Abstract
Background
Intrathecal hydrophilic opioids decrease systemic opioid consumption after abdominal surgery and potentially facilitate enhanced recovery. A meta-analysis is needed to quantify associated risks and benefits.
Methods
A systematic search was performed to find RCTs investigating intrathecal hydrophilic opioids in abdominal surgery. Caesarean section and continuous regional or neuraxial techniques were excluded. Several subgroup analyses were prespecified. A conventional meta-analysis, meta-regression, trial sequential analysis, and provision of GRADE scores were planned.
Results
The search yielded 40 trials consisting of 2500 patients. A difference was detected in ‘i.v. morphine consumption’ at Day 1 {mean difference [MD] −18.4 mg, (95% confidence interval [CI]: −22.3 to −14.4)} and Day 2 (MD −25.5 mg [95% CI: −30.2 to −20.8]), pain scores at Day 1 in rest (MD −0.9 [95% CI: −1.1 to −0.7]) and during movement (MD −1.2 [95% CI: −1.6 to −0.8]), length of stay (MD −0.2 days [95% CI: −0.4 to −0.1]) and pruritus (relative risk 4.3 [95% CI: 2.5–7.5]) but not in nausea or sedation. A difference was detected for respiratory depression (odds ratio 5.5 [95% CI: 2.1–14.2]) but not when two small outlying studies were excluded (odds ratio 1.4 [95% CI: 0.4–5.2]). The level of evidence was graded as high for morphine consumption, in part because the required information size was reached.
Conclusions
This study showed important opioid-sparing effects of intrathecal hydrophilic opioids. Our data suggest a dose-dependent relationship between the risk of respiratory depression and the dose of intrathecal opioids. Excluding two high-dose studies, intrathecal opioids have a comparable incidence of respiratory depression as the control group.
Clinical trial registration
PROSPERO-registry: CRD42018090682.
Keywords: analgesics, enhanced recovery, intrathecal, laparoscopy, laparotomy, opioids, spinal injections
Editor's key points.
-
•
In this meta-analysis of 40 studies (2500 subjects), the authors investigated the analgesia provided following abdominal surgery by the use of intrathecal hydrophilic opioids.
-
•
They found that opioid consumption and pain scores were reduced when intrathecal hydrophilic opioids were used, while pruritus was increased. Late respiratory depression occurred more often, but not when lower doses were used.
-
•
The findings imply that use of low-dose intrathecal hydrophilic opioids provides analgesic and opioid-sparing effects in abdominal surgery, and that side-effects are limited.
-
•
This technique may complement enhanced recovery programs.
Enhanced recovery programs (ERPs) are accompanied by multiple recommendations, one of which is sufficient postoperative analgesia.1 A promising analgesic approach is the use of intrathecal hydrophilic opioids, which have been used for decades, and renewed interest was caused by a recent study that was able to show an enhanced recovery in abdominal surgery.2,3 Still, the risks and benefits need to be quantified before the widespread use in abdominal surgery can be advocated.
The benefits of intrathecal hydrophilic opioids, compared with i. v. administration, are believed to be caused by a higher potency and a prolonged action, because of a small distribution volume of the CSF and a slow diffusion, respectively.4 Used as a single bolus technique, intrathecal hydrophilic opioids have an i. v. opioid-sparing effect, facilitate mobilisation and—because of a lack of peripheral vasodilation—a restrictive fluid management can easily be achieved.5 These properties may lead to a faster recovery after abdominal surgery.
The risks, however, are pruritus, nausea, and late respiratory depression. Especially the fear for the latter has limited the use of intrathecal hydrophilic opioids. Meylan and colleagues 6 performed a meta-analysis regarding intrathecal morphine, and they found higher rates of pruritus and respiratory depression. However, that meta-analysis involved predominantly studies in cardiac surgery and a wide range of dosages were used. This limits the transfer of the found risks and benefits to abdominal surgery, which requires a meta-analysis of its own.
Therefore, we performed a meta-analysis to quantify the risks and benefits of intrathecal hydrophilic opioids. Our study had two goals: firstly, we set out to identify the studies published in the last decade in order to come to an updated evaluation of the benefits and risks of intrathecal morphine. Secondly, we focused on a particular patient group (i.e. abdominal surgery patients undergoing both open and laparoscopic procedures). Furthermore, in recent years trial sequential analysis (TSA) has emerged as a statistical technique that maintains the Type 1 error-rate in meta-analyses at a prespecified level, which contributes to the certainty of a conclusion in a meta-analysis.7 This technique was applied to the data obtained from trials on intrathecal hydrophilic opioids for abdominal surgery.
Methods
Our meta-analysis was performed in accordance with the PRISMA statement.8 The meta-analysis was registered at PROSPERO with registration number CRD42018090682.
A systemic literature search was performed in December 2019. We searched the databases of Medline, Embase, CINAHL, LILACS, Cochrane CENTRAL, Web of Science, ClinicalTrials.gov, and Google Scholar. Filters or language restriction were not applied. The search combined terms for ‘intrathecal’, ‘hydrophilic opioid’, and ‘abdominal surgery (see Supplementary material). Morphine, hydromorphone, diamorphine, pethidine, and dihydromorphine were considered hydrophilic opiates. The search was managed with EndNote and duplicates were removed. Bibliographies of selected studies were also screened for studies of interest. The search included trial registers and these records were checked for completion and publication.
Inclusion and exclusion criteria were defined a priori, and only randomised trials were considered. The inclusion criteria were defined according to a PICO-search, in which the Patients were adults undergoing abdominal surgery, the Intervention was the administration of intrathecal hydrophilic opioids, with or without additives, such as local anaesthetics, the Comparator was analgesia without intrathecal hydrophilic opioids. The primary outcome measures were i. v. morphine-equivalents consumption at 24 and 48 h. The secondary outcome measures were: pain scores in rest and during movement at 24 and 48 h; time to fit for discharge; length of hospital stay; time to first analgesic request; intraoperative sufentanil-equivalent consumption; and incidence of nausea, pruritus, sedation, and respiratory depression.
Exclusion criteria were Caesarean section and the use of concomitant continuous regional anaesthesia or neuraxial anaesthesia.
Two authors (MVK and MK) screened the abstracts for eligible studies. Full texts of these studies were analysed, and data were extracted if the study was considered includable. The extracted data were authors, year of publication, type of surgery, details of intervention, details of control, postoperative analgesia, and urinary catheter management. If the mean and standard deviation were not reported in the paper, we derived the mean and standard deviation from the median and range using the formula by Hozo and colleagues.9 Morphine equivalents were calculated. The conversion factor for piritramide was 0.7,10 for papaveretum 0.665,11 for fentanyl 100,12 for pethidine 0.133,13 and for tramadol 0.1.12 The conversion factor to calculate fentanyl into sufentanil equivalents for intraoperative analgesia was 0.1.14 If multiple groups with intrathecal morphine were compared, we combined those groups and used the mean dose of intrathecal morphine. If a trial used multiple groups that could serve as control groups (i.e. without intrathecal hydrophilic opioids), the group with the control treatment most similar to the intervention group was used. The continuous outcome measures of such a study were the mean values of the groups and the largest standard deviation of the groups. Additions of events and patients were used for binary data.
The methodological quality of each study was evaluated by two authors (MVK and MH) based on the Cochrane Risk of Bias tool.15 This tool includes assessment of the risks of selection bias (random sequence generation, allocation concealment), performance bias (blinding of participant and personnel), detection bias (blinding of assessor), attrition bias, and other biases (e.g. multiple treatment groups, comparable baseline values, and number of participants).
We used Review Manager (RevMan, version 5.1, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for meta-analysis. We considered meta-analyses worthwhile only if at least three studies with at least 100 patients per treatment arm were available for analysis. In order to deal with the expected clinical and methodological heterogeneity across studies, a random effects model with inverse variance was applied. For dichotomous data, the Mantel-Haenszel-method was used. Risk ratio and 95% confidence interval (95% CI) were calculated for binary outcome and mean difference (MD) and 95% CI were calculated for continuous outcomes. The Peto odds ratio was used to analyse the risk of respiratory depression, because of the low incidence. The I2 statistic was used to assess heterogeneity and an I2>50% was considered important heterogeneity.16 A P-value of <0.05 was taken to indicate statistical significance. We performed the following prespecified subgroup analyses: laparoscopic surgery, laparotomic surgery, addition of bupivacaine to the intrathecal hydrophilic opioids, solely intrathecal hydrophilic opioids, studies with an ERP, and studies with a sham procedure in the control group for blinding purposes. For the latter, only studies with a lumbar needle insertion in the control group, either s. c. or intrathecally and regardless if medication was administered, were included in this subgroup.
Asymmetry in conventional funnel plots can exist without true asymmetry, and reasons other than publication bias can result in asymmetry.17,18 For this reason, contour-enhanced funnel plots were performed. This was done if there were 10 or more studies in the meta-analyses of the outcomes.15 We used the test described by Egger and colleagues 19 to test for plot asymmetry.
We hypothesised that the effect of the dose of intrathecal opioid could influence the outcome variables. To test for possible heterogeneity, we performed mixed-effects meta-regression (unrestricted maximum likelihood) to determine the effect of the dose of intrathecal opioid. R version 3.1.3 with the ‘meta’ package (version 4.2–0) and ‘metafor’ package (version 1.9–7) was used.
Furthermore, similar to interim analyses of primary clinical trials, meta-analyses have been found to be prone to Type 1 (falsely positive results) and Type 2 error (falsely negative results) during statistical analysis.20,21. TSA is a method to avoid Type 1 errors and was performed for the primary outcomes of our meta-analyses, in order to consider the risk of random error and better estimate the uncertainty in our findings.22,23 TSA methodology was described elsewhere.24 Sequential monitoring boundaries are made to decide whether a trial could be terminated early because of a sufficiently small P-value. When the cumulative z-curve crosses the monitoring boundaries, an acceptable small chance of a false-positive result can be assumed. We calculated the required information size allowing for a Type 1 error of 0.05, and Type 2 error of 0.20, with the MD from the effect estimate from the conventional random effects model,25 and heterogeneity estimated by the diversity (D2) in the included trials. For the analyses we used TSA Viewer (Version 0.9.5.10 Beta, Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, 2016).
In order to rate the quality of evidence and strength of recommendation of our primary outcomes, the Grading of Recommendations Assessment, Development, and Evaluation system (GRADE) was used.26 We assessed the following criteria: risk of bias, inconsistency, indirectness, imprecision, and publication bias. When one of the earlier-mentioned items was assessed as a risk, the evidence was downgraded by two levels (very serious risk) or one level (serious risk). In addition, when the required information size was not reached or the sequential boundary was not crossed, the evidence was downgraded one level as well. One of the following four grades was assigned: high quality (further research is very unlikely to alter the confidence in the estimate of the effect); moderate quality (further research is likely to alter the confidence in the estimate of the effect); low quality (further research is very likely to alter the confidence in the estimate of the effect); or very low quality (the confidence in the effect estimate is very little).
Results
The flow chart of our literature search is presented in Fig 1. A total of 40 studies was included in the quantitative analysis and study characteristics are presented in Table 1. Only Child and Kaufman,37 Day and colleagues,39 and Levy and colleagues5 used diamorphine; all others used morphine as intrathecal opioid. The dose varied between 100 and 800 μg of morphine and except for two studies that administered a body weight adjusted dose of 15 μg kg−1 and 50 μg kg−1 morphine.47,55.
Fig 1.
Flow diagram of study selection.
Table 1.
Characteristics of included studies. PCA, patient-controlled analgesia; POD, postoperative day.
| First author, year of publication, reference | Type of surgery | Number of (intervention vs control) | Intervention | Comparator | Postoperative analgesic regimen | Sham procedure | Subgroup | Urinary catheter |
|---|---|---|---|---|---|---|---|---|
| Abd El-Rahman, 201827 | Major abdominal cancer surgery | 30 vs 30 | 300 μg morphine, 10 mg bupivacaine, 0.1 mg kg−1 ketamine | 10 mg bupivacaine, 0.1 mg kg−1 ketamine | PCA morphine | Intrathecal medication | A | Unspecified |
| Abdel-Ghaffar, 201628 | Major abdominal cancer surgery | 30 vs 30 | 500 μg morphine, 10 mg bupivacaine | 10 mg bupivacaine | PCA morphine | Intrathecal medication | A | Urinary catheter removed on POD1 |
| Andreoni, 200229 | Percutaneous nephrolithotomy | 9 vs 11 | 0.3–0.5 μg kg−1 morphine | Local infiltration with ropivacaine | Unspecified | None | B | Nephrostomy catheter, no urinary catheter |
| Andrieu, 200930 | Retropubic radical prostatectomy | 17 vs 16 | 4 μg kg−1 morphine, maximum of 300 μg | No additional medication | Paracetamol, PCA morphine | None | B, D | Unspecified |
| Bae, 201731 | Robotic-assisted laparoscopic prostatectomy | 15 vs 15 | 300 μg morphine | No additional medication | PCA morphine, pethidine rescue dose | None | B, C | Urinary catheter for 1 week |
| Beaussier, 200632 | Colonic surgery | 26 vs 26 | 300 μg morphine | No additional medication | Paracetamol, PCA morphine | S.C. saline | B | Unspecified |
| Beltrutti, 200233 | Hysterectomy | 15 vs 14 | 4.3 μg kg−1 morphine | 1.3 μg kg−1 buprenorphine i.v. | I.V. buprenorphine | Intrathecal saline | B, D | No postoperative urinary catheter in a part of the patients |
| Blay, 200634 | Abdominal aortic surgery | 15 vs 15 | 200 μg morphine | No additional medication | Paracetamol, nefopam, morphine rescue dose | S.C. saline | B, D | Urinary catheter of unknown duration |
| Boonmak, 200735 | Kidney surgery | 40 vs 40 | 300 μg morphine | No additional medication | PCA morphine | None | B, D | Unspecified |
| Brown, 200436 | Radical prostatectomy | 49 vs 50 | 200 μg morphine, 15 mg bupivacaine, 75 μg clonidine | 15 mg bupivacaine, 75 μg clonidine | Paracetamol, ketorolac, PCA morphine | SC saline | A, D | Unspecified |
| Child, 198537 | Colonic surgery | 8 vs 8 | 50 μg kg−1 diamorphine | 3–5 μg kg−1 fentanyl i.v. | Unspecified | None | B, D | Unspecified |
| Colibaseanu, 201938 | Colorectal surgery | 98 vs 102 | 100 μg morphine | Bilateral TAP-block with liposomal bupivacaine | Multimodal analgesia, unspecified | None | B, E | Unspecified |
| Day, 201539 | Colorectal surgery | 60 vs 60 | 250 μg diamorphine, 12.5 mg bupivacaine | 10 mg morphine i.v. and PCA morphine | Tramadol and morphine p.o. as needed, diclofenac, paracetamol | None | A, C | Urinary catheter removed on POD1 |
| Devys, 200340 | Mixed abdominal surgery | 30 vs 30 | 300–400 μg morphine | No additional medication | PCA morphine | None | B | Unspecified |
| Dichtwald, 201741 | Hepatopancreatic surgery | 23 vs 26 | 4 μg kg−1 morphine | I.V. loading dose of 0.15 μg kg−1 morphine | PCA morphine, paracetamol, and dypirone rescue doses | None | B, D | Urinary catheter of unknown duration |
| Downing, 198542 | Cholecystectomy | 10 vs 10 | 800 μg morphine | I.V. titration of papaveretum during surgery | I.V. papaveretum rescue dose | None | B, D | Unspecified |
| Drasner, 198843 | Major gynaecological surgery | 10 vs 10 | 750 μg morphine | I.M. 750 μg morphine | Unspecified | None | B, D | Unspecified |
| El-Sherif, 201644 | Laparoscopic bariatric surgery | 50 vs 50 | 300 μg morphine, 6 mg bupivacaine | Intrathecal 6 mg bupivacaine and saline | Paracetamol, ketorolac, PCA morphine, wound infiltration with ropivacaine | Intrathecal medication | A, C | Removal of urinary catheter after surgery |
| Fléron, 200345 | Abdominal aortic surgery | 102 vs 115 | 8 μg kg−1 morphine, 1 μg kg−1 sufentanil | Continuous i.v. sufentanil | Paracetamol, PCA morphine | None | D | Urinary catheter of unspecified duration |
| Hein, 201246 | Abdominal hysterectomy | 102 vs 34 | Mean 200 μg morphine, 12 mg bupivacaine | Intrathecal 12 mg bupivacaine | Paracetamol, PCA morphine | Intrathecal medication | A, D | Unspecified |
| Houweling, 199347 | Abdominal aortic surgery | 18 vs 18 | 50 μg kg−1 morphine | Intrathecal 150 μg sufentanil | 500 μg morphine intrathecal | Intrathecal medication | B, D | Urinary catheter of unspecified duration |
| Kang, 201948 | Laparoscopic partial hepatectomy | 27 vs 27 | 400 μg morphine | Bilateral ESP-block with ropivacaine | Paracetamol, ibuprofen, PCA fentanyl, i.v. meperidine | None | B, C, E | Urinary catheter of unspecified duration |
| Kara, 201249 | Major gynaecological surgery | 30 vs 30 | 300 μg morphine | No additional medication | PCA morphine | S.C. needle introduction | B | Unspecified |
| Karaman, 200650 | Abdominal hysterectomy | 12 vs 12 | 5 μg kg−1 morphine | No additional medication | Diclofenac, PCA morphine | None | B, D | Unspecified |
| Kim, 201651 | Kidney surgery | 22 vs 23 | 300 μg morphine | No additional medication | PCA morphine, pethidine rescue dose | None | B, D | Unspecified |
| Ko, 200952 | Liver transplantation donors | 20 vs 20 | 400 μg morphine | No additional medication | PCA fentanyl | None | B, D | Urinary catheter of unspecified duration |
| Kong, 200253 | Laparoscopic colorectal surgery | 18 vs 17 | 200 μg morphine, 15 mg bupivacaine | 15 mg bupivacaine | PCA morphine | Intrathecal medication | A, C | Unspecified |
| Koning, 20183 | Laparoscopic colonic surgery | 27 vs 29 | 300 μg morphine, 12.5 mg bupivacaine | I.V. 0.1 mg kg−1 piritramide | Paracetamol, diclofenac, PCA piritramide | SC lidocaine | A, C | Urinary catheter removed on POD1 |
| Koning, 201954 | Robot-assisted radical prostatectomy | 76 vs 79 | 300 μg morphine, 12.5 mg bupivacaine | I.V. 0.1 mg kg−1 morphine | Paracetamol, diclofenac, PCA morphine | SC lidocaine | A, C | Urinary catheter for one week |
| Levy, 20115 | Laparoscopic colorectal surgery | 31 vs 30 | 250 μg diamorphine, 12.5 mg bupivacaine | I.V. 10 mg morphine | Paracetamol, diclofenac, tramadol, or morphine | None | A, C | Urinary catheter removed on POD1 |
| Licina, 199155 | Mixed abdominal surgery | 12 vs 12 | 15 μg kg−1 morphine | No additional medication | Unspecified | SC saline | B, D | Unspecified |
| Marion, 201056 | Abdominal hysterectomy | 35 vs 32 | 200 μg morphine, 10 μg fentanyl, 12.5 mg bupivacaine | Intrathecal 10 μg fentanyl, 12.5 mg bupivacaine | Paracetamol, diclofenac, and PCA ketobemidone | Intrathecal medication | A, D | Unspecified |
| Motamed, 200057 | Laparoscopic cholecystectomy | 17 vs 17 | 100 μg morphine, 5 mg bupivacaine | No additional medication | PCA morphine, paracetamol, and ketoprofen rescue doses | SC saline | A, C | No catheterisation |
| Nuri Deniz, 201358 | Retropubic radical prostatectomy | 28 vs 28 | 200 μg morphine | No additional medication | PCA tramadol, paracetamol, and diclofenac rescue doses | None | B, D | Unspecified |
| Ray, 201759 | Major abdominal surgery | 46 vs 46 | 750 μg morphine, 10 mg bupivacaine | I.V. 0.2 mg kg−1 morphine, s.c. 0.1 mg kg−1 morphine | Paracetamol, SC morphine | Intrathecal saline | A | Urinary catheter of unspecified duration |
| Roy, 200660 | Partial hepatic resections | 10 vs 10 | 500 μg morphine, 15 μg fentanyl | No additional medication | PCA morphine | S.C. needle introduction | D | Unspecified |
| Sarma, 199361 | Abdominal hysterectomy | 60 vs 20 | Mean 300 μg morphine | No additional medication | Pethidine rescue dose | Intrathecal saline | B, D | Urinary catheter removed on POD1 |
| Selvam, 201862 | Laparoscopic hysterectomy | 16 vs 15 | 200 μg morphine, 5 mg bupivacaine | Intrathecal 5 mg bupivacaine | Paracetamol, PCA fentanyl | Intrathecal medication | A, C | Unspecified |
| Togal, 200463 | Abdominal hysterectomy | 25 vs 25 | 100 μg morphine | No additional medication | PCA morphine | Intrathecal saline | B, D | Urinary catheter removed on POD1 |
| Wongyingsinn, 201264 | Laparoscopic colonic resection | 24 vs 25 | 200 μg morphine, 10 mg bupivacaine | PCA morphine | Paracetamol, naproxen, oxycodone | None | A, C | Urinary catheter removed on POD1 |
‘No additional medication’ under Comparator means that no additional medication to the postoperative analgesic regimen was administered.
A, addition of bupivacaine to intrathecal hydrophilic opioids; B, only intrathecal hydrophilic opioids; C, laparoscopic procedures; D, open procedures; E, regional anaesthesia.
Risk of bias analysis is presented in Fig 2. Main limitations were allocation concealment and blinding of personnel and participants.
Fig 2.
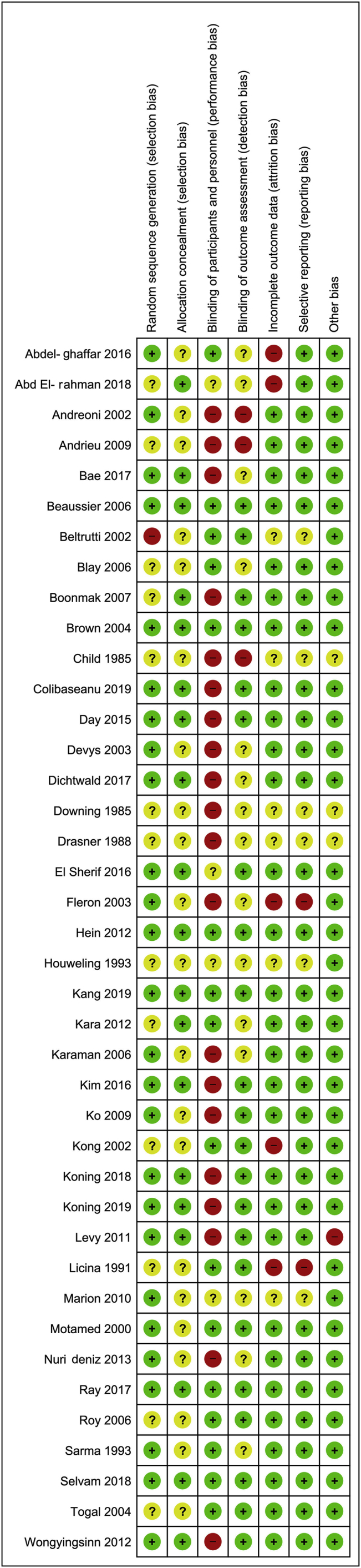
Risk of bias assessment for included studies.
Primary outcomes
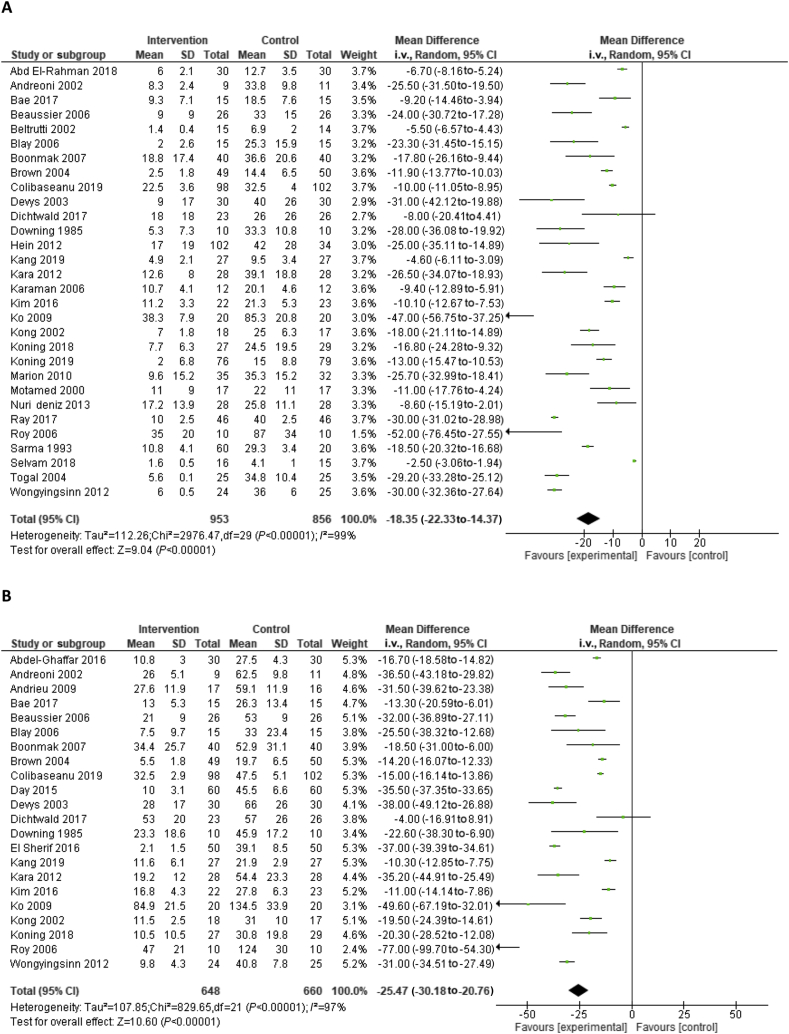
Meta-analysis showed an MD in i. v. morphine equivalent consumption after 24 and 48 h of −18.4 mg (95% CI −22.3 to −14.4) and −25.5 mg (95% CI −30.2 to −20.8), respectively, in favour of the intrathecal opioids (Fig 3).
Fig 3.
Forest plot of (a) morphine-equivalent consumption after 24 h and (b) 48 h. CI, confidence interval; SD, standard deviation.
Secondary outcomes (Table 2)
Table 2.
Summary of the meta-analyses. I2 describes the heterogeneity. RIS, required information size as measured by trial sequential analysis, Egger test describes the risk for publication bias.
| Variable | Studies (n) | Participants (n) | Value (95% CI) | I2 (%) | RIS | Egger test | Grade |
|---|---|---|---|---|---|---|---|
| Benefit | Mean difference | ||||||
| Morphine consumption day 1 (mg) | 30 | 1809 | −18.4 (−22.3 to −14.4) | 99 | 266 | 0.03 | High |
| Morphine consumption day 2 (mg) | 22 | 1309 | −25.5 (−30.2 to −20.8) | 97 | 103 | 0.21 | High |
| Pain scores in rest, day 1 (NRS) | 33 | 2164 | −0.9 (−1.1 to −0.7) | 93 | 0.03 | ||
| Pain in exertion, day 1 (NRS) | 19 | 1099 | −1.2 (−1.6 to −0.8) | 79 | 0.79 | ||
| Pain scores in rest, day 2 (NRS) | 19 | 1114 | −0.4 (−0.7 to −0.1) | 97 | 0.94 | ||
| Pain in exertion, day 2 (NRS) | 13 | 639 | −0.4 (−0.7 to −0.1) | 50 | 0.14 | ||
| Intraoperative sufentanil use (μg) | 11 | 625 | −12.9 (−19.3 to −6.5) | 91 | 0.07 | ||
| Time to first analgesic request (h) | 8 | 309 | 9.7 (4.9–14.5) | 99 | 0.01 | ||
| Time to fit-for-discharge (days) | 4 | 233 | −0.3 (−0.5 to −0.1) | 28 | 0.80 | ||
| Length of hospital stay (days) | 17 | 1416 | −0.2 (−0.4 to −0.1) | 88 | 0.12 | ||
| Risk | Risk ratio | ||||||
| Incidence of nausea | 25 | 1412 | 1.1 (0.9–1.4) | 48 | 0.12 | ||
| Incidence of pruritus | 23 | 1282 | 4.3 (2.5–7.5) | 57 | 0.05 | ||
| Incidence of sedation | 12 | 644 | 0.7 (0.5–1.1) | 2 | 0.53 | ||
| Incidence of respiratory depression | 31 | 1862 | 5.5 (2.1–14.2) | 14 | 0.17 | ||
| Incidence of respiratory depression (<500 μg) | 26 | 1473 | 1.1 (0.2–8.2) | 21 | N/A |
MD, mean difference; 95% CI, 95% confidence interval; NRS, numeric rating scale; RIS, required information size; RR, relative risk.
The pain scores (converted to a range of 0–10) both in rest and during exertion were reduced in the intrathecal opioid group after 24 h. The lower pain scores persisted during exertion after 48 h, but were no longer different in rest. Intraoperative sufentanil-equivalents consumption was reduced, and time-to-first analgesic request was prolonged in the intrathecal opioid group.
No increased risk for nausea or sedation was detected. The risk for pruritus was increased. Only Boonmak and colleagues 35 reported the incidence of pruritus over different timepoints during the first two postoperative days, thus no data on duration and timing could be retrieved. All other studies reported an incidence of pruritus and monitored over 20–48 h.
Because of the heterogeneity in definition of respiratory depression, only the cases in which medication was administered or mechanical ventilation was necessary were scored as respiratory depression in the meta-analysis. An increased risk for respiratory depression was found between intrathecal and i. v. opioids (Peto odds ratio 5.49 [95% CI: 2.12–14.24]). The incidence of respiratory depression was 18/974 in the intrathecal opioids group vs 4/888 in the control group. The timing of respiratory depression after administration of intrathecal opioids was only reported by Dichtwald and colleagues,41 which was after a mean of 6 h after injection. Licina and colleagues55 and Houweling and Joosten47 reported the highest incidence of respiratory depression with 11/12 patients and 2/18 patients, respectively. Both studies also used a much higher dose of intrathecal morphine than the other studies (15 μg kg−1 and 50 μg kg−1, respectively, resulting in 1200 μg and 4000 μg in a 80 kg patient).
However, when those two outlying high-dose studies were excluded,47,55 the incidence of respiratory depression was 5/944 for the intrathecal opioids group and 4/858 for the control group. This led to a Peto odds ratio of 1.39 (95% CI 0.37–5.21).
The length of hospital stay was reduced with an MD of −0.2 days (95% CI −0.4 to −0.1). In addition, patients in the intervention group were earlier fit-for-discharge as well (−0.3 days [95% CI −0.5 to −0.1]).
Management of urinary catheter was reported in 19 studies (Table 1). The majority inserted a catheter for at least 1 day or for an unspecified duration. These studies reported no interventions for urine retention after removal of the urinary catheter. More specifically, the studies that removed the catheter after 24 h did not report any recatheterisation,3,5,28,39,61,63 Three studies used no postoperative urinary catheter, which allowed evaluation for urinary retention.33,44,57 El Sheriff and colleagues44 found no urinary retention in 50 patients. Beltrutti and colleagues33 found urinary retention in four of seven patients in the intervention group vs three of nine patients in the control group, although none required recatheterisation. Motamed and colleagues57 found four of 17 patients in the intervention group vs one of 17 patients in the control group with urinary retention. Of the four of the intervention group, two were managed with naloxone and two were managed with a urinary catheter.
Publication bias
The search included trial registries and yielded 26 trial registrations of which 12 were published and already included. Six trials were still recruiting. Two trials were completed and added to the database.38,54 Two other, completed studies were of potential interest but no publication could be found (NCT03620916 and NCT03675646).
Contour-enhanced funnel plots were generated and only 24 h i. v. morphine equivalent consumption pain scores in rest after 24 h and time to first analgesic request had Egger tests with a P-value <0.05 (Fig 4). Asymmetry in the 24 h i. v. morphine equivalents and pain score in rest after 24 h seemed to originate from the lack of studies with low standard error with a large effect size or from the lack of small studies. Based on visual inspection of the two contour-enhanced funnel plots, the asymmetry was unlikely to exaggerate the effect size, which makes a small study effect unlikely. The lack of studies with a large benefit and a small standard error is unlikely to be caused by publication bias. Time to first analgesic request included eight studies, which limits its power. The funnel plots are presented in the Supplementary material. Based on these findings, the risk of publication bias seems low.
Fig 4.
Contour-enhanced funnel plot of A. 24 hour morphine equivalent consumption and B. pain score at rest after 24 hours. NRS, numeric rating scale.
Subgroup analyses (see Supplementary material)
Five subgroup analyses were performed, which were solely intrathecal hydrophilic opioids, the addition of intrathecal bupivacaine, laparoscopic surgical procedures, laparotomies, and studies that involved an ERP. The first four mentioned subgroups showed no difference to the general comparison (see Supplementary material). Five studies described use of an ERP.3,5,38,39,64 In these studies the length of stay was −0.2 days (95% CI: −0.5 to 0.1), I2 93%. Fit-for-discharge had too few subjects (82 vs 84) to produce a reliable analysis. In addition, a sensitivity analysis was performed including only studies with a patient-blinding procedure in the control group for the outcomes ‘pain scores’, morphine consumption, nausea, and pruritus.3,27,28,32, 33, 34,36,44,46,47,49,53, 54, 55, 56, 57,59, 60, 61, 62, 63 This analysis showed comparable outcomes to the general comparison.
Meta-regression
Meta-regression analyses were performed to detect a dose-dependent effect in 24 h and 48 h i. v. morphine equivalents consumption, pain scores in rest and during movement, nausea, pruritus, sedation, and respiratory depression (see Supplementary material). The variation in doses was limited since the most commonly used dose was 300 μg and all but six studies varied between 100 and 400 μg of intrathecal morphine. A dose dependency was observed only for pain scores in rest after 48 h (slope of 0.006/μg morphine [95% CI: 0.001–0.011]) and incidence of pruritus (slope of 0.005/μg morphine [95% CI: 0.002–0.007]) (see Supplementary material).
Trial sequential analysis
TSA showed a required information size of n=266 for 24 h i. v. morphine equivalent consumption, n=103 for 48 h i. v. morphine equivalent consumption.
GRADE recommendations
GRADE recommendations were made for the outcomes ‘i.v. morphine equivalent consumption after 24 h’, ‘i.v. morphine equivalent consumption after 48 h’. Inconsistency was detected, since conventional meta-analyses showed an I2>74% and a P-value for heterogeneity >0.05. The inconsistency was not explained by subgroup analysis or by different types of studies since all studies were prospective randomised trials. Moreover, no studies were in the opposite direction, thus important clinical inconsistency was deemed unlikely. Since the CIs of the outcomes were within a clinical useful range, we did not downgrade the level of evidence because of inconsistency. No publication bias was detected by contour-enhanced funnel plots and all outcomes were directly measured. The risk of bias was high because of limited blinding of participants or outcome assessors in a number of studies, but the sensitivity analysis of only blinded studies with a sham procedure did not show different results. Therefore, insufficient blinding probably had a limited effect and the level of evidence was not downgraded. The required information size was reached for both outcomes. Therefore, we graded the outcomes of 24 and 48 h i. v. morphine equivalent consumption as a high level of evidence.
Discussion
Our meta-analysis of 40 studies including 2500 patients found a reduced postoperative i. v. morphine equivalent consumption of −18.4 mg (95% CI −22.3 to −14.4) in the first 24 and −25.5 mg (95% CI −30.2 to −20.8) in the first 48 h in the intrathecal hydrophilic opioids group. Moreover, we found clinically relevant reductions by intrathecal hydrophilic opioids for the following secondary outcomes: pain scores in rest and during movement after 24 h, pain scores during movement after 48 h, time to first analgesic request, length of hospital stay, and intraoperative sufentanil equivalent consumption. The risk of pruritus was increased, and a dose-dependent effect was found. Overall, the risk of respiratory depression was increased (Peto odds ratio 5.49 [95% CI: 2.12–14.24]), but when two outlying studies of doses >1000 μg of intrathecal morphine were excluded, a similar incidence of respiratory depression as the control group was found (Peto odds ratio of 1.39 [95% CI 0.37–5.21]). Subgroup analysis for laparoscopic, laparotomic, addition of bupivacaine, and solely hydrophilic intrathecal opioids yielded no substantial differences compared with the total group for all the outcomes.
These results led to different conclusions than the results of a previous meta-analysis.6 This meta-analysis shows that the use of intrathecal hydrophilic opioids in abdominal surgery has several benefits including the reduced systemic opioid consumption, lower pain scores, and a slightly reduced length of stay. The risks consist mostly of pruritus. Urinary retention was not evaluated in the majority of the included trials. The risk of respiratory depression was not increased when the studies with a dose more than 1000 μg were excluded. It appeared that a specific indication (i.e. abdominal surgery), a specific definition of respiratory depression, and more recent studies led to an acceptable safety profile. While in the other meta-analysis it was suggested to abandon this analgesic technique, this study shows the positive effects may be substantial in abdominal surgery and the risks are limited.6
The reduction in i. v. morphine equivalents consumption may not come as a surprise, since this effect has already been described for many years.65 However, we feel that our finding of a reduction in postoperative morphine consumption of 18.4 mg (95% CI −22.3 to −14.4) in the first 24 h is clinically relevant. In addition, difference in morphine consumption further increased to 25.5 mg (95% CI −30.2 to −20.8) after 48 h, a finding that is unique in our study and which was not shown by Meylan and co-workers.6 These findings are based on sufficient data, as displayed by TSA.
In addition, the mean morphine equivalent consumption allows a comparison of this method with other opioid-sparing techniques such as i. v. lidocaine (−4.5 mg [95% CI: −6.3 to −2.8]),66 high dose pregabalin (−13.4 mg [95% CI: −22.8 to −4.0]),67 and ketamine (−10.3 [95% CI −13.8 to −6.8]).68 This is not a direct scientific comparison, so it should be interpreted with caution, but it may provide an intuitive effect size. Of importance is that the opioid-sparing effect in our meta-analysis is in addition to paracetamol and NSAIDs, since most studies used this medication as a basal multimodal analgesia regimen. We believe that the use of additional opioid-sparing strategies, such as intrathecal hydrophilic opioids, i. v. lidocaine, pregabalin, or ketamine, should be regarded as addition to the use of paracetamol and NSAIDs, since these are most consolidated in clinical practice.
This work supports the use of intrathecal hydrophilic opioids within an ERP, since the lower pain scores during movement caused by intrathecal hydrophilic opioids may facilitate early mobilisation.69 Additionally, other goals such as to minimise systemic opioids and still produce low pain scores are achieved as well.70 This mechanism could explain the reduced postoperative length of stay. In line with previous research, we interpreted the difference in length of stay as one out of every five patients leaves the hospital a day earlier, because in most studies the length of stay was scored per full day and not in half or quarter days. Still, this outcome must be interpreted with caution, because the subgroup analysis of studies which implemented an ERP did not show any difference and length of stay may be influenced by non-medical issues, making fit-for-discharge perhaps a better variable for reflecting recovery.3
Other studies reported that the use of intrathecal hydrophilic opioids was associated with adverse effects, such as urinary retention, pruritus, nausea, and the risk of late respiratory depression.71 By contrast, our meta-analysis was unable to detect a difference in nausea. Urinary retention was not measured since the majority of the included studies used an urinary catheter for at least the first postoperative day. Interestingly, none of these studies reported a case of recatheterisation or urinary retention beyond that period.
The most common side-effect of intrathecal hydrophilic opioids is pruritus and we found a dose-dependent effect for pruritus in the range of 100–800 μg of intrathecal morphine. We have to point out that a previous meta-analysis of Meylan and colleagues6 did not detect a dose-dependent effect, which may be attributable to the lower number of studies in that analysis. Studies that have purposely investigated the relationship between the dose and the incidence of pruritus were able to detect a correlation.72 Theoretically, severe pruritus might delay hospital discharge, albeit the pruritus probably lasts shorter than the time for recovery. The duration of pruritus was only investigated in the study of Boonmak and colleagues35 over 48 h, which showed a decline of incidence after 24 h. This is in accordance with other studies.3,73
Late respiratory depression is an adverse effect of concern and probably limits the widespread use of intrathecal hydrophilic opioids.74 Since only one study explicitly investigated the time to respiratory depression, we are unable to draw conclusions on this aspect.35 In our analysis we detected similar incidences of respiratory depression (5/944 for the intrathecal opioids group and 4/844 in the control group) by the use of intrathecal opioids in low dosage. This led to a markedly different conclusion than a previous meta-analysis, which found 6/504 in the intrathecal morphine group and 0/440 in the control group. This difference can be explained by a different definition of respiratory depression, the difference in dosage, and the different type of surgery (i.e. abdominal vs cardiac surgery).
The definition of respiratory depression varies amongst studies, which makes the incidence and severity of respiratory depression less than clear.75 For our analysis, respiratory depression was only scored when a medical intervention (i.e. mechanical ventilation or medication) was installed. This is a high threshold to score respiratory depression, but we believe that this definition excludes respiratory failure as a result of other pathology (e.g. atelectasis, diaphragm dysfunction, pneumothorax, or haemothorax). Meylan and colleagues6 used a different definition and included patients after cardiac surgery, who have higher incidences of this type of pathology than abdominal surgery. Although the upside of a high threshold for scoring is that only the clinically important respiratory depression is scored, the downside is the risk of missing respiratory depression that does not require a medical intervention, but still may impact the clinical course of the patients.
Gehling and Tryba76 found a dose-dependent effect for respiratory depression with a cut-off of 300 μg. In our meta-regression a dose-dependent effect was visible, but the CI was too wide for statistical significance. In our analysis with the exclusion of two outlying studies, the incidence of respiratory depression that required a medical intervention was still similar to the control group. When excluding these two outlying high-dose studies, the maximum dose included in our analysis was 800 μg, but the majority of the studies used a dose less than 500 μg. For safety measures, we would recommend using doses less than 500 μg, because these doses were predominantly investigated.
The incidence of respiratory depression in our control group seems to be in line with reported incidences in patient-controlled analgesia (PCA) opioids in a Cochrane review.77 Still, the Cochrane review used a lower threshold for scoring respiratory depression, making this comparison to be interpreted with caution. However, because the incidences of respiratory depression are likely to be within the same range for low dose intrathecal morphine as for PCA opioids, we suggest that the same monitoring as for patients with PCA opioids should be applied.77,78 The ERAS society recommends this as well.1 Nonetheless, coadministration of benzodiazepines and routinely administered systemic opioids should be avoided during the first 24 h, since respiratory depression may occur because of interaction.79.
This meta-analysis contains a high level of heterogeneity, which was not explained by the subgroup analysis, meta-regression, or methodological differences of the included studies. The differences in type of surgery is a likely cause of heterogeneity, but further subgroup analysis was not prespecified and could increase the chance of a Type 1 error. The postoperative analgesic regimen consisted in most studies of paracetamol, NSAID, and PCA opioids, but variation adds to heterogeneity as well. Still, the CIs are within clinical significant limits and the effects of individual studies were predominantly in the same direction, therefore we did not alter the GRADE level of evidence based on heterogeneity.
Besides the inherent downside of a meta-analysis by the methodological limitations of the included studies, an additional limitation of this study is the probability of missing studies. We were unable to retrieve a full text of Toǧal and colleagues.80 Another issue is the low number of patients for some outcomes. Of importance is the respiratory depression, for which no increased ratio was found. This too could be because of the low number of events and patients. Some outcomes have been reported in dichotomous and continuous variables, such as patient satisfaction and sedation, which limited the ability to pool the data. A third limitation is the pooling of various types of abdominal surgery, which adds to heterogeneity. We mentioned in the introduction that only similar types of surgery should be analysed and even though only abdominal surgery was included, a variance within abdominal surgery is still expected. Subgroup analyses were performed to restrict this limitation. Fourth, not all included studies described characteristics of the recovery phase such as time to oral feeding, mobilisation, and extent of mobilisation and therefore no comments regarding this subject can be made. Finally, high levels of bias for blinding and allocation concealment in the individual studies cause limitations for the meta-analysis as well.
In conclusion, intrathecal hydrophilic opioids reduce intraoperative and postoperative opioid consumption, pain scores, and length of hospital stay in abdominal surgery. These properties make it a potentially important contributor to the overall effects of an ERP, and we feel this technique should be considered more frequently. The risk for pruritus is increased in a dose-dependent fashion. In our opinion, anaesthesiologists are reluctant to administer intrathecal morphine because of fear of respiratory depression. An increased incidence of respiratory depression was found, but this was predominantly caused by two studies using high doses of intrathecal morphine. When these two studies were excluded, this rare complication was not more common in the intervention group than in the control group with systemic opioids. Still, the majority of the studies used a dose less than 500 μg, thus the evidence is predominantly based on this range of doses. We recommend taking similar precautions as with the use of systemically administered opioids for the duration of at least 12 h.
Authors' contributions
Designed the study: MVK, MK, MAH
Selected the studies: MVK, MK
Extracted the data: MVK, MAH
Performed the analyses: MVK, KR
Interpreted the data: all authors
Drafted the manuscript: MVK
Co-authored the manuscript: MK, KR, RJS, MAH
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We are grateful to M.F.M. Engel and G. De Jonge, Biomedical Information Specialists, Medical Library, Erasmus University Medical Centre, Rotterdam, The Netherlands for support with the systematic electronic literature search and S.E. Hoeks, Epidemiologist, Erasmus University Medical Centre, Rotterdam, The Netherlands for statistical advice.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.05.061.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gustafsson U.O., Scott M.J., Hubner M. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS(®)) society recommendations. World J Surg. 2018;43:659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.K., Nauss L.A., Thomas J.E. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Koning M.V., Teunissen A.J.W., Van Der Harst E., Ruijgrok E.J., Stolker R.J. Intrathecal morphine for laparoscopic segmental colonic resection as part of an enhanced recovery protocol: a randomized controlled trial. Reg Anesth Pain Med. 2018;43:166–173. doi: 10.1097/AAP.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ummenhofer W.C., Arends R.H., Shen D.D., Bernards C.M. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Levy B.F., Scott M.J., Fawcett W., Fry C. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011;98:1068–1078. doi: 10.1002/bjs.7545. [DOI] [PubMed] [Google Scholar]
- 6.Meylan N., Elia N., Lysakowski C., Tramer M.R. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth. 2009;102:156–167. doi: 10.1093/bja/aen368. [DOI] [PubMed] [Google Scholar]
- 7.Wetterslev J., Jakobsen J.C., Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Biotechnology Information . 2019. PubChem compound database; CID=9331.https://pubchem.ncbi.nlm.nih.gov/compound/9331 Available from: [Google Scholar]
- 11.Keeri-Szanto M. Papaveretum for anaesthesia and its comparison with morphine. Anaesthetic time/dose curves VIII. Can Anaesth Soc J. 1976;23:239–243. doi: 10.1007/BF03005699. [DOI] [PubMed] [Google Scholar]
- 12.Symons J.M.P., Mehra R., Ball C. Perioperative medicine for the junior clinician. In: Symons J.M.P., Mehra R., Ball C., editors. John Wiley & Sons, Ltd.; West Sussex, United Kingdom: 2015. [Google Scholar]
- 13.Norman P.D.D.M., Kowalski A. Postoperative analgesia for thoracotomy patients: a current review. In: Franco K.L.P.J., editor. BC Decker Inc.; New York: 2005. pp. 1–3114. (Advanced therapy in thoracic surgery). [Google Scholar]
- 14.Monk J.P., Beresford R., Ward A. Sufentanil. A review of its pharmacological properties and therapeutic use. Drugs. 1988;36:286–313. doi: 10.2165/00003495-198836030-00003. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Smith A.F., Carlisle J. Reviews, systematic reviews and Anaesthesia. Anaesthesia. 2015;70:644–650. doi: 10.1111/anae.13108. [DOI] [PubMed] [Google Scholar]
- 18.Choi S.W., Lam D.M. Heterogeneity in meta-analyses. Comparing apples and oranges? Anaesthesia. 2017;72:532–534. doi: 10.1111/anae.13832. [DOI] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brok J., Thorlund K., Wetterslev J., Gluud C. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38:287–298. doi: 10.1093/ije/dyn188. [DOI] [PubMed] [Google Scholar]
- 21.Afshari A., Wetterslev J., Smith A.F. Can systematic reviews with sparse data be trusted? Anaesthesia. 2017;72:12–16. doi: 10.1111/anae.13730. [DOI] [PubMed] [Google Scholar]
- 22.Thorlund K., Devereaux P.J., Wetterslev J. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38:276–286. doi: 10.1093/ije/dyn179. [DOI] [PubMed] [Google Scholar]
- 23.Wetterslev J., Thorlund K., Brok J., Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Heesen M., Klimek M., Imberger G., Hoeks S.E., Rossaint R., Straube S. Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth. 2018;120:212–227. doi: 10.1016/j.bja.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 25.Wetterslev J., Thorlund K., Brok J., Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9:86. doi: 10.1186/1471-2288-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abd El-Rahman A.M., Mohamed A.A., Mohamed S.A., Mostafa M.A.M. Effect of intrathecally administered ketamine, morphine, and their combination added to bupivacaine in patients undergoing major abdominal cancer surgery a randomized, double-blind study. Pain Med. 2018;19:561–568. doi: 10.1093/pm/pnx105. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Ghaffar H.S., Mohamed S.A.B., Fares K.M. Combined intrathecal morphine and dexmedetomidine for postoperative analgesia in patients undergoing major abdominal cancer surgery. Pain Med. 2016;17:2109–2118. doi: 10.1093/pm/pnw031. [DOI] [PubMed] [Google Scholar]
- 29.Andreoni C., Olweny E.O., Portis A.J., Sundaram C.P., Monk T., Clayman R.V. Effect of single-dose subarachnoid spinal anesthesia on pain and recovery after unilateral percutaneous nephrolithotomy. J Endourol. 2002;16:721–725. doi: 10.1089/08927790260472863. [DOI] [PubMed] [Google Scholar]
- 30.Andrieu G., Roth B., Ousmane L. The efficacy of intrathecal morphine with or without clonidine for postoperative analgesia after radical prostatectomy. Anesth Analg. 2009;108:1954. doi: 10.1213/ane.0b013e3181a30182. 7. [DOI] [PubMed] [Google Scholar]
- 31.Bae J., Kim H.C., Hong D.M. Intrathecal morphine for postoperative pain control following robot-assisted prostatectomy: a prospective randomized trial. J Anesth. 2017;31:565–571. doi: 10.1007/s00540-017-2356-9. [DOI] [PubMed] [Google Scholar]
- 32.Beaussier M., Weickmans H., Parc Y. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg Anesth Pain Med. 2006;31:531–538. doi: 10.1016/j.rapm.2006.06.250. [DOI] [PubMed] [Google Scholar]
- 33.Beltrutti D., Niv D., Ben-Abraham R., Di Santo S., Weinbroum A.A. Late antinociception and lower untoward effects of concomitant intrathecal morphine and intravenous buprenorphine in humans. J Clin Anesth. 2002;14:441–446. doi: 10.1016/s0952-8180(02)00397-5. [DOI] [PubMed] [Google Scholar]
- 34.Blay M., Orban J.C., Rami L. Efficacy of low-dose intrathecal morphine for postoperative analgesia after abdominal aortic surgery: a double-blind randomized study. Reg Anesth Pain Med. 2006;31:127–133. doi: 10.1016/j.rapm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Boonmak S., Boonmak P., Bunsaengjaroen P., Srichaipanha S., Thincheelong V. Comparison of intrathecal morphine plus PCA and PCA alone for post-operative analgesia after kidney surgery. J Med Assoc Thailand. 2007;90:1143–1149. [PubMed] [Google Scholar]
- 36.Brown D.R., Hofer R.E., Patterson D.E. Intrathecal anesthesia and recovery from radical prostatectomy: a prospective, randomized, controlled trial. Anesthesiology. 2004;100:926–934. doi: 10.1097/00000542-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Child C.S., Kaufman L. Effect of intrathecal diamorphine on the adrenocortical, hyperglycaemic and cardiovascular responses to major colonic surgery. Br J Anaesth. 1985;57:389–393. doi: 10.1093/bja/57.4.389. [DOI] [PubMed] [Google Scholar]
- 38.Colibaseanu D.T., Osagiede O., Merchea A. Randomized clinical trial of liposomal bupivacaine transverse abdominis plane block versus intrathecal analgesia in colorectal surgery. Br J Surg. 2019;106:692–699. doi: 10.1002/bjs.11141. [DOI] [PubMed] [Google Scholar]
- 39.Day A.R., Smith R.V., Scott M.J., Fawcett W.J., Rockall T.A. Randomized clinical trial investigating the stress response from two different methods of analgesia after laparoscopic colorectal surgery. Br J Surg. 2015;102:1473–1479. doi: 10.1002/bjs.9936. [DOI] [PubMed] [Google Scholar]
- 40.Devys J.M., Mora A., Plaud B. Intrathecal + PCA morphine improves analgesia during the first 24 hr after major abdominal surgery compared to PCA alone. Can J Anesth. 2003;50:355–361. doi: 10.1007/BF03021032. [DOI] [PubMed] [Google Scholar]
- 41.Dichtwald S., Ben-Haim M., Papismedov L., Hazan S., Cattan A., Matot I. Intrathecal morphine versus intravenous opioid administration to impact postoperative analgesia in hepato-pancreatic surgery: a randomized controlled trial. J Anesth. 2017;31:237–245. doi: 10.1007/s00540-016-2286-y. [DOI] [PubMed] [Google Scholar]
- 42.Downing R., Davis I., Black J., Windsor C.W.O. When do patients given intrathecal morphine need postoperative systemic opiates? Ann R Coll Surg Engl. 1985;67:251–253. [PMC free article] [PubMed] [Google Scholar]
- 43.Drasner K., Bernards C.M., Ozanne G.M. Intrathecal morphine reduces the minimum alveolar concentration of halothane in humans. Anesthesiology. 1988;69:310–312. doi: 10.1097/00000542-198809000-00004. [DOI] [PubMed] [Google Scholar]
- 44.El Sherif F.A., Othman A.H., Abd El-Rahman A.M., Taha O. Effect of adding intrathecal morphine to a multimodal analgesic regimen for postoperative pain management after laparoscopic bariatric surgery: a prospective, double-blind, randomized controlled trial. Br J Pain. 2016;10:209–216. doi: 10.1177/2049463716668904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fléron M.H., Weiskopf R.B., Bertrand M. A comparison of intrathecal opioid and intravenous analgesia for the incidence of cardiovascular, respiratory, and renal complications after abdominal aortic surgery. Anesth Analg. 2003;97:2–12. doi: 10.1213/01.ane.0000066355.07482.0c. [DOI] [PubMed] [Google Scholar]
- 46.Hein A., Rösblad P. Wiley Online Library; 2012. Low dose intrathecal morphine effects on post-hysterectomy pain: a randomized placebo-controlled study. [DOI] [PubMed] [Google Scholar]
- 47.Houweling P.L., Joosten W. A haemodynamic comparison of intrathecal morphine and sufentanil supplemented with general anaesthesia for abdominal aortic surgery. Eur J Vasc Surg. 1993;7:283–290. doi: 10.1016/s0950-821x(05)80010-6. [DOI] [PubMed] [Google Scholar]
- 48.Kang R., Chin K.J., Gwak M.S. Bilateral single-injection erector spinae plane block versus intrathecal morphine for postoperative analgesia in living donor laparoscopic hepatectomy: a randomized non-inferiority trial. Reg Anesth Pain Med. 2019;44:1059–1065. doi: 10.1136/rapm-2019-100902. [DOI] [PubMed] [Google Scholar]
- 49.Kara I., Apiligullari S., Oc B. The effects of intrathecal morphine on patient-controlled analgesia, morphine consumption, postoperative pain and satisfaction scores in patients undergoing gynaecological oncological surgery. J Int Med Res. 2012;40:666–672. doi: 10.1177/147323001204000229. [DOI] [PubMed] [Google Scholar]
- 50.Karaman S., Kocabas S., Uyar M., Zincircioglu C., Firat V. Intrathecal morphine: effects on perioperative hemodynamics, postoperative analgesia, and stress response for total abdominal hysterectomy. Adv Ther. 2006;23:295–306. doi: 10.1007/BF02850135. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.C., Bae J.Y., Kim T.K. Efficacy of intrathecal morphine for postoperative pain management following open nephrectomy. J Int Med Res. 2016;44:42–53. doi: 10.1177/0300060515595650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko J.S., Choi S.J., Gwak M.S. Intrathecal morphine combined with intravenous patient-controlled analgesia is an effective and safe method for immediate postoperative pain control in live liver donors. Liver Transplant. 2009;15:381–389. doi: 10.1002/lt.21625. [DOI] [PubMed] [Google Scholar]
- 53.Kong S.K., Onsiong S.M.K., Chiu W.K.Y., Li M.K.W. Use of intrathecal morphine for postoperative pain relief after elective laparoscopic colorectal surgery. Anaesthesia. 2002;57:1168–1173. doi: 10.1046/j.1365-2044.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 54.Koning M.V., de Vlieger R., Teunissen A.J.W. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomised controlled trial. Anaesthesia. 2019;75:599–608. doi: 10.1111/anae.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Licina M.G., Schubert A., Tobin J.E., Nicodemus H.F., Spitzer L. Intrathecal morphine does not reduce minimum alveolar concentration of halothane in humans: results of a double-blind study. Anesthesiology. 1991;74:660–663. doi: 10.1097/00000542-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Marion E.K., Hansen K., Tegerstedt G.E., Svensen C.H., Andrijauskas A., Drobin D. Spinal blocks with and without morphine in women undergoing hysterectomies - a randomized study. Sri Lankan J Anaesthesiol. 2010;18:23–28. [Google Scholar]
- 57.Motamed C., Bouaziz H., Franco D., Benhamou D. Analgesic effect of low-dose intrathecal morphine and bupivacaine in laparoscopic cholecystectomy. Anaesthesia. 2000;55:118–124. doi: 10.1046/j.1365-2044.2000.055002118.x. [DOI] [PubMed] [Google Scholar]
- 58.Nuri Deniz M., Erhan E., Ugur G. Intrathecal morphine reduces postoperative tramadol consumption in patients undergoing radical retropubic prostatectomy: a randomized trial. Eur Rev Med Pharmacol Sci. 2013;17:834–838. [PubMed] [Google Scholar]
- 59.Ray S., Kirtania J. Randomised double-blind study of intrathecal bupivacaine-morphine versus systemic morphine analgesia for major abdominal surgery in a resource poor setting. J Evol Med Dent Sci. 2017;6:5345–5352. [Google Scholar]
- 60.Roy J.D., Massicotte L., Sassine M.P., Seal R.F., Roy A. A comparison of intrathecal morphine/fentanyl and patient-controlled analgesia with patient-controlled analgesia alone for analgesia after liver resection. Anesth Analg. 2006;103:990–994. doi: 10.1213/01.ane.0000238040.41872.7e. [DOI] [PubMed] [Google Scholar]
- 61.Sarma V.J., Bostrom U.V. Intrathecal morphine for the relief of post-hysterectomy pain - a double-blind, dose-response study. Acta Anaesthesiol Scand. 1993;37:223–227. doi: 10.1111/j.1399-6576.1993.tb03705.x. [DOI] [PubMed] [Google Scholar]
- 62.Selvam V., Subramaniam R., Baidya D.K. Safety and efficacy of low-dose intrathecal morphine for laparoscopic hysterectomy: a randomized, Controlled Pilot Study. J Gynecol Surg. 2018;34:77–83. [Google Scholar]
- 63.Togal T., Demirbilek S., Gulhas N., Koroglu A. Combination of low-dose (0.1 mg) intrathecal morphine and patient-controlled intravenous morphine in the management of postoperative pain following abdominal hysterectomy. Pain Clinic. 2004;16:335–341. [Google Scholar]
- 64.Wongyingsinn M., Baldini G., Stein B., Charlebois P., Liberman S., Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012;108:850–856. doi: 10.1093/bja/aes028. [DOI] [PubMed] [Google Scholar]
- 65.Gjessing J., Tomlin P.J. Postoperative pain control with intrathecal morphine. Anaesthesia. 1981;36:268–276. doi: 10.1111/j.1365-2044.1981.tb10199.x. [DOI] [PubMed] [Google Scholar]
- 66.Weibel S., Jelting Y., Pace N.L. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J., Ho K.Y., Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106:454–462. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
- 68.Brinck E.C., Tiippana E., Heesen M. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12:CD012033. doi: 10.1002/14651858.CD012033.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helander E.M., Webb M.P., Bias M., Whang E.E., Kaye A.D., Urman R.D. Use of regional anesthesia techniques: analysis of institutional enhanced recovery after surgery protocols for colorectal surgery. J Laparoendosc Adv Surg Tech A. 2017;27:898–902. doi: 10.1089/lap.2017.0339. [DOI] [PubMed] [Google Scholar]
- 70.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 71.Chaney M.A. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903. doi: 10.1007/BF03011037. [DOI] [PubMed] [Google Scholar]
- 72.Slappendel R., Weber E.W., Benraad B., van Limbeek J., Dirksen R. Itching after intrathecal morphine. Incidence and treatment. Eur J Anaesthesiol. 2000;17:616–621. doi: 10.1046/j.1365-2346.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 73.Akhan A., Subasi F.D., Bosna G. Comparison of mirtazapine, gabapentin and ondansetron to prevent intrathecal morphine-induced pruritus. North Clin Istanb. 2016;3:53–59. doi: 10.14744/nci.2016.38233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sultan P., Gutierrez M.C., Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71:1807–1819. doi: 10.2165/11596250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 75.Ko S., Goldstein D.H., VanDenKerkhof E.G. Definitions of "respiratory depression" with intrathecal morphine postoperative analgesia: a review of the literature. Can J Anaesth. 2003;50:679–688. doi: 10.1007/BF03018710. [DOI] [PubMed] [Google Scholar]
- 76.Gehling M., Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009;64:643–651. doi: 10.1111/j.1365-2044.2008.05817.x. [DOI] [PubMed] [Google Scholar]
- 77.McNicol E.D., Ferguson M.C., Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2015:CD003348. doi: 10.1002/14651858.CD003348.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macintyre P.E. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36–46. doi: 10.1093/bja/87.1.36. [DOI] [PubMed] [Google Scholar]
- 79.Dworzak H., Fuss F., Buttner T. [Persisting respiratory depression following intrathecal administration of morphine and simultaneous sedation with midazolam] Anaesthesist. 1999;48:639–641. doi: 10.1007/s001010050764. [DOI] [PubMed] [Google Scholar]
- 80.Toǧal T., Türköz A., Durmuş M., Şahin S., Yilmaz S., Ersoy M.Ö. Effect of intratechal morphine on postoperative stress response and postoperative analgesic requirements on cardiac patients in major abdominal surgery. Turk Anesteziyol Reanim. 2000;28:492–499. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.