Abstract
Background and Aims
Nonalcoholic fatty liver disease (NAFLD) encompasses a range of conditions, from simple steatosis to nonalcoholic steatohepatitis. Studies in the United States have reported an increased mortality risk among individuals with NAFLD; therefore, the population attributable fractions (PAFs) for mortality were examined.
Approach and Results
A total of 12,253 adult individuals with ultrasound assessment of NAFLD from the Third National Health and Nutrition Examination Survey and mortality follow‐up through 2015 were included in the analysis. Cox proportional hazard regression was used to estimate multivariable‐adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for NAFLD in association with all‐cause and cause‐specific mortality. Overall, sex‐ and race/ethnicity‐specific PAFs and 95% CIs were estimated. In the current study, presence of NAFLD was associated with a 20% increased risk of all‐cause mortality (HR, 1.20; 95% CI, 1.08, 1.34). The overall PAF for all‐cause mortality associated with NAFLD was 7.5% (95% CI, 3.0, 12.0). The PAF for diabetes‐specific mortality was 38.0% (95% CI, 13.1, 63.0) overall, 40.8% (95% CI, 2.1, 79.6) in men, and 36.8% (95% CI, 6.6, 67.0) in women. The PAF for liver disease (LD)‐specific mortality was notably higher in men (68.3%; 95% CI, 36.3, 100.0) than women (3.5%; 95% CI, −39.7, 46.8). In the race‐specific analysis, the PAFs of NAFLD for all‐cause mortality (9.3%; 95% CI, 4.0, 14.6) and diabetes‐specific mortality (44.4%; 95% CI, 10.8, 78.0) were significantly greater than zero only for whites.
Conclusions
In the United States, approximately 8% of all‐cause mortality and more than one‐third of LD‐ and diabetes‐specific deaths are associated with NAFLD. With these high percentages, efforts are needed to reduce the burden of NAFLD in the United States.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CLD
chronic liver disease
- CVD
cardiovascular disease
- LD
liver disease
- HR
hazard ratio
- HS
hepatic steatosis
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NCHS
National Center for Health Statistics
- NDI
National Death Index
- NHANES III
Third National Health and Nutrition Examination Survey
- PAF
population attributable fraction
Nonalcoholic fatty liver disease (NAFLD) encompasses a range of histopathological conditions, from mild steatosis to severe nonalcoholic steatohepatitis (NASH).1 NAFLD has become the most common cause of chronic liver disease (CLD) worldwide and can lead to serious sequelae, such as end‐stage liver disease and hepatocellular carcinoma (HCC).2, 3, 4 In the United States, prevalence of NAFLD has been previously estimated to be 30% in the general population, affecting almost 100 million individuals.5, 6 NASH, characterized by the presence of lobular inflammation and hepatocyte ballooning degeneration with or without fibrosis, has an estimated prevalence of approximately 4% in the U.S. population.7
NAFLD has been associated with increased risks of all‐cause mortality as well as mortality attributable to liver disease (LD), cardiovascular disease (CVD), cancer, and diabetes.8, 9, 10, 11, 12, 13 Study results have varied, however. A Swedish study reported increased risks of all‐cause mortality, CVD, and LD.8 In contrast, a Korean study reported increased mortality risks attributed to all causes, cancer, CVD, and LDs among women, but not among men.9 In the United States, a recently reported community‐based study found that persons with NAFLD had an increased risk of mortality after a 20‐year follow‐up period.11 Other U.S. studies have analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III) and have reported inconsistent results, with an earlier analysis finding no increased risks of mortality6, 14 and a later analysis reporting a significantly increased risk of LD mortality.10 In addition, early analyses in NHANES III found no association between NASH (defined as NAFLD with elevated liver enzymes) and risk of mortality,6 whereas a more recent analysis reported an increased risk of LD mortality in association with severe hepatic steatosis (HS) and elevated liver enzymes.15 Consistent with this finding was that of a large UK study, which found that all‐cause mortality was higher among individuals with NASH than NAFLD.16
Given that prevalence of NAFLD has increased over the past decades,17 the proportion of deaths attributed to NAFLD (i.e., the population attributable fraction [PAF]) would also be expected to increase.18 Therefore, the goal of the current study was to assess the PAFs for all‐cause and cause‐specific mortality using data from NHANES III conducted between 1988 and 1994. Because attributable fractions may vary by sex and race/ethnicity, PAFs for each group were also calculated.
Participants and Methods
NHANES III is a nationally representative survey of the U.S. civilian, noninstitutionalized population that was conducted from 1988 to 1994 using a complex multistage, stratified, cluster probability sample.19 The survey consisted of a cross‐sectional household interview as well as clinical and laboratory examinations, including an abdominal ultrasound, of 14,797 adults aged 20‐74 years.19 The ultrasounds were originally performed to assess gallbladder disease using a Toshiba SSA‐90A machine (3.75 and 5.0 MHz transducer; Toshiba America Medical Systems, Tustin, CA).20 Between 2009 and 2010, archived videotapes of the ultrasounds were reviewed to assess the presence of steatosis within the hepatic parenchyma using five standard criteria: (1) parenchymal brightness; (2) liver to kidney contrast; (3) deep beam attenuation; (4) bright vessel walls; and (5) gallbladder wall definition. A detailed description of this protocol has been reported elsewhere.20 Degree of steatosis was categorized as none, mild, moderate, or severe. All participants signed informed consent. The NHANES III was approved by the institutional review board of the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC). All participants signed informed consent.
For the current analysis, participants were excluded if there was no image from the ultrasound examination or an ungradable image (n = 941), high alcohol consumption (≥2 drinks for men and ≥1 drink for women per day in the past year or if they reported a period in life where they drank ≥5 drinks almost every day; n = 1,241), viral hepatitis (n = 324), iron overload (n = 28), or no follow‐up data on mortality (n = 10). Thus, the total analytical sample consisted of 12,253 persons (Fig. 1).
Figure 1.
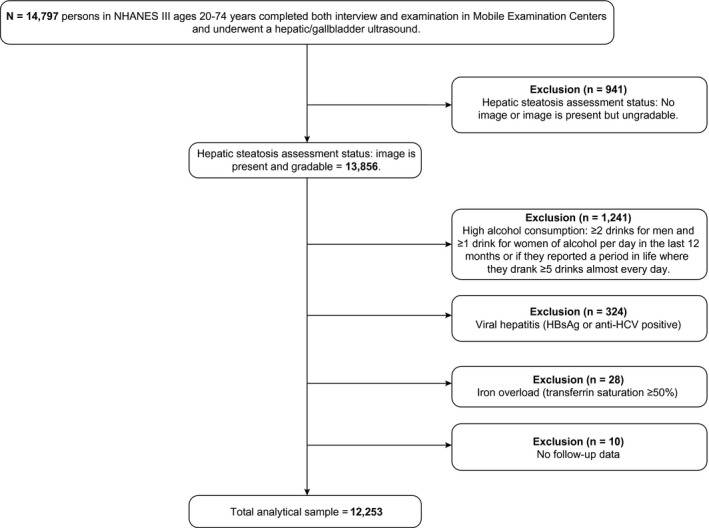
Flowchart of NHANES III participants for the current analysis. Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
NASH Ascertainment
In addition to the overall analysis, analysis was conducted after stratifying individuals with NAFLD into two groups based on elevation of liver enzyme levels. Presence of elevated enzyme levels among persons with NAFLD was considered a proxy variable for having NASH. Elevation was defined as having aspartate aminotransferase ≥37 U/L in men or ≥31 U/L in women or alanine aminotransferase ≥40 U/L in men or ≥31 U/L in women.
Mortality Follow‐up
Individuals were followed up for mortality by linkage to the National Death Index (NDI), which is maintained by the NCHS of the CDC. Follow‐up time started from the date of NHANES III participation and extended to December 31, 2015. For those persons who survived past December 31, 2015, follow‐up time was censored. Cause of death was determined using the underlying cause of death, which was created by the NCHS and includes 113 categories. For the current analysis, all‐cause mortality and selected cause‐specific mortality (CVD, cancer, diabetes, and LD) were analyzed. Liver cancer was included in the LD group rather than the cancer group. Supporting Table S1 shows the causes of death and International Classification of Diseases (ICD), Tenth Revision (ICD‐10) codes for each category of cause‐specific mortality.
Baseline Covariates
Baseline sociodemographic characteristics, clinical data, and lifestyle factors were available in NHANES III. Potential confounders were identified a priori and included in the final models: age; sex; race/ethnicity (non‐Hispanic white, non‐Hispanic black, Mexican American, or other); years of education (<9, 9‐11, 12, and ≥13); physical activity score (0, >0‐<100, 100‐<250, and ≥250, derived by multiplying metabolic equivalent tasks [METs] for each specific activity by the number of times per month the activity was performed and then summing over all the activities); cigarette smoking (never, former, or current); moderate alcohol consumption (categorized in tertiles of alcohol per day during the past year, with <2 drinks in men or <1 drink in women); and body mass index (BMI; <18.5, 18.5‐<25, 25‐<30, 30‐<35, and ≥35 kg/m2). Other variables considered, but not included, in the final models were physician‐reported health status (excellent, very good, good, fair, or poor); self‐reported health status (excellent, very good, good, fair, or poor); diabetes, hypertension, and metabolic syndrome (MetS) defined as having three or more of the following conditions: elevated waist circumference (≥88 cm for women and ≥102 cm for men); elevated triglycerides (≥150 mg/dL) or drug treatment for elevated triglycerides; low high‐density lipoprotein (HDL) cholesterol (<40 mg/dL for men and <50 mg/dL for women) or drug treatment for low HDL cholesterol; elevated blood pressure (systolic ≥130 mm Hg or diastolic ≥85 mm Hg or both) or antihypertensive drug treatment for a history of hypertension; and elevated fasting glucose (≥100 mg/dL) or drug treatment for elevated glucose. MetS or other metabolic conditions (i.e., diabetes) that are strongly associated with NAFLD were not included in the final models to avoid overadjustment.
Statistical Analysis
Baseline characteristics of NHANES III participants were compared by NAFLD status (mild‐to‐severe HS [NAFLD] vs. no NAFLD). Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for all‐cause and cause‐specific mortality. Sex‐specific, race/ethnicity‐specific, and liver‐enzyme‐level–specific HRs were also calculated. All analyses used the NHANES III sampling weights and accounted for the other aspects of the complex survey design. The NHANES III sampling design and computation of the sample weights have been described elsewhere.21 In brief, the sample weights are the product of three component weights: (1) inverse of the probabilities of selection at each stage of selection (e.g., counties, segments, households, and individuals); (2) a nonresponse adjustment weight for sampled individuals who did not participate in either the household interview or exams; and (3) a poststratification adjustment weight to match the 1990 U.S. Census population totals for designated subdomains to estimate weighted total of those subdomains from the NHANES III sample.21 All analyses are sample weighted using individuals who completed both the household interview and examination in the Mobile Examination Centers.
The adjusted PAFs of NAFLD for all‐cause and cause‐specific mortality were estimated using a method for complex‐weighted sample designs, as described.22 Standard errors estimated from adjusted PAFs were used to compute 95% CIs. In addition to the overall PAF, PAFs by sex and race/ethnicity were calculated. The number of deaths associated with NAFLD in the 2015 U.S. population was calculated by multiplying the PAFs by the total number of deaths. The total number of U.S. deaths was obtained from vital records data available from the CDC.23 Because NAFLD is not an exogenous exposure, the PAFs in the current study should be interpreted as measures of association rather than causation.
Because of the small number of deaths among individuals with NASH (i.e., NAFLD with elevated liver enzymes), PAFs and the estimated number of deaths were not calculated, but the HRs for all‐cause and LD mortality are reported (Supporting Table S2). HRs and other quantities used for estimating the PAFs were computed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC) callable add‐on, SUDAAN (version 10.0.3; Research Triangle Institute, Research Triangle Park, NC). All statistical analyses were conducted in SAS software (version 9.4; SAS Institute).
Results
Demographic and clinical characteristics of study participants are presented in Table 1. Overall weighted prevalence of NAFLD was 32.8% (95% CI, 30.3, 35.2). Weighted prevalence was similar among men (34.7%) and women (31.0%), but varied by race/ethnicity, with Mexican Americans having a higher prevalence (41.2%) than whites (32.5%) or blacks (29.1%). Persons with NAFLD were more likely to be older, have a greater BMI, be less well educated and physically active, and have MetS or the component conditions (e.g., obesity, diabetes, or hypertension). Both by self‐report and physician report, persons with NAFLD were less likely to be classified as having excellent health. During a median follow‐up of 23.3 years (interquartile range [IQR], 21.4‐25.1), 3,509 individuals died.
Table 1.
Baseline Characteristics of the Study Participants From NHANES III (1988‐1994)
| NAFLD | No NAFLD | |
|---|---|---|
| n (%*) | n (%*) | |
| 4,355 (32.8) | 7,898 (67.2) | |
| Age | ||
| Median (IQR) | 43 (42, 44) | 38 (37, 39) |
| Sex | ||
| Female | 2,369 (50.6) | 4,442 (54.9) |
| Male | 1,986 (49.4) | 3,456 (45.1) |
| Race/ethnicity | ||
| Non‐Hispanic white | 1,533 (74.8) | 2,958 (75.8) |
| Non‐Hispanic black | 1,067 (9.7) | 2,498 (11.4) |
| Mexican American | 1,587 (6.9) | 2,088 (4.8) |
| Other | 168 (8.6) | 354 (8.0) |
| Years of education† | ||
| <9 | 1,226 (13.2) | 1,467 (8.7) |
| 9‐11 | 719 (13.9) | 1,228 (11.7) |
| 12 | 1,303 (35.7) | 2,544 (33.9) |
| ≥13 | 1,085 (37.1) | 2,601 (45.7) |
| BMI† | ||
| <18.5 | 68 (2.0) | 168 (2.4) |
| 18.5‐<25.0 | 997 (25.8) | 3,445 (49.9) |
| 25.0‐<30.0 | 1,445 (32.3) | 2,782 (32.8) |
| 30.0‐<35.0 | 1,037 (22.9) | 1,041 (10.7) |
| ≥35.0 | 793 (17.0) | 452 (4.2) |
| Physical activity‡ | ||
| 0 | 1,086 (17.1) | 1,451 (12.0) |
| >0‐<100 | 1,897 (46.1) | 3,436 (44.9) |
| 100‐<250 | 995 (25.8) | 2,072 (30.2) |
| ≥250 | 377 (11.1) | 939 (12.9) |
| Cigarette smoking† | ||
| Never | 2,210 (46.6) | 4,113 (48.4) |
| Former | 1,192 (30.0) | 1,659 (23.0) |
| Current | 952 (23.4) | 2,126 (28.6) |
| Alcohol consumption † , § | ||
| Never | 2,572 (53.0) | 4,203 (45.8) |
| Tertile 1 | 654 (20.1) | 1,287 (19.9) |
| Tertile 2 | 506 (13.6) | 1,126 (18.7) |
| Tertile 3 | 467 (13.3) | 1,031 (15.7) |
| Physician impression health status† | ||
| Excellent | 1,396 (35.9) | 3,661 (54.2) |
| Very good | 1,090 (26.4) | 1,841 (23.7) |
| Good | 1,271 (27.2) | 1,725 (18.4) |
| Fair | 415 (9.1) | 399 (3.3) |
| Poor | 72 (1.5) | 41 (0.3) |
| Self‐reported health status† | ||
| Excellent | 558 (17.5) | 1,411 (23.5) |
| Very good | 926 (29.4) | 2,035 (32.9) |
| Good | 1,631 (34.7) | 2,836 (31.2) |
| Fair | 1,007 (15.0) | 1,359 (10.3) |
| Poor | 232 (3.4) | 256 (2.1) |
| MetS conditions† | ||
| 0 | 725 (19.4) | 2,923 (42.5) |
| 1 | 1,031 (23.9) | 2,488 (30.5) |
| 2 | 1,075 (23.3) | 1,463 (17.5) |
| 3 | 968 (23.0) | 750 (7.7) |
| 4 or 5 | 523 (10.5) | 220 (1.9) |
| Diabetes mellitus | ||
| Yes | 991 (16.8) | 848 (7.4) |
| No | 3,364 (83.2) | 7,050 (92.6) |
| Hypertension† | ||
| Yes | 1,696 (35.8) | 2,053 (21.6) |
| No | 2,657 (64.2) | 5,843 (78.4) |
Percentages are weighted by the sample weights.
Categories do not sum to the totals because of missing data.
Physical activity score derived by multiplying METs for each specific activity by the number of times per month the activity was performed, then summing over all the activities.
For men: tertile 1, 0.00‐0.28 drinks per day; tertile 2, 0.29‐0.85 drinks per day; tertile 3, 0.86‐2.00 drinks per day. For women: tertile 1, 0.00‐0.13 drinks per day; tertile 2, 0.14‐0.30 drinks per day; tertile 3, 0.31‐1.00 drink per day.
Table 2 shows the adjusted HRs and 95% CIs for all‐cause and cause‐specific mortality by NAFLD status. Risk of all‐cause mortality was 20% higher among individuals with NAFLD than those without (95% CI, 1.08, 1.34). Risk of diabetes‐specific mortality was >2‐fold higher among individuals with NAFLD (HR, 2.25; 95% CI, 1.21, 4.18). Cause‐specific mortality was elevated for the other causes examined, but the results were not statistically significant: CVD (HR, 1.05; 95% CI, 0.89, 1.24); cancer (HR, 1.13; 95% CI, 0.91, 1.39); and LD (HR, 2.66; 95% CI, 0.83, 8.60). Mortality risks were similar among men and women, with the sole exception of LD mortality, which was significantly higher among men with NAFLD (HR, 8.83; 95% CI, 2.29, 34.03), but not among women with NAFLD (HR, 1.10; 95% CI, 0.34, 3.49).
Table 2.
Overall and Sex‐Specific HRs of All‐Cause and Cause‐Specific Mortality Associated With NAFLD, NHANES III
| All | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | HR* | 95% CI | Deaths | HR* | 95% CI | Deaths | HR* | 95% CI | |
| All causes | |||||||||
| No NAFLD | 2,016 | Ref | 1,038 | Ref | 978 | Ref | |||
| NAFLD | 1,493 | 1.20 | (1.08, 1.34) | 775 | 1.16 | (1.00, 1.33) | 718 | 1.27 | (1.10, 1.47) |
| CVD | |||||||||
| No NAFLD | 706 | Ref | 358 | Ref | 348 | Ref | |||
| NAFLD | 517 | 1.05 | (0.89, 1.24) | 284 | 1.02 | (0.81, 1.29) | 233 | 1.11 | (0.82, 1.51) |
| Cancer | |||||||||
| No NAFLD | 440 | Ref | 224 | Ref | 216 | Ref | |||
| NAFLD | 278 | 1.13 | (0.91, 1.39) | 139 | 0.92 | (0.71, 1.19) | 139 | 1.37 | (0.96, 1.96) |
| Diabetes | |||||||||
| No NAFLD | 67 | Ref | 30 | Ref | 37 | Ref | |||
| NAFLD | 88 | 2.25 | (1.21, 4.18) | 38 | 2.43 | (0.94, 6.32) | 50 | 2.16 | (1.02, 4.58) |
| LD | |||||||||
| No NAFLD | 26 | Ref | 10 | Ref | 16 | Ref | |||
| NAFLD | 29 | 2.66 | (0.83, 8.60) | 13 | 8.83 | (2.29, 34.03) | 16 | 1.10 | (0.34, 3.49) |
Adjusted by age, sex, race/ethnicity, education, physical activity, smoking, alcohol, and BMI.
Mortality risks by race/ethnicity were similar (Table 3). There was an increased risk of all‐cause mortality among all three groups, but the risk only attained statistical significance among whites (HR, 1.25; 95% CI, 1.09, 1.44). This was also the case with diabetes mortality, which was elevated in all three groups, but only attained statistical significance (HR, 2.61; 95% CI, 1.03, 6.59) among whites. LD mortality was also elevated in all groups, but risk of mortality was not significantly increased in any of the three.
Table 3.
Race/Ethnic‐Specific HRs of All‐Cause and Cause‐Specific Mortality Associated With NAFLD, NHANES III
| White | Black | Mexican American | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | HR* | 95% CI | Deaths | HR* | 95% CI | Deaths | HR* | 95% CI | |
| All causes | |||||||||
| No NAFLD | 885 | Ref | 625 | Ref | 453 | Ref | |||
| NAFLD | 651 | 1.25 | (1.09, 1.44) | 338 | 1.18 | (0.98, 1.41) | 473 | 1.04 | (0.88, 1.24) |
| CVD | |||||||||
| No NAFLD | 311 | Ref | 240 | Ref | 133 | Ref | |||
| NAFLD | 236 | 1.10 | (0.90, 1.36) | 118 | 0.90 | (0.69, 1.16) | 154 | 0.98 | (0.74, 1.30) |
| Cancer | |||||||||
| No NAFLD | 194 | Ref | 139 | Ref | 96 | Ref | |||
| NAFLD | 129 | 1.21 | (0.95, 1.55) | 57 | 1.17 | (0.80, 1.72) | 87 | 0.99 | (0.69, 1.41) |
| Diabetes | |||||||||
| No NAFLD | 9 | Ref | 31 | Ref | 27 | Ref | |||
| NAFLD | 25 | 2.61 | (1.03, 6.59) | 26 | 1.77 | (0.96, 3.26) | 34 | 1.48 | (0.74, 2.97) |
| LD | |||||||||
| No NAFLD | 5 | Ref | 8 | Ref | 12 | Ref | |||
| NAFLD | 6 | 3.97 | (0.79, 20.07) | 5 | 1.71 | (0.39, 7.56) | 17 | 1.44 | (0.59, 3.56) |
Adjusted by age, sex, race/ethnicity, education, physical activity, smoking, alcohol, and BMI.
To examine whether mortality risks were particularly high in association with NASH, persons with NAFLD, both with and without elevated enzymes, were compared to persons without NAFLD (Supporting Table S2). The analysis found that persons with NAFLD without elevated enzymes had a higher risk of all‐cause mortality (HR, 1.17; 95% CI, 1.03, 1.33), as did persons with NAFLD with elevated enzymes (HR, 1.58; 95% CI, 1.21, 2.08). In contrast, risk of LD mortality was higher only among the group with elevated enzyme levels (HR, 7.75; 95% CI, 3.20, 18.77), but not among the group without (HR, 1.03; 95% CI, 0.44, 2.42). In the analyses stratified on sex, women with NAFLD had mortality risks attributed to all causes regardless of enzyme levels, whereas men with NAFLD only had increased risk of overall mortality if they had elevated enzyme levels. Among both men and women with NAFLD, risk of LD mortality was only elevated in persons with elevated enzymes. In the analyses stratified on race/ethnicity, whites with NAFLD had an increased risk of all‐cause mortality regardless of enzyme level, whereas blacks were only at increased risk if they had elevated enzyme levels, and Mexican Americans had no increased risks. For LD mortality, risks were significantly increased among whites and blacks, but not among Mexican Americans.
Table 4 shows the PAFs of NAFLD overall and by sex. Overall, the PAF associated with all‐cause mortality was 7.5% (95% CI, 3.0, 12.0), which equates to 200,115 (range, 79,461‐320,769) deaths in the U.S. population in 2015. For cause‐specific mortality, the PAF was highest for diabetes (38.0%; 95% CI, 13.1, 63.0), which equated to 30,220 deaths. A similarly high PAF was found for LD mortality (36.0%; 95% CI, −2.2, 74.1), but the 95% CI included zero. PAFs for CVD (2.2%) and cancer (4.7%) were smaller, and the 95% CI included zero. Examining sex‐specific PAFs, the PAFs for all‐cause mortality were similar among women (9.1%; 95% CI, 3.5, 14.7) and men (6.4%; 95% CI, 0.2, 12.6), as were the PAFs for diabetes‐specific mortality (women: 36.8%; 95% CI, 6.6, 67.0; men: 40.8%; 95% CI, 2.1, 79.6). In contrast, men had a notably elevated PAF for LD mortality (68.3%; 95% CI, 36.3, 100.0), but women did not (3.5%; 95% CI, −39.7, 46.8). Neither men nor women had elevated PAFs for CVD or cancer.
Table 4.
Overall and Sex‐Specific PAFs of NAFLD to All‐Cause and Cause‐Specific Mortality, NHANES III, and Total Number of Deaths Associated With NAFLD in the U.S. Population in 2015
| All | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| PAF* % | 95% CI | Total Deaths | Range | PAF* % | 95% CI | PAF* % | 95% CI | |
| All causes | 7.5 | (3.0, 12.0) | 2,00,115 | (79,461, 3,20,769) | 6.4 | (0.2, 12.6) | 9.1 | (3.5, 14.7) |
| CVD | 2.2 | (−5.1, 9.5) | 14,168 | (−31,950, 60,286) | 1.1 | (−9.6, 11.8) | 4.3 | (−7.6, 16.1) |
| Cancer | 4.7 | (−3.3, 12.7) | 23,434 | (−16,462, 63,329) | −3.6 | (−14.2, 7.0) | 11.5 | (−1.0, 23.9) |
| Diabetes mellitus | 38.0 | (13.1, 63.0) | 30,220 | (10,390, 50,051) | 40.8 | (2.1, 79.6) | 36.8 | (6.6, 67.0) |
| LD | 36.0 | (−2.2, 74.1) | 23,735 | (−1,420, 48,890) | 68.3 | (36.3, 100.0) | 3.5 | (−39.7, 46.8) |
Adjusted by age, sex, race/ethnicity, education, physical activity, smoking, alcohol, and BMI.
Examining race/ethnicity‐specific results (Table 5), the PAFs of NAFLD for all‐cause mortality (9.3%; 95% CI, 4.0, 14.6) and diabetes‐specific mortality (44.4%; 95% CI, 10.8, 78.0) were increased in whites, but not in blacks or Mexican Americans.
Table 5.
Race/Ethnic‐Specific PAFs of NAFLD to All‐Cause and Cause‐Specific Mortality, NHANES III
| White | Black | Mexican American | ||||
|---|---|---|---|---|---|---|
| PAF* % | 95% CI | PAF* % | 95% CI | PAF* % | 95% CI | |
| All causes | 9.3 | (4.0, 14.6) | 5.7 | (−0.1, 11.5) | 2.1 | (−6.7, 10.9) |
| CVD | 4.4 | (−5.1, 13.9) | −4.1 | (−13.6, 5.3) | −0.9 | (−17.7, 15.9) |
| Cancer | 7.6 | (−3.7, 18.8) | 4.9 | (−7.6, 17.4) | −0.7 | (−20.1, 18.7) |
| Diabetes | 44.4 | (10.8, 78.0) | 23.3 | (−2.6, 49.2) | 21.3 | (−10.0, 52.6) |
| LD | 47.1 | (−0.5, 94.8) | 21.5 | (−36.5, 79.6) | 18.0 | (−24.8, 60.7) |
Adjusted by age, sex, race/ethnicity, education, physical activity, smoking, alcohol, and BMI.
Discussion
In the current study, NAFLD was associated overall with significantly increased risks of all‐cause mortality and diabetes mortality; and among men, with LD mortality. The attributable risk of NAFLD for all‐cause mortality was 7.5%. Based on this, NAFLD is estimated to account for more than 2,00,000 deaths in the U.S. population in 2015. The attributable risk of NAFLD for diabetes mortality was 38%, which accounted for more than 30,000 deaths in the same time period. The PAFs were similar among men and women for all‐cause mortality and diabetes mortality, but the PAF of NAFLD for LD mortality was notably higher among men (68%) than women (3.5%).
Earlier studies using NHANES III data (1988‐1994) have reported conflicting results concerning the association between NAFLD and mortality. Unlike the current analysis, an earlier analysis with follow‐up to 2006 found no increased risk of death with ultrasound‐defined NAFLD.6 The longer follow‐up time in the current analysis with the corresponding increase in the number of deaths may explain the discrepancy in findings. In addition, the former analysis defined NAFLD as moderate‐to‐severe steatosis, whereas the current analysis defined NAFLD as mild‐to‐severe steatosis in order to cover the entire spectrum of NAFLD.1 The current analysis also did not adjust for conditions of MetS given that they are strongly related to NAFLD, and we believe that adjusting for these conditions could result in overadjustment that would bias the association between NAFLD and mortality. A 2018 analysis of NHANES III data, which measured NAFLD by several liver fat scores (LFSs) rather than ultrasonography, reported that higher scores with the U.S. Fatty Liver Index and the NAFLD‐LFS were associated with increased risk of LD mortality, but not mortality attributed to other causes.10 Comparisons with the current analysis are not straightforward, however, because there were a number of differences in the definition of NAFLD, definition of the analytical sample, and covariates used for adjustment in the analysis.
NAFLD, which is associated with MetS, obesity, and diabetes,24 has increased in prevalence in parallel with epidemics of diabetes and obesity in the United States.5 Obesity may play a role in both initiation of liver steatosis and progression of NAFLD.25 Furthermore, prevalence of NAFLD is highest among persons with diabetes,26 with studies reporting a prevalence of nearly 70%.27 In the current study, weighted prevalence of NAFLD among individuals with diabetes was approximately 54%. In addition, NAFLD has been reported to increase the risk of morbidity and mortality in persons with diabetes,28, 29, 30 which is consistent with the increased risk of diabetes mortality and large proportion of diabetes deaths associated with NAFLD noted in the current study. Similarly, NAFLD has been reported to increase the risk of LD progression and mortality. A long‐term follow‐up study of NAFLD and disease‐specific mortality found that persons with NAFLD had elevated risk of death from LDs, including cirrhosis and HCC.8 In agreement, a recent U.S. study that analyzed data from the NCHS reported that the leading causes of CLD‐related deaths are NAFLD (41%) and alcohol‐associated LD (32%).31
The current study found that the PAF of NAFLD for CVD mortality and cancer mortality was <10%. This relatively small percentage may be because factors other than NAFLD are known to contribute substantially to those deaths. For example, the proportion of CVD deaths attributable to smoking has been reported to be approximately 20%, and the proportion attributable to the combination of smoking and hypertension is >40%.32 Similarly, the proportion of cancer deaths attributable to smoking in the United States is almost 30%.33 Other factors, including alcohol intake, poor diet (defined as consumption of red and processed meat and low consumption of fruits/vegetables, dietary fiber, and dietary calcium), physical inactivity, and excess body weight, are also major contributors to cancer mortality.33
Among women in the current study, 9.1% of all deaths were associated with NAFLD, whereas among men, 6.4% of all deaths were associated with NAFLD, although the estimates had overlapping CIs. Whether there are sex differences in the association of NAFLD and mortality, however, is uncertain. In the current study, risk of CVD mortality was approximately the same among men and women, but the PAF was higher among women (4.3%) than men (1.1%). A large Korean study found that NAFLD was associated with increased risk of all‐cause mortality and mortality attributable to cancer, CVD, and LD among women, but not men.9 Similarly, a study using NHANES III and NHANES 1999‐2014 data found that risk of 5‐year mortality was higher among women with NAFLD compared to women without NAFLD.34 Although NAFLD is more common among men, risk and progression from simple steatosis to NASH has been suggested to increase among women after menopause.35, 36, 37, 38, 39 In the current study, however, prevalence of NASH among women aged ≥55 years (3.3%) was similar to that of women aged <55 years (3.4%). In contrast, prevalence of NASH among men aged ≥55 years was 2.9%, whereas prevalence among men aged <55 years was 5.2%. These data indicate that risk of NASH may remain stable among women, but decline with age among men. Why this would be the case is not certain, but it may suggest that, among men, the higher prevalence of NASH at younger ages brings a higher mortality risk. In support of this hypothesis, the current study found that the PAF of NAFLD for LD mortality was notably higher among men (68.3%) than women (3.5%), a finding which was driven by a much greater risk of LD mortality among men as well as a higher prevalence of NAFLD.
Racial/ethnic disparities in NAFLD prevalence and severity may be influenced by multiple factors, including environmental and genetic factors as well as socioeconomic status and less access to health care.40 The current analysis found higher PAFs for mortality attributable to all causes, diabetes, and LD among whites in comparison to blacks and Mexican Americans. These results are consistent with some, but not all, earlier studies. A recent review of 34 studies found that, although some studies reported higher risks of all‐cause mortality among blacks compared to whites, other studies have reported higher risks of all‐cause mortality and LD mortality among whites than among other racial/ethnic groups.41
The current study estimated that more than 2,00,000 deaths were associated with NAFLD in 2015. A recent study reported that among individuals with NAFLD, total deaths were expected to increase 44% and liver‐related deaths 178% between 2015 and 2030 in the United States.18 In addition, the study projected that 40% of deaths among persons with NAFLD will occur among those with NASH,18 which will result in a 3% increase in all‐cause mortality among persons with NASH by 2030.18 In the current study, PAFs for NASH could not be calculated because of the small number of events, but there was a higher risk of all‐cause and LD mortality among individuals with NASH.
The study had several strengths, including the use of a large, nationally representative sample that permitted the results to be extrapolated to the general population of U.S. adults. In addition, the analysis was able to account for a wide variety of sociodemographic, clinical, and lifestyle factors. Finally, the long duration of follow‐up (more than two decades) provided sufficient time for mortality to occur, and the ascertainment of vital status using the NDI is almost 100%.15
In addition to the strengths, there were also some limitations. The study relied on ultrasonography rather than liver biopsy for the determination of HS. However, in 2011, a systematic review and meta‐analysis reported that ultrasonography can accurately detect steatosis with high sensitivity (84.8%) and specificity (93.6%) and an area under the curve of 0.93.42 More recently, a study that evaluated the validity of ultrasound versus magnetic resonance spectroscopy found a sensitivity of 96% and a specificity of 94%.43 Moreover, both studies reported that abdominal ultrasound is a valid method for detecting HS in both clinical and population settings.42, 43 Furthermore, it would not be feasible to conduct routine liver biopsies as part of a population survey. Another limitation of the current study was the lack of validation of cause of death. However, a recent study validating causes of death in the NDI versus the Department of Defense’s Armed Forces Medical Examiner Systems found good agreement between the sources of information.44 In addition, the current analysis was based on a single measurement of NAFLD, and changes could have occurred over time. In addition, although there was an attempt to control for important confounders in the analysis, residual confounding could still have affected the results, as is the case for any epidemiology study. Furthermore, because we only have U.S. estimates of the prevalence of NAFLD jointly with the other risk factors and covariates at the time of NHANES III, the calculation of the associated number of deaths for 2015 uses these prevalences as an approximation of what they would be in 2015. Finally, some of the estimates had limited statistical power because of small numbers of events, particularly in analyses stratified by race/ethnicity or sex.
In the current study, almost 8% of all‐cause mortality and more than one‐third of LD and diabetes‐specific deaths in the United States were associated with NAFLD in 2015. Regardless of sex or racial/ethnic group, prevalence of NAFLD is high in the population and is increasing; thus, the number of sequelae will continue to grow. NAFLD is already a major cause for liver transplantation,45, 46 underscoring the importance for effective interventions to reduce the prevalence of NAFLD and other metabolic conditions in the United States.
Author Contributions
C.S.A., K.A.M. and B.I.G. conceived and designed the study. C.S.A. and J.E.T. acquired the data. C.S.A., K.A.M. and B.I.G. analyzed and interpreted the data. C.S.A. drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Supporting information
Acknowledgment
We thank the NCHS, CDC, for the NHANES III Linked Mortality Files. In particular, we thank Mr. Dieudonne Nahigombeye and Mr. Ray Kuntz for assistance in using the NCHS Research Data Center.
Supported by the National Institutes of Health Intramural Research Program.
Potential conflict of interest: Nothing to report.
REFERENCES
Author names in bold designate shared co‐first authorship.
- 1. Benedict M, Zhang X. Non‐alcoholic fatty liver disease: an expanded review. World J Hepatol 2017;9:715‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 3. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2019;16:377‐386. [DOI] [PubMed] [Google Scholar]
- 4. Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995;346:987‐990. [DOI] [PubMed] [Google Scholar]
- 5. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 6. Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non‐alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younossi ZM, Marchesini G, Pinto‐Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation 2019;103:22‐27. [DOI] [PubMed] [Google Scholar]
- 8. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 9. Hwang YC, Ahn HY, Park SW, Park CY. Nonalcoholic fatty liver disease associates with increased overall mortality and death from cancer, cardiovascular disease, and liver disease in women but not men. Clin Gastroenterol Hepatol 2018;16:1131‐1137. [DOI] [PubMed] [Google Scholar]
- 10. Unalp‐Arida A, Ruhl CE. Liver fat scores predict liver disease mortality in the United States population. Aliment Pharmacol Ther 2018;48:1003‐1016. [DOI] [PubMed] [Google Scholar]
- 11. Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year‐community study. Hepatology 2018;67:1726‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 13. Mahady S, Wong G, Turner R, Craig J, George J. Non‐alcoholic fatty liver disease and all‐cause and cardiovascular mortality: a population based cohort study. J Gastroenterol Hepatol 2014;29:93.25521740 [Google Scholar]
- 14. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646‐650. [DOI] [PubMed] [Google Scholar]
- 15. Unalp‐Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology 2016;63:1170‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mann JP, Armstrong MJ, Uppal H, Chandran S, Newsome PN, Potluri R. The burden of cardiovascular disease and mortality across a spectrum of non‐alcoholic fatty liver disease: a 14‐year follow‐up population study of 9,29,465 individuals. J Hepatol 2015;62:S215. [Google Scholar]
- 17. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23:8263‐8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Center for Health Statistics, Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey (NHANES) III. October 2018. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/Default.aspx. Accessed April 3, 2019. [Google Scholar]
- 20. National Center for Health Statistics, Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey (NHANES) III: Hepatic Steatosis Ultrasound Images Assessment Procedure Manual. November 2010. https://www.cdc.gov/nchs/data/nhanes/nhanes3/Hepatic_Steatosis_Ultrasound_Procedures_Manual.pdf. Accessed April 3, 2019. [Google Scholar]
- 21. Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 1992;2:1‐35. [PubMed] [Google Scholar]
- 22. Graubard BI, Flegal KM, Williamson DF, Gail MH. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Stat Med 2007;26:2639‐2649. [DOI] [PubMed] [Google Scholar]
- 23. National Center for Health Statistics . Underlying Cause of Death 1999‐2017 on CDC Wonder Online Database. December 2018. http://wonder.cdc.gov/ucd‐icd10.html. Accessed April 3, 2019. [Google Scholar]
- 24. Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 2015;19:361‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 2019;92:82‐97. [DOI] [PubMed] [Google Scholar]
- 26. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 27. Leite NC, Salles GF, Araujo AL, Villela‐Nogueira CA, Cardoso CR. Prevalence and associated factors of non‐alcoholic fatty liver disease in patients with type‐2 diabetes mellitus. Liver Int 2009;29:113‐119. [DOI] [PubMed] [Google Scholar]
- 28. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non‐alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262‐265. [DOI] [PubMed] [Google Scholar]
- 30. McGurnaghan S, Blackbourn LAK, Mocevic E, Haagen Panton U, McCrimmon RJ, Sattar N, et al. Cardiovascular disease prevalence and risk factor prevalence in type 2 diabetes: a contemporary analysis. Diabet Med 2019;36:718‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paik J, Golabi P, Sayiner M, Biswas R, Alqahtani S, Venkatesan C, et al. The increase in mortality related to chronic liver disease is explained by non‐alcoholic fatty liver disease (NAFLD). Hepatology 2018;68:453a. [Google Scholar]
- 32. Hozawa A. Attributable fractions of risk factors for cardiovascular diseases. J Epidemiol 2011;21:81‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31‐54. [DOI] [PubMed] [Google Scholar]
- 34. Arshad T, Golabi P, Paik J, Mishra A, Younossi ZM. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun 2019;3:74‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in NAFLD: state of the art and identification of research gaps. Hepatology 2019;70:1457‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung GE, Yim JY, Kim D, Lim SH, Yang JI, Kim YS, et al. The influence of metabolic factors for nonalcoholic fatty liver disease in women. Biomed Res Int 2015;2015:131528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014;59:1406‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016;64:85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel SS, Siddiqui MS. Current and emerging therapies for non‐alcoholic fatty liver disease. Drugs 2019;79:75‐84. [DOI] [PubMed] [Google Scholar]
- 41. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2018;16:198‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011;54:1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Lucia Rolfe E, Brage S, Sleigh A, Finucane F, Griffin SJ, Wareham NJ, et al. Validity of ultrasonography to assess hepatic steatosis compared to magnetic resonance spectroscopy as a criterion method in older adults. PLoS One 2018;13:e0207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skopp NA, Smolenski DJ, Schwesinger DA, Johnson CJ, Metzger‐Abamukong MJ, Reger MA. Evaluation of a methodology to validate National Death Index retrieval results among a cohort of U.S. service members. Ann Epidemiol 2017;27:397‐400. [DOI] [PubMed] [Google Scholar]
- 45. Calzadilla Bertot L, Adams LA. The natural course of non‐alcoholic fatty liver disease. Int J Mol Sci 2016;17:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


