Abstract
Background
Impressive progress in new therapeutic options has been made for psoriasis. Treatments include topical steroids, phototherapy, conventional, synthetic disease‐modifying drugs and an expanding list of biologics.
Objective
The primary objective of this work was to collect evidence for the creation of practice guidelines for systemic treatment of psoriasis (BETA‐PSO: Belgian Evidence‐based Treatment Advice in Psoriasis).
Methods
Evidence‐based recommendations were formulated using a quasi‐Delphi methodology after a systematic search of the literature and a consensus procedure involving 8 psoriasis experts.
Results
In this part, the use of systemic treatment in different age groups, during pregnancy, in metabolic syndrome, in patients with mental health problems, in different psoriasis subtypes and in previously systemically treated patients treatment is discussed.
Conclusion
Guidance on therapeutic choice in specific clinical situations in psoriasis is provided in order to facilitate the decision‐making in clinical practice.
Introduction
The therapeutic arsenal of psoriasis has quickly risen to the widest of any inflammatory skin disease. While this is very promising for our patients, it complicates the ‘right’ personalized therapeutic choice of the clinician. Due to the vast amount of literature, it has even for psoriasis experts become almost impossible to be aware of all studies that might be relevant in each clinical context. Patient characteristics such as age, weight or comorbidities such as diabetes or cardiovascular diseases may interact with efficacy and/or development of adverse events. Special psoriasis subtypes such as nail psoriasis, pustular psoriasis and erythrodermic psoriasis require a different approach. Despite evidence of efficacy, some drugs are licensed in all age groups (e.g. children), and additionally, previous systemic drugs may influence the outcome of subsequent treatment.
The BETA‐PSO (Belgian Evidence‐based Treatment Advice in Psoriasis) project was initiated by the Royal Belgian Society of Dermatology and Venerology (under the presidency of Jo Lambert, first author) with the intention to provide a practical aid for dermatologists to facilitate a well‐informed therapeutic decision for each patient. Although the context and reimbursement criteria of Belgium were taken into account, these recommendations are likely to be valuable for all dermatologists treating psoriasis patients worldwide.
In this project, relevant clinical questions on the treatment of psoriasis were formulated and a systematic search was performed. Subsequently, a group of 8 Belgian psoriasis experts discussed the data, rated the evidence and made specific appropriate recommendations.
Material and methods
The clinical recommendations were developed using a quasi‐Delphi consensus methodology as follows: an expert group (EG) of 8 Belgian dermatologists who treat patients with psoriasis, discussed and agreed on the recommendation of the type of systemic treatment which was considered to be advisable in a particular clinical context based on the existing evidence. The experts identified during a full‐day face‐to‐face meeting on 16 January 2019 a list of 38 questions related to real‐world situations frequently faced by clinicians when managing patients with psoriasis in their clinics.
Each expert was assigned a separate topic to summarize based on a systematic search of the literature in PubMed. Articles (including randomized controlled trials, case–control studies, observational studies, systematic reviews, meta‐analyses, case reports but excluding letters and opinion papers) on psoriasis patients treated with systemic treatments for psoriasis (conventional, synthetic and biological) were included that reported data on:
the influence of metabolic comorbidities on the outcome (efficacy on psoriasis, side‐effects) or the influence of the treatment on metabolic comorbidities
the influence of the treatment on specific clinical situations such as nail psoriasis, erythrodermic psoriasis and pustular psoriasis
the effect of being biological experienced or not
the influence of age on the outcome (efficacy, side‐effects) and the specific use of the considered drugs in defined age groups (efficacy, side‐effects)
the influence of the treatment on mental health
The following sixteen drugs for the systemic treatment of psoriasis were considered in this practical guidance:
conventional antipsoriatic drugs: acitretin, cyclosporine, dimethylfumarate, methotrexate
synthetic antipsoriatic drugs: apremilast
biological antipsoriatic drugs: tumour necrosis factor (TNF) antagonists: adalimumab, certolizumab pegol, etanercept, infliximab; interleukin (IL) 12/23 inhibitor: ustekinumab; IL23/p19 inhibitors: guselkumab, risankizumab, tildrakizumab; IL17 receptor blocker: brodalumab; IL17 inhibitors: ixekizumab, secukinumab
Searches were performed on 27 November 2018 and were restricted to publications during previous 5 years, in English language. The search was simultaneously performed for all clinical situations (BETA‐PSO part 1 and 2) (Figure S1 and Table S1). Additionally, SmPCs (Summary of Product Characteristics) of the concerned drugs were screened. An updated literature search was performed 25 January 2019.
The studies identified through electronic searches were subjected to screening of the title and abstracts to find relevant publications. During first pass, all the references were screened by single analyst as per specified population, intervention, comorbidities and outcome (PICO) criteria provided in Table 1.
Table 1.
Recommendations for systemic psoriasis treatments according to age and pregnancy/breastfeeding
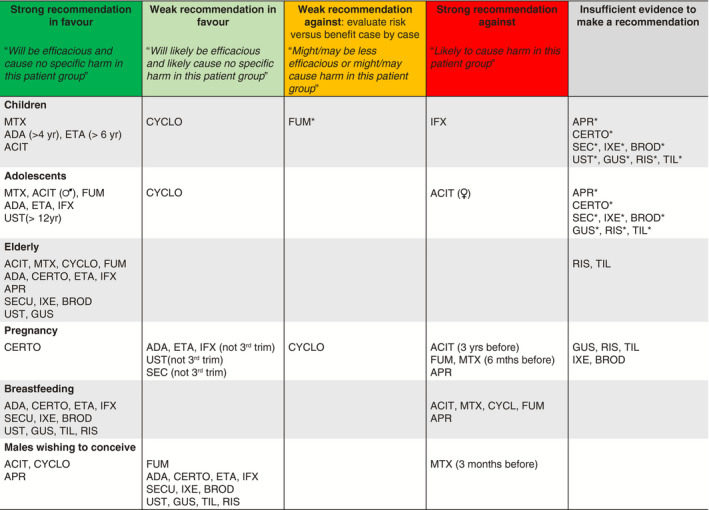
ACIT, acitretin; ADA, adalimumab; APR, apremilast; BROD, brodalumab; CERT, certolizumab pegol; CYCLO, cyclosporin; ETA, etanercept; GUS, guselkumab; IFX, infliximab; IXE, ixekizumab; RIS, risankizumab; SEC, secukinumab; TIL, tildrakizumab; UST, ustekinumab.
Unlicensed for this indication.
In addition to a comprehensive literature search of the available published evidence, pharmaceutical manufacturers of treatments currently licensed in Belgium for the systemic treatment of psoriasis were invited to provide the latest peer‐reviewed published materials on their drugs. The experts were allowed to add any additional relevant articles if deemed necessary (e.g. if published before 5 years).
The quality of the evidence (A: high, B: moderate and C: low) and the strength of the recommendations (strong vs weak) were categorized according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) criteria. 1 A high level of evidence (A) was given for data from well‐sized randomized clinical trials or extensive experience in clinical practice. Moderate evidence (B) was considered in case of observational studies, small‐sized randomized clinical trials or moderate experience in clinical practice. Low evidence (C) was attributed when only case series, retrospective studies without controls were available or there was only limited experience in clinical practice.
The outcome of the identified studies was classified as indicating (i) that the efficacy of the drug was preserved without causing increased adverse events or worsening of the comorbidity; (ii) a limited risk of decreased efficacy of the drug and/or limited risk of increased adverse events or worsening of the comorbidity, (iii) a moderate risk of decreased efficacy of the drug and/or moderate risk of increased adverse events or worsening of the comorbidity and (iv) an important risk of decreased efficacy of the drug and/or moderate risk of increased adverse events or worsening of the comorbidity.
Subsequently, the 38 clinical questions were answered by each expert via an online digital platform. The participants were able to review all comments and the supporting published evidence. For each clinical situation, the experts agreed on a ‘strong’ or ‘weak’ recommendation in favour or against the use of the concerned systemic treatments. The consolidated answers generated were summarized into clinical recommendations and reviewed by the expert group. The experts were then invited to review wording of the draft clinical recommendations and to vote (agree/disagree) (8 March 2019 to 19 April 2019) concerning the final wording of the clinical recommendations. Where there was disagreement with the draft wording, the chairperson contacted the expert to understand and clarify the issue. The recommendation was then amended on 17 September 2019 to all members’ satisfaction and agreed upon via a final voting step (agree/disagree) on 10 January 2020.
Results
Clinical recommendations
Age
Paediatric patients
In children below 12 years from an efficacy and safety perspective, we recommend that the following biological drugs, adalimumab and etanercept, and the conventional systemic drugs, methotrexate and cyclosporine (short‐term use only), are used to treat paediatric psoriasis patients. Some advisors report good results with acitretin therapy in paediatric psoriasis patients, in doses of 0, 3–0 and 5 mg/kg. However, we advise caution when using acitretin due to occasional reports of bone changes in children using retinoids. 2 A far more recent review of bone toxicity of retinoids in psoriasis (albeit not in children) did not show evidence for bone toxicity. 3
We do not recommend using infliximab in paediatric psoriasis patients due to higher reported rates of malignancies associated with infliximab use compared to the general paediatric population. 4
Some reports mention the use of fumarates although it is currently not licensed in this age group in Belgium. 5
Although there is evidence to show that the synthetic drug apremilast, the TNF inhibitor certolizumab pegol, the IL17 inhibitors, brodalumab, ixekizumab and secukinumab; the IL12/23 inhibitor ustekinumab (and other new biological drugs such as the IL23/p19 inhibitors: guselkumab, risankizumab, tildrakizumab) are efficacious in paediatric patients, we are – due to a lack of safety data – unable to recommend their use in paediatric psoriasis patients at this point in time. We also note that these drugs are not currently licensed for use in this age group.
Based on the manufacturers’ information, we note that the following age limits apply: adalimumab is recommended for the treatment of psoriasis patients above 4 years and etanercept in patients above 6 years of age. 6 , 7
Adolescent patients
In young people with psoriasis, from an efficacy and safety perspective, we recommend the biological drugs, adalimumab, etanercept, infliximab and ustekinumab, and the conventional drugs acitretin, cyclosporine (for short‐term use only), fumarates and methotrexate, for the treatment of adolescent patients, 12–18 years.
However, we do not recommend acitretin in adolescent girls with psoriasis due to the risk of teratogenicity and the need for contraception for 3 years following stopping acitretin treatment.
Similar to paediatric patients, apremilast, the TNF inhibitor certolizumab pegol, the IL17 inhibitors, brodalumab, ixekizumab and secukinumab, and the IL23/p19 inhibitors, guselkumab, risankizumab and tildrakizumab, can currently not be recommend in these patients due to limited data and lack of licence for use in children.
Based on the manufacturers’ information, we note that the following age limits apply: ustekinumab is recommended for the treatment of psoriasis patients greater than 12 years. 8
Elderly patients
From a safety and efficacy perspective in elderly psoriasis patients, we recommend the following biological drugs adalimumab, certolizumab pegol, etanercept and infliximab; brodalumab, ixekizumab and secukinumab; ustekinumab, guselkumab, risankizumab, tildrakizumab and, the synthetic drug, apremilast.
We also recommend the conventional drugs, methotrexate, cyclosporine, and fumarates and acitretin are used to treat patients (greater than 65 years) with psoriasis.
Pregnancy/Lactation
Female patients of childbearing age
Based on the available placental transfer and pregnancy outcomes evidence, our recommendation for female patients with psoriasis wishing to conceive or who may be pregnant and where treatment is clinically needed is to use of the Fc‐free biological drug certolizumab pegol as first‐line treatment, followed by either adalimumab or etanercept. 9 , 10 , 11
However, the use of Fc‐containing biologics (including etanercept, adalimumab) during the third trimester is not recommended. We also advise that infliximab, secukinumab, ixekizumab, brodalumab and ustekinumab may be used in female patients wishing to conceive; however, data are limited with these biological drugs. 12 , 13 , 14
Regarding the conventional drugs, cyclosporine should not be used during pregnancy unless the potential benefit to the mother justifies the potential risk to the foetus. 15 We also recommend that female psoriasis patients who may wish to conceive to have completed acitretin treatment at least 3 years before conception due to the high teratogenicity risk associated with this drug. Likewise, we advise female patients to complete treatment with fumarates and methotrexate for at least 6 months before conception. 16 , 17 Regarding the newer biological drugs, we note from the manufacturer’s information that pregnancy should be avoided for 17 weeks after treatment with tildrakizumab, for 12 weeks with guselkumab and for 21 weeks with risankizumab. 18 Finally, apremilast is contraindicated in female psoriasis patient wishing to conceive or who may be pregnant. This is based on animal data indicating apremilast can cause foetal loss in mice and monkeys. 19
Breastfeeding
From a safety perspective, we advise that the majority of the biological drugs may be safely used to treat female psoriasis patients who are also breastfeeding, as they are denatured in the gastro‐intestinal tract of the infant. 20
Apremilast, and fumarates, methotrexate, cyclosporine and acitretin are contraindicated during lactation due to adverse risk posed to the feeding infant. Therefore, we recommend that they should be avoided while breastfeeding.
Males wishing to conceive
From a safety perspective, we recommend that cyclosporine, acitretin and apremilast and fumarates can be used to treat male psoriasis patients who are wishing to conceive. The only formal contraindication is methotrexate with a recommendation to stop 6 months prior to conception. However, this is not evidenced by clear data on paternal‐mediated teratogenicity. 21
On the use of biologics (TNF inhibitors, IL17 blockers, IL17 receptor blocker and IL23 blockers) in males wishing to conceive, there is currently no clear evidence pointing to an increased risk. 22
Mental health
Psychiatric disorders
Patients with psoriasis are more affected by depression, anxiety, suicidal ideation behaviour (SIB), lack of confidence, insomnia and poor quality of life (QOL), and we also know that these symptoms are reduced with effective treatment in these patients. 23 Therefore, we recommend that the rapidity of onset of action with effective treatment to improve the QOL in psoriasis patients with psychiatric issues may be an advantage. Large studies showed a sustained benefit of biologics in reducing antidepressant use among psoriasis patients. The beneficial effect was more significant with continuous treatment. 24 , 25 Biological treatments seem more effective in reducing depression and insomnia than DMARDs. 24 , 26 However, there is a lack of robust comparative data between the different biological drugs. Although adalimumab, etanercept and ustekinumab were associated with a statistically significant reduction in depressive symptoms, comparison between the drugs could not be made due to different rating scales being used. 27 One study found greater improvements in anxiety and depression with guselkumab vs adalimumab. 23 Studies have shown that the IL‐17 antagonists, secukinumab and ixekizumab improve patients’ QOL and alleviate depression in 40% patients, respectively. 28 , 29 Fumarates have also been shown to reduce depressive symptoms in patients. 30
The TNF antagonists: infliximab, adalimumab, certolizumab pegol and etanercept; the IL12/23 inhibitor: ustekinumab; the IL23/p19 inhibitors: guselkumab, risankizumab and tildrakizumab; the IL17 inhibitors: secukinumab and ixekizumab; and conventional drugs, including methotrexate, cyclosporin, fumarates and acitretin, have been shown to be effective when used as systemic treatments for psoriasis patients with psychiatric issues such as depression.
Although the AMAGINE studies also confirm the improvement of patients' quality of life with brodalumab, we would advise caution, however, when using the IL17 receptor blocker, brodalumab, in patients with a history of depression as suicidal behaviour has been reported in patients treated with an FDA‐mandated black box warning regarding suicide. 31 However, the EMA, Health Canada and the FDA, as well as recent reports, cannot confirm a causal relationship between brodalumab and suicidal ideation and behaviour. 32 We would also advise caution using the synthetic drug, apremilast, due to an increased risk of psychiatric disorders. Despite several studies showing an improvement in patients’ QOL with treatment, an increased risk of mental disorders has been associated with its use in psoriasis patients. 33 , 34 , 35 The risks and benefits of starting or continuing treatment with apremilast should be carefully assessed whether patients report previous or existing psychiatric symptoms or whether concomitant treatment with other medicinal products likely to cause psychiatric events is intended. 19
Metabolic disorders
Metabolic syndrome
We advise that the TNF antagonists: adalimumab, certolizumab pegol, etanercept and infliximab; the IL12/23 inhibitor: ustekinumab; the IL23/p19 inhibitors: guselkumab, risankizumab and tildrakizumab; the IL17 receptor blocker: brodalumab; the IL17 inhibitors: ixekizumab and secukinumab; as well as the synthetic small molecule drug, apremilast, and the conventional drugs, methotrexate, cyclosporine, fumarates and acitretin can all be used as systemic treatments for adult psoriasis patients with metabolic syndrome. This is primarily because they provide effective treatment to reduce the symptoms of psoriasis and improve patients’ quality of life. Secondly, some of the drugs might also offer some beneficial impact on the cardiovascular risk factors associated with the metabolic syndrome. 36
There is currently an ongoing debate concerning improvement in physiological measures of metabolic syndrome resulting from various newer treatments such as TNF inhibitors for psoriasis. In fact, weight may increase in patients with their use. 37 Specifically, with cyclosporine treatment, atherogenic dyslipidaemia, arterial hypertension and glucose intolerance may worsen, and with acitretin use, atherogenic dyslipidaemia may worsen. In patients with increased risk for liver or renal toxicity, caution is also recommended for methotrexate. 38 Therefore, we recommend careful monitoring and follow‐up of patients treated with these drugs.
We also advise increased surveillance of the markers of metabolic syndrome in psoriasis patients including increasing waistline, elevated blood pressure, raised triglyceride levels, reduced HDL cholesterol and raised fasting glycemia. We also advise patients with metabolic syndrome receive obesity management and smoking cessation advice, as relevant.
Type II diabetes and/or insulin resistance
From a safety perspective, none of the biological and non‐biological systemic treatments available for patients with psoriasis are specifically contraindicated in those patients who also have type 2 diabetes and/or insulin resistance. It is difficult to make meaningful clinical recommendations due to a lack of comparative data between these drugs. Some studies suggest that the use of anti‐TNF drugs, such as infliximab, adalimumab, certolizumab pegol and etanercept, is associated with decreased insulin resistance. 39 , 40 , 41 Another study, however, has shown no benefit with anti‐TNF drugs in combination with methotrexate versus methotrexate alone on HbA1C or fasting blood glucose in psoriasis patients. 42 Methotrexate and acitretin have also been linked to a decreased insulin resistance in psoriasis. 43 We do advise caution with the use of cyclosporine and methotrexate in these patients due to an increased risk of liver and renal toxicity. 38
All of the systemic treatments considered can be used to treat psoriasis patients who also have type 2 diabetes and/or insulin resistance. We advise that the TNF antagonists: adalimumab, certolizumab pegol, etanercept and infliximab; the IL12/23 inhibitor: ustekinumab; the IL23/p19 inhibitors: guselkumab, risankizumab and tildrakizumab; the IL17 receptor blocker: brodalumab; the IL17 inhibitors: ixekizumab and secukinumab; as well as the synthetic drug, apremilast, and the conventional drugs, fumarates and acitretin, can be used as systemic treatments for psoriasis patients with type II diabetes and/or insulin resistance. Cyclosporine and methotrexate can also be used although more caution is advisable.
Obesity
For obese psoriasis patients, we advise that the TNF antagonists: adalimumab, certolizumab pegol, etanercept and infliximab; the IL12/23 inhibitor: ustekinumab; the IL23/p19 inhibitors: guselkumab, risankizumab and tildrakizumab; the IL17 receptor blocker: brodalumab; the IL17 inhibitors: ixekizumab and secukinumab; as well as the synthetic drug, apremilast, and the conventional drugs, fumarates and acitretin, be used as systemic treatments. Diet interventions should be encouraged as they result in improved treatment outcomes using biologic therapy. 44
From an efficacy perspective, studies have shown that the biological drugs, ustekinumab, infliximab, adalimumab and etanercept, and most conventional drugs require higher dosing in obese psoriatic patients, compared with healthy‐weight patients. Therefore, it is our opinion that weight‐adjusted dosing with these drugs may be required in obese patients. Currently, only infliximab and ustekinumab allow a specific higher dosing by weight. 45 , 46 Nonetheless, this trend is not yet clear with the newer biologics. Latest trial data on risankizumab, guselkumab, ixekizumab and brodalumab show a less pronounced effect of weight on their efficacy. 47 , 48 , 49 , 50 A few studies have reported an increase in weight associated with treatment with some of the biologics (such as TNF antagonists) although results remain contradictory to date. 51 , 52 Apremilast rather leads to weight loss. 53
From a safety perspective, we advise caution, however, with weight‐dependent dosing of the conventional drugs, methotrexate and cyclosporine, due to increased risk of renal and liver toxicities associated with increased dosing.
Cardiovascular risk factors
It is well known that psoriasis patients have an elevated risk of atherosclerosis, characterized by endothelial dysfunction. The features of metabolic syndrome, including hypertension and dyslipidaemia, are associated with endothelial activation in patients with moderate‐to‐severe psoriasis.
In patients with psoriasis and cardiovascular risk factors, we advise that the biologics including adalimumab, certolizumab pegol, etanercept and infliximab; ustekinumab; guselkumab, risankizumab and tildrakizumab; brodalumab; ixekizumab and secukinumab; as well as the synthetic drug, apremilast, and the conventional drugs, including cyclosporine, methotrexate, fumarates and acitretin, can be used from an efficacy and safety perspective as systemic treatment.
We suggest that the TNF antagonist drugs are primarily used to treat psoriasis patients with cardiovascular risk factors. Studies show that adalimumab therapy leads to a reduction in the endothelial activation biomarker, soluble (s) E‐selectin (sE‐selectin) levels, and a decrease in intima–media thickness as an indicator of atherosclerosis has been reported. 54 , 55 Nonetheless, only few preliminary findings suggest a clinical significance. 56 No difference in cardiovascular events or atrial fibrillation was found between TNFi therapy and ustekinumab was found in a large cohort study. 57 There is also evidence to suggest that the anti‐IL17 drug, secukinumab, might have a beneficial effect on CV risk by improving the endothelial function of patients with psoriasis. 58 A recent observational study shows that biologic therapy in severe psoriasis was associated with favourable modulation of coronary plaque indices by coronary computed tomography angiography. 59 These findings highlight the importance of systemic inflammation in coronary artery disease and larger, randomized trials with all the biological drugs are required.
We advise caution with cyclosporine and acitretin treatment in psoriasis patients with cardiovascular risk factors as they have been shown to increase the risk of hypertension and dyslipidaemia, and hyperlipidaemia alone, respectively. However, these side‐effects are manageable with appropriate treatment and should not form a formal contraindication for its use.
We note that several of the anti‐TNF drugs are contraindicated in psoriasis patients with moderate or severe heart failure (NYHA class III/IV) including adalimumab, certolizumab pegol and infliximab and that the entire class should be used with caution in patients with mild heart failure (NYHA class I/II).
Non‐alcoholic fatty liver disease
From an efficacy and safety perspective, most biological and non‐biological systemic drugs for psoriasis can be used effectively to treat psoriasis patients who also have non‐alcoholic fatty liver disease. However, there is no evidence to show whether one of the listed drugs is more or less efficacious compared with another in a patient with psoriasis and fatty liver disease. This is due to a lack of comparative studies between the drugs in this patient group.
In psoriasis patients with non‐alcoholic fatty liver disease, we recommend that the biological drugs, infliximab, adalimumab, certolizumab pegol and etanercept; ustekinumab, guselkumab, risankizumab and tildrakizumab; brodalumab, ixekizumab and secukinumab; the synthetic drug, apremilast; and the conventional drug, cyclosporine, are used from an efficacy and safety perspective.
From a safety perspective, methotrexate has been shown to cause elevated liver function tests in patients. Caution is warranted with methotrexate but also with fumarates and acitretin. This is because methotrexate has been shown to increase liver function tests in these patients, although no cases of liver failure have been observed with methotrexate treatment. 60 Several cases of liver toxicity have been described with fumarates in patients with multiple sclerosis although severe liver injury has not been reported in psoriasis. 61 , 62 Regarding acitretin, serum aminotransferase elevation has been noted, but is usually self‐limiting. 63
Psoriasis subtypes
Nail psoriasis
From an efficacy perspective, we recommend in psoriasis patients with nail disease the TNF antagonists: infliximab, adalimumab, certolizumab pegol and etanercept; the IL12/23 inhibitor: ustekinumab; the IL23/p19 inhibitors: guselkumab and risankizumab; the IL17 receptor blocker: brodalumab; the IL17 inhibitors: ixekizumab and secukinumab; and the synthetic drug, apremilast. 64 , 65 , 66 , 67 Also, the conventional drugs dimethylfumarate, cyclosporine and methotrexate are used based on clinical trial data. There is less robust evidence supporting the use of the conventional drugs cyclosporin, methotrexate and acitretin; however, it is our opinion that these drugs also provide benefit to psoriasis patients with nail disease especially in patients with limited skin involvement (PASI/BSA < 10). 68 There is also limited evidence supporting the use of dimethylfumarate. 69 There was no recommendation of the experts on the use of tildrakizumab in these patients, mainly due to a paucity of supporting data.
Generalized pustular psoriasis (GPP)
We recommend the use of acitretin for the treatment of patients with generalized pustular psoriasis (GPP), as it is the only drug licensed for this indication. There is also evidence supporting the use of methotrexate, adalimumab, ixekizumab, secukinumab, brodalumab and guselkumab to treat GPP patients and, based on our collective experience, we advise their use in these patients. 70 , 71 , 72 , 73 , 74 There is less robust evidence based on limited case series and case reports supporting the use of infliximab, etanercept, ustekinumab, risankizumab, tildrakizumab, apremilast and cyclosporine in GPP patients. 75 , 76 We do not recommend the use of fumarates in the systemic treatment of GPP patients, due their slow mode of action and potential hypersensitivity reactions worsening the disease.
Erythrodermic psoriasis
We agree that cyclosporine and infliximab appear to be the most rapidly acting agents for the treatment of erythrodermic psoriasis. Acitretin and methotrexate are also appropriate first‐line choices, although they usually work more slowly. 77
There is also clinical evidence supporting the use of the biological IL12/23 inhibitor, ustekinumab for the treatment of erythrodermic psoriasis. 78 , 79 Beneficial results with secukinumab, ixekizumab, brodalumab, guselkumab, adalimumab, etanercept, apremilast and cyclosporine have been observed to treat erythrodermic psoriasis patients. However, this is based on some published open‐label studies and case series mostly in Japanese patients. 72 , 73 , 74 , 80 Based on our collective experience, we advise their use. No data on use of risankizumab nor tildrakizumab were found.
We do not recommend the use of fumarates, however, in the systemic treatment of erythrodermic psoriasis patients, due to their slow mode action and potential hypersensitivity reactions worsening the disease.
Practical use of biologics
Biologic‐experienced patients
In biologic‐experienced patients, drug survival is better if switch is performed between as opposed to within biologic classes. 81 However, in case a deliberate choice was made for a certain biologic class based on efficacy, side‐effects or comorbidities, evidence indicates that switch within class is also a good option if a biologic with a higher efficacy is chosen. 82 The general consensus of the expert discussion was to consider a switch to another biologic class or to opt for the biologic within the same class exhibiting the highest efficacy in clinical trials. Overall, recent data suggest that newer biologics are less affected by a history of previous failure to another biologic. 83
Intermittent treatment
Regarding biologics, we recommend continuous systemic treatment for patients with psoriasis when the patient is still receiving benefit. Should a psoriasis patient wish to stop and then restart systemic treatment, we advise that it is possible to do so, particularly with etanercept and ustekinumab. 84 , 85 Conventional drugs and synthetic drug apremilast seem well suited for intermittent treatment. There is robust evidence that demonstrates these drugs can be stopped and then restarted with equivalent efficacy and without increased risk of flare of disease. For adalimumab, certolizumab pegol, guselkumab, risankizumab and tildrakizumab, ixekizumab, secukinumab and brodalumab, the evidence is less clear. Fumarates can also be used intermittently although they exhibit a slow onset of action. 86 This observation is based on our collective opinion and experience.
There is currently insufficient evidence to support stopping and then restarting treatment with other biological drugs, including adalimumab, certolizumab pegol and tildrakizumab. We advise caution with this approach until these data are available. For guselkumab, risankizumab, ixekizumab and secukinumab, promising results when retreating after drug withdrawal were obtained in clinical trial settings indicating that intermittent treatment might be an option with the newer IL23/p19 inhibitors and IL17 blockers. 87 , 88
However, we recommend against stopping and restarting infliximab due to reduced efficacy on restarting treatment as a result of the development of antidrug antibodies. From a safety perspective, there is also an increased risk of serious infusion reactions with intermittent infliximab dosing. 46 , 89
Use of biosimilars
From an efficacy and safety perspective, we advise that it is possible to switch from TNF antagonist reference drugs, infliximab, adalimumab and etanercept, to their respective biosimilar. 90 However, this advice is based on some but not all of the groups’ experience with switching.
Discussion
Systemic psoriasis treatments result in a variable clinical efficacy and adverse event rate depending on pre‐existing patients’ characteristics. The decision aid based on the current evidence in Tables 1, 2 and 3 can be a valuable tool in clinical practice to guide the therapeutic choice. Unfortunately, evidence is in a substantial part limited to ‘level C’, indicating case series, case reports or limited experience in clinical practice. In case of new biologics, data are often still lacking. Head‐to‐head comparison of drugs is very limited, especially in less common disease presentations such as generalized pustular psoriasis. Nonetheless, obvious ‘red’ and ‘orange’ flags should be recognized by every physician before a treatment is initiated.
Table 2.
Recommendations for systemic psoriasis treatments according to comorbidities, psoriasis subtypes and intermittent treatment
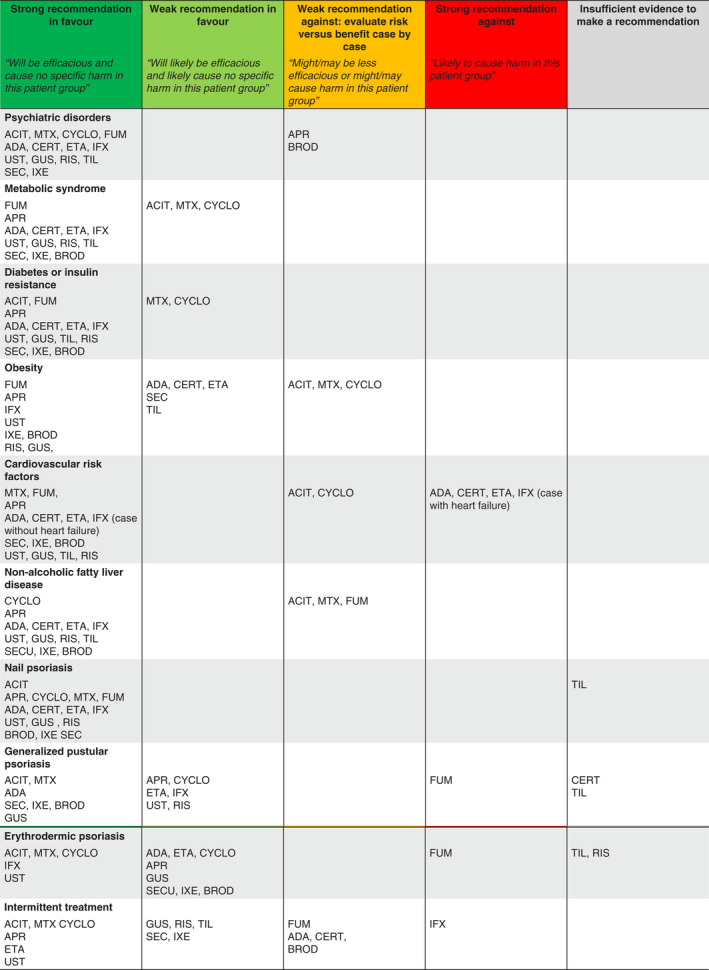
ACIT, acitretin; ADA, adalimumab; APR, apremilast; BROD, brodalumab; CERT, certolizumab pegol; CYCLO, cyclosporin; ETA, etanercept; GUS, guselkumab; IFX, infliximab; IXE, ixekizumab; RIS, risankizumab; SEC, secukinumab; TIL, tildrakizumab; UST, ustekinumab.
Green: will be efficacious and cause no specific harm in this patient group; Light green: will likely be efficacious and likely cause no specific harm in this patient group; Orange: might/may be less efficacious or might/may cause harm in this patient group; Red: likely to cause harm in this patient group; Grey: insufficient evidence to make a recommendation.
Table 3.
Evidence of systemic treatments for psoriasis in different clinical conditions
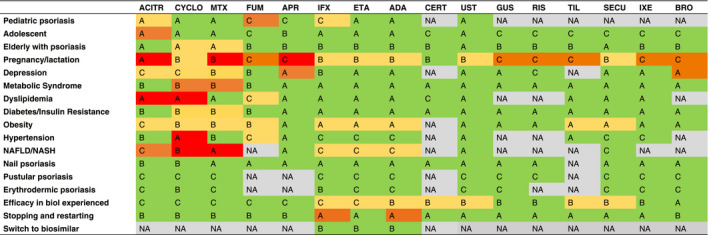
Levels of evidence: A (high level of evidence), B (moderate level of evidence), C (low level of evidence).
Results of the studies: 1. Green: preserved efficacy without increased adverse events or worsening of the comorbidity; 2. Yellow: limited risk of decreased efficacy and/or limited risk of increased adverse events or worsening of the comorbidity, 3. Orange: moderate risk of decreased efficacy and/or moderate risk of increased adverse events or worsening of the comorbidity, 4. Red: important risk of decreased efficacy and/or moderate risk of increased adverse events or worsening of the comorbidity.
ACIT, acitretin; ADA, adalimumab; APR, apremilast; BROD, brodalumab; CERT, certolizumab pegol; CYCLO, cyclosporin; ETA, etanercept; GUS, guselkumab; IFX, infliximab; IXE, ixekizumab; RIS, risankizumab; SEC, secukinumab; TIL, tildrakizumab; UST, ustekinumab.
Regarding age, many drugs are not licensed for use in children such as ustekinumab (>12 years), fumarates or apremilast (>18 years). Some experts chose acitretin as first choice in order to avoid long‐term immunosuppressive effects despite the concern of bone changes based on data with long‐term use of etretinate. 2 Older age, pre‐existing renal or liver injury may increase the risk of adverse events of conventional drugs such as methotrexate and cyclosporin. 91 Furthermore, the risk of drug–drug interactions is increased in (elderly) patients with polypharmacy using methotrexate or cyclosporine and apremilast. 19 , 92
In metabolic syndrome, several conventional drugs including cyclosporine, acitretin and methotrexate exhibit an unfavourable effect on lipids, hypertension and liver injury. However, as most issues are manageable by accurate intervention (e.g. diet, statins, antihypertensive medication), this does not preclude their use. Nonetheless, apremilast and biologics seem often a more favourable choice in patients with metabolic syndrome.
No clear data have shown a convincing different response of systemic treatments in nail psoriasis compared to psoriasis vulgaris. However, a BSA and/or PASI> 10 is often not reached in patients with nail psoriasis limiting the use of biologics in these patients. In Belgium, an extensive BSA involvement is not a requirement for reimbursement of fumarates which makes it a reasonable option despite limited reports on nail psoriasis.
Pustular and erythrodermic psoriasis display a different disease pattern requiring a tailored approach. Acitretin is the only licensed drug in generalized pustular psoriasis, although often combination therapy with corticosteroids is necessary, and it is contraindicated in women of childbearing age. Regarding erythrodermic psoriasis, one expert raised the concern of the differential diagnosis with cutaneous T‐cell lymphoma. In the latter case, acitretin may be the safest option until the final diagnosis is established.
A limitation of these recommendations is that only the viewpoint of dermatologists was taken into account. The expert group emphasized the need for a multidisciplinary approach in patients with important comorbidities. These guidelines do not replace the need for shared decision‐making as patients may balance efficacy versus side‐effects differently.
Given the rapid evolution of the therapeutic landscape of psoriasis, readers should be aware that this project is a living guideline that will require a regular update based on new data. This is certainly the case for the new class of specific IL‐23 inhibitors which have currently limited available data.
Supporting information
Figure S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram for screening and selection of the literature search in patients with psoriasis.
Table S1. Eligibility criteria for study screening.
Acknowledgements
We wish to thank the other board members of the Royal Belgian Society of Dermatology and Venerology : Josette André, Bernard Bouffioux, Véronique del Marmol, Marjan Garmyn, Jan Gutermuth, Stéphanie Ryckaert, Mark Vandaele and Katrien Vossaert, for their advise in the design and their continuous support of this work.
Funding source
Funding for this project was provided to the Royal Belgian Society of Dermatology and Venerology by six pharmaceutical companies: Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and UCB. These companies were, however, not involved in the making of the manuscript.
Conflict of Interest
Authors have no conflict of interest with regard to the topic of this manuscript.
References
- 1. Guyatt GH, Oxman AD, Vist GE et al GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halkier‐Sørensen L, Laurberg G Andresen J. Bone changes in children on long‐term treatment with etretinate. J Am Acad Dermatol 1987; 16(5 Pt 1): 999–1006. [DOI] [PubMed] [Google Scholar]
- 3. Sbidian E, Maza A, Montaudié H et al Efficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature review. J Eur Acad Dermatol Venereol 2011; 25(Suppl 2): 28–33. [DOI] [PubMed] [Google Scholar]
- 4. Diak P, Siegel J, Grenade LL, Choi L, Lemery S, McMahon A. Tumor necrosis factor α blockers and malignancy in children: Forty‐eight cases reported to the food and drug administration. Arthritis Rheum 2010; 62: 2517–2524. [DOI] [PubMed] [Google Scholar]
- 5. van Geel MJ, van de Kerkhof PCM, Oostveen AM, de Jong EMGJ, Seyger MMB. Fumaric acid esters in recalcitrant pediatric psoriasis: a prospective, daily clinical practice case series. J Dermatolog Treat 2016; 27: 214–220. [DOI] [PubMed] [Google Scholar]
- 6. Humira 40 mg solution for injection in pre‐filled syringe ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/2150/smpc (last accessed: 25 January 2020).
- 7. Enbrel 25 mg powder and solvent for solution for injection ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/3837/smpc (last accessed: 25 January 2020).
- 8. STELARA 45 mg solution for injection (vials) ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/4413/smpc (last accessed: 25 January 2020).
- 9. Ghalandari N, Dolhain RJEM, Hazes JMW et al The pre‐ and post‐authorisation data published by the European Medicines Agency on the use of Biologics during pregnancy and lactation. Br J Clin Pharmacol 2019; 86: 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clowse MEB, Scheuerle AE, Chambers C et al Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol 2018; 70: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariette X, Förger F, Abraham B et al Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018; 77: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis JR, Mariette X, Gaujoux‐Viala C et al Long‐term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open 2019; 5: e000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plachouri K‐M, Georgiou S. Special aspects of biologics treatment in psoriasis: management in pregnancy, lactation, surgery, renal impairment, hepatitis and tuberculosis. J Dermatolog Treat 2019; 30: 668–673. [DOI] [PubMed] [Google Scholar]
- 14. Warren RB, Reich K, Langley RG et al Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol 2018; 179: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 15. Neoral Soft Gelatin Capsules ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/1034/smpc (last accessed: 25 January 2020).
- 16. Tecfidera 120mg gastro‐resistant hard capsules ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/5256/smpc#PREGNANCY (last accessed: 25 January 2020).
- 17. Methotrexate 2.5 mg Tablets ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/511/smpc (last accessed 25 January 2020).
- 18. Ilumetri 100 mg solution for injection in pre‐filled syringe ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/9819/smpc (last accessed: 25 January 2020).
- 19. Otezla 30 mg Film‐Coated Tablets ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/3648/smpc (last accessed 25 January 2020).
- 20. Witzel SJ. Lactation and the use of biologic immunosuppressive medications. Breastfeed Med 2014; 9: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutierrez JC, Hwang K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin Drug Metab Toxicol 2017; 13: 51–58. [DOI] [PubMed] [Google Scholar]
- 22. Mouyis M, Flint JD, Giles IP. Safety of anti‐rheumatic drugs in men trying to conceive: a systematic review and analysis of published evidence. Semin Arthritis Rheum 2019; 48: 911–920. [DOI] [PubMed] [Google Scholar]
- 23. Gordon KB, Armstrong AW, Han C et al Anxiety and depression in patients with moderate‐to‐severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol 2018; 32: 1940–1949. [DOI] [PubMed] [Google Scholar]
- 24. Wu C‐Y, Chang Y‐T, Juan C‐K et al Depression and insomnia in patients with psoriasis and psoriatic arthritis taking tumor necrosis factor antagonists. Medicine (Baltimore) 2016; 95: e3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strober B, Gooderham M, de Jong EMGJ et al Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol 2018; 78: 70–80. [DOI] [PubMed] [Google Scholar]
- 26. Salame N, Ehsani‐Chimeh N, Armstrong AW. Comparison of mental health outcomes among adults with psoriasis on biologic versus oral therapies: a population‐based study. J Dermatolog Treat 2019; 30: 135–140. [DOI] [PubMed] [Google Scholar]
- 27. Fleming P, Roubille C, Richer V et al Effect of biologics on depressive symptoms in patients with psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2015; 29: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 28. Griffiths CEM, Fava M, Miller AH et al Impact of Ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate‐to‐severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom 2017; 86: 260–267. [DOI] [PubMed] [Google Scholar]
- 29. Mease P, Lebwohl M, Gilloteau I et al Secukinumab treatment of psoriatic arthritis and moderate to severe psoriasis relieves anxiety/depression up to 52 weeks: an overview from secukinumab phase 3 clinical trials. Arthritis Rheumatol 2017; 69(Suppl 10): 607. [Google Scholar]
- 30. Schmieder A, Poppe M, Hametner C et al Impact of fumaric acid esters on cardiovascular risk factors and depression in psoriasis: a prospective pilot study. Arch Dermatol Res 2015; 307: 413–424. [DOI] [PubMed] [Google Scholar]
- 31. Lebwohl MG, Papp KA, Marangell LB et al Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol 2018; 78: 81–89.e5. [DOI] [PubMed] [Google Scholar]
- 32. Koo J, Ho RS Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis 2019; 104: 361–365. [PubMed] [Google Scholar]
- 33. Crowley J, Thaçi D, Joly P et al Long‐term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol 2017; 77: 310–317.e1. [DOI] [PubMed] [Google Scholar]
- 34. Thaçi D, Kimball A, Foley P et al Apremilast, an oral phosphodiesterase 4 inhibitor, improves patient‐reported outcomes in the treatment of moderate to severe psoriasis: results of two phase III randomized, controlled trials. J Eur Acad Dermatol Venereol 2017; 31: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmutz J‐L. Apremilast: beware of suicidal ideation and behaviour. Ann Dermatol Venereol 2017; 144: 243–244. [DOI] [PubMed] [Google Scholar]
- 36. Gelfand JM, Shin DB, Alavi A et al A phase IV, randomized, double‐blind, placebo‐controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP‐U Trial). J Invest Dermatol 2019; 140: 85–93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu M‐Y, Yu C‐L, Yang S‐J, Chi C‐C. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta‐analysis. J Am Acad Dermatol 2019; 82: 101–109. [DOI] [PubMed] [Google Scholar]
- 38. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol 2018; 36: 21–28. [DOI] [PubMed] [Google Scholar]
- 39. Costa L, Caso F, Atteno M et al Impact of 24‐month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol 2014; 33: 833–839. [DOI] [PubMed] [Google Scholar]
- 40. Chen D‐Y, Chen Y‐M, Hsieh T‐Y, Hsieh C‐W, Lin C‐C, Lan J‐L. Significant effects of biologic therapy on lipid profiles and insulin resistance in patients with rheumatoid arthritis. Arthritis Res Ther 2015; 17: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al‐Mutairi N Shabaan D. Effects of tumor necrosis factor α inhibitors extend beyond psoriasis: insulin sensitivity in psoriasis patients with type 2 diabetes mellitus. Cutis 2016; 97: 235–241. [PubMed] [Google Scholar]
- 42. Wu JJ, Rowan CG, Bebchuk JD Anthony MS. No association between TNF inhibitor and methotrexate therapy versus methotrexate in changes in hemoglobin A1C and fasting glucose among psoriasis, psoriatic arthritis, and rheumatoid arthritis patients. J Drugs Dermatol 2015; 14: 159–166. [PubMed] [Google Scholar]
- 43. Karadag AS, Ertugrul DT, Kalkan G et al The effect of acitretin treatment on insulin resistance, retinol‐binding protein‐4, leptin, and adiponectin in psoriasis vulgaris: a noncontrolled study. Dermatology 2013; 227: 103–108. [DOI] [PubMed] [Google Scholar]
- 44. Al‐Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther 2014; 14: 749–756. [DOI] [PubMed] [Google Scholar]
- 45. STELARA 45 mg solution for injection (vials) ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/4413/smpc (last accessed: 25 January 2020).
- 46. Remicade 100mg powder for concentrate for solution for infusion ‐ Summary of Product Characteristics (SmPC) ‐ (emc). URL https://www.medicines.org.uk/emc/product/3831/smpc#POSOLOGY (last accessed: 25 Jan 2020).
- 47. Timmermann S, Hall A. Population pharmacokinetics of brodalumab in patients with moderate to severe plaque psoriasis. Basic Clin Pharmacol Toxicol 2019; 125: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gordon KB, Blauvelt A, Foley P et al Efficacy of guselkumab in subpopulations of patients with moderate‐to‐severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol 2018; 178: 132–139. [DOI] [PubMed] [Google Scholar]
- 49. Khatri A, Eckert D, Oberoi R et al Pharmacokinetics of risankizumab in Asian healthy subjects and patients with moderate to severe plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis. J Clin Pharmacol 2019; 59: 1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reich K, Puig L, Mallbris L, Zhang L, Osuntokun O, Leonardi C. The effect of bodyweight on the efficacy and safety of ixekizumab: results from an integrated database of three randomised, controlled Phase 3 studies of patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1196–1207. [DOI] [PubMed] [Google Scholar]
- 51. Mahé E, Reguiai Z, Barthelemy H et al Evaluation of risk factors for body weight increment in psoriatic patients on infliximab: a multicentre, cross‐sectional study. J Eur Acad Dermatol Venereol 2014; 28: 151–159. [DOI] [PubMed] [Google Scholar]
- 52. Owczarczyk‐Saczonek A, Placek W Rybak‐d’Obyrn J, Wygonowska E. Influence of ustekinumab on body weight of patients with psoriasis: an initial report. Postepy Dermatol Alergol 2014; 31: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ighani A, Georgakopoulos JR, Zhou LL, Walsh S, Shear N, Yeung J. Efficacy and safety of apremilast monotherapy for moderate to severe psoriasis: retrospective study. J Cutan Med Surg 2018; 22: 290–296. [DOI] [PubMed] [Google Scholar]
- 54. Genre F, Armesto S, Corrales A et al Significant sE‐Selectin levels reduction after 6 months of anti‐TNF‐α therapy in non‐diabetic patients with moderate‐to‐severe psoriasis. J Dermatolog Treat 2017; 28: 726–730. [DOI] [PubMed] [Google Scholar]
- 55. Jókai H, Szakonyi J, Kontár O et al Impact of effective tumor necrosis factor‐alfa inhibitor treatment on arterial intima‐media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol 2013; 69: 523–529. [DOI] [PubMed] [Google Scholar]
- 56. Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor‐α inhibitors versus methotrexate. J Am Acad Dermatol 2017; 76: 81–90. [DOI] [PubMed] [Google Scholar]
- 57. Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A Kim SC. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol 2019; 155: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Stebut E, Reich K, Thaçi D et al Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol 2019; 139: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 59. Elnabawi YA, Dey AK, Goyal A et al Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res 2019; 115: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Conway R, Low C, Coughlan RJ, O’Donnell MJ, Carey JJ. Risk of liver injury among methotrexate users: a meta‐analysis of randomised controlled trials. Semin Arthritis Rheum 2015; 45: 156–162. [DOI] [PubMed] [Google Scholar]
- 61. Jüngst C, Kim Y‐J, Lammert F. Severe drug‐induced liver injury related to therapy with dimethyl fumarate. Hepatology 2016; 64: 1367–1369. [DOI] [PubMed] [Google Scholar]
- 62. Muñoz MA, Kulick CG, Kortepeter CM, Levin RL, Avigan MI. Liver injury associated with dimethyl fumarate in multiple sclerosis patients. Mult Scler 2017; 23: 1947–1949. [DOI] [PubMed] [Google Scholar]
- 63. Chularojanamontri L, Silpa‐Archa N, Wongpraparut C Limphoka P. Long‐term safety and drug survival of acitretin in psoriasis: a retrospective observational study. Int J Dermatol 2019; 58: 593–599. [DOI] [PubMed] [Google Scholar]
- 64. Crowley JJ, Weinberg JM, Wu JJ, Robertson AD Van Voorhees AS. Treatment of nail psoriasis: best practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol 2015; 151: 87–94. [DOI] [PubMed] [Google Scholar]
- 65. Foley P, Gordon K, Griffiths CEM et al Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol 2018; 154: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Elewski BE, Okun MM, Papp K et al Adalimumab for nail psoriasis: Efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo‐controlled trial. J Am Acad Dermatol 2018; 78: 90–99.e1. [DOI] [PubMed] [Google Scholar]
- 67. Papp KA, Blauvelt A, Bukhalo M et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 68. Rigopoulos D, Baran R, Chiheb S et al Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: A dermatologist and nail expert group consensus. J Am Acad Dermatol 2019; 81: 228–240. [DOI] [PubMed] [Google Scholar]
- 69. Frambach Y, Galli E, Mohr M, Zillikens D, Ludwig R.Fun or no fun? Efficacy of fumaric acid esters in nail psoriasis: results of the FUN study. Poster presented at 23th World Congress of Dermatology (Vancouver); 2015.
- 70. Morita A, Yamazaki F, Matsuyama T et al Adalimumab treatment in Japanese patients with generalized pustular psoriasis: results of an open‐label phase 3 study. J Dermatol 2018; 45: 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Imafuku S, Honma M, Okubo Y et al Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: A 52‐week analysis from phase III open‐label multicenter Japanese study. J Dermatol 2016; 43: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 72. Saeki H, Nakagawa H, Nakajo K et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamasaki K, Nakagawa H, Kubo Y Ootaki K. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study. Br J Dermatol 2017; 176: 741–751. [DOI] [PubMed] [Google Scholar]
- 74. Sano S, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, a human interleukin‐23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52‐week, phase 3, multicenter, open‐label study. J Dermatol 2018; 45: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kearns DG, Chat VS, Zang PD, Han G, Wu JJ. Review of treatments for generalized pustular psoriasis manuscript. J Dermatolog Treat 2019: 1–13. [DOI] [PubMed] [Google Scholar]
- 76. Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult‐onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol 2014; 53: 676–684. [DOI] [PubMed] [Google Scholar]
- 77. Rosenbach M, Hsu S, Korman NJ et al Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 62: 655–662. [DOI] [PubMed] [Google Scholar]
- 78. Singh RK, Lee KM, Ucmak D et al Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl) 2016; 6: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pescitelli L, Dini V, Gisondi P et al Erythrodermic psoriasis treated with ustekinumab: an Italian multicenter retrospective analysis. J Dermatol Sci 2015; 78: 149–151. [DOI] [PubMed] [Google Scholar]
- 80. Mateu‐Puchades A, Santos‐Alarcón S, Martorell‐Calatayud A, Pujol‐Marco C, Sánchez‐Carazo J‐L. Erythrodermic psoriasis and secukinumab: our clinical experience. Dermatol Ther 2018; 31: e12607. [DOI] [PubMed] [Google Scholar]
- 81. Cozzani E, Wei Y, Burlando M, Signori A, Parodi A. Serial biologic therapies in psoriasis patients: A 12‐year, single‐center, retrospective observational study. J Am Acad Dermatol 2019; 82: 37–44. [DOI] [PubMed] [Google Scholar]
- 82. Kimmel G, Chima M, Kim HJ et al Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti‐interleukin 17A therapies have failed. J Am Acad Dermatol 2019; 81: 857–859. [DOI] [PubMed] [Google Scholar]
- 83. Wang T‐S Tsai T‐F. Biologics switch in psoriasis. Immunotherapy 2019; 11: 531–541. [DOI] [PubMed] [Google Scholar]
- 84. Segaert S, Ghislain P‐D, Boone C. An observational study of the real‐life management of psoriasis patients treated with etanercept according to the new reimbursement criteria (in Belgium). J Dermatolog Treat 2016; 27: 103–109. [DOI] [PubMed] [Google Scholar]
- 85. Choi CW, Choi JY, Kim BR, Youn SW. Economic burden can be the major determining factor resulting in short‐term intermittent and repetitive ustekinumab treatment for moderate‐to‐severe psoriasis. Ann Dermatol 2018; 30: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tzaneva S, Geroldinger A, Trattner H, Tanew A. Fumaric acid esters in combination with a 6‐week course of narrowband ultraviolet B provides an accelerated response compared with fumaric acid esters monotherapy in patients with moderate‐to‐severe plaque psoriasis: a randomized prospective clinical study. Br J Dermatol 2018; 178: 682–688. [DOI] [PubMed] [Google Scholar]
- 87. Langley R, Blauvelt A, Gooderham M et al Efficacy and safety of continuous Q12W risankizumab versus treatment withdrawal: results from the phase 3 IMMhance Trial. J Am Acad Dermatol 2019; 81: AB52. [Google Scholar]
- 88. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 89. Reich K, Wozel G, Zheng H, van Hoogstraten HJF, Flint L, Barker J. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate‐to‐severe plaque psoriasis: results of a randomized, long‐term extension trial (RESTORE2). Br J Dermatol 2013; 168: 1325–1334. [DOI] [PubMed] [Google Scholar]
- 90. Jørgensen KK, Olsen IC, Goll GL et al Switching from originator infliximab to biosimilar CT‐P13 compared with maintained treatment with originator infliximab (NOR‐SWITCH): a 52‐week, randomised, double‐blind, non‐inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 91. Bennett WM. Drug‐related renal dysfunction in the elderly In Oreopoulos DG, Hazzard WR, Luke R, eds. Nephrology and Geriatrics Integrated: Proceedings of the Conference on Integrating Geriatrics into Nephrology held in Jasper, Alberta, Canada, July 31‐August 5, 1998. Springer Netherlands, Dordrecht, 2000: 45–50. 10.1007/978-94-011-4088-1_5 [DOI] [Google Scholar]
- 92. Saurat J‐H, Guérin A, Yu AP et al High prevalence of potential drug‐drug interactions for psoriasis patients prescribed methotrexate or cyclosporine for psoriasis: associated clinical and economic outcomes in real‐world practice. Dermatology 2010; 220: 128–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram for screening and selection of the literature search in patients with psoriasis.
Table S1. Eligibility criteria for study screening.


