Summary
Background
Eczema phenotypes and emotional and behavioural problems are highly prevalent in childhood, but their mutual relationship is not fully clear.
Objectives
To examine the associations of eczema phenotypes with school‐age emotional and behavioural problems, and the bidirectional associations of eczema and emotional and behavioural problems from birth until 10 years.
Methods
This study among 5265 individuals was embedded in a prospective population‐based cohort study. Never, early transient, mid‐transient, late transient and persistent eczema phenotypes were identified based on parent‐reported, physician‐diagnosed eczema from age 6 months until 10 years. Emotional (internalizing) and behavioural (externalizing) problems were measured repeatedly using the Child Behavior Checklist from age 1·5 to 10 years. Cross‐lagged models were applied for bidirectional analyses.
Results
All eczema phenotypes were associated with more internalizing problems and attention problems at age 10 years, compared with never having eczema: range of Z‐score differences 0·14 [95% confidence interval (CI) 0·01–0·27] to 0·39 (95% CI 0·18–0·60). Children with early transient eczema had more aggressive behaviour symptoms at age 10 years (Z = 0·16, 95% CI 0·05–0·27). Bidirectional analysis showed that eczema at 0–2 years was associated with more internalizing and externalizing problems at ages 3–6 and 10 years, while, inversely, only internalizing problems at 0–2 years were associated with an increased risk of eczema at age 10 years.
Conclusions
Eczema phenotypes are very modestly associated with more somatic symptoms and attention problems at school age. Early transient eczema is associated with more aggressive behaviour symptoms. Directional effects seem to occur from early‐life eczema to later‐life internalizing and externalizing problems, rather than the reverse.
Short abstract
What's already known about this topic?
Previous cohort studies using non‐data‐driven methods to define eczema phenotypes observed that children with early‐onset and persistent eczema had a higher risk of emotional and behavioural problems in preadolescence.
Alternatively, previous cohort studies showed that children with emotional and behavioural problems had more severe eczema and eczema exacerbations in childhood.
The direction of effects between eczema and emotional and behavioural problems is not fully clear.
What does this study add?
Taking the variability of eczema onset and persistence within and between children over time into account, all identified eczema phenotypes were very modestly associated with more somatic symptoms and attention problems at school age.
Directional effects seem to occur from eczema leading to emotional and behavioural problems, rather than the reverse.
Future research should focus on the effect of early optimal eczema management on mental health disorders in children later in life.
Plain language summary available online
Eczema is a common skin disorder in early life. Both genetic and environmental early‐life factors affect the risk of childhood eczema.1, 2 Psychopathological problems also seem to be involved.3, 4, 5 We previously showed that maternal psychiatric symptoms during pregnancy were associated with an increased risk of childhood eczema, independently of maternal psychiatric symptoms after birth or paternal psychiatric symptoms.6 Stress, as a proxy of these problems, could shift the balance towards type 2 T helper cells via the hypothalamic–pituitary–adrenal axis and sympathetic adrenomedullary system, leading to more susceptibility to atopic diseases.7 It is not clear whether a child's emotional and behavioural problems affect the risk of eczema.
Previous systematic reviews and large survey studies showed that children in all age groups with eczema had more emotional problems, anxiety, depression, attention deficit hyperactivity disorders and conduct disorders5, 8, 9, 10 than children who never had eczema. Taking the onset of eczema into account, it was shown that children with early‐onset, transient and chronic eczema had increased risks of emotional and behavioural problems at age 10–15 years.11, 12 Alternatively, previous studies showed that children with emotional and behavioural problems had more severe eczema and eczema exacerbations at ages 3–18 years.3, 4, 9, 10 Thus, the effects between eczema and emotional and behavioural problems could be in both directions.9
Eczema phenotypes take into account the variability of eczema onset and persistence within and between individuals over time.13 Using eczema phenotypes might clarify which children with eczema in early life are most at risk of emotional and behavioural problems later in life. Additionally, bidirectional analyses could reveal whether eczema leads to emotional and behavioural problems or the reverse. Therefore, we aimed to examine the associations of eczema phenotypes from birth until age 10 years with school‐age emotional and behavioural problems among 5265 individuals in a population‐based prospective cohort study. Next, we examined the associations of eczema with emotional and behavioural problems from birth until age 10 years bidirectionally.
Patients and methods
Study design
This study was embedded in the Generation R Study, a population‐based prospective cohort study from early fetal life onwards in Rotterdam, the Netherlands. Eligible women were those who were living in Rotterdam and who had an expected delivery date from April 2002 to January 2006, as described previously.14 The children form a prenatally recruited birth cohort that will be followed at least until young adulthood. Around the age of 10 years, all children were invited to visit our research centre in the Erasmus MC Sophia Children's Hospital to participate in hands‐on measurements, advanced imaging modalities, behavioural observations and biological sample collection. The study was approved by the medical ethical committee of the Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. Written informed consent was obtained from parents or legal guardians. For the current analysis, children were included if they had available information on eczema on at least three timepoints to create eczema phenotypes, and any assessment of emotional and behavioural problems from birth until the age of 10 years. In total, 5265 children were included (Fig. S1; see Supporting Information).
Eczema phenotypes
Information on physician‐diagnosed eczema was obtained from parental‐reported questionnaires at the ages of 6 months and 1, 2, 3, 4 and 10 years: ‘Was your child diagnosed with eczema in the last 6 months/last year by a general practitioner or physician in the hospital?’ (no; yes). In children with available data on physician‐diagnosed eczema on at least three timepoints, we previously identified five eczema phenotypes, namely never, early transient, mid‐transient, late transient and persistent eczema.13 The children were assigned to the eczema phenotype for which they had the highest posterior probability. Data on ever having eczema were collected by parent‐reported questionnaires at 10 years of age: ‘Has your child ever had eczema diagnosed by a doctor?’.
Emotional and behavioural problems
The Child Behavior Checklist (CBCL) was used to assess parent‐reported child emotional and behavioural problems. This instrument has proven to be reliable and valid in various population samples from different countries and cultures.15, 16 The symptoms were measured at median (interquartile range) ages of 1·5 (1·5–1·6), 3·0 (3·0–3·1), 6·0 (5·8–6·2) and 9·7 (9·6–9·8) years. At the ages of 1·5, 3 and 6 years, the preschool CBCL version for children aged 1·5–5 years (CBCL/1·5–5) was used.17 At the age of 10 years, the school‐age version of the CBCL for children aged 6–18 years was used (CBCL/6–18).
The CBCL consists of empirically derived broadband scales and syndrome scales. We used two broadband scales and five syndrome scales based on the strongest associations with eczema observed in previous studies.3, 4, 5, 8, 9, 10, 11, 12 We examined the broadband scale of internalizing problems, a measure of emotional problems, and the three comprising syndrome scales of anxious‐depressed, withdrawn‐depressed and somatic symptoms (e.g. constipation, dizziness, headaches). We also examined the broadband scale externalizing problems, a measure of behavioural problems, and the two comprising syndrome scales of attention problems and aggressive behaviour symptoms at ages 1·5, 3 and 5 years, and only aggressive behaviour symptoms at age 10 years. In CBCL/6–18, attention problems are not part of externalizing problems due to their sizeable factor in both broadband scales in factor analysis.15 Scale scores were log, quadratic and square‐root transformed and then standardized because of non‐normal distributions.
Covariates
Information on maternal age, parity (multiparous; nulliparous), education (primary or secondary school; higher than secondary school) and parental history eczema, allergy or asthma (no; yes) was available from questionnaires obtained at enrolment. Maternal psychiatric symptoms during pregnancy were defined using the Global Severity Index.18 The child's sex, gestational age at birth and birthweight were obtained from midwives and hospital records, and ethnic origin (European; non‐European) was defined based on the parents’ country of birth according to Statistics Netherlands.19 Postnatal questionnaires provided information on breastfeeding at 2, 6 and 12 months after birth, which was combined into never vs. ever, and on sleep problems at 2 months after birth (no; yes).
Statistical analysis
We compared the characteristics of those included and not included in our study using Pearson's χ2‐test, an independent‐samples t‐test and the Mann–Whitney U‐test. We examined the associations of eczema phenotypes with internalizing and externalizing problems, and comprising syndrome scales, at age 10 years using linear regression models. Next, cross‐lagged models were used to examine bidirectional associations of eczema with internalizing and externalizing problems, and comprising syndrome scales, from birth until 10 years. Cross‐lagged models allow associations between two repeatedly measured variables to be examined in both directions simultaneously while accounting for continuity between the repeated measures over time. With this method, we were able to disentangle the predominant direction of the observed association between eczema and emotional and behavioural problems.
In order to make a more balanced model, three age categories (0–2, 3–6 and 10 years) were defined based on the prevalence of physician‐diagnosed eczema and distribution of CBCL scales. We examined cross‐lagged effects, cross‐sectional effects and stability effects. A conceptual model of the studied cross‐lagged associations is presented in Figure S2 (see Supporting Information). Bidirectional associations of withdrawn‐depressed symptoms were not studied because this scale was not assessed by CBCL/1·5–5. All analyses were adjusted for potential confounders, which were selected from the literature based on whether they were strongly related to eczema and internalizing and externalizing problems, and were not in the causal pathway. Therefore maternal age; parity; history of eczema, allergy or asthma; and child's birthweight were not included in the model. We assumed that data were missing at random. The amount of missing data in covariates was ≤ 21%, and 125 datasets were created using multiple imputation by chained equations.
The class assignment was sampled using the participant‐specific posterior class probabilities determined by the latent class growth model in regression modelling based on the posterior probabilities, in order to take into account the uncertainty of eczema phenotype class assignment. The size and direction of the effect estimates were similar when we used complete‐case analyses, and therefore we present only the results based on imputed data. For more easy interpretation, we also examined the associations of eczema phenotypes with borderline clinical cutoffs of the internalizing and the externalizing problem scales (< 84th vs. ≥ 84th percentile) and syndrome scales (< 80th vs. ≥ 80th percentile).15
All research questions, including defined exposure definitions and outcomes, and statistical analyses, were determined a priori, discussed with the principal investigators and study team members, and adapted where appropriate. No post hoc analyses were performed. We did not adjust for multiple testing, because the used syndrome scales were related to each other and examined under the same hypothesis. All measures of association are presented as Z‐score differences or odds ratios (ORs), together with their corresponding 95% confidence intervals (CIs). Imputation and regression analyses were performed using the packages ‘mice’ (version 3·3·0) and ‘stats’ (version 3·5·2) in R version 3·5·2,20, 21 and cross‐lagged analyses were performed in Mplus version 8·2.22
Results
Characteristics of the cohort
The characteristics of the children and their mothers are shown in Table 1. Rates of physician‐diagnosed eczema ranged from 16% at age 6 months to 6% at age 10 years, and 23% of children had ever had eczema at age 10 years. Compared with children who were included in the analysis, those not included had mothers who had lower education and more often had psychiatric symptoms and a history of asthma, allergy or eczema (Table S1; see Supporting Information). Children not included were more often of non‐European ethnicity and never breastfed, and had a lower gestational age and birthweight.
Table 1.
Characteristics of the children and their mothers
| All patients (N = 5265) | Never eczema phenotype (n = 3995) | Ever eczema phenotypea (n = 1270) | |
|---|---|---|---|
| Maternal characteristics | |||
| Age at enrolment (years), mean ± SD | 31·5 ± 4·6 | 31·5 ± 4·6 | 31·4 ± 4·6 |
| Parity, nulliparous, n (%) | 3072 (58) | 2280 (57) | 792 (62) |
| Maternal education, higher, n (%) | 3001 (57) | 2277 (57) | 724 (57) |
| Maternal psychiatric symptom scale, median (IQR) | 0·1 (0·1–0·3) | 0·1 (0·1–0·3) | 0·2 (0·1–0·3) |
| Child characteristics | |||
| Sex, female, n (%) | 2652 (50) | 2041 (51) | 611 (48) |
| Gestational age at birth (weeks), median (IQR) | 40·1 (39·0–41·0) | 40·1 (39·0–41·0) | 40·1 (39·0–41·0) |
| Birthweight (g), mean ± SD | 3446 ± 567 | 3454 ± 569 | 3422 ± 559 |
| Ethnicity, non‐European, n (%) | 1331 (25) | 984 (25) | 347 (27) |
| Breastfeeding, ever, n (%) | 4857 (92) | 3695 (93) | 1162 (92) |
| Sleep problems at 2 months, yes, n (%) | 615 (12) | 447 (11) | 168 (13) |
| Eczema phenotypes, n (%)b | |||
| Never | 3995 (76) | 3995 (100) | 0 |
| Early transient | 434 (8) | 0 | 434 (34) |
| Mid‐transient | 302 (6) | 0 | 302 (24) |
| Late transient | 412 (8) | 0 | 412 (32) |
| Persistent | 122 (2) | 0 | 122 (10) |
| Emotional and behavioural problems at age 10 years, median item score (IQR)b | |||
| Internalizing problems | 3 (1–7) | 3 (1–6) | 4 (2–7) |
| Anxious‐depressed symptoms | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| Withdrawn‐depressed symptoms | 1 (0–2) | 0 (0–2) | 1 (0–2) |
| Somatic symptoms | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Externalizing problems | 2 (0–5) | 2 (0–5) | 3 (1–6) |
| Aggressive behaviour symptoms | 2 (0–4) | 1 (0–4) | 2 (0–5) |
| Attention problems | 2 (1–5) | 2 (1–5) | 3 (1–6) |
Values are presented after imputation. aEver eczema phenotype consists of early transient, mid‐transient, late transient and persistent eczema phenotypes. bData were not imputed, and were missing for ever/never eczema at age 10 years (29%), and emotional and behavioural problems at age 1·5 (12%), 3 (12%), 6 (13%) and 10 years (26%).
Eczema phenotypes and internalizing problems in school‐age children
Compared with children who had never had eczema, those who had ever had eczema had more internalizing problems, including more anxious‐depressed and somatic symptoms at age 10 years – Z‐score differences (95% CI) 0·21 (0·14–0·29), 0·10 (0·02–0·17) and 0·29 (0·22–0·37), respectively (Table 2) – but not withdrawn‐depressed symptoms. Compared with the never eczema phenotype, all eczema phenotypes were associated with more internalizing problems [range 0·14 (95% CI 0·01–0·27) to 0·30 (95% CI 0·10–0·51)] and an increased risk of somatic symptoms [range 0·13 (95% CI 0·01–0·24) to 0·39 (95% CI 0·18–0·60)]. When we used borderline clinical cutoffs, children with early transient, late transient and persistent eczema had the highest risks of somatic symptoms at age 10 years: ORs 1·61 (95% CI 1·26–2·04), 1·43 (95% CI 1·12–1·83) and 2·37 (95% CI 1·53–3·66), respectively (Table S2; see Supporting Information).
Table 2.
Associations of eczema phenotypes with internalizing problems at age 10 years
| Internalizing problems | Anxious‐depressed symptoms | Withdrawn‐depressed symptoms | Somatic symptoms | |
|---|---|---|---|---|
| Never | Reference | Reference | Reference | Reference |
| Ever | 0·21 (0·14–0·29)* | 0·10 (0·02–0·17)* | 0·05 (−0·02–0·12) | 0·29 (0·22–0·37)* |
| Never | Reference | Reference | Reference | Reference |
| Early transient | 0·20 (0·09–0·31)* | 0·10 (−0·02–0·21) | 0·09 (−0·02–0·20) | 0·23 (0·12–0·34)* |
| Mid‐transient | 0·14 (0·01–0·27)* | 0·12 (−0·01–0·25) | 0·12 (−0·01–0·25) | 0·15 (0·02–0·27)* |
| Late transient | 0·14 (0·03–0·25)* | 0·07 (−0·04–0·19) | 0·09 (−0·03–0·20) | 0·13 (0·01–0·24)* |
| Persistent | 0·30 (0·10–0·51)* | 0·18 (−0·03–0·38) | 0·00 (−0·20–0·21) | 0·39 (0·18–0·60)* |
Values are average standardized regression coefficients (Z‐scores) (95% confidence intervals) from linear regression models. The reference group is the never eczema (phenotype) group. Models were adjusted for maternal education and psychiatric symptoms, and child's sex, gestational age, ethnicity, breastfeeding and sleep disturbances. *P < 0·05.
Eczema phenotypes and externalizing or attention problems in school‐age children
Compared with children who had never had eczema, those who had ever had eczema had more externalizing problems, including more symptoms of aggressive behaviour, and attention problems: Z‐score differences (95% CI) 0·13 (0·06–0·21), 0·12 (0·05–0·19) and 0·15 (0·08–0·23), respectively (Table 3). Compared with the never eczema phenotype, only early transient eczema was associated with more externalizing problems, including more symptoms of aggressive behaviour: Z‐scores 0·16 (95% CI 0·04–0·27) and 0·16 (95% CI 0·05–0·27). All eczema phenotypes were associated with more attention problems: Z‐score range 0·15 (95% CI 0·04–0·26) to 0·24 (95% CI 0·04–0·45). When we used borderline clinical cutoffs, children with early transient, mid‐transient and persistent eczema had the highest risks of attention problems: OR range 1·36 (95% CI 1·02–1·81) to 1·74 (95% CI 1·08–2·79) (Table S3; see Supporting Information).
Table 3.
Associations of eczema phenotypes with externalizing problems at age 10 years
| Externalizing problems | Aggressive behaviour symptoms | Attention problems | |
|---|---|---|---|
| Never | Reference | Reference | Reference |
| Ever | 0·13 (0·06–0·21)* | 0·12 (0·05–0·19)* | 0·15 (0·08–0·23)* |
| Never | Reference | Reference | Reference |
| Early transient | 0·16 (0·04–0·27)* | 0·16 (0·05–0·27)* | 0·20 (0·09–0·31)* |
| Mid‐transient | 0·12 (−0·01–0·25) | 0·12 (−0·01–0·25) | 0·21 (0·08–0·34)* |
| Late transient | 0·08 (−0·03–0·20) | 0·10 (−0·01–0·22) | 0·15 (0·04–0·26)* |
| Persistent | 0·05 (−0·16–0·26) | 0·04 (−0·17–0·24) | 0·24 (0·04–0·45)* |
Values are average standardized regression coefficients (Z‐scores) (95% confidence intervals) from linear regression models. The reference group is the never eczema (phenotype) group. Models were adjusted for maternal education and psychiatric symptoms, and child's sex, gestational age, ethnicity, breastfeeding and sleep disturbances. *P < 0·05.
Direction of associations between eczema and emotional and behavioural problems
Figure 1 and Table S4 (see Supporting Information) show the bidirectional associations between eczema and internalizing problems. Cross‐lagged effects showed that compared with children without eczema at ages 0–2 years, those with eczema at ages 0–2 years had more internalizing problems at ages 3–6 years and 10 years: Z‐score differences 0·08 (95% CI 0·02–0·15) and 0·09 (95% CI 0·02–0·16) (Fig. 1a). This included more anxious‐depressed symptoms at ages 3–6 years (Z‐score difference 0·08, 95% CI 0·02–0·15) (Fig. 1b) and more somatic symptoms at ages 3–6 years and 10 years (Z‐score differences 0·11, 95% CI 0·04–0·18, and 0·13, 95% CI 0·05–0·20) (Fig. 1c). Reversely, cross‐lagged effects showed that children with more internalizing problems at ages 0–2 years had an increased risk of eczema only at age 10 years: OR 1·19 (95% CI 1·00–1·40) per point increase in internalizing problem scale) (Fig. 1a). No associations of anxious‐depressed or somatic symptoms with later eczema were observed (Fig. 1b, c).
Figure 1.
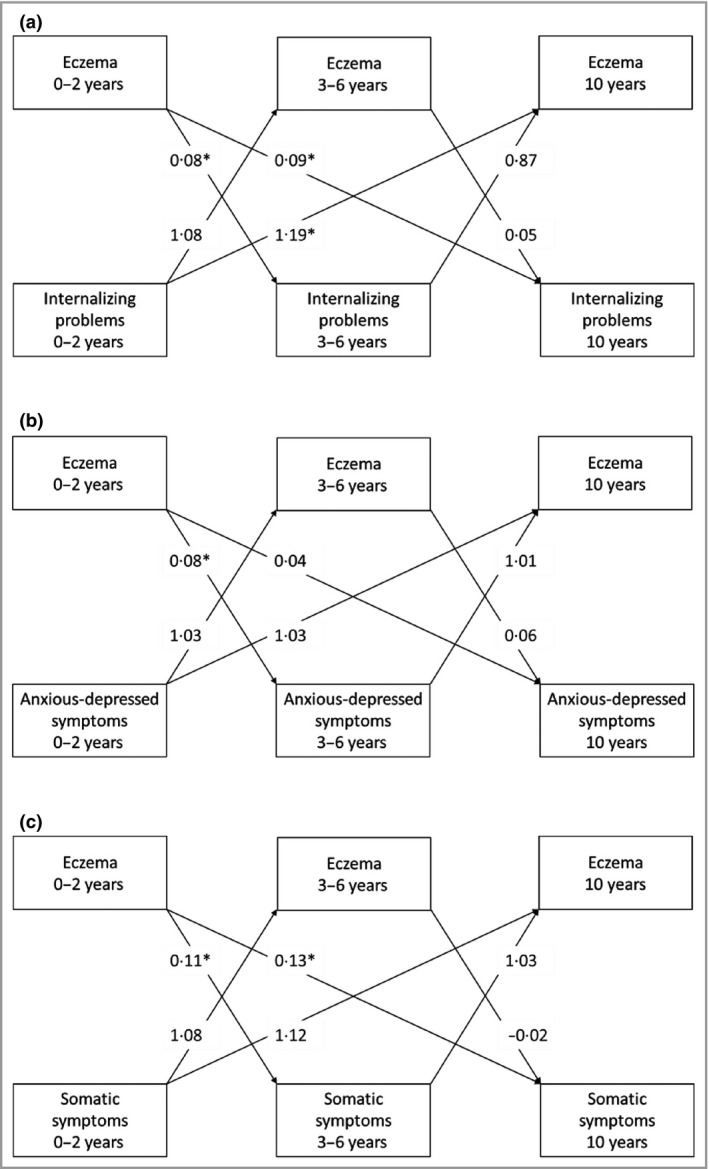
Bidirectional associations between eczema and internalizing problems. Directions of associations between physician‐diagnosed eczema and (a) internalizing problems, (b) anxious‐depressed symptoms and (c) somatic symptom from birth until age 10 years. The arrows indicate the directions of the associations. Values are average standardized regression coefficients for internalizing problems as the outcome, and odds ratios for eczema as the outcome (reference group: no eczema) derived from linear or logistic regression models, using cross‐lagged modelling. Models were adjusted for maternal education and psychiatric symptoms, and child's sex, gestational age, ethnicity, breastfeeding and sleep disturbances. *P < 0·05. The corresponding 95% confidence intervals of the cross‐lagged effects, and the effect estimates of the cross‐sectional and stability effects are shown in Table S4 (see Supporting Information).
Figure 2 and Table S4 (see Supporting Information) show the bidirectional associations between eczema and externalizing problems. Cross‐lagged effects showed that compared with children without eczema at ages 0–2 years, children with eczema at ages 0–2 years had more externalizing problems at age 10 years only (Z‐score difference 0·08, 95% CI 0·01–0·14) (Fig. 2a), including more aggressive behaviour at age 10 years (Z‐score difference 0·08, 95% CI 0·01–0·14) (Fig. 2b). Compared with children without eczema at ages 0–2 and 3–6 years, children with eczema in those age groups had more attention problems at age 10 years [Z‐score differences 0·09 (95% CI 0·02–0·15) and 0·10 (95% CI 0·01–0·19), respectively] (Fig. 2c). Reversely, no associations of early externalizing problems or the related syndrome scales with later eczema were observed (Fig. 2). The full results of the cross‐sectional and stability effects of eczema with internalizing and externalizing problems are presented in Table S4 (see Supporting Information).
Figure 2.
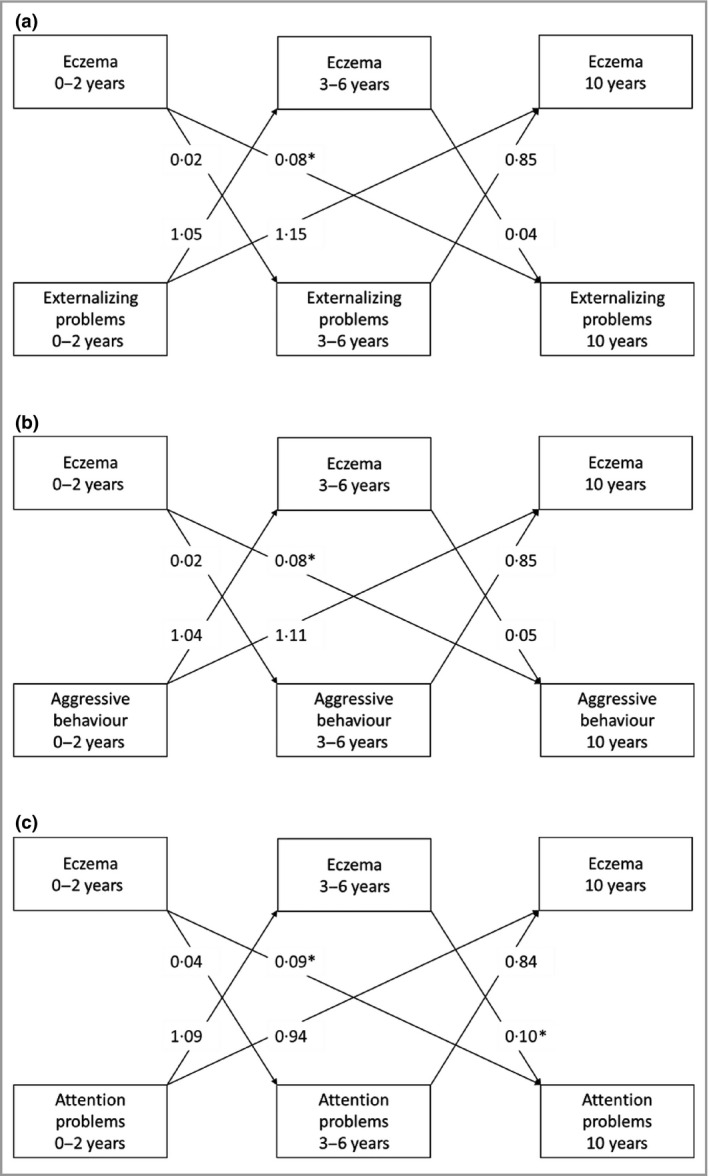
Bidirectional association between eczema and externalizing problems. Direction of associations between physician‐diagnosed eczema and (a) externalizing problems, (b) aggressive behaviour symptoms and (c) attention problems from birth until age 10 years. The arrows indicate the directions of the associations. Values are average standardized regression coefficients for externalizing problems as the outcome and odds ratios for eczema as the outcome (reference group: no eczema) derived from linear or logistic regression models, using cross‐lagged modelling. Models were adjusted for maternal education and psychiatric symptoms, and child's sex, gestational age, ethnicity, breastfeeding and sleep disturbances. *P < 0·05. The corresponding 95% confidence intervals of the cross‐lagged effects, and the effect estimates of the cross‐sectional and stability effects are shown in Table S4 (see Supporting Information).
Discussion
In this population‐based prospective cohort study, we observed that children who had ever had eczema had more emotional (internalizing) and behavioural (externalizing) problems at the age of 10 years than children without eczema. All eczema phenotypes were consistently but very modestly associated with more somatic symptoms and attention problems at school age. Additionally, children with early transient eczema had more aggressive behaviour symptoms at school age. The directions of effects were predominantly present for eczema until 2 years leading to increased internalizing and externalizing problems at school age rather than the reverse.
We observed that children who had ever had eczema had more internalizing and externalizing problems at school age. These results are in line with previous meta‐analyses.3, 4, 5, 10 Concerning the internalizing problems, we observed that children who had ever had eczema had more anxious‐depressed symptoms only, but not when we grouped children based on the onset and persistence over time using data‐driven analysis. In contrast, previous meta‐analyses showed that children with eczema had more anxious and depressed symptoms.4, 5 The differences in results might partly be explained by our study population, which consists of relatively healthy and young children until age 10 years, compared with previous studies consisting of patients and children until 18 years. Those studies observed stronger effect estimates of eczema with anxious and depressed symptoms in adults compared with children.4 Also, differences in sample size, definition of eczema and measurement tools for anxiety‐ and depression‐related symptoms might have played a role.
Furthermore, we observed that all eczema phenotypes were most consistently associated with more somatic symptoms and attention problems at school age. Previous cohort studies using non‐data‐driven methods to define eczema phenotypes, such as grouping of children by researchers’ experiences, observed that children with early‐onset and persistent eczema had a higher risk of emotional and behavioural problems in preadolescence.4, 9, 10, 11 While data‐driven analyses appear to be less biased, the best method to define eczema phenotypes depends on the study population, data distribution and availability, and specific research aim.
To the best of our knowledge, our study is the first to apply cross‐lagged modelling for bidirectional analyses, and we observed that eczema until age 2 years was associated with more emotional and behavioural problems at school age more dominantly than reversely. These findings contribute to the disentanglement of the complex bidirectional relationship of eczema with emotional and behavioural problems and suggest that the directional effects are from early‐life eczema to later‐life internalizing and externalizing problems rather than the reverse.
The modest effect sizes of the observed associations of eczema phenotypes with emotional and behavioural problems imply that, on a population‐based level, most children with eczema are mentally healthy. However, the results might be different on an individual or hospital‐based level. When we used subclinical cutoffs, the effect estimates were greater and consistent for the association of early transient eczema with the risk of emotional and behavioural problems. Therefore, part of our results are in line with the current European guidelines, suggesting that children with moderate or recurrent eczema should receive psychosomatic counselling.23 Future studies are needed to examine the role of such eczema treatment on the development of emotional and behavioural problems later in life, while taking severity and genetic susceptibility into account.
Several hypotheses exist on the relationship of eczema with emotional and behavioural problems. Eczema and related symptoms such as chronic itchiness, red patchy skin appearance and disturbed sleep could negatively affect mental health via social isolation, low self‐image, lack of concentration and more irritability, and possibly also via low‐grade inflammation and blood–brain barrier disruption.10, 24, 25, 26 Conversely, stress, as a proxy of these problems, could shift the balance towards type 2 T helper cells via the hypothalamic–pituitary–adrenal axis and sympathetic adrenomedullary system, leading to more susceptibility to atopic inflammation and diseases, resulting in a vicious cycle.7
Another explanation is based on shared pathogenesis between eczema and mental health disorders, as both skin cells and neurons originate from the ectoderm.27 Shared common genetic variants were found for skin barrier defects and mental health disorders that are involved in both histamine and immune response regulation, and the dopaminergic system.28, 29, 30, 31, 32 The immune system has been associated with many mental health disorders in adults, but the underlying pathways remain unclear.33 Expression of proinflammatory factors in adults with atopic diseases and autism spectrum disorders was shown to disrupt the blood–brain barrier.34 Therefore, chronic inflammation, specifically in early life when maturation of the nervous system and the immune system is still ongoing, might increase susceptibility for mental health disorders.
The strengths of this study are its prospective design with detailed, repeated information on eczema and emotional and behavioural problems, and the use of data‐driven defined eczema phenotypes. By using sampling based on class assignment probabilities, we achieved less misclassification bias and more precise effect estimates. However, some methodological limitations need to be addressed. The exclusion of some children resulted in a selection towards a healthier and more affluent population. Secondly, reporting of emotional and behavioural problems by parents of children with eczema might be different from that by parents of children without eczema. We tried to minimize this misclassification by using validated questionnaires.15, 35 Thirdly, residual confounding might be present as not all factors associated with eczema and emotional and behavioural problems were measured or included in the analysis, such as severity of eczema, sleep problems in later life, and other (atopic) comorbidities.10 No information was available to determine the severity of cases of eczema. Fourthly, the clinical impact might be minimal due to the observed very modest effect sizes in Z‐score differences and the use of subclinical cutoffs for the associations of eczema phenotypes with emotional and behavioural problems in this population‐based study. Lastly, we cannot exclude that the observations from cross‐lagged models might be the result of the natural course of the studied conditions.2, 36 However, cross‐lagged models take the natural course into account by adjusting for the stability effects.
In conclusion, all eczema phenotypes were very modestly associated with more somatic symptoms and attention problems at school age. Children with early transient eczema had more symptoms of aggressive behaviour. The very modest effect sizes of the observed associations of eczema phenotypes with emotional and behavioural problems imply that, on a population‐based level, most children with eczema are mentally healthy. The directional effects occur from eczema in early life leading to internalizing and externalizing problems in later life, rather than the reverse. Therefore, future research should focus on the effect of early optimal eczema management on mental health disorders in children later in life.
Supporting information
Fig S1. Flowchart of participants included in the analysis.
Fig S2. Conceptual cross‐lagged model of eczema and emotional and behavioural problems.
Table S1 Characteristics of children and their mothers for those included and not included in the analyses.
Table S2 Associations of eczema phenotypes with borderline clinical cutoffs of internalizing problems at age 10 years.
Table S3 Associations of eczema phenotypes with borderline clinical cutoffs of externalizing problems at age 10 years.
Table S4 Direction of associations between eczema and emotional and behavioural problems from birth until age 10 years.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam area, Rotterdam; the Rotterdam Homecare Foundation, Rotterdam; and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR‐MDC), Rotterdam. We gratefully acknowledge the contribution of the children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. We would like to thank Ivonne P.M. Derks and Yllza Xerxa from the Generation R Study Group and Department of Child and Adolescent Psychiatry/Psychology at Erasmus MC, University Medical Center Rotterdam, for advice on the statistical analyses.
Funding sources The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. L.D. received funding from the European Union's Horizon 2020 cofunded programme ERA‐Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project no. 696295; 2017), ZonMW the Netherlands (no. 529051014; 2017) and the European Union's Horizon 2020 research and innovation programme (LIFECYCLE project, grant agreement no. 733206; 2016). The study sponsors had no role in the study design, data analysis, interpretation of data or writing of this report.
Conflicts of interest None to declare.
Plain language summary available online
References
- 1. Paternoster L, Standl M, Waage J et al Multi‐ancestry genome‐wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet 2015; 47:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deckert S, Kopkow C, Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy 2014; 69:37–45. [DOI] [PubMed] [Google Scholar]
- 3. Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta‐analysis. Psychosom Med 2008; 70:102–16. [DOI] [PubMed] [Google Scholar]
- 4. Ronnstad ATM, Halling‐Overgaard AS, Hamann CR et al Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta‐analysis. J Am Acad Dermatol 2018; 79:448–56. [DOI] [PubMed] [Google Scholar]
- 5. Patel KR, Immaneni S, Singam V et al Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta‐analysis. J Am Acad Dermatol 2019; 80:402–10. [DOI] [PubMed] [Google Scholar]
- 6. Elbert NJ, Duijts L, den Dekker HT et al Maternal psychiatric symptoms during pregnancy and risk of childhood atopic diseases. Clin Exp Allergy 2017; 47:509–19. [DOI] [PubMed] [Google Scholar]
- 7. Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 2004; 1024:138–46. [DOI] [PubMed] [Google Scholar]
- 8. Ahn HJ, Shin MK, Seo JK et al Cross‐sectional study of psychiatric comorbidities in patients with atopic dermatitis and nonatopic eczema, urticaria, and psoriasis. Neuropsychiatr Dis Treat 2019; 15:1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag 2018; 11:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schans JV, Çiçek R, de Vries TW et al Association of atopic diseases and attention‐deficit/hyperactivity disorder: a systematic review and meta‐analyses. Neurosci Biobehav Rev 2017; 74:139–48. [DOI] [PubMed] [Google Scholar]
- 11. Schmitt J, Apfelbacher C, Chen CM et al Infant‐onset eczema in relation to mental health problems at age 10 years: results from a prospective birth cohort study (German Infant Nutrition Intervention plus). J Allergy Clin Immunol 2010; 125:404–10. [DOI] [PubMed] [Google Scholar]
- 12. Teyhan A, Galobardes B, Henderson J. Child allergic symptoms and well‐being at school: findings from ALSPAC, a UK cohort study. PLOS ONE 2015; 10:e0135271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu C, Duijts L, Erler NS et al Most associations of early‐life environmental exposures and genetic risk factors poorly differentiate between eczema phenotypes: the Generation R Study. Br J Dermatol 2019; 181:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaddoe VW, van Duijn CM, Franco OH et al The Generation R Study: design and cohort update 2012. Eur J Epidemiol 2012; 27:739–56. [DOI] [PubMed] [Google Scholar]
- 15. Achembach TM, Rescorla LA. Manual for the ASEBA School‐age Forms & Profiles. Burlington, VT: University of Vermont, 2001. [Google Scholar]
- 16. Ivanova MY, Achenbach TM, Rescorla LA et al Preschool psychopathology reported by parents in 23 societies: testing the seven‐syndrome model of the Child Behavior Checklist for ages 1.5–5. J Am Acad Child Adolesc Psychiatry 2010; 49:1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basten MM, Althoff RR, Tiemeier H et al The dysregulation profile in young children: empirically defined classes in the Generation R study. J Am Acad Child Adolesc Psychiatry 2013; 52:841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983; 13:595–605. [PubMed] [Google Scholar]
- 19. Kooijman MN, Kruithof CJ, van Duijn CM et al The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016; 31:1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Buuren S, Groothuis‐Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45:1–67. [Google Scholar]
- 21. R Core Team . R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 22. Muthén LK, Muthén BO. Mplus User's Guide, 8th edn Los Angeles, CA: Muthén & Muthén, 2017. [Google Scholar]
- 23. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32:850–78. [DOI] [PubMed] [Google Scholar]
- 24. Ramirez FD, Chen S, Langan SM et al Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019; 173:e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev 2012; 16:129–36. [DOI] [PubMed] [Google Scholar]
- 26. Hurtado‐Alvarado G, Domínguez‐Salazar E, Pavon L et al Blood–brain barrier disruption induced by chronic sleep loss: low‐grade inflammation may be the link. J Immunol Res 2016; 2016:4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elshazzly M, Caban O. Embryology, central nervous system In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2019. Available at: https://www.ncbi.nlm.nih.gov/books/NBK526024 (last accessed 21 November 2019). [PubMed] [Google Scholar]
- 28. Wu J, Xiao H, Sun H et al Role of dopamine receptors in ADHD: a systematic meta‐analysis. Mol Neurobiol 2012; 45:605–20. [DOI] [PubMed] [Google Scholar]
- 29. Mori T, Kabashima K, Fukamachi S et al D1‐like dopamine receptors antagonist inhibits cutaneous immune reactions mediated by Th2 and mast cells. J Dermatol Sci 2013; 71:37–44. [DOI] [PubMed] [Google Scholar]
- 30. Nakagome K, Imamura M, Okada H et al Dopamine D1‐like receptor antagonist attenuates Th17‐mediated immune response and ovalbumin antigen‐induced neutrophilic airway inflammation. J Immunol 2011; 186:5975–82. [DOI] [PubMed] [Google Scholar]
- 31. Stevenson J, Sonuga‐Barke E, McCann D et al The role of histamine degradation gene polymorphisms in moderating the effects of food additives on children's ADHD symptoms. Am J Psychiatry 2010; 167:1108–15. [DOI] [PubMed] [Google Scholar]
- 32. Casaca VI, Illi S, Klucker E et al STAT6 polymorphisms are associated with neonatal regulatory T cells and cytokines and atopic diseases at 3 years. Allergy 2013; 68:1249–58. [DOI] [PubMed] [Google Scholar]
- 33. Mondelli V, Vernon AC, Turkheimer F et al Brain microglia in psychiatric disorders. Lancet Psychiatry 2017; 4:563–72. [DOI] [PubMed] [Google Scholar]
- 34. Theoharides TC, Tsilioni I, Patel AB, Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry 2016; 6:e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silverberg JI, Patel N, Immaneni S et al Assessment of atopic dermatitis using self‐report and caregiver report: a multicentre validation study. Br J Dermatol 2015; 173:1400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Polanczyk GV, Salum GA, Sugaya LS et al Annual research review: a meta‐analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry 2015; 56:345–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Flowchart of participants included in the analysis.
Fig S2. Conceptual cross‐lagged model of eczema and emotional and behavioural problems.
Table S1 Characteristics of children and their mothers for those included and not included in the analyses.
Table S2 Associations of eczema phenotypes with borderline clinical cutoffs of internalizing problems at age 10 years.
Table S3 Associations of eczema phenotypes with borderline clinical cutoffs of externalizing problems at age 10 years.
Table S4 Direction of associations between eczema and emotional and behavioural problems from birth until age 10 years.
Powerpoint S1 Journal Club Slide Set.


