Abstract
Diurnal oscillations in energy metabolism are linked to the activity of biological clocks and contribute to whole‐body glucose homeostasis. Postprandially, skeletal muscle takes up approximately 80% of circulatory glucose and hence is a key organ in maintenance of glucose homeostasis. Dysregulation of molecular clock components in skeletal muscle disrupts whole‐body glucose homeostasis. Next to light‐dark cycles, nonphotic cues such as nutrient intake and physical activity are also potent cues to (re)set (dys)regulated clocks. Physical exercise is one of the most potent ways to improve myocellular insulin sensitivity. Given the role of the biological clock in glucose homeostasis and the power of exercise to improve insulin sensitivity, one can hypothesize that there might be an optimal time for exercise to maximally improve insulin sensitivity and glucose homeostasis. In this review, we aim to summarize the available information related to the interaction of diurnal rhythm, glucose homeostasis, and physical exercise as a nonphotic cue to correct dysregulation of human glucose metabolism.
Study Importance.
What is already known?
-
►
Most of the cells of the human body express a molecular clock machinery that is able to drive circadian oscillations in multiple metabolic process throughout the day. Disrupted molecular clock expression in human skeletal muscle is associated with metabolic disarrangements, such as insulin resistance and type 2 diabetes. Exercise and diet are the main nonphotic cues to entrain the peripheral clocks and first-line treatments for metabolic disorders. Previous human‐based reports displayed that human skeletal muscle mitochondrial function and insulin‐stimulated plasma glucose uptake exhibit diurnal oscillations.
What does this review add?
-
►
The skeletal muscle peripheral clock might interact with insulin‐sensitizing effects of exercise and nutritional status of subjects, subsequently triggering different metabolic responses to exercise according to time of the day.
Introduction
Whole‐body glucose homeostasis is under the control of multiple hormones, including insulin, glucagon, cortisol, and adrenergic hormones. Insulin resistance of liver, skeletal muscle, and white adipose tissue is an early hallmark of type‐2 diabetes mellitus pathogenesis. Interestingly, whole‐body glycemic regulation and insulin sensitivity exhibit diurnal variations (1, 2). These diurnal variations are mainly driven by circadian changes in circulatory glucoregulatory hormones, whole‐body substrate oxidation and tissue‐specific insulin responsiveness (3, 4). While circadian changes in hormonal patterns have been appreciated for some time, recent work has identified the molecular machinery present in mammalian cells that is able to control a roughly 24‐hour pattern of mRNA expression. Thus, the importance of chronobiology and tissue‐specific biological clocks in the regulation of metabolic health in humans has become an area of increasing focus during the past decade.
Virtually all cells of the human body have an internal timekeeping system (5) composed by the master molecular clock located in the hypothalamic superchiasmatic nucleus of the brain. This clock is primarily entrained by light. In the remainder of this paper, we will refer to the master molecular clock as the “master clock” to clearly make the distinction from the peripheral biological clock referred to as “peripheral clock,” with reference to the relevant tissues/organs, such as adipose tissue, liver, and skeletal muscle, that also possess biological clocks (6). The cell‐autonomous autoregulatory mechanism of the master clock relies on transcriptional and post‐translational negative feedback loops. The transcriptional factors Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT‐Like (BMAL)‐1 (or Aryl hydrocarbon receptor nuclear translocator like [ARNTL]) constitute the positive regulation of these feedback loops, which activate their target genes cryptochrome‐2 (CRY), period (PER), and nuclear receptor subfamily 1 group D member 1 (Rev‐erbA‐α), which, in turn, are negative regulators of clock feedback loops that eventually supress transcriptional activity (7). Recent results from animal models have indicated that the peripheral clock in skeletal muscle orchestrates a temporal organization of metabolic gene expression, thereby affecting glucose homeostasis (8). Similar observations have been reported in humans; laboratory‐induced circadian misalignment interventions affect the diurnal pattern of peripheral clock gene expression in skeletal muscle (9). Moreover, in night‐shift workers, master and peripheral clock disruption has been reported to coincide with an elevated risk for type 2 diabetes development (10).
Increased physical activity is a clinically proven first‐line strategy to improve glucose homeostasis. Most of the benefits noted in skeletal muscle that are associated with increased physical activity emerge from an upregulation of multiple components in the myocellular insulin signaling cascade after exercise training that jointly promote postprandial glucose clearance (11). In this regard, animal studies have revealed that the peripheral muscle clock is involved in the regulation of insulin‐stimulated glucose disposal and exercise‐induced changes in whole‐body glucose homeostasis (12). Exercise is an external nonphotic (not related to light) modulator of the expression and rhythmicity of the peripheral muscle clock (13). This has led to the suggestion that peripheral clock dysfunction and its related metabolic effects may be corrected by resetting peripheral clocks in metabolically compromised participants. Considering the interplay between the peripheral clock in skeletal muscle and exercise as a nonphotic cue tool to readjust the clock, an emerging area of research aims to maximize the beneficial effects of exercise for glucose homeostasis by optimizing timing of the performed exercise (14).
In this review, we summarize current information related to the interaction of diurnal rhythm, glucose homeostasis, and physical exercise as a nonphotic cue to correct dysregulation of glucose metabolism.
Diurnal Rhythmicity in Substrate Metabolism
Postprandial glucose disposal possesses a diurnal pattern in healthy normoglycemic individuals as well as in patients with impaired glucose tolerance. Plasma glucose clearance rates are higher in the morning compared with the evening in response to similar glucose loads (3, 15). Elegant studies using tissue‐specific catheterization and continuous glucose infusion techniques have revealed that glycemic dysregulation during evening time originates from a progressive impairment of skeletal muscle insulin responsiveness (4). Moreover, pancreatic insulin secretion exhibits rhythmicity in humans, with a lower rate of insulin secretion at evening hours (16). Thus, temporal fluctuation of skeletal muscle insulin sensitivity and a time‐dependent insulin secretion rate both contribute to a diurnal pattern in whole‐body glucose metabolism in humans. Additionally, diurnal fluctuations in key metabolic hormones such as cortisol and melatonin (17), as well as diurnal elevations of nonesterified free fatty acids (NEFA) concentration in blood plasma (18), will affect whole‐body glucose homeostasis. White adipose tissue also possesses a peripheral clock, contributing to oscillations in lipase activity (19, 20), triacylglycerol turnover (21) and plasma NEFA levels. Tracer studies using continuous intravenous infusion of [2H2]‐Palmitate reveal that postprandial storage of fatty acids in adipose tissue (NEFA buffering capacity) is higher during evening versus morning hours in lean participants (22). Interestingly, the postprandial NEFA buffering capacity of white adipose tissue is compromised in participants with obesity who do exhibit different diurnal variations upon consecutive meals throughout the day, variation patterns of which are similar to those noted in lean participants (22). Lower buffering capacity of adipose tissue in the evening contributes to a timing‐dependent NEFA spillover to other peripheral tissues. This, combined with timing‐dependent disinhibition of adipose tissue lipolysis (23), promotes intracellular substrate competition as well as lipid storage in ectopic tissues such as muscle. Augmented myocellular lipid storage often is associated with compromised insulin signaling. Thus, compromised adipose tissue NEFA buffering capacity and disinhibition of adipose tissue lipolysis may contribute to rhythmicity in insulin sensitivity.
Insulin‐stimulated intramyocellular glucose disposal comprises oxidative and nonoxidative glucose disposal. While nonoxidative glucose disposal predominantly reflects glucose storage as muscle glycogen or conversion to lactate (24), oxidative glucose disposal is partly determined by mitochondrial oxidative capacity. We have shown rhythmicity in skeletal muscle mitochondrial oxidative capacity in humans, with peak oxidative capacity in the evening (23:00 hours). The peak in oxidative capacity coincided with a peak resting energy expenditure predominantly relying on carbohydrate oxidation (25). Whether this temporal variation of human skeletal muscle oxidative capacity and preferential glucose oxidation at evening times represents an intrinsic circadian‐related oscillation or is merely a reflection of behavioral cues such as meal times or physical activity needs to be determined. Furthermore, if peak skeletal muscle mitochondrial function and predominant glucose oxidation in the evening are triggered to counteract impaired evening glycemic regulation to maintain glucose homeostasis warrants further investigation.
Noninvasive measurement of muscle and hepatic glycogen content by 13C magnetic resonance spectroscopy indicated that glycogen content in liver and muscle was higher in the evening versus morning in healthy individuals (26). Although feeding‐induced hyperglycemia per se upregulates both intracellular glucose disposal pathways (oxidation and storage), animal studies have highlighted a crucial role of muscle‐ (12) or liver‐specific peripheral clocks (27) in the circadian regulation of glucose metabolism. Muscle‐specific ablation of BMAL‐1 not only disrupted glucose metabolism in muscle but also profoundly compromised whole‐body glucose homeostasis (12). Liver‐specific ablation of CLOCK dampened circadian variation in hepatic glycogen storage (27). Jointly, these observations coincide with fasting hyperglycemia and impaired glucose tolerance (12, 27) and stress the importance of peripheral clocks in the maintenance of glucose homeostasis.
Thus, the clock could play a role in orchestrating the anticipation of multiple insulin‐responsive peripheral tissues to timely promote glucose disposal and avoid undue peaks in blood glucose. Eating behavior throughout wakefulness could contribute to maintain diurnal responses of peripheral clocks in different insulin target organs, which may serve to fine tune circadian regulation of plasma glucose clearance over the day. Nevertheless, more tightly controlled studies exploring the prime involvement of peripheral clocks and relevant downstream targets in maintaining glucose homeostasis in humans are required to elucidate whether the clock can be endorsed as a therapeutic target to improve glucose homeostasis.
The Peripheral Clock in Skeletal Muscle
Expression of clock genes in human skeletal muscle exhibits a profound day‐night rhythmicity with alternating peak‐expression of the positive (BMAL1 and CLOCK) and negative (PER and cryptochrome‐2) clock components (25). In addition, multiple clock‐controlled genes related with substrate oxidation (pyruvate dehydrogenase complex), tricarboxylic acid cycle/respiratory electron transport chain (NADH dehydrogenase complexes), and ATP synthase (ATP5F1, ATP5G3, ATP5A1, and ATP5L) possess periodic expression, concomitant with an oscillatory mitochondrial respiratory capacity and mitochondrial dynamic in healthy individuals over a 24‐hour period (25). Whether the temporal changes in expression of the peripheral muscle clock and its target genes in human skeletal muscle reflect an intrinsic circadian rhythmicity or originate from the interaction of the master clock with external cues such as meal times and daily physical activity is hard to disentangle. Furthermore, the physiological relevance of a peripheral clock in human skeletal muscle requires sequential muscle biopsies and hence is not straightforward to accomplish. Nevertheless, current findings indicate the peripheral muscle clock might sense and anticipate diurnal feeding‐fasting and activity cycles, likely orchestrating diurnal gene expression and substrate selection in a circadian fashion (28). In addition, clock‐governed events such as peak mitochondrial oxidative capacity match with a peak in carbohydrate oxidation (25) and a peak in human physical performance (29) (Figure 1). This discloses new avenues of research pointing toward the timing of interventions to prime skeletal muscle metabolic capacity and optimizes buffering responses to subsequent daily challenges such as feeding‐fasting and physical activity–rest transitions.
Figure 1.
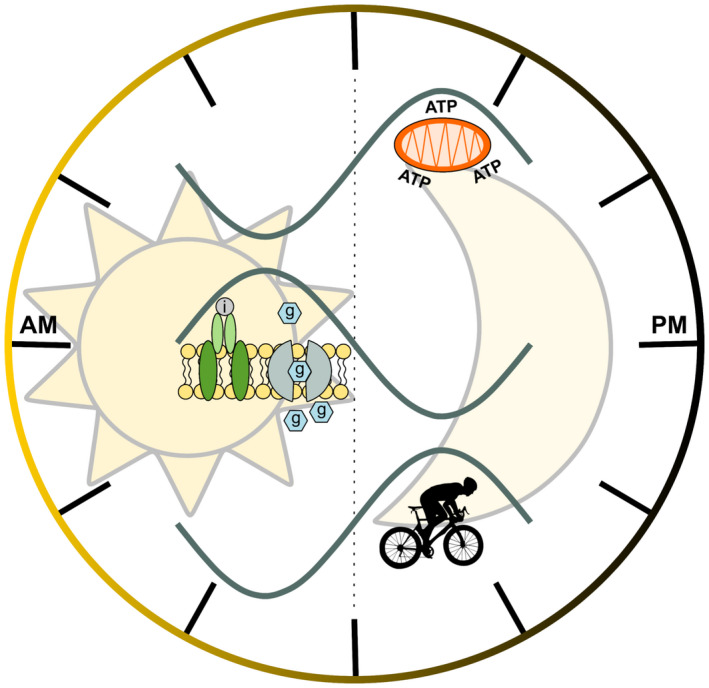
The master clock drives circadian variations in human metabolism. The intracellular timekeeping system composed by the clock orchestrates temporal organization of organismal homeostasis. In humans, skeletal muscle mitochondrial oxidation capacity, whole‐body insulin sensitivity, and maximal aerobic performance (upper, middle, and lower rhythms, respectively) all exhibit diurnal variations. Mitochondrial oxidative capacity is highest at evening time (23:00 hours) and coincides with peak resting energy expenditure predominantly fueled by glucose oxidation (25). Whole‐body insulin sensitivity possesses diurnal fluctuation related to changes in glucoregulatory hormones and oscillations in glycogen content and glycolytic gene expression (52) with diurnal fluctuations in insulin sensitivity as a consequence. Furthermore, maximal aerobic capacity displays diurnal variations, with a peak in maximal exercise performance at evening hours. As mitochondrial oxidative capacity affects maximal exercise performance and is paralleled by a shift in substrate selection (9), it can be speculated that distinct times throughout the day the muscle can more readily meet its metabolic demands than during other periods.
Consistent with the results from human muscle biopsy samples, previous data has shown circadian rhythmicity of peripheral clock genes and other energy cellular sensor enzymes in primary human muscle cells after in vitro synchronization (30, 31). Remarkably, a dampened gene expression of the clock gene Rev‐erb‐α and NAD‐dependent deacetylase Sirtuin (SIRT)1 in cultured myotubes from metabolically compromised donors was reported (31). The blunted oscillation was in sharp contrast to profound fluctuations observed in the same genes in myotubes cultured from endurance‐trained individuals (31). Particularly, the nuclear receptor Rev‐erb‐α and SIRT1 can modulate the oxidative phenotype in skeletal muscle. Importantly, both rev‐erb‐α and SIRT1 are highly sensitive to feeding‐fasting transitions and strongly associate with glucose homeostasis (32). Emerging evidence from animal studies has indicated that functional expression of peripheral clocks in muscle controls insulin‐stimulated skeletal muscle glucose uptake, not only via transcriptional regulation of insulin signaling–related proteins (TBC1 domain family member 1 and Glucose Transporter [GLUT]4) but also via conserving a diurnal expression of glycolytic rate‐limiting enzymes (8). Robust cellular metabolic networks that are under the control of the peripheral clock have recently been discovered. These interconnected cellular pathways anticipate time‐dependent nutrient availability along with transient alterations in fuel oxidation upon feeding/exercise in mice skeletal muscle (33). In more detail, peripheral clock genes in muscle are involved in the regulation of the class I histone deacetylase Histone Deacetylation 3 to adjust glycolysis and plasma glucose clearance along with muscle performance during exercise (33). Collectively, one can hypothesize that disruptions of the peripheral clock in skeletal underlies the pathophysiology of insulin resistance and related compromised metabolic health (10, 34). In this regard, our group previously showed that short‐term laboratory‐induced day‐night shift resulted in circadian misalignment of both the master and peripheral clock, which was accompanied by lowered muscle insulin sensitivity in healthy volunteers (9). The compromised insulin sensitivity was mainly explained for by a deteriorated nonoxidative glucose disposal pathway, while oxidative glucose disposal remained unaltered. Clearly, more well‐controlled human intervention studies are needed to examine if restoring master and peripheral clocks can be used as interventions aiming to promote insulin sensitivity and glucose homeostasis.
Exercise and the Peripheral Clock
The intimate interplay between the peripheral muscle clock and substrate metabolism indicates that intramyocellular clock functioning might dictate different periodic responses to exercise and modulates exercise‐induced changes in gene expression. This interplay raises two different questions. First, how much and in what context (for example diet) can exercise affect the peripheral muscle clock? Second, one may ask if the muscle clock dictates the effect of a given exercise bout (and thereby is a determinant of the outcome of an exercise training intervention). The latter notion is triggered by previous reports showing that muscle contractile bioenergetics and fiber‐type–specific gene expression upon exercise are regulated by peripheral clocks in mice muscle cells (12, 35). In humans, exercise‐induced hormonal responses are highly affected by the time of the day; this supports the notion that timing of exercise may also affect the hormonal responses and thereby energy metabolism and substrate selection postexercise (36). Although the impact of timing of exercise on exercise‐induced improvements in glucose homeostasis remains to be determined, it is appealing to hypothesize that the therapeutic potential to optimize exercise timing can be used to maximize health outcomes in a clinical context. In this regard, one can argue for timing exercise when mitochondrial oxidative capacity peaks, as at that time, the muscle is primed to meet the energy requirements of contraction. Consequently, premature muscle fatigue may be diminished, and long‐term adherence might be promoted. Considering that mitochondrial oxidative capacity in skeletal muscle plays a crucial role determining exercise performance, timing of exercise training might also be relevant to optimize (health) performance benefits. Alternatively, it could be hypothesized that exercising at times when mitochondrial oxidative capacity is lowest provides a relatively stronger trigger for adaptation, thus creating more room for improvement than applying the same type of exercise when mitochondrial capacity peaks.
Improvements in glucose metabolism in multiple peripheral tissues upon exercise training have partly been attributed to endocrine properties of the skeletal muscle via release of contraction‐induced myokines into systemic circulation (37). In fact, the synthesis of myokines interleukin 6, Monocyte chemoattractant protein‐1, and interleukin 8 with a putative glucoregulatory role is under control of the peripheral muscle clock, as was shown in cultured human myotubes (38). This indicates that humoral effects of exercise via skeletal muscle might be affected by the status of the peripheral muscle clock and indeed display oscillatory changes over a 24 hour period. If the ability to secrete myokines upon muscle contraction indeed exhibits diurnal variation warrants further investigation.
By mutating the CLOCK gene in somatic cells (39), it has become clear that disrupting the master clock has metabolic consequences as well as affects exercise performance (39). In a more tissue‐specific manner, it has also been shown that deletion of CLOCK and BMAL‐1 in pancreatic islet cells resulted in hypoinsulinemia and aberrations in glucose homeostasis (40). Muscle‐specific loss of BMAL‐1 resulted in compromised glucose uptake in muscle with disturbed systemic glucose homeostasis as a consequence (12). Earlier studies using global knockout models of CLOCK (41) or BMAL‐1 (42) revealed that life‐long ablation of CLOCK was paralleled by hyperphagia and a sustained positive energy balance (41), while the global and life‐long absence of BMAL‐1 affected timing of locomotor behavior and reduced spontaneous activity (42). It could hence be argued that some of the effects of clock disruption observed may be secondary to developmental defects and/or to the lifelong absence of a functional clock. However, it is important to note that mice with inducible muscle‐specific ablation of BMAL‐1 showed compromised insulin‐stimulated glucose uptake in muscle, partly originating from downregulation of key insulin‐signaling genes and lower levels of the main glucose transporter GLUT4 (8). Interestingly, compelling evidence from animal and human studies has illustrated that exercise is a potent nonphotic cue, able to reset the peripheral clock in muscle by modulating the expression of master‐clock proteins (43). Day versus night exercise was also shown to differentially phase‐shift peaks in the expression of PER2 in explants of muscle and lungs but not in suprachiasmatic nucleus explants, indicating that peripheral clock gene expression is responsive to exercise and the timing of exercise and suggesting that exercise can contribute to synchronization of peripheral and master clocks (13).
In line with these animal data, one‐legged acute resistance exercise was also shown to trigger a rhythmic phase shift of peripheral clock genes in muscle along with changes in glucose metabolism and mitochondrial function (43). These data indicate interaction of exercise timing and metabolism in humans and highlight the role exercise might have in the restoration of a transiently disturbed clock (e.g., upon traveling time zones and during shift work). In line with this, master‐clock and clock‐controlled gene expression in cultured human primary cells reflects the metabolic condition of the donors and fundamentally preserves a robust intrinsic rhythmicity in muscle cells upon long‐term training of the donors (31, 38). Furthermore, the peripheral clock in cultured human myotubes displayed a strong association with in vivo insulin sensitivity (31, 38). It was also observed that the amplitude of oscillations of the clock gene Rev‐Erb Alpha was blunted in patients with type 2 diabetes compared with trained lean young individuals (31). This may suggest that regular exercise training endows the intracellular milieu with a tightly regulated time‐keeping system that enables dampening fluctuations in substrate availability in a 24‐hour cycle, thereby promoting insulin sensitivity. In summary, these findings indicate it is worth exploring if the interplay between exercise and circadian rhythmicity can be used to sustain and promote glucose homeostasis. To this end, we will need to integrate the knowledge from the chronobiology field with respect to the timing of exercise with the prescription of exercise regimes meant to improve, for example, glucose homeostasis predominantly in metabolically deprived individuals. Future studies should examine if, indeed, appropriate timing of exercise helps to maximize exercise responsiveness (Figure 2).
Figure 2.
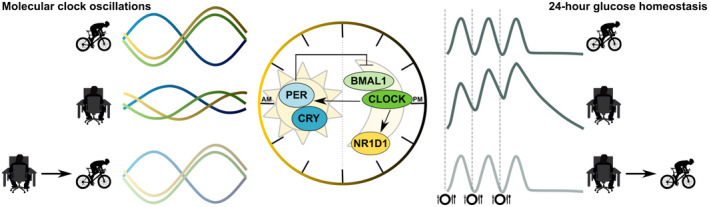
Exercise is a nonphotic cue able to reset peripheral clocks. Expression of clock genes in human skeletal muscle exhibits a profound day‐night rhythmicity with alternating peak expression of the positive (BMAL1 and CLOCK) and negative (PER and CRY) clock components. The amplitude of these oscillations is more profound in physically fit, lean, young individuals (upper left‐hand part) and is blunted in patients with type 2 diabetes (middle left‐hand part). We hypothesize that exercise, as an external nonphotic (not related to light) modulator of the expression and rhythmicity of peripheral (muscle) clocks, can restore the amplitude in clock genes (lower left‐hand part). At the right‐hand side of the image, postprandial glucose peaks in physically fit, lean, young individuals (upper right‐hand part) and in patients with type 2 diabetes (middle right‐hand part), and the hypothetical exercise‐mediated restoration of postprandial glucose peaks is shown schematically (lower right‐hand part).
Classically, the insulin‐sensitizing effects of exercise are explained via 5’‐Adenosine Mono Phosphate–activated protein kinase (AMPK)‐mediated GLUT4 translocation to the subsarcolemmal membrane, thereby promoting insulin‐stimulated myocellular glucose uptake (44). Interestingly, phosphorylation of specific catabolic (α) and regulatory (β and γ) AMPK‐containing subunits exhibit circadian variation in mammalian muscle cells (45), and mice lacking the gamma 3 subunit of AMPK do not display diurnal changes in the respiratory exchange ratio between the light and dark phase that are commonly observed in wild‐type mice (46). In addition, some fundamental clock‐network elements possess AMPK target activation sites (45, 47), providing a molecular mechanism for exercise‐stimulated insulin sensitivity to interact with the clock genes. Bearing in mind that phosphorylation of specific AMPK subunit complexes upon exercise is highly dependent on intensity/duration and exercise type (48, 49), dosing precise exercise methodologies at certain times of the day might be crucial to maximize glycemic outcome in metabolically compromised participants.
Exercise Timing
Timing of exercise can be related to clock time, timing relative to meals, timing relative to light exposure, and timing relative to substrate stores. Obviously, these tightly intertwined factors hamper making the distinction between the contributions of the individual components. In this section, we aim to summarize the effects of timing of exercise without the aim to disentangle the individual contributing components.
Studies in humans consistently revealed that interaction of exercise‐induced AMPK phosphorylation with subsequent TBC1D4 (AS160)‐GLUT4 activation upon insulin stimulation mitigates 24‐hour hyperglycemia (50, 51). Time of day–specific responses to an acute exercise bout have been noted at both the transcriptomic and the metabolomics level in mice (52). Almost 25% of skeletal muscle transcripts responded to exercise in the early active phase. In the early rest phase, however, only around 5% of the transcripts responded to an exercise stimulus (52). In individuals with prediabetes, timing of exercise relative to meal time differentially affects blood glucose control; short‐term (6 × 1 minute) intense (90% maximal heart rate) 30 minutes of exercise prior to each meal was found to attenuate postmeal glucose peaks more profoundly than one 30‐minute exercise session of moderate intensity (30% maximal heart rate) before dinner (53). It has, however, also been shown that low‐intensity exercise after a meal lowers postprandial glucose excursions (54), indicating that timing of the exercise relative to nutrient intake is not the sole determinant of the timing effects of exercise. In fact, nutritional status before as well as after exercise dictates substrate availability and hormonal concentration in plasma, consequently affecting the resynchronization of whole‐body glucose homeostasis. For instance, performing short cycling bouts while being fasted triggers elevated synthesis of stress‐related hormones in individuals with type 2 diabetes and does not result in improved glucose homeostasis directly postexercise (55). Notably, such findings do not match the previously reported diminished postprandial glucose excursions when participants with type 2 diabetes perform similar high‐intensity intermittent cycling bouts at fed condition (56).
In patients with type 2 diabetes, afternoon high‐intensity interval training more effectively improved 48‐hour blood glucose profile than morning training, which in fact had deleterious effects on blood glucose values (57). These findings are in line with mice data showing that glycolytic activation is specific to exercise at the early active phase rather than the early rest phase (52), suggesting that time of day affects the beneficial health effects of exercise. In a recent study (58), normoglycemic individuals with overweight and patients with type 2 diabetes followed a 12‐week training program of 30 minutes of moderate‐intensity walking combined with four resistance‐based exercises performed in the morning or afternoon. While the training program improved all glycemic markers, no differences were observed between morning and afternoon exercise (58). This may indicate that timing effects of exercise might be (partly) related to exercise intensity, but clearly more research is needed on this topic.
Twelve weeks of multimodal exercise training improved glycemic control and postprandial glycemic responses in individuals with overweight with or without type 2 diabetes. However, no distinct glycemic benefits or alterations in circadian rhythm were associated with morning versus evening exercise when performed three times per week in this cohort.
Another crucial factor likely influencing temporal fluctuations on exercise‐induced glycemic control is skeletal muscle glycogen content. It has been previously reported that skeletal muscle glycogen content per se stimulates glucose uptake by a negative feedback loop (high muscle glycogen content, low glucose uptake) (59, 60). Moreover, glycogen acts as a signaling molecule (61), altering, for instance, exercise‐induced myokines synthesis (62, 63, 64) and mitochondrial‐related gene expression (65). Subsequently, one can hypothesize that the beneficial effects of exercise on blood glucose homeostasis are more pronounced after an overnight fast (when glycogen depots may be lowered) relative to exercise in the postprandial state. On the other hand, it is important to highlight that exercise‐induced skeletal muscle glycogen turnover is governed by absolute glycogen content (66, 67), which is expected to be higher in the evening. If exercise in the evening indeed amplifies exercise‐induced blood glucose homeostasis in the morning via higher rates of muscle glycogen degradation is currently unknown.
Exercise‐induced catecholamine synthesis is the main humoral factor promoting white adipose tissue and visceral lipolysis (68). Catecholamines possess circadian variation with a peak during the day under resting conditions and two peaks in the morning and the evening upon exercise (69) indicating involvement of the master clock in catecholamine reactivity to exercise. Thus, interplay between the master clock and exercise triggers timing‐dependent metabolic responses (Figure 3). On the one hand, it could be argued that performing exercise when the adrenergic responsiveness is greatest might maximize the exercise‐induced lipolytic response and could lead to a higher Free fatty acids overflow, thereby stimulating ectopic fat turnover. Consequently, peripheral fat accumulation might be reduced and aberrations in lipid‐induced insulin signaling alleviated. On the other hand, however, the stimulatory effects of catecholamines on hepatic glucose output and inhibitory effects on peripheral glucose uptake may translate into elevated blood glucose levels (55, 70). Thus, it is currently unknown if alignment of exercise with the adrenergic circadian peak will be beneficial for glucose homeostasis.
Figure 3.
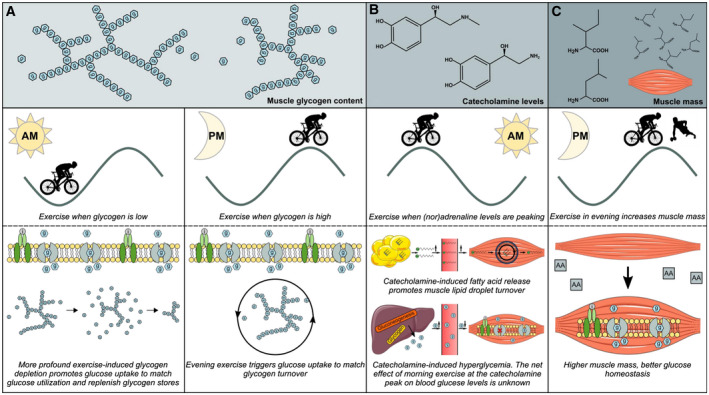
Exercise timing and hypothetical effects on glucose homeostasis. (A) In morning exercise, low glycogen content promotes glucose uptake to match exercise‐mediated glucose utilization and to replenish glycogen stores. Evening exercise triggers glucose uptake to match glycogen turnover. (B) In morning exercise, catecholamine levels are peaking. This induces free fatty acid release from white adipose tissue and may promote uptake and utilization of plasma fatty acids by skeletal muscle, thus preventing ectopic fat storage and putatively ameliorating insulin resistance. Also, catecholamine‐induced hyperglycemia caused by increased hepatic glucose output and reduced peripheral insulin sensitivity can be alleviated by exercise. At the same time, it should be noted that exercise induces a second peak of catecholamines later in the day. The net effect on blood glucose homeostasis of exercise timed at the catecholamine peak remains to be elucidated. (C) Muscle mass is a major determinant of insulin‐stimulated glucose and glucose homeostasis. For optimal maintenance of muscle mass and to promote muscle mass accretion, data indicate that evening premeal resistance exercise promotes muscle protein synthesis by increasing diet‐derived amino acid incorporation in muscle. If this effect is due to the mode of exercise, is due to the timing of the evening relative to clock time, or is relative to the meal is currently unknown.
Preservation of muscle mass throughout the lifespan is crucial for the maintenance or improvement of glucose homeostasis. In this regard, it is important to note that there is a synergistic interaction between resistance‐type exercise training and protein (or protein hydrolysates) ingestion to stimulate muscle mass gain in healthy individuals (71) and/or in individuals at risk for or diagnosed with type 2 diabetes (72). Resistance exercise training stimulates muscle protein synthesis via mammalian Target of Rapamycin‐p70s6K signaling (73), which interacts with the peripheral clock to regulate cell growth in a rhythmic fashion (74). A study in male elderly patients showed that the overnight response of muscle protein synthesis to presleep protein ingestion was stimulated by evening exercise (75). Interestingly, the stimulatory effect of exercise on muscle mass accretion was found to be more prominent in the evening than in the morning (76). In contrast, a recent meta‐analysis concluded that the gain in muscle mass upon resistance exercise training does not differ when exercise is performed in the morning versus the evening. It should be noted, though, that the putative interaction between food ingestion and exercise timing was not taken into account in this meta‐analysis (77), possibly explaining the difference with the study by Kuusmaa et al. (76), in which the participants were instructed to adhere to the national dietary guidelines These findings suggest an interaction between exercise‐induced skeletal muscle adaptations and the peripheral clock in humans.
As dietary‐derived amino acids constitute the main substrate for a positive protein balance postexercise (78, 79), it could be suggested that timed ingestion of amino acids can result in optimized amino acid supply to peripheral organs, thus stimulating muscle mass accretion and maintenance (80). Different regimes for diet and exercise timing have been explored with the aim to identify the optimal timing and mode of exercise to promote or maintain muscle mass (81, 82, 83). Resistance exercise performed in the evening followed by dietary amino acids ingested before sleep promotes de novo myofibrillar protein synthesis and results in a positive net protein balance during overnight sleep in young as well as older participants (75, 84).
Conclusion and Future Perspectives
Glucose homeostasis, and the regulation thereof, exhibits diurnal variation. Specifically, reduced skeletal muscle insulin responsiveness toward the evening coincides with dysregulation of plasma glucose. Alignment of master and peripheral clocks is most likely essential to maintain glucose homeostasis. Exercise training is a first‐line prevention/treatment strategy to circumvent glycemic dysregulation in humans. Acute metabolic exercise‐derived responses and transcriptional adaptations display periodic oscillations over the day. Thus, timing of the exercise session relative to clock time and meals is likely to affect (and possibly maximize) the consequences for glucose homeostasis. Dosing exercise at different times of the day translates into a natural interplay with the nutritional status of patients in a 24‐hour cycle. Hence, we would like to stress the need to integrate the new knowledge from the chronobiology with the aim to understand how the synergistic interplay between exercise and nutritional cues can modulate human (patho)physiology. Importantly, exercise training and nutrition are two potent nonphotic cues that can affect the master as well as peripheral clocks. Conversely, the master and peripheral clocks also affect exercise‐mediated responses. Thus, for optimal alignment of the cellular microenvironment to oscillatory metabolic challenges throughout the day, more information on how the biological clock and physical exercise bidirectionally interact is needed.
Funding agencies
RMF is supported by the National Commission of Scientific and Technological Research (CONICYT) PhD scholarship (Resolucion Extenta number 4426, 2016).
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Anne Gemmink for her assistance on elaborating the images for the present manuscript.
References
- 1. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991;88:934‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab 2014;3:372‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibson T, Stimmler L, Jarrett RJ, Rutland P, Shiu M. Diurnal variation in the effects of insulin on blood glucose, plasma non‐esterified fatty acids and growth hormone. Diabetologia 1975;11:83‐88. [DOI] [PubMed] [Google Scholar]
- 4. Verrillo A, De Teresa A, Martino C, et al. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am J Physiol 1989;257(4 Pt 1):E459‐E465. [DOI] [PubMed] [Google Scholar]
- 5. Panda S. Circadian physiology of metabolism. Science 2016;354:1008‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaix A, Zarrinpar A, Panda S. The circadian coordination of cell biology. J Cell Biol 2016;215:15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckel‐Mahan K, Sassone‐Corsi P. Metabolism and the circadian clock converge. Physiol Rev 2013;93:107‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dyar KA, Ciciliot S, Wright LE, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 2014;3:29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wefers J, van Moorsel D, Hansen J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A 2018;115:7789‐7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta‐analysis of observational studies. Occup Environ Med 2015;72:72‐78. [DOI] [PubMed] [Google Scholar]
- 11. Sjoberg KA, Frosig C, Kjobsted R, et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 2017;66:1501‐1510. [DOI] [PubMed] [Google Scholar]
- 12. Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle‐specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 2016;6:12. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc 2012;44:1663‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabriel BM, Zierath JR. Circadian rhythms and exercise ‐ re‐setting the clock in metabolic disease. Nat Rev Endocrinol 2019;15:197‐206. [DOI] [PubMed] [Google Scholar]
- 15. Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications 2014;28:836‐843. [DOI] [PubMed] [Google Scholar]
- 16. Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691‐2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plat L, Leproult R, L'Hermite‐Baleriaux M, et al. Metabolic effects of short‐term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 1999;84:3082‐3092. [DOI] [PubMed] [Google Scholar]
- 18. Yoshino J, Almeda‐Valdes P, Patterson BW, et al. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole‐body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab 2014;99:E1666‐E1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shostak A, Meyer‐Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013;62:2195‐2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiehn JT, Tsang AH, Heyde I, et al. Circadian rhythms in adipose tissue physiology. Compr Physiol 2017;7:383‐427. [DOI] [PubMed] [Google Scholar]
- 21. Stenvers DJ, Jongejan A, Atiqi S, et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019;62:704‐716. [DOI] [PubMed] [Google Scholar]
- 22. McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr Rev 2018;39:261‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009;32(suppl 2):S157‐S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Moorsel D, Hansen J, Havekes B, et al. Demonstration of a day‐night rhythm in human skeletal muscle oxidative capacity. Mol Metab 2016;5:635‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macauley M, Smith FE, Thelwall PE, Hollingsworth KG, Taylor R. Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin Sci (Lond) 2015;128:707‐713. [DOI] [PubMed] [Google Scholar]
- 27. Doi R, Oishi K, Ishida N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. J Biol Chem 2010;285:22114‐22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinouchi K, Magnan C, Ceglia N, et al. Fasting imparts a switch to alternative daily pathways in liver and muscle. Cell Rep 2018;25:3299‐3314.e3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezagouri S, Zwighaft Z, Sobel J, et al. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab 2019;30:78‐91.e4. [DOI] [PubMed] [Google Scholar]
- 30. Perrin L, Loizides‐Mangold U, Chanon S, et al. Transcriptomic analyses reveal rhythmic and CLOCK‐driven pathways in human skeletal muscle. Elife 2018;7. doi: 10.7554/eLife.34114.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansen J, Timmers S, Moonen‐Kornips E, et al. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci Rep 2016;6:35047. doi: 10.1038/srep35047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woldt E, Sebti Y, Solt LA, et al. Rev‐erb‐alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 2013;19:1039‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong S, Zhou W, Fang B, et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat Med 2017;23:223‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Laurenti MC, Dalla Man C, et al. Glucose metabolism during rotational shift‐work in healthcare workers. Diabetologia 2017;60:1483‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodge BA, Wen Y, Riley LA, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 2015;5:17. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandes AL, Lopes‐Silva JP, Bertuzzi R, et al. Effect of time of day on performance, hormonal and metabolic response during a 1000‐M cycling time trial. PLoS One 2014;9:e109954. doi: 10.1371/journal.pone.0109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care 2016;19:270‐275. [DOI] [PubMed] [Google Scholar]
- 38. Perrin L, Loizides‐Mangold U, Skarupelova S, et al. Human skeletal myotubes display a cell‐autonomous circadian clock implicated in basal myokine secretion. Mol Metab 2015;4:834‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pastore S, Hood DA. Endurance training ameliorates the metabolic and performance characteristics of circadian clock mutant mice. J Appl Physiol 2013;114:1076‐1084. [DOI] [PubMed] [Google Scholar]
- 40. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010;466:627‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000;103:1009‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zambon AC, McDearmon EL, Salomonis N, et al. Time‐ and exercise‐dependent gene regulation in human skeletal muscle. Genome Biol 2003;4:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kjobsted R, Munk‐Hansen N, Birk JB, et al. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 2017;66:598‐612. [DOI] [PubMed] [Google Scholar]
- 45. Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009;326:437‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vieira E, Nilsson EC, Nerstedt A, et al. Relationship between AMPK and the transcriptional balance of clock‐related genes in skeletal muscle. Am J Physiol Endocrinol Metab 2008;295:E1032‐E1037. [DOI] [PubMed] [Google Scholar]
- 47. Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol 2013;366:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform‐specific and exercise intensity‐dependent activation of 5'‐AMP‐activated protein kinase in human skeletal muscle. J Physiol 2000;528(Pt 1):221‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1‐ but not alpha2beta2gamma3‐AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 2007;292:E715‐E722. [DOI] [PubMed] [Google Scholar]
- 50. Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes 1997;46:1775‐1781. [DOI] [PubMed] [Google Scholar]
- 51. Wojtaszewski JF, Hansen BF, Gade J, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 2000;49:325‐331. [DOI] [PubMed] [Google Scholar]
- 52. Sato S, Basse AL, Schönke M, et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab 2019;30:92‐110.e114. [DOI] [PubMed] [Google Scholar]
- 53. Francois ME, Baldi JC, Manning PJ, et al. 'Exercise snacks' before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 2014;57:1437‐1445. [DOI] [PubMed] [Google Scholar]
- 54. Manohar C, Levine JA, Nandy DK, et al. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care 2012;35:2493‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kjaer M, Hollenbeck CB, Frey‐Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non‐insulin‐dependent diabetes. J Appl Physiol 1990;68:2067‐2074. [DOI] [PubMed] [Google Scholar]
- 56. Larsen JJ, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia 1999;42:1282‐1292. [DOI] [PubMed] [Google Scholar]
- 57. Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 2019;62:233‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teo SYM, Kanaley JA, Guelfi KJ, Marston KJ, Fairchild TJ. The effect of exercise timing on glycemic control: a randomized clinical trial. Med Sci Sports Exerc 2020;52:323‐334. [DOI] [PubMed] [Google Scholar]
- 59. Jensen J, Jebens E, Brennesvik EO, et al. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab 2006;290:E154‐E162. [DOI] [PubMed] [Google Scholar]
- 60. Derave W, Hansen BF, Lund S, Kristiansen S, Richter EA. Muscle glycogen content affects insulin‐stimulated glucose transport and protein kinase B activity. Am J Physiol Endocrinol Metab 2000;279:E947‐E955. [DOI] [PubMed] [Google Scholar]
- 61. Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab 2012;302:E1343‐E1351. [DOI] [PubMed] [Google Scholar]
- 62. Steensberg A, Febbraio MA, Osada T, et al. Interleukin‐6 production in contracting human skeletal muscle is influenced by pre‐exercise muscle glycogen content. J Physiol 2001;537:633‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL‐6 receptor expression in human skeletal muscle. J Appl Physiol 2013;99:2075‐2079. [DOI] [PubMed] [Google Scholar]
- 64. MacDonald C, Wojtaszewski JF, Pedersen BK, Kiens B, Richter EA. Interleukin‐6 release from human skeletal muscle during exercise: relation to AMPK activity. J Appl Physiol 2003;95:2273‐2277. [DOI] [PubMed] [Google Scholar]
- 65. Pilegaard H, Keller C, Steensberg A, et al. Influence of pre‐exercise muscle glycogen content on exercise‐induced transcriptional regulation of metabolic genes. J Physiol 2002;541:261‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shearer J, Marchand I, Tarnopolsky MA, Dyck DJ, Graham TE. Pro‐ and macroglycogenolysis during repeated exercise: roles of glycogen content and phosphorylase activation. J Appl Physiol 2001;90:880‐888. [DOI] [PubMed] [Google Scholar]
- 67. Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol 1995;78:288‐292. [DOI] [PubMed] [Google Scholar]
- 68. Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 1990;85:893‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 2010;107:20541‐20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest 1980;65:682‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. Am J Clin Nutr 2007;85:1005‐1013. [DOI] [PubMed] [Google Scholar]
- 72. Campbell WW, Kim JE, Amankwaah AF, Gordon SL, Weinheimer‐Haus EM. Higher total protein intake and change in total protein intake affect body composition but not metabolic syndrome indexes in middle‐aged overweight and obese adults who perform resistance and aerobic exercise for 36 weeks. J Nutr 2015;145:2076‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17:162‐184. [DOI] [PubMed] [Google Scholar]
- 74. Ramanathan C, Kathale ND, Liu D, et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet 2018;14:e1007369. doi: 10.1371/journal.pgen.1007369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Holwerda AM, Kouw IW, Trommelen J, et al. Physical activity performed in the evening increases the overnight muscle protein synthetic response to presleep protein ingestion in older men. J Nutr 2016;146:1307‐1314. [DOI] [PubMed] [Google Scholar]
- 76. Kuusmaa M, Schumann M, Sedliak M, et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab 2016;41:1285‐1294. [DOI] [PubMed] [Google Scholar]
- 77. Grgic J, Lazinica B, Garofolini A, Schoenfeld BJ, Saner NJ, Mikulic P. The effects of time of day‐specific resistance training on adaptations in skeletal muscle hypertrophy and muscle strength: a systematic review and meta‐analysis. Chronobiol Int 2019;36:449‐460. [DOI] [PubMed] [Google Scholar]
- 78. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein‐derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322‐331. [DOI] [PubMed] [Google Scholar]
- 79. Witard OC, Tieland M, Beelen M, Tipton KD, van Loon LJ, Koopman R. Resistance exercise increases postprandial muscle protein synthesis in humans. Med Sci Sports Exerc 2009;41:144‐154. [DOI] [PubMed] [Google Scholar]
- 80. Mamerow MM, Mettler JA, English KL, et al. Dietary protein distribution positively influences 24‐h muscle protein synthesis in healthy adults. J Nutr 2014;144:876‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tipton KD, Rasmussen BB, Miller SL, et al. Timing of amino acid‐carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 2001;281:E197‐E206. [DOI] [PubMed] [Google Scholar]
- 82. Areta JL, Burke LM, Ross ML, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 2013;591:2319‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moore DR, Areta J, Coffey VG, et al. Daytime pattern of post‐exercise protein intake affects whole‐body protein turnover in resistance‐trained males. Nutr Metab (Lond) 2012;9:91. doi: 10.1186/1743-7075-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Snijders T, Res PT, Smeets JS, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance‐type exercise training in healthy young Men. J Nutr 2015;145:1178‐1184. [DOI] [PubMed] [Google Scholar]


