Abstract
N‐Acetyltransferases play critical roles in the deactivation and clearance of xenobiotics, including clinical drugs. NAT2 has been classified as an arylamine N‐acetyltransferase that mainly converts aromatic amines, hydroxylamines, and hydrazines. Herein, we demonstrate that the human arylamine N‐acetyltransferase NAT2 also acetylates aliphatic endogenous amines. Metabolomic analysis and chemical synthesis revealed increased intracellular concentrations of mono‐ and diacetylated spermidine in human cell lines expressing the rapid compared to the slow acetylator NAT2 phenotype. The regioselective N 8‐acetylation of monoacetylated spermidine by NAT2 answers the long‐standing question of the source of diacetylspermidine. We also identified selective acetylation of structurally diverse alkylamine‐containing drugs by NAT2, which may contribute to variations in patient responses. The results demonstrate a previously unknown functionality and potential regulatory role for NAT2, and we suggest that this enzyme should be considered for re‐classification.
Keywords: drug metabolism, mass spectrometry, metabolomics, N-acetyltransferases, polyamines
Same NAT2, new substr8: NAT2 has been classified as an arylamine N‐acetyltransferase that mainly converts aromatic amines, hydroxylamines, and hydrazines. Through a combination of metabolomics, chemical synthesis, and mass spectrometry it is demonstrated that unknown endogenous, aliphatic metabolites, and aliphatic amine‐containing commonly used drugs also act as substrates for NAT2.
The human body clears xenobiotics, drugs, and other metabolites that are not part of the human metabolic pathways mainly through excretion via urine. This detoxification process involves different phase I and phase II enzymes that increase the hydrophilicity of these compounds.1 N‐Acetyltransferases play a critical role in the phase II clearance of aromatic amines, hydrazines, and hydroxylamines by transferring an acetyl group from acetyl coenzyme A (CoA) (Figure 1 a). Two isozymes, arylamine N‐acetyltransferase 1 (NAT1) and arylamine N‐acetyltransferase 2 (NAT2), are encoded in the human genome. While NAT1 is ubiquitous, NAT2 is expressed primarily in the liver and intestines.2 The NAT2 gene is highly polymorphic, with over 100 human alleles identified to date.3 The correlation between the NAT2 haplotype and the encoded acetylator phenotype allows for the classification of individuals into rapid, intermediate, or slow acetylators. Seven common single nucleotide polymorphisms (SNPs) define the different NAT2 allele groups. The wild‐type allele NAT2*4 and the allele groups NAT2*11, NAT2*12, and NAT2*13 encode enzymatic variants with rapid acetylator phenotype, whereas the allele groups NAT2*5, NAT2*6, NAT2*7, and NAT2*14 encode slow acetylator variants.4 Consequently, intermediate acetylators possess one copy of a rapid and one copy of a slow acetylator allele. Analysis of NAT2 allele frequencies in 2054 individuals indicates that the wild‐type NAT2*4 allele is present in circa 20 % of the global population, and that circa 36 % of individuals have an intermediate NAT2 acetylator phenotype.5
Figure 1.
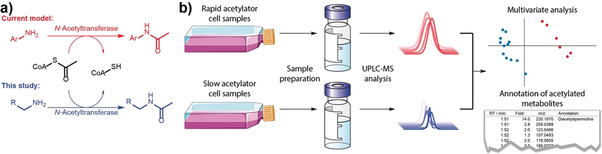
a) Our study reveals acetylation of unknown aliphatic amines by N‐Acetyltransferase NAT2 that is in contrast to the known acetylation of aromatic amines (Ar‐NH2). b) Metabolomics workflow.
The different NAT2 genotypes have substantial effects on the processing of enzyme‐specific substrates as subjects with two rapid NAT2 alleles clear the tuberculosis drug isoniazid almost twice as quickly as individuals with two slow alleles.6 Similarly, individuals with slow NAT2 acetylator phenotypes have impaired processing of the antibiotic dapsone.7 Therefore, NAT2 genotype resolution can predict patient response to drug treatment. The relationship between the NAT2 genotype and disease is more controversial. For example, NAT2‐catalyzed acetylation has been reported to inactivate some carcinogens and the slow acetylator phenotype has been associated with lung and liver cancers.8 In contrast, the rapid acetylator phenotype has been linked to an increased risk of colorectal cancer through the generation of reactive species, which can form irreversible DNA adducts.9 NAT enzymes have historically been studied from the perspective of xenobiotic metabolism and it has been assumed that these enzymes have no endogenous substrates. Herein, we report that NAT2 acetylates a wider range of compounds than previously known including aliphatic amines found in a series of endogenous metabolites as well as several clinically used drugs prescribed in large numbers. These findings may have implications for the regulation of cell signaling molecules and help to predict individual responses to drug therapy.
To uncover metabolic differences between rapid and slow acetylators, human colorectal cancer RKO cell lines transfected with constructs encoding rapid (NAT2*4) and slow (NAT2*6) NAT2 alleles were analyzed using state‐of‐the‐art mass spectrometry‐based metabolomics (Figure 1).10 One of the features found to be significantly more abundant in cells with rapid NAT2 was identified as N 1,N 8‐diacetylspermidine (1, Figure 2 a–c). This observation was surprising, as NAT2 is currently classified as an acetylator of aromatic amines (EC 2.3.1.5). The substrate spermidine has not only two primary aliphatic amines, but the metabolite scaffold also lacks any aromatic moiety and had not previously been reported as a NAT2 substrate. In addition, monoacetylated spermidine (2 a, Figure 2 b,c) was also significantly upregulated, while spermidine (3) itself was significantly reduced by about 30 % in rapid acetylator cells. To validate the metabolite identities, we synthesized N 1‐acetylspermidine (2 a), N 8‐acetylspermidine (2 b) and 1 (Supporting Information, Schemes S1 and S2). Monoacetylated spermidine analogues 2 a and 2 b were distinguished based on their differences in retention times and characteristic high‐resolution mass spectrometric fragmentation (Figure 2 d–f). Co‐spiking of these metabolites confirmed that the N 1‐acetylspermidine isomer was regioselectively produced in the rapid NAT2 cells (Figure S1). Next, we quantified spermidine and acetylated spermidine derivatives using commercially available (D8)spermidine (4) and we chemically synthesized N 1‐(D3)acetylspermidine (5) and N,N‐(D6)diacetylspermidine (6) as stable isotopically labeled internal standards (Schemes S1 and S3). Calibration curves were prepared for precise quantification and each standard was spiked into cell samples (Figure S2).11 Metabolites were then extracted from each sample and analyzed using ultra‐performance liquid chromatography coupled to mass spectrometry (UPLC‐MS). This quantification validated the results of the exploratory data analysis. Significant depletion of spermidine (3) by 50 % in cells with the rapid acetylator phenotype was observed, while the concentration of 1 was found to be 6‐fold higher and N 1‐acetylspermidine (2 a) 3‐fold higher in rapid acetylator cells (Figure 3 a).
Figure 2.
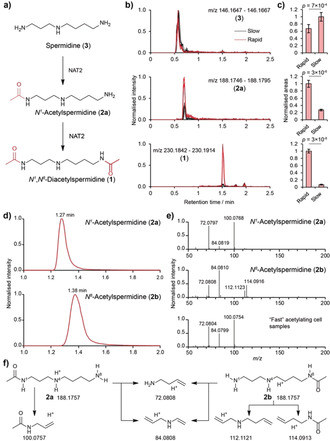
a) Mass spectrometric analysis of acetylation of spermidine (3) to form N 1‐acetylspermidine (2 a) and diacetylspermidine (1) in cell lines. b,c) Extracted ion chromatograms (EICs) and peak areas [Welch's t‐test (N=6)]. d) EICs for the N 1‐ (2 a) and N 8‐acetylated (2 b) isomers of spermidine. e) Comparison of MS/MS fragmentation pattern of synthetic 2 a/2 b and cell extracts. f) Annotated MS fragments for 2 a/2 b.
Figure 3.
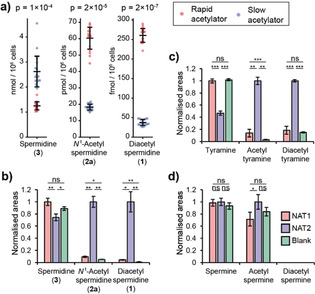
a) Precise quantification of spermidine (3), N 1‐acetylspermidine (2 a) and diacetylspermidine (1) in cell lines with the rapid and slow acetylator phenotypes. Enzymatic acetylation experiments of b) spermidine (3), c) tyramine, and d) spermine. Areas are normalized to the maximum area. Error bars: SD (N=3; ns=not significant). Welch's t‐test, *: p<0.05, **: p<0.01, ***: p<0.001; details in Table S2.
To confirm that the acetylation of spermidine (3) was catalyzed by NAT2, we performed an in vitro enzymatic assay using human recombinant NAT2 followed by UPLC‐MS analysis. Mass spectrometric analysis validated selective conversion of spermidine to N 1‐acetylspermidine and N 1,N 8‐diacetylspermidine by NAT2 (Figure 3 b). We also confirmed acetylation of synthetic monoacetylated N 1‐ as well as N 8‐acetylspermidine (2 a/b) to form diacetylspermidine (1, Figure S3). This observation is of particular importance as the identity of the enzyme that acetylates the N 8‐position in N 1‐acetylspermidine to produce N 1,N 8‐diacetylspermidine has been sought for the past 30 years as no enzyme has yet been identified that performs this reaction.12 In contrast, spermidine/spermine N 1‐acetyltransferase (SSAT) has been reported to acetylate the N 1‐position of spermidine. These results are the first example of NAT2‐catalyzed acetylation of endogenous, aliphatic metabolites as previous reports have been restricted to two studies of a small number of synthetic drug candidates, all of which possessed an aromatic moiety and are not endogenous metabolites.13
To investigate whether this unexpected substrate selectivity of NAT2 is limited to spermidine, we analyzed additional endogenous aliphatic amines and the positive control aniline (Figure S4).14 The two polyamines cadaverine and putrescine were also mono‐ and diacetylated by recombinant NAT2 (Figure S5). Furthermore, the trace amines tyramine and phenethylamine were acetylated by NAT2 (Figure 3 c).11b, 15 Surprisingly, NAT2 can also catalyze O‐acetylation as demonstrated for N‐acetylated tyramine. Interestingly, the polyamine spermine was the only tested polyamine that was not found to be acetylated by NAT2 (Figure 3 d). Taken together, our analysis revealed that NAT2 acetylates different endogenous aliphatic amines and monoacetylated polyamines.
As the next step, we investigated NAT2‐dependent acetylation by quantification of 1 and 2 a in human plasma samples, which were selected from a population‐based biobank according to specific NAT2 alleles. Of these 113 samples, 38 were classified as rapid (two NAT2*4 alleles), 38 as intermediate (one NAT2*4 allele), and 37 as slow (no NAT2*4 alleles) acetylators. No traces of N 8‐acetylspermidine were detected in these samples. The determined quantitative values for all samples were 1.7±0.6 nm for 1 and 0.6±0.2 μm for 2 a (Figure 4 a). No significant quantitative differences were observed for any of the two metabolites by comparing the three sample sets. This result is in contrast with the quantitative differences observed in intracellular concentrations in cell lines (Figure 3 a) but consistent with previous reports on the tightly regulated polyamine metabolism and catabolism (Figure 4 b).
Figure 4.
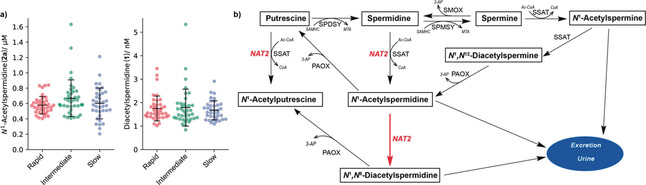
a) Quantification of N 1‐acetylspermidine (2 a) and diacetylspermidine (1) in human plasma samples. b) Spermidine main homeostasis pathways. The newly identified acetylation of spermidine is highlighted in red. SPMSY=spermine synthase; SMOX=spermine oxidase; PAOX=polyamine oxidase; 3‐AP=3‐aminoacetopropanal.
Polyamines play a role in fundamental cellular processes, such as proliferation, gene expression and response to stress, and their cellular concentrations are strictly regulated.16 The depletion of intracellular spermidine has been reported to halt protein synthesis and growth, and consumption of polyamines by NAT2 can either be an indirect or direct role for NAT2 in regulatory cell processes.17 Beyond these functions, spermidine (3) has beneficial cardioprotective and neuroprotective effects and administration of spermidine stimulates autophagy and mitophagy.18 Spermidine (3) is produced from dietary sources by the microbiome and has also been identified as a key metabolite in aging and longevity.19 Increased levels have been correlated with reduced cardiovascular and cancer‐related mortality in humans, while spermidine concentrations in tissue decrease with higher age.18a, 20 Polyamine metabolism including acetylation pathways have been extensively studied and it has been demonstrated that this metabolic network is tightly controlled to keep concentrations of each polyamine and corresponding metabolites constant in healthy individuals (Figure 4 b).21 This is supported by our quantification experiments in plasma samples that concentrations of 1 and 2 a lie within a narrow range. The polyamine derivatives diacetylspermidine and diacetylspermine have also been proposed as urinary markers for various cancer types. Despite the amount of attention these molecules have received, the enzyme which catalyzes the formation of diacetylspermidine has not been identified. Our cell line and enzymatic assays clearly demonstrate that NAT2 is capable of acetylating the N 8‐position of N 1‐acetylated spermidine and that it is a source of 1. The ability of the rapid NAT2 isozyme to deplete intracellular levels of spermidine and the regioselectivity of the monoacetylation to form 2 a suggests an unknown regulatory function for this acetylation process.
The role of NAT2 in drug metabolism has been extensively studied and different isozymes of NAT2 have been reported to significantly affect the rate of clearance of drug molecules, with the antitubercular drug isoniazid being the archetypical example.6 To explore the ability of NAT2 to modulate and alter drug efficacy, we decided to test a panel of eight representative compounds possessing aliphatic amines to determine whether they are acetylated by NAT2 (Figure S6). N‐Acetylation of these drugs would alter their hydrogen bond acceptor and donor properties and lead to faster clearance, consequently reducing their efficacy.22 We identified acetylation of the calcium channel blocker amlodipine (7), the serotonin‐norepinephrine inhibitor duloxetine (8), the beta blockers nebivolol (9), and carvedilol (10) selectively by NAT2 (Figure 5 and Figure S7). Acetylated structures were confirmed through tandem mass spectrometry (Figures S8–11). These compounds are prescription drugs that are used to treat and prevent prevalent conditions such as high blood pressure, stroke, fibromyalgia, anxiety, and depression. In contrast, we did not observe any NAT2 mediated acetylation of the drugs salbutamol (Figure 5 d), cinacalcet, thyroxine, and varenicline (Figure S7). Steric hindrance may be a factor for the selectivity of NAT2 identified towards different substrates; for example, amlodipine (7) and duloxetine (8) have aliphatic primary and secondary amine functionalities similar to the endogenous metabolites. In contrast, the amine in salbutamol (11) is capped by a bulky tert‐butyl moiety and was not converted by NAT2. This result could be validated in future human metabolism studies to investigate NAT2‐dependent drug metabolism. The results presented clearly reveal that acetylation by NAT2 may play a greater role in the metabolism, efficacy, and clearance of common drugs than previously thought and should be considered in future metabolism and ADME‐Tox studies in personal medication. In 2016, the drugs acetylated by NAT2 in this study were prescribed over 124 million times in the USA alone.23 Furthermore, 21 % of the 200 most prescribed drugs in the USA contain aliphatic amines, representing almost 900 million prescriptions. Differential metabolism of drugs according to NAT2 genotype therefore has the potential to affect a major part of the population. These findings suggest that knowledge of the patient's NAT2 genotype can aid in optimizing drug dosage to maximize efficacy and minimize side effects.
Figure 5.
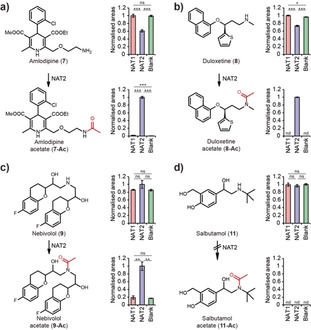
Acetylation experiments of commonly prescribed drugs a) amlodipine (7), b) duloxetine (8), c) nebivolol (9), and d) salbutamol (11) after incubation with recombinant enzymes for 24 h at 37 °C.
In summary, cell‐based and in vitro assays revealed acetylation of several endogenous metabolites and major drugs that have not previously been described as substrates of this enzyme. This previously unknown enzymatic activity extends NAT2 acetylation beyond aromatic xenobiotics to the modification of aliphatic amine‐containing endogenous metabolites and drugs, implying that circa 10 % of commonly prescribed drugs can be metabolized by NAT2. We therefore postulate that NAT2 acetylator phenotype affects the efficacy and clearance of commonly used drugs with non‐aromatic amines and propose that the catalytic activity of NAT2 should be re‐classified to encompass acetylation of both aryl‐ and alkylamines.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This study was funded by the Swedish Research Council (VR 2016‐04423), Carl Tryggers Foundation (CTS 2018:820) and start‐up grant from SciLifeLab to DG; VR 2016‐01890 and the Cancer Foundation (CAN 2018/772) to TS. We also thank the Västerbotten Intervention Programme, the MONICA study and the County Council of Västerbotten for providing NSHDS data and samples. Acknowledged are also the contribution from Biobank Sweden (VR 2017‐00650) and Natallia Rameika for support with human samples.
L. P. Conway, V. Rendo, M. S. P. Correia, I. A. Bergdahl, T. Sjöblom, D. Globisch, Angew. Chem. Int. Ed. 2020, 59, 14342.
Contributor Information
Prof. Dr. Tobias Sjöblom, Email: Tobias.sjoeblom@igp.uu.se.
Prof. Dr. Daniel Globisch, Email: Daniel.globisch@scilifelab.uu.se.
References
- 1. Hanson S. R., Best M. D., Wong C.-H., Angew. Chem. Int. Ed. 2004, 43, 5736–5763; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 5858–5886. [Google Scholar]
- 2. Husain A., Zhang X. Y., Doll M. A., States J. C., Barker D. F., Hein D. W., Drug Metab. Dispos. 2007, 35, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh E. M., Boukouvala S., Aklillu E., Hein D. W., Altman R. B., Klein T. E., Pharmacogenet. Genomics 2014, 24, 409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Grant D. M., Hughes N. C., Janezic S. A., Goodfellow G. H., Chen H. J., Gaedigk A., Yu V. L., Grewal R., Mutat. Res. 1997, 376, 61–70; [DOI] [PubMed] [Google Scholar]
- 4b. Hein D. W., Mutat. Res. 2002, 506–507, 65–77. [DOI] [PubMed] [Google Scholar]
- 5. Genomes Project C., Auton A., Brooks L. D., Durbin R. M., Garrison E. P., Kang H. M., Korbel J. O., Marchini J. L., McCarthy S., McVean G. A., Abecasis G. R., Nature 2015, 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kinzig-Schippers M., Tomalik-Scharte D., Jetter A., Scheidel B., Jakob V., Rodamer M., Cascorbi I., Doroshyenko O., Sorgel F., Fuhr U., Antimicrob. Agents Chemother. 2005, 49, 1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palamanda J. R., Hickman D., Ward A., Sim E., Romkes-Sparks M., Unadkat J. D., Drug Metab. Dispos. 1995, 23, 473–477. [PubMed] [Google Scholar]
- 8. Agundez J. A. G., Curr. Drug Metab. 2008, 9, 520–531. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Matejcic M., Vogelsang M., Wang Y. B., Parker I. M., BMC Cancer 2015, 15, 150; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Ying X. J., Dong P., Shen B., Wang J., Wang S., Wang G., J. Cancer Res. Clin. Oncol. 2011, 137, 1661–1667. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Rendo V., et al., Nat. Commun. 2020, 11, 1308; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Johnson C. H., Ivanisevic J., Siuzdak G., Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Globisch D., Pearson D., Hienzsch A., Brückl T., Wagner M., Thoma I., Thumbs P., Reiter V., Kneuttinger A. C., Müller M., Sieber S. A., Carell T., Angew. Chem. Int. Ed. 2011, 50, 9739–9742; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 9913–9916; [Google Scholar]
- 11b. Globisch D., Moreno A. Y., Hixon M. S., Nunes A. A. K., Denery J. R., Specht S., Hoerauf A., Janda K. D., Proc. Natl. Acad. Sci. USA 2013, 110, 4218–4223; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c. Brückl T., Globisch D., Wagner M., Müller M., Carell T., Angew. Chem. Int. Ed. 2009, 48, 7932–7934; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 8074–8077. [Google Scholar]
- 12.
- 12a. Kawakita M., Hiramatsu K., J. Biochem. 2006, 139, 315–322; [DOI] [PubMed] [Google Scholar]
- 12b. Hiramatsu K., Sugimoto M., Kamei S., Hoshino M., Kinoshita K., Iwasaki K., Kawakita M., J. Biochem. 1995, 117, 107–112; [DOI] [PubMed] [Google Scholar]
- 12c. Umemori Y., Ohe Y., Kuribayashi K., Tsuji N., Nishidate T., Kameshima H., Hirata K., Watanabe N., Clin. Chim. Acta 2010, 411, 1894–1899. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Rioux N., Mitchell L. H., Tiller P., Plant K., Shaw J., Frost K., Ribich S., Moyer M. P., Copeland R. A., Chesworth R., Waters N. J., Drug Metab. Dispos. 2015, 43, 936–943; [DOI] [PubMed] [Google Scholar]
- 13b. Meyer M. R., Robert A., Maurer H. H., Toxicol. Lett. 2014, 227, 124–128. [DOI] [PubMed] [Google Scholar]
- 14. Liu L., Von Vett A., Zhang N. X., Walters K. J., Wagner C. R., Hanna P. E., Chem. Res. Toxicol. 2007, 20, 1300–1308. [DOI] [PubMed] [Google Scholar]
- 15. Garg N., Conway L. P., Ballet C., Correia M. S. P., Olsson F. K. S., Vujasinovic M., Lohr J. M., Globisch D., Angew. Chem. Int. Ed. 2018, 57, 13805–13809; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 14001–14005. [Google Scholar]
- 16.
- 16a. Miller-Fleming L., Olin-Sandoval V., Campbell K., Ralser M., J. Mol. Biol. 2015, 427, 3389–3406; [DOI] [PubMed] [Google Scholar]
- 16b. Erb T. J., Evans B. S., Cho K., Warlick B. P., Sriram J., Wood B. M., Imker H. J., Sweedler J. V., Tabita F. R., Gerlt J. A., Nat. Chem. Biol. 2012, 8, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandal S., Mandal A., Johansson H. E., Orjalo A. V., Park M. H., Proc. Natl. Acad. Sci. USA 2013, 110, 2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Madeo F., Eisenberg T., Pietrocola F., Kroemer G., Science 2018, 359, eaan2788; [DOI] [PubMed] [Google Scholar]
- 18b. Pietrocola F., Castoldi F., Kepp O., Carmona-Gutierrez D., Madeo F., Kroemer G., Autophagy 2019, 15, 362–365; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18c. Eisenberg T., et al., Nat. Med. 2016, 22, 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Gupta V. K., et al., Nat. Neurosci. 2013, 16, 1453–1460; [DOI] [PubMed] [Google Scholar]
- 19b. Fernández A. F., et al., Nature 2018, 558, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madeo F., Carmona-Gutierrez D., Kepp O., Kroemer G., Aging 2018, 10, 2209–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.
- 21a. R. A. Casero, Jr. , Stewart T. M., Pegg A. E., Nat. Rev. Cancer 2018, 18, 681–695; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21b. Minois N., Carmona-Gutierrez D., Madeo F., Aging 2011, 3, 716–732; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21c. Pegg A. E., Chem. Res. Toxicol. 2013, 26, 1782–1800; [DOI] [PubMed] [Google Scholar]
- 21d. Ou Y., Wang S. J., Li D., Chu B., Gu W., Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.
- 22a. Ma M. K., Woo M. H., McLeod H. L., Am. J. Health-Syst. Pharm. 2002, 59, 2061–2069; [DOI] [PubMed] [Google Scholar]
- 22b. Omiecinski C. J., Vanden Heuvel J. P., Perdew G. H., Peters J. M., Toxicol. Sci. 2011, 120, S49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.S. P. Kane, Vol. 2019, https://clincalc.com/blog/2018/11/the-top-200-drugs-of-2019/, ClinCalc, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


