Abstract
Objective
The objective of this study was to examine if patients with migraine who responded sufficiently to acute treatment were significantly different from those who did not in terms of patient characteristics, treatment patterns, and patient level of impairment, and to identify characteristics associated with insufficient response.
Background
Migraine is highly prevalent and impacts functional ability substantially. Current treatment approaches are not sufficiently meeting the needs of patients, and inadequate response to acute treatment is reported by at least 56% of patients with migraine in the United States.
Methods
Data were obtained from the 2014 Adelphi Migraine Disease‐Specific Program, a cross‐sectional survey. Using logistic regression, we assessed the association between patient factors and insufficient response. Responders were defined as patients with migraine who achieved pain freedom within 2 hours of acute treatment in ≥4 of 5 attacks, while insufficient responders achieved it in ≤3 of 5 attacks.
Results
Of 583 patients included, insufficient responders to acute treatment constituted 34.3% (200/583) of the study population. A statistically significantly larger proportion of insufficient responders vs responders had ≥4 migraine headache days/month (46.3% [88/190] vs 31% [114/368]), had ever been prescribed ≥3 unique preventive treatment regimens (11.7% [21/179] vs 6.3% [22/347]), and had chronic migraine, medication‐overuse headaches, and comorbid depression (all P values ≤.05). Patient level of impairment was statistically significantly greater among insufficient responders vs responders. Factors associated with insufficient response after adjusting for covariates included Migraine Disability Assessment total score (odds ratio [OR] = 1.04, 95% CI [1.02, 1.05]), time of administration of acute treatment (OR = 1.83, 95% CI [1.15, 2.92]), depression (OR = 1.98, 95% CI [1.21, 3.23]), sensitivity to light not listed as current most troublesome symptom (OR = 2.30, 95% CI [1.21, 4.37]), and change in the average headache days per month before being prescribed an acute treatment vs now (OR = 1.75, 95% CI [1.05, 2.90]).
Conclusions
Clinical characteristics, treatment patterns, and health‐related quality of life measures are statistically significantly different between insufficient responders and responders to acute treatment in patients with migraine.
Keywords: migraine, responder, insufficient responder, real‐world, acute treatment
Abbreviations
- AMPP
American Migraine Prevalence and Prevention
- DSP
Disease‐Specific Program
- EQ‐5D
EuroQoL‐5 dimensions
- HDPM
headache days per month
- HIPAA
Health Insurance Portability and Accountability Act
- HITECH
Health Information Technology for Economic and Clinical Health
- MIDAS
Migraine Disability Assessment
- NSAID
nonsteroidal anti‐inflammatory drug
- OTC
over the counter
- PRF
patient record form
- PSC
patient self‐completion form
- SD
standard deviation
- US
United States
- VAS
visual analog scale
- WPAI
work productivity and activity impairment
Introduction
Migraine is a chronic, disabling neurological disease that is characterized by attacks of moderate‐to‐severe headache associated with nausea, vomiting, photophobia, and phonophobia. The prevalence of migraine in the United States (US) and Europe ranges from 11% to 12%, with higher rates among women than men. 1 , 2 It is the second‐highest cause of years lost due to disability globally and has a substantial impact on day‐to‐day functioning, and causes a significant interference with occupational, household, educational, family, and social responsibility. 3 The high prevalence of this disease and substantial negative functional impacts highlight the importance of having treatments that effectively mitigate the intense pain and associated symptoms and consequential negative impacts on daily life for those suffering from such attacks.
Migraine is characterized by recurrent headaches lasting 4 to 72 hours, with unilateral, pulsating, moderate or severe pain and is associated with nausea and/or photophobia and phonophobia. 4 The desired level of medication effectiveness for acute treatment of migraine is pain‐freedom within 2 hours of acute medication intake. 5 Various treatments are approved for the acute treatment of migraine, with the most recommended treatments being analgesics and triptans. 6 , 7 Besides pain relief, some of the other outcomes that determine effectiveness of treatments include consistency of relief 8 and consistency of response. 9 However, current treatments have varying efficacy response rates, with a substantial proportion of patients not responding to each individual treatment. Common classes of drugs that are used for the acute treatment of migraine include analgesics, antiemetics, and specific antimigraine medications. 10 , 11 , 12 Triptans are the most commonly prescribed treatment, and the rates of pain freedom post dose range from 20% to 40%, 13 with about 30% to 40% of patients not experiencing an adequate response to treatment. 9 Simple analgesics (nonsteroidal anti‐inflammatory drugs [NSAIDs], acetaminophen) and combinations are frequently used for less‐severe migraine attacks. These medications have similar or lower efficacy than triptans, with pain‐free rates in the range of 20% to 25%, 13 and they lack evidence of efficacy on other symptoms of migraine attacks. Opioids and barbiturates, while not recommended as first‐line treatment in the US guidelines, are also used for the acute treatment of migraine. However, opioid and barbiturate treatments are associated with the development of chronic migraine, and opioids reduce responsiveness to other acute migraine medications. 10 , 14 , 15
There is evidence that current treatment approaches are not sufficiently meeting the needs of patients. A large survey conducted in the American Migraine Prevalence and Prevention (AMPP) study revealed that 40.7% of the more than 5000 participants identified themselves in at least 1 of 5 defined unmet need categories. 16 Other treatment gaps relate to those patients who are unable to tolerate side effects of current treatment options. Patient compliance is influenced not only by efficacy, but also by tolerability; and in some cases, patients do not refill their acute medication due to adverse side effect or safety concerns leading to patients delaying or avoiding medication use. 17 , 18 , 19 Inadequately managed migraine poses a risk of medication overuse headache, chronification of migraine, and inefficiency in health care resource utilization. 20 Thus, new acute treatment options are needed for patients with migraine.
Inadequate response to acute treatment is reported by at least 56% of patients with migraine in the US. 21 The predictors of inadequate response that have been previously identified based on 2006 data from the AMPP study include gender (male), higher body mass index, cutaneous allodynia, higher headache pain intensity, more headache days per month (HDPM), depression, and not using preventive migraine medications. 21 As treatment paradigms and understanding of disease mechanisms have shifted over the last decade, analyses of more current data on predictors of response are needed.
The objective of this study was to examine if patients with migraine who responded sufficiently to acute treatment were significantly different from those who do not in terms of patient characteristics, treatment patterns, and patient level of impairment, and to identify characteristics associated with insufficient response using the 2014 US Adelphi Migraine Disease‐Specific Program (DSP) survey data. This study aims to build on prior work by Lipton et al (2016), 21 by providing additional information on key patient‐focused outcomes such as the Migraine Disability Assessment (MIDAS), recommended treatments, most troublesome symptoms, and timing of acute administration.
Methods
This research was a retrospective analysis of survey data obtained from the Adelphi Migraine DSP, a real‐world, point‐in‐time, cross‐sectional survey of primary care physicians, neurologists, and their consulting patients with migraine in the US (index dates of January‐March 2014) which was conducted independently by Adelphi Real World (Bollington, UK). The retrospective analysis was preplanned with the protocol prepared. Adelphi Migraine DSP are large, multinational, cross‐sectional observational studies of clinical practice providing valuable data on a range of common chronic diseases to supplement the findings of larger population‐based studies and uses a standardized methodology. 22
DSP Survey Data
The population included in this study consisted of licensed physicians (including neurologists and primary care physicians) and their consulting patients. The physicians were identified from public lists of health care professionals, who actively managed patients with migraine. There were 100 primary care physicians or internists, and 50 neurologists of which 20 were specialists in headache. Each physician was instructed to recruit 9 consecutive patients with a migraine diagnosis, and to achieve a 10% oversampling goal, the 10th patient had to be on at least their second line of preventive treatment. The 10th patient may not have been consecutive. Physicians were compensated to participate in the DSP according to fair market research rates consistent with the amount of time involved. For each patient with a migraine diagnosis, the physician completed a Patient Record Form (PRF) where data are collected on patient demographics, diagnoses, severity of condition, number of headache days, concomitant conditions, tests performed for diagnosis and monitoring, treatment history, drivers of treatment choice, and compliance and general patient management.
Patients for whom the physician completed a PRF were invited to complete a confidential patient self‐completion form (PSC). Patients answered questions on demographics, current conditions, level of treatment satisfaction, compliance, and health insurance status. Patients also provided data relating to emotional and physical impact of their condition using the EuroQoL‐5 Dimensions (EQ‐5D) 23 and the MIDAS test 24 , 25 and provided information related to the impact of migraine on their impact on work using the Work Productivity and Activity Impairment questionnaire (WPAI). 26
Study Population
All patients who participated in the migraine DSP and were currently prescribed an acute treatment for migraine were eligible for this study. Treatment response was determined based on participants’ reply to the following Adelphi DSP PSC question regarding acute treatment: “In approximately how many migraine attacks would you say your prescription acute medicine stops the migraine pain entirely within 2 hours of taking the medication?” Response options were 0, 1, 2, 3, 4, or 5 out of every 5 attacks. In our study, responders were defined as patients with migraine who achieved pain freedom within 2 hours of acute treatment in ≥4 of 5 attacks while insufficient responders achieved pain freedom in ≤3 of 5 attacks based on the response to this survey question. Two subgroups were created according to the definition. Patients who did not respond to the question were excluded from the analysis. After 2 subgroups were defined, the information collected in the PRF was combined and linked to the information from the PSC. Chronic migraine was defined as ≥15 HDPM over the last 3 months while episodic migraine was defined as <15 HDPM. All responses were de‐identified to protect participant confidentiality. Adelphi DSP data were collected in accordance with Adelphi Real‐World procedures, which are compliant with the Health Information Technology for Economic and Clinical Health (HITECH) Act and the Health Insurance Portability and Accountability Act (HIPAA).
Adelphi Real World follows the European Pharmaceutical Market Research Association (EphMRA) Code of Conduct. The Code of Conduct states that ethical approval was not necessary as the goal of research was to improve understanding rather than to test a hypothesis.
All patients provided written informed consent to participate by ticking a box on the front of the PSC.
Patient Reported Outcomes Measures
The MIDAS instrument is designed to quantify headache‐related disability over a 3‐month period, and it consists of 5 items that reflect the number of days reported as missing, or with reduced productivity at work or home and social events. It is weighted to produce scores in which a higher value is indicative of more disability. For clinical interpretability, 4 categorical grades were developed based on the total score: Grade I (0 to 5) is for little or no disability, Grade II (6 to 10) is for mild disability, Grade III (11 to 20) is for moderate disability, and Grade IV (21+) is for severe disability. This instrument is considered reliable and valid and is correlated with clinical judgment regarding the need for medical care. 24 , 25
The EQ‐5D is a multidimensional, generic health‐related quality of life (QoL) instrument which has 2 parts, a health‐status profile and a visual analog scale (VAS) which rates global health‐related QoL. With this profile, patients can rate their health state on that day within the domains of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. 27 There are 3 levels of severity, including no, some, and severe problems. A single score is generated for each domain with all 5 domains being mapped to a single index through an algorithm. The index ranges between 0 and 1 with the higher score indicating a better health state perceived by the patient. The VAS is a measurement instrument that provides an overall patient rating of health status on that day. It has a range from 0 (worst imaginable health state) to 100 (best imaginable health state).
Scores can be converted into weighted health‐state preferences for quality‐adjusted life year models.
The WPAI is a valid and reliable instrument that measures work productivity as missed work time and impairment of work and usual activities. 26 The outcomes of the WPAI are expressed as impairment percentages, with higher numbers indicating more impairment and less productivity. 28 In this study, the WPAI captured the implications on work productivity and impairment of usual activity due to headaches.
Statistical Analysis
No statistical power calculation was conducted prior to the study, as these were retrospective analyses on the available Adelphi DSP data. We assume patients in the analyses are independent from one another. Descriptive analyses included reporting mean and standard deviation (SD) for continuous variables and number of patients and percentages (%) for categorical measures for each subgroup. To compare 2 subgroups, insufficient responders vs responders in demographics, migraine clinical characteristics, comorbidities, migraine acute treatment pattern, symptoms causing the most trouble to patients, burden of migraine, QoL, and work productivity, a bivariate test was conducted by a 2‐sample t‐test for continuous variables if the normality assumption was held or by nonparametric Wilcoxon Rank‐sum test for non‐normally distributed variables and chi‐square/Fisher’s exact test for categorical variables. For questions of current symptom severity, each question was scored on an ordinal scale from none to severe (1‐4), and the Wilcoxon Rank‐sum test was applied to test median differences in symptom severity between the 2 groups. Summary statistical information was based on nonmissing data.
Stepwise logistic regression with insufficient responders as outcome was used as exploratory analysis to identify factors associated with insufficient response controlling for patient demographics and clinical characteristics. The variables we considered to include in the stepwise logistic regression were either based on statistical significance from bivariate tests or some measures that are clinically relevant to patients with migraine. The covariates included age, gender, ethnicity, employment status, living status (living alone or with a friend), smoking status, time to migraine diagnosis in weeks, depression, other psychological conditions, neuropathic pain, cardiovascular disease, insurance plan, doctor specialty, current most troublesome symptom: sensitivity to light, current most troublesome symptom: vomiting, currently prescribed acute treatment, time of administration of acute treatment, migraine with aura, currently prescribed recommended Level A, B, or C acute treatments, change in average HDPM before prescribed acute treatment, patient level of impairment last 6 months, migraine HDPM, number of unique treatment regimens for acute treatment, number of unique treatment regimens for preventive, and MIDAS total score. In stepwise selection, a significance level of .3 was required to allow a variable into the model, and a significance level of .35 was required for a variable to stay in the model. The C‐statistics and P value of Hosmer‐Lemeshow test for logistic regression were reported to show the discrimination and goodness of fit. All statistical tests were conducted at a 2‐sided 5% significance level. No adjustments for multiplicity were conducted. All analyses were conducted using SAS® enterprise guide 7. 9.3 (SAS Institute Inc., Cary, NC).
Results
The study population consisted of 583 patients of which 200 (34.3%) were insufficient responders and 383 (65.7%) were responders (Fig. 1).
Fig. 1.
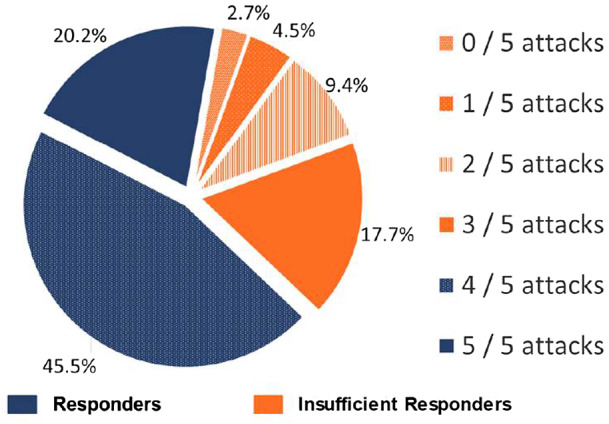
Distribution of insufficient responders and responders to acute treatment for migraine: pain freedom within 2 hours.
Age and gender were balanced between insufficient responder and responder groups. There was a statistically significantly larger proportion of insufficient responders vs responders among patients who consulted a neurologist (P = .011), had chronic migraine (P = .001), had medication‐overuse headaches (P < .001) (Table 1), and did not take acute medication at the first sign of a migraine attack (P = .008) (Table 2). There was a statistically significantly smaller proportion of insufficient responders vs responders who were employed (P = .044) (Table 1).
Table 1.
Patient Characteristics in Insufficient Responders and Responders to Acute Treatment
| Insufficient Responders (N = 200) | Responders (N = 383) | Total (N = 583) | P Value | |
|---|---|---|---|---|
| Age (years, mean [SD]) | 39.7 (12.7) | 40.1 (13.3) | 40.0 (13.1) | .850 |
| Female, n (%) | 160 (80.0) | 287 (74.9) | 447 (76.7) | .170 |
| White/Caucasian, n (%) | 144 (72.0) | 294 (76.8) | 438 (75.1) | .279 |
| Smoking status: current or prior smoker, n (%) | 63 (32.1) | 139 (36.4) | 202 (34.9) | .311 |
| Doctor specialty, n (%)† | .011 | |||
| Primary care/Internist consultation | 131 (65.5) | 289 (75.5) | 420 (72.0) | |
| Neurologist consultation | 69 (34.5) | 94 (24.5) | 163 (28.0) | |
| Employed, n (%) | 130 (65.0) | 279 (73.0) | 409 (70.3) | .044 |
| Insurance plan, n (%) | .219 | |||
| Commercial | 137 (82.5) | 289 (87.3) | 426 (85.7) | |
| Medicare/Medicaid/Medical | 26 (15.7) | 34 (10.3) | 60 (12.1) | |
| Others | 3 (1.8) | 8 (2.4) | 11 (2.2) | |
| Migraine with aura, n (%) | 69 (34.7) | 163 (42.7) | 232 (39.9) | .062 |
| Migraine type based on headache days, n (%) | .001 | |||
| Chronic migraine (15+ headache days) | 23 (11.6) | 17 (4.5) | 40 (6.9) | |
| Episodic migraine (<15 headache days) | 176 (88.4) | 365 (95.5) | 541 (93.1) | |
| Migraine Headache Days/Month, n (%) | <.001 | |||
| 0‐3 | 102 (53.7) | 254 (69.0) | 356 (63.8) | |
| ≥4 | 88 (46.3) | 114 (31.0) | 202 (36.2) | |
| 4‐7 | 51 (26.8) | 87 (23.6) | 138 (24.7) | .406 |
| 8‐14 | 24 (12.6) | 22 (6.0) | 46 (8.2) | .007 |
| 15+ | 13 (6.8) | 5 (1.4) | 18 (3.2) | <.001 |
| Rebound headaches or medication‐overuse headaches, n (%) | 31 (15.5) | 25 (6.5) | 56 (9.6) | <.001 |
| Tension headaches, n (%) | 89 (44.5) | 141 (36.8) | 230 (39.5) | .071 |
| Family history with migraine: parent, n (%) | 88 (44.4) | 155 (40.6) | 243 (41.9) | .371 |
| Comorbidities (patient background) | ||||
| Depression | 72 (37.5) | 82 (22.0) | 154 (27.3) | <.001 |
| Anxiety | 59 (30.7) | 126 (33.8) | 185 (32.7) | .464 |
| Other psychological conditions‡ | 16 (8.3) | 14 (3.8) | 30 (5.3) | .021 |
| Neuropathic pain | 9 (4.7) | 9 (2.4) | 18 (3.2) | .145 |
All calculations were based on nonmissing values. Bold font in the P values column indicates statistically significant.
Consultation from which the patient was enrolled in the survey. For categorical measures, chi‐square or Fisher’s exact test was used. For continuous measures, Wilcoxon Rank‐sum test or 2‐sample t‐test was used.
Other psychological conditions include panic disorder, obsessive compulsive disorder, other psychological or psychiatric symptoms except for depression and anxiety.
Table 2.
Change in Average Headache Days/Month Before Prescribed an Acute Treatment vs Now and Treatment Regimens in Insufficient Responders and Responders to Acute Treatment
| Insufficient Responders (N = 200) | Responders (N = 383) | Total (N = 583) | P Value | |
|---|---|---|---|---|
| Number of unique preventive† treatment regimens, n (%) | .085 | |||
| 0 | 60 (33.5) | 141 (40.6) | 201 (38.2) | .112 |
| 1 | 62 (34.6) | 126 (36.3) | 188 (35.7) | .704 |
| 2 | 36 (20.1) | 58 (16.7) | 94 (17.9) | .335 |
| 3+ | 21 (11.7) | 22 (6.3) | 43 (8.2) | .033 |
| Number of unique acute treatment regimens, n (%) | .029 | |||
| 0 | 11 (5.6) | 6 (1.6) | 17 (3.0) | .008 |
| 1 | 94 (47.7) | 195 (52.4) | 289 (50.8) | .286 |
| 2 | 49 (24.9) | 105 (28.2) | 154 (27.1) | .392 |
| 3+ | 43 (21.8) | 66 (17.7) | 109 (19.2) | .239 |
| Number of unique triptans, n (%) | .695 | |||
| 0 | 22 (11.0) | 41 (10.7) | 63 (10.8) | .913 |
| 1 | 117 (58.5) | 239 (62.4) | 356 (61.1) | .359 |
| 2 | 47 (23.5) | 84 (21.9) | 131 (22.5) | .667 |
| 3+ | 14 (7.0) | 19 (5.0) | 33 (5.7) | .312 |
| Change in average headache days/month before prescribed an acute treatment vs now, n (%) | .008 | |||
| Headache days increased | 33 (17.8) | 43 (11.8) | 76 (13.8) | |
| Headache days decreased | 97 (52.4) | 240 (65.9) | 337 (61.4) | |
| No change | 55 (29.7) | 81 (22.3) | 136 (24.8) | |
All calculations were based on nonmissing values. Bold font in the P values column indicates statistically significant.
Please note: these data are influenced by the inclusion of a 10% oversampling of patients who had failed at least one line of preventive treatment. P values were from chi‐square or Fisher’s exact test.
The analyses of patients with comorbidities in insufficient responders and responders to acute treatment demonstrated that there was a statistically significantly higher proportion of insufficient responders compared with responders who had comorbid depression (P < .001) and other psychological conditions (P = .021) (Table 1). There was also a statistically significantly larger proportion of insufficient responders vs responders who had ≥4 migraine HDPM (46.3% [88/190] vs 31% [114/368]) (P < .001) (Table 1). For treatment regimens, there was also a statistically significantly larger proportion of insufficient responders vs responders who had ever been prescribed ≥3 unique preventive treatment regimens (11.7% [21/179] vs 6.3% [22/347]) (P = .033) (Table 2) (note that these data are influenced by the inclusion of a 10% oversampling of patients who had failed at least 1 line of preventive treatment). There was no difference in the proportion of insufficient responders vs responders who had been prescribed 0 (P = .913), 1 (P = .359), 2 (P = .667), or ≥3 (P = .312) unique triptans. Evaluation of current and previous acute treatments in insufficient responders and responders to acute treatment demonstrated that the insufficient responders vs responders was associated with an increased use of over the counter (OTC) medications (P = .012), opioids (P = .003), and NSAIDS (P = .018) (Table 3). There were also differences seen in the acute treatment patterns of insufficient responders and responders to acute treatment (P < .001) (Table 4). Evaluation of current symptom severity in insufficient responders and responders to acute treatment demonstrated the median response of symptom severity of patients who had bilateral pain (P = .016), sensitivity to smell (P = .039), vomiting (P < .001), pain that worsened with activity (P < .001), or muscle weakness/fatigue (P = .007) was statistically significantly higher in insufficient responders compared with responders (Table 5).
Table 3.
Current and Select Previous Acute Treatments in Insufficient Responders and Responders to Acute Treatment
| Acute Treatments, n (%) | Insufficient Responders (N = 200) | Responders (N = 383) | Total (N = 583) | P Value |
|---|---|---|---|---|
| Current acute treatments use | ||||
| Patient currently taking over the counter (OTC) medications | 72 (50.3) | 111 (37.8) | 183 (41.9) | .012 |
| Currently taking OTC and/or prescribed acute treatment | .031 fe | |||
| OTC and prescribed acute | 72 (50.3) | 110 (37.4) | 182 (41.6) | |
| OTC only | – | 1 (0.3) | 1 (0.2) | |
| Prescribed acute only | 71 (49.7) | 180 (61.2) | 251 (57.4) | |
| Neither OTC nor prescribed acute | – | 3 (1.0) | 3 (0.7) | |
| Opioid analgesics | 31 (15.5) | 29 (7.6) | 60 (10.3) | .003 |
| NSAIDs (including in Combinations) | 57 (28.5) | 76 (19.8) | 133 (22.8) | .018 |
| Butalbital/caffeine/acetaminophen | 3 (1.5) | 6 (1.6) | 9 (1.5) | 1.000 |
| Ibuprofen | 24 (12.0) | 31 (8.1) | 55 (9.4) | .126 |
| Naproxen | 27 (13.5) | 41 (10.7) | 68 (11.7) | .318 |
| Triptans | 167 (83.5) | 316 (82.5) | 483 (82.8) | |
| Sumatriptan | 88 (44.0) | 167 (43.6) | 255 (43.7) | .927 |
| Rizatriptan | 34 (17.0) | 81 (21.1) | 115 (19.7) | .232 |
| Eletriptan | 22 (11.0) | 30 (7.8) | 52 (8.9) | .203 |
| Zolmitriptan | 19 (9.5) | 23 (6.0) | 42 (7.2) | .121 |
| Frovatriptan | 6 (3.0) | 21 (5.5) | 27 (4.6) | .176 |
| Almotriptan | 6 (3.0) | 11 (2.9) | 17 (2.9) | .931 |
| Naratriptan | 4 (2.0) | 5 (1.3) | 9 (1.5) | .501 |
| Naproxen/sumatriptan | 10 (5.0) | 22 (5.7) | 32 (5.5) | .708 |
| Select previous acute treatments use, n (%) | ||||
| Triptans | 79 (69.9) | 135 (67.8) | 214 (68.6) | .705 |
| NSAIDs (including in combinations) | 43 (38.1) | 77 (38.7) | 120 (38.5) | .911 |
| Opioid Analgesics (including in combinations) | 21 (18.6) | 33 (16.6) | 54 (17.3) | .653 |
All calculations were based on nonmissing values. Bold font in the P values column indicates statistically significant.
P values were from chi‐square or Fisher’s exact test (fe).
Table 4.
Acute Treatment Patterns in Insufficient Responders and Responders to Acute Treatment
| Insufficient Responders (N = 200) | Responders (N = 383) | Total (N = 583) | P Value | |
|---|---|---|---|---|
| Time of administration of acute treatment, n (%) | <.001 | |||
| At first sign of a migraine attack | 108 (56.3) | 277 (73.7) | 385 (67.8) | |
| When/after the pain starts | 84 (43.8) | 99 (26.3) | 183 (32.2) | |
| Continue using your currently prescribed acute medication? n (%) | <.001 | |||
| Definitely yes | 69 (35.9) | 234 (63.2) | 303 (53.9) | |
| Probably yes | 93 (48.4) | 120 (32.4) | 213 (37.9) | |
| Do not know | 16 (8.3) | 13 (3.5) | 29 (5.2) | |
| Probably not | 12 (6.3) | 2 (0.5) | 14 (2.5) | |
| Definitely not | 2 (1.0) | 1 (0.3) | 3 (0.5) | |
| Do you ever need to take extra doses to relieve the pain? n (%) | <.001 | |||
| Yes | 130 (70.7) | 164 (45.7) | 294 (54.1) | |
| No | 54 (29.3) | 195 (54.3) | 249 (45.9) | |
| Number of times extra doses of a prescribed acute medication was taken for the last 10 migraine attacks, mean (SD) | 4.295 (2.396) | 2.442 (1.637) | 3.26 (2.206) | <.001 |
| Number of extra doses taken when extra doses had to be taken, n (%) | <.001 | |||
| 1 extra dose | 88 (70.4) | 143 (89.9) | 231 (81.3) | |
| 2 extra doses | 34 (27.2) | 14 (8.8) | 48 (16.9) | |
| 3 extra doses | 3 (2.4) | 2 (1.3) | 5 (1.8) | |
For categorical measures, chi‐square or Fisher’s exact test was used. For continuous measures, Wilcoxon Rank‐sum test was used.
All calculations were based on nonmissing values. Bold font in the P values column indicates statistically significant.
Table 5.
Current Symptom Severity in Insufficient Responders and Responders to Acute Treatment
| Insufficient Responders (N = 200) | Responders (N = 383) | Total (N = 583) | P Value | ||
|---|---|---|---|---|---|
| Unilateral pain | |||||
| Median (Q1, Q3) | 3 (1, 4) | 3 (1, 3) | 3 (1, 4) | .583 | |
| None, n (%) | 61 (30.5) | 103 (26.9) | 164 (28.1) | ||
| Mild, n (%) | 13 (6.5) | 39 (10.2) | 52 (8.9) | ||
| Moderate, n (%) | 67 (33.5) | 152 (39.7) | 219 (37.6) | ||
| Severe, n (%) | 59 (29.5) | 89 (23.2) | 148 (25.4) | ||
| Bilateral pain | |||||
| Median (Q1, Q3) | 2 (1, 3) | 1 (1, 3) | 1 (1, 3) | .016 | |
| None, n (%) | 94 (47.0) | 215 (56.1) | 309 (53.0) | ||
| Mild, n (%) | 18 (9.0) | 31 (8.1) | 49 (8.4) | ||
| Moderate, n (%) | 52 (26.0) | 95 (24.8) | 147 (25.2) | ||
| Severe, n (%) | 36 (18.0) | 42 (11.0) | 78 (13.4) | ||
| Pulsating/throbbing pain | |||||
| Median (Q1, Q3) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | .791 | |
| None, n (%) | 37 (18.5) | 59 (15.4) | 96 (16.5) | ||
| Mild, n (%) | 21 (10.5) | 42 (11.0) | 63 (10.8) | ||
| Moderate, n (%) | 82 (41.0) | 186 (48.6) | 268 (46.0) | ||
| Severe, n (%) | 60 (30.0) | 96 (25.1) | 156 (26.8) | ||
| Sensitivity to light (photophobia) | |||||
| Median (Q1, Q3) | 3 (2, 3) | 3 (1, 3) | 3 (2, 3) | .199 | |
| None, n (%) | 42 (21.0) | 100 (26.1) | 142 (24.4) | ||
| Mild, n (%) | 34 (17.0) | 54 (14.1) | 88 (15.1) | ||
| Moderate, n (%) | 85 (42.5) | 172 (44.9) | 257 (44.1) | ||
| Severe, n (%) | 39 (19.5) | 57 (14.9) | 96 (16.5) | ||
| Sensitivity to sound (phonophobia) | |||||
| Median (Q1, Q3) | 2 (1, 3) | 1 (1, 3) | 2 (1, 3) | .061 | |
| None, n (%) | 86 (43.0) | 198 (51.7) | 284 (48.7) | ||
| Mild, n (%) | 25 (12.5) | 42 (11.0) | 67 (11.5) | ||
| Moderate, n (%) | 67 (33.5) | 106 (27.7) | 173 (29.7) | ||
| Severe, n (%) | 22 (11.0) | 37 (9.7) | 59 (10.1) | ||
| Sensitivity to smell | |||||
| Median (Q1, Q3) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | .039 | |
| None, n (%) | 134 (67.0) | 285 (74.4) | 419 (71.9) | ||
| Mild, n (%) | 20 (10.0) | 39 (10.2) | 59 (10.1) | ||
| Moderate, n (%) | 34 (17.0) | 44 (11.5) | 78 (13.4) | ||
| Severe, n (%) | 12 (6.0) | 15 (3.9) | 27 (4.6) | ||
| Pain worsened by activity | |||||
| Median (Q1, Q3) | 3 (1, 3) | 1 (1, 3) | 2 (1, 3) | <.001 | |
| None, n (%) | 77 (38.5) | 210 (54.8) | 287 (49.2) | ||
| Mild, n (%) | 21 (10.5) | 26 (6.8) | 47 (8.1) | ||
| Moderate, n (%) | 65 (32.5) | 105 (27.4) | 170 (29.2) | ||
| Severe, n (%) | 37 (18.5) | 42 (11.0) | 79 (13.6) | ||
| Sensory aura (pins and needles/numbness) | |||||
| Median (Q1, Q3) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | .145 | |
| None, n (%) | 156 (78.0) | 320 (83.6) | 476 (81.6) | ||
| Mild, n (%) | 17 (8.5) | 13 (3.4) | 30 (5.1) | ||
| Moderate, n (%) | 21 (10.5) | 41 (10.7) | 62 (10.6) | ||
| Severe, n (%) | 6 (3.0) | 9 (2.3) | 15 (2.6) | ||
| Nausea | |||||
| Median (Q1, Q3) | 3 (2, 3) | 2 (1, 3) | 3 (1, 3) | .069 | |
| None, n (%) | 44 (22.0) | 105 (27.4) | 149 (25.6) | ||
| Mild, n (%) | 43 (21.5) | 98 (25.6) | 141 (24.2) | ||
| Moderate, n (%) | 93 (46.5) | 141 (36.8) | 234 (40.1) | ||
| Severe, n (%) | 20 (10.0) | 39 (10.2) | 59 (10.1) | ||
| Vomiting | |||||
| Median (Q1, Q3) | 1 (1, 3) | 1 (1, 2) | 1 (1, 2) | <.001 | |
| None, n (%) | 109 (54.5) | 281 (73.4) | 390 (66.9) | ||
| Mild, n (%) | 38 (19.0) | 45 (11.7) | 83 (14.2) | ||
| Moderate, n (%) | 41 (20.5) | 41 (10.7) | 82 (14.1) | ||
| Severe, n (%) | 12 (6.0) | 16 (4.2) | 28 (4.8) | ||
| Visual aura/sight disturbance | |||||
| Median (Q1, Q3) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | .633 | |
| None, n (%) | 130 (65.0) | 261 (68.1) | 391 (67.1) | ||
| Mild, n (%) | 27 (13.5) | 33 (8.6) | 60 (10.3) | ||
| Moderate, n (%) | 32 (16.0) | 69 (18.0) | 101 (17.3) | ||
| Severe, n (%) | 11 (5.5) | 20 (5.2) | 31 (5.3) | ||
| Speech disturbance / problems | |||||
| Median (Q1, Q3) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | .182 | |
| None, n (%) | 184 (92.0) | 363 (94.8) | 547 (93.8) | ||
| Mild, n (%) | 9 (4.5) | 12 (3.1) | 21 (3.6) | ||
| Moderate, n (%) | 5 (2.5) | 3 (0.8) | 8 (1.4) | ||
| Severe, n (%) | 2 (1.0) | 5 (1.3) | 7 (1.2) | ||
| Muscle weakness / fatigue | |||||
| Median (Q1, Q3) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | .007 | |
| None, n (%) | 151 (75.5) | 323 (84.3) | 474 (81.3) | ||
| Mild, n (%) | 16 (8.0) | 27 (7.0) | 43 (7.4) | ||
| Moderate, n (%) | 25 (12.5) | 23 (6.0) | 48 (8.2) | ||
| Severe, n (%) | 8 (4.0) | 10 (2.6) | 18 (3.1) | ||
| Light‐headedness | |||||
| Median (Q1, Q3) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | .550 | |
| None, n (%) | 142 (71.0) | 282 (73.6) | 424 (72.7) | ||
| Mild, n (%) | 25 (12.5) | 40 (10.4) | 65 (11.1) | ||
| Moderate, n (%) | 26 (13.0) | 48 (12.5) | 74 (12.7) | ||
| Severe, n (%) | 7 (3.5) | 13 (3.4) | 20 (3.4) | ||
P values were from Wilcoxon Rank‐sum test for median differences in symptom severity between the 2 groups by considering 4 levels of severity as an ordinal scale with none, mild, moderate, and severe response (1‐4). The 4 levels of severity are none, mild, moderate, and severe, which together add up to 100%.
All calculations were based on nonmissing values. Bold font in the P values column indicates statistically significant.
For the health‐related QoL measures (Fig. 2) in insufficient responders and responders to acute treatment, disability was statistically significantly higher among insufficient responders vs responders (higher scores on MIDAS indicate worse disability) (P < .001). EQ‐5D overall VAS score was statistically significantly higher (P < .001) (indicating better health utility) among responders vs insufficient responders, and patient level of impairment (P < .001) and impact of headache on work (WPAI) were statistically significantly greater (P < .05) among insufficient responders vs responders.
Fig. 2.
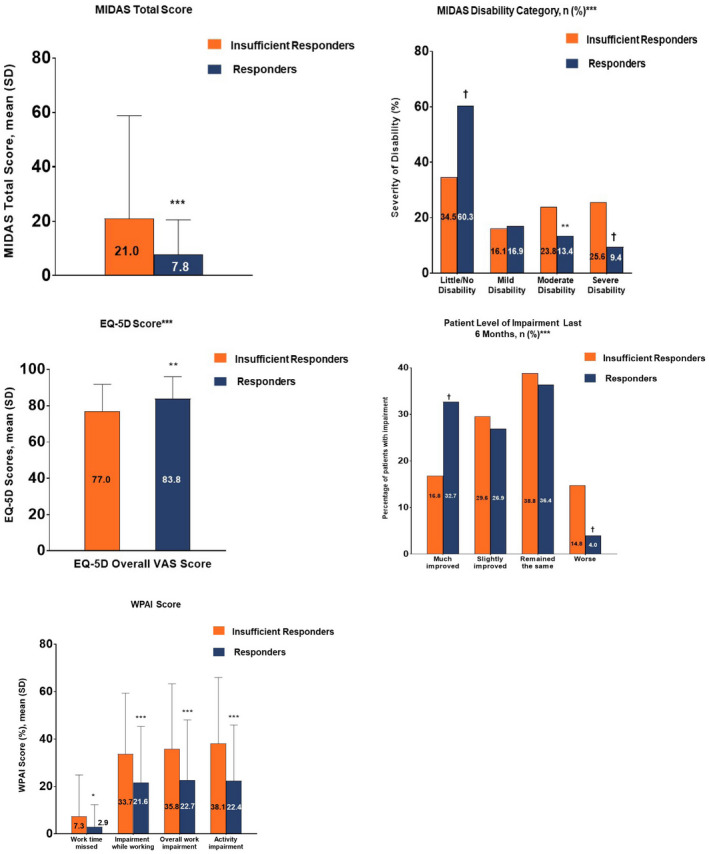
Health‐related quality of life (QoL) measures in insufficient responders and responders to acute treatment. *P ≤ .05, **P ≤ .01, ***P ≤ .001, †P < .0001. For categorical measures, chi‐square or Fisher’s exact test was used. For continuous measures, 2‐sample t‐test was used. EQ‐5D = Euro‐QuoL‐5 dimensions; MIDAS = Migraine Disability Assessment; VAS = visual analog scale, WPAI = work productivity and activity impairment. All calculations were based on nonmissing values.
There were 116 of 583 patients excluded from the logistic regression model due to incomplete data on one of the covariates included in the model. The exploratory analysis of using stepwise logistic regression with insufficient responders as the outcome revealed the statistically significant factors (P < .05) positively associated with being an insufficient responder (Table 6). The C‐statistics of final logistic regression was .78 and the P value for Hosmer‐Lemeshow test was 0.399. When compared to patients without depression, patients with depression were more likely to be insufficient responders to acute treatments (OR = 1.98; 95% CI [1.21, 3.23]; P = .006). Compared with acute treatment taken at first sign of a migraine attack, the odds of insufficient response were statistically significantly higher for those patients who take acute treatments when or after the pain started (OR = 1.83; 95% CI [1.15, 2.92]; P = .011). Patients with a higher MIDAS total score, that is, more severe disability, were more likely to be insufficient responders, with increasing odds of 3.5% per 1‐unit increase in MIDAS total score (OR = 1.04; 95% CI [1.02, 1.05]; P < .001). Patients without photophobia as one of their current most troublesome symptoms were more likely to be insufficient responders to acute treatment compared to those who did report photophobia as a most troublesome symptom (OR = 2.30; 95% CI [1.21, 4.37]; P = .011). Patients who reported no change in average HDPM before being prescribed acute treatment had a higher likelihood of being an insufficient responder than those who had decreased average HDPM before prescribed acute treatment (OR = 1.75; 95% CI [1.05, 2.90]; P = .031).
Table 6.
Factors Associated With Insufficient Responders to Acute Treatment vs Responders
| Factors | Odds Ratio | 95 Confidence Interval | P Value | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Ethnicity (White/Caucasian vs Other) | 0.73 | 0.45 | 1.19 | .208 |
| Living alone (Yes vs No) | 1.45 | 0.85 | 2.47 | .171 |
| Living with friend (Yes vs No) | 0.52 | 0.19 | 1.44 | .209 |
| Had depression (Yes vs No) | 1.98 | 1.21 | 3.23 | .006 |
| Had neuropathic pain (Yes vs No) | 2.01 | 0.55 | 7.29 | .289 |
| Time of administration acute treatment (When/After the pain starts vs at first sign of a migraine) | 1.83 | 1.15 | 2.92 | .011 |
| MIDAS total score | 1.04 | 1.02 | 1.05 | <.001 |
| Currently prescribed recommended acute treatments (Yes vs No) | 3.74 | 0.71 | 19.62 | .119 |
| Change in average HDPM before prescribed acute treatment (increase vs decrease) | 1.25 | 0.64 | 2.46 | .515 |
| Current most troublesome symptom: Sensitivity to light (No vs Yes) | 2.30 | 1.21 | 4.37 | .011 |
| Patient level of impairment last 6 months (Much improved vs Remained the same) | 0.59 | 0.34 | 1.05 | .072 |
| Patient level of impairment last 6 months (Slightly improved vs Remained the same) | 1.01 | 0.59 | 1.72 | .965 |
| Patient level of impairment last 6 months (Worse vs Remained the same) | 1.50 | 0.60 | 3.72 | .386 |
| Migraine Headache Days per month 15+ vs 0‐3 | 4.01 | 0.98 | 16.39 | .053 |
| Migraine Headache Days per month 4‐7 vs 0‐3 | 1.06 | 0.63 | 1.78 | .831 |
| Migraine Headache Days per month 8‐14 vs 0‐3 | 1.34 | 0.58 | 3.10 | .489 |
| Change in average HDPM before prescribed acute medication and now (no change vs decrease) | 1.75 | 1.05 | 2.90 | .031 |
The results in the table were from stepwise logistic regression with selection criteria a significance level of .3 to allow a variable into the model and a significance level of .35 for a variable to stay in the model. Odds ratio >1 with its 95% CI not including 1 indicates as a significant factor associated with acute treatment insufficient‐response while <1 with its 95% CI not including 1 is a significant factor for acute treatment response. Bold font in the P values column indicates statistically significant.
HDPM = number of headache days per month; MIDAS = Migraine Disability Assessment.
Discussion
In this study utilizing a cross‐sectional survey of primary care physicians, neurologists, and their consulting patients with migraine in the US (Adelphi Migraine US DSP), patient demographics were generally representative of the migraine population. 29 , 30 Insufficient responders to acute treatment (pain freedom at 2 hours in ≤3 out of 5 attacks) constituted 34.3% of the study population, which is consistent with previously reported literature 9 , 31 , 32 but lower than Lipton et al (2016) who used a different definition of insufficient response. 21
In regards to their treatment regiment, insufficient responders to acute treatment vs responders had increased use of opioids, NSAIDs, and OTC medications. However, there was no difference in the proportion of insufficient responders vs responders who had been prescribed 0, 1, 2, or ≥3 unique triptans. This suggests that there is no likely benefit in having patients try more than 2 triptans, consistent with recent guidance issued by the American Headache Society that new acute therapies should be considered in patients who have failed at least 2 oral triptans. 33 As an increasing number of acute medication switches is associated with healthcare resource utilization increases, 34 future research should also look at how better managing the types/numbers of switches in insufficient responders could benefit the healthcare system in addition to individual patients.
Insufficient responders experienced more severe symptoms, a higher number of comorbidities, and worse health‐related QoL compared to responders. Since both episodic and chronic migraine are associated with a decrease in productivity and work‐related activities, 35 it is not surprising that the patient level of impairment and impact of headache on work was statistically significantly greater among insufficient responders vs responders, and there was a statistically significantly greater percentage of insufficient responders compared to responders who were not employed. Some previously identified predictors of inadequate response include the male sex, higher body mass index, higher headache pain intensity, cutaneous allodynia, more HDPM, the comorbidity of depression, and not using preventive migraine medications. 21 The only predictor of insufficient response which overlaps from the Lipton et al (2016) 21 study is comorbidity of depression. Our study also found other factors associated with insufficient response such as taking acute medication when or after the pain started vs at the first sign of a migraine attack (ie, premonitory phase), higher MIDAS total scores which indicated more severe disability, and not reporting photophobia as the current most troublesome symptom. Taken together with prior work, the current findings seem to suggest that with more severe or intense headaches, typical oral medications may not be as effective.
Various outcomes such as pain relief or pain freedom at 2 hours, recurrence rate, or sustained response for 24 hours have been used to determine the effectiveness of treatments; therefore, various definitions of insufficient response are found in the literature. Pain free at 2 hours is considered to be a robust endpoint that is desirable by patients, when determining response to treatment. 8 Consistency of relief is also another important treatment attribute. 8 Response has been defined as a positive outcome in at least 2 out of 3 treated attacks 9 or 3 out of 4 attacks. 36 The current study authors synthesized these findings and operationalized a definition of insufficient response anchored on pain freedom at 2 hours that also reflected consistency of response at a threshold above the most conservative estimates in the literature (ie, 4 out of 5 attacks). Given that pain free at 2 hours and consistency of response is such an important outcome to patients, we believe that 4 out of 5 was an appropriate choice for the definition of response.
Limitations
Certain limitations need to be taken into consideration for this study. The results from the study are limited by the cross‐sectional nature of the survey; therefore, causal inferences cannot be made. The purpose of this study was exploratory and to identify an association rather than for future prediction because the collinearity among the variables may have not been well handled in stepwise selection of logistic regression. Furthermore, the study sites were selected based on the volume of patients with migraine routinely seen. Therefore, consulting physicians were experienced with treating migraine and may not reflect the standard of care seen in more general practice. Additionally, patients recruited were from a diagnosed population and were actively seeking medical care. This limits the generalization of these results to all patients with migraine. Due to the survey being cross‐sectional, it is unknown if any of the characteristics are the cause of the insufficient response to acute treatments or if the insufficient response to acute treatments resulted in the characteristics such as chronic migraine, increased disability, and increased comorbidities like depression. Further longitudinal research would help elucidate the interactions of these factors.
Conclusion
The results suggest that clinical characteristics, treatment patterns, and QoL measures are significantly different between insufficient responders and responders to acute treatment in patients with migraine. The results validate what we know about insufficient responders as well as present new information. New findings that insufficient responders were among patients who consulted a neurologist, had a medication‐overuse headache component, and were taking their acute medication too late suggests that these patients may benefit from education on how and when to use current treatments. The difference in clinical characteristics and QoL measures also presents insufficient responders as more medically and psychosocially complex. Optimizing a treatment regimen using available medications is likely to be challenging and highlights the need for efforts to personalize treatment regimens as well as developing new medications and interventions that could reduce the number of insufficient responders. Identifying insufficient responder patterns/factors in a clinical setting may contribute to optimal management of patients with migraine.
Statement of Authorship
Category 1
(a) Conception and Design
Wenyu Ye, James Jackson, Sarah Cotton
(b) Acquisition of Data
James Jackson, Sarah Cotton
(c) Analysis and Interpretation of Data
Louise Lombard, Wenyu Ye, Russell Nichols, James Jackson, Sarah Cotton, Shivang Joshi
Category 2
(a) Drafting the Manuscript
Louise Lombard
(b) Revising It for Intellectual Content
Louise Lombard, Wenyu Ye, Russell Nichols, James Jackson, Sarah Cotton, Shivang Joshi
Category 3
(a) Final Approval of the Completed Manuscript
Louise Lombard, Wenyu Ye, Russell Nichols, James Jackson, Sarah Cotton, Shivang Joshi
Acknowledgments
The Adelphi Migraine Disease‐Specific Program (DSP) was an independent survey conducted by Adelphi Real World. Lilly subscribed to the dataset from which this analysis and publication are derived. All authors had full access to all data and had final responsibility for the decision to submit for publication. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Conflict of Interest: Louise Lombard, Wenyu Ye, and Russell Nichols are employees and shareholders of Eli Lilly and Company, Indianapolis, IN, USA. James Jackson, and Sarah Cotton are employees of Adelphi Real World, Adelphi Mill, Bollington, UK. Shivang Joshi has received honoraria for contribution to an advisory board and speaker’s bureau from Eli Lilly.
Funding: The Adelphi Migraine Disease‐Specific Program (DSP) was an independent survey conducted by Adelphi Real World. Eli Lilly and Company, Indianapolis, IN, USA. subscribed to the dataset from which this analysis and publication are derived.
References
- 1. Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 2. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta‐analysis of community‐based studies involving 6 million participants. J Neurol Sci. 2017;372:307‐315. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2016. Global Burden of Disease Study 2016: Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. Available at https://www.ichd-3.org/wp-content/uploads/2018/01/The-International-Classification-of-Headache-Disorders-3rd-Edition-2018.pdf. Accessed May 1, 2020. [DOI] [PubMed] [Google Scholar]
- 5. Tfelt‐Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: A guide for investigators. Cephalalgia. 2012;32:6‐38. [DOI] [PubMed] [Google Scholar]
- 6. Silberstein SD, Holland S, Freitag F, et al. Evidence‐based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Physician. 2011;83:271‐280. [PubMed] [Google Scholar]
- 8. Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache. 2002;42(Suppl. 1):S3‐S9. [DOI] [PubMed] [Google Scholar]
- 9. Viana M, Genazzani AA, Terrazzino S, Nappi G, Goadsby PJ. Triptan nonresponders: Do they exist and who are they? Cephalalgia. 2013;33:891‐896. [DOI] [PubMed] [Google Scholar]
- 10. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3‐20. [DOI] [PubMed] [Google Scholar]
- 11. Malone CD, Bhowmick A, Wachholtz AB. Migraine: Treatments, comorbidities, and quality of life, in the USA. J Pain Res. 2015;8:537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: A review of statistics from national surveillance studies. Headache. 2013;53:427‐436. [DOI] [PubMed] [Google Scholar]
- 13. Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: A systematic review and network meta‐analysis. Headache. 2015;55(Suppl. 4):221‐235. [DOI] [PubMed] [Google Scholar]
- 14. Bigal ME, Borucho S, Serrano D, Lipton RB. The acute treatment of episodic and chronic migraine in the USA. Cephalalgia. 2009;29:891‐897. [DOI] [PubMed] [Google Scholar]
- 15. Friedman BW. Managing migraine. Ann Emerg Med. 2017;69:202‐207. [DOI] [PubMed] [Google Scholar]
- 16. Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1300‐1311. [DOI] [PubMed] [Google Scholar]
- 17. Lipton RB, Stewart WF. Acute migraine therapy: Do doctors understand what patients with migraine want from therapy? Headache. 1999;39(Suppl. 2):S20‐S26. [Google Scholar]
- 18. Sheftell FD, Fox AW. Acute migraine treatment outcome measures: A clinician’s view. Cephalalgia. 2000;20(Suppl. 2):14‐24. [DOI] [PubMed] [Google Scholar]
- 19. Gallagher RM, Kunkel R. Migraine medication attributes important for patient compliance: Concerns about side effects may delay treatment. Headache. 2003;43:36‐43. [DOI] [PubMed] [Google Scholar]
- 20. Casucci G, Cevoli S. Controversies in migraine treatment: Opioids should be avoided. Neurol Sci. 2013;34(Suppl. 1):S125‐S128. [DOI] [PubMed] [Google Scholar]
- 21. Lipton RB, Munjal S, Buse DC, Fanning KM, Bennett A, Reed ML. Predicting inadequate response to acute migraine medication: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2016;56:1635‐1648. [DOI] [PubMed] [Google Scholar]
- 22. Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real‐world physician and patient behaviour across countries: Disease‐specific programmes—A means to understand. Curr Med Res Opin. 2008;24:3063‐3072. [DOI] [PubMed] [Google Scholar]
- 23. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population‐based sample of headache sufferers. Cephalalgia. 1999;19:107‐114. [DOI] [PubMed] [Google Scholar]
- 25. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache‐related disability. Neurology. 2001;56(Suppl. 1):S20‐S28. [DOI] [PubMed] [Google Scholar]
- 26. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353‐365. [DOI] [PubMed] [Google Scholar]
- 27. Brooks R. EuroQol: The current state of play. Health Policy. 1996;37:53‐72. [DOI] [PubMed] [Google Scholar]
- 28. Reilly Associates WPAI Scoring [Internet]. Margaret Reilly Associates, Inc; 2002. [cited 2019 May 1]. Available from: http://www.reillyassociates.net/WPAI_Scoring.html [Google Scholar]
- 29. Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428‐432. [DOI] [PubMed] [Google Scholar]
- 30. Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: Results from the American Migraine Prevalence and Prevention study. Headache. 2007;47:355‐363. [DOI] [PubMed] [Google Scholar]
- 31. Landy SH, Tepper SJ, Schweizer E, Almas M, Ramos E. Outcome for headache and pain‐free nonresponders to treatment of the first attack: A pooled post‐hoc analysis of four randomized trials of eletriptan 40 mg. Cephalalgia. 2014;34:376‐381. [DOI] [PubMed] [Google Scholar]
- 32. Oliver RL, Taylor A. Treatment‐resistant migraines. Pract Pain Manag. 2012;4 Available at https://www.practicalpainmanagement.com/pain/headache/migraine/treatment-resistant-migraines. Accessed May 4, 2020. [Google Scholar]
- 33. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 34. Lombard L, Schroeder K, Nichols R, Kar‐Chan Choong C, Ye W. Characteristics, treatment patterns, and healthcare resource utilization in patients with migraine who initiated a triptan. Headache. 2018;58(Suppl. 2):182‐183. [Google Scholar]
- 35. D’Amico D, Grazzi L, Curone M, et al. Difficulties in work activities and the pervasive effect over disability in patients with episodic and chronic migraine. Neurol Sci. 2015;36(Suppl. 1):S9‐S11. [DOI] [PubMed] [Google Scholar]
- 36. Ho AP, Dahlof CG, Silberstein SD. Randomized, controlled trial of telcagepant over four migraine attacks. Cephalalgia. 2010;30:1443‐1457. [DOI] [PubMed] [Google Scholar]


