oxytocin and vasopressin function is at present a barrier to using these nonapeptides as medicines. Furthermore, as mentioned above, the oxytocin and vasopressin-like molecules exist in several endogenous forms, and the signaling functions of precursors or fragments of these peptides have only begun to be described.
XI. Genetics and Epigenetics
A reduction or upregulation in the availability of the oxytocin or vasopressin receptors can reflect both heritable genetic variation (King et al., 2016; Baker et al., 2017) and/or epigenetic tuning (Okhovat et al., 2015; Baker et al., 2017; Okhovat et al., 2018; Towers et al., 2018; Perkeybile et al., 2019) of this system. The variable expression in tissues of OXTR is also epigenetically tuned by early life experience (Baker et al., 2017; Krol et al., 2019a; Perkeybile et al., 2019), increasing the capacity of peptides to have complex adaptive functions. Evidence in humans for the functional importance of the oxytocin genetic-epigenetic systems at present comes primarily from correlations among genetic and epigenetic variations in the OXTR gene and individual differences in behavior, physiology, and brain anatomy (Rodrigues et al., 2009; Tost et al., 2010; Jack et al., 2012; Puglia et al., 2015; Lancaster et al., 2018b).
Genetic variations in the OXTR, indexed by single-nucleotide polymorphisms, were originally related to autism spectrum disorders (for example, variant rs2254298 G>A and rs53676 G>A) (Jacob et al., 2007). The OXTR gene can be silenced via DNA methylation, thus reducing the expression of the oxytocin receptor (Kusui et al., 2001). Functional relationships between methylation of the oxytocin receptor gene and behavior were first detected in autism (Gregory et al., 2009) and recently validated (Andari et al., 2020). Such relationships have also been found in other conditions, including postpartum depression (Bell et al., 2015) and schizophrenia (Rubin et al., 2014). In neurotypical humans, methylation status of the OXTR also has been shown to strongly predict measures of social perception in both college-aged adults and infants (Jack et al., 2012; Puglia et al., 2015, 2018; Krol et al., 2019b). Specific single-nucleotide polymorphisms could be implicated in a variety of functions through epigenetic and intergenerational effects on the expression of OXTR (Bell et al., 2015; Chagnon et al., 2015). Below, we discuss how experiences in early life, including parental care and oxytocin administration in the context of obstetric care, can program epigenetically the OXTR system to produce lifelong changes in this system.
XII. Early Experience and Context
A. Emotional Context Matters
This is in part because context can influence physiology, helping to create individual differences in the capacity to react to environmental events. As one example, behavioral changes induced by early experience may reflect changes in the oxytocin system. Human research, especially studies conducted in individuals with a history of personal adversity, suggest that in some contexts, exogenous oxytocin can have asocial or negative consequences (Bartz et al., 2010), including the increased perception of threat in the presence of individuals from other social groups (De Dreu, 2012). In terms of early experience, positive parenting (vs. neglect) has lasting beneficial consequences for behavior and health in the offspring, possibly also through retuning of the oxytocin/vasopressin pathways.
B. Adversity Versus High Nurture
Animal research also suggests that the nature of early experience can influence the development of oxytocin/vasopressin pathways and subsequent long-term behavioral outcomes. For instance, adversity in early life has the capacity to sensitize the vasopressin system and, in some cases, upregulate AVPR1A in brain regions involved with defensive behaviors (Carter, 2017). Alternatively, early life exposure to highly nurturant parenting or as little as a single exposure to exogenous oxytocin produces epigenetic changes in Oxtr (Kenkel et al., 2019; Krol et al., 2019a; Perkeybile et al., 2019). Receptor changes such as these can have long-lasting consequences for the capacity for sociality and emotional regulation (Carter, 2003; Carter et al., 2009; Kenkel et al., 2019).
C. Vasopressin Systems as Likely Targets for Adversity and Stress in Early Life
Expression of vasopressin or the vasopressin receptors also are likely to be affected by negative social experiences, including neglect or maltreatment in early life (Bunck et al., 2009; Hernandez et al., 2016). Under conditions in which the vasopressin system is sensitized, especially in a context of fear or danger, we can hypothesize that oxytocin could stimulate the vasopressin receptor. Thus, early adversity or maltreatment might dysregulate or upregulate vasopressin, the vasopressin receptor, or other factors regulating this system, including catecholamines. This switch from oxytocinergic to vasopressinergic stimulation in turn would alter the ability to cope with a challenge. In later life, it could encourage either a more active or mobilized defensive or aggressive behavioral strategy possibly accompanied by anxiety. In cases of severe traumatic stress, a more energetically conservative “shut-down” strategy might emerge, characterized by immobility due to fear rather than mobilization. It is likely that dynamic interactions between oxytocin and vasopressin allow behavioral, autonomic, and emotional-state shifts between anxiety and depression, such as those that characterize a history of trauma (see below).
D. Early Exposure to Peptides May Modulate Perceived Context
In individuals with a history of adversity or trauma, the effects of exogenous oxytocin may appear to be detrimental [for example, Walsh et al. (2018)]. Once more, we can hypothesize that the apparently paradoxical effects of oxytocin in responses to extreme stressors represent, in part, the capacity of oxytocin to stimulate AVPR1A and AVPR1B (Fig. 3) (Caldwell, 2017; Carter, 2017). This may be of particular importance in individuals primed by negative experience. For example, even small amounts of oxytocin might be capable of activating vasopressin receptors, with effects that involve mobilization and potentially defensive emotional or behavioral responses. Based on data from oxytocin knockout mice, in which the vasopressin system is sensitized (Ragnauth et al., 2004), we can also hypothesize that individuals with low concentrations of endogenous oxytocin might be more likely to experience increased vasopressin-like activities when given oxytocin (Carter, 2017).
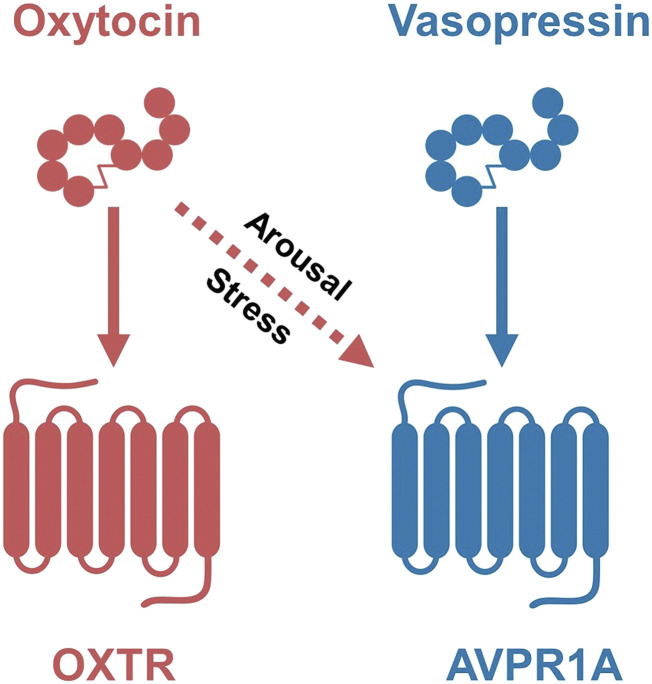
Fig. 3.
The effects of oxytocin and vasopressin can be context-dependent. Sensitivity of the AVPR1A or OXTR to either oxytocin or vasopressin may be altered by a history of adversity or by positive experiences, especially during early life. For example, as illustrated here, arousal or stress may increase the release of oxytocin and/or increase sensitivity of AVPR1A to oxytocin [reviewed Carter (2017)].
E. Birth and Epigenetics
Data from animal models and human epidemiology indicate that widely used medical interventions (i.e., exogenous oxytocin, such as Pitocin given to facilitate labor, opioid medications that block the oxytocin system, or cesarean sections that alter exposure to endogenous oxytocin), have lasting consequences for the offspring and/or mother (Hayes and Weinstein, 2008; Kroll-Desrosiers et al., 2017; Kingsbury and Bilbo, 2019). Such exposures hold the potential to have epigenetic effects on the oxytocin systems, including changes in DNA methylation (Kenkel et al., 2019). These changes in turn would have lasting effects on the expression of receptors for oxytocin, leaving individuals differentially able to respond to oxytocin and also possibly to the effects of vasopressin. For instance, in a recent study intended to model labor induction, voles exposed indirectly to oxytocin at birth (via maternal administration) show changes in Oxtr methylation patterns in the fetal brain, a gregarious phenotype of social behavior, and sex- and region-specific changes in OXTR and AVPR1A densities in the brain as adults (Kenkel et al., 2019). Deconstructing the developmental benefits of endogenous oxytocin and the implications of exogenous exposure to this peptide has much to teach us about the biology of health versus disease.
XIII. The Autonomic Nervous System as a Target for Oxytocin
A. Neural and Peptide Interactions Allow a Wide Range of Adaptive Responses
Understanding the complex actions of oxytocin requires awareness that oxytocin regulates not only the brain and reproductive system but also the immune (see below) and autonomic nervous systems. Effects on the autonomic nervous system include shifts in state and emotional regulation and can be seen as acute changes as well as chronic adjustments that affect the entire body. For example, evidence supporting the coordinated effects of acetylcholine (the major neurotransmitter in the parasympathetic nervous system) and oxytocin comes from colocalization of these molecules and their receptors in tissues, including hypothalamic and brainstem areas, that regulate the autonomic nervous system (Freeman and Young, 2016; Quintana et al., 2019). Interactions between oxytocin and the autonomic nervous system are also relevant under conditions in which the system is manipulated pharmacologically. For example, treatment with exogenous oxytocin induces increases in vagal activity as measured by respiratory sinus arrhythmia (Quintana et al., 2013).
B. The Autonomic Nervous System Consists of Sympathetic and Parasympathetic Components
The sympathoadrenal axis is associated with flight-fight responses. Under extreme stress or life-threatening situations, both oxytocin and vasopressin activate sympathetic and parasympathetic pathways, with a more variable autonomic response in the face of milder stressors (Porges, 2012; Yee et al., 2016).
The parasympathetic nervous system and specifically vagal pathways have important consequences for brain and cognitive function. The vagus also plays a particularly important role in the neural regulation of the mammalian immune system (Dantzer, 2018). It is likely that these peptides integrate the activity of different branches of the autonomic nervous system, which helps to explain the importance of social attachment in protecting against or modulating different forms of emotion dysregulation. These same peptides, together with the vagus nerve, have effects on the immune and metabolic systems across the lifespan, which also helps to explain the lasting effects of early emotional experiences on physical health and well-being (Hammock, 2015; Amini-Khoei et al., 2017; Kingsbury and Bilbo, 2019).
C. The Polyvagal Hypothesis
The autonomic nervous system supports the physiologic and metabolic demands of the cerebral cortex and is necessary for social behavior (Porges, 2009, 2012). Embedded within the mammalian parasympathetic nervous system/vagus nerve are two systems: a more ancient unmyelinated branch of the vagus with cells that originate within the brainstem region known as the dorsal vagal complex and with pathways to subdiaphramatic organs but also innervating the heart. In addition, encased within the vagus nerve, as it exits the brainstem, is a more evolutionarily modern myelinated vagal system; cells for this branch originate in the ventral-vagal complex, with pathways to the heart and bronchi. The ventral-vagal pathways interact with the brainstem nuclei that innervate the striated muscles regulating the face and head; these may be of particular relevance to social communication as well as the anti-inflammatory actions of oxytocin (Dantzer, 2018, Kingsbury and Bilbo, 2019).
Oxytocin receptors are especially abundant in brainstem regions, including the dorsal vagal complex or dorsal motor nucleus of the vagus (DMX) that regulates subdiaphramatic organs. Stimulation of the DMX slows the heart. Vagal regulation is also implicated in sexual and social behaviors and is linked with motivation and reward circuitry (Han et al., 2018). Oxytocin may act on these ancient and highly conserved brainstem regions to permit mammalian patterns of maternal and sexual behavior that require immobility without fear (Porges, 1998; Kenkel et al., 2013).
D. The Hypothalamus as a Site for Coordination of Vagal-Peptide Interactions
The PVN of the hypothalamus is both a major site of oxytocin synthesis and a key area that responds to oxytocin, presumably via input from oxytocin receptors (Quintana et al., 2019). Furthermore, the PVN is a regulatory center for autonomic functions, particularly vagal functions (Herman et al., 2012). The visceral target organs of the autonomic nervous system, such as the heart, digestive, and immune systems, also contain abundant receptors for oxytocin (Welch et al., 2005; Gutkowska and Jankowski, 2012; Jurek and Neumann, 2018; Wang et al., 2019). Thus, it is not surprising that in the face of stress oxytocin can protect the functions of the digestive system (Yang et al., 2019), heart (Gutkowska and Jankowski, 2012; Buemann and Uvnas-Moberg, 2020), and immune system (Kingsbury and Bilbo, 2019).
E. Social Communication and Myelinated Ventral-Vagal Pathways
The muscles of the mammalian face and head are regulated by the same brainstem area that also regulates the myelinated vagal pathway, thus linking through bidirectional communication the heart with facial expressions and intonation of vocalizations. This integrated social engagement system provides mechanisms through which emotional experience and physiologic states are involved in social communication. Autonomic actions of oxytocin as well as vasopressin also influence the capacity for and expression of social bonds that arise in response to challenge or adversity (DeVries et al., 1996; Cho et al., 1999).
Oxytocin acting via the autonomic nervous system and acting on the cortex and brainstem has been repeatedly implicated in human social engagement, facially expressed emotions, and eye gaze (Kemp et al., 2012; Quintana et al., 2013; Freeman and Young, 2016). Evidence from rodents indicates that the oxytocin receptor plays an important role in cortical functions necessary for social cognition (Ophir, 2017), social reward (Song et al., 2014), and social affective behavior (Rogers-Carter et al., 2018; Rogers-Carter and Christianson, 2019). At least some of these effects also involve interactions between oxytocin and dopamine (Quintana et al., 2019; Martins et al., 2020). Moreover, the modulation of social approach behavior by PVN oxytocin release onto ventral tegmental area dopaminergic neurons is dependent on gut-brain signaling via the vagus nerve (Sgritta et al., 2019).
F. Cortical Integration of Peptide-Autonomic Interactions
The insular cortex, a multisensory processing region that also contains a rich population of OXTRs, functions to integrate external sensory stimuli with internal visceral, emotional, and motivational states (Rogers-Carter and Christianson, 2019). Specifically, the “visceral insular cortex,” which receives input from gastric mechanoreceptors, arterial chemoreceptors, and cardiovascular baroreceptors, respiratory centers, and the vagus nerve, plays a central role in interoception (Barnabi and Cechetto, 2001). This region of cortex is able to detect changes in internal state, influencing appropriate social responses, such as approach versus avoidance behavior with a stressed conspecific (Rogers-Carter et al., 2018).
XIV. Oxytocin and “Stress Coping”
Many of the emotional, visceral, and immune effects of oxytocin and vasopressin rely on interactive actions of these molecules on the autonomic pathways. These effects are adaptive in the face of stressful experiences, although the functions of these peptides as well as hormones of the HPA axis are different under conditions of acute versus chronic stress (Table 1).
A. Acute Stress Responses
Adaptive responses to acute stressors have a distinct physiology from those elicited by chronic stress (Pournajafi-Nazarloo et al., 2011; Bosch and Young, 2018). In many cases reactions to acute stressors include the release of oxytocin, possibly in anticipation of more chronic situations that may follow (Lang et al., 1983; Gibbs, 1986; Engert et al., 2016). These anticipatory responses also may include increases in social behavior and transient changes in other organ systems, including the heart (Grippo et al., 2012; Kemp et al., 2012).
B. Chronic or Traumatic Stress
Oxytocin has particular importance in the face of chronic stress when “stress-coping” effects may take precedent. Oxytocin can facilitate passive forms of coping and protect against shutting down or “immobilization with fear” (Porges, 1998). Behavioral, physiologic, and anatomic data from rodents (Grippo et al., 2009) and humans (Grewen and Light, 2011) suggest that chronic, antistress effects of social support can downregulate the sympathetic nervous system, allowing the expression of protective and restorative functions of the vagal systems (Thompson et al., 2008; Yee et al., 2016).
C. Myelinated Ventral-Vagal Pathways and Passive Coping
Studies in prairie voles have revealed that oxytocin has protective actions on the autonomic functions of the ventral, myelinated vagus, as measured by changes in neutrally regulated heart rate variability (respiratory sinus arrhythmia) (Grippo et al., 2009). Oxytocin also may directly co-opt the older unmyelinated vagus through receptors in the dorsal vagal complex, protecting mammals from responding to threat with a more primitive “reptile-like” freezing pattern and evacuation of the lower bowel (Porges, 2012).
Immobility with fear or freezing, associated with traumatic experiences, can be a component of despair and depression. This response is adaptive in conserving energy. But freezing in fear is not optimal for other functions, such as active social behaviors that rely on mobilization and high levels of metabolic activity (Porges, 2012). The capacity of oxytocin to protect this system is critical to the types of social engagements that characterize mammalian social behavior, including birth and parenting.
Mammals, with their comparatively large brains, are particularly vulnerable to the need for oxygen. Under traumatic conditions, the functions of oxytocin may allow a shift to those necessary for protection of the survival of cortical processing. However, when oxygen is insufficient, psychologic dissociation (Li et al., 2020) or even loss of consciousness can occur (Carter, 2014). As one example, oxytocin levels were exceptionally high in women with a history of trauma who showed a pattern of dissociation, supporting the hypothesis that elevations in oxytocin can be adaptive in trauma (Seng et al., 2013).
As described below, oxytocin, in part through effects on the vagus nerve, has direct and indirect effects on the immune and metabolic systems across the lifespan. These actions also help to explain the lasting effects of early emotional experiences on physical health and well-being (Hammock, 2015; Engert et al., 2016; Amini-Khoei et al., 2017; Quintana et al., 2017; Kingsbury and Bilbo, 2019).
XV. Immunologic and Anti-Inflammatory Effects of Oxytocin
Oxytocin has central roles in immune surveillance and immunologic homeostasis and supports resistance to a whole host of immune challenges, stressors, and insults across a range of body tissues (Li et al., 2017b; Buemann and Uvnas-Moberg, 2020). Many of the protective effects of oxytocin observed across the lifespan appear to be mediated through the OXTR and occur in response to both normal adaptive stressors during development (e.g., birth, gut microbial colonization) and in response to injury and trauma (Kingsbury and Bilbo, 2019). Furthermore, although oxytocin can influence immune-system development (Geenen, 2006; Murgatroyd et al., 2016; Rotondo et al., 2016; Li et al., 2017b), such as being highly concentrated in the thymus and playing a role in the “education” of thymic cells for self-tolerance (Geenen, 2006), we focus here on oxytocin’s anti-inflammatory roles in the context of immune-system function and homeostatic cellular processes.
A. Oxytocin and Neuroinflammation
The role of oxytocin as an important regulator of the mammalian immune system (Li et al., 2017b; Kingsbury and Bilbo, 2019) that ties social behavior and experiences with the capacity to heal in the face of stress or trauma is especially evident within the nervous system (Fig. 4). In an adult animal model of ischemic stroke, animals subjected to focal cerebral ischemia have reduced tissue damage, increased antioxidant activity, and decreased oxidative stress if they are socially housed during the recovery period, as opposed to animals that recover in social isolation (Karelina et al., 2011). These effects of social housing are mediated by oxytocin, as the administration of exogenous oxytocin to experimental animals recovering in social isolation recapitulates the neuroprotective effects; OXTR antagonism blocks this protection in both socially housed and socially isolated animals (Karelina et al., 2011). Similarly, in a model of acute brain inflammation induced by a systemic injection of the bacterial endotoxin lipopolysaccharide (LPS), intranasal administration of oxytocin 1 hour prior to the LPS injection reduces microglial activation, the levels of proinflammatory cytokines TNF-α and IL-1β, and the levels of proinflammatory mediators cyclooxygenase-2 and iNOS. These effects were detected in the prefrontal cortex of LPS-treated adult male mice, as compared with LPS-treated males that received intranasal vehicle (Yuan et al., 2016). Interestingly, oxytocin has no effect on these inflammatory measures in the absence of LPS exposure, suggesting that oxytocin’s anti-inflammatory actions require the presence of a stressor. Remarkably similar effects of oxytocin were observed in healthy men subjected to an immune challenge (Clodi et al., 2008). Compared with men administered LPS alone, men who received intravenous infusion of LPS plus oxytocin had an attenuated endocrine, cytokine, and chemokine response to the immune challenge. Taken together, these findings demonstrate that oxytocin can lessen the inflammatory response induced by LPS across species.
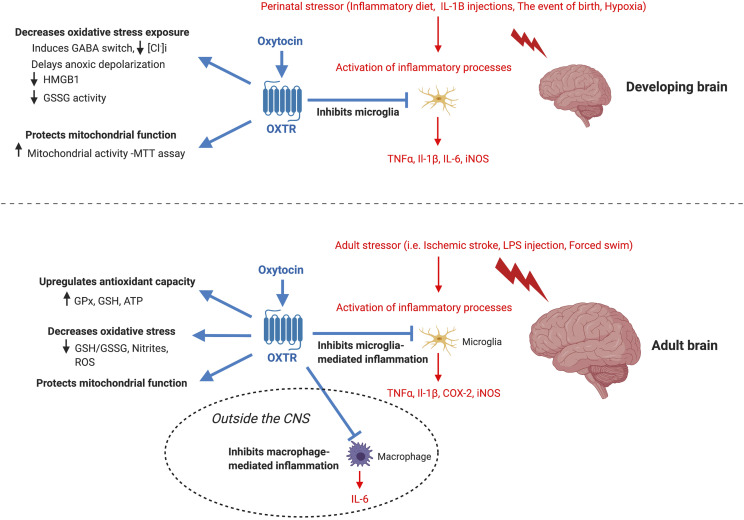
Fig. 4.
Oxytocin acts as an anti-inflammatory molecule for the nervous system in the presence of stressors. For the developing brain, oxytocin confers neuroprotection in the presence of a stressor by inhibiting the release of proinflammatory cytokines by microglia by decreasing oxidative stress exposure and by protecting mitochondrial function. For the adult brain, oxytocin confers similar neuroprotection in the presence of a stressor by inhibiting the release of proinflammatory cytokines by microglia, by decreasing oxidative stress exposure, by protecting mitochondrial function, and by increasing antioxidant capacity. Moreover, similar to its inhibition of microglia-mediated inflammatory cascades, oxytocin inhibits macrophage-mediated proinflammatory cascades outside of the central nervous system during an immune challenge with LPS. Although oxytocin mediates many aspects of social behavior and cognition, the known social and cognitive functions that oxytocin protects in the presence of inflammation are shown here for the developing and adult brain. Inflammatory cascades are shown in purple and oxytocin signaling is shown in red. All of oxytocin’s protective effects shown here are believed to be mediated by oxytocin binding to the OXTR. COX-2, cyclooxygenase-2; GPx, glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidized glutathione; HMGB1, high-mobility group box 1.
B. Early Experience and Inflammation
In animals subjected to early life adversity, oxytocin also has protective effects (i.e., boosts resilience) later in life against subsequent stressors or immune challenges through a reduction in brain inflammatory processes (Amini-Khoei et al., 2017; Mairesse et al., 2019) (Fig. 4). For instance, male mice subjected to maternal separation stress during the first 2 weeks of life are characterized by a depressive-like phenotype in adulthood during a forced swim test, sucrose preference test, and grooming assay. This depressive phenotype is accompanied by impaired mitochondrial function, reduced antioxidant activity, increased oxidative stress, and increased gene expression for immune-proinflammatory markers, all within the hippocampus (Amini-Khoei et al., 2017). Importantly, the depressive-like phenotype and the accompanying inflammation were attenuated if male mice were injected intracerebroventricularly with oxytocin in adulthood but not if the OXTR antagonist, atosiban, was administered alongside oxytocin. Similar findings were observed for mice subjected to a low-protein diet during gestation to simulate fetal growth restriction, a condition associated with the development of cerebral palsy, cognitive, and behavior deficits. In this model, subsequent postnatal injections of the inflammatory cytokine IL-1β cause increased expression of classic and nonclassic proinflammatory genes within microglia, decreased myelination, decreased brain connectivity, and increased anxiety-like behavior (Mairesse et al., 2019). When carbetocin, a long-lasting OXTR agonist, was administered postnatally alongside IL-1β, it attenuated the microglial proinflammatory gene expression, reversed the myelination and connectivity deficits, and normalized anxiety-like behavior.
C. Microglia and Macrophage
Many of oxytocin’s anti-inflammatory actions appear to be, at least in part, through the attenuation of inflammatory processes mediated by microglia and macrophages (Karelina et al., 2011; Yuan et al., 2016; Li et al., 2017b; Szeto et al., 2017; Mairesse et al., 2019) (Fig. 4). OXTR is present on the cell surface of microglia as well as neurons and astrocytes; the addition of oxytocin to microglia cultures stimulated with LPS dampens the expression of major histocompatibility complex class II, a gene that is induced within activated microglia (Karelina et al., 2011). Similarly, both Oxtr mRNA and OXTR protein are enriched in primary microglia stimulated with LPS, and treatment of these microglial cultures with oxytocin suppresses the LPS-induced production of TNF-α and IL-1β (Yuan et al., 2016). After an LPS immune challenge in vivo, oxytocin also reduces the expression and production of proinflammatory cytokines TNF-α and IL-1β as well as the number of prefrontal cortex cells labeled with ionized calcium-binding adapter molecule 1, a microglial marker (Yuan et al., 2016). In microglia cultures isolated from both animals exposed to an inflammatory low-protein diet during gestation and control animals injected with IL-1β and interferon α (proinflammatory mediators), the oxytocin agonist carbetocin reduces the morphologic changes associated with microglia activation as well the expression of proinflammatory mediators IL-6, TNF-α, and iNOS (Mairesse et al., 2019). These effects are mediated through OXTR, as addition of an OXTR antagonist (L-368899) prevents the microglial morphologic changes and the attenuation of IL-6, TNF-α, and iNOS expression. Given the ancient conserved functions of oxytocin, it is perhaps not surprising that carbetocin has similar immune suppressive effects on microglia morphology and cytokine release in zebrafish in vivo after an inflammatory stimulus (Mairesse et al., 2019). Similarly, an additional ancient function of oxytocin is the protection against painful stimuli, including a behavior response that alleviates stress. Within zebrafish, hypothalamic oxytocin neurons drive nocifensive behavior after the exposure to a noxious stimulus, suggesting a conserved stress-coping mechanism of oxytocin (Wee et al., 2019). Moreover, in mammals, oxytocin cells in the PVN control acute inflammatory pain perception, whereas oxytocin in the spinal cord controls nociception, and oxytocin in the amygdala regulates the processing of emotional pain (Poisbeau et al., 2018).
Whereas oxytocin dampens inflammatory processes mediated by microglia, the tissue-resident macrophages of the CNS, similar effects are observed for oxytocin acting on macrophages outside of the CNS (Fig. 4). For instance, THP-1 and human primary macrophages stimulated with LPS upregulate OXTR expression and protein production relative to nonstimulated cells (Szeto et al., 2017). LPS treatment also upregulates IL-6 mRNA and protein in THP-1 macrophages and murine macrophages (including tissue-resident murine peritoneal macrophages), and this effect is attenuated when THP-1 cells are pretreated with oxytocin (Szeto et al., 2017). Furthermore, blocking NF-κB activation prevents this upregulation of Oxtr and IL-6 expression, suggesting that the OXTR acts as an acute phase protein that reduces macrophage-mediated inflammatory responses both within and outside of the CNS. Interestingly, LPS challenge downregulates AVPR2 in primary human macrophages, and pretreatment of THP-1 cells with vasopressin has no effect on LPS-induced IL-6 secretion, highlighting the anti-inflammatory function of oxytocin as opposed to vasopressin (Szeto et al., 2017).
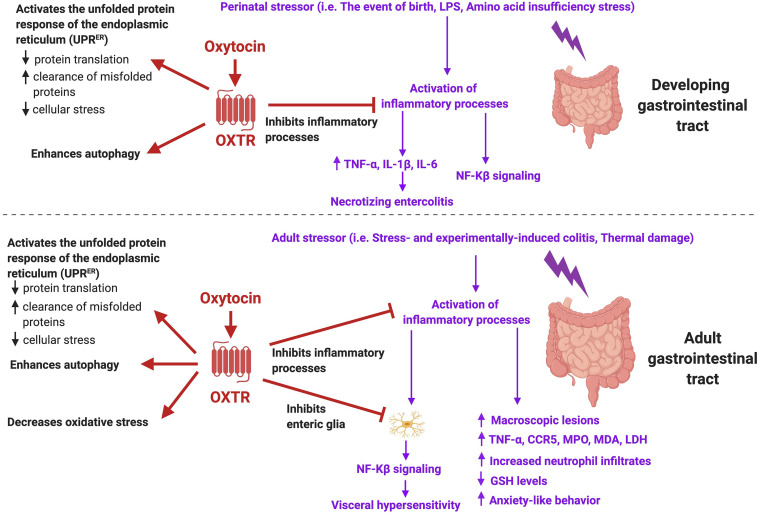
D. Oxytocin Protects against Inflammation Associated with Birth
Oxytocin also plays a significant anti-inflammatory role at birth protecting the fetal brain against the hypoxic-like conditions that accompany labor (reviewed in Kingsbury and Bilbo (2019); Fig. 4). It also likely preserves mitochondrial function (Kaneko et al., 2016), potentially by acting on the mitochondrial unfolded protein response (Bordt et al., 2019). The process of parturition is considered an inflammatory/immune event in which offspring are exposed to reduced oxygen and blood flow (Lagercrantz and Slotkin, 1986; Tyzio et al., 2006; Maron et al., 2010), a surge in stress hormones (Lagercrantz and Slotkin, 1986), elevated fetal cytokines (Castillo-Ruiz et al., 2018), and antigen colonization (Costello et al., 2012; Castillo-Ruiz et al., 2018). Under hypoxic-ischemic conditions, oxytocin has been shown to confer neuroprotection to fetal tissue on the day of birth by reducing the metabolic demand of neurons through a dramatic and precise switch in GABAergic signaling that attenuates the elevation of intracellular chloride (Tyzio et al., 2006; Khazipov et al., 2008). This oxytocin-mediated switch delays the time to anoxic depolarization, a process of accelerated cell death that is characterized by significant increases in oxidative stress and reactive oxygen species (Tyzio et al., 2006; Khazipov et al., 2008). In rodent models of autism wherein this oxytocin-mediated switch is absent at birth, offspring are characterized by altered social behaviors and neuronal hyperexcitability, demonstrating that oxytocin’s neuroprotective effects during a major inflammatory event give rise to appropriate social behaviors in adolescence (Tyzio et al., 2014).
However, oxytocin appears to have dose-dependent effects at birth, particularly when administered exogenously, with higher doses conferring less neuroprotection in vitro (Ceanga et al., 2010) as well as an increased risk for autism for male offspring (Soltys et al., 2020). Possible explanations to explain the loss of oxytocin’s protection when administered exogenously at high concentrations are oxytocin-induced receptor desensitization (Plested and Bernal, 2001; Robinson et al., 2003) and/or oxygen desaturation of the fetal blood supply (i.e., greater hypoxia) that can occur after synthetic oxytocin-induced uterine hyperstimulation (Simpson and James, 2008). A third explanation may be the promiscuous binding of oxytocin to the vasopressin receptor AVPR1A when administered exogenously at high doses, as AVPR1A can mediate proinflammatory signaling (Bordt et al., 2019) and exacerbate neural toxicity during hypoxia/hypoglycemia (Tanaka et al., 1994; Spoljaric et al., 2017). Thus, the protective nature of endogenous versus exogenous oxytocin as well as its dose-dependent effects at birth require further study given the widespread use of synthetic oxytocin to manage labor.
E. Oxytocin, Oxidative Stress, and Mitochondria
Oxytocin also protects neural cells against hypoxic-ischemic conditions by preserving mitochondrial function, reducing oxidative stress, and decreasing a chromatin protein that is released during inflammation (Kaneko et al., 2016), which can activate microglia through the receptor for advanced glycation end products (RAGE) (Massey et al., 2019; Yamamoto and Higashida, 2020). All of these effects are abolished in the presence of OXTR antagonism (Tyzio et al., 2006; Ceanga et al., 2010; Kaneko et al., 2016) and are consistent with the anti-inflammatory and neuroprotective functions of oxytocin signaling during hypoxic-ischemic events, including birth. Moreover, the serum of pregnant women administered the OXTR antagonist atosiban for preterm labor displays an increase in oxidative stress and a decrease in antioxidant capacity compared with women in preterm labor who did not receive atosiban (Grzesiak et al., 2018). Similarly, the plasma of offspring born to pregnant rats treated with atosiban is characterized by increased oxidative stress compared with the plasma of offspring born to saline-injected mothers (Simsek et al., 2012). Finally, term babies born to mothers who underwent elective cesarean sections at term are characterized by significantly lower levels of serum oxytocin at birth as compared with vaginally delivered infants (Marchini et al., 1988), suggesting a reduction in the protective, anti-inflammatory actions of oxytocin with cesarean deliveries.
F. Oxytocin and Gastrointestinal Inflammation
A rich literature demonstrates that oxytocin also acts as an anti-inflammatory protective molecule for the adult and developing gastrointestinal system, including the period of gut microbial colonization at birth and lactation during which the fetal gut is exposed to significant antigen stimulation from thousands of bacterial species (reviewed in Kingsbury and Bilbo (2019)) (Fig. 5). Within adult rodents, stress- and/or chemical-induced colitis increases intestinal damage, oxidative stress, neutrophil infiltration, the expression of inflammatory pathway genes, and anxiety-related behavior (Iseri et al., 2008; Cetinel et al., 2010; Welch et al., 2014). Oxytocin treatment dampens the severity of intestinal damage and alleviates anxiety, whereas coadministration of an OXTR antagonist or the use of Oxtr knockout mice prevents oxytocin’s protective effects (Iseri et al., 2008; Cetinel et al., 2010; Welch et al., 2014). Similar to offering neuroprotection to the brain during hypoxic-ischemic events, oxytocin appears to protect the gut during reduced intestinal blood flow within a model of burn-induced gastric injury (Iseri et al., 2008). Thermal burns produce transient splanchnic vasoconstriction followed by oxidative and nitrosative intestinal injury and increased reperfusion injury and, if severe enough, increased intestinal permeability, sepsis, and organ failure (Iseri et al., 2008). Whereas rats exposed to a thermal burn followed by a subcutaneous saline injection are characterized by greater levels of malondialdehyde and myeloperoxidase activity, greater gastric neutrophil infiltration, and greater microscopic damage scores of the gastric mucosa and skin, experimental rats that received two subcutaneous injections of oxytocin had a significant attenuation of skin damage and burn-induced oxidative damage of the intestinal mucosa (Iseri et al., 2008). Furthermore, Erdman and colleagues have shown that the gut bacterium, Lactobacillus reuteri, acting through vagal nerve stimulation promotes the endogenous release of oxytocin, which functions to accelerate wound healing of the skin by activating CD4+Foxp3+CD25+–immune regulatory T cells (Poutahidis et al., 2013; Varian et al., 2017). Moreover, in a rodent model of necrotizing enterocolitis induced by formula feeding, maternal separation, and intermittent hypoxia, oxytocin administration decreases the severity of intestinal inflammation, whereas the OXTR antagonist exacerbates it (Gross Margolis et al., 2017). Oxytocin also reduces visceral hypersensitivity, a feature of intestinal bowel syndrome (Xu et al., 2018) and diabetic polyneuropathy (Erbas et al., 2017), and attenuates stress-induced GI motility disorders (Yang et al., 2019).
Fig. 5.
Oxytocin acts as an anti-inflammatory molecule for the gastrointestinal system in the presence of stressors. For the developing gut, oxytocin confers protection in the presence of a stressor by inhibiting the activation of proinflammatory cascades by activating the UPRER and by enhancing autophagy. For the adult gut, oxytocin confers protection in the presence of a stressor by inhibiting proinflammatory cascades by inhibiting enteric glia and NF-κB signaling by activating the UPRER by enhancing autophagy and by decreasing oxidative stress. Although oxytocin mediates many aspects of social behavior and cognition after stress-aggravated colitis, oxytocin has been shown to decrease anxiety-like behavior (Cetinel et al., 2010). Inflammatory cascades are shown in purple, and oxytocin signaling is shown in red. All of oxytocin’s protective effects shown here are believed to be mediated by oxytocin binding to the OXTR. CCR5, C-C chemokine receptor-5; GSH, reduced glutathione; LDH, lactate dehydrogenase; MDA, malondialdehyde; MPO, myeloperoxidase.
G. Oxytocin Is Protective to the Developing Gut
The increased expression of OXTR in enteric neurons and enterocytes in intestinal tissue coincides with the periods of birth and lactation, a time when the fetal gut is simultaneously exposed to both endogenous maternal oxytocin (via maternal release at birth and during breastfeeding) and inflammatory processes, such as amino acid–insufficiency stress, bacterial antigen stimulation, and microbial colonization (Welch et al., 2009; Klein et al., 2017; Kingsbury and Bilbo, 2019). The absence of Oxtr during development in Oxtr knockout mice results in increased intestinal inflammation in addition to increased macromolecular gut permeability, increased gut motility, and disruption of mucosal maintenance (Welch et al., 2014), indicating that the oxytocin-signaling system has important functions for the establishment of the gastrointestinal system. Specifically, oxytocin signaling has been shown to reduce cellular stress within Caco2BB cells, a human gut cell line used as an enterocyte model, by modulating the unfolded protein response (UPR) in the endoplasmic reticulum (UPRER)) (Klein et al., 2013, 2014, 2016). The UPRER can be activated during inflammation or immune challenges or in response to other stressors (Bordt et al., 2019) and is a cellular stress response that occurs when there is a buildup of unfolded and/or misfolded proteins within the endoplasmic reticulum of cells. This accumulation of proteins activates the UPRER, which serves to trigger signaling cascades that halt protein synthesis, reduce protein load, and restore cellular homeostasis (Schroder and Kaufman, 2005; Hetz et al., 2011; Hetz, 2012). In addition to the UPRER, oxytocin also modulates autophagy, a process in which cellular components are degraded and recycled to restore homeostasis (Klein et al., 2014).
H. Oxytocin and Breast Milk
In an effort to simulate the exposure of developing enterocytes to bacterial endotoxin via breast milk, Klein et al. (2016) treated Caco2BB cells with LPS. Whereas LPS increased NF-κB proinflammatory signaling and suppressed the UPRER within Caco2BB cells, oxytocin treatment prevented these LPS-mediated effects (Klein et al., 2016). This led Klein and associates (Klein et al., 2017) to propose that, during the lactation period, oxytocin in colostrum and breast milk both protects enterocytes from apoptotic cell death amid low level antigen microbial stimulation and modulates cellular metabolism via phasic UPR runs to promote enterocyte cell differentiation. Klein et al. (2017) further showed that both colostrum oxytocin and exogenous oxytocin protect primary intestinal rat villi at birth from cellular stress induced by amino acid starvation, a period of nutrient insufficiency between birth and first feeding. Specifically, oxytocin dampens inflammatory process, halts protein translation, and promotes autophagy in newborn intestinal villi.
I. Oxytocin and the Healing of Damaged Tissues
In addition to its protective role for developing and adult gastrointestinal tissue, endogenous oxytocin, at high levels, has been implicated in human wound healing (Gouin et al., 2010) and is protective against cardiovascular dysfunction via a suppression of inflammatory processes mediated by neutrophils, macrophages, T lymphocytes, TNF-α, and IL-6 (Jankowski et al., 2016; Reiss et al., 2019). Among other commonly studied models in which oxytocin has been shown to be anti-inflammatory are those involving the healing of tissue flaps (Xu et al., 2017) and ovarian cancer (Cuneo et al., 2019). Within human patients, higher levels of ascites oxytocin were associated with lower levels of the proinflammatory cytokine IL-6 and a significant survival advantage. Moreover, oxytocin inhibited IL-6 production in cancer cell lines (Cuneo et al., 2019). Oxytocin also may restore tissue after procedures that damage the nervous system, intestines, and cardiovascular system (Karelina and DeVries, 2011; Bordt et al., 2019; Kingsbury and Bilbo, 2019). Interestingly, some of oxytocin’s wound-healing properties are mediated via vagal nerve stimulation by the intestinal bacteria L. reuteri, which causes an elevation in PVN oxytocin followed by an increase in systemic oxytocin (Varian et al., 2017).
J. Oxytocin and Developmentally Induced Inflammation
Benefits of oxytocin are often seen in developmental rodent models, such as maternal separation or pharmacological or genetic models capable of creating animals with atypical patterns of social behavior and associated elevations in inflammation. Neonatal rodents respond with increases in inflammation after nutrient insufficiency, maternal separation, or injections that induce inflammation (Klein et al., 2018). In all of these cases, there is evidence that oxytocin is protective. Atypical behaviors, tissue damage, and microglial activation can be reduced by oxytocin treatments (Wang et al., 2018; Zinni et al., 2018; Mairesse et al., 2019).
K. Oxytocin, CD38, Immune Function, and Social Behavior
Previous as well as recent findings suggest that some of oxytocin’s protective effects on immune function and social behaviors are mediated by CD38. CD38 is a well-known immune marker that is expressed by a range of immune cells (T cells, B cells, macrophages, natural killer cells), is involved in the proliferation and immune responses of lymphocytes, and serves as a diagnostic marker for lymphoma, leukemia, myeloma, and human immunodeficiency virus infection (Malavasi et al., 2008). Within the brain, CD38 is a transmembrane receptor on hypothalamic neurons that catalyzes the formation of cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate from NAD+ and NAD phosphate (Deaglio and Malavasi, 2006). These molecules play an integral role in the regulation of intracellular Ca2+ and control highly conserved processes, such as egg fertilization, muscle contraction, hormone secretion, and immune function (Malavasi et al., 2008). In the hypothalamus, both cADPR and nicotinic acid adenine dinucleotide phosphate serve to increase intracellular Ca2+, thereby triggering the central and peripheral release of oxytocin that is important for a variety of social behaviors (Higashida et al., 2012) Within CD38 knockout mice, neuroanatomical findings are consistent with deficits in hypothalamic oxytocin secretion, and females show defects in maternal care, whereas males display social memory impairments (Jin et al., 2007). These social behaviors were rescued by subcutaneous OT injections or CD38 re-expression (Jin et al., 2007). Within humans, single-nucleotide polymorphisms of the CD38 gene have been associated with oxytocin inhibition and an increased risk for autism (Lerer et al., 2010; Higashida et al., 2012), a disorder characterized by immune dysfunction (McDougle et al., 2015). Moreover, human lymphoblastoid cell lines derived from autistic patients show reduced CD38 expression compared with those derived from nonautistic controls (Lerer et al., 2010). Recent research also suggests that autism risk associated with vitamin A deficiency may be mediated through the CD38-oxytocin pathway (Gamliel et al., 2016; Lai et al., 2018). In an intriguing review by Higashida et al. (2018), evidence is put forth that hypothalamic oxytocin secretion is increased after social stress in subordinate animals via a CD38-cADPR–dependent mobilization of Ca2+ from intracellular stores and after hyperthermia (triggered by LPS endotoxin exposure, social stress, or stress coping) via a transient receptor potential melastatin-2–dependent influx of Ca2+. Higashida et al. (2018) elaborate on how stress-induced oxytocin release caused by fever may explain the transient recovery of social functions within autistic individuals during hyperthermia (Zhong et al., 2016), similar to how exogenous OT administration has been reported to improve social behavioral deficits within autistic patients and animal models (Higashida et al., 2018). Intriguingly, this model helps explain our earlier findings of greater hypothalamic oxytocin-Fos colocalization in subordinate and nonsocial stressed finches (Goodson et al., 2015) as well as the facilitation of partner preference formation in LPS-injected female prairie voles (Bilbo et al., 1999).
L. Oxytocin Regulates Probiotic Bacteria
Other treatments that may act through oxytocin include the benefits of probiotic bacteria, such as L. reuteri (Erdman and Poutahidis, 2016; Sgritta et al., 2019), found in colostrum and breast milk (Klein et al., 2018). For instance, adequate levels of intestinal L. reuteri stimulate adequate oxytocin PVN production that is necessary for typical social affiliation (Sgritta et al., 2019), particularly after prenatal exposure to an inflammatory maternal high-fat diet (Buffington et al., 2016). Some of these in vivo anti-inflammatory benefits of oxytocin are autonomically mediated by the vagal pathways (Garrott et al., 2017; Reyes-Lagos et al., 2019). Importantly, anti-inflammatory and beneficial behavioral effects of oxytocin have been detected both in vivo and in vitro (Szeto et al., 2008; Gutkowska and Jankowski, 2012; Szeto et al., 2017), indicating that the benefits of oxytocin are not limited to indirect neural or endocrine actions.
M. Vasopressin and Inflammation
Vasopressin and AVPR1A have also been implicated in immune function with effects that, in general, are proinflammatory and often opposite in direction from those of oxytocin (Li et al., 2017b; Bordt et al., 2019). For instance, vasopressin signaling exacerbates inflammatory processes in the brain after ischemic stroke and traumatic brain injury (Szmydynger-Chodobska et al., 2010, 2011). Furthermore, like oxytocin, vasopressin has roles for immune-system development, yet vasopressin may play a more limited role in immune-system regulation and in some cases can function to augment oxytocin’s related immune effects (Li et al., 2017b).
N. Sex Differences in the Immune System
As detailed below, recent research also links sex differences and sexual differentiation to the immune system (McCarthy et al., 2017; Dantzer, 2018, 2019). These effects differ according to the social history of the individual, contributing to what has been termed “context” (Bartz et al., 2010; Carter, 2017). These effects also differ in physiology, with females more protected than males in the context of stressors, such as birth, because of their perinatal hormonal status and an inherently greater antioxidant capacity within their brains (Kingsbury and Bilbo, 2019). Thus, these effects likely give rise to differences in how males and females respond to the protective functions of endogenous and exogenous oxytocin.
XVI. Sex Differences
Oxytocin and vasopressin are produced by both males and females; however, the functions of these peptides vary between sexes and among species (Carter and Perkeybile, 2018). Because of the adaptive nature of these hormonal systems and the fact that sexual differentiation is influenced by genetics, epigenetics, and physiologic processes (including inflammation) across the lifespan (McCarthy et al., 2017), the relationship between sex and peptides is not very well-understood. Steroids, including estrogen and testosterone of either endogenous or exogenous origins, can influence both oxytocin and vasopressin as well as the expression of their receptors (Jirikowski et al., 2018). The study of sex differences in oxytocin and vasopressin is further complicated by dynamic changes in steroids that are experienced in both sexes across the lifecycle.
A thorough description of factors regulating sex differences is beyond the scope of this review, although this topic is discussed in some depth in earlier reviews from our group (Carter, 2003, 2014, 2017; Carter et al., 2009; Carter and Perkeybile, 2018; Kenkel et al., 2019). However, knowledge of sex differences with regard to the functions of oxytocin and vasopressin is greatly needed in the development of peptide-based treatments for mental and physical disorders, including substance abuse, schizophrenia, and trauma (Macdonald, 2013). This is particularly important since, for several of these disorders, one sex often has greater vulnerability for social and cognitive dysfunction.
A. Sex Differences in Vasopressin
Effects of vasopressin are often more apparent in males versus females. Of particular relevance to understanding interactions between oxytocin and vasopressin is the consistent finding that vasopressin in the nervous system is affected by androgens. In rodent brains, endogenous vasopressin synthesis is androgen-dependent and sexually dimorphic within an axis that modulates self-defensive behavior (de Vries and Forger, 2015; Dumais and Veenema, 2016). Vasopressin receptors also vary regionally between males and females (DiBenedictis et al., 2017).
Responses to exogenous vasopressin also can differ in males and females (Dantzer, 1998; Thompson et al., 2006; Carter et al., 2009). However, vasopressin also functions in conjunction with oxytocin to facilitate selective social behaviors, including social bonds and selective aggression (Cho et al., 1999; Carter, 2017).
B. Developmental Effects of Oxytocin and Vasopressin Are Often Sexually Dimorphic
Adding to the complexity of the sex differences in these peptides are findings that indicate that the presence of oxytocin or vasopressin during development can modify both of these peptides and their receptors. During development, the vasopressin receptors appear to be particularly sensitive to tuning by exogenous peptides, with effects that also differ between males and females. For example, research in voles indicates that vasopressin and its receptors can be developmentally regulated by exposure to exogenous oxytocin, thus contributing to altered capacity to form pair bonds (Bales et al., 2007). In both sexes, exposure to a relatively low dose of exogenous oxytocin shortly after birth reduces the expression of AVPR1A in several other brain areas (Bales et al., 2007). However, in male prairie voles, the same treatment increases the expression of vasopressin receptors in the ventral pallidum, a brain region associated with reward and pair bonding. In another experiment in prairie voles using a wide range of doses of vasopressin, dose-dependent increases in vasopressin in the 1st week of life were followed by increases in aggression in adulthood. These effects on aggression, necessary for mate guarding, also were most pronounced in males (Stribley and Carter, 1999).
C. Sex Differences in Response to Stress and Trauma
Sex differences in the oxytocin-vasopressin system often are most apparent in the face of stressful experiences, including social and hormonal experiences in early life (Carter et al., 2009; Kenkel et al., 2019). Glucocorticoids and other hormones released during stressful experiences also can have major effects on oxytocin, and oxytocin can act to moderate the release of and effects of glucocorticoids. For example, glucocorticoids and exposure to stressors have been shown to upregulate the oxytocin receptor (Liberzon and Young, 1997).
D. Sex Differences in Strategies Used to Manage Challenges
We can speculate that activation of central vasopressin or its receptors and associated increases in the sympathetic nervous system would allow more active or mobilized responses to emotional and physiologic challenge including the capacity for aggression and physical violence. For example, it is possible that a male-biased dependence on vasopressin might help explain the capacity of male prairie voles to form social bonds in the face of extreme challenges, whereas stress inhibits heterosexual pair-bond formation in females (DeVries et al., 1996). Dependence on vasopressin would leave males more capable of responding to challenges associated with defending the family using mobilized responses. However, if generalized to other species, this sex difference may also leave males more vulnerable to disorders or injury associated with increased aggression and risk taking (Carter, 1998, 2007; Dantzer, 2019). Concurrently, males appear to be protected against shut-down responses, especially in life-threatening situations, via vasopressin and associated increases in alertness or arousal in the face of threats.
E. Females and Vasopressin
Females also produce and rely on the physiologic effects of vasopressin. However, females are significantly more vulnerable to disorders associated with shut-down responses or immobilization, such as depression and post-traumatic stress disorder. In general, in adulthood females also may be more dependent on estrogen and estrogen-oxytocin interactions. Thus, in the presence of deficiencies in either oxytocin or its receptor, females may be more vulnerable to disorders, including symptoms of depression, characterized by either generalized anxiety or passive responses. For example, female humans also are at least twice as likely to be victims of trauma or other adversities, which in turn can increase later vulnerability to emotional disorders (Altemus et al., 2014).
F. Sex Differences in Response to Exogenous Peptides
The consequences of treatment with exogenous peptides can vary by sex in humans (Thompson et al., 2006; Domes et al., 2010; Taylor et al., 2010), nonhuman primates (Jiang and Platt, 2018), and other mammals (De Vries and Panzica, 2006; Carter, 2007; Albers, 2015; Okhovat et al., 2018). In postmenopausal women, increases in endogenous oxytocin have been associated with “gaps in social relationships” (Taylor et al., 2006). Releasing oxytocin may be a component of a self-regulatory process that helps mammals deal with isolation, stress coping, and recovery from trauma. For example, female prairie voles subjected to a 1-hour immobilization stress recovered faster in the presence of their male partner as opposed to recovering alone, displaying less anxiety-like behavior, lower levels of the stress hormone corticosterone, and increased PVN oxytocin release (Smith and Wang, 2014). Furthermore, the benefits of this oxytocin-mediated social buffering were recapitulated in stressed females that were subsequently isolated after they received injections of oxytocin in the PVN (rather than access to their partner) (Smith and Wang, 2014). These hormonal responses could also facilitate subsequent social engagement and relationships as well as boost the immune system (see below), functions that would be especially adaptive in females who may be less able than males to survive alone (Taylor et al., 2010).
G. The Stress of Birth
Oxytocin may be of special relevance in helping infants (Tyzio et al., 2006, 2008) and mothers recover from the trauma of birth while simultaneously engaging in mother-infant bonding (Nissen et al., 1995). For instance, there are peaks in serum oxytocin immediately postpartum in women that correspond with the expulsion of the placenta, contractions to reduce uterine bleeding, and an early “sensitive” period for mother-infant bonding (Nissen et al., 1995).
H. Gonadal Steroids
Species variation also reflects differential sensitivity to gonadal steroids among mammals. For example, behavioral patterns in socially monogamous species may be particularly reliant on peptides, including oxytocin and vasopressin, whereas fewer social species, especially males of nonmonogamous species, seem more behaviorally dependent on gonadal steroids, including androgens (Carter and Perkeybile, 2018). Adaptive variations in the relative importance of steroids and peptides are critical to understanding the origins of species and sex differences but also may help in understanding individual differences in the consequences of treatments with exogenous molecules.
XVII. Implications for Oxytocin in Mental and Physical Health
A. Social Support and Perceived Safety
The effects of endogenous oxytocin and vasopressin on brain-body connections, including the immune and autonomic nervous system may help to explain the profound health-related effects of social relationships or their absence. Despite the complexity associated with using exogenous peptides as medicines, there has been considerable optimism generated by short-term behavioral effects of oxytocin. Oxytocin appears to be of special relevance to physical and mental protective adaptations that involve high levels of sociality or social support and a sense of psychologic safety as well as emotional regulation and autonomic stability that are necessary for mental health and higher levels of cognitive functions (Carter, 2014).
B. Oxytocin in Psychologic Functioning
A recurring issue in attempts to use oxytocin as a medicine is the need to understand its role in behavioral and physiologic adaptations and resilience in the face of stressors and trauma. Nervous system activity, neural connectivity, and responses to social stimuli have been related to peripheral peptide concentrations in both neurotypical individuals (Lancaster et al., 2018a) and those diagnosed with various mental illnesses, such as schizophrenia or bipolar disorder (Rubin et al., 2018). Peripheral levels of oxytocin and vasopressin or variations in their receptors have been associated with function in Williams syndrome (Dai et al., 2012), autism spectrum disorders (Oztan et al., 2018b), schizophrenia (Rubin et al., 2014, 2018), depression (Cyranowski et al., 2008; Goekoop et al., 2011), postpartum depression (Zelkowitz et al., 2014; Bell et al., 2015), borderline personality disorders (Brune, 2016), eating disorders (Aulinas et al., 2019), and substance abuse (Buisman-Pijlman et al., 2014).
XVIII. Examples of the Complexity Encountered in Treating Disease with Oxytocin-Like Drugs
A large scientific literature, beyond the scope of this review, has implicated oxytocin in both health and illness. However, the relationships between endogenous and responses to exogenous oxytocin have not been easily identified. In some individuals and for a subset of disorders, the effects of exogenous oxytocin have been negative. Below, we offer examples of the complexity associated with the use of oxytocin as a therapeutic in postpartum depression and cancer. Among the sources of variation in outcomes are genetics, epigenetics, and especially adversity in early life. In addition, as described below in the case of cancer, effects of oxytocin on different subcellular signaling pathways will need to be considered in creating safe medicines based on oxytocin-like molecules.
A. Postpartum Depression
Because of its central role in birth and lactation, oxytocin was a logical target as an explanation for and treatment of postpartum depression. We hypothesized that endogenous oxytocin, including that released as a consequence of lactation, would be protective against the development of postpartum mood shifts, possibly in part by reducing susceptibility to social or environmental stressors (Carter and Altemus, 1997). There is increasing experimental evidence for this hypothesis (Gust et al., 2020).
Comparatively lower concentrations of plasma oxytocin, both prenatally and postpartum, have been associated with increased symptomology (Skrundz et al., 2011; Stuebe et al., 2013; Cox et al., 2015; Garfield et al., 2015). Specific polymorphisms within both OXT (Jonas et al., 2013) and OXTR (Apter-Levy et al., 2013; Mileva-Seitz et al., 2013) also appear to confer vulnerability to developing postpartum depression. Women with higher reported levels of psychosocial stress in early pregnancy and postpartum had lower depressive symptoms if they had higher levels of circulating oxytocin (Zelkowitz et al., 2014). In addition, epigenetic modification of OXTR via DNA methylation at specific CpG sites in the promoter region also increased the risk of postpartum depression, with effects that were expressed in a genotype-specific manner (Bell et al., 2015).
However, there is considerable individual variation in oxytocin concentrations and in the oxytocin receptor in pregnancy and postpartum. It is possible that knowledge of these differences, especially in the context of individual variations in life history, as well as the specific levels of peptides and receptors for both oxytocin and vasopressin, can be used to predict or treat disorders, such as postpartum depression. Furthermore, in humans it appears that the adult response to exogenous oxytocin is moderated by early life experiences including trauma (Walsh et al., 2018). The release of endogenous oxytocin also has been related to early childhood abuse, with generally lower levels in individuals who have experienced greater emotional adversity in early life (Heim et al., 2009; Toepfer et al., 2017). Moreover, the release of oxytocin differs according to the adult attachment style of the individual. In one study, oxytocin levels were elevated in trauma survivors, supporting the hypothesis that oxytocin serves a “stress-coping” function. However, a lower level of increase in oxytocin in response to a stressor was seen in individuals with a “dismissive” attachment style compared with more securely attached individuals (Pierrehumbert et al., 2012). However, “secure” attachment or a “sense of safety,” as used in psychologic research, is a theoretical construct, which must be translated into physiologic states. The relationship between oxytocin and trauma in adulthood has not yielded consistent results (Engel et al., 2019), and perhaps a fuller consideration of early life experience could resolve this. Thus, psychologic context can manifest as physiology, with consequences for the actions of oxytocin and vasopressin; this of course complicates the attempts to study these peptides in clinical populations.
The effects of the relationship between oxytocin and the symptoms of postpartum depression are particularly obvious in women experiencing stress (Zelkowitz et al., 2014; Massey et al., 2016) or with a history of adversity (Walsh et al., 2018). Furthermore, early life stress and the emotional history of an individual can influence the reaction to exogenous oxytocin. For example, in a placebo-controlled experiment, intranasal oxytocin was administered to women diagnosed with postpartum depression (Walsh et al., 2018). In that study, oxytocin decreased symptoms in women who did not have a history of early life stress but increased symptoms in women with a history of stress.
Depression, used in a more general sense, also has proven difficult to reliably relate to endogenous oxytocin. Several studies show a decrease in peripheral oxytocin with increased depressive symptoms (Frasch et al., 1995; Scantamburlo et al., 2007; Ozsoy et al., 2009; Seay et al., 2014). However, individuals with increased depressive symptoms sometimes exhibit increased levels of oxytocin (Parker et al., 2010; Holt-Lunstad et al., 2011; Seay et al., 2014). Furthermore, high levels of oxytocin-staining cells have been detected in postmortem tissue; people with major depressive disorder had a greater number of oxytocin and vasopressin-immunoreactive cells in the PVN compared with controls (Purba et al., 1996). There are also reports of increased variability in oxytocin levels in people with depressive symptoms compared with controls (van Londen et al., 1997; Cyranowski et al., 2008). Studies such as these suggest dysregulation in the oxytocin-vasopressin system possibly associated with stressful experiences across the lifespan and the emotional experiences described as major depression (Bao and Swaab, 2018). These studies also illustrate the challenges associated with using oxytocin as therapy for behavioral disorders.
B. Oxytocin and Cancer: a Complex Relationship
The use of oxytocin as a pharmaceutical also requires attention to possible physical side effects of prolonged exposure to this molecule. In many physical disorders, including cancer, social support is associated with reduced risk of disease progression (Hinzey et al., 2016). Lactation has long been associated with reductions in breast cancer (Lerman et al., 2018). In these cases, oxytocin is a plausible protective mechanism. For example, a variety of experiments, including animal research and in vitro studies supports the hypothesis that oxytocin can inhibit tumor growth in breast cancer. In addition, breast tumor growth is inhibited (or perhaps prevented) by exercise, another effect that has been attributed to the capacity of exercise to release oxytocin (Alizadeh et al., 2018).
In another example, the finding of exceptionally high levels of oxytocin in samples taken from ovarian cancers is particularly intriguing. Levels of oxytocin measured within tumors were associated with inflammatory markers, including IL-6, as well as patient survival. Thus, tissues challenged by cancer may produce healing levels of oxytocin (Cuneo et al., 2019).
However, the effect of oxytocin may not be universally beneficial. For example, there is conflicting evidence from several studies that prostate cancer, breast cancer, uterine cancer, and osteosarcomas can be either increased or decreased by oxytocin (Lerman et al., 2018). Among the types of cancer for which there are indications that exogenous oxytocin can increase proliferation and thus could be dangerous are prostate, uterine, and small cell lung cancer.
The complex relationships between oxytocin and cancer provide insight into the specificity of oxytocin’s effects. The local availability of oxytocin or its receptor, the tumor cell type, and the differential activation of subcellular signaling pathways downstream of OXTR can give rise to a unique oxytocin effect. For instance, there are three different subtypes of the guanine nucleotide–binding protein-α (Gα) (Gαi, Gαs, and Gαq) that couple to OXTR and that have separable functions. Gαq has been implicated in muscle contractions and cellular proliferation, whereas Gαi and Gαs may be antiproliferative (Lerman et al., 2018). Thus, effects on different subcellular signaling pathways could have opposite effects on cancer. Observations like these illustrate the challenges for creating drugs that optimize the benefits of oxytocin while reducing risks.
XIX. Pharmacology and Oxytocin: Issues in Therapeutic Applications
A. The Effects of Oxytocin Are Dose-Dependent
Doses of oxytocin currently used in human research have been restricted by availability and reliable access. Moreover, doses that were available were based on historical applications of oxytocin as an intranasal “lactational aid” or as an intravenous treatment in labor induction. Both of these applications were originally used in women who were pregnant or who had just given birth. Whether these doses and treatment regimens extrapolate to men and nonpregnant subjects, including children, is not well-studied.
Studies using different amounts of oxytocin are needed to examine possible threshold differences among individuals as a function of sex, experience, and emotional lability (Goldman et al., 2011). Different doses of a given peptide can produce different effects (Ceanga et al., 2010; Klein et al., 2014; Borland et al., 2019), and dose-response curves are only just appearing in the in vivo literature (Price et al., 2017).
B. Individual and Sex Differences Are to Be Expected in Both the Production of and Response to Oxytocin
As discussed above, the production of oxytocin varies among individuals, between males and females (Borland et al., 2019), and across the lifespan as well as in the capacity to respond to either endogenous or exogenous hormones (Plasencia et al., 2019). For example, studies of the effects of oxytocin on neural responses measured by functional magnetic resonance imaging have revealed marked sex differences in the effects of even a single intranasal treatment (Domes et al., 2010). Connectivity in the orbital frontal cortex, which is important in emotion processing, was correlated with oxytocin in women and vasopressin in men (Rubin et al., 2018). The developmental effects of these peptides are of special concern and are different in males and females (Carter, 2003; Bales et al., 2007) and in the face of adversity and stressful experiences.
C. The Basic Biochemistry of Oxytocin, Including Its Capacity to Bond to Other Molecules, Creates Special Challenges
The crosstalk between oxytocin, vasopressin, and their receptors (Figs. 2 and 3) has complicated attempts to create a medicine based on one or the other of these peptides (Caldwell, 2017; Carter, 2017; Song and Albers, 2018; Wang et al., 2019). The evolutionary basis of the oxytocin-vasopressin system permits a large number of functional permutations and creates significant obstacles to the creation of “medicines” based on these molecules. These interactions need to be considered if oxytocin-based molecules are to be used as the basis of a medicine (Feng et al., 2015; Li et al., 2017a). Moreover, as discussed here, developing effective pharmaceuticals based on oxytocin requires awareness of functional issues associated with oxytocin-vasopressin interactions and the biologic factors through which experience alters the functions of these molecules. As just one example, as described above, our research in prairie voles has revealed that small changes in experience or hormonal exposure, especially in early life, have epigenetic consequences for the expression of Oxtr (Kenkel et al., 2019; Perkeybile et al., 2019).
XX. Methods for Administering Oxytocin or Stimulating the Oxytocin System
Studies of the effects of peripherally administered oxytocin offered a new perspective on the role of oxytocin in human behavior and aroused great interest in therapeutic applications of oxytocin (Kosfeld et al., 2005; Macdonald and Feifel, 2013). Dozens of clinical trials are now examining the functional effects of OXTR agonists, including oxytocin and related drugs, such as the selective oxytocin receptor agonist carbetocin. Clinical studies typically report positive outcomes in at least a subset of patients.
In behavioral studies oxytocin has been most typically administered by intranasal spray. However, the behavioral effects of oxytocin have been questioned by complex findings from those studies, as detailed in many reviews (De Dreu, 2012; Macdonald and Feifel, 2013; Bethlehem et al., 2014; Bernaerts et al., 2017; Hurlemann and Grinevich, 2018). Moreover, even trials based on the classic nine–amino acid oxytocin molecule are often limited by the use of acute treatment protocols; in most cases a single treatment is used. Analysis of repeated and long-term effects of oxytocin or oxytocin-like molecules are rare, although even in adults, the effects of oxytocin-like drugs differ by the duration and dose used, and the effects may last for weeks beyond the time of treatment (Moy et al., 2019). Interpretation of these findings needs to be considered in the context of early life experience and the capacity of oxytocin to interact with vasopressin receptors (Carter, 2017) as well as other features of oxytocin’s functions detailed in the current review.
A. Does Peripherally Administered Oxytocin Reach the Brain?
In its classic nine–amino acid form, it was traditionally argued that oxytocin did not readily cross the blood-brain barrier (Leng and Ludwig, 2016). However, in studies done in monkeys, intranasally administered oxytocin labeled with deuterium was detected in cerebrospinal fluid (Lee et al., 2018) and in brain regions where oxytocin activity influences motivated behaviors (Lee et al., 2020). This argument also is diminished by recent awareness of the capacity of RAGE to act as an oxytocin-binding protein facilitating the transport of oxytocin across the blood-brain barrier and through other tissues (Yamamoto and Higashida, 2020). RAGE exists in a soluble form in blood and is also bound to cell membranes. Of particular interest to the functions of oxytocin, the directionality of this transport is 5–10 times higher from the blood to the brain, in comparison with brain to blood transport. Furthermore, RAGE is a member of the immunoglobulin superfamily with a role in regulating inflammation—once more implicating oxytocin in the body’s adaptive response to immune challenges (Figs. 4 and 5).
Imaging studies in rats comparing the effects of oxytocin given intravenously versus intracerebral infusion revealed that these two methods had very different consequences for blood oxygen level–dependent activity within the nervous system (Ferris et al., 2015). Imaging studies in humans also reveal that different methods of administration of oxytocin can produce different outcomes. For example, intranasal spray versus a nebulizer elicited responses, albeit in different brain areas and possibly in different regions of the olfactory tract (Martins et al., 2020). These findings support the potential usefulness of intranasal administration but leave the mechanisms of action open to further investigation. It also remains to be determined which methods of administration of exogenous oxytocin might be most functionally comparable to the effects of endogenously released peptides.
B. Placental Transport of Oxytocin
The weak penetrance of oxytocin through both the placenta and blood-brain barrier has been used as a justification for the wide use of Pitocin in labor induction. However, the evidence for transplacental passage of oxytocin has been mixed, with both positive and negative results reported [reviewed Kenkel et al. (2014)]. Interestingly, levels of oxytocin in cord blood measured within 1 minute of birth were significantly elevated in infants born via a vaginal birth (without maternal exogeneous oxytocin administration) as compared with infants born via elective cesarean section before labor started (Marchini et al., 1988). Further complicating understanding of their mechanisms of action, peptide receptors exist throughout the viscera, such as in the digestive tract (Welch et al., 2014). These receptors may allow oxytocin-like molecules to stimulate vagal afferents, thereby circumventing the blood-brain barrier in the transmission of information back to the brain.
C. Oral Oxytocin
Oxytocin is present in milk, and oral treatments with oxytocin, especially in young animals, may be an important route for influencing this system. Recent research in mice demonstrates that oxytocin is resistant to digestion (Yamamoto and Higashida, 2020) and is active in milk and colostrum, with effects on brain and behavior, especially in preweaning animals (Welch et al., 2014; Higashida et al., 2017; Klein et al., 2018; Tabbaa and Hammock, 2020). For example, Tabbaa and Hammock (2020) report that orally administered oxytocin affects the nervous system in young mice. Furthermore, RAGE transports oxytocin readily across the intestinal epithelium during the first postnatal week in mice when the intestinal barrier is still fairly permeable (Higashida et al., 2017). Although oxytocin absorption from the gut lumen to the blood can occur in adults, a 10-fold higher concentration of oxytocin is required (Higashida et al., 2017). As described above, comparable processes that are dependent on RAGE appear to occur in the nervous system, permitting crossing of the blood-brain barrier (Yamamoto et al., 2019). Knowledge of RAGE suggests yet another method for regulating the availability of and transport of oxytocin (Yamamoto and Higashida, 2020). For example, individual differences in RAGE could help to predict cellular access to oxytocin and might also facilitate access to oxytocin under conditions of stress or illness (Higashida et al., 2018; Yamamoto et al., 2019).
D. Increasing the Bioavailability of Oxytocin
Recent studies using methods to increase oxytocin’s access to the nervous system have employed nanoparticle encapsulation (Oppong-Damoah et al., 2019). This method of drug-delivery increases bioavailability of the oxytocin molecule both in cell-culture models of the blood-brain barrier and in a mouse model of social behavior. Intranasal application of nanoparticle-encapsulated of oxytocin had larger prosocial effects and behavioral changes that were sustained for at least 3 days after treatment. It is likely that new methods for delivering oxytocin will have dramatic effects on this field in the near future (Gulliver et al., 2019).
XXI. Summary
Oxytocin has effects on the regulation of physiologic processes and emotional states associated with coping and healing, with increasing evidence for beneficial actions mediated by the autonomic and immune systems. Oxytocin has special relevance for mammals, linking individuals to each other, with beneficial effects on reproduction, social behavior, and stress coping.
Endogenous oxytocin and vasopressin are integral components of the body’s capacity to cope with stress. However, the effects of exogenous oxytocin are context- and dose-dependent (Huang et al., 2014). In individuals with a history of extreme stress or trauma, the effects of oxytocin have been especially unpredictable (Walsh et al., 2018). The reasons for this are unclear, but it is possible that stimulation of the vasopressin receptor(s) occurs, producing more primitive forms of response, such as defensive behaviors or even behavioral or metabolic shutdown (Porges and Furman, 2011).
Oxytocin and its receptors are comparatively modern molecules with particular relevance in mammalian evolution, physiology, and social behaviors (Carter, 2014). Oxytocin, possibly acting via the autonomic nervous system, seems to play a central role in supportive relationships and psychologic safety, helping to protect and heal in the face of stress and adversity (Carter, 1998; Karelina and DeVries, 2011; Porges and Furman, 2011; Hinzey et al., 2016). As describe above, oxytocin also has powerful immune consequences (Figs. 4 and 5), may promote longevity, and could have played a central role in human evolution (Carter, 2014). However, attempts to create safe drugs based on this molecule must take into account oxytocin’s unique evolutionary history and complex integrative adaptive properties that span the physiologic and social realms.
Acknowledgments
We are very grateful to Katie Krol, Josh Danoff, and Pat Kratochwell for editorial assistance.
Abbreviations
- AVP
arginine vasopressin
- AVPR
AVP receptor
- cADPR
cyclic ADP-ribose
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- DMX
dorsal motor nucleus of the vagus
- Gα
guanine nucleotide–binding protein-α
- GPCR
G-protein–coupled receptor
- HPA
hypothalamic-pituitary-adrenal
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IRAP
insulin-regulated aminopeptidase
- LPS
lipopolysaccharide
- MIF-1
melanocyte-inhibiting factor-1
- MOR
μ-opioid receptor
- MS
mass spectrometry
- NF-κB
nuclear factor-κB
- OT-X
precursor or extended forms of oxytocin
- OXT
oxytocin
- OXTR
OXT receptor
- PVN
paraventricular nucleus
- RAGE
receptor for advanced glycation end products
- TNF-α
tumor necrosis factor-α
- UPR
unfolded protein response
- UPRER
UPR in the endoplasmic reticulum
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Carter, Kenkel, MacLean, Wilson, Perkeybile, Yee, Ferris, Nazarloo, Porges, Davis, Connelly, Kingsbury.
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of National Institutes of Health [Grants F32-HD092051, R21-HD095217, R01-HD098117, and P01-HD075750]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Acher R, Chauvet J, Chauvet MT. (1995) Man and the chimaera. Selective versus neutral oxytocin evolution. Adv Exp Med Biol 395:615–627. [PubMed] [Google Scholar]
- Aguilera G, Subburaju S, Young S, Chen J. (2008) The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res 170:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. (2015) Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36:49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh AM, Heydari Z, Rahimi M, Bazgir B, Shirvani H, Alipour S, Heidarian Y, Khalighfard S, Isanejad A. (2018) Oxytocin mediates the beneficial effects of the exercise training on breast cancer. Exp Physiol 103:222–235. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C. (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Khoei H, Mohammadi-Asl A, Amiri S, Hosseini MJ, Momeny M, Hassanipour M, Rastegar M, Haj-Mirzaian A, Mirzaian AH, Sanjarimoghaddam H, et al. (2017) Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog Neuropsychopharmacol Biol Psychiatry 76:169–178. [DOI] [PubMed] [Google Scholar]
- Andari E, Nishitani S, Kaundinya G, Caceres GA, Morrier MJ, Ousley O, Smith AK, Cubells JF, Young LJ. (2020) Epigenetic modification of the oxytocin receptor gene: implications for autism symptom severity and brain functional connectivity. Neuropsychopharmacology 45:1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. (2013) Impact of maternal depression across the first 6 years of life on the child’s mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry 170:1161–1168. [DOI] [PubMed] [Google Scholar]
- Arrowsmith S, Wray S. (2014) Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol 26:356–369. [DOI] [PubMed] [Google Scholar]
- Aulinas A, Plessow F, Pulumo RL, Asanza E, Mancuso CJ, Slattery M, Tolley C, Thomas JJ, Eddy KT, Miller KK, et al. (2019) Disrupted oxytocin-appetite signaling in females with anorexia nervosa. J Clin Endocrinol Metab 104:4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanti C, Hinrichs WLJ, Casini A, Eissens AC, Van Dam A, Kedrov A, Driessen AJM, Frijlink HW, Permentier HP. (2013) The formation of oxytocin dimers is suppressed by the zinc-aspartate-oxytocin complex. J Pharm Sci 102:1734–1741. [DOI] [PubMed] [Google Scholar]
- Baker M, Lindell SG, Driscoll CA, Zhou Z, Yuan Q, Schwandt ML, Miller-Crews I, Simpson EA, Paukner A, Ferrari PF, et al. (2017) Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc Natl Acad Sci USA 114:11769–11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. (2007) Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. (2018) The human hypothalamus in mood disorders: the HPA axis in the center. IBRO Rep 6:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabi F, Cechetto DF. (2001) Neurotransmitters in the thalamus relaying visceral input to the insular cortex in the rat. Am J Physiol Regul Integr Comp Physiol 281:R1665–R1674. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. (2010) Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci USA 107:21371–21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S. (1986) Effect of oxytocin on calcium influx and efflux in the rat myometrium. Eur J Pharmacol 120:57–61. [DOI] [PubMed] [Google Scholar]
- Bell AF, Carter CS, Steer CD, Golding J, Davis JM, Steffen AD, Rubin LH, Lillard TS, Gregory SP, Harris JC, et al. (2015) Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front Genet 6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. (2018) Oxytocin and vasopressin, and the GABA developmental shift during labor and birth: friends or foes? Front Cell Neurosci 12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaerts S, Prinsen J, Berra E, Bosmans G, Steyaert J, Alaerts K. (2017) Long-term oxytocin administration enhances the experience of attachment. Psychoneuroendocrinology 78:1–9. [DOI] [PubMed] [Google Scholar]
- Bernhard A, van der Merwe C, Ackermann K, Martinelli A, Neumann ID, Freitag CM. (2018) Adolescent oxytocin response to stress and its behavioral and endocrine correlates. Horm Behav 105:157–165. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. (2014) The oxytocin paradox. Front Behav Neurosci 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Klein SL, DeVries AC, Nelson RJ. (1999) Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiol Behav 68:151–156. [DOI] [PubMed] [Google Scholar]
- Bocheva A, Dzambazova-Maximova E. (2004) Antiopioid properties of the TYR-MIF-1 family. Methods Find Exp Clin Pharmacol 26:673–677. [DOI] [PubMed] [Google Scholar]
- Bordt EA, Smith CJ, Demarest TG, Bilbo SD, Kingsbury MA. (2019) Mitochondria, oxytocin, and vasopressin: unfolding the inflammatory protein response. Neurotox Res 36:239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Rilling JK, Frantz KJ, Albers HE. (2019) Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology 44:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ. (2018) Oxytocin and social relationships: from attachment to bond disruption. Curr Top Behav Neurosci 35:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. (2011) Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One 6:e27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, Leknes S, Lundanes E, Wilson SR. (2016) Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep 6:31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. (2016) On the role of oxytocin in borderline personality disorder. Br J Clin Psychol 55:287–304. [DOI] [PubMed] [Google Scholar]
- Buemann B, Uvnäs-Moberg K. (2020) Oxytocin may have a therapeutical potential against cardiovascular disease. Possible pharmaceutical and behavioral approaches. Med Hypotheses 138:109597. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. (2016) Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165:1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman-Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, Tops M. (2014) Individual differences underlying susceptibility to addiction: role for the endogenous oxytocin system. Pharmacol Biochem Behav 119:22–38. [DOI] [PubMed] [Google Scholar]
- Bunck M, Czibere L, Horvath C, Graf C, Frank E, Kessler MS, Murgatroyd C, Müller-Myhsok B, Gonik M, Weber P, et al. (2009) A hypomorphic vasopressin allele prevents anxiety-related behavior. PLoS One 4:e5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP, Bohus B, Kovács GL, Van Nispen JW, Greven HM, De Wied D. (1983) Oxytocin is a precursor of potent behaviourally active neuropeptides. Eur J Pharmacol 94:125–131. [DOI] [PubMed] [Google Scholar]
- Burbach JP, De Kloet ER, De Wied D. (1980) Oxytocin biotransformation in the rat limbic brain: characterization of peptidase activities and significance in the formation of oxytocin fragments. Brain Res 202:401–414. [DOI] [PubMed] [Google Scholar]
- Burns P, Bowditch J, McFadyen J, Loiacono R, Albiston AL, Pham V, Chai SY. (2019) Social behaviour is altered in the insulin-regulated aminopeptidase knockout mouse. Behav Brain Res 376:112150. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Chini B. (2018) Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr Top Behav Neurosci 35:3–29. [DOI] [PubMed] [Google Scholar]
- Caldwell HK. (2017) Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist 23:517–528. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. (2016) Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Curr Top Behav Neurosci 27:51–103. [DOI] [PubMed] [Google Scholar]
- Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, Jackson LP, Sumiyoshi RD, Howerton CL, Hannah SL, et al. (2015) Arginine vasopressin is a blood-based biomarker of social functioning in children with autism. PLoS One 10:e0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. (1998) Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23:779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS. (2003) Developmental consequences of oxytocin. Physiol Behav 79:383–397. [DOI] [PubMed] [Google Scholar]
- Carter CS. (2007) Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res 176:170–186. [DOI] [PubMed] [Google Scholar]
- Carter CS. (2014) Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol 65:17–39. [DOI] [PubMed] [Google Scholar]
- Carter CS. (2017) The oxytocin-vasopressin pathway in the context of love and fear. Front Endocrinol (Lausanne) 8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Altemus M. (1997) Integrative functions of lactational hormones in social behavior and stress management. Ann N Y Acad Sci 807:164–174. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. (2009) Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci 31:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Perkeybile AM. (2018) The monogamy paradox: what do love and sex have to do with it? Front Ecol Evol 6:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. (2007) Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci 1098:312–322. [DOI] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Mosley M, George AJ, Mussaji LF, Fullerton EF, Ruszkowski EM, Jacobs AJ, Gewirtz AT, Chassaing B, Forger NG. (2018) The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav Immun 67:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceanga M, Spataru A, Zagrean AM. (2010) Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neurosci Lett 477:15–18. [DOI] [PubMed] [Google Scholar]
- Cetinel S, Hancioğlu S, Sener E, Uner C, Kiliç M, Sener G, Yeğen BC. (2010) Oxytocin treatment alleviates stress-aggravated colitis by a receptor-dependent mechanism. Regul Pept 160:146–152. [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Potvin O, Hudon C, Préville M. (2015) DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. Front Genet 6:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Verhage M, Grinevich V. (2017) The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol Sci 38:982–991. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. (1999) The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci 113:1071–1079. [DOI] [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. (2008) Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab 295:E686–E691. [DOI] [PubMed] [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. (2012) The application of ecological theory toward an understanding of the human microbiome. Science 336:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EQ, Stuebe A, Pearson B, Grewen K, Rubinow D, Meltzer-Brody S. (2015) Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology 55:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo MG, Szeto A, Schrepf A, Kinner EM, Schachner BI, Ahmed R, Thaker PH, Goodheart M, Bender D, Cole SW, et al. (2019) Oxytocin in the tumor microenvironment is associated with lower inflammation and longer survival in advanced epithelial ovarian cancer patients. Psychoneuroendocrinology 106:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. (2008) Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med 70:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Carter CS, Ying J, Bellugi U, Pournajafi-Nazarloo H, Korenberg JR. (2012) Oxytocin and vasopressin are dysregulated in Williams Syndrome, a genetic disorder affecting social behavior. PLoS One 7:e38513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH. (1906) On some physiological actions of ergot. J Physiol 34:163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. (1998) Vasopressin, gonadal steroids and social recognition. Prog Brain Res 119:409–414. [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2018) Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 98:477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. (2019) Can immunopsychiatry help in understanding the basis of sex differences in major depressive disorder? Biol Psychiatry Cogn Neurosci Neuroimaging 4:606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Malavasi F. (2006) The CD38/CD157 mammalian gene family: an evolutionary paradigm for other leukocyte surface enzymes. Purinergic Signal 2:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. (2012) Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav 61:419–428. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. (1996) The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci USA 93:11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Forger NG. (2015) Sex differences in the brain: a whole body perspective. Biol Sex Differ 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. (2006) Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D, Elands J, Kovács G. (1991) Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor? Proc Natl Acad Sci USA 88:1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. (2017) Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol 525:2549–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G. (2015) Autism: oxytocin, serotonin, and social reward. Soc Neurosci 10:450–465. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. (2010) Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35:83–93. [DOI] [PubMed] [Google Scholar]
- du Vigneaud V, Gish DT, Katsoyannis PG, Hess GP. (1958) Synthesis of the pressor-antidiuretic hormone, arginine vasopressin. J Am Chem Soc 80:3355–3358. [Google Scholar]
- du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG. (1954) The synthesis of oxytocin. J Am Chem Soc 76:3115–3121. [Google Scholar]
- Duchemin A, Seelke AM, Simmons TC, Freeman SM, Bales KL. (2017) Localization of oxytocin receptors in the prairie vole (Microtus ochrogaster) neocortex. Neuroscience 348:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. (2016) Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol 40:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Lin T, Muradoglu M, Weir DH, Plasencia GM, Lillard TS, Pournajafi-Nazarloo H, Cohen RA, Sue Carter C, Connelly JJ. (2019) Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int J Psychophysiol 136:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrensing RH. (2015) An extraordinary relationship involving MIF-1 and other peptides. Peptides 72:73–74. [DOI] [PubMed] [Google Scholar]
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. (1990) The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept 28:199–214. [DOI] [PubMed] [Google Scholar]
- Engel S, Klusmann H, Laufer S, Pfeifer AC, Ditzen B, van Zuiden M, Knaevelsrud C, Schumacher S. (2019) Trauma exposure, posttraumatic stress disorder and oxytocin: a meta-analytic investigation of endogenous concentrations and receptor genotype. Neurosci Biobehav Rev 107:560–601. [DOI] [PubMed] [Google Scholar]
- Engert V, Koester AM, Riepenhausen A, Singer T. (2016) Boosting recovery rather than buffering reactivity: higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology 74:111–120. [DOI] [PubMed] [Google Scholar]
- Erbas O, Taşkıran D, Oltulu F, Yavaşoğlu A, Bora S, Bilge O, Çınar BP, Peker G. (2017) Oxytocin provides protection against diabetic polyneuropathy in rats. Neurol Res 39:45–53. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T. (2016) Microbes and oxytocin: benefits for host physiology and behavior. Int Rev Neurobiol 131:91–126. [DOI] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. (2016) Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry 79:174–184. [DOI] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. (2015) Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav 9:754–764. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Yee JR, Kenkel WM, Dumais KM, Moore K, Veenema AH, Kulkarni P, Perkybile AM, Carter CS. (2015) Distinct BOLD activation profiles following central and peripheral oxytocin administration in awake rats. Front Behav Neurosci 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi S, Vignozzi L, Vannelli GB, Ledda F, Forti G, Maggi M. (2003) Role of oxytocin in the ejaculatory process. J Endocrinol Invest 26 (Suppl):82–86. [PubMed] [Google Scholar]
- Franke AA, Li X, Menden A, Lee MR, Lai JF. (2019) Oxytocin analysis from human serum, urine, and saliva by orbitrap liquid chromatography-mass spectrometry. Drug Test Anal 11:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch A, Zetzsche T, Steiger A, Jirikowski GF. (1995) Reduction of plasma oxytocin levels in patients suffering from major depression. Adv Experim Med Biol 395:257–258. [PubMed] [Google Scholar]
- Freeman SM, Palumbo MC, Lawrence RH, Smith AL, Goodman MM, Bales KL. (2018) Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Transl Psychiatry 8:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. (2016) Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J Neuroendocrinol 28:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H. (2012) Cell-type specific expression of oxytocin and vasopressin genes: an experimental odyssey. J Neuroendocrinol 24:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galina ZH, Kastin AJ. (1986) Existence of antiopiate systems as illustrated by MIF-1/Tyr-MIF-1. Life Sci 39:2153–2159. [DOI] [PubMed] [Google Scholar]
- Gamliel M, Anderson KL, Ebstein RP, Yirmiya N, Mankuta D. (2016) The oxytocin-CD38-vitamin A axis in pregnant women involves both hypothalamic and placental regulation. J Matern Fetal Neonatal Med 29:2685–2690. [DOI] [PubMed] [Google Scholar]
- Garfield L, Giurgescu C, Carter CS, Holditch-Davis D, McFarlin BL, Schwertz D, Seng JS, White-Traut R. (2015) Depressive symptoms in the second trimester relate to low oxytocin levels in African-American women: a pilot study. Arch Womens Ment Health 18:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. (2012) Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science 338:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, Mendelowitz D, Kay MW. (2017) Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc Res 113:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen V. (2006) Thymus-dependent T cell tolerance of neuroendocrine functions: principles, reflections, and implications for tolerogenic/negative self-vaccination. Ann N Y Acad Sci 1088:284–296. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. (1986) Vasopressin and oxytocin: hypothalamic modulators of the stress response: a review. Psychoneuroendocrinology 11:131–139. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683. [DOI] [PubMed] [Google Scholar]
- Gobrogge K, Wang Z. (2016) The ties that bond: neurochemistry of attachment in voles. Curr Opin Neurobiol 38:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Jia X, Liu Y, Wang Z. (2017) Neurochemical mediation of affiliation and aggression associated with pair-bonding. Biol Psychiatry 81:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop JG, de Winter RF, Wolterbeek R, van Kempen GM, Wiegant VM. (2011) Evidence of vasopressinergic-noradrenergic mechanisms in depression with above-normal plasma vasopressin concentration with and without psychotic features. J Psychopharmacol 25:345–352. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. (2011) Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 216:101–110. [DOI] [PubMed] [Google Scholar]
- Goodson JL. (2013) Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 38:465–478. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. (2006) Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav 50:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA. (2012) Evolving nonapeptide mechanisms of gregariousness and social diversity in birds. Horm Behav 61:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Kingsbury MA. (2015) Oxytocin mechanisms of stress response and aggression in a territorial finch. Physiol Behav 141:154–163. [DOI] [PubMed] [Google Scholar]
- Gottlieb MM. (2016) Pitocin and autism: an analysis of oxytocin receptor desensitization in the fetus. Behav Brain Res 298 (Pt B):246–247. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. (2010) Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 35:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. (2001) Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry 50:609–613. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, et al. (2009) Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Light KC. (2011) Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol Psychol 87:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. (2016) Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol Psychiatry 79:155–164. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, Trahanas DM, McNeal N, Clarke DA, Porges SW, Sue Carter C. (2012) Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress 15:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. (2009) Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34:1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross Margolis K, Vittorio J, Talavera M, Gluck K, Li Z, Iuga A, Stevanovic K, Saurman V, Israelyan N, Welch MG, et al. (2017) Enteric serotonin and oxytocin: endogenous regulation of severity in a murine model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 313:G386–G398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald R, Seelke AM, Perkeybile AM, Cooke DF, Bales KL, Krubitzer L. (2012) Effects of Early Parenting Experience on Cortical Connections in Prairie Voles (Microtus Ochrogaster), Society for Neuroscience, New Orleans, LA. [Google Scholar]
- Grzesiak M, Gaj Z, Kocyłowski R, Suliburska J, Oszukowski P, Horzelski W, von Kaisenberg C, Banach M. (2018) Oxidative stress in women treated with atosiban for impending preterm birth. Oxid Med Cell Longev 2018:3919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver D, Werry E, Reekie TA, Katte TA, Jorgensen W, Kassiou M. (2019) Targeting the oxytocin system: new pharmacotherapeutic approaches. Trends Pharmacol Sci 40:22–37. [DOI] [PubMed] [Google Scholar]
- Gust K, Caccese C, Larosa A, Nguyen TV. (2020) Neuroendocrine effects of lactation and hormone-gene-environment interactions. Mol Neurobiol 57:2074–2084. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. (2012) Oxytocin revisited: its role in cardiovascular regulation. J Neuroendocrinol 24:599–608. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, Antunes-Rodrigues J. (2014) The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res 47:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA. (2015) Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40:24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. (2005) Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308:1630–1634. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018) A neural circuit for gut-induced reward. Cell 175:665–678.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. (1984) Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides 5 (Suppl 1):121–138. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Wang YF. (2008) Neural mechanisms underlying the milk ejection burst and reflex. Prog Brain Res 170:155–166. [DOI] [PubMed] [Google Scholar]
- Hayes EJ, Weinstein L. (2008) Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol 198:622.e1-622.e7. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. (2009) Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry 14:954–958. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. (2012) Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res 45:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández VS, Hernández OR, Perez de la Mora M, Gómora MJ, Fuxe K, Eiden LE, Zhang L. (2016) Hypothalamic vasopressinergic projections innervate central amygdala gabaergic neurons: implications for anxiety and stress coping. Front Neural Circuits 10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. [DOI] [PubMed] [Google Scholar]
- Hetz C, Martinon F, Rodriguez D, Glimcher LH. (2011) The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev 91:1219–1243. [DOI] [PubMed] [Google Scholar]
- Hicks C, Ramos L, Reekie TA, Narlawar R, Kassiou M, McGregor IS. (2015) WAY 267,464, a non-peptide oxytocin receptor agonist, impairs social recognition memory in rats through a vasopressin 1A receptor antagonist action. Psychopharmacology (Berl) 232:2659–2667. [DOI] [PubMed] [Google Scholar]
- Higashida H, Furuhara K, Yamauchi AM, Deguchi K, Harashima A, Munesue S, Lopatina O, Gerasimenko M, Salmina AB, Zhang JS, et al. (2017) Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Sci Rep 7:7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Kikuchi M, Munesue T. (2012) CD38 and its role in oxytocin secretion and social behavior. Horm Behav 61:351–358. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yuhi T, Akther S, Amina S, Zhong J, Liang M, Nishimura T, Liu HX, Lopatina O. (2018) Oxytocin release via activation of TRPM2 and CD38 in the hypothalamus during hyperthermia in mice: implication for autism spectrum disorder. Neurochem Int 119:42–48. [DOI] [PubMed] [Google Scholar]
- Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC. (2016) Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res 18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham W, Light KC. (2011) The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 36:1249–1256. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, et al. (2014) Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39:1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Grinevich V. (2018) Behavioral Pharmacology of Neuropeptides: Oxytocin, Springer, Cham, Switzerland. [Google Scholar]
- Işeri SO, Gedik IE, Erzik C, Uslu B, Arbak S, Gedik N, Yeğen BC. (2008) Oxytocin ameliorates skin damage and oxidant gastric injury in rats with thermal trauma. Burns 34:361–369. [DOI] [PubMed] [Google Scholar]
- Jack A, Connelly JJ, Morris JP. (2012) DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci 6:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr. (2007) Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett 417:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M, Broderick TL, Gutkowska J. (2016) Oxytocin and cardioprotection in diabetes and obesity. BMC Endocr Disord 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Platt ML. (2018) Oxytocin and vasopressin increase male-directed threats and vocalizations in female macaques. Sci Rep 8:18011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, et al. (2007) CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446:41–45. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Ochs SD, Caldwell JD. (2018) Oxytocin and steroid actions. Curr Top Behav Neurosci 35:77–95. [DOI] [PubMed] [Google Scholar]
- Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy JL, Sokolowski M, Meaney MJ, Fleming AS, Steiner M, MAVAN Research Team (2013) Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav 12:681–694. [DOI] [PubMed] [Google Scholar]
- Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, Jurek B, Bosch OJ, Hellhammer J, Neumann ID. (2015) Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 62:381–388. [DOI] [PubMed] [Google Scholar]
- Jurek B, Neumann ID. (2018) The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev 98:1805–1908. [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. (2013) Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol 25:668–673. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Pappas C, Tajiri N, Borlongan CV. (2016) Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci Rep 6:35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, DeVries AC. (2011) Modeling social influences on human health. Psychosom Med 73:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, DeVries AC. (2011) Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke 42:3606–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. (2014) Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol 35:512–529. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Kuhnert RL, Griffiths K, Hickie IB, Guastella AJ. (2012) Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One 7:e44014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. (1987) Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology 46:56–61. [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Lewis GF, Yee JR, Pournajafi-Nazarloo H, Grippo AJ, Porges SW, Carter CS. (2013) Autonomic substrates of the response to pups in male prairie voles. PLoS One 8:e69965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, Yee JR, Pournajafi-Nazarloo H, Lillard TS, Ferguson EF, Wroblewski KL, Ferris CF, Carter CS, Connelly JJ. (2019) Behavioral and epigenetic consequences of oxytocin treatment at birth. Sci Adv 5:eaav2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Yee JR, Carter CS. (2014) Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development. J Neuroendocrinol 26:739–749. [DOI] [PubMed] [Google Scholar]
- Khan RS, Yu C, Kastin AJ, He Y, Ehrensing RH, Hsuchou H, Stone KP, Pan W. (2010) Brain activation by peptide Pro-Leu-Gly-NH(2) (MIF-1). Int J Pept 2010:537639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Tyzio R, Ben-Ari Y. (2008) Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog Brain Res 170:243–257. [DOI] [PubMed] [Google Scholar]
- Kheterpal I, Kastin AJ, Mollah S, Yu C, Hsuchou H, Pan W. (2009) Mass spectrometric quantification of MIF-1 in mouse brain by multiple reaction monitoring. Peptides 30:1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. (2016) Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol Psychiatry 80:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Bilbo SD. (2019) The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Front Neuroendocrinol 55:100794. [DOI] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Anwar M, Ludwig RJ, Kaidbey JH, Glickstein SB, Welch MG. (2018) Assessing cellular stress and inflammation in discrete oxytocin-secreting brain nuclei in the neonatal rat before and after first colostrum feeding. J Vis Exp 141:e58341. [DOI] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Hirschberg DL, Glickstein SB, Ludwig RJ, Welch MG. (2014) Oxytocin modulates markers of the unfolded protein response in Caco2BB gut cells. Cell Stress Chaperones 19:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Hirschberg DL, Glickstein SB, Welch MG. (2013) Oxytocin modulates mTORC1 pathway in the gut. Biochem Biophys Res Commun 432:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Hirschberg DL, Ludwig RJ, Glickstein SB, Myers MM, Welch MG. (2016) Oxytocin opposes effects of bacterial endotoxin on ER-stress signaling in Caco2BB gut cells. Biochim Biophys Acta 1860:402–411. [DOI] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Ludwig RJ, Glickstein SB, Welch MG, Anwar M. (2017) Colostrum oxytocin modulates cellular stress response, inflammation, and autophagy markers in newborn rat gut villi. Biochem Biophys Res Commun 487:47–53. [DOI] [PubMed] [Google Scholar]
- Klein BY, Tamir H, Welch MG. (2011) PI3K/Akt responses to oxytocin stimulation in Caco2BB gut cells. J Cell Biochem 112:3216–3226. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. (2014) Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. (2005) Oxytocin increases trust in humans. Nature 435:673–676. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Bohus B, Versteeg DH, de Kloet ER, de Wied D. (1979) Effect of oxytocin and vasopressin on memory consolidation: sites of action and catecholaminergic correlates after local microinjection into limbic-midbrain structures. Brain Res 175:303–314. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. (2004) Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can J Zool 82:1194–1200. [Google Scholar]
- Krol KM, Moulder RG, Lillard TS, Grossmann T, Connelly JJ. (2019a) Epigenetic dynamics in infancy and the impact of maternal engagement. Sci Adv 5:eaay0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol KM, Puglia MH, Morris JP, Connelly JJ, Grossmann T. (2019b) Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev Cogn Neurosci 37:100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll-Desrosiers AR, Nephew BC, Babb JA, Guilarte-Walker Y, Moore Simas TA, Deligiannidis KM. (2017) Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year. Depress Anxiety 34:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, Azuma C, Murata Y. (2001) DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun 289:681–686. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Slotkin TA. (1986) The “stress” of being born. Sci Am 254:100–107. [DOI] [PubMed] [Google Scholar]
- Lai X, Wu X, Hou N, Liu S, Li Q, Yang T, Miao J, Dong Z, Chen J, Li T. (2018) Vitamin A deficiency induces autistic-like behaviors in rats by regulating the RARβ-CD38-oxytocin axis in the hypothalamus. Mol Nutr Food Res 62:1–10. [DOI] [PubMed] [Google Scholar]
- Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, Davis JM, Morris JP, Connelly JJ. (2015) Plasma oxytocin explains individual differences in neural substrates of social perception. Front Hum Neurosci 9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K, Goldbeck L, Pournajafi-Nazarloo H, Connelly JJ, Carter CS, Morris JP. (2018a) The role of endogenous oxytocin in anxiolysis: structural and functional correlates. Biol Psychiatry Cogn Neurosci Neuroimaging 3:618–625. [DOI] [PubMed] [Google Scholar]
- Lancaster K, Goldbeck L, Puglia MH, Morris JP, Connelly JJ. (2018b) DNA methylation of OXTR is associated with parasympathetic nervous system activity and amygdala morphology. Soc Cogn Affect Neurosci 13:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25:150–176. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil JWE, Ganten D, Hermann K, Unger T, Rascher W. (1983) Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 37:314–316. [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB. (2018) Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry 23:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Shnitko TA, Blue SW, Kaucher AV, Winchell AJ, Erikson DW, Grant KA, Leggio L. (2020) Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat Commun 11:2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. (2016) Intranasal oxytocin: myths and delusions. Biol Psychiatry 79:243–250. [DOI] [PubMed] [Google Scholar]
- Leng G, Russell JA. (2019) The osmoresponsiveness of oxytocin and vasopressin neurones: mechanisms, allostasis and evolution. J Neuroendocrinol 31:e12662. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, Nurit Y, Ebstein RP. (2010) Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res 3:293–302. [DOI] [PubMed] [Google Scholar]
- Lerman B, Harricharran T, Ogunwobi OO. (2018) Oxytocin and cancer: an emerging link. World J Clin Oncol 9:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang B, Cao H, Liu W, Guo F, Shen F, Ye B, Liu H, Li Y, Liu Z. (2020) Oxytocin Exerts Antidepressant-like effect by potentiating dopaminergic synaptic transmission in the mPFC. Neuropharmacology 162:107836. [DOI] [PubMed] [Google Scholar]
- Li T, Chen X, Mascaro J, Haroon E, Rilling JK. (2017a) Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Horm Behav 93:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang P, Wang SC, Wang YF. (2017b) Approaches mediating oxytocin regulation of the immune system. Front Immunol 7:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Young EA. (1997) Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 22:411–422. [DOI] [PubMed] [Google Scholar]
- Lockard MA, Ebert MS, Bargmann CI. (2017) Oxytocin mediated behavior in invertebrates: an evolutionary perspective. Dev Neurobiol 77:128–142. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Feifel D. (2013) Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald KS. (2013) Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Front Neurosci 6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K. (1911) An experimental investigation of the mechanism of milk secretion, with special reference to the action of animal extracts. Q J Exp Physiol 4:305–330. [Google Scholar]
- MacLean EL, Gesquiere LR, Gee NR, Levy K, Martin WL, Carter CS. (2017a) Effects of affiliative human-animal interaction on dog salivary and plasma oxytocin and vasopressin. Front Psychol 8:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Gesquiere LR, Gee N, Levy K, Martin WL, Carter CS. (2018) Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J Neurosci Methods 293:67–76. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Gesquiere LR, Gruen ME, Sherman BL, Martin WL, Carter CS. (2017b) Endogenous oxytocin, vasopressin, and aggression in domestic dogs. Front Psychol 8:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. (2019) Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology 107:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse J, Zinni M, Pansiot J, Hassan-Abdi R, Demene C, Colella M, Charriaut-Marlangue C, Rideau Batista Novais A, Tanter M, Maccari S, et al. (2019) Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 67:345–359. [DOI] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88:841–886. [DOI] [PubMed] [Google Scholar]
- Mann A, Verma V, Basu D, Skoblenick KJ, Beyaert MGR, Fisher A, Thomas N, Johnson RL, Mishra RK. (2010) Specific binding of photoaffinity-labeling peptidomimetics of Pro-Leu-Gly-NH2 to the dopamine D2L receptor: evidence for the allosteric modulation of the dopamine receptor. Eur J Pharmacol 641:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. (2008) Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res 170:473–512. [DOI] [PubMed] [Google Scholar]
- Marchini G, Lagercrantz H, Winberg J, Uvnäs-Moberg K. (1988) Fetal and maternal plasma levels of gastrin, somatostatin and oxytocin after vaginal delivery and elective cesarean section. Early Hum Dev 18:73–79. [DOI] [PubMed] [Google Scholar]
- Maron JL, Johnson KL, Parkin C, Iyer L, Davis JM, Bianchi DW. (2010) Cord blood genomic analysis highlights the role of redox balance. Free Radic Biol Med 49:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins DA, Mazibuko N, Zelaya F, Vasilakopoulou S, Loveridge J, Oates A, Maltezos S, Mehta M, Wastling S, Howard M, et al. (2020) Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat Commun 11:1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey N, Puttachary S, Mahadev-Bhat S, Kanthasamy AG, Charavaryamath C. (2019) HMGB1-RAGE signaling plays a role in organic dust-induced microglial activation and neuroinflammation. Toxicol Sci 169:579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, Carter CS. (2016) Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. Arch Women Ment Health 19:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM. (2017) Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci 18:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ. (2013) Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev 37:1485–1492. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Landino SM, Vahabzadeh A, O’Rourke J, Zurcher NR, Finger BC, Palumbo ML, Helt J, Mullett JE, Hooker JM, et al. (2015) Toward an immune-mediated subtype of autism spectrum disorder. Brain Res 1617:72–92. [DOI] [PubMed] [Google Scholar]
- Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, Ogino T, Fujii Y, Manabe S, Eto M, Nonaka M, et al. (2018) Neuropeptide oxytocin enhances μ opioid receptor signaling as a positive allosteric modulator. J Pharmacol Sci 137:67–75. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS. (2013) Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One 8:e61443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Teng BL, Nikolova VD, Riddick NV, Simpson CD, Van Deusen A, Janzen WP, Sassano MF, Pedersen CA, Jarstfer MB. (2019) Prosocial effects of an oxytocin metabolite, but not synthetic oxytocin receptor agonists, in a mouse model of autism. Neuropharmacology 144:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Hicks-Nelson A, Fink A, Beamer G, Gurel K, Elnady F, Pittet F, Nephew BC. (2016) Effects of chronic social stress and maternal intranasal oxytocin and vasopressin on offspring interferon-γ and behavior. Front Endocrinol (Lausanne) 7:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. (2013) Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan Y, Demirkhanyan L, Cabezas-Bratesco D, Oakes V, Kusuda R, Dawson T, Sun X, Cao C, Cohen AM, Chelluboina B, et al. (2017) Oxytocin modulates nociception as an agonist of pain-sensing TRPV1. Cell Rep 21:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. (2016) Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry 79:213–221. [DOI] [PubMed] [Google Scholar]
- Nissen E, Lilja G, Widström AM, Uvnäs-Moberg K. (1995) Elevation of oxytocin levels early post partum in women. Acta Obstet Gynecol Scand 74:530–533. [DOI] [PubMed] [Google Scholar]
- Ocampo Daza D, Lewicka M, Larhammar D. (2012) The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, inclucing two distinct V2 subtypes. Gen Comp Endocrinol 175:135–143. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. (2015) Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350:1371–1374. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Chen IC, Dehghani Z, Zheng DJ, Ikpatt JE, Momoh H, Phelps SM. (2018) Genetic variation in the developmental regulation of cortical avpr1a among prairie voles. Genes Brain Behav 17:36–48. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Maguire SM, Phelps SM. (2017) Methylation of avpr1a in the cortex of wild prairie voles: effects of CpG position and polymorphism. R Soc Open Sci 4:160646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG. (2017) Navigating monogamy: nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Front Neurosci 11:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppong-Damoah A, Zaman RU, D’Souza MJ, Murnane KS. (2019) Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm Behav 108:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Esel E, Kula M. (2009) Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res 169:249–252. [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Constantino JN, Parker KJ. (2020) Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc Natl Acad Sci USA 117:10609–10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, Thurm A, Swedo SE, Parker KJ. (2018a) Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann Neurol 84:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Jackson LP, Libove RA, Sumiyoshi RD, Phillips JM, Garner JP, Hardan AY, Parker KJ. (2018b) Biomarker discovery for disease status and symptom severity in children with autism. Psychoneuroendocrinology 89:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. (2010) Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res 178:359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, et al. (2017) Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci USA 114:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr. (1979) Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA 76:6661–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, Karaoli T, Gregory SG, Mohammadi N, Epstein L, et al. (2019) Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Burbach H, Adan RA, Lolait SJ, van Leeuwen FW, Mezey E, Palkovits M, Barberis C. (1995) Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cell Mol Neurobiol 15:573–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Ansermet F, Borghini A, Halfon O. (2012) Adult attachment representations predict cortisol and oxytocin responses to stress. Attach Hum Dev 14:453–476. [DOI] [PubMed] [Google Scholar]
- Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, Ebner NC. (2019) Plasma oxytocin and vasopressin levels in young and older men and women: functional relationships with attachment and cognition. Psychoneuroendocrinology 110:104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested CP, Bernal AL. (2001) Desensitisation of the oxytocin receptor and other G-protein coupled receptors in the human myometrium. Exp Physiol 86:303–312. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Grinevich V, Charlet A. (2018) Oxytocin signaling in pain: cellular, circuit, system, and behavioral levels. Curr Top Behav Neurosci 35:193–211. [DOI] [PubMed] [Google Scholar]
- Porges SW. (1998) Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology 23:837–861. [DOI] [PubMed] [Google Scholar]
- Porges SW. (2009) The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 76 (Suppl 2):S86–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. (2012) The polyvagal theory: neurophysiological foundations of emotions, attachment, communication, self-regulation, J Can Acad Child Adolesc Psychiatry 21, pp 313–314. [Google Scholar]
- Porges SW, Furman SA. (2011) The early development of the autonomic nervous system provides a neural platform for social behavior: a polyvagal perspective. Infant Child Dev 20:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Yee J, Stevenson J, Sanzenbacher L, Kenkel W, Mohsenpour SR, Hashimoto K, Carter CS. (2011) Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology 36:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. (2013) Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One 8:e78898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Burris D, Cloutier A, Thompson CB, Rilling JK, Thompson RR. (2017) Dose-dependent and lasting influences of intranasal vasopressin on face processing in men. Front Endocrinol (Lausanne) 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Connelly JJ, Morris JP. (2018) Epigenetic regulation of the oxytocin receptor is associated with neural response during selective social attention. Transl Psychiatry 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. (2015) Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci USA 112:3308–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJG, Hofman MA, Swaab DF. (1996) Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 53:137–143. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Dieset I, Elvsåshagen T, Westlye LT, Andreassen OA. (2017) Oxytocin system dysfunction as a common mechanism underlying metabolic syndrome and psychiatric symptoms in schizophrenia and bipolar disorders. Front Neuroendocrinol 45:1–10. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ. (2020) An allostatic theory of oxytocin. Trends Cogn Sci 24:515–528. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Kemp AH, Alvares GA, Guastella AJ. (2013) A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front Neurosci 7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Córdova-Palomera A, Dieset I, Andreassen OA, Westlye LT. (2019) Oxytocin pathway gene networks in the human brain. Nat Commun 10:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Smerud KT, Mahmoud RA, Andreassen OA, Djupesland PG. (2018) Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm Behav 102:85–92. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Goodwillie A, Brewer C, Muglia LJ, Pfaff DW, Kow LM. (2004) Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology 80:92–99. [DOI] [PubMed] [Google Scholar]
- Reiss AB, Glass DS, Lam E, Glass AD, De Leon J, Kasselman LJ. (2019) Oxytocin: potential to mitigate cardiovascular risk. Peptides 117:170089. [DOI] [PubMed] [Google Scholar]
- Reyes-Lagos JJ, Ledesma-Ramírez CI, Pliego-Carrillo AC, Peña-Castillo MA, Echeverría JC, Becerril-Villanueva E, Pavón L, Pacheco-López G. (2019) Neuroautonomic activity evidences parturition as a complex and integrated neuro-immune-endocrine process. Ann N Y Acad Sci 1437:22–30. [DOI] [PubMed] [Google Scholar]
- Robinson C, Schumann R, Zhang P, Young RC. (2003) Oxytocin-induced desensitization of the oxytocin receptor. Am J Obstet Gynecol 188:497–502. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. (2009) Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA 106:21437–21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Christianson JP. (2019) An insular view of the social decision-making network. Neurosci Biobehav Rev 103:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, Christianson JP. (2018) Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci 21:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondo F, Butz H, Syro LV, Yousef GM, Di Ieva A, Restrepo LM, Quintanar-Stephano A, Berczi I, Kovacs K. (2016) Arginine vasopressin (AVP): a review of its historical perspectives, current research and multifunctional role in the hypothalamo-hypophysial system. Pituitary 19:345–355. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, et al. (2014) Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull 40:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Li S, Yao L, Keedy SK, Reilly JL, Hill SK, Bishop JR, Sue Carter C, Pournajafi-Nazarloo H, Drogos LL, et al. (2018) Peripheral oxytocin and vasopressin modulates regional brain activity differently in men and women with schizophrenia. Schizophr Res 202:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saameli K. (1963) An indirect method for the estimation of oxytocin blood concentration and half-life in pregnant women near term. Am J Obstet Gynecol 85:186–192. [DOI] [PubMed] [Google Scholar]
- Saníger MA, Ramírez-Expósito MJ, de la Chica S, Carrera-González MP, Mayas MD, Manuel Martínez-Martos J. (2011) Alpha-1-adrenergic receptor blockade modifies insulin-regulated aminopeptidase (IRAP) activity in rat prostate and modulates oxytocin functions. Drug Metab Lett 5:192–196. [DOI] [PubMed] [Google Scholar]
- Sannino S, Chini B, Grinevich V. (2017) Lifespan oxytocin signaling: maturation, flexibility, and stability in newborn, adolescent, and aged brain. Dev Neurobiol 77:158–168. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. (1985) Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc 44:221–227. [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M, Legros JJ. (2007) Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 32:407–410. [DOI] [PubMed] [Google Scholar]
- Scharrer E, Scharrer B. (1939) Secretory cells within the hypothalamus. Research Publications - Association for Research in Nervous and Mental Disease 20:170–194. [Google Scholar]
- Schröder M, Kaufman RJ. (2005) ER stress and the unfolded protein response. Mutat Res 569:29–63. [DOI] [PubMed] [Google Scholar]
- Seay JS, Lattie E, Schneiderman N, Antoni MH, Fekete EM, Mendez AJ, Szeto A, Fletcher MA. (2014) Linear and quadratic associations of plasma oxytocin with depressive symptoms in ethnic minority women living with HIV. J Appl Biobehav Res 19:70–78. [Google Scholar]
- Seelke AM, Perkeybile AM, Grunewald R, Bales KL, Krubitzer LA. (2016a) Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). J Comp Neurol 524:564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AM, Yuan SM, Perkeybile AM, Krubitzer LA, Bales KL. (2016b) Early experiences can alter the size of cortical fields in prairie voles (Microtus ochrogaster). Environ Epigenet 2:dvw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng J, Miller J, Sperlich M, van de Ven CJ, Brown S, Carter CS, Liberzon I. (2013) Exploring dissociation and oxytocin as pathways between trauma exposure and trauma-related hyperemesis gravidarum: a test-of-concept pilot. J Trauma Dissociation 14:40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. (2019) Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101:246–259.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KR, James DC. (2008) Effects of oxytocin-induced uterine hyperstimulation during labor on fetal oxygen status and fetal heart rate patterns. Am J Obstet Gynecol 199:34.e1-5. [DOI] [PubMed] [Google Scholar]
- Simsek Y, Celik O, Karaer A, Yılmaz E, Gul M, Ozerol E, Bilgic S, Celik N. (2012) Elevated cardiac oxidative stress in newborn rats from mothers treated with atosiban. Arch Gynecol Obstet 285:655–661. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. (2011) Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 36:1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. (2014) Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry 76:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys SM, Scherbel JR, Kurian JR, Diebold T, Wilson T, Hedden L, Groesch K, Diaz-Sylvester PL, Botchway A, Campbell P, et al. (2020) An association of intrapartum synthetic oxytocin dosing and the odds of developing autism. Autism 24:1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE. (2018) Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol 51:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Larkin TE, Malley MO, Albers HE. (2016) Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus). Horm Behav 81:20–27. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK, IV, Larkin TE, II, Huhman KL, Albers HE. (2014) Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoljaric A, Seja P, Spoljaric I, Virtanen MA, Lindfors J, Uvarov P, Summanen M, Crow AK, Hsueh B, Puskarjov M, et al. (2017) Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc Natl Acad Sci USA 114:E10819–E10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. (2012) The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav 61:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. (2012) Neuromodulation by oxytocin and vasopressin. Neuron 76:142–159. [DOI] [PubMed] [Google Scholar]
- Stoop R, Hegoburu C, van den Burg E. (2015) New opportunities in vasopressin and oxytocin research: a perspective from the amygdala. Annu Rev Neurosci 38:369–388. [DOI] [PubMed] [Google Scholar]
- Stribley JM, Carter CS. (1999) Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA 96:12601–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. (2013) Association between maternal mood and oxytocin response to breastfeeding. J Womens Health (Larchmt) 22:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraev AS, Bowen MT, Ali SO, Hicks C, Ramos L, McGregor IS. (2014) Adolescent exposure to oxytocin, but not the selective oxytocin receptor agonist TGOT, increases social behavior and plasma oxytocin in adulthood. Horm Behav 65:488–496. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. (2011) Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 73:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. (2008) Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab 295:E1495–E1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Sun-Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. (2017) Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab 312:E183–E189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A. (2010) Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma 27:1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Zink BJ, Chodobski A. (2011) Multiple sites of vasopressin synthesis in the injured brain. J Cereb Blood Flow Metab 31:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Hammock EAD. (2020) Orally administered oxytocin alters brain activation and behaviors of pre-weaning mice. Horm Behav 118:104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati C, Carvalho JCA, Luca A, Balki M. (2019) The effect of intermittent oxytocin pretreatment on oxytocin-induced contractility of human myometrium in vitro. Anesth Analg 128:671–678. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Shishido Y, Shibata S, Watanabe S. (1994) Facilitatory effect of vasopressin on the ischemic decrease of the CA1 presynaptic fiber spikes in rat hippocampal slices. Brain Res 644:343–346. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. (2006) Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med 68:238–245. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. (2010) Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci 21:3–7. [DOI] [PubMed] [Google Scholar]
- Theodosis DT. (2002) Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol 23:101–135. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. (2006) Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA 103:7889–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Walton JC, Bhalla R, George KC, Beth EH. (2008) A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur J Neurosci 27:2285–2293. [DOI] [PubMed] [Google Scholar]
- Toepfer P, Heim C, Entringer S, Binder E, Wadhwa P, Buss C. (2017) Oxytocin pathways in the intergenerational transmission of maternal early life stress. Neurosci Biobehav Rev 73:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. (2010) A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA 107:13936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers AJ, Tremblay MW, Chung L, Li XL, Bey AL, Zhang W, Cao X, Wang X, Wang P, Duffney LJ, et al. (2018) Epigenetic dysregulation of Oxtr in Tet1-deficient mice has implications for neuropsychiatric disorders. JCI Insight 3:120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hübner CA, Represa A, Ben-Ari Y, Khazipov R. (2006) Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314:1788–1792. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Minlebaev M, Rheims S, Ivanov A, Jorquera I, Holmes GL, Zilberter Y, Ben-Ari Y, Khazipov R. (2008) Postnatal changes in somatic gamma-aminobutyric acid signalling in the rat hippocampus. Eur J Neurosci 27:2515–2528. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, et al. (2014) Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343:675–679. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs Moberg K, Handlin L, Kendall-Tackett K, Petersson M. (2019) Oxytocin is a principal hormone that exerts part of its effects by active fragments. Med Hypotheses 133:109394. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. (1998) Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139:5015–5033. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Hammock EAD. (2020) Oxytocin receptor gene loss influences expression of the oxytocin gene in C57BL/6j mice in a sex- and age-dependent manner. J Neuroendocrinol 32:e12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GMJ, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D. (1997) Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17:284–292. [DOI] [PubMed] [Google Scholar]
- Varian BJ, Poutahidis T, DiBenedictis BT, Levkovich T, Ibrahim Y, Didyk E, Shikhman L, Cheung HK, Hardas A, Ricciardi CE, et al. (2017) Microbial lysate upregulates host oxytocin. Brain Behav Immun 61:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak AP, Skoblenick K, Thomas N, Mishra RK, Johnson RL. (2007) Allosteric modulation of the dopamine receptor by conformationally constrained type VI beta-turn peptidomimetics of Pro-Leu-Gly-NH2. J Med Chem 50:6725–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros ATM, Jatzkowski A, Komen J. (2003) Effects of oxytocin on semen release response in African catfish (Clarias gariepinus). Theriogenology 59:1905–1917. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Eisenlohr-Moul TA, Pedersen CA, Rubinow DR, Girdler SS, Dichter GS. (2018) Early life abuse moderates the effects of intranasal oxytocin on symptoms of premenstrual dysphoric disorder: preliminary evidence from a placebo-controlled trial. Front Psychiatry 9:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang SC, Yang H, Lv C, Jia S, Liu X, Wang X, Meng D, Qin D, Zhu H, et al. (2019) Therapeutic potential of oxytocin in atherosclerotic cardiovascular disease: mechanisms and signaling pathways. Front Neurosci 13:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao S, Liu X, Zheng Y, Li L, Meng S. (2018) Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed Pharmacother 107:262–269. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. (2004) Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav 83:319–328. [DOI] [PubMed] [Google Scholar]
- Wee CL, Nikitchenko M, Wang WC, Luks-Morgan SJ, Song E, Gagnon JA, Randlett O, Bianco IH, Lacoste AMB, Glushenkova E, et al. (2019) Zebrafish oxytocin neurons drive nocifensive behavior via brainstem premotor targets. Nat Neurosci 22:1477–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Margolis KG, Li Z, Gershon MD. (2014) Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am J Physiol Gastrointest Liver Physiol 307:G848–G862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. (2009) Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol 512:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Welch-Horan TB, Anwar M, Anwar N, Ludwig RJ, Ruggiero DA. (2005) Brain effects of chronic IBD in areas abnormal in autism and treatment by single neuropeptides secretin and oxytocin. J Mol Neurosci 25:259–274. [DOI] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. (2009) Detection of salivary oxytocin levels in lactating women. Dev Psychobiol 51:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. (1994) Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J Neuroendocrinol 6:247–250. [DOI] [PubMed] [Google Scholar]
- Wilson LC, Goodson JL, Kingsbury MA. (2016) Seasonal variation in group size is related to seasonal variation in neuropeptide receptor density. Brain Behav Evol 88:111–126. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Lnsel TR. (1991) Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J Neuroendocrinol 3:155–161. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. (1990) Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol Biochem Behav 37:63–69. [DOI] [PubMed] [Google Scholar]
- Wrzal PK, Devost D, Pétrin D, Goupil E, Iorio-Morin C, Laporte SA, Zingg HH, Hébert TE. (2012a) Allosteric interactions between the oxytocin receptor and the β2-adrenergic receptor in the modulation of ERK1/2 activation are mediated by heterodimerization. Cell Signal 24:342–350. [DOI] [PubMed] [Google Scholar]
- Wrzal PK, Goupil E, Laporte SA, Hébert TE, Zingg HH. (2012b) Functional interactions between the oxytocin receptor and the β2-adrenergic receptor: implications for ERK1/2 activation in human myometrial cells. Cell Signal 24:333–341. [DOI] [PubMed] [Google Scholar]
- Xu PF, Fang MJ, Jin YZ, Wang LS, Lin DS. (2017) Effect of oxytocin on the survival of random skin flaps. Oncotarget 8:92955–92965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Qin B, Shi A, Zhao J, Guo X, Dong L. (2018) Oxytocin inhibited stress induced visceral hypersensitivity, enteric glial cells activation, and release of proinflammatory cytokines in maternal separated rats. Eur J Pharmacol 818:578–584. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Higashida H. (2020) RAGE regulates oxytocin transport into the brain. Commun Biol 3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Liang M, Munesue S, Deguchi K, Harashima A, Furuhara K, Yuhi T, Zhong J, Akther S, Goto H, et al. (2019) Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun Biol 2:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Kitano T. (2013) Molecular evolution of the oxytocin-oxytocin receptor system in eutherians. Mol Phylogenet Evol 67:520–528. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yu H, Babygirija R, Shi B, Sun W, Zheng X, Zheng J. (2019) Intranasal administration of oxytocin attenuates stress responses following chronic complicated stress in rats. J Neurogastroenterol Motil 25:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Kenkel WM, Frijling JL, Dodhia S, Onishi KG, Tovar S, Saber MJ, Lewis GF, Liu W, Porges SW, et al. (2016) Oxytocin promotes functional coupling between paraventricular nucleus and both sympathetic and parasympathetic cardioregulatory nuclei. Horm Behav 80:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z. (2016) Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation 13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P. (2014) Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav 66:351–360. [DOI] [PubMed] [Google Scholar]
- Zhong J, Amina S, Liang M, Akther S, Yuhi T, Nishimura T, Tsuji C, Tsuji T, Liu HX, Hashii M, et al. (2016) Cyclic ADP-ribose and heat regulate oxytocin release via CD38 and TRPM2 in the hypothalamus during social or psychological stress in mice. Front Neurosci 10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinni M, Colella M, Batista Novais AR, Baud O, Mairesse J. (2018) Modulating the oxytocin system during the perinatal period: a new strategy for neuroprotection of the immature brain? Front Neurol 9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]