SUMMARY
The broad host range necrotrophic fungus Sclerotinia sclerotiorum is a devastating pathogen of many oil and vegetable crops. Plant genes conferring complete resistance against S. sclerotiorum have not been reported. Instead, plant populations challenged by S. sclerotiorum exhibit a continuum of partial resistance designated as quantitative disease resistance (QDR). Because of their complex interplay and their small phenotypic effect, the functional characterization of QDR genes remains limited. How broad host range necrotrophic fungi manipulate plant programmed cell death is for instance largely unknown. Here, we designed a time‐resolved automated disease phenotyping pipeline enabling high‐throughput disease lesion measurement with high resolution, low footprint at low cost. We could accurately recover contrasted disease responses in several pathosystems using this system. We used our phenotyping pipeline to assess the kinetics of disease symptoms caused by seven S. sclerotiorum isolates on six A. thaliana natural accessions with unprecedented resolution. Large effect polymorphisms common to the most resistant A. thaliana accessions identified highly divergent alleles of the nucleotide‐binding site leucine‐rich repeat gene LAZ5 in the resistant accessions Rubezhnoe and Lip‐0. We show that impaired LAZ5 expression in laz5.1 mutant lines and in A. thaliana Rub natural accession correlate with enhanced QDR to S. sclerotiorum. These findings illustrate the value of time‐resolved image‐based phenotyping for unravelling the genetic bases of complex traits such as QDR. Our results suggest that S. sclerotiorum manipulates plant sphingolipid pathways guarded by LAZ5 to trigger programmed cell death and cause disease.
Keywords: quantitative disease resistance, fungal pathogen, Sclerotinia sclerotiorum, NBS‐LRR, plant phenotyping, technical advance
Significance Statement
We developed an image‐based method to measure plant quantitative disease resistance over time with high resolution and throughput. We applied it to reveal Arabidopsis LAZ5 as a susceptibility gene to the necrotrophic fungal pathogen Sclerotinia sclerotiorum using only six plant genotypes. LAZ5 belongs to a family of typical resistance genes, suggesting that S. sclerotiorum exploits classical resistance pathways to cause disease.
INTRODUCTION
The fungal pathogen Sclerotinia sclerotiorum is the causal agent of Sclerotinia stem rot (SSR), also designated as white mold disease, on numerous crop and vegetable species, including rapeseed, soybean, sunflower, and tomato. S. sclerotiorum can be among the most damaging pathogens of rapeseed and soybean when conditions are favourable (Peltier et al., 2012; Derbyshire and Denton‐Giles, 2016). S. sclerotiorum penetrates plant tissues through wounds, natural openings, or actively forming compound appressoria (Bolton et al., 2006). It employs a typical necrotrophic strategy to colonize host tissues, rapidly triggering plant cell death (Kabbage et al., 2015; Mbengue et al., 2016). As genetic sources of resistance to SSR are lacking for most crop species, adapted cultural practices, the use of fungicides, and biological control methods are frequently employed to limit damages due to S. sclerotiorum (Derbyshire and Denton‐Giles, 2016). Instead of a clear demarcation between resistant and susceptible genotypes, plants challenged with S. sclerotiorum generally show a continuum of resistance levels designated as quantitative disease resistance (QDR) phenotype (Perchepied et al., 2010; Roux et al., 2014). The molecular bases of QDR in plants remain largely elusive (Poland et al., 2009; Roux et al., 2014). Whereas resistance (R) genes mediating complete disease resistance all belong to the nucleotide‐binding site leucine‐rich repeat (NLR) family, genes underlying QDR discovered to date span a broad range of molecular functions (Poland et al., 2011; Roux et al., 2014; Corwin and Kliebenstein, 2017). Molecular function of genes associated with QDR include for instance transporters (Krattinger et al., 2009), kinases (Fu et al., 2009; Huard‐Chauveau et al., 2013), peptidases (Poland et al., 2011; Badet et al., 2017b), or actin‐related proteins (Moscou et al., 2011). NLRs (Debieu et al., 2015; Lee et al., 2016) and signalling components typically associated with R‐mediated resistance (Iakovidis et al., 2016) can also mediate QDR, illustrating the tight integration of QDR and R‐mediated immunity components.
Because of their complex interplay and their small phenotypic effect, the functional characterization of QDR genes is challenging. With large collections of mutant lines available, studies in the model plant A. thaliana enable the rapid functional characterization of individual candidate QDR genes (Huard‐Chauveau et al., 2013; Corwin et al., 2016; Rajarammohan et al., 2018). Fully quantitative readouts are often required to reveal the contribution of individual A. thaliana genes to QDR against S. sclerotiorum, such as ethylene and ROS detection (Perchepied et al., 2010; Zhang et al., 2013), lesion area measurements, and estimation of fungal biomass (Zhang et al., 2013; Badet et al., 2017b). To this end, quantitative image analysis can be used as a proxy to evaluate disease severity (Baranowski et al., 2015; Mutka et al., 2016; Karisto et al., 2017; Badet et al., 2017b) and the impact of stress on plant fitness (Chen et al., 2014; Nelson et al., 2018; Czedik‐Eysenberg et al., 2018). Increasing the accuracy and robustness of such quantitative phenotyping often involves increasing the number of measurements. This allowed for instance to reveal the role of individual effectors from the bacterial pathogen Xanthomonas axonopodis pv. manihotis in virulence (Mutka et al., 2016). High‐throughput image‐based plant phenotyping proved valuable in crop breeding and for research purposes (Araus and Cairns, 2014; Fahlgren et al., 2015; Coppens et al., 2017). There is therefore a need to improve our ability to generate large datasets of quantitative plant disease measurements at low cost, low footprint, and reduced human intervention (Czedik‐Eysenberg et al., 2018). Because it is generally non‐destructive, image‐based disease measurement also gives access to the dynamics of disease progression. This enabled for instance to study the spatial and temporal distribution of pathogens in plants tissues (Mutka et al., 2016) and distinguish between infected and non‐infected plants long before qualitative symptoms are visible (Czedik‐Eysenberg et al., 2018).
In spite of remarkable progress in methods and technology in recent years, fundamental advances in plant pathology enabled by automated plant phenotyping are still relatively limited. A method for the image‐based phenotyping of plant disease symptoms combining high throughput, high resolution, low footprint, and low cost is currently lacking. This design is crucial to identify novel QDR genes and advance our conceptual understanding of this complex trait. Here, we present the Navautron system, an integrated hardware and software solution capable of automatically calculating disease lesion area over time on detached leaves inoculated by pathogens causing necrotic lesions. As a proof of concept, we compared the susceptibility of sunflower genotypes inoculated by the fungal pathogen S. sclerotiorum and Nicotiana benthamiana leaves inoculated by the oomycete pathogen Phytophthora infestans. In its current design, the Navautron system is able to capture 800 A. thaliana leaves per m2. Its automatic leaf and lesion tracking features allow discriminating disease symptom dynamics with differences in lesion doubling time, as small as 12 min, with nearly no human intervention.
To illustrate how the Navautron system can advance our fundamental understanding in plant pathology, we used it to demonstrate that A. thaliana NLR gene LAZ5 (At5g44870), but not its close relative constitutive shade avoidance 1 (CSA1), confers susceptibility to S. sclerotiorum. For this, we precisely assessed the kinetics of disease caused by seven S. sclerotiorum strains on six A. thaliana natural accessions. We found that the speed of lesion growth was highly dependent on the host plant genotype and a good indicator of QDR level. We hypothesized that large effect polymorphisms common to the two most resistant A. thaliana accessions, but absent from the four other accessions, would point towards disease susceptibility genes. We identified highly divergent alleles of LAZ5 in the resistant accessions Rubezhnoe and Lip‐0, while identical alleles existed in the other A. thaliana accessions analyzed. As expected, two LAZ5‐deficient mutant lines in the Col‐0 genetic background showed enhanced QDR to S. sclerotiorum, whereas plants mutated in the closely related CSA1 gene responded like the wild‐type. Considering that the ectopic activation of LAZ5 triggers cell death (Palma et al., 2010), our results suggest that the broad host range necrotrophic fungus S. sclerotiorum exploits this R gene induced plant cell death to its benefit.
RESULTS
Quantification of disease resistance by automated time‐resolved image analysis
To generate massive, quantitative, and kinetic measurements of disease caused by S. sclerotiorum, we designed mobile imaging cabinets (Navigable automatized phytotron, Navautron) and the associated automatized image analysis pipeline for detached leaves (Figure 1a). A typical phenotyping experiment on detached leaves inoculated by S. sclerotiorum involves: (i) plants cultivation in growth chambers, (ii) leaves cutting, inoculation and display, (iii) automated imaging every 10 min over 3 days (36 h are usually sufficient), (iv) automated recognition of disease lesions on individual leaves, and (v) estimation of disease parameters. A Navautron unit (Figures 1b and S1) is composed of a poly(methyl‐methacrylate) box of internal dimensions 53 (length) × 38.6 (width) × 45 (height) cm equipped with a Raspberry Pi microcomputer, a high‐definition (HD) camera, and a light‐emitting diode (LED) flashlight. The low footprint of this system allows using it in various environments such as growth chambers, growth cabinets, and incubators. It can be assembled from widely available standard parts making it very affordable (typically under 300€ per unit). A single Navautron unit allows imaging simultaneously up to 120–270 detached leaves of 4‐ to 5‐week‐old A. thaliana (Figure S1). Alternatively, although not optimized for this application, the system can image 24–32 whole A. thaliana plants simultaneously. A high‐definition webcam located at the centre of the lid of Navautron boxes is set to image the bottom tray carrying inoculated leaves every 10 min. We designed a bioinformatics pipeline to extract automatically the size of disease lesions and parameters describing disease kinetics. The first step of the analysis pipeline consists in a python script called INFEST (kINematic oF lESion development) which calculates lesion areas for each leaf in a series of images (Figure 1c and Boxes 1 and 2). The script requires: (i) a time series of images taken by a Navautron box, all located in the same folder, and (ii) a layout text file providing an identifier and bounding rectangle coordinates for each leaf. For a typical experiment with A. thaliana and S. sclerotiorum (120 leaves imaged for 36 h), a single Navautron will yield c. 26 000 lesion measurements with almost no human intervention.
Figure 1.
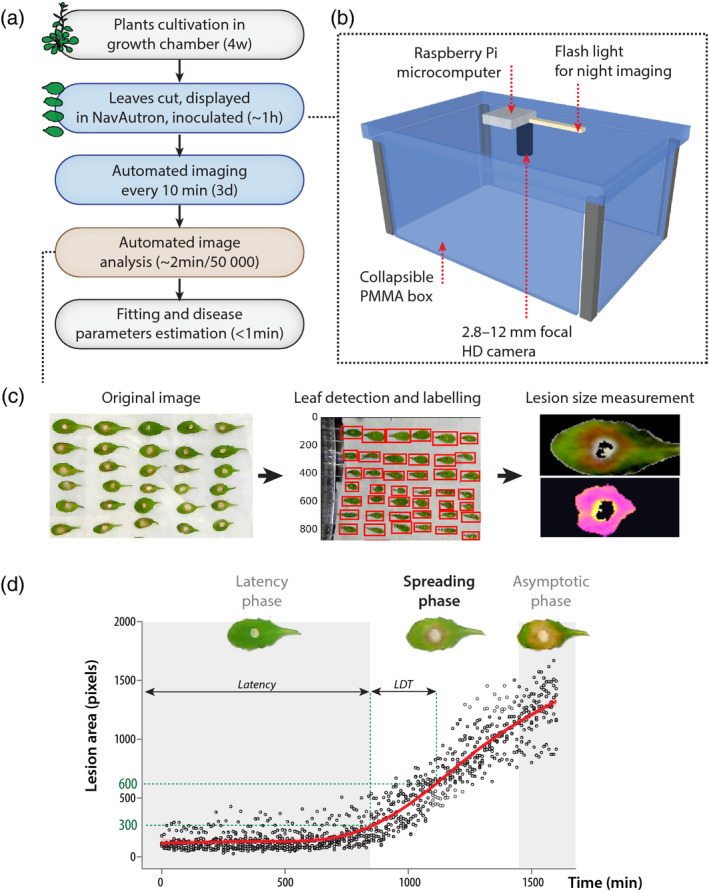
Analysis of quantitative disease resistance (QDR) against Sclerotinia sclerotiorum with the Navautron system.
(a) Pipeline describing the experiments reported in this manuscript. Detached leaves were analyzed with the Navautron through automated imaging, automated image analysis, curve fitting, and QDR parameters estimation. Approximate duration for each step is indicated (d, days; w, weeks).
(b) The Navautron setup: Each Navautron consists of a transparent plastic box (poly(methyl‐methacrylate), PMMA) equipped with a Raspberry Pi microcomputer, a high‐definition (HD) camera and a LED flash light.
(c) The three major steps of the automated image analysis.
(d) Typical kinetics of S. sclerotiorum disease lesion development on A. thaliana, illustrating the latency phase, spreading phase, and asymptotic phase. Characteristic values are the duration of latency phase and the lesion doubling time (LDT). Data shown correspond to values collected on five leaves of A. thaliana Col‐0, the red curve shows fitted average.
Box 1. Installation guide for INFEST, the Navautron image analysis tools.
A complete tutorial and updates can be found at https://github.com/A02l01/INFEST. Infest requires python and conda installed on your machine. Major steps of the installation procedure are:
-
1
Clone the infest repository
$ git clone https://github.com/A02l01/INFEST.git
-
2
Create and activate the INFEST conda environment using the yaml file
$ conda env create ‐n INFEST ‐f env_Infest.yml
-
3
Analyze pictures contained in a directory after activating INFEST conda environment
$ conda activate INFEST $ python infest.py path_to_picture.
Numbers of the first and last picture to analyze could be specified e.g.
$ python infest.py path_to_picture ‐f 0 ‐l 400.
Infest command line
usage: infest.py [‐h] [‐f FIRST] [‐l LAST] mpath positional arguments: mpath Path to the directory containing pictures optional arguments: ‐h, ‐‐help show this help message and exit ‐f FIRST, ‐‐first FIRST Number of the first picture ‐l LAST, ‐‐last LAST Number of the last picture
Box 2. User guide for disease lesion analysis with the INFEST script.
Pre‐requisites: Image files composing a time series must all be located in the same directory with images named by an integer e.g. 1.jpg to N.jpg in chronological order (https://github.com/A02l01/tuto/tree/master/data_tuto/pictures for example). Gaps between pictures are admitted.
-
1
Determine bounding rectangles coordinates for each leaf (in pixels). To this end, we recommend opening one image file in ImageJ software, tracing a rectangular ‘Region of Interest’ around each leaf and retrieving the corresponding coordinates. For accurate lesion size measurement, leaves position must remain identical through the whole duration of the experiment (usually the case when images are acquired through the Navautron system) and that possible distortion due to camera lens are corrected.
-
2
Create a grid_layout/ subdirectory in the image directory
-
3
Create a grid_layout.layout file within the grid_layout/ directory. This should be text file containing one line per leaf to be analyzed, and on each line five items separated by tabs: (i) a leaf identifier, (ii) the minimum coordinate of the bounding rectangle on y‐axis, (iii) the minimum coordinate of the bounding rectangle on x‐axis, (iv) the maximum coordinate of the bounding rectangle on y‐axis, and (v) the maximum coordinate of the bounding rectangle on x‐axis. For an example of the layout file, see the Github page of the INFEST script here: https://github.com/A02l01/tuto/tree/master/data_tuto/pictures/grid_layout. Note that placing leaves at the same position when setting up experiments greatly accelerates this step.
-
4
Run the INFEST script in conda environment with command
$ conda activate INFEST $ python infest.py path_to_picture ‐f first_picture ‐l last_picture
where
path_containing_images is the full path of the directory containing pictures (e.g. /home/foo/bar/). Pictures must be named by an integer corresponding to time (e.g. 1.jpg, 100.jpg). path_containing_images is a positional argument.
‐f first (optional, default min(image_name)) is an integer corresponding to the first image to consider.
‐l last (optional, default max(image_name)) is an integer corresponding to the last image to consider.
-
5
The output will be an analysis of the .txt file created in the path_containing_images directory containing three columns: the Id of leaf, the time extracted from pictures name and the lesions size.
-
6
Deactivate conda environment
$ conda deactivate
A complete tutorial and updates can be found at https://github.com/A02l01/INFEST
We distinguished three major phases along the kinetics of lesions development (Figure 1d): (i) a latency phase, during which no necrotic lesion was detected, (ii) a spreading phase, during which the area of necrotic lesions grew exponentially to reach (iii) an asymptotic phase, when lesions have spread over the whole leaf surface. The overall kinetics are therefore depicted by a logistic function. The asymptotic phase was dependent only on leaf size which sets the value of the asymptote and then did not provide information on disease progression. Although exponential, the spreading phase can be approximated classically by a polynomial function through Taylor’s development. The disease lesion doubling time (LDT, in minutes) is deduced from the slope of the lesion size curve during the spreading phase. Importantly, the LDT is determined at the beginning of the spreading phase, before lesions reach the edge of infected leaves, to reduce bias due to leaf shape and size. The LDT is the characteristic value for the infection since it is sufficient to characterize completely the spreading phase.
The computation of an accurate LDT required the post‐treatment of the kinematics of lesion obtains by image analysis (Box 3). To limit the potential effect of noise and the rate of image acquisition on the computation of the LDT, kinematics of lesion development was first fitted by a 4‐degree polynomial. Note that the fit of the lesion kinematics allows also the computation of the LDT even if a few data points are missing. As A. thaliana leaves had areas over 1000 pixels, the LDT was computed as the time required by the infection to increase from 300 to 600 px. For the purpose of comparing kinetics, we recommend the transformation by natural logarithm Log(LDT), yielding a distribution closer to normality and increasing the validity of the associated statistical analyses.
Box 3. User guide for the computation of the LDT by the fit_INFEST script.
usage: fit_INFEST.py [‐h] [‐ft FIRST] [‐g] path_in path_out.
positional arguments
path_inthe path to the file containing temporal data computed by INFEST
path_outthe path to the file containing LDT and Latency
optional arguments
‐h, ‐‐help show this help message and exit
‐ft FIRST, ‐‐first FIRST the first time to consider to compute the LDT
‐g, ‐‐graph monitoring the fit of the curve
Pre‐requisites:
an analyse.txt file computed by the infest script.
-
1
activate INFEST conda environment
$ conda activate INFEST
-
2
Run fit_INFEST script
$ python fit_INFEST.py path_to_picture/analyse.txt path_to_somewhere/ldt.txt ‐g ‐ft 400
Assessment of the Navautron system versatility
Our lesion detection algorithm is based on the intensity of the red channel relative to the green channel in leaf images, which proved successful in detecting disease lesions caused by S. sclerotiorum on A. thaliana. To verify that this approach is not sensitive to the physiology of detached leaves, we compared lesion detection in A. thaliana leaves inoculated by S. sclerotiorum and by a sterile agar plug as a mock control (Figure 2a). The signal collected from mock inoculated leaves remained very low for the whole duration of the experiment, allowing to discriminate unambiguously disease symptoms from general stress in detached leaves. To verify that our lesion detection algorithm is not sensitive to the type of leaf we first analyzed the kinetics of S. sclerotiorum lesion development on two sunflower (Helianthus annuus) genotypes. Previous field trials have reported that S. sclerotiorum induced larger lesions on sunflower genotype XRQ than on genotype PSC8, which was selected for capitulum resistance to S. sclerotiorum (Bert et al., 2002). We quantified S. sclerotiorum LDT on A. thaliana Col‐0 accession and sunflower genotypes XRQ and PSC8 using the Navautron system (Figure 2b). Lesions were detected reliably on both plant species with the same settings. We obtained an average Log(LDT) = 6.00 (doubling time 6.91 h) on A. thaliana Col‐0. Log(LDT) was 5.84 (doubling time 6.31 h) on the resistant sunflower genotype PSC8 and 5.40 (doubling time 4.54 h) on the susceptible sunflower genotype XRQ (Wilcoxon’s test P‐value = 3.51e−08). Second, we analyzed images of Nicotiana benthamiana leaves inoculated by the oomycete pathogen Phytophthora infestans obtained from (Bozkurt et al., 2014), taken with a conventional digital camera. We applied the INFEST algorithm to measure the size of lesions 8 days post inoculation on lines accumulating the REM1.3 protein to different levels (Figure 2c). Lesions were on average c. 5.2 times larger on lines overexpressing REM1.3 (OX) than on wild‐type (WT) (Wilcoxon’s test P‐value = 7.40e−07). By contrast, lesions were c. 1.8‐fold smaller on plants silenced for REM1.3 (VIGS) than on wild‐type (P‐val = 0.0023). These results correctly recapitulated the findings by that REM1.3 accumulation enhances P. infestans infection. We conclude that our plant phenotyping setup is suitable to track necrotic lesions caused by diverse pathogens over time on multiple plant species and should prove useful to progress in our understanding of the mechanisms underlying plant–pathogen interactions.
Figure 2.
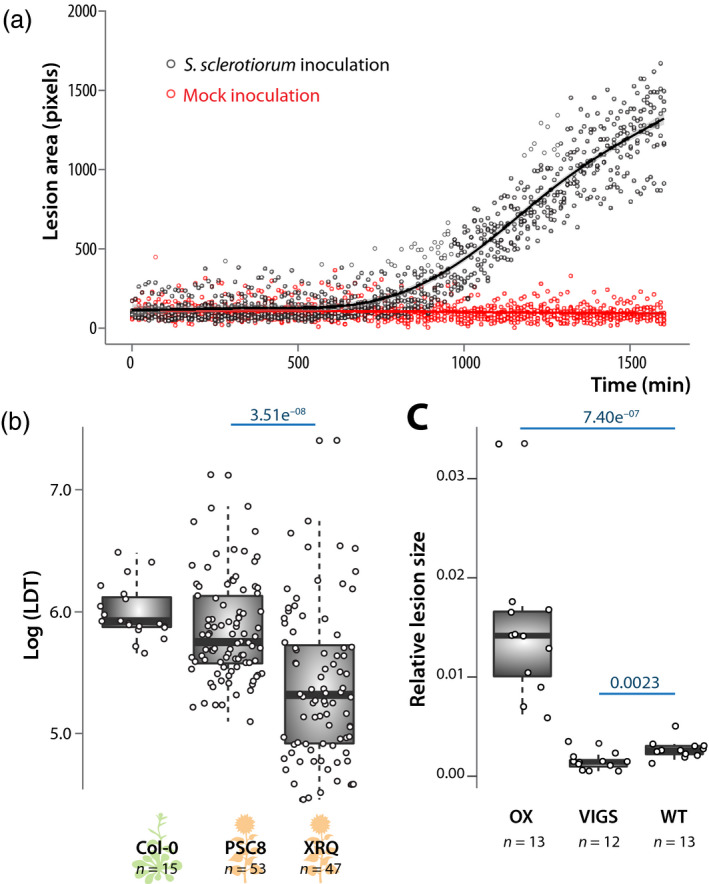
Assessment of sensitivity and versatility of the Navautron system for quantifying plant disease symptoms.
(a) Kinetics of disease lesion development of leaves inoculated by S. sclerotiorum (black) and mock inoculated (sterile agar plug, red). Data shown correspond to values collected on five leaves of A. thaliana Col‐0, lines show fitted average.
(b) Lesion doubling time (LDT) measured on leaves of A. thaliana Col‐0 accession and sunflower PSC8 and XRQ genotypes after inoculation by Sclerotinia sclerotiorum. LDT was measured on n = 15 to 53 mature leaves per genotype in three independent biological replicates. Statistical difference between LDT on the two sunflower genotypes was assessed by Student’s t‐test with P‐value indicated in blue.
(c) Relative size of necrotic lesions measured on leaves of Nicotiana benthamiana plants overexpressing the REM1.3 remorin protein (OX), silenced for rem1.3 by virus‐induced gene silencing (VIGS), and wild‐type (WT), after inoculation by Phytophthora infestans. Measurements were performed on 12 or 13 infection sites per genotype. Statistical difference between LDT was assessed using a Wilcoxon’s test with P‐value indicated in blue. Boxplots show 1st and 3rd quartiles (box), median (thick line) and the most dispersed values within 1.5 times the interquartile range (whiskers).
Grouping of plant and fungal genotypes according to QDR phenotypes
To document the diversity of QDR phenotypes in the A. thaliana–S. sclerotiorum pathosystem, we quantified the area of necrotic lesions caused by seven isolates of S. sclerotiorum (Badet et al., 2017a) on detached leaves from six natural accessions of A. thaliana (Figure 3a). In the 42 interactions tested, the duration of the latency phase and the speed of lesion size increase during the spreading phase were independent (R 2 = 0.07). The duration of the latency phase was mainly dependent on S. sclerotiorum genotype (P = 1.2 × 10−21) with a significant but weaker effect of plant genotype (P = 2.95 × 10−4). This suggested that the latency phase was primarily determined by fungal strains virulence. Necrotic disease lesions were detected after a latency phase of 1 to 1.5 days in average, mostly determined by S. sclerotiorum genotypes. Post hoc pairwise t‐tests with Benjamini–Hochberg P‐value correction (Benjamini and Hochberg, 1995) revealed four groups of S. sclerotiorum isolates with distinct latency phases (Figure 3b). Isolates 1980 and p314 had the shorter latency phase (average 22.6 and 23.2 h), isolates FrB5 and C014 had an intermediate latency phase (average 24.4 and 25.5 h), isolates C104 and S55 had a longer latency phase (average 26.4 and 26.7 h) and isolate P163 had the longest latency phase (29.8 h). The smallest significant difference in latency phase that could be detected in this experiment was about 1h. The ranking of isolates according to their latency phase was identical on all A. thaliana accession and therefore not dependent on the plant genotype.
Figure 3.
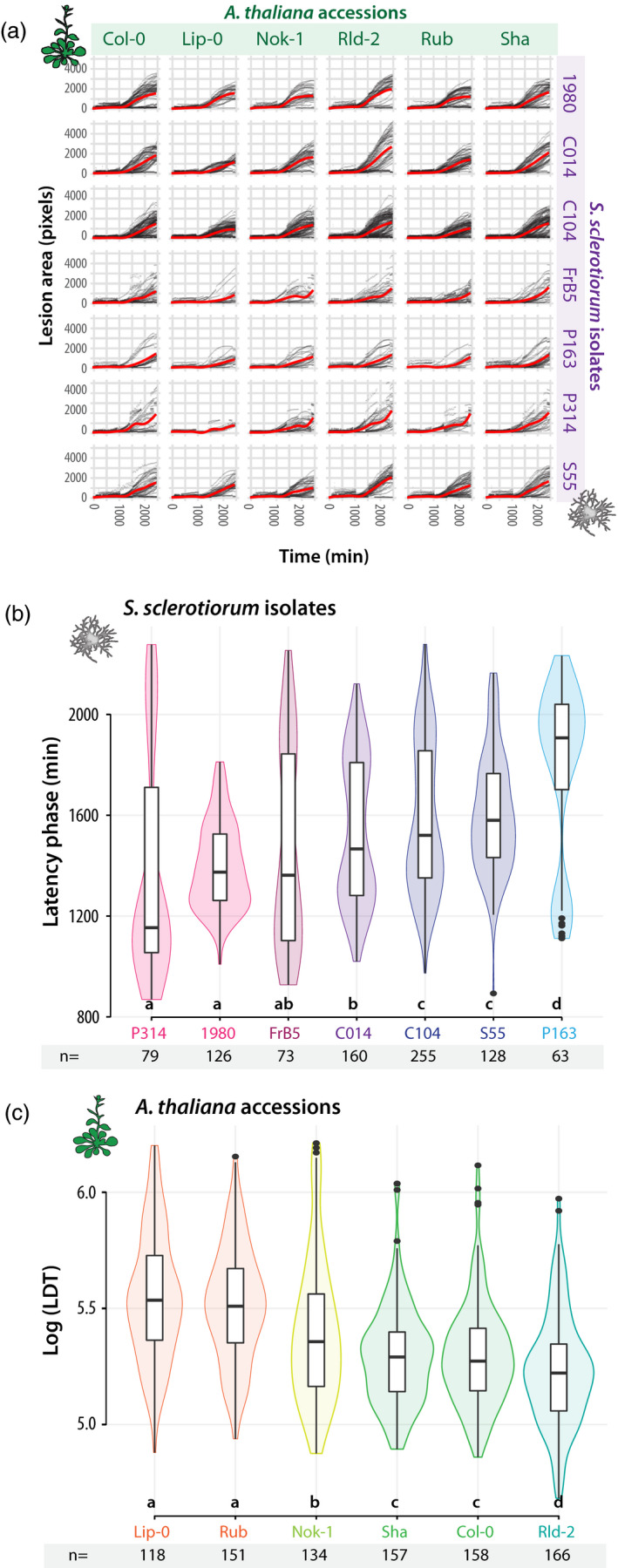
Characteristic values describing disease symptom dynamics in the interaction between seven Sclerotinia sclerotiorum isolates and six A. thaliana accessions.
(a) Kinetics of disease lesion development for 42 different combinations of A. thaliana natural accessions (columns) and S. sclerotiorum isolates (lines). Red curves show smooth fitting curves for 1500 to 12 250 measurements.
(b) The duration of latency phase (Y‐axis) was mostly dependent on S. sclerotiorum isolates (x‐axis), ranked from the most (P314) to the least virulent (P163). Duration of the latency phase was measured n = 63 to 255 times for each isolate.
(c) The lesion doubling time (LDT, y‐axis) was mostly dependent on A. thaliana accessions (y‐axis), ranked from the most (Lip‐0) to the least resistant (Rld‐2). LDT was measured n = 118 to 158 times on each accession. Letters and colours indicate groups of significance determined by post hoc pairwise t‐tests. Boxplots show 1st and 3rd quartiles (box), median (thick line) and the most dispersed values within 1.5 times the interquartile range (whiskers).
By contrast with latency, Log(LDT) was mostly dependent on the A. thaliana genotype (P = 7.16.10−37) and weakly function of S. sclerotiorum isolates (P = 2.39.10−3). We conclude that the Log(LDT) provides an appropriate measure of QDR to S. sclerotiorum. The average LDT ranged between 3.08 and 4.24 h, corresponding to Log(LDT) of 5.22 to 5.54 (Figure 3c), mostly determined by A. thaliana genotypes. Post hoc pairwise t‐tests with Benjamini–Hochberg P‐value correction (Benjamini and Hochberg, 1995) revealed three groups of resistance (Figure 3c). With an average Log(LDT) 5.55 and 5.51, Lip‐0 and Rubezhnoe (Rub) accessions were substantially more resistant than Nok‐1, Col‐0 and Shahdara (Sha) (average Log(LDT) 5.40, 5.30 and 5.30 respectively), whereas Rld‐2 exhibited the highest susceptibility to any S. sclerotiorum strain tested (average Log(LDT) 5.23). The smallest significant difference in LDT that could possibly be detected in this experiment was 13.3 min (Log = 0.069). The ranking of A. thaliana accessions according to LDT was identical with all S. sclerotiorum genotypes. Although the precise ranking of accessions based on LDT differs from the ranking obtained previously with disease severity index on whole plants with S. sclerotiorum strains S55 (Perchepied et al., 2010) and 1980 (Badet et al., 2017b), we consistently found Lip‐0 and Rub among the most resistant accessions and Rld‐2 among the most susceptible ones.
Identification of candidate QDR genes in A. thaliana by an association approach
We hypothesized that enhanced QDR in Lip‐0 and Rub accessions could result from disruptive mutations in QDR‐relevant genes. To rapidly pinpoint such candidate QDR genes, we screened the genome of A. thaliana for genes harbouring non‐synonymous mutations both in Lip‐0 and Rub accessions but not in any of the other four accessions analyzed (Nok‐1, Col‐0, Sha, and Rld‐2) (Figure 4a). For this, we screened 213 624 positions genotyped in the six A. thaliana accessions selected (Atwell et al., 2010). We found a total 57 716 single nucleotide polymorphisms (SNPs) present both in Lip‐0 and Rub, among which 2312 were not found in Nok‐1, Rld‐2, or Sha (unique to Rub and Lip‐0). Among those, 298 were non‐synonymous SNPs unique to Lip‐0 and Rub. The density of non‐synonymous SNPs unique to Lip‐0 and Rub accessions per gene followed an exponentially decreasing distribution (Figure 4b). Three genes harboured at least four non‐synonymous SNPs in Lip‐0 and Rub but not any non‐synonymous SNP in Nok‐1, Sha, or Rld‐2 (Figure 4c and Table 1). This included AT1G23935, encoding an uncharacterized protein with similarity to apoptosis inhibitory proteins, AT2G33090, encoding an uncharacterized member of the transcription elongation factor IIS family, and AT5G44870 encoding LAZ5, a disease resistance protein of the TIR‐NBS‐LRR (NLR) family with similarity to RPS4 and CSA1 (Palma et al., 2010). In a suppressor screen, single point mutations in LAZ5 resulted in a dominant negative phenotype (Palma et al., 2010). LAZ5 is expressed and induced 2.32 fold during the infection of A. thaliana by S. sclerotiorum (Badet et al., 2017b), and was given the highest priority for functional validation.
Figure 4.
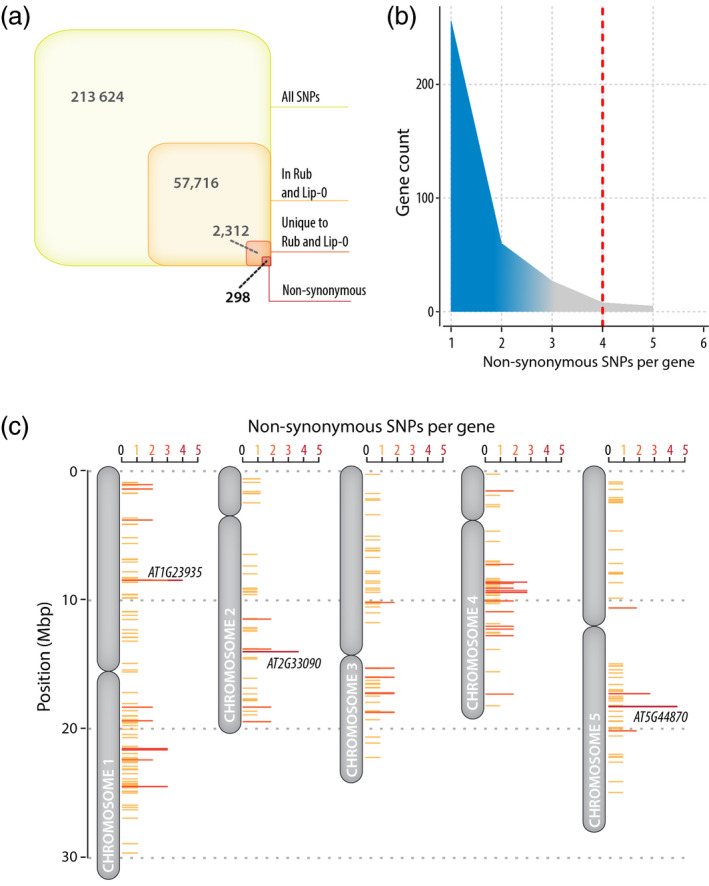
Distribution of single nucleotide polymorphisms (SNPs) in selected A. thaliana accessions and identification of candidate disease‐relevant genes.
(a) Distribution of SNPs genotyped by (Atwell et al., 2010) through our pipeline for finding candidate disease‐relevant genes. There were 2312 SNPs common to Lip‐0 and Rub but not present in Nok‐1, Rld‐2, and Sha, among which 298 were non‐synonymous SNPs.
(b) Number of non‐synonymous SNP per gene in the list of 298 SNPs identified in (a). We report on the three genes including at least four non‐synonymous SNPs in Lip‐0 and Rub but no SNP in Nok‐1, Rld‐2, and Sha (red dotted line).
(c) Map of A. thaliana chromosomes showing genes with non‐synonymous SNPs in Lip‐0 and Rub but no SNP in Nok‐1, Rld‐2, and Sha. Genes discussed in the text are labelled on the figure.
Table 1.
List of genes harbouring at last four non‐synonymous SNPs in Lip‐0 and Rub but none in Nok‐1, Sha, and Rld2
| Gene ID | Description | Chr. | SNP pos. | From | to | SNP type | Gene LFC |
|---|---|---|---|---|---|---|---|
| AT1G23935 | Apoptosis inhibitory protein | 1 | 8462159 | C | G | not_syn |
3.93 P = 0 |
| 1 | 8462342 | C | G | not_syn | |||
| 1 | 8463263 | T | C | not_syn | |||
| 1 | 8465002 | G | A | not_syn | |||
| AT2G33090 | Transcription elongation factor (TFIIS) family protein | 2 | 14035515 | C | G | not_syn |
2.97 P = NA |
| 2 | 14035545 | G | T | not_syn | |||
| 2 | 14035721 | C | G | not_syn | |||
| 2 | 14035944 | C | G | not_syn | |||
| AT5G44870 | Disease resistance protein (TIR‐NBS‐LRR class) family (LAZ5) | 5 | 18115125 | T | C | not_syn |
1.21 P = 0 |
| 5 | 18115243 | A | G | not_syn | |||
| 5 | 18116419 | C | G | not_syn | |||
| 5 | 18116946 | A | G | not_syn | |||
| 5 | 18118097 | T | A | not_syn |
Chr., chromosome; LFC, Log2 fold gene of gene expression upon inoculation by S. sclerotiorum, with the adjusted P‐value for differential expression. NA, not applicable; pos. position.
Disruption of LAZ5 increases quantitative resistance to S. sclerotiorum
To test for a role of LAZ5 as a susceptibility gene against S. sclerotiorum, we analyzed the phenotype of two LAZ5 insertion mutant lines in the Col‐0 background during infection with S. sclerotiorum 1980. The laz5‐1 null mutant (SALK_087262C) carries a T‐DNA insertion in the second exon of LAZ5 and shows dominant suppression of autoimmune cell death in an acd11‐2 background (Palma et al., 2010). The laz5‐3 mutant (SALK_068316) carries a T‐DNA insertion c. 300 bp upstream of LAZ5 start codon (Figure 5a andData S1). Using our Navautron system, we also determined the resistance to S. sclerotiorum in csa1‐2 mutant plants defective in AT5G17880, a TIR‐NBS‐LRR gene closely related to LAZ5 (Faigón‐Soverna et al., 2006). As opposed to laz5‐1, the csa1‐2 mutant showed enhanced susceptibility to avirulent strains of the bacterial pathogen Pseudomonas syringae (Faigón‐Soverna et al., 2006; Palma et al., 2010). Consistent with our previous measurements (Figure 3), the average Log(LDT) was 5.35 on Col‐0, similar to the Log(LDT) on the csa1‐2 mutant (Student’s t‐test P‐value = 0.68) (Figure 5b). The laz5‐1 and laz5‐3 mutants had an average Log(LDT) of 5.5 and 5.44, significantly higher than Col‐0 (P‐value = 1.3e−05 and 0.01 respectively). This represented an average LDT of 3.51 h in Col‐0 and 4.08 h in laz5‐1, equivalent to a 16% gain of QDR in the laz5‐1 mutants compared with wild‐type. The automated analysis of 74–104 plants per genotype thanks to the Navautron setup allowed assessing this quantitative variation robustly. In agreement with our previous measurements (Figure 3), the Lip‐0 accession showed an average Log10(LDT) of 5.7, significantly higher than the laz5 mutants (P‐value = 3.0e−07 and 1.6e−10).
Figure 5.
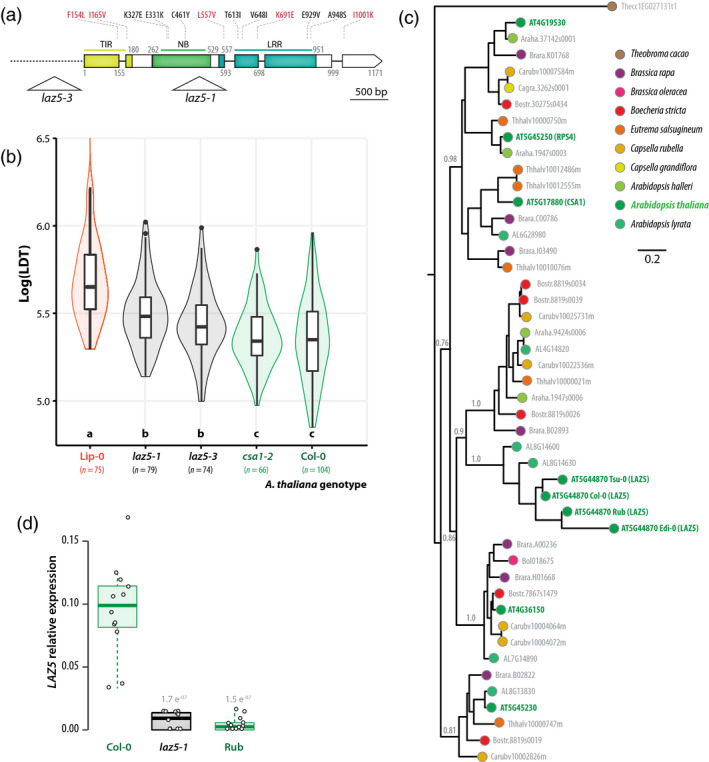
Disruption of the NLR gene LAZ5 reduces lesion doubling time upon Sclerotinia sclerotiorum challenge.
(a) Schematic map of the LAZ5 gene showing the position of T‐DNA insertion in the laz5‐1 and laz5‐3 mutant lines (triangles). Exons are shown as boxes, introns as plain lines, upstream non‐coding region as a dotted line. Domains encoded by exons are colour coded and labelled TIR, NB, and LRR. Positions are given as amino acid numbers. Non‐synonymous mutations known in Lip‐0 allele are indicated above boxes, in red when present in (Atwell et al., 2010), in black otherwise.
(b) Lesion doubling time (LDT, Y‐axis) in the most resistant accession Lip‐0, two laz5 mutant lines, the csa1‐2 mutant, and Col‐0 wild‐type. LDT was measured n = 74 to 104 times on each accession. Letters and colours indicate groups of significance determined by post hoc pairwise t‐tests.
(c) Phylogenetic relationship of LAZ5 and its 42 closest homologues in the phytozome 12.1 database. LAZ5 closest homologue outside of the Brassicaceae family (Theobroma cacao 1EG027131) was included as outgroup, and alleles from A. thaliana Edi‐0, Rub, and Tsu‐0 natural accessions to represent infraspecific diversity. The tree obtained by a maximum likelihood analysis is shown, with the number of substitution per site used as branch length, and branch support determined by an approximate likelihood‐ratio test (grey labels). Terminal nodes are colour coded according to plant species.
(d) Relative expression of the LAZ5 gene determined by quantitative RT‐PCR in healthy plants. Values shown correspond to three independent biological samples analyzed through four technical repeats each. Statistical difference from expression in Col‐0 plants was assessed with Student’s t‐tests. Boxplots show 1st and 3rd quartiles (box), median (thick line) and the most dispersed values within 1.5 times the interquartile range (whiskers).
In the dataset from (Atwell et al., 2010), five non‐synonymous SNPs were found in the TIR domain, the LRR domain and the C‐terminal extension of LAZ5 (Figure 5a). Data from http://signal.salk.edu/atg1001/ revealed seven additional non‐synonymous SNPs in the NB and LRR domains of the Lip‐0 allele of LAZ5 and indicated that several accessions, including Rub and Edi‐0, harboured large deletions within LAZ5 coding sequence. To document natural diversity at the LAZ5 locus, we analyzed phylogenetic relationships between A. thaliana Col‐0 LAZ5 and its 42 closest homologues retrieved from the Phytozome 12.1 database (Goodstein et al., 2012) (Figure 5c and Data S2). We included the closest homologue retrieved outside the Brassicaceae family (Theobroma cacao 1EG027131) as outgroup, as well as alleles from A. thaliana Edi‐0, Rub, and Tsu‐0 natural accessions to represent infraspecific diversity. LAZ5 belonged to a monophyletic clade clearly separated from its closest A. thaliana relatives CSA1, RPS4, At4G19530, At4G36150, and At5G45230. LAZ5 clade was only represented in A. thaliana and A. lyrata, while its sister clade is represented in most Brassicaceae species but not A. thaliana. Together with long tree branches in LAZ5 clade, this indicates strong sequence divergence among Arabidopsis accessions at the LAZ5 locus. We used quantitative RT‐PCR to assess the expression of three LAZ5 alleles in healthy A. thaliana plants (Figure 5d and Data S1). We found that LAZ5 expression was strongly impaired in the laz5.1 mutant (c. 13‐fold reduction, Student’s t‐test P‐value 1.7e−07) and in Rub natural accession (c. 21.3‐fold reduction, P‐value 1.5e−07). These results indicate that reduced expression of mutant and natural alleles of LAZ5 correlate with enhanced QDR to S. sclerotiorum and suggest that the Rub allele of LAZ5 diverged functionally from its Col‐0 counterpart. We conclude that disruption of the LAZ5 gene contributes to the enhanced QDR against S. sclerotiorum measured in Lip‐0 and Rub accessions compared with Col‐0. This identifies LAZ5 as a TIR‐NBS‐LRR gene candidate conferring susceptibility to S. sclerotiorum through its Col‐0 allele.
DISCUSSION
Quantitative disease resistance is a complex trait governed by multiple genes of small to moderate effect (Poland et al., 2009; Roux et al., 2014; Corwin and Kliebenstein, 2017). Revealing the phenotypic contribution of such small‐effect genes challenges our ability to quantify precisely and robustly the level of QDR in diverse plant genotypes. In Botrytis cinerea interaction with A. thaliana, most plant genes were associated with QDR against a specific B. cinerea isolate (Corwin et al., 2016), emphasizing pathogen genetic diversity as a determinant of QDR phenotype. Previous studies used disease index (Perchepied et al., 2010; Rajarammohan et al., 2018), ethylene production (Zhang et al., 2013), camalexin production (Corwin et al., 2016), and lesion area (Corwin et al., 2016; Badet et al., 2017b) to quantify QDR against necrotrophic fungi in natural accessions and mutants of A. thaliana. Here we chose lesion area measurement to assess QDR for being: (i) a continuous parameter (by contrast with discrete disease indices), (ii) non‐destructive and therefore giving access to disease kinetics, and (iii) amenable to automated image‐based measurement, opening the way to user‐independent high‐throughput quantification.
For the experiments reported in this manuscript, we conducted S. sclerotiorum inoculations and QDR measurement on A. thaliana detached leaves to reach 120 samples analyzed with a single Navautron cabinet. This approach does not provide a complete characterization of plant QDR as discrepancies may exist between detached leaves and whole plant disease resistance (Liu et al., 2007), lesion area may differ from area colonized by the fungus (Kabbage et al., 2015). This could explain differences in the ranking of A. thaliana accessions according to resistance between this study and (Perchepied et al., 2010). Our Navautron pipeline gives access to the kinetics of symptoms development during plant‐fungal pathogen interactions, which offers several advantages over single end‐point measurements. First, it allows uncoupling LDT and latency duration. Since latency appeared mostly independent from plant genotype in our analysis, LDT provides a more direct measurement of plant QDR potential than end‐point measurements. This approach would allow untangling plant and pathogen genetic factors contributing to quantitative immunity (Corwin et al., 2016). Second, the resolution and data richness offered by time‐resolved image‐based phenotyping allows detecting small phenotypic effects that are not accessible to classical approaches, revealing for instance the virulence function of single pathogen effectors (Mutka et al., 2016). Here we could detect robustly variations as small as 1h (about 3.9%) in latency period and 12 min (about 5.24%) in LDT. Finally, LDT is independent of leaf shape and size and allows comparison of QDR in plants with contrasted leaf architectures.
In this initial study, A. thaliana accessions ranked identically for their LDT against seven S. sclerotiorum isolates, arguing against specific plant–pathogen genotype interactions below the species level. Previous studies reported genetic determinants of plant QDR specific of pathogen genotypes (i.e. evidence for ‘pathotypes’) in the interaction of B. cinerea with A. thaliana interaction (Corwin et al., 2016) and S. sclerotiorum with Brassica napus and B. juncea (Ge et al., 2012; Barbetti et al., 2014). These pathotypes may result from the combined effect of fungal genetic determinants of latency duration and plant determinants of LDT. Future experiments will expand the diversity of S. sclerotiorum isolates and A. thaliana accessions screened by time‐resolved phenotyping to search for specific plant × pathogen interactions affecting LDT.
Like most pathogens, fungi secrete molecules, often termed ‘effectors’, to manipulate host physiology and cause disease (Doehlemann et al., 2014; Lo Presti et al., 2015; Schornack et al., 2009). Some pathogen effectors are recognized by specific plant R genes, leading to a rapid and efficient immune response designated as effector‐triggered immunity (ETI) (Dodds and Rathjen, 2010). In some plant–pathogen interactions, plant resistance is triggered only in plant genotypes carrying an R gene enabling the specific recognition of an avirulence effector produced by the pathogen, according to a gene‐for‐gene model (Flor, 1956). However, this model rarely applies to plant interactions with necrotrophic pathogens. ETI often involves the rapid programmed death of host cells at the site of infection, a process designated as the hypersensitive response (HR) (Mur et al., 2008). Although very efficient to control the spread of biotrophic pathogens, the HR can instead favour the colonization of plants by necrotrophic fungi (Govrin and Levine, 2000). A number of necrotrophic fungal pathogen specialized to infect a few plant species evolved effectors designated as host‐specific toxins (HSTs) that trigger cell death specifically in some plant genotypes (Friesen et al., 2008; Oliver and Solomon, 2010). Typical HST examples are the victorin peptides produced by the necrotrophic Victoria Blight fungal pathogen Cochliobolus victoriae. Victorin is critical for C. victoriae virulence, which is mediated through the specific recognition by the plant LOV1 protein belonging to the NLR class (Lorang et al., 2007). Functional and structural analyses of LOV1 suggest that it corresponds to a typical R gene that has been hijacked by C. victoriae to trigger cell death and facilitate infection (Lorang et al., 2012; Wolpert and Lorang, 2016). The wheat Tsn1 gene belongs to the NLR class and recognizes the ToxA peptide produced by the necrotrophic fungus Stagonospora nodorum, conferring effector‐triggered susceptibility (ETS) to this pathogen (Faris et al., 2010). Another effector produced by S. nodorum, SnTox1, triggers programmed cell death when recognized by the wheat gene Snn1 encoding a wall‐associated kinase (WAK) (Liu et al., 2012; Shi et al., 2016). WAKs contribute to resistance against biotrophic and hemibiotrophic fungal pathogens (Zuo et al., 2015; Hurni et al., 2015) providing another example of a biotrophic pathogen defence mechanism hijacked by a specialized necrotrophic fungus. Our knowledge on whether and how broad host range necrotrophic fungi manipulate plant programmed cell death remains nevertheless limited.
The analysis of HR‐deficient Arabidopsis thaliana mutants suggested that the broad host range necrotrophic fungi S. sclerotiorum and Botrytis cinerea can benefit from HR cell death (Govrin and Levine, 2000). Similarly, inactivation of the BIK1 kinase enhances A. thaliana resistance to avirulent bacterial pathogens but increases susceptibility to B. cinerea and Alternaria brassicicola (Veronese et al., 2006). Several molecules secreted by broad host range necrotrophic fungi trigger cell death in plants. Examples include B. cinerea endo‐arabinase BcAra1 (Nafisi et al., 2014), xyloglucanase BcXYG1 (Zhu et al., 2017), the S. sclerotiorum necrosis, and ethylene‐inducing peptide SsNEP1 (Dallal Bashi et al., 2010) or Alternaria tenuissima Hrip1 protein elicitor (Kulye et al., 2012). Cell death induction by these elicitors is often due to direct toxic effects on plant cells independently of the manipulation of plant programmed cell death (Lenarčič et al., 2017). Conversely, the A. thaliana aspartyl protease APCB1 cleaves the BAG6 cochaperone, triggering autophagy, and restricting B. cinerea colonization (Li et al., 2016). This findings points towards a role for autophagy cell death in resistance to necrotrophic fungi (Lai et al., 2011; Lenz et al., 2011) and highlight the complex role of plant programmed cell death in the interaction with necrotrophic pathogens.
Time‐resolved image‐based phenotyping with the Navautron system allowed identifying LAZ5 as a susceptibility gene to S. sclerotiorum. LAZ5 belongs to the TIR‐NBS‐LRR family and is required for ACD11‐mediated cell death (Palma et al., 2010). Overexpression of LAZ5 results in hypersensitive cell death, while LAZ5 mutant alleles suppress the autoimmune phenotype of acd11 mutants (Palma et al., 2010). This analysis identifies LAZ5 as a prime example of a TIR‐NBS‐LRR gene probably controlling susceptibility to a broad host range necrotrophic fungus. Whether effector molecules secreted by S. sclerotiorum interfere directly or indirectly with LAZ5 function remains to be determined. Testing for enhanced resistance of laz5 mutants to diverse S. sclerotiorum isolates and necrotrophic fungal species should be a promising future direction to address this question.
Broad host range fungal pathogens invest a substantial fraction of their cellular energy in secreted proteins to counter plant defences and extract nutrients from plant cells (Badet et al., 2017a). Manipulation of the LAZ5 pathway may be part of S. sclerotiorum strategy to trigger plant cell death actively. Autoimmune mutants such as acd11 were proposed to be altered in functions guarded by NB‐LRR genes such as LAZ5 (Palma et al., 2010; Tong et al., 2017). ACD11 encodes a ceramide‐1‐phosphate transfer protein, which led to the hypothesis that LAZ5 could guard sphingolipid metabolic pathways targeted by pathogen effectors (Simanshu et al., 2014). Necrosis and ethylene‐inducing peptide 1–like (NLP) are toxins secreted by diverse plant pathogens that target plant sphingolipids (Lenarčič et al., 2017). S. sclerotiorum produces NLPs (Dallal Bashi et al., 2010) the activity of which could be guarded by LAZ5. A. thaliana mutants in the dihydrosphingosine‐1‐phosphate lyase AtDLP1 are more resistant to B. cinerea (Magnin‐Robert et al., 2015) supporting the view that sphingolipids are important mediators of QDR against necrotrophic fungi. Alternatively, LAZ5 activity could be costly for plants so that the inactivation of this gene would be beneficial even in the absence of pathogen. However, we did not detect growth defects in laz5 mutants, arguing against this hypothesis. The similar LDT measured in csa1‐2 and wild‐type plants upon S. sclerotiorum inoculation also pleads for a relatively specific role of LAZ5 in QDR against this fungus. CSA1 and RPS4 are two TIR‐NBS‐LRR genes closely related to LAZ5 that confer resistance to avirulent strains of the bacterial pathogen Pseudomonas syringae (Gassmann et al., 1999; Faigón‐Soverna et al., 2006), whereas LAZ5 does not (Palma et al., 2010), supporting LAZ5 specific function.
Inactivation of LAZ5 contributed c. 30% of the reduced LDT in Lip‐0 accession, indicating that Lip‐0 harbours other genetic variants positively affecting QDR. Lip‐0 also shows high QDR against B. cinerea, Plectosphaerella cucumerina, and Fusarium oxysporum (Llorente et al., 2005), making it a useful natural resource for studying the determinants of QDR. The Navautron system is flexible allowing for variations and improvements. Future experiments will include imaging whole plants to detect putative spatial bottlenecks to disease progression, age‐related factors associated with QDR and trade‐offs between QDR and plant growth.
EXPERIMENTAL PROCEDURES
Plant material and cultivation
Six natural accessions of A. thaliana were chosen to cover the range of resistance to S. sclerotiorum (Perchepied et al., 2010): Lip‐0 (CS76542; ecotype ID: 8325), Rubezhnoe‐1 (CS76594; 7323), Nok‐1 (CS78282; 7270), Shahdara (CS78397; 6962), Col‐0 (CS76778; 6909), and Rld2 (CS78349; 7457). Plants were grown at 22°C, 9 h light period at 120 μmol m−2 sec−1 during 4 weeks before inoculation. Arabidopsis insertion mutant lines in the Col‐0 background were obtained from the Nottingham Arabidopsis Stock Centre (NASC). Mutant lines in LAZ5 (AT5G44870) were SALK_087262C (laz5‐1) and SALK_068316 (laz5‐3), the csa1‐2 mutant line was SALK_023219C. Homozygous T‐DNA insertion was verified by PCR for each line following recommendations from the NASC website. Sunflower genotypes PSC8 and XRQ were obtained from the Centre of Biological Resources for sunflower (crb.tournesol-toulouse@inrae.fr) and grown in 22°C, 9 h light for 16 days before inoculation.
Fungal cultivation and inoculation
The seven isolates of S. sclerotiorum used in this work are described in (Badet et al., 2017a). Four isolates (C014, C104, P314, and P163) were obtained from a rapeseed field population collected in Blois (France) in 2010, isolate FrB5 was obtained from (Vleugels et al., 2013), isolate S55 was obtained from (Perchepied et al., 2010) and isolate 1980 is S. sclerotiorum reference strain (Derbyshire et al., 2017). S. sclerotiorum isolates were grown on Potato Dextrose Agar (PDA) plates for 5 days at 23°C in the dark before inoculation. In total, 288–308 combinations of A. thaliana accessions and S. sclerotiorum isolates were distributed in two Navautrons in a fully randomized design. Leaves were cut with a scalpel blade at the time of inoculation, placed adaxial face up on wet paper towel overlaid at the bottom of Navautrons. Inoculations were performed as described in Badet et al. (2017b), using PDA plugs of 5 mm diameter (A. thaliana) and 8 mm diameter (sunflower) colonized by the fungus placed upside down on the adaxial surface of leaves (mycelium in contact with the leaf). The first true leaves were used for experiments with sunflower. The experiment was repeated three times independently and results from the three replicates were combined for analysis. Statistical analyses were performed using R software and the ‘car’ library for type II anova and pairwise t‐test with Benjamini–Hochberg P‐value correction (Benjamini and Hochberg, 1995; R Core Team, 2014). Distributions of LDT were normalized by natural logarithm transformation. Plots were made using the ‘ggplot2’ library (Wickham, 2010).
Design of the Navautron phenotyping cabinets
Five‐mm‐wide PMMA plates were cut on a Trotec Speedy 500 Laser cutting machine according to plans provided as Data S1. Holes were drilled at the centre of the upper panel to place full HD 1080p USB cameras with 2.8–12 mm focal length (model ELP‐USBFHD05MT‐FV‐F1 manufactured by ELP, China). The cameras were plugged to Raspberry Pi 3 Model B motherboards equipped with Waveshare 3.2 inch thin‐film transistor touchscreens. Autofocus and automatic white balance were disabled. For each experiment, pictures were taken every 10 min during 4 days and stored on secure digital cards. To obtain uniform light exposure during image acquisition, a LED flash light is turned on for 5 sec during both day and night time. Day and night images could then be processed identically. Navautrons were placed in Percival AR‐41L2 growth chambers at 22°C with c. 90% humidity under 9 h light period.
Image analysis pipeline
The area of necrotic disease lesions was computed by the INFEST python script available at https://github.com/A02l01/INFEST. Briefly, the script relies on a thresholding method based on colour analysis using the scikit‐image python image analysis toolbox (van der Walt et al., 2014). The method splits images of an infected leaf into three layers corresponding to the background, the lesion, and the leaf. The ‘background’ corresponded to pixels with a saturation value superior or equal to 0.3 in HSV colour space. The lesion corresponded to pixels with red component higher than green value in RGB colour space. The area of disease lesions was computed as the sum of pixels in the ‘lesion’ layer. The remaining pixels were attributed to the ‘leaf’ layer. Image analysis allowed the collection of lesion area values over time. Every kinetics was fitted by a 4‐degree polynomial regression to limit the effect of noise on the computation of the LDT. Fit of the kinematics of lesions development was computed by the numpy polytfit library. The latency time and LDT were extracted directly from the fit. The latency time was defined as the time needed to reach a lesion area of 300 pixels. The LDT was computed as the difference between the time when lesion area reached 600 pixels and the time when lesion area reached 300 pixels. Note that these values have been set for the present data analysis but can be changed easily in python script provided at https://github.com/A02l01/INFEST. Kinetics that exhibited excessive signal to noise ratio were automatically excluded from the analysis as follows: we excluded leaves of size <300 pixels and leaves with a maximum lesion size less than the variance of lesion size during the latency period. About three leaves were excluded per Navautron representing <2.5% of all leaves. Images of N. benthamiana leaves 8 days post inoculation by P. infestans were obtained from (Bozkurt et al., 2014). Detached leaves were inoculated by droplets of c. 500 zoospores of P. infestans strain 88069 tdtomato. Overexpressing plants were stably transformed with a p35S‐YFP:StREM1.3 construct (Raffaele et al., 2009), REM1.3 silencing was achieved by virus‐induced gene silencing (VIGS) with the tobacco rattle virus vector pTV00.
Analysis of diversity and identification of candidate genes
We used a dataset of 213 624 SNPs on 1196 A. thaliana accessions (Atwell et al., 2010; Horton et al., 2012) to search for non‐synonymous mutations present in Lip‐0 and Rubezhnoe accessions but not in Col‐0, Nok‐1, Shahdara, and Rld‐2. The functional consequences of SNPs were predicted automatically using a script derived from the BioPython library (Cock et al., 2009). For this, gene models from tair version 9 were used. Predictions were verified manually for the top candidates using expasy translate tool and http://signal.salk.edu/atg1001/ genome browser. LAZ5 closest homologues were identified using a BlastP search against phytozome 12.1 database. A protein sequence alignment of 350 positions was used as input for phylogenetic tree reconstruction by maximum likelihood method in phylogeny.fr (Dereeper et al., 2008). Branch support was obtained by approximate likelihood‐ratio test using the WAG substitution model, four substitution rate categories, gamma = 1.794, proportion of invariants = 0.041, and gaps automatically removed from alignment. The input sequences, alignment, and resulting tree in newick format are provided in Data S2. Total RNA extraction was performed with Macherey‐Nagel Nucleospin RNA extraction kit following the manufacturer’s instructions. One µg of total RNA was used for cDNA synthesis in a 20‐µl reaction according to Roche Transcriptor Reverse Transcriptase protocol, using 0.5 µl of SuperScript II reverse transcriptase. Quantitative RT‐PCR reactions were performed on a LightCycler 480 apparatus (Roche Diagnostics, Basel, Switzerland, https://diagnostics.roche.com/global/en/home.html) as described in (Badet et al., 2015), with primers given in Data S1. Relative gene expression corresponds to the ratio between the expression of LAZ5 over expression of the reference gene At2g28390, with runs yielding Cp>32 for At2g28390 excluded.
AUTHORS CONTRIBUTIONS
AB, ON, and SR conceived the original screening and research plans; AB, ON, MM, and SR supervised the experiments; MB, LG, MK, and AL performed most of the experiments; AB and SR designed the experiments and analyzed the data; AB and SR conceived the project and wrote the article with contributions of all the authors; AB and SR agree to serve as the authors responsible for contact and ensure communication.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests to disclose.
Supporting information
Figure S1. Illustration of the Navautron box design and use. (a) Template for PMMA pieces making a Navautron box, with dimensions indicated in mm. (b) A Navautron bottom tray filled with 270 A. thaliana leaves inoculated by an agar plug colonized by S. sclerotiorum, at a start of an experiment. (c) Navautron phenotyping experiment running, with LED flashlights on.
Data S1. Representative PCR analysis for the genotyping of one T‐DNA mutant line. The file includes the list of the oligonucleotide primers used in this work and a map of the LAZ5 locus showing the position of T‐DNA insertions (triangles) and primers (red arrows). Exons are shown are boxes, with the encoded protein domain labelled, introns are shown as plain lines. The expected size of PCR amplicons is indicated in red italic fonts. Picture of a representative agarose gel used to separate PCR products generated for screening three plants (#1–3) of line SALK_068316C. Sizes are indicated in base pairs. Primers used for each PCR reactions are indicated below the corresponding lane.
Data S2. Fasta sequence, multisequence alignment (FASTA format), and phylogenetic tree (NEWICK format) of LAZ5 and its 42 closest homologues (.txt file).
ACKNOWLEDGEMENTS
We are grateful to past and present members of the QIP laboratory for stimulating discussions and suggestions. We thank the two anonymous reviewers for their comments that helped significantly improve our manuscript. This work was supported by a starting grant of the European Research Council (ERC‐StG 336808 project VariWhim) to SR and the French Laboratory of Excellence project TULIP (ANR‐10‐LABX‐41; ANR‐11‐IDEX‐0002‐02).
Contributor Information
Adelin Barbacci, Email: adelin.barbacci@inrae.fr.
Sylvain Raffaele, Email: sylvain.raffaele@inrae.fr.
DATA AVAILABILITY STATEMENT
Arabidopsis Genome Initiative locus identifiers for the genes in this article are as follows: At5g44870 (LAZ5); AT5G17880 (CSA1). The code and tutorials for the software presented in this manuscript are available from https://github.com/A02l01/INFEST
REFERENCES
- Araus, J.L. and Cairns, J.E. (2014) Field high‐throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 19, 52–61. [DOI] [PubMed] [Google Scholar]
- Atwell, S. , Huang, Y.S. , Vilhjálmsson, B.J. et al . (2010) Genome‐wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature, 465, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet, T. , Peyraud, R. and Raffaele, S. (2015) Common protein sequence signatures associate with sclerotinia borealis lifestyle and secretion in fungal pathogens of the sclerotiniaceae . Front. Plant Sci. 6, 776 10.3389/fpls.2015.00776ISSN=1664-462X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet, T. , Peyraud, R. , Mbengue, M. , Navaud, O. , Derbyshire, M. , Oliver, R.P. , Barbacci, A. and Raffaele, S. (2017a) Codon optimization underpins generalist parasitism in fungi. Elife, 6, e22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet, T. , Voisin, D. , Mbengue, M. , Barascud, M. , Sucher, J. , Sadon, P. , Balagué, C. , Roby, D. and Raffaele, S. (2017b) Parallel evolution of the POQR prolyl oligo peptidase gene conferring plant quantitative disease resistance. PLoS Genet. 13, e1007143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski, P. , Jedryczka, M. , Mazurek, W. , Babula‐Skowronska, D. , Siedliska, A. and Kaczmarek, J. (2015) Hyperspectral and thermal imaging of oilseed rape (Brassica napus) response to fungal species of the genus Alternaria R. A. Wilson, ed. PLoS ONE, 10, e0122913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbetti, M.J. , Banga, S.K. , Fu, T.D. , Li, Y.C. , Singh, D. , Liu, S.Y. , Ge, X.T. and Banga, S.S. (2014) Comparative genotype reactions to Sclerotinia sclerotiorum within breeding populations of Brassica napus and B. juncea from India and China. Euphytica, 197, 47–59. [Google Scholar]
- Benjamini, Y. and Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B, 57, 289–300. [Google Scholar]
- Bert, P.‐F. , Jouan, I. , de Labrouhe, T.D. , Serre, F. , Nicolas, P. and Vear, F. (2002) Comparative genetic analysis of quantitative traits in sunflower (Helianthus annuus L.) 1. QTL involved in resistance to Sclerotinia sclerotiorum and Diaporthe helianthi . Theor. Appl. Genet. 105, 985–993. [DOI] [PubMed] [Google Scholar]
- Bolton, M.D. , Thomma, B.P.H.J. and Nelson, B.D. (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 7, 1–16. [DOI] [PubMed] [Google Scholar]
- Bozkurt, T.O. , Richardson, A. , Dagdas, Y.F. , Mongrand, S. , Kamoun, S. and Raffaele, S. (2014) The plant membrane‐associated REMORIN1.3 accumulates in discrete perihaustorial domains and enhances susceptibility to Phytophthora infestans. Plant Physiol. 165, 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. , Neumann, K. , Friedel, S. , Kilian, B. , Chen, M. , Altmann, T. and Klukas, C. (2014) Dissecting the phenotypic components of crop plant growth and drought responses based on high‐throughput image analysis. Plant Cell Online, 26, 4636–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock, P.J.A. , Antao, T. , Chang, J.T. et al . (2009) Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics, 25, 1422–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens, F. , Wuyts, N. , Inzé, D. and Dhondt, S. (2017) Unlocking the potential of plant phenotyping data through integration and data‐driven approaches. Curr. Opin. Syst. Biol. 4, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin, J.A. and Kliebenstein, D.J. (2017) Quantitative Resistance: more than just perception of a pathogen. Plant Cell, 29, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin, J.A. , Copeland, D. , Feusier, J. , Subedy, A. , Eshbaugh, R. , Palmer, C. , Maloof, J. and Kliebenstein, D.J. (2016) The quantitative basis of the arabidopsis innate immune system to endemic pathogens depends on pathogen genetics. PLoS Genet. 12, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czedik‐Eysenberg, A. , Seitner, S. , Güldener, U. , Koemeda, S. , Jez, J. , Colombini, M. and Djamei, A. (2018) The ‘PhenoBox’, a flexible, automated, open‐source plant phenotyping solution. New Phytol. 219, 808–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallal Bashi, Z. , Hegedus, D.D. , Buchwaldt, L. , Rimmer, S.R. and Borhan, M.H. (2010) Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptides (NEPs). Mol. Plant Pathol. 11, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debieu, M. , Huard‐Chauveau, C. , Genissel, A. , Roux, F. , Roby, D. , Huard‐Chauveau, C. , Genissel, A. , Roux, F. and Roby, D. (2015) Quantitative disease resistance to the bacterial pathogen Xanthomonas campestris involves an Arabidopsis immune receptor pair and a gene of unknown function. Mol. Plant Pathol. 17, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire, M.C. and Denton‐Giles, M. (2016) The control of sclerotinia stem rot on oilseed rape (Brassica napus): current practices and future opportunities. Plant Pathol. 65, 859–877. [Google Scholar]
- Derbyshire, M. , Denton‐Giles, M. , Hegedus, D. et al . (2017) The complete genome sequence of the phytopathogenic fungus Sclerotinia sclerotiorum reveals insights into the genome architecture of broad host range pathogens. Genome Biol. Evol. 9, 593–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper, A. , Guignon, V. , Blanc, G. et al . (2008) Phylogeny. fr: robust phylogenetic analysis for the non‐specialist. Nucleic Acids Res. 36, W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Doehlemann, G. , Requena, N. , Schaefer, P. , Brunner, F. , O’Connell, R. and Parker, J.E. (2014) Reprogramming of plant cells by filamentous plant‐colonizing microbes. New Phytol. 204, 803–814. [DOI] [PubMed] [Google Scholar]
- Fahlgren, N. , Gehan, M.A. and Baxter, I. (2015) Lights, camera, action: high‐throughput plant phenotyping is ready for a close‐up. Curr. Opin. Plant Biol. 24, 93–99. [DOI] [PubMed] [Google Scholar]
- Faigón‐Soverna, A. , Harmon, F.G. , Storani, L. et al . (2006) A constitutive shade‐avoidance mutant implicates TIR‐NBS‐LRR proteins in Arabidopsis photomorphogenic development. Plant Cell, 18, 2919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris, J.D. , Zhang, Z. , Lu, H. et al . (2010) A unique wheat disease resistance‐like gene governs effector‐triggered susceptibility to necrotrophic pathogens. Proc. Natl Acad. Sci. USA, 107, 13544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H.H. (1956) The complementary genic systems in flax and flax rust. Adv. Genet. 8, 29–54. [Google Scholar]
- Friesen, T.L. , Faris, J.D. , Solomon, P.S. and Oliver, R.P. (2008) Host specific toxins: effectors of necrotrophic pathogenicity. Cell Microbiol. 10, 1421–1428. [DOI] [PubMed] [Google Scholar]
- Fu, D. , Uauy, C. , Distelfeld, A. , Blechl, A. , Epstein, L. , Chen, X. , Sela, H. , Fahima, T. and Dubcovsky, J. (2009) A kinase‐START gene confers temperature‐dependent resistance to wheat stripe rust. Science, 323, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, W. , Hinsch, M.E. and Staskawicz, B.J. (1999) The Arabidopsis RPS4 bacterial‐resistance gene is a member of the TIR‐NBS‐LRR family of disease‐resistance genes. Plant J. 20, 265–77. [DOI] [PubMed] [Google Scholar]
- Ge, X.T. , Li, Y.P. , Wan, Z.J. et al . (2012) Delineation of Sclerotinia sclerotiorum pathotypes using differential resistance responses on Brassica napus and B. juncea genotypes enables identification of resistance to prevailing pathotypes. Field Crop Res. 127, 248–258. [Google Scholar]
- Goodstein, D.M. , Shu, S. , Howson, R. et al. (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40(D1), D1178–D1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Curr. Biol. 10, 751–757. [DOI] [PubMed] [Google Scholar]
- Horton, M.W. , Hancock, A.M. , Huang, Y.S. et al . (2012) Genome‐wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the RegMap panel. Nat. Genet. 44, 212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard‐Chauveau, C. , Perchepied, L. , Debieu, M. , Rivas, S. , Kroj, T. , Kars, I. , Bergelson, J. , Roux, F. and Roby, D. (2013) An atypical kinase under balancing selection confers broad‐spectrum disease resistance in Arabidopsis. PLoS Genet. 9, e1003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurni, S. , Scheuermann, D. , Krattinger, S.G. et al . (2015) The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall‐associated receptor‐like kinase. Proc. Natl Acad. Sci. 112, 8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakovidis, M. , Teixeira, P.J.P.L. , Exposito‐Alonso, M. et al . (2016) Effector triggered immune response in Arabidopsis thaliana is a quantitative trait. Genetics, 204, 337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbage, M. , Yarden, O. and Dickman, M.B. (2015) Pathogenic attributes of Sclerotinia sclerotiorum: switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 233, 53–60. [DOI] [PubMed] [Google Scholar]
- Karisto, P. , Hund, A. , Yu, K. , Anderegg, J. , Walter, A. , Mascher, F. , McDonald, B.A. and Mikaberidze, A. (2017) Ranking quantitative resistance to Septoria tritici blotch in elite wheat cultivars using automated image analysis. Phytopathology, 108, 568–581. [DOI] [PubMed] [Google Scholar]
- Krattinger, S.G. , Lagudah, E.S. , Spielmeyer, W. , Singh, R.P. , Huerta‐Espino, J. , McFadden, H. , Bossolini, E. , Selter, L.L. and Keller, B. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science, 323, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Kulye, M. , Liu, H. , Zhang, Y. , Zeng, H. , Yang, X. and Qiu, D. (2012) Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence‐related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 35, 2104–2120. [DOI] [PubMed] [Google Scholar]
- Lai, Z. , Wang, F. , Zheng, Z. , Fan, B. and Chen, Z. (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 66, 953–968. [DOI] [PubMed] [Google Scholar]
- Lee, T.G. , Diers, B.W. and Hudson, M.E. (2016) An efficient method for measuring copy number variation applied to improvement of nematode resistance in soybean. Plant J. 88, 143–153. [DOI] [PubMed] [Google Scholar]
- Lenarčič, T. , Albert, I. , Böhm, H. et al . (2017) Eudicot plant‐specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science, 358, 1431–1434. [DOI] [PubMed] [Google Scholar]
- Lenz, H.D. , Haller, E. , Melzer, E. et al . (2011) Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. 66, 818–830. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Kabbage, M. , Liu, W. and Dickman, M.B. (2016) Aspartyl protease mediated cleavage of AtBAG6 is necessary for autophagy and fungal resistance in plants. Plant Cell, 28, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. , Kennedy, R. , Greenshields, D.L. , Peng, G. , Forseille, L. , Selvaraj, G. and Wei, Y. (2007) Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol. Plant. Microb. Interact. 20, 1308–1319. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Zhang, Z. , Faris, J.D. et al . (2012) The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines Harboring Snn1 B. Tyler, ed. PLoS Pathog. 8, e1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente, F. , Alonso‐Blanco, C. , Sánchez‐Rodriguez, C. , Jorda, L. and Molina, A. (2005) ERECTA receptor‐like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina . Plant J. 43, 165–80. [DOI] [PubMed] [Google Scholar]
- Lorang, J.M. , Sweat, T.A. and Wolpert, T.J. (2007) Plant disease susceptibility conferred by a “resistance” gene. Proc. Natl Acad. Sci. USA, 104, 14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorang, J. , Kidarsa, T. , Bradford, C.S. , Gilbert, B. , Curtis, M. , Tzeng, S.C. , Maier, C.S. and Wolpert, T.J. (2012) Tricking the guard: exploiting plant defense for disease susceptibility. Sci. Signal. 338, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin‐Robert, M. , Le Bourse, D. , Markham, J. , Dorey, S. , Clément, C. , Baillieul, F. and Dhondt‐Cordelier, S. (2015) Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiol. 169, 2255–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbengue, M. , Navaud, O. , Peyraud, R. , Barascud, M. , Badet, T. , Vincent, R. , Barbacci, A. and Raffaele, S. (2016) Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum . Front. Plant Sci. 7, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M.J. , Lauter, N. , Steffenson, B. and Wise, R.P. (2011) Quantitative and qualitative stem rust resistance factors in barley are associated with transcriptional suppression of defense regulons. PLoS Genet. 7, e1002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur, L.A.J. , Kenton, P. , Lloyd, A.J. , Ougham, H. and Prats, E. (2008) The hypersensitive response; The centenary is upon us but how much do we know? J. Exp. Bot. 59, 501–520. [DOI] [PubMed] [Google Scholar]
- Mutka, A.M. , Fentress, S.J. , Sher, J.W. , Berry, J.C. , Pretz, C. , Nusinow, D.A. and Bart, R. (2016) (2016) Quantitative, image‐based phenotyping methods provide insight into spatial and temporal dimensions of plant disease. Plant Physiol. 172, 00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafisi, M. , Stranne, M. , Zhang, L. , van Kan, J.A.L. and Sakuragi, Y. (2014) The endo‐arabinanase BcAra1 is a novel host‐specific virulence factor of the necrotic fungal phytopathogen Botrytis cinerea . Mol. Plant‐Microbe Interact. 27, 781–792. [DOI] [PubMed] [Google Scholar]
- Nelson, J.M. , Hauser, D.A. , Hinson, R. and Shaw, A.J. (2018) A novel experimental system using the liverwort Marchantia polymorpha and its fungal endophytes reveals diverse and context‐dependent effects. New Phytol. 218, 1217–1232. [DOI] [PubMed] [Google Scholar]
- Oliver, R.P. and Solomon, P.S. (2010) New developments in pathogenicity and virulence of necrotrophs. Curr. Opin. Plant Biol. 13, 415–419. [PubMed] [Google Scholar]
- Palma, K. , Thorgrimsen, S. , Malinovsky, F.G. , Fiil, B.K. , Nielsen, H.B. , Brodersen, P. , Hofius, D. , Petersen, M. and Mundy, J. (2010) Autoimmunity in arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathog. 6, e1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier, A.J. , Bradley, C.A. , Chilvers, M.I. , Malvick, D.K. , Mueller, D.S. , Wise, K.A. and Esker, P.D. (2012) Biology, yield loss and control of Sclerotinia stem rot of soybean. J. Integr. Pest Manag. 3, B1–B7. [Google Scholar]
- Perchepied, L. , Balagué, C. , Riou, C. , Claudel‐Renard, C. , Rivière, N. , Grezes‐Besset, B. and Roby, D. (2010) Nitric oxide participates in the complex interplay of defense‐related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana . Mol. Plant‐Microbe Interact. 23, 846–860. [DOI] [PubMed] [Google Scholar]
- Poland, J.A. , Balint‐Kurti, P.J. , Wisser, R.J. , Pratt, R.C. and Nelson, R.J. (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 14, 21–29. [DOI] [PubMed] [Google Scholar]
- Poland, J.A. , Bradbury, P.J. , Buckler, E.S. and Nelson, R.J. (2011) Genome‐wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc. Natl Acad. Sci. 108, 6893–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti, L.Lo , Lanver, D. , Schweizer, G. , Tanaka, S. , Liang, L. , Tollot, M. , Zuccaro, A. , Reissmann, S. and Kahmann, R. (2015) Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 66, 513–545. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. Austria: RA Language and Environment for Statistical Computing. [Google Scholar]
- Raffaele, S. , Bayer, E. , Lafarge, D. et al (2009) Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell, 21, 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarammohan, S. , Pradhan, A.K. , Pental, D. and Kaur, J. (2018) Genome‐wide association mapping in Arabidopsis identifies novel genes underlying quantitative disease resistance to Alternaria brassicae . Mol. Plant Pathol. 19, 1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, F. , Voisin, D. , Badet, T. , Balagué, C. , Barlet, X. , Huard‐Chauveau, C. , Roby, D. and Raffaele, S. (2014) Resistance to phytopathogens e tutti quanti: placing plant quantitative disease resistance on the map. Mol. Plant Pathol. 15, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack, S. , Huitema, E. , Cano, L.M. et al . (2009) Ten things to know about oomycete effectors. Mol. Plant Pathol. 10, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, G. , Zhang, Z. , Friesen, T.L. et al . (2016) The hijacking of a receptor kinase‐driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2, e1600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanshu, D.K. , Zhai, X. , Munch, D. et al. (2014) Arabidopsis accelerated cell death 11, ACD11, is a ceramide‐1‐phosphate transfer protein and intermediary regulator of phytoceramide levels. Cell Rep. 6, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, M. , Kotur, T. , Liang, W. et al . (2017) E3 ligase SAUL1 serves as a positive regulator of PAMP‐triggered immunity and its homeostasis is monitored by immune receptor SOC3. New Phytol. 215, 1516–1532. [DOI] [PubMed] [Google Scholar]
- Veronese, P. , Nakagami, H. , Bluhm, B. , Abuqamar, S. , Chen, X. , Salmeron, J. , Dietrich, R.A. , Hirt, H. and Mengiste, T. (2006) The membrane‐anchored BOTRYTIS‐INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell, 18, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugels, T. , Baert, J. and Bockstaele, E.Van (2013) Morphological and pathogenic characterization of genetically diverse Sclerotinia isolates from European red clover crops (Trifolium pratense L.). J. Phytopathol. 161, 254–262. [Google Scholar]
- van der Walt, S. , Schönberger, J.L. , Nunez‐Iglesias, J. , Boulogne, F. , Warner, J.D. , Yager, N. , Gouillart, E. and Yu, T. (2014) scikit‐image: image processing in Python. PeerJ, 2, e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2010) A Layered grammar of graphics. J. Comput. Graph. Stat. 19, 3–28. [Google Scholar]
- Wolpert, T.J. and Lorang, J.M. (2016) Victoria Blight, defense turned upside down. Physiol. Mol. Plant Pathol. 95, 8–13. [Google Scholar]
- Zhang, W. , Fraiture, M. , Kolb, D. et al . (2013) Arabidopsis receptor‐like protein30 and receptor‐like kinase suppressor of BIR1‐1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell, 25, 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Ronen, M. , Gur, Y. et al . (2017) BcXYG1, a secreted Xyloglucanase from Botrytis cinerea, triggers both cell death and plant immune responses. Plant Physiol. 175, 438–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, W. , Chao, Q. , Zhang, N. et al . (2015) A maize wall‐associated kinase confers quantitative resistance to head smut. Nat. Genet. 47, 151–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Illustration of the Navautron box design and use. (a) Template for PMMA pieces making a Navautron box, with dimensions indicated in mm. (b) A Navautron bottom tray filled with 270 A. thaliana leaves inoculated by an agar plug colonized by S. sclerotiorum, at a start of an experiment. (c) Navautron phenotyping experiment running, with LED flashlights on.
Data S1. Representative PCR analysis for the genotyping of one T‐DNA mutant line. The file includes the list of the oligonucleotide primers used in this work and a map of the LAZ5 locus showing the position of T‐DNA insertions (triangles) and primers (red arrows). Exons are shown are boxes, with the encoded protein domain labelled, introns are shown as plain lines. The expected size of PCR amplicons is indicated in red italic fonts. Picture of a representative agarose gel used to separate PCR products generated for screening three plants (#1–3) of line SALK_068316C. Sizes are indicated in base pairs. Primers used for each PCR reactions are indicated below the corresponding lane.
Data S2. Fasta sequence, multisequence alignment (FASTA format), and phylogenetic tree (NEWICK format) of LAZ5 and its 42 closest homologues (.txt file).
Data Availability Statement
Arabidopsis Genome Initiative locus identifiers for the genes in this article are as follows: At5g44870 (LAZ5); AT5G17880 (CSA1). The code and tutorials for the software presented in this manuscript are available from https://github.com/A02l01/INFEST


