Abstract
Honokiol is a phytochemical isolated from the Magnolia plant. It exhibits significant antitumor activity against a variety of cancer cell types via targeting of critical mediators of tumor progression, stromal remodeling, and chemoresistance. However, poor bioavailability and inefficient tumor uptake remain some of the hurdles in its translation as a therapeutically useful drug. Here, we developed a nanoformulation of honokiol using mesenchymal stem cell-derived exosomes, which are nonimmunogenic and express surface markers to support their tumor-targeted delivery. Maximum entrapment of honokiol occurred when it was mixed in a 1:4 weight ratio with exosomes and subjected to six cycles of sonication. Dynamic light scattering analysis demonstrated that the average size (∼175.3 nm), polydispersity (∼0.11), and integrity (∼12.9 mV) of exosomes remained in the desirable range post honokiol encapsulation. Exosome-encapsulated honokiol exhibited significantly higher therapeutic efficacy over the free honokiol in WST-1 growth and long-term clonogenicity assays. Flow cytometry-based cell cycle and live/dead cell assay, respectively, confirmed the enhanced effect of exosomal honokiol formulation on cell cycle arrest and apoptosis induction. More significant alterations in the expression of cell cycle- and survival-associated proteins were also observed in cancer cells treated with exosomal honokiol over free honokiol. Higher intracellular accumulation of honokiol was recorded in cancer cells treated with equivalent doses of honokiol as compared to the free honokiol. Together, our work is the first demonstration of exosomal encapsulation of honokiol and its improved antitumor efficacy resulting from improved cellular uptake.
Introduction
Honokiol is a polyphenol isolated from the Magnolia officinalis/grandiflora plant.1 It has been documented to have enormous therapeutic potential against several pathological conditions, including cancer. We demonstrated, for the first time, the antitumor activity of honokiol against pancreatic cancer cells, and it also sensitized them to gemcitabine toxicity.2 In an orthotopic mouse model, honokiol inhibited pancreatic tumor growth, metastasis, and desmoplasia by interfering with tumor-stromal crosstalk.3 Honokiol has also been shown to suppress the growth and metastasis of breast, prostate, lung, and renal cancer as well by targeting of various cancer-relevant cell signaling pathways.4−6 Despite having remarkable antitumor properties, translation of honokiol as a therapeutic drug has not been possible due to its hydrophobic nature, which makes it less water soluble. Further, honokiol also suffers from poor bioavailability like many other natural agents due to systemic metabolization.1,7
Several types of synthetic nanodelivery systems, such as metal nanoparticles, liposomes, and carbon nanotubes, have been developed to enhance drug bioavailability in the systemic circulation and reduce nontargeted cell toxicity.8−10 However, these nanoformulations pose several clinical concerns, including immunogenicity and liver and kidney toxicity.11,12 Exosomes are nanosized (30–150 nm) membrane vesicles shed by nearly all types of cells. They are the natural delivery vehicles that actively transfer biomaterials (protein, DNA, RNA, miRNAs, etc.) from one cell to another to facilitate intercellular communications.13,14 Exosomes can travel to distant locations in the body and execute functional changes in the recipient cells.15,16 Exosomes are currently being investigated as a drug delivery system, and exosomes derived from different cell types vary in their loading capacity, yield, and antitumor efficacy.17,18 More importantly, exosomes derived from certain cell types, such as mesenchymal stem cells (MSCs), are nonimmunogenic and express surface proteins that could facilitate their tumor-targeted delivery.19−21
In this study, we isolated exosomes from MSCs and loaded them with honokiol by the sonication method. Honokiol-loaded exosomes were characterized for membrane integrity, desirable size, and polydispersity. Further, antitumor efficacy of exosomal honokiol was evaluated in several cancer cell types and compared with the equivalent doses of free honokiol. The effect of exosomal honokiol on cell cycle arrest, apoptosis induction, and associated molecular changes was also examined. The difference in intracellular accumulation of the free drug and exosomal honokiol was also assessed. Together, our findings present the first description of exosomal honokiol preparation and demonstrate its enhanced antitumor efficacy resulting from increased cellular uptake by the cancer cells.
Results
Exosomal Preparation and Optimization of Honokiol Loading in Exosomes
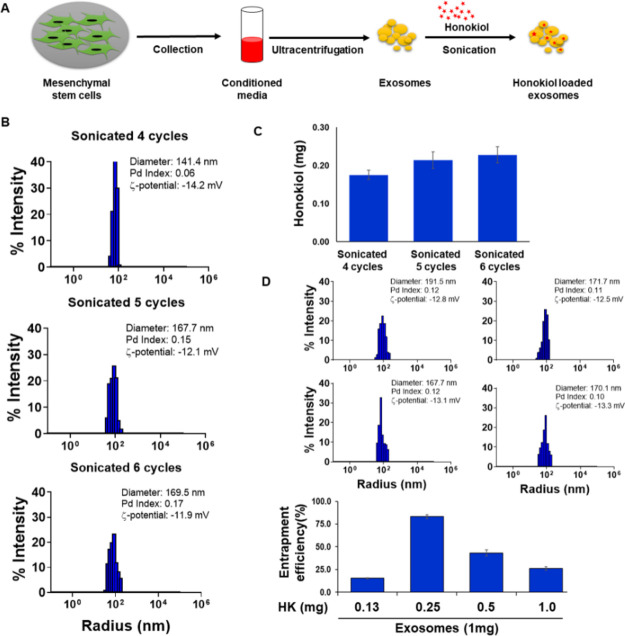
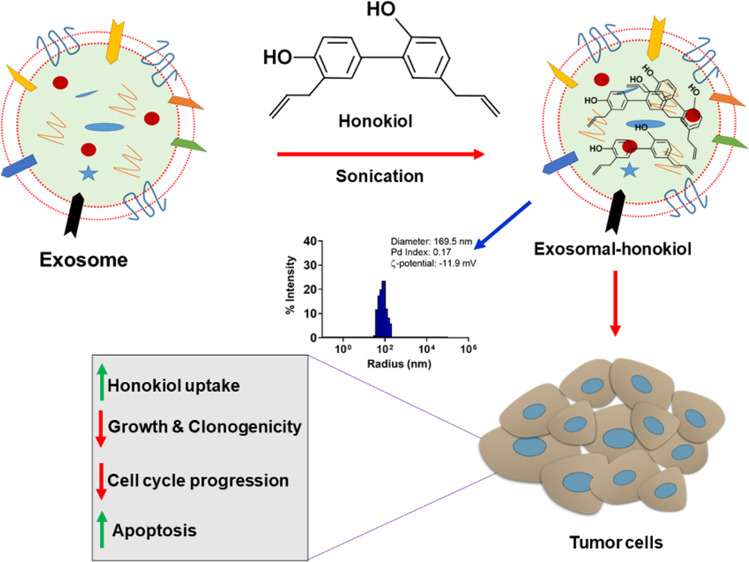
Exosomes were isolated from the conditioned media of mesenchymal stem cells (MSCs) through ultracentrifugation and characterized, as previously described.15 Honokiol loading into the exosomes was achieved by the sonication method (Figure 1a). We first examined the effect of sonication duration and frequency on exosome integrity and size. For this, exosomes were subjected to sonication (30 s pulse followed by 30 s pause) for varying number of cycles (4, 5, 6, 7, and 8) and subsequently analyzed by dynamic light scattering (DLS) for changes in their size and integrity. Exosomes maintained their integrity during the different number of cycles of sonication as depicted by their zeta potential values (−13.9 to −12.6 mV), which were comparable with that (−14.6 mV) of unsonicated exosomes. However, the size of exosomes increased gradually with increasing sonication frequency. The size of unsonicated exosomes was 120.5 nm, which increased to ∼174.2 nm after six cycles of sonication and reached up to ∼236.2 nm after eight cycles of sonication (Figure S1). Therefore, in subsequent optimization steps for honokiol loading, we used 4–6 cycles of sonication and mixed the honokiol with exosomes in 1:1 ratios (1.0 mg exosomes and 1.0 mg honokiol). DLS analysis demonstrated that the size of exosomes remained in the 141.4–169.5 nm range, and they maintained their integrity (zeta potential range: −14.2 to −11.9 mV) as well (Figure 1b). HPLC analyses showed that the maximum loading of honokiol occurred after six cycles of sonication (Figure 1c). Next, we optimized exosomes and honokiol ratio to achieve maximum loading efficiency. Varying amounts of honokiol (0.125, 0.25, 0.5, and 1.0 mg) were mixed with a fixed amount of exosomes (1.0 mg protein) and subjected to six cycles of sonication. Maximum entrapment (∼80%) of honokiol was recorded when honokiol and exosomes were used in 1:4 ratios (0.25 mg honokiol:1.0 mg exosomes). The size of these honokiol-loaded exosomes was ∼171.7 nm, with a zeta potential of −12.5 mV (Figure 1d).
Figure 1.
Optimization of honokiol loading on exosomes and their characterization. (a) Schematic describing our strategy for exosome (Exo) isolation and honokiol (HK) loading. Mesenchymal stem cells (MSCs) were seeded in tissue culture plates and allowed to grow to near confluence. Conditioned media were collected from subconfluent cells and subjected to ultracentrifugation for exosome isolation. Honokiol loading was achieved by sonication method. (b) Exosomes were sonicated at different cycles in the presence of HK, and their size and integrity were monitored by dynamic light scattering (DLS). (c) Loading of HK in the sonicated exosomes was measured by HPLC equipped with a UV detector. (d) To optimize loading efficiency, various amounts of HK (0.125, 0.25, 0.5, and 1.0 mg) were used with a constant amount of exosomes (1.0 mg protein). Exosome size and integrity were measured by DLS, and HK loading was determined by HPLC.
Exosomal Honokiol Formulation Shows Superior Antitumor Activity Than the Equivalent Doses of Free Honokiol
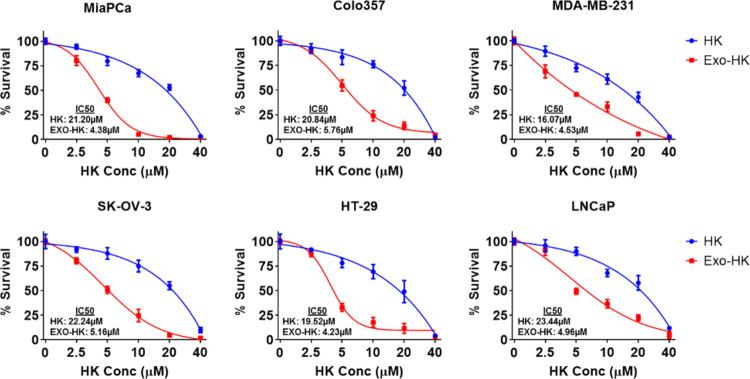
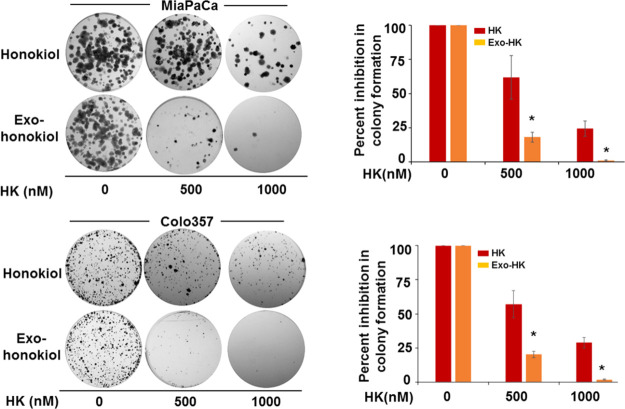
To compare the effect of exosomal loading of honokiol on its antitumor efficacy, we used multiple cancer cell lines (pancreatic, breast, ovarian, colon, and prostate). The cells were treated with equivalent doses of free and exosomal honokiol (0–40 μM) for 72 h, and their effect on cell survival was examined. A comparison of IC50 values demonstrates that the exosomal honokiol was nearly 4–5 times more effective in killing cancer cells than the free honokiol (Figure 2). To further examine the effect in longer term treatment assays, we examined the effect of exosomal or free honokiol on the clonogenic potential of two pancreatic cancer cell lines (MiaPaCa and Colo357). Cells were treated either with free or exosomal honokiol in a dose range of 0–1000 nM and incubated for two weeks. Our data demonstrate that the plating efficiency of MiaPaCa and Colo357 cells decreased gradually with the increasing concentrations of exosomal or free honokiol. However, a greater decrease in clonogenicity of cancer cells was reported in cells treated with exosomal honokiol as compared to those treated with equivalent doses of free honokiol (Figure 3).
Figure 2.
Cytotoxicity of exosomal honokiol on human cancer cells. Pancreatic cancer (MiaPaCa, Colo357), breast cancer (MDA-MB-231), ovarian cancer (SK-OV-3), colon cancer (HT-29), and prostate cancer (LNCaP) cells (5 × 103/well) were seeded in 96-well plates and treated with either vehicle, empty exosomes, or equivalent doses of free or exosome-encapsulated HK (0–40 μM) for 72 h. Viability of cells was determined by the WST-1 assay. The data is presented as mean ± SD (n = 3) after normalization with vehicle control (for free honokiol) or empty exosomes (for exosomal honokiol). Signficant differences (p < 0.05) in cell survival were observed at 5–20 μM treatment doses of free and exosomal honokiol.
Figure 3.
Effect of exosomal honokiol on clonogenicity of pancreatic cancer cells. MiaPaCa and Colo357 cells were seeded in a six-well plate (1 × 103/well), and after 24 h, treated with different doses (0, 500, and 1000 nM) of free HK or exo-HK. Cells were allowed to form colonies for two weeks, and after that, colonies were fixed, stained, and photographed. The total number of colonies were counted using image analysis software (Gene Tools; Syngene, Frederick, MD). Data are shown as percent inhibition of the clonogenic survival of HK and exo-HK treated cells. Bars represent the mean ± SD (n = 3); *p < 0.05.
Exosomal Honokiol Has a More Potent Effect on Cell Cycle Arrest and Apoptosis Than the Free Honokiol
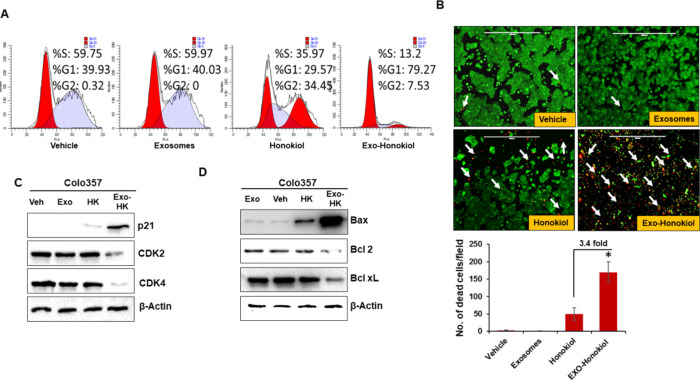
Next, we examined the effect of free and exosomal honokiol on cell cycle progression and apoptosis in pancreatic cancer cells (Colo357). Cells were treated with the IC50 concentration of exosomal honokiol or an equivalent dose of free honokiol (4 μM) for 24 h. Cell cycle distribution of control (vehicle and exosomes only) and free and exosomal honokiol-treated cells was determined by propidium iodide (PI)/RNase staining followed by flow cytometry. The data demonstrate that the treatment with exosomal honokiol resulted in enhanced accumulation of pancreatic cancer cells in the G1 phase as compared to that observed with free honokiol along with a concomitant decrease in the number of cells in the replicative S-phase. No effect of exosomes only was observed on the cell cycle when compared to the vehicle-treated cells (Figure 4a). To examine the effect of free and exosomal honokiol on cell survival, we performed live/dead assay. The green fluorescence signal of calcein dye suggests the viability of the cells while dead cells display red ethidium bromide florescence. The data demonstrate that exosomal honokiol-treated pancreatic tumor cells generated ∼3.4-fold higher red fluorescence relative to those treated with free honokiol. However, exosomes or vehicle-treated pancreatic tumor cells did not affect tumor cell viability (Figure 4b).
Figure 4.
Effect of exosomal honokiol on cell cycle progression and cell survival. (a) Pancreatic tumor cells, Colo357 (1 × 106 cells/well) were seeded and allowed to grow for 24 h, and then cultured in serum-deprived media for 72 h for synchronization. Subsequently, media was replaced with serum-containing media, and cells were treated with IC50 doses of exosomal honokiol or equivalent free honokiol for 24 h. The distribution of cells in different phases of the cell cycle was analyzed by propidium iodide (PI) staining followed by flow cytometry. (b) Pancreatic tumor cells were treated with exosomal honokiol or equivalent free HK and subjected to live/dead cell staining with calcein-AM and ethidium homodimer-1. Live and dead cells were monitored by fluorescence imaging using an EVOS FL Imaging System. A green color indicates live cells, while a red color is indicative of dead cells (arrow). The number of dead cells was counted in six random fields and presented as mean ± SD. *p value < .05. (c,d) Total protein was isolated from the vehicle, exosome, free, and encapsulated honokiol-treated tumor cells and subjected to immunoblot analysis for analyzing the expresson of cell cycle-associated (CDK2, CDK4, and p21) and antiapoptotic/proapoptotic proteins (Bcl-2 and Bcl-xL/Bax). β-actin was used as a loading control.
We next examined the effect of free or exosomal honokiol treatment on the expression of proteins associated with cell cycle and survival. Cells were treated with equivalent doses of free and exosomal honokiol for 48 h, and protein lysates prepared. The changes in the expression of cell cycle- and survival-associated proteins were examined by western blotting. The data demonstrate that exosomal honokiol treatment leads to far greater suppression of cyclin-dependent kinases (CDK2 and CDK4) as compared to free honokiol at equivalent doses. Similarly, we also observed a significantly higher increase in the expression of cyclin-dependent kinase inhibitor, p21, in pancreatic tumor cells treated with exosomal honokiol as compared to those treated with free honokiol (Figure 4c). Furthermore, a significantly higher expression of a proapoptotic protein, Bax, along with a concomitant decrease in the expression of Bcl-2 and Bcl-xL was observed in exosomal honokiol-treated pancreatic cancer cells as compared to those that were treated with an equivalent dose of free honokiol (Figure 4d). Altogether, our findings demonstrate that exosomal honokiol is more effective in reducing tumor cell growth by altering cell cycle progression and survival as compared to free honokiol.
Enhanced Efficacy of Exosomal-Loaded Honokiol Is Associated with Its Improved Cellular Uptake
Since exosomes are natural carriers and efficient delivery vehicles, we examined if exosomal formulation more effectively delivered honokiol into the cancer cells over its free form. To determine this, we treated the tumor cells either with exosomal or free honokiol for 4 h. Subsequently, cell lysates were made, and intracellular honokiol accumulation was quantified by mass spectrometry. The data reveal that the intracellular accumulation of honokiol in cancer cells treated with the exosomal honokiol is significantly higher [MiaPaCa (3.64-fold) and Colo357 (4.68-fold)] as compared to those treated with free honokiol (Figure 5). These data suggest that enhanced cytotoxic potential of exosomal honokiol is due to its efficient delivery of the drug to tumor cells.
Figure 5.
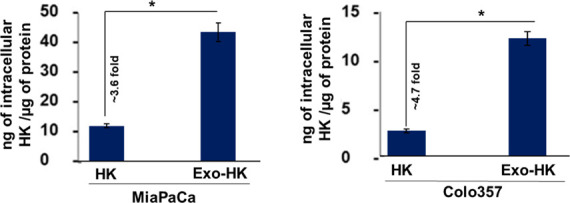
Mass spectrometric analysis was performed to examine intracellular honokiol. Pancreatic tumor cells, MiaPaCa and Colo357, were treated either with exosomal honokiol or equivalent free honokiol for 4 h. After that, cells were washed to remove extracellular honokiol, and cell lysates were made. The level of honokiol in tumor cells was determined by LC–MS/MS analysis. Bars represent mean intracellular levels of HK (ng) per μg of protein ± SD. *p < 0.05.
Discussion
This study presented the data to support that honokiol can be efficiently loaded into the exosomes derived from MSCs. Furthermore, far superior antitumor efficacy of the exosomal formulation was reported over the free honokiol against a variety of tumor cell types resulting from its enhanced uptake by the tumor cells (Figure 6). Several lines of evidence suggest the antitumor activity of honokiol in a variety of cancer cell types. Importantly, honokiol is shown to target critical mediators of tumor progression, metastasis, and chemoresistance.2,3,5,22 Its effect on stromal remodeling has also been demonstrated.3 Since honokiol has poor water solubility and limited systemic bioavailability,7,23 efforts have been made to overcome these pharmacological barriers in its therapeutic translation. Like, nanomicellar formulation of honokiol showed increased bioavalibility of honokiol with profound anticancer effects in triple negative breast cancer.7 Fang et al., developed a thermosensitive hydrogel-loaded honokiol nanoparticle and demonstrated anticancer effects in lung cancer.24
Figure 6.
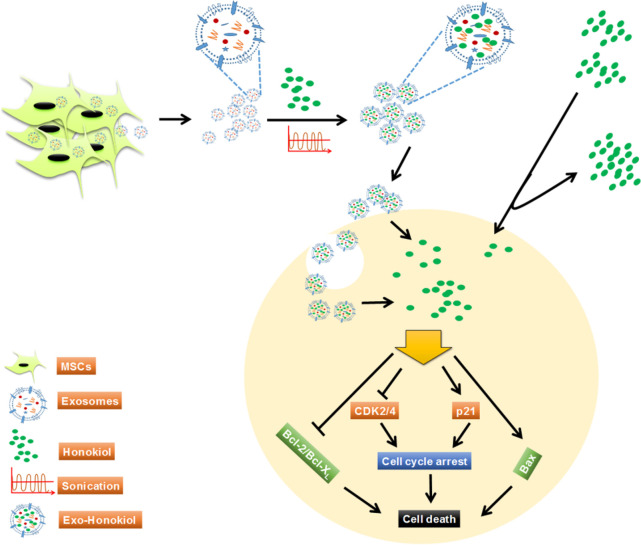
Schematic summarizing honokiol loading into the exosomes, its uptake, and the mechanism of action. Honokiol encapsulated in MSC-derived exosomes through sonication accumulates inside the cells more efficiently over the free honokiol. Mechanistic findings suggest that increased honokiol accumulation more effectively causes cell cycle arrest and apoptosis by modulating the expression of the cell cycle- (CDK2/4, p21) and apoptosis- (Bcl-2, Bcl-xL, and Bax)associated proteins in the tumor cells.
We successfully developed an exosomal nanoformulation of honokiol by using the sonication method. Various other methods, such as extrusion, freeze–thaw cycles, electroporation, and saponin permeabilization, have been employed for drug loading into the exosomes and other extracellular vehicles.25,26 But these methods have certain limitations such as the freeze–thaw method induces degradation and aggregation of exosomes. It also has poor drug loading ability compared to extrusion or sonication methods.25,26 Similarly, electroporation can cause exosome instability and use of saponin in nanoformulation is associated with the risk of hemolytic activity in in vivo study.27,28 It was shown in a study that the catalase could be loaded most efficiently into the exosomes by sonication as compared to other methods.26 We were also able to efficiently load honokiol in the exosomes by the sonication method without having much effect on their integrity and physical features (size and zeta potential). However, we did observe that the size and polydispersity index of exosomes continued to increase slightly with increasing number of sonication cycles, but no or minimal change in the zeta potential was recorded. The increase in size and polydispersity index of exosomes could be due to exosomal fusion and/or their mild aggregation. Similar observations were also made in another study where the loading of paclitaxel in exosomes through sonication increased exosomal size without altering the surface charge.29 We chose six or lesser cycles of sonication for optimization of honokiol loading as size remained under 200 nm at these cycles. Studies have suggested that the nanoparticles in the 100–200 nm size range have better cellular uptake and enhanced permeability and retention (EPR) effect with low risk of immature clearance from the body.30 We also observed highest entrapment efficiency (∼83%) of honokiol when honokiol and exosomes were used in 1:4 weight ratio suggesting that while sufficient availability of honokiol is important for optimal loading, its excess may destabilize exosomes and reduce entrapment. In another study, optimal gadolinium loading into the exosomes was achieved at 1:1 ratio.31 Therefore, it appears that the entrapment efficiency likely depends on the chemical nature of the drug loaded into the exosomes.
We observed that exosomal honokiol formulation was more potent than the free honokiol in inhibiting tumor cell growth. As expected, the increased efficacy of exosomal honokiol resulted from its enhanced cellular uptake. The utility of exosomes in promoting delivery and enhanced tumor uptake of various other anticancer drugs and natural compounds such as paclitaxel, doxorubicin, and curcumin has also been demonstrated.29,32,33 Exosomes are the natural carriers and delivery vehicles that carry and deliver biomolecules from one cell to another.13−15 They bear the surface proteins that allow their desired interaction with the recipient cells leading to efficient uptake. Exosome uptake could be through clathrin-dependent endocytosis or clathrin-independent mechanisms such as lipid raft-mediated internalization, macropinocytosis, and phagocytosis.13,34 Therefore, it will be interesting to investigate the route and associated mechanisms of exosomal uptake in our system.
Intracellular accumulation of a drug is directly proportional to the biological activity.35 Accordingly, we observed that exosomal honokiol arrested pancreatic tumor cells in the G1 phase of cell cycle and promoted apoptosis more effectively than the free honokiol. Similarly, the decrease in cyclin-dependent kinases (CDK2 and CDK4) and antiapoptotic proteins (Bcl-2 and Bcl-xL) and increase in p21 and proapoptotic Bax expression was more potent in cancer cells treated with exosomal honokiol as compared to free honokiol. These findings provided further support to our previous data on the antitumor activity of honokiol.2,3 Moreover, our data also suggest that developing exosomal nanoformulation of honokiol could be an attractive approach that could be exploited for its therapeutic translation. Also, exosomes can be further surface modified using biological or chemical manipulations to improve their uptake and tumor-targeted delivery. Altogether, our study is the first step demonstrating a novel exosomal formulation of honokiol with superior antitumor activity resulting from enhanced uptake by the tumor cells. Further improvement and in vivo evaluation in animal models are warranted before realizing the full potential of honokiol as an antitumor drug in the clinics.
Materials and Methods
Reagents and Antibodies
Dulbecco’s modified Eagle medium (DMEM) and Roswell Park Memorial Institute medium (RPMI-1640) were procured from Hyclone Laboratory (Logan, UT), and fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Mesenchymal stem cell basal media and growth kit were obtained from American Type Culture Collection (Manassas,VA). Penicillin and streptomycin were from Invitrogen (Carlsbad, CA), and western blotting SuperSignal West Femto Maximum Sensitivity Substrate Kit and LIVE/DEAD Viability/Cytotoxicity Kit were from Thermo Fisher Scientific (Logan, UT). Propidium iodide/RNAse staining buffer was from BD Bioscience (San Diego, CA). Antibodies against Bcl-2 and Bax (rabbit polyclonal), Bcl-xL (rabbit monoclonal) were from Cell Signaling Technology (Beverly, MA) and those against p21, CDK4 (mouse monoclonal), and CDK2 (rabbit polyclonal) as well as horseradish peroxidase (HRP)-labeled secondary antibodies were from Santa Cruz Biotechnology (Dallas, TX). Biotinylated anti-β-actin (mouse monoclonal) was from Sigma-Aldrich (St. Louis, MO).
Cell Lines and Culture Conditions
Pancreatic cancer cell lines (MiaPaCa and Colo357), breast cancer cell line (MDA-MB-231), colon cancer cell line (HT-29), prostate tumor cell line (LNCaP), and ovarian cancer cell line (SKOV-3) were procured, maintained, and authenticated as previously described.36−40 Mesenchymal stem cells (MSCs) were procured from American Type Culture Collection (Manassas, VA) and maintained in special mesenchymal stem cell basal media that was supplemented with growth factors. All the cells were routinely tested for mycoplasma contamination in our in-house facility.
Isolation of Exosomes
Exosomes were isolated from MSCs using the ultracentrifugation method, as previously described.16 In brief, the conditioned media from subconfluent MSCs were collected and centrifuged at 300 g for 10 min to remove cell debris. After that, centrifugation at 16,500 g was done for 30 min to remove apoptotic bodies and other medium- to large-sized vesicles. Finally, the resulting supernatant was centrifuged for 2 h at a speed of 120,000 g to collect exosomes. The exosomal pellet was washed by resuspending in 5 mL of PBS followed by centrifugation at 120,000 g for 2 h. Exosomes were aliquoted and stored at −80 °C until further use.
Size Determination and Zeta Potential of Exosomes by Dynamic Light Scattering
Resuspended exosomes (1 μL) were added to 999 μL of deionized water and subjected to dynamic light scattering (DLS) analysis using DelsaMax PRO (Beckman Coulter, Brea, CA, USA). We also performed the phase analysis light scattering (PALS) to determine the zeta potential in water as per the manufacturer’s recommendations.
Loading of Honokiol in Exosomes
Honokiol was mixed with exosomes in various ratios and sonicated (10% amplitude, 4/5/6/7/8 cycles by 30 sec pulse/30 sec pause) on ice using Misonix sonicator (Farmingdale, NY). Free honokiol was removed by ultracentrifugation at 120,000 g for 2 h, and loaded exosome pallets were quantified based on protein content using protein DC assay kit as described earlier.41
Determination of Honokiol Loading by High-Performance Liquid Chromatography
Honokiol loading in exosomes was determined by high-performance liquid chromatography (HPLC) that was equipped with a UV detector. Honokiol standards of concentrations ranging from 1 to 250 μM were prepared using a 35:65 mixture of water/acetonitrile. A standard curve was plotted using the area under the curve of the standards. Subsequently, 5.0 μL of exosomal formulation was digested, injected onto a Kromasil C18 column using an isocratic flow of 1.5 mL/min at 35 °C, and honokiol was detected using UV absorption at 220 nm.
Cytotoxicity Assay
Cells were seeded in 96-well plates at a density of 5 × 103/well and after 24 h were treated with vehicle, unloaded exosomes, free honokiol, or exosomal honokiol (0–40 μM) for 72 h. Cell viability was measured using the WST-1 assay as previously described.16 For live/dead assay, tumor cells (2.5 × 105/well) were seeded in six-well plates, treated with either vehicle, unloaded exosomes, free honokiol, or exosomal honokiol (0–40 μM) for 72 h and stained with calcein-AM and ethidium homodimer-1 as described earlier.36
Plating Efficiency Assay
Single-cell suspensions of cancer cells were seeded at low density (1 × 103 cells/well) in six-well plates and allowed to grow for 24 h. The media was replaced the next day, and cells were treated with vehicle, exosomes only, free honokiol, or exosomal honokiol and allowed to form colonies for two weeks. After the end of the incubation period, colonies were fixed with methanol, stained with crystal violet, photographed, and counted using image analysis software (ImageJ, National Institutes of Health).
Cell Cycle Analysis
Cell cycle analysis was performed as previously described.2 Briefly, cells (5 × 105cells/well) were synchronized and incubated with vehicle, unloaded exosomes, free honokiol, or exosomal honokiol for 24 h. Subsequently, cells were harvested, washed with 1X PBS, and fixed with 70% methanol overnight at 4 °C. For post fixation, cells were rewashed with 1X PBS and stained with propidium iodide using PI/RNase kit. Flow cytometry was done on a BD FACSCanto II cytometer (BD Biosciences, San Diego, CA). The percentage of cell population in various phases of the cell cycle was calculated using ModFit LT software (Verity Software House, Topsham, ME).
Western Blot Analysis
Cells were harvested, and total protein was isolated from the treated tumor cells as described earlier.36 Proteins were resolved on 10–12% (sodium dodecyl sulfate) SDS-polyacrylamide gel by electrophoresis and probed with specific antibodies against Bcl-2, Bcl-xL, Bax, CDK2, CDK4, and p21 (1:200) and β-actin (1:20000). All respective secondary antibodies were used at 1:2000 dilutions. Signals were recorded using West Femto Maximum Sensitivity Substrate kit under Bio-Rad ChemiDoc Imager (Hercules, CA).
Determination of Intracellular Honokiol
Pancreatic tumor cells (Colo357 and MiaPaCa) were seeded in a six-well plate (1 × 106 cells/well) and allowed to grow for 24 h. Thereafter, cells were treated with free or exosomal honokiol for 4 h, and trypsinized and cell pellets were collected. Ice cold methanol (200 μL) was added to the cell pellets, and they were subjected to bead-beating homogenization for 30 s with 0.5 mm glass beads. The samples were then centrifuged at 10,000 rpm for 10 min, and the supernatant was transferred to an HPLC vial and brought to dry. Samples were resuspended in 100 μL acetonitrile and water mixture (1:1) of which 95 μL was injected onto a C18 analytical column (2.1 × 150mm, 5 μm). Fractions were collected from 4.5 to 7 min and centrifuged, and the supernatant was analyzed by mass spectrometry according to an earlier study.42
Statistical Analysis
All the experiments were performed at least three times and data were expressed as mean ± SD. Wherever appropriate, the data were also subjected to unpaired two-tailed Student’s t test and two-way ANOVA. *p < 0.05 was considered as significant.
Acknowledgments
Authors would like to acknowledge the funding from NIH/NCI [R01CA224306, R01CA175772, and U01CA185490 (to APS);R01CA204801and R01CA231925 (to SS)]; and USAMCI (to APS and SS).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03136.
DLS analysis of empty exosomes after different cycles of sonication (PDF)
Author Contributions
⊥ These authors contributed equally to this manuscript
Author Contributions
R.K., S.S., and A.P.S. conceived the idea; R.K., M.A.K., and S.K.S. performed the experiments; R.K., M.A.K., S.K.D, and, S.K.S. analyzed data; R.K., M.A.K., M.K., and S.K.D. wrote the original draft; and A.P.S. and S.S. supervised the study.
The authors declare no competing financial interest.
Supplementary Material
References
- Arora S.; et al. Honokiol: a novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012, 12, 1244–1252. 10.2174/156652412803833508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S.; et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One 2011, 6, e21573 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averett C.; et al. Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor-stromal cross-talk. Carcinogenesis 2016, 37, 1052–1061. 10.1093/carcin/bgw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-d.; Shang Y.; Li Y.; Chen S.-z. Honokiol inhibits breast cancer cell metastasis by blocking EMT through modulation of Snail/Slug protein translation. Acta Pharmacol. Sin. 2019, 40, 1219–1227. 10.1038/s41401-019-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen M.; et al. Honokiol induces apoptosis of lung squamous cell carcinoma by targeting FGF2-FGFR1 autocrine loop. Cancer Med. 2018, 7, 6205–6218. 10.1002/cam4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan M.; Chakraborty S.; Flynn E.; Zurakowski D.; Pal S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci. Rep. 2017, 7, 5900. 10.1038/s41598-017-05455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godugu C.; Doddapaneni R.; Singh M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC). Colloids Surf., B 2017, 153, 208–219. 10.1016/j.colsurfb.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleanu D. M.; Chircov C.; Grumezescu A. M.; Volceanov A.; Teleanu R. I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. 10.3390/pharmaceutics10040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y.; Yin M.; Zhao L.; Meng F.; Luo L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. 10.20892/j.issn.2095-3941.2017.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son K. H.; Hong J. H.; Lee J. W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, Volume 11, 5163–5185. 10.2147/IJN.S112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong W. H.; Borm P. J. A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski A.; et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M.; Martin-Jaular L.; Lavieu G.; Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Patel G. K.; Patton M. C.; Singh S.; Khushman M.; Singh A. P. Pancreatic Cancer Exosomes: Shedding Off for a Meaningful Journey. Pancreatic Disord. Ther. 2016, 06, e148 10.4172/2165-7092.1000e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton M. C.; Zubair H.; Khan M. A.; Singh S.; Singh A. P. Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival. J. Cell. Biochem. 2020, 121, 828–839. 10.1002/jcb.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. K.; et al. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme. DCK. Br. J. Cancer 2017, 116, 609–619. 10.1038/bjc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanapally R.; et al. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int. J. Nanomed. 2019, Volume 14, 531–541. 10.2147/IJN.S191313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson H. C.; Svensson K. J.; van Kuppevelt T. H.; Li J.-P.; Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 17380–17385. 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzás E. I.; Tóth E. Á.; Sódar B. W.; Szabó-Taylor K. É. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. 10.1007/s00281-018-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K.; Wang S.; Zhao R. C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Res. 2019, 7, 8. 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets N.; et al. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. 10.1021/acs.nanolett.8b04148. [DOI] [PubMed] [Google Scholar]
- Pan J.; et al. Honokiol Decreases Lung Cancer Metastasis through Inhibition of the STAT3 Signaling Pathway. Cancer Prev. Res. 2017, 10, 133–141. 10.1158/1940-6207.CAPR-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.; et al. Synthesis, characterization and in vivo evaluation of honokiol bisphosphate prodrugs protects against rats’ brain ischemia-reperfusion injury. Asian J. Pharm. Sci. 2019, 14, 640–648. 10.1016/j.ajps.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.; et al. Honokiol nanoparticles in thermosensitive hydrogel: therapeutic effects on malignant pleural effusion. ACS Nano 2009, 3, 4080–4088. 10.1021/nn900785b. [DOI] [PubMed] [Google Scholar]
- Luan X.; et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. J.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Controlled Release 2015, 207, 18–30. 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolak I.; Galanty A.; Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem. Rev. 2010, 9, 425–474. 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen K. B.; et al. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016, 68, 2125–2138. 10.1007/s10616-016-9952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antimisiaris S. G.; Mourtas S.; Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abello J.; Nguyen T. D. T.; Marasini R.; Aryal S.; Weiss M. L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345. 10.7150/thno.30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan J. E.; et al. Exosomes as Drug Carriers for Cancer Therapy. Mol. Pharmaceutics 2019, 16, 1789–1798. 10.1021/acs.molpharmaceut.9b00104. [DOI] [PubMed] [Google Scholar]
- Zhuang X.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. A.; Pink R. C.; Carter D. R. F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. E.; Relling M. V. Clinical pharmacokinetics-pharmacodynamics of anticancer drugs. Clin. Pharmacokinet. 1989, 16, 327–336. 10.2165/00003088-198916060-00001. [DOI] [PubMed] [Google Scholar]
- Khan M. A.; et al. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J. Biol. Chem. 2020, 295, 8413–8424. 10.1074/jbc.RA119.011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh S. K.; et al. Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett. 2017, 396, 21–29. 10.1016/j.canlet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir R.; et al. Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ. 2002, 9, 636–642. 10.1038/sj.cdd.4401012. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A.; et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget 2014, 5, 11490–11500. 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Qiu Z.; Jin Q.; Chen G.; Guo M. Cell Cycle Arrest and Apoptosis in HT-29 Cells Induced by Dichloromethane Fraction From Toddalia asiatica (L.) Lam. Front. Pharmacol. 2018, 9, 629. 10.3389/fphar.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. K.; et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-T.; Lin L.-C.; Tsai T.-H. Simultaneous determination of honokiol and magnolol in Magnolia officinalis by liquid chromatography with tandem mass spectrometric detection. Biomed. Chromatogr. 2006, 20, 1076–1081. 10.1002/bmc.644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.